Abstract
The cholinergic system in the brain modulates patterns of activity involved in general arousal, attention processing, memory and consciousness. In the present study we determined the effects of selective cholinergic lesions of the medial septum area (MS) or nucleus basalis magnocellularis (NBM) on amplitude and phase characteristics of event related oscillations (EROs). A time–frequency based representation was used to determine ERO energy, phase synchronization across trials, recorded within a structure (phase lock index, PLI), and phase synchronization across trials, recorded between brain structures (phase difference lock index, PDLI), in the frontal cortex (Fctx), dorsal hippocampus (DHPC) and central amygdala (Amyg). Lesions in MS produced: (1) decreases in ERO energy in delta, theta, alpha, beta and gamma frequencies in Amyg, (2) reductions in gamma ERO energy and PLI in Fctx, (3) decreases in PDLI between the Fctx–Amyg in the theta, alpha, beta and gamma frequencies, and (4) decreases in PDLI between the DHPC–Amyg and Fctx–DHPC in the theta frequency bands. Lesions in NBM resulted in: (1) increased ERO energy in delta and theta frequency bands in Fctx, (2) reduced gamma ERO energy in Fctx and Amyg, (3) reductions in PLI in the theta, beta and gamma frequency ranges in Fctx, (4) reductions in gamma PLI in DHPC and (5) reduced beta PLI in Amyg. These studies suggest that the MS cholinergic system can alter phase synchronization between brain areas whereas the NBM cholinergic system modifies phase synchronization/phase resetting within a brain area.
Keywords: Event-related potential, Electroencephalogram, Cholinergic system, Event related oscillation, Phase lock index, Phase difference lock index
1. Introduction
The cholinergic system in the brain modulates the pattern of activity involved in general arousal, attentional processing, motivation, memory formation and consciousness (Bauer et al., 2012; Butt and Hodge, 1995; Deiana et al., 2011; Everitt and Robbins, 1997; Muir et al., 1993; Wenk, 1997; Woolf, 1996, 2006; Yener et al., 2013). Central cholinergic pathways regulate global functions that rely upon the cerebral cortex and subcortical regions (Woolf, 1991, 1996). Two groups of cholinergic neurons in the basal forebrain play a substantial role in this process, the medial septal group (medial septal nucleus and vertical diagonal band) and the nucleus basalis group (nucleus basalis, substantia innominata and horizontal diagonal band) (Wenk, 1997; Wenk et al., 1994b; Woolf, 1991; Woolf et al., 1986).
Studies investigating the role of cholinergic neurons from the basal forebrain in animals have demonstrated that excitotoxic lesions can produce behavioral impairments (see Baxter and Bucci (2013) for review). Highly selective 192 IgG-saporin lesions to the medial septum and vertical limb of the diagonal band or bilaterally into the nucleus basalis magnocellularis and substantia innominata have been found to produce mild impairments in performance of a memory task in rats (Baxter et al., 2013). Cholinergic hypofunction has also been related to a number of cognitive disorders in humans including: the progressing memory deficits associated with aging, Alzheimer’s disease (AD), Parkinson’s disease, Down-syndrome, progressive supranuclear palsy, Jakob–Creutzfeld disease, Korsakoff’s syndrome and traumatic brain injury (for review see Niewiadomska et al. (2009), Schliebs and Arendt (2011), Woolf (2006) and Woolf and Butcher, (2011)). Activity of the enzyme choline acetyl transferase (ChAT) is typically used as a marker for the loss of cholinergic neurons (Wenk et al., 1994a, 1994b). Although it has been suggested in AD that post-mortem assays of ChAT might not be representative of the extracellular levels of Ach and cholinergic neural activity, there have been significant validation of the functional loss of cholinergic neural activity using several bio-markers such as structural/amyloid imaging, cerebrospinal fluid measurements, and glucose positron emission tomography (Frings et al., 2013; Wirth et al., 2013). However, there is still a need for a functional biomarker that would reflect the changes in brain dynamics that might be associated with cholinergic hypofunction. Recently spontaneous electroencephalography (EEG), sensory-evoked oscillations, and event-related oscillations (EROs) have emerged as potential functional biomarkers for neuropsychiatric diseases in disorders such as: attention deficit hyperactivity disorder, Alzheimer’s disease, bipolar disorder, schizophrenia (for review see Basar et al. (2013) and Yener and Basar (2013a)), and alcoholism (Andrew and Fein, 2010; Criado and Ehlers, 2009, 2010a, 2010b; Ehlers et al., 2010, 2012; Kovacevic et al., 2012; Rangaswamy and Porjesz, 2008). Thus these electrophysiological measures may also be capable of indexing hypofunction of the cholinergic system.
The generation of cortical event-related potentials (ERPs) in the rat has been previously demonstrated to involve cholinergic innervation (Pirch et al., 1986). We have also previously shown that lesions of the nucleus basalis magnocellularis (NBM) induce changes in several components of ERPs elicited by an auditory discrimination task, especially the amplitude of N1 and P2 components recorded in the frontal cortex and amygdala respectively (Ehlers et al., 1998; Robledo et al., 1998). The present study extended our initial analyses of neurophysiological endophenotypes observed in measures of ERPs after NBM lesions in rats, to the evaluation of event-related oscillations.
The stimuli that evoke ERPs components influence oscillatory changes within the dynamics of ongoing EEG rhythms (Basar-Eroglu et al., 1991; Demiralp and Ademoglu, 2001; Ehlers et al., 2012; Karakas et al., 2000a, 2000b; Schurmann and Basar, 2001; Yordanova et al., 2002), and this synchronization or enhancement of ongoing EEG oscillations by a time locked cognitive and/or sensory process is termed event-related oscillations (Basar et al., 2000; Begleiter and Porjesz, 2006; Roach and Mathalon, 2008). EROs are typically estimated by a decomposition of the EEG signal into phase and magnitude information for a range of frequencies and then changes in those frequencies are characterized with respect to their energy (amplitude) and phase relationships over a millisecond time scale with respect to task events (Ehlers et al., 1994). EROs have been demonstrated to be sensitive measures of both normal (Basar et al., 1999; Gevins, 1998) and abnormal cognitive functioning (Begleiter and Porjesz, 2006; Criado and Ehlers, 2009, 2010b; Ehlers et al., 2012; Porjesz and Begleiter, 2003).
The purpose of the present study was to examine whether selective cholinergic lesions of the medial septum area (MS) or the nucleus basalis magnocellularis (NBM) influence the amplitude and phase characteristics of ERO oscillatory activity in the delta, theta, alpha, beta and gamma frequency bands in the frontal cortex, DHPC and central amygdala elicited passively with an acoustic oddball paradigm in adult rats.
2. Results
2.1. MS-lesion induces lower ChAT activity in dorsal hippocampus and NBM-lesion induces lower ChAT activity in frontal cortex
Choline acetyltransferase (ChAT) activity (nmoles Ach/hr/mg prot) was measured in the frontal cortex and dorsal hippocampus from sham operated, MS-lesion and NBM-lesion rats. Results of those determinations were analyzed using a two way Analysis of Variance (ANOVA) that revealed a significant main effect of group (sham, MS-lesion and NBM-lesion) (F=4.33, p=0.01), region (frontal cortex, dorsal hippocampus) (F=18.71, p<0.001), and a group × region interaction (F=4.59, p=0.01). Post-hoc analyses revealed significant differences (Tukey LSD p<0.05) between sham and lesion groups, but there were no significant differences observed between MS-sham and NBM-sham groups. Therefore, for all subsequent analyses, MS sham and NB sham groups were collapsed into a common sham group.
Compared to the sham group, the MS-lesion was not found to produce significant changes in frontal cortex ChAT activity (8.69±5.66%; p=0.878, sham 47.84±2.89 vs. MS-lesion 43.68±2.7 nmoles Ach/h/mg prot) whereas it did induce a significant reduction in DHPC ChAT activity (29.4±5.72%; p<0.001, sham 59.29±3.43 vs. MS-lesion 41.87±3.39 nmoles Ach/h/mg prot). Compared to the sham group, the NBM-lesion was found to significantly reduce cortical ChAT activity (27.08±4.31%; p=0.002, sham 47.84±2.89 vs. NBM lesion 34.88±2.06 nmoles Ach/h/mg prot) but did not produce a significant change in DHPC ChAT activity (7.85±3.68%; p=0.641, sham 59.29±3.43 vs. NBM-lesion 54.64±2.17 nmoles Ach/h/mg prot). These results indicate that compared to the sham group the MS-lesion induced a significant reduction in hippocampal ChAT activity (Tukey post-hoc, p<0.01) whereas, the NBM-lesion induced a significant reduction in frontal cortex ChAT activity (Tukey post-hoc, p<0.01).
2.2. Rare (infrequent) tones have higher PLI than standard (frequent) tones
A two-way ANOVA was use to determine if the values for phase locking index (PLI) for the three electrode locations in the rat (Frontal cortex (FCTX), dorsal hippocampus (DHPC), amygdala (AMYG)) were higher following the rare (infrequent) tone as compared to the standard (frequent) tone within the ROI frequencies and time intervals under sham, MS-lesion and NBM-lesion conditions. When collapsing all bands across frontal cortex, DHPC and amygdala (Fig. 1a) there was a significant main effects of tone (F=105.68, p<0.0001), lesion (F=8.81, p=0.0004), although tone × lesion interaction was not significant (F=1.26, p=0.28). Post-hoc pairwise comparisons (Fig. 1a) showed higher PLI in response to rare tones (infrequent tone) compared to standard tones (frequent tone) (Tukey post-hoc, p<0.01). Thus, to reduce multiple comparisons EROs to the rare tones were used for all subsequent analyses.
Fig. 1.
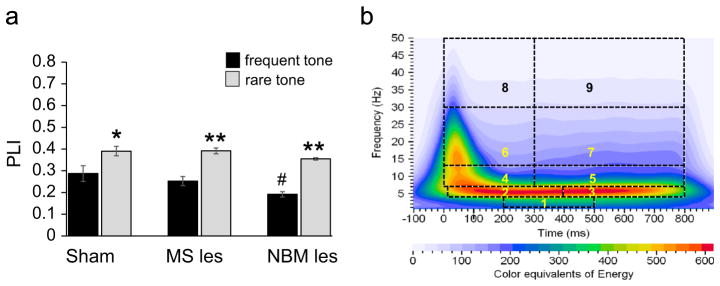
(a) Grand mean values for the phase locking index (PLI) of event-related oscillations in sham operated, MS- and NBM-lesion rats. ANOVA revealed that the rare (infrequent) tone (gray bars), as compared to the standard (frequent) tone (black bars) produce significant increase in phase locking in ROI. (b) schematic representations of ROI: ROI1 (delta band, 1–4 Hz, 200–500 ms), ROI2 (theta band, 4–7 Hz, 10–400 ms), ROI3 (theta band, 4–7 Hz, 400–800 ms), ROI4 (alpha band, 7–13 Hz, 0–300 ms), ROI5 (alpha band, 7–13 Hz, 300–800 ms), ROI6 (beta band, 13–30 Hz, 0–300 ms), ROI7 (beta band13–30 Hz, 300–800 ms), ROI8 (gamma band, 30–50 Hz, 0–300 ms), and ROI9 (gamma band, 30–50 Hz, 300–800 ms). Post-hoc Tukey pairwise comparisons indicate the following: frequent tone vs. rare tone (*p<0.05) in sham operated group; frequent tone vs. rare tone (**p<0.01) in septal lesion (MS-les) and NBM lesion (NBM-les) groups. Pairwise comparisons using frequent tone indicate significant reduction in the NBM lesion group (#p<0.05, sham vs. NBM lesion).
2.3. MS-lesion induced changes in ERO energy associated to phase synchronization between frontal cortex and amygdala and between dorsal hippocampus and amygdala
A two way ANOVA with ERO energy as a dependent variable, and brain region (hippocampus, frontal cortex, amygdala) and lesion (sham, NBM lesion, MS lesion) as independent variables, revealed significant main effects of brain region (F=22, p<0.0001) and lesion (F=4.84, p=0.0096), on ERO energy, although the brain region × lesion interaction was not significant (F=1.18, p=0.32). Grand averages of the color equivalent of energy values for the entire group of subjects (n=42) including sham operated, MS-lesion and NBM-lesion rats are presented in Fig. 2. Compared to sham operated rats, lesions in MS and NBM decreased ERO energy in frontal cortex in the gamma frequency band in the 0–300 ms time interval (Fig. 3a), but did not cause a significant effect in DHPC in any of the frequency bands and time intervals (Fig. 3b). However the most significant effects were observed in the amygdala in all frequency bands (see Fig. 3c). Compared to sham operated rats, MS lesions significantly reduced ERO energy in the delta frequencies in the 200–500 ms time interval (F=3.03, df=2,39, p=0.05, post-hoc p<0.01), in theta frequencies in the 10–400 ms time interval (F=6.09, df=2,39. p<0.005, post-hoc p<0.01), in theta frequencies in the 400–800 ms time interval (F=7.42, df=2,39, p<0.01, post-hoc p<0.01), and in alpha (F=5.31, df=2,39, p<0.01, post-hoc p<0.05), beta (F=3.85, df=2,39, p<0.03, post-hoc p<0.05) and gamma frequency bands (F=16.39, df=2,39, p<0.001, post-hoc p<0.01) in the 0–300 ms time interval, and, alpha (F=6.75, df=2,39, p<0.01, post-hoc p<0.01), beta (F=6.77, df=2,39, p<0.01, post-hoc p<0.01) and gamma frequency bands (F=6.14, df=2,39, p<0.01, post-hoc p<0.01) in the 300–800 ms time interval.
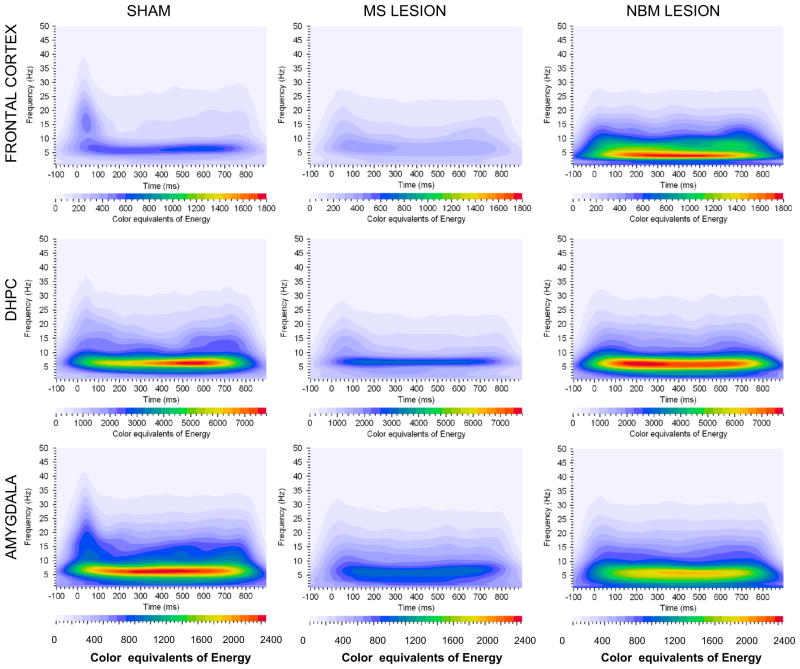
Fig. 2.
Grand averages of event related oscillations energy color equivalent for sham-operated, MS-lesion (MS les) and NBM-lesion (NBM les). Each graph depicts a time–frequency representation of ERO energy values in the delta, theta, alpha, beta and gamma bands following the rare tone in Frontal cortex, dorsal hippocampus (DHPC) and Amygdala electrode locations. In each graph frequency (Hz) is presented on the Y-axis, time regions of interest on the X-axis (ms) and ERO energy is presented as color equivalents of energy as indicated in the color bar at the bottom of each graph. NBM-lesion produced increases in color equivalents in frontal cortex (color equivalents in sham and MS les was adjusted to NBM les scale for visual purposes) and MS-lesion produced decreases in color equivalents in amygdala (color equivalents in NBM les and MS les was adjusted sham scale for visual purposes).
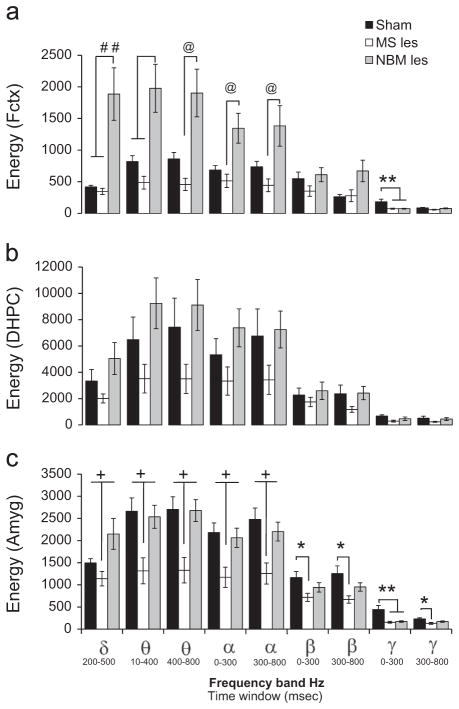
Fig. 3.
MS-lesion reduces ERO energy in amygdala and NBM-lesion increases ERO energy in Frontal cortex. Grand mean values for the event-related oscillations energy equivalents in sham operated (black bars), MS-lesion (white bars) and NBM-lesion (gray bars) rats for the rare tone in ROI1 (delta band,1–4 Hz, 200–500 ms), ROI2 (theta band, 4–7 Hz, 10–400 ms), ROI3 (theta band, 4–7 Hz, 400–800 ms), ROI4 (alpha band, 7–13 Hz, 0–300 ms), ROI5 (alpha band, 7–13 Hz, 300–800 ms), ROI6 (beta band, 13–30 Hz, 0–300 ms), ROI7 (beta band, 13–30 Hz, 300–800 ms), ROI8 (gamma band, 30–50 Hz, 0–300 ms), and ROI9 (gamma band, 30–50 Hz, 300–800 ms). Energy was calculated in: (a) the frontal cortex (Fctx), (b) dorsal hippocampus (DHPC), and (c) amygdala (Amyg). Compared to sham lesions, lesions in MS decreased ERO energy in frontal cortex only in the gamma frequency band (ROI8, see (a)) and reduced the ERO energy in the theta (ROI2, ROI3), alpha (ROI4, ROI5), beta (ROI6, ROI7), and gamma (ROI8, ROI9), frequency bands in amygdala (see (c)). Compared to sham lesions, lesions in NBM significantly increased ERO energy in frontal cortex in the delta (ROI1), and theta (ROI2) frequency bands and significantly reduced ERO energy in the gamma (ROI8) frequency band (see (a)); in addition, lesions in NBM induced a reduction in ERO energy in the gamma (ROI8) frequencies in the amygdala (see (c)). ERO energy in the alpha frequency band was significantly increased when the NBM lesion group was compared to MS lesion group but not to the sham operated controls (see (a)). Post-hoc Tukey pairwise comparisons indicate the following: sham vs. MS–les (*p<0.05), sham vs. MS-les and NBM–les (**p<0.05), MS-les vs. sham and NBM=les (+p<0.05), NBM-les vs. sham and MS-les (#p<0.05) and NBM–les vs. MS-les (@p<0.05).
Compared to sham operated rats, lesions in NBM significantly increased ERO energy in frontal cortex in the delta frequencies in the 200–500 ms time interval (F=7.12, df=2,39, p<0.01, post-hoc p<0.01), in theta frequencies in the 10–400 ms time interval (F=6.56, df=2,39, p<0.01, post-hoc p<0.05) and significantly reduced ERO energy in the gamma frequency band (F=13.19, df=2,39, p<0.001, post-hoc p<0.01) in the 0–300 ms time interval. ERO energy in frontal cortex in the alpha frequency band was significantly increased in the NBM lesion group when it was compared to MS lesion group but not in comparison to the sham operated controls (F=4.9, df=2,39, p<0.05, post-hoc p<0.05, see Fig. 3a) in the 0–300 ms and 300–800ms (F=3.41, df=2,39, p<0.05, post-hoc p<0.05) time interval and in theta frequencies in the 400–800 ms time interval (F=6.04, df=2,39, p<0.001, post-hoc p<0.01). The NBM lesion was not found to produce significant effects in the DHPC in any of the frequency bands (see Fig. 3b). The effect of NBM lesions on ERO energy in amygdala was restricted only to the gamma frequency band (F=16.39, df=2,39, p<0.001, post-hoc p<0.01, Fig. 3c) in the 0–300 ms time interval.
Two-way ANOVA with ERO phase difference locking index (PDLI) as the dependent variable, and brain region and lesion as independent variables, revealed a significant main effect of lesion (F=7.01, p=0.0013) but not brain region (F=1.69, p=0.189) or brain region × lesion interaction (F=0.69, p=0.6). Compared to sham operated rats, lesions in MS significantly reduced PDLI in the electrode pair Fctx–DHPC in the theta frequencies at 400–800 ms time interval (F=3.56, df=2,39, p<0.05, post-hoc p<0.05, Fig. 4a). Reductions in PDLI in the electrode pair Fctx–Amyg were also seen in the theta frequencies at the 10–400 ms time interval (F=9.64, df=2,39, p<0.001, post-hoc p<0.01), and the 400–800 ms time interval (F=11.79, df=2,39, p<0.001, post-hoc p<0.05), and in the alpha (F=3.61, df=2,39, p<0.05, post-hoc p<0.05), beta (F=7.21, df=2,39, p=0.002, post-hoc p<0.01) and gamma (F=7.69, df=2,39, p=0.001, post-hoc p<0.05) frequency bands in the 0–300 ms time interval (Fig. 4b). Analysis on the 300–800 ms interval for PDLI revealed that in the electrode pair Fctx–Amyg a reduction in alpha (F=10.29, df=2,39, p<0.01, post-hoc p<0.01) and beta (F=8.64, df=2,39, p=0.01, post-hoc p<0.01) frequencies were found. The PDLI for the electrode pair DHPC–Amyg shows a signicant reduction in the theta (F=5.25, df=2,39, p=0.009, post-hoc p<0.05) frequency band at 10–400 ms time interval (see Fig. 4c).
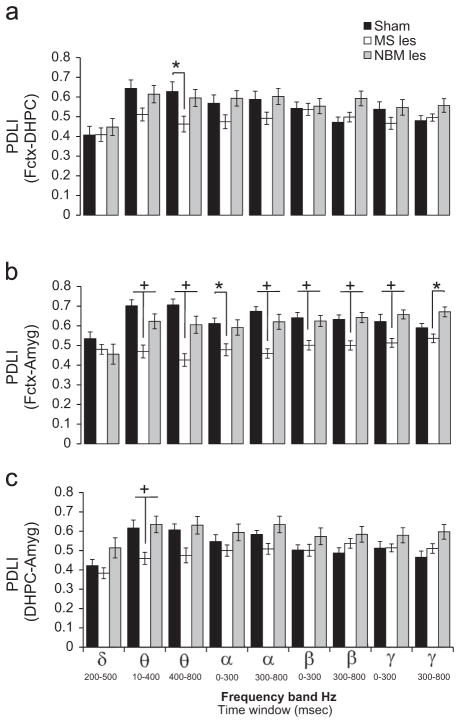
Fig. 4.
MS lesion reduces phase synchronization between Frontal cortex-amygdala and between dorsal hippocampus–amygdala. Grand mean values for the phase difference lock index (PDLI) of event-related oscillations (EROs) in sham operated (black bars), MS-lesion (white bars) and NBM-lesion (gray bars) rats for the rare tone in ROI1 (delta band, 1–4 Hz, 200–500 ms), ROI2 (theta band, 4–7 Hz, 10–400 ms), ROI3 (theta band, 4–7 Hz, 400–800 ms), ROI4 (alpha band, 7–13 Hz, 0–300 ms), ROI5 (alpha band, 7–13 Hz, 300–800 ms), ROI6 (beta band, 13–30 Hz, 0–300 ms), ROI7 (beta band, 13–30 Hz, 300–800 ms), ROI8 (gamma band, 30–50 Hz, 0–300 ms), and ROI9 (gamma band, 30–50 Hz, 300–800 ms). In the upper graph (a), phase difference was calculated between frontal cortex (Fctx) and dorsal hippocampus (DHPC); in the middle graph (b), phase difference was calculated between Fctx and amygdala (Amyg); and in the bottom graph (c), phase difference was calculated between DHPC and Amyg. MS lesions reduced phase synchronization between frontal cortex and DHPC (Fctx–DHPC) in the theta (ROI3) frequency bands. In addition, MS lesions reduced phase synchronization between frontal cortex and amygdala (Fctx–Amyg) in theta (ROI2 and ROI3), alpha (ROI4 and ROI5), beta (ROI6 and ROI7) and gamma (ROI8) frequency bands and reduced phase synchronization between dorsal hippocampus–amygdala (DHPC–Amyg) in theta (ROI2) frequency band. Post-hoc Tukey pairwise comparisons indicate the following: sham vs. MS-les or MS-less vs. NBM (*p<0.05), and MS-les vs. sham and NBM–les (+p<0.05).
Compared to sham operated rats, lesions in the NBM did not cause significant changes in any of the electrode pairs; Fctx–DHPC, Fctx–Amyg, DHPC–Amyg (Fig. 4a–c).
2.4. NBM-lesions induce reductions in phase synchronization in frontal cortex, dorsal hippocampus and amygdala
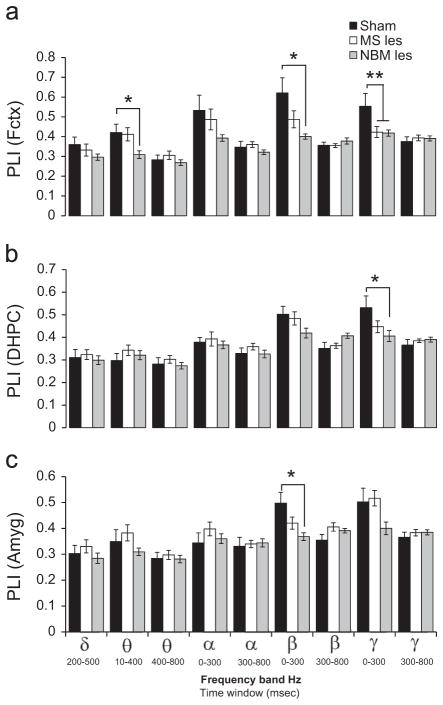
Two-way ANOVA, with ERO phase locking index (PLI) as the dependent variable, and brain region and lesion as independent variables, revealed significant effects of brain region (F=3.63, p<0.03) and lesion (F=8.97, p=0.0002) but no brain region × lesion interactions (F=1.45, p=0.222). Grand averages of the PLI values for the entire group of subjects (n=42) for sham operated, MS lesion, and NBM lesioned rats are presented in Fig. 5. Compared to sham operated rats, lesions in MS or NBM were associated with a reduction in evoked gamma PLI in the Fctx (F=5.37, df=2,39, p<0.01, post-hoc p<0.05, see Fig. 6a) in the 0–300 ms time interval. NBM lesion produced a significant reduction in theta in the 10–400 ms time interval (F=4.03, df=2,39. p<0.03, post-hoc p<0.05) and in beta (F=7.15, df=2,39, p<0.01, post-hoc p<0.01) PLI in frontal cortex in the 0–300 ms time interval (Fig. 6a). In addition, NBM lesions induced a reduction in gamma phase locking (F=3.9, df=2,39, p<0.03; post-hoc p<0.05) in DHPC, as well as a reduction in beta phase locking (F=6.74, df=2,39 p<0.01; post-hoc p<0.01) in the 0–300 ms time interval in amygdala (Fig. 6b and c).
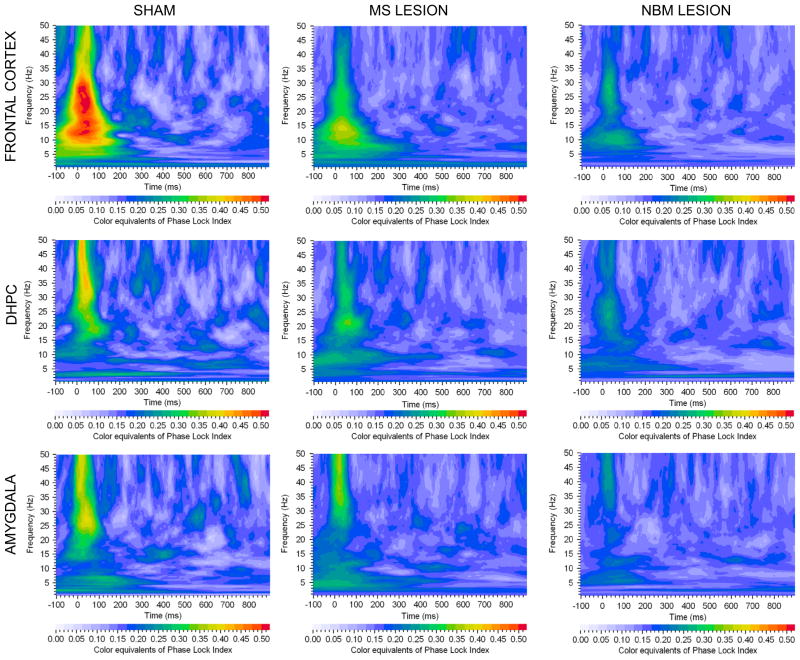
Fig. 5.
Grand averages of phase locking index values of event related oscillations for sham-operated, MS-lesion and NBM-lesion rats. Each graph depicts a time–frequency representation of PLI values in the delta, theta, alpha, beta and gamma bands following the rare tone in Frontal cortex, dorsal hippocampus (DHPC) and Amygdala electrode locations. In each graph frequency (Hz) is presented on the Y-axis, time regions of interest on the X-axis (ms) and PLI is presented as color equivalents as indicated in the bar at the bottom of each graph. NBM lesion shows reduction in color equivalents of phase locking at all three electrode sites.
Fig. 6.
Effects of NBM-lesions on phase synchronization in frontal cortex, dorsal hippocampus or amygdala. Grand mean values for the phase locking index (PLI) of event-related oscillations in sham operated (black bars), MS-lesion (white bars) and NBM-lesion (gray bars) rats for the rare tone in ROI1 (delta band, 1–4 Hz, 200–500 ms), ROI2 (theta band, 4–7 Hz, 10–400 ms), ROI3 (theta band, 4–7 Hz, 400–800 ms), ROI4 (alpha band, 7–13 Hz, 0–300 ms), ROI5 (alpha band, 7–13 Hz, 300–800 ms), ROI6 (beta band, 13–30 Hz, 0–300 ms), ROI7 (beta band, 13–30 Hz, 300–800 ms), ROI8 (gamma band, 30–50 Hz, 0–300 ms), and ROI9 (gamma band, 30–50 Hz, 300–800 ms). Phase locking index was calculated in: (a) the frontal cortex (Fctx), (b) dorsal hippocampus (DHPC), and (c) amygdala (Amyg). Compared to sham operated rats, MS-lesions (MS les) induced a significant reduction in gamma PLI in Fctx. Compared to sham operated rats NBM-lesion (NBM les) induced a significant reduction in gamma phase locking in Fctx and DHPC and a significant reduction in beta phase locking in Fctx and Amyg and theta phase locking in Fctx. Post-hoc Tukey pairwise comparisons indicate the following: sham vs. NBM-les (*p<0.05) and sham vs. MS-les and NBM-les (**p<0.05).
3. Discussion
The purpose of the present study was to investigate oscillatory activity in the delta, theta, alpha, beta and gamma frequency bands in the frontal cortex, DHPC and central amygdala of adult rats after selective cholinergic lesions of the medial septum or nucleus basalis magnocellularis. Network oscillations provide a mechanism to functionally link ensembles of neurons from discrete and regulatory pathways into complex interplay during information processing and represent neurophysiological correlates of human information processing and cognitive function (Basar et al., 1999, 2001c; Karakas et al., 2000b). ERO energy and phase locking of frequency specific, neuro-oscillatory activity within and between neural assemblies may underlie the processes whereby the brain organizes and communicates information (Barutchu et al., 2013; Basar et al., 1999; Roach and Mathalon, 2008; Sauseng et al., 2007), and represents a methodology whereby neuronal synchrony and/or phase resetting can be quantified and compared among experimental conditions in both man and animals providing thereby a translatable measure with which to explore the neural basis of behavior (Basar and Guntekin, 2008; Sazonov et al., 2009; Thatcher, 2012).
Using a simple auditory task we found that the infrequently presented (rare) stimulus produced a robust and highly significant increase in phase locking of EROs. The most likely explanation of this finding is that it represents a change in neural state associated with attending to a more novel, possibly environmentally relevant noise. Our findings are consistent with a previous study that evaluated phase locking of EROs using a complex motor-learning task (Sauseng et al., 2007). In that task, long-range theta phase coherence was stronger in the novel condition compared to learned sequences, independent of task-difficulty. Based on these data it has been suggested that the processing of sensory information, such as those used in our simple auditory task, is primarily guided by automatic “bottom up” processes that do not require active mental processing (Klimesch et al., 2007).
3.1. MS-lesion induced changes in ERO energy associated to phase synchronization between brain areas
Medial septal lesions were associated with reductions in ERO energy in the delta, theta, alpha, beta and gamma bands in the amygdala and the gamma band energy in the frontal cortex. In contrast to the NBM lesions, MS lesions produced decreases in evoked gamma phase locking index only in the frontal cortex. However, lesions in the medial septum, while not producing decreases in synchronization within brain areas as shown by PLI, did decrease phase synchronization between brain areas as shown by PDLI. MS lesions induced a reduction in synchronization between Fctx and Amyg in theta, alpha, beta and gamma frequency bands, reduction in synchronization between Fctx and DHPC in theta frequency bands, and between DHPC and Amyg in theta frequency band. However, it is not clear whether the principal action of MS lesions is a reduction in Amyg oscillatory energy within the Amyg, or phase synchronization between brain regions, since it is theoretically possible that reductions in power within the Amyg may reduce the strength of the structure to entrain other brain areas.
Since the MS is critically involved in the rhythm generation of theta frequency output to cortical targets (Petsche et al., 1962), the reduction of synchronization seen between brain regions following loss of cholinergic tone still suggests a possible modulation of MS cholinergic system in synchronization between brain regions, and this observation is supported by pharmacological studies in humans and animal models. For instance, aging or administration of cholinergic M1 antagonists have been shown to reduce interregional phase synchronization between premotor/prefrontal cortex and the medial temporal lobe in humans using functional MRI and EEG (Wink et al., 2006). Comparable reports of cholinergic modulation of EEG coherence (Kikuchi et al., 2000), age-related change in hippocampal connectivity measured using fMRI (Grady et al., 2003) and evidence for age- and Alzheimer-related changes in hippocampal connectivity (Greicius et al., 2004) support the cholinergic involvement in interregional phase synchronization.
3.2. NBM-lesion induced changes in ERO energy are associated with phase synchronization within a brain area
This study demonstrated that NBM lesions increase ERO energy in the delta and theta frequency bands in Fctx and reduce gamma ERO energy in Fctx and Amyg. NBM provides the major source of cholinergic projections to the cortex (Amaral and Kurz, 1985; Bigl et al., 1982; Harati et al., 2008; Mesulam, 1995, 2004, 2013), the olfactory bulbs, and the amygdala (Wenk, 1997; Woolf, 1991). The reduction observed in ChAT activity in frontal cortex after lesioning NBM may account for the redistribution of ERO energy: increasing ERO energy in low frequencies (delta, theta and alpha bands) and decreasing ERO energy in high frequencies (such as gamma band). Although we did not measure ChAT activity in the amygdala other studies have shown that AMPA lesion into the MS/NBM induced up to 20% reduction compared to control (Waite et al., 1994), and the deficit of cholinergic projection from NBM could be involved in the decrease in ERO energy in gamma band in amygdala.
These studies may also be of theoretical interest in understanding several disorders that are associated with cholinergic hypofunction or loss. One important component of the pathophysiology of AD is degeneration of the cholinergic system (Perry et al., 1978; Sabbagh and Cummings, 2011) where an overall 55% loss of cortical cholinergic fibers has been reported in the basal forebrain in humans (Geula and Mesulam, 1995, 1996). Spontaneous activity in the EEG of AD patients reveals a topographically changed pattern of oscillations characterized by decrease of fast and increase of slow frequencies (Yener and Basar, 2010, 2013b; Yener et al., 2012). ERO studies in humans also show decreased responses in fronto-central regions of the brain in delta and theta frequencies (Yener and Basar, 2013b). While EEG synchronization between different brain regions, in mild AD subjects, appears to be mostly intact, event-related synchronization between brain regions is decreased in alpha, theta, and delta frequency ranges. Alpha synchronization also seems to be sensitive to cholinergic treatment in AD (Yener and Basar, 2013b). Thus human studies suggest that ERO may be a good index of both cholinergic loss and cognitive deficit in AD.
Several authors have suggested that oscillations in specific frequency ranges may underlie specific mental functions. Some studies have demonstrated that cholinergic neuromodulation contributes to gamma oscillation production in vivo and in vitro (Bauer et al., 2012; Buhl et al., 1998; Hentschke et al., 2007; Liljenstrom and Hasselmo, 1995; Pafundo et al., 2013; Tiesinga et al., 2001). Synchronized gamma band (30–80 Hz) oscillations may be an index of ACh signaling during cognitive tasks, since gamma band power increases in relation to working memory load (Roux et al., 2012) and abnormal gamma oscillations are associated with cognitive deficits (Sun et al., 2012; Uhlhaas and Singer, 2006; Uhlhaas et al., 2011). For instance, event-related alpha oscillations have been attributed to attentional resources, semantic memory, and stimulus processing (Basar et al., 1997; Klimesch et al., 1994, 2004) whereas, beta and gamma oscillations have been associated with sensory integrative processes (Basar, 2013; Basar et al., 2001b; Schurmann et al., 1997). Oscillations in the delta and theta frequency ranges have been associated with signal detection, decision-making, conscious awareness, recognition memory and episodic retrieval (Basar et al., 2001a, 2013; Gevins et al., 1998; Klimesch et al., 2005). It has been suggested that high frequency oscillations (above 30 Hz) reflect synchronization of neuronal ensembles that are interacting over short distances in response to primarily sensory processes (Bressler and Freeman, 1980; Ohl et al., 2003) whereas, lower frequency oscillations (1–4 Hz) are generated by synchronization of ensembles interacting at longer distances during higher cognitive processing (Kopell et al., 2000; Lubar, 1997).
There is evidence for a functional dissociation between medial septum and nucleus basalis cholinergic systems in aspects of cognitive function (Lehmann et al., 2003). In general, NBM lesions have shown to impair performance on a variety of tasks involving sustained attention and discrimination (Dunnett, 1991; Harati et al., 2008; Lehmann et al., 2003; Motohashi et al., 1986; Nieto-Escamez et al., 2002; Risbrough et al., 2002; Santucci and Haroutunian, 1989). Lesions of medial septum impair learning and memory and generation of hippocampal theta rhythm (Brito and Brito, 1990; Colom et al., 1991; Easton et al., 2011; Harati et al., 2008; Martin et al., 2008). The cholinergic innervation of the hippocampus plays a key role in spatial reference memory processes involved in place navigation (Hagan et al., 1988) and rats with lesions of cholinergic medial septum are impaired in a task that requires the association of places with contexts (Easton et al., 2011). Combined lesions in the medial septum/diagonal band and nucleus basalis magno-cellularis (NBM) in rats induces spatial memory impairment (Waite et al., 1994) which is qualitatively similar to nucleus basalis lesions alone involving decreasing of the attentional load (Lehmann et al., 2003).
In this study selective cholinergic lesions produced changes in energy, PLI and PDLI depending on the area of the lesion (see Table 1). Whether the electrophysiological results obtained were due exclusively to the loss of cholinergic tone or whether they reflect a compensation or adaption of other brain systems to the loss of cholinergic tone is not clear at the time. Independent of the exact mechanisms of the effects observed, we have demonstrated that loss of cholinergic modulation can influence the output of brain oscillations in this rat model. These findings could theoretically contribute to the understanding of the role of cholinergic function in a number of cognitive disorders including: Alzheimer diseases, Parkinson diseases, other cognitive disorders including Down-syndrome, progressive supranuclear palsy, Jakob–Creutzfeld disease, Korsakoff’s syndrome and traumatic brain injury and alcoholism.
Table 1.
Effects of MS or NBM lesions on energy, phase-locking index (PLI), and phase difference lock index (PDLI).
| MS lesion
|
NBM lesion
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency band | δ |
θ
|
α
|
β
|
γ
|
δ |
θ
|
α
|
β
|
γ
|
||||||||
| Time window (ms) | 200– 500 |
10– 400 |
400– 800 |
0– 300 |
300– 800 |
0– 300 |
300– 800 |
0– 300 |
300– 800 |
200– 500 |
10– 400 |
400– 800 |
0– 300 |
300– 800 |
0– 300 |
300– 800 |
0– 300 |
300– 800 |
| Region of interest | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| Energy | ||||||||||||||||||
| Fctx | ↓ | ↑ | ↑ | ↓ | ||||||||||||||
| DHPC | ||||||||||||||||||
| Amyg | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||
| PLI | ||||||||||||||||||
| Fctx | ↓ | ↓ | ↓ | ↓ | ||||||||||||||
| DHPC | ↓ | |||||||||||||||||
| Amyg | ↓ | |||||||||||||||||
| PDLI | ||||||||||||||||||
| Fctx–DHPC | ↓ | |||||||||||||||||
| Fctx–Amyg | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | |||||||||||
| DHPC–Amyg | ↓ | |||||||||||||||||
Effects of medial septal (MS) or nucleus basalis magnocellularis (NBM) lesions on measures of event-related oscillations (EROs) (Energy; PLI, phase locking index; PDLI, phase difference lock index) in 9 time frequency regions of interest for three brain areas: frontal cortex (Fctx), dorsal hippocampus (DHPC), and amygdala (Amyg). Arrows indicate direction of change in the lesion group as compared to the sham lesion group.
4. Conclusions
These studies suggest that MS lesions to cholinergic neurons are associated with phase synchronization across stimulus trials between brain areas whereas NBM lesions to cholinergic neurons contribute to phase synchronization/phase resetting across stimulus trials within a brain area.
5. Experimental procedures
5.1. Animal subjects
Forty-two (42) rats weighing 250–410 g were used. All rats had ad libitum access to food and water. A detailed description of the environmental conditions of rats can be found in previous reports (Ehlers et al., 1998; Robledo et al., 1998). The work described herein adheres to the guidelines stipulated in the NIH Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revised 1996) and was reviewed and approved by The Scripps Research Institute’s Institutional Animal Care and Use Committee.
5.2. Surgical and electrophysiological recording procedures
Surgical and electrophysiological recording procedures performed in this study have been previously described (Ehlers et al., 1994; Robledo et al., 1998). Briefly, rats were divided into three groups (sham-operated controls, MS-lesion and NBM-lesion groups). Rats were deeply anesthetized with Nembutal (50 mg/kg, intraperitoneally) and surgically implanted with bilateral cannula above the nucleus basalis magnocellularis (NBM: AP −0.8; ML±2.5; DV −4.3 mm) or medial septal nucleus (MS: AP 0.6; ML 0.0; DV −4.3 mm) together with stainless steel single-wire recording electrodes in the frontal cortical area (AP: −3.0 mm, ML: ±3.0 mm, DV: −3.0 mm), DHPC (AP: −4.2 mm, ML:±3.0 mm, DV: −3.0 mm) and amygdala (AP: 1.0 mm, ML: ±5.3 mm, DV: −8.5 mm). Additional screw electrodes were placed in the skull overlying the frontal (AP: 3.0 mm, ML: ±3.0 mm, FR1) and parietal (AP: −3.0 mm, ML: ±4.0 mm, PAR3) cortices using the Paxinos and Watson atlas (1986). A midline screw “reference” electrode was placed 3 mm posterior to lambda in the skull overlying the cerebellum. Electrode connections were made to a multi-pin amphenol connector and the assembly was anchored to the skull with dental acrylic and anchor screws. EEG signals were recorded, using a unipolar montage, with a band pass of 0.5–70 Hz with a 60-Hz notch filter in. ERPs were elicited by auditory stimuli that were presented through a small speaker centered approximately 20 cm above the rat’s head. ERPs were elicited passively with an acoustic “oddball” paradigm as described previously (Ehlers and Chaplin, 1992; Ehlers et al., 1994) and consisted of 200 individual tone presentations. The tones were generated by a programmable multiple-tone generator. Two tone types were presented: standard tones (1000 Hz square wave, duration 20 ms, 71 dB, 83.5% probability) and rare tones (2000 Hz square wave, duration 20 ms, 71 dB, 16.5% probability). Individual trials were 1000 ms in duration (100 ms pre-stimulus+900 ms post-stimulus) and were separated by variable intervals ranging from 500 to 1000 ms. Rare tones were interspersed with standards such that no two rare tones occurred successively. The EEG amplifier input range corresponding to the full range of the 12-bit analog-to-digital converter was about ±250 μV. Periodic calibration results were used to scale the digitized EEG to microvolts. An artifact rejection program was utilized to eliminate individual trials in which the EEG exceeded +400 μV. Potential artifacts identified by computer software were excluded only after visual analysis of raw EEG. Trials containing excessive movement artifact were eliminated prior to averaging (<5% of the trials). ERPs trials were digitized at a rate of 256 Hz.
5.3. ERO energy and PLI analyses
Data from single trials generated by the stimuli were entered into the time frequency analyses algorithm. The S-transform (ST), a generalization of the Gabor transform (Gabor, 1946), was used (see Stockwell et al., 1996).
The S transform mathematically resembles the continuous wavelet transform but it uses Gaussian windows which do not meet a requirement of wavelet analysis, and it includes a “phase correction” that is not part of wavelet analysis. The actual use of the S-transform was simplified by performing first a forward Fourier transform of the time series. Then, for each frequency of the Fourier transform, summing the results of multiplication by a set of Fourier transforms of Gaussian windows of varying width. Finally, for each of these sums, taking the inverse Fourier transform. The equation for calculation of the S-transform of discrete time series h(kT) at time jT and frequency n/NT is where T is the sample period of the discrete time series, j is the sample index, N is the number of samples in the time series, n is the frequency index, and H[ ] is the Fourier spectrum of the discrete time series. The S-transform results in a time–frequency representation of the data. The exact code we used is a C language, S-transform subroutine available from the NIMH MEG Core Facility web site (http://kurage.nimh.nih.gov/meglab/). This code is specifically for use with real time series, so it sets the input imaginary values, required by the S-transform, to zero, and it always uses the Hilbert transform so that each of the complex output time series is an analytic signal.
To reduce anomalies in the S-transform output at the beginning and the end of the output time series, we used a Hanning window over the initial and final 100 ms of the input time series. The output of the transform for each stimuli and electrode site was calculated by averaging the individual trials containing the time–frequency energy distributions. To quantify S-transform magnitudes, a region of interest (ROI) was identified by specifying the band of frequencies and the time interval contained in the rectangular ROI. The time–frequency points saved from each S transformation are from 100 ms before to 900 ms after the onset of the stimulus, and from 1 Hz to 50 Hz at intervals of 0.5 Hz. Energy is the square of the magnitude of the S-transform output in a time frequency region of interest. The S-transform output for a time/frequency ROI, for a specific EEG lead, is proportional to the input voltage of the lead over the time/frequency interval. The S-transform magnitude squared for a time/frequency interval is therefore proportional to volts squared. These analyses are similar to what has been previously described (Jones (2004)).
An S transformation at time t and frequency f has real and imaginary parts:
where i is the square root of −1. The cosine and sine of the phase angle at this time–frequency point are
where the vertical bar pair indicates magnitude, here and below.
ERP trials are averaged by summing separately the real and imaginary parts of the S transform outputs, and dividing each by the sum over trials of the magnitudes of S transform outputs. The sums over trials of the real and imaginary parts of the S transform outputs are the sides of a right triangle and the sum of magnitudes is the hypotenuse. From this, the angles of the triangle of the sums are calculated:
where an angle bracket pair indicates mean value from S transforms, and a vertical bar pair indicates magnitude, here and below.
PLI is a measure of synchrony of phase angle over trials, as a function of frequency and of time relative to the start of the stimulus for each trial. The range of PLI is from zero to 1.0, with high values at a time and frequency indicating little variation, among trials, of phase angle at that time and frequency. PLI is defined as follows:
where the angle bracket pair indicates mean value over eligible trials, here and below. Eligibility depends on the stimulus type and absence of significant artifact. This definition is mathematically equivalent to the definition in Schack and Klimesch (2002).
PDLI is a measure of constancy over trials of the difference in phase angle between two channels, as a function of frequency and of time relative to the start of the stimulus for each trial. The range of PDLI is from zero to 1.0, with high values at a time and frequency indicating little variation, among trials, of phase angle difference between channels of the pair, at that time and frequency. PDLI is defined for frequency f at time t as
where φA and φB are phase angles of channels A and B, respectively. This definition of PDLI is equivalent to a definition of PLV, phase lock value, in Brunner et al. (2005). By means of some standard trigonometric identities the equation above is equivalent to the following, which, as for PLI, does not require that the phase angles be calculated:
Rectangular regions of interest (ROIs) were defined within the time–frequency analysis plane by specifying, for each ROI, a band of frequencies and a time interval relative to the stimulus onset time. Time 0 in these definitions is the onset of the stimulus. The 9 ROIs were: ROI1 (delta band,1–4 Hz, 200–500 ms), ROI2 (theta band, 4–7 Hz, 10–400 ms), ROI3 (theta band, 4–7 Hz, 400–800 ms), ROI4 (alpha band, 7–13 Hz, 0–300 ms), ROI5 (alpha band, 7–13 Hz, 300–800 ms), ROI6 (beta band, 13–30 Hz, 0–300 ms), ROI7 (beta band, 13–30 Hz, 300–800 ms), ROI8 (gamma band, 30–50 Hz, 0–300 ms), and ROI9 (gamma band, 30–50 Hz, 300–800 ms) (see Fig. 1b). These regions were chosen apriori to co-inside with the major EEG frequencies and the latency windows of the N1 and P3 components in the rat. Using mean values over trials, the maximum values were calculated for each ROI, for each electrode location or, for PDLI, for a pair of electrode locations (FZ–PZ) for energy (E), PLI amplitude, and PDLI amplitude.
5.4. Statistical analyses
Statistical analyses were performed by using One-Way Analysis of Variance and Two-Way Analysis of Variance on line (www.vassarstats.net). Values are mean±standard error of the mean (SEM). Analyses were performed on data generated from trials in response to the infrequently presented (rare) tone collected from sham-operated, MS-lesion and NBM-lesion rats. Energy, PLI and PDLI for the 9 ROIs. Group (sham-operated, NBM-lesion and MS-lesion rats) was assessed as a between subject variable. Lesion effects were determined using a two way ANOVA (lesion × brain region) (sham-operated, NBM-lesion and MS-lesion rats), brain region (frontal cortex, DHPC, amygdala) and lesion × brain region interaction was computed. Post-hoc analyses (Tukey LSD p<0.05) between sham and lesioned groups was determined. One-way ANOVA was used to assess lesion site differences. For these analyses, P-value was set at p<0.05 to determine the levels of statistical significance. ANOVA with Tukey post-hoc analyses were utilized to statistically evaluate ChAT activity.
5.5. Lesion and perfusion
Lesion and perfusions were conducted as previously described (Robledo et al., 1998). Under Nembutal anesthesia (1 ml/kg) rats were infused through previously implanted guide cannula with 0.5 ul per side 0.01 M AMPA (a-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; Cambridge Research Biochemicals, UK) (Muir et al., 1994) into the NBM (3 mm below cannula at DV= −7.3), the MS (3 mm below cannula at DV= −7.3), and the controls were infused with vehicle (0.2 M PB). ERPs trials were conducted 10 days following the sham/lesion surgery. At the end of the study rats were euthanized and their brains were extracted rapidly and dissected on wet ice. The frontal and parietal cortices and hippocampus were removed and choline acetyltransferase (ChAT) activity was measured. In brief, extracted tissue was sonicated in 400 μl of 50 nM phosphate buffer (pH 7.4). ChAT activity was measured by the incorporation of 14C-acetyl coenzyme A into 14C-Ach. The assay is based on the transfer of the radiolabeled acetyl moiety from acetyl CoA to choline, and separation of radiolabeled 14C-acetyl CoA from the radiolabeled product, 14C-Ach (Fonnum, 1969).
Acknowledgments
Role of funding source
This study was supported in part by the National Institutes of Health (NIH), National Institute on Alcoholism and Alcohol Abuse grants, AA006059 and AA019969 awarded to CLE. NIAAA had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors thank Jose Criado, Anita Desikan, Phil Lau and Shirley Sanchez for their assistance in data collection, analyses and editing.
Abbreviations
- Ach
Acetylcholine
- AD
Alzheimer’s disease
- AMYG
Amygdala
- ANOVA
Analysis of Variance
- ChAT
Choline acetyltransferase
- DHPC
Dorsal Hippocampus
- EEG
Electroencephalogram
- ERO
Event-related oscillations
- ERP
Event-related potentials
- FCTX
Frontal Cortex
- MS
Medial Septum
- NBM
nucleus basalis magnocellularis
- PDLI
phase difference lock index
- PLI
phase locking index
- ROI
region of interest
Footnotes
Contributors
All authors have given intellectual contributions to the study and the paper and have approved the final manuscript. Manuel Sanchez-Alavez, Patricia Robledo and Cindy L. Ehlers and Jim Havstad were responsible for the study design and prepared de manuscript. Derek Wills was responsible for collecting and coding the data. Jim Havstad was responsible for developing all software for ERO analyses.
Conflict of interest
Dr. Ehlers work has been funded by the NIH. She has received compensation as a consultant from Neurocrine Biosciences and Raptor Pharmaceutical Corp. in capacities not related to the subject of the report. Dr. Manuel Sanchez-Alavez, Dr. Patricia Robledo Dr. Jim Havstad and Derek Wills declare no potential conflicts of interest.
References
- Amaral DG, Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat 240, 37–59J. Comp Neurol. 1985;240:37–59. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- Andrew C, Fein G. Event-related oscillations versus event-related potentials in a P300 task as biomarkers for alcoholism. Alcohol Clin Exp Res. 2010;34:669–680. doi: 10.1111/j.1530-0277.2009.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barutchu A, Freestone DR, Innes-Brown H, Crewther DP, Crewther SG. Evidence for enhanced multisensory facilitation with stimulus relevance: an electrophysiological investigation. PLoS One. 2013;8:e52978. doi: 10.1371/journal.pone.0052978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basar-Eroglu C, Basar E, Schmielau F. P300 in freely moving cats with intracranial electrodes. Int J Neurosci. 1991;60:215–226. doi: 10.3109/00207459109167034. [DOI] [PubMed] [Google Scholar]
- Basar E. A review of gamma oscillations in healthy subjects and in cognitive impairment. Int J Psychophysiol. 2013;90:99–117. doi: 10.1016/j.ijpsycho.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Basar E, Guntekin B. A review of brain oscillations in cognitive disorders and the role of neurotransmitters. Brain Res. 2008;1235:172–193. doi: 10.1016/j.brainres.2008.06.103. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Basar-Eroglu C, Karakas S. Alpha oscillations in brain functioning: an integrative theory. Int J Psychophysiol. 1997;26:5–29. doi: 10.1016/s0167-8760(97)00753-8. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Are cognitive processes manifested in event-related gamma, alpha, theta and delta oscillations in the EEG? Neurosci Lett. 1999;259:165–168. doi: 10.1016/s0304-3940(98)00934-3. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Brain oscillations in perception and memory. Int J Psychophysiol. 2000;35:95–124. doi: 10.1016/s0167-8760(99)00047-1. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001a;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Basar-Eroglu C, Demiralp T. Selectively distributed gamma band system of the brain. Int J Psychophysiol. 2001b;39:129–135. doi: 10.1016/s0167-8760(00)00136-7. [DOI] [PubMed] [Google Scholar]
- Basar E, Schurmann M, Demiralp T, Basar-Eroglu C, Ademoglu A. Event-related oscillations are ‘real brain responses’—wavelet analysis and new strategies. Int J Psychophysiol. 2001c;39:91–127. doi: 10.1016/s0167-8760(00)00135-5. [DOI] [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Guntekin B, Yener GG. Brain’s alpha, beta, gamma, delta, and theta oscillations in neuropsychiatric diseases: proposal for biomarker strategies. Suppl Clin Neurophysiol. 2013;62:19–54. doi: 10.1016/b978-0-7020-5307-8.00002-8. [DOI] [PubMed] [Google Scholar]
- Bauer M, Kluge C, Bach D, Bradbury D, Heinze HJ, Dolan RJ, Driver J. Cholinergic enhancement of visual attention and neural oscillations in the human brain. Curr Biol. 2012;22:397–402. doi: 10.1016/j.cub.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MG, Bucci DJ. Selective immunotoxic lesions of basal forebrain cholinergic neurons: twenty years of research and new directions. Behav Neurosci. 2013;127:611–618. doi: 10.1037/a0033781. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Bucci DJ, Gorman LK, Wiley RG, Gallagher M. Selective immunotoxic lesions of basal forebrain cholinergic cells: effects on learning and memory in rats. Behav Neurosci. 2013;127:619–627. doi: 10.1037/a0033939. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Genetics of human brain oscillations. Int J Psychophysiol. 2006;60:162–171. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Bigl V, Woolf NJ, Butcher LL. Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital, and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull. 1982;8:727–749. doi: 10.1016/0361-9230(82)90101-0. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Freeman WJ. Frequency analysis of olfactory system EEG in cat, rabbit, and rat. Electroencephalogr Clin Neurophysiol. 1980;50:19–24. doi: 10.1016/0013-4694(80)90319-3. [DOI] [PubMed] [Google Scholar]
- Brito GN, Brito LS. Septohippocampal system and the prelimbic sector of frontal cortex: a neuropsychological battery analysis in the rat. Behav Brain Res. 1990;36:127–146. doi: 10.1016/0166-4328(90)90167-d. [DOI] [PubMed] [Google Scholar]
- Brunner C, Graimann B, Huggins JE, Levine SP, Pfurtscheller G. Phase relationships between different subdural electrode recordings in man. Neurosci Lett. 2005;375:69–74. doi: 10.1016/j.neulet.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Tamas G, Fisahn A. Cholinergic activation and tonic excitation induce persistent gamma oscillations in mouse somatosensory cortex in vitro. J Physiol. 1998;513 (Pt. 1):117–126. doi: 10.1111/j.1469-7793.1998.117by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AE, Hodge GK. Acquisition, retention, and extinction of operant discriminations in rats with nucleus basalis magnocellularis lesions. Behav Neurosci. 1995;109:699–713. doi: 10.1037//0735-7044.109.4.699. [DOI] [PubMed] [Google Scholar]
- Colom LV, Nassif-Caudarella S, Dickson CT, Smythe JW, Bland BH. In vivo intrahippocampal microinfusion of carbachol and bicuculline induces theta-like oscillations in the septally deafferented hippocampus. Hippocampus. 1991;1:381–390. doi: 10.1002/hipo.450010406. [DOI] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Event-related oscillations as risk markers in genetic mouse models of high alcohol preference. Neuroscience. 2009;163:506–523. doi: 10.1016/j.neuroscience.2009.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Effects of adolescent ethanol exposure on event-related oscillations (EROs) in the hippocampus of adult rats. Behav Brain Res. 2010a;210:164–170. doi: 10.1016/j.bbr.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Event-related oscillations in the parietal cortex of adult alcohol-preferring (P) and alcohol-nonpreferring rats (NP) Alcohol. 2010b;44:335–342. doi: 10.1016/j.alcohol.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiana S, Platt B, Riedel G. The cholinergic system and spatial learning. Behav Brain Res. 2011;221:389–411. doi: 10.1016/j.bbr.2010.11.036. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Ademoglu A. Decomposition of event-related brain potentials into multiple functional components using wavelet transform. Clin Electroencephalogr. 2001;32:122–138. doi: 10.1177/155005940103200307. [DOI] [PubMed] [Google Scholar]
- Dunnett S. Cholinergic grafts, memory and ageing. Trends Neurosci. 1991;14:371–376. doi: 10.1016/0166-2236(91)90166-r. [DOI] [PubMed] [Google Scholar]
- Easton A, Fitchett AE, Eacott MJ, Baxter MG. Medial septal cholinergic neurons are necessary for context-place memory but not episodic-like memory. Hippocampus. 2011;21:1021–1027. doi: 10.1002/hipo.20814. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI. Long latency event related potentials in rats: the effects of changes in stimulus parameters and neurochemical lesions. J Neural Transm Gen Sect. 1992;88:61–75. doi: 10.1007/BF01245037. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Kaneko WM, Robledo P, Lopez AL. Long-latency event-related potentials in rats: effects of task and stimulus parameters. Neuroscience. 1994;62:759–769. doi: 10.1016/0306-4522(94)90474-x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Somes C, Lopez AL, Robledo P. Long latency event-related potentials in rats: response of amygdala, nucleus accumbens, dorsal hippocampus and frontal cortex to changes in reward characteristics of conditioned stimuli. Brain Res. 1998;780:138–142. doi: 10.1016/s0006-8993(97)01294-8. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR, Phillips E, Wilhelmsen KC. EEG alpha phenotypes: linkage analyses and relation to alcohol dependence in an American Indian community study. BMC Med Genet. 2010;11:43. doi: 10.1186/1471-2350-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Wills DN, Havstad J. Ethanol reduces the phase locking of neural activity in human and rodent brain. Brain Res. 2012;1450:67–79. doi: 10.1016/j.brainres.2012.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Radiochemical micro assays for the determination of choline acetyltransferase and acetylcholinesterase activities. Biochem J. 1969;115:465–472. doi: 10.1042/bj1150465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings L, Spehl TS, Weber WA, Hull M, Meyer PT. Amyloid-beta load predicts medial temporal lobe dysfunction in Alzheimer dementia. J Nucl Med. 2013;54:1909–1914. doi: 10.2967/jnumed.113.120378. [DOI] [PubMed] [Google Scholar]
- Gabor D. Theory of Communication. J Inst Electr Eng. 1946;93:429–457. [Google Scholar]
- Geula C, Mesulam MM. Cholinesterases and the pathology of Alzheimer disease. Alzheimer Dis Assoc Disord. 1995;9 (Suppl 2):S23–S28. doi: 10.1097/00002093-199501002-00005. [DOI] [PubMed] [Google Scholar]
- Geula C, Mesulam MM. Systematic regional variations in the loss of cortical cholinergic fibers in Alzheimer’s disease. Cereb Cortex. 1996;6:165–177. doi: 10.1093/cercor/6.2.165. [DOI] [PubMed] [Google Scholar]
- Gevins A. The future of electroencephalography in assessing neurocognitive functioning. Electroencephalogr Clin Neurophysiol. 1998;106:165–172. doi: 10.1016/s0013-4694(97)00120-x. [DOI] [PubMed] [Google Scholar]
- Gevins A, Smith ME, Leong H, McEvoy L, Whitfield S, Du R, Rush G. Monitoring working memory load during computer-based tasks with EEG pattern recognition methods. Hum Factors. 1998;40:79–91. doi: 10.1518/001872098779480578. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13:572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JJ, Salamone JD, Simpson J, Iversen SD, Morris RG. Place navigation in rats is impaired by lesions of medial septum and diagonal band but not nucleus basalis magnocellularis. Behav Brain Res. 1988;27:9–20. doi: 10.1016/0166-4328(88)90105-2. [DOI] [PubMed] [Google Scholar]
- Harati H, Barbelivien A, Cosquer B, Majchrzak M, Cassel JC. Selective cholinergic lesions in the rat nucleus basalis magnocellularis with limited damage in the medial septum specifically alter attention performance in the five-choice serial reaction time task. Neuroscience. 2008;153:72–83. doi: 10.1016/j.neuroscience.2008.01.031. [DOI] [PubMed] [Google Scholar]
- Hentschke H, Perkins MG, Pearce RA, Banks MI. Muscarinic blockade weakens interaction of gamma with theta rhythms in mouse hippocampus. Eur J Neurosci. 2007;26:1642–1656. doi: 10.1111/j.1460-9568.2007.05779.x. [DOI] [PubMed] [Google Scholar]
- Jones BE. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog Brain Res. 2004;145:157–169. doi: 10.1016/S0079-6123(03)45011-5. [DOI] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E. A new strategy involving multiple cognitive paradigms demonstrates that ERP components are determined by the superposition of oscillatory responses. Clin Neuropathol. 2000a;111:1719–1732. doi: 10.1016/s1388-2457(00)00418-1. [DOI] [PubMed] [Google Scholar]
- Karakas S, Erzengin OU, Basar E. The genesis of human event-related responses explained through the theory of oscillatory neural assemblies. Neurosci Lett. 2000b;285:45–48. doi: 10.1016/s0304-3940(00)01022-3. [DOI] [PubMed] [Google Scholar]
- Kikuchi M, Wada Y, Koshino Y, Nanbu Y, Hashimoto T. Effects of scopolamine on interhemispheric EEG coherence in healthy subjects: analysis during rest and photic stimulation. Clin Electroencephalogr. 2000;31:109–115. doi: 10.1177/155005940003100210. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schimke H, Schwaiger J. Episodic and semantic memory: an analysis in the EEG theta and alpha band. Electroencephalogr Clin Neurophysiol. 1994;91:428–441. doi: 10.1016/0013-4694(94)90164-3. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Schabus M, Doppelmayr M, Gruber W, Sauseng P. Phase-locked alpha and theta oscillations generate the P1–N1 complex and are related to memory performance. Brain Res Cognit Brain Res. 2004;19:302–316. doi: 10.1016/j.cogbrainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Sauseng P. The functional significance of theta and upper alpha oscillations. Exp Psychol. 2005;52:99–108. doi: 10.1027/1618-3169.52.2.99. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S, Gruber W, Freunberger R. Event-related phase reorganization may explain evoked neural dynamics. Neurosci Biobehav Rev. 2007;31:1003–1016. doi: 10.1016/j.neubiorev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci USA. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic S, Azma S, Irimia A, Sherfey J, Halgren E, Marinkovic K. Theta oscillations are sensitive to both early and late conflict processing stages: effects of alcohol intoxication. PLoS One. 2012;7:e43957. doi: 10.1371/journal.pone.0043957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann O, Grottick AJ, Cassel JC, Higgins GA. A double dissociation between serial reaction time and radial maze performance in rats subjected to 192 IgG-saporin lesions of the nucleus basalis and/or the septal region. Eur J Neurosci. 2003;18:651–666. doi: 10.1046/j.1460-9568.2003.02745.x. [DOI] [PubMed] [Google Scholar]
- Liljenstrom H, Hasselmo ME. Cholinergic modulation of cortical oscillatory dynamics. J Neurophysiol. 1995;74:288–297. doi: 10.1152/jn.1995.74.1.288. [DOI] [PubMed] [Google Scholar]
- Lubar JF. Neocortical dynamics: implications for understanding the role of neurofeedback and related techniques for the enhancement of attention. Appl Psychophysiol Biofeedback. 1997;22:111–126. doi: 10.1023/a:1026276228832. [DOI] [PubMed] [Google Scholar]
- Martin MM, Winter SS, Cheatwood JL, Carter LA, Jones JL, Weathered SL, Wagner SJ, Wallace DG. Organization of food protection behavior is differentially influenced by 192 IgG-saporin lesions of either the medial septum or the nucleus basalis magnocellularis. Brain Res. 2008;1241:122–135. doi: 10.1016/j.brainres.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Cholinergic pathways and the ascending reticular activating system of the human brain. Ann NY Acad Sci. 1995;757:169–179. doi: 10.1111/j.1749-6632.1995.tb17472.x. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. The cholinergic innervation of the human cerebral cortex. Prog Brain Res. 2004;145:67–78. doi: 10.1016/S0079-6123(03)45004-8. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Cholinergic circuitry of the human nucleus basalis and its fate in alzheimer’s disease. J Comp Neurol. 2013;521:1424–1444. doi: 10.1002/cne.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi N, Dubois A, Scatton B. Lesion of nucleus basalis magnocellularis decreases [3H]hemicholinium-3 binding (as measured by autoradiography) in the amygdala and frontal cortex of the rat. Neurosci Lett. 1986;71:7–12. doi: 10.1016/0304-3940(86)90248-x. [DOI] [PubMed] [Google Scholar]
- Muir JL, Page KJ, Sirinathsinghji DJ, Robbins TW, Everitt BJ. Excitotoxic lesions of basal forebrain cholinergic neurons: effects on learning, memory and attention. Behav Brain Res. 1993;57:123–131. doi: 10.1016/0166-4328(93)90128-d. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. AMPA-induced excitotoxic lesions of the basal forebrain: a significant role for the cortical cholinergic system in attentional function. J Neurosci. 1994;14:2313–2326. doi: 10.1523/JNEUROSCI.14-04-02313.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Escamez FA, Sanchez-Santed F, de Bruin JP. Cholinergic receptor blockade in prefrontal cortex and lesions of the nucleus basalis: implications for allocentric and egocentric spatial memory in rats. Behav Brain Res. 2002;134:93–112. doi: 10.1016/s0166-4328(01)00458-2. [DOI] [PubMed] [Google Scholar]
- Niewiadomska G, Baksalerska-Pazera M, Riedel G. The septo-hippocampal system, learning and recovery of function. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:791–805. doi: 10.1016/j.pnpbp.2009.03.039. [DOI] [PubMed] [Google Scholar]
- Ohl FW, Deliano M, Scheich H, Freeman WJ. Analysis of evoked and emergent patterns of stimulus-related auditory cortical activity. Rev Neurosci. 2003;14:35–42. doi: 10.1515/revneuro.2003.14.1-2.35. [DOI] [PubMed] [Google Scholar]
- Pafundo DE, Miyamae T, Lewis DA, Gonzalez-Burgos G. Cholinergic modulation of neuronal excitability and recurrent excitation–inhibition in prefrontal cortex circuits: implications for gamma oscillations. J Physiol. 2013;591:4725–4748. doi: 10.1113/jphysiol.2013.253823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Sydney, Australia: 1986. [Google Scholar]
- Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J. 1978;2:1457–1459. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsche H, Stumpf C, Gogolak G. The significance of the rabbit’s septum as a relay station between the midbrain and the hippocampus. I The control of hippocampus arousal activity by the septum cells. Electroencephalogr Clin Neurophysiol. 1962;14:202–211. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- Pirch JH, Corbus MJ, Rigdon GC, Lyness WH. Generation of cortical event-related slow potentials in the rat involves nucleus basalis cholinergic innervation. Electroencephalogr Clin Neurophysiol. 1986;63:464–475. doi: 10.1016/0013-4694(86)90128-8. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Alcoholism and human electrophysiology. Alcohol Res Health. 2003;27:153–160. [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. From event-related potential to oscillations: genetic diathesis in brain (dys)function and alcohol dependence. Alcohol Res Health. 2008;31:238–242. [PMC free article] [PubMed] [Google Scholar]
- Risbrough V, Bontempi B, Menzaghi F. Selective immunolesioning of the basal forebrain cholinergic neurons in rats: effect on attention using the 5-choice serial reaction time task. Psychopharmacology. 2002;164:71–81. doi: 10.1007/s00213-002-1170-7. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophr Bull. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo P, Somes C, Winkler J, Thal LJ, Ehlers CL. Long latency event-related potentials in rats: effects of nucleus basalis magnocellularis lesions. Int J Neurosci. 1998;96:23–44. [Google Scholar]
- Roux F, Wibral M, Mohr HM, Singer W, Uhlhaas PJ. Gamma-band activity in human prefrontal cortex codes for the number of relevant items maintained in working memory. J Neurosci. 2012;32:12411–12420. doi: 10.1523/JNEUROSCI.0421-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh M, Cummings J. Progressive cholinergic decline in Alzheimer’s disease: consideration for treatment with donepezil 23 mg in patients with moderate to severe symptomatology. BMC Neurol. 2011;11:21. doi: 10.1186/1471-2377-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci AC, Haroutunian V. Nucleus basalis lesions impair memory in rats trained on nonspatial and spatial discrimination tasks. Physiol Behav. 1989;45:1025–1031. doi: 10.1016/0031-9384(89)90233-3. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gruber WR, Hanslmayr S, Freunberger R, Doppelmayr M. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146:1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Sazonov AV, Ho CK, Bergmans JW, Arends JB, Griep PA, Verbitskiy EA, Cluitmans PJ, Boon PA. An investigation of the phase locking index for measuring of interdependency of cortical source signals recorded in the EEG. Biol Cybern. 2009;100:129–146. doi: 10.1007/s00422-008-0283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schack B, Klimesch W. Frequency characteristics of evoked and oscillatory electroencephalic activity in a human memory scanning task. Neurosci Lett. 2002;331:107–110. doi: 10.1016/s0304-3940(02)00846-7. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011;221:555–563. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar E. Functional aspects of alpha oscillations in the EEG. Int J Psychophysiol. 2001;39:151–158. doi: 10.1016/s0167-8760(00)00138-0. [DOI] [PubMed] [Google Scholar]
- Schurmann M, Basar-Eroglu C, Basar E. Gamma responses in the EEG: elementary signals with multiple functional correlates. Neuroreport. 1997;8:531–534. doi: 10.1097/00001756-199701200-00030. [DOI] [PubMed] [Google Scholar]
- Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: the S transform. IEEE Trans Signal Process. 1996;44:998–1001. [Google Scholar]
- Sun L, Grutzner C, Bolte S, Wibral M, Tozman T, Schlitt S, Poustka F, Singer W, Freitag CM, Uhlhaas PJ. Impaired gamma-band activity during perceptual organization in adults with autism spectrum disorders: evidence for dysfunctional network activity in frontal–posterior cortices. J Neurosci. 2012;32:9563–9573. doi: 10.1523/JNEUROSCI.1073-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher RW. Coherence, phase differences, phase shift, and phase lock in EEG/ERP analyses. Dev Neuropsychol. 2012;37:476–496. doi: 10.1080/87565641.2011.619241. [DOI] [PubMed] [Google Scholar]
- Tiesinga PH, Fellous JM, Jose JV, Sejnowski TJ. Computational model of carbachol-induced delta, theta, and gamma oscillations in the hippocampus. Hippocampus. 2001;11:251–274. doi: 10.1002/hipo.1041. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Uhlhaas PJ, Pipa G, Neuenschwander S, Wibral M, Singer W. A new look at gamma? High- (>60 Hz) gamma-band activity in cortical networks: function, mechanisms and impairment. Prog Biophys Mol Biol. 2011;105:14–28. doi: 10.1016/j.pbiomolbio.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Waite JJ, Chen AD, Wardlow ML, Thal LJ. Behavioral and biochemical consequences of combined lesions of the medial septum/diagonal band and nucleus basalis in the rat when ibotenic acid, quisqualic acid, and AMPA are used. Exp Neurol. 1994;130:214–229. doi: 10.1006/exnr.1994.1200. [DOI] [PubMed] [Google Scholar]
- Wenk GL. The nucleus basalis magnocellularis cholinergic system: one hundred years of progress. Neurobiol Learn Mem. 1997;67:85–95. doi: 10.1006/nlme.1996.3757. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Danysz W, Mobley SL. Investigations of neurotoxicity and neuroprotection within the nucleus basalis of the rat. Brain Res. 1994a;655:7–11. doi: 10.1016/0006-8993(94)91590-3. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Stoehr JD, Quintana G, Mobley S, Wiley RG. Behavioral, biochemical, histological, and electrophysiological effects of 192 IgG-saporin injections into the basal forebrain of rats. J Neurosci. 1994b;14:5986–5995. doi: 10.1523/JNEUROSCI.14-10-05986.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink AM, Bernard F, Salvador R, Bullmore E, Suckling J. Age and cholinergic effects on hemodynamics and functional coherence of human hippocampus. Neurobiol Aging. 2006;27:1395–1404. doi: 10.1016/j.neurobiolaging.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Wirth M, Villeneuve S, Haase CM, Madison CM, Oh H, Landau SM, Rabinovici GD, Jagust WJ. Associations between Alzheimer disease biomarkers, neurodegeneration, and cognition in cognitively normal older people. JAMA Neurol. 2013;70:1512–1519. doi: 10.1001/jamaneurol.2013.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. The critical role of cholinergic basal forebrain neurons in morphological change and memory encoding: a hypothesis. Neurobiol Learn Mem. 1996;66:258–266. doi: 10.1006/nlme.1996.0068. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Acetylcholine, cognition, and consciousness. J Mol Neurosci. 2006;30:219–222. doi: 10.1385/JMN:30:1:219. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems mediate action from movement to higher consciousness. Behav Brain Res. 2011;221:488–498. doi: 10.1016/j.bbr.2009.12.046. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Hernit MC, Butcher LL. Cholinergic and non-cholinergic projections from the rat basal forebrain revealed by combined choline acetyltransferase and Phaseolus vulgaris leucoagglutinin immunohistochemistry. Neurosci Lett. 1986;66:281–286. doi: 10.1016/0304-3940(86)90032-7. [DOI] [PubMed] [Google Scholar]
- Yener GG, Basar E. Sensory evoked and event related oscillations in Alzheimer’s disease: a short review. Cognit Neurodyn. 2010;4:263–274. doi: 10.1007/s11571-010-9138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yener GG, Basar E. Brain oscillations as biomarkers in neuropsychiatric disorders: following an interactive panel discussion and synopsis. Suppl Clin Neurophysiol. 2013a;62:343–363. doi: 10.1016/b978-0-7020-5307-8.00016-8. [DOI] [PubMed] [Google Scholar]
- Yener GG, Basar E. Biomarkers in Alzheimer’s disease with a special emphasis on event-related oscillatory responses. Suppl Clin Neurophysiol. 2013b;62:237–273. doi: 10.1016/b978-0-7020-5307-8.00020-x. [DOI] [PubMed] [Google Scholar]
- Yener GG, Guntekin B, Orken DN, Tulay E, Forta H, Basar E. Auditory delta event-related oscillatory responses are decreased in Alzheimer’s disease. Behav Neurol. 2012;25:3–11. doi: 10.3233/BEN-2012-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yener GG, Kurt P, Emek-Savas DD, Guntekin B, Basar E. Reduced visual event-related delta oscillatory responses in amnestic mild cognitive impairment. J Alzheimers Dis. 2013;37:759–767. doi: 10.3233/JAD-130569. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Kolev V, Heinrich H, Woerner W, Banaschewski T, Rothenberger A. Developmental event-related gamma oscillations: effects of auditory attention. Eur J Neurosci. 2002;16:2214–2224. doi: 10.1046/j.1460-9568.2002.02286.x. [DOI] [PubMed] [Google Scholar]