Abstract
Significant attention has recently been drawn to the potential link between head trauma and the development of neurodegenerative disease, namely chronic traumatic encephalopathy (CTE). The acute neurotrauma associated with sports-related concussions in athletes and blast-induced traumatic brain injury in soldiers elevates the risk for future development of chronic neurodegenerative diseases such as CTE. CTE is a progressive disease distinguished by characteristic tau neurofibrillary tangles (NFTs) and, occasionally, transactive response DNA binding protein 43 (TDP43) oligomers, both of which have a predilection for perivascular and subcortical areas near reactive astrocytes and microglia. The disease is currently only diagnosed postmortem by neuropathological identification of NFTs. A recent workshop sponsored by National Institute of Neurological Disorders and Stroke emphasized the need for premortem diagnosis, to better understand disease pathophysiology and to develop targeted treatments. In order to accomplish this objective, it is necessary to discover the mechanistic link between acute neurotrauma and the development of chronic neurodegenerative and neuropsychiatric disorders such as CTE. In this review, we briefly summarize what is currently known about CTE development and pathophysiology, and subsequently discuss injury-induced pathways that warrant further investigation. Understanding the mechanistic link between acute brain injury and chronic neurodegeneration will facilitate the development of appropriate diagnostic and therapeutic options for CTE and other related disorders.
Key words: : chronic traumatic encephalopathy, neurofibrillary tangles
Introduction
Neurotrauma is one of the most common injuries in contact sports and military conflicts.1 Each year in the United States alone, >1,700,000 traumatic brain injuries (TBIs) occur.2 Many of these TBIs are related to participation in contact sports, such as football and hockey, but a high rate of neurotrauma has also been reported for civilian and military populations in war zones.3 In fact, the United States Department of Defense labeled blast-induced TBI (bTBI) as the “signature injury” of the recent wars in Iraq and Afghanistan.1 The estimated annual cost of treatment for bTBI in the United States is 2.5 billion dollars.4 The enormous economic burden is caused, in part, by the progressive development of cognitive, motor, and psychiatric problems in blast-exposed veterans and civilians.5 These clinical symptoms, emerging in former athletes and soldiers alike, are often the first measurable signs for the development of a chronic neurodegenerative disease such as chronic traumatic encephalopathy (CTE).3 Clinical presentation of CTE has recently been divided into two categories: young age of onset with primarily psychiatric and behavioral problems, and older age of onset with primarily cognitive and motor deficits.6 Increased awareness about CTE has prompted widespread investigation into the progression and pathophysiology of this disease.7
The two populations at greatest risk for development of CTE are professional athletes and soldiers.5 It appears that individuals with one or two copies of the apolipoprotein ɛ4 (APOɛ4) allele have poorer outcome following head trauma and are at increased risk for developing CTE following TBI.8 Athletes exposed to subconcussive and concussive injury, as well as soldiers exposed to even a single blast, can develop behavioral and psychiatric problems within a single year following injury.9 An area in need of further investigation is how acute neurotrauma relates to and/or causes chronic neurodegenerative diseases in susceptible individuals. In this review, we examine tau-based CTE pathophysiology and disease progression while discussing potential mechanistic pathways that may link acute neurotrauma with chronic neurodegenerative disease development and neurofibrillary tangle (NFT) formation.
Neuropathological Findings in CTE
Postmortem examination is currently the only widely utilized and accepted method by which CTE is diagnosed clinically, although in vivo approaches have been identified and are currently under development for diagnosis and tracking premortem.3,10 Common neuropathological findings of CTE include NFTs and transactive response DNA binding protein 43 (TDP43), as well as microglial and astrocyte activation.11 Although the mechanistic link responsible for these pathological outcomes is not fully known, the current understanding of the pathological progression will be discussed in the following paragraphs.6
NFTs
Normal tau binds to tubulin and stabilizes microtubule fibrils in neurons, thereby facilitating neurite outgrowth. When tau becomes hyperphosphorylated, it binds to other normal tau proteins, which leads to aggregation.12 Tau hyperphosphorylation in the central nervous system (CNS) is common after TBI and other brain injuries.13 TBI can cause normal tau to dissociate from tubulin, thereby exposing multiple phosphorylation sites.14 Hyperphosphorylated tau is no longer able to bind to tubulin, and translocates from the axon to the neuron soma.12 A primary reason for this translocation is that normal tau is soluble, whereas hyperphosphorylated tau becomes insoluble, therefore favoring a paired helical filament arrangement that is too large to function in axons.13 The paired helical arrangement also leads to poor clearance of hyperphosphorylated tau from the neuron.14 Accumulation of insoluble tau within neurons contributes to the development of tau oligomers.15 Tau oligomers are granular intracellular buildups of mutated tau, which precede the development of NFTs.14 When tau phosphatases can no longer dephosphorylate oligomers efficiently, NFTs grow and eventually mature.16 NFT maturation involves the acetylation of the lysine residue 280.15 Once NFTs fully mature, they affect large projecting neurons in a progressive hierarchical pattern.17 NFTs can spread to surrounding at-risk neurons through trans-synaptic propagation or extracellular secretion as depicted in Figure 1.18 Propagation can occur by direct seeding of tau oligomers into the lipid rafts of cell membranes, thus increasing cell permeability and allowing access for the spread of larger secreted NFTs.16 After NFTs propagate, the post-translational modifications become finalized, and behavioral and motor symptoms begin to surface in patients.14 A likely reason for the symptomatic changes is that NFTs cause neurons to become de-innervated, in part, because of decreased neurite outgrowth, which ultimately leads to neuronal death.15
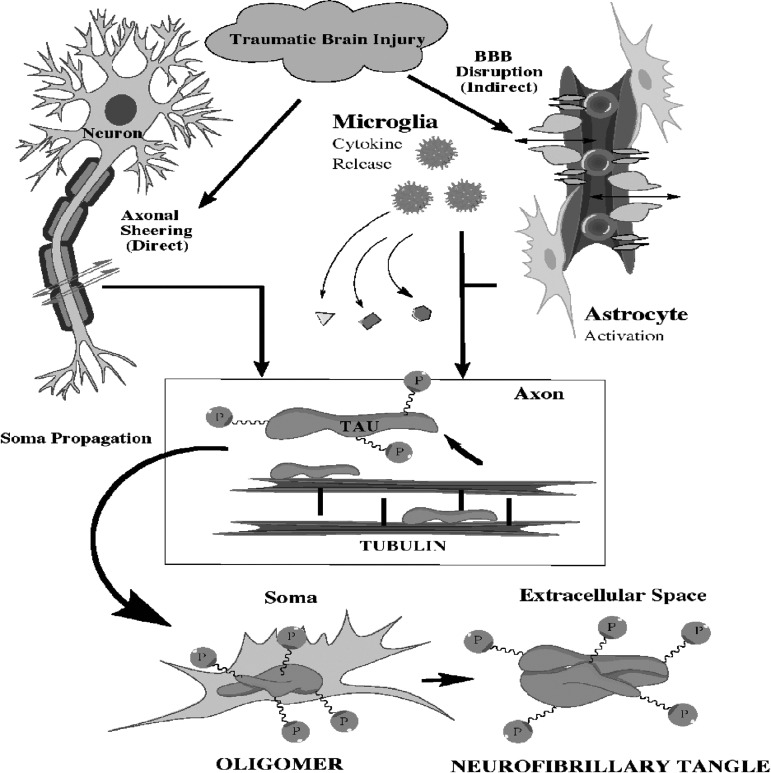
FIG. 1.
Traumatic brain injury can lead to diffuse traumatic axonal injury and blood–brain barrier disruption. Shearing of axons results in the disruption of tau binding to tubulin. Subsequent hyperphosphorylation of tau leads to formation of tau oligomers in the neuronal soma. Eventually, neurofibrillary tangles form and are secreted into the extracellular milieu or spread to other neurons via trans-synaptic propagation. Concurrent with axonal shearing, traumatic brain injury can cause a rapid blood pressure spike resulting in blood–brain barrier disruption. The disruption leads to an inflammatory cascade as well as microglia and astrocyte activation. Microglia and astrocyte activation in conjunction with tauopathy contribute to the pathology of chronic traumatic encephalopathy.
TDP43
Wild-type TDP43 is a nuclear RNA/DNA binding protein that regulates the transcription of thousands of genes.19 TDP43 is predominantly found in large motor neurons throughout the CNS.20 TBI causes an upregulation of Ca2+-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, which in turn lead to carboxy-terminal-cleaved TDP43 fragments.21 These fragments translocate to the cytosol, mediated in part by the process of ubiquitination.19 The fragments form intracellular aggregates that are representative of several neurodegenerative diseases including: Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, and CTE.20 Aggregates sequester RNA leading to pronounced neurotoxicity.21 One mechanism by which neurotoxicity occurs is TDP43 aggregate-induced misfolding of Cu/Zn superoxide dismutase (SOD1), which predisposes surrounding cells to free-radical damage.20 Associated clinical symptoms of TDP43 pathology include cognitive and motor impairment.21 Cognitive impairment may take the form of apathy, poor impulse control, and lack of overall executive judgment.22 It has yet to be determined exactly how TDP43 aggregates coincide and interact with NFTs to produce the wide spectrum of clinical CTE presentation seen in patients.18
Astrocyte activation
Astrocytes in a healthy brain provide a supporting role for neurons.23 If brain injury occurs, astrocytes become responsive by increasing expression of a microfilament known as glial fibrillary acidic protein (GFAP), while simultaneously releasing cytokines that activate nearby neurons to increase nociceptive receptivity.24 Astrocytes also trigger self-renewing neurospheres that may help in brain recovery.23 Blast-induced TBI (bTBI), in particular, causes increased astrocyte activation and GFAP levels within 24 h post-injury.25 Peripheral GFAP is absorbed and sequestered from the plasma immediately following bTBI, leading to an initial decrease in serum levels at 6 h, but GFAP is subsequently increased by augmented gene expression and changes in membrane permeability at 24 h.26 The process of astrocyte activation involves an increase in astrocyte size, number, and motility that primarily occurs in the white matter following brain or spinal injury.24 The activation is most pronounced in the corpus callosum, motor, and somatosensory cortex leading to symptoms of increased impulsive behavior and cognitive dysfunction.25 One mechanism by which astrocytes are activated following brain injury is microglia-mediated crosstalk via pro-inflammatory cytokines.24 These immune cells release interleukin-1β and other cytokines that act on toll-like receptors (TLRs) in astrocytes, thereby inducing astrocytes to release reactive oxygen species (ROS).27 ROS indirectly cause excitotoxicity by decreasing the ability of astrocytes to uptake glutamate.28 This excitotoxicity was shown to cause changes in exploratory behavior and distinct motor deficits in mice exposed to cortical impact TBI.29
Microglial activation
Microglia are ramified immune cells of the brain that are inactive in healthy brains.30 Following repetitive TBI, localized cell death activates microglia in the striatum and thalamus, which can be measured by the markers OX6 and CD68.31 Activated microglia have a bushy appearance with thickened processes and enlarged cell bodies.30 Furthermore, the microglia foster the spread of neuroinflammation.32 Short- term activation of microglia is neuroprotective, while chronic activation is involved in neurodegeneration.31 Chronic activation becomes more common in an aged brain, accounting in part for the progressive nature of neurodegenerative diseases.33 Glial tangles (GT), for example, are prominent in the frontal and temporal lobes of CTE brains many years after initial injury.3 A primary reason for the persistence of microglia activation is the presence of diffuse traumatic axonal injury (dTAI).34 dTAI can cause the upregulation of surface antigens on microglia, which ultimately triggers the release of inflammatory cytokines.32 Sensorimotor behavioral deficits following dTAI have been reported, possibly as a result of microglia-induced inflammatory tissue damage.34
Background on Tau
Tau isoforms and tau mutations
The gene responsible for encoding tau is microtubule-associated protein tau (mapt) on chromosome 17.35 After tau is encoded, six isoforms can form by various splicing of exons 2, 3, or 10 on the microtubule-binding domain of pre-mRNA.36 The exon 10 splice variant, hTau40, is the most important in neurodegenerative disease development, and is found specifically in the central nervous system.37 The isoforms consist of three (3R) or four (4R) tau repeats inserted at the carboxyl terminus.38 Ideally, the ratio between 3R and 4R isoforms in the brain is maintained at 1:1.39 When mutations occur in the exon 10 splice variant, the ratio is shifted to favor an increase in the 3R isoforms.37 Normally, apolipoprotein E in the brain helps catalyze the proteolytic breakdown of mutated tau and restore the ideal 3R to 4R ratio, but the APOɛ4 allele produces an apolipoprotein that is ineffective in this reaction.3 When mutations persist, they play an important role in NFT development and the activation of astrocytes.8 The altered tau proteins have widespread pathological consequences.18
Tau kinase overview
Tau has 79 potential binding sites, and phosphorylation of these sites plays an important role in embryonic CNS development.40 Thirty functional sites on the normal tau protein can be phosphorylated in the adult brain as depicted in Figure 2, but the amount of phosphorylation is kept to a minimum by tau phosphatases.41 In the adult brain, tau is regulated through multisite phosphorylation at serine/threonine residues by proline kinases such as extracellular signal-regulated kinases (ERK1/2), cycline-dependent kinase 5 (CDK5), and glycogen synthase kinase 3-β (GSK3-β).42 Other non- proline kinases such as protein kinase C (PKC), c-Jun kinase (JNK), Akt, and various tyrosine kinases play a secondary role in tau phosphorylation.43 Following TBI, tau hyperphosphorylation is increased because of an elevation in kinases compared with phosphatases, as depicted in Figure 3, marking an initial pathological change that indicates future development of chronic neurodegeneration.44 The key tau kinases are described in Table 1, and discussed in further detail with relation to changes caused by TBI in the following paragraphs.
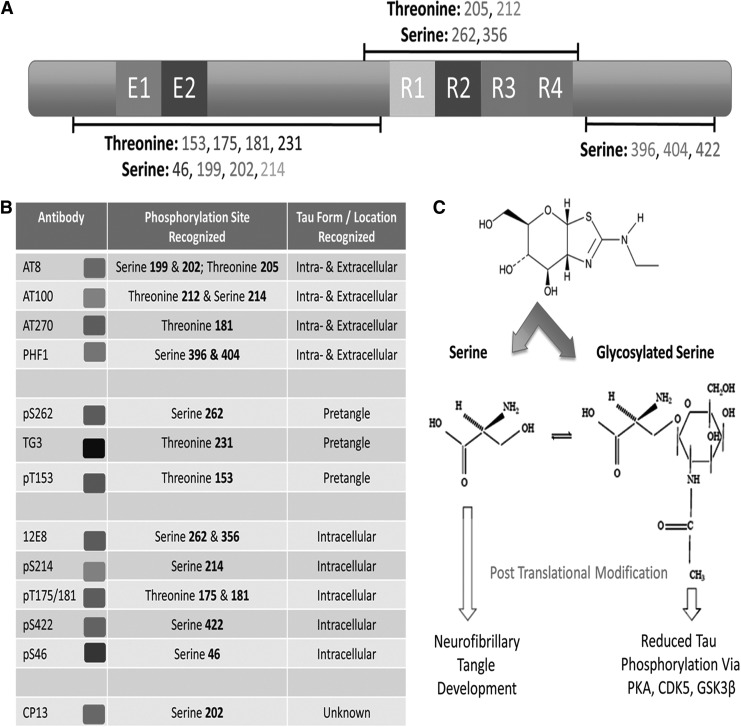
FIG. 2.
Tau is regulated by multiple biochemical processes including: nitration, glycosylation, ubiquitination, acetylation, sumoylation, and phosphorylation. (A) We highlight some of the key regulation sites potentially involved in the pathophysiology of chronic traumatic encephalopathy (CTE). (B) Each site has a specific antibody of interest that can be used to detect changes in intracellular/extracellular tau. (C) Deglycosylation allows for conversion of tau tangles into bundles of straight filaments, thus increasing the accessibility of remaining tau located at microtubule edges. Glycosylation, however, reduces phosphorylation of protein kinase A (PKA), cycline-dependent kinase 5 (CDK5), and glycogen synthase kinase-3β (GSK3β) decreasing formation of neurofibrillary tangles. This example shows how post-translational modification of tau can regulate tau phosphorylation and ultimately lead to the development of neurofibrillary tangles, a hallmark of CTE.
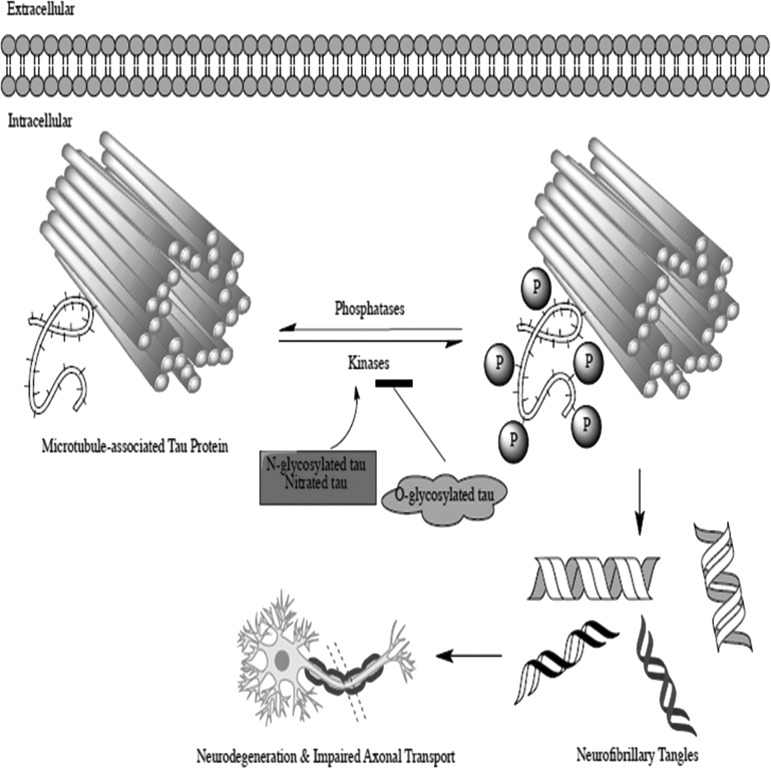
FIG. 3.
Neurofibrillary tangle formation involves an imbalance between tau kinase and tau phosphatase activity. If tau kinase activity is increased, and the phosphatase activity is decreased, hyperphosphorylation persists and can result in the formation of neurofibrillary tangles. Neurofibrillary tangles contribute to poor outcome by disrupting axonal transport and eventually causing the hierarchical spread of neurodegeneration. Neurodegeneration ultimately causes the classic symptoms seen in patients suspected of having chronic traumatic encephalopathy.
Table 1.
List of Tau Kinases and the Physiologic Roles in which They Function, also Highlighting if the Overall Levels of these Kinases Are Altered by Traumatic Brain Injury (TBI)
| Kinase name | Site of regulation | Physiological role | Activated by TBI |
|---|---|---|---|
| Extracellular signal-regulated kinases (ERK1/2) | Phosphorylation of threonine-x-tyrosine motif | Important role in growth factor signaling, cell survival, and apoptosis | Yes45 |
| Cycline-dependent kinase 5 (CDK5) | Binding to CDK Receptor 1 or CDK Receptor 2 | Plays a role in neural development, pain signaling, and sensory processing | Yes52 |
| Glycogen synthase kinase 3-β (GSK3-β) | Requires priming kinase to phosphorylate a substrate prior to phosphorylation at tyrosine-216. Phosphorylation at serine-9, however, hides the active site | Implicated in neuronal development, glucose homeostasis, and body pattern organization | Yes57 |
| Protein kinase C (PKC) | 3 categories based on binding at C-terminal: conventional requires diacylglycerol and calcium for activation, novel requires diacylglycerol, and atypical does not require calcium or diacylglycerol. Once active, the receptors for activated C-kinase bind PKC and help translocate it to the plasma membrane | PKC activity is involved with learning and memory, regulation of transcription, controlling cell growth, and mediating immune responses | Yes68 |
| c-Jun kinase (JNK) | Diphosphorylation of the threonine-proline-tyrosine motif | JNKs participate in multiple stress cascades, the inflammation response, and reactive oxygen species formation | Yes70 |
| Akt | Akt binds to phosphatidylinositol (3,4,5)-triphosphate on the cell membrane and then is phosphorylated at threonine 308 by phosphoinosotide kinase 1 | Akt plays a role in apoptosis, cellular metabolism, and cell migration | Yes75 |
ERK1/2
ERK1/2 is dephosphorylated following a single mild TBI.45 The dephosphorylated ERK1/2 triggers apoptosis via caspase3 activation.46 Administration of estrone after TBI triggers ERK1/2 phosphorylation and pro-survival.47 This hormone warrants further investigation with regard to its potential role in ameliorating tau hyperphosphorylation. Estrone may initiate a neuroprotective priming response that protects against subsequent injury.47 Other kinases, such as p70S6K and mitogen and stress-activated protein kinase 1, may also be involved in this neuroprotective process.48,49 Facilitation of ERK1/2 via the compound PD90859 can additionally foster cell survival after brain injury.50 In addition to mediating the complex balance between cell survival and apoptosis, ERK1/2 plays another unique role by regulating the cytoskeleton of activated astrocytes following TBI.51 ERK1/2 is, therefore, important not only for tau hyperphosphorylation but also in the process of reactive astrogliosis.
CDK5
Following TBI, CDK5 acutely binds to its receptor and activates a pro-apoptotic cascade.52 Furthermore, CDK5 triggers cell-cycle activation and microglia activation following controlled cortical impact TBI.53 The extent and duration of microglia activation mediated by CDK5 requires further investigation. It is known that inhibition of CDK5 with the roscovitine derivative, CR8, promotes neuroprotection and decreased apoptosis after controlled cortical impact.54 Additionally, roscovitine itself can improve cognitive and motor function in Sprague–Dawley rats after TBI.55 CDK5 inhibitors even improve outcome when administered several hours to days after TBI.56 How these inhibitors alter the progression of tauopathies has yet to be investigated.
GSK3-β
Protein kinase B (PKB) and serum and glucocorticoid-regulated kinase (SGK) are activated following TBI, which both subsequently phosphorylate GSK3-β.57,58 GSK3-β activation via its phosphorylation has been linked to apoptosis and tau hyperphosphorylation.59 Furthermore, GSK3-β upregulates N-Methyl d-aspartate (NMDA) receptors following brain injury, causing an exacerbation of glutamate excitotoxicity.60 Inhibition of GSK3-β consequently reduces apoptosis and the extent of excitotoxicity.61 Humanin is a potential inhibitor of GSK3-β that increases neuroprotection following brain injury, but further studies are still needed to elucidate the mechanism of action.62 GSK3-β is also functionally significant in microglial migration, translocation of monocytes across the blood–brain barrier (BBB), and inflammatory cascades following TBI.63 GSK3-β may, therefore, be a key target in discovering the link between acute brain injury and chronic neurodegeneration, because of its primary roles in both microglia migration and tau hyperphosphorylation.
PKC
The five most common isoforms of PKC (α, δ, ɛ, ζ, η) play various supporting roles as serine/threonine (Ser/Thr) kinases throughout multiple tissues in the body.64 Activation of specific PKC isoforms (α, δ, and ζ) is associated with perturbations in tight junction proteins following brain injury, which ultimately leads to increased BBB permeability.65 The disruption in the BBB further increases PKC activity, thereby triggering the tau kinase, GSK3-β.66 PKCη additionally activates the pro-survival tau kinase, Akt, at several days post-injury.67 After hyperphosphorylation occurs, PKCα maintains the phosphorylation changes by inhibiting tau phosphatases.65 PKC prompts signal cascades that work in conjunction with altered calcium homeostasis to propel the development of NFTs.66 Because PKC involvement is intimately associated with tau hyperphosphorylation and NFT formation, it seems reasonable to investigate the role of selective PKC inhibitors/activators, such as bryostatin and balonol, in the prevention of chronic tauopathies such as CTE.68,69
JNK
JNK is increased in damaged axons following TBI.70 JNK activity is also markedly increased in neurons and astrocytes of the hippocampus following TBI.71 JNK signaling can cause post-traumatic cellular damage within the brain following injury.70 JNK additionally may phosphorylate p53, which enhances neuronal autophagy.72 When JNK is inhibited, the extent of abnormal tau hyperphospharylation is lessened.70 Furthermore, glucagon has been used to inhibit JNK signaling immediately after TBI, causing a decrease in intracranial cerebrovasodilation.73 Maintaining JNK signaling within a tightly controlled range is not only important for tau regulation but also for maintaining blood flow to the brain following TBI.74
Akt
Akt produces an interesting effect following brain injury, by phosphorylating tau at Ser212, but also inhibiting GSK3-β.75 GSK3-β activation may, therefore, be necessary for initial tau hyperphosphorylation; however, Akt activity maintains hyperphosphorylation at later time points.43 By inhibiting GSK3-β, it is thought that Akt triggers an antiapoptotic pathway allowing for damaged cells to survive and propagate NFTs.75 If Akt is inhibited, cell death will occur.76 Histone deacetylase inhibitors, such as scriptaid, prevent the dephosphorylation of Akt, and, therefore, increase the number of surviving neurons after TBI.77 Akt regulation warrants further investigation to tease out the level of activation that is necessary for maintaining neuroprotective properties while avoiding the spread of NFTs.
Tau phosphatases
Two phosphatases, protein phosphatase 1 and 2A, are responsible for maintaining tau in a non-hyperphosphorylated state.41 If these two phosphatases become dysfunctional or decreased, the hyperphosphorylated tau is quickly ubiquitinated, which then predisposes the neuron to increased NFT formation.78 TBI can cause a decrease in tau phosphatases.79 In particular, protein phosphatase 2A is decreased in the hippocampus for several weeks post-TBI, which results in dysfunctional hippocampus plasticity.80 Tau phosphatase activity must drop by half before NFTs will begin to develop.78 Compounds that increase tau phosphatases, such as sodium selenate, may prove promising in slowing the progression of tauopathies.81
Discussion
Although the postmortem pathology of CTE has been well described, the mechanism by which acute TBI leads to initial tau hyperphosphorylation and the eventual development of neurofibrillary tangles remains poorly understood. Because the term CTE was only recently reintroduced into the medical literature in 2005, understanding disease pathophysiology is in its infancy.82 Despite the link between TBI and CTE being associational rather than mechanistic at this point, the growing prevalence of this disease among soldiers, football players, wrestlers, and other athletes exposed to brain injuries increases the urgency for finding a causative mechanism, and also for locating pharmacological targets for treating this devastating disease. In the following paragraphs, we discuss a few molecular pathways previously associated with other forms of brain injury that warrant further investigation following TBI.
Endoplasmic reticulum (ER) stress
The ER is responsible for the correct folding and sorting of proteins.83 Following brain injury, the ER becomes dysfunctional, as is evidenced by changes in bound intracellular calcium, leading to the accumulation of unfolded proteins within the cell.84 The increase in unfolded proteins is known as the “ER stress response.”85 Ischemic stroke and TBI can both cause acute activation of the ER stress response.86 Three arms of the ER stress response (protein kinase-like ER kinase [PERK], inositol requiring enzyme 1α [IRE1α], and activating transcription factor 6 [ATF6]) regulate the amount of pro-apoptotic activity following injury.83 All three arms affect the protein expression of C/EBP-homologous protein (CHOP).84 CHOP is noteworthy for its ability to trigger apoptosis via the activation of caspase12.86 If CHOP is maintained below threshold by the PERK arm, neuronal apoptosis does not occur.85 When CHOP is pushed beyond threshold through activation of the IRE1α and ATF6 arms, neuronal apoptosis does occur, and tau hyperphosphorylation results from GSK3-β.87 Furthermore, two downstream targets of the ATF6 arm of the ER stress pathway are mitogen-activated protein kinase (MAPK) and JNK, which may subsequently be involved in tau hyperphosphorylation as well.88 In light of these findings, it may prove beneficial to utilize a pharmacological agent that attenuates the ER stress response in a model of TBI. Salubrinal can increase activity of the neuroprotective PERK arm of the ER stress response and inhibit the pro-apoptotic activity of the IRE1α arm of the ER stress response.89 Because GSK3-β and caspase12 are increased following brain injury, it may also be worth investigating the GSK3-β peptide inhibitors, L803-mts and TDZD-8, and the role they may play in preventing the development of NFTs.87,90
Glutamate excitotoxicity
Glutamate excitotoxicity is triggered following brain injury, and results in elevated intracellular calcium, formation of ROS, and mitochondrial failure.91 Ischemia and other forms of brain injury can cause an increase in calcium that activates α-calcium/calmodulin protein kinase II, leading to memory impairment via increased AMPA receptor activity in the hippocampus.92 The increased calcium also leads to intracellular accumulation in neuronal mitochondria, making the organelle dysfunctional.91 Activated microglia and astrocytes concurrently release interleukin-6, which triggers a further increase in intracellular calcium within neurons and sensitizes NMDA receptors.93 Sensitized NMDA receptors promote auxiliary excitotoxicity and foster the release of ROS from the mitochondria, which can eventually cause neuronal destruction.28 Caspase3, a pro-apoptotic factor, is increased following glutamate- induced mitochondria dysfunction.86 Caspase3 can cause tau cleavage and predisposes the neuron to NFT development.87 To stem the tide of neuronal destruction and progressive tau changes, it seems fitting to investigate compounds that are known to decrease the amount of ROS such as the nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase inhibitor, apocynin.27 Furthermore, targeting mitochondrial dysfunction through p38 inhibitors may also prove beneficial following head trauma.94 By targeting key downstream pathways of glutamate excitotoxicity, it may be possible to alleviate the potential progression to neurodegeneration.
Microglial and astrocyte regulators
Neurotrauma can result in a dynamic equilibrium between classically activated (M1) and alternatively activated (M2) microglia.31 M1 microglia are pro-inflammatory, whereas M2 microglia are anti-inflammatory.34 Targeting the activation of M2 microglia immediately following injury may prove beneficial in preventing neurodegeneration.95 Alternatively, acutely inhibiting M1 microglia with the noncompetitive cholinesterase inhibitor, donepezil, has also decreased neuroinflammation and apoptosis after TBI.96 Similarly, neurotrauma triggers two distinct responses, pro-survival or apoptosis, in activated astrocytes, depending upon the extent and duration of injury.25 If astrocyte activation extends several days post-injury, it was found that nitration of tau occurs, which may lead to a more rapid development of NFTs.97 Furthermore, mutations in tau may be occurring in activated astrocytes, resulting in tau oligomers being subsequently secreted into the extracellular milieu.17 Future studies are needed to characterize the time course of astrocyte activation following TBI, and, more importantly, at what point it is ideal to inhibit astrocyte activation.
Clinical Relevance and Conclusions
The Veterans Affairs Healthcare System reported that patients exposed to repetitive blast waves have quantitative electroencephalogram changes that are comparable to concussive injury.98 Similarly, the detection of repetitive concussions in athletes has increased significantly over the past 20 years.10 The duration between injuries may account for why certain individuals develop rapidly progressive neurodegeneration and increased phospho-tau expression.13 The Department of Defense has recently invested $700,000,000 into improving clinical diagnosis and care for the 266,810 bTBI patients who were injured from 2001 onwards.98 Likewise, the National Football League has recently organized a new medical committee to investigate the issue of TBI, and has started a multiprong approach for making football safer for the players.99 Increased investigation into understanding the pathology of CTE will hopefully aid in premortem diagnosis, as well as finding viable treatment options. The pathways mentioned in this review (ER stress, glutamate excitotoxicty, and microglia and astrocyte modulation) appear promising in understanding the link between acute bTBI and CTE development. Discovering the process of tau hyperphosphorylation and NFT development following TBI will likely provide a key for unlocking the unknown mysteries of similar progressive neurodegenerative diseases.
Acknowledgment
We thank Ann Noelle Lucke-Wold for her help in editing this manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Goldstein L.E., Fisher A.M., Tagge C.A., Zhang X.L., Velisek L., Sullivan J. A., Upreti C., Kracht J.M., Ericsson M., Wojnarowicz M.W., Goletiani C.J., Maglakelidze G.M., Casey N., Moncaster J.A., Minaeva O., Moir R.D., Nowinski C. J., Stern R.A., Cantu R.C., Geiling J., Blusztajn J.K., Wolozin B.L., Ikezu T., Stein T.D., Budson A.E., Kowall N.W., Chargin D., Sharon A., Saman S., Hall G.F., Moss W.C., Cleveland R.O., Tanzi R.E., Stanton P.K., and McKee A.C. (2012). Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 4, 134–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gavett B.E., Stern R.A., and McKee A.C. (2011). Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin. Sports Med. 30, 179–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern R.A., Riley D.O., Daneshvar D.H., Nowinski C.J., Cantu R.C., and McKee A.C. (2011). Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM R 3, S460–S467 [DOI] [PubMed] [Google Scholar]
- 4.Shenton M.E., Hamoda H.M., Schneiderman J.S., Bouix S., Pasternak O., Rathi Y., Vu M.A., Purohit M.P., Helmer K., Koerte I., Lin A.P., Westin C.F., Kikinis R., Kubicki M., Stern R.A., and Zafonte R. (2012). A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 6, 137–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peskind E.R., Brody D., Cernak I., McKee A., and Ruff R.L. (2013). Military- and sports-related mild traumatic brain injury: clinical presentation, management, and long-term consequences. J. Clin. Psychiatry 74, 180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern R.A., Daneshvar D.H., Baugh C.M., Seichepine D.R., Montenigro P.H., Riley D.O., Fritts N.G., Stamm J.M., Robbins C.A., McHale L., Simkin I., Stein T.D., Alvarez V.E., Goldstein L.E., Budson A.E., Kowall N.W., Nowinski C.J., Cantu R.C., and McKee A.C. (2013). Clinical presentation of chronic traumatic encephalopathy. Neurology 81, 1122–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKee A.C., Cantu R.C., Nowinski C.J., Hedley–Whyte E.T., Gavett B.E., Budson A.E., Santini V.E., Lee H., Kubilus C.A., and Stern R.A. (2009). Chronic traumatic encephalopathy in athletes. J. Neuropathol. Exp. Neurol. 68, 709–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spillantini M.G., Lovino M., and Vuono R. (2011). Release of growth factors by neuronal precursor cells as a treatment for diseases with tau pathology. Arch. Ital. Biol. 149, 215–223 [DOI] [PubMed] [Google Scholar]
- 9.Bailes J.E., Petraglia A.L., Omalu B.I., Nauman E., and Talavage T. (2013). Role of subconcussion in repetitive mild traumatic brain injury. J. Neurosurg. 119, 1235–1245 [DOI] [PubMed] [Google Scholar]
- 10.Small G. W., Kepe V., Siddarth P., Ercoli L.M., Merrill D.A., Donoghue N., Bookheimer S.Y., Martinez J., Omalu B., Bailes J., and Barrio J.R. (2013). PET scanning of brain tau in retired national football league players: preliminary findings. Am. J. Geriatr. Psychiatry 21, 138–144 [DOI] [PubMed] [Google Scholar]
- 11.Lakis N., Corona R.J., Toshkezi G., and Chin L.S. (2013). Chronic traumatic encephalopathy – neuropathology in athletes and war veterans. Neurol. Res. 35, 290–299 [DOI] [PubMed] [Google Scholar]
- 12.Iqbal K., Gong C.X., and Liu F. (2013). Hyperphosphorylation–induced tau oligomers. Front. Neurol. 4, 112–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mannix R., Meehan W.P., Mandeville J., Grant P.E., Gray T., Berglass J., Zhang J., Bryant J., Rezaie S., Chung J.Y., Peters N.V., Lee C., Tien L.W., Kaplan D.L., Feany M., and Whalen M. (2013). Clinical correlates in an experimental model of repetitive mild brain injury. Ann. Neurol. 73, 65–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker K.R., and Tesco G. (2013). Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Front. Aging Neurosci. 5, 29–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowan C.M., and Mudher A. (2013). Are tau aggregates toxic or protective in Tauopathies? Front. Neurol. 4, 114–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whittington R.A., Bretteville A., Dickler M.F., and Planel E. (2013). Anesthesia and tau pathology. Prog. Neuropsychopharmacol. Biol. Psychiatry 4, S0278–5846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Calignon A., Polydoro M., Suarez–Calvet M., William C., Adamowicz D.H., Kopeikina K.J., Pitstick R., Sahara N., Ashe K.H., Carlson G.A., Spires–Jones T.L., and Hyman B.T. (2012). Propagation of tau pathology in a model of early Alzheimer's disease. Neuron 73, 685–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le M.N., Kim W., Lee S., McKee A.C., and Hall G.F. (2012). Multiple mechanisms of extracellular tau spreading in a non-transgenic tauopathy model. Am. J. Neurodegener. Dis. 1, 316–333 [PMC free article] [PubMed] [Google Scholar]
- 19.Hebron M.L., Lonskaya I., Sharpe K., Weerasinghe P.P., Algarzae N.K., Shekoyan A.R., and Moussa C.E. (2013). Parkin ubiquitinates Tar-DNA binding protein-43 (TDP-43) and promotes its cytosolic accumulation via interaction with histone deacetylase 6 (HDAC6). J. Biol. Chem. 288, 4103–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bandyopadhyay U., Cotney J., Nagy M., Oh S., Leng J., Mahajan M., Mane S., Fenton W.A., Noonan J.P., and Horwich A.L. (2013). RNA-Seq profiling of spinal cord motor neurons from a presymptomatic SOD1 ALS mouse. PLoS One 8, e53575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashita T., Hideyama T., Hachiga K., Teramoto S., Takano J., Iwata N., Saido T.C., and Kwak S. (2012). A role for calpain-dependent cleavage of TDP-43 in amytrophic lateral sclerosis pathology. Nat. Commun. 3, 1307–1321 [DOI] [PubMed] [Google Scholar]
- 22.McKee A.C., Gavett B.E., Stern R.A., Nowinski C.J., Cantu R.C., Kowall N.W., Perl D.P., Hedley–Whyte E.T., Price B., Sullivan C., Morin P., Lee H.S., Kubilus C. A., Daneshvar D.H., Wulff M., and Budson A.E. (2010). TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J. Neuropathol. Exp. Neurol. 69, 918–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sirko S., Behrendt G., Johansson P.A., Tripathi P., Costa M., Bek S., Heinrich C., Tiedt S., Colak D., Dichgans M., Fischer I.R., Plesnila N., Staufenbiel M., Haass C., Snapyan M., Saghatelyan A., Tsai L.H., Fischer A., Grobe K., Dimou L., and Götz M. (2013). Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. Cell Stem Cell 12, 426–439 [DOI] [PubMed] [Google Scholar]
- 24.Hochman S., Wang W., Wang W., Mei X., Huang J., Wei Y., Wang Y., Wu S., and Li Y. (2009). Crosstalk between spinal astrocytes and neurons in nerve injury-induced neuropathic pain. PLoS ONE 4, e6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kochanek P.M., Dixon C.E., Shellington D.K., Shin S.S., Bayır H., Jackson E. K., Kagan V.E., Yan H.Q., Swauger P.V., Parks S.A., Ritzel D.V., Bauman R., Clark R.S., Garman R.H., Bandak L., Ling G., and Jenkins L.W. (2013). Screening of biochemical and molecular mechanisms of secondary injury and repair in the brain after experimental blast-induced traumatic brain injury in rats. J. Neurotrauma 30, 920–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arun P., Abu–Taleb R., Oguntayo S., Tanaka M., Wang Y., Valiyaveettil M., Long J.B., Zhang Y., and Nambiar M.P. (2013). Distinct patterns of expression of traumatic brain injury biomarkers after blast exposure: Role of compromised cell membrane integrity. Neurosci. Lett. 552, 87–91 [DOI] [PubMed] [Google Scholar]
- 27.Ferreira A.P.O., Rodrigues F.S., Della–Pace I.D., Mota B.C., Oliveira S.M., Velho Gewehr C.d.C., Bobinski F., de Oliveira C.V., Brum J.S., Oliveira M.S., Furian A.F., de Barros C.S.L., Ferreira J., Santos A.R.S.d., Fighera M.R., and Royes L.F.F. (2013). The effect of NADPH-oxidase inhibitor apocynin on cognitive impairment induced by moderate lateral fluid percussion injury: Role of inflammatory and oxidative brain damage. Neurochem. Int. 63, 583–593 [DOI] [PubMed] [Google Scholar]
- 28.Qureshi G.A., Baig S., Sarwar M., and Parvez S.H. (2004). Neurotoxicity, oxidative stress and cerebrovascular disorders. Neurotoxicology 25, 121–138 [DOI] [PubMed] [Google Scholar]
- 29.Madathil S.K., Carlson S.W., Brelsfoard J.M., Ye P., D'Ercole A.J., and Saatman K.E. (2013). Astrocyte-specific overexpression of insulin-like growth factor-1 protects hippocampal neurons and reduces behavioral deficits following traumatic brain injury in mice. PLoS One 8, e67204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai A.Y., Dibal C.D., Armitage G.A., Winship I.R., and Todd K.G. (2013). Distinct activation profiles in microglia of different ages: A systematic study in isolated embryonic to aged microglial cultures. Neuroscience 254, 185–195 [DOI] [PubMed] [Google Scholar]
- 31.Acosta S.A., Tajiri N., Shinozuka K., Ishikawa H., Grimmig B., Diamond D., Sanberg P.R., Bickford P.C., Kaneko Y., and Borlongan C.V. (2013). Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PLoS One 8, e53376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernandez–Ontiveros D.G., Tajiri N., Acosta S., Giunta B., Tan J., and Borlongan C.V. (2013). Microglia activation as a biomarker for traumatic brain injury. Front. Neurol. 4, 30–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar A., Stoica B.A., Sabirzhanov B., Burns M.P., Faden A.I., and Loane D.J. (2013). Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiol. Aging 34, 1397–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith C. (2013). Review: the long-term consequences of microglial activation following acute traumatic brain injury. Neuropathol. Appl. Neurobiol. 39, 35–44 [DOI] [PubMed] [Google Scholar]
- 35.Rossi G., Bastone A., Piccoli E., Morbin M., Mazzoleni G., Fugnanesi V., Beeg M., Favero E.D., Cantù L., Motta S., Salsano E., Pareyson D., Erbetta A., Elia A., Silani V., Morelli C., Salmona M., and Tagliavini F. (2013). Different mutations at V363 MAPT codon are associated with atypical clinical phenotypes and show unusual structural and functional features. Neurobiol. Aging,35, 408–417 [DOI] [PubMed] [Google Scholar]
- 36.Avale M.E., Rodriguez–Martin T., and Gallo J.M. (2013). Trans-splicing correction of tau isoform imbalance in a mouse model of tau mis-splicing. Hum. Mol. Genet. 22, 2603–2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwal P.K., Raz Y., and Miller Y. (2013). Interactions between Aß and mutated tau lead to polymorphism and induce aggregation of aß-mutated tau oligomeric complexes. PLoS ONE 8, e73303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takuma H., Arawaka S., and Mori H. (2003). Isoforms changes of tau protein during development in various species. Dev. Brain Res. 142, 121–127 [DOI] [PubMed] [Google Scholar]
- 39.Sadik G., Tanaka T., Kato K., Yanagi K., Kudo T., and Takeda M. (2009). Differential interaction and aggregation of 3-repeat and 4-repeat tau isoforms with 14-3-3ζ protein. Biochem. Biophys. Res. Commun. 383, 37–41 [DOI] [PubMed] [Google Scholar]
- 40.Kanemaru K., Takio K., Miura R., Titani K., and Ihara Y. (1992). Fetal-type phosphorylation of the tau in paired helical filament. J. Neurochem. 58, 1667–1675 [DOI] [PubMed] [Google Scholar]
- 41.Billingsley M.L., and Kincaid R.L. (1997). Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem. J. 323, 577–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsujio I., Tanaka T., Kudo T., Nishikawa T., Shinozaki K., Grundke-Iqbal I., Iqbal K., and Takeda M. (2000). Inactivation of glycogen synthase kinase-3 by protein kinase C δ: implications for regulation of τ phosphorylation. FEBS Lett. 469, 111–117 [DOI] [PubMed] [Google Scholar]
- 43.Zeng K., Ko H., Yang H.O., and Wang X. (2010). Icariin attenuates β-amyloid-induced neurotoxicity by inhibition of tau protein hyperphosphorylation in PC12 cells. Neuropharmacology 59, 542–550 [DOI] [PubMed] [Google Scholar]
- 44.Liu Y., Su Y., Wang J., Sun S., Wang T., Qiao X., Run X., Li H., and Liang Z. (2013). Rapamycin decreases tau phosphorylation at Ser214 through regulation of cAMP-dependent kinase. Neurochem. Int. 62, 458–467 [DOI] [PubMed] [Google Scholar]
- 45.Kuo J.R., Cheng Y.H., Chen Y.S., Chio C.C., and Gean P.W. (2013). Involvement of extracellular signal regulated kinases in traumatic brain injury-induced depression in rodents. J. Neurotrauma 30, 1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao Y., Luo P., Guo Q., Li S., Zhang L., Zhao M., Xu H., Yang Y., Poon W., and Fei Z. (2012). Interactions between SIRT1 and MAPK/ERK regulate neuronal apoptosis induced by traumatic brain injury in vitro and in vivo. Exp. Neurol. 237, 489–498 [DOI] [PubMed] [Google Scholar]
- 47.Gatson J.W., Liu M.M., Abdelfattah K., Wigginton J.G., Smith S., Wolf S., Simpkins J.W., and Minei J.P. (2012). Estrone is neuroprotective in rats after traumatic brain injury. J. Neurotrauma 29, 2209–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen S., Atkins C.M., Liu C.L., Alonso O.F., Dietrich W.D., and Hu B.R. (2007). Alterations in mammalian target of rapamycin signaling pathways after traumatic brain injury. J. Cereb. Blood Flow Metab. 27, 939–949 [DOI] [PubMed] [Google Scholar]
- 49.Li Z., Zhao L., Hang H., Zhu N., Ning B., and Lv Z. (2013). Spatiotemporal patterns and essential role of msk1 expression after rat spinal cord injury. Neurochem Res 38, 2581–2587 [DOI] [PubMed] [Google Scholar]
- 50.Mori T., Wang X., Jung J. C., Sumii T., Singhal A.B., Fini M.E., Dixon C.E., Alessandrini A., and Lo E.H. (2002). Mitogen-activated protein kinase inhibition in traumatic brain injury: in vitro and in vivo effects. J. Cereb. Blood Flow Metab. 22, 444–452 [DOI] [PubMed] [Google Scholar]
- 51.Kramerov A.A., Golub A.G., Bdzhola V.G., Yarmoluk S.M., Ahmed K., Bretner M., and Ljubimov A.V. (2011). Treatment of cultured human astrocytes and vascular endothelial cells with protein kinase CK2 inhibitors induces early changes in cell shape and cytoskeleton. Mol. Cell Biochem. 349, 125–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li A., Zou F., Fu H., Cui G., Yan Y., Wu Q., and Gu X. (2013). Upregulation of CRM1 relates to neuronal apoptosis after traumatic brain injury in adult rats. J. Mol. Neurosci. 51, 208–218 [DOI] [PubMed] [Google Scholar]
- 53.Kabadi S.V., Stoica B.A., Byrnes K.R., Hanscom M., Loane D.J., and Faden A.I. (2012). Selective CDK inhibitor limits neuroinflammation and progressive neurodegeneration after brain trauma. J. Cereb. Blood Flow Metab. 32, 137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kabadi S.V., Stoica B.A., Hanscom M., Loane D.J., Kharebava G., Murray M. G., Cabatbat R.M., and Faden A.I. (2012). CR8, a selective and potent CDK inhibitor, provides neuroprotection in experimental traumatic brain injury. Neurotherapeutics 9, 405–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hilton G.D., Stoica B.A., Byrnes K.R., and Faden A.I. (2008). Roscovitine reduces neuronal loss, glial activation, and neurologic deficits after brain trauma. J. Cereb. Blood Flow Metab. 28, 1845–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson S.M., Torrice C.D., Bell J.F., Monahan K.B., Jiang Q., Wang Y., Ramsey M.R., Jin J., Wong K.K., Su L., Zhou D., and Sharpless N.E. (2010). Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK4/6 inhibition. J. Clin. Invest. 120, 2528–2536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shapira M., Licht A., Milman A., Pick C.G., Shohami E., and Eldar–Finkelman H. (2007). Role of glycogen synthase kinase-3β in early depressive behavior induced by mild traumatic brain injury. Mol. Cell Neurosci. 34, 571–577 [DOI] [PubMed] [Google Scholar]
- 58.Wu X., Mao H., Liu J., Xu J., Cao J., Gu X., and Cui G. (2013). Dynamic change of SGK expression and its role in neuron apoptosis after traumatic brain injury. Int. J. Clin. Exp. Pathol. 6, 1282–1293 [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J.Z., Xia Y.Y., Grundke–Iqbal I., and Iqbal K. (2013). Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J. Alzheimers Dis. 33, 123–139 [DOI] [PubMed] [Google Scholar]
- 60.Deng Y., Xiong Z., Chen P., Wei J., Chen S., and Yan Z. (2013). β-Amyloid impairs the regulation of N-methyl-D-aspartate receptors by glycogen synthase kinase 3. Neurobiol. Aging 35, 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dash P.K., Johnson D., Clark J., Orsi S.A., Zhang M., Zhao J., Grill R.J., Moore A.N., and Pati S. (2011). Involvement of the glycogen synthase kinase-3 signaling pathway in TBI pathology and neurocognitive outcome. PLoS One 6, e24648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang T., Huang Y., Zhang M., Wang L., Wang Y., Zhang L., Dong W., Chang P., Wang Z., Chen X., and Tao L. (2013). [Gly14]-Humanin offers neuroprotection through glycogen synthase kinase-3β inhibition in a mouse model of intracerebral hemorrhage. Behav. Brain Res. 247, 132–139 [DOI] [PubMed] [Google Scholar]
- 63.Lyman M., Lloyd D. G., Ji X., Vizcaychipi M.P., and Ma D. (2014). Neuroinflammation: The role and consequences. Neurosci. Res. 79C, 1–12 [DOI] [PubMed] [Google Scholar]
- 64.Kim M.H., Jung Y., Moon C., Jeong E., Lee S.H., Baik E.J., and Moon C. (2003). Isoform-specific induction of PKC-ɛ by high glucose protects heart-derived H9c2 cells against hypoxic injury. Biochem. Biophys. Res. Commun. 309, 1–6 [DOI] [PubMed] [Google Scholar]
- 65.Combs C.K., Coleman P.D., and O'Banion M.K. (1998). Developmental regulation and PKC dependence of Alzheimer's-type tau phosphorylations in cultured fetal rat hippocampal neurons. Dev. Brain Res. 107, 143–158 [DOI] [PubMed] [Google Scholar]
- 66.Martin L., Latypova X., Wilson C.M., Magnaudeix A., Perrin M., Yardin C., and Terro F. (2013). Tau protein kinases: Involvement in Alzheimer's disease. Ageing Res. Rev. 12, 289–309 [DOI] [PubMed] [Google Scholar]
- 67.Cho C. (2011). Fronteir of epilepsy research-mTOR signaling pathway. Exp. Mol. Med. 43, 231–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zohar O., Lavy R., Zi X., Nelson T.J., Hongpaisan J., Pick C.G., and Alkon D.L. (2011). PKC activator therapeutic for mild traumatic brain injury in mice. Neurobiol. Dis. 41, 329–337 [DOI] [PubMed] [Google Scholar]
- 69.Yaragorla S., and Muthyala R. (2010). Formal total synthesis of (−)-balanol: a potent PKC inhibitor. Tetrahedron Lett. 51, 467–470 [Google Scholar]
- 70.Tran H.T., Sanchez L., and Brody D.L. (2012). Inhibition of JNK by a peptide inhibitor reduces traumatic brain injury-induced tauopathy in transgenic mice. J. Neuropathol. Exp. Neurol. 71, 116–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Otani N., Nawashiro H., Fukui S., Nomura N., Yano A., Miyazawa T., and Shima K. (2002). Differential activation of mitogen-activated protein kinase pathways after traumatic brain injury in the rat hippocampus. J. Cereb. Blood Flow Metab. 22, 327–334 [DOI] [PubMed] [Google Scholar]
- 72.Hong M.Y., Gao J.L., Cui J.Z., Wang K.J., Tian Y.X., Li R., Wang H.T., and Wang H. (2012). Effect of c-Jun NH2-terminal kinase-mediated p53 expression on neuron autophagy following traumatic brain injury in rats. Chin. Med. J. 125, 2019–2024 [PubMed] [Google Scholar]
- 73.Armstead W.M., Riley J., Cines D.B., and Higazi A.A. (2012). Combination therapy with glucagon and a novel plasminogen activator inhibitor-1-derived peptide enhances protection against impaired cerebrovasodilation during hypotension after traumatic brain injury through inhibition of ERK and JNK MAPK. Neurol. Res. 34, 530–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Armstead W.M., Kiessling J.W., Riley J., Cines D.B., and Higazi A.A. (2011). tPA contributes to impaired NMDA cerebrovasodilation after traumatic brain injury through activation of JNKMAPK. Neurol. Res. 33, 726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ksiezak–Reding H., Pyo H.K., Feinstein B., and Pasinetti G.M. (2003). Akt/PKB kinase phosphorylates separately Thr212 and Ser214 of tau protein in vitro. Biochim. Biophys. Acta 1639, 159–168 [DOI] [PubMed] [Google Scholar]
- 76.Farook J.M., Shields J., Tawfik A., Markand S., Sen T., Smith S.B., Brann D., Dhandapani K.M., and Sen N. (2013). GADD34 induces cell death through inactivation of Akt following traumatic brain injury. Cell Death Dis. 4, e754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang G., Jiang X., Pu H., Zhang W., An C., Hu X., Liou A.K., Leak R.K., Gao Y., and Chen J. (2013). Scriptaid, a novel histone deacetylase inhibitor, protects against traumatic brain injury via modulation of PTEN and AKT pathway: scriptaid protects against TBI via AKT. Neurotherapeutics 10, 124–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lambrecht C., Haesen D., Sents W., Ivanova E., and Janssens V. (2013). Structure, regulation, and pharmacological modulation of PP2A phosphatases. Methods Mol. Biol. 1053, 283–305 [DOI] [PubMed] [Google Scholar]
- 79.Kane M.J., Angoa–Pérez M., Briggs D.I., Viano D.C., Kreipke C.W., and Kuhn D.M. (2012). A mouse model of human repetitive mild traumatic brain injury. J. Neurosci. Methods 203, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bales J.W., Ma X., Yan H.Q., Jenkins L.W., and Dixon C.E. (2010). Expression of protein phosphatase 2B (calcineurin) subunit A isoforms in rat hippocampus after traumatic brain injury. J. Neurotrauma 27, 109–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Corcoran N.M., Martin D., Hutter–Paier B., Windisch M., Nguyen T., Nheu L., Sundstrom L.E., Costello A.J., and Hovens C.M. (2010). Sodium selenate specifically activates PP2A phosphatase, dephosphorylates tau and reverses memory deficits in an Alzheimer's disease model. J. Clin. Neurosci. 17, 1025–1033 [DOI] [PubMed] [Google Scholar]
- 82.Omalu B.I., DeKosky S.T., Minster R.L., Kamboh M.I., Hamilton R.L., and Wecht C. H. (2005). Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 57, 128–134 [DOI] [PubMed] [Google Scholar]
- 83.Rubovitch V., Shachar A., Werner H., and Pick C.G. (2011). Does IGF-1 administration after a mild traumatic brain injury in mice activate the adaptive arm of ER stress? Neurochem. Int. 58, 443–446 [DOI] [PubMed] [Google Scholar]
- 84.Osada N., Kosuge Y., Kihara T., Ishige K., and Ito Y. (2009). Apolipoprotein E-deficient mice are more vulnerable to ER stress after transient forebrain ischemia. Neurochem. Int. 54, 403–409 [DOI] [PubMed] [Google Scholar]
- 85.Cullinan S.B., and Diehl J.A. (2006). Coordination of ER and oxidative stress signaling: The PERK/Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 38, 317–332 [DOI] [PubMed] [Google Scholar]
- 86.Srinivasan K., and Sharma S.S. (2012). 3-Bromo-7-nitroindazole attenuates brain ischemic injury in diabetic stroke via inhibition of endoplasmic reticulum stress pathway involving CHOP. Life Sci. 90, 154–160 [DOI] [PubMed] [Google Scholar]
- 87.Hoozemans J.J.M., van Haastert E.S., Nijholt D.A.T., Rozemuller A.J.M., Eikelenboom P., and Scheper W. (2009). The unfolded protein response is activated in pretangle neurons in Alzheimer's disease hippocampus. Am. J. Pathol. 174, 1241–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krajewska M., Xu L., Xu W., Krajewski S., Kress C.L., Cui J., Yang L., Irie F., Yamaguchi Y., Lipton S.A., and Reed J.C. (2011). Endoplasmic reticulum protein BI-1 modulates unfolded protein response signaling and protects against stroke and traumatic brain injury. Brain Res. 1370, 227–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gong T., Wang Q., Lin Z., Chen M., and Sun G. (2012). Endoplasmic reticulum (ER) stress inhibitor salubrinal protects against ceramide-induced SH-SY5Y cell death. Biochem. Biophys. Res. Commun. 427, 461–465 [DOI] [PubMed] [Google Scholar]
- 90.King M.K., Pardo M., Cheng Y., Downey K., Jope R.S., and Beurel E. (2014). Glycogen synthase kinase-3 inhibitors: rescuers of cognitive impairments. Pharmacol. Ther. 141, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wagner A.K., and Zitelli K.T. (2013). A Rehabilomics focused perspective on molecular mechanisms underlying neurological injury, complications, and recovery after severe TBI. Pathophysiology 20, 39–48 [DOI] [PubMed] [Google Scholar]
- 92.Atkins C.M., Chen S., Alonso O.F., Dietrich W.D., and Hu B.R. (2006). Activation of calcium/calmodulin-dependent protein kinases after traumatic brain injury. J. Cereb. Blood Flow Metab. 26, 1507–1518 [DOI] [PubMed] [Google Scholar]
- 93.Ma D., Jin S., Li E., Doi Y., Parajuli B., Noda M., Sonobe Y., Mizuno T., and Suzumura A. (2013). The neurotoxic effect of astrocytes activated with toll-like receptor ligands. J. Neuroimmunol. 254, 10–18 [DOI] [PubMed] [Google Scholar]
- 94.Huang L., Wan J., Chen Y., Wang Z., Hui L., Li Y., Xu D., and Zhou W. (2013). Inhibitory effects of p38 inhibitor against mitochondrial dysfunction in the early brain injury after subarachnoid hemorrhage in mice. Brain Res. 1517, 133–140 [DOI] [PubMed] [Google Scholar]
- 95.Kuo C.L., Ho F.M., Chang M.Y., Prakash E., and Lin W.W. (2008). Inhibition of lipopolysaccharide-induced inducible nitric oxide synthase and cyclooxygenase-2 gene expression by 5-aminoimidazole-4-carboxamide riboside is independent of AMP-activated protein kinase. J. Cell Biochem. 103, 931–940 [DOI] [PubMed] [Google Scholar]
- 96.Hwang J., Hwang H., Lee H., and Suk K. (2010). Microglia signaling as a target of donepezil. Neuropharmacology 58, 1122–1129 [DOI] [PubMed] [Google Scholar]
- 97.Reyes J.F., Reynolds M.R., Horowitz P.M., Fu Y., Guillozet–Bongaarts A.L., Berry R., and Binder L.I. (2008). A possible link between astrocyte activation and tau nitration in Alzheimer's disease. Neurobiol. Dis. 31, 198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tompkins P., Tesiram Y., Lerner M., Gonzalez L.P., Lightfoot S.A., Rabb C.H., and Brackett D.J. (2013). Brain injury: neuro-inflammation, cognitive deficit & MRI in a model of blast induced TBI. J. Neurotrauma 30, 1888–1897 [DOI] [PubMed] [Google Scholar]
- 99.Ellenbogen R.G., Berger M.S., and Batjer H.H. (2010). The National Football League and concussion: leading a culture change in contact sports. World Neurosurg. 74, 560–565 [DOI] [PubMed] [Google Scholar]