Abstract
Background
Increasing evidence shows chemotherapy in combination with VEGF inhibition is a clinically active therapy for patients with metastatic melanoma (MM).
Methods
A phase II trial was conducted in chemotherapy naïve patients with unresectable stage IV MM who were randomized to temozolomide (200 mg/m2 on d. 1–5) and bevacizumab (10mg/kg IV d. 1 and 15) every 28 days (Regimen temozolomide/bevacizumab [TB]) or nab-paclitaxel (100mg/m2 [80 mg/m2 post addendum 5-secondary to toxicity] days 1, 8 and 15), bevacizumab (10mg/kg on days 1 and 15), and carboplatin (AUC 6 day 1 [ AUC 5 post addendum 5]) every 28 days (Regimen ABC). Accrual goal was 41 patients per regimen. The primary aim of this study was to estimate progression-free survival rate at 6 months (PFS6) in each regimen. A regimen would be considered promising if its PFS6 rate was > 60%.
Results
Ninety-three eligible patients (42 TB and 51 ABC) were enrolled. The majority of patients had M1c disease (20- TB & 26 ABC). The median PFS and overall survival (OS) times with ABC were 6.7 months and 13.9 months, respectively. Median PFS time and median OS with TB were 3.8 months and 12.3 months, respectively. The most common severe toxicities (≥grade 3) in both regimens were cytopenias, fatigue, and thrombosis. Among the first 41 patients enrolled onto each regimen, PFS6 rate was 32.8% (95% CI: 21.1–51.2%) for TB and 56.1% (90% CI: 44.7–70.4%) for ABC.
Conclusions
The addition of bevacizumab to nab-paclitaxel and carboplatin shows promising activity despite tolerability issues.
Keywords: metastatic melanoma, chemotherapy, VEGF inhibition, combination therapy, unresectable metastatic melanoma
INTRODUCTION
Melanoma affected approximately 60,000 people in the US in 20101, with approximately 8000 deaths.1 Until very recently, the Food and Drug Administration (FDA) had only approved two drugs, dacarbazine (DTIC) and interleukin-2 (IL-2) for clinical use in patients with metastatic melanoma (MM).2, 3 In 2011, two new agents received FDA approval for MM: ipilimumab (anti-CTLA4 antibody) and vemurafenib (BRAF V600E inhibitor). Both agents were approved in the US based on completed phase III clinical trials demonstrating superior survival endpoints, overall survival (OS); or progression free survival (PFS). In the case of ipilimumab, an OS advantage was observed over that of a peptide vaccine (gp100)4 and in the case of vemurafenib, a PFS advantage was observed over that of DTIC.5, 6
Over the past several years, our research team has engaged in an effort to assess the clinical utility of combinational therapeutics involving cytotoxic chemotherapy and inhibitors of angiogenesis in patients with MM. Vascular endothelial growth factor (VEGF) has been shown to play a significant role in the natural history of malignant melanoma.7, 8 The role of VEGF appears particularly in the context of melanoma therapy with cytotoxic agents. Laboratory evidence demonstrates that malignant melanocytes exposed to conventional cytotoxic agents (DTIC) dramatically up-regulate VEGF production.9, 10 Thus, the addition of a VEGF blocking agent in the context of systemic chemotherapy for MM may yield anti-tumor benefits beyond those of chemotherapy alone.
Bevacizumab is a recombinant humanized murine monoclonal antibody to VEGF- A that blocks the binding of VEGF- A to its receptors thereby inhibiting its biologic activity.11 In 2009 we reported that the combination of bevacizumab with paclitaxel and carboplatin for patients with MM resulted in modest clinical benefit in a single arm phase II clinical trial.12 A randomized comparison of paclitaxel/carboplatin/bevacizumab (PCB) to paclitaxel/carboplatin (PC) in patients with MM reported a trend towards a survival benefit of PCB over PC13, 14 even though the study did not reach its primary objective of statistically significant PFS advantage in the PCB arm. Thus, in an effort to improve upon these observations, we sought to identify a more effective chemotherapy regimen that in combination with bevacizumab would yield greater clinical benefit. As such, we conducted a randomized phase II clinical trial in chemotherapy naïve patients with MM to assess the anti-tumor activity and safety profiles of nab-paclitaxel (Abraxane®, Celgene, NJ)/bevacizumab/carboplatin (ABC) and temozolomide/bevacizumab (TB) regimens.
PATIENTS AND METHODS
This phase II clinical trial randomized patients previously untreated patients with MM to either regimen TB: temozolomide 200 mg/m2 orally days 1–5 and bevacizumab 10/kg IV days 1 and 15 of a 28 day cycle repeated until disease progression or regimen ABC: nab-paclitaxel 100mg/m2 IV days 1, 8, and 15, bevacizumab 10 mg/kg IV days 1 and 15, and carboplatin at AUC of 6 IV on day 1, of a 28 day cycle until disease progression. A stratified randomization procedure was employed (when both regimens were open to enrollment) using performance status (PS) and M sub-stage (M1a, b, c) to assign patients in equal number to the two regimens. The primary aim of this study was to independently assess the anti-tumor activity and safety profile of each regimen. The study was not designed to compare efficacy between the two regimens. The approach of conducting two concurrent phase II clinical trials (with randomization) in one study was done to streamline the protocol development process, eliminate potential selection bias, and ensure consistent evaluation and surveillance. This study was conducted as a multi-institution cooperative group trial through the North Central Cancer Treatment Group (NCCTG). All patients provided signed informed written consent. This study was approved by the institutional review boards (IRB) of all participating institutions.
The study data safety monitoring plan included criteria for suspending enrollment to any given regimen for an unacceptable toxicity. For a given regimen, if 2 or more of the first 5 patients enrolled or 30% or more thereafter developed ≥ grade 3 (CTCAE v. 3.0) non-hematologic or hematologic toxicity (excluding grade 3 neutropenia or thrombocytopenia) possibly, probably or definitely related to treatment, enrollment would be suspended and a trial recommendation would be formulated and submitted for review to the Cancer Therapy Evaluation Program/National Cancer Institute (CTEP/NCI) and institutional IRBs. Under these conditions, enrollment to regimen ABC was suspended from June 22, 2009 to August 14, 2009 when 9 of the first 28 patients randomized to regimen ABC developed severe thrombosis (3 patients), hypertension (2 patients), dyspnea and fatigue (2 patients), hemorrhage (1 patient), and oral mucositis (1 patient). The study was amended to reduce the starting dose of both nab-paclitaxel and carboplatin to 80mg/m2 and AUC 5, respectively.
Enrollment to regimen TB closed prior to that of regimen ABC due to suspension of accrual to regimen ABC, secondary to toxicity. As regimen ABC closed in on its accrual goal of 41 patients, proper notice was sent to the memberships stating that enrollment would close in 3 weeks. An unexpected jump in accrual rate occurred, leading to 52 total patients being enrolled to this regimen. The 23 patients who were enrolled to regimen ABC after addendum 5 was put into place will be referred to as the “post-addendum 5” patients (postadd 5) for the purposes of this report.
Eligibility
Eligible patients had to be ≥ 18 years of age with unresectable, histologically confirmed MM. Additional eligibility criteria included: measurable disease by the Response Evaluation Criteria in Solid Tumors (RECIST v 1.0), Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1, adequate hematologic, renal and hepatic function, life expectancy of ≥4 months, and no prior chemotherapy for MM. Exclusion criteria included prior exposure to taxanes, agents disrupting VEGF activity in the adjuvant setting; peripheral neuropathy ≥ grade 2; major surgical procedure, open biopsy, or significant trauma ≤4 weeks prior to randomization; brain metastases per magnetic resonance imaging (MRI), active infection requiring parenteral antibiotics, poorly controlled hypertension, New York Heart Association Class II–IV congestive heart failure, serious cardiac arrhythmia, myocardial infarction or unstable angina (≤6 months), clinically significant peripheral vascular disease, deep venous thrombosis or pulmonary embolus ≤1 year, need for full-dose anticoagulation therapy, active bleeding or known condition that carries a high risk of bleeding, non-healing wound, history of abdominal fistula, gastrointestinal perforation, or intra-abdominal abscess ≤6 months, central nervous system (CNS) disease (primary brain tumor, vascular abnormalities), stroke or transient ischemic attack (≤6 months), uncontrolled seizures, radiographically documented invasion of adjacent organs (duodenum, stomach) or tumor invading major blood vessels, known to be HIV positive or have hepatitis. Women who were pregnant or breast feeding were also not eligible for participation.
Assessments
Within 14 days of registration, patients underwent a complete physical exam, assessment of performance status, complete blood counts with differential, comprehensive metabolic panel (including lactate dehydrogenase [LDH]), urinalysis for proteinuria, and tumor assessment by conventional computerized axial tomography (CT), spiral CT or MRI. Within 28 days of registration, patients underwent MRI of the brain (CT if MRI not able to be performed) to assess for the presence of brain metastasis. Prior to each cycle of treatment, patients underwent a physical exam, toxicity assessments (Common Terminology Criteria for Adverse Events [CTCAE] v. 3.0) and laboratory (urine and blood) testing. Tumor status was assessed every 8 weeks until progression (RECIST v 1.0). All patients received standard supportive care, including antiemetics, antibiotics, transfusions, and growth factor support as clinically appropriate.
For patients on regimen TB, treatment was held on day 1 of the cycle if their absolute neutrophil count (ANC) was <1500 mm3, platelet count (PLT) was < 75,000 mm3 or the patient experienced >grade 3 non-hematologic toxicity. Once blood counts recovered to above these levels and/or other non-hematologic adverse events resolved to ≤ grade 1, patients were eligible for retreatment at a lower dose level of temozolomide (reduction of 50mg/m2/day). There were no dose reductions allowed on either regimen for toxicities related to bevacizumab. If a patient had a toxicity that was felt to be related to bevacizumab, further dosing was held or discontinued based on the specific toxicity. Treatment could be held for up to 4 weeks.
For patients on regimen ABC, treatment was held on day 1 of the cycle if ANC < 1500 mm3; PLT < 75,000 mm3; ≥grade 2 AST, alkaline phosphatase, or peripheral neuropathy; or any other ≥grade 3 non-hematologic toxicity. Patients could be retreated at a lower dose level (20 % dose reduction of nab-paclitaxel and carboplatin decreased by 1 AUC). As with regimen TB, there were no dose reductions allowed for toxicities related to bevacizumab. If a patient had a toxicity that was felt to be related to bevacizumab, patients were to have their dose held or discontinued based on the specific toxicity. Treatment could be held for up to 4 weeks.
Statistical Analysis
The primary endpoint for evaluating each regimen was 6 month progression-free survival rate. Under the assumption that all 41 patients randomized to a given regimen will be followed at least 6 months (or until death), a one-sided α=0.10 one sample test of proportions will have a 90% chance of detecting that the true 6 month PFS rate with that regimen is at least 60% when the true 6 month PFS rate for the regimen is greater than 40%. If any patients were lost to follow-up within 6 months of registration, the 6 month PFS rate would be estimated using the Kaplan-Meier method. If a patient died without documentation of progression, the patient was considered to have progressed at death.
Quantification of plasma VEGF levels
Plasma levels of VEGF-A and VEGF-D were measured using Duoset antibodies from R and D Systems (Minneapolis, MN) as per manufacturer’s instructions. Briefly, 96 well plates were coated overnight at 4°C with capture antibodies at 1.0ug/ml and 2.0ug/ml for VEGF-A and VEGF-D, respectively. The plates were washed and blocked for 1 hour at room temperature with reagent diluent and 50ul of undiluted plasma was added to wells in duplicate and incubated for 2 hours at room temperature. The plates were washed with PBS and 0.5% Tween-20 and 100ng/ml VEGF-A and 200ng/ml VEGF-D biotinylated detection antibodies were added for 1 hour at room temperature and was followed by incubation with a streptavidin horse radish peroxidase complex. Color was developed with tetramethylbenzidine (TMB) substrate and optical density was measured at 450nm. VEGF-A and VEGF-D concentration was determined using a standard curve ranging from 0–2000pg/ml.
RESULTS
Patient Characteristics
Ninety-five patients (regimen TB: 43 patients; regimen ABC: 52 patients) were enrolled onto this trial between August 15, 2008–January 12, 2010 (Table 1). One patient randomized to regimen TB was found ineligible at the time of registration due to brain metastasis. One patient assigned to regimen ABC withdrew consent prior to study treatment. Therefore regimen TB included 42 patients (57.1% male) ages 25–82 years (median age 57 years). Regimen ABC included 51 patients (56.9% male) ages 22–83 years (median age: 57 years). The characteristics of the patients were well balanced between the treatment regimens (Table 1).
Table 1.
Demographic and Baseline Characteristics
| Patient Characteristics | Regimen A (TB) n=43 | Regimen B (ABC) n=51 |
|---|---|---|
|
| ||
| Median Age (range) | 57 (range: 25–82) | 57 (range: 22–83) |
|
| ||
| Male | 58.1% | 56.9% |
|
| ||
| Prior radiation therapy | 25.6% | 17.6% |
|
| ||
| Prior Systemic Therapies | ||
| None | 65.1% | 62.7% |
| Immunotherapy | 32.6% | 35.3% |
| Vaccine therapy | ---- | 2.0% |
| Immuno- and vaccine therapy | 2.3% | ---- |
|
| ||
| ECOG Performance Status | ||
| 0 | 74.4% | 64.7% |
| 1 | 25.6% | 35.3% |
|
| ||
| M1 sub stage | ||
| M1a | 23.3% | 23.5% |
| M1b | 30.2% | 25.5% |
| M1c | 46.5% | 51.0% |
|
| ||
| Elevated LDH (> ULN) | 47% | 51% |
|
| ||
| Pre-existing Signs and Symptoms | ||
| Grade 3 hypertension | ---- | 2.0% |
| Grade 2 hypertension | 4.7% | 3.9% |
| Grade 2 fatigue | 11.6% | 5.9% |
| Grade 2 muscle pain | 4.7% | 5.9% |
| Grade 2 joint pain | 0.0% | 7.8% |
| Grade 2 anemia | 2.3% | 2.0% |
Treatment Course and Toxicities
Regimen TB
At the time of this report all but 1 patient had discontinued study treatment. The median number of cycles administered was 4 cycles (total, ≥247 cycles, range 1 to >29). There were 3 patients who had dose reductions of temozolomide due to hematologic adverse events. The most common severe (≥grade 3) toxicities (possibly, probably, or definitely related to treatment) included: vomiting (12%), fatigue (10%), neutropenia (10%), and leukopenia (10%) (Table 2). Three patients discontinued treatment due to the following grade 3 adverse events: pulmonary embolism (1), dyspnea (1), ileal obstruction with abdominal hemorrhage (1). Two patients died while on study treatment including a 72 year old woman with bone metastases previously treated with radiation who died during cycle 1 from complications of bone marrow suppression and a 47 year old woman who died during cycle 1 due to rapid disease progression. The reasons for treatment discontinuation among the remaining 36 patients are disease progression (32), refusal (3), and other medical condition (1).
Table 2.
More Common Toxicities Reported to be probably, possibly or definitely related to study treatment
| Regimen A (TB) (n=42) | Regimen B (ABC) Pre Add5 (n=28) | Regimen B (ABC) Post Add5 (n=23) | ||||
|---|---|---|---|---|---|---|
| Toxicity | any grade | ≥grade 3 | any grade | ≥grade 3 | any grade | ≥grade 3 |
| Anemia | 57% | 5% | 96% | 21% | 83% | 17% |
| Leukopenia | 36% | 10% | 75% | 29% | 74% | 26% |
| Neutropenia | 19% | 10% | 75% | 54% | 65% | 43% |
| Thrombocytopenia | 48% | 5% | 57% | 21% | 65% | 17% |
| Dyspnea | 2% | 2% | 18% | 14% | 9% | 0% |
| Fatigue | 88% | 10% | 93% | 21% | 87% | 9% |
| Hypertension | 52% | 2% | 29% | 7% | 17% | 0% |
| Nausea | 76% | 7% | 57% | 0% | 57% | 4% |
| Thrombosis | 5% | 5% | 18% | 14% | 9% | 9% |
| Vomiting | 43% | 12% | 11% | 4% | 30% | 0% |
| Neuropathy-sensory | 10% | 0% | 54% | 0% | 35% | 0% |
Regimen ABC
At the time of this report all but 1 patient had discontinued study treatment. The median number of cycles administered is 4 cycles (total >310 cycles, range 1 to >33). The most common severe (≥grade 3) toxicities reported included: neutropenia (49%), leukopenia (27%), thrombocytopenia (20%), anemia (20%), fatigue (16%), and thrombosis (12%) as shown in (Table 2). Twelve patients (8 pre addendum 5 and 4 post addendum 5) discontinued study treatment due to the following ≥grade 3 adverse events: thrombosis/thrombus/embolism (preadd5: 5 patients; postadd5: 1 patient) neutropenia, thrombocytopenia, leukopenia, with renal failure (preadd5: 1 patient.), pleural effusion and pancreatitis (preadd5: 1 patient), thrombocytopenia (preadd5: 1 patient.), infection (postadd5: 2 patients.), and persistent fatigue (postadd5: 1 patient.). The reasons for treatment discontinuation among the other 38 patients were disease progression (28), patient refusal (3), desire for alternative therapy (6), and unacceptable treatment delay secondary to port-a-cath placement (1).
Clinical Efficacy
Regimen TB
All but one patient (who refused further follow-up) were followed for a minimum of 16 months or until death. At last contact, 3 patients were alive without disease progression, 5 patients are alive with disease progression and 34 patients have died of their disease.
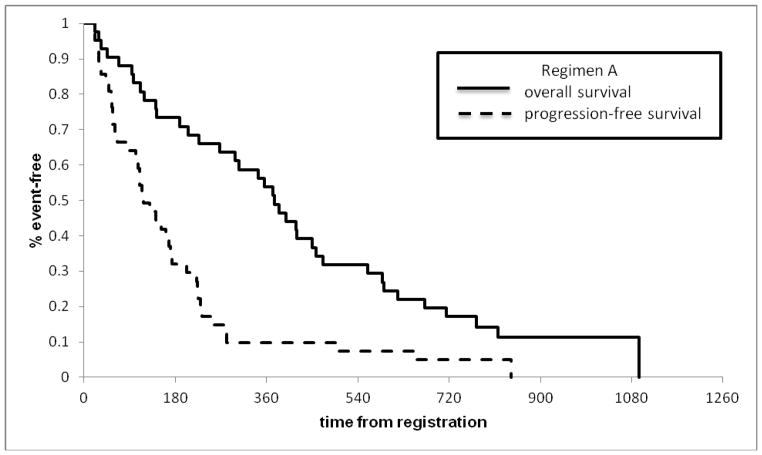
Among the first 41 eligible patients randomized to TB, the Kaplan-Meier estimate for the 6 month PFS rate was 32.8% (95% CI: 21.1–51.2%); thus, ruling out a 6 month PFS rate of 60% or more. Considering all 42 eligible patients randomized to Regimen TB, the tumor response rate (TRR) was 23.8% (95% CI 12.1–39.5%), with 9 partial responses and one complete response. The median PFS and OS times were estimated to be 3.8 months (95% CI: 3.0–6.2 months) and 12.3 months (95% CI: 9.3–15.3 months), respectively (Figure 1).
Figure 1.
Survival and progression curves for Regimen A
Regimen ABC
All 51 patients were followed for a minimum of 16.5 months or until death. At last contact, 4 patients were alive without disease progression, 9 patients were alive with disease progression, and 37 patients had died of their disease.
Among the first 41 eligible patients randomized to ABC, the Kaplan-Meier estimate for the 6 month PFS rate was 56.1% (90% CI: 44.7–70.4%), thereby not excluding a 6 month PFS rate of 60% or more. Considering all 51 eligible patients randomized to Regimen ABC, the overall 6 month PFS rate was 54.9% (90% CI: 42.8–70.4%) with a PFS6 in the preadd 5 and postadd 5 cohorts of 60.7% (90%CI: 47.3–78.0%) and 47.8% (90% CI 33.4–68.4%) respectively. There were 17 partial responses and no complete responses yielding an overall TRR of 33.3% (95% CI 20.8–47.9%). The TRR among the preadd 5 and postadd 5 cohorts was 35.7% (95% CI: 18.6–55.9%) and 30.4% (95% CI: 13.2–52.9%) respectively.
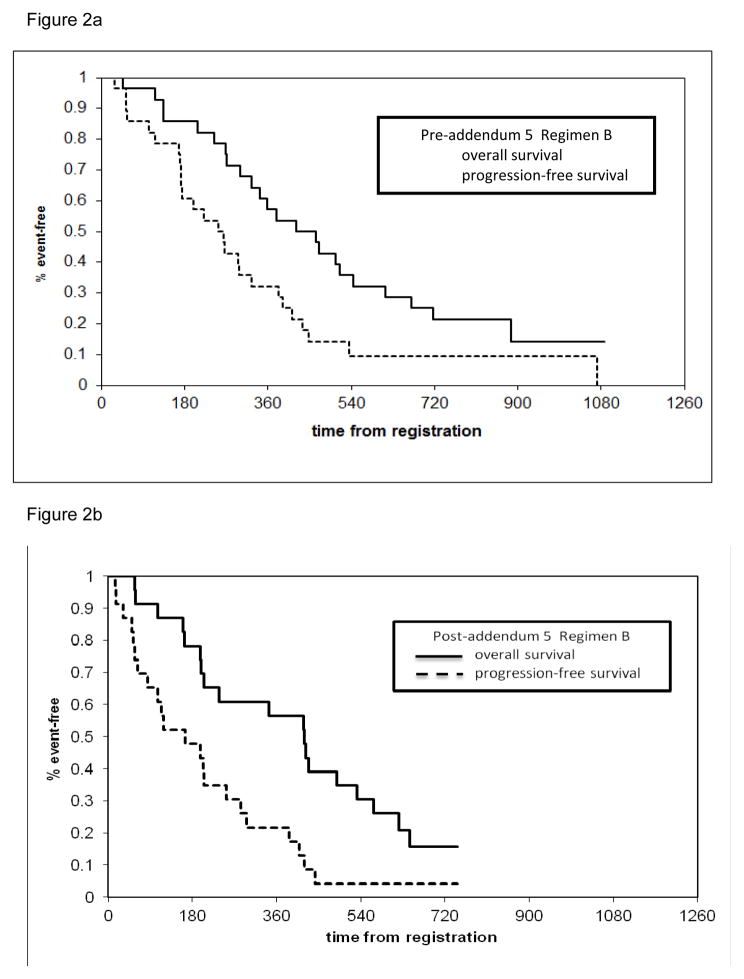
The overall median PFS time was estimated to be 6.7 months (95% CI: 5.6–9.5 months). Additionally the median PFS in the preadd 5 and postadd5 cohorts was 9.4 months (95% CI: 5.7–11.6 months) and 5.4 months (95% CI: 3.1–8.8 months) respectively. The overall median OS was estimated to be 13.9 months (95% CI: ). Finally the preadd5 and postadd 5 OS rates were 14.5 months and 13.8 months respectively. (Figures 2a & b).
Figure 2.
Survival and progression curves for Regimen B
Changes in plasma VEGF A and D levels
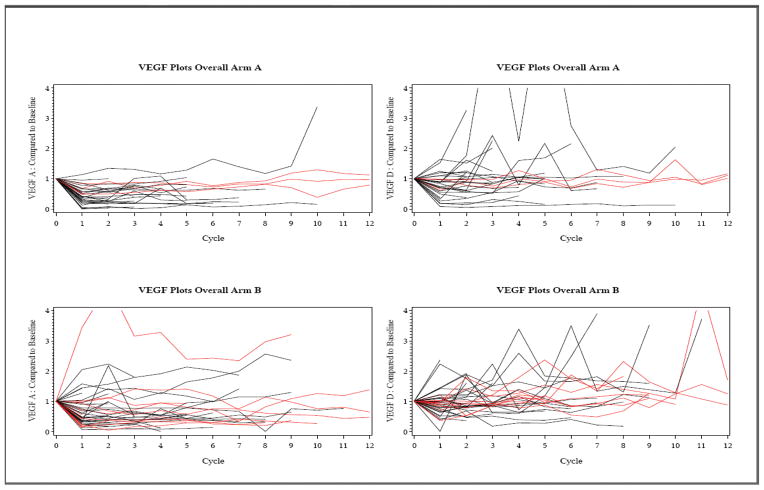
Serial measurements of plasma VEGF-A and VEGF-D levels were obtained prior to each treatment as well as at time of progression. Bevacizumab is a primarily VEGF-A neutralizing antibody and we sought to understand whether or not its use affected the levels of VEGF-D as well. Emerging evidence suggests that VEGF-D plays a central role in intratumoral endothelial cell survival.15 Our data demonstrated that among the 30 patients on regimen TB and 44 patients on regimen ABC who had at least one post-registration determination of VEGF-A and VEGF-D, there were decrements in VEGF-A levels in a majority of the patients after one cycle of treatment (Figure 3). However, changes in VEGF-A or VEGF-D levels during treatment did not correlate with clinical response to treatment.
Figure 3.
VEGF A and D levels
DISCUSSION
The current study trial was primarily built on the findings from our previous phase II trial of nab-paclitaxel and carboplatin (N057E) that reported a median PFS time of 4.3 months and median OS of 11.1 months among chemotherapy naïve patients, and 4.2 months and 10.9 months among those previously treated.16 Toxicities encountered included nausea, vomiting, peripheral neuropathy, and cytopenias necessitating the lower starting doses of nab-paclitaxel and carboplatin. In the current trial, the addition of bevacizumab to nab-paclitaxel and carboplatin (ABC regimen) shows promising activity in both PFS (6.7 months) and OS (13.9 months) despite tolerability issues, relative to N057E as well as historical benchmarks of other therapies for MM tested in the NCI Cooperative Groups.17 Of note, the initial dosing schedule of nab-paclitaxel 100mg/m2 IV days 1, 8, and 15, carboplatin at AUC of 6 IV on day 1 and bevacizumab 10 mg/kg IV days 1 and 15 of a 28 day cycle induced higher incidence of cytopenias, fatigue, hypertension, nausea, thrombosis, and neuropathy than expected which required lower starting doses of nab-paclitaxel and carboplatin to 80mg/m2 and AUC 5, respectively. While there was some difference in PFS (9.4 months- preadd5 and 5.4 months-postadd 5) OS was largely unaffected (14.5 months-preadd5 and 13.8 months-postadd5). The ABC regimen of our current study also compares favorably to two prior phase II studies evaluating bevacizumab combinations with paclitaxel and carboplatin which demonstrated median PFS and OS times in the 5 and 12 month range respectively.12,13
In our present study we also evaluated the utility of the addition of bevacizumab to temozolomide (regimen TB), a commonly used chemotherapeutic agent in MM. The TB regimen resulted in a median PFS time of 3.8 months similar to the median PFS of 4.2 months reported by Dummer et al 18 confirming their observation. The TB regimen was well tolerated in both trial populations.
In conclusion, the last several years have witnessed a tremendous effort in developmental therapeutics for MM resulting in the FDA approval of two new agents, ipilimumab and vemurafenib in 2011.5, 19 Our current study found that the addition of bevacizumab to nab-paclitaxel and carboplatin (regimen ABC) shows promising activity in terms of both PFS and OS that is comparable to that of ipilimumab and vemurafenib. Toxicity on this regimen was somewhat higher than was seen on the TB arm with more grade 3 toxicities, but with careful monitoring this is still a generally acceptable safety profile. Its place in the treatment armamentarium for metastatic melanoma should be explored in the phase III clinical trial setting.
Footnotes
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group and Mayo Clinic and was supported in part by Public Health Service grants CA-25224, CA-37404, CA-35267, CA-35113, CA-35090, CA-60276, CA-35448, CA-63848, CA-63849, CA-35101, CA-35415, CA-35431, CA-35269, CA-37417, CA-35103, CA-52352 from the National Cancer Institute, Department of Health and Human Services.The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
Additional participating institutions include: Geisinger Clinic and Medical Center CCOP, Danville, PA 17822 (Albert M. Bernath, Jr, M.D.); Collumbus CCOP, Columbus, OH 53215 (J. Philip Kuebler, M.D., Ph.D.); Grand Rapids Clinical Oncology Program, Grand Rapids, MI 49503 (Martin J. Bury, M.D.); Michigan Cancer Research Consortium, Ann Arbor, MI 48106 (Philip J. Stella, M.D.); Iowa Oncology Rsearch Association CCOP, Des Moines, IA 50309-1014 (Robert J. Behrens, M.D.); Duluth CCOP, Duluth, MN 55805 (Daniel A. Nikcevich, M.D.); Meritcare Hospital CCOP, Fargo, ND 58122 (Preston D. Steen, M.D.); Lehigh Valley Hospital, Allentown PA 18103 (Suresh Nair, M.D.); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403 (Martin Wiesenfeld, M.D.); Colorado Cancer Research Program, Denver, CO 80224 (Eduardo R. Pajon, Jr, M.D.); Missouri Valley Cancer Consortium Omaha, NE 68106 (Gamini S. Soori, M.D.); Toledo Community Hospital Oncology Program CCOP, Toledo, OH 43623 (Rex B. Mowat, M.D.); Wichita Community Clinical Oncology Program, Wichita, KS 67214-3882 (Shaker R. Dakhil, M.D.); Northern Indiana Cancer Research Consortium CCOP, South Bend, IN 46601 (Robin T. Zon, M.D.); Rapid City Regional Oncology Group, Rapid City, SD 57709 (Richard C. Tenglin, M.D.); Sioux Community Cancer Consortium, Sioux Falls, SD 57105 (Miroslaw Mazurczak, M.D.); Montana Cancer Consortium Billings, MT 59101 (Benjamin T. Marchello, M.D.); Cancer Research for the Ozarks, Springfield, MO 65804 (Robert L Carolla, M.D.); CentraCare Clinic, St. Cloud, MN 56301 (Donald J. Jurgens, M.D.); Virgina Mason CCOP, Seattle, WA 98101 (Jacqueline Vuky, M.D.)
This study was presented at the 2011 ASCO Annual Meeting as a poster discussion
Financial Disclosures: None
References
- 1.Society AC. [[accessed 1-25-2011, 2011].];Cancer Facts and Figures. Available from URL: http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-and-figures-2010.
- 2.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 3.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol. 2000;18:158–166. doi: 10.1200/JCO.2000.18.1.158. [DOI] [PubMed] [Google Scholar]
- 4.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salven P, Heikkila P, Joensuu H. Enhanced expression of vascular endothelial growth factor in metastatic melanoma. Br J Cancer. 1997;76:930–934. doi: 10.1038/bjc.1997.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ugurel S, Rappl G, Tilgen W, Reinhold U. Increased serum concentration of angiogenic factors in malignant melanoma patients correlates with tumor progression and survival. J Clin Oncol. 2001;19:577–583. doi: 10.1200/JCO.2001.19.2.577. [DOI] [PubMed] [Google Scholar]
- 9.Lev DC, Onn A, Melinkova VO, et al. Exposure of melanoma cells to dacarbazine results in enhanced tumor growth and metastasis in vivo. J Clin Oncol. 2004;22:2092–2100. doi: 10.1200/JCO.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 10.Lev DC, Ruiz M, Mills L, McGary EC, Price JE, Bar-Eli M. Dacarbazine causes transcriptional up-regulation of interleukin 8 and vascular endothelial growth factor in melanoma cells: a possible escape mechanism from chemotherapy. Mol Cancer Ther. 2003;2:753–763. [PubMed] [Google Scholar]
- 11.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 12.Perez DG, Suman VJ, Fitch TR, et al. Phase 2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab in patients with unresectable stage IV melanoma: a North Central Cancer Treatment Group study, N047A. Cancer. 2009;115:119–127. doi: 10.1002/cncr.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Day SKK, Sosman J, Peterson A, Feng S, et al. BEAM: A randomized phase II study evaluating the activity of BEvacizumab in combination with carboplatin plus paclitaxel in patients with previously untreated Advanced Melanoma. European Journal of Cancer Supplements. 2009;7:13. doi: 10.1200/JCO.2011.34.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim KBS, JA, Minor DR, Fruehauf JP, Linette GP, et al. BEAM: A Randomized Phase II Study Evaluating the Activity of Bevacizumab in Combination with Carboplatin Plus Paclitaxel in Patients with Previously Untreated Advanced Melanoma. Journal Of Clinical Oncology (accepted) 2011 doi: 10.1200/JCO.2011.34.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papiewska-Pajak I, Boncela J, Przygodzka P, Cierniewski CS. Autocrine effects of VEGF-D on endothelial cells after transduction with AD-VEGF-D(DeltaNDeltaC) Experimental cell research. 2010;316:907–914. doi: 10.1016/j.yexcr.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Kottschade LA, Suman VJ, Amatruda T, 3rd, et al. A phase II trial of nab-paclitaxel (ABI-007) and carboplatin in patients with unresectable stage IV melanoma : a north central cancer treatment group study, N057E1. Cancer. 2010 doi: 10.1002/cncr.25659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 18.Dummer RM, Seifert OB, Ochsenbein A, Cathomas R, et al. First-line temozolomide (TEM) combined with bevacizumab (BEV) in metastatic melanoma (MM): A multicenter phase II trial (SAKK 50/07) Journal of Clinical Oncology. 2010:28. doi: 10.1093/annonc/mdr126. [DOI] [PubMed] [Google Scholar]
- 19.Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009;27:2823–2830. doi: 10.1200/JCO.2007.15.7636. [DOI] [PubMed] [Google Scholar]