Abstract
Phospholipase A2(PLA2) are esterases that cleave glycerophospholipids to release fatty acids and lysophospholipids. Several studies demonstrate that PLA2 regulate growth and signaling in several cell types. However, few of these studies have focused on Ca2+-independent phospholipase A2(iPLA2 or Group VI PLA2). This class of PLA2 was originally suggested to mediate phospholipid remodeling in several cell types including macrophages. As such, it was labeled as a housekeeping protein and thought not to play as significant of roles in cell growth as its older counterparts cytosolic PLA2(cPLA2 or Group IV PLA2) and secretory PLA2(sPLA2 or Groups I–III, V and IX–XIV PLA2). However, several recent studies demonstrate that iPLA2 mediate cell growth, and do so by participating in signal transduction pathways that include epidermal growth factor receptors (EGFR), mitogen activated protein kinases (MAPK), mdm2, and even the tumor suppressor protein p53 and the cell cycle regulator p21. The exact mechanism by which iPLA2 mediates these pathways are not known, but likely involve the generation of lipid signals such as arachidonic acid, lysophosphatidic acid (LPA) and lysophosphocholines (LPC). This review discusses the role of iPLA2 in cell growth with special emphasis placed on their role in cell signaling. The putative lipid signals involved are also discussed.
Keywords: Phospholipase A2, Ca2+-independent phospholipase A2, Cell growth, Cell signaling, Arachidonic acid, Lysophosphatidic acid
1. Introduction
Phospholipase A2(PLA2) are esterases that cleave glycerophospholipids at the sn-2 ester bond, releasing a fatty acid and a lysophospholipid [1]. They are broadly defined into five different types; secretory PLA2(sPLA2), cytosolic PLA2(cPLA2), Ca2+-independent PLA2(iPLA2), platelet-activating factor hydrolases (PAF-AH) and lysosomal PLA2[2-4]. sPLA2 are the oldest class of PLA2, typically the smallest (13-18 kDa) and use histidine to hydrolyze the sn-2 ester bond [3]. In contrast, cPLA2 and iPLA2 are larger in size, typically 66–90 kDa, and use a serine to catalyze the hydrolysis of the sn-2 ester bond [3]. PAF-AH hydrolyze acetyl groups from PAF using a catalytic serine, range in size from 26 to 45 kDa, and include lipoprotein-associated PLA2[4]. Lysosomal PLA2 are among the newest class of PLA2 to be identified. Only one group member is known to exist, which is 45 kDa in size and utilizes a catalytic serine to hydrolyze the sn-2 bond at the C-1 position of ceramide [4]. As its name implies, lysosomal PLA2 localizes to lysosomes [4].
A newer classification system organizes PLA2 based on their genetic sequence into 15 groups (designated by a roman numeral), encompassing over 20 individual members (designated by Arabic letters) [4]. Several recent reviews discuss PLA2 as a group, including those dealing with their classification, function and roles in the brain, heart and cancer cells [1,5,6]. Some even discuss roles of PLA2, in general, on cell growth and death [2,7]. This review focuses on the specific role of iPLA2 in cell growth and signaling.
Several studies, and some reviews, have previously suggested roles for iPLA2 in cell regulation, growth and death [6-8]. Some have even suggested roles for iPLA2 in cell signaling [7,8]. However, several recent studies, all published within the last 2 years, suggest novel roles for iPLA2 in signal transduction pathways that include EGFR, MAPK, the tumor suppressor protein p53 and the cell cycle regulator p21 [9-13]. These new studies suggest that iPLA2 play more significant roles in cell growth than previous thought, and suggest that iPLA2 may be therapeutic targets for alteration of a number of pathologies including cancer, heart disease, renal failure and atherosclerosis.
2. Classification and function of iPLA2
2.1. Types of iPLA2
iPLA2 are referred to as Group VI PLA2 using the newer classification system and there are at least six known members [4,14] (Table 1). Group VIA-1 and A-2 are splice variants of the same gene and are expressed in the cytosol [15]. Group VIA-1 is commonly referred to as iPLA2 while A-2 is commonly called iPLA2β. Group VIB is a distinct gene product localized to the endoplasmic, peroxisomal, and mitochondrial membranes [16,17] and is usually called iPLA2γ.
Table 1.
Classification and inhibitors of Group VI PLA2a
| Group | Common name | Molecular weight (kDa) | Inhibitors | Suggested IC50b (μM) |
|---|---|---|---|---|
| VIA-1 | iPLA2 | 84–85 | AAOCF3c | 0.3/10 |
| MAFP | 0.5/10 | |||
| S-BEL | ||||
| BEL | 0.5/5 | |||
| VIA-2 | iPLA2β | 88–90 | AAOCF3 | 0.3/10 |
| MAFP | 0.5/10 | |||
| BEL | 0.5/5 | |||
| S-BEL | 1/2.5 | |||
| VIB | iPLA2γ | 88–91 | AAOCF3 | 0.3/10 |
| MAFP | 0.5/10 | |||
| BEL | 0.5/2.5 | |||
| R-BEL | 2/2.5 | |||
| VIC | iPLA2δ | 146 | BEL Organophosphorus esters | 0.9/5 0.03-0.2/1-10 |
| VID | iPLA2ε | 53 | BEL | 1–5 |
| VIE | iPLA2ζ | 57 | BEL | 1–5 |
| VIF | iPLA2η | 28 | BEL | 1–5 |
Adapted from Cummings et al. (2007), and Schaloske and Dennis (2007).
x/y: x = reported IC50/y = dose commonly used in cells and tissues, when known.
AAOCF3 = arachidonyl triflouromethylketone; BEL = bromoenol lactone ((E)-6-(1-bromoethyl) tetrahydro-3-(1-naphthalenyl)-2H-pyran-2-one)); R-BEL = R enantiomer of BEL, S-BEL—S enantiomer of BEL, MAFP = methyl arachidonyl flourophosphonate.
The most recent iPLA2 to be discovered are Group VIC-F and are referred to as iPLA2δ, iPLA2ε, iPLA2ζ and iPLA2η, respectively [4,18,19]. For the purposes of this review we will now refer to iPLA2 using their properly designated group numbering system [4] (Table 1). Differences in the function of each of these enzymes have recently been reviewed [4], and will not be re-covered here. However, a discussion of general functions of Group VI PLA2 is warranted.
2.2. General functions of Group VI PLA2
A majority of what we know about the function of Group VI PLA2 comes from studies in macrophages and mostly applies to Groups VIA and VIB PLA2. These studies suggest that these enzymes are involved in the maintenance of membrane phospholipids under normal conditions [2,20,21]. This includes the generation of lysophospholipid acceptors that are re-incorporated with fatty acids. This is one way by which Group VI PLA2 regulate fatty acyl turnover. Group VI PLA2 also produce arachidonic acid and lysophospholipids in several cell lines [14,21-24], and recent studies suggest roles for Group VI PLA2 in the generation of LPA [25,26].
The ability of Group VI PLA2 to generate lysophospholipid acceptors and regulate fatty acyl-turnover resulted in them being labeled as “housekeeping” proteins. As a result it was originally thought that they did not play as prominent of roles in the generation of arachidonic acid as Group IV PLA2 or Groups I-III, V and IX-XIV PLA2(sPLA2) [6,27-32]. The reality of this situation is that Group VI PLA2 do play “housekeeping” roles by facilitating phospholipid remodeling and maintaining phosphatidylcholines [21,33-36]. Group VI PLA2 complete these tasks while releasing relatively low level of arachidonic acid or LPA, which limits inflammation and cell death.
It should be noted that Group VI PLA2 roles as housekeeping enzymes might not apply to all cells. In fact, studies in INS-1 cells suggest that Group VI PLA2 may play more significant roles in cell signaling than phospholipid remodeling [33,35,37]. Thus, the functions of Group VI PLA2 appear cell dependent.
2.3. Methods for inhibition of Group VI PLA2
Much of what we know about the function of Group VI PLA2 in cell physiology is derived from studies using pharmacological and molecular inhibitors. The pharmacological inhibitor of choice for Group VI PLA2 is bromoenol lactone (BEL or (E)-6-(1-bromoethyl) tetrahydro-3-(1-naphthalenyl)-2H-pyran-2-one)). Other inhibitors include methyl arachidonyl flourophosphonate (MAFP) and arachidonyl triflouromethylketone (AAOCF3). These inhibitors are available from a variety of sources including Cayman Chemical Company (Ann Arbor, MI) and Sigma (St. Louis, MO). The selectivity and specificity of these agents for Group VI PLA2 have been recently, and thoroughly, reviewed [1,4], and will not be covered in detail here. However, it is important to note that MAFP and AAOCF3 both inhibit Group IV PLA2(cPLA2) at concentrations around 5–10 μM [1,38] (Table 1).
BEL selectively inhibits Group VI PLA2 in several cell models at concentrations around 2–5 μM [1,39]. BEL also inhibits phosphatidate phosphohydrolase (PAP-1) [36]. However, PAP-1 is inhibited by propranolol, which does not inhibit Group VI PLA2. Using a combination of MAFP, AAOCF3, BEL and propranolol will allow one to distinguish between events mediated by Group IV and VI PLA2, and eliminate roles for PAP-1 [4].
Recently R- and S-enantiomers of BEL were developed and validated to selectively inhibit Group VIB and VIA PLA2, respectively [40,41]. The mechanisms involved in their selectivity are under study; however, they have similar IC50’s in cells as racemic BEL (50:50 mixture of R- and S-BEL, 2–5 μM). R- and S-BEL have proven extremely useful as tools to distinguish between Group VIA and VIB [40-42]. However, they still may inhibit PAP-1 and should be used with propranolol when studying roles of Group VI PLA2 in cell growth and signaling.
Several molecular techniques exist for inhibition and activation of Group VI PLA2. Multiple studies have used siRNA or anti-sense oligonucleotides to inhibit Group VIA PLA2[11,13,21,42,43], as well as Group VIB PLA2[42,44]. In addition, studies using mice null for Group VIA [45-47] have been published. To date no studies are available describing the use of knockout animals for Group VIB-F PLA2.
In addition to knockdown studies, several studies have over expressed Group VIA and VIB PLA2 and studied cellular function [34,48-50]. Relatively, few of these studies address the affect of Group VI PLA2 over expression on cell growth or signaling. It should be noted that both Group VIA and VIB are constitutively active in several cell types. Thus, increasing Group VI PLA2 activity may not have as great of consequence on cell growth or signaling as inhibiting its activity.
3. Group VI PLA2 and cell growth and signaling
3.1. Evidence that Group VI PLA2 mediate cell growth
Recent studies using both pharmacological and molecular inhibition strategies demonstrate that Group VI PLA2 mediate growth, cell cycle regulation and signaling in a variety of models, and do so independently of Group IV PLA2(Table 2, [9-12,42,51]). Models used in these studies include HEK293 cells, fibroblasts, macrophages, insulinomas, ovarian, colon, pancreatic and prostate cancer cells [9-11,13,23,51,52]. A majority of these studies focused on Group VIA PLA2, although a significant amount of work focused on Group VIB PLA2 as well [40,50,53].
Table 2.
Cells in which Group VI PLA2 mediates cell growth
| Cell origin | Cell type | Reference |
|---|---|---|
| Cancerous | ||
| Prostate | PC-3 and LNCaP | [9] |
| Ovarian | OVCAR-3, SKOV-3, DOV-13 | [10] |
| Insulinoma | INS-1 | [37,51] |
| Kidney | Caki-1 | [42] |
| Colon | HCT | [11] |
| Fibroblast | 3T6 | [52] |
| Macrophages | Mouse peritoneal macrophages | [23] |
| Muscle | Rat vascular smooth muscle | [43] |
| Endothelial | HUVEC and HDMEC | [55] |
Some of the first evidence that Group VI PLA2-mediated cell growth was derived from studies in macrophages. For example, macrophage spreading was more effectively inhibited by BEL than MAFP, suggesting that Group VI PLA2 have larger roles in macrophage growth than Group IV PLA2[23]. This hypothesis was supported by subsequent studies in ovarian cancer cells [10,26], fibroblasts [54], smooth muscle cells [43], endothelial cells [55], HEK293 and Caki-1 cells [42], insulinoma cells [13,51] and colon cancer HCT116 cells [12].
Evidence that Group VI PLA2 mediate cell growth is also derived from studies demonstrating that these enzymes regulate cell death. For example, inhibition of Group VI PLA2 altered Fas-induced apoptosis in U937 cells [20,24], and chemotherapeutic-induced apoptosis in primary cultures of renal proximal tubules [53], HEK293 cells and Caki-1 cells [56]. Further, Group VI PLA2 inhibition accelerated oxidant-induced necrosis in renal cells [17,57].
Group VIA PLA2 is believed to be the primary isoform involved in Fas-induced apoptosis in U937 cells. In contrast, Group VIB PLA2 appears to be more involved in mediation of renal cell apoptosis. This hypothesis is supported by studies demonstrating that expression of shRNA (plasmid forms or siRNA) against Group VIB PLA2 increased lipid peroxidation and induced apoptosis in primary cultures of renal cells [44]. Further, renal cells appear to express higher level of Group VIB PLA2, compared to Group VIA PLA2[17,53,56].
3.2. Mechanisms by which Group VI PLA2 regulate cell growth
While the above studies support the hypothesis that Group VI PLA2 mediate cell growth, they do not identify the mechanisms or signaling pathways involved. It’s possible, at least in some studies, that alterations in cell death alone can explain decreases in cell growth. However, several reports demonstrate that inhibition of Group VI PLA2 decreases cell growth in the absence of cell death [9,10,12,42].
Inhibition of Group VI PLA2 decreased prostate cancer cell growth in correlation with G1 and G2/M arrests, decreased EGFR activation and increased p53 expression [9]. Decreases in cell growth were not accompanied by increases in cell death as determined by annexin V and PI staining and nuclear morphology. This study confirmed early reports in HEK293 cells and human colon carcinomas, which demonstrated that inhibition of Group VIA PLA2 increased p53 expression [11,12]. These studies also demonstrated that p53 induction increased the expression of p21, which induced G1 arrest. The mechanism of p53 induction may involve down regulation of the p53 antagonist mdm2 [9]. Further, Ma and co-workers demonstrated that inhibition of Group VI PLA2-induced p53 via activation of ataxia-telangiectasia and Rad-3-related (ATR) kinase [12].
The above studies suggest that one mechanism by which Group VI PLA2 mediate cell growth involves regulation of p53. However, studies in p53-null cells [9], or genetically modified cells lacking p53 [10], suggest that Group VI PLA2 can mediate cell growth independently of p53. The signaling pathways involved are not exactly known, but may involve alteration of growth factors and cell stress signaling pathways such as EGFR and MAPK [9,10,43,58,59].
3.3. Group VI PLA2 and EGFR
EGFR are integral membrane tyrosine kinases. They are members of the ErbB family of receptors, which contains four members. Upon activation, these receptors form dimers and recruit other proteins to their cytosolic domain [60]. Ligands that activate these receptors include neuropeptides like EGF, transforming growth factor-α (TGF-α), heparin-binding EGF-like growth factor (HB-EGF), amphiregulin, betacellulin, epiregulin and epigen [61]. GPCR ligands such as LPA also activate EGFR via transactivation mechanisms described below [62-71].
Two recent studies suggest that Group VI PLA2 mediate the transactivation of EGFR in prostate [9] and ovarian cancer cells [10]. Other studies have suggested links between EGFR and PLA2; however, most of these studies focused on Group VI PLA2[63,65,72]. Inhibition of Group VI PLA2 altered EGFR activation in both p53 positive and negative cells [9,10], suggesting that the ability of Group VI PLA2 to regulate EGFR is p53-independent.
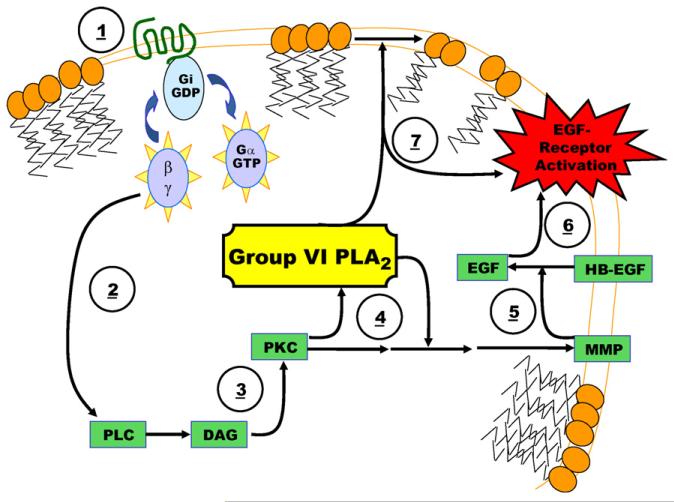
How exactly Group VI PLA2 regulates EGFR is not known. EGFR transactivation can be dependent on GPCR and activation of PKC, presumably via activation of phospholipase C isoforms by Gα and βγ [71,73]. These signaling events, through unknown intermediate steps, result in the activation of membrane bound matrix metalloproteinases (MMPs), which cleave heparin-bound epidermal growth factor (HB-EGF) on the extracellular surface to release free EGF, which can in turn bind and activate EGFR [74,75] (Fig. 1).
Fig. 1.
Proposed signaling pathways by which Group VI PLA2 mediate cell growth. (1) Group VI PLA2 may mediate G-protein coupled receptors (GPCR), such as those activated by lysophosphatidic acid (LPA), by either altering substrate generation for these receptors, or by altering the activity of their downstream targets. (2) GPCR activation results in the release of Ga and bg subunits, which can activate numerous signaling pathways, including phospholipase C (PLC). (3) PLC can produce diacylglycerol (DAG) and activate protein kinase C (PKC). (4) Interactions between PKC and Group VI PLA2 are reported in several studies and may alter the activation of several downstream targets. (5) The targets of PKC include matrix metalloproteinase (MMP). (6) Alteration in MMP activity may alter the activation of epidermal growth factor-receptors (EGF-receptors) by altering the cleavage of heparin-bound-EGF (ProHB-EGF) to EGF. (7) Alternatively, EGF-receptors, GPCR and MMP are integral membrane proteins, or are associated with the membrane by lipid modification. Thus, the ability of Group VI PLA2 to alter the signaling of these proteins may simply result from its ability to alter the plasma membrane lipid environment.
Several potential sites of regulation for Group VI PLA2 exist within the above pathway. First, the majority of the signaling molecules involved are either integral membrane proteins, or are associated with the membrane by lipid modification. Thus, the ability of Group VI PLA2 to alter the plasma membrane lipid environment may alter the regulation of EGFR by GPCR, MMP or HB-EGF. More specifically, it has been clearly demonstrated that PKC can activate Group VI PLA2[53,76]. Thus, Group VI PLA2 may contribute to the missing link in the pathway between PKC activation and MMP activation.
3.4. Group VI PLA2 and MAPK activation
Group VI PLA2 may also mediate cell growth by regulating MAPK. Several studies demonstrate that Group VI PLA2 inhibition alters MAPK activity. For example, treatment of rabbit ventricular myocytes with BEL inhibits thrombin stimulated p42/44 and p38 activation (two distinct types of MAPK) [59]. Activation of these MAPK appeared to be mediated by Group VI PLA2-mediated release of LPC, and LPC exposure increased the activity of both p42/44 and p38.
It’s also possible that Group VI PLA2 regulate MAPK using arachidonic acid. Studies in vascular smooth muscle cells demonstrated that thrombin-induced DNA synthesis correlated to arachidonic acid release, which were both inhibited by BEL [43]. However, in contrast to data in ventricular myocytes, inhibition of MAPK, specifically p38, decreased Group VI PLA2 activity, arachidonic acid release and DNA synthesis. These data suggest that the interaction of MAPK and Group VI PLA2 are cell-dependent.
Another class of lipid signals that may mediate MAPK activation in tandem with Group VI PLA2 is oxidized-low density lipoprotein (Ox-LDL). Ox-LDL result when reactive oxygen species oxidize the lipid constituents of LDL [77]. Ox-LDL induces p42/p44 in vascular smooth muscle cells using PKC and GPCR-mediated pathways [78]. Ox-LDL also induce MAPK p42/p44 and p38 in macrophages [79]. Few studies exist linking Group VI PLA2 to Ox-LDL. The one study that could be found suggested that Ox-LDL activates Group VI PLA2 and increases its expression in correlation with activation of MAPK [80].
4. Possible lipid signals by which Group VI PLA2 regulate cell growth and signaling
A majority of the above studies demonstrate that alterations in EGFR and MAPK, as well as p53 and mdm2, occur downstream of Group VI PLA2; however, these studies do not identify the signals involved. Like all PLA2, Group VI PLA2 can produce arachidonic acid and lysophospholipids [6,14,21-24], and recent studies even suggest roles in LPA production [25,26]. All of these lipids can mediate cell growth and signaling.
4.1. Group VI PLA2, arachidonic acid, LPA and cell growth and signaling
Several studies demonstrate that inhibition of PLA2 alters arachidonic acid and LPA release [1]. However, the majority of these studies focus on Group IV PLA2. Further, many of these studies used MAFP or AAOCF3. As mentioned above, these compounds decrease both Groups IV and VI PLA2 activity [1,14,22,81]. Several recent studies demonstrate that selective inhibition of Group VI PLA2 using BEL, siRNA or anti-sense oligonucleotides, decreases cell growth, alters cell cycle and cell signaling [9-12,42,51]. While, these studies demonstrated direct roles for Group VI PLA2 in cell growth, only one addressed the lipid signals involved [12].
Inhibition of Group VI PLA2 may decrease the release of both arachidonic acid and LPA. This could decrease the basal activation of EGFR and MAPK, as well as GPCR pathways (Fig. 1). These events would decrease cell growth by a variety of mechanisms [1,6,82], the majority of which center around the downstream metabolism of each lipid. For example, arachidonic acid is metabolized by cyclooxygenases, lipoxygenases and cytochrome P450 monooxygenases to numerous compounds, most of which are known to induce cell growth [1], as well as EGFR and MAPK activation [59,63-66]. Lysophospholipids can be metabolized by lyso-phospholipase D (lysoPLD) to form lysophosphatidic acid (LPA). LPA activates GPCR-pathways, EGFR and MAPK in several cell types [69-71,83,84].
4.2. Alternative lipid pathways by which Group VI PLA2 may mediate cell growth
Studies presented above suggest two hypotheses by which inhibition of Group VI PLA2 alters cell growth (1) decreased arachidonic acid release and (2) decreased LPA production. It’s also possible that the role of Group VI PLA2 in cell growth is simply a result of its housekeeping function with regard to cellular phospholipids. This hypothesis is supported by studies in HEK293 and INS-1 cells, which demonstrate that inhibition of Group VIA and VIB PLA2 altered several phospholipids in correlation with decreased cell growth and p53 activation [12,42,51].
A recent study demonstrated that Group VI PLA2-mediated activation of p53 and cell cycle arrest correlated to increases in phospholipids containing poly-unsaturated fatty acids and decreases in those containing saturated fatty acids [12]. This study also demonstrated that direct addition of 18:2-phosphatidylcholine activated p53. This study suggests that Group VI PLA2 inhibition alters cell signaling independently of either arachidonic acid or LPA.
Another Group VI PLA2 metabolite that may mediate cell growth is LPC. As mentioned above, one of the primary functions of Group VI PLA2 is to generate LPC acceptors. This LPC does not have to be generated from arachidonic acid containing phospholipids. Thus, inhibition of Group VI PLA2 could decrease basal levels of LPC without altering arachidonic acid levels. LPC is suggested to play roles in cell signaling[6,51,82,85], which can be independent of it’s conversion to LPA [6,82]. The mechanisms involved in LPC stimulated cell growth are not well understood, and few studies suggest roles for Group VI PLA2 in LPC-mediated cell growth.
5. Roles for novel Group VI PLA2 in cell growth
Almost all of the above studies focus on Group VIA or VIB PLA2 in cell growth and signaling. In contrast, few studies exist addressing roles for Group VIC, VID, VIE and VIF PLA2 in these same processes. Several functions for these newer Group VI PLA2 are reported (see [4] for review). For example, Group VIC PLA2 is also called neuropathy target esterase and may mediate membrane homeostasis in axons [4,19]. Group VID, VIE and VIF PLA2 all possess triacylglycerol lipase and acylglycerol transacylase activity and may mediate energy homeostasis [18]. Importantly, Group VID, VIE and VIF PLA2 are all inhibited by BEL. This fact must be taken into account when attempting to identify the role of different Group VI PLA2 in cell growth. It’s clear that studies are needed examining the role of the newer Group VI PLA2 in cell signaling and growth.
6. Summary and conclusions
Roles for Group VI PLA2 in cell signaling and growth are becoming more apparent in several cell types. Several studies demonstrate that Group VI PLA2 are more than just mere “housekeeping” proteins. The irony of this situation is that the housekeeping role of Group VI PLA2 may be key to their ability to mediate cell growth. Their ability to function in the absence of Ca2+, and comparatively lower selectivity for arachidonic acid, makes them suitable for controlling the level of several types of lipid signals under normal conditions.
It is important to note that few studies suggest that Group VI PLA2 supplicates, or circumvents those of Group IV or the Groups that correspond to sPLA2 (I–III, V, IX–XIV). Indeed, it appears that Group VI PLA2 activity actually declines under conditions in which these later two enzymes are induced. For example high levels of ROS formation and lipid peroxidation inactivate Group VI PLA2[57,86]. Yet, these same conditions can activate Groups IV and II PLA2[87,88]. Thus, these enzymes appear to have distinct roles in cell growth and death.
The bulk of studies, addressing roles of Group VI PLA2 in cell growth, signaling and death focus on one enzyme. This is almost out of necessity, and these studies are paramount to our understanding of the role of individual Group VI PLA2 in these processes. Nevertheless, studies are needed addressing the differential roles of Group VI PLA2 isoforms in cell growth and signaling in common models.
The ability of Group VI PLA2 to mediate cell growth suggests that it may be a drug target in several pathologies including cardiovascular diseases, renal pathologies, atherosclerosis and cancer. This hypothesis is supported by the fact that many of these diseases are characterized by deregulated cell growth. For example proliferation of endothelial cells in atherosclerosis may be decreased by Group VI PLA2 inhibitors. Further, the ability of Group VI PLA2 inhibitors to decrease growth in cancer cells suggest that it may be a target for chemotherapeutic intervention [1]. Studies are needed correlating the activity and expression of Group VI PLA2 to alterations in the above pathologies in vivo.
In closing, Group VI PLA2 mediate growth and signaling in numerous cell types. As such, they may offer unique targets for treatment of a variety of pathologies whose etiology involves the generation of lipid signals. Future studies are needed focusing on alteration of Group VI PLA2 expression and activity in vivo to realize this goal. Indeed, such studies are already underway using genetically modified animals lacking Group VIA PLA2[45-47]. Studies are also needed developing permeable, non-toxic and selective Group VI PLA2 pharmacological inhibitors for in vivo use.
Acknowledgements
This work was supported by a Georgia Cancer Coalition Distinguished Scholar Grant to BSC and Elsa U. Pardee Foundation Grant to SBH.
Abbreviations
- AAOCF3
arachidonyl triflouromethylketone
- BEL
bromoenol lactone ((E)-6-(1-bromoethyl) tetrahydro-3-(1-naphthalenyl)-2H-pyran-2-one))
- cPLA2
cytosolic phospholipase A2
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor(s)
- GPCR
G-protein coupled receptor(s)
- MAPF
methyl arachidonyl flourophosphonate
- Ox-LDL
oxidized-low density lipoproteins
- PAF-HA
platelet activating factor-acetylhydrolase
- PLA2
phospholipase A2
- PKC
protein kinase C
- sPLA2
secretory phospholipase A2
REFERENCES
- [1].Cummings BS. Phospholipase A(2) as targets for anti-cancer drugs. Biochem Pharmacol. 2007;74((7):949–59. doi: 10.1016/j.bcp.2007.04.021. [DOI] [PubMed] [Google Scholar]
- [2].Cummings BS, McHowat J, Schnellmann RG. Phospholipase A(2)s in cell injury and death. J Pharmacol Exp Ther. 2000;294:793–9. [PubMed] [Google Scholar]
- [3].Balsinde J, Winstead MV, Dennis EA. Phospholipase A(2) regulation of arachidonic acid mobilization. FEBS Lett. 2002;531:2–6. doi: 10.1016/s0014-5793(02)03413-0. [DOI] [PubMed] [Google Scholar]
- [4].Schaloske RH, Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–59. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- [5].Farooqui AA, Horrocks LA. Phospholipase A2-generated lipid mediators in the brain: the good, the bad, and the ugly. Neuroscientist. 2006;12:245–60. doi: 10.1177/1073858405285923. doi: 10.1177/107385805285923. [DOI] [PubMed] [Google Scholar]
- [6].McHowat J, Creer MH. Catalytic features, regulation and function of myocardial phospholipase A2. Curr Med Chem Cardiovasc Hematol Agents. 2004;2:209–18. doi: 10.2174/1568016043356282. [DOI] [PubMed] [Google Scholar]
- [7].Balsinde J, Balboa MA. Cellular regulation and proposed biological functions of group VIA calcium-independent phospholipase A2 in activated cells. Cell Signal. 2005;17:1052–62. doi: 10.1016/j.cellsig.2005.03.002. [DOI] [PubMed] [Google Scholar]
- [8].Akiba S, Sato T. Cellular function of calcium-independent phospholipase A2. Biol Pharm Bull. 2004;27:1174–8. doi: 10.1248/bpb.27.1174. [DOI] [PubMed] [Google Scholar]
- [9].Sun B, Zhang X, Talathi S, Cummings B. Inhibition of Ca2+-independent phospholipase A2 decreases prostate cancer cell growth by p53-dependent and - independent mechanisms. J Pharmacol Exp Ther. 2008;326((1):59–69. doi: 10.1124/jpet.108.138958. [DOI] [PubMed] [Google Scholar]
- [10].Song Y, Wilkins P, Hu W, Murthy KS, Chen J, Lee Z, et al. Inhibition of calcium-independent phospholipase A2 suppresses proliferation and tumorigenicity of ovarian carcinoma cells. Biochem J. 2007;406((3):427–36. doi: 10.1042/BJ20070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang XH, Zhao C, Seleznev K, Song K, Manfredi JJ, Ma ZA. Disruption of G1-phase phospholipid turnover by inhibition of Ca2+-independent phospholipase A2 induces a p53-dependent cell-cycle arrest in G1 phase. J Cell Sci. 2006;119:1005–15. doi: 10.1242/jcs.02821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang XH, Zhao C, Ma ZA. The increase of cell-membranous phosphatidylcholines containing polyunsaturated fatty acid residues inducesphosphorylation of p53 through activation of ATR. J Cell Sci. 2007;120:4134–43. doi: 10.1242/jcs.015834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lei X, Zhang S, Bohrer A, Bao S, Song H, Ramanadham S. The group VIA calcium-independent phospholipase A2 participates in ER stress-induced INS-1 insulinoma cell apoptosis by promoting ceramide generation via hydrolysis of sphingomyelins by neutral sphingomyelinase. Biochemistry. 2007;46:10170–85. doi: 10.1021/bi700017z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Balsinde J. Roles of various phospholipases A2 in providing lysophospholipid acceptors for fatty acid phospholipid incorporation and remodelling. Biochem J. 2002;364:695–702. doi: 10.1042/BJ20020142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ma Z, Wang X, Nowatzke W, Ramanadham S, Turk J. Human pancreatic islets express mRNA species encoding two distinct catalytically active isoforms of group VI phospholipase A2 (iPLA2) that arise from an exon-skipping mechanism of alternative splicing of the transcript from the iPLA2 gene on chromosome 22q13.1. J Biol Chem. 1999;274:9607–16. doi: 10.1074/jbc.274.14.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mancuso DJ, Jenkins CM, Gross RW. The genomic organization, complete mRNA sequence, cloning, and expression of a novel human intracellular membrane-associated calcium-independent phospholipase A(2) J Biol Chem. 2000;275:9937–45. doi: 10.1074/jbc.275.14.9937. [DOI] [PubMed] [Google Scholar]
- [17].Cummings BS, McHowat J, Schnellmann RG. Role of an endoplasmic reticulum Ca(2+)-independent phospholipase A(2) in oxidant-induced renal cell death. Am J Physiol Renal Physiol. 2002;283:F492–8. doi: 10.1152/ajprenal.00022.2002. [DOI] [PubMed] [Google Scholar]
- [18].Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–75. doi: 10.1074/jbc.M407841200. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- [19].Glynn P. Neuropathy target esterase and phospholipid deacylation. Biochim Biophys Acta. 2005;1736:87–93. doi: 10.1016/j.bbalip.2005.08.002. [DOI] [PubMed] [Google Scholar]
- [20].Perez R, Melero R, Balboa MA, Balsinde J. Role of group VIA calcium-independent phospholipase A2 in arachidonic acid release, phospholipid fatty acid incorporation, and apoptosis in U937 cells responding to hydrogen peroxide. J Biol Chem. 2004;279:40385–91. doi: 10.1074/jbc.M402562200. [DOI] [PubMed] [Google Scholar]
- [21].Balsinde J, Balboa MA, Dennis EA. Antisense inhibition of group VI Ca2+-independent phospholipase A2 blocks phospholipid fatty acid remodeling in murine P388D1 macrophages. J Biol Chem. 1997;272:29317–21. doi: 10.1074/jbc.272.46.29317. [DOI] [PubMed] [Google Scholar]
- [22].Lio YC, Reynolds LJ, Balsinde J, Dennis EA. Irreversible inhibition of Ca(2+)-independent phospholipase A2 by methyl arachidonyl fluorophosphonate. Biochim Biophys Acta. 1996;1302:55–60. doi: 10.1016/0005-2760(96)00002-1. [DOI] [PubMed] [Google Scholar]
- [23].Teslenko V, Rogers M, Lefkowith JB. Macrophage arachidonate release via both the cytosolic Ca(2+)-dependent and -independent phospholipases is necessary for cell spreading. Biochim Biophys Acta. 1997;1344:189–99. doi: 10.1016/s0005-2760(96)00137-3. [DOI] [PubMed] [Google Scholar]
- [24].Atsumi G, Tajima M, Hadano A, Nakatani Y, Murakami M, Kudo I. Fat-induced arachidonic acid release is mediated by Ca2+-independent phospholipase A2 but not cytosolic phospholipase A2, which undergoes proteolytic inactivation. J Biol Chem. 1998;273:13870–7. doi: 10.1074/jbc.273.22.13870. [DOI] [PubMed] [Google Scholar]
- [25].Ren J, Xiao Y-j, Singh LS, Zhao X, Zhao Z, Feng L, et al. Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells. Cancer Res. 2006;66:3006–14. doi: 10.1158/0008-5472.CAN-05-1292. %R 101158/0008-5472CAN-05-1292. [DOI] [PubMed] [Google Scholar]
- [26].Shen Z, Belinson J, Morton RE, Xu Y. Phorbol 12-myristate 13-acetate stimulates lysophosphatidic acid secretion from ovarian and cervical cancer cells but not from breast or leukemia cells. Gynecol Oncol. 1998;71:364–8. doi: 10.1006/gyno.1998.5193. [DOI] [PubMed] [Google Scholar]
- [27].Andresen TL, Jensen SS, Kaasgaard T, Jorgensen K. Triggered activation and release of liposomal prodrugs and drugs in cancer tissue by secretory phospholipase A2. Curr Drug Deliv. 2005;2:353–62. doi: 10.2174/156720105774370203. [DOI] [PubMed] [Google Scholar]
- [28].Aoki J. Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol. 2004;15:477–89. doi: 10.1016/j.semcdb.2004.05.001. [DOI] [PubMed] [Google Scholar]
- [29].Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J Lipid Res. 2004;45:205–13. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- [30].Kudo I, Murakami M. Phospholipase A2 enzymes. Prostaglandins Other Lipid Mediat. 2002;68-69:3–58. doi: 10.1016/s0090-6980(02)00020-5. [DOI] [PubMed] [Google Scholar]
- [31].Kramer RM, Stephenson DT, Roberts EF, Clemens JA. Cytosolic phospholipase A2 (cPLA2) and lipid mediator release in the brain. J Lipid Mediat Cell Signal. 1996;14:3–7. doi: 10.1016/0929-7855(96)01501-5. [DOI] [PubMed] [Google Scholar]
- [32].Bonventre JV. Roles of phospholipases A2 in brain cell and tissue injury associated with ischemia and excitotoxicity. J Lipid Mediat Cell Signal. 1996;14:15–23. doi: 10.1016/0929-7855(96)00503-2. [DOI] [PubMed] [Google Scholar]
- [33].Ramanadham S, Hsu FF, Bohrer A, Ma Z, Turk J. Studies of the role of group VI phospholipase A2 in fatty acid incorporation, phospholipid remodeling, lysophosphatidylcholine generation, and secretagogue-induced arachidonic acid release in pancreatic islets and insulinoma cells. J Biol Chem. 1999;274:13915–27. doi: 10.1074/jbc.274.20.13915. [DOI] [PubMed] [Google Scholar]
- [34].Ma Z, Ramanadham S, Wohltmann M, Bohrer A, Hsu F-F, Turk J. Studies of insulin secretory responses and of arachidonic acid incorporation into phospholipids of stably transfected insulinoma cells that overexpress group VIA phospholipase A2 (iPLA2beta) indicate a signaling rather than a housekeeping role for iPLA2beta. J Biol Chem. 2001;276:13198–208. doi: 10.1074/jbc.M010423200. [DOI] [PubMed] [Google Scholar]
- [35].Ma Z, Ramanadham S, Wohltmann M, Bohrer A, Hsu FF, Turk J. Studies of insulin secretory responses and of arachidonic acid incorporation into phospholipids of stably transfected insulinoma cells that overexpress group VIA phospholipase A2 (iPLA2beta) indicate a signaling rather than a housekeeping role for iPLA2beta. J Biol Chem. 2001;276:13198–208. doi: 10.1074/jbc.M010423200. [DOI] [PubMed] [Google Scholar]
- [36].Balsinde J, Dennis EA. Bromoenol lactone inhibits magnesium-dependent phosphatidate phosphohydrolase and blocks triacylglycerol biosynthesis in mouse P388D1 macrophages. J Biol Chem. 1996;271:31937–41. doi: 10.1074/jbc.271.50.31937. [DOI] [PubMed] [Google Scholar]
- [37].Ma Z, Bohrer A, Wohltmann M, Ramanadham S, Hsu FF, Turk J. Studies of phospholipid metabolism, proliferation, and secretion of stably transfected insulinoma cells that overexpress group VIA phospholipase A2. Lipids. 2001;36:689–700. doi: 10.1007/s11745-001-0774-9. [DOI] [PubMed] [Google Scholar]
- [38].Ackermann EJ, Kempner ES, Dennis EA. Ca(2+)-independent cytosolic phospholipase A2 from macrophage-like P388D1 cells. Isolation and characterization. J Biol Chem. 1994;269:9227–33. [PubMed] [Google Scholar]
- [39].Balsinde J, Bianco ID, Ackermann EJ, Conde-Frieboes K, Dennis EA. Inhibition of calcium-independent phospholipase A2 prevents arachidonic acid incorporation and phospholipid remodeling in P388D1 macrophages. Proc Natl Acad Sci USA. 1995;92:8527–31. doi: 10.1073/pnas.92.18.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jenkins CM, Han X, Mancuso DJ, Gross RW. Identification of calcium-independent phospholipase A2 (iPLA2) beta, and not iPLA2gamma, as the mediator of arginine vasopressin-induced arachidonic acid release in A-10 smooth muscle cells. Enantioselective mechanism-based discrimination of mammalian iPLA2s. J Biol Chem. 2002;277:32807–14. doi: 10.1074/jbc.M202568200. [DOI] [PubMed] [Google Scholar]
- [41].Kinsey GR, Cummings BS, Beckett CS, Saavedra G, Zhang W, McHowat J, et al. Identification and distribution of endoplasmic reticulum iPLA2. Biochem Biophys Res Commun. 2005;327:287–93. doi: 10.1016/j.bbrc.2004.12.016. [DOI] [PubMed] [Google Scholar]
- [42].Saavedra G, Zhang W, Peterson B, Cummings BS. Differential roles for cytosolic and microsomal Ca2+-independent phospholipase A2 in cell growth and maintenance of phospholipids. J Pharmacol Exp Ther. 2006;318:1211–9. doi: 10.1124/jpet.106.105650. doi: 10.1124/jpet.106.105650. [DOI] [PubMed] [Google Scholar]
- [43].Yellaturu CR, Rao GN. A requirement for calcium-independent phospholipase A2 in thrombin-induced arachidonic acid release and growth in vascular smooth muscle cells. J Biol Chem. 2003;278:43831–7. doi: 10.1074/jbc.M301472200. [DOI] [PubMed] [Google Scholar]
- [44].Kinsey GR, Blum JL, Covington MD, Cummings BS, McHowat J, Schnellmann RG. Decreased iPLA2gamma expression induces lipid peroxidation, cell death, and sensitizes cells to oxidant-induced apoptosis. J Lipid Res. 2008;324((1):376–82. doi: 10.1194/jlr.M800030-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bao S, Miller DJ, Ma Z, Wohltmann M, Eng G, Ramanadham S, et al. Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J Biol Chem. 2004;279:38194–200. doi: 10.1074/jbc.M406489200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ramanadham S, Yarasheski KE, Silva MJ, Wohltmann M, Novack DV, Christiansen B, et al. Age-related changes in bone morphology are accelerated in group VIA phospholipase A2 (iPLA2beta)-null mice. Am J Pathol. 2008;172:868–81. doi: 10.2353/ajpath.2008.070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shinzawa K, Sumi H, Ikawa M, Matsuoka Y, Okabe M, Sakoda S, et al. Neuroaxonal dystrophy caused by group VIA phospholipase A2 deficiency in mice: a model of human neurodegenerative disease. J Neurosci. 2008;28:2212–20. doi: 10.1523/JNEUROSCI.4354-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Xie Z, Gong MC, Su W, Turk J, Guo Z. Group VIA phospholipase A2 (iPLA2 beta) participates in angiotensin II-induced transcriptional up-regulation of regulator of G-protein signaling-2 in vascular smooth muscle cells. J Biol Chem. 2007;282:25278–89. doi: 10.1074/jbc.M611206200. %R 101074/jbcM611206200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ramanadham S, Song H, Hsu FF, Zhang S, Crankshaw M, Grant GA, et al. Pancreatic islets and insulinoma cells express a novel isoform of group VIA phospholipase A2 (iPLA2 beta) that participates in glucose-stimulated insulin secretion and is not produced by alternate splicing of the iPLA2 beta transcript. Biochemistry. 2003;42:13929–40. doi: 10.1021/bi034843p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Murakami M, Masuda S, Ueda-Semmyo K, Yoda E, Kuwata H, Takanezawa Y, et al. Group VIB Ca2+-independent phospholipase A2gamma promotes cellular membrane hydrolysis and prostaglandin production in a manner distinct from other intracellular phospholipases A2. J Biol Chem. 2005;280:14028–41. doi: 10.1074/jbc.M413766200. [DOI] [PubMed] [Google Scholar]
- [51].Bao S, Bohrer A, Ramanadham S, Jin W, Zhang S, Turk J. Effects of stable suppression of group VIA phospholipase A2 expression on phospholipid content and composition, insulin secretion, and proliferation of INS-1 insulinoma cells. J Biol Chem. 2006;281:187–98. doi: 10.1074/jbc.M509105200. doi: 10.1074/jbc.M509105200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sanchez T, Moreno JJ. Role of phospholipases A(2) in growth-dependent changes in prostaglandin release from 3T6 fibroblasts. J Cell Physiol. 2001;189:237–43. doi: 10.1002/jcp.10020. [DOI] [PubMed] [Google Scholar]
- [53].Cummings BS, McHowat J, Schnellmann RG. Role of an endoplasmic reticulum Ca2+-independent phospholipase A2 in cisplatin-induced renal cell apoptosis. J Pharmacol Exp Ther. 2004;308:921–8. doi: 10.1124/jpet.103.060541. [DOI] [PubMed] [Google Scholar]
- [54].Sanchez T, Moreno JJ. The effect of high molecular phospholipase A2 inhibitors on 3T6 fibroblast proliferation. Biochem Pharmacol. 2001;61:811–6. doi: 10.1016/s0006-2952(01)00555-x. [DOI] [PubMed] [Google Scholar]
- [55].Herbert SP, Walker JH. Group VIA calcium-independent phospholipase A2 mediates endothelial cell S phase progression. J Biol Chem. 2006;281:35709–16. doi: 10.1074/jbc.M600699200. [DOI] [PubMed] [Google Scholar]
- [56].Zhang L, Peterson BL, Cummings BS. The effect of inhibition of Ca2+-independent phospholipase A2 on chemotherapeutic-induced death and phospholipid profiles in renal cells. Biochem Pharmacol. 2005;70:1697–706. doi: 10.1016/j.bcp.2005.09.008. [DOI] [PubMed] [Google Scholar]
- [57].Cummings BS, Gelasco AK, Kinsey GR, McHowat J, Schnellmann RG. Inactivation of endoplasmic reticulum bound Ca2+-independent phospholipase A2 in renal cells during oxidative stress. J Am Soc Nephrol. 2004;15:1441–51. doi: 10.1097/01.asn.0000127923.57438.ec. [DOI] [PubMed] [Google Scholar]
- [58].Hsu MF, Lu MC, Tsao LT, Kuan YH, Chen CC, Wang JP. Mechanisms of the influence of magnolol on eicosanoid metabolism in neutrophils. Biochem Pharmacol. 2004;67:831–40. doi: 10.1016/j.bcp.2003.09.040. [DOI] [PubMed] [Google Scholar]
- [59].Beckett CS, Pennington K, McHowat J. Activation of MAPKs in thrombin-stimulated ventricular myocytes is dependent on Ca2+-independent PLA2. Am J Physiol Cell Physiol. 2006;290:C1350–4. doi: 10.1152/ajpcell.00487.2005. [DOI] [PubMed] [Google Scholar]
- [60].Kim ES, Khuri FR, Herbst RS. Epidermal growth factor receptor biology (IMC-C225) Curr Opin Oncol. 2001;13:506–13. doi: 10.1097/00001622-200111000-00014. [DOI] [PubMed] [Google Scholar]
- [61].Harris RC, Chung E, Coffey RJ. EGF receptor ligands. Exp Cell Res. 2003;284:2–13. doi: 10.1016/s0014-4827(02)00105-2. [DOI] [PubMed] [Google Scholar]
- [62].Hernandez M, Barrero MJ, Crespo MS, Nieto ML. Lysophosphatidic acid inhibits Ca2+ signaling in response to epidermal growth factor receptor stimulation in human astrocytoma cells by a mechanism involving phospholipase C(gamma) and a G(alpha) protein. J Neurochem. 2000;75:1575–82. doi: 10.1046/j.1471-4159.2000.0751575.x. [DOI] [PubMed] [Google Scholar]
- [63].Hassan S, Carraway RE. Involvement of arachidonic acid metabolism and EGF receptor in neurotensin-induced prostate cancer PC3 cell growth. Regul Pept. 2006;133:105–14. doi: 10.1016/j.regpep.2005.09.031. [DOI] [PubMed] [Google Scholar]
- [64].Choudhury QG, McKay DT, Flower RJ, Croxtall JD. Investigation into the involvement of phospholipases A(2) and MAP kinases in modulation of AA release and cell growth in A549 cells. Br J Pharmacol. 2000;131:255–65. doi: 10.1038/sj.bjp.0703573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Xu L, Han C, Wu T. A novel positive feedback loop between peroxisome proliferator-activated receptor-delta and prostaglandin E2 signaling pathways for human cholangiocarcinoma cell growth. J Biol Chem. 2006;281:33982–96. doi: 10.1074/jbc.M600135200. [DOI] [PubMed] [Google Scholar]
- [66].Xu J, Weng YI, Simonyi A, Krugh BW, Liao Z, Weisman GA, et al. Role of PKC and MAPK in cytosolic PLA2 phosphorylation and arachadonic acid release in primary murine astrocytes. J Neurochem. 2002;83:259–70. doi: 10.1046/j.1471-4159.2002.01145.x. [DOI] [PubMed] [Google Scholar]
- [67].Rao TS, Lariosa-Willingham KD, Lin FF, Yu N, Tham CS, Chun J, et al. Growth factor pre-treatment differentially regulates phosphoinositide turnover downstream of lysophospholipid receptor and metabotropic glutamate receptors in cultured rat cerebrocortical astrocytes. Int J Dev Neurosci. 2004;22:131–5. doi: 10.1016/j.ijdevneu.2004.03.005. [DOI] [PubMed] [Google Scholar]
- [68].Piazza GA, Ritter JL, Baracka CA. Lysophosphatidic acid induction of transforming growth factors alpha and beta: modulation of proliferation and differentiation in cultured human keratinocytes and mouse skin. Exp Cell Res. 1995;216:51–64. doi: 10.1006/excr.1995.1007. [DOI] [PubMed] [Google Scholar]
- [69].Xu KP, Yin J, Yu FS. Lysophosphatidic acid promoting corneal epithelial wound healing by transactivation of epidermal growth factor receptor. Invest Ophthalmol Vis Sci. 2007;48:636–43. doi: 10.1167/iovs.06-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Liu Z, Armant DR. Lysophosphatidic acid regulates murine blastocyst development by transactivation of receptors for heparin-binding EGF-like growth factor. Exp Cell Res. 2004;296:317–26. doi: 10.1016/j.yexcr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- [71].Zhao Y, He D, Saatian B, Watkins T, Spannhake EW, Pyne NJ, et al. Regulation of lysophosphatidic acid-induced epidermal growth factor receptor transactivation and interleukin-8 secretion in human bronchial epithelial cells by protein kinase Cdelta, Lyn kinase, and matrix metalloproteinases. J Biol Chem. 2006;281:19501–1. doi: 10.1074/jbc.M511224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wendum D, Comperat E, Boelle PY, Parc R, Masliah J, Trugnan G, et al. Cytoplasmic phospholipase A2 alpha overexpression in stromal cells is correlated with angiogenesis in human colorectal cancer. Mod Pathol. 2005;18:212–20. doi: 10.1038/modpathol.3800284. [DOI] [PubMed] [Google Scholar]
- [73].Bookout AL, Finney AE, Guo R, Peppel K, Koch WJ, Daaka Y. Targeting Gbetagamma signaling to inhibit prostate tumor formation and growth. J Biol Chem. 2003;278:37569–73. doi: 10.1074/jbc.M306276200. [DOI] [PubMed] [Google Scholar]
- [74].Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–8. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- [75].Santiskulvong C, Rozengurt E. Galardin (GM 6001), a broad-spectrum matrix metalloproteinase inhibitor, blocks bombesin- and LPA-induced EGF receptor transactivation and DNA synthesis in rat-1 cells. Exp Cell Res. 2003;290:437–46. doi: 10.1016/s0014-4827(03)00355-0. [DOI] [PubMed] [Google Scholar]
- [76].Meyer MC, Kell PJ, Creer MH, McHowat J. Calcium-independent phospholipase A2 is regulated by a novel protein kinase C in human coronary artery endothelial cells. Am J Physiol Cell Physiol. 2005;288:C475–82. doi: 10.1152/ajpcell.00306.2004. [DOI] [PubMed] [Google Scholar]
- [77].Young IS, McEneny J. Lipoprotein oxidation and atherosclerosis. Biochem Soc Trans. 2001;29:358–62. doi: 10.1042/0300-5127:0290358. [DOI] [PubMed] [Google Scholar]
- [78].Yang CM, Chiu CT, Wang CC, Chien CS, Hsiao LD, Lin CC, et al. Activation of mitogen-activated protein kinase by oxidized low-density lipoprotein in canine cultured vascular smooth muscle cells. Cell Signal. 2000;12:205–14. doi: 10.1016/s0898-6568(99)00087-x. [DOI] [PubMed] [Google Scholar]
- [79].Taketa K, Matsumura T, Yano M, Ishii N, Senokuchi T, Motoshima H, et al. Oxidized low density lipoprotein activates peroxisome proliferator-activated receptor-{alpha} (PPAR{alpha}) and PPAR{gamma} through MAPK-dependent COX-2 expression in macrophages. J Biol Chem. 2008;283:9852–62. doi: 10.1074/jbc.M703318200. %R 101074/jbcM703318200. [DOI] [PubMed] [Google Scholar]
- [80].Lupo G, Nicotra A, Giurdanella G, Anfuso CD, Romeo L, Biondi G, et al. Activation of phospholipase A(2) and MAP kinases by oxidized low-density lipoproteins in immortalized GP8.39 endothelial cells. Biochim Biophys Acta. 2005;1735:135–50. doi: 10.1016/j.bbalip.2005.05.008. [DOI] [PubMed] [Google Scholar]
- [81].Balboa MA, Balsinde J, Jones SS, Dennis EA. Identity between the Ca2+-independent phospholipase A2 enzymes from P388D1 macrophages and Chinese hamster ovary cells. J Biol Chem. 1997;272:8576–80. doi: 10.1074/jbc.272.13.8576. [DOI] [PubMed] [Google Scholar]
- [82].Yan W, Jenkins CM, Han X, Mancuso DJ, Sims HF, Yang K, et al. The highly selective production of 2-arachidonoyl lysophosphatidylcholine catalyzed by purified calcium-independent phospholipase A2{gamma}: identification of a novel enzymatic mediator for the generation of a key branch point intermediate in eicosanoid signaling. J Biol Chem. 2005;280:26669–7. doi: 10.1074/jbc.M502358200. doi: 10.1074/jbc.M502358200. [DOI] [PubMed] [Google Scholar]
- [83].Marrache AM, Gobeil F, Zhu T, Chemtob S. Intracellular signaling of lipid mediators via cognate nuclear G protein-coupled receptors. Endothelium. 2005;12:63–72. doi: 10.1080/10623320590933815. [DOI] [PubMed] [Google Scholar]
- [84].Mori K, Kitayama J, Shida D, Yamashita H, Watanabe T, Nagawa H. Lysophosphatidic acid-induced effects in human colon carcinoma DLD1 cells are partially dependent on transactivation of epidermal growth factor receptor. J Surg Res. 2006;132:56–61. doi: 10.1016/j.jss.2005.07.040. [DOI] [PubMed] [Google Scholar]
- [85].McHowat J, Creer MH. Lysophosphatidylcholine accumulation in cardiomyocytes requires thrombin activation of Ca2+-independent PLA2. Am J Physiol. 1997;272:H1972–80. doi: 10.1152/ajpheart.1997.272.4.H1972. [DOI] [PubMed] [Google Scholar]
- [86].Song H, Bao S, Ramanadham S, Turk J. Effects of biological oxidants on the catalytic activity and structure of group VIA phospholipase A2. Biochemistry. 2006;45:6392–406. doi: 10.1021/bi060502a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Balsinde J, Dennis EA. Distinct roles in signal transduction for each of the phospholipase A2 enzymes present in P388D1 macrophages. J Biol Chem. 1996;271:6758–65. doi: 10.1074/jbc.271.12.6758. [DOI] [PubMed] [Google Scholar]
- [88].Muralikrishna Adibhatla R, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40:376–87. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]