Abstract
Flt3 signaling plays a crucial role in regulating the survival and differentiation of lymphoid progenitors into B cell precursors (BCPs) in bone marrow. To define further the role of Flt3 signaling in lymphoid progenitor survival, mice deficient in Flt3 ligand that also expressed a Bcl2 transgene (Eμ-bcl2tg flt3l−/−) were generated. Intracellular flow cytometry established transgene expression in primitive hematopoietic progenitors, including lineage-negative Sca-1+ c-kit+ (LSK+) CD27− cells enriched for functional hematopoietic stem cells. Compared with flt3l−/− mice, Eμ-bcl2tg flt3l−/− mice had significantly increased multipotential progenitors (MPPs), IL-7R+ common lymphoid progenitors, and B cell precursors. To determine whether forced expression of Bcl2 was sufficient to restore lymphoid priming in the absence of Flt3 signaling Eμ-bcl2tg flt3l−/− rag1-gfp+ mice were generated. Analysis of Eμ-bcl2tg flt3l−/− rag1-gfp+ mice revealed that the Bcl2 transgene had no effect on lymphoid priming before CD19 expression. Thus, forced expression of a survival gene can bypass the requirement for threshold levels of Flt3 signaling requisite for lymphoid priming. Temporal Flt3 ligand (FL) replacement therapy in flt3l−/− mice revealed specific requirements for Flt3 signaling in the expansion and maintenance of Flt3+hi MPP and Flt3+ all lymphoid progenitors, but not Flt3+ B lymphoid progenitors (BLPs), the immediate precursors of BCPs. BCPs were restored after temporal in vivo FL treatment, albeit with delayed kinetics. Together, these results show that Flt3 regulates the proliferation, and survival, and maintenance of developmental stage–specific hematopoietic progenitors that give rise to BCPs.
B cell development from hematopoietic stem cells (HSCs) requires the concerted activities of transcription factors that regulate cell fate decisions and signaling molecules that temporally control the differentiation, proliferation, and survival of select progenitor subsets. Fms-like tyrosine kinase (Flt3) and its ligand, Flt3 ligand (FL), play a critical role in regulation of the multipotential progenitor (MPP) and common lymphoid progenitor (CLP) hematopoietic progenitor pools from which B cell precursors are derived [1–3]. While it is widely appreciated that Flt3 signaling is critical for lymphoid lineage and B-cell development, much remains to be learned concerning the roles of this growth factor receptor–ligand pair in their regulation. Indeed, precise cellular targets and mechanisms by which Flt3 signaling regulates the MPP and CLP pools, or their differentiation into B cell precursors (BCPs), remains largely unknown.
The generation of BCPs from HSCs proceeds through a series of developmental intermediates that initiate a series of molecular events that culminate in the activation of a genetic program that selectively orchestrates B cell differentiation. Upregulation of Flt3 receptor density in MPPs correlates with lymphoid-myeloid restriction [4–6]. A small subset of Flt3+ MPPs express high levels of Flt3 (Flt3hi MPPs) and exhibit a lymphoid-biased genetic program and differentiation potential in vitro and in vivo [4–6]. The significance of high Flt3 receptor density on lymphoid-biased progenitors remains to be elucidated, but it is consistent with our previous findings that threshold levels of Flt3 signaling are required for lymphoid development.
Flt3hi MPPs express il7ra transcripts and are the presumed immediate precursors of IL-7R+ Flt3+ CLPs [2,6]. A recent study determined that a subset of IL-7R+ Flt3+ CLPs express the surface marker Ly6D [7]. In vitro and in vivo assays revealed that the Ly6D− fraction of CLPs retained all lymphoid potential, while the Ly6D+ fraction was enriched for B and dendritic cell potentials. Based on these findings, IL-7R+ Flt3+ Ly6D− CLPs are now referred to as all lymphoid progenitors (ALPs), and IL-7R+ Flt3+ Ly6D+ CLPs are known as B lineage-restricted B lymphoid progenitors (BLPs). IL-7R signaling in BLPs induces expression of early B cell factor (EBF), a transcription factor critical for specification and commitment to the B cell fate [8–10].
Cytokine signaling also contributes to hematopoiesis by promoting progenitor expansion and survival. Although we did not find a proliferation defect in vivo in mice deficient for FL (flt3l−/−), in vivo administration of FL expands MPPs with lymphoid–myeloid differentiation potential [11]. It is well established that FL synergizes with other early-acting cytokines in the expansion of MPPs [12–15]. The combination of FL, stem cell factor, and IL-7 is widely used to generate lymphoid–B lineage cells from MPPs [16–18]. In addition to proliferation, Flt3 signaling regulates hematopoietic progenitor survival. Flt3l−/− mice have increased percentages of Annexin V+ lineage-negative Sca-1+ c-kit+ (LSK+) Flt3+ cells and reduced levels of the prosurvival protein Mcl-1, supporting a role for Flt3 signaling in the survival of primitive hematopoietic progenitors [3].
In this study, we sought to pinpoint developmental stage–specific roles for Flt3 signaling in the proliferation, survival, and maintenance of Flt3+ MPPs into BCPs in vivo. We show that enforced expression of the antiapoptotic protein Bcl2 largely restored LSK+, Flt3+ CLP, and select BCP deficiencies in flt3l−/− mice. We crossed our Eμ-bcl2tg flt3l− mice to rag1-gfp+ mice to determine whether lymphoid priming was restored in Lin− progenitor subsets. Strikingly, the frequency of GFP+ cells within Flt3+hi MPP, ALP, and BLP in Eμ-bcl2tg flt3l−/− rag1-gfp+ was similar to the flt3l−/− rag1-gfp+ mice, indicating that restoration was independent of lymphoid priming. Importantly, temporal FL administration in vivo expanded Flt3+ LSK+ and Flt3+ ALP subsets enriched for multilymphoid lineage differentiation potential, but only minimally affected Flt3+ BLPs. Regardless of the minor effect of FL administration on BLP, BCP numbers were normalized after temporal FL administration. Together, these experimental findings show that Flt3 signaling regulates the survival and expansion of Flt3+ LSK+ into BCPs. Furthermore, these results suggest that IL-7R signaling coupled with enforced expression of a survival gene can circumvent the requirement for Flt3 signaling in B cell development.
Methods
Mice
All mice in this study have been maintained on a C57Bl/6 genetic background for greater than 10 generations. WT (C57Bl/6) mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Flt3l−/− mice were obtained from Taconic Farms (Germantown, NY, USA) and then bred and maintained in our colony. Eμ-Bcl2-36 transgenic (Eμ-bcl2tg) mice have been described previously and were provided by Paul W. Kincade (Oklahoma Medical Research Foundation, Oklahoma City, OK, USA) [19]. Flt3l−/− and Eμ-bcl2tg mice were bred to generate Eμ-bcl2tg flt3l−/− mice. Eμ-bcl2tg flt3l−/− mice were crossed to rag1-gfp+ mice to generate Eμ-bcl2tg flt3l−/− rag1-gfp+ progeny. Rag1-gfp+and flt3l−/− rag1-gfp+ mice have been described [3,48]. Age-matched or littermate controls were used for individual experiments, and all mice analyzed in this study ranged from 8–12 weeks of age. All animals were bred and maintained at the Mayo Clinic animal facility, and experiments were performed according to the Mayo Clinic Institutional Animal Care and Use Committee guidelines.
Genotyping
Eμ-bcl2tg flt3l−/− mice were identified by separate genotyping reactions with two primer sets: flt3l and bcl2tg. Eμ-bcl2tg flt3l−/− rag1-gfp+ were identified by separate genotyping reactions using four primer sets: rag1, gfp, flt3l, and bcl2tg. PCR sequences and conditions for genotyping rag1, gfp, and flt3l−/− mice have been described [3]. Bcl2tg genotyping was performed using primer combinations and PCR conditions found on The Jackson Laboratory website, mouse stock number 002321.
Flow cytometry
Methods for flow cytometry and progenitor isolation have been described [20,49,50]. BM or spleen was harvested and stained with combinations of the following antibodies: c-kit (APC, APC-eFluor 780), Sca-1 PerCP-Cy5.5, Flt3 PE, CD34 eFluor 450, CD150 PE-Cy7, IL-7Rα (bio or PE-Cy7), Ly6D (APC, eFluor 450), IgM APC-Cy7, CD19 (APC, PE-Cy7), B220 APC, CD43 PerCP-Cy5.5, and a FITC- or biotin-labeled (for rag1- gfp+ mice) lineage cocktail (B220, CD3ε, CD11b/Mac-1, Gr-1, and Ter119) to exclude Lin+ cells. Incubation with streptavidin- PE-Cy7 or streptavidin-APC was used to visualize bio-IL-7Rα or bio-lineage markers, respectively. For intracellular staining of the Bcl2 transgene in LSK+ cells, BM was stained with a biotin-labeled lineage mixture (B220, CD19, Gr-1, Ter119, CD3ε, NK1.1, IgM, and CD8α) and depleted of Lin+ cells using streptavidin magnetic bead depletion. Lin−lo cells were surface stained with c-kit APC, Sca-1 PE, and CD27 APC-eFluor 780, fixed, and permeabilized using Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA, USA), followed by intracellular staining with hamster anti-human Bcl2 FITC or PE (for rag1-gfp+ mice) or isotype control FITC or PE (BD Biosciences, San Jose, CA, USA). All antibodies were obtained from eBioscience (San Diego, CA, USA), BD Biosciences, or BioLegend (San Diego, CA, USA). Flow cytometric analysis was performed on the LSRII or Canto cytometers (BD Biosciences). Data were analyzed using FlowJo software (Tree Star, Ashland, OR, USA).
Progenitor frequency and absolute number calculations
Progenitor frequencies were calculated by multiplying percentages of sequential gated populations. Absolute numbers were calculated by multiplying mononuclear cell counts obtained after BM harvest by progenitor frequencies. The frequencies and number calculations reflect mononuclear and doublet exclusion gates.
Flt3 ligand replacement therapy
Flt3 ligand was obtained from PeproTech (Rocky Hill, NJ, USA). Two cohorts of flt3l−/− mice were administered Flt3 ligand five times over the course of an 8-day injection schedule. Each injection, spaced 2 days apart, consisted of 10 μg of FL delivered in 200 μL of PBS. For controls, WT and flt3l−/− mice were similarly injected with equivalent volumes (200μL) of PBS. Two days following the last injection, one cohort of mice were euthanized, BM harvested, and progenitor populations were analyzed by flow cytometry. The second cohort of mice was euthanized, and BM was harvested 5 days after the last injection for flow cytometric analysis.
Statistical analysis
Statistical analysis was performed using the Student t test; p ≤ 0.05 was significant. All numerical data are presented as mean ± SEM.
Results
Enforced expression of Bcl2 rescues LSK+ cells, but not deficiencies in Flt3+hi MPPs in flt3l−/− mice
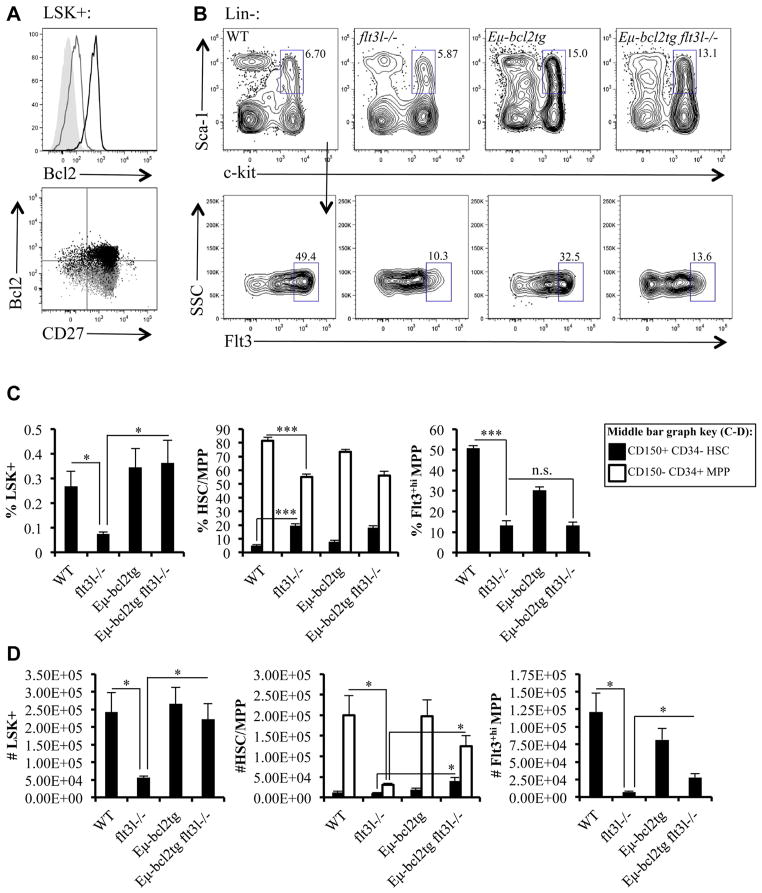
We previously reported increased percentages of Annexin V+ Flt3+hi MPPs and decreased intracellular levels of the prosurvival protein Mcl-1 in flt3l−/− mice compared with their wild type (WT) counterparts [3]. To further elucidate the role of Flt3 signaling in hematopoietic progenitor survival in vivo, we generated Eμ-bcl2tg flt3l−/− mice. Eμ-Bcl2 line 36 transgenic mice have been described previously [19]. In these mice, the Eμ enhancer drives expression of human Bcl2, primarily in the B lineage. We previously showed that the Eμ enhancer is active in LSK+ cells [20]. FL is required from an early stage in hematopoiesis; therefore, it was important to establish that the Bcl2 transgene was expressed early enough to effect the survival of Flt3+ MPPs. As shown in Figure 1A, the Bcl2 transgene is expressed in LSK+ cells and initiates before upregulation of CD27, a marker acquired as HSC transition into MPPs (Fig. 1A) [21].
Figure 1.
Enforced expression of Bcl2 rescues LSK+ cells, but not deficiencies in Flt3+hi MPPs in flt3l−/− mice. (A) Intracellular human Bcl2 expression in LSK+ cells in Eμ-bcl2tg mice. The filled histogram represents the unstained control, the gray line represents the isotype control, and the black line represents Bcl2 staining (top panel). Staining of WT B6 mice with the human Bcl-2 antibody gave identical staining pattern to the isotype control. The bottom panel depicts human Bcl2 versus CD27 staining. The overlaid gray and black dot plots represent isotype control and Bcl2 staining, respectively. Data are representative of three Eμ-bcl2tg mice and three independent experiments. (B) Flow cytometric analysis of LSK+ (pregated on Lin−, top panels) from a representative WT, flt3l−/−, Eμ-bcl2tg, and Eμ-bcl2tg flt3l−/− mouse further stained to examine Flt3 expression (bottom panels). (C, D) Bar graphs illustrating the frequency (C) and numbers (D) of LSK+, CD150+CD34− HSCs (black bars) and CD150−CD34+ MPPs (white bars) within LSK+ (middle bar graphs) and Flt3+hi MPPs within LSK+ across the four genotypes. Supplementary Figure E1 (online only, available at www.exphem.org) displays HSC and MPP gating strategy. Data are representative of four or five mice per genotype and four independent experiments (B–D). Error bars represent mean ± SEM. *p ≤ 0.05, ***p < 0.0001, Student t test, between the means of different genotypes. n.s. = nonsignificant differences between genotypes measured using Student t test at p > 0.05.
Bone marrow (BM) cellularity is reduced in FL-deficient mice, and this was not significantly altered by expression of a Bcl2 transgene (Table 1). Percentages and absolute cell numbers of LSK+ cells are significantly reduced in flt3l−/− mice, and this deficiency was restored by expression of the Bcl2 transgene (Fig. 1B, C, and D). MPPs can be discriminated from HSCs within the LSK+ compartment by differential expression of CD150 and CD34. HSCs are LSK+CD150+CD34− and MPPs are LSK+CD150−CD34+ (Supplementary Figure E1, online only, available at www.exphem.org) [22,23]. Consistent with our previous report, percentages of LSK+CD150+CD34− HSCs are increased in flt3l−/− mice, and this is not altered by expression of the Bcl2 transgene (Fig. 1C, D, and Supplementary Figure E1) [24]. Indeed, the most significantly upregulated subset of LSK+ cells affected by expression of Bcl2 are LSK+CD150−CD34+ MPPs (Fig. 1D). Although forced expression of the Bcl2 transgene corrected the flt3l−/− deficiency in LSK+ cells, it did not restore frequencies of Flt3+hi MPPs enriched for lymphoid-biased progenitors (Fig. 1B and C).
Table 1.
Bone marrow cellularitya
| Miceb | No. of BM cells (×107)c |
|---|---|
| Wild type | 9.5 ± 0.4 |
| ftl3l−/− | 7.5 ± 0.4 |
| Eμ-bcl2tg | 8.1 ± 0.5 |
| Eμ-bcl2tg ftl3l−/− | 7.3 ± 0.5 |
Cell counts taken from four hind leg bones per mouse.
n = 7–11 mice per genotype.
Mean ± SEM.
Due to the impact of the Bcl2 transgene on the LSK+ compartment, we observed statistically significant increases in absolute numbers of LSK+CD150−CD34+ MPPs, Flt3+hi MPPs, and LSK+CD150+CD34− HSCs, in the Eμ-bcl2tg flt3l−/− mice compared with flt3l−/− mice (LSK+CD150−CD34+ MPPs: 1.24 × 105 ± 2.60 × 104 vs. 3.12 × 104 ± 3.42 × 103, p = 0.012; Flt3+hi MPPs: 2.79 × 104 ± 4.97 × 103 vs. 7.29 × 103 ± 1.04 × 103, p = 0.0066; LSK+CD150+CD34− HSCs: 3.96 × 104 ± 8.25 × 103 vs. 1.09 × 104 ± 9.00 × 102, p = 0.013; Fig. 1D). However, the Bcl2 transgene did not alter Flt3 receptor density on LSK+ of Eμ-bcl2tg flt3l−/− mice (Fig. 1B). Consequently, the Flt3+hi MPP subset was not restored. To determine whether upregulation of Flt3 is important for lymphoid priming, we crossed the Eμ-bcl2tg flt3l−/− mice to rag1-gfp+ mice. RAG1 expression is a hallmark of lymphoid priming [25]. rag1-gfp+ mice have GFP knocked into the RAG1 locus, facilitating tracking of RAG1 induction via GFP expression. In agreement with our previous study, flt3l−/− rag1-gfp+ mice had a severe reduction in frequencies of GFP+ cells in the Flt3+hi MPP subset (Table 2) [3]. Strikingly, the percentage of GFP+ cells in the Flt3+hi MPP subset from Eμ-bcl2tg flt3l−/− rag1-gfp+ was similar to the flt3l−/− rag1-gfp+ mice (Table 2). Thus, forced expression of a survival protein rescues the LSK+ deficiency, but not the immunophenotypic Flt3+hi MPP subset in flt3l−/− mice or lymphoid priming in the LSK+ compartment.
Table 2.
Frequencies of RAG1-GFP+ in hematopoietic progenitor populationsa
| Miceb | Flt3+hi MPPc | ALP | BLP | Pro B | Naïve Bd | Mature Bd |
|---|---|---|---|---|---|---|
| WT GFP | 14.1 ± 3.2 | 58.1 ± 7.0 | 83.0 ± 3.5 | 94.0 ± 0.5 | 83.7 ± 0.5 | 16.3 ± 0.5 |
| flt3l−/− GFP | 2.2 ± 0.4 | 20.1 ± 0.6 | 40.2 ± 2.7 | 81.8 ± 0.9 | 61.9 ± 4.6 | 38.2 ± 4.6 |
| bcl2tg GFP | 7.8 ± 2.8 | 40.4 ± 4.2 | 81.6 ± 5.2 | 94.8 ± 3.5 | 73.8 ± 2.0 | 26.2 ± 2.0 |
| bcl2tg flt3l−/− GFP | 1.7 ± 0.8 | 18.5 ± 0.3 | 38.6 ± 10.0 | 92.7 ± 1.8 | 62.7 ± 6.6 | 37.4 ± 6.6 |
Frequencies reflect mean ± SEM of two independent experiments.
n = 2 mice per genotype.
Flt3+hi multipotential progenitor defined as LSK+Flt3+hi.
Naive B cells are CD19+IgM+GFP+ whereas mature B cells are CD19+IgM+GFP−.
Enforced expression of Bcl2 partially rescues Flt3+ CLPs in flt3l−/− mice
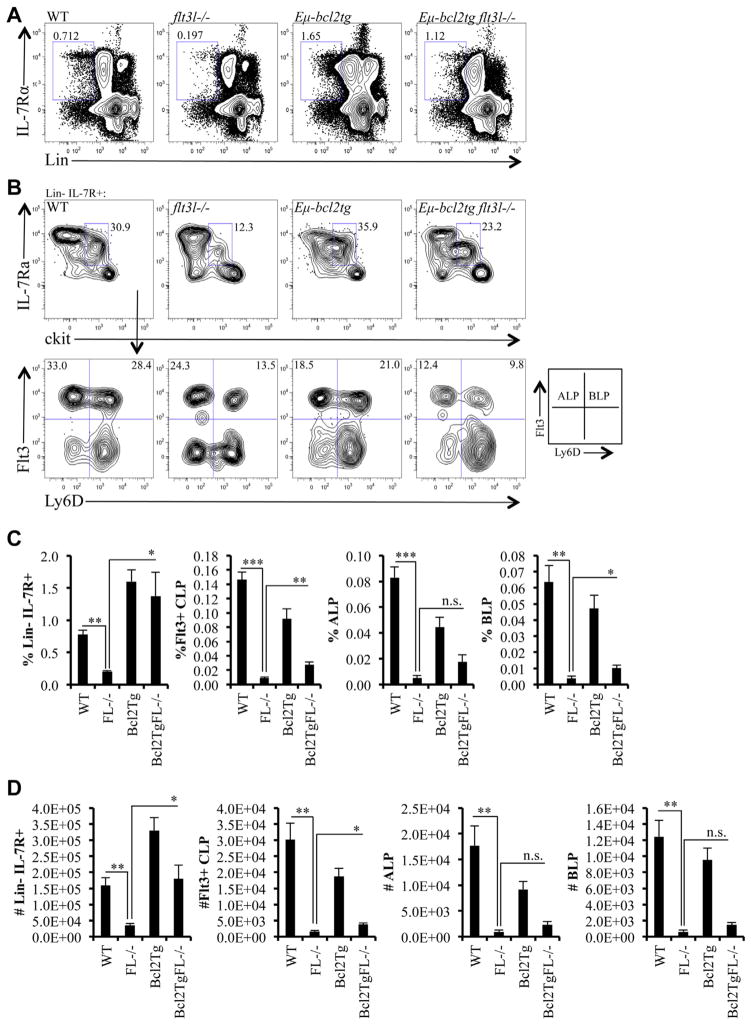
LSK+ cells that express high levels of Flt3 have increased abundance of lymphoid lineage-associated transcripts, including il7ra, compared to LSK+ cells expressing lower levels of Flt3 [6]. Flt3hi LSK+ cells are the presumed precursors of Flt3+ CLPs, the lymphoid-restricted progenitor subset that gives rise to BCPs [26]. To determine whether upregulation of Flt3 in LSK+ cells is an obligate step in the generation of Flt3+ CLPs, we examined the Flt3+ CLP compartment in Eμ-bcl2tg flt3l−/− mice. Consistent with previous findings, percentages and numbers of Lin−IL7R+ CLPs were significantly reduced in flt3l−/− mice (Fig. 2A) [1,3]. In contrast, frequencies and absolute numbers of Lin− IL-7R+ CLPs in Eμ-bcl2tg flt3l−/− mice were comparable to controls (Fig. 2A, C, and D). Numbers of Flt3+ CLPs increased 2.4-fold in the Eμ-bcl2tg flt3l−/− mice compared with flt3l−/− mice (3.74 × 103 ± 4.69 × 102 vs. 1.57 × 103 ± 4.04 × 102, p = 0.013; Fig. 2D). Flt3+ CLPs can be fractionated into Flt3+ Ly6D− ALPs and Flt3+ Ly6D+ BLPs that we and others have shown have upregulated expression of combinations of genes that accompany B cell fate specification and commitment [7,27]. Percentages of ALPs and BLPs were both increased approximately twofold to threefold in Eμ-bcl2tg flt3l−/− mice, compared with flt3l−/− mice (ALP: 0.017 ± 0.006 vs. 0.005 ± 0.002, p = 0.079; BLP: 0.010 ± 0.002 vs. 0.004 ± 0.001, p = 0.029; Fig. 2C). Similarly, numbers of ALPs and BLPs were elevated twofold to threefold in the Eμ-bcl2tg flt3l−/− mice compared with flt3l−/− mice (ALP: 2.30 × 103 ± 6.20 × 102 vs. 9.48 × 102 ± 3.44 × 102, p = 0.10; BLP: 1.44 × 103 ± 2.99 × 102 vs. 6.18 × 102 ± 2.04 × 102, p = 0.063; Fig. 2D). Although the increases in ALP and BLP did not reach statistical significance, they do reflect a similar proportional increase (approximately threefold) that we observed in Flt3+hi MPPs in the Eμ-bcl2tg flt3l−/− mice. To determine whether Flt3+ CLP subsets in the Eμ-bcl2tg flt3l−/− mice exhibited lymphoid priming compared with flt3l−/− mice, we compared the frequencies of GFP+ ALPs and GFP+ BLPs in Eμ-bcl2tg flt3l−/− rag1-gfp+ to flt3l−/− rag1-gfp+ mice. Similar to findings observed in GFP+ Flt3+hi MPPs, percentages of GFP+ cells in ALPs or BLPs were comparable in these two mice (Table 2). These data show that forced expression of a survival protein restores IL-7R+ CLPs and partially restores Flt3+ CLP subsets in flt3l−/− mice, but does not alter lymphoid priming in the ALP or BLP compartments.
Figure 2.
Enforced expression of Bcl2 rescues CLPs, but not the deficiency in Flt3+ CLPs in flt3l−/− mice. (A) Flow cytometric analysis of Lin−IL-7Rα+ from a representative WT, flt3l−/−, Eμ-bcl2tg, and Eμ-bcl2tg flt3l−/− mouse. (B) c-kitlo IL-7Rα+ CLP compartment (pregated on Lin−IL-7Rα+, top panels) across the four genotypes further stained with Ly6D and Flt3 to examine Ly6D− ALPs and Ly6D+ BLPs (bottom panels). (C, D) Bar graphs illustrating the frequency (C) and numbers (D) of Lin−IL-7Rα+, Flt3+CLPs, ALPs, and BLPs across the four genotypes. Data are representative of four to seven mice per genotype and four to five independent experiments. Error bars represent mean ± SEM. *p ≤ 0.05, **p ≤ 0.005, ***p < 0.0001, Student’s t-test at, between the means of different genotypes. n.s. = nonsignificant differences between genotypes measured using Student t test at p > 0.05.
Enforced expression of Bcl2 restores BCP in flt3l−/− mice
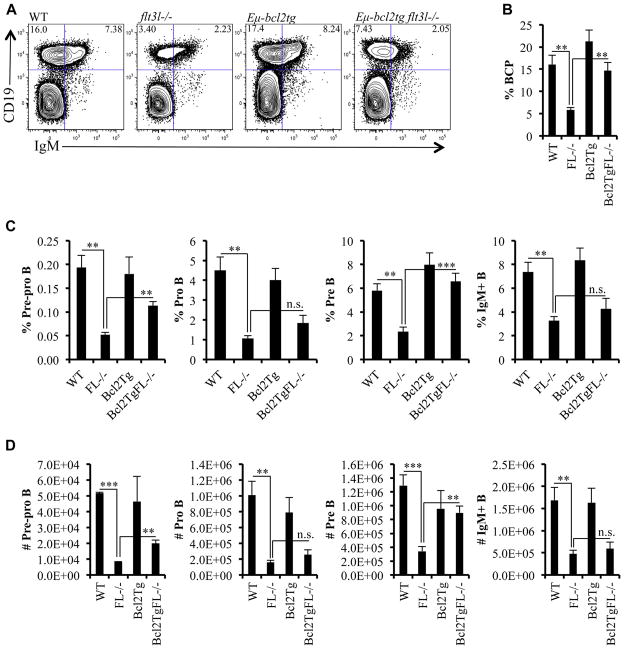
The B lineage–restricted progeny of Flt3+ BLPs are B220+CD19−Ly6D+PDCA1− Pre-pro B, CD19+B220+ CD43+loIgM− Pro-B, CD19+B220+CD43−IgM− Pre-B, and CD19+IgM+ B cells. Flt3l−/− mice have significant reductions in all B lineage progeny (Fig. 3) [3,27]. Bcl2 can functionally substitute for Mcl-1 deficiency in Pro-B cell lines [28]. Furthermore, the same study showed a Bcl2 transgene partially restored numbers of BCP in IL-7R−/− mice. Therefore, we examined whether forced expression of Bcl2 corrected the B lineage deficiencies in flt3l−/− mice.
Figure 3.
Enforced expression of Bcl2 restored BCP in flt3l−/− mice. (A) Flow cytometric analysis of BCPs (CD19+IgM−) and CD19+IgM+ B cells from a representative WT, flt3l−/−, Eμ-bcl2tg, and Eμ-bcl2tg flt3l−/− mouse. (B) Bar graph showing the frequency of BCPs across the four genotypes. (C, D) Bar graphs illustrating the frequency (C) and numbers (D) of pre-pro B (gated on B220+CD19−AA4.1+Ly6D+PDCA-1−), pro B, pre B, and IgM+ B cells across the four genotypes. Supplementary Figure E2 (online only, available at www.exphem.org) displays pro B and pre B gating strategy. With the exception of the pre-pro B cell stain (three mice per genotype and two independent experiments), data are representative of six to nine mice per genotype and five independent experiments. Error bars represent mean ± SEM. **p ≤ 0.005, ***p<0.0001, Student t test, between the means of different genotypes. n.s. = nonsignificant differences between genotypes measured using Student t test at p > 0.05.
Pre-pro B cells are discriminated from BLP by surface expression of CD45R/B220 [27]. Forced expression of the Bcl2 transgene partially restored percentages and absolute numbers of Pre-pro B cells in flt3l−/− mice (Fig. 3C and D). CD19+B220+CD43+loIgM− Pro-B cells were also increased, but the increase was not statistically significant (Fig. 3C, D, and Supplementary Figure E2 [online only, available at www.exphem.org]). In contrast to the severe decreases in percentages of GFP+ cells within Flt3+hi MPP, ALP, and BLP in the flt3l−/− rag1-gfp+ mice, regardless of expression of the Bcl2 transgene, the frequency of Pro B cells from Eμ-bcl2tg flt3l−/− rag1-gfp+ was similar to that from rag1-gfp+ control mice (Table 2). Pre-B defined as CD19+ B220+ CD43−IgM− were significantly increased in Eμbcl2tg flt3l−/− mice (Fig. 3C, D, and Supplementary Figure E2). Interestingly, mature B cells in the marrow, identified as CD19+ IgM+, which includes naive and recirculating B cells, were not substantially altered by expression of the Bcl2 transgene. Naive B cells can be discriminated from mature B cells within the CD19+IgM+ subset by GFP expression. As Table 2 illustrates, the decrease in GFP+ naive B cells in the flt3l−/− rag1-gfp+ mice was not altered by expression of the Bcl2 transgene.
To determine whether the decrease in IgM+ B cells in the marrow mirrored the peripheral B cell pool, spleens were harvested from WT, flt3l−/−, Eμ-bcl2tg, Eμ-bcl2tg flt3l−/−, and numbers of IgM+ B cells were determined. As we and others reported previously, B cell numbers in the spleen are reduced in flt3l−/− mice [24,29,30]. Splenic cellularity was dramatically increased in Eμ-bcl2tg mice (data not shown) [31]. Importantly, numbers of IgM+ splenic B cells were reduced in Eμ-bcl2tg flt3l−/− compared with control Eμ-bcl2tg mice (Supplementary Figure E3, online only, available at www.exphem.org). These data suggest that FL deficiency does not affect survival pathways in peripheral B cells. Taken together, these experimental findings show that forced expression of a survival gene selectively affects developmental stage− specific B cell precursor subsets in the bone marrow of flt3l−/− mice.
Flt3 signaling is required for the expansion and maintenance of LSK+ Flt3+hi MPPs
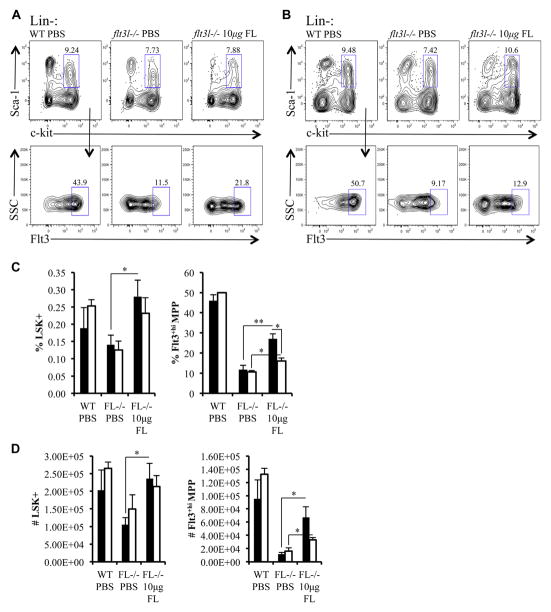
Previous studies using flt3l-deficient mice implicated Flt3 signaling in the regulation of Flt3+hi MPPs and CLPs [1,2]. In vivo administration of FL expands hematopoietic progenitors, which could reflect roles in proliferation or maintenance. Reductions in Flt3+hi MPPs or CLPs in flt3l−/− mice during homeostasis does not distinguish roles for Flt3 signaling in maintenance of Flt3+ progenitor subsets. To make these distinctions in vivo, we performed temporal FL replacement therapy. Ten micrograms of recombinant murine FL was administered to flt3l−/− mice over an 8-day period (five injections spaced 2 days apart). WT and flt3l−/− mice injected with phosphate-buffered saline (PBS) using the same injection schedule served as a control group. Two cohorts of mice were analyzed. The first set of mice was analyzed 2 days after the last injection to determine the requirement for Flt3 signaling in the expansion of Flt3+ progenitor subsets. The second set was analyzed 5 days after the final injection of FL to determine the requirement for Flt3 signaling in the maintenance of Flt3+ progenitor subsets.
Two days after cessation of FL administration, the LSK+ compartment in flt3l−/− mice remained comparable with PBS-treated WT mice (Fig. 4A, C, and D). FL administration significantly increased percentages and numbers of Flt3+hi MPPs within the LSK+ subset compared with PBS-injected flt3l−/− mice (Fig. 4A, C, and D). We noted that frequencies and numbers of Flt3+hi MPPs increased in FL injected flt3l−/− mice, but did not reach WT levels following FL injection. This result likely reflects the in vivo half-life of FL [32]. The increase in LSK+ Flt3+hi MPPs 2 days after FL administration was significantly decreased 5 days after the cessation of FL administration (Fig. 4B–D). These in vivo results support roles for Flt3 signaling in the expansion and maintenance of LSK+ Flt3+hi MPPs.
Figure 4.
Flt3 signaling is required for the expansion and maintenance of LSK+ Flt3+hi MPPs. (A, B) Flow cytometric analysis of BM taken 2 days (A) or 5 days (B) after either the last injection of PBS (control mice) or FL. LSK+ cells (pregated on Lin−, top panels) from PBS-injected WT mice (WT PBS), PBS-injected flt3l−/− mice (flt3l−/− PBS), and FL-injected flt3l−/− mice (flt3l−/− 10 μg FL) further stained to examine Flt3+hi MPP (bottom panels). (C, D) Bar graphs illustrating the frequency (C) and numbers (D) of LSK+ and Flt3+hi MPPs within LSK+ across the three conditions. The bars represent data collected 2 days (black) or 5 days (white) following cessation of PBS or FL injection schedule. Data are representative of four to six mice per genotype and two to three independent experiments. Error bars represent mean ± SEM. *p ≤ 0.05, **p ≤ 0.005, Student t test, between the means of different conditions.
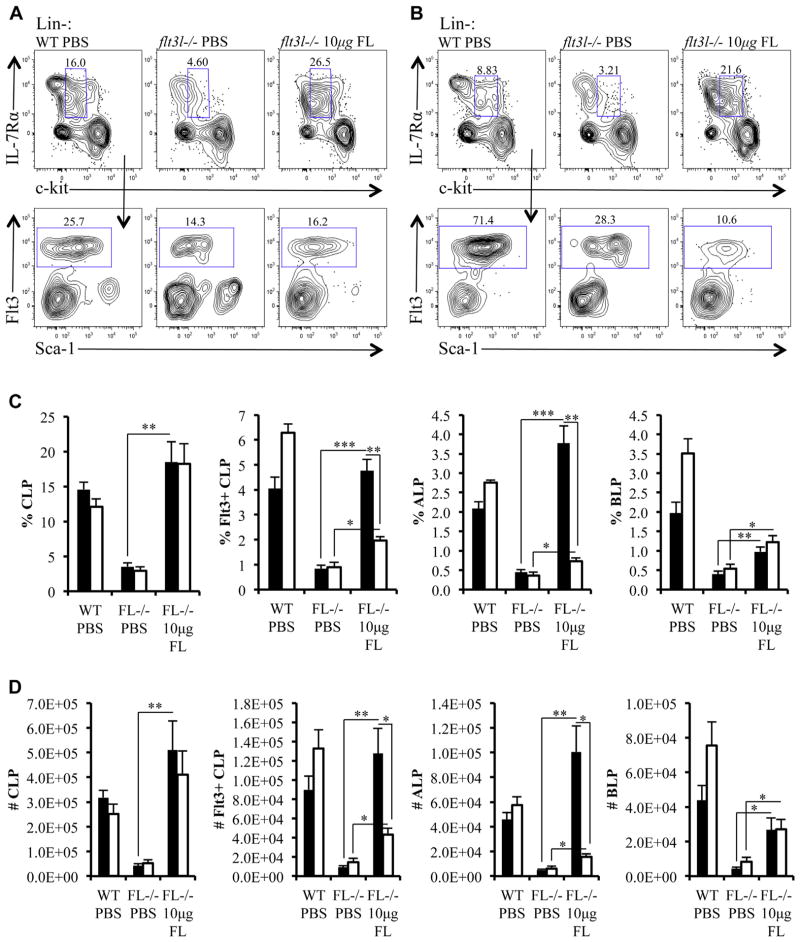
Differential requirements for Flt3 signaling in expansion and maintenance of Flt3+ CLP subsets
Next, we examined whether FL administration affected the expansion and maintenance of Flt3+ CLPs. Similar to the LSK+ subset, FL administration in flt3l−/− mice increased total Lin− IL-7Rα+ c-kit+lo CLPs and Flt3+ CLPs comparable to PBS-injected WT mice (Fig. 5A, C, and D). However, 5 days after FL administration, percentages and numbers of Flt3+ CLPs resembled that of flt3l−/− mice (Fig. 5B–D). To define FL-sensitive CLP subsets, we fractionated Flt3+ CLPs into Ly6D− ALPs and Ly6D+ BLPs. ALPs were highly sensitive to FL replacement therapy, whereas BLPs were not (Fig. 5C and D). We observed a sharp increase in ALPs 2 days after FL treatment, coupled with a decline in this population 3 days later (Fig. 5C and D). In contrast, frequencies and numbers of BLPs were more refractory to FL administration than ALPs in flt3l−/− FL-injected mice compared with PBS-injected flt3l−/− control mice (% BLP: flt3l−/− FL, 1.0 ± 0.1; flt3l−/− PBS, 0.4 ± 0.1, p = 0.0025 (to flt3l−/− PBS); no. of BLPs: flt3l−/− FL, 2.7 × 104 ± 7.0 × 103; flt3l−/− PBS, 4.3 × 103 ± 8.3 × 102, p = 0.01 (to flt3l−/− PBS; Fig. C and D). In addition, we observed that BLPs remain stable between 2 and 5 days after FL treatment (Fig. 5C and D). Overall, these data indicate that FL is critical for the expansion and maintenance of Flt3+ ALPs, but not Flt3+ BLPs.
Figure 5.
Differential requirements for Flt3 signaling in expansion and maintenance of Flt3+ CLP subsets. (A, B) Flow cytometric analysis of bone marrow taken 2 days (A) or 5 days (B) after either the last injection of PBS (control mice) or FL. Bone marrow (pregated on Lin−) is stained with antibodies against c-kit and IL-7Rα to visualize CLP compartment in PBS-injected WT mice (WT PBS), PBS-injected flt3l−/− mice (flt3l−/− PBS), and FL-injected flt3l−/− mice (flt3l−/− 10 μg FL). Flt3+ CLPs are shown (bottom panels). (C, D) Bar graphs illustrating the frequency (C) and numbers (D) of total Lin−c-kit+lo IL-7Rα+ CLPs, Flt3+ CLPs, Ly6D− ALPs, and Ly6D+ BLPs across the three conditions. The bars represent data collected 2 days (black) or 5 days (white) after cessation of PBS or FL injection schedule. Data are representative of four to six mice per genotype and two to three independent experiments. Error bars represent mean ± SEM. *p ≤ 0.05, **p ≤ 0.005, and ***p < 0.0001 Student t test, between the means of different conditions.
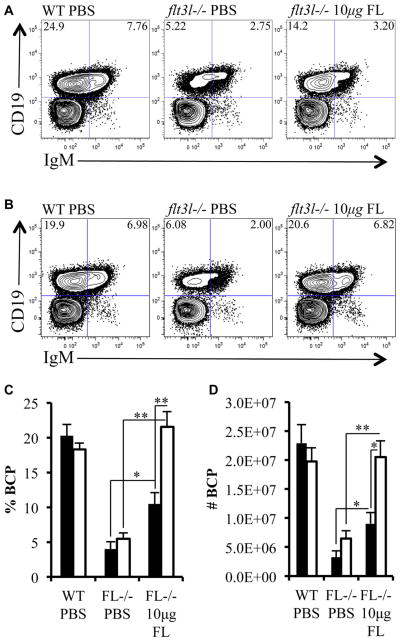
Delayed recovery of BCPs after temporal FL administration
FL replacement therapy primarily targeted Flt3+ MPPs and ALPs. BLPs, the immediate precursor of BCPs, were less sensitive to FL. This observation suggested that the temporal in vivo administration model could be informative regarding the kinetics of B cell genesis from Flt3+ hematopoietic progenitors. First, we determined whether BCPs were restored within the time frame evaluated. Two days after cessation of FL administration, BCPs were increased approximately twofold (Fig. 6A, C, and D). Interestingly, at this time point, the increase in BCPs was directly proportional to the increase in BLPs (% BCP: flt3l−/− FL, 10 ± 2; flt3l−/− PBS, 4 ± 1, p = 0.0075 (to flt3l−/− PBS); no. of BCPs: flt3l−/− FL, 9.0 × 106 ± 1.9 × 106; flt3l−/− PBS, 3.3 × 106 ± 1.1 × 106, p = 0.025 (to flt3l−/− PBS; Fig. 6A, C, and D). These data are consistent with BLPs being the immediate precursor of BCPs. In contrast, 5 days after cessation of FL, the BCP compartment was fully restored in flt3l−/− mice (Fig. 6B–D). The complete restoration of BCPs five days after cessation of FL administration suggests that approximately 72 hours is sufficient for ALPs to differentiate into CD19+IgM− BCPs.
Figure 6.
Delayed recovery of BCPs after temporal FL administration. (A, B) Flow cytometric analysis of bone marrow taken 2 days (A) or 5 days (B) after either the last injection of PBS (control mice) or FL. Bone marrow is stained with antibodies against CD19 and IgM to visualize BCP (CD19+IgM−) and CD19+IgM+ B cells in PBS-injected WT mice (WT PBS), PBS-injected flt3l−/− mice (flt3l−/− PBS), and FL-injected flt3l−/− mice (flt3l−/− 10μg FL). (C, D) Bar graphs illustrating the frequency (C) and numbers (D) of BCPs across the three conditions. The bars represent data collected 2 days (black) or 5 days (white) following cessation of PBS or FL injection schedule. Data are representative of four to six mice per genotype and two to three independent experiments. Error bars represent mean ± SEM. *p ≤ 0.05, **p ≤ 0.005, Student t test, between the means of different conditions.
Discussion
In this study, we sought to elucidate the roles of Flt3 signaling in the proliferation, survival, and maintenance of Flt3+ hematopoietic progenitor subsets into BCPs. We generated Eμ-bcl2tg flt3l−/− mice to determine whether Flt3 signaling is critical for the survival of Flt3+hi MPPs and Flt3+ CLPs. Forced expression of Bcl2 at the transition of HSC to MPP, the stage where Flt3 signaling initializes, restored the LSK+ and Lin−IL-7R+ CLP compartments [33,34]. Furthermore, CD19+ IgM− BCPs in Eμ-bcl2tg flt3l−/− mice were comparable to WT. Importantly, restoration of the BCP compartment by forced expression of Bcl2 was independent of lymphoid priming at the earliest stages of lymphopoiesis. Cytokines also regulate the proliferation and maintenance of developmental stage–specific hematopoietic progenitor subsets [35]. Temporal FL administration in vivo substantially expanded Flt3+hi MPPs and Flt3+ ALPs in flt3l−/− mice. However, these subsets quickly declined, establishing the importance of continuous Flt3 signaling in their maintenance. In contrast, Flt3+ BLPs were more refractory to Flt3 signaling. We conclude from these new in vivo experimental findings that Flt3 signaling is critical for the proliferation, maintenance, and survival of Flt3+ MPPs and ALPs. In contrast, BLPs that are poised to downregulate Flt3 and become BCPs are less dependent on signals from Flt3.
Forced expression of a survival gene has been shown to rescue lineage-specific developmental blocks imposed by cytokine deficiencies [36–40]. High-density Flt3 expression is commonly used to resolve lymphoid-biased MPPs (Flt3hi MPP) from MPPs with combined lymphoid-myeloid potential that express lower levels of Flt3 [2,3,5,6,41]. Interestingly, restoration of the LSK+ subset was not accompanied by upregulation of Flt3, unlinking Flt3 signaling activated survival pathways to receptor upregulation. We interpret this finding to suggest that in the absence of sufficient levels of Flt3 receptor expression necessary to activate survival pathways, lymphoid-primed MPPs are susceptible to apoptosis. Indeed, analysis of EuBcl2tg flt3l−/− rag1-gfp+ mice revealed no rescue or increase in GFP reporter expression in the LSK+ or CLP subsets. Thus, forced expression of a survival gene bypasses the requirement for threshold levels of Flt3 signaling to activate lymphoid lineage-specific survival pathways and lymphoid priming. Importantly, IL-7R, once expressed, can largely compensate for reduced Flt3-regulated survival [28]. B cell differentiation does not proceed past the CLP stage in mice deficient for IL-7R signaling underscoring the importance of this signaling pathway in B lymphopoiesis [9,27,42]. The BCP compartment in Eμ-bcl2tg flt3l−/− mice was largely comparable to WT controls; thus, IL-7R signaling, coupled with forced expression of a survival gene, can bypass the requirement for threshold levels of Flt3 signaling in the generation of BCP from residual Flt3+ CLPs.
The restoration of numbers of select hematopoietic progenitor subsets that we document in this study strongly supports a role for Flt3 signaling in the regulation of cell survival pathways. However, the restoration for all lymphoid–B lineage subsets downstream of the LSK+ compartment was partial. In addition to regulating cell survival, Bcl2 transgene expression reduces the proliferation of B and T lineage lymphocytes [43,44]. Indeed, a previous study showed that overexpression of Bcl2 reduces the proliferation of all cycling B lymphocytes [44]. Furthermore, Bcl2 overexpression retards the transition between quiescent and cycling states regardless of differentiation state. The effect of Bcl2 overexpression on proliferation provides an explanation for why we document only a partial rescue of Flt3+IL-7R+ CLPs and BCP subsets in Eμ-bcl2tg flt3l−/− mice.
This study determined that Flt3 signaling is essential for the expansion and maintenance of Flt3+hi MPPs and Flt3+ ALPs. Temporal FL administration to flt3l−/− mice selectively expanded Flt3+hi MPPs and Flt3+ ALPs. Three days after restoration, the increases in these two subsets diminished. These data support and extend previous in vitro and in vivo findings by others that Flt3 signaling augments hematopoietic progenitor proliferation [11,12,15,45]; hhowever, to our knowledge they show for the first time, the select sensitivity of Flt3+hi MPPs and Flt3+ ALPs to Flt3 signaling. In stark contrast, Flt3+ BLPs that have initiated B cell fate specification and commitment are more refractory to Flt3 signaling [7]. Indeed, in contrast to ALPs, BLPs have detectable transcripts for the B cell commitment factor Pax5 [7]. Importantly, Pax5 is a negative regulator of flt3 transcription [46]. Thus, Pax5 modulation of flt3 in BLPs likely contributes to the differential sensitivity of BLPs to FL administration.
FL administration in flt3l−/− mice restored BCP. We note that the kinetics of restoration of the BCP pool was delayed compared with Flt3+hi MPPs and Flt3+ ALPs. We suggest that these data reflect the timing of two critical events in B lymphopoiesis regulated by Flt3 signaling. First, signaling via FL plays an active role in upregulating levels of Flt3 in MPPs. Flt3+hi MPPs are initiating immunoglobulin gene recombination, and this event is coupled to cell cycle regulation [20,25,47]. Thus, strong survival signals provided by Flt3 at this stage are likely critical to protect lymphoid progenitors until sufficient upregulation of IL-7R. Second, Flt3 signaling augments the proliferation of Flt3+hi MPPs and Flt3+ ALPs, expanding the pool of lymphoid progenitors that can generate BCP.
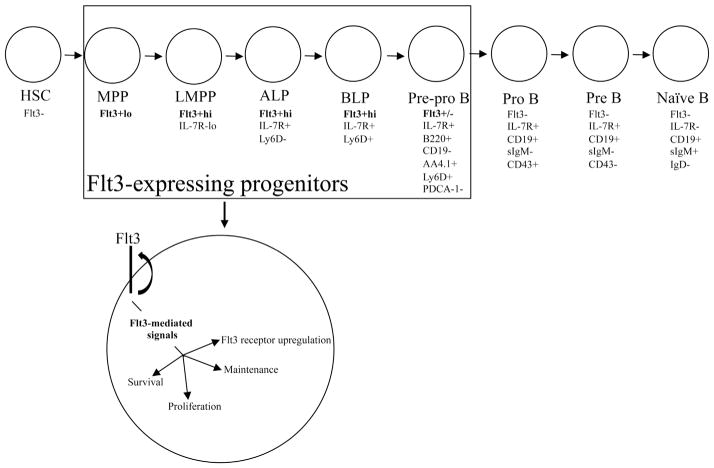
Overall, this study provides new information concerning how Flt3 signaling regulates early steps in lymphoid–B lineage development in BM (Fig. 7). We show in vivo, that Flt3 signaling is critical for the survival, expansion, and maintenance of developmental stage–specific hematopoietic progenitor subsets that give rise to BCPs. Finally, our findings suggest that FL plays an active role in upregulating and maintaining Flt3 expression in Flt3+hi MPPs, establishing an autoregulatory role for FL in regulation of Flt3 density.
Figure 7.
Model illustrating Flt3 signaling in regulating the proliferation, survival, and maintenance of Flt3+ hematopoietic progenitors. In mice, Flt3 is initially expressed at low levels in MPPs. Flt3 is upregulated in LMPP to high levels, where it remains in ALPs and BLPs until Flt3 is downregulated in pre-pro B cells and silenced at the pro B cell stage of B cell development. Once bound by its ligand, Flt3-ligand, Flt3 sends critical signals necessary for the proliferation, survival, and maintenance of Flt3-expressing hematopoietic progenitors. In addition, Flt3 signaling works in an autoregulatory manner to upregulate and maintain high levels of Flt3 receptor density, ensuring threshold levels of Flt3 signaling are present to maintain the pool of Flt3+ progenitors that give rise to B cell precursors.
Supplementary Material
Acknowledgments
We thank Virginia Smith Shapiro for helpful discussions and critical comments on the manuscript, and the Mayo Clinic Flow Cytometry Core Facility for technical expertise. This work was supported by National Heart, Lung, and Blood Institute grant R01HL096108 (to K.L.M.) and National Institutes of Health training grant T32AI07425 to the Department of Immunology (to J.J.D.)
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.exphem.2014.01.001.
Conflict of interest disclosure
No financial interest/relationships with financial interest relating to the topic of this article have been declared.
References
- 1.Sitnicka E, Bryder D, Theilgaard-Monch K, Buza-Vidas N, Adolfsson J, Jacobsen SE. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 2002;17:463–472. doi: 10.1016/s1074-7613(02)00419-3. [DOI] [PubMed] [Google Scholar]
- 2.Sitnicka E, Buza-Vidas N, Ahlenius H, et al. Critical role of FLT3 ligand in IL-7 receptor independent T lymphopoiesis and regulation of lymphoid-primed multipotent progenitors. Blood. 2007;110:2955–2964. doi: 10.1182/blood-2006-10-054726. [DOI] [PubMed] [Google Scholar]
- 3.Dolence JJ, Gwin K, Frank E, Medina KL. Threshold levels of Flt3-ligand are required for the generation and survival of lymphoid progenitors and B-cell precursors. Eur J Immunol. 2011;41:324–334. doi: 10.1002/eji.201040710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adolfsson J, Mansson R, Buza-Vidas N, et al. Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Mansson R, Hultquist A, Luc S, et al. Molecular evidence for hierarchical transcriptional lineage priming in fetal and adult stem cells and multipotent progenitors. Immunity. 2007;26:407–419. doi: 10.1016/j.immuni.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Lai AY, Kondo M. Asymmetrical lymphoid and myeloid lineage commitment in multipotent hematopoietic progenitors. J Exp Med. 2006;203:1867–1873. doi: 10.1084/jem.20060697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inlay MA, Bhattacharya D, Sahoo D, et al. Ly6d marks the earliest stage of B-cell specification and identifies the branchpoint between B-cell and T-cell development. Genes Dev. 2009;23:2376–2381. doi: 10.1101/gad.1836009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kikuchi K, Kasai H, Watanabe A, Lai AY, Kondo M. IL-7 specifies B cell fate at the common lymphoid progenitor to pre-proB transition stage by maintaining early B cell factor expression. J Immunol. 2008;181:383–392. doi: 10.4049/jimmunol.181.1.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kikuchi K, Lai AY, Hsu CL, Kondo M. IL-7 receptor signaling is necessary for stage transition in adult B cell development through up-regulation of EBF. J Exp Med. 2005;201:1197–1203. doi: 10.1084/jem.20050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsapogas P, Zandi S, Ahsberg J, et al. IL-7 mediates Ebf-1-dependent lineage restriction in early lymphoid progenitors. Blood. 2011;118:1283–1290. doi: 10.1182/blood-2011-01-332189. [DOI] [PubMed] [Google Scholar]
- 11.Ceredig R, Rauch M, Balciunaite G, Rolink AG. Increasing Flt3L availability alters composition of a novel bone marrow lymphoid progenitor compartment. Blood. 2006;108:1216–1222. doi: 10.1182/blood-2005-10-006643. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsen SE, Okkenhaug C, Myklebust J, Veiby OP, Lyman SD. The FLT3 ligand potently and directly stimulates the growth and expansion of primitive murine bone marrow progenitor cells in vitro: synergistic interactions with interleukin (IL) 11, IL-12, and other hematopoietic growth factors. J Exp Med. 1995;181:1357–1363. doi: 10.1084/jem.181.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramsfjell V, Borge OJ, Veiby OP, et al. Thrombopoietin, but not erythropoietin, directly stimulates multilineage growth of primitive murine bone marrow progenitor cells in synergy with early acting cytokines: distinct interactions with the ligands for c-kit and FLT3. Blood. 1996;88:4481–4492. [PubMed] [Google Scholar]
- 14.Veiby OP, Jacobsen FW, Cui L, Lyman SD, Jacobsen SE. The flt3 ligand promotes the survival of primitive hemopoietic progenitor cells with myeloid as well as B lymphoid potential. Suppression of apoptosis and counteraction by TNF-alpha and TGF-beta. J Immunol. 1996;157:2953–2960. [PubMed] [Google Scholar]
- 15.Yonemura Y, Ku H, Lyman SD, Ogawa M. In vitro expansion of hematopoietic progenitors and maintenance of stem cells: comparison between FLT3/FLK-2 ligand and KIT ligand. Blood. 1997;89:1915–1921. [PubMed] [Google Scholar]
- 16.Medina KL, Strasser A, Kincade PW. Estrogen influences the differentiation, proliferation, and survival of early B-lineage precursors. Blood. 2000;95:2059–2067. [PubMed] [Google Scholar]
- 17.Pongubala JM, Northrup DL, Lancki DW, et al. Transcription factor EBF restricts alternative lineage options and promotes B cell fate commitment independently of Pax5. Nat Immunol. 2008;9:203–215. doi: 10.1038/ni1555. [DOI] [PubMed] [Google Scholar]
- 18.Ikawa T, Kawamoto H, Wright LY, Murre C. Long-term cultured E2A-deficient hematopoietic progenitor cells are pluripotent. Immunity. 2004;20:349–360. doi: 10.1016/s1074-7613(04)00049-4. [DOI] [PubMed] [Google Scholar]
- 19.Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- 20.Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat Immunol. 2001;2:718–724. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- 21.Wiesmann A, Phillips RL, Mojica M, et al. Expression of CD27 on murine hematopoietic stem and progenitor cells. Immunity. 2000;12:193–199. doi: 10.1016/s1074-7613(00)80172-7. [DOI] [PubMed] [Google Scholar]
- 22.Morita Y, Ema H, Nakauchi H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. J Exp Med. 2010;207:1173–1182. doi: 10.1084/jem.20091318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson A, Laurenti E, Oser G, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 24.Gwin KA, Shapiro MB, Dolence JJ, Huang ZL, Medina KL. Hoxa9 and Flt3 signaling synergistically regulate an early checkpoint in lymphopoiesis. J Immunol. 2013;191:745–754. doi: 10.4049/jimmunol.1203294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 26.Karsunky H, Inlay MA, Serwold T, Bhattacharya D, Weissman IL. Flk2+ common lymphoid progenitors possess equivalent differentiation potential for the B and T lineages. Blood. 2008;111:5562–5570. doi: 10.1182/blood-2007-11-126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medina KL, Tangen S, Gwin K, Thapa P, Seaburg L, Shapiro VS. PDCA-1 separates plasmacytoid dendritic cells from B-cell-biased lymphoid progenitor (BLP) and Pre-pro B cells. PLoS One. 2013;8:e78408. doi: 10.1371/journal.pone.0078408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malin S, McManus S, Cobaleda C, et al. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro B cell development. Nat Immunol. 2010;11:171–179. doi: 10.1038/ni.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buza-Vidas N, Cheng M, Duarte S, Nozad H, Jacobsen SE, Sitnicka E. Crucial role of FLT3 ligand in immune reconstitution after bone marrow transplantation and high-dose chemotherapy. Blood. 2007;110:424–432. doi: 10.1182/blood-2006-09-047480. [DOI] [PubMed] [Google Scholar]
- 30.McKenna HJ, Stocking KL, Miller RE, et al. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 2000;95:3489–3497. [PubMed] [Google Scholar]
- 31.Strasser A, Whittingham S, Vaux DL, et al. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson SN, Chavez JM, Pisarev VM, et al. Delivery of Flt3 ligand (Flt3L) using a poloxamer-based formulation increases biological activity in mice. Bone Marrow Transplant. 2003;31:361–369. doi: 10.1038/sj.bmt.1703816. [DOI] [PubMed] [Google Scholar]
- 33.Boyer SW, Schroeder AV, Smith-Berdan S, Forsberg EC. All hematopoietic cells develop from hematopoietic stem cells through Flk2/Flt3-positive progenitor cells. Cell Stem Cell. 2011;9:64–73. doi: 10.1016/j.stem.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buza-Vidas N, Woll P, Hultquist A, et al. FLT3 expression initiates in fully multipotent mouse hematopoietic progenitor cells. Blood. 2011;118:1544–1548. doi: 10.1182/blood-2010-10-316232. [DOI] [PubMed] [Google Scholar]
- 35.Socolovsky M, Lodish HF, Daley GQ. Control of hematopoietic differentiation: lack of specificity in signaling by cytokine receptors. Proc Natl Acad Sci U S A. 1998;95:6573–6575. doi: 10.1073/pnas.95.12.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 37.Kondo M, Akashi K, Domen J, Sugamura K, Weissman IL. Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common gamma chain-deficient mice. Immunity. 1997;7:155–162. doi: 10.1016/s1074-7613(00)80518-x. [DOI] [PubMed] [Google Scholar]
- 38.Maraskovsky E, O’Reilly LA, Teepe M, Corcoran LM, Peschon JJ, Strasser A. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell. 1997;89:1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 39.Malin S, McManus S, Cobaleda C, et al. Role of STAT5 in controlling cell survival and immunoglobulin gene recombination during pro-B cell development. Nat Immunol. 2010;11:171–179. doi: 10.1038/ni.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kee BL, Bain G, Murre C. IL-7Ralpha and E47: independent pathways required for development of multipotent lymphoid progenitors. EMBO J. 2002;21:103–113. doi: 10.1093/emboj/21.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dias S, Mansson R, Gurbuxani S, Sigvardsson M, Kee BL. E2A proteins promote development of lymphoid-primed multipotent progenitors. Immunity. 2008;29:217–227. doi: 10.1016/j.immuni.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dias S, Silva H, Jr, Cumano A, Vieira P. Interleukin-7 is necessary to maintain the B cell potential in common lymphoid progenitors. J Exp Med. 2005;201:971–979. doi: 10.1084/jem.20042393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Reilly LA, Harris AW, Strasser A. bcl-2 transgene expression promotes survival and reduces proliferation of CD3-CD4-CD8- T cell progenitors. Int Immunol. 1997;9:1291–1301. doi: 10.1093/intimm/9.9.1291. [DOI] [PubMed] [Google Scholar]
- 44.O’Reilly LA, Harris AW, Tarlinton DM, Corcoran LM, Strasser A. Expression of a bcl-2 transgene reduces proliferation and slows turnover of developing B lymphocytes in vivo. J Immunol. 1997;159:2301–2311. [PubMed] [Google Scholar]
- 45.Wils EJ, Braakman E, Verjans GM, et al. Flt3 ligand expands lymphoid progenitors prior to recovery of thymopoiesis and accelerates T cell reconstitution after bone marrow transplantation. J Immunol. 2007;178:3551–3557. doi: 10.4049/jimmunol.178.6.3551. [DOI] [PubMed] [Google Scholar]
- 46.Holmes ML, Carotta S, Corcoran LM, Nutt SL. Repression of Flt3 by Pax5 is crucial for B-cell lineage commitment. Genes Dev. 2006;20:933–938. doi: 10.1101/gad.1396206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin WC, Desiderio S. Cell cycle regulation of V(D)J recombination-activating protein RAG-2. Proc Natl Acad Sci U S A. 1994;91:2733–2737. doi: 10.1073/pnas.91.7.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuwata N, Igarashi H, Ohmura T, Aizawa S, Sakaguchi N. Cutting edge: absence of expression of RAG1 in peritoneal B-1 cells detected by knocking into RAG1 locus with green fluorescent protein gene. J Immunol. 1999;163:6355–6359. [PubMed] [Google Scholar]
- 49.Gwin K, Frank E, Bossou A, Medina KL. Hoxa9 regulates Flt3 in lymphohematopoietic progenitors. J Immunol. 2010;185:6572–6583. doi: 10.4049/jimmunol.0904203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medina KL, Pongubala JM, Reddy KL, et al. Assembling a gene regulatory network for specification of the B cell fate. Dev Cell. 2004;7:607–617. doi: 10.1016/j.devcel.2004.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.