SUMMARY
The number and types of venom components that affect ion-channel function are reviewed. These are the most important venom components responsible for human intoxication, deserving medical attention, often requiring the use of specific anti-venoms. Special emphasis is given to peptides that recognize Na+-, K+- and Ca++-channels of excitable cells. Knowledge generated by direct isolation of peptides from venom and components deduced from cloned genes, whose amino acid sequences are deposited into databanks are now adays in the order of 1.5 thousands, out of an estimate biodiversity closed to 300,000. Here the diversity of components is briefly reviewed with mention to specific references. Structural characteristic are discussed with examples taken from published work. The principal mechanisms of action of the three different types of peptides are also reviewed. Na+-channel specific venom components usually are modifier of the open and closing kinetic mechanisms of the ion-channels, whereas peptides affecting K+-channels are normally pore blocking agents. The Ryanodine Ca++-channel specific peptides are known for causing sub-conducting stages of the channels conductance and some were shown to be able to internalize penetrating inside the muscle cells.
Keywords: Biodiversity, ion-channel, functional effect, scorpion toxin, structural features
1. INTRODUCTION
Scorpion stings are a public health problem in certain countries, with an estimate over one million accidents in humans, annually (Chippaux and Goyffon, 2008), which certainly requires medical attention, due to high number of fatalities, which is approximately 2600 per year (Chippaux, 2012). Scorpions have been classified into 18 different families (Prendini and Wheeler, 2005) of which 30 genera belonging to the family Buthidae presents human treat, and they constitute circa 25% of the world biodiversity (possible in the order of 2,000 different species). The most important species causing human accidents are scorpions of the genus: Androctonus, Buthus, and Leiurus, in North Africa and Middle East, Centruroides and Tityus in the American continent, Mesobuthus in Asia, Parabuthus in South Africa (Caliskan et al., 2013).
Scorpion venoms are a complex mixture of substances, among which are: inorganic salts, free amino acids, heterocyclic components, peptides and proteins, mainly enzymes, which are used by the scorpions for defense and capture of prays. Each scorpion has its own arsenal of components for these purposes. The known number of different components in their venoms varies from 72 (Androctonus mauretanicus mauretanicus) to over 600 (Mesobuthus tumulus, and Tityus serrulatus) (Oukkache et al., 2008; Newton et al., 2007; Batista et al., 2007). But the world biodiversity is in the order of 300,000, if we assume each scorpion having 150 different components, from which approximately 750 proteins are registered in The Animal Annotation Program of UniProt (www.uniprot.org/program/Toxins), and at least the double of this number is known after translating the known nucleotide sequences of genes cloned from scorpions. Yet, this is less than 1% of the total expected number of distinct components to exist in scorpion venoms. The best known components are peptides that modify ion-channel permeability of both excitable and non excitable cells, belonging to a single structural class built around a scaffold known as Cystine-Sabilized α/β (CS-α/β) motif. Most of the data mentioned above can easily be revised in the recent publication, dealing with the mining aspects of scorpion venom components (Rodriguez de la Vega et al., 2013). However, due to the increment of new strategies of proteome analysis and gene cloning from transcriptomes, the number of identified components has increased significantly. The new approaches have indicated that many other structural motifs and possible functions are being found and will be identified in the near future (Rodriguez de la Vega et al., 2013).
The General classification of scorpion toxins (Possani et al., 1999; Tytgat et al., 1999, Rodriguez de la Vega and Possani, 2004; Tan et al., 2006a) is based on four different criteria: the ion-channel involved (sodium, potassium, calcium and chlorine), the specific receptor to which the toxin binds to, the three-dimensional structure of the toxin and the type of response induced (activation/inactivation of the receptor).
In this review will focus our attention to the classical peptides, normally called toxins that modify the gating mechanism of Na+-channels, block K+-channel or modulate the function of calcium channel sensitive to Ryanodine.
2. Na+-CHANNEL SPECIFIC TOXINS
The venom of scorpions contains several types of toxins that may interact with each other, modulating the function of ion channels, usually being responsible for the known multiple symptoms of poisoning. On the medical viewpoint, the toxins that bind to sodium channels of mammals, particularly humans, are the most important ones. These toxins are polypeptides of 61–76 amino acid residues in length, tightly bound by four disulfide bridges (Possani et al., 1999) and currently are classified into two categories, based on their physiological effects on channel gating and their binding properties: alpha-toxins (α-NaScTxs), which bind at receptor site 3 on the extracellular surface of the channel and inhibit the fast inactivation process (Couraud et al., 1982; Meves et al., 1986 and reviewed in Bosmans and Tytgat, 2007), and beta-toxins (β-NaScTxs), which bind to receptor site 4 and shift the threshold of the channel activation to more negative membrane potentials (reviewed in Rodriguez de la Vega and Possani, 2007 and Weinberger et al., 2010).
2.1 SCORPION α-NaScTxs
Scorpion α-NaScTxs were initially described for species collected in the “Old World” (Africa and Asia), but latter also described for scorpions of the “New World” (America) (reviewed in Gordon et al., 2003). The α-NaScTxs are subdivided into distinct groups (Catterall, 1992; Gordon et al., 1998 and reviewed in Gordon et al., 2007):
Classical α-toxins, are highly active only in mammalian voltage-gated sodium channels (VGSCs) with high affinity (Kd in the range of 0.2–5 nM) to rat brain synaptosomes. Among these toxins are: Aah2, Aah1 and Aah3 from Androctonus australis Hector, Lqq5 from Leiurus quinquestriatus quinquestriatus and Bot3 from Bothus occitanus tunetatus, peptides purified from North African scorpions (Martin-Eauclaire and Couraud, 1995; Froy and Gurevitz, 2003). From the venom of scorpions of the ‘New World’ peptides isolated from Tityus serrulatus and Centruroides sculpturatus have also been classified as α-NaScTxs (Meves et al., 1984; Possani et al., 1999).
Anti-insect α-NaScTXs, that are highly active only on insect VGSCs. Examples of these toxins are: LqhαIT (Eitan et al., 1990), Lqq3 (Kopeyan et al., 1993), and BotIT1 (Borchani et al., 1997), which bind with high affinity to insect neuronal preparations (0.06–1 nM).
α-Like toxins, active on both insect and mammalian VGSCs. Examples are: Lqh3 and Lqh6 (from Leiurus quinquestriatus hebraeus), Bom3 and Bom4 (from Buthus occitanus mardochei), and BmK M1 (from Buthus martensii Karsch).
2.2 SCORPION β-NaScTxs
β-NaScTxs are classified into four well supported phylogenetic branches or subclasses (reviewed in Pedraza Escalona and Possani, 2013): (1) anti-mammalian β-toxins exclusively found in scorpions of the genus Centruroides. Examples are Cn2 from Centruroides noxius and Css4 from Centruroides suffusus suffusus (Vazquez, et al., 1995; Martín, et al., 1987); (2) β-toxins active on both insect and mammalian VGSCs, such as Ts1 from Tityus serrulatus (Possani, et al., 1985) and Lqhβ1 from Leiurus quinquestriatus hebraeus (Gordon, et al., 2003); (3) anti-insect selective excitatory β-toxins such as AahIT from Androctonus australis Hector and Bj-xtrIT from Hotentota judaica, that causes contraction paralysis in fly larvae (Pélate and Zlotkin, 1982; Zlotkin, et al., 1985; Froy, et al., 1999); and finally (4) anti-insect selective depressant toxins, which induce flaccid paralysis upon injection. Example is peptide LqhIT2 from Leiurus quinquestriatus hebraeus (Zuo and Ji, 2004, Gurevitz, et al., 2007).
2.3 AMINO ACID SEQUENCES OF NA+-CHANNELS TOXINS
The first scorpion toxins sequenced were purified from North African scorpions of the genus Androctonus (Rochat et al., 1967), but also from American scorpions (Babin et al., 1975). The work was performed after chromatographic separation of components of venoms and the amino acid sequence determination was usually by Edman degradation procedures. More recently, the amino acid sequence of several Na+-channel toxins have been obtained by sequencing cDNA clones, either by direct search or by analysis of transcriptomes from scorpion venom glands (reviewed in Quintero-Hernández, et al., 2011). Recent examples of sequences of Na+-channels toxins obtained from cDNA by specific search are: the Bu1 toxin, the most potent toxin from the Turkish scorpion Buthacus macrocentrus (Caliskan et al., 2012), and the potent Acra4 toxin from Androctonus crassicauda scorpion venom (Caliskan et al., 2013). However, many Na+-channel specific peptides sequences were obtained, by comparison of similarities of sequences using transcriptome analysis of scorpion glands, as it will be discusse below.
The Na+-channel toxins from scorpions are 6500–8500 Da polypeptides composed of 58–76 amino acids residues (Rodríguez de la Vega and Possani, 2005).
Presently, the UniProt Knowledgebase (UniProtKB) database of the Universal Protein Resource (UniProt; www.uniprot.org) provides more than 300 protein entries for sodium channel scorpion toxins alone (including putatives sodium channel toxins).
2.4 THREE-DIMENSIONAL STRUCTURE OF NA+-CHANNEL TOXINS
Figure 1 shows some examples of the three-dimensional structures of a few known toxins. The core structure contains six cysteines which form three conserved disulfide bridges and a fourth disulfide bridge can be formed in three different arrangements (Mouhat et al., 2004). These toxins have an essential three-dimensional structure highly conserved comprising an α-helix and three or four-stranded anti-parallel β-sheets (Gordon et al., 1998) that are connected by solvent-exposed irregular loops and stabilized by four spatially conserved disulfide bridges. The scorpion toxin fold families include the βαββ–family (Housset et al., 1994) and the βααββα-family (Oren et al., 1998).
Figure 1. Three-dimensional structures of Na-ScTxs.
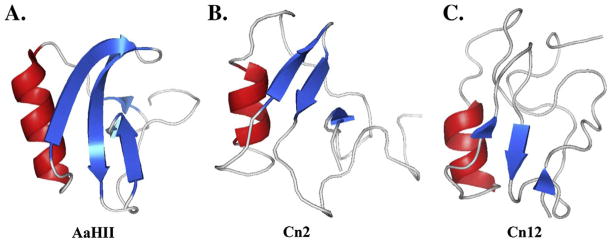
The structures shown are: α-NaScTx AaHII from Androctonus australis Hector (pdb 1SEG), β-NaScTxs Cn2 (pdb 1CN2) and Cn12 (pdb 1PEA) from Centruroides noxius Hoffmann). The structures were obtained from Protein databank (www.pdb.org) and were displayed with PyMOL (www.pymol.org). AaHII is the prototype of α-NaScTxs and Cn2 is a typical β-NaScTx; however, Cn12 is structurally a β-NaScTx, but it has a α-NaScTx effect. All these toxins have a three-dimensional structure highly conserved comprising an α-helix (red) and three or four-stranded anti-parallel β-sheets (blue).
The helix motif is linked to the β3 strand by two of the four disulfide bonds. The cysteine pair of the α-helix motif is spaced by a tripeptide, whereas the pair of cysteine residues of the β3 strand is separated by only one amino acid residue (Possani, 1999).
By numbering the cysteine residues progressively from the N- to C-terminus, the α-helix would contain the residues Cys3 and Cys4 and the β3 strand would include residues Cys6 and Cys7, forming two conserved disulfide bridges: Cys3-Cys6 and Cys4-Cys7. These structural elements are conserved in all sodium channel scorpion toxins described until now. A third structurally conserved disulfide bond is the one between the Cys residue located at the β2 strand, Cys5, and Cys2, except for the excitatory insect toxins, in which there are two contiguous Cys residues in their corresponding β2 strand (Possani, et al., 1999). The three first conserved disulfide bridges are involved in the stabilization of a structurally conserved core of scorpion toxins. The fourth disulfide bridge in the majority of toxins is established between the most N- and C-terminal cysteine residues (Cys1 and Cys8). In excitatory toxins the fourth disulfide bridge is established in an atypical way between the contiguous Cys residue in their β2 strand and the cysteine residue at the C-terminal end of the toxin (Cys8) (Possani, et al., 1999). Therefore, toxins with a disulfide bridge connecting their β2 strand to the C-terminal end, and α B loop of minimal length, are excitatory insect toxins.
For Old World scorpion toxins, α B loop of maximal length is linked to an α-type activity. An intermediate B loop is related to depressant insect toxins. New World scorpion toxins are mainly β-type. However, there are many exceptions: toxin V from Centruroides sculpturatus is pharmacologically α-type but “structurally” β-type. Toxin IV-5 from Tityus serrulatus, has also been shown to be α-type (competes for AaHII binding). Toxin CsEV does not compete for AaHII binding, indicating that they can be classified as α-like toxins. Toxins TsIV-5 and CsEV, typical α-type toxins of New World scorpions, have in common α B loop of intermediate size, sharing this characteristic with Old World depressant insect toxins (reviewed in Possani, et al., 1999). Another case is the peptide Cn12 from the scorpion Centruroides noxius, which is structurally similar to the β-NaScTx, but has an α-NaScTx effect (Del Rio Portilla et al., 2004).
From all these examples, we can conclude that it is difficult to classify toxins based solely on its three dimensional structure. It seems clear that electrophysiological characterization of their function is also required.
2.5 FUNCTIONAL SURFACE OF THE α-NaScTxs
A functional surface called “NC-domain” was identified in α-NaScTxs (revised in Kahn et al., 2009 Chen and Chung, 2012 and Gurevitz, 2012). This domain comprises the five-residue turn, that interlaces with the C-terminal segment (residues 8–12) and the C-terminus (residues 56–64) and a core-domain formed by several residues (positively-charged and hydrophobic amino acids at the short loops connecting the conserved secondary structure elements of the molecule core) spatially in close proximity to the residue at position 18. The NC- and core-domains are interconnected by the linker-domain in residues 8–18.
The NC-domain varies in amino acid composition and spatial arrangement and very likely dictates toxin selectivity (Gordon and Gurevitz, 2003; Karbat et al., 2007; Kahn et al., 2009).
Moreover, a recent study examined the binding modes between two α-toxins, the anti-mammalian AahII and the anti-insect LqhαIT, and the voltage-sensing domain of rat NaV1.2, a subtype of NaV channels expressed in nerve cells, using for this purpose atomistic molecular dynamics simulations (Chen and Chung, 2012). AahII and LqhαIT toxins were docked to the extracellular side of the voltage-sensing domain of NaV1.2 using molecular dynamics simulations, with the linker-domain assumed to wedge into the binding pocket. Several salt bridges and hydrophobic clusters were observed to form between the NC- and core-domains of the toxins and NaV1.2 and stabilize the toxin-channel complexes (Chen and Chung, 2012). The binding modes predicted were consistent with available mutagenesis data and can readily explain the relative affinities of AahII and LqhαIT for NaV1.2. The models demonstrated that the functional surface of anti-mammalian scorpion α-toxins is centered on the linker-domain, similar to that of β-toxins (Chen and Chung, 2012).
2.6 FUNCTIONAL SURFACE OF THE β-NaScTxs
Four common functional sections of the β-NaScTxs were identified: 1) a central “pharmacopore region” involved in the receptor binding site, consisting of a negatively charged residue, located in the α-helix which is flanked by diverse solvent-exposed hydrophobic residues; 2) a “solvent-exposed aromatic cluster” that is critical for activity and is generally located in the β2 and β3 strands; 3) some residues localized in the center of the N-groove region, which are involved in voltage sensor trapping; and 4) C-terminal residues that increase the binding affinity to the receptor binding site (reviewed in Pedraza Escalona and Possani, 2013).
However, it is important to mention that there are structural differences, between the β-toxins, located in the variable loops that connect the main secondary structure elements of the toxin core and in the arrangement of the C-tail. For such reason, each toxin has particular patherns for the location of functional regions, which explains the high selectivity for a specific target. Four motifs for identification of functional regions of the β-NaScTxs subclasses have been proposed (Tan et al., 2006b; Pedraza Escalona and Possani, 2013):
-
Motif of anti-mammalian subclass:
KxGYxVx(4)GCKxxCxxLGxNxxCxxECx(9)GYCYxFxCxCxxLx(7)PlxxKxC (Tan et al., 2006b);
-
Motif of anti-insect excitatory subclass:
KKxGxxxDxxGKxxECx(4,9)YCxxxCTKVxYAxxGYCCxxxCYCxGLxDDKx(9)KxxCD (Tan et al., 2006b);
-
Motif of anti-insect depressant subclass:
DGY[IP][KR]x(2)[DNS]GC[KR]x[ADS]Cx(2,3)Nx(2,3)Cx(3)Cx(3)G[AG]x[FY]GYCW[AGT]WGLACWC[EQ][GN]LP[ADE] (Pedraza Escalona and Possani, 2013), and
-
Motif of anti-mammalian/insect subclass:
GCK[FLV]xC[FV][IP][NR][NP][AES][EGS]x[CGN] (Pedraza Escalona and Possani, 2013).
In these four motifs the letter “x” means any amino acid, and the numbers between parentheses mean the number of residues in that particular space of the sequence.
2.7 ELECTROPHYSIOLOGICAL EFFECTS OF α-NaScTxs
The α-NaScTxs toxins bind with high affinity to the resting state of Na+ channels and inhibit fast inactivation by interacting with channel receptor site 3 located in the S3–S4 extracellular loop in domain IV and in the S5–S6 extracellular linker domain I of NaV channels (Catteral, et al., 2000). The binding of α-scorpions is reversed by depolarizations that activate Na+ channels (Catterall, 1979). The interaction induces prolonged depolarization overcomes the effect of toxin binding and drives dissociation as the S4 segment moves outward (Catterall, et al., 2007 and reviewed in Wang et al., 2011). The amino acid residue E1613 in S3–S4 in domain IV is involved in binding of α-scorpion toxins (Rogers, et al., 1996). Amino acid residues in the SS2-S6 of loop in domain I, S1–S2 of loop in domain IV and S3 segment in domain IV were identified as components of neurotoxin receptor site 3 (Wang, et al., 2011).
2.8 ELECTROPHYSIOLOGICAL EFFECTS OF β-NaScTxs
The β-scorpion toxins bind at neurotoxin receptor site 4 and enhance activation of NaV channels (Caterall, et al., 2007). After binding to receptor site 4, the β-NaScTxs toxins induce a reduction of the peak sodium current amplitude and shift the voltage dependence of Nav channel activation towards a more hyperpolarized membrane potential (Meves, et al., 1982). The β-NaScTxs toxins bind to receptor site in the extracellular loops connecting transmembrane segments S3 and S4 and the S1 and S2 segments in domain II of brain NaV 1.2 channels (Cestèle, et al., 1998; Cestèle, et al., 2006).
β-NaScTxs toxins are thought to act via a voltage-sensor trapping mechanism in which the toxin binds to its receptor site in the inactivated state of the voltage sensor, which produces an extended period of inactivation at negative membrane potentials (Cestèle, et al., 1998; Cestèle, et al., 2001; Cestèle, et al., 2006)
Four amino acid residues in the SS2-S6 extracellular loop of domain III of Nav channel contribute to toxin binding and efficacy (Zhang, et al., 2012), which leads at the conclusion that the pore module of domain III and the voltage-sensing module of domain II form the receptor site and this means that β-NaScTxs toxins make a three-point interaction with NaV channels and alter voltage sensor function (Zhang, et al., 2012),
2.9. PUTATIVE NA+-CHANNEL TOXINS OF cDNA LIBRARIES FROM SCORPION VENOM GLAND
Recently, the identification of venom components have been obtained by sequencing cDNA clones of cDNA libraries of venomous glands obtained from scorpions of different families (Schwartz et al., 2007; Kozminsky-Atias et al., 2008; D’Suze et al., 2009; Ma et al., 2009; Roeding et al., 2009; Silva et al., 2009; Ma et al., 2010; Ruiming et al., 2010; Morgenstern et al., 2011; Almeida 2012; Diego-García 2012; Ma et al., 2012; Rendón-Anaya 2012; Luna-Ramírez et al., 2013).
The results obtained by this more recent methodology, using cDNA libraries, indicate the presence of a rich biodiversity and variability of components in the species studied. Apart from the peptides that modify ion-channel permeability, initially isolated and characterized by classical biochemical methods, many other components were placed in evidence, such as: factors that active lipolysis, phospholipase A2, serine-proteases, metalloproteinases, protein homologs of tick salivary glands, precursors of cytolytic peptides, proteins rich in cysteine contents and a great number of proteins and peptides deduced from the ESTs for which the function is still unknown (Soudani et al., 2005; Fletcher et. al., 2010; reviewed in Rodríguez de la Vega et al., 2013). The results of cDNA analysis also show that the venomous glands of scorpions have many components related to cellular processes, protein synthesis, protein trafficking and others (Schwartz et al., 2007; Kozminsky-Atias et al., 2008; Silva et al., 2009; D’Suze et al., 2009; Ma et al., 2009; Ruiming et al., 2010; Ma et al., 2010; Morgenstern et al., 2011).
One of the major differences between buthidae and non-buthidae scorpion venoms is the abundance and dominance of sodium channel specific toxins (NaScTxs) in buthidae venoms compared with the almost complete lack of them in non-buthidae scorpions. This has been emphasized quantitatively in transcriptomic analysis of buthidae venoms (Kozminsky-Atias et al., 2008; D’Suze et al., 2009; Ruiming, et al., 2010; Morgenstern, et al., 2011; Almeida, et al., 2012; Ma, et al., 2012; Rendón-anaya, et al., 2012).
Reviewing the information available concerning Na-ScTx peptides the numbers of putative peptides are:
In the transcriptome of Buthus occitanus israelis, 39 toxin sequences were predicted to be Na+ channel modifiers, from which 17 are putative α-type, 4 are β-like, 3 are excitatory and 15 are depressant (Kozminsky-Atias 2008).
Fourteen sequences of the cDNA phagemid library from Tityus discrepans encode for putative peptides that recognize or affect Na+-channel function (D’Suze et al., 2009).
A comparative analysis of venom transcriptomes of the scorpion Lychas mucronatus, from different geographical regions, showed that no significant differences were observed in the NaScTxs types from two scorpion populations of this scorpion species. NaScTxs corresponds almost to 16% (53 NaScTxs transcripts grouped in 16 clusters) of all toxin-like peptides in Hainan-sourced population, while 11% (43 NaScTxs transcripts grouped in 12 clusters) was detected in Yunnan-sourced population (Ruiming, et al., 2010).
From the scorpion Hottentotta judaicus only six novel NaScTx transcripts were detected, most of which belong to the β-depressant toxin family (Morgenstern et al., 2011).
From the transcriptome of Tityus stigmurus, forty-one percent of the ESTs belong to toxin-coding sequences, which 0.93% (3 clusters) corresponded to β-NaScTx-like and 0.37% (2 clusters) to α-NaScTx-like (Almeida et al., 2012).
In the transcriptomes of Lychas mucronatus and Isometrus maculates (Ma et al., 2012), 9.6% of ESTs corresponded to NaScTx sequences which 9 were α-NaScTxs and 12 were β-NaScTxs in the case of Lychas mucronatus and 8.9 % of ESTs were NaScTx sequences which included 1 sequence of α-NaScTxs and 12 sequences of β-NaScTxs.
Finally, the global transcriptome analysis of the scorpion Centruroides noxius (Rendon-Anaya et al., 2012) was obtained by means of the platform of massive pyrosequencing, using genes obtained from the cDNAs of venomous glands, as well as cDNAs from the entire body of this species. In the order of three million readings were obtained and assembled, from which 72 different toxin-like isogroups containing peptides similar to toxins previously reported for other scorpions were identified. Twenty seven isogroups corresponded to sodium channel toxins, which 12 sequences of known toxins were identified and 22 new putative NaScTxs with variable identity values to other NaScTxs of different species were obtained.
In summary, to date more that 140 new sequences of putative NaScTxs have been identified in the scorpion transcriptomes. It is expected that with the new sequencing methodology (Next Generation -Next-Gen- sequencing), the future transcriptomes will reveal more information about sequences coding for NaScTxs toxins and of course, for other types of peptides contained in the venom glands. This, coupled with proteomic analysis and classical biochemical characterization, will allow us to discover a greater number of peptides from the venom glands of scorpions.
3. K+-CHANNEL SPECIFIC SCORPION TOXINS
Scorpion venom is a rich source of K+ channel specific toxins (KTx), which have been used in the structural and functional characterization of various K+-channels. Based on primary amino acid sequences and cysteine pairing, KTx have been classified into four families α-, β-, γ- and κ-KTx (Tytgat et al., 1999; Rodríguez de la Vega and Possani, 2004). There are two main types of structural motifs in these peptides: (1) the common motif comprising one or two short α-helices connected to a triple-stranded antiparallel β-sheet stabilized by three or four disulfide bonds, named CSαβ (Rodríguez de la Vega and Posssani, 2004; Mouhat et al., 2004), (2) the α-helix-loop-helix (CSαα) fold consists of two short α-helices connected by a β-turn; only the kappa toxins adopt this fold (Srinivasan et al., 2002; Chagot et al., 2005; Camargos et al., 2011; Saucedo et al., 2012).
3. 1. ALPHA-KTx FAMILY
The α-KTx family is considered as the largest of potassium channel toxin family (Rodriguez de la Vega and Possani, 2004), known about 140 different peptides comprised in 30 subfamilies based on differences in their amino acid sequences (http://www.uniprot.org/docs/scorpktx; Chen et al., 2012; Zhen et al., 2012; Diego-García et al., 2013). These peptides are composed of 23–42 residues with three or four disulfide bridges, adopt a typical CSαβ fold and are known to show two different modes of interaction with the K+ channels: (1) the “pore plugging mode”, ion conduction through the selectivity filter of Kv1, KCa2 and KCa3 channels is blocked by a peptide consisting of a Lysine residue assisted by an aromatic side chain located some 7 Å apart. Other residues facing the channel vestibule also contribute to the binding (Rodríguez de la Vega et al., 2013). This interaction has been determined in studies of structure and function of various toxins such as Agitoxin, Charybdotoxin, Noxiustoxin (Bergeron and Bingham, 2012); (2) the “intermediate” mode, where a patch of the basic residues of the toxin (Tamapin from M. tamulus) make contacts with a negatively charged extracellular loop of KCa2 channel stabilizing the binding (Rodriguez de la Vega et al., 2013; Andreotti et al., 2005).
In the last two years new peptides have been described and classified within this family. In the transcriptomic analysis from M. martensii two new peptides were found BmKcug1 and BmKcug2, called α-KTx1.14 and α-KTx1.15, respectively. Both toxins show a high identity with the BmTx1, BmTx2 and IbTx, therefore are surmised to have similar activity on the K+ channels. Other peptide described was BmKcugx, belonging to the α-KTx22 subfamily along with others three sequences from H. judaicus (Hj1a, Hj2a and Hj3a) and three from B. occitanus (Tx308, Tx773 and Tx790; Zeng et al., 2012). Likewise, three new were found from M. gibbsous and called MegKTx1, MegKTx2 and MegKTx3 (α-KTx3.15, α-KTx9.11, α-KTx16.7, respectively). The peptide α-KTx3.15 showed an effect on Kv1.1, Kv1.3 and Kv1.6 channels similar to other members of the α-KTx3.x subfamily. In the case of α-KTx9.11 toxin at a concentration of 1 μM blocks the Kv1.1, Kv1.2 and Kv1.3 channels, whereas the α-KTx16.7 affect the Kv1.3 channel with an IC50 value of 112 nM (Diego-Garcia et al., 2013). A new subfamily is the α-KTx23, formed by Vm23 and Vm24, two new peptides purified from the scorpion Vaejovis mexicanus smithi. These peptides are potent blockers of Kv1.3 channel of human lymphocytes with an IC50 in the order of 3 pM (Gurrola et al., 2012). Other blockers identified for the Kv 1.3 channel were: OdK2 from Odonthobuthus doriae, with an IC50 = 7.2 nM (Abdel-Mottaleb et al., 2008), OSK1 from Orthochirus scrobiculosus, with an IC50 = 14 pM (Mouhat et al., 2005), ADWX-1, Moka1 with an IC50 = 1 nM (Takacs et al., 2009) and ImKTx 88 from Isometrus maculates, showing an IC50 = 91 pM (Han et al., 2008; Han et al., 2011). In this family of toxins there are important blockers of Kv channels that could potentially play a major role for the development of future biopharmaceuticals.
The toxin Ts16 is classified as α-KTx20.1 by primary sequence similitary, but has a tridimentional structure that is characteristic of the κ-KTxs toxins. This toxin inhibit the K+ current of the Kv1.2 and Kv1.3 channels, showing an IC50 of 100 and 7 nM, respectively (Saucedo et al., 2012).
3.2 BETA-KTx FAMILY
The β-KTxs are long-chain toxins of 50–75 amino acid residues and can be subdivided into three groups. The first group comprises TsTX-Kβ-related peptides, such as TsTx-Kβ from T. serrulatus, TtrβKTx, TdiβKTx, TstβKTx, Tco 42.14 from T. serrulatus, T. trivittatus, T. discrepans, T. stigmurus, T. costatus, respectively, and MeuTXKβ1, MeuTXKβ2 from B. eupeus, AaTXKβ from A. australis and BuTXKβ from B. occitanus. The only peptide characterized to a certain extend is TsTx-Kβ from T. serrulatus. This peptide is a blocker of Kv 1.1 channel with IC50 values of 96 nM (Diego-García et al., 2008), whereas the rest of the reported peptides are transcripts that encode for putative homolog of TsTX-Kβ (Feng et al., 2013; Ma et al., 2012). The second group is made up of peptides homologous to BmTXKβ. The peptides of this group were identified from the scorpion of the families Buthidae, among which are BmTXKβ, BmTXKβ2, TdiKIK, TtrKIK, TcoKIK and TstKMK (Diego-García et al., 2008; Almeida et al., 2013) and Caraboctonidae such as HgeβKTx (Diego-Garcia et al., 2008. BmTXKβ, TtrKIKβ and HgeβKTx peptides were functionally characterized. The recombinant version of BmTXKβ showed an inhibition of the transient outward K+ current (Ito) of the rabbit atrial myocytes rabbit (Cao et al., 2003), whereas HegβTKx and TtrKIK peptides tested in cells expressing the Kv1.1, 1.2, 1.3 and 1.4 channels showed no blocking activity at 500 nM and 1 μM, respectively (Diego-Garcia et al., 2008). The last group of this family is formed by the Scorpine-like peptides, also known as “orphan” peptides. They possess two structural and functional domains: a N-terminal α-helix (with cytolytic and/or antimicrobial activity like the insect defensins) and a tightly folded C-terminal region with a CSαβ motif, displaying K+ channel-blocking activity (Diego-Garcia et al., 2007). Such peptides have been identified in the proteome and transcriptome from buthids, caraboctonids, euscorpiids, liochelids and scorpionids (Ma et al., 2012), including scorpine (Conde et al., 2000), Opiscorpine1–4 (Zhu and Tytgat, 2004), HgeScplp1 and 2 (Diego-Garcia et al., 2007), Heteroscorpine 1 (HS-1; Uawonggul et al., 2007), SJE005C, SJE056C1 and 2 (Ma et al., 2009), Ev37 (Feng et al., 2012), Tco 41.46-2 (Diego-Garcia et al., 2005), UySCl1 and 2 (Luna-Ramírez et al., 2013). The Scorpine homologs exhibit strong antimicrobial effects as well as cytolytic activity against eukaryotic cells. However, the C-terminus of HgeScplp1, which forms a CSαβ structure, blocks Kv1.1 channel currents (IC50 88 nM), while the full-length of HgeScplp1 shows cytolytic activity at 200 nM (oocytes and erythrocytes) and inhibit the grown of B. subtilis at 2 μM concentration (Diego-García et. al., 2008). Likewise, although Ev37 showed a sequences identity of 55% to HgeScplp1, the recombinant version of Ev37 did not show any antimicrobial or hemolytic activity at 20 and 10 μM, respectively; but it is able of inhibit the Kv1.3 channel current with an IC50 value of 1 μM (Feng et al., 2013). Additionally, these peptides were tested for their possible effects on parasites that causesl malaria, in special the Scorpine, which has been recombinantly expressed in Anopheles gambie cells showed antibacterial activity against B. subtilis and K. pneumonia, at 5 and 10 μM, respectively. It also produced 98% mortality in sexual stages of Plasmodium berghei (ookinetes) at 15 μM and 100% reduction in P. falciparum parasitemia at 5 μM (Carballar-Lejarazú et al., 2008). Moreover, the overexpression and secretion of the scorpine into the hemolymph from transgenic mosquitoes reduced sporozoite counts by 98%, just a few days after a Plasmodium-infected blood meal, suggesting that it could be a powerful weapon for combating malaria (Carballar-Lejarazú et al., 2008; Fang et al., 2011).
3.3 GAMMA-KTx FAMILY
The structural signature of these peptides family also is defined by the presence of the CSαβ motif-containing short chain peptides. Until now 29 sequences are known and were obtained either by isolation of the peptides from venom or inferred from cDNA sequences. These peptides were found in scorpions of the genus Centruroides, Mesobuthus and Buthus (Zeng et al., 2006; Jiménez-Vargas et al., 2012). The first peptide isolated was ErgTx1 (C. noxius; Gurrola et al., 1999a), followed by the BeKm-1 (B. eupeus; Korolkova et al., 2001); other 26 members of this subfamily were Ergtoxin-like peptides isolated from scorpions of the genus Centruroides (Lecchi et al., 2002; Nastainzky et al., 2002; Corona et al., 2002; Coronas et al., 2005; Restano-Cassulini et al., 2008; Jiménez-Vargas et al., 2012) and BmKKx2 (from M. martensii; Zeng et al., 2006). The γ-KTxs were described as mainly targeting hERG channels (Coronas et al., 2002). For the case of the ErgTx1, BeKm-1, ErgTx2, CeErg4, CeErg5 their activities were assayed using the three isoforms of ERG channels of human and rat origin. The data reveal that ErgTx2 eliminates the slow component of the rERG2 current without affecting rERG3 and weakly blocking rERG1 (IC50 400 nM; Lecchi et al., 2002; Restano-Cassulini et al., 2006). ErgTx1 inhibit human and rat ERG1 in a similar manner (IC50 4–7 nM), however, it was unable to affect human ERG2 current while completely blocked the rat ERG2 channel with poor recuperation (~IC50 1 nM). Using the isoform ERG3, this toxin showed preference for the human channel, eliminating the current completely and without recuperation (IC50 0.18 nM), whereas for rat ERG3 channel causes a weak blockade (IC50 38 nM). The blocking effects of CeErg4 and CeErg5 on ERG channels of human and rats are similar to ErgTx1 ((IC50 9–13 nM). BeKm-1 discriminates well within the three rat channels, with KD values in the range 1.5 – 757 nM. Contrary to the human channels, the effects on ERG1 and ERG3 are similar, but on ERG2 channel the effect is weaker (Jiménez-Vargas et al., 2012). The HERG channels are associated in the cell cycle and proliferation of several cancers, therefore the use of specific blockers of the channel could inhibit the proliferation of tumor cells. For example, toxin ErgTx1 was shown to inhibit the proliferatin of ovarian cancer cells (SK-OV-3) when used at concentration of 100 nM, but it was not effective on breast cancer cells using up to 300 nM concentration (Asher et al., 2011).
3.4. KAPPA-KTx FAMILY
The κ-KTx toxins are formed by two parallel α-helices linked by two disulfide bridges CSαα. Eighteen sequences were identified by transcriptomic and proteomic analysis (http://www.uniprot.org/docs/scorpktx). The peptides characterized were isolated from venoms of H. fulvipes (κ-Hefutoxin 1 and 2), H. spinifer (κ-KTx1.3, HeTx203, HeTx204), O. madagascariensis (OmTx1, 2, 3 and 4) and O. cayaporum (OcyC8 and OcyC9; Camargos et al., 2011; Chen et al., 2012). The κ-KTx toxins were subdivided in four sub-families: κ-KTx1, 2, 3 and 4. In the κ-KTx1, the first peptide characterized was κ-Hefutoxin 1 (κ-KTx1.1) that blocks Kv1.2 and 1.3 channels currents with an IC50 values of 150 and 40 μM, respectively. Apparently their interaction with voltage-gated K+ channels is similar to α-KTx toxins, consisting of the presence of the a Lys and a hydrophobic residue (mostly Phe or Tyr), that are fully exposed from a flat surfaces and interact with the channel (Srinivasan et al., 2002). Subsequently, the peptide κ-KTx1.3 was isolated and shown to have 60% identity with the κ-KTx1, and had blocking activity on Kv1.1, 1.2 and 1.3 channels (Nirthanan et al., 2005). The κ-KTx2 subfamily is composed by the Om-Toxins (κ-KTx2.1, 2.2, 2.3 and 2.4), OcyC8, OcyC9, HeTx203 and HeTx204. This subfamily shows 20% identity with respect to κ-KTx1; among themselves the identity is in the order of 60%. The κ-KTx2.3 induces a 70% reduction of K+ currents in Kv1.3 channels at 500 μM (Chagot et al., 2005). OcyC8 (κ-KTx2.5) inhibits the K+ currents of the Kv1.1 and Kv1.4 channels showing IC50 values of 217 and 71, respectively (Camargos et al., 2011). The peptide HeTx204 reduces 35 and 45 % of the K+ current of the Kv 1.3 and KCNQ1 channels at the concentration of 1 and 10 μM, respectively, whereas peptide HeTx203 alone reduces the K+ current of the KCNQ1 channel by about 18% at 10 μM (Chen et al., 2012).
4. Ca++RELEASE-CHANNEL SPECIFIC PEPTIDES (CALCINS)
Calcium channels have been classified into various subfamilies including: voltage-gated channels, voltage-independent channels and ligand-activated channels. Ligand-activated channels in turn include the ryanodine receptors (RyRs). Ryanodine receptors release calcium from the intracellular stores located in the endo/sarcoplasmic reticulum. Different isoforms of ryanodine-sensitive calcium channels designated as RyR1, RyR2 and RyR3 are found. In mammals RyR1 is expressed predominantly in skeletal muscle while RyR2 is expressed predominantly in cardiac muscle. RyR3 appears to be localized in brain smooth muscle and epithelial cells; although low levels of expression of RyR1 and RyR2 are also found in some of these tissues. The RyRs are activated by calcium or by an allosteric coupling to the L type calcium channel called the dihydropyridine receptor (DHPR) (Frazini-Armstrong and Protasi, 1997). This receptor has a cytoplasmic loop of 138 amino acids between repeats II and III of alpha 1 subunit which interacts with a region from the ryanodine receptor (Leong and MacLennan, 1998, Kimlicka and Van Petegem, 2011, Van Petegem F. 2012).
Small amounts of specific natural ligands, besides ryanodine, that bind to the RyRs have been characterized. These ligands have been found in scorpion venoms which have become a very helpful tool in the study of ionic channels (see reviews Possani et al., 1999, Rodríguez de la Vega and Possani, 2004, Rodríguez de la Vega and Possani, 2005 and Gurevitz, 2012). The first peptides known to have activity over the ryanodine receptor were found in the venom of the scorpion Buthotus hottentota. A peptide fraction of 4–7 KD increases the binding of [3H] ryanodine and induces a subconductance state in the skeletal muscle’s RyRs (Valdivia et al., 1991). In the venom of the scorpion Buthotus judaicus, a peptide of 11 KD, named ryanotoxin, was found. Such peptide increases the release of Ca2+ from the sarcoplasmic reticulum vesicles inducing the subconductance state of RyRs (Morrisette et al., 1996). Two other peptides were also found in Buthotus judaicus scorpion’s venom; they both have 28 amino acids and are named BjtX-1 and BjtX-2 respectively. They both increase the binding of [3H]ryanodine in sarcoplasmic reticulum membranes of skeletal muscle but do not have any effect in liver or cardiac microsomes (Zhu et al., 2004). In the scorpion Butus martensis Krash’s venom two peptides of 66 amino acids each one have been reported: toxins Bmk-AS and Bmk-AS-1, which significantly stimulate the binding of ryanodine to the RyR in rabbit skeletal muscle (Ji et al., 1997). The imperatoxins (IpTxi and IpTxa), both peptides of 15 KD and 3.7 KD respectively, were the first peptides reported to have a high affinity for the RyR. They receive their name from the scorpion Pandinus imperator whose venom yielded those toxins (Valdivia et al., 1992). The IpTxi is a heterodimeric protein that has phospholipase A2 activity which indirectly inhibits the skeletal and cardiac RyR; the inhibitory mechanism involves the fatty acids released from phospholipid hydrolysis caused by the toxin’s activity (Zamudio et al., 1997). Imperatoxin A (IpTxa) selectively increases the binding of [3H]ryanodine to the skeletal but not the cardiac RyR with a Kd ~6nM (El-Hayek et al., 1995b).
4.1. IMPERATOXIN A (IpTxa): STRUCTURE - FUNCTION RELATIONSHIP
Imperatoxin A was shown to be an agonist with high affinity for RyR1 (El-Hayek et al., 1995b, Valdivia et al., 1992). Nanomolar concentrations of IpTxa increase the binding of [3H]ryanodine and induce a fast release of calcium from sarcoplasmic reticulum vesicles. More recent studies show that IpTxa prolongs the lifetime of the Ca2+ sparks in frog skeletal muscle (Shtifman et al., 2000, Gonzalez et al., 2000) and significantly increases the amplitude and rate of Ca2+ release in developing skeletal muscle (Nabhani et al., 2002). In single channel recordings, IpTxa stabilizes prolonged subconductance states in RyRs of skeletal and cardiac muscle, having 43 and 28% of the channel’s conductance at the holding potentials of -40 and +40 mV, respectively (Trypathy et al., 1998).
IpTxa is a basic peptide of 33 amino acids stabilized by three disulfide bridges with a molecular weight of 3759 Da. This toxin has peculiar structural properties, since it does not possess any of the consensus motifs reported for other scorpion toxins (Bontems et al., 1991, Bonmatin et al., 1992); this means that they do not fold as the alpha/beta conformation typically found in the toxins that affect the potassium, sodium, and chloride channels (Miller C. 1995, Darbon H. 1999). Instead, the cysteines form a structural motif that belongs to the conformation known as ‘inhibitor cysteine knot’ which is found in toxins acting on voltage dependent calcium channels, such as spider and sea snail toxins μ-agatoxins and ω-conotoxins respectively (Narasimhan et al., 1994, Pallaghy et al., 1994).
The molecular structure of IpTxa (Lee et al., 2004) consists of two antiparallel β strands connected by four reverse chains (Figure 3). Both β strands are formed by the residues K20-R23 (β strand I) and K30-R33 (β strand II). The first reverse chain is formed by the residues P5-K8, which forms a β turn type IV. The second and third reverse chains are formed by the residues D13–D15 and C16-K19 and form a 310 helical turn and a type 1 β turn respectively. These two structures are found inside the loop between the first turn and the first β strand. The last reverse turn is between the residues R24 and E29 and serves to reverse the backbone between β strands I and II, this loop is ill-defined and presents the highest grade of structural disorder (Lee et al., 2004).
Figure 3. Structure of IpTxa.
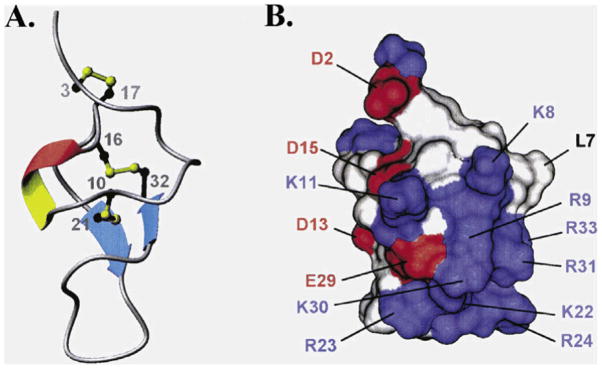
(A) Schematic diagram of IpTxa, illustrating the location of the β strands (blue), the 310 helical turn (red), and the disulfide bridges (yellow). (B) Profile of surfaces, negatively charged residues (acid) are shown in red, positively charged residues (basic) are shown in blue, and the residues not charged or hydrophobic are in white (Figure taken from Lee et al., 2004).
The domain inside the II-III loop of the DHPR that is essential for the activity of the RyR1 has been found in the region between T671 and L690 and has been denominated peptide A (El-Hayek et al., 1995a, El-Hayek and Ikemoto 1998, Bannister et al., 2009). The mechanism by which peptide A alters the RyR function has not been entirely defined. Even though the exact role of this region of the DHPR in the activation of RyR is debated, (El-Hayek and Ikemoto 1998, Proenza et al., 2000, Ahern et al., 2001, Cui et al 2009) the binding of this peptide to RyR1 has been demonstrated (Gurrola et al., 1999b, Dulhunty et al., 2004, Casarotto et al., 2001, Green et al., 2003, Porta et al., 2009).
It has been proposed that IpTxa mimics the action of peptide A, based in the similar properties of activation of the RyR1, probably through a common effector site, that consists of a grouping of basic amino acid residues (KKCKRR) in the positions 19–24 of the IpTxa and in the positions 681–685 (RKRRK) in peptide A (Gurrola et al., 1999b, Porta et al., 2008). Mutations within these regions in the IpTxa and peptide A, indicate that the basic amino acids followed by a hydroxylated amino acid, (T26 for IpTxa and S687 for peptide A) form a crucial domain necessary for the binding of both peptides to RyR.
Through a series of experiments using a group of IpTxa analogues where every amino acid was substituted by alanine (alanine-scanning) Lee et al found that the residues responsible for the activation of the RyR1 are located in the C-terminal region and correspond to R24, R31 and R33 as well as to K22 and R23. They also show that the Leucine found in position 7 plays a very important role, since it functions as a point of hydrophobic interaction with the RyR. Mutations of some acidic residues (e.g., D2, D13, and E29) also reduced the effects of the toxin on channel modification. These results suggest that the charged amino acid distributed on the surface of IpTxa contribute to the stimulatory action of the toxin and to its interaction with RyR1 (Seo et al., 2011)
4.3 SPECIFICITY AND STOICHIOMETRY OF IMPERATOXIN A
IpTxa increases the binding of [3H]ryanodine in RyR1 but not in the RyR2, even though it induces the appearance of subconductance states in both types of receptors (El-Hayek et al., 1995b, Trypathy et al., 1998). IpTxa stimulates the binding of [3H]ryanodine to RyR3 even though this response is smaller than that observed in RyR1 and induces the appearance of substates in RyR3 similar to those induced in RyR1 and RyR2 channels (Simeoni et al., 2001).
At least three separate effects of IpTxa over the cardiac and skeletal receptor have been reported. They can be distinguished by their affinity, their reversibility and by their ability to compete with peptide A. Dulhunty et al show that peptide A competes with IpTxa for a site of high affinity that activates the channel, however, peptide A does not prevent the characteristic substates induced by the toxin from happening. These results suggest that at least a common binding site as well as an independent binding site exist for both the IpTxa and peptide A (Dulhunty et al., 2004).
Studies in fibers of frog skeletal muscle led us to conclude that one molecule of IpTxa interact with one RyR during the induction of the long events of calcium release (Shtifman et al., 2000). Nevertheless, [3H]ryanodine binding (Gurrola et al., 1999b) and electronic microscopy (Samso et al., 1999) experiments indicate a binding stoichiometry of four molecules of IpTxa per RyR channel, that is one IpTxa per RyR monomer. So it is possible that the binding of IpTxa to one of the four possible sites could be enough to activate the channel. Since the mean open time of the events induced by the toxin is independent from the concentration of the toxin, it is possible that the binding of more than one molecule of the toxin will not alter the conductance control of the first toxin molecule (Shtifman et al., 2000).
4.4. OTHER CALCINS
Maurocalcin (MCa), a peptide of 33 amino acids purified from the venom of the scorpion Scorpio maurus palmatus, shares 82% sequence identity with IpTxa (Fajloun et al., 2000). As expected from the high grade of sequence similarity, both toxins present a very closely related 3D structure. The 3D structure of MCa shows that it consists of three β strands and four reverse chains (Mosbah et al., 2000). The 3D structure also shows a surface made up of basic residues (K20-R24) with an opposite surface constituted of acidic residues (E12, D15 and E29) which is also noticed in the outline of IpTxa surfaces. The main structural differences between IpTxa and MCa are found near the amino terminal region, where MCa forms an additional peripheral β strand (residues 9 to 11) that participates in the β sheet formed by the triple β strand.
As performed with IpTxa, Esteve et al, conducted experiments using synthetic analogues of MCa. They identified the R24 as an amino acid crucial for the toxin’s activity. The replacement of the R24 with Alanine produces a complete loss in the toxin’s activity. The loss of activity of this analogue is due to the fact that it is not able to bind to the RyR. Other synthetic analogues (K8A, K19A, K20A, K22A, R23A, and T26A) maintain effects similar to those of the native toxin albeit with reduced capacity; therefore, the changed residues in these analogues do not appear crucial for the binding of MCa to the RyR1 (Esteve et al., 2003).
Hemicalcin (HCa) was purified from the venom of the Iranian scorpion Hemiscorpus lepturus. It is a highly basic 33-amino-acid peptide that is cross-linked by three disulfide bridges. It shares approximately 90% sequence identity with MCa and 88% with IpTxa. Like these two related toxins, HCa also shares sequence similarity with peptide A.
A common binding site on RyR1 that can bind to peptide A as well as to MCa indicates that the binding site of these toxins on RyR1 involves the region of identity between peptide A and the toxins (Altafaj et al., 2005). The molecular model of HCa suggests that it also possesses a highly basic domain that presents the same surface orientation as seen in IpTx A and MCa. HCa stimulates [3H]ryanodine binding to RyR1 and produces Ca2+ release from SR vesicles. In single channel experiments the peptide induces an increase in open probability (Po) and, at higher concentrations, stabilizes a prolonged subconductance state of RyR1. These results show that the activity of HCa on RyR1 is similar to those observed with IpTxa and MCa, indicating that these toxins have similar mechanisms of action on RyR1 (Shahbazzadeh et al., 2007).
Hadrucalcin (HdCa) was isolated from the venom of Hadrurus gertschi. This toxin shares 68% and 78% sequence identity with IpTxa and MCa, respectively. Furthermore, the sequence of HdCa contains a Serine instead of a Lysine in a region previously shown to be critical for the affinity of the toxin-RyR interaction (Lee et al, 2004, Mabrouk et al., 2007) and the globular structure of the toxin was considerably less amphipathic than in other calcins. Despite these differences, HdCa was found to enhance the binding of [3H] ryanodine to RyR1 with an ED50 of ~37nM: only slightly higher than IpTxa (10 nM) and MCa (25 nM), but lower than HCa (71 nM; Schwartz et al., 2009).
In single channel studies HdCa induced the prolonged subconductance state that has become a distinctive characteristic of the calcin family. The amplitude of the subconductance state constituted 35% of the total conductance in RyR1 similar to HCa (38%) and IpTxa (25–30%), and slightly lower than MCa (~50%). However in cardiac single channels the response was quite different; in those channels the amplitude of the subconductance state was ~50% of the total conductance, making HdCa the first calcin known to induce subconductance states of differing amplitude in the skeletal and cardiac RyR isoforms (Schwartz et al., 2009).
Other calcins, such as Opicalcin 1 and 2 have been deduced from a nucleotide sequence of venom libraries. Those calcins belong to scorpion Opistophthalmus carinatus’s venom (Zhu et al., 2003) but have not yet been purified nor tested for biological activity. Inferences based in their sequences are therefore provisional and await experimental confirmation.
4.5. CALCINS AS CELL-PENETRATING PEPTIDES
It is known that charged peptidic toxins are not capable of crossing the plasmatic membrane, and because of this, they are not known to have intracellular targets. Nevertheless, calcins are extremely basic peptides, they possess a net charge of +7 at physiological pH and, like classical cell-penetrating peptides, and these properties make them good candidates to permeate the membrane (Philippe et al., 2003, Lindgren et al., 2000). It is also known that the highly basic peptides translocate to the cytosol by locally perturbing the integrity of the lipidic bilayer (Mabrouk et al., 1991). This crossing through the membrane could be the result of the interaction of the positive charges of the basic residues with the negatively charged polar heads of fatty acids of the plasmatic membrane.
It has been shown that MCa is able to induce intracellular calcium release in intact myotubes in the absence of external calcium, demonstrating that the increased citoplasmatic calcium concentration is due to the calcium release from the internal stores by the action of MCa (Esteve et al., 2003). Esteve et al labeled MCa with biotin, coupled it to fluorescent streptavidine and showed that the entire complex could translocate across the membrane of CHO cells (Esteve et al., 2005).
It has been demonstrated that IpTxa, both in its native state and as a fluorescent derivative (Alexa-IpTxa), has the ability to cross the plasma membrane of intact cardiomyocytes to modulate RyRs, its intracellular target. The penetration of native IpTxa into living, isolated cardiomyocytes is fast (few seconds) and functionally consequential, as assessed by the visualization of [Ca2+]i transients when perfused directly with IpTxa. Permeation of Alexa-IpTxa on the other hand is slower (minutes), possibly due to the insertion of the membrane-impermeable fluorophore. These results demonstrate that IpTxa is capable of crossing cell membranes to alter the release of Ca2+ in vivo, and has the ability to carry a large, membrane-impermeable cargo across the plasma membrane (Gurrola et al., 2010).
Perfusion of HdCa onto the surface of intact cardiomyocytes dramatically modified the amplitude of the [Ca2+]i transient, initially increasing its amplitude and later producing a lower steady state with spontaneous Ca2+ release. The initial effect had a rapid onset (~2 s) and was the best indicator that the peptide penetrates the plasmatic membrane of cardiomyocytes with remarkably rapid kinetics. (Schwartz et al., 2009).
5. GENERAL CONCLUSIONS
In this communication, we reviewed three different types of toxic peptides isolated from scorpion venoms, or assumed to be present in their venoms by cloning genes of venomous glands. The peptides specific for Na+-channels are the most important for their medical relevance. They are the ones that recognize mammalian Na+-channels and can produce an anomalous depolarization of excitable cells. The known number of these peptides is in excess of 300. There are two principal types of physiological effects that these peptides can produce: either prolonging the action potential or making the channels to open at lower voltage. These effects are responsible for a series of intoxication symptoms in animals, including man. If not treated on time can cause fatality of the affected subject. The K+-channel specific peptides are also important for their participation in many of the intoxication symptoms, because they are blockers of potassium permeability. Although not so important as life threatening, they certainly produce discomfort and cause electrophysiological problems to the affect subject. The amount of the known K+-channels is in excess of 140.
Finally the calcins are a family of peptide toxins isolated from the venom of scorpions, including Imperatoxin, Maurocalcin, Hemicalcin, Hadrucalcin and Opicalcin 1 and 2, capable or recognizing Ca++-channels. Three of the calcins have been shown to translocate across cell membranes to directly interact with ryanodine receptors, making the calcins attractive candidates for novel drug design.
To counteract the deleterious effects of these peptides, specific anti-venoms have been prepared, mainly using horses for production of immunoglobulins that can neutralize the toxicity of these three types of different peptides found in scorpion venoms.
Figure 2. Structures of scorpion toxins affecting K+ channels.
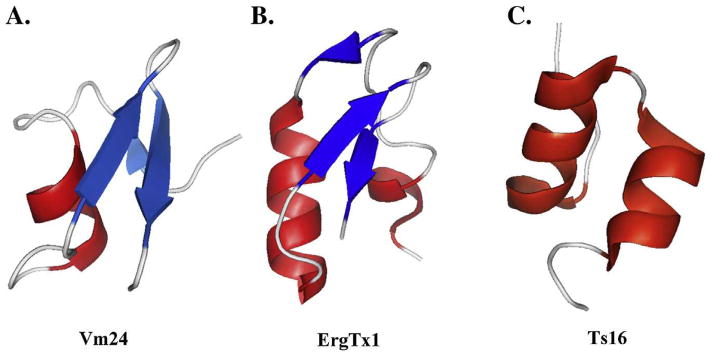
This figure shows three examples of toxins belonging to: (A) the α-KTx23 subfamily (Vm24; pdb 2K9O), (B) the γ-KTx1 subfamily (ErgTx1; pdb 1PX9) and (C) the α-KTx20 subfamily (Ts16; pdb 2LO7). The latter toxin has a CSαα motif, typical of the κ-KTxs, however, was classified by its primary sequence into of the α-KTx family. The structures were displayed with Pymol (www.pymol.org) and shown in red the α-helix and in blue the β-sheets.
Acknowledgments
The authors hold a National Institutes of Health grant (RO1-HL108175). Partial support came also from grant Dirección General de Asuntos del Personal Academico of UNAM, grant number IN-200113 to LDP.
References
- Abdel-Mottaleb Y, Vandendriessche T, Clynen E, Landuyt B, Jalali Vatanpour H, et al. OdK2, a Kv1.3 channel-selective toxin from the venom Iranian scorpion Odonthobuthus doriae. Toxicon. 2008;51:1424–30. doi: 10.1016/j.toxicon.2008.03.027. [DOI] [PubMed] [Google Scholar]; Acta Trop. 107:71–79. [Google Scholar]
- Ahern CA, Bhattacharya D, Mortenson L, Coronado R. A component of excitation-contraction coupling triggered in the absence of the T671-L690 and L720-Q765 regions of the II-III loop of the dehydropyridine receptor alpha(ls) pore subunit. Biophys J. 2001;81(6):3294–307. doi: 10.1016/S0006-3495(01)75963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DD, Scortecci KC, Kobashi LS, ALF, Medeiros SR, Silva-Junior AA, De Junqueira-de-Azevedo I, de Fernandes-Pedrosa MF. Profiling the resting venom gland of the scorpion Tityus stigmurus through a transcriptomic survey. BioMed Central. 2012;13:362. doi: 10.1186/1471-2164-13-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DD, Torres TM, Barbosa EG, Lima JPMS, Fernandes-Pedrosa MF. Molecular approaches for structural characterization of a new potassium channel blocker from Tityus stigmurus venom: cDNA cloning, homology modeling, dynamic simulations and docking. Biochemical and Biophysical Research Communications. 2013;430:113–118. doi: 10.1016/j.bbrc.2012.11.044. [DOI] [PubMed] [Google Scholar]
- Altafaj X, Cheng E, Estève E, Urbani J, Grunwald D, Sabatier J-M, Coronado R, De Waard M, Ronjat M. Maurocalcine and domain A of the II–III loop of the dihydroprydine receptor Cav1.1 subunit share common binding sites on the skeletal ryanodine receptor. J Biol Chem. 2005;280:4013–4016. doi: 10.1074/jbc.C400433200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreotti N, di Luccio E, Sampieri F, De Waard M, Sabatier JM. Molecular modeling and docking simulations of scorpion toxins and related analogs on human SKCa2 and SKCa3 channels. Peptides. 2005;26:1095–1108. doi: 10.1016/j.peptides.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Asher V, Warren A, Shaw R, Sowtere H, Bali A, Khan R. The role of Eag and HERG channels in cell proliferation and apoptotic cell death in SK-OV-3 ovarian cancer cell line. Cancer Cell International. 2011;11(6):1–7. doi: 10.1186/1475-2867-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babin DR, Watt DD, Goos SM, Mlejnek RV. Amino acid sequence of neurotoxin I from Centruroides sculpturatus Ewing. Arch Biochem Biophys. 1975;166:125–134. doi: 10.1016/0003-9861(75)90371-9. [DOI] [PubMed] [Google Scholar]
- Bannister RA, Papadopoulos S, Haarmann CS, Beam KG. Effects of inserting fluorescent proteins into the alpha1S II-III loop: insights into excitation-contraction coupling. J Gen Physiol. 2009;134(1):35–51. doi: 10.1085/jgp.200910241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista CV, Román-González SA, Salas-Castillo SP, Zamudio FZ, Gómez-Lagunas F, Possani LD. Proteomic analysis of the venom from the scorpion Tityus stigmurus: biochemical and physiological comparison with other Tityus species. Comp Biochem Physiol C Toxicol Pharmacol. 2007;146:147–157. doi: 10.1016/j.cbpc.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Bergeron ZL, Bingham JP. Scorpion Toxins Specific for Potassium (K+) Channels: A Historical Overview of Peptide Bioengineering. Toxins. 2012;4:1082–1119. doi: 10.3390/toxins4111082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonmatin JM, Bonnat JL, Gallet X, Vovelle F, Ptak M, Reichhart JM, Hoffmann JA, Keppi E, Legrain M, Achstetter T. Two-dimensional 1H NMR study of recombinant insect defensin A in water: resonance assignments, secondary structure and global folding. J Biomol NMR. 1992;2(3):235–56. doi: 10.1007/BF01875319. [DOI] [PubMed] [Google Scholar]
- Bontems F, Roumestand C, Gilquin B, Menez A, Toma F. Refined structure of charybdotoxin: common motifs in scorpion toxins and insect defensins. Science. 1991;254(5037):1521–3. doi: 10.1126/science.1720574. [DOI] [PubMed] [Google Scholar]
- Borchani L, Stankiewicz M, Kopeyan C, Mansuelle P, Kharrat R, Cestèle S, Karoui H, Rochat H, Pelhate M, El Ayeb M. Purification, structure and activity of three insect toxins from Buthus occitanus tunetanus venom. Toxicon. 1997;35:365–382. doi: 10.1016/s0041-0101(96)00173-0. [DOI] [PubMed] [Google Scholar]
- Bosmans F, Tytgat J. Voltage-gated sodium channel modulation by scorpion α-toxins. Toxicon. 2007;49:142–158. doi: 10.1016/j.toxicon.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliskan F, Quintero-Hernández V, Restano-Cassulini R, Batista CV, Zamudio FZ, Coronas FI, Possani LD. Turkish scorpion Buthacus macrocentrus: general characterization of the venom and description of Bu1, a potent mammalian Na+-channel α-toxin. Toxicon. 2012;59:408–415. doi: 10.1016/j.toxicon.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Caliskan F, Quintero-Hernández V, Restano-Cassulini R, Coronas-Valderrama FI, Corzo G, Possani LD. Molecular cloning and biochemical characterization of the first Na(+)-channel α-type toxin peptide (Acra4) from Androctonus crassicauda scorpion venom. Biochimie. 2013 doi: 10.1016/j.biochi.2013.01.015. In press. [DOI] [PubMed] [Google Scholar]
- Camargos TS, Restano-Cassulini R, Possani LD, Peigneur S, Tytgat J, Schwartz CA, Alves EMC, de Freitas SM, Schwartz EF. The new kappa-KTx 2.5 from the scorpion Opisthacanthus cayaporum. Peptides. 2011;32:1509–1517. doi: 10.1016/j.peptides.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Cao Z, Xian F, Peng F, Jiang D, Mao X, Liu H, Li W, Hu D, Wang T. Expression, purification and functional characterization of a recombinant scorpion venom peptide BmTXKbeta. Peptides. 2003;24:187–192. doi: 10.1016/s0196-9781(03)00025-1. [DOI] [PubMed] [Google Scholar]
- Carballar-Lejarazú R, Rodríguez MH, de la Cruz Hernández-Hernández F, Ramos-Castañeda J, Possani LD, Zurita-Ortega M, Reynaud-Garza E, Hernández-Rivas R, Loukeris T, Lycett G, Lanz-Mendoza H. Recombinant scorpine: a multifunctional antimicrobial peptide with activity against different pathogens. Cell Mol Life Sci. 2008;65:3081–3092. doi: 10.1007/s00018-008-8250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarotto MG, Gibson F, Pace SM, Curtis SM, Mulcair M, Dulhunty AF. A structural requirement for activation of skeletal ryanodine receptors by peptides of the dihydropyridine receptor II-III loop. J Biol Chem. 2001;275(16):11631–11637. doi: 10.1074/jbc.275.16.11631. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Binding of scorpion toxin to receptor sites associated with sodium channels in frog muscle. Correlation of voltage-dependent binding with activation. J Gen Physiol. 1979;74:375–391. doi: 10.1085/jgp.74.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev. 1992;72:S15–S48. doi: 10.1152/physrev.1992.72.suppl_4.S15. [DOI] [PubMed] [Google Scholar]
- Catterall WA. From Ionic Currents to Molecular Mechanisms: The Structure and Function of Voltage-Gated Sodium Channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Cestèle S, Yarov-Yarovoy V, Yu FH, Konoki K, Scheuer T. Voltage-gated ion channels and gating modifier toxins. Toxicon. 2007;49:124–141. doi: 10.1016/j.toxicon.2006.09.022. [DOI] [PubMed] [Google Scholar]
- Cestèle S, Qu Y, Rogers JC, Rochat H, Scheuer T, Catterall WA. Voltage sensor-trapping: enhanced activation of sodium channels by beta-scorpion toxin bound to the S3–S4 loop in domain II. Neuron. 1998;21:919–931. doi: 10.1016/s0896-6273(00)80606-6. [DOI] [PubMed] [Google Scholar]
- Cestèle S, Scheuer T, Mantegazza M, Rochat H, Catterall WA. Neutralization of gating charges in domain II of the sodium channel alpha subunit enhances voltage-sensor trapping by a beta-scorpion toxin. J Gen Physiol. 2001;118:291–302. doi: 10.1085/jgp.118.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cestèle S, Yarov-Yarovoy V, Qu Y, Sampieri F, Scheuer T, Catterall WA. Structure and function of the voltage sensor of sodium channels probed by a beta-scorpion toxin. J Biol Chem. 2006;281:21332–21344. doi: 10.1074/jbc.M603814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagot B, et al. An unusual fold for potassium channel blockers: NMR structure of three toxins from the scorpion Opisthacanthus madagascariensis. Biochem J. 2005;388:263–271. doi: 10.1042/BJ20041705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Chung SH. Binding modes and functional surface of anti-mammalian scorpion α-toxins to sodium channels. Biochemistry. 2012;51:7775–7782. doi: 10.1021/bi300776g. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Zeng DY, Hu YT, He YW, Pan N, Ding JP, Cao ZJ, Liu ML, Li WX, Yi H, Jiang L, Wu YL. Structural and Functional Diversity of Acidic Scorpion Potassium Channel Toxins. Plos One. 2012;7 (4):1–10. doi: 10.1371/journal.pone.0035154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux JP. Emerging options for the management of scorpion stings. Drug Des Devel Ther. 2012;6:165–73. doi: 10.2147/DDDT.S24754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chippaux JP, Goyffon M. Epidemiology of scorpionism: a global appraisal. Acta Trop. 2008;107:71–79. doi: 10.1016/j.actatropica.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Conde R, Zamudio FZ, Rodriguez MH, Possani LD. Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett. 2000;471:165–168. doi: 10.1016/s0014-5793(00)01384-3. [DOI] [PubMed] [Google Scholar]
- Corona M, Gurrola GB, Merino E, Cassulini RR, Valdez-Cruz NA, García B, Ramírez-Domínguez ME, Coronas FI, Zamudio FZ, Wanke E, Possani LD. A large number of novel Ergtoxin-like genes and ERG K+-channels blocking peptides from scorpions of the genus Centruroides. FEBS Lett. 2002;532:121–126. doi: 10.1016/s0014-5793(02)03652-9. [DOI] [PubMed] [Google Scholar]
- Coronas FI, Balderas C, Pardo-Lopez L, Possani LD, Gurrola GB. Amino acid sequence determination and chemical synthesis of CllErg1 (γ-KTx1.5), a K+ channel blocker peptide isolated from the scorpion Centruroides limpidus limpidus. J Braz Chem Soc. 2005;16(3A):404–411. [Google Scholar]
- Couraud F, Jover E, Dubois JM, Rochat H. Two types of scorpion receptor sites, one related to the activation, the other to the inactivation of the action potential sodium channel. Toxicon. 1982;20:9–16. doi: 10.1016/0041-0101(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Cui Y, Tae HS, Norris NC, Karunasekara Y, Pouliquin P, Board PG, Dulhunty AF, Casarotto MG. A dihydropyridine receptor alpha 1s loop region critical for skeletal muscle contraction is intrinsically unstructured and binds to a SPRY domain of the type 1 ryanodine receptor. Int J Biochem Cell Biol. 2009;41(3):677–686. doi: 10.1016/j.biocel.2008.08.004. [DOI] [PubMed] [Google Scholar]
- D’Suze G, Schwartz EF, García-Gómez BI, Sevcik C, Possani LD. Molecular cloning and nucleotide sequence analysis of genes from a cDNA library of the scorpion Tityus discrepans. Biochimie. 2009;91:1010–1019. doi: 10.1016/j.biochi.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Darbon H. Animal toxin and ion channels. J Soc Biol. 1999;193(6):445–50. [PubMed] [Google Scholar]
- Del Rio-Portilla F, Hernández-Marín E, Pimienta G, Coronas FV, Zamudio FZ, Rodríguez de la Vega RC, Wanke E, Possani LD. NMR solution structure of Cn12, a novel peptide from the Mexican scorpion Centruroides noxius with a typical α-toxin sequence but with β-like physiological activity. Eur J Biochem. 2004;271:2504–2516. doi: 10.1111/j.1432-1033.2004.04181.x. [DOI] [PubMed] [Google Scholar]
- Diego-García E, Abdel Mottaleb Y, Schwartz EF, de la Vega RC, Tytgat J, Possani LD. Cytolytic and K+ cannel blocking activities of beta-KTx and scorpine-like peptides purified from scorpion venoms. Cell Mol Life Sci. 2008;65(1):187–200. doi: 10.1007/s00018-007-7370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diego-Garcia E, Batista CV, Garcia-Gomez BI, Lucas S, Candido DM, Gomez-Lagunas F, Possani LD. The Brazilian scorpion Tytius costatus Karsch: Genes, peptides and function. Toxicon. 2005;45:273–283. doi: 10.1016/j.toxicon.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Diego-García E, Peigneur S, Clynen E, Marien T, Czech L, Schoofs L, Tytgat J. Molecular diversity of the telson and venom components from Pandinus cavimanus (Scorpionidae Latreille 1802): transcriptome, venomics and function. Proteomics. 2012;12:313–328. doi: 10.1002/pmic.201100409. [DOI] [PubMed] [Google Scholar]
- Diego-García E, Peigneur S, Debaveye S, Gheldof E, Tytgat J, Caliskan F. Novel potassium channel blocker venom peptides from Mesobuthus gibbosus (Scorpiones: Buthidae) Toxicon. 2013;61:72–82. doi: 10.1016/j.toxicon.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Diego-García E, Schwartz EF, D’Suze G, González SAR, Batista CVF, García BI, Rodriguez de la Vega RC, Possani LD. Wide phylogenetic distribution of Scorpine and long-chain β-KTx-like peptides in scorpion venoms: identification of”orphan”components. Peptides. 2007;28:31–37. doi: 10.1016/j.peptides.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Dulhunty AF, Curtis SM, Watson S, Cengia L, Casarotto MG. Multiple actions of imperatoxin A on ryanodine receptors: Interactions with the II-III loop “A” fragment. J Biol Chem. 2004;279(12):11853–11862. doi: 10.1074/jbc.M310466200. [DOI] [PubMed] [Google Scholar]
- Eitan M, Fowler E, Herrmann R, Duval A, Pelhate M, Zlotkin E. A scorpion venom neurotoxin paralytic to insects that affects sodium current inactivation: purification, primary structure, and mode of action. Biochemistry. 1990;29:5941–5947. doi: 10.1021/bi00477a009. [DOI] [PubMed] [Google Scholar]
- El-Hayek R, Ikemoto N. Identification of the minimum essential region in the II-III loop of the dihydropyridine receptor alpha 1 subunit required for activation of skeletal muscle-type excitation-contraction coupling. Biochemistry. 1998;37(19):7015–20. doi: 10.1021/bi972907o. [DOI] [PubMed] [Google Scholar]
- El-Hayek R, Antoniu B, Wang J, Hamilton SL, Ikemoto N. Identification of calcium release-triggering and blocking regions of the II-III loop of the skeletal muscle dihydropyridine receptor. J Biol Chem. 1995a;270(38):22116–8. doi: 10.1074/jbc.270.38.22116. [DOI] [PubMed] [Google Scholar]
- El-Hayek R, Lokuta AJ, Arevalo C, Valdivia HH. Peptide probe of ryanodine receptor function. Imperatoxin A, a peptide from the venom of the scorpion Pandinus imperator, selectively activates skeletal-types ryanodine receptor isoforms. J Biol Chem. 1995b;270(48):28696–704. doi: 10.1074/jbc.270.48.28696. [DOI] [PubMed] [Google Scholar]
- Estève E, Mabrouk K, Dupuis A, Smida-Rezgui S, Altafaj X, Grunwald D, Platel JC, Andreotti N, Marty I, Sabatier JM, Ronjat M, De Waard M. Transduction of the Scorpion Toxin Maurocalcine into Cells: Evidence that the toxin crosses the plasma membrane. J Biol Chem. 2005;280(13):12833–12839. doi: 10.1074/jbc.M412521200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteve E, Smida-Rezgi S, Sarkozi S, Szegedi C, Regaya I, Chen L, Altafaj X, Rochat H, Allen P, Pessah IN, Marty I, Sabatier JM, Jona I, Waard M, Ronjat M. Critical amino acid residues determine the binding affinity and the Ca2+ release efficacy of maurocalcine in skeletal muscle cells. J Biol Chem. 2003;278(39):37822–37831. doi: 10.1074/jbc.M305798200. [DOI] [PubMed] [Google Scholar]
- Fajloun Z, Kharrat R, Chen L, Lecomte C, Di Luccio E, Bichet D, El Ayeb M, Rochat H, Allen PD, Pessah IN, Dewaard M, Sabatier JM. Chemical synthesis and characterization of maurocalcine, a scorpion toxin that activates Ca2+-release channel/ryanodine receptor. FEBS Lett. 2000;469:179–185. doi: 10.1016/s0014-5793(00)01239-4. [DOI] [PubMed] [Google Scholar]
- Fang W, Vega-Rodríguez J, Ghosh A, Jacobs-Lorena M, Kang A, Leger RJS. Development of Transgenic Fungi That Kill Human Malaria Parasites in Mosquitoes. Science. 2011;331 (6020):1074–1077. doi: 10.1126/science.1199115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Yu C, Wang, Li Z, Wu Y, Cao Z, Li W, He X, Han S. Expression and characterization of a novel scorpine-like peptide Ev37, from the scorpion Euscorpiops validus. Protein Expression and Purification. 2013;88:127–133. doi: 10.1016/j.pep.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Fletcher PL, Fletcher MD, Weninger K, Anderson TE, Martin BM. Vesicle-associated membrane protein (VAMP) cleavage by a new metalloprotease from the Brazilian scorpion Tityus serrulatus. J Biol Chem. 2010;285:7405–7416. doi: 10.1074/jbc.M109.028365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol Rev. 1997;77(3):699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- Froy O, Zilberberg N, Gordon D, Turkov M, Gilles N, Stankiewicz M, Pelhate M, Loret E, Oren DA, Shaanan B, Gurevitz M. The putative bioactive surface of insect-selective scorpion excitatory neurotoxins. J Biol Chem. 1999;274:5769–5776. doi: 10.1074/jbc.274.9.5769. [DOI] [PubMed] [Google Scholar]
- Froy O, Gurevitz M. New insight on scorpion divergence inferred from comparative analysis of toxin structure, pharmacology and distribution. Toxicon. 2003;42:549–555. doi: 10.1016/s0041-0101(03)00236-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Kirsch WG, Shirokova N, Pizarro G, Brum G, Pessah IN, Stern MD, Cheng H, Rios E. Involvement of multiple intracellular release channels in calcium sparks of skeletal muscle. Proc Natl Acad Sci USA. 2000;97(8):4380–4385. doi: 10.1073/pnas.070056497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D, Gurevitz M. The selectivity of scorpion alpha-toxins for sodium channel subtypes is determined by subtle variations at the interacting surface. Toxicon. 2003;41:25–28. doi: 10.1016/s0041-0101(02)00294-5. [DOI] [PubMed] [Google Scholar]
- Gordon D, Ilan N, Zilberberg N, Gilles N, Urbach D, Cohen L, Karbat I, Froy O, Gaathon A, Kallen RG, Benveniste M, Gurevitz M. An ‘Old World’ scorpion α-toxin that recognizes both insect and mammalian sodium channels A possible link towards diversification of α-toxins. Eur J Biochem. 2003;270:2663–2670. doi: 10.1046/j.1432-1033.2003.03643.x. [DOI] [PubMed] [Google Scholar]
- Gordon D, Karbat I, Ilan N, Cohen L, Kahn R, Gilles N, Dong K, Stühmer W, Tytgat J, Gurevitz M. The differential preference of scorpion alpha-toxins for insect or mammalian sodium channels: implications for improved insect control. Toxicon. 2007;49:452–472. doi: 10.1016/j.toxicon.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Gordon D, Savarin P, Gurevitz M, Zinn-Justin S. Functional anatomy of scorpion toxins affecting sodium channels. J Toxicol Toxin Rev. 1998;17:131–159. [Google Scholar]
- Green D, Pace S, Curtis SM, Sakowska M, Lamb GD, Dulhunty AF. The three-dimensional structural surface of two b-sheet scorpion toxins mimics that of an a-helical dihydropyridin receptor segment. Biochem J. 2003;370:517–527. doi: 10.1042/BJ20021488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevitz M, Karbat I, Cohen L, Ilan N, Kahn R, Turkov M, Stankiewicz M, Stühmer W, Dong K, Gordon D. The insecticidal potencial of scorpion beta-toxins. Toxicon. 2007;49:473–489. doi: 10.1016/j.toxicon.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Gurevitz M. Mapping of scorpion toxin receptor sites at voltage-gated sodium channels. Toxicon. 2012;60:502–511. doi: 10.1016/j.toxicon.2012.03.022. [DOI] [PubMed] [Google Scholar]
- Gurrola GB, Capes EM, Zamudio FZ, Possani LD, Valdivia HH. Imperatoxin A, a Cell-Penetrating Peptide from Scorpio Venom, as a Probe of Ca-Release Channels/Ryanodine receptors. Pharmaceuticals (Basel) 2010;3(4):1093–1107. doi: 10.3390/ph3041093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurrola GB, Hernández-López RA, Rodríguez de la Vega RC, Varga Z, Batista CV, Salas-Castillo SP, Panyi G, del Río-Portilla F, Possani LD. Structure, function, and chemical synthesis of Vaejovis mexicanus peptide 24: a novel potent blocker of Kv1.3 potassium channels of human T lymphocytes. Biochemistry. 2012;51 (19):4049–4061. doi: 10.1021/bi300060n. [DOI] [PubMed] [Google Scholar]
- Gurrola GB, Rosati B, Rocchetti M, Pimienta G, Zaza A, Arcangeli A, Olivotto M, Possani LD, Wanke E. A toxin to nervous, cardiac, and endocrine ERG K+ channels isolated from Centruroides noxius scorpion venom. FASEB J. 1999a;13:953–962. [PubMed] [Google Scholar]
- Gurrola GB, Arevalo C, Sreekumar R, Lokuta AJ, Walker JW, Valdivia HH. Activation of ryanodine receptor by imperatoxin A and a peptide segment of the II-III loop of the dihydropyridine receptor. J Biol Chem. 1999b;274:7879–86. doi: 10.1074/jbc.274.12.7879. [DOI] [PubMed] [Google Scholar]
- Han S, Hu Y, Zhang R, Yi H, Wei J, Wu Y, et al. ImKTx88, a novel selective Kv1.3 channel blocker derived from the scorpion Isometrus maculates. Toxicon. 2011;57:348–55. doi: 10.1016/j.toxicon.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Han S, Yi H, Yin SJ, Chen ZY, Liu H, Cao ZJ, et al. Structural basis of a potent peptide inhibitor designed for Kv1.3 channel, a therapeutic target of autoinmune disease. J Biol Chem. 2008;283:19058–65. doi: 10.1074/jbc.M802054200. [DOI] [PubMed] [Google Scholar]
- Housset D, Habersetzer-Rochat C, Astier JP, Fontecilla-Camps JC. Crystal structure of toxin II from the scorpion Androctonus australis Hector refined at 1.3 Å resolution. J Mol Biol. 1994;238:88–103. doi: 10.1006/jmbi.1994.1270. [DOI] [PubMed] [Google Scholar]
- Ji YH, Liu Y, Yu K, Ohishi T, Hoshino M, Mochizuki T, Yanaihara N. Amino acid sequence of an novel activator of ryanodine receptor on skeletal muscle. Chin Sci Bull. 1997;42(11):952–956. [Google Scholar]
- Jiménez-Vargas JV, Restano-Cassulini R, Possani LD. Toxins modulators and blockers of hERG K+ channels. Toxicon. 2012;60:492–501. doi: 10.1016/j.toxicon.2012.03.024. [DOI] [PubMed] [Google Scholar]
- Kahn R, Karbat I, Ilan N, Cohen L, Sokolov S, Catterall WA, Gordon D, Gurevitz M. Molecular requirements for recognition of brain voltage-gated sodium channels by scorpion α-toxins. J Biol Chem. 2009;284:20684–20691. doi: 10.1074/jbc.M109.021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbat I, Kahn R, Cohen L, Ilan N, Gilles N, Corzo G, Froy O, Gur M, Albrecht G, Heinemann SH, Gordon D, Gurevitz M. The unique pharmacology of the scorpion a-like toxin Lqh3 is associated with its flexible C-tail. FEBS J. 2007;274:1918–31. doi: 10.1111/j.1742-4658.2007.05737.x. [DOI] [PubMed] [Google Scholar]
- Kimlicka L, Van Petegem F. The structural biology of ryanodine receptors. Sci China life Sci. 2011;54(8):712–24. doi: 10.1007/s11427-011-4198-2. [DOI] [PubMed] [Google Scholar]
- Kopeyan C, Mansuelle P, Martin-Eauclaire MF, Rochat H, Miranda F. Characterization of toxin III of the scorpion Leiurus quinquestriatus quinquestriatus: a new type of alpha-toxin highly toxic both to mammals and insects. Natural toxins. 1993;1:308–312. doi: 10.1002/nt.2620010510. [DOI] [PubMed] [Google Scholar]
- Korolkova YV, Kozlov SA, Lipkin AV, Pluzhnikov KA, Hardley JK, Filippov AK, Brown DA, Angelo K, Strobaek D, Jespersen T, Olesen SP, Jensen BS, Grishin EV. An ERG channel inhibitor from the scorpion Buthus eupeus. J Biol Chem. 2001;276:9868–9876. doi: 10.1074/jbc.M005973200. [DOI] [PubMed] [Google Scholar]
- Kozminsky-Atias A, Bar-shalom A, Mishmar D, Zilberberg N. Assembling an arsenal, the scorpion way. BioMed Central. 2008;8:333. doi: 10.1186/1471-2148-8-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecchi M, Redaelli E, Rosati B, Gurrola G, Florio T, Crociani O, Curia G, Restano-Cassulini R, Masi A, Arcangeli A, et al. Isolation of a long-lasting ERG-type K+ current in MMQ lactotrophs and its accommodating role during slow firing and prolactin release. J Neurosci. 2002;22:3414–3425. doi: 10.1523/JNEUROSCI.22-09-03414.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Lee EH, Takeuchi K, Takahashi H, Shimada I, Sato K, Shin SY, Kim do H, Kim JI. Molecular basis for the high-affinity activation of skeletal ryanodine receptor Ca2+-release channel by imperatoxin a. Biochem J. 2004;377:385–94. doi: 10.1042/BJ20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong P, MacLennan DH. A 37-amino acid sequence in the skeletal muscle ryanodine receptor interacts with the cytoplasmic loop between domains II and III in the skeletal muscle dihydropyridine receptor. J Biol Chem. 1998;273:7791–7794. doi: 10.1074/jbc.273.14.7791. [DOI] [PubMed] [Google Scholar]
- Lindgren M, Hallbrink M, Prochiantz A, Langel U. Cell-penetrating peptides. Trends Pharmacol Sci. 2000;21(3):99–103. doi: 10.1016/s0165-6147(00)01447-4. [DOI] [PubMed] [Google Scholar]
- Luna-Ramírez K, Quintero-Hernández V, Vargas-Jaimes L, Batista CV, Winkel KD, Possani LD. Characterization of the venom from the Australian scorpion Urodacus yaschenkoi: molecular mass analysis of components, cDNA sequences and peptides with antimicrobial activity. Toxicon. 2013;63:44–54. doi: 10.1016/j.toxicon.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Ma Y, He Y, Zhao R, Wu Y, Li W, Cao Z. Extreme diversity of scorpion venom peptides and proteins revealed by transcriptomic analysis: implication for proteome evolution of scorpion venom arsenal. J Proteomics. 2012;75:1563–1576. doi: 10.1016/j.jprot.2011.11.029. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhao R, He Y, Li S, Liu J, Wu Y, Cao Z, Li W. Transcriptome analysis of the venom gland of the scorpion Scorpiops jendeki: implication for the evolution of the scorpion venom arsenal. BMC Genomics. 2009;10:290. doi: 10.1186/1471-2164-10-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Zhao Y, Zhao R, Zhang W, He Y, Wu Y, Cao Z, Guo L, Li W. Molecular diversity of toxic components from the scorpion Heterometrus petersii venom revealed by proteomic and transcriptome analysis. Proteomics. 2010;10:2471–2485. doi: 10.1002/pmic.200900763. [DOI] [PubMed] [Google Scholar]
- Mabrouk K, Ram N, Boisseau S, Strappazzon F, Rehaim A, Sadoul R, Darbon H, Roniat M, De Waard M. Critical amino acid residues of maurocalcine involved in pharmacology, lipid interaction and cell penetration. Biochim Biophys Acta. 2007;1768(10):2528–2540. doi: 10.1016/j.bbamem.2007.06.030. [DOI] [PubMed] [Google Scholar]
- Mabrouk K, Van Rietschoten J, Vives E, Darbon H, Rochat H, Sabatier JM. Lethal neurotoxicity in mice of the basic domains of HIV and SIV Rev proteins. Study of these regions by circular dichroism. FEBS Lett. 1991;289:13–17. doi: 10.1016/0014-5793(91)80898-d. [DOI] [PubMed] [Google Scholar]
- Martin MF, García y Pérez LG, el Ayeb M, Kopeyan C, Bechis G, Jover E, Rochat H. Purification and chemical and biological characterizations of seven toxins from the Mexican scorpion, Centruroides suffusus suffusus. J Biol Chem. 1987;262:4452–4459. [PubMed] [Google Scholar]
- Martin-Eauclaire MF, Couraud F. Scorpion neurotoxins: effects and mechanisms. In: Chang LW, Dyer RS, editors. Handk Neurotoxicology. Marcel Dekker; NY: 1995. pp. 683–716. [Google Scholar]
- Meves H, Rubly N, Watt DD. Effect of toxins isolated from the venom of the scorpion Centruroides sculpturatus on the Na currents of the node of Ranvier. Pflugers Arch. 1982;393:56–62. doi: 10.1007/BF00582392. [DOI] [PubMed] [Google Scholar]
- Meves H, Rubly N, Watt DD. Voltage-dependent effect of a scorpion toxin on sodium current inactivation. Eur J Physiol Pflugers Arch. 1984;402:24–33. doi: 10.1007/BF00584827. [DOI] [PubMed] [Google Scholar]
- Meves H, Simard JM, Watt DD. Interactions of scorpion toxins with the sodium channel. Ann N Y Acad Sci. 1986;479:113–132. doi: 10.1111/j.1749-6632.1986.tb15565.x. [DOI] [PubMed] [Google Scholar]
- Miller C. The charybdotoxin family of K+ channel-blocking peptides. Neuron. 1995;15:5–10. doi: 10.1016/0896-6273(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Morgenstern D, Rohde BH, King GF, Tzachy T, Sher D, Zlotkin E. The tale of a resting gland: Transcriptome of a replete venom gland from the scorpion Hottentotta judaicus. Toxicon. 2011;57:695–703. doi: 10.1016/j.toxicon.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Morrissette J, Beurg M, Sukhareva M, Coronado R. Purification and characterization of ryanotoxin, a peptide with actions similar to those of ryanodine. Biophys J. 1996;71(2):707–21. doi: 10.1016/S0006-3495(96)79270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosbah A, Kharrat R, Faijloun Z, Renisio JG, Blanc E, Sabatier JM, El Ayeb M, Darbon H. A new fold in the scorpion toxin family, associated with an activity on a ryanodine-sensitive calcium channel. PROTEINS: Structure, Function and Genetics. 2000;40:436–442. doi: 10.1002/1097-0134(20000815)40:3<436::aid-prot90>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Mouhat S, Teodorescu G, Homerick D, Visan V, Wulff H, Wu Y, et al. Pharmacological profiling of Orthochirus scrobiculosus toxin 1 analogs with a trimmed N-terminal domain. Mol Pharmacol. 2005;9:354–62. doi: 10.1124/mol.105.017210. [DOI] [PubMed] [Google Scholar]
- Mouhat S, Jouirou B, Mosbah A, De Waard M, Sabatier JM. Diversity of folds in animal toxins acting on ion channels. Biochem J. 2004;378:717–726. doi: 10.1042/BJ20031860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabhani T, Zhu X, Simeoni I, Sorrentino V, Valdivia HH, Garcia J. Imperatoxin a enhances Ca(2+) release in developing skeletal muscle containing ryanodine receptor type 3. Biophys J. 2002;82(3):1319–28. doi: 10.1016/S0006-3495(02)75487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan L, Singh J, Humblet C, Guruprasad K, Blundell T. Snail and spider toxins share a similar tertiary structure and ‘cystine motif’. Nat Struct Biol. 1994;1(12):850–2. doi: 10.1038/nsb1294-850. [DOI] [PubMed] [Google Scholar]
- Nastainczyk W, Meves H, Watt DD. A short-chain peptide toxin isolated from Centruroides sculpturatus scorpion venom inhibits ether-a-go-go-related gene K(+) channels. Toxicon. 2002;40:1053–1058. doi: 10.1016/s0041-0101(02)00100-9. [DOI] [PubMed] [Google Scholar]
- Newton KA, Clench MR, Deshmukh R, Jeyaseelan K, Strong PN. Mass fingerprinting of toxic fractions from the venom of the Indian red scorpion, Mesobuthus tamulus: biotope-speciic variation in the expression of venom peptides. Rapid Commun Mass Spectrom. 2007;21:3467–3476. doi: 10.1002/rcm.3240. [DOI] [PubMed] [Google Scholar]
- Nirthanan S, Pil J, Abdel-Mottaleb Y, Sugahara Y, Gopalakrishnakone P, Joseph JS, Sato K, Tytgat J. Assignment of voltage-gated potassium cannel blocking activity to κ-KTx1.3, a non-toxic homologue of κ-hefutoxin-1, from Heterometrus spinifer venom. Biochemical Pharmacology. 2005;69:669–678. doi: 10.1016/j.bcp.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Oren DA, Froy O, Amit E, Kleinberger-Doron N, Gurevitz M, Shaanan B. An excitatory scorpion toxin with a distinctive feature: an additional alpha helix at the C terminus and its implications for interaction with insect sodium channels. Structure. 1998;6:1095–1103. doi: 10.1016/s0969-2126(98)00111-7. [DOI] [PubMed] [Google Scholar]
- Oukkache N, Rosso JP, Alami M, Ghalim N, Saïle R, Hassar M, Bougis PE, Martin-Eauclaire MF. New analysis of the toxic compounds from the Androctonus mauritanus mauritanus scorpion venom. Toxicon. 2008;51:835–52. doi: 10.1016/j.toxicon.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Pallaghy PK, Nielsen KJ, Craik DJ, Norton RS. A common structural motif incorporating a cystine knot and a triple-stranded beta-sheet in toxic and inhibitory polypeptides. Protein Sci. 1994;3:1833–1839. doi: 10.1002/pro.5560031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedraza Escalona M, Possani LD. Scorpion beta-toxins and voltage-gated sodium channels: interactions and effects. Front Biosci Landmark. 2013;18:572–587. doi: 10.2741/4121. [DOI] [PubMed] [Google Scholar]
- Pélate M, Zlotkin E. Actions of insect toxin and other toxins derived form the venom of the scorpion Androctonus australis on isolated giant axons of the cockroach (Periplaneta americana) J Exp Biol. 1982;97:67–77. doi: 10.1242/jeb.97.1.67. [DOI] [PubMed] [Google Scholar]
- Philippe JR, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B. Cell-penetrating peptides. A reevaluation of the mechanism of cellular uptake. The Journal of Biological Chemistry. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- Porta M, Diaz-Sylvester PL, Nani A, Ramos-Franco J, Copello JA. Ryanoids and imperatoxin affect the modulation of cardiac ryanodine receptors by dihydropyridine receptor Peptide A. Biochim Biophys Acta. 2008;778(11):2469–2479. doi: 10.1016/j.bbamem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possani LD, Becerril B, Delepierre M, Tytgat J. Scorpion toxins specific for Na+-channels. Eur J Biochem. 1999;264:287–300. doi: 10.1046/j.1432-1327.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- Possani LD, Martin BM, Svendsen I, Rode GS, Erickson BW. Scorpion toxins form Centruroides noxius and Tityus serrulatus. Primary structures and sequence comparison by metric analysis. Biochem. 1985;229:739–750. doi: 10.1042/bj2290739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendini L, Wheeler WC. Scorpion higher phylogeny and classification, taxonomic anarchy, and standards for peer review in online publishing. Cladistics. 2005;21:446–494. doi: 10.1111/j.1096-0031.2005.00073.x. [DOI] [PubMed] [Google Scholar]
- Proenza C, Wilkens CM, Beam KG. Excitation-contraction coupling is not affected by scrambled sequence in residues 681–690 of the dihydropyridine receptor II-III loop. J Biol Chem. 2000;275(39):29935–29937. doi: 10.1074/jbc.C000464200. [DOI] [PubMed] [Google Scholar]
- Quintero-Hernández V, Ortiz E, Rendón-Anaya M, Shwartz EF, Becerril B, Corzo G, Possani LD. Scorpion and spider venom peptides: Gene cloning and peptide expression. Toxicon. 2011;58:644–663. doi: 10.1016/j.toxicon.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Rendón-Anaya M, Delaye L, Possani LD, Herrera-Estrella A. Global transcriptome analysis of the scorpion Centruroides noxius: new toxin families and evolutionary insights from an ancestral scorpion species. PloS One. 2012;7:e43331. doi: 10.1371/journal.pone.0043331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restano-Cassulini R, Korolkova YK, Diochot S, Gurrola G, Guasti L, Possani L, Lasdunski M, Grishin EV, Arcangeli A, Wanke E. Species diversity and peptides toxins blocking selectivity of Ether-á-go-go-related gene subfamily K+ channels in the central nervous system. Mol Pharmacol. 2006;69:1673–1683. doi: 10.1124/mol.105.019729. [DOI] [PubMed] [Google Scholar]
- Restano-Cassulini R, Olamendi-Portugal T, Zamudio F, Becerril B, Possani LD. Two novel Ergtoxins, blockers of K+ channels, purified from the Mexican scorpion Centruroides elegans elegans. Neurochem Res. 2008;33:1525–1533. doi: 10.1007/s11064-008-9634-8. [DOI] [PubMed] [Google Scholar]
- Rochat C, Rochat H, Miranda F, Lissitzky S. Purification and Some Properties of the Neurotoxins of Androctonus australis Hector. Biochemistry. 1967;6:578–585. doi: 10.1021/bi00854a028. [DOI] [PubMed] [Google Scholar]
- Rodríguez de la Vega RC, Possani LD. Current views on scorpion toxins specific for K+-channels. Toxicon. 2004;43:865–875. doi: 10.1016/j.toxicon.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Rodríguez de la Vega RC, Possani LD. Overview of scorpion toxins specific for Na+ channels and related peptides: biodiversity, structure–function relationships and related peptides: biodiversity and evolution. Toxicon. 2005;46:831–44. doi: 10.1016/j.toxicon.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Rodríguez de la Vega RC, Possani LD. Novel paradigms on scorpion toxins that affect the activating mechanism of sodium channels. Toxicon. 2007;49:171–180. doi: 10.1016/j.toxicon.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Rodríguez de la Vega RC, Vidal N, Lourival D, Possani LD. Scorpion peptides. In: Sabatier JM, editor. Handbook of Biologically Active Peptides. 2. Chapter 59. Academic Press; 2013. pp. 423–429. Scorpion venom peptides., section ed. [Google Scholar]
- Roeding F, Borner J, Kube M, Klages S, Reinhardt R, Burmester T. A 454 sequencing approach for large scale phylogenomic analysis of the common emperor scorpion (Pandinus imperator) Mol Phylogenet Evol. 2009;53:826–834. doi: 10.1016/j.ympev.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Rogers JC, Qu Y, Tanada TN, Scheuer T, Catterall WA. Molecular determinants of high affinity binding of alpha-scorpion toxin and sea anemone toxin in the S3–S4 extracellular loop in domain IV of the Na+ channel alpha subunit. J Biol Chem. 1996;271:15950–15962. doi: 10.1074/jbc.271.27.15950. [DOI] [PubMed] [Google Scholar]
- Ruiming Z, Yibao M, Yawen H, Zhiyong D, Yingliang W, Zhijian C, Wenxin L. Comparative venom gland transcriptome analysis of the scorpion Lychas mucronatus reveals intraspecific toxic gene diversity and new venomous components. BMC Genomics. 2010;11:452. doi: 10.1186/1471-2164-11-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samso M, Trujillo R, Gurrola BG, Valdivia HH, Wagenknecht T. Three-dimensional Location of the Imperatoxin A binding site on the ryanodine receptor. The Journal of Cell Biology. 1999;146(2):493–499. doi: 10.1083/jcb.146.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucedo AL, Flores-Solís D, Rodríguez de la Vega RC, Ramírez-Cordero B, Hernández-López R, Cano-Sánchez P, Noriega-Navarro R, García-Valdes J, Coronas-Valderrama F, de Roodt A, Brieba LG, Possani LD, Del Rio-Portilla F. New tricks of an old pattern: structural versatility of scorpion toxins with common cysteine spacing. J Biol Chem. 2012;287:12321–12330. doi: 10.1074/jbc.M111.329607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EF, Diego-Garcia E, Rodríguez de la Vega RC, Possani LD. Transcriptome analysis of the venom gland of the Mexican scorpion Hadrurus gertschi (Arachnida: Scorpiones) BMC Genomics. 2007;8:119. doi: 10.1186/1471-2164-8-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EF, Capes EM, Diego-García E, Zamudio FZ, Fuentes O, Possani LD, Valdivia HH. Characterization of hadrucalcin, a peptide from Hadrurus gertschi scorpion venom with pharmacological activity on ryanodine receptors. Br J Pharmacol. 2009;57(3):392–403. doi: 10.1111/j.1476-5381.2009.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo IR, Kang DE, Song DW, Kim DH. Both Basic and Acidic Amino Acid Residues of IpTxa Are Involved in Triggering Substate of RyR1. J Biomed Biotechnol. 2011;2011:386384. doi: 10.1155/2011/386384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbazzadeh D, Srairi-Abid N, Feng W, Ram N, Borchani L, Ronjat M, Akbari A, Pessah IN, De Waard M, El-Ayeb M. Hemicalcin, a new toxin from the Iranian scorpion Hemiscorpius lepturus which is active on ryanodine-sensitive Ca2+ channels. Biochem J. 2007;404(1):89–96. doi: 10.1042/BJ20061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtifman A, Ward CW, Wang J, Valdivia HH, Schneider F. Effects of imperatoxin A on local sarcoplasmic reticulum Ca(2+) release in frog skeletal muscle. Biophys J. 2000;79(2):814–27. doi: 10.1016/S0006-3495(00)76338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CN, Camargos TS, Maranha AQ, Schwartz EF, Silva LP, Possani LD. Cloning and characterization of cDNA sequences encoding for new venom peptides of the Brazilian scorpion Opisthacanthus cayaporum. Toxicon. 2009;54:252–261. doi: 10.1016/j.toxicon.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Simeoni I, Rossi D, Zhu X, Garcia J, Valdivia HH. Imperatoxin A (IpTxa) from Pandinus imperator stimulates [3H]ryanodine binding to RyR3 channels. FEBS Letters. 2001;508:5–10. doi: 10.1016/s0014-5793(01)03013-7. [DOI] [PubMed] [Google Scholar]
- Soudani N, Gharbi-Chihi J, Srairi-Abid N, Kaabi H, Margotat A, Torresani J, El Ayeb M. Identification of second lipolysis activating protein from scorpion Buthus occitanus tunetanus. Arch Inst Pasteur Tunis. 2005;82:39–46. [PubMed] [Google Scholar]
- Srinivasan KN, Sivaraja V, Huys I, Sasaki T, Cheng B, Kumar TK, Sato K, Tytgat J, Yu C, San BC, Ranganathan S, Bowie HJ, Kini RM, Gopalakrishnakone P. kappa-Hefutoxin1, a novel toxin from the scorpion Heterometrus fulvipes with unique structure and function. Importance of the functional diad in potassium channel selectivity. J Biol Chem. 2002;277:30040–30047. doi: 10.1074/jbc.M111258200. [DOI] [PubMed] [Google Scholar]
- Takacs Z, Toups M, Kollewe A, Johnson E, Cuello LG, Driessens G, Biancalana M, Koide A, Ponte CG, Perozo E, et al. A designer ligand specific for Kv1.3 channels from a scorpion neurotoxin-based library. Proc Natl Acad Sci USA. 2009;106:22211–22216. doi: 10.1073/pnas.0910123106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan PTJ, Ranganathan S, Brusic V. Deduction of functional peptide motifs in scorpion toxins. J Peptide Sci. 2006b;12:420–427. doi: 10.1002/psc.744. [DOI] [PubMed] [Google Scholar]
- Tan PTJ, Veeramani A, Srinivasan KN, Ranganathan S, Brusic V. SCORPION2: A database for structure–function analysis of scorpion toxins. Toxicon. 2006a;47:356–363. doi: 10.1016/j.toxicon.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Trypathy A, Resch W, Xu L, Valdivia HH, Meissner G. Imperatoxin A induces subconductance states in Ca2+ release channels (ryanodine receptors) of cardiac and skeletal muscle. J Gen Physiol. 1998;III(5):679–90. doi: 10.1085/jgp.111.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat J, Chandy KG, Garcia ML, Gutman GA, Martin-Eauclaire MF, van der Walt JJ, Possani LD. A unified nomenclature for short-chain peptides isolated from scorpion venoms: alpha-KTx molecular subfamilies. Trends Pharmacol Sci. 1999;20:444–447. doi: 10.1016/s0165-6147(99)01398-x. [DOI] [PubMed] [Google Scholar]
- Uawonggul N, Thammasirirak S, Chaveerach A, Arkaravichien T, Bunyatratchata W, Ruangjirachuporn W, Jearranaiprepame P, Nakamura T, Matsuda M, Kobayashi M, Hattori S, Daduang S. Purification and characterization of Heteroscorpine-1 (HS-1) toxin from Heterometrus laoticus scorpion venom. Toxicon. 2007;49:19–29. doi: 10.1016/j.toxicon.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Vacher H, Romi-Lebrun R, Mourre C, Lebrun B, et al. A new class of scorpion toxin binding sites related to an A-type K+ channel: pharmacological characterization and localization in rat brain. FEBS Lett. 2001;501:31–36. doi: 10.1016/s0014-5793(01)02620-5. [DOI] [PubMed] [Google Scholar]
- Valdivia HH, Fuentes O, El-Hayek R, Morrissette J, Coronado R. Activation of the ryanodine receptor Ca 2+-release channel of sarcoplasmic reticulum by a novel scorpion venom. J Biol Chem. 1991;266(29):19135–8. [PubMed] [Google Scholar]
- Valdivia HH, Kirby MS, Lederer WJ, Coronado R. Scorpion toxins targeted against the sarcoplasmic reticulum Ca2+-release channel of skeletal and cardiac muscle. Proc Natl Acad Sci USA. 1992;89(24):12185–9. doi: 10.1073/pnas.89.24.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez A, Tapia JV, Eliason WK, Martin BM, Lebreton F, Delepierre M, Possani LD, Becerril B. Cloning and characterization of the cDNAs encoding Na+ channel specific toxins 1 and 2 of the scorpion Centruroides noxius Hoffman. Toxicon. 1995;33:1161–70. doi: 10.1016/0041-0101(95)00058-t. [DOI] [PubMed] [Google Scholar]
- Wang J, Yarov-Yarovoy V, Kahn R, Gordon D, Gurevitz M, Scheuer T, Catterall WA. Mapping the receptor site for α-scorpion toxins on a Na+ channel voltage sensor. Proc Natl Acad Sci USA. 2011;108:15426–15431. doi: 10.1073/pnas.1112320108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger H, Moran Y, Gordon D, Turkov M, Kahn R, Gurevitz M. Positions under positive selection--key for selectivity and potency of scorpion alpha-toxins. Mol Biol Evol. 2010;27:1025–34. doi: 10.1093/molbev/msp310. [DOI] [PubMed] [Google Scholar]
- Zamudio FZ, Conde R, Arevalo C, Becerril B, Martin BM, Valdivia HH, Possani LD. The mechanism of inhibition of ryanodine receptor channels by imperatoxin I, a heterodimeric protein from the scorpio Pandinus imperator. J Biol Chem. 1997;272(18):11886–94. doi: 10.1074/jbc.272.18.11886. [DOI] [PubMed] [Google Scholar]
- Zeng XC, Luo F, Li WX. Molecular dissection of venom from Chinese scorpion Mesobuthus martensii: Identification and characterization of four novel disulfide-bridged venom peptides. Peptides. 2006;27:1745–1754. doi: 10.1016/j.peptides.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Zeng XC, Zhang L, Nie Y, Luo X. Identification and molecular characterization of three new K+-channel specific toxins from the Chinese scorpion Mesobuthus martensii Karsch revealing intronic number polymorphism and alternative splicing in duplicated genes. Peptides. 2012;34:311–323. doi: 10.1016/j.peptides.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Zhang JZ, Yarov-Yarovoy V, Scheuer T, Karbat I, Cohen L, Gordon D, Gurevitz M, Catterall WA. Mapping the interaction site for a β-scorpion toxin in the pore module of domain III of voltage-gated Na+ channels. J Biol Chem. 2012;36:30719–30728. doi: 10.1074/jbc.M112.370742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XC, Luo F, Li WX. Molecular dissection of venom from Chinese scorpion Mesobuthus martensii: Identification and characterization of four novel disulfide-bridged venom peptides. Peptides. 2006;27(7):1746–1754. doi: 10.1016/j.peptides.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Zhu S, Tytgat J. The scorpine family of defensins: Gene structure, alternative polyadenylation and fold recognition. Cell Mol Life Sci. 2004;61:1751–1763. doi: 10.1007/s00018-004-4149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Darbon H, Dyason K, Verdonck F, Tytgat J. Evolutionary origin of inhibitor cystine knot peptides. The FASEB Journal. 2003;12:1765–1667. doi: 10.1096/fj.02-1044fje. [DOI] [PubMed] [Google Scholar]
- Zhu X, Zamudio FZ, Olbinski BA, Possani LD, Valdivia HH. Activation of skeletal ryanodine receptors by two novel scorpion toxins from Buthotus judaicus. Journal of Biological Chemistry. 2004;279(25):26588–26596. doi: 10.1074/jbc.M403284200. [DOI] [PubMed] [Google Scholar]
- Zlotkin E, Kadouri D, Gordon D, Pelhate M, Martin MF, Rochat H. An excitatory and a depressant insect toxin from scorpion venom both affect sodium conductance and possess a common binding site. Arch Biochem Biophys. 1985;240:877–887. doi: 10.1016/0003-9861(85)90098-0. [DOI] [PubMed] [Google Scholar]
- Zuo XP, Ji YH. Molecular mechanism of scorpion neurotoxins acting on sodium channels: insight into their diverse selectivity. Mol Neurobiol. 2004;30:265–278. doi: 10.1385/MN:30:3:265. [DOI] [PubMed] [Google Scholar]


