Abstract
Objective
This study examined the effects of chronic focal lesions to the lateral prefrontal cortex (LPFC) or orbitofrontal cortex (OFC) on neuropsychological test performance and self-reported executive functioning in everyday living.
Methods
Fourteen adults with OFC lesions were compared to 10 patients with LPFC injuries and 21 healthy controls. Neuropsychological tests with emphasis on measures of cognitive executive function were administered along with the Behavior Rating Inventory of Executive Functions (BRIEF-A) and a psychiatric screening instrument.
Results
The LPFC group differed from healthy controls on neuropsychological tests of sustained mental effort, response inhibition, working memory and mental switching, while the BRIEF-A provided more clinically important information on deficits in everyday life in the OFC group compared to the LPFC group. Correlations between neuropsychological test results and BRIEF-A were weak, while the BRIEF-A correlated strongly with emotional distress.
Conclusions
It was demonstrated that LPFC damage is particularly prone to cause cognitive executive deficit, while OFC injury is more strongly associated with self-reported dysexecutive symptoms in everyday living. The study illustrates the challenge of identifying executive deficit in individual patients and the lack of strong anatomical specificity of the currently employed methods. There is a need for an integrative methodological approach where standard testing batteries are supplemented with neuropsychiatric and frontal-specific rating scales.
Keywords: Executive functioning, frontal lobe, neuropsychological, assessment, cognition
Introduction
Wilful control of thoughts, emotions and behaviour is an intrinsically human capacity and a prerequisite for adaptive functioning. This capacity relies on complex higher-order mental processes typically denoted as executive functions. Executive functioning is called for in non-routine situations where habitual responses and prior experiences are not sufficient. When the ability to maintain meta-control over mental processes breaks down, the information processing system is rendered inflexible and increasingly stimulus-bound [1]. Executive deficit is a common result of acquired brain injury and is a potent negative predictor for long-term outcome [2].
There is emerging consensus that executive functioning is not a unitary function, but a set of inter-related capacities resulting from activity in a collection of anatomically and functionally independent, but closely interacting networks. Widespread brain regions are involved, with the prefrontal cortex (PFC) playing a central role [3]. An important anatomical distinction is that between the dorsolateral and ventral/orbital divisions of the PFC [3-6]. The dorsolateral PFC (DLPFC) is primarily associated with cognitive executive functions such as controlled attention, working memory, strategic memory, conceptual reasoning, goal selection, planning, sequencing and set shifting [7]. Injury to the orbitofrontal cortex (OFC) is more strongly associated with altered self-regulatory behaviour such as poor emotional modulation and real-life decision-making. OFC damage tends to affect the ability to utilize cues in the environment to predict future rewarding or aversive events and choose appropriate responses in the context of changing reinforcement contingencies [6, 8]. A recent study of patients with neurodegenerative disease demonstrated this double dissociation as OFC volume loss predicted results on measures of behavioural regulation, while DLPFC volume loss predicted performance on tests of executive cognitive function [9]. Commonly used neuropsychological tests of cognitive executive control are more sensitive to DLPFC injury compared to injury to the ventral parts of the PFC [5]. Thus, while patients with OFC lesions can be spared the cognitive executive problems caused by lateral prefrontal cortex (LPFC) injury, they might experience devastating behavioural regulation deficit in everyday living, resulting in poor interpersonal and occupational functioning. The effects of frontal lobe damage in general tend to result in reduced but within normal range neuropsychological performance [10]. However, capturing the consequences of OFC lesions in formal assessments poses a particularly great clinical challenge and highlights the need for standardized measures with predictive value in relation to everyday functioning [11].
A questionnaire that aims at identifying executive problems in everyday living is the Behavior Rating Inventory of Executive Functions [12]. It exists in a child (BRIEF) and adult (BRIEF-A) version and has gained increased clinical popularity over recent years, as it is widely presumed that this inventory helps in detecting signs of executive deficit that may not be exhibited in neuropsychological test protocols. The BRIEF has been investigated more rigorously than the BRIEF-A [13]. One interesting finding, however, is that self-reported working memory problems on the BRIEF-A were related to smaller bifrontal volume in patients with schizophrenia [14]. A study of patients with mild cognitive impairment suggests that the BRIEF-A is sensitive in detecting subjectively experienced executive deficit that is not detected with neuropsychological tests [15]. BRIEF-A scores were unrelated to IQ. However, informant-, but not self-report, was related to neuropsychological test results in brain injured patients evaluated for work capacity [16]. An association between focal frontal lobe injury and a variety of BRIEF scores has been demonstrated in children [17, 18]. To the authors’ knowledge, similar studies have not been conducted in relation to the BRIEF-A, rendering the assumed connection between BRIEF-A scores and frontal systems pathology largely theoretical in patients with acquired brain injury. Furthermore, the authors are not aware of studies exploring lesions to different anatomical sub-divisions of the PFC relative to the BRIEF or the BRIEF-A.
In the current study, sub-groups of adult patients with chronic focal frontal lobe lesions in the LPFC or OFC were compared to each other and to healthy controls on neuropsychological tests, the BRIEF-A, and a psychiatric screening instrument. Consistent with previous reports, it was anticipated that LPFC lesions would predominantly affect cognitive executive control, while OFC lesions were predicted to be more strongly related to indices of self-regulatory behaviour. Thus, it was hypothesized that impaired executive functioning in the LPFC group would be detectable with neuropsychological measures of executive functioning. The study aimed at exploring whether the BRIEF-A would aid in identifying problem areas resulting from prefrontal injury in general and OFC damage in particular. Moreover, the relationship was examined between neuropsychological performance measures, executive functioning in everyday living and emotional distress. In accord with previous studies [15, 18], a weak association between the BRIEF-A and performance measures of executive functioning was expected.
Methods
Participants
Twenty-four patients and 21 healthy controls were included. The OFC group consisted of 14 and the LPFC group of 10 patients (see Figures 1 and 2).
Figure 1.
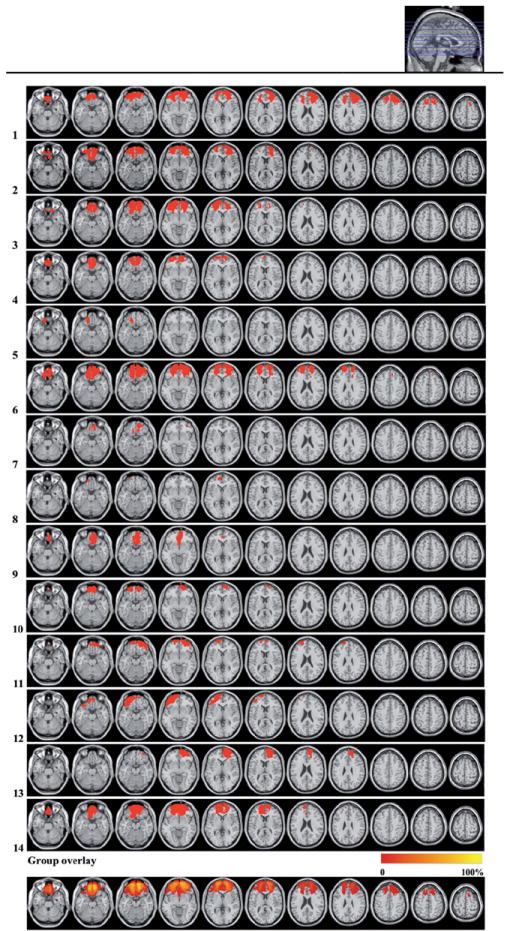
Lesion reconstructions for the OFC group. Individual patients (1–14) and group overlay (bottom row). Eighty-four percent of the cortical lesion volume was within Brodmann Areas 10, 11 and 47.
Figure 2.
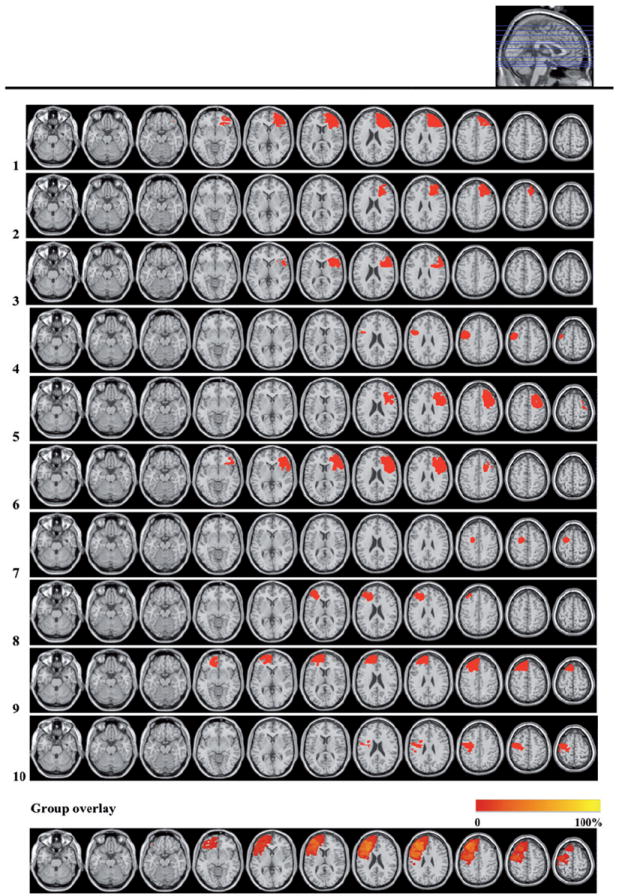
Lesion reconstructions of the LPFC group. Individual patients (1–10) and group overlay (bottom row). Seventy-one percent of the cortical lesion volume was within Brodmann Areas 6, 8, 9, 44, 45 and 46.
* Note that the group overlay presents all patients as having left hemisphere lesions to aid comparison
Patient inclusion was based on focal frontal brain lesions indicated on pre-existing computer tomography (CT) and/or Magnetic Resonance Imaging (MRI) scans. Lesion reconstructions were based on structural MRIs obtained after inclusion and were verified by the neuroradiologist (PDT), neurologist (RTK) and neurosurgeon (TRM) in the research group. Lesion characteristics are displayed in Table I. Patients were matched with healthy controls by age, sex and years of education (Table II). Participants with a history of serious psychiatric disease, drug or alcohol abuse requiring treatment, pre-morbid head injury, pre-/comorbid neurological disease, IQ < 85, substantial aphasia, visual neglect or marked sensory impairment were excluded from participation. Testing took place at least 6 months after injury or surgery. The OFC group was tested at a mean of 32 (±17.4) months after injury/surgery and the LPFC group at a mean of 49 (±36.4) months. This difference was non-significant. With the exception of one healthy control and one OFC patient, all participants were right-handed.
Table I.
Patient sample description. Patient characteristics: Injury aetiology, time since injury, lesion size and affected Brodmann areas.*
| Subject | Injury aetiology | Months post-injury | Lesion size (ccm)* | BA right hemisphere | BA left hemisphere |
|---|---|---|---|---|---|
| OFC group | |||||
| 1 | TBI | 45 | 139.5 | 6, 8, 9–11, 32, 45–47 | 8–11, 32, 46, 47 |
| 2 | Meningioma | 13 | 69.1 | 10, 11, 32, 46, 47 | 10, 11, 47 |
| 3 | Meningioma | 49 | 79.8 | 10, 11, 47 | 10, 11, 46, 47 |
| 4 | Meningioma | 13 | 39.7 | 10, 11 | 10, 11, 47 |
| 5 | Meningioma | 19 | 5.1 | 11 | |
| 6 | Meningioma | 43 | 134.8 | 9–11, 32, 46, 47 | 9–11, 32, 46, 47 |
| 7 | Meningioma | 27 | 7.2 | 11, 47 | 11 |
| 8 | Meningioma | 44 | 2.9 | 10, 11 | |
| 9 | LGG | 7 | 28.6 | 10, 11, 25 | 11, 25 |
| 10 | TBI | 44 | 23.6 | 10, 11 | 11 |
| 11 | TBI | 59 | 33.3 | 10, 11, 47 | 10, 11, 46 |
| 12 | TBI | 15 | 41.1 | 11 | 10, 11, 38, 45–47 |
| 13 | Meningioma | 52 | 48.7 | 9–11, 32, 46, 47 | |
| 14 | Meningioma | 20 | 96.8 | 10, 11, 47 | 10, 11, 24, 25, 32, 47 |
| Mean lesion size (SD) | 53.6 (4.5) | 29.8 | 23.9 | ||
| LPFC group | |||||
| 1 | LGG | 33 | 80 | 9–11, 32, 44–47 | |
| 2 | LGG | 30 | 34.4 | 8, 9, 32, 44–46 | |
| 3 | LGG | 51 | 29.3 | 6, 44, 45 | |
| 4 | LGG | 27 | 24.8 | 4, 6, 9, 44 | |
| 5 | LGG | 68 | 60.1 | 4, 6, 8, 9, 32, 44–46 | |
| 6 | LGG | 112 | 72.8 | 6, 9, 32, 44–47 | |
| 7 | LGG | 9 | 10.1 | 6 | |
| 8 | LGG | 54 | 25.0 | 9, 10, 32, 45–47 | |
| 9 | LGG | 6 | 77.5 | 6, 8–11, 24, 32, 46, 47 | |
| 10 | LGG | 104 | 39.9 | 3, 4, 6 | |
| Mean lesion size (SD) | 45.4 (2.5) | 55.3 | 35.5 | ||
Lesions that comprise < 0.5 ccm in any given BA are not reported.
Table II.
Patient sample description. Demographic characteristics.*
| Control | OFC | LPFC | ANOVA, p-values | |
|---|---|---|---|---|
| n (female) | 21 (10) | 14 (8) | 10 (5) | 0.86 |
| Years of age | 42.9 (11.9) | 47.1 (10.7) | 42.9 (9.8) | 0.50 |
| Years of education | 13.5 (2.3) | 12.9 (2.3) | 12.7 (2.4) | 0.62 |
| Months since injury | na | 32 (17.4) | 49.4 (36.4) | 0.13 |
| GOS-E | na | 6.4 (1.1) | 5.6 (0.5) | 0.08 |
Values are means (SD).
BA, Brodmann areas; TBI, traumatic brain injury; LGG, low grade glioma.
Participants gave informed consent to participation. Controls received 500 NOK (~80 US$) for participation in the entire research programme (neuropsychological assessment, EEG, structural and functional MRI). The study was approved by the Norwegian Regional Committee for Medical Research Ethics, Region South.
Functional outcome
Functional outcome of the patients was classified using the Glasgow Outcome Scale Extended (GOS-E) [19]. GOS-E is a hierarchical scale in which the patient’s overall functional outcome is estimated based on the lowest item score obtained.
Neuropsychological tests
Computations of full scale, verbal and performance IQ were based on the four sub-tests of the Wechsler Abbreviated Scale of Intelligence (WASI) [20]; Vocabulary, Similarities, Block Design and Matrix Reasoning. Digit Span and Letter-Number Sequencing from the Wechsler Adult Intelligence Scale Third Edition (WAIS-III) [21] were included as measures of working memory. Verbal learning and memory was assessed with the California Verbal Learning Test Second Edition (CVLT-II) [22]. Statistical analyses were performed on all available measures in the computerized scoring program for the CVLT-II [23]. Visuospatial learning and memory were examined with the Brief Visuospatial Memory Test-Revised (BVMT-R) [24]. The following sub-tests from the Delis-Kaplan Executive Function System (D-KEFS) [25] were included: Trail Making Test (TMT), Design Fluency, Verbal Fluency and Colour–Word Interference Test (CWI).
Questionnaires
Self-reported symptoms of executive problems in everyday living were assessed with the BRIEF-A [12]. A close relative of the patient completed the informant version. The BRIEF-A states 75 behaviours to be rated as often, sometimes or never being a problem over the past 4 weeks. Scores result in nine sub-scale scores, one global executive composite (GEC) and two index scores indicating problems with metacognition (MI) and behavioural regulation (BRI). The Symptom Checklist 90 Revised (SCL-90-R) [26] was used to screen for emotional distress, with a clinical cut-off at t-scores higher than 65.
Statistical methods
SPSS 18 for Windows (SPSS Inc.) was used. Demographic, test- and questionnaire data were analysed using one-way ANOVA with Group (Control, OFC and LPFC groups) as between-subjects factor. In cases of significant differences between patient groups, lesion volume was entered as a covariate in an ANCOVA. As all LPFC cases were unilateral lesions, ANOVAs were repeated for left and right lesioned patients separately. Bonferroni corrected p-values are reported in post-hoc analyses. Effect size was computed using partial eta-squared. There were only two cases in which the application of non-parametric statistics (independent samples Kruskal-Wallis test) on non-normally distributed variables (demonstrated by the Shapiro-Wilks test) changed the result. In these cases, the non-parametric statistics are reported. The relationship between measures was explored with Pearson two-tailed correlation coefficients. Results are reported with a significance level of ≤ 0.05.
Results
Gross functional outcome
Scores on the GOS-E categorized both patient groups as ‘Moderate Recovery, Upper level’ an outcome level that characterizes patients who are capable of living an independent life despite having disabilities due to brain injury [27]. The somewhat higher score in the OFC group (6.4) compared to the LPFC group (5.6) did not reach significance (p < 0.08).
Performance-based neuropsychological tests
Group means and statistical comparisons on neuropsychological test measures are reported in Table III.
Table III.
Neuropsychological performance measures. Group means and statistical comparisons.a
| Control
|
OFC
|
LPFC
|
ANOVA
|
Part. Eta2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | F-value (df 2,42) | p < | ||
| 2A. The WASI IQ scores, WASI sub-tests and the Digit Span and Letter-Number Sequencing from the WAIS-III | |||||||||
| Total IQ | 112.8d | 8.7 | 106.9 | 11.9 | 98.9b | 11.0 | 6.3 | ** | 0.23 |
| Verbal IQ | 108.8d | 8.8 | 102.8 | 12.0 | 96.0b | 16.2 | 4.1 | * | 0.17 |
| Vocabulary | 63.5d | 5.4 | 58.4 | 6.4 | 55.1b | 11.3 | 4.9 | ** | 0.19 |
| Similarities | 38.3d | 3.9 | 36.3 | 5.9 | 33.0b | 5.3 | 4.0 | * | 0.16 |
| Performance IQ | 113.2 | 9.0 | 109.2 | 12.9 | 103.6 | 9.4 | 2.9 | n.s. | 0.12 |
| Block Design | 50.4 | 11.5 | 48.0 | 13.0 | 36.8 | 12.4 | 4.4 | * | 0.17 |
| Matrix Reasoning | 27.2d | 3.4 | 27.0 | 3.5 | 25.0b | 4.7 | 1.3 | n.s. | 0.06 |
| Digit Span Total | 16.8 | 4.0 | 14.7 | 3.2 | 13.7 | 2.9 | 3.0 | n.s. | 0.13 |
| Digit Span Forward | 6.1 | 1.4 | 5.7 | 0.8 | 5.4 | 1.1 | 1.3 | n.s. | 0.06 |
| Digit Span Backward | 5.4 | 1.2 | 4.8 | 1.5 | 4.5 | 1.2 | 1.9 | n.s. | 0.08 |
| Letter-Number Sequencing | 12.0d | 2.6 | 10.0 | 2.9 | 9.5b | 1.2 | 4.4 | * | 0.17 |
| 2B. The Delis-Kaplan Executive Function System (D-KEFS): Trail Making Test (TMT), Design Fluency, Verbal Fluency and Colour-Word Interference Test (CWI) | |||||||||
| TMT 1 | 20.5 | 3.8 | 21.2 | 5.9 | 22.4 | 6.1 | 0.5 | n.s. | 0.02 |
| TMT 2 | 32.4 | 9.7 | 32.6 | 12.8 | 35.9 | 9.6 | 0.4 | n.s. | 0.02 |
| TMT 3 | 32.2 | 11.3 | 33.2 | 11.9 | 35.9 | 8.6 | 0.4 | n.s. | 0.02 |
| TMT 4 | 71.3 | 21.1 | 75.9 | 29.0 | 97.1 | 32.9 | 3.3 | n.s. | 0.14 |
| TMT 5 | 24.7 | 10.6 | 23.9 | 9.4 | 30.3 | 9.5 | 1.4 | n.s. | 0.06 |
| Design Fluency 1 | 10.0 | 2.9 | 8.9 | 3.7 | 7.7 | 2.5 | 1.9 | n.s. | 0.08 |
| Design Fluency 2 | 11.5 | 3.9 | 10.6 | 4.8 | 8.8 | 2.8 | 1.6 | n.s. | 0.07 |
| Design Fluency 3 | 10.2 | 2.7 | 9.4 | 3.2 | 7.9 | 2.1 | 2.4 | n.s. | 0.10 |
| Verbal Fluency 1 – Phonemic | 44.8 | 10.4 | 41.3 | 15.0 | 32.4b | 8.4 | 3.8 | * | 0.15 |
| Verbal Fluency 2 – Semantic | 40.4 | 10.4 | 47.0 | 9.4 | 41.7 | 6.3 | 3 | n.s. | 0.12 |
| Verbal Fluency 3 – Switching | 14.5 | 2.6 | 14.7 | 3.5 | 13 | 3.5 | 1.1 | n.s. | 0.05 |
| Verbal Fluency 3 correct switches | 13.8 | 2.4 | 14.1 | 3.8 | 12.2 | 3.6 | 1.9 | n.s. | 0.08 |
| CWI 1 – Colour naming | 29.4d | 4.5 | 28.8d | 4.9 | 37.2b | 6.8 | 9.4 | ** | 0.31 |
| CWI 2 – Word reading | 22.4 | 3.5 | 22.0 | 4.0 | 24.0 | 6.6 | 0.6 | n.s. | 0.03 |
| CWI 3 – Inhibition | 51.1d | 10.7 | 50.5d | 18.2 | 68.4b | 21.2 | 4.7 | * | 0.18 |
| CWI 4 – Inhibition/switching | 60.5d | 13.7 | 62.0 | 19.6 | 82.0b | 32.9 | 3.9 | * | 0.16 |
| 2C. The California Verbal Learning Test (CVLT-II) | |||||||||
| Total learning trial 1–5 | 54.1 | 9.8 | 51.9 | 9.1 | 48.8 | 10.8 | 1.0 | n.s. | 0.05 |
| Short-term free recall | 11.5 | 3.5 | 11.3 | 2.8 | 10.1 | 3.6 | 0.6 | n.s. | 0.03 |
| Short-term recall interference list | 6.6 | 3.1 | 5.6 | 1.9 | 5.2 | 1.5 | 1.4 | n.s. | 0.06 |
| Long-term free recall | 12.4 | 3.0 | 12.4 | 2.5 | 12.1 | 3.4 | 1.2 | n.s. | 0.05 |
| 2D. The Brief Visuospatial Memory Test-Revised (BVMT-R) | |||||||||
| Learning trial 1 | 5.3 | 1.9 | 4.4 | 1.8 | 4.2 | 2.0 | 1.6 | n.s. | 0.07 |
| Learning trial 2 | 9.0 | 1.5 | 7.3 | 2.8 | 7.2 | 1.9 | 3.6 | * | 0.15 |
| Learning trial 3 | 10.8c, d | 0.9 | 9.3b | 2.1 | 8.5b | 2.3 | 7.0 | ** | 0.25 |
| Total learning trial 1–3 | 25.1d | 3.1 | 21.2 | 6.2 | 19.6b | 5.2 | 5.4 | ** | 0.21 |
| Learning | 5.5 | 2.0 | 4.6 | 1.3 | 4.2 | 1.8 | 2.0 | n.s. | 0.09 |
| Delayed recall | 10.7d | 1.1 | 9.2 | 2.3 | 8.7b | 2.0 | 5.5 | ** | 0.21 |
| Percentage recall | 98.3 | 6.0 | 98.4 | 14.7 | 100.3 | 9.9 | 0.1 | n.s. | 0.01 |
| Recognition hits | 5.8 | 0.4 | 5.6 | 0.7 | 5.7 | 4.8 | 1.2 | n.s. | 0.06 |
| Recognition discrimination | 5.6c, d | 1.3 | 2.9b | 3.1 | 3.1b | 2.9 | 6.8 | ** | 0.25 |
| Response bias | 0.5 | 0.1 | 0.4 | 0.1 | 0.5 | 0.2 | 0.7 | n.s. | 0.03 |
Values given are means and Standard Deviations; SD. Mean values for WASI and WAIS-III sub-tests are given in raw scores, while IQ measures are reported in IQ scores.
sig diff from Control;
sig diff from OFC;
sig diff from LPFC.
p < 0.05,
p < 0.01,
p < 0.001.
LPFC lesions were associated with significantly lower Total and Verbal IQ than controls. The LPFC group differed significantly from controls on all but the Matrix Reasoning WASI sub-scale and on the WAIS-III Letter-Number Sequencing test (Table III).
The OFC group did not differ from controls on any D-KEFS measures, while the LPFC group performed worse than controls on the Phonemic Verbal Fluency task (condition 1) and was slower than controls on the CWI colour naming (condition 1), response inhibition (condition 3) and on the combined response inhibition and switching task (condition 4). The LPFC group differed from the OFC group on the CWI conditions 1 and 3, representing the only cases of significant differences between the patient groups. The OFC and LPFC groups still differed significantly when lesion volume was entered as covariate in the analyses (CWI 1: F(1,23) = 13.54, p < 0.001, η2 = 0.39; CWI 3: F(1,23) = 5.51, p < 0.03, η2 = 0.21). Although the difference between the controls and the LPFC group on the TMT 4 (number–letter switching) no longer reached significance in non-parametric analysis, the raw data indicated that the LPFC group was markedly slower than both other groups, a difference that was significant in the ANOVA analysis (F(2,42) = 3.31, p < 0.05).
Both patient groups performed significantly worse than controls on the 3rd learning trial and recognition discrimination (indicating more false positives) of the BVMT-R. Only the LPFC group differed significantly from controls on the Total learning and Delayed recall measures. No significant group effects were seen on the CVLT-II.
Questionnaires
Behaviour rating inventory of executive functions (BRIEF-A)
Self-report
The OFC group reported more symptoms than healthy controls on all three main indexes (GEC, MI and BRI) of the BRIEF-A and the four sub-scales Initiate, Working Memory, Shift and Emotional Control. The LPFC group deviated significantly from controls on the Working Memory and Emotional Control sub-scales only (Table IV). The BRIEF-A includes three validity scales indicating whether respondents tend to have a negative response style (Negativity scale), report highly unlikely symptoms (Infrequency scale) or tend to be inconsistent in their report style (Inconsistency scale). In Table IV it can be seen that the LPFC group had a significantly lower mean Infrequency scale score (0.5) than the control group (1.6), while the OFC group had a significantly higher mean score (2.9) than the controls (1.3) on the Inconsistency scale. The BRIEF-A manual [12] states that Infrequency scale scores below 6 and Inconsistency scale scores below 8 indicate valid questionnaire profiles. All three groups performed well within these limits, thus indicating that there is no reason to suspect validity issues in the study sample.
Table IV.
The Behavior Rating Inventory of Executive Function (BRIEF-A)–Self-Report Form and the Symptom Checklist 90 Revised (SCL-90-R). Mean scores and group comparisons.a
| Control
|
OFC
|
LPFC
|
ANOVA
|
Part. Eta2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | F | P < | ||
| 3A. BRIEF-A | |||||||||
| Global Executive Composite (GEC) | 42.9c | 9.2 | 51.9b | 7.8 | 50.8 | 10.9 | 4.8 | ** | 0.19 |
| Metacognition Index (MI) | 44.1c | 8.6 | 52.6b | 10.1 | 50.5 | 9.5 | 3.9 | * | 0.16 |
| Initiate | 43.9c | 7.2 | 53.2b | 14.1 | 52.0 | 8.1 | 4.4 | * | 0.17 |
| Working Memory | 45.8c, d | 7.5 | 59.3b | 10.3 | 55.8b | 11.2 | 9.8 | ** | 0.32 |
| Plan/Organize | 44.4 | 7.1 | 51.5 | 9.0 | 50.0 | 12.7 | 2.9 | * | 0.12 |
| Task Monitor | 46.3 | 10.2 | 51.4 | 9.4 | 49.8 | 9.2 | 1.3 | n.s. | 0.06 |
| Organization of Materials | 44.9 | 10.0 | 45.4 | 6.4 | 45.4 | 6.1 | 0.01 | n.s. | 0.00 |
| Behavioral Regulation Index (BRI) | 42.5c | 9.1 | 51.9b | 7.8 | 51.3 | 12.8 | 4.5 | * | 0.18 |
| Inhibit | 43.7 | 9.1 | 45.4 | 5.3 | 48.7 | 8.5 | 1.4 | n.s. | 0.06 |
| Shift | 43.8c | 6.2 | 54.6b | 11.6 | 50.4 | 14.1 | 4.9 | ** | 0.19 |
| Emotional Control | 44.4c, d | 8.7 | 54.6b | 9.2 | 55.4b | 15.7 | 5.4 | ** | 0.21 |
| Self-Monitor | 42.8 | 9.1 | 47.3 | 7.9 | 46.7 | 8.3 | 1.4 | n.s. | 0.06 |
| Validity Scales | |||||||||
| Negativity | 0.1 | 0.5 | 0.4 | 0.6 | 0.5 | 0.7 | 1.4 | n.s. | 0.06 |
| Infrequency | 1.6d | 1.4 | 0.9 | 0.9 | 0.5b | 0.9 | 3.6 | * | 0.15 |
| Inconsistency | 1.3c | 1.6 | 2.9b | 1.8 | 2.6 | 1.1 | 4.9 | ** | 0.19 |
| 3B. SCL-90-R | |||||||||
| Global Severity Index (GSI) | 46.6c, d | 7.5 | 55.4b | 8.9.0 | 58.3b | 14.0 | 6.3 | ** | 0.23 |
| Somatization Scale (SOM) | 47.5d | 5.9 | 54.0 | 11.8 | 56.7b | 11.3 | 4.0 | * | 0.17 |
| Obsessive-Compulsive Scale (O-C) | 47.9c, d | 8.2 | 60.8b | 11.3 | 63.1b | 15.8 | 8.8 | ** | 0.28 |
| Interpersonal Sensitivity Scale (I-S) | 47.0 | 7.0 | 54.3 | 8.6 | 54.3 | 12.7 | 3.8 | * | 0.13 |
| Depression Scale (DEP) | 47.3d | 8.4 | 55.6 | 8.7 | 59.0b | 16.6 | 4.9 | ** | 0.19 |
| Anxiety Scale (ANX) | 48.0 | 6.7 | 54.9 | 11.7 | 55.1 | 12.9 | 2.7 | n.s. | 0.11 |
| Hostility Scale (HOS) | 45.5c, d | 5.2 | 53.2b | 8.6 | 55.2b | 11.8 | 6.4 | ** | 0.27 |
| Phobic Anxiety Scale (PHOB) | 47.7c | 5.1 | 56.3b | 14.3 | 54.3 | 9.9 | 3.6 | * | 0.14 |
| Paranoid Ideation Scale (PAR) | 46.7 | 7.1 | 52.4 | 9.3 | 52.2 | 13.4 | 2.0 | n.s. | 0.07 |
| Psychotisism Scale (PSY) | 48.3d | 9.1 | 50.0 | 5.8 | 62.7b | 24.4 | 4.1 | * | 0.17 |
Values given are mean t-scores and Standard Deviations; SD.
sig diff from Control;
sig diff from OFC;
sig diff from LPFC.
p < 0.05,
p < 0.01,
p < 0.001.
Informant report
No significant differences were observed between BRIEF-A informant responses in the OFC and LPFC groups, neither did difference-variables for self- and informant-reports differ significantly between groups. The OFC patients reported significantly more problems than their informants with regard to emotional control (self: 54.6, SD = 9.2; informant: 47.4, SD = 7.9; p < 0.02) and working memory (self: 59.3, SD = 10.2; informant: 52.6, SD = 11.7; p < 0.03).
Symptom checklist 90 revised (SCL-90-R)
As reported in Table IV, both patient groups differed significantly from controls on the Global Severity Index (GSI) and additional sub-scales, but the patient groups did not score in the clinical range or differ from each other.
Correlations between cognitive test measures, BRIEF-A and SCL-90-R
The relationship between the BRIEF-A and neuropsychological test results was weak. With the exception of significant correlations between Digit Span total score and self-reported GEC and MI (both r = −0.6, p < 0.03) and Digit Span forward and MI (r = −0.5, p < 0.05) for the OFC group, there were no significant correlations between the self- or informant reported BRIEF-A indexes and any IQ measures, WASI sub-scales or tests of executive functioning (CWI 1 and 3, TMT 4 and Verbal Fluency 1). On the other hand, the self-reported Global Executive Composite (GEC) of the BRIEF-A correlated strongly (r = 0.64–0.98, p < 0.001) with the Global Severity Index (GSI) of the SCL-90-R for all three groups.
Individual test performance and self-report
Table V displays the proportion of individuals in each patient group that performed 1.5 SD worse than the mean of the control group on the measures involving significant group effects. Overall, a relatively larger proportion of patients with LPFC injury were impaired on neuropsychological performance measures, whereas a larger proportion of the OFC patients reported executive problems on the BRIEF-A relative to the LPFC group. LPFC injury was more likely to result in reduced scores on the CWI 1 and 3, whereas the BRIEF-A GEC, Shift and Working Memory scales were most apt to be enhanced in OFC patients.
Table V.
The proportion of individuals in each patient group scoring 1.5 SD worse than the mean score of healthy controls on the measures with significant group differences on the ANOVA.
| OFC, % (n) | LPFC, % (n) | |
|---|---|---|
| WASI & WAIS-III | ||
| Total IQ | 28 (4) | 40 (4) |
| Verbal IQ | 21 (3) | 60 (6) |
| Vocabulary | 35 (5) | 60 (6) |
| Similarities | 28 (4) | 40 (4) |
| Block Design | 14 (2) | 30 (3) |
| Letter-Number Sequencing | 28 (4) | 10 (1) |
| D-KEFS | ||
| TMT 4 | 28 (4) | 40 (4) |
| Verbal Fluency 1 | 28 (2) | 40 (4) |
| CWI – Colour naming | 14 (2) | 50 (5) |
| CWI – Inhibition | 14 (2) | 50 (5) |
| CWI – Inhibition/switching | 21 (3) | 30 (3) |
| BVMT-R | ||
| Learning trial 2 | 49 (7) | 40 (4) |
| Learning trial 3 | 49 (7) | 60 (6) |
| Total learning trial 1–3 | 56 (8) | 50 (5) |
| Delayed recall | 42 (6) | 70 (7) |
| Recognition discrimination | 49 (7) | 50 (5) |
| BRIEF-A | ||
| GEC | 35 (5) | 20 (2) |
| MI | 42 (6) | 40 (4) |
| Initiate | 35 (5) | 40 (4) |
| Working Memory | 63 (9) | 40 (4) |
| BRI | 21 (3) | 20 (2) |
| Shift | 56 (8) | 20 (2) |
| Emotional Control | 49 (7) | 40 (4) |
Discussion
Neuropsychological performance and self-reported executive functioning in everyday living was studied in patients with chronic focal lesions to the orbital or lateral sub-divisions of the PFC. As hypothesized, the effects of LPFC lesions were predominantly observed on tests of cognitive executive function, with reductions on tasks demanding sustained mental effort, working memory, response inhibition and mental switching. OFC injury was more strongly associated with indices of self-regulatory behaviour in everyday living. While the LPFC group differed significantly from healthy controls on 15 neuropsychological test measures, the OFC patients differed from controls on two performance measures only. Conversely, the OFC patients differed from healthy controls on seven measures of self-reported symptoms of executive impairment, compared to two measures for the LPFC group.
Lesion effects on cognitive performance measures
The CWI test was the only task where the LPFC group differed significantly from OFC patients. Lesion volume did not explain this effect, giving strong support to the CWI colour naming and inhibition conditions being particularly prone to be reduced after LPFC damage [10]. Of note, 50% of the LPFC group performed more than 1.5 SD below the control group mean on the CWI compared to 14% of the OFC group, adding relevance in individual clinical assessments.
The LPFC group performed worse than controls on the Phonemic but not the Semantic Fluency sub-test, in accord with prior studies showing impaired phonemic and normal semantic fluency after lateral and posterior medial PFC lesions [28]. Reduced performance on the TMT-4 in the LPFC group is also in accord with earlier studies [29-31].
Both patient groups performed worse than controls on learning and recognition discrimination of the BVMT-R, the latter due to patients giving more false positive responses. Normal score on the first learning trial and normal percentage recall indicates unaffected short- and long-term visual memory. Thus, reduced learning and recognition performances might reflect disturbed attentional effort canalized to the task, in line with the PFC being involved in organizational and strategic aspects of memory [32]. This was not a uniform finding, however. In contrast to other studies [33, 34], significant group effects were not observed in relation to verbal learning and memory on the CVLT-II.
The LPFC group in this study had lower Total and Verbal IQ than healthy controls. While Tranel et al. [35] did not find IQ effects when comparing patients with ventromedial or dorsolateral lesions to patients with extrafrontal injury, Anderson et al. [17] found lower Full Scale IQ and no laterality effects in children with focal PFC lesions compared to healthy controls. In the present study, all three groups had IQ scores within the normal range and patients and healthy controls were well matched with regard to age and educational level. The Verbal IQ of the LPFC sub-group with left hemisphere lesions was the same (mean = 96, SD = 16) as in the sub-group with right hemisphere lesions (mean = 96, SD = 18), suggesting that the lower IQ-measures were not driven by a left-hemisphere language impairment. The cognitive effects seen in the LPFC group were not undifferentiated, rather there was a selective reduction of executive cognitive functions and IQ. The IQ effects found are thus understood as related to the injury.
Self-reported executive functions in everyday living
Although neuropsychological test results in patients with OFC injury rarely differed significantly from those of healthy controls, the patients reported more difficulties in everyday executive function. The OFC group differed from healthy controls on all three main indexes of the BRIEF-A as well as on four sub-scales, while the LPFC group had elevated scores on two sub-scales only. On an individual level, the BRIEF-A added additional information across lesion sites as between 20–63% of patients in both groups rated themselves more than 1.5 SD above the healthy control group on all BRIEF-A measures. The GEC, Shift and Working Memory sub-scales were particularly apt to be increased in OFC patients, as 35, 56 and 63% of the patients fell above 1.5 SD of the scores of the controls on these measures compared to 20, 20 and 40%, respectively, for the LPFC group. Of note, the reported difficulties of shifting and working memory on the BRIEF-A were not accompanied by impaired performance on neuropsychological tests of these functions in the OFC group.
As the BRI index is intended to reflect behavioural dysexecutive symptoms and the MI measures cognitive executive control, one might have expected the two indexes to differentiate between the patient groups. The results did not support such a hypothesis. It was confirmed that the BRIEF-A did not covary with IQ [16] or with standardized measures of executive function. This is in line with several investigations of the children’s BRIEF [18, 36, 37] and suggests that the BRIEF-A as intended taps into other aspects of executive functions than neuropsychological test measures. On the other hand, the high correlation between the BRIEF-A GEC and the GSI of the SCL-90-R reveals a strong association between the BRIEF-A and general emotional distress and possibly a lack of specificity to executive functions. A similar concern has been raised in relation to the children’s version of the BRIEF [38].
The only difference found between informants’ and patients’ reports on the BRIEF-A was that the OFC group reported more symptoms than their relatives on the Emotional Control and Working Memory sub-scales. This could be related to injury aetiology, as nine of 14 OFC patients suffered meningioma, where tumour resection typically results in marked functional improvement. This might cause the relatives to report the remaining symptoms as relatively less burdensome than if the symptoms occurred abruptly. The use of self-rating tools is prone to result in inaccurate reports of executive deficit in patient groups known to exhibit reduced subjective awareness of their problems [8]. Although informant reports are helpful in the measurement of self-awareness issues after brain injury, it does not represent a ‘gold standard’ of actual functioning [39]. Insight is a complex multi-level phenomenon and the risk of under-reporting of symptoms is not in itself a valid argument for the exclusion of self-report measures as part of a multilevel assessment after PFC lesions. In fact, the data reported here indicate that self-reported symptoms on the BRIEF-A were in agreement with informant reports and provided valuable information on areas of perceived impairment in patients with PFC lesions.
The use of US norms resulted in mean healthy control scores ranging from 0.5–1 SD below the assumed mean of 50. This suggests that a score of ~40 might be normal in the healthy Norwegian population, rendering a patient score of 55–60 of potential clinical interest. Clinical samples used for validation purposes tended to differ between 0.5–1 SD from the normative mean (see BRIEF-A manual [12]), a difference size in line with the present study. Cultural differences in the perception of problem behaviour, demographic and socioeconomic factors might contribute. Firm conclusions await future non-US studies with larger samples of healthy controls, but these preliminary data indicate a need to establish non-US norms.
Both patient groups had higher scores than controls on the GSI and several sub-scales of the SCL-90-R. This indicates increased levels of emotional distress, although the scores were below clinical cutoff values and did not indicate that the patients represented a clinical psychiatric sample. It is well known that neurological populations tend to display elevations on the SCL-90-R [40], which may be interpreted as reflecting the concerted effect of structural lesions and concern regarding experienced changes in level of functioning. For the brain tumour patients in particular, treatment effects and uncertainty about future prognosis will add to the emotional strain [40].
Study limitations
The sample size of this study was limited, although comparable to previous studies of focal frontal lobe injuries [29, 30, 34, 41]. Obtaining large sample sizes with focal frontal damage is challenging, as there is no predisposition of a particular neurological disease to the frontal lobes [3]. By international standards, the OFC cohort presented here represents a substantial group. In the research programme that this work forms part of, efforts are made to establish an international multi-centre cohort of patients, including an extension of the LPFC group as well as establishment of a group with medial PFC lesions. The main goal of lesion studies is to contribute to knowledge of what distinct anatomical areas are necessary for normal performance, rendering anatomical specificity as important as sample size. However, it is acknowledged that, given the sample size, with relatively many variables being explored and a significance level of 0.05, one cannot exclude the possibility of Type 1 errors. On the other hand, the results are in accord with the main hypotheses that was explored in this study, as it was expected that impaired executive functioning in the LPFC group would be detectable with neuropsychological measures. With regard to the lesion-effects on BRIEF-A, the study was of a more exploratory nature and there is need of replication of findings in larger samples. Related to the issue of sample size is a potential confounding between lesion aetiology and anatomical sub-groups, as nine of 14 OFC patients had suffered meningiomas and all LPFC patients had low-grade gliomas. In the chronic stages of brain injury, there is however reason to expect lesion location to be more important than aetiology [3]. Although some overlap did exist between the patient groups in ventrolateral areas, the core lesioned areas were clearly within functional neuroanatomical sub-divisions of OFC and LPFC. An additional issue related to anatomy is lesion volume. This potential caveat is reduced by the fact that mean lesion size did not differ significantly between groups. Additionally, in the only case of significant patient group differences, the CWI test, entering lesion volume as a co-variate did not diminish the group effect, supporting a distinct effect of lesion location on performance. Finally, a note of methodological selection is warranted. Although the patients in this study were subjected to a broad neuropsychological assessment, it would have been of interest to contrast the results with not only a rating scale such as the BRIEF-A, but also to performance-based measures of complex planning, strategic reasoning and prospective memory [42].
Clinical implications and conclusions
Diagnosing executive deficit represents one of the most complex assessment issues for clinical neuro-psychologists. The findings of this study support that while patients with frontal lobe injury typically perform worse than healthy controls, the results are often within normal limits [10]. The results largely demonstrate the complexity of diagnosing executive dysfunction in individual patients and the lack of strong anatomical specificity of the currently employed methods. However, the study showed that LPFC damage is particularly prone to cause cognitive executive deficit [5], while OFC injury is more strongly associated with self-reported dysexecutive problems in everyday living [4]. The effects of LPFC injury were seen in tasks demanding sustained mental effort, response inhibition, working memory and mental switching. It was furthermore demonstrated that the BRIEF-A provides important information not conveyed by neuropsychological tests, particularly in the OFC group. Gioia et al. [43] note that, while the BRIEF is helpful in capturing executive profiles in clinical groups, it is not diagnostic in its own right and should be used in a broad clinical context. As it is unlikely that a single measure will capture the range of executive functions supported by OFC, the need for an integrative approach where standard testing batteries are supplemented with neuropsychiatric and frontal-specific rating scales has been stressed [11]. It has also been noted that experimental tasks related to executive functioning could be of diagnostic value in the future given the establishment of adequate standardization and normative data. Of note, this group has recently demonstrated that both LPFC and OFC patients exhibit marked electrophysiological indices of altered novelty detection in an Event-related Potentials study, indicating disturbances of basic aspects of attentional control after PFC injury [44].
Acknowledgments
We would like to thank Clay Campbell Clayworth and Haakon Engen for support in establishing routines for lesion reconstructions. We thank Maya Tomstad and Martin Seem Sundal for coding of data.
This research is supported by the South-Eastern Norway Regional Health Authority (grants SUN-001-SS and 2008047) and the Research Council of Norway (grant no. 186504/V50 to author AKS) as well as the National Institute of Neurological Disorders (NINDS; grant NS21135 to author RTK). The work forms part of a doctoral thesis to be submitted to the Department of Psychology, University of Oslo, Norway.
Footnotes
Declaration of Interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Fernandez-Duque D, Baird JA, Posner MI. Executive attention and metacognitive regulation. Consciousness and Cognition. 2000;9:288–307. doi: 10.1006/ccog.2000.0447. [DOI] [PubMed] [Google Scholar]
- 2.Draper K, Ponsford J. Cognitive functioning ten years following traumatic brain injury and rehabilitation. Neuropsychology. 2008;22:618–625. doi: 10.1037/0894-4105.22.5.618. [DOI] [PubMed] [Google Scholar]
- 3.Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues in Clinical Neuroscience. 2007;9:141–151. doi: 10.31887/DCNS.2007.9.2/rbonelli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stuss DT. New approaches to frontal lobe testing. In: Miller BL, Cummings JL, editors. The human frontal lobes Functions and disorders. New York: The Guilford Press; 2007. pp. 292–305. [Google Scholar]
- 6.Stuss DT, Levine B. Adult clinical neuropsychology: Lessons from studies of the frontal lobes. Annual Review of Psychology. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- 7.Royall DR. Executive control function: A review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. The Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14:377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- 8.Koenigs M, Tranel D. Pseudopsychopathy: A perspective from cognitive neuroscience. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. New York: Oxford University Press; 2006. pp. 597–619. [Google Scholar]
- 9.Krueger CE, Laluz V, Rosen HJ, Neuhaus JM, Miller BL, Kramer JH. Double dissociation in the anatomy of socio-emotional disinhibition and executive functioning in dementia. Neuropsychology. 2011;25:249–259. doi: 10.1037/a0021681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvarez JA, Emory E. Executive function and the frontal lobes: A meta-analytic review. Neuropsychological Review. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 11.Zald DH, Andreotti C. Neuropsychological assessment of the orbital and ventromedial prefrontal cortex. Neuropsychologia. 2010;48:3377–3391. doi: 10.1016/j.neuropsychologia.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Roth RM, Isquith PK, Gioia GA. Behavioral rating inventory of executive function-adult version. Lutz, FL: Psychological Assessment Resources, Inc; 2005. [Google Scholar]
- 13.Gioia GA, Kenworthy L, Isquith PK. Executive Function in the Real World: BRIEF lessons from Mark Ylvisaker. Journal of Head Trauma Rehabilitation. 2010;25:433–439. doi: 10.1097/HTR.0b013e3181fbc272. [DOI] [PubMed] [Google Scholar]
- 14.Garlinghouse MA, Roth RM, Isquith PK, Flashman LA, Saykin AJ. Subjective rating of working memory is associated with frontal lobe volume in schizophrenia. Schizophrenia Research. 2010;120:71–75. doi: 10.1016/j.schres.2010.02.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabin LA, Roth RM, Isquith PK, Wishart HA, Nutter-Upham KE, Pare N, Flashman LA, Saykin AJ. Self- and informant reports of executive function on the BRIEF-A in MCI and older adults with cognitive complaints. Archives of Clinical Neuropsychology. 2006;21:721–732. doi: 10.1016/j.acn.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Matheson L. Executive dysfunction, severity of traumatic brain injury, and IQ in workers with disabilities. Work. 2010;36:413–422. doi: 10.3233/WOR-2010-1043. [DOI] [PubMed] [Google Scholar]
- 17.Anderson V, Jacobs R, Harvey AS. Prefrontal lesions and attentional skills in childhood. Journal of the International Neuropsychological Society. 2005;11:817–831. doi: 10.1017/s1355617705051052. [DOI] [PubMed] [Google Scholar]
- 18.Anderson VA, Anderson P, Northam E, Jacobs R, Mikiewicz O. Relationships between cognitive and behavioral measures of executive function in children with brain disease. Child Neuropsychology. 2002;8:231–240. doi: 10.1076/chin.8.4.231.13509. [DOI] [PubMed] [Google Scholar]
- 19.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. Journal of Neurotrauma. 1998;15:573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 21.Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 22.Delis D, Kaplan E, Kramer J, Ober B. California Verbal Learning Test. 2. San Antonio, TX: The Psychological Corporation; 2000. [Google Scholar]
- 23.Pearson Education Inc. California Verbal Learning Test. 2. (CVLT–II) Comprehensive Scoring System; San Antonio, TX: 2000. [Google Scholar]
- 24.Benedict RHB. Brief Visuospatial Memory Test - Revised: Professional manual. Odessa, FL: Psychological Assessment Recourses, Inc; 1997. [Google Scholar]
- 25.Delis D, Kaplan E, Kramer JH. Examiner’s manual of the Delis-Kaplan Executive Functioning System. The Psychological Corporation place; San Antonio, TX: 2001. [Google Scholar]
- 26.Derogatis LR. Symptom Checklist-90-R: Administrative scoring and procedures manual. 3. Mineapolis, MN: National Computer Systems Inc; 1994. [Google Scholar]
- 27.Teasdale GM, Pettigrew LE, Wilson JT, Murray G, Jennett B. Analyzing outcome of treatment of severe head injury: A review and update on advancing the use of the Glasgow Outcome Scale. Journal of Neurotrauma. 1998;15:587–597. doi: 10.1089/neu.1998.15.587. [DOI] [PubMed] [Google Scholar]
- 28.Troyer AK, Moscovitch M, Winocur G, Alexander MP, Stuss D. Clustering and switching on verbal fluency: The effects of focal frontal- and temporal-lobe lesions. Neuropsychologia. 1998;36:499–504. doi: 10.1016/s0028-3932(97)00152-8. [DOI] [PubMed] [Google Scholar]
- 29.Stuss DT, Alexander MP, Hamer L, Palumbo C, Dempster R, Binns M, Levine B, Izukawa D. The effects of focal anterior and posterior brain lesions on verbal fluency. Journal of the International Neuropsychologicl Society. 1998;4:265–278. [PubMed] [Google Scholar]
- 30.Stuss DT, Bisschop SM, Alexander MP, Levine B, Katz D, Izukawa D. The Trail Making Test: A study in focal lesion patients. Psychological Assessment. 2001;13:230–239. [PubMed] [Google Scholar]
- 31.Stuss DT, Floden D, Alexander MP, Levine B, Katz D. Stroop performance in focal lesion patients: Dissociation of processes and frontal lobe lesion location. Neuropsychologia. 2001;39:771–786. doi: 10.1016/s0028-3932(01)00013-6. [DOI] [PubMed] [Google Scholar]
- 32.Shimamura AP. Memory and the prefrontal cortex. Annals of the New York Academy of Sciences. 1995;769:151–159. doi: 10.1111/j.1749-6632.1995.tb38136.x. [DOI] [PubMed] [Google Scholar]
- 33.Alexander MP, Stuss DT, Fansabedian N. California Verbal Learning Test: Performance by patients with focal frontal and non-frontal lesions. Brain. 2003;126:1493–1503. doi: 10.1093/brain/awg128. [DOI] [PubMed] [Google Scholar]
- 34.Baldo JV, Delis D, Kramer J, Shimamura AP. Memory performance on the California Verbal Learning Test-II: Findings from patients with focal frontal lesions. Journal of the International Neuropsychological Society. 2002;8:539–546. doi: 10.1017/s135561770281428x. [DOI] [PubMed] [Google Scholar]
- 35.Tranel D, Manzel K, Anderson SW. Is the prefrontal cortex important for fluid intelligence? A neuropsychological study using Matrix Reasoning. Clinical Neuropsychology. 2008;22:242–261. doi: 10.1080/13854040701218410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mangeot S, Armstrong K, Colvin AN, Yeates KO, Taylor HG. Long-term executive function deficits in children with traumatic brain injuries: Assessment using the Behavior Rating Inventory of Executive Function (BRIEF) Child Neuropsychology. 2002;8:271–284. doi: 10.1076/chin.8.4.271.13503. [DOI] [PubMed] [Google Scholar]
- 37.Vriezen ER, Pigott SE. The relationship between parental report on the BRIEF and performance-based measures of executive function in children with moderate to severe traumatic brain injury. Child Neuropsychology. 2002;8:296–303. doi: 10.1076/chin.8.4.296.13505. [DOI] [PubMed] [Google Scholar]
- 38.McAuley T, Chen S, Goos L, Schachar R, Crosbie J. Is the behavior rating inventory of executive function more strongly associated with measures of impairment or executive function? Journal of the International Neuropsychological Society. 2010;16:495–505. doi: 10.1017/S1355617710000093. [DOI] [PubMed] [Google Scholar]
- 39.Prigatano GP. Disturbances of self-awareness and rehabilitation of patients with traumatic brain injury: A 20-year perspective. Journal of Head Trauma Rehabilitation. 2005;20:19–29. doi: 10.1097/00001199-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Kaplan CP, Miner ME, Mervis L, Newton H, McGregor JM, Goodman JH. Interpretive risks: The use of the Hopkins Symptom Checklist 90-Revised (SCL 90-R) with brain tumour patients. Brain Injury. 1998;12:199–204. doi: 10.1080/026990598122674. [DOI] [PubMed] [Google Scholar]
- 41.Yochim B, Baldo J, Nelson A, Delis DC. D-KEFS Trail Making Test performance in patients with lateral prefrontal cortex lesions. Journal of the International Neuropsychological Society. 2007;13:704–709. doi: 10.1017/S1355617707070907. [DOI] [PubMed] [Google Scholar]
- 42.Kliegel M, Mackinlay R, Jager T. Complex prospective memory: Development across the lifespan and the role of task interruption. Developmental Psychology. 2008;44:612–617. doi: 10.1037/0012-1649.44.2.612. [DOI] [PubMed] [Google Scholar]
- 43.Gioia GA, Isquith PK, Kenworthy L, Barton RM. Profiles of everyday executive function in acquired and developmental disorders. Child Neuropsychology. 2002;8:121–137. doi: 10.1076/chin.8.2.121.8727. [DOI] [PubMed] [Google Scholar]
- 44.Løvstad M, Funderud I, Lindgren M, Endestad T, Due-Tonnessen P, Meling T, Voytek B, Knight RT, Solbakk AK. Contribution of subregions of human frontal cortex to novelty processing. Journal of Cognitive Neuroscience. 2012;24:378–395. doi: 10.1162/jocn_a_00099. [DOI] [PMC free article] [PubMed] [Google Scholar]


