Abstract
Cognitive flexibility has been measured with inductive reasoning or explicit rule tasks in individuals with autism spectrum disorders (ASD). The Flexible Item Selection Task (FIST) differs from previous cognitive flexibility tasks in ASD research by giving children an abstract, ambiguous rule to switch. The ASD group (N=22; Mean age=8.28 years, SD=1.52) achieved a lower shift percentage than the typically developing verbal mental-age control group (N=22; Mean age=6.26 years, SD=0.82). There was a significant positive correlation between verbal mental age and shift percentage for children with ASD. Group differences on the FIST converge and extend prior evidence documenting an impaired ability to adapt rapidly to changes in task demands for individuals with ASD.
Cognitive flexibility is the process of adapting thoughts and behavior in response to situational demands (Geurts, Corbett, & Solomon, 2009). Cognitive flexibility is impaired in adults and children with autism spectrum disorders (ASD) when performance is measured in the laboratory (Lopez, Lincoln, Ozonoff, & Lai, 2005; Maes, Eling, Wezenberg, Vissers, & Kan, 2010; Ozonoff et al., 2004; Schmitz et al., 2006; Solomon, Ozonoff, Cummings, & Carter, 2008; Solomon et al., 2008; South, Ozonoff, & McMahon, 2007; Yerys et al., 2009; but see Goldberg et al., 2005; Happé, Booth, Charlton, & Hughes, 2006; Liss et al., 2001; Poljac et al., 2010). Furthermore, parents' reports of cognitive flexibility in everyday settings suggest significant impairments in children with ASD (Boyd, McBee, Holtzclaw, Baranek, & Bodfish, 2009; Gioia, Isquith, Kenworthy, & Barton, 2002; Kenworthy, Black, Harrison, Della Rosa, & Wallace, 2009). For example, inflexible behaviors included repeating the same approach to solving a problem even if it isn't working, or failing to incorporate or accept new methods to solving a problem (Gioia et al., 2002; Kenworthy, Yerys, Anthony, & Wallace, 2008).
Cognitive flexibility tasks assess executive function processes – a set of processes that aid behavior regulation and goal-directed behavior (Welsh & Pennington, 1988). Recent efforts in delineating separable components of executive function have examined these executive processes with latent factor analyses and would most likely classify cognitive flexibility tasks within the “shifting” factor (Miyake et al., 2000). Although one cognitive flexibility task, the Wisconsin Card Sorting Task (WCST), falls into the “working memory” factor for children (Huizinga, Dolan, & van der Molen, 2006), many cognitive flexibility tasks are conceptualized as loading on the shifting factor.
Cognitive flexibility tasks typically used in ASD research generally fall into two broad categories: 1) inductive reasoning tasks and 2) explicit rule tasks. `Inductive' tasks include the WCST (Heaton, Chelune, Talley, Kay, & Curtis, 1993) and the intradimensional/extradimensional shift task (Cambridge Cognition, 1996). These tasks require individuals to induce a general rule from a specific event (e.g., discover a shape rule after positive reinforcement for sorting a card with a red circle into a target pile with three blue circles). The advantage of inductive tasks is that participants must engage in a problem-solving strategy that allows for reinterpretation of a stimulus by a different feature (e.g., “red” instead of “circle”) for inducing a shift rule. One disadvantage is that poor performance may be due to problems with the process of identifying new rules (i.e., reasoning that the rule has switched from shape to color) and not with cognitive flexibility per se. `Explicit' tasks include the dimensional change card sort task (Zelazo, 2006), the “preparing to overcome prepotency” task (Solomon et al., 2008, 2009), and oddball tasks (Shafritz, Dichter, Baranek, & Belger, 2008). These tasks require individuals to switch rules after being given an explicit cue (e.g., red square means switch responses and press the “left button” when the target stimulus [`→'] is shown). The main advantage of explicit tasks is that knowing the new rule and having unambiguous stimuli minimizes the influence of other cognitive processes besides flexibility. One disadvantage is that this simplified form of cognitive flexibility may lack ecological validity (Burgess et al., 2006).
While individuals with ASD have performed significantly worse than controls on both task types (Geurts, Verté, Oosterlaan, Roeyers, & Sergeant, 2004; Kenworthy et al., 2008; B. F. Pennington & Ozonoff, 1996; Sergeant, Geurts, & Oosterlaan, 2002), these findings have significant limitations. Having a computer providing feedback has yielded better inductive reasoning cognitive flexibility performance in ASD relative to controls than human feedback (Ozonoff, 1995; Pascualvaca, Fantie, Papageorgiou, & Mirsky, 1998), and co-varying verbal intelligence even when ASD and controls are matched on this variable has been shown to eliminate group differences on inductive reasoning tasks (Liss et al., 2001). The simplified format of explicit tasks may explain why there is less consistency in identifying impairments for individuals with ASD (Bíró & Russell, 2001; Schmitz et al., 2006; Solomon et al., 2008, 2009). A recent review has charged the field to develop tasks that better capture cognitive flexibility impairments characteristic of ASD (Geurts et al., 2009). An alternative cognitive flexibility task is one that explicitly requires a shift without providing explicit information on the new rule or category. This more ambiguous form of switching may tap into difficulties observed by parents, such as repeating the same approach to problem-solving.
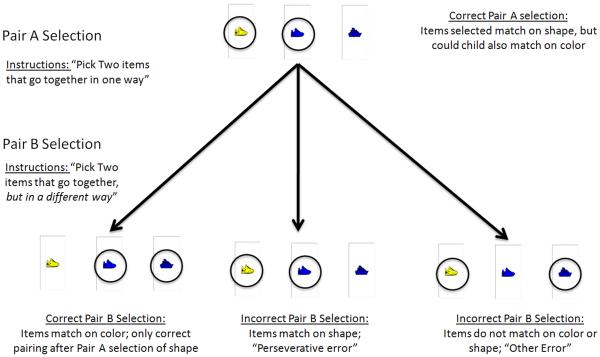
The Flexible Item Selection Task (FIST), a simplified version of the Visual-Verbal task (Feldman & Drasgow, 1951), is a cognitive flexibility task that provides a shift cue without explicit information for the new rule (Jacques & Zelazo, 2001). In the FIST, children are first shown an array of three simple pictures (yellow shoe, blue shoe, blue boat; See Figure 1) where one pivot item (e.g., blue shoe) shares a feature with each of the other two items in the array. To identify “Pair A,” children are asked by the examiner to “show me two things that go together in one way.” Children are then asked by the examiner to “pick two things that go together, but in a different way” to identify “Pair B.” Children do not receive feedback during the FIST's 15 trials (two pair selections on each trial). The FIST combines the advantages of both inductive and explicit rule tasks, and would likely fall in the shifting factor identified in executive function latent factor analyses (Miyake et al., 2000). It engages children in an endogenous problem-solving strategy to establish and switch sets, but in line with explicit rule tasks, the FIST attempts to reduce the influence of other cognitive processes. For example, working memory/inhibition processes are hypothesized as reduced, because switching occurs right after establishing a set on each trial rather than after establishing a strong prepotent response over several trials and maintaining that rule across time.
Figure 1.
An example of a single trial from the Flexible Item Selection Task. Note the middle stimulus (blue shoe) is the pivot item, because it matches each of the other two stimuli on one feature (shape or color). All three stimuli remain on the screen for both Pair A and Pair B selections.
The goal of the present study was to examine cognitive flexibility in an exclusively preadolescent ASD sample using the FIST. We predicted children with ASD would perform significantly worse in their accuracy of selecting Pair B relative to a typically developing (TD) control group matched on verbal mental-age and gender. We also examined shifting by evaluating Pair B accuracy only if the Pair A selection was correct (shift percentage), and we predicted the ASD group would have a significantly lower shift percentage compared to the TD group. We explored whether FIST performance improves across verbal mental age in children with ASD, as this has been observed in typical development.
Method
Participants
Forty-four children between 5 –11 years were enrolled in the present study. The ASD group (n=22) was comprised of High-functioning Autism (n=19), Asperger Syndrome (n=2), and Pervasive Developmental Disorder-Not Otherwise Specified (n=1), while the typically developing (TD) comparison group (TD) included children who were significantly younger matched on verbal mental age (n=22), but non-verbal mental age was significantly lower for the subset of TD that had scores. The FIST has a significant relationship with verbal mental abilities but not nonverbal mental abilities (Jacques, Landry, Sutton, Russo, & Burack, 2002), thus our goal was to match only on verbal mental abilities. Children in the TD group were significantly younger chronologically See Table 1 for participant characteristics.
Table 1.
Participant Demographics and Task Performance by Diagnosis.
| ASD (n=22) | TD (n=22) | p-value | |
|---|---|---|---|
| Chronological Age (Years) M (SD) | 8.48 (1.52) | 6.26 (0.82) | <0.01 |
| Verbal Mental Age M (SD) | 7.92 (1.90) | 7.16 (1.18) | 0.12 |
| Nonverbal Mental Age* M (SD) | 9.08 (1.76) | 7.55 (1.32) | <0.05 |
| Gender (male/female) | 18/4 | 16/6 | 0.47 |
| ADI-R (n=20) | |||
| Social | |||
| M (SD) | 15.60 (3.57) | -------- | -------- |
| Communication | |||
| M (SD) | 11.00 (2.45) | -------- | -------- |
| Repetitive Behaviors | |||
| M (SD) | 5.90 (1.25) | -------- | -------- |
| ADOS Module 2 (n=2) | |||
| Social + Communication Score | |||
| M (SD) | 11.50 (4.95) | -------- | -------- |
| ADOS Module 3 (n=20) | |||
| Social + Communication Score | |||
| M (SD) | 7.85 (3.15) | -------- | -------- |
| Flexible Item Selection Task (FIST) | |||
| Pair A Selection % | |||
| M (SD) | 96.36 (7.90) | 98.18 (3.04) | 0.44 |
| Pair B Selection % | |||
| M (SD) | 75.15 (29.18) | 89.39 (10.01) | <0.001 |
| Shift Percentage | |||
| M (SD) | 76.08 (29.33) | 89.48 (9.77) | <0.001 |
ASD N=19; TD N=13
ASD Inclusion criteria
Current diagnosis of ASD based upon independent chart review by two clinical psychologists reviewing previously administered autism diagnostic measures [Autism Diagnostic Observation Schedule (ADOS), Autism Diagnostic Interview (ADI), and DSM-IV checklist] and a current score on the appropriate module of the ADOS at or above the cutoff for autism spectrum. Case conferences were held to resolve differences in clinical opinion. Furthermore, children with a history of a traumatic brain injury, severe prematurity, a known genetic abnormality (e.g., Fragile X Syndrome), or a specific neurological finding that suggested possible alternative explanations (e.g., absent corpus callosum) were excluded from the present study. The ASD group was recruited from a larger study occurring at the primary outpatient clinical site which specialized in assessing autism and other developmental disorders, as well as clinics serving families with children with developmental disorders, parent/advocacy groups, and community-based service providers.
TD Inclusion Criteria
The TD group participated in the same cognitive and executive function assessments as the ASD group. These children were recruited from a participant pool maintained at a collaborating university, as well as through flyers at pediatrician offices and local schools. TD participants were screened for developmental and physical delays, ASD symptoms via the social communication questionnaire (Rutter, Bailey, & Lord, 2003), learning and mood disorders, and significant neurological or other medical conditions.
Tasks and Materials
The Flexible Item Selection Task (FIST; (Jacques & Zelazo, 2001) was used to measure cognitive flexibility. Children were shown the three pictures on a computer screen (See Figure 1). Children first observed the computer highlight two pictures that “go together in one way” (Pair A), and then observed the selection of two pictures that “go together, but in a different way” (Pair B). Children then completed two practice trials, and 15 test trials. Feedback was provided on the practice trials, but not the test trials. Counterbalancing of stimuli was identical to the original FIST, with the notable exception that only one stimuli order from Jacques and Zelazo (2001) was used in the present study, because no order effects emerged in the original study. We examined the percentage of correct Pair A and Pair B selections, the percentage of correct Pair B selections after correct Pair A selections (hereafter, “shift percentage”), and whether children selected Pair B correctly after making an error on Pair A. Pair A accuracy indexes establishing a cognitive set (e.g., `color'), and Pair B accuracy indexes switching to a new cognitive set (e.g., `shape').
Verbal cognitive ability was assessed with one of several measures including the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999), the Wechsler Preschool and Primary Scale of Intelligence – Third Edition (Wechsler, 2002), and due to difficulty in scheduling one child for a second visit the Peabody Picture Vocabulary Test – Third Edition (Dunn & Dunn, 1997) was used. Diagnostic tools included the ADOS (Lord, Risi, et al., 2000) as a direct observation measure of social, communication, and restricted, repetitive behaviors and interest symptoms.
Procedures
IRB approval was obtained prior to data collection. Parental consent and child assent were obtained prior to data collection. Data collection included administration of the tasks described above during two sessions lasting about two hours; task order was counterbalanced within the testing session to reduce fatigue effects. Participants and families were compensated $10/hour.
Data Analysis Plan
We compared the number of correct Pair A and Pair B responses and the shift percentage between the two groups and controlled for differences in chronological age in a univariate analysis of covariance (ANCOVA). We also explored whether correct selection of Pair B trials after an error on Pair A influenced results. Pearson's correlation was computed to examine the relationship of shift percentage with verbal mental age.
Results
We examined the data for significant: (1) kurtosis, (2) skew, and (3) outliers. We also assessed whether minor manipulations in task administration format (computerized/human) or task instructions (naming the category of first pair selected), incorporated as part of a separate study, affected performance. All variables met the assumptions of normality, allowing us to conduct parametric analyses. Significant outliers included scores greater than 2.5 standard deviations (SD) from their own diagnostic group mean. Group means were calculated without the potential outlier score. No significant outliers were identified. Furthermore, chronological age was used as a covariate of interest in the ANCOVA, and the results remained significant whether the covariate was included or not; to reduce redundancy, we only report the results with age as a covariate. The task presentation manipulations had no effect on performance and thus are not discussed further. Both groups' performances on selecting Pair A (i.e., establishing a set) were near ceiling, with the mean percentage of correct scores over 95%, and no difference between groups (See Table 1 for means). The groups did not differ in their selection of stimulus category (color, shape, or size) for the first pair on any trial, χ2(N=27)<5.3, p>0.15. Only 17 of 660 Pair A selections were errors (TD=5; ASD=12) for the two groups combined. Further, on all 17 of these trials, participants made a correct Pair B selection, which may have lead to an inflated Pair B total score. These two issues raise the importance of controlling for Pair A performance prior to examining Pair B performance, which we accomplished by calculating the shift percentage.
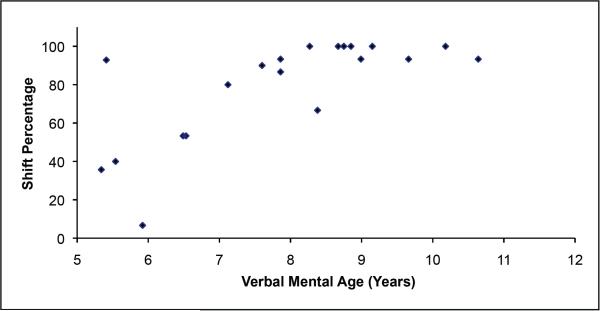
As shown in Table 1, the ASD group had a lower percentage for correct Pair B selections, F(1,41)=27.29,p<0.001, and compiled a lower shift percentage, F(1,41)=24.13,p<0.001, compared to the TD group while controlling for differences in chronological age. Shift percentage was positively correlated with verbal mental age in the ASD group, r(22)=0.69,p<0.001 (See Figure 2); this relationship was not significant in the TD group, r(22)=0.17,p=0.459. However, the range of verbal mental age scores for the TD group was 4.14 (6.16 – 10.30) relative to the 7.37 (4.81–12.18) in the ASD group, and this reduced range may limit our ability to detect a correlation in the TD group.
Figure 2.
Scatterplot of shift percentage score and verbal mental age in the autism spectrum disorder group.
Discussion
The main goal of this study was to examine cognitive flexibility in school-aged children with ASD on a task that combined the benefits of inductive and explicit flexibility tasks (i.e., requiring a problem-solving strategy while also limiting the influence of other cognitive processes). Children with ASD demonstrated poorer performance on Pair B accuracy and the shift percentage scores. Cognitive flexibility performance was also positively correlated with verbal mental age in our ASD group.
With respect to previous cognitive flexibility studies, the most developed literature in this age range is with two inductive reasoning tasks: the Intradimensional/Extradimensional task and the WCST (Hill, 2004; Kenworthy et al., 2008; Pennington & Ozonoff, 1996; Sergeant et al., 2002). The results from the present study converge with those of prior investigations demonstrating cognitive flexibility impairments in ASD (Lopez et al., 2005; Maes et al., 2010; Ozonoff et al., 2004; Solomon et al., 2008, 2008; South et al., 2007; Yerys et al., 2009; but see Happé et al., 2006) and extends past findings to a novel task. The FIST attempts to capture different aspects of flexibility, because flexible thinking is not always reliant upon the two conditions of either inductive reasoning or following explicit rules. Furthermore, applying rules in a general way has often been a core training component of evidence-based social interventions for children with ASD (Rogers, 2000), and therefore the FIST may better capture the cognitive flexibility weaknesses observed by clinicians and parents for individuals with ASD (Geurts et al., 2009; Gioia et al., 2002; Kenworthy et al., 2008).
As discussed earlier, inductive tasks like the WCST, have been shown in latent variable factor analyses to load more on working memory than a shifting factor in TD children (Huizinga et al., 2006). The FIST's construction overlaps to some degree with the WCST, but also differs in ways that align it with more explicit tasks like the preparing to overcome prepotency task (Solomon et al., 2008). Thus, while the FIST is conceptualized primarily as a cognitive flexibility/shifting task, it is possible that impaired performance by the ASD group on this task may reflect some combination of cognitive flexibility/shifting and working memory impairments. Future investigations may seek to conduct latent variable factor analyses with complex cognitive flexibility tasks other than the WCST to determine whether the loading of WCST on working memory factors is specific to the task or characteristic of all complex cognitive flexibility tasks.
The correlation between shift performance and verbal mental age is not likely explained by language delay because the groups were matched on verbal mental age. This finding also converges with previous FIST studies documenting age-related improvements, specifically related to increases in verbal mental age, in typical development and ASD (Jacques & Zelazo, 2001, 2005; Jacques et al., 2002). The present study's ASD sample's age range extends beyond that of previous typical development studies; therefore we speculate these data are consistent with a delayed developmental trajectory for ASD in cognitive flexibility. It is also possible that different cognitive and neural mechanisms may support successful performance on cognitive flexibility measures for TD and ASD populations; the limited range of verbal mental age in the TD group restricted our ability to detect meaningful correlations.
This study has potential limiting factors. First, the verbal mental age of our participants was higher than that of participants in the original FIST studies (i.e., 4–5 years) (Jacques & Zelazo, 2001), and Pair A selection rates were near ceiling in both groups. Ceiling performance limits the ability to observe differences in Pair A selection, but not Pair B as accuracy was in the 75–90% range which is higher than previous TD studies (Jacques & Zelazo, 2001), but still not at ceiling for the ASD group However, our goal was to examine the ability of children to flexibly shift from a correct Pair A selection to a correct Pair B selection, thus ceiling performance on Pair A is a desirable result because it confirms that both groups reliably establish a cognitive set. Next, we did not correlate FIST performance with restricted, repetitive, behavior and interest symptoms. Cognitive flexibility is likely to relate to the insistence on sameness and behavioral rigidity observed in ASD, and the ADOS only includes two items (D4 and D5) that assess this collection of autistic behaviors. At the time of data collection we were unable to include more comprehensive measures like the repetitive behavior scale – revised (Lam & Aman, 2007). Future research will be needed to explore how cognitive flexibility, as indexed by the FIST, relates to these symptoms, as well as other performance-based and ecologically-valid measures of flexibility. Our ASD and TD samples were matched on verbal mental age rather than non-verbal mental age or overall mental age. Non-verbal mental age tends to be greater in those with ASD relative to verbal mental age (Joseph, Tager-Flusberg, & Lord, 2002). As a result, the estimate of cognitive flexibility in the ASD group may be overly conservative. That is, if we had matched on overall cognitive ability or non-verbal mental age, the ASD group may have demonstrated even poorer overall performance, inflating the observed differences. Detecting significant group differences despite this conservative matching procedure suggests a meaningful impairment in cognitive flexibility in children with ASD. Finally, use of a covariate that correlates with the dependent variable of interest may artificially inflate group differences statistically (see Dennis et al., 2009 for an example of IQ as a covariate), and verbal mental age correlates with FIST performance in our study and previously (Jacques & Zelazo, 2001; Jacques et al., 2002). This concern is mitigated by our findings of significant group differences when chronological age is not included as a covariate. Thus, even though the ASD group is chronologically older and matched on verbal mental age relative to controls, they performed worse in the FIST's flexibility condition. This sample is generally considered to be high functioning as all children in the ASD group except one had a developmental quotient (Developmental Quotient = Mental Age ÷ Chronological Age).greater than 0.70). The one child with a developmental quotient below 0.70, had a verbal mental age above 4 years, and his inclusion did not affect the results.
Finally, this study was conducted prior to the publication of evidence that reduced novelty processing may contribute to poor cognitive flexibility in ASD (Maes et al., 2010). This study suggests individuals with ASD are inflexible when previously irrelevant stimuli become relevant during the switch (termed “learned irrelevance”). When the previously irrelevant stimuli are removed and individuals with ASD must shift to a novel stimulus attribute, they perform similarly to controls. The FIST could be adapted to include a fourth dimension (e.g., number) that is only present during Pair B in substitution for an ignored dimension in Pair A selection. This additional stimulus dimension would be the “novel” switch category in comparison to the standard FIST.
The need for sameness is both highly heritable (Smith et al., 2009) and worsens across age in children with ASD (Richler, Huerta, Bishop, & Lord, 2010). As such, the importance of understanding the underlying genetic, neural and cognitive basis of cognitive flexibility, and then translating this information into meaningful interventions, is a high priority for improving the health outcomes of individuals with ASD.
References
- Bíró S, Russell J. The execution of arbitrary procedures by children with autism. Development and Psychopathology. 2001;13(1):97–110. doi: 10.1017/s0954579401001079. doi:10.1017/S0954579401001079. [DOI] [PubMed] [Google Scholar]
- Boyd B, McBee M, Holtzclaw T, Baranek G, Bodfish J. Relationships among repetitive behaviors, sensory features, and executive functions in high functioning autism. Research in Autism Spectrum Disorders. 2009;3(4):959–966. doi: 10.1016/j.rasd.2009.05.003. doi:10.1016/j.rasd.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess PW, Alderman N, Forbes C, Costello A, Coates LM-A, Dawson DR, Anderson ND, et al. The case for the development and use of “ecologically valid” measures of executive function in experimental and clinical neuropsychology. Journal of the International Neuropsychological Society: JINS. 2006;12(2):194–209. doi: 10.1017/S1355617706060310. doi:10.1017/S1355617706060310. [DOI] [PubMed] [Google Scholar]
- Cambridge Cognition . CANTAB. Cambridge Cognition Ltd; Cambridge: 1996. [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society: JINS. 2009;15(3):331–343. doi: 10.1017/S1355617709090481. doi:10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn, Lloyd M, Dunn LM. Examiner's Manual for the PPVT-III, Peabody Picture Vocabulary Test. AGS: 1997. [Google Scholar]
- Feldman MJ, Drasgow J. A Visual-Verbal Test for schizophrenia. Psychiatric Quarterly. 1951;25(Suppl):55–64. [Google Scholar]
- Geurts HM, Corbett B, Solomon M. The paradox of cognitive flexibility in autism. Trends in Cognitive Sciences. 2009;13(2):74–82. doi: 10.1016/j.tics.2008.11.006. doi:10.1016/j.tics.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts HM, Verté S, Oosterlaan J, Roeyers H, Sergeant JA. How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2004;45(4):836–54. doi: 10.1111/j.1469-7610.2004.00276.x. doi:15056314. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Isquith PK, Kenworthy L, Barton RM. Profiles of everyday executive function in acquired and developmental disorders. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence. 2002;8(2):121–37. doi: 10.1076/chin.8.2.121.8727. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Mostofsky SH, Cutting LE, Mahone EM, Astor BC, Denckla MB, Landa RJ. Subtle executive impairment in children with autism and children with ADHD. Journal of Autism and Developmental Disorders. 2005;35(3):279–93. doi: 10.1007/s10803-005-3291-4. doi:16119469. [DOI] [PubMed] [Google Scholar]
- Happé F, Booth R, Charlton R, Hughes C. Executive function deficits in autism spectrum disorders and attention-deficit/hyperactivity disorder: examining profiles across domains and ages. Brain and Cognition. 2006;61(1):25–39. doi: 10.1016/j.bandc.2006.03.004. doi:S0278-2626(06)00069-8. [DOI] [PubMed] [Google Scholar]
- Heaton R, Chelune G, Talley J, Kay C, Curtis G. WCST Manual: Revised and Expanded. Psychological Assessment Resources Inc; USA: 1993. [Google Scholar]
- Hill EL. Evaluating the theory of executive dysfunction in autism. Developmental Review. 2004;24(2):189–233. [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia. 2006;44(11):2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. doi:10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Jacques S, Zelazo PD. The Flexible Item Selection Task (FIST): a measure of executive function in preschoolers. Developmental Neuropsychology. 2001;20(3):573–591. doi: 10.1207/S15326942DN2003_2. [DOI] [PubMed] [Google Scholar]
- Jacques S, Zelazo PD. On the possible roots of cognitive flexibility. In: Homer BD, Tamis-LeMonda CS, editors. The development of social cognition and communication. Lawrence Erlbaum Associates Publishers; Mahwah, NJ US: 2005. pp. 53–81. [Google Scholar]
- Jacques S, Landry O, Sutton M, Russo N, Burack JA. Language and cognitive flexibility in people with autism and down syndrome. Presented at the International Meeting for Autism Research; Orlando, FL: 2002. [Google Scholar]
- Jacques S, Zelazo PD, Lourenco SF, Sutherland AE. The roles of labeling and abstraction in the development of cognitive flexibility. Unpublished Data. 2005 [Google Scholar]
- Joseph RM, Tager-Flusberg H, Lord C. Cognitive profiles and social-communicative functioning in children with autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2002;43(6):807–21. doi: 10.1111/1469-7610.00092. doi:12236615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenworthy L, Black DO, Harrison B, Della Rosa A, Wallace GL. Are executive control functions related to autism symptoms in high-functioning children? Child Neuropsychology. 2009;15(5):425–440. doi: 10.1080/09297040802646983. doi:10.1080/09297040802646983. [DOI] [PubMed] [Google Scholar]
- Kenworthy L, Yerys BE, Anthony LG, Wallace GL. Understanding executive control in autism spectrum disorders in the lab and in the real world. Neuropsychology Review. 2008;18(4):320–38. doi: 10.1007/s11065-008-9077-7. doi:10.1007/s11065-008-9077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KSL, Aman MG. The Repetitive Behavior Scale-Revised: independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(5):855–866. doi: 10.1007/s10803-006-0213-z. doi:10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Liss M, Fein D, Allen D, Dunn M, Feinstein C, Morris R, Waterhouse L, et al. Executive functioning in high-functioning children with autism. Journal of Child Psychology and Psychiatry. 2001;42(2):261–270. [PubMed] [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. Journal of Autism and Developmental Disorders. 2005;35(4):445–60. doi: 10.1007/s10803-005-5035-x. doi:10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Lord Catherine, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–23.. doi:11055457. [PubMed] [Google Scholar]
- Maes JHR, Eling PATM, Wezenberg E, Vissers CTWM, Kan CC. Attentional set shifting in autism spectrum disorder: Differentiating between the role of perseveration, learned irrelevance, and novelty processing. Journal of Clinical and Experimental Neuropsychology. 2010:1–8.. doi: 10.1080/13803395.2010.501327. doi:10.1080/13803395.2010.501327. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cognitive Psychology. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. doi:10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Ozonoff S. Reliability and validity of the wisconsin card sorting test in studies of autism. Neuropsychology. 1995;9(4):491–500. [Google Scholar]
- Ozonoff S, Cook I, Coon H, Dawson G, Joseph RM, Klin A, McMahon WM, et al. Performance on Cambridge Neuropsychological Test Automated Battery subtests sensitive to frontal lobe function in people with autistic disorder: evidence from the Collaborative Programs of Excellence in Autism network. Journal of Autism and Developmental Disorders. 2004;34(2):139–150. doi: 10.1023/b:jadd.0000022605.81989.cc. [DOI] [PubMed] [Google Scholar]
- Pascualvaca DM, Fantie BD, Papageorgiou M, Mirsky AF. Attentional capacities in children with autism: is there a general deficit in shifting focus? Journal of Autism and Developmental Disorders. 1998;28(6):467–478. doi: 10.1023/a:1026091809650. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of Child Psychology and Psychiatry. 1996;37(1):51–87. doi: 10.1111/j.1469-7610.1996.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Poljac E, Simon S, Ringlever L, Kalcik D, Groen WB, Buitelaar JK, Bekkering H. Impaired task switching performance in children with dyslexia but not in children with autism. The Quarterly Journal of Experimental Psychology. 2010;63(2):401–416. doi: 10.1080/17470210902990803. doi:10.1080/17470210902990803. [DOI] [PubMed] [Google Scholar]
- Richler J, Huerta M, Bishop SL, Lord C. Developmental trajectories of restricted and repetitive behaviors and interests in children with autism spectrum disorders. Development and Psychopathology. 2010;22(1):55–69. doi: 10.1017/S0954579409990265. doi:10.1017/S0954579409990265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ. Interventions that facilitate socialization in children with autism. Journal of Autism and Developmental Disorders. 2000;30(5):399–409. doi: 10.1023/a:1005543321840. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire Manual. Westerm Psychological Services; 2003. [Google Scholar]
- Schmitz N, Rubia K, Daly E, Smith A, Williams S, Murphy DGM. Neural correlates of executive function in autistic spectrum disorders. Biological Psychiatry. 2006;59(1):7–16. doi: 10.1016/j.biopsych.2005.06.007. doi:10.1016/j.biopsych.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Geurts HM, Oosterlaan J. How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behavioural Brain Research. 2002;130(1–2):3–28. doi: 10.1016/s0166-4328(01)00430-2. doi:11864714. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Dichter GS, Baranek GT, Belger A. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biological Psychiatry. 2008;63(10):974–980. doi: 10.1016/j.biopsych.2007.06.028. doi:10.1016/j.biopsych.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Lang CM, Kryzak L, Reichenberg A, Hollander E, Silverman JM. Familial associations of intense preoccupations, an empirical factor of the restricted, repetitive behaviors and interests domain of autism. Journal of Child Psychology and Psychiatry. 2009;50(8):982–990. doi: 10.1111/j.1469-7610.2009.02060.x. doi:10.1111/j.1469-7610.2009.02060.x. [DOI] [PubMed] [Google Scholar]
- Solomon M, Ozonoff S, Cummings N, Carter CS. Cognitive control in autism spectrum disorders. International Journal of Developmental Neuroscience. 2008;26(2):239–47. doi: 10.1016/j.ijdevneu.2007.11.001. doi:S0736-5748(07)00161-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Ozonoff S, Ursu S, Ravizza S, Cummings N, Ly S, Carter CS. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47(12):2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. doi:10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M, Ozonoff S, McMahon WM. The relationship between executive functioning, central coherence, and repetitive behaviors in the high-functioning autism spectrum. Autism. 2007;11(5):437–51. doi: 10.1177/1362361307079606. doi:10.1177/1362361307079606. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scales of Intelligence. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. Third Edition Psychological Corporation; San Antonio, TX: 2002. [Google Scholar]
- Welsh M, Pennington B. Assessing frontal-lobe functioning in children - views from developmental-psychology. DEVELOPMENTAL NEUROPSYCHOLOGY. 1988;4(3):199–230. [Google Scholar]
- Yerys BE, Wallace GL, Harrison B, Celano MJ, Giedd JN, Kenworthy L. Set-shifting in children with autism spectrum disorders: reversal shifting deficits on the Intradimensional/Extradimensional Shift Test correlate with repetitive behaviors. Autism: The International Journal of Research and Practice. 2009;13(5):523–538. doi: 10.1177/1362361309335716. doi:10.1177/1362361309335716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelazo PD. The Dimensional Change Card Sort (DCCS): a method of assessing executive function in children. Nature Protocols. 2006;1(1):297–301. doi: 10.1038/nprot.2006.46. doi:10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]