Letter to the Editor
Acute myeloid leukemia (AML) is a malignant disease of cells arising from the myeloid lineage. A heterogeneous disease, AML consists of many different subtypes, each with different treatment sensitivities and prognoses. Despite advances in care, AML remains a difficult to treat disease, with overall survival (OS) being approximately 25% and 65% in the adult and pediatric populations, respectively.1, 2 A major factor contributing to treatment failure in AML is resistance to standard chemotherapy, consisting primarily of a combination of cytarabine (ara-C) and an anthracycline.
Overexpression of anti-apoptotic Bcl-2 family proteins (Bcl-2, Bcl-xL, Mcl-1, etc.) has been shown to cause resistance to chemotherapy in many cancer types, including AML.3–5 While inhibitors of this family have been promising, inhibition of Bcl-xL has been associated with platelet death and subsequent thrombocytopenia.5, 6 Fortunately, the recently developed ABT-199 is specific for Bcl-2, making it an attractive potential option for treatment of AML.7–9
This study was designed to identify genetic subgroups which may respond to ABT-199 favorably and biomarkers predicting ABT-199 sensitivity in AML. First, we screened 11 AML cell lines (CMS, CTS, HL-60, MOLM-13, MV4-11, NB4, OCI-AML3, THP-1, U937, CMK, and CMY, Table S1) for Bcl-2 family protein and mRNA expression (Figure S1A–C). In general, gene expression did not appear to follow any subtype distribution. Most cell lines expressed all three proteins, albeit at variable levels. We then tested the sensitivity of these cell lines to single-agent ABT-199 (Table S1). The cell lines exhibited a wide range of sensitivities, with IC50s ranging from 97 nM (HL-60) to 15 μM (OCI-AML3). Protein and transcript levels for the individual anti-apoptotic Bcl-2 family genes did not correlate with ABT-199 IC50 (data not shown).
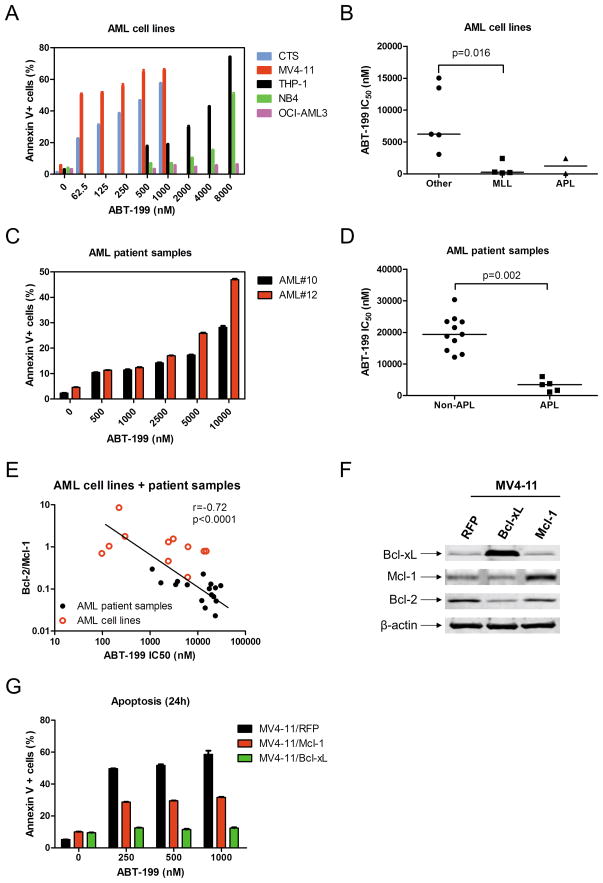
To confirm that ABT-199 caused cell death and not merely growth inhibition, Annexin V and propidium iodide (PI) dual staining was used to measure apoptosis in five AML cell lines. ABT-199 was able to induce apoptosis in a dose-dependent manner in four of the five cell lines tested. In contrast, apoptosis induced by up to 8 μM ABT-199 in OCI-AML3 cell line (the most resistant cell line determined by MTT assays) was minimal (Figure 1A). The relative magnitude of this induction was in agreement with the ABT-199 sensitivities shown in Table S1. Interestingly, we found that AML cell lines harboring MLL fusion genes were especially sensitive to ABT-199 compared to those that did not (median ABT-199 IC50s were 260 nM and 6.2 μM, respectively, p=0.016, Figure 1B). Further, the two acute promyelocytic leukemia (APL, FAB M3) cell lines (HL-60 and NB4) tended to be more sensitive to ABT-199 compared to the other AML cell lines.
Figure 1.
Panel A: Cell lines were treated with ABT-199 for 24 h. Apoptotic events (Annexin V+) were determined by Annexin V/PI staining and flow cytometry analyses. Panel B: AML cell lines were cultured for 72 h in the presence of variable concentrations of ABT-199 and viable cell numbers were determined using MTT reagent and a microplate reader. IC50 values were calculated as drug concentration necessary to inhibit 50% proliferation compared to untreated control cells. The horizontal lines indicate the median. Panel C: Freshly isolated AML patient samples were purified by standard Ficoll-Hypaque density centrifugation. AML patient samples #10 and #12 were treated with ABT-199 for 24 h and apoptotic events were determined by Annexin V/PI staining and flow cytometry analyses. Panel D: Ex vivo ABT-199 sensitivity was determined using MTT assays. The horizontal lines indicate median ABT-199 IC50s in each group of AML patient samples (Panel D). Panel E: Total RNAs were isolated and gene transcript levels were determined by Real-time RT-PCR. Transcript levels were normalized to GAPDH and relative expression levels were calculated using the comparative Ct method (comparing all samples to the CMS cell line expression levels). The Bcl-2/Mcl-1 ratios for the AML cell line and patient samples were graphed against the ABT-199 IC50. Panel F: MV4-11 cells were infected with Precision LentiORF Mcl-1, Bcl-xL, or red fluorescent protein control (designated MV4-11/Mcl-1, MV4-11/Bcl-xL, and MV4-11/RFP respectively) lentivirus overnight, washed, and incubated for 48 h prior to adding selection drug (blasticidin) to the culture medium. Whole cell lysates were subjected to Western blotting and probed with anti-Bcl-xL, -Mcl-1, -Bcl-2 or –β-actin antibody. Panel G: MV4-11/Mcl-1, MV4-11/Bcl-xL, and MV4-11/RFP were treated with ABT-199 for 24 h and apoptotic events were determined by Annexin V/PI staining and flow cytometry analyses. The p values were calculated using the Mann-Whitney U test. The relationship between the Bcl-2 family transcript levels/ratios and ABT-199 sensitivity were determined by the nonparametric Spearman rank correlation coefficient.
To confirm that ABT-199 is effective against primary AML patient samples, we tested the drug ex vivo in freshly isolated AML blast samples (n=16). Similar to the cell line results, ABT-199 was able to induce a dose-dependent increase in apoptosis in two AML patient samples (Figure 1C). The ABT-199 sensitivities for the primary AML samples were comparable to the cell lines (Tables 1&S1). Interestingly, AML patient samples with an APL phenotype were significantly more sensitive to single-agent ABT-199 compared to non-APL AML patient samples (median ABT-199 IC50s were 3.4 μM and 19 μM, respectively, p=0.002, Figure 1D).
Table 1.
Patient characteristics and ABT-199 sensitivity for the primary acute myeloid leukemia patient samples
| Patient | Gender | Age (year) | Disease | Disease status | FAB subtype | WBC (×103/μL) | Blast purity (%) | Cytogenetics | Fusion gene/ Gene mutation | ABT-199 IC50 (nM) |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 | Female | 50 | AML | At diagnosis | M4 | 22.66 | 52 | 46, XX, del(5q), add(12p) | dupMLL | 23305 |
| #2 | Male | 20 | AML | At diagnosis | M2 | 23.55 | 42 | 45, X, -Y, t(8;21)(q22; q22) | AML1-ETO | 30350 |
| #3 | Male | 4 | AML | At diagnosis | M3 | 4.71 | 75 | 46, XY, t(15;17)(q22; q21) | PML-RARα | 1108 |
| #4 | Male | 55 | AML | At relapse | M2 | 5.64 | 14 | 46, XY, t(8;21)(q22; q22) | AML1-ETO | 24320 |
| #5 | Female | 57 | AML | At relapse | M2 | 22.16 | 70 | 46, XX | FLT3 ITD | 18795 |
| #6 | Male | 60 | AML | At diagnosis | M3 | 1.83 | 86 | 46, XY, t(15;17)(q22; q21) | PML-RARα | 3443 |
| #7 | Female | 53 | AML | At relapse | M4 | 3.87 | 36 | 46, XX | 21520 | |
| #8 | Male | 55 | AML | At diagnosis | M2 | 17.71 | 32 | 46, XY | 19395 | |
| #9 | Female | 35 | AML | At relapse | M5 | 25.89 | 87 | 47, XX, +10, t(16;21)(p11; q22), add(11p) | TLS-ERG | 23420 |
| #10 | Male | 23 | AML | At diagnosis | M2 | 25.12 | 53 | 46, XY, del(9q) | 13000 | |
| #11 | Female | 43 | AML | At diagnosis | M2 | 93.99 | 91 | 46, XX | 12138 | |
| #12 | Male | 6 | AML | At diagnosis | M3 | 1.65 | 85 | 46, XY, t(15;17)(q22; q21) | PML-RARα | 3760 |
| #13 | Male | 52 | AML | At diagnosis | M2 | 8.84 | 27 | 46, XY, t(11;15;17)(q25; q15; q21) | 14270 | |
| #14 | Female | 18 | AML | At diagnosis | M3 | 1.62 | 64 | 46, XX | 6021 | |
| #15 | Female | 63 | AML | At diagnosis | M2 | 0.6 | 23 | 46, XX | 17384 | |
| #16 | Male | 38 | AML | At diagnosis | M3 | 2.27 | 90 | 46, XY, t(15;17)(q22; q21) | PML-RARα | 1680 |
Efforts were then undertaken to identify biomarkers predicting ABT-199 sensitivity in AML cells. As previously reported for multiple myeloma,8 ABT-199 sensitivity strongly and inversely correlated with the ratios of Bcl-2/Mcl-1 transcripts in the combined cohort of AML cell lines and patient samples (r=−0.72, p<0.0001, Figure 1E). Interestingly, ABT-199 IC50s also correlated positively with Mcl-1 transcript levels (r=0.46, p=0.015, Figure S1D) and negatively with the ratios of Bcl-2/Bcl-xL transcripts (r=0.−46, p=0.018, Figure S1E), however to a much lesser extent. These results suggest that besides Bcl-2, Bcl-xL and Mcl-1 play important roles in ABT-199 sensitivity in AML. Finally, to confirm that Bcl-xL and Mcl-1 affect ABT-199 sensitivity, we ectopically overexpressed Mcl-1 and Bcl-xL in MV4-11 cells. Western blot analysis confirmed overexpression of Bcl-xL or Mcl-1. Interestingly, MV4-11 cells ectopically overexpressing Bcl-xL had a decrease in Bcl-2 protein expression (Figure 1F). When treated with ABT-199 for 24 h, the MV4-11/RFP control cells showed a similar dose-dependent induction of apoptosis as compared to the parental cells (Figure 1G&A). As expected, overexpression of Bcl-xL and Mcl-1 attenuated ABT-199 induced apoptosis (Figure 1G).
In conclusion, we provide evidence supporting the further clinical investigation of ABT-199 in AML. While the AML cell lines demonstrated a range of sensitivities to single-agent ABT-199 therapy, one group appears to be especially sensitive. Cell lines harboring MLL fusion genes were more sensitive, suggesting that MLL fusion genes play a common role in Bcl-2 mediated anti-apoptotic effects. Unfortunately, we were unable to obtain any patient samples with these abnormalities to confirm this finding. Based on the cell line data, patients, such as those with infant AML,10 whose blast cells have a MLL gene rearrangement, may benefit from treatment with ABT-199, however confirmation in patient samples is warranted.
Importantly, we found that primary AML samples with the APL phenotype are especially sensitive to single-agent ABT-199 suggesting a possible role for this agent in APL patients who do not respond to all-trans retinoic acid and/or arsenic trioxide based therapies. This finding, while thus far only in a limited number of samples, has potential importance as patients with APL meet the exclusion criteria for the current phase II clinical trial investigating ABT-199 in AML (NCT01994837, clinicaltrials.gov). Further, our data from both AML cell lines and primary patient samples suggest that the ratios of Bcl-2/Mcl-1 transcripts may represent a good biomarker predicting ABT-199 sensitivity in acute leukemia. Our results further support the clinical development of ABT-199 for treatment of AML.
Supplementary Material
Acknowledgments
This study was support by a Start-up Fund from Jilin University, Changchun, China, and grants from the National Natural Science Foundation of China (NSFC 31271477 and 81200363). Mr. JTC is a predoctoral trainee supported by T32 CA009531 from the National Cancer Institute.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Supplementary information is available at Leukemia’s website (www.nature.com/leu)
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63 (1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. The lancet oncology. 2010;11(6):543–52. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saxena A, Viswanathan S, Moshynska O, Tandon P, Sankaran K, Sheridan DP. Mcl-1 and Bcl-2/Bax ratio are associated with treatment response but not with Rai stage in B-cell chronic lymphocytic leukemia. American journal of hematology. 2004;75(1):22–33. doi: 10.1002/ajh.10453. [DOI] [PubMed] [Google Scholar]
- 4.Lauria F, Raspadori D, Rondelli D, Ventura MA, Fiacchini M, Visani G, et al. High bcl-2 expression in acute myeloid leukemia cells correlates with CD34 positivity and complete remission rate. Leukemia. 1997;11(12):2075–8. doi: 10.1038/sj.leu.2400854. [DOI] [PubMed] [Google Scholar]
- 5.Davids MS, Letai A. Targeting the B-cell lymphoma/leukemia 2 family in cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(25):3127–35. doi: 10.1200/JCO.2011.37.0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goard CA, Schimmer AD. An evidence-based review of obatoclax mesylate in the treatment of hematological malignancies. Core evidence. 2013;8:15–26. doi: 10.2147/CE.S42568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nature medicine. 2013;19(2):202–8. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 8.Touzeau C, Dousset C, Le Gouill S, Sampath D, Leverson JD, Souers AJ, et al. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia. 2014;28(1):210–2. doi: 10.1038/leu.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 Inhibition by ABT-199 Causes On Target Cell Death in Acute Myeloid Leukemia. Cancer Discovery. 2013 doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felix CA, Lange BJ. Leukemia in Infants. The Oncologist. 1999;4(3):225–240. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.