Abstract
The invited special lecture at the 76th Annual Scientific Meeting of the Japanese Circulation Society focused on the central role of inflammation in vascular injury and repair. Early studies pioneered the concept that mechanical injury, such as balloon angioplasty and endovascular stent deployment, elicits an inflammatory response from the vessel wall. This hypothesis was developed and substantiated at a time when the prevailing dogma viewed restenosis following angioplasty as a primarily proliferative smooth muscle cell disease. Antibody targeting of Mac-1 reduced leukocyte accumulation and limited neointimal formation following balloon injury or stent implantation. Genetic absence of Mac-1 resulted in diminished leukocyte accumulation and neointimal thickening after carotid artery injury in mice. In the course of those studies, our laboratory made fundamental discoveries regarding the mechanism of leukocyte recruitment at sites of vascular injury and identified platelet glycoprotein (GP) Ibα, a component of the GPIb-IX-V complex, as the previously unknown platelet counter-receptor for Mac-1. Follow-on studies have focused extensively on the structure, function, and signaling of the leukocyte integrin Mac-1. The binding site for GPIbα in Mac-1 has been mapped and subsequently showed that leukocyte engagement of platelet GPIbα via Mac-1 is critical not only for the biological response to vascular injury, but also for thrombosis, vasculitis, glomerulonephritis, and multiple sclerosis, thereby advancing the hypothesis that virtually all inflammation is platelet-dependent. Furthermore, ligand engagement of Mac-1 initiates a novel gene program that promotes inflammation by activating NFκB and downregulating the expression of the forkhead transcription factor Foxp1 that controls monocyte differentiation. Small molecule inhibitors of Mac-1 function have been pursued, including targeting of Mac-1-GPIbα binding or the downstream tyrosine kinase spleen tyrosine kinase. Drs Teruo Inoue, Koichi Node, Tatsuya Fukotomi, Masashi Sakuma, Toshifumi Morooka, and Kohsuke Nakajima, valued Japanese collaborators and post-doctoral fellows, have contributed enormously to these discoveries.
Keywords: Inflammation, Leukocytes, Platelets, Vascular injury
Inflammation and Vascular Injury
The biological repair response to mechanical vascular injury served as the initial focal point of investigation of our group, providing a disease model to dissect molecular and cellular mechanisms.1 From a historical perspective, Forrester and coworkers proposed a paradigm for neointimal hyperplasia as a general wound-healing response based largely on observations from animal studies.2 Platelet aggregation, inflammatory cell infiltration, release of growth factors, medial smooth muscle cell (SMC) modulation and proliferation, proteoglycan deposition, and extracellular matrix (ECM) remodeling were identified as the major milestones in the temporal sequence of this response. In 1992, Dr Peter Libby and colleagues first advanced the principle that inflammation could be central in this process, proposing that autocrine or paracrine mediators (eg, interleukin [IL]-1β and tumor necrosis factor [TNF]-α), the expressions of which are triggered by vascular injury, contribute to deranged SMC behavior during restenosis.3 On the basis of experimental observations of many investigators spanning greater than a decade, Welt and Rogers4 proposed an integrated view of the molecular and cellular events of instent restenosis. A series of events are initiated immediately after balloon inflation or stent deployment. The initial consequences immediately after stent placement are endothelial denudation, crush of the plaque (often with dissection into the tunica media and occasionally adventitia), and stretching of the entire artery. A layer of platelets and fibrin is then deposited at the injured site. Activated platelets on the surface expressing P-selectin attach to circulating leukocytes bearing PSGL-1 (P-selectin glycoprotein ligand) and begin a process of rolling along the injured surface. Leukocytes then bind tightly to the surface through the leukocyte β2-integrin Mac-1 (αMβ2, CD11b/CD18) via direct attachment to platelet receptors such as glycoprotein (GP) Ibα. Migration of leukocytes across the platelet-fibrin layer and diapedesis into the tissue is driven by chemical gradients of chemokines released from SMCs and resident macrophages.
Next is a granulation or cellular proliferation phase. Growth factors are subsequently released from platelets, leukocytes, and SMCs, which stimulate migration of SMCs from the media into the neointima. The resultant neointimal consists of SMCs, ECM, and macrophages recruited over several weeks. Cellular division takes place in this phase, which appears to be essential for the subsequent development of restenosis. The ECM is composed of various collagen subtypes and proteoglycans5 and constitutes the major component of the mature restenotic plaque.6 Over longer time periods, the artery enters a phase of ECM remodeling with a shift to fewer cellular elements and greater production of ECM. In the artery subjected to balloon angioplasty, reorganization of the ECM, replacing hydrated molecules with collagen, may lead to shrinkage of the entire artery and negative remodeling.7 In the stented artery, this phase has less effect, because of minimal negative remodeling, although constituents of ECM such as hyaluronan, fibronectin, osteopontin and vitronectin also facilitate SMC migration.8,9 In both balloon-angioplastied and stented arteries, re-endothelialization of at least part of the injured vessel surface occurs.
Animal Model and Human Evidence for the Role of Inflammation in Restenosis
Experimental and clinical data indicate that leukocytes are central to intimal growth after mechanical arterial injury. In animal models of vascular injury, leukocytes are recruited as a precursor to intimal thickening.10,11 In animal models in which a stent is deployed to produce deep vessel wall trauma, a brisk early inflammatory response is induced with abundant surface-adherent neutrophils and monocytes.11 Days and weeks later, macrophages accumulate within the developing neointima and are observed clustering around stent struts. The number of vessel wall monocytes/macrophages positively correlates with the neointimal area, suggesting a possible causal role for monocytes in restenosis. My group and others have shown that blockade of early monocyte recruitment results in reduced late neointimal thickening.12–14 Leukocytes most likely modulate vascular repair through multiple mechanisms. Inflammatory cells may contribute to neointimal thickening because of their direct bulk within the intima,15 generation of injurious reactive oxygen intermediates,16 elaboration of growth and chemotactic factors,17 or production of enzymes (eg, matrix metalloproteinases, cathepsin S) capable of degrading extracellular constituents and thereby facilitating cell migration.18,19
Quantitative immunohistochemical analysis of directional coronary atherectomy specimens from humans has shown that the number of macrophages present in the tissue at the time of angioplasty predicts future restenosis.15 Farb et al reported findings from pathological studies of 116 stents from 56 patients after PCI.20 A strong link between the extent of medial damage, inflammation, and neointimal thickness was observed. Systemic markers of inflammation also appear to be predictive of restenosis after balloon angioplasty. Stenting of patients with stable angina and low levels of C-reactive protein (CRP) at baseline is associated with a transient rise in CRP that returns to baseline within 48–72 h.21 Sustained elevations of CRP are associated with an increased risk of clinical and angiographic restenosis. Using flow cytometry, several groups have reported independently that balloon angioplasty and stenting are associated with upregulation of neutrophil CD11b, which positively correlated with clinical restenosis and late lumen loss,22–24 and that cell activation occurred across the mechanically injured vessel.
Molecular Mechanisms of Inflammation
Leukocyte-platelet interactions are critical in the initiation and progression of atherosclerosis,25 as well as restenosis.26 Platelet deposition precedes inflammatory cell accumulation in mouse models of atherogenesis, and inhibition of platelet adhesion dramatically reduces atherosclerotic lesion formation.25 However, the specific receptors responsible for mediating adhesive interactions between neutrophils and platelets in vivo are incompletely defined.
Recruitment of circulating leukocytes to vascular endothelium requires multistep adhesive and signaling events, including selectin-mediated attachment and rolling, leukocyte activation, and integrin-mediated firm adhesion and diapedesis that result in the infiltration of inflammatory cells into the blood vessel wall.27 Firm attachment is mediated by members of the β2-integrin family, LFA-1 (αLβ2, CD11a/CD18), Mac-1 (αMβ2, CD11b/CD18), and p150,95 (αxβ2, CD11c/CD18), which bind to endothelial counter ligands (eg, intercellular adhesion molecule-1 [ICAM-1]), to endothelial-associated ECM proteins (eg, fibrinogen), or to glycosaminoglycans.28
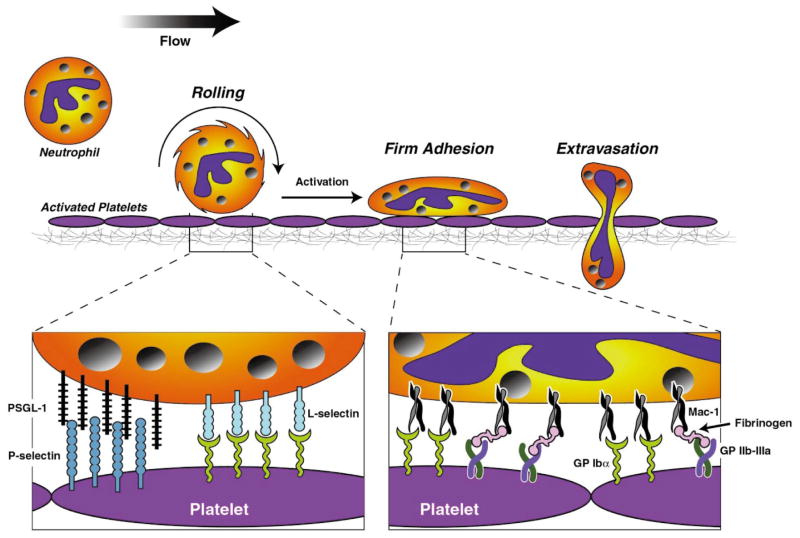
Importantly, leukocyte recruitment also occurs at sites of vascular injury where the lining endothelial cells have been activated/denuded and platelets and fibrin have been deposited. A similar sequential adhesion model of leukocyte attachment to and transmigration across surface-adherent platelets has been proposed29 (Figure 1). The initial tethering and rolling of leukocytes on platelet P-selectin30 are followed by their firm adhesion and transplatelet migration, processes that are dependent on Mac-1.29
Figure 1.
Leukocyte recruitment at site vascular injury with endothelial denudation and platelet deposition. Sequential adhesion cascade of leukocyte attachment to and transmigration across surface-adherent platelets involves the initial tethering and rolling of leukocytes on platelet P-selectin and is followed by firm leukocyte adhesion and transplatelet migration, processes that are dependent on leukocyte Mac-1 and platelet receptors, including GPIbα, JAM-3, and fibrinogen bound to GPIIb/IIIa.
Experimental observations support a causal relationship between inflammation and experimental restenosis. Antibody-mediated blockade12 or selective absence of Mac-126 diminished leukocyte accumulation and limited neointimal thickening after experimental angioplasty or stent implantation. Targeting earlier, selectin-mediated interactions between platelets and leukocytes also markedly reduces leukocyte recruitment and neointimal thickening in a variety of animal models.31 Blockade of the monocyte chemoattractant protein-1 (MCP-1) receptor, CCR2, has been shown to reduce neointimal thickening within stented arterial segments.32 Interestingly, MCP-1 is upregulated after percutaneous coronary intervention in humans, and MCP-1 levels correlate with the risk for restenosis.33
Importantly, the precise cellular and molecular mechanisms of inflammation after arterial injury are highly dependent on the specific type of injury (ie, stent vs. balloon and mechanical vs. atherogenesis). For example, experimental stent deployment in animal arteries causes sustained elevation of MCP-1 after injury (14 days) compared with balloon-injured arteries (24 h).34 Correspondingly, antibody-mediated blockade of CCR2 markedly diminished neointimal thickening after stent-induced but not balloon-induced injury in non-human primates.32 In contrast to targeting Mac-1, which reduces neointimal thickening after experimental angioplasty,12,26,32 but does not attenuate atherogenesis,35 targeting MCP-1 also appears to benefit arteries affected by either mechanical injury or atherogenesis. Mice genetically deficient in MCP-136 or CCR237 demonstrated significant reductions in aortic lipid content, monocyte accumulation, and atherosclerotic lesion development, as well as neointimal thickening after experimental angioplasty.38
Our research group has focused on identifying the platelet counter-receptor for Mac-1/αMβ2. Evaluation of the structural features of integrins provides insight into candidate platelet counter-receptors for Mac-1/αMβ2. Integrins are heterodimeric proteins composed of one α- and one β-subunit. A subset of integrin α-subunits, including αM, contains an inserted domain (I-domain) of 200 amino acids that is implicated in ligand binding39 and is strikingly similar to the A- domains of von Willebrand factor (vWf),40 one of which, A1, mediates the interaction of vWf with its platelet receptor, the glycoprotein (GP) Ib-IX-V complex. Because of the similarity of the vWf A1 domain and the αMI-domain, we hypothesized that GPIbα might also be able to bind Mac-1/αMβ2 and reported that GP Ibα is indeed a constitutively expressed counter-receptor for Mac-1/αMβ2.41 Furthermore, under the conditions used in those studies, the predominant interaction between neutrophils and platelets appeared to be between Mac-1/αMβ2 and GPIbα.
The αMI-domain contributes broadly to the recognition of ligands by αMβ239 and specifically to the binding of GPIbα.41 This region has also been implicated in the binding of ICAM-1,42 C3bi,43 and fibrinogen.42 My group localized the binding site for GPIbα within the αMI-domain segment αMP201-K217 using a strategy based on the differences in the binding of GPIbα to the αMI- and αLI-domains, which involved several independent approaches, including screening of mutant cells, synthetic peptides, site-directed mutagenesis, and gain-in-function analyses.44 The grafting of only 2 amino acids within this segment into the αLI-domain converted it to a GPIbα binding protein. Thus, a small segment that has a defined structure within the αMI-domain is necessary and sufficient for GPIbα binding.
Although Mac-1/αMβ2 has a broad ligand binding repertoire, the precise ligand responsible for leukocyte accumulation at sites of platelet deposition remained to be defined. Central to unraveling the precise biological roles of Mac-1/αMβ2 was defining the biologically relevant ligand(s) for this integrin. We went on to show that antibody targeting of αMP201-K217 (termed anti-M2) reduced Mac-1/αMβ2-dependent adhesion to GPIbα, but not other ligands, in vitro and leukocyte accumulation after vascular injury in vivo.45 In a mouse femoral artery injury model, treatment with anti-M2 antibody was accompanied by inhibition of cellular proliferation and neointimal thickening.
Mac-1 Signaling
Pluripotent hematopoietic stem cells undergo progressive restriction in their lineage potential to give rise to mature terminally differentiated cells. The process of hematopoietic differentiation is thought to follow a developmentally ordered pattern of gene expression. Transcription factors play a key role in the lineage determination and maturation of hematopoietic cells.46 Identification of these regulators and determining the mechanism of how they activate their target genes are important for understanding the development of monocytes and macrophages. In the case of monocyte differentiation, several transcription factors, including PU.1, C/EBPβ, AML1, EGR-1, MafB, IRF-8/ICSBP (interferon regulatory factor-8/interferon consensus sequence-binding protein), and others, have been implicated based on experimental evidence obtained from knockdown and gain-in-function strategies both in vitro and in vivo.47 The transcriptional regulation of the c-fms gene, which encodes for the macrophage colony-stimulating factor receptor (M-CSFR), is a focal point of investigation because it is required for the differentiation, proliferation, and survival of monocytic phagocytes.48,49
However, the precise external signals that control differentiation of peripheral blood monocytes to tissue macrophages are incompletely defined. Monocytes leave the bone marrow and travel through peripheral blood vessels. Once they reach a tissue, possibly in response to MCSF, GM-CSF, MCP-1, and/or IL-3, they differentiate into macrophages by growing in size and increasing their lysosomal compartment, the amount of hydrolytic enzymes and the number and size of mitochondria, and the extent of their energy metabolism.50
My group was intrigued by the possibility that cell adhesion molecules participating in the firm arrest and transmigration of blood-borne monocytes across endothelial and ECM barriers could provide these signals. Integrins mediate adhesion of cells to extracellular matrices, as well as intercellular interactions that are central to inflammation, immunity, hemostasis, and tumor metastasis.51 These adhesive interactions transduce “outside-in” signals that control complex cell functions, such as proliferation, differentiation, and survival, and require the regulation of gene expression.52 Neutrophil and monocyte recruitment in acute inflammation are mediated in part by the β2-integrin family of receptors. Engagement of β2-integrins by a broad repertoire of ligands generates “outside-in” signals leading to inflammatory cell activation and induction of genes encoding for IL-1β, TNF-α, and tissue factor.53,54 The cytoplasmic tail of LFA-1 interacts with the transcriptional co-activator JAB1 and modulates AP-1 activity by regulating JAB1 nuclear localization.55 Mac-1 associates with IRAK1 (IL-1 receptor-associated kinase) and promotes activation of NF-κB activity in a cascade involving TRAF6 (TNF receptor-associated factor 6) and TAK1 (TGF-β activated kinase 1).56
We described a new mechanism by which integrin engagement orchestrates monocyte differentiation signals through the forkhead transcription factor (Foxp1).57 Based on prior observations from our laboratory that antibody12 and gene26 targeting of Mac-1 attenuate the biological inflammatory and neo-intimal thickening responses to vascular injury, we hypothesized that clustering and activation of Mac-1 may initiate a novel gene program that promotes vascular inflammation. We cloned an 85-kDa forkhead transcription factor (originally termed Mac-1-regulated forkhead, found subsequently to be identical to Foxp1) using differential display polymerase chain reaction that is downregulated in Mac-1-clustered compared to -non-clustered monocytic THP-1 cells.57 Foxp1 is expressed in untreated HL-60 cells and its expression was markedly reduced during phorbol ester-induced monocyte differentiation. Over-expression of Foxp1 markedly attenuated phorbol ester-induced expression of c-fms and was accompanied by decreased CD11b expression, cell adhesiveness, and phagocytosis. Using electromobility shift and reporter assays, we established that Foxp1 binds to forkhead binding sites within the c-fms promoter and functions as a transcriptional repressor. Importantly, deficiency of Mac-1 is associated with altered regulation of Foxp1 and monocyte maturation in vivo. Taken together, these observations suggest that downregulation of Foxp1 by integrin engagement is essential for the control of monocyte differentiation.
In follow-on studies, we directly tested whether Foxp1 plays a critical role in monocyte differentiation and macrophage functions in vivo by generating transgenic mice expressing human Foxp1 in monocyte/macrophage lineage cells using the CD68 promoter (macFoxp1tg).58 We found that macrophage functions were globally impaired in macFoxp1tg compared with wild-type cells. Osteoclastogenesis and bone resorption activity were also attenuated in macFoxp1tg mice. In models of chemical and bacterial peritonitis, macFoxp1tg mice exhibited reduced macrophage accumulation, bacterial clearance, and survival. These studies delineated important physiologic roles for Mac-1 and Foxp1 in monocyte differentiation and macrophage function.
General Role for Leukocyte-Platelet Interactions in Inflammation
A particularly important development of these studies has been the unexpected observation that leukocyte-platelet interactions broadly regulate inflammation and tissue injury in diverse models of human disease. To examine mechanisms of inflammation-induced thrombosis, for example, the laboratory of Dr Tanya Myadas developed a murine model of thrombotic glomerulonephritis, a known cause of acute renal failure in patients.59 This model, induced by lipopolysaccharide and antibody to the glomerular basement membrane, led to rapid glomerular neutrophil recruitment, thrombotic glomerular lesions with endothelial cell injury, and renal dysfunction. In mice immunodepleted of neutrophils or lacking the leukocyte-specific integrin Mac-1, neutrophil recruitment, endothelial injury, glomerular thrombosis, and acute renal failure were markedly attenuated, despite the robust generation of renal cytokines. Importantly, platelets accumulated in glomerular capillaries within 4 h of thrombotic glomerulonephritis before evidence of thrombosis. Targeting Mac-1–GPIbα binding with anti-M2 antibody attenuated the severity of glomerular thrombus formation and fibrin deposition, proteinuria and rise in serum creatinine. Another surprising example of the importance of leukocyte-platelet interactions was observed recently in a model of multiple sclerosis (MS). A major hallmark of MS and of its mouse model, experimental autoimmune encephalomyelitis (EAE), is the infiltration of inflammatory cells into the central nervous system (CNS). The laboratories of Drs Harald Langer and Triantafyllos Chavakis demonstrated that platelets were present in a human chronic active MS lesion, as well as in the CNS of mice subjected to EAE, but not in the CNS from control non-diseased mice.60 Platelets contributed significantly to the pathogenesis of EAE, as platelet depletion resulted in significantly ameliorated EAE development in mice associated with reduced recruitment of leukocytes to the inflamed CNS. In vitro, platelets interacted with inflammatory cells via the binding of the leukocyte Mac-1 to the platelet GPIbα. Consistently, GPIbα-deficient mice displayed reduced macrophage recruitment to the spinal cord after induction of EAE and decreased disease severity. Moreover, blockade of Mac-1–GPIbα interaction with anti-M2 antibody attenuated EAE in mice. Thus, platelets contribute to the pathogenesis of EAE by promoting recruitment of inflammatory cells to the inflamed CNS and may, therefore, represent an important new therapeutic target in MS.
Translational Therapeutic Approaches
Experimental and clinical studies support close interrelationship between inflammation and thrombosis.61 Leukocyte-platelet interactions induce bidirectional signals that amplify pro-inflammatory and pro-thrombotic cellular responses62 and are critical, for example, in the initiation and progression of atherosclerosis,25 as well as restenosis26 and in thrombotic events.63 Antithrombotic agents developed to date provide substantial benefits via inhibition of thrombosis, but do not appear to affect the progression of underlying vascular disease.64,65
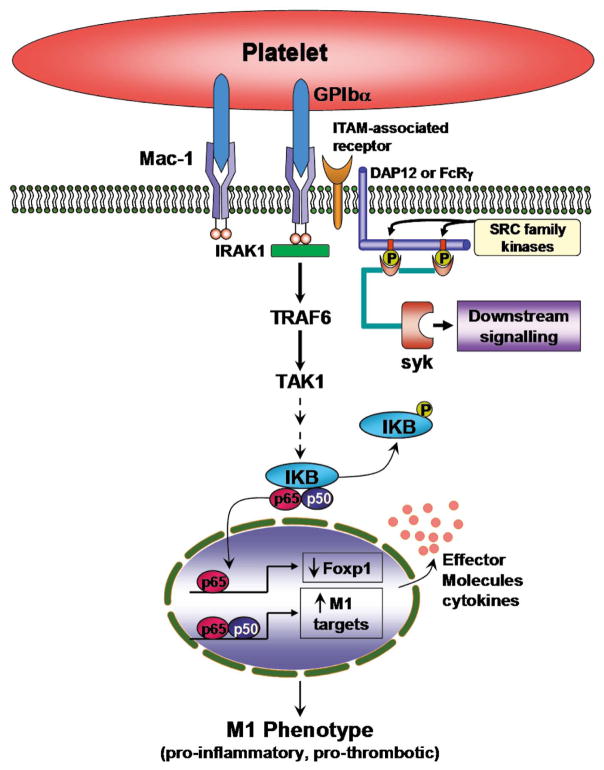
Several in vitro lines of evidence suggest that inflammatory and thrombotic signaling pathways converge on spleen tyrosine kinase (Syk), a 72-kDa signaling protein with kinase and scaffolding activities (Figure 2). In platelets, phosphorylation of Syk has been reported following activation by multiple receptors (eg, GPVI, which mediates platelet adhesion and activation to vascular collagen,66 GPIbα and GPIIb–IIIa67). Syk is also a mediator of signaling events induced by high shear stress,68 following engagement of FcγRIIA, FcRγ, FcαRI, and the C-type lectin receptor (CLEC-2).66,69–72 In leukocytes, Syk promotes the recruitment of these cells to both inflamed and injured blood vessels. In the presence of activated endothelium, Syk regulates selectin-dependent leukocyte rolling.73 At sites of vascular injury with endothelial denudation and platelet deposition, leukocyte recruitment is mediated by the leukocyte β2-integrin Mac-1 and the platelet counter-receptor GPIbα,26,45 both of which signal through Syk.74
Figure 2.
Mac-1 signaling, monocyte differentiation and pro-inflammatory gene expression. Mac-1 engagement and clustering recruits a Toll/IL-1 receptor family-like cascade involving IRAK1 (IL-1 receptor-associated kinase), TRAF-6 (TNF- receptor-associated factor 6) and TAK-1 (TGF-β-activated kinase) to modulate NFκB activity. Clustering of Mac-1 also leads to downregulation of Foxp1, which functions as a transcriptional repressor of the MCSF-1 (macrophage colony-stimulating factor) receptor, c-fms. Leukocyte integrin signaling pathways converge on spleen tyrosine kinase (Syk), a 72-kDa signaling protein with kinase and scaffolding activities.
Despite a well-described role for Syk in platelet and leukocyte biology, our understanding of the contribution of Syk to platelet-mediated thrombosis and vascular inflammation in vivo remains limited. This is likely explained by the severe phenotype associated with Syk deficiency (embryonic lethality, anemia and petechial hemorrhages throughout the gut, impairment of B-cell development75,76) and the lack of a highly selective Syk inhibitor, which led to contradictory results. For example, although the results obtained with pharmacologic inhibitor R406 support a role for Syk downstream of both GPVI and CLEC-2 in human washed platelets,77 oral administration of R406 blocked Fc receptor signaling and reduced immune complex-mediated inflammation in whole blood, but had no significant effect on collagen-induced platelet aggregation in platelet rich plasma.78 Furthermore, the selectivity of R406 for Syk is open to question, because it inhibits multiple tyrosine kinases.78,79 Similarly, the lack of a selective pharmacologic agent with favorable pharmacokinetic properties has also limited the evaluation of Syk kinase activity in atherogenesis and restenosis.
Studies performed in my group’s laboratory in collaboration with investigators from Portola Pharmaceuticals using a novel and highly selective pharmacologic inhibitor of the spleen tyrosine kinase Syk (PRT060318; 2-((1R,2S)-2-aminocyclohexylamino)-4-(m-tolylamino)pyrimidine-5-carboxamide) coupled with genetic experiments, demonstrate that Syk inhibition ameliorates both the acute and chronic response to vascular injury without affecting hemostasis.80 Specifically, lack of Syk (murine radiation chimeras) attenuated shear-induced thrombus formation ex vivo, and PRT060318 strongly inhibited arterial thrombosis in vivo in multiple animal species while having minimal effect on bleeding. Furthermore, leukocyte-platelet-dependent responses to vascular injury, including inflammatory cell recruitment and neointima formation, were markedly inhibited by PRT060318. Because leukocyte-platelet interactions are critical in the initiation and progression of atherosclerosis,25 as well as restenosis,45 we examined the development of atherosclerotic lesions in apolipoprotein E-deficient mice consuming a high-fat diet from 8 to 28 weeks of age. Mice were treated with PRT060318 (30 mg/kg) or vehicle control administered via oral gavage twice daily for 3 weeks followed by 1 week off from 8 weeks of age until 24 weeks of age for a total of 16 weeks. Plasma lipid profiles did not differ significantly between vehicle control- and PRT060318-treated mice on the high-fat diet. Treatment with PRT060318 did not affect the total white blood cell count, hemoglobin, or platelet count. Aortas were harvested at 28 weeks of age for atherosclerotic lesion analysis. Inhibition of Syk activity with PRT060318 resulted in a 36% reduction in lesion area in en face analysis of Sudan IV stained thoraco-abdominal aortas. Thus, Syk controls acute and long-term responses to arterial vascular injury. The therapeutic potential of Syk may be exemplary of a new class of antiatherothrombotic agents that target the interface between thrombosis and inflammation.
The search for a small molecule inhibitor of the Mac-1–GPIbα binding interaction is a second translational therapeutic approach under active investigation. The interaction of the leukocyte integrin Mac-1 and platelet GPIbα has been implicated in a variety of cardiovascular diseases, including atherothrombosis, neointimal formation,45 vasculitis, and other disease, including glomerulonephritis,59 and demyelinating diseases.60 The anti-M2 antibody raised to the αMP201–K217 sequence in the I-domain of integrin blocks binding of GPIbα and platelets to the integrin and to leukocytes.45 Importantly, anti-M2 antibody blocks Mac-1–GPIbα binding, but does not inhibit binding of other Mac-1 ligands. This behavior establishes the precedence that selective, small molecule inhibitors of Mac-1–GPIbα binding can be developed and may have therapeutic utility. For initial screening of a compound library, a binding assay using recombinant αMI-domain, the region of Mac-1 that binds GPIbα, was developed. The αMI-domain was purified and fluorescently labeled, and its binding to soluble GPIbα or CHO cells expressing GPIbα was assessed. After validating assay sensitivity and specificity, a number of small molecule inhibitors have been identified and are the focus of ongoing studies.
Conclusions
Leukocyte-platelet interactions govern inflammation in diverse disease models. Investigation of the biological repair response to mechanical vascular injury has provided a robust disease model for dissecting the molecular and cellular mechanisms. Identification of the leukocyte integrin Mac-1 as a critical determinant of vascular inflammation and neointimal formation drove studies investigating Mac-1’s structure, function, and signaling. Translational studies are underway to leverage these basic discoveries in human health and disease.
Acknowledgments
This work was supported in part by a National Institutes of Health grant to DIS (HL57506 MERIT Award).
Footnotes
Competing Interests/Disclosures
DIS is a co-inventor of technology related to Mac-1-GPIbα that is assigned to Case Western Reserve University. DIS receives honoraria from Cordis/Johnson & Johnson and Medtronic Vascular for advisory board activities and from Abbott Vascular for speaker activities.
References
- 1.Costa MA, Simon DI. Molecular basis of restenosis and drug-eluting stents. Circulation. 2005;111:2257– 2273. doi: 10.1161/01.CIR.0000163587.36485.A7. [DOI] [PubMed] [Google Scholar]
- 2.Forrester JS, Fishbein M, Helfant R, Fagin J. A paradigm for restenosis based on cell biology: Clues for the development of new preventive therapies. J Am Coll Cardiol. 1991;17:758– 769. doi: 10.1016/s0735-1097(10)80196-2. [DOI] [PubMed] [Google Scholar]
- 3.Libby P, Schwartz D, Brogi E, Tanaka H, Clinton S. A cascade model for restenosis: A special case of atherosclerosis progression. Circulation. 1992;86(Suppl):III47–III52. [PubMed] [Google Scholar]
- 4.Welt FG, Rogers C. Inflammation and restenosis in the stent era. Arterioscler Thromb Vasc Biol. 2002;22:1769– 1776. doi: 10.1161/01.atv.0000037100.44766.5b. [DOI] [PubMed] [Google Scholar]
- 5.Riessen R, Isner JM, Blessing E, Loushin C, Nikol S, Wight TN. Regional differences in the distribution of the proteoglycans biglycan and decorin in the extracellular matrix of atherosclerotic and restenotic human coronary arteries. Am J Pathol. 1994;144:962– 974. [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz RS, Huber KC, Murphy JG, Edwards WD, Camrud AR, Vlietstra RE, et al. Restenosis and the proportional neointimal response to coronary artery injury: Results in a porcine model. J Am Coll Cardiol. 1992;19:267– 274. doi: 10.1016/0735-1097(92)90476-4. [DOI] [PubMed] [Google Scholar]
- 7.Strauss BH, Robinson R, Batchelor WB, Chisholm RJ, Ravi G, Natarajan MK, et al. In vivo collagen turnover following experimental balloon angioplasty injury and the role of matrix metalloproteinases. Circ Res. 1996;79:541– 550. doi: 10.1161/01.res.79.3.541. [DOI] [PubMed] [Google Scholar]
- 8.Bauters C, Marotte F, Hamon M, Oliviero P, Farhadian F, Robert V, et al. Accumulation of fetal fibronectin mRNAs after balloon denudation of rabbit arteries. Circulation. 1995;92:904– 911. doi: 10.1161/01.cir.92.4.904. [DOI] [PubMed] [Google Scholar]
- 9.Farb A, Kolodgie FD, Hwang JY, Burke AP, Tefera K, Weber DK, et al. Extracellular matrix changes in stented human coronary arteries. Circulation. 2004;110:940– 947. doi: 10.1161/01.CIR.0000139337.56084.30. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka H, Sukhova GK, Swanson SJ, Clinton SK, Ganz P, Cybulsky MI, et al. Sustained activation of vascular cells and leukocytes in the rabbit aorta after balloon injury. Circulation. 1993;88:1788– 1803. doi: 10.1161/01.cir.88.4.1788. [DOI] [PubMed] [Google Scholar]
- 11.Rogers C, Welt FG, Karnovsky MJ, Edelman ER. Monocyte recruitment and neointimal hyperplasia in rabbits: Coupled inhibitory effects of heparin. Arterioscler Thromb Vascr Biol. 1996;16:1312– 1318. doi: 10.1161/01.atv.16.10.1312. [DOI] [PubMed] [Google Scholar]
- 12.Rogers C, Edelman ER, Simon DI. A monoclonal antibody to the b2-leukocyte integrin Mac-1 (CD11b/CD18) reduces intimal thickening after angioplasty or stent implantation in rabbits. Proc Natl Acad Sci USA. 1998;95:10134– 10139. doi: 10.1073/pnas.95.17.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishop GG, McPherson JA, Sanders JM, Hesselbacher SE, Feldman MJ, McNamara CA, et al. Selective alpha(v)beta(3)-receptor blockade reduces macrophage infiltration and restenosis after balloon angioplasty in the atherosclerotic rabbit. Circulation. 2001;103:1906– 1911. doi: 10.1161/01.cir.103.14.1906. [DOI] [PubMed] [Google Scholar]
- 14.Barringhaus KG, Phillips JW, Thatte JS, Sanders JM, Czarnik AC, Bennett DK, et al. Alpha4beta1 integrin (VLA-4) blockade attenuates both early and late leukocyte recruitment and neointimal growth following carotid injury in apolipoprotein E (−/−) mice. J Vasc Res. 2004;41:252– 260. doi: 10.1159/000078646. [DOI] [PubMed] [Google Scholar]
- 15.Moreno PR, Bernardi VH, Lopez-Cuellar J, Newell JB, McMellon C, Gold HK, et al. Macrophage infiltration predicts restenosis after coronary intervention in patients with unstable angina. Circulation. 1996;94:3098– 3102. doi: 10.1161/01.cir.94.12.3098. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Keaney JF, Jr, Schulz E, Levison B, Shan L, Sakuma M, et al. Decreased neointimal formation in Nox2-deficient mice reveals a direct role for NADPH oxidase in the response to arterial injury. Proc Natl Acad Sci USA. 2004;101:13014– 13019. doi: 10.1073/pnas.0405389101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assoian RK, Fleurdelys BE, Stevenson HC, Miller PJ, Madtes DK, Raines EW, et al. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA. 1987;84:6020– 6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garbisa S, Ballin M, Daga-Gordini D, Fastelli G, Naturale M, Negro A, et al. Transient expression of type IV collagenolytic metalloproteinase produced by human mononuclear phagocytes. J Biol Chem. 1986;261:2369– 2375. [PubMed] [Google Scholar]
- 19.Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastinolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576– 583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farb A, Weber DK, Kolodgie FD, Burke AP, Virmani R. Morphological predictors of restenosis after coronary stenting in humans. Circulation. 2002;105:2974– 2980. doi: 10.1161/01.cir.0000019071.72887.bd. [DOI] [PubMed] [Google Scholar]
- 21.Gaspardone A, Crea F, Versaci F, Tomai F, Pellegrino A, Chiariello L, et al. Predictive value of C-reactive protein after successful coronary-artery stenting in patients with stable angina. Am J Cardiol. 1998;82:515– 518. doi: 10.1016/s0002-9149(98)00370-1. [DOI] [PubMed] [Google Scholar]
- 22.Inoue T, Sakai Y, Morooka S, Hayashi T, Takayanagi K, Takabatake Y. Expression of polymorphonuclear leukocyte adhesion molecules and its clinical significance in patients treated with percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1996;28:1127– 1133. doi: 10.1016/S0735-1097(96)00308-7. [DOI] [PubMed] [Google Scholar]
- 23.Mickelson JK, Lakkis NM, Villarreal-Levy G, Hughes BJ, Smith CW. Leukocyte activation with platelet adhesion after coronary angioplasty: A mechanism for recurrent disease? J Am Coll Cardiol. 1996;28:345– 353. doi: 10.1016/0735-1097(96)00164-7. [DOI] [PubMed] [Google Scholar]
- 24.Neumann FJ, Ott I, Gawaz M, Puchner G, Schomig A. Neutrophil and platelet activation at balloon-injured coronary artery plaque in patients undergoing angioplasty. J Am Coll Cardiol. 1996;27:819– 824. doi: 10.1016/0735-1097(95)00563-3. [DOI] [PubMed] [Google Scholar]
- 25.Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887– 896. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon DI, Chen Z, Seifert P, Edelman ER, Ballantyne CM, Rogers C. Decreased neointimal formation in Mac-1(−/−) mice reveals a role for inflammation in vascular repair after angioplasty. J Clin Invest. 2000;105:293– 300. doi: 10.1172/JCI7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301– 314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 28.Plow EF, Zhang L. A MAC-1 attack: Integrin functions directly challenged in knockout mice. J Clin Invest. 1997;99:1145– 1146. doi: 10.1172/JCI119267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diacovo TG, Roth SJ, Buccola JM, Bainton DF, Springer TA. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood. 1996;88:146– 157. [PubMed] [Google Scholar]
- 30.McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100:S97– S103. [PubMed] [Google Scholar]
- 31.Bienvenu JG, Tanguay JF, Theoret JF, Kumar A, Schaub RG, Merhi Y. Recombinant soluble P-selectin glycoprotein ligand-1-Ig reduces restenosis through inhibition of platelet-neutrophil adhesion after double angioplasty in swine. Circulation. 2001;103:1128– 1134. doi: 10.1161/01.cir.103.8.1128. [DOI] [PubMed] [Google Scholar]
- 32.Horvath C, Welt FG, Nedelman M, Rao P, Rogers C. Targeting CCR2 or CD18 inhibits experimental instent restenosis in primates: Inhibitory potential depends on type of injury and leukocytes targeted. Circ Res. 2002;90:488– 494. doi: 10.1161/hh0402.105956. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt AM, Stern DM. Chemokines on the rise: MCP-1 and restenosis. Arterioscler Thromb Vasc Biol. 2001;21:297– 299. doi: 10.1161/01.atv.21.3.297. [DOI] [PubMed] [Google Scholar]
- 34.Welt FG, Tso C, Edelman ER, Kjelsberg MA, Paolini JF, Seifert P, et al. Leukocyte recruitment and expression of chemokines following different forms of vascular injury. Vasc Med. 2003;8:1– 7. doi: 10.1191/1358863x03vm462oa. [DOI] [PubMed] [Google Scholar]
- 35.Kubo N, Boisvert WA, Ballantyne CM, Curtiss LK. Leukocyte CD11b expression is not essential for the development of atherosclerosis in mice. J Lipid Res. 2000;41:1060– 1066. [PubMed] [Google Scholar]
- 36.Gu L, Okada Y, Clinton SK, Gerard C, Sukhova GK, Libby P, et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol Cell. 1998;2:275– 281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- 37.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894– 897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 38.Roque M, Kim WJ, Gazdoin M, Malik A, Reis ED, Fallon JT, et al. CCR2 deficiency decreases intimal hyperplasia after arterial injury. Arterioscler Thromb Vasc Biol. 2002;22:554– 559. doi: 10.1161/hq0402.105720. [DOI] [PubMed] [Google Scholar]
- 39.Diamond M, Garcia-Aguiliar J, Bickford J, Corbi A, Springer T. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct ligands. J Cell Biol. 1993;120:1031– 1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadler JE, Shelton-Inloes BB, Sorace JM, Harlan JM, Titanti K, Davie EW. Cloning and characterization of two cDNAs coding for human von Willebrand factor. Proc Natl Acad Sci USA. 1985;82:6394. doi: 10.1073/pnas.82.19.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon DI, Chen Z, Xu H, Li CQ, Dong J, McIntire LV, et al. Platelet glycoprotein Ib alpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Exp Med. 2000;192:193– 204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou L, Lee DH, Plescia J, Lau CY, Altieri DC. Differential ligand binding specificities of recombinant CD11b/CD18 integrin I-domain. J Biol Chem. 1994;269:17075– 17079. [PubMed] [Google Scholar]
- 43.Ueda T, Rieu P, Brayer J, Arnaout MA. Identification of the complement iC3b binding site in the beta 2 integrin CR3 (CD11b/CD18) Proc Natl Acad Sci USA. 1994;91:10680– 10684. doi: 10.1073/pnas.91.22.10680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ehlers R, Ustinov V, Chen Z, Zhang X, Rao R, Luscinskas FW, et al. Targeting platelet-leukocyte interactions: Identification of the integrin Mac-1 binding site for the platelet counter receptor glycoprotein Ibalpha. J Exp Med. 2003;198:1077– 1088. doi: 10.1084/jem.20022181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Sakuma M, Chen Z, Ustinov V, Shi C, Croce K, et al. Leukocyte engagement of platelet glycoprotein Ibalpha via the integrin Mac-1 is critical for the biological response to vascular injury. Circulation. 2005;112:2993– 3000. doi: 10.1161/CIRCULATIONAHA.105.571315. [DOI] [PubMed] [Google Scholar]
- 46.Tenen DG, Hromas R, Licht JD, Zhang DE. Transcription factors, normal myeloid development, and leukemia. Blood. 1997;90:489– 519. [PubMed] [Google Scholar]
- 47.Friedman AD. Transcriptional regulation of granulocyte and monocyte development. Oncogene. 2002;21:3377– 3390. doi: 10.1038/sj.onc.1205324. [DOI] [PubMed] [Google Scholar]
- 48.Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, Jr, Ahmed-Ansari A, Sell KW, Pollard JW, et al. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA. 1990;87:4828– 4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai XM, Ryan GR, Hapel AJ, Dominguez MG, Russell RG, Kapp S, et al. Targeted disruption of the mouse colony-stimulating factor 1 receptor gene results in osteopetrosis, mononuclear phagocyte deficiency, increased primitive progenitor cell frequencies, and reproductive defects. Blood. 2002;99:111– 120. doi: 10.1182/blood.v99.1.111. [DOI] [PubMed] [Google Scholar]
- 50.Valledor AF, Borras FE, Cullell-Young M, Celada A. Transcription factors that regulate monocyte/macrophage differentiation. J Leukoc Biol. 1998;63:405– 417. doi: 10.1002/jlb.63.4.405. [DOI] [PubMed] [Google Scholar]
- 51.Hynes RO. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11– 25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 52.Giancotti FG. Integrin signaling: Specificity and control of cell survival and cell cycle progression. Curr Opin Cell Biol. 1997;9:691– 700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- 53.Fan ST, Edgington TS. Coupling of the adhesive receptor CD11b/CD18 to functional enhancement of effector macrophage tissue factor response. J Clin Invest. 1991;87:50– 57. doi: 10.1172/JCI115000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rezzonico R, Chicheportiche R, Imbert V, Dayer JM. Engagement of CD11b and CD11c beta2 integrin by antibodies or soluble CD23 induces IL-1beta production on primary human monocytes through mitogen-activated protein kinase-dependent pathways. Blood. 2000;95:3868– 3877. [PubMed] [Google Scholar]
- 55.Bianchi E, Denti S, Granata A, Bossi G, Geginat J, Villa A, et al. Integrin LFA-1 interacts with the transcriptional co-activator JAB1 to modulate AP-1 activity. Nature. 2000;404:617– 621. doi: 10.1038/35007098. [DOI] [PubMed] [Google Scholar]
- 56.Shi C, Zhang X, Chen Z, Robinson MK, Simon DI. Leukocyte integrin Mac-1 recruits toll/interleukin-1 receptor superfamily signaling intermediates to modulate NF-kappaB activity. Circ Res. 2001;89:859– 865. doi: 10.1161/hh2201.099166. [DOI] [PubMed] [Google Scholar]
- 57.Shi C, Zhang X, Chen Z, Sulaiman K, Feinberg MW, Ballantyne CM, et al. Integrin engagement regulates monocyte differentiation through the forkhead transcription factor Foxp1. J Clin Invest. 2004;114:408– 418. doi: 10.1172/JCI21100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi C, Sakuma M, Mooroka T, Liscoe A, Gao H, Croce KJ, et al. Down-regulation of the forkhead transcription factor Foxp1 is required for monocyte differentiation and macrophage function. Blood. 2008;112:4699– 4711. doi: 10.1182/blood-2008-01-137018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirahashi J, Hishikawa K, Kaname S, Tsuboi N, Wang Y, Simon DI, et al. Mac-1 (CD11b/CD18) links inflammation and thrombosis after glomerular injury. Circulation. 2009;120:1255– 1265. doi: 10.1161/CIRCULATIONAHA.109.873695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Langer HF, Choi EY, Zhou H, Schleicher R, Chung KJ, Tang Z, et al. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circ Res. 2012;110:1202– 1210. doi: 10.1161/CIRCRESAHA.111.256370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Libby P, Simon DI. Inflammation and thrombosis: The clot thickens. Circulation. 2001;103:1718– 1720. doi: 10.1161/01.cir.103.13.1718. [DOI] [PubMed] [Google Scholar]
- 62.McEver RP. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost. 2001;86:746– 756. [PubMed] [Google Scholar]
- 63.Palabrica T, Lobb R, Furie BC, Aronovitz M, Benjamin C, Hsu YM, et al. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992;359:848– 851. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- 64.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): Developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:e148– e304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 65.Kushner FG, Hand M, Smith SC, Jr, King SB, 3rd, Anderson JL, Antman EM, et al. 2009 Focused Updates: ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction (updating the 2004 Guideline and 2007 Focused Update) and ACC/AHA/SCAI Guidelines on Percutaneous Coronary Intervention (updating the 2005 Guideline and 2007 Focused Update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271– 2306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 66.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J Thromb Haemost. 2005;3:1752– 1762. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 67.Gibbins JM. Platelet adhesion signalling and the regulation of thrombus formation. J Cell Sci. 2004;117:3415– 3425. doi: 10.1242/jcs.01325. [DOI] [PubMed] [Google Scholar]
- 68.Feng S, Lu X, Resendiz JC, Kroll MH. Pathological shear stress directly regulates platelet alphaIIb beta3 signaling. Am J Physiol Cell Physiol. 2006;291:C1346– C1354. doi: 10.1152/ajpcell.00559.2005. [DOI] [PubMed] [Google Scholar]
- 69.Yanaga F, Poole A, Asselin J, Blake R, Schieven GL, Clark EA, et al. Syk interacts with tyrosine-phosphorylated proteins in human platelets activated by collagen and cross-linking of the Fc gamma-IIA receptor. Biochem J. 1995;311:471– 478. doi: 10.1042/bj3110471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gibbins J, Asselin J, Farndale R, Barnes M, Law CL, Watson SP. Tyrosine phosphorylation of the Fc receptor gamma-chain in collagen-stimulated platelets. J Biol Chem. 1996;271:18095– 18099. doi: 10.1074/jbc.271.30.18095. [DOI] [PubMed] [Google Scholar]
- 71.Poole A, Gibbins JM, Turner M, van Vugt MJ, van de Winkel JG, Saito T, et al. The Fc receptor gamma-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. Embo J. 1997;16:2333– 2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suzuki-Inoue K, Fuller GL, Garcia A, Eble JA, Pohlmann S, Inoue O, et al. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542– 549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 73.Abbal C, Lambelet M, Bertaggia D, Gerbex C, Martinez M, Arcaro A, et al. Lipid raft adhesion receptors and Syk regulate selectin-dependent rolling under flow conditions. Blood. 2006;108:3352– 3359. doi: 10.1182/blood-2006-04-013912. [DOI] [PubMed] [Google Scholar]
- 74.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387– 402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303– 306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 76.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, et al. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298– 302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 77.Spalton JC, Mori J, Pollitt AY, Hughes CE, Eble JA, Watson SP. The novel Syk inhibitor R406 reveals mechanistic differences in the initiation of GPVI and CLEC-2 signaling in platelets. J Thromb Haemost. 2009;7:1192– 1199. doi: 10.1111/j.1538-7836.2009.03451.x. [DOI] [PubMed] [Google Scholar]
- 78.Braselmann S, Taylor V, Zhao H, Wang S, Sylvain C, Baluom M, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319:998– 1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 79.Pamuk ON, Tsokos GC. Spleen tyrosine kinase inhibition in the treatment of autoimmune, allergic and autoinflammatory diseases. Arthritis Res Ther. 2011;12:222. doi: 10.1186/ar3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Andre P, Morooka T, Sim D, Abe K, Lowell C, Nanda N, et al. Critical role for Syk in responses to vascular injury. Blood. 2011;118:5000– 5010. doi: 10.1182/blood-2011-06-360743. [DOI] [PMC free article] [PubMed] [Google Scholar]