Abstract
Observational studies have associated patent ductus arteriosus (PDA) ligation in preterm infants with increased chronic lung disease (CLD), retinopathy of prematurity, and neurodevelopmental impairment at long-term follow-up. Although the biological rationale for this association is incompletely understood, there is an emerging secular trend toward a permissive approach to the PDA. However, insufficient adjustment for postnatal, pre-ligation confounders, such as intraventricular hemorrhage and the duration and intensity of mechanical ventilation, suggests the presence of residual bias due to confounding by indication, and obliges caution in interpreting the ligation-morbidity relationship. A period of conservative management after failure of medical PDA closure may be considered to reduce the number of infants treated with surgery. Increased mortality and CLD in infants with persistent symptomatic PDA suggests that surgical ligation remains an important treatment modality for preterm infants.
Keywords: Chronic lung disease, confounding by indication, conservative, mortality, neurodevelopmental impairment, neurosensory impairment, preterm, retinopathy of prematurity
INTRODUCTION
Patent ductus arteriosus (PDA) occurs in up to 60% of preterm infants born at <29 weeks gestational age (GA).[1,2] It is associated with mortality and severe morbidity, including intraventricular hemorrhage (IVH), necrotizing enterocolitis (NEC), prolonged ventilator dependency, and an increased incidence of chronic lung disease (CLD)[3] and retinopathy of prematurity (ROP).[4] The associations with morbidity have motivated over three decades of research trials and clinical focus aimed at mitigating the potential negative effects of the ductal shunt. Treatments targeting ductal closure include pharmacotherapy (cyclooxygenase inhibitors or more recently, acetaminophen), and surgical ligation.[5] Surgical PDA ligation is usually only considered when medical treatments have either failed or were contraindicated.[6]
Controlled observational studies have associated PDA ligation with increased CLD,[7,8,9,10,11] ROP,[7,8] and neurodevelopmental impairment (NDI) at 18-24 month follow-up.[8,9] However, the biological rationale for this association is incompletely understood. In addition, the predominant clinical utility of ligation as a “rescue” treatment may have resulted in confounding by indication, where ligated infants may be more likely to have increased pretreatment morbidity. This potential source of bias threatens the validity of these studies. This review will examine the major studies and biological plausibility associating ligation with increased morbidities, the impact of potential bias on the validity of these observational studies, and the recently reported effects of treatment strategies that avoid surgical PDA closure.
PATENT DUCTUS ARTERIOSUS LIGATION AND THE ASSOCIATION WITH SEVERE MORBIDITIES OF PREMATURITY
In a large retrospective cohort study of preterm infants born <32 weeks GA with a symptomatic PDA, Mirea et al. compared neonatal outcomes according to PDA treatment assignment.[7] After adjustment for confounders, infants treated with surgical ligation had lower mortality but higher odds of CLD and ROP, compared with infants treated with medical management alone [Table 1]. Similarly, in a retrospective review of 426 extremely low (<1000 g) birth-weight (ELBW) infants, Kabra et al. detected higher CLD and ROP in 110 infants who underwent surgical ligation compared to 316 infants who received medical management only [Table 1].[8] Higher CLD among infants with PDA ligation was also reported by Madan et al.[9] in a review of 2,838 ELBW infants who received treatment for a symptomatic PDA. In a post hoc analysis of a small randomized controlled trial[12] that compared early prophylactic surgical ligation (95% ligated) with delayed selective ligation (56% ligated) in ELBW infants, the prophylactic ligation group had a higher-risk of moderate to severe CLD (48% vs. 21%, P < 0.05).[10]
Table 1.
Odds ratios for neonatal and neurodevelopmental outcomes for infants with a patent ductus arteriosus treated with surgical ligation compared with medical management
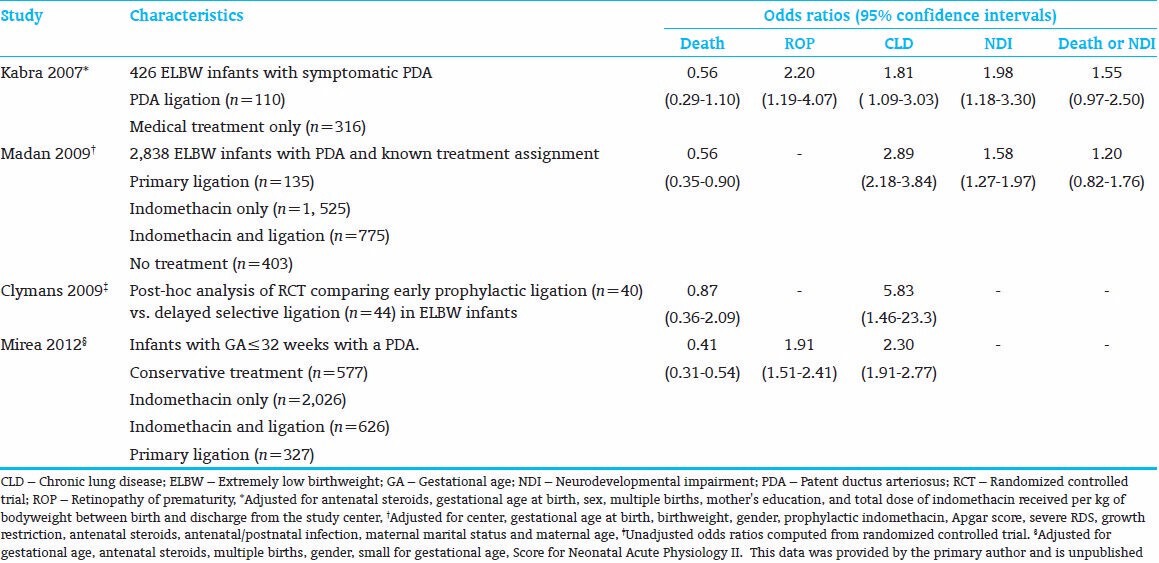
Several studies have associated PDA ligation with increased NDI[8,9] [Table 1]. Kabra et al. found increased cognitive delay and a composite of NDI at 18-month follow-up in ligated compared with medically treated infants, after adjusting for perinatal covariates and the total dose of indomethacin received during hospitalization.[8] Madan et al. reported increased NDI among infants treated with indomethacin and ligation, compared with infants treated with indomethacin alone, after adjusting for perinatal covariates and postnatal sepsis.[9]
BIOLOGICAL AND CLINICAL EVIDENCE FOR A CAUSAL EFFECT OF PATENT DUCTUS ARTERIOSUS LIGATION
The pathogeneses of CLD, ROP, and NDI are a complex interplay of the immediate and longitudinal effects of perinatal and postnatal insults on pulmonary, cardiac, immunological, metabolic and cerebral physiology and development. Surgical PDA ligation is a comparatively brief exposure in the context of the neonatal intensive care unit (NICU) course of an extremely preterm infant. To be causally related to severe morbidities, exposure to ligation must therefore induce a profound and lasting cascade of adverse end-organ effects.
Several intra- and post-operative factors potentially support a biological association of ligation and severe morbidities of prematurity. Operative management involves invasive mechanical ventilation, the need for analgesia, administration of induction and maintenance anesthetic agents, posterolateral thoracotomy, retraction of the left lung, and the application of a clip or ligature to the ductus arteriosus. Immediate complications include bleeding, chylothorax, pneumothorax, and inadvertent occlusion of the left main bronchus, left pulmonary artery or aorta.[13,14] Postoperatively, poor tolerance of the increased left ventricular afterload following surgical interruption of the ductal shunt places up to half of preterm infants at risk of acute left ventricular dysfunction and associated cardiorespiratory failure, termed post ligation cardiac syndrome (PLCS).[15] Left vocal cord paresis (VCP) has been reported to occur in 1-50% of preterm infants after PDA ligation, and presents with signs of upper airway obstruction and respiratory failure at the time of endotracheal extubation.[14,16,17] It is plausible that the collective cardiopulmonary and systemic adverse effects of these complications may initiate or enhance morbidity in preterm infants.
Chronic lung disease
The pathophysiology of CLD involves pulmonary immaturity, oxidative stress, and an imbalance of proteases/anti-proteases and oxidants/anti-oxidants. Combined with alveolar inflammation due to chorioamnionitis or the effects of mechanical ventilation, there is a culmination of heterogeneous areas of atelectasis, overdistension, fibrosis, disrupted alveolarization, and decreased microvascular development.
Animal studies support a link between ligation and the development of CLD. While pharmacologic PDA closure reduces the detrimental effects of preterm delivery on pulmonary function and alveolar development, ligation of a moderate PDA in preterm baboons has not been found to improve alveolar surface area or pulmonary mechanics.[18] Waleh et al. identified that compared with untreated subjects, preterm baboons treated with ligation produced a significant increase in the expression of genes involved in pulmonary inflammation and a decrease in alveolar fluid clearance.[19] These findings support the biological plausibility of a causal effect of ligation and CLD.
In contrast, the clinical complications of surgical ligation have been variably associated with increased risk of CLD. PLCS has not been associated with CLD,[20] and the effect of anesthesia has not been evaluated. While pneumothorax, chylothorax, and obstruction of a great vessel or bronchus may result in lung injury or prolonged mechanical ventilation, their collective incidence is low (<3%) and are unlikely to account for increased CLD.[14,21]
Left VCP may result in upper airway obstruction and aspiration of gastric or upper airway contents, with resultant difficulty weaning from invasive mechanical ventilation. In a retrospective analysis of 60 ELBW infants treated with ligation, 22 of the surviving 55 infants were diagnosed with left VCP. Left VCP was associated with higher adjusted odds of CLD.[16] In a prospective study of 61 ligated preterm infants who were successfully extubated and underwent routine flexible laryngoscopy, 7 (11.5%) were identified as having left VCP, of which two were symptomatic.[22] VCP was associated with a longer duration of mechanical ventilation (49 vs. 27 days, P = 0.05). Importantly, this study excluded four infants who required tracheostomy due to extubation failure, which may have underestimated the adverse effect of VCP in this study.[22] Jawa et al. retrospectively reviewed 98 ligated preterm infants, of whom 32 (33%) had left VCP. After adjusting for GA, there was a trend toward increased CLD in infants with VCP (adjusted odds ratio: 7.14, 95% confidence intervals: 0.87-100), but no effect on the number of days of mechanical ventilation.[23]
Clinical trials of PDA ligation have randomized infants to routine versus selective ligation, and therefore only permit estimating the difference in risk between these two approaches. Gersony et al. randomized 154 infants with a persistent PDA after conservative therapy (fluid restriction, diuretics, and digoxin) to routine ligation versus indomethacin with ligation as a back-up. All but one infant (99%) in the routine ligation group underwent surgery compared with 33% of the indomethacin with ligation back-up group. Pneumothorax occurred more often in the routine ligation group (39 vs. 15%, P < 0.0001), but there was no difference in the risk of CLD (39% vs. 31%, P = 0.30), duration of invasive mechanical ventilation (P = 0.20), or duration of nasal continuous positive airway pressure (P = 0.20).[24] In the only randomized trial of prophylactic surgical ligation in ELBW infants, the prophylactic ligation group had a higher-risk of moderate-severe CLD compared with the control group in whom surgical ligation was selectively performed to treat a symptomatic PDA.[10] Importantly, both of these trials were small and conducted over 30 years ago. The interim evolution of neonatal care practices likely limits the contemporary relevance of these findings.
Retinopathy of prematurity
The pathogenesis of ROP proceeds in two phases and is governed by vascular endothelial growth factor (VEGF) and insulin-like growth factor 1 (IGF-1).[25] First, preterm birth results in a reduction in circulating IGF-1, and high oxygen exposure suppresses VEGF, leading to cessation of normal retinal vascular growth and a demarcation line of retinal vascularization. Subsequently, hypoxia-induced increases in retinal and vitreal VEGF combine with maturity-related increases in IGF-1 to promote retinal neovascularization at the demarcation line at approximately 32-34 weeks GA. This neovascular proliferation may result in fibrosis and eventual retinal detachment.
A direct effect of PDA ligation on retinal angiogenesis, VEGF or IGF-1 has not been evaluated. While PDA and surgical ligation have been associated with perturbations in cerebral oxygen saturation and perfusion,[26,27,28,29,30,31,32] no studies have investigated the role of ischemia-reperfusion injury on ROP. In lieu, increased hypoxia, likely in parallel with the factors contributing to CLD, forms the dominant biological rationale for the association between ligation and ROP. Thus complications, such as pneumothorax, VCP, and postoperative cardiorespiratory instability may be risks for ROP. In the trial by Gersony et al., infants with a persistent PDA randomized to routine ligation had increased risk of ROP (15% vs. 4%, P = 0.02) compared with the group treated with ligation after failure of medical closure with indomethacin.[24] In contrast, Cassady et al. reported no difference in ROP between infants randomized to early prophylactic ligation versus selective ligation for persistent symptomatic PDA (5% vs. 14%, P = 0.62), though the trial was not powered to assess this outcome. No difference in ROP has been detected between infants with and without VCP after ligation,[23,33] and the effect of PLCS on ROP has not been evaluated.
Neurodevelopmental impairment
Neurodevelopmental impairment in preterm infants is commonly defined as a composite outcome comprising neuromotor, neurocognitive and neurosensory impairment.[8,9] Cerebral injury/dysmaturation and subsequent NDI is likely the final common pathway after cardiorespiratory instability leads to hypoxia-ischemia-reperfusion injury, inflammation, or arrested development of sensitive, immature white and grey matter [Figure 1].[34,35,36,37,38,39,40,41,42]
Figure 1.
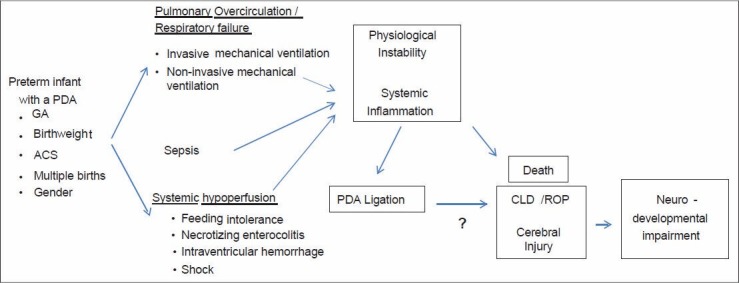
Directed acyclic graph depicting the relationship between perinatal and postnatal factors, physiological instability and systemic inflammation, patent ductus arteriosus ligation and neonatal and neurodevelopmental outcomes (ACS – Antenatal corticosteroids, CLD – Chronic lung disease; GA – Gestational age, ROP – Retinopathy of prematurity, PDA – Patent ductus arteriosus)
Predictive models have identified risk factors for death and NDI at different time points during NICU care. At birth, GA, birth-weight (BW), multiple gestation, antenatal corticosteroids, intra-uterine growth restriction and gender are the most important prognostic perinatal risk factors for death and NDI.[43,44] At the time of NICU discharge, neonatal morbidities of prematurity such as CLD, ROP, sepsis and major brain injury can reliably predict NDI at 18-24 months corrected age.[44,45] The increased CLD and ROP associated with ligation may therefore represent a credible causal pathway linking ligation and increased NDI.
Other aspects of PDA ligation that may contribute to the risk of NDI include the effects of anesthesia and postoperative hemodynamic compromise. Recent cohort studies, including sibling studies, have associated the administration of anesthesia in infancy and early childhood with NDI.[46,47,48,49,50,51] This association was weakened after adjustment for key covariates, such as perinatal characteristics (e.g. prematurity), the indication for anesthesia, and socioeconomic factors, but remained significant for repeated exposure to anesthesia. While PLCS may result in cerebral hypoperfusion and injury,[15,52,53,54,55,56] a recent retrospective study found no association between PLCS and NDI.[20] Infants who are <28 days old or <1000 g at the time of ligation are at highest-risk for PLCS, likely due to decreased adaptability of the preterm myocardium to altered loading conditions.[57,58] This suggests that the timing of ligation may contribute to the risk of NDI; however, a post hoc analysis of the trial of indomethacin prophylaxis in preterm infants found no association between the timing of PDA ligation and NDI.[8] Vocal cord paresis, while variably associated with CLD, has not been associated with increased adjusted odds of cerebral palsy or hearing loss.[23]
CONFOUNDING BY INDICATION: A SOURCE OF RESIDUAL BIAS
A serious concern in observational studies is bias arising when treatment assignment is not independent of baseline prognostic factors. The methodology used in the observational studies described above suggests that the authors did not adequately address confounding by indication. Multivariable analyses were performed that predominantly controlled for antenatal or perinatal covariates [Table 1]. This set of covariates, if complete, would be sufficient to balance baseline prognostic factors for interventions that occur shortly after birth. However, PDA ligation typically occurs several weeks after birth, and the interval accumulation of PDA-related postnatal comorbidities influences both treatment assignment and outcomes.
Patent ductus arteriosus ligation may be a surrogate marker for increased illness severity, as “sicker” infants may be more likely to be referred for ligation. Medical or surgical treatments to close the PDA are typically considered for infants dependent on mechanical ventilation, with surgical ligation reserved for infants with persistent PDA after medical treatment has failed or was contraindicated. A survey of American neonatologists confirmed that clinicians are much more willing to treat with ligation in infants dependent on invasive mechanical ventilation, compared with those supported noninvasively (91% vs. 37%). The same group was comparatively more willing (73%) to treat noninvasively ventilated infants with indomethacin or ibuprofen.[59] Studies to date have inadequately addressed this confounding by indication-that infants referred for ligation may be more “ill” at the time of the decision to treat with surgery, compared with infants who are treated with medical management alone.
Illness severity, characterized by dependence on mechanical ventilation, IVH, and sepsis, predicts both neonatal morbidities and NDI.[34,36,45,60,61] Severe IVH is a true confounder as it is associated with both PDA ligation and NDI,[62,63,64,65,66] and is not on the causal pathway. Although IVH has been found to occur after treatment with ligation and indomethacin,[67] most (90%) IVH occurs in the 1st week of life,[68] preceding the timing of surgical ligation reported by most studies.[67,69,70,71,72] Other studies have demonstrated that IVH does not worsen after PDA treatment with indomethacin or ligation.[73,74,75] Postnatal sepsis and NEC are other important potential postnatal confounders, which increase illness severity and are associated with both PDA ligation and death or NDI.[61,76,77,78,79]
However, these morbidities can occur both before, during, or after PDA treatments and thus may be confounders in some infants, and outcomes in others. It is clear that only morbidities arising during or after surgery may be attributable to a complication of ligation. It follows that morbidities (or the precursors of them) that existed prior to ligation, if they influence treatment assignment and outcomes, are potential or true confounders. It is therefore necessary to obtain data on the timing and severity of respiratory failure, IVH, NEC, and sepsis relative to surgical ligation, to correct for possible bias due to these potential confounders in multivariable analyses examining the impact of surgical PDA ligation.
The reported association of PDA ligation and CLD is perhaps the most problematic. While several studies performed multivariable analyses examining the effect of ligation on CLD, most studies did not adjust for illness severity or postnatal morbidities (including respiratory) beyond the 1st day of life.[7,8] Persistent dependence on mechanical ventilation is commonly considered the sine qua non of treating with surgical ligation,[70,71,80,81,82] yet it is also a risk factor for CLD. Persistent postnatal respiratory support at 7 or 28 days of life is a more accurate predictor of CLD than perinatal or early postnatal characteristics (e.g. GA, Score For Neonatal Acute Physiology).[83] This difference in predictive ability may help quantify the residual confounding in studies that only adjusted for perinatal covariates. While some studies have adjusted for postnatal sepsis,[9,76] and NEC,[76] known risk factors for CLD,[84] they did not differentiate whether these morbidities occurred before or after ligation.
Confounding by indication may have also resulted in biased estimates of the association between ligation, and ROP and NDI. Increased post-ligation cardiorespiratory morbidity may be an important pathophysiological route of causality for both ROP and NDI, but may be as confounded as the outcome of CLD. It is noteworthy that in a retrospective study that adjusted for IVH, hypotension in the 1st week of life, and respiratory failure severity at the time of surgery, ligation was found to be protective against the outcome of death or NDI compared with non-surgically treated infants with PDA.[85] In another retrospective study of 446 extremely preterm infants treated with routine early ligation after failure of indomethacin treatment, PDA ligation was not associated with increased NDI compared with infants without a PDA, after adjustment for GA, severe IVH and periventricular leukomalacia.[76] The finding of a lack of harm associated with ligation, after adjusting for postnatal pre-ligation morbidities, supports the possibility of confounding by indication in studies that only accounted for perinatal covariates.
Other implicated aspects of surgical ligation, such as anesthesia effects and PLCS, are biologically plausible but inconsistently supported.[20,48,51] In addition, the use of targeted milrinone prophylaxis has significantly reduced the incidence of PLCS.[15] Any contribution of PLCS to increased NDI in prior studies may be diminished under contemporary practices.
PERMISSIVE APPROACH TO THE PATENT DUCTUS ARTERIOSUS: ARE WE ACHIEVING WHAT WE WANT?
In light of reports associating therapies aimed at PDA closure with increased neonatal morbidities and NDI, some authors have advocated avoiding cyclooxygenase inhibitors and surgical ligation.[59,86,87,88] This has been accompanied by a secular trend away from surgical ligation and toward a permissive (or “conservative”) approach to the PDA, incorporating the use of fluid restriction, diuretics and increased positive end-expiratory pressure. The goal is to limit shunt volume and/or improve an infant's physiological tolerance of the PDA without trying to achieve ductal closure using medication or surgery. The permissive approach still recognizes that some ductal shunts have demonstrable circulatory effects and should not be ignored, but rather managed using intensive care strategies. Many of the studies advocating this approach to the PDA still employed pharmacotherapy and/or surgical ligation as a “back-up,” suggesting that a proportion of the persistent PDA shunts were considered pathological.
Studies have reported that strategies avoiding or more selectively administering ductal closure treatments resulted in outcomes that are no worse (and perhaps better) than outcomes where infants were more aggressively treated with medication or surgery.[89,90,91,92] Jhaveri et al. reported similar neonatal outcomes with a late, selective ligation strategy compared with an early ligation approach for infants with a persistent PDA after medical therapy failure.[91] This same group subsequently reported improved neurodevelopmental outcomes in the delayed selective ligation group.[89] Another single-center study reported 100% spontaneous PDA closure and no increased neonatal complications among infants <30 weeks GA treated with a conservative approach consisting of fluid restriction and increased positive end-expiratory pressure.[90]
However, recent studies have associated a conservative approach to the PDA with increased mortality and morbidity. A retrospective study of very low birthweight infants compared the neonatal outcomes across epochs, after moving to a permissive approach from a strategy of early indomethacin and/or ligation.[93] In the period where conservative measures were used, the outcomes of bronchopulmonary dysplasia (BPD) alone, and the composite outcome of death or BPD were both increased.[93] Brooks et al. investigated the outcomes of preterm infants ≤28 weeks GA with a persistent PDA after failure of indomethacin treatment, for whom surgical ligation was unavailable.[92] Compared with infants whose PDA had closed with indomethacin, infants with a persistent PDA had similar rates of CLD, IVH and NEC, but higher mortality. Noori et al. have reported a similar increase in mortality among infants with a persistent PDA compared with infants with a closed ductus, after adjusting for GA, 5-min Apgar and clinical risk index for babies scores, severe IVH, NEC, sepsis and COX inhibitor exposure.[94]
Lower mortality in ligated versus non-surgically treated infants was confirmed in a recent systematic review and meta-analysis.[95] Although the possibility of survival bias necessitates caution in interpreting this finding, it suggests that the association of ligation with increased morbidity may be influenced by improved survival of higher-risk infants treated with ligation. This is supported by the absence of a difference in the composite outcome of death or NDI among ligated versus medically treated infants [Table 1].[95]
In summary, early routine surgical ligation subjects many infants to the risks of surgery whose PDAs may close spontaneously, and is unwarranted. A period of conservative management for a PDA in preterm infants may be a useful adjunct to ductal closure, either as primary therapy or after failure of medical treatment, though the optimal duration of “watchful waiting” requires further study. Nonetheless, increased mortality and CLD associated with persistent symptomatic PDA indicates that the ductal shunt is potentially hazardous, should continue to be considered in the routine prescription of intensive care support, and that surgical ligation remains an important treatment modality.
CONCLUSION
Observational studies have associated PDA ligation with increased CLD, ROP and NDI, and contributed to a trend toward a permissive approach to the PDA. Failure to adjust for postnatal, pre-ligation confounders, such as IVH, the duration and intensity of mechanical ventilation, NEC, and sepsis, confers a risk of residual bias and obliges caution in interpreting these findings. A period of conservative management after failure of medical PDA closure may be considered to reduce the number of infants treated with surgery. However, increased mortality and CLD in infants with persistent symptomatic PDA suggests that the ductal shunt should not be neglected if medical therapy fails, and that surgical ligation remains an important treatment option for preterm infants.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Giliberti P, De Leonibus C, Giordano L, Giliberti P. The physiopathology of the patent ductus arteriosus. J Matern Fetal Neonatal Med. 2009;22(Suppl 3):6–9. doi: 10.1080/14767050903198215. [DOI] [PubMed] [Google Scholar]
- 2.Hamrick SE, Hansmann G. Patent ductus arteriosus of the preterm infant. Pediatrics. 2010;125:1020–30. doi: 10.1542/peds.2009-3506. [DOI] [PubMed] [Google Scholar]
- 3.Shimada S, Kasai T, Hoshi A, Murata A, Chida S. Cardiocirculatory effects of patent ductus arteriosus in extremely low-birth-weight infants with respiratory distress syndrome. Pediatr Int. 2003;45:255–62. doi: 10.1046/j.1442-200x.2003.01713.x. [DOI] [PubMed] [Google Scholar]
- 4.Tsui I, Ebani E, Rosenberg JB, Lin J, Angert RM, Mian U. Patent ductus arteriosus and indomethacin treatment as independent risk factors for plus disease in retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2013;50:88–92. doi: 10.3928/01913913-20130108-03. [DOI] [PubMed] [Google Scholar]
- 5.Sehgal A, Coombs P, Tan K, McNamara PJ. Spectral Doppler waveforms in systemic arteries and physiological significance of a patent ductus arteriosus. J Perinatol. 2011;31:150–6. doi: 10.1038/jp.2010.83. [DOI] [PubMed] [Google Scholar]
- 6.Malviya M, Ohlsson A, Shah S. Surgical versus medical treatment with cyclooxygenase inhibitors for symptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev. 2003;3:CD003951. doi: 10.1002/14651858.CD003951. [DOI] [PubMed] [Google Scholar]
- 7.Mirea L, Sankaran K, Seshia M, Ohlsson A, Allen AC, Aziz K, et al. Treatment of patent ductus arteriosus and neonatal mortality/morbidities: Adjustment for treatment selection bias. J Pediatr. 2012;161:689–941. doi: 10.1016/j.jpeds.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Kabra NS, Schmidt B, Roberts RS, Doyle LW, Papile L, Fanaroff A, et al. Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: Results from the Trial of Indomethacin Prophylaxis in Preterms. J Pediatr. 2007;150:229–34, 234.e1. doi: 10.1016/j.jpeds.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 9.Madan JC, Kendrick D, Hagadorn JI, Frantz ID., 3rd National Institute of Child Health and Human Development Neonatal Research Network. Patent ductus arteriosus therapy: Impact on neonatal and 18-month outcome. Pediatrics. 2009;123:674–81. doi: 10.1542/peds.2007-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clyman R, Cassady G, Kirklin JK, Collins M, Philips JB., 3rd The role of patent ductus arteriosus ligation in bronchopulmonary dysplasia: Reexamining a randomized controlled trial. J Pediatr. 2009;154:873–6. doi: 10.1016/j.jpeds.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jawa G, Husein M, Macrae D, Coughlin K, Seabrook J, da Silva O. Short and long term outcomes following PDA ligation in VLBW infants. Paediatr Child Health. 2013;18(Suppl A):49A. [Google Scholar]
- 12.Cassady G, Crouse DT, Kirklin JW, Strange MJ, Joiner CH, Godoy G, et al. A randomized, controlled trial of very early prophylactic ligation of the ductus arteriosus in babies who weighed 1000 g or less at birth. N Engl J Med. 1989;320:1511–6. doi: 10.1056/NEJM198906083202302. [DOI] [PubMed] [Google Scholar]
- 13.Harris LL, Krishnamurthy R, Browne LP, Morales DL, Friedman EM. Left main bronchus obstruction after patent ductus arteriosus ligation: An unusual complication. Int J Pediatr Otorhinolaryngol. 2012;76:1855–6. doi: 10.1016/j.ijporl.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Mandhan P, Brown S, Kukkady A, Samarakkody U. Surgical closure of patent ductus arteriosus in preterm low birth weight infants. Congenit Heart Dis. 2009;4:34–7. doi: 10.1111/j.1747-0803.2008.00241.x. [DOI] [PubMed] [Google Scholar]
- 15.Jain A, Sahni M, El-Khuffash A, Khadawardi E, Sehgal A, McNamara PJ. Use of targeted neonatal echocardiography to prevent postoperative cardiorespiratory instability after patent ductus arteriosus ligation. J Pediatr. 2012;160:584–5891. doi: 10.1016/j.jpeds.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin JR, Smith PB, Cotten CM, Jaggers J, Goldstein RF, Malcolm WF. Long-term morbidities associated with vocal cord paralysis after surgical closure of a patent ductus arteriosus in extremely low birth weight infants. J Perinatol. 2010;30:408–13. doi: 10.1038/jp.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clement WA, El-Hakim H, Phillipos EZ, Coté JJ. Unilateral vocal cord paralysis following patent ductus arteriosus ligation in extremely low-birth-weight infants. Arch Otolaryngol Head Neck Surg. 2008;134:28–33. doi: 10.1001/archoto.2007.2. [DOI] [PubMed] [Google Scholar]
- 18.Chang LY, McCurnin D, Yoder B, Shaul PW, Clyman RI. Ductus arteriosus ligation and alveolar growth in preterm baboons with a patent ductus arteriosus. Pediatr Res. 2008;63:299–302. doi: 10.1203/PDR.0b013e318163a8e4. [DOI] [PubMed] [Google Scholar]
- 19.Waleh N, McCurnin DC, Yoder BA, Shaul PW, Clyman RI. Patent ductus arteriosus ligation alters pulmonary gene expression in preterm baboons. Pediatr Res. 2011;69:212–6. doi: 10.1203/PDR.0b013e3182084f8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Dyk J, Lepointe A, Young J, El-Khuffash AF, Ly L, Asztalos E, et al. Preoperative hemodynamic instability is associated with poor neurodevelopmental outcome or death before discharge in preterm infants undergoing patent ductus arteriosus ligation. Pediatric Academic Societies. 2012;4528:469. [Google Scholar]
- 21.Niinikoski H, Alanen M, Parvinen T, Aantaa R, Ekblad H, Kero P. Surgical closure of patent ductus arteriosus in very-low-birth-weight infants. Pediatr Surg Int. 2001;17:338–41. doi: 10.1007/s003830000515. [DOI] [PubMed] [Google Scholar]
- 22.Pereira KD, Webb BD, Blakely ML, Cox CS, Jr, Lally KP. Sequelae of recurrent laryngeal nerve injury after patent ductus arteriosus ligation. Int J Pediatr Otorhinolaryngol. 2006;70:1609–12. doi: 10.1016/j.ijporl.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Jawa G, Husein M, Macrae D, Coughlin K, Seabrook J, da Silva O. Does vocal cord paralysis following PDA ligation in VLBW infants worsen outcome? Paediatr Child Health. 2013;18(Suppl A):39A–40. [Google Scholar]
- 24.Gersony WM, Peckham GJ, Ellison RC, Miettinen OS, Nadas AS. Effects of indomethacin in premature infants with patent ductus arteriosus: Results of a national collaborative study. J Pediatr. 1983;102:895–906. doi: 10.1016/s0022-3476(83)80022-5. [DOI] [PubMed] [Google Scholar]
- 25.Smith LE. Pathogenesis of retinopathy of prematurity. Semin Neonatol. 2003;8:469–73. doi: 10.1016/S1084-2756(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 26.Lemmers PM, Toet MC, van Bel F. Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics. 2008;121:142–7. doi: 10.1542/peds.2007-0925. [DOI] [PubMed] [Google Scholar]
- 27.Chock VY, Ramamoorthy C, Van Meurs KP. Cerebral autoregulation in neonates with a hemodynamically significant patent ductus arteriosus. J Pediatr. 2012;160:936–42. doi: 10.1016/j.jpeds.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrova A, Bhatt M, Mehta R. Regional tissue oxygenation in preterm born infants in association with echocardiographically significant patent ductus arteriosus. J Perinatol. 2011;31:460–4. doi: 10.1038/jp.2010.200. [DOI] [PubMed] [Google Scholar]
- 29.Chock VY, Ramamoorthy C, Van Meurs KP. Cerebral oxygenation during different treatment strategies for a patent ductus arteriosus. Neonatology. 2011;100:233–40. doi: 10.1159/000325149. [DOI] [PubMed] [Google Scholar]
- 30.Lemmers PM, Molenschot MC, Evens J, Toet MC, van Bel F. Is cerebral oxygen supply compromised in preterm infants undergoing surgical closure for patent ductus arteriosus? Arch Dis Child Fetal Neonatal Ed. 2010;95:F429–34. doi: 10.1136/adc.2009.180117. [DOI] [PubMed] [Google Scholar]
- 31.Vanderhaegen J, De Smet D, Meyns B, Van De Velde M, Van Huffel S, Naulaers G. Surgical closure of the patent ductus arteriosus and its effect on the cerebral tissue oxygenation. Acta Paediatr. 2008;97:1640–4. doi: 10.1111/j.1651-2227.2008.01021.x. [DOI] [PubMed] [Google Scholar]
- 32.Hoodbhoy SA, Cutting HA, Seddon JA, Campbell ME. Cerebral and splanchnic hemodynamics after duct ligation in very low birth weight infants. J Pediatr. 2009;154:196–200. doi: 10.1016/j.jpeds.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 33.Rukholm G, Farrokhyar F, Reid D. Vocal cord paralysis post patent ductus arteriosus ligation surgery: Risks and co-morbidities. Int J Pediatr Otorhinolaryngol. 2012;76:1637–41. doi: 10.1016/j.ijporl.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 34.Dammann O, Naples M, Bednarek F, Shah B, Kuban KC, O'Shea TM, et al. SNAP-II and SNAPPE-II and the risk of structural and functional brain disorders in extremely low gestational age newborns: The ELGAN study. Neonatology. 2010;97:71–82. doi: 10.1159/000232588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fortes Filho JB, Dill JC, Ishizaki A, Aguiar WW, Silveira RC, Procianoy RS. Score for neonatal acute physiology and perinatal extension II as a predictor of retinopathy of prematurity: Study in 304 very-low-birth-weight preterm infants. Ophthalmologica. 2009;223:177–82. doi: 10.1159/000197114. [DOI] [PubMed] [Google Scholar]
- 36.Mattia FR, deRegnier RA. Chronic physiologic instability is associated with neurodevelopmental morbidity at one and two years in extremely premature infants. Pediatrics. 1998;102:E35. doi: 10.1542/peds.102.3.e35. [DOI] [PubMed] [Google Scholar]
- 37.Volpe JJ. Brain injury in the premature infant: Overview of clinical aspects, neuropathology, and pathogenesis. Semin Pediatr Neurol. 1998;5:135–51. doi: 10.1016/s1071-9091(98)80030-2. [DOI] [PubMed] [Google Scholar]
- 38.Leviton A, Allred E, Kuban KC, Dammann O, O'Shea TM, Hirtz D, et al. Early blood gas abnormalities and the preterm brain. Am J Epidemiol. 2010;172:907–16. doi: 10.1093/aje/kwq222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Msall ME. Physiological stress and brain vulnerability: Understanding the neurobiology of connectivity in preterm infants. Ann Neurol. 2011;70:523–4. doi: 10.1002/ana.22614. [DOI] [PubMed] [Google Scholar]
- 40.O'Shea TM, Allred EN, Kuban KC, Dammann O, Paneth N, Fichorova R, et al. Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. J Pediatr. 2012;160:395–4014. doi: 10.1016/j.jpeds.2011.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Shea TM, Shah B, Allred EN, Fichorova RN, Kuban KC, Dammann O, et al. Inflammation-initiating illnesses, inflammation-related proteins, and cognitive impairment in extremely preterm infants. Brain Behav Immun. 2013;29:104–12. doi: 10.1016/j.bbi.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith GC, Gutovich J, Smyser C, Pineda R, Newnham C, Tjoeng TH, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 2011;70:541–9. doi: 10.1002/ana.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tyson JE, Parikh NA, Langer J, Green C, Higgins RD. National Institute of Child Health and Human Development Neonatal Research Network. Intensive care for extreme prematurity - Moving beyond gestational age. N Engl J Med. 2008;358:1672–81. doi: 10.1056/NEJMoa073059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schlapbach LJ, Adams M, Proietti E, Aebischer M, Grunt S, Borradori-Tolsa C, et al. Outcome at two years of age in a Swiss national cohort of extremely preterm infants born between 2000 and 2008. BMC Pediatr. 2012;12:198. doi: 10.1186/1471-2431-12-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bassler D, Stoll BJ, Schmidt B, Asztalos EV, Roberts RS, Robertson CM, et al. Using a count of neonatal morbidities to predict poor outcome in extremely low birth weight infants: Added role of neonatal infection. Pediatrics. 2009;123:313–8. doi: 10.1542/peds.2008-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–61. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ing C, DiMaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, et al. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–85. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 48.DiMaggio C, Sun LS, Ing C, Li G. Pediatric anesthesia and neurodevelopmental impairments: A Bayesian meta-analysis. J Neurosurg Anesthesiol. 2012;24:376–81. doi: 10.1097/ANA.0b013e31826a038d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–51. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojaniæ K, et al. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–9. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Xu Z, Miao CH. Current clinical evidence on the effect of general anesthesia on neurodevelopment in children: An updated systematic review with meta-regression. PLoS One. 2014;9:e85760. doi: 10.1371/journal.pone.0085760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El-Khuffash AF, Jain A, Dragulescu A, McNamara PJ, Mertens L. Acute changes in myocardial systolic function in preterm infants undergoing patent ductus arteriosus ligation: A tissue Doppler and myocardial deformation study. J Am Soc Echocardiogr. 2012;25:1058–67. doi: 10.1016/j.echo.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 53.El-Khuffash AF, Jain A, McNamara PJ. Ligation of the patent ductus arteriosus in preterm infants: Understanding the physiology. J Pediatr. 2013;162:1100–6. doi: 10.1016/j.jpeds.2012.12.094. [DOI] [PubMed] [Google Scholar]
- 54.El-Khuffash AF, McNamara PJ. The patent ductus arteriosus ligation decision. J Pediatr. 2011;158:1037–8. doi: 10.1016/j.jpeds.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 55.Sehgal A, Francis JV, James A, McNamara PJ. Patent ductus arteriosus ligation and post-operative hemodynamic instability: Case report and framework for enhanced neonatal care. Indian J Pediatr. 2010;77:905–7. doi: 10.1007/s12098-010-0137-7. [DOI] [PubMed] [Google Scholar]
- 56.Sehgal A, McNamara PJ. Coronary artery perfusion and myocardial performance after patent ductus arteriosus ligation. J Thorac Cardiovasc Surg. 2012;143:1271–8. doi: 10.1016/j.jtcvs.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 57.Teixeira LS, Shivananda SP, Stephens D, Van Arsdell G, McNamara PJ. Postoperative cardiorespiratory instability following ligation of the preterm ductus arteriosus is related to early need for intervention. J Perinatol. 2008;28:803–10. doi: 10.1038/jp.2008.101. [DOI] [PubMed] [Google Scholar]
- 58.McNamara PJ, Stewart L, Shivananda SP, Stephens D, Sehgal A. Patent ductus arteriosus ligation is associated with impaired left ventricular systolic performance in premature infants weighing less than 1000 g. J Thorac Cardiovasc Surg. 2010;140:150–7. doi: 10.1016/j.jtcvs.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Clyman RI, Couto J, Murphy GM. Patent ductus arteriosus: Are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. 2012;36:123–9. doi: 10.1053/j.semperi.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barrington KJ. Hypotension and shock in the preterm infant. Semin Fetal Neonatal Med. 2008;13:16–23. doi: 10.1016/j.siny.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Chau V, Brant R, Poskitt KJ, Tam EW, Synnes A, Miller SP. Postnatal infection is associated with widespread abnormalities of brain development in premature newborns. Pediatr Res. 2012;71:274–9. doi: 10.1038/pr.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brouwer A, Groenendaal F, van Haastert IL, Rademaker K, Hanlo P, de Vries L. Neurodevelopmental outcome of preterm infants with severe intraventricular hemorrhage and therapy for post-hemorrhagic ventricular dilatation. J Pediatr. 2008;152:648–54. doi: 10.1016/j.jpeds.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Klebermass-Schrehof K, Czaba C, Olischar M, Fuiko R, Waldhoer T, Rona Z, et al. Impact of low-grade intraventricular hemorrhage on long-term neurodevelopmental outcome in preterm infants. Childs Nerv Syst. 2012;28:2085–92. doi: 10.1007/s00381-012-1897-3. [DOI] [PubMed] [Google Scholar]
- 64.Srinivasakumar P, Limbrick D, Munro R, Mercer D, Rao R, Inder T, et al. Posthemorrhagic ventricular dilatation-impact on early neurodevelopmental outcome. Am J Perinatol. 2013;30:207–14. doi: 10.1055/s-0032-1323581. [DOI] [PubMed] [Google Scholar]
- 65.van Zanten SA, de Haan TR, Ursum J, van Sonderen L. Neurodevelopmental outcome of post-hemorrhagic ventricular dilatation at 12 and 24 months corrected age with high-threshold therapy. Eur J Paediatr Neurol. 2011;15:487–92. doi: 10.1016/j.ejpn.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 66.Vohr BR, Allan WC, Westerveld M, Schneider KC, Katz KH, Makuch RW, et al. School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2003;111:e340–6. doi: 10.1542/peds.111.4.e340. [DOI] [PubMed] [Google Scholar]
- 67.Koehne PS, Bein G, Alexi-Meskhishvili V, Weng Y, Bührer C, Obladen M. Patent ductus arteriosus in very low birthweight infants: Complications of pharmacological and surgical treatment. J Perinat Med. 2001;29:327–34. doi: 10.1515/JPM.2001.047. [DOI] [PubMed] [Google Scholar]
- 68.Kadri H, Mawla AA, Kazah J. The incidence, timing, and predisposing factors of germinal matrix and intraventricular hemorrhage (GMH/IVH) in preterm neonates. Childs Nerv Syst. 2006;22:1086–90. doi: 10.1007/s00381-006-0050-6. [DOI] [PubMed] [Google Scholar]
- 69.Lee LC, Tillett A, Tulloh R, Yates R, Kelsall W. Outcome following patent ductus arteriosus ligation in premature infants: A retrospective cohort analysis. BMC Pediatr. 2006;6:15. doi: 10.1186/1471-2431-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moore GP, Lawrence SL, Maharajh G, Sumner A, Gaboury I, Barrowman N, et al. Therapeutic strategies, including a high surgical ligation rate, for patent ductus arteriosus closure in extremely premature infants in a North American centre. Paediatr Child Health. 2012;17:e26–31. doi: 10.1093/pch/17.4.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O'Donovan DJ, Baetiong A, Adams K, Chen A, Smith EO, Adams JM, et al. Necrotizing enterocolitis and gastrointestinal complications after indomethacin therapy and surgical ligation in premature infants with patent ductus arteriosus. J Perinatol. 2003;23:286–90. doi: 10.1038/sj.jp.7210911. [DOI] [PubMed] [Google Scholar]
- 72.Vida VL, Lago P, Salvatori S, Boccuzzo G, Padalino MA, Milanesi O, et al. Is there an optimal timing for surgical ligation of patent ductus arteriosus in preterm infants? Ann Thorac Surg. 2009;87:1509–15. doi: 10.1016/j.athoracsur.2008.12.101. [DOI] [PubMed] [Google Scholar]
- 73.Szymonowicz W, Yu VY. Periventricular haemorrhage: Association with patent ductus arteriosus and its treatment with indomethacin or surgery. Aust Paediatr J. 1987;23:21–5. doi: 10.1111/j.1440-1754.1987.tb02170.x. [DOI] [PubMed] [Google Scholar]
- 74.Maher P, Lane B, Ballard R, Piecuch R, Clyman RI. Does indomethacin cause extension of intracranial hemorrhages: A preliminary study. Pediatrics. 1985;75:497–500. [PubMed] [Google Scholar]
- 75.Ment LR, Oh W, Ehrenkranz RA, Phillip AG, Vohr B, Allan W, et al. Low-dose indomethacin therapy and extension of intraventricular hemorrhage: A multicenter randomized trial. J Pediatr. 1994;124:951–5. doi: 10.1016/s0022-3476(05)83191-9. [DOI] [PubMed] [Google Scholar]
- 76.Chorne N, Leonard C, Piecuch R, Clyman RI. Patent ductus arteriosus and its treatment as risk factors for neonatal and neurodevelopmental morbidity. Pediatrics. 2007;119:1165–74. doi: 10.1542/peds.2006-3124. [DOI] [PubMed] [Google Scholar]
- 77.Schlapbach LJ, Aebischer M, Adams M, Natalucci G, Bonhoeffer J, Latzin P, et al. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics. 2011;128:e348–57. doi: 10.1542/peds.2010-3338. [DOI] [PubMed] [Google Scholar]
- 78.Bonnard A, Zamakhshary M, Ein S, Moore A, Kim PC. The use of the score for neonatal acute physiology-perinatal extension (SNAPPE II) in perforated necrotizing enterocolitis: Could it guide therapy in newborns less than 1500 g? J Pediatr Surg. 2008;43:1170–4. doi: 10.1016/j.jpedsurg.2008.02.051. [DOI] [PubMed] [Google Scholar]
- 79.Sonntag J, Grimmer I, Scholz T, Metze B, Wit J, Obladen M. Growth and neurodevelopmental outcome of very low birthweight infants with necrotizing enterocolitis. Acta Paediatr. 2000;89:528–32. doi: 10.1080/080352500750027790. [DOI] [PubMed] [Google Scholar]
- 80.Little DC, Pratt TC, Blalock SE, Krauss DR, Cooney DR, Custer MD. Patent ductus arteriosus in micropreemies and full-term infants: The relative merits of surgical ligation versus indomethacin treatment. J Pediatr Surg. 2003;38:492–6. doi: 10.1053/jpsu.2003.50086. [DOI] [PubMed] [Google Scholar]
- 81.Natarajan G, Chawla S, Aggarwal S. Short-term outcomes of patent ductus arteriosus ligation in preterm neonates: Reason for concern? Am J Perinatol. 2010;27:431–7. doi: 10.1055/s-0029-1243367. [DOI] [PubMed] [Google Scholar]
- 82.Perez CA, Bustorff-Silva JM, Villasenor E, Fonkalsrud EW, Atkinson JB. Surgical ligation of patent ductus arteriosus in very low birth weight infants: Is it safe? Am Surg. 1998;64:1007–9. [PubMed] [Google Scholar]
- 83.Laughon MM, Langer JC, Bose CL, Smith PB, Ambalavanan N, Kennedy KA, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183:1715–22. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hentschel J, Berger TM, Tschopp A, Müller M, Adams M, Bucher HU, et al. Population-based study of bronchopulmonary dysplasia in very low birth weight infants in Switzerland. Eur J Pediatr. 2005;164:292–7. doi: 10.1007/s00431-005-1623-1. [DOI] [PubMed] [Google Scholar]
- 85.Qureshi M, Al-Sufayan F, Kwiatkowski K, Moddemann D, Seshia M, Baier RJ. Is PDA ligation responsible for the adverse outcome in very low birthweight (VLBW) infants with PDA? Pediatric Academic Societies. 2009:476. [Google Scholar]
- 86.Benitz WE. Treatment of persistent patent ductus arteriosus in preterm infants: Time to accept the null hypothesis? J Perinatol. 2010;30:241–52. doi: 10.1038/jp.2010.3. [DOI] [PubMed] [Google Scholar]
- 87.Benitz WE. Patent ductus arteriosus: To treat or not to treat? Arch Dis Child Fetal Neonatal Ed. 2012;97:F80–2. doi: 10.1136/archdischild-2011-300381. [DOI] [PubMed] [Google Scholar]
- 88.Benitz WE. Learning to live with patency of the ductus arteriosus in preterm infants. J Perinatol. 2011;31(Suppl 1):S42–8. doi: 10.1038/jp.2010.175. [DOI] [PubMed] [Google Scholar]
- 89.Wickremasinghe AC, Rogers EE, Piecuch RE, Johnson BC, Golden S, Moon-Grady AJ, et al. Neurodevelopmental outcomes following two different treatment approaches (early ligation and selective ligation) for patent ductus arteriosus. J Pediatr. 2012;161:1065–72. doi: 10.1016/j.jpeds.2012.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vanhaesebrouck S, Zonnenberg I, Vandervoort P, Bruneel E, Van Hoestenberghe MR, Theyskens C. Conservative treatment for patent ductus arteriosus in the preterm. Arch Dis Child Fetal Neonatal Ed. 2007;92:F244–7. doi: 10.1136/adc.2006.104596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jhaveri N, Moon-Grady A, Clyman RI. Early surgical ligation versus a conservative approach for management of patent ductus arteriosus that fails to close after indomethacin treatment. J Pediatr. 2010;157:381–7, 3871. doi: 10.1016/j.jpeds.2010.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brooks JM, Travadi JN, Patole SK, Doherty DA, Simmer K. Is surgical ligation of patent ductus arteriosus necessary. The Western Australian experience of conservative management? Arch Dis Child Fetal Neonatal Ed. 2005;90:F235–9. doi: 10.1136/adc.2004.057638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaempf JW, Wu YX, Kaempf AJ, Kaempf AM, Wang L, Grunkemeier G. What happens when the patent ductus arteriosus is treated less aggressively in very low birth weight infants? J Perinatol. 2012;32:344–8. doi: 10.1038/jp.2011.102. [DOI] [PubMed] [Google Scholar]
- 94.Noori S, McCoy M, Friedlich P, Bright B, Gottipati V, Seri I, et al. Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics. 2009;123:e138–44. doi: 10.1542/peds.2008-2418. [DOI] [PubMed] [Google Scholar]
- 95.Weisz DE, More K, McNamara PJ, Shah PS. PDA ligation and health outcomes: A meta-analysis. Pediatrics. 2014;133:e1024–46. doi: 10.1542/peds.2013-3431. [DOI] [PubMed] [Google Scholar]


