Abstract
It is known that the phospholipids of the brain cells of fish are altered during cold adaptation. In particular, the 1-monounsaturated 2-polyunsaturated phosphatidylethanolamines (PEs) increase 2- to 3-fold upon adaptation to cold. One of the most striking changes is in the 18:1/22:6 species of PE. We determined how this lipid affected the bilayer-to-hexagonal-phase transition temperature of 16:1/16:1 PE. We found that it was more effective in lowering this transition temperature than were other, less unsaturated, PE species. In addition, it was not simply the presence of the 18:1/22:6 acyl chains which caused this effect, since the 18:1/22:6 species of phosphatidylcholine had the opposite effect on this transition temperature. Zwitterionic substances that lower the bilayer-to-hexagonal-phase transition temperature often cause an increase in the activity of protein kinase C (PKC). Indeed, the 18:1/22:6 PE caused an increase in the rate of histone phosphorylation by PKC which was greater than that caused by other, less unsaturated, PEs. The 18:1/22:6 phosphatidylcholine had no effect on this enzyme. The stimulation of the activity of PKC by the 18:1/22:6 PE is a consequence of this lipid's increasing the partitioning of PKC to the membrane.
Full text
PDF
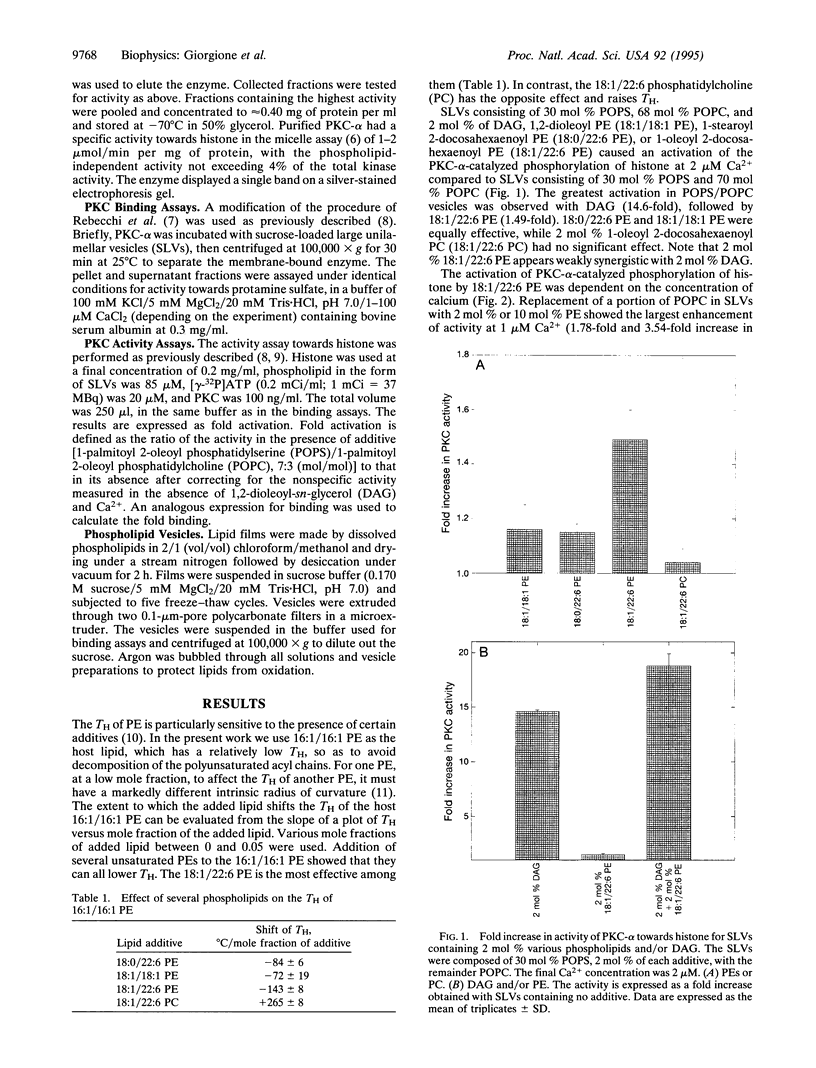
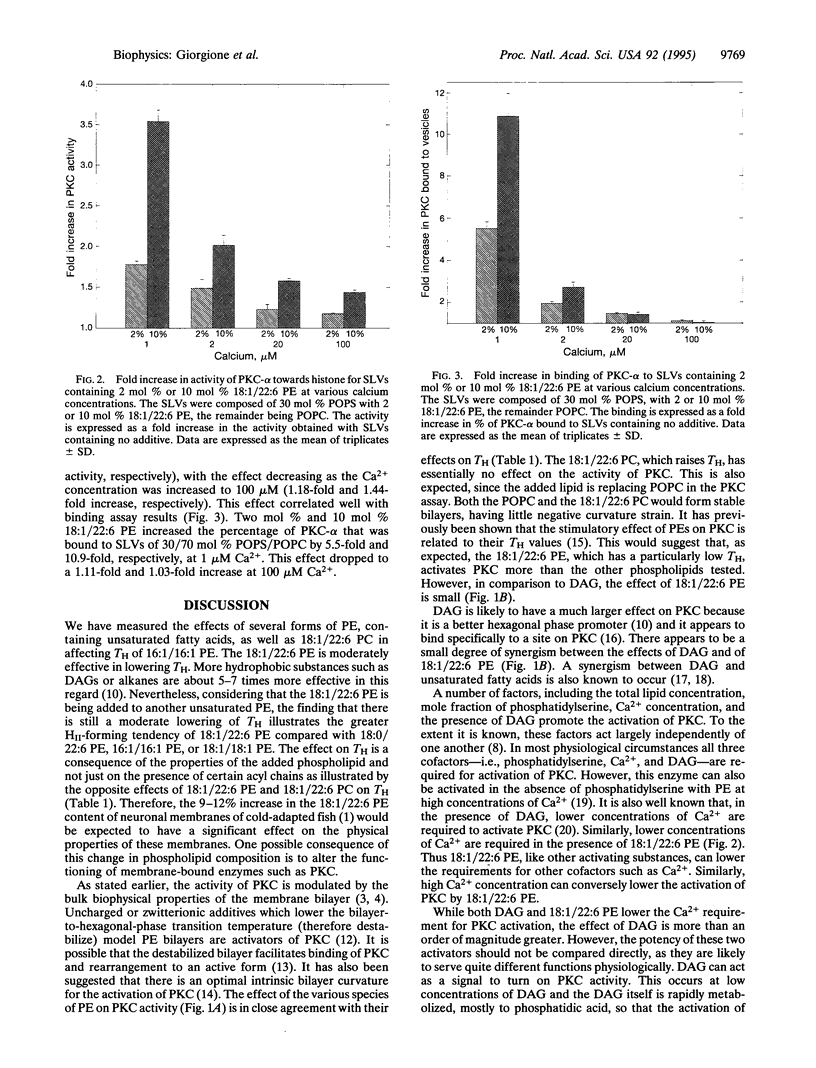

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazzi M. D., Youakim M. A., Nelsestuen G. L. Importance of phosphatidylethanolamine for association of protein kinase C and other cytoplasmic proteins with membranes. Biochemistry. 1992 Feb 4;31(4):1125–1134. doi: 10.1021/bi00119a022. [DOI] [PubMed] [Google Scholar]
- Buda C., Dey I., Balogh N., Horvath L. I., Maderspach K., Juhasz M., Yeo Y. K., Farkas T. Structural order of membranes and composition of phospholipids in fish brain cells during thermal acclimatization. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8234–8238. doi: 10.1073/pnas.91.17.8234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell R. B. Regulation of CTP:phosphocholine cytidylyltransferase by lipids. 2. Surface curvature, acyl chain length, and lipid-phase dependence for activation. Biochemistry. 1991 Jun 18;30(24):5881–5888. doi: 10.1021/bi00238a011. [DOI] [PubMed] [Google Scholar]
- Epand R. M. Diacylglycerols, lysolecithin, or hydrocarbons markedly alter the bilayer to hexagonal phase transition temperature of phosphatidylethanolamines. Biochemistry. 1985 Dec 3;24(25):7092–7095. doi: 10.1021/bi00346a011. [DOI] [PubMed] [Google Scholar]
- Epand R. M., Lester D. S. The role of membrane biophysical properties in the regulation of protein kinase C activity. Trends Pharmacol Sci. 1990 Aug;11(8):317–320. doi: 10.1016/0165-6147(90)90234-y. [DOI] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Bell R. M. Activation of protein kinase C by Triton X-100 mixed micelles containing diacylglycerol and phosphatidylserine. J Biol Chem. 1985 Aug 25;260(18):10039–10043. [PubMed] [Google Scholar]
- Janz S., Gawrisch K., Lester D. S. Translocation and activation of protein kinase C by the plasma cell tumor-promoting alkane pristane. Cancer Res. 1995 Feb 1;55(3):518–524. [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Nishizuka Y. Cooperative roles of various membrane phospholipids in the activation of calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1981 Jul 25;256(14):7146–7149. [PubMed] [Google Scholar]
- Lester D. S., Collin C., Etcheberrigaray R., Alkon D. L. Arachidonic acid and diacylglycerol act synergistically to activate protein kinase C in vitro and in vivo. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1522–1528. doi: 10.1016/0006-291x(91)91745-x. [DOI] [PubMed] [Google Scholar]
- Mallampalli R. K., Salome R. G., Spector A. A. Regulation of CTP:choline-phosphate cytidylyltransferase by polyunsaturated n-3 fatty acids. Am J Physiol. 1994 Dec;267(6 Pt 1):L641–L648. doi: 10.1152/ajplung.1994.267.6.L641. [DOI] [PubMed] [Google Scholar]
- Mosior M., Epand R. M. Mechanism of activation of protein kinase C: roles of diolein and phosphatidylserine. Biochemistry. 1993 Jan 12;32(1):66–75. doi: 10.1021/bi00052a010. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Rando R. R., Young N. The stereospecific activation of protein kinase C. Biochem Biophys Res Commun. 1984 Jul 31;122(2):818–823. doi: 10.1016/s0006-291x(84)80107-2. [DOI] [PubMed] [Google Scholar]
- Rebecchi M., Peterson A., McLaughlin S. Phosphoinositide-specific phospholipase C-delta 1 binds with high affinity to phospholipid vesicles containing phosphatidylinositol 4,5-bisphosphate. Biochemistry. 1992 Dec 29;31(51):12742–12747. doi: 10.1021/bi00166a005. [DOI] [PubMed] [Google Scholar]
- Rilfors L., Hauksson J. B., Lindblom G. Regulation and phase equilibria of membrane lipids from Bacillus megaterium and Acholeplasma laidlawii strain A containing methyl-branched acyl chains. Biochemistry. 1994 May 24;33(20):6110–6120. doi: 10.1021/bi00186a010. [DOI] [PubMed] [Google Scholar]
- Senisterra G., Epand R. M. Role of membrane defects in the regulation of the activity of protein kinase C. Arch Biochem Biophys. 1993 Jan;300(1):378–383. doi: 10.1006/abbi.1993.1051. [DOI] [PubMed] [Google Scholar]
- Shinomura T., Asaoka Y., Oka M., Yoshida K., Nishizuka Y. Synergistic action of diacylglycerol and unsaturated fatty acid for protein kinase C activation: its possible implications. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5149–5153. doi: 10.1073/pnas.88.12.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater S. J., Kelly M. B., Taddeo F. J., Ho C., Rubin E., Stubbs C. D. The modulation of protein kinase C activity by membrane lipid bilayer structure. J Biol Chem. 1994 Feb 18;269(7):4866–4871. [PubMed] [Google Scholar]
- Stabel S., Schaap D., Parker P. J. Expression of protein kinase C isotypes using baculovirus vectors. Methods Enzymol. 1991;200:670–673. doi: 10.1016/0076-6879(91)00179-z. [DOI] [PubMed] [Google Scholar]


