Abstract
Mesenchymal stromal cells (mSCs) are presently studied for the prophylaxis and therapy of a variety of diseases such as acute graft-versus-host disease after allogeneic stem cell transplantation, cardiac indications, bone degeneration, Crohn's disease, and organ rejection, as well as prevention of acute renal failure in high-risk situations. mSCs appear to function through paracrine mechanisms that exert immunosuppressive, anti-inflammatory, anti-apoptotic, mitogenic, and other organ-protective and repair-stimulating actions. mSCs are either cultured in the presence of fetal calf serum (FCS) or platelet lysate (PL). PL lysate-generated mSCs exhibit faster doubling times, different gene expression profiles, and more potent immunosuppressive activity compared with FSC-generated mSCs. The utility of mSCs in the treatment of chronic inflammatory diseases is being evaluated in prospective studies.
Keywords: fusion, organ injury, paracrine actions, plasticity
mSCs IN REGENERATIVE MEDICINE
Recent interest in cell-based therapies has renewed the research on adult stem cells (SCs) derived from bone marrow (BM). Mononuclear cells of the BM contain SCs that can be purified by density centrifugation. These non-hematopoietic SCs have been characterized through plastic adherence and termed ‘mesenchymal' stem or multipotent stromal cells (mSCs). This cell population was first described by Friedenstein et al.1, 2, 3 in 1966. These cells exhibit in vitro a fibroblastoid phenotype and have been characterized as progenitors of adipocytes, chondrocytes, and osteocytes. Caplan, Prockop, and Pittinger further described them as mutlipotent progenitors for connective tissues.4, 5, 6
The ‘Mesenchymal and Tissue Stem Cell Committee of the International Society for cellular Therapy' published in a consensus statement the main criteria that define mSCs: (1) mSCs are plastic adherent; (2) they are negative for the hematopoietic markers CD34, CD45, CD14, and major histocompatibility complex (MHC)-II, and positive for CD90, CD105, CD73, and MHC-I; and (3) they differentiate into adipo-, osteo-, and chondrogenic lineages in vitro.7
Approximately 10 years ago, several publications reported on the ability of mSCs to differentiate across germ-layer lineages and boundaries; specifically, they were found to differentiate into brain, liver, kidney, heart, and muscle cells (Table 1).8, 9, 10, 11 Similar results were obtained with hematopoietic SCs (Table 2).12, 13, 14, 15, 16 In vitro studies showed that induced antigen expression did match that of targeted tissues or organs. However, this de novo expression of tissue-specific antigens was achieved by treating mSCs with nonspecific demethylating agents such as 5-azacytidine. However, not entirely unexpectedly, the reproduction of these in vitro findings in vivo proved difficult, whereas organ-protective and regenerative effects of administrated mSCs were still observed in injured organs.
Table 1. Proposed differentiation of mSCs across tissue lineage boundaries.
| mSCs into brain | Azizi et al.8 |
| mSCs into liver | Avital et al.9 |
| mSCs into kidney | Jiang et al.10 |
| mSCs into heart | Toma et al.11 |
Abbreviation: mSC, mesenchymal stromal cell.
Table 2. Proposed differentiations of hematopoietic stem cells across tissue lineage boundaries.
The mechanisms that were proposed to explain the observed effects of cellular therapy included plasticity (Table 3), that is, differentiation of cells beyond their lineage boundaries, cell fusion, and paracine effects. Several of the data suggesting ‘plasticity' of adult SCs were subsequently explained by the phenomenon of fusion of stem with target cells.17, 18, 19, 20 Terada et al.17 showed that BM cells adopt the phenotype of other cells by spontaneous cell fusion. Vassilopolous et al.18 and Wang et al.19 showed that cell fusion is the principal mechanism whereby BM-derived ‘hepatocytes' affect liver repair, whereas Villenbrink et al.20 demonstrated that myelomonocytic cells were sufficient to support repair of the diseased liver via cell fusion.
Table 3. Possible mediator mechanism that explain beneficial effects of mSCs in cellular therapy.
| 1 | Plasticity |
| 2 | Contamination with multi-/pluripotent stem cells |
| 3 | Dedifferentiation |
| 4 | Fusion (e.g., liver, muscle) |
| 5 | Paracrine effects |
Abbreviation: mSC, mesenchymal stromal cell.
ISOLATION AND GENERATION OF mSCs: EXPANSION OF mSCs WITH FETAL CALF SERUM OR WITH HUMAN PLATELET LYSATE
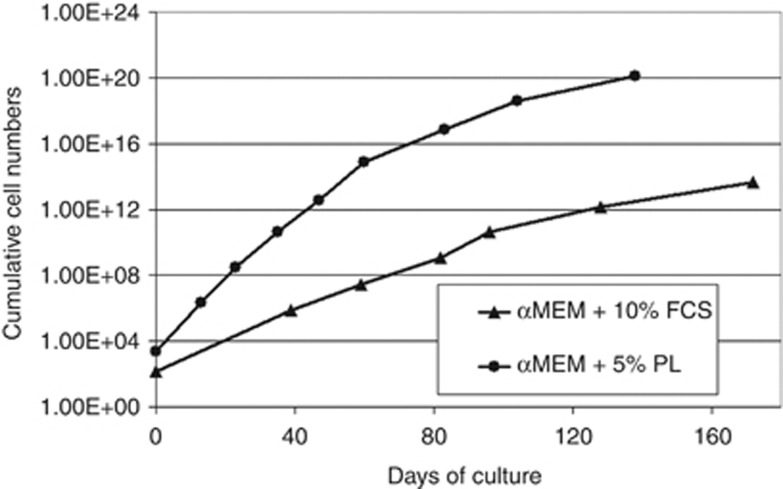
Expansion of mSCs with fetal calf serum (FCS) carries the risk of infectious bovine contaminants, and they are potentially antigenic as FCS is stored in mSCs. mSCs grown with human platelet lysate (PL) have the advantage that platelet donors are tested according to strict blood transfusion standards. Comparison of the two expansion methods revealed that FCS in vitro is a strong antigen even at low concentrations, whereas such a response is not seen with mSCs grown with PL. Comparative doubling times of PL-expanded MSCs are much shorter (Figure 1), and the gene expression profile of PL-grown mSCs shows lower expression of MHC II genes, such as MHC-II DP beta 1 (HLA-DPB1), MHC-II DM alpha (HLA-DMA), MHC-II DR alpha (HLA-DRA), MHC-II DP alpha 1 (HLA-DPA1), and MHC-II DR beta 1 (Dw14). These expression profiles explain, at least in part, the weaker antigenicity of PL-expanded mSCs I allogeneic settings.
Figure 1.
Comparative growth characteristics of human mesenchymal stromal cells (mSCs) when expanded with fetal calf serum or platelet lysate. αMEM, α-minimum essential Eagle's medium.
PRECLINICAL OBSERVATIONS WITH mSC THERAPY IN REGENERATIVE MEDICINE
An example in which in vitro and in vivo results were incongruent was reported by Jaquet et al.21 Pretreatment of mSCs in vitro with 5-azacytidine induced both smooth-muscle actin expression, a protein of immature cardiomyocytes, and troponin T, a protein of mature cardiomyocytes. However, no beating or contracting myogenic fibers were detected. When these rat mSCs were injected adjacent to ventricular cryolesions of 3 × 6 mm size, an in vivo model for acute myocardial infarction/injury, which resulted in a significant reduction of myocardial scar area. Significantly, there was no myogenic differentiation of injected mSCs, that is, no mature sarcomeric organization or intercalated disk formation by these cells, and there was no endothelial differentiation. The authors concluded, therefore, that paracrine actions of mSCs appeared to mediate regeneration of this type of myocardial injury.
Similar observations were made in a rat model of ischemia/reperfusion acute kidney injury.22, 23, 24, 25 Significant functional improvement was shown, leading to an earlier normalization of serum creatinine levels, and better long-term survival after mSC infusion. mSCs were labeled with superparamagnetic particles of iron oxide for in vivo tracking. Animals with acute renal failure were given superparamagnetic particles of iron oxide-labeled mSCs and scanned in a whole-body scanner.25 Rat mSCs were detected immediately after administration in the cortex of both kidneys, as revealed by signal extinction, on magnetic resonance imaging, at these sites. The renal signal of iron-labeled cells disappeared within 3 days of administration. On histological examination of kidneys at 3 days post injury and mSC infusion, no iron-labeled cells had differentiated into tubular or endothelial cells. Despite the rapid disappearance of administered mSCs from the kidneys, gene expression studies comparing mSC to vehicle-treated kidney tissues revealed strikingly altered gene expression profiles. Specifically, the kidneys of mSC-treated animals showed increased expression of anti-apoptotic Bcl-2, anti-inflammatory interleukin-10, mitogenic tissue growth factor-α, and vasculogenic basic fibroblast growth factor. In addition, proinflammatory genes IL-1β, TNF-α and IFN-γ, and nitric oxide synthase were downregulated.24 In conclusion, these data provide further clear evidence for the paracrine mode of action of mSCs in the cytoprotection and repair of the injured kidney.
Claims regarding the plasticity of ‘adult' SCs came from investigations in BM transplantation. Specifically, following transplantation, cells of donor origin were detected in other tissues such as liver, lung, and skin, by using the Y chromosome as a marker of male donor cells. In subsequent studies, however, BM DNA of donor cells was found to be transported into mature recipient cells by cell fusion or DNA transfer,26 largely invalidating the hypothesis of transdifferentiation.
On the basis of the above and other data, an important conclusion can be drawn: mSCs appear not to function directly by replacing destroyed cells following their differentiation into tissue-resident cells, but rather release factors that support endogeneous regeneration by decreasing the inflammation of injured tissue, inhibition of apoptosis, and stimulation of mitogenesis of viable cells.
CURRENT CLINICAL STUDIES WITH mSCs
At least 60 clinical studies evaluating mSCs as prophylaxis and therapy for various diseases are currently under way (Table 4; http://www.clinicaltrials.gov). Major indications are prevention and therapy of acute graft-versus-host disease (n=12), cardiac indications (n=12), bone generation (n=7), treatment of BM and organ rejection (n=4), and Crohn's disease (n=4). Other indications include multiple sclerosis, liver regeneration, and diabetes mellitus.
Table 4. Current clinical trials with mSCs.
| Disease | Phase |
|---|---|
| Transplant rejection (GvHD, kidney transplant) | I–III |
| Morbus Crohn | III |
| Acute renal failure/acute kidney injury | I |
| Lupus nephritis | I/II |
| Diabetes mellitus | I/II–II |
| Chronic obstructive pulmonary disease | II |
| Liver failure | I/II |
| Multiple sclerosis | I/II |
| Cardiac disease | I/II–II |
| Bone and cartilage defects | I/II–II |
| Osteogenesis imperfecta | I |
| Cord blood expansion | I/II |
Abbreviations: GvHD, graft-versus-host disease; mSC, mesenchymal stromal cell.
The working hypothesis of virtually all ongoing mSC-based clinical studies is based on their paracrine modes of action that collectively effect, in injured organs, immunosuppressive, anti-inflammatory, anti-apoptotic, vasculoprotective, and mitogenic responses, together resulting in organ protection and repair. Finally, it is presently unknown whether the administration of mSCs that are first predifferentiated into phenotypes of an injured organ is advantageous.
All authors are consultants to Allocure. CL has received consulting fees from Nephrogen. CW received a Veterans Administration merit grant.
Footnotes
TO CITE THIS ARTICLE: Zander AR, Lange C, Westenfelder C. Mesenchymal stromal cells: main factor or helper in regenerative medicine? Kidney inter., Suppl. 2011; 1: 74–76.
References
- Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381–390. [PubMed] [Google Scholar]
- Owen M. The origin of bone cells. Int Rev Cytol. 1970;28:213–238. doi: 10.1016/s0074-7696(08)62544-9. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ, Deriglasova UF, Kulagina NN, et al. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp Hematol. 1974;2:83–92. [PubMed] [Google Scholar]
- Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Azizi SA, Stokes D, Augelli BJ, et al. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—similarities to astrocyte grafts. Proc Natl Acad Sci USA. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avital I, Inderbitzin D, Aoki T, et al. Isolation, characterization, and transplantation of bone marrow-derived hepatocyte stem cells. Biochem Biophys Res Commun. 2001;288:156–164. doi: 10.1006/bbrc.2001.5712. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Toma C, Pittenger MF, Cahill KS, et al. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- Theise ND, Nimmakayalu M, Gardner R, et al. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Bjornson CR, Rietze RL, Reynolds BA, et al. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- Mezey E, Chandross KJ, Harta G, et al. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- Terada N, Hamazaki T, Oka M, et al. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature. 2002;416:542–545. doi: 10.1038/nature730. [DOI] [PubMed] [Google Scholar]
- Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- Willenbring H, Bailey AS, Foster M, et al. Myelomonocytic cells are sufficient for therapeutic cell fusion in liver. Nat Med. 2004;10:744–748. doi: 10.1038/nm1062. [DOI] [PubMed] [Google Scholar]
- Jaquet K, Krause KT, Denschel J, et al. Reduction of myocardial scar size after implantation of mesenchymal stem cells in rats: what is the mechanism. Stem Cells Dev. 2005;14:299–309. doi: 10.1089/scd.2005.14.299. [DOI] [PubMed] [Google Scholar]
- Lange C, Tögel F, Ittrich H, et al. Administered mesenchymal stem cells enhance recovery from ischemia/reperfusion-induced acute renal failure in rats. Kidney Int. 2005;68:1613–1617. doi: 10.1111/j.1523-1755.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- Tögel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- Tögel F, Weiss K, Yang Y, et al. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626–F1635. doi: 10.1152/ajprenal.00339.2006. [DOI] [PubMed] [Google Scholar]
- Ittrich H, Lange C, Tögel F, et al. In vivo magnetic resonance imaging of iron oxide-labeled, arterially-injected mesenchymal stem cells in kidneys of rats with acute ischemic kidney injury: detection and monitoring at 3T. J Magn Reson Imaging. 2007;25:1179–1191. doi: 10.1002/jmri.20925. [DOI] [PubMed] [Google Scholar]
- Metaxas Y, Spyridonidis A, Bertz H, et al. Donor-derived mucosal epithelial cells after human hematopoietic cell transplantation are not derived from the CD34-positive fraction of the graft. Leukemia. 2007;21:2214–2216. doi: 10.1038/sj.leu.2404759. [DOI] [PubMed] [Google Scholar]