Abstract
AIM: To detect multiple H pylori antibodies in serum samples of individuals who carryH pylori by protein array.
METHODS: Recombinant H pylori antigens, urease B subunit (UreB), vacuolating toxin A (VacA) and cytotoxin associated gene A protein (CagA), were prepared and immobilized in matrixes on nitrocellulose membrane by robotics to bind the specific immunoglobulin G (IgG) antibodies in serum. Staphylococcus protein A (SPA) labeled by colloid gold was used to integrate the immuno-complex and gave red color signal. The scanner based on charge-coupled device (CCD) could collect the image signal and convert it into digital signal.
RESULTS: When human IgG was printed on the membrane in increasing concentrations and incubated with immunogold, a linear dose response curve was obtained and the detection limit for IgG was about 0.025 ng. The cutoff values, which were defined as the mean grey level plus 3 times of standard deviation, were 27.183, 28.546 and 27.402, for anti-UreB IgG, anti-CagA IgG and anti-VacA IgG, respectively, as 400 human serum samples with negative H pylori antibodies were detected by the protein array. When 180 serum samples from patients in hospital were employed for detection of IgG against UreB, CagA and VacA, the sensitivity of the protein array was 93.4%, 95.4%, 96.0%, and the specificity was 94.8%, 94.4% and 97.5%, respectively, as compared with the results obtained by ELISA. The assay also showed high reproducibility, uniformity and stability, and the results were available within 30 min.
CONCLUSION: The protein array is a very practical method for rapid detection of multiple antibodies in serum samples. It is especially useful for large scale epidemiological investigation of the infection of H pylori.
Keywords: Helicobacter pylori, Protein array, Antibody, Immunogold
INTRODUCTION
H pylori chronically infect more than half of the world population and are associated with chronic gastritis, peptic ulcer and even gastric cancer[1-7]. Several virulence factors of H pylori, such as urease, vacuolating toxin A(VacA) and cytotoxin associated gene A protein(CagA) have been characterized[8-11]. Urease is produced by all H pylori and functions to hydrolyze urea into CO2 and NH3 which can buffer the acid environment and permit the bacterium survival in stomach. All H pylori strains carry vacA gene and only 50% of them express VacA protein, which assembles into a flower-shaped oligomer, alters intracellular vesicular trafficking and induces vacuole formation in eukaryotic cells. The most important feature that distinguishes H pylori strains is the presence or absence of the cag pathogenicity island (cag PAI). It contains about 30 genes and codes for a type IV secretion machinery system (TFSS), through which CagA is introduced into host cell[12,13]. Phosphorylated CagA in cytoplasm dephosphorylates host cell protein (cortactin), altering cytoskeletal structure and forming a hummimgbird phenotype[14,15]. CagA is implicated in host cell signal transduction system[16,17], and CagA positive H pylori are much more closely related with peptic ulcer and gastric cancer in western country[4,6]. When H pylori infect human being, multiple antibodies are generated against various antigenic compounds and an antibody library may form in serum. Therefore, screening the antibodies against these virulence factors in serum of H pylori infected individual is useful for detection and classification of the pathogen.
Serological assays for diagnosis of H pylori infection are included among the noninvasive methods recommended by the European H pylori study group[18,19]. At present, the common method to detect H pylori antibodies in serum is enzyme-linked immunosorbent assay (ELISA). The procedure is time-consuming especially when multiple antibodies are detected at the same time. Although there are reports regarding the detection of multiple proteins in an antibody-based protein microarray system[20-21], the labor-intensive procedures and the expensive instrumentations have limited its application[22]. Based on the previous work, we developed a low-cost protein array system for rapid detection of multiple H pylori antibodies in serum samples.
MATERIALS AND METHODS
Preparation of antigens
Recombinant urease B subunit (UreB, 40 Ka), N-terminal fragment (amino acid: 1-284,38 Ka) of CagA and middle region fragment (amino acid: 579-907, 30 Ka) of VacA were produced in our institute previously[23]. The purity of these proteins was 95%, 96% and 96%, respectively, as identified by coomassie stained gel and evaluated by dual-wave length flying-spot scanner CS-9000 (Shimadzu). The antigenicity was defined as the dilution titer of the antigen to give 1.0 OD in ELISA when the standard positive serum samples (Xinkang Company, Shenzhen) were used. If the original concentration was 1 mg/mL, the dilution titer of UreB, CagA, and VacA was 1:800,1:1000, and 1:600, respectively.
Preparation of protein array
Nitrocellulose membrane (Pharmacia) with a pore diameter of 0.45 μm was immersed in 0.05mol/L carbonate buffer (pH 9.0) and dried at room temperature. The antigens (0.1%) in 0.01 mol/L phosphate-buffered saline (PBS, pH 7.0) were transferred from the micro-well plate to the membrane by use of the stainless steel solid pin (0.7 mm in diameter) and doted on the nitrocellulose membrane by using computer-controlled high speed robotics, MicroGrid II (BioRobotics). The printing was performed in a cabinet at 4°C,with 60% humidity. Each pin was estimated to transfer 10 nL of the solution. The protein array consisted of 4 matrixes, in three of which the antigens were printed in 9 replicates. Another one was the control matrix printed of rabbit myosin (R.M, Sigma) and human IgG (H. IgG, Sigma) (Figure 1A). The membrane was blocked by 5% bovine serum albumin (BSA, Sigma) at 4°C for 2 h and then washed twice by weakly shaking in the wash buffer for 10 min. After drying at room temperature, the membrane was cut into small pieces (1.2 cm × 1.2 cm) according to the positions properly marked and assembled into the H pylori protein array apparatus. The longitudinal section of the protein array apparatus was illustrated in Figure 2.
Figure 1.
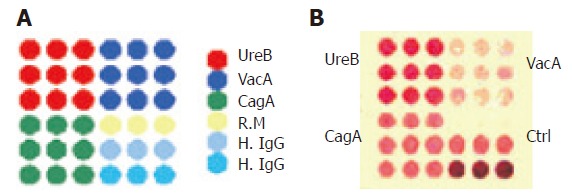
A: Schematic map of protein distribution on the protein array. Four squares were involved and different proteins were printed in different areas: UreB in upper left area; VacA in upper right area; CagA in left bottom area; R.M (10 ng) in the upper row of right bottom area for negative control (Ctrl), H. IgG in the middle row for positive control and at the bottom row for strong positive control; B: positive results of anti-UreB IgG, anti-CagA IgG and anti-VacA IgG detected by the protein array.
Figure 2.
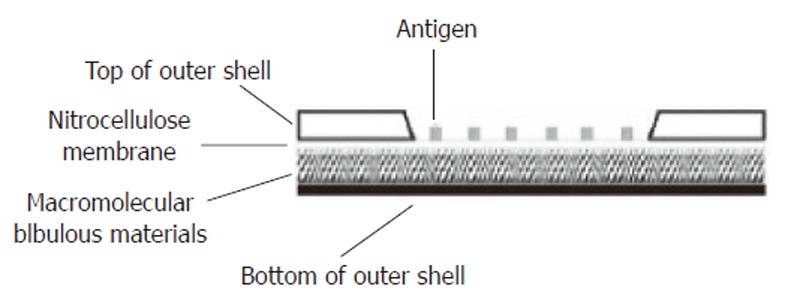
Longitudinal section of the protein array apparatus. The shell of the apparatus was made of plastic materials. A window was opened on the top of the outer shell and the margin of the nitrocellulose membrane printed with antigens was sealed to the inner surface of the window. Under the nitrocellulose membrane was the macromolecular bibulous material.
Preparation of immunogold
Colloid gold was made according to the sodium citrate reduction method[23]. In brief, 200 mL of 0.01% chloroauric acid (analytical grade) was heated to the boiling point and 8 mL of the 1% sodium citrate (analytical grade) was then added under vigorously stirring condition. When the solution became dark red in color, the boiling was lasted further for 5 min. The colloid gold should have the highest light absorbance at 525 nm. The shape and size were observed and the diameter (15 nm) was measured under transmission electron microscope. One hundred microlitres of colloidal gold was taken and the pH value was adjusted to 6.2 by 0.1 mol/L potassium carbonate. Staphylococcus protein A (SPA) was added to the final concentration of 1.5 mg% under stirring condition. Ten minutes later, 10 mL of 5% BSA was added and the colloidal gold was stirred for 5 min, making sure that there was no bubble. The immunogold was centrifuged at 4000 g, 4°C for 5 min. The supernatant was then loaded to the Sephacryl S-400 column (Pharmacia) and eluted with 0.02 mol/L tris-buffered saline (pH 7.4). The main red portion was collected and centrifuged at 12 000 g, 4°C for 10 min. The pellets were then suspended in 100 mL solution (0.01 mol/L PBS, 1% BSA) and stored at -20°C for further use.
Procedures to detect antibodies in serum samples
The procedures are as follows: Firstly, 4 drops of the washing solution were added to the center of the nitrocellulose membrane to immerse it evenly. Secondly, 100 μL of the five times diluted serum was added onto the membrane, and 6 drops of the washing solution were dripped onto it to wash the residual serum 5 min later. Thirdly, 100 μL of the immunogold was added onto the membrane, and again, 6 drops of the washing solution were dripped onto it 5 min later. Finally, the protein array was placed into the scanner, which adopts charge-coupled device (CCD) camera to collect the grey level of the central part (0.5 mm in diameter) of every spot and converts the image signal into digital signal. The results were given according to the positions of the matrixes and the mean grey levels of the spots in the matrixes.
Detection of the antibodies in serum samples
Eight hundred and sixty-five serum samples were collected from blood centers in Xi’an and Shenzhen. H pylori antibodies (anti-UreB IgG, anti-CagA IgG and anti-VacA IgG) negative serum samples were screened by using the ELISA kits (kit to detect IgG against H pylori CagA, Jingying Company, Shanghai; kits to detect IgG against H pylori CagA and VacA, Xingkang Company, Shenzhen). The cutoff values of anti-UreB IgG, anti-CagA IgG and anti-VacA IgG were then determined. One hundred and eighty serum samples, collected from patients with different background in Xi’an Center Hospital, were employed to evaluate the sensitivity and specificity of the protein array in detection of H pylori antibodies. Rapid urease test (RUT) and ELISA were also used to detect the infection of H pylori.
Statistical analysis
All experiments were performed in replicate. χ2 and Student’s t test were used for statistic analysis.
RESULTS
Limit of detection
Human IgG was printed in 6 replicates in increasing concentrations and incubated with 100 μL of immuno-gold on nitrocellulose membrane. A linear dose response curve was obtained (Figure 3) and the detection limit for IgG was about 0.025 ng bound on membrane in this system.
Figure 3.
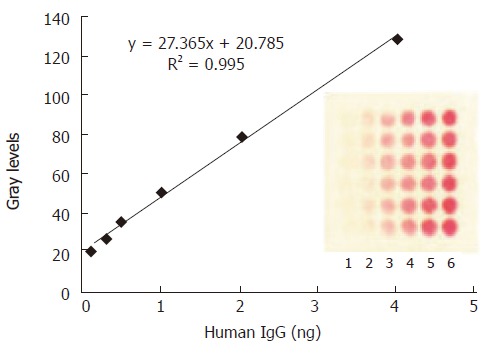
The mean grey level of the spot against various amount of H.IgG immobilized on the protein array. The concentrations of human IgG spotted from lane 1 to lane 6 are as follows: 1.25, 2.5, 5, 10, 20, 40 μg/mL. The optimized amount of SPA binding to colloid gold was 12 μg/mL. The data in the plot were obtained from the image of CCD by averaging the mean grey levels of the 6 replicate spots.
Amount of antigen immobilized
Each antigen with different concentrations ranging from 0.1 to 2.0 ng/nL was printed on the membrane to make the protein array. Five serum samples with H pylori antibody titer of 1:32 in ELISA were used as references to detect the relevant antibody by protein array. The least amount of antigen that defined all the samples as positive was designated as the optimized one. In this way, the amount of antigen chosen to immobilize on membrane was 12, 10, and 16 ng for UreB, CagA and VacA, respectively.
Determination of the cutoff values
The cutoff values, which were defined as the mean grey level plus 3 SD, were 27.183, 28.546 and 27.402, for anti-UreB IgG, anti-CagA IgG and anti-VacA IgG, respectively, as 400 serum samples with negative H pylori antibodies were detected by the protein array (Table 1).
Table 1.
Cutoff values determined in H pylori antibody negative sera
| Items | Number | Mean grey values | SD | Cutoff values |
| Anti-UreB IgG | 400 | 18.060 | 3.041 | 27.183 |
| Anti-CagA IgG | 400 | 17.992 | 3.518 | 28.546 |
| Anti-VacA IgG | 400 | 17.730 | 3.224 | 27.402 |
Uniformity and reproducibility
When a protein array was randomly chosen from the same batch and subjected to detection of the antibodies in a serum sample, the coefficient variation (CV) of the grey values of the 9 spots in every matrix was less than 8%. When 10 protein arrays were randomly chosen to detect the antibodies in a serum sample, the mean grey value of every matrix in every protein array was obtained and the CV of the mean grey values from the ten relevant matrixes was less than 10%. Ten quality control serum samples, 5 positive and 5 negative for the three antibodies detected by ELISA, were used to screen the antibody profiles by protein array. The results were the same when the test was repeated 5 times.
Sensitivity and specificity
For the 180 serum samples collected from clinical patients, the antibodies were screened by both ELISA and protein array. The results showed that, of these samples, 117, 108 and 99 were positive for anti-UreB IgG, anti-CagA IgG and anti-VacA IgG respectively by protein array (positive results of the three antibodies are shown in Figure 1B), and 122, 109 and 101 were positive respectively by ELISA. The sensitivity of protein array in detection of anti-UreB IgG, anti-CagA IgG and anti-VacA IgG was 93.4%, 95.4%, and 96.0%, and the specificity was 94.8%, 94.4% and 97.5% respectively, compared with the results detected by ELISA (Table 2).
Table 2.
Results detected by ELISA and protein array
| Protein array |
ELISA |
|||||
| Anti-UreB IgG | Anti-CagA IgG | Anti-VacA IgG | ||||
| P | N | P | N | P | N | |
| P | 114 | 3 | 104 | 4 | 97 | 2 |
| N | 8 | 55 | 5 | 67 | 4 | 77 |
P : positive; N: negative.
Relation of H pylori antibody profiles to diseases
The positive rates of anti-UreB IgG in groups of gastric cancer (GC, 26/32) and peptic ulcer (PUD, 39/45) were significantly higher than that in group of non-ulcer dyspepsia (NUD, 52/103) (P < 0.005). There was no significant difference in the positive rates of anti-CagA IgG or anti-VacA IgG between different patient groups (Table 3).
Table 3.
Relation of diseases to H pylori antibody (IgG) profiles
| Diseases |
Numbers |
||||
| Total | RUT | Anti-UreB | Anti-CagA | Anti-VacA | |
| GC | 32 | 30 | 26a | 25 | 22 |
| PUD | 45 | 43 | 39c | 37 | 36 |
| NUD | 103 | 58 | 52 | 46 | 41 |
P < 0.005 vs NUD;
P < 0.005 vs NUD.
DISCUSSION
Biochip technique is an advanced tool that enables the binding of multiple molecules in a small area at one time[24,25]. The molecules (protein, nucleic acid or amino acid) can be immobilized on a piece of support substance such as glass, silica, plastic, nitrocellulose and even metal. The fixation of the molecules to the support substance is by means of non-specific absorption, affinity absorption or covalent attachment, depending on the purpose to design the biochip. Methods based on protein array by non-specific absorption are successfully used for serodiagnosis of infectious diseases in some laboratories[26-28]. The protein array in the current investigation was designed in accordance with the recent trends including mini-turization of assays and the simultaneous measurement of a panel of antibodies in a single assay.
Actually, this protein array is an immunoassay based on dot immunogold filtration assay. Nitrocellulose membrane was chosen as the chip and the recombinant antigens were immobilized on it by non-specific adsorption. The membrane can bind more amount of protein compared with the glass surface, as each pore of the membrane can be considered as a column and the antibodies can be accumulated when they pass through the membrane. Moreover, the proteins in the membrane are in three-dimensional structure and the binding of antigen and antibody is highly efficient[29]. Colloid gold was used to label SPA as it was red in color and did not need any substance to produce signal for detection. The reader, which was CCD based video collection system to convert image signal into digital signal, made the results more objective. The entire system offers the simultaneous determination of several antibodies in one test with low cost and faster kinetic. It takes less than 30 min to finish the whole procedures. The stability was quite good and the specificity and sensitivity were close to those of ELISA when H pylori antibody profiles of 180 serum samples were detected. Therefore, it is the most practical means of obtaining accurate and precise results. All these make the method a promising future for application, especially for large scales investigation of H pylori infection.
In western countries, about 50% to 60% H pylori isolates are cagA positive, and the rates are much higher in Asia-Pacific areas[30-32]. In this study, the positive rates of anti-UreB IgG in groups of GC and PUD were significantly higher than that in group of NUD, indicating that H pylori infection was more closely related with GC and PUD, whereas the role of anti-CagA IgG or anti-VacA IgG in prediction of specific diseases was limited.
In the pathogenesis study of the H pylori, we know very well that the living conditions of this bacterium in vivo and in vitro are quite different. Some proteins contributing to virulence of the bacterium may not be expressed under in vitro culture conditions, and their expression may depend on certain in vivo stimuli, such as, cell to cell contact[33]. So antibody profile may be a reliable indication to reflect the host immune response to the bacterium. Since two genomes of this bacterium have already been sequenced, functional study of these genes is becoming the most important target and many studies have been carried out in the disease related gene screening and identification[34-37]. With more H pylori pathogenic genes being known, more proteins may be added onto the protein array. Obviously, to optimize the binding condition of antigen and antibody and to select one that is suitable for a panel of reactions are the key to success. By accumulating more data about the clinical background of the patients and the antibody profiles in sera, we can draw a clear picture of the relationship between the two aspects.
In summary, detection of multiple H pylori antibodies in serum by protein array has the prospect in clinical application, especially in developing countries or small hospitals. Further studies are still going on to increase the density of the protein array.
Footnotes
Supported by National 863 Research Project of China, No. 2002AA232031
S- Editor Wang J L- Editor Zhu LH E- Editor Liu Y
References
- 1.Israel DA, Peek RM. pathogenesis of Helicobacter pylori-induced gastric inflammation. Aliment Pharmacol Ther. 2001;15:1271–1290. doi: 10.1046/j.1365-2036.2001.01052.x. [DOI] [PubMed] [Google Scholar]
- 2.Sanders MK, Peura DA. Helicobacter pylori-Associated Diseases. Curr Gastroenterol Rep. 2002;4:448–454. doi: 10.1007/s11894-002-0019-x. [DOI] [PubMed] [Google Scholar]
- 3.Dawsey SM, Mark SD, Taylor PR, Limburg PJ. Gastric cancer and H pylori. Gut. 2002;51:457–458. doi: 10.1136/gut.51.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaser MJ. Linking Helicobacter pylori to gastric cancer. Nat Med. 2000;6:376–377. doi: 10.1038/74627. [DOI] [PubMed] [Google Scholar]
- 5.Unger Z, Molnár B, Prónai L, Szaleczky E, Zágoni T, Tulassay Z. Mutant p53 expression and apoptotic activity of Helicobacter pylori positive and negative gastritis in correlation with the presence of intestinal metaplasia. Eur J Gastroenterol Hepatol. 2003;15:389–393. doi: 10.1097/00042737-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Deng CS, Peng JZ, Wong BC, Lam SK, Xia HH. Effect of Helicobacter pylori on apoptosis and apoptosis related genes in gastric cancer cells. Mol Pathol. 2003;56:19–24. doi: 10.1136/mp.56.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meining A, Riedl B, Stolte M. Features of gastritis predisposing to gastric adenoma and early gastric cancer. J Clin Pathol. 2002;55:770–773. doi: 10.1136/jcp.55.10.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ching CK, Wong BC, Kwok E, Ong L, Covacci A, Lam SK. Prevalence of CagA-bearing Helicobacter pylori strains detected by the anti-CagA assay in patients with peptic ulcer disease and in controls. Am J Gastroenterol. 1996;91:949–953. [PubMed] [Google Scholar]
- 10.Cover TL, Blaser MJ. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- 11.Prinz C, Hafsi N, Voland P. Helicobacter pylori virulence factors and the host immune response: implications for therapeutic vaccination. Trends Microbiol. 2003;11:134–138. doi: 10.1016/s0966-842x(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 12.Covacci A, Telford JL, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka J, Suzuki T, Mimuro H, Sasakawa C. Structural definition on the surface of Helicobacter pylori type IV secretion apparatus. Cell Microbiol. 2003;5:395–404. doi: 10.1046/j.1462-5822.2003.00286.x. [DOI] [PubMed] [Google Scholar]
- 14.Selbach M, Moese S, Hurwitz R, Hauck CR, Meyer TF, Backert S. The Helicobacter pylori CagA protein induces cortactin dephosphorylation and actin rearrangement by c-Src inactivation. EMBO J. 2003;22:515–528. doi: 10.1093/emboj/cdg050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci U S A. 2000;97:1263–1268. doi: 10.1073/pnas.97.3.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Churin Y, Al-Ghoul L, Kepp O, Meyer TF, Birchmeier W, Naumann M. Helicobacter pylori CagA protein targets the c-Met receptor and enhances the motogenic response. J Cell Biol. 2003;161:249–255. doi: 10.1083/jcb.200208039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mimuro H, Suzuki T, Tanaka J, Asahi M, Haas R, Sasakawa C. Grb2 is a key mediator of helicobacter pylori CagA protein activities. Mol Cell. 2002;10:745–755. doi: 10.1016/s1097-2765(02)00681-0. [DOI] [PubMed] [Google Scholar]
- 18.Cohen H, Rose S, Lewin DN, Retama B, Naritoku W, Johnson C, Bautista L, Crowe H, Pronovost A. Accuracy of four commercially available serologic tests, including two office-based tests and a commercially available 13C urea breath test, for diagnosis of Helicobacter pylori. Helicobacter. 1999;4:49–53. doi: 10.1046/j.1523-5378.1999.09025.x. [DOI] [PubMed] [Google Scholar]
- 19.De Arruda SM, Passaro DJ, Parsonnet J. Variability of serologic testing for H. pylori using U.S. and Peruvian antigens. Gastroenterology. 2001;120:325–326. doi: 10.1053/gast.2001.21387. [DOI] [PubMed] [Google Scholar]
- 20.Wiese R, Belosludtsev Y, Powdrill T, Thompson P, Hogan M. Simultaneous multianalyte ELISA performed on a microarray platform. Clin Chem. 2001;47:1451–1457. [PubMed] [Google Scholar]
- 21.Mendoza LG, McQuary P, Mongan A, Gangadharan R, Brignac S, Eggers M. High-throughput microarray-based enzyme-linked immunosorbent assay (ELISA) Biotechniques. 1999;27:778–780, 782-786, 788. doi: 10.2144/99274rr01. [DOI] [PubMed] [Google Scholar]
- 22.Liang RQ, Tan CY, Ruan KC. Colorimetric detection of protein microarrays based on nanogold probe coupled with silver enhancement. J Immunol Methods. 2004;285:157–163. doi: 10.1016/j.jim.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Han FC, Yan XJ, Su CZ. Expression of the CagA gene of H. pylori and application of its product. World J Gastroenterol. 2000;6:122–124. doi: 10.3748/wjg.v6.i1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Templin MF, Stoll D, Schrenk M, Traub PC, Vöhringer CF, Joos TO. Protein microarray technology. Trends Biotechnol. 2002;20:160–166. doi: 10.1016/s0167-7799(01)01910-2. [DOI] [PubMed] [Google Scholar]
- 25.Stoll D, Templin MF, Schrenk M, Traub PC, Vöhringer CF, Joos TO. Protein microarray technology. Front Biosci. 2002;7:c13–c32. doi: 10.2741/stoll. [DOI] [PubMed] [Google Scholar]
- 26.Bacarese-Hamilton T, Mezzasoma L, Ardizzoni A, Bistoni F, Crisanti A. Serodiagnosis of infectious diseases with antigen microarrays. J Appl Microbiol. 2004;96:10–17. doi: 10.1046/j.1365-2672.2003.02111.x. [DOI] [PubMed] [Google Scholar]
- 27.Bacarese-Hamilton T, Ardizzoni A, Gray J, Crisanti A. Protein arrays for serodiagnosis of disease. Methods Mol Biol. 2004;264:271–283. doi: 10.1385/1-59259-759-9:271. [DOI] [PubMed] [Google Scholar]
- 28.Mezzasoma L, Bacarese-Hamilton T, Di Cristina M, Rossi R, Bistoni F, Crisanti A. Antigen microarrays for serodiagnosis of infectious diseases. Clin Chem. 2002;48:121–130. [PubMed] [Google Scholar]
- 29.Angenendt P. Progress in protein and antibody microarray technology. Drug Discov Today. 2005;10:503–511. doi: 10.1016/S1359-6446(05)03392-1. [DOI] [PubMed] [Google Scholar]
- 30.Yakoob J, Fan XG, Peng XN, Hu GL, Zhang Z. Helicobacter pylori cagA and vacA cytotoxin genes in Changsha, China. Br J Biomed Sci. 2002;59:150–153. doi: 10.1080/09674845.2002.11783652. [DOI] [PubMed] [Google Scholar]
- 31.Hua J, Zheng PY, Yeoh KG, Ho B. The status of the cagA gene does not predict Helicobacter pylori-associated peptic ulcer disease in Singapore. Microbios. 2000;102:113–120. [PubMed] [Google Scholar]
- 32.Maeda S, Ogura K, Yoshida H, Kanai F, Ikenoue T, Kato N, Shiratori Y, Omata M. Major virulence factors, VacA and CagA, are commonly positive in Helicobacter pylori isolates in Japan. Gut. 1998;42:338–343. doi: 10.1136/gut.42.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimmel B, Bosserhoff A, Frank R, Gross R, Goebel W, Beier D. Identification of immunodominant antigens from Helicobacter pylori and evaluation of their reactivities with sera from patients with different gastroduodenal pathologies. Infect Immun. 2000;68:915–920. doi: 10.1128/iai.68.2.915-920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Björkholm BM, Oh JD, Falk PG, Engstrand LG, Gordon JI. Genomics and proteomics converge on Helicobacter pylori. Curr Opin Microbiol. 2001;4:237–245. doi: 10.1016/s1369-5274(00)00197-1. [DOI] [PubMed] [Google Scholar]
- 35.Salama N, Guillemin K, McDaniel TK, Sherlock G, Tompkins L, Falkow S. A whole-genome microarray reveals genetic diversity among Helicobacter pylori strains. Proc Natl Acad Sci U S A. 2000;97:14668–14673. doi: 10.1073/pnas.97.26.14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blaser MJ, Berg DE. Helicobacter pylori genetic diversity and risk of human disease. J Clin Invest. 2001;107:767–773. doi: 10.1172/JCI12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Israel DA, Salama N, Arnold CN, Moss SF, Ando T, Wirth HP, Tham KT, Camorlinga M, Blaser MJ, Falkow S, et al. Helicobacter pylori strain-specific differences in genetic content, identified by microarray, influence host inflammatory responses. J Clin Invest. 2001;107:611–620. doi: 10.1172/JCI11450. [DOI] [PMC free article] [PubMed] [Google Scholar]


