Abstract
A plausible process for non-enzymatic RNA replication would greatly simplify models of the transition from prebiotic chemistry to simple biology. However, all known conditions for the chemical copying of an RNA template result in the synthesis of a complementary strand containing a mixture of 2′–5′ and 3′–5′ linkages, rather than the selective synthesis of only 3′–5′ linkages as found in contemporary RNA. Here we show that such backbone heterogeneity is compatible with RNA folding into defined three-dimensional structures that retain molecular recognition and catalytic properties and, therefore, would not prevent the evolution of functional RNAs such as ribozymes. Moreover, the same backbone heterogeneity lowers the melting temperature of RNA duplexes that would otherwise be too stable for thermal strand separation. By allowing copied strands to dissociate, this heterogeneity may have been one of the essential features that allowed RNA to emerge as the first biopolymer.
The ability of RNA molecules to fold into defined three-dimensional structures with exquisitely specific molecular recognition and catalytic properties is the conceptual basis of the RNA World hypothesis, an early stage in the evolution of life in which RNA served not only as the polymer of inheritance, but as the central functional polymer of biochemistry1–3. This model is most strikingly supported by the observation that all modern proteins are synthesized by the peptidyl transferase ribozyme at the heart of the ribosome4,5. With the RNA World hypothesis so strongly supported by this and other evidence1, the central question in the origin of life field concerns the pathway from the prebiotic chemistry of the early Earth to the emergence of simple forms of cellular life containing RNA genomes coding for RNA enzymes. While there has been considerable recent progress towards the elucidation of potentially prebiotic pathways for ribonucleotide synthesis6–8 and the assembly of activated nucleotides into oligonucleotides9,10, the non-enzymatic replication of RNA oligonucleotides remains problematic. A series of seemingly intractable difficulties continues to make a robust system for the chemical replication of RNA elusive11–14. These problems include the slow rate, poor fidelity and low regioselectivity of non-enzymatic RNA template copying; in addition, activated substrates typically hydrolyze on the same timescale as polymerization. The importance of the latter point is highlighted by the efficient copying that can be attained by flowing fresh substrates over immobilized templates13. Other issues are the lack of primers in prebiotic scenarios, the difficulty of strand-separation that is a consequence of the high thermal stability of long RNA duplexes, and the fast re-annealing of separated strands. Finally, RNA template copying requires high Mg2+ concentrations, while prebiotically likely protocell membranes composed of fatty acids are sensitive to low (10−3 M) concentrations of Mg2+, making these two key components of primitive cells appear to be mutually incompatible. As a result of these unresolved issues, considerable effort has been devoted to the synthesis and characterization of alternative polymers that might have served as progenitors of RNA15–22. Nevertheless, the recent advances in understanding potential prebiotic routes to RNA have motivated us to revisit the unsolved problems of RNA replication.
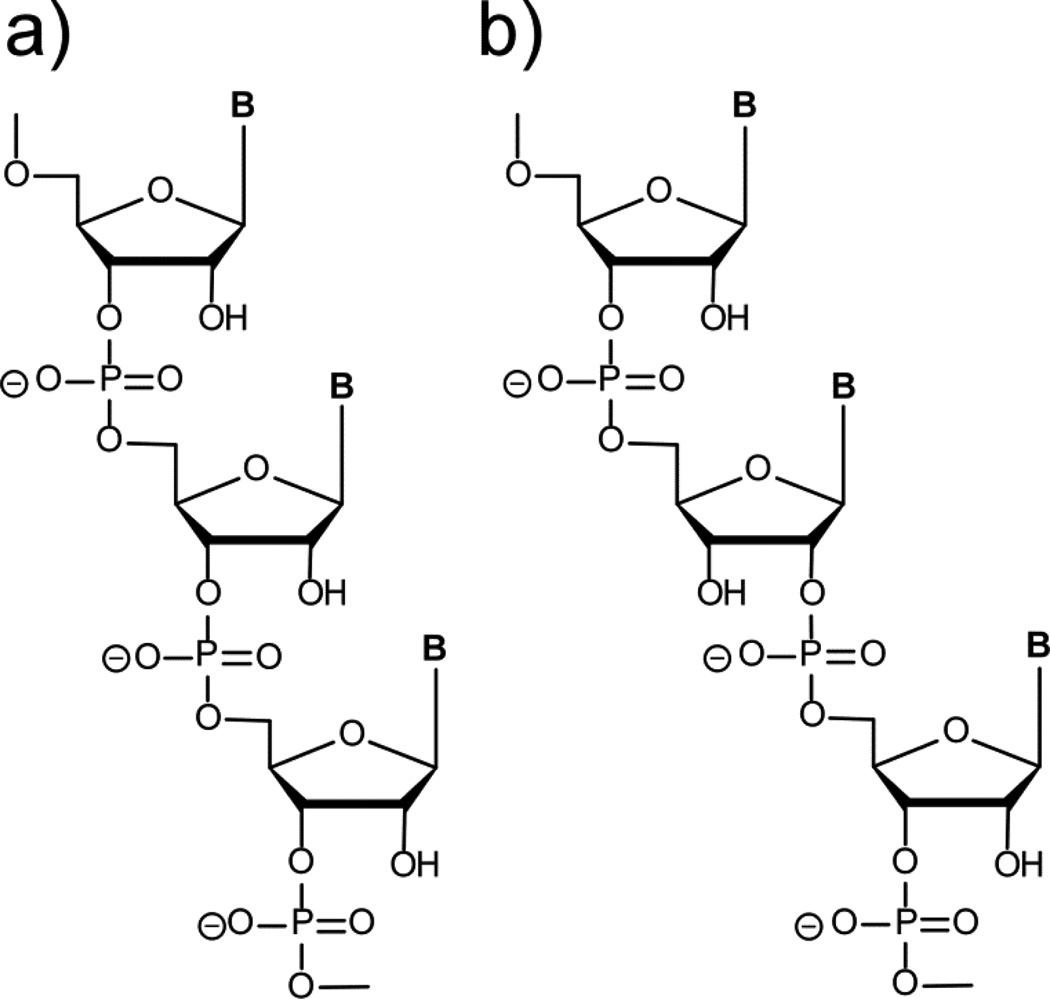
Here we address two of the above eight problems that have so far prevented a demonstration of complete cycles of chemically driven RNA replication. First, we consider the issue of regiospecificity. In contrast to contemporary life, in which the transcription of DNA templates generates exclusively 3′–5′ phosphodiester linked RNA, prebiotically plausible syntheses of RNA in nonenzymatic template copying reactions generate a mixture of 2′–5′ and 3′–5′ linkages as a result of the proximity and similar nucleophilicity of the 2′ and 3′ hydroxyls of the ribose moiety (Figure 1). The regioselectivity of non-enzymatic template-directed RNA synthesis can be improved by the use of oligomer (as opposed to monomer) substrates, or by varying the metal ion or leaving group used for monomer activation, or by exploiting the differential stability of the 2′–5′ and 3′–5′ linkages in an RNA double helix, but no chemical means of template-directed RNA synthesis reported to date matches the complete regiospecificity achieved in enzymatic RNA synthesis23–28. As a result, RNA strands generated by non-enzymatic template-directed copying inevitably contain a random mixture of non-heritable 2′–5′ and 3′–5′ backbone linkages. The presence of 2′–5′ linkages is not a barrier to subsequent rounds of replication, since Switzer and colleagues have demonstrated that 2′–5′ linked RNA, as well as mixed 2′–5′/3′–5′ RNA, can template primer extension reactions, albeit more slowly than 3′–5′ RNA29. Based on the reasonable assumption that precise three-dimensional interactions are required for macromolecular folding and catalysis, the presence of randomly distributed 2′–5′ linkages would be expected to interfere with the reproducible formation of the folded three-dimensional structures required for ribozyme catalytic activity, thus preventing the emergence of primitive life forms based on RNA catalysis. However, recent work from this laboratory has shown that aptamers with highly specific molecular recognition properties can be evolved from libraries of polynucleotides containing randomly interspersed ribo- and deoxyribo-nucleotides30. Since non-heritable ribo/deoxyribo backbone heterogeneity did not prevent the evolution of functional RNAs, we decided to investigate whether this tolerance for structural heterogeneity might not extend to 2′–5′- vs. 3′–5′ backbone heterogeneity. Here, we show that RNAs containing remarkably high proportions of randomly distributed 2′–5′ linkages retain the ability to fold, recognize ligands, and catalyze reactions.
Figure 1.
RNA is enzymatically synthesized with complete regiospecificity to generate a uniform 3′–5′-phosphodiester backbone. Prebiotically plausible non-enzymatic syntheses of RNA result in backbone heterogeneity, and a randomly distributed mixture of 3′–5′ and 2′–5′ linkages. a) Homogeneous 3′–5′ linked RNA. b) Heterogeneous RNA containing one 2′–5′-phosphodiester linkage. B=nucleobase.
A second major problem with the chemical replication of RNA is that RNA duplexes >20–30 nucleotides in length are difficult or impossible to thermally denature under template copying conditions (most significantly, the presence of high concentrations of Mg2+ ions). Since strand separation is required to allow for repeated cycles of template copying, the accurate copying of a template strand would generate a dead-end RNA duplex product. However, it has been known for some time that 2′–5′ linkages destabilize RNA duplexes with respect to thermal denaturation31,32. Here, we show that 2′–5′ linkages can destabilize long RNA duplexes to the point that thermal strand separation could occur under reasonable geophysical conditions. We therefore propose that 2′–5′ linkages in RNA, far from being problematic, were in fact an essential feature that allowed RNA to emerge as the first genetic polymer of life.
Results
We examined the effects of 2′–5′ substitution on two functional RNAs, an aptamer and a ribozyme, both of which had evolved as all 3′–5′ linked RNA (Figures 2a, 3a). The aptamer we chose to study was generated by in vitro directed evolution, with selection for binding to flavin mononucleotide (FMN)33. The FMN aptamer is formed of two stems flanking a central binding pocket, in which its ligand intercalates between a base pair and base triple, with one residue making a hydrogen bonding contact with the ligand34. The catalytic RNA we studied is the hammerhead ribozyme, which cleaves a substrate strand at a defined site. A minimized trans-acting consensus sequence has been described, which we have employed here35.
Figure 2.
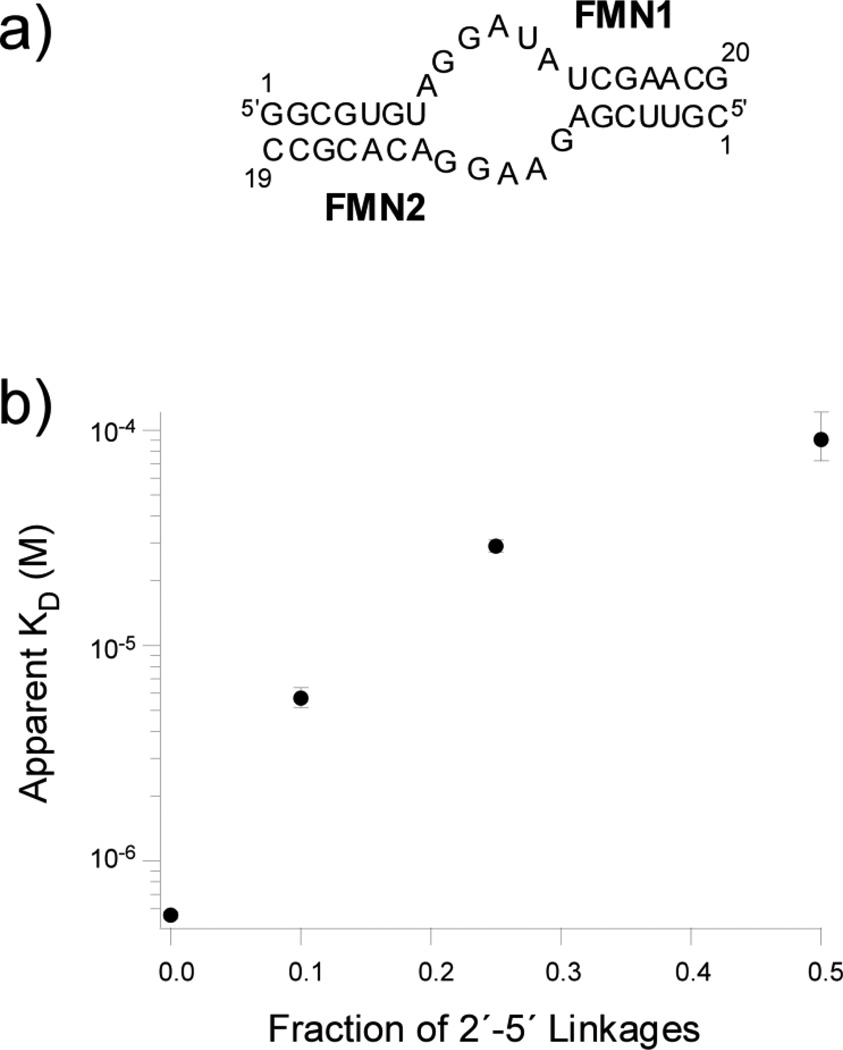
A pool of FMN aptamers containing moderate levels of 2′–5′ linkages is observed to retain FMN binding activity. a) Schematic representation of FMN aptamer. b) Apparent KD of FMN aptamer vs. fraction of 2′–5′ linkages present in the aptamer pool. Apparent KD represents the average value for a heterogeneous population of RNAs, in which 2′–5′ linkages are randomly positioned. Error bars are ±1 S.D. from curve fit.
Figure 3.
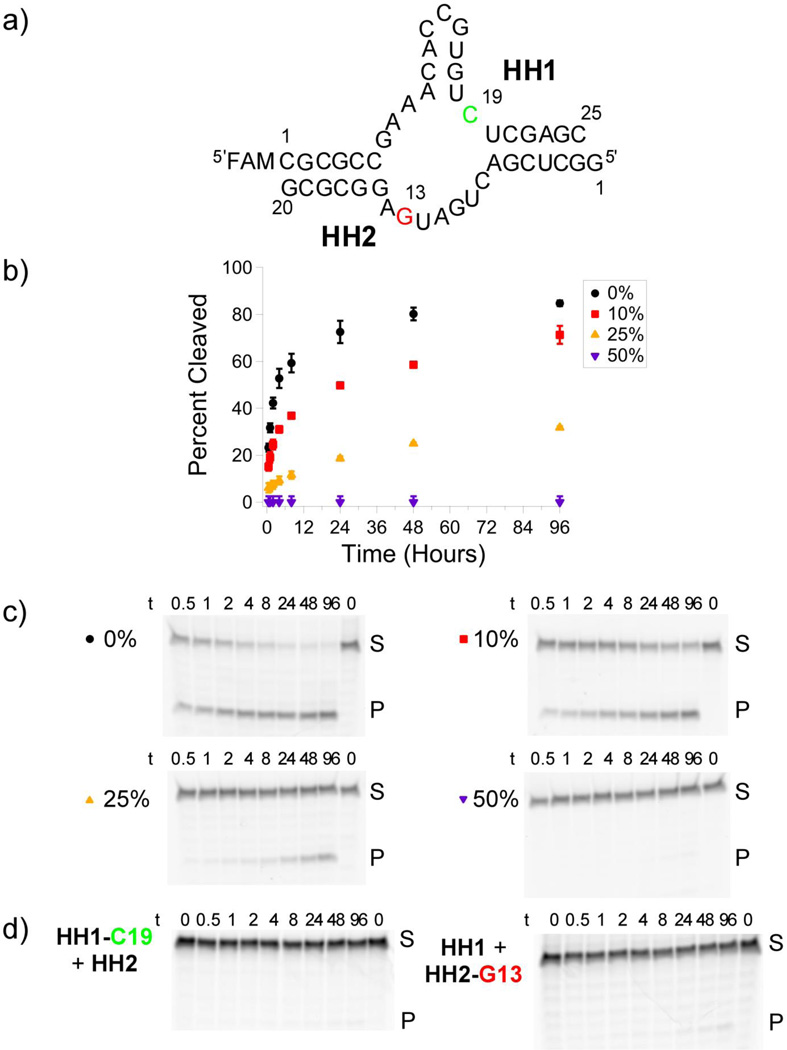
A pool of hammerhead ribozymes containing up to 25% randomly distributed 2′–5′ linkages is observed to retain catalytic activity. a) Schematic representation of the hammerhead ribozyme construct used in these studies. The ribozyme is assembled from the two oligonucleotides HH1 and HH2. HH1 contains the self-cleavage site, C19, colored green, and is labeled with a 5′-fluorophore. b) Fraction of hammerhead cleavage vs. time for fully 3′–5′-linked and 2′–5′-doped hammerhead ribozymes. Error bars are ±S.E.M. from triplicate measurement, except the 50% 2′–5′-doped strand, which is given as 0% +2.5%/–0% yield, representing cleavage yields below the limit of detection. c) Representative electropherograms from b). d) Electropherograms of point-2′–5′-substituted hammerhead ribozyme reactions. 2′–5′ linkages were incorporated at C19, the site of cleavage in HH1, colored green in Figure 3a, or at G13 in HH2, colored red in Figure 3a.
We examined the effect of 2′–5′ linkages on FMN aptamer functionality by chemically synthesizing a series of variants of the aptamer. We first prepared two fully 3′–5′ linked RNAs by chemical synthesis, corresponding to the two halves of the FMN aptamer. Upon mixing in buffer, the two RNA oligonucleotides spontaneously assembled to generate the aptamer structure. We measured the affinity of the two-piece aptamer for FMN by titrating increasing amounts of this RNA into a solution containing 1 µM FMN, and monitoring the resulting quenching of FMN fluorescence. The two-piece FMN aptamer exhibited a KD for FMN of 560 nM, in line with previously reported values for this complex (Figure 2b).33 We then synthesized versions of both oligonucleotides with 10, 25 and 50% of randomly distributed 2′–5′ linkages, which were introduced by mixing 3′–TBDMS (tert-butyldimethylsilyl)-protected 2′-phosphoramidites at the specified ratios with the standard 2′-TBDMS protected 3′-phosphoramidites. In every case, the replacement of one strand with a variant of the same sequence but with a higher extent of 2′–5′ substitution resulted in a functional aptamer with a moderately higher KD (Figure 2b). When the aptamer was assembled with both strands containing 10% 2′–5′ linkages (a plausibly prebiotic scenario, given the previously-achieved regioselectivity described by Orgel and coworkers24,25,36), the apparent (population average) KD for FMN was 5.7µM, or an average penalty of only +1.3 kcal/mol. Even the aptamer in which both strands contained an equal number of randomly distributed 2′–5′ and 3′–5′ linkages exhibited clear FMN binding, with an apparent KD of 90µM, corresponding to a penalty of +2.8 kcal/mol to the energy of ligand binding. Thus, this aptamer, which was evolved to function as an entirely 3′–5′ linked RNA, exhibited a remarkable tolerance to 2′–5′/3′–5′ backbone heterogeneity.
We then examined the effect of increasing levels of 2′–5′ linkages on the self-cleavage activity of the hammerhead ribozyme. We began by chemically synthesizing two RNA oligonucleotides that would self-assemble into the hammerhead structure, such that one of the oligonucleotides would self-cleave at a specific site. By synthesizing the substrate oligonucleotide with a 5′-fluorescent dye, we were able to monitor the self-cleavage activity of the two component ribozyme by PAGE (polyacrylamide gel electrophoresis) separation of intact from cleaved oligonucleotide, with fluorescence detection of substrate and product oligonucleotides. The two-stranded hammerhead ribozyme with all 3′–5′ linkages self-cleaved to 50% of maximal extent in 30 minutes, with a maximal extent of cleavage of 81% at 96 hours (Figure 3b). As before, we chemically synthesized both strands with 10, 25, and 50% of randomly distributed 2′–5′ linkages. Oligonucleotides containing increasing levels of 2′–5′ linkages resulted in slower self-cleavage and a lower maximal extent of self-cleavage (Figures 3b, 3c). With both strands containing 10, 25, or 50% 2′–5′ linkages, the maximal extent of selfcleavage fell to 69, 34, and <2% respectively, at 96 hours (Figure 3b). At the prebiotically plausible 10% level of 2′–5′ linkages, the ribozyme retained 85% of maximal self-cleavage activity, while the rate of self-cleavage slowed from a t1/2 of ca. 0.5 hours to ca. 2 hours. The decreasing maximal extent of self-cleavage with increasing fraction of 2′–5′ linkages suggests that some specific linkages (or combinations of linkages) are critical for catalytic activity, while the slower rate of self-cleavage suggests that other linkages slow, but do not prevent, self-cleavage. Consistent with this, we have found that two specific point substitutions of 2′–5′ linkages within the catalytic core of the enzyme result in substantial or total diminution of hammerhead activity (Figure 3d).
In our pools of RNA with randomly distributed 2′–5′ linkages, a binomial distribution of linkages is likely present, in proportion to the level of 2′–5′ monomer employed in the synthesis. Not surprisingly, such a distribution predicts that, even at the 10% doping level, only ca. 2% of our 39-nucleotide FMN aptamer and only ca. 1% of the 45-nucleotide hammerhead would have no 2′–5′ linkages. Essentially no strands at higher doping levels would have no 2′–5′ linkages (see Supplementary Figure S1a online). Clearly, the observed aptamer and ribozyme activities cannot be due to complexes that lack, or even contain very few, 2′–5′ linkages. However, given the ability of 2′–5′-linked RNAs to form a duplex, it is more likely that 2′–5′ substitution is only fatal to ribozyme or aptamer function at a few critical sites. Even at highly conserved positions, a 2′–5′ linkage may have little effect if the RNA conformation is largely determined by hydrogen-bonding and stacking interactions between the bases, while the backbone plays a relatively passive role. Given a critical core of 5 linkages in a hypothetical ribozyme or aptamer, 59% of RNAs at the 10% doping level would have no 2′–5′ linkages, while 24% at the 25% doping level and 3% at the 50% doping level would have no 2′–5′ linkages (see Supplementary Figure S1b online). These numbers are in qualitative agreement with the fraction of maximum cleavage obtained with hammerhead ribozymes at these doping levels (Figure 3b). In a library of functional RNAs with a low-to-moderate level of randomly distributed 2′–5′ linkages, only a fraction would be fatally inactivated by 2′–5′ linkages at critical residues, consistent with our observations and with previous work on ribozymes containing point substitutions of these linkages37,38.
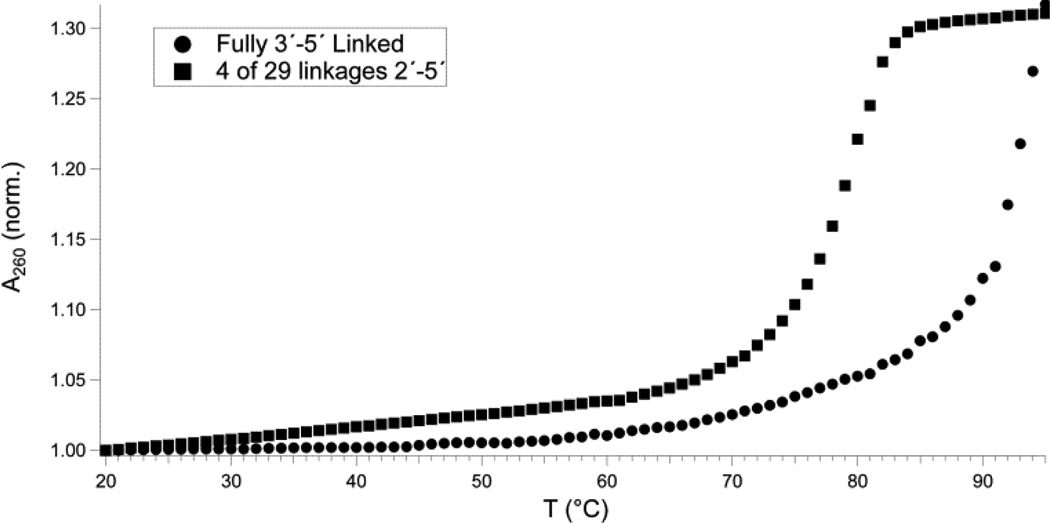
It has been known for over a decade that 2′–5′ linkages destabilize RNA duplexes, based on melting studies of short duplexes containing 2′–5′ linkages at defined sites31. To test the idea that low to moderate (10–25%) levels of 2′–5′ linkages would lower the melting temperature of an RNA duplex of sufficient length to exhibit function enough to allow for thermal strand separation, and, thus, repeated cycles of non-enzymatic replication, we examined the melting behavior of longer duplexes containing 2′–5′ linkages in the presence of 50 mM Mg2+, to represent typical RNA template copying conditions. A 30-nt duplex containing four 2′–5′ linkages in each strand exhibited a Tm of 79°C, while the same sequence in fully 3′–5′ linked strands was not fully denatured, even at 95°C (Figure 4). The duplex containing 2′–5′ linkages exhibits diminished hyperchromism on heating, consistent with the possibility that diminished stacking interactions contribute to the decreased thermal stability of duplexes containing 2′–5′ linkages.
Figure 4.
An RNA duplex containing 14% 2′–5′ linkages is observed to denature at a temperature at least 15 °C lower than the corresponding homogeneous 3′–5′ linked duplex, facilitating thermal strand separation under geochemically plausible conditions. 30-mer RNA duplexes were formed by annealing oligonucleotides Duplex30-1 and Duplex30-2. Strands were fully 3′–5′-linked (filled circles) or contained four 2′–5′ linkages on each strand (filled squares).
Discussion
The most important implication of our finding that functional RNAs can tolerate 2′–5′ vs. 3′–5′ backbone heterogeneity is that this heterogeneity is not necessarily an undesirable consequence of non-enzymatic template-copying chemistry. Therefore, it is not required that backbone heterogeneity must be eliminated through improvements to the chemistry. Indeed, when combined with the long-standing observation that 2′–5′ linkages lower the melting temperature of RNA duplexes, our observations suggest that the generation of 2′–5′ vs. 3′–5′ heterogeneity may have facilitated an essential aspect of primordial RNA replication, since it would allow repeated cycles of replication to occur as a result of thermal strand separation. Although a number of environmental factors such as high pH, low salt or the presence of denaturants, such as urea or formamide, would lower the Tm of long RNA duplexes, these conditions might also interfere with either non-enzymatic RNA replication or with the function of folded RNAs such as ribozymes. Our results suggest that the presence of 10–25% 2′–5′ linkages is a very effective means of lowering the melting temperature of RNA duplexes that is also compatible with both non-enzymatic RNA copying23 and with ribozyme activity. A more speculative extension of this idea derives from the randomness of the incorporation of 2′–5′ linkages during non-enzymatic RNA copying, which implies that different copies of the same sequence would contain 2′–5′ linkages in different locations. Some copies might, by chance, contain most or all 2′–5′ linkages in positions with minimal effects on, e.g., ribozyme function. As well-folded structures, however, these copies would be poor templates for subsequent rounds of replication. Other copies would have some 2′–5′ linkages at positions that interfered with the formation of well folded structures, and these copies would be poor ribozymes but much better replication templates. Thus, the generation of 2′–5′ vs. 3′–5′ linkage heterogeneity could result in the formation of a heterogeneous set of copies of every sequence in a protocell, with some being better for function (phenotype) and others being better for replication (genotype). In effect, this structural heterogeneity would result in a primitive and incomplete separation of genotype from phenotype within one polymer system. This new way of looking at chemical RNA replication raises a number of questions and priorities for experimental directions, which we discuss below.
The aptamer and the ribozyme we examined evolved to function as all 3′–5′ linked RNA; therefore, it is unsurprising they exhibited diminished aptamer affinity and ribozyme activity with increasing incorporation of 2′–5′ linkages in the RNA backbone. A better mechanistic understanding of how functional RNAs that contain 2′–5′ linkages retain function, or conversely, lose function when 2′–5′ linkages are present at critical locations, will ultimately emerge from the comparison of high-resolution structures of native RNAs and RNAs containing 2′–5′ linkages. It is possible that structures that are even more tolerant of 2′–5′ vs. 3′–5′ heterogeneity would be found if evolutionary optimization were to occur within a mixed-backbone system. At present, no enzymes are known that provide a means to transcribe, reverse-transcribe, or replicate mixed-sequence 2′–5′ RNA backbone linkages, although reverse transcriptase can read through isolated 2′–5′ linkages39. Recent advances in directed evolution may provide the means to generate enzymes that would enable the in vitro directed evolution experiments with mixed backbone RNA populations that would be required to test the above hypothesis40.
If primordial RNA was indeed characterized by considerable 2′–5′ vs. 3′–5′ heterogeneity, what selective forces might have contributed to the subsequent evolution of the machinery enabling replication of fully 3′–5′-linked RNA? We have previously proposed that the energy required for strand separation could come from transient exposure to high temperatures, possibly as a result of entrainment in the streams of hot water emanating from hydrothermal vents in surface ponds in geothermally active areas41,42. Such an environmental scenario implies a strong selective advantage for the evolution of strand-separating ribozymes that would enable continued replication under more isothermal conditions, thus enabling the colonization of new environmental niches. Once strand-separating ribozymes (e.g., helicases or strand-displacing polymerases) had evolved, the requirement for 2′–5′ linkages to lower the duplex melting point could be eliminated. The gradual nature of the effects of changing the regioisomer composition of the RNA backbone on functional RNAs (as measured by ribozyme activity or aptamer KD) and duplex RNAs (as measured by Tm) suggests that the transition from mixed to homogeneous backbone RNAs could also have occurred gradually, in similar fashion to the gradually increasing incorporation of phospholipids into (proto)cell membranes43. Thus, as helicases or polymerases with increasing strand displacement activity emerged, the advantage of being able to optimize function in structurally homogeneous RNA transcripts could be realized. Additional advantages of an all 3′–5′-linked backbone might include greater stability to hydrolysis in the duplex state22, and faster and/or more accurate replication23,37.
The results reported in this paper, along with other recent advances13 are encouraging with respect to the search for a non-enzymatic RNA replication process. Future effort must focus on removing the remaining barriers to chemical RNA replication, including the slow rate and poor fidelity of non-enzymatic template-copying, the difficulty of maintaining substrates in a chemically activated state, replication without added primers, the fast reannealing of separated strands, and the incompatibility of RNA copying chemistry with protocell membranes. If these problems can be solved, it should be possible to assemble and study self-replicating protocells, in a laboratory recapitulation of the emergence of cellular life on the early Earth.
Methods
RNAs were synthesized in-house on an ABI Expedite 8909, by IDT, Oligos, Etc., or the Keck Biotechnology Resource Laboratory, using 3′-or 2′-TBDMS phosphoramidites obtained from ChemGenes. Libraries of RNA strands containing randomly interspersed 2′–5′ linkages were synthesized using mixtures of 3′-or 2′-TBDMS phosphoramidites at the specified stoichiometry. We synthesized a GpG dinucleotide using a 1:1 mixture of these phosphoramidites, and used NMR analysis of the resulting mixture of products to show that the 3′-and the 2′-TBDMS phosphoramidites exhibited essentially identical coupling efficiencies (see Supplementary Figure S2 online). Oligonucleotides were purified either chromatographically (cartridge or HPLC) or by PAGE. Oligonucleotides synthesized in-house, by Oligos, Etc., or Keck were analyzed by high-resolution LC/MS; in all cases, the deconvoluted monoisotopic mass was found to be within <20 ppm of the nominal value (see Supplementary Table S1 online).
Functional RNAs were comprised of the following sequences: FMN aptamer: FMN1, r(GGC GUG UAG GAU AUC GAA CG) and FMN2, r(CGU UCG AGA AGG ACA CGC C); Hammerhead ribozyme: HH1, r(FAM-CG CGC CGA AAC ACC GUG UCU CGA GC) and HH2, r(GGC UCG ACU GAU GAG GCG CG). The hammerhead strands with point substitutions, HH1-C19, r(FAM-CG CGC CGA AAC ACC GUG UC*U CGA GC) and HH2-G13, r(GGC UCG ACU GAU G*AG GCG CG), contained a single 2′–5′ linkage, denoted by an asterisk. The 30mer duplex used in thermal denaturation was comprised of the following sequences: Duplex30-1, r(GAA G*UC AGU AC*G CCA UUC* GAG AUC CUC* AUG) and Duplex30-2, r(CAU G*AG GAU C*UC GAA UGG* CGU ACU GAC* UUC). These sequences were synthesized in two versions: an all 3′–5′ linked form, and a version in which the four linkages denoted with asterisks were 2′–5′.
Binding constant studies for the FMN aptamer-FMN complex were performed on a Molecular Devices SpectraMax EM in a 384-well plate by preparing 16 samples in 100mM sodium HEPES, pH 7.5, 10mM MgCl2, 100mM NaCl, 1mM EDTA, with a fixed 1µM concentration of ligand, and 0–50µM FMN1–FMN2 aptamer complex. The sample was held at 8°C, excited from the bottom at 444 nm, and the emission was read at 538 nm. The fluorescence emission was background corrected by subtracting the average value of neighboring empty wells, which were essentially the same as non-neighboring cells, indicating neighboring wells did not interact significantly (see Supplementary Figure S3 online). The resulting fluorescence 14 values were fit to a nonlinear single binding site model in Igor Pro (Wavemetrics), by which they were well-described44.
For hammerhead ribozyme reactions, ribozymes comprised of oligonucleotides HH1 and HH2 containing the specified fraction of 2′–5′ linkages were incubated at room temperature in 50mM sodium HEPES, pH 7.5, 10mM MgCl2, 100µM EDTA. Reactions were quenched by addition to 8M urea, 250mM EDTA, separated by denaturing PAGE, imaged by fluorescence scanning (Typhoon) and quantitated by integration (ImageQuant).
Thermal denaturation was performed using a 1 mm quartz Starna cell in an Agilent Cary 60 UV spectrophotometer with a Quantum Northwest LC 600 temperature controller. The temperature reported is the block temperature. The second heating trace after an initial annealing heat/cool cycle is shown (Figure 4). 30-mer melts were 5µM in Duplex30-1 and Duplex30-2 in 100mM sodium HEPES, pH 7.5, 50mM MgCl2, 100mM NaCl, 200mM sodium citrate, 1mM sodium EDTA. For both melts, at each 1°C temperature step, the system was allowed to equilibrate at the setpoint until it was within 0.25°C of the setpoint for 30 seconds, at which point a spectrum was collected. This resulted in a temperature increase of ca. 0.45°C/min.
Supplementary Material
Acknowledgements
J.W.S. is an Investigator of the Howard Hughes Medical Institute. A.E.E. is supported by an appointment to the NASA Postdoctoral Program, administered by Oak Ridge Associated Universities through a contract with NASA. M.W.P. was an HHMI Research Associate. This work was supported in part through NSF Grant CHE-0809413 to J.W.S. We thank J. Craig Blain for oligonucleotide mass spectrometry, Katarzyna Adamala for advice with RNA melting experiments, and Noam Prywes for helpful discussions and assistance with figure preparation.
Footnotes
Author Contributions
All authors contributed to the design of the experiments and to writing the paper. Experiments were conducted by A.E.E. and M.W.P.
Competing Financial Interests Statement
The authors declare no competing financial interests.
References
- 1.Gilbert W. The RNA world. Nature. 1986;319:618. [Google Scholar]
- 2.Joyce GF. RNA evolution and the origins of life. Nature. 1989;338:217–224. doi: 10.1038/338217a0. [DOI] [PubMed] [Google Scholar]
- 3.Joyce GF, Orgel LE. In: The RNA World, Second Edition: The Nature of Modern RNA Suggests a Prebiotic RNA World. Gesteland RF, Atkins JF, editors. Cold Spring Harbor Laboratory Press; 1999. pp. 49–77. [Google Scholar]
- 4.Ban N, Nissen P, Hansen J, Moore PB, Steitz TA. The complete atomic structure of the large ribosomal subunit at 2.4 Ångstrom resolution. Science. 2000;289:905–920. doi: 10.1126/science.289.5481.905. [DOI] [PubMed] [Google Scholar]
- 5.Nissen P, Hansen J, Ban N, Moore PB, Steitz TA. The structural basis of ribosome activity in peptide bond synthesis. Science. 2000;289:920–930. doi: 10.1126/science.289.5481.920. [DOI] [PubMed] [Google Scholar]
- 6.Powner MW, Gerland B, Sutherland JD. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459:239–242. doi: 10.1038/nature08013. [DOI] [PubMed] [Google Scholar]
- 7.Powner MW, Sutherland JD, Szostak JW. Chemoselective multicomponent one-pot assembly of purine precursors in water. J. Am. Chem. Soc. 2010;132:16677–16688. doi: 10.1021/ja108197s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powner MW, Sutherland JD. Phosphate-mediated interconversion of ribo- and arabino-configured prebiotic nucleotide intermediates. Angew. Chem. Int. Ed. Engl. 2010;49:4641–4643. doi: 10.1002/anie.201001662. [DOI] [PubMed] [Google Scholar]
- 9.Ferris JP, Jr, A RH, Liu R, Orgel LE. Synthesis of long prebiotic oligomers on mineral surfaces. Nature. 1996;381:59–61. doi: 10.1038/381059a0. [DOI] [PubMed] [Google Scholar]
- 10.Kanavarioti A, Monnard PA, Deamer DW. Eutectic phases in ice facilitate nonenzymatic nucleic acid synthesis. Astrobiology. 2001;1:271–281. doi: 10.1089/15311070152757465. [DOI] [PubMed] [Google Scholar]
- 11.Hill AJ, Orgel L, Wu T. The limits of template-directed synthesis with nucleoside-5'-phosphoro(2-methyl)imidazolides. Origins Life Evol. Biospheres. 1993;23:285–290. doi: 10.1007/BF01582078. [DOI] [PubMed] [Google Scholar]
- 12.Orgel LE. Prebiotic chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Biol. 2004;39:99–123. doi: 10.1080/10409230490460765. [DOI] [PubMed] [Google Scholar]
- 13.Deck C, Jauker M, Richert C. Efficient enzyme-free copying of all four nucleobases templated by immobilized RNA. Nat. Chem. 2011;3:603–608. doi: 10.1038/nchem.1086. [DOI] [PubMed] [Google Scholar]
- 14.Szostak JW. The eightfold path to non-enzymatic RNA replication. J. Syst. Chem. 2012;3:2. [Google Scholar]
- 15.Joyce GF, Schwartz AW, Miller SL, Orgel LE. The case for an ancestral genetic system involving simple analogues of the nucleotides. Proc. Natl. Acad. Sci. USA. 1987;84:4398–4402. doi: 10.1073/pnas.84.13.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egholm M, et al. PNA hybridizes to complementary oligonucleotides obeying the Watson–Crick hydrogen-bonding rules. Nature. 1993;365:566–568. doi: 10.1038/365566a0. [DOI] [PubMed] [Google Scholar]
- 17.Richert C, Roughton AL, Benner SA. Nonionic analogs of RNA with dimethylene sulfone bridges. J. Am. Chem. Soc. 1996;118:4518–4531. [Google Scholar]
- 18.Eschenmoser A. Chemical etiology of nucleic acid structure. Science. 1999;284:2118–2124. doi: 10.1126/science.284.5423.2118. [DOI] [PubMed] [Google Scholar]
- 19.Chaput JC, Switzer C. Nonenzymatic oligomerization on templates containing phosphodiester-linked acyclic glycerol nucleic acid analogues. J. Mol. Evol. 2000;51:464–470. doi: 10.1007/s002390010109. [DOI] [PubMed] [Google Scholar]
- 20.Bean HD, Anet FAL, Gould IR, Hud NV. Glyoxylate as a backbone linkage for a prebiotic ancestor of RNA. Origins Life Evol. Biospheres. 2006;36:39–63. doi: 10.1007/s11084-005-2082-4. [DOI] [PubMed] [Google Scholar]
- 21.Chen JJ, Cai X, Szostak JW. N2′→P3′ phosphoramidate glycerol nucleic acid as a potential alternative genetic system. J. Am. Chem. Soc. 2009;131:2119–2121. doi: 10.1021/ja809069b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelhart AE, Hud NV. Primitive genetic polymers. Cold Spring Harbor Perspect. Biol. 2010;2 doi: 10.1101/cshperspect.a002196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Usher DA, McHale AH. Hydrolytic stability of helical RNA - Selective advantage for natural 3′,5′-bond. Proc. Natl. Acad. Sci. USA. 1976;73:1149–1153. doi: 10.1073/pnas.73.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bridson PK, Orgel LE. Catalysis of accurate poly(C)-directed synthesis of 3′–5′-linked oligoguanylates by Zn2+ J. Mol. Biol. 1980;144:567–577. doi: 10.1016/0022-2836(80)90337-x. [DOI] [PubMed] [Google Scholar]
- 25.Inoue T, Orgel LE. Oligomerization of (guanosine-5′-phosphor)-2-methylimidazole on poly(C) - an RNA-polymerase model. J. Mol. Biol. 1982;162:201–217. doi: 10.1016/0022-2836(82)90169-3. [DOI] [PubMed] [Google Scholar]
- 26.Rohatgi R, Bartel DP, Szostak JW. Nonenzymatic, template-directed ligation of oligoribonucleotides is highly regioselective for the formation of 3′–5′ phosphodiester bonds. J. Am. Chem. Soc. 1996;118:3340–3344. doi: 10.1021/ja9537134. [DOI] [PubMed] [Google Scholar]
- 27.Ekland EH, Bartel DP. RNA-catalysed RNA polymerization using nucleoside triphosphates. Nature. 1996;382:373–376. doi: 10.1038/382373a0. [DOI] [PubMed] [Google Scholar]
- 28.Johnston WK, Unrau PJ, Lawrence MS, Glasner ME, Bartel DP. RNA-catalyzed RNA polymerization: accurate and general RNA-templated primer extension. Science. 2001;292:1319–1325. doi: 10.1126/science.1060786. [DOI] [PubMed] [Google Scholar]
- 29.Prakash TP, Roberts C, Switzer C. Activity of 2′,5′-linked RNA in the template-directed oligomerization of mononucleotides. Angew. Chem., Int. Ed. Engl. 1997;36:1522–1523. [Google Scholar]
- 30.Trevino SG, Zhang N, Elenko MP, Luptak A, Szostak JW. Evolution of functional nucleic acids in the presence of nonheritable backbone heterogeneity. Proc. Natl. Acad. Sci. USA. 2011;108:13492–13497. doi: 10.1073/pnas.1107113108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giannaris PA, Damha MJ. Oligoribonucleotides containing 2′,5′-phosphodiester linkages exhibit binding selectivity for 3′,5′-RNA over 3′,5′-ssDNA. Nucleic Acids Res. 1993;21:4742–4749. doi: 10.1093/nar/21.20.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wasner M, et al. Physicochemical and biochemical properties of 2′,5′-linked RNA and 2′,5′-RNA: 3′,5′-RNA"hybrid" duplexes. Biochemistry. 1998;37:7478–7486. doi: 10.1021/bi980160b. [DOI] [PubMed] [Google Scholar]
- 33.Burgstaller P, Famulok M. Isolation of RNA aptamers for biological cofactors by invitro selection. Angew. Chem., Int. Ed. Engl. 1994;33:1084–1087. [Google Scholar]
- 34.Fan P, Suri AK, Fiala R, Live D, Patel DJ. Molecular recognition in the FMN-RNA aptamer complex. J. Mol. Biol. 1996;258:480–500. doi: 10.1006/jmbi.1996.0263. [DOI] [PubMed] [Google Scholar]
- 35.Birikh KR, Heaton PA, Eckstein F. The structure, function and application of the hammerhead ribozyme. Eur. J. Biochem. 1997;245:1–16. doi: 10.1111/j.1432-1033.1997.t01-3-00001.x. [DOI] [PubMed] [Google Scholar]
- 36.Hiraki S, Orgel LE. Oligonucleotide synthesis catalyzed by the Zn2+ ion. J. Am. Chem. Soc. 1975;97:3532–3533. doi: 10.1021/ja00845a050. [DOI] [PubMed] [Google Scholar]
- 37.Burlina F, Fourrey J, Lefort V, Favre A. Cleavage activity of a hammerhead ribozyme domain containing 2′,5′-phosphodiester linkages. Tetrahedron Lett. 1999;40:4559–4562. [Google Scholar]
- 38.Shih I, Been MD. Ribozyme cleavage of a 2′,5′-phosphodiester linkage: Mechanism and a restricted divalent metal-ion requirement. RNA. 1999;5:1140–1148. doi: 10.1017/s1355838299990763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorsch JR, Bartel DP, Szostak JW. Reverse transcriptase reads through a 2'–5' linkage and a 2'-thiophosphate in a template. Nucleic Acids Res. 1995;23:2811–2814. doi: 10.1093/nar/23.15.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pinheiro VB, et al. Synthetic genetic polymers capable of heredity and evolution. Science. 2012;336:341–344. doi: 10.1126/science.1217622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricardo A, Szostak JW. Life on earth. Scientific American. 2009;301:54–61. doi: 10.1038/scientificamerican0909-54. [DOI] [PubMed] [Google Scholar]
- 42.Budin I, Szostak JW. Expanding roles for diverse physical phenomena during the origin of life. Annu. Rev. Biophys. 2010;39:245–263. doi: 10.1146/annurev.biophys.050708.133753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Budin I, Szostak JW. Physical effects underlying the transition from primitive to modern cell membranes. Proc. Natl. Acad. Sci. USA. 2011;108:5249–5254. doi: 10.1073/pnas.1100498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qu XG, Chaires JB. Analysis of drug-DNA binding data. Method. Enzymol. 2000;321:353–369. doi: 10.1016/s0076-6879(00)21202-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.