Abstract
TDP1 and TDP2 were discovered and named based on the fact they process 3′- and 5′-DNA ends by excising irreversible protein tyrosyl-DNA complexes involving topoisomerases I and II, respectively. Yet, both enzymes have an extended spectrum of activities. TDP1 not only excises trapped topoisomerases I (Top1 in the nucleus and Top1mt in mitochondria), but also repairs oxidative damage-induced 3′-phosphoglycolates and alkylation damage-induced DNA breaks, and excises chain terminating anticancer and antiviral nucleosides in the nucleus and mitochondria. The repair function of TDP2 is devoted to the excision of topoisomerase II- and potentially topoisomerases III-DNA adducts. TDP2 is also essential for the life cycle of picornaviruses (important human and bovine pathogens) as it unlinks VPg proteins from the 5′-end of the viral RNA genome. Moreover, TDP2 has been involved in signal transduction (under the former names of TTRAP or EAPII). The DNA repair partners of TDP1 include PARP1, XRCC1, ligase III and PNKP from the base excision repair (BER) pathway. By contrast, TDP2 repair functions are coordinated with Ku and ligase IV in the non-homologous end joining pathway (NHEJ). This article summarizes and compares the biochemistry, functions, and post-translational regulation of TDP1 and TDP2, as well as the relevance of TDP1 and TDP2 as determinants of response to anticancer agents. We discuss the rationale for developing TDP inhibitors for combinations with topoisomerase inhibitors (topotecan, irinotecan, doxorubicin, etoposide, mitoxantrone) and DNA damaging agents (temozolomide, bleomycin, cytarabine, and ionizing radiation), and as novel antiviral agents.
Keywords: Topoisomerases, Poly(ADPribose) polymerases (PARP), Homologous Recombination (HR), Non-homologous end joining (NHEJ), Chemotherapy
1. Introduction
Tyrosyl-DNA phosphodiesterases (TDP1 and TDP2) are among the most recently discovered DNA repair enzymes. Ongoing studies point to their relevance for the repair of trapped topoisomerase-DNA complexes and for the processing of diverse 3′- and 5′-blocking groups at DNA ends. First, we will provide background for the physiological, environmental and pharmacological conditions leading to the trapping of topoisomerases on DNA (Fig. 1 and Tables 1 and 2). After which we will place TDP1 and TDP2 in the context of the different pathways involved in the removal of topoisomerase-DNA complexes (Fig. 2). We will review the biochemistry, structure, functions, substrates and regulation of both TDP1 and TDP2 (Table 3 and Figs. 3–7). Finally, we will discuss the rationale and approaches for the discovery of TDP1 and TDP2 inhibitors.
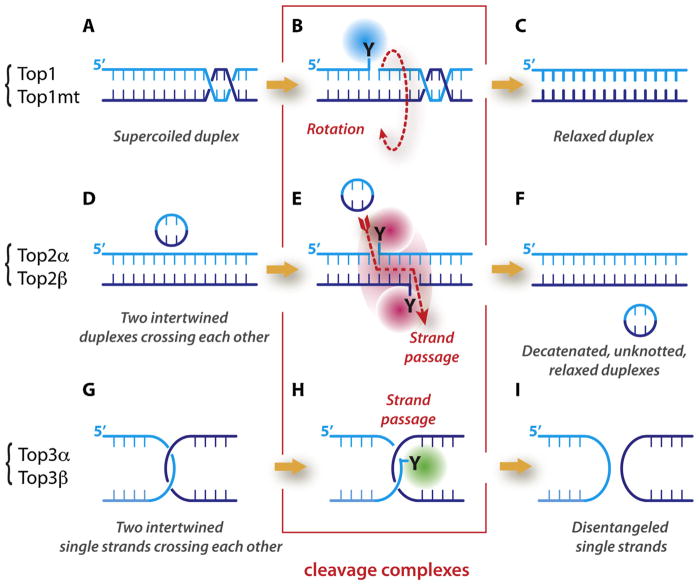
Fig. 1.
Mechanisms of action of topoisomerases and schematic representation of the cleavage complexes. (A)–(C) Topoisomerases I (Top1 for nuclear DNA and Top1mt for mitochondrial DNA) relax supercoiled DNA (A) by reversibly cleaving one DNA strand, forming a covalent bond between the enzyme catalytic tyrosine and the 3′ end of the nicked DNA (the Top1 cleavage complex: Top1cc; (B) This reaction allows the swiveling of the broken strand around the intact strand. Rapid religation allows the dissociation of Top1. (D)–(F) Topoisomerases II (Top2α and Top2β) act on two DNA duplexes (D) They act as homodimers, cleaving both strand, forming a covalent bond between their catalytic tyrosine and the 5′ end of the DNA break (Top2cc; (E)). This reaction allows the passage of the intact duplex through the Top2 homodimer (red dotted arrow; (E)). Religation and ATP hydrolysis allow the dissociation of Top2 (F). (G)–(I) Topoisomerases III (Top3α and Top3β) act on intertwined single-strands (DNA for Top3α and DNA and RNA for Top3β. They cleave one strand, forming a covalent bond between their catalytic tyrosine and the 5′ end of the nucleic acid (Top3cc; (H)). This reaction allows the passage of the intact strand through the Top3 monomer. Religation allows the dissociation of Top3 (I).
Table 1.
Exogenous and endogenous factors producing Top1 cleavage complexes and relevant for TDP1.
| Endogenous DNA lesions | Notes | Refs. [163,164] |
|---|---|---|
| Single base mismatches | Polymerase and mismatch defects | [163,165] |
| Mismatched loops | Mismatch deficiencies | [165] |
| Abasic sites | Abasic sites; BER* | [165] |
| 8-Oxoguanosine | Free radicals | [166] |
| 5-Hydroxycytosine | Free radicals | [166] |
| Single-strand breaks | Free radicals; base excision repair | [16,167] |
| Cytosine methylation | Physiological | [168] |
| Triple helix formation | Physiological | [169] |
| Apoptotic chromatin fragmentation | Ubiquitous during apoptosis | [164,170–172] |
| Exogenous DNA lesions | [163] | |
|
| ||
| UV lesions | Dimers and 6,4-photoproducts | [173,174] |
| IR-induced DNA breaks | Both single- and double-strand breaks | [16] |
| O6- or N7- or O3-methylguanine | Alkylating drugs (MNNG, MMS, TMZ)* | [45,53,175] |
| O6-dA-benzo[a]pyrene adducts | Intercalated carcinogenic adducts | [176] |
| N2-dG-benzo[a]pyrene adducts | Minor groove carcinogenic adducts | [177,178] |
| N2-dG-benzo[c]phenanthrene adducts | Intercalated carcinogenic adducts | [178] |
| N6-ethenoadenine | Carcinogenic vinyl adduct | [179] |
| N2-dG-ethyl adducts | Produced by acetaldehyde (alcohol) | [180] |
| N2-dG-crotonaldehyde adducts | Exogenous carcinogens and endogenous | [181] |
BER: base excision repair; MNNG: methylnitronitrosoguanine; MMS: methylmethanesulfonate; TMZ: temozolomide.
Table 2.
Exogenous and endogenous factors producing Top2 cleavage complexes and relevant to TDP2.
| Endogenous DNA lesions | Notes | Refs. [182] |
|---|---|---|
| Abasic sites | Apurinic and apyrimidinic sites; BER* | [183–186] |
| Processed abasic sites | BER intermediates | [187] |
| 8-oxoguanine/8-oxoadenine | Free radicals; weak topoisomerase II poison | [185] |
| O6-methylguanine | Free radicals; weak topoisomerase II poison | [185] |
| Deoxyuracil | Cytosine deamination | [188] |
| Base mismatches | DNA replication | [188,189] |
| Ribonucleotides | DNA replication | [190,191] |
| 1,N6-ethenodeoxyadenine | Lipid peroxidation; industrial chemicals | [185,186] |
| 1,N2-ethenodeoxyguanosine | Lipid peroxidation; industrial chemicals | [186] |
| 3,N4-ethenodeoxycytidine | Lipid peroxidation; industrial chemicals | [186] |
| 3,N4-ethenodeoxycytidine | Lipid peroxidation; industrial chemicals | [186] |
| M1dG | Lipid peroxidation; industrial chemicals | [186] |
| Single-stranded DNA breaks | Free radicals; cellular processes | [192,193] |
| Exogenous DNA lesions
| ||
| UV lesions | Cyclobutane dimers | [194] |
| Cytosine arabinoside | Cytarabine-induced DNA lesions | [195] |
| Ethenobase adducts | Chloracetaldehyde treatment of cells | [186] |
| benzo[a]pyrene 7,8-diol 9,10-epoxide deoxyadenosine | Intercalated carcinogenic adducts | [196] |
BER: base excision repair.
Fig. 2.
Tyrosyl-phosphodiesterase vs endonuclease pathways for the removal of irreversible topoisomerase cleavage complexes: (A) Top1; (B) Top2. TDP1 and TDP2 (yellow highlights) hydrolyze the tyrosyl-DNA links following proteolysis and/or denaturation of Top1 or Top2. The alternative endonuclease pathways shown in green remove the DNA segment covalently attached to topoisomerases. For Top1, they include XPF-ERCC1 [29,55], Mre11-Rad50-Nibrin/Nbs1 (MRN) [148], SLX4 [161] and CtIP [149,150,162]. For Top2, they include MRN and CtIP [reviewed in [140]]. Double-arrows and orange text indicate the pathways that finalize DNA repair. In the case of Top1 (A), homologous recombination (HR including the BRCA proteins) is critical for repairing the double-strand ends generated by replication run-off [102]. For Top2 (B), end-joining (EJ) with Ku and ligase IV are essential downstream from TDP2 [27,131,132] whereas HR is linked to the endonuclease pathway [140].
Table 3.
Comparison of TDP1 and TDP2.
| TDP1 | TDP2 | |
|---|---|---|
| Family | Phospholipase D (PLD) | Mg2+/Mn2+-dependent phosphodiesterases (MDP) |
| Substrates | DNA-phosphotyrosines (Top1) 3′-phosphoglycolates 3′-nucleosides |
DNA-phosphotyrosines (Top2, Top3?) RNA-phosphotyrosine (VPg unlinkase) |
| Polarity | 3′ ≫ 5′ [45,68] | 5′ ≫ 3′ [134] |
| Catalytic mechanism | Covalent intermediate [73] | Two-metal catalysis [134] |
| Amino acids | 608 residues | 362 residues |
| Molecular weight | 68 kDa | 41 kDa |
| Genomic location | 14q32.11 | 6p22.3-p22.1 |
| Associated disease | SCAN1 [79] | Reading disability [197–199] Parkinson disease [200–202] |
| Knockout mice | Yes [51,203] | Yes [132] |
| Crystal structure with substrate | Yes [75] | Yes [137] |
Fig. 3.
TDP1 processes a wide range of substrates that represent 3′-blocking DNA (or RNA) lesions to convert them to 3′-hydroxyl-ends (see Fig. 5). The red arrows indicate the cleavage sites. (A) Canonical substrate corresponding to Top1 [39,40] and Top1mt [43]. (B) TDP1 can also hydrolyze 3′-phosphoamide groups such as those generated by mutated TDP1 with the SCAN-1 mutation [58]. (C) TDP1 can also excise (less efficiently) 3′-base (3′-nucleosidase activity) [58]. (D) TDP1 is involved in the excision repair of 3′-chain-terminating anticancer and antiviral nucleosides (pink) including acyclovir (ACV), cytarabine (Ara-C) and zidovudine (AZT) [69]. (E) TDP1 excises 3′-blocking lesions (brown) resulting from base alkylation: 3′-abasic and 3′-deoxyribose phosphate ends [45,53,58] or oxidation (3-glycolates) [58,62,63]. (F) Yeast TDP1 and less efficiently human TDP1 can hydrolyze 5′-tyrosyl ends [45,60]. (G) A large number of substrates with 3′-phospho-linkage have been used to screen for TDP1 inhibitors: 4-methyl- and 4-nitro-phenol [70], 4-methyl-umbelferone [71], biotin linker [58], 3′-ruthenium-BV-tag [72], 3′-FITC [152], 6-FAM [69] and BHQ [155].
Fig. 7.

Proposed reaction mechanism for phosphodiester bond cleavage by TDP2. (A) Top2-derived peptide (trapped topoisomerase) linked to 5′-DNA via a phosphotyrosyl bond. (B) Upon binding of the covalent peptide-DNA substrate in the Tdp2 active site, two magnesium ions are coordinated by the TDP2 catalytic residues for nucleophilic attack of the phosphotyrosyl bond (black arrows) [134]. (C) Cleavage products consisting in the topoisomerase polypeptide (top) and the liberated DNA with a 5′-phosphate end.
2. Topoisomerase cleavage complexes
Large nucleic acid molecules (DNA and RNA) are typically densely packaged in the cell nucleus. Yet, local relaxation of nucleic acids is required for transcription, replication and proper chromosomal segregation. These vital cellular processes generate torsional stresses in the form of supercoiling, as well as DNA and RNA entanglements that are resolved by topoisomerases.
2.1. Overview of human topoisomerases
Topoisomerases are ubiquitous and essential. They solve topological problems by cutting and religating nucleic acids without assistance of additional enzymes (i.e. they are endowed with nucleic acid cutting-pasting properties). Human cells contain six topoisomerase genes. Topoisomerases have been numbered historically (see Fig. 1 in Ref. [1]), and the corresponding polypeptides (Fig. 1, left brackets) are abbreviated: Top1 for topoisomerases I (Top1mt for the mitochondrial topoisomerase I, whose gene is encoded in the nuclear genome), Top2 for topoisomerases II, and Top3 for topoisomerases III.
Top1 (Fig. 1A–C) and Top3 (Fig. 1G–I) act by cleaving-religating a single-strand of DNA (or RNA in the case of Top3β), whereas Top2 enzymes (Fig. 1D–F) cleave both strands of a DNA duplex, generating a 4 base pair reversible staggered cut (Fig. 1E). It is convenient to remember that odd-numbered topoisomerases (Top1 and Top3) cleave and religate one strand, whereas the even-numbered topoisomerases (Top2) cleave and relegate both strands.
2.2. Topoisomerization mechanisms: Swiveling vs strand passage, DNA vs RNA topoisomerases
Topoisomerases use two main mechanisms to change the topology of nucleic acids. Unique to Top1, the first mechanism consists in “untwisting” the DNA duplex. To do so, Top1 generates a single-strand break by forming a covalent DNA-enzyme intermediate that allows the broken strand to rotate around the intact one until DNA supercoiling is dissipated (Fig. 1B). At which point, the stacking of adjacent DNA bases realigns the broken ends and the 5′-hydroxyl end attacks the 3′-phosphotyrosyl bond, thereby allowing DNA religation (Fig. 1C). A remarkable feature of the Top1 untwisting mechanism is its extreme efficiency. The rotation speed has been estimated by single molecule analyses at about 6000 rpm irrespective of torque, thereby allowing full relaxation of DNA supercoils [2].
The second mechanism used by the other topoisomerases is by “strand passage”. It allows the passage of a double- or a single-stranded DNA (or RNA) through the cleavage complexes. Top2α and Top2β both act by allowing the passage of an intact DNA duplex through the DNA double-strand break (DSB) generated by enzyme homodimers (Fig. 1E). Afterwards, Top2 religates the broken duplex (Fig. 1F). Such reactions permit DNA decatenation, unknotting and the relaxation of supercoils [1,3]. Top3 enzymes pass only one strand through the single-strand break generated by the enzymes (Fig. 1G–I). In the case of Top3α, the substrate is a single-stranded DNA segment (such as a double-Holliday junction) [4–6], whereas in the case of Top3β, the substrate can be a single-stranded RNA segment with Top3β acting as a RNA topoisomerase [7,8]. Topoisomerases have distinct biochemical requirements. Top1 and Top1mt are the simplest topoisomerases, acting as monomers in the absence of any cofactors at temperatures as low as 0 °C. Top2 enzymes are complex molecular machines, working as dimers, requiring ATP binding, ATP hydrolysis and divalent metals (Mg2+) for catalysis. Top3 enzymes also require Mg2+ but function as monomers without ATP.
2.3. Cleavage complexes are the key catalytic intermediates of topoisomerases
The DNA (and RNA) cleavage/relegation mechanism of topoisomerases operates by the attack of catalytic tyrosine residues acting as nucleophiles to generate covalent tyrosine–nucleic acid intermediates. These catalytic intermediates are referred to as cleavage complexes (outlined in red in Fig. 1B, E and H). The reverse religation reactions are carried out by attacks of the deoxyribose (or ribose) hydroxyl ends toward the tyrosyl-phosphodiester bonds.
Top1 (and Top1mt) attaches to the 3′-end of the scissile phosphate, whereas the other topoisomerases (Top2 and Top3) have opposite polarity and covalently attach to the 5′-end of the scissile phosphate (Fig. 1B, E and H). Notably, the DNA substrates differ for Top3 enzymes. Whereas Top1 and Top2 both process double-stranded DNA, the Top3 enzymes act on single-stranded nucleic acids (DNA for Top3α and RNA for Top3β) [4,7,8].
2.4. Trapping of topoisomerase cleavage complexes by endogenous and exogenous DNA lesions and Top1-induced DNA nicks at ribonucleotides embedded in DNA
Tables 1 and 2 summarize the ubiquitous DNA modifications and lesions generated by endogenous and exogenous factors leading to the trapping of Top1 and Top2 cleavage complexes (Table 1 for Top1cc and Table 2 for Top2cc). Both tables include corresponding references. Two points are particularly relevant to TDP1 and TDP2. First, trapping of Top1cc and Top2cc can occur independently of drugs, consistent with the fact that TDP1 genes are present in all eukaryotic cells (from yeast to humans). Second, Top1cc trapping is likely to be frequent and biologically relevant, especially in neurons because of their high oxidative metabolism and long transcripts [9,10]. Abasic sites resulting from spontaneous depurination and base excision repair (BER) intermediates have been estimated at a frequency of ≈ 104 per cell per day [11].
A special form of abortive Top1 cleavage complexes has recently been the focus of investigations because it has been linked to Top1-mediated mutations in yeast [12]. Top1 converts ribonucleotide embedded in cellular DNA into nicks with 2′–3′-cyclophosphate and 5′-hydroxyl ends [13]; a property of Top1 that was first observed in biochemical systems [14]. The biological relevance of this RNA nicking activity of Top1 could represent a previously unrecognized repair pathway for misincorporated ribonucleotides during DNA replication as an alternative to RNase II [15]. It could also be detrimental to the genome by initiating short base deletions [12,13] and by generating nicks, which can then trap Top1cc [16].
2.5. Topoisomerase inhibitors trap topoisomerase cleavage complexes by a common interfacial mechanism
Drug binding at the enzyme–DNA interface misaligns the DNA ends, which precludes religation and results in the stabilization of topoisomerase cleavage complexes (Top1cc and Top2cc). Crystal structures of drug-bound cleavage complexes have generalized this mechanism for both Top1- and Top2-targeted drugs (see Figs. 1–3 and citations in Ref. [17]).
The cytotoxic mechanism of topoisomerase inhibitors requires the drugs to trap the topoisomerase cleavage complexes rather than to block catalytic activity. This sets apart topoisomerase inhibitors from classical competitive enzyme inhibitors. Indeed, knocking out Top1 renders yeast cells immune to camptothecin [18,19], and reducing enzyme levels in cancer cells confers drug resistance [20]. Conversely, in breast cancers, amplification of TOP2A, which is on the same locus as HER2, contributes to the efficacy of doxorubicin [21]. Also, mutations of Top1 and Top2 that interfere with the trapping of cleavage complexes produce high resistance to Top1 or Top2 inhibitors [22]. Based on their characteristic trapping mechanism, topoisomerase inhibitors should be viewed as topoisomerase cleavage complex-targeted drugs. Another example of trapping mechanism was recently discovered for poly(ADP-ribose) polymerase (PARP) inhibitors, which trap PARP1 and PARP2 on damaged DNA [23].
2.6. Top1cc damage DNA by replication fork and transcription collisions
Top1 cleavage complexes damage the genome by generating DNA double-strand ends upon replication and transcription fork collisions (Fig. 2A) (reviewed in [24]). This explains why the cytotoxicity of camptothecins is primarily related to the duration of drug exposure and why arresting DNA replication protects cells from camptothecin [25,26]. Such collisions have two main consequences (Fig. 2A): 1/DSBs (replication and transcription run-off) and 2/irreversible Top1-DNA adducts. Replication-induced DSBs are repaired by homologous recombination, which explains the hypersensitivity of BRCA-deficient cancer cells to Top1cc-targeted drugs [27]. Top1-DNA covalent complexes can be removed by two pathways: 1/excision of Top1 by TDP1 [28], and 2/DNA cleavage by 3′-flap endonucleases such as XPF-ERCC1 [29] (Fig. 2A). In addition, drug-trapped Top1cc may directly generate DSBs when they are within 10 base pairs on opposite strands of the DNA duplex or when they occur next to a preexisting single-strand break on the opposite strand [16,30]. Finally, it is not excluded that topological defects contribute to the cytotoxicity of Top1cc-targeted drugs [accumulation of supercoils [31] and formation of alternative structures such as R-loops] [32].
2.7. Top2cc-induced DNA damage
Unlike camptothecins, Top2 inhibitors (e.g. etoposide and doxorubicin) kill cancer cells even in the absence of replication. Thirty minute exposure to doxorubicin and other Top2cc-targeted drugs can kill over 99% of the cells, which is in large excess of the fraction of S-phase cells in tissue culture (generally less than 50%) [33,34]. In the case of Top2cc-targeted drugs, stalled transcription complexes triggers proteolysis of both Top2 and RNA polymerase II [35], which then leads to DSBs by disruption of the Top2 dimer interface. Alternatively, the Top2 homodimer interface could be disjoined by mechanical tension arising from transcription and replication collisions. Yet, up to 90% of the Top2cc trapped by etoposide are not “concerted” consisting of Top2-linked single-strand breaks [1,36,37]. This is different from doxorubicin, which produces mostly DSBs by trapping both Top2 monomers simultaneously [38]. Finally, topological defects resulting from drug-induced Top2 sequestration may contribute to the cytotoxicity of Top2cc-targeted drugs. Such topological defect would include persistent DNA knots and catenanes, potentially leading to chromosome breaks during mitosis.
3. Tyrosyl-DNA phosphodiesterase 1 (TDP1)
3.1. TDP1 substrates and functions
Nash and coworkers first identified TDP1 in yeast Saccharomyces cerevisiae [39,40], based on an activity that specifically hydrolyzed phosphotyrosyl bonds at DNA 3′-DNA ends (Fig. 3A). As 3′-tyrosyl substrates correspond to trapped Top1cc, tdp1 was shown to repair Top1cc in S. cerevisiae [40,41]. Because TDP1 generates 3′-phosphate ends, its cellular activity needs to be coupled with polynucleotide kinase phosphatase (PNKP) to generate 3′-hydroxyl ends that can be extended by polymerases. TDP1 orthologs exist in all organisms and act both in the nucleus and vertebrate mitochondria (where Top1mt is present [42]) [43–46]. Genetic inactivation of TDP1 confers hypersensitivity to camptothecins in human [47–50], murine [51,52], and chicken cells [27,45,53], as well as in Leishmania donovani (trypanosome) [46], and in yeast Saccharomyces pombe [54], and S. cerevisiae [41,55].
TDP1 hydrolyzes 3′-tyrosine (Figs. 2 and 3A) in a variety of DNA structures with preference for single-stranded DNA. It retains activity for DNA segments as short as 4 nucleotides [56] and double-stranded substrates, especially at gaps, blunt ends, frayed-and tailed-ends [39,57]. While TDP1 cannot remove full-length native Top1, proteolytic digestion or denaturation of Top1 enables hydrolysis by TDP1 [39,56,58]. TDP1 can process 3′-peptides ranging from one to more than 100 residues [56,58,59]. However, it hydrolyzes longer oligonucleotide and shorter peptides more efficiently [56,59]. TDP1 can resolve 5′-phosphotyrosyl bonds (Fig. 3F) [45,60], albeit much less efficiently than TDP2, which implicates TDP1 only as in a back-up pathway for Top2cc repair [45,60].
Other than phosphotyrosyl bonds, TDP1 readily hydrolyzes a wide range of physiological and pharmacological 3′-blocking lesions (Fig. 3C–E). The importance of TDP1 outside Top1cc repair was first shown for 3′-phosphoglycolate ends and 3′-deoxyribose phosphate ends (Fig. 3E), which are common products of oxidative DNA damage as well as from radiomimetic drugs such as bleomycin [45,52,61–65]. Accordingly, TDP1 knockout cells are deficient in repairing oxidative DNA damage both in mitochondria and the nucleus [43,66,67]. The nucleosidase activity of TDP1 can remove 3′-terminal deoxyribo- and ribo-nucleotides when they are not phosphorylated at their 3′-end (Fig. 3C) [58,68]. The fact that TDP1 cannot process 3′-phosphate ends limits its activity to the removal of only one nucleoside from DNA ends. We recently showed that the nucleosidase activity of TDP1 removes widely used anti-viral and anti-cancer chain-terminating nucleoside analogs, such as acyclovir (ACV), zidovudine (AZT) and cytarabine (Ara-C) (Fig. 3D) in biochemical and cellular repair assays [69]. Anti-viral and anti-cancer nucleosides act by generating 3′-blocking lesions at replication sites, suggesting the importance of TDP1 in the repair of replication-associated lesions in the nucleus and mitochondria [69].
TDP1 also efficiently hydrolyzes 3′-deoxyribose lesions resulting from base alkylation after AP lyase processing [45,65]. This activity is particularly relevant for the repair of DNA lesions induced by monofunctional alkylating agents including methylmethanesulfonate and temozolomide, and ionizing radiations [45,53]. In such cases, TDP1 can act both by directly removing the 3′-end blocking lesions and by repairing Top1 covalent complexes that have been trapped at DNA nicks [16,45,53]. Finally, the potent phosphodiesterase activity of TDP1 allows hydrolysis of a wide range of synthetic DNA adducts attached to 3′-phosphate ends, such as biotin and a variety of fluorophores (Fig. 3G) [58,68–72], which have been particularly valuable for screening TDP1 inhibitors and for detailed mechanistic studies (see Section 5).
3.2. TDP1 structure
Human TDP1 is a 68-kDa polypeptide comprising 608 amino acid residues. It consists of two domains (Fig. 4A). The N-terminus (white in Fig. 4A) is dispensable for enzymatic activity but regulates TDP1 recruitment and protein stability (see Section 3.4). The C-terminus catalytic domain belongs to the phospholipase D family (PLD) [73] as it contains two catalytic HKN motifs separated from each other by 210 amino acid residues (for human TDP1: H263K265N283 and H493K495N516, Fig. 4A). TDP1 differs from other PLD family members because the aspartates in the HKD motifs are replaced by asparagines (HKN motifs). Truncated human TDP1 (Δ1–148) has been co-crystallized with single-stranded DNA substrates attached to a four amino acid tyrosyl peptide and a vanadate ion mimicking the phosphate group linked to the Top1 catalytic tyrosine [74,75]. TDP1 acts as a monomer with the two HKN motifs in close proximity to form the catalytic site located inside an asymmetric substrate-binding channel (Fig. 4C), which is relatively narrow and positively charged to bind the single-stranded DNA substrate. The channel widens in its portion receiving the polypeptide, where its charge distribution is more mixed (Fig. 4C). Both HKN motifs stabilize the vanadate used as transition intermediate by six H-bonds between the nitrogen atoms of the HKN residues and the oxygen atoms of the vanadate (Fig. 4E). The active site asparagine residues (N283 and N516) stabilize the neighboring lysine side chain by hydrogen bonding between their side chain oxygen atoms and the ε-amino groups of the lysines. The single-stranded DNA substrate is held in place by a network of polar interactions involving the 5′-phosphate groups of the 3 nucleotides upstream from the phosphotyrosine bond (T-1, T-2 and G-3, Fig. 4E). Residues involved in this stabilization include S400, S403, K469, S518, K519 and A520 (Fig. 4E). Residues F259, P461 and W590 are also engaged in hydrophobic interactions with the DNA (Fig. 4E). Because substrate stabilization is made via either hydrophobic or polar interactions with the DNA phosphodiester backbone, the TDP1 DNA substrate recognition is not restricted to particular base sequences. This is consistent with the fact that Top1 cleavage complexes form at many different genomic sites, and that TDP1 needs to be effective irrespective of the DNA sequence environment, including at 3′-blocking lesions besides Top1-DNA adducts [76,77] (Table 3).
Fig. 4.
Crystal structures of TDP1 (left) and TDP2 (right). (A) and (B) Ribbon representation of TDP1 (A) and TDP2 (B) polypeptides. N-terminal (white) segments (residues 1–148 for TDP1 and 1–120 for TDP2) are absent in the crystal structures shown below. UBA in TDP2: ubiquitin binding associated domain. Conserved catalytic segments are highlighted in yellow. (C) and (D) Surface representations of the crystal structures for TDP1 ((C) PDB ID 1NOP) and TDP2 ((D) PDB ID 4F1H). Proteins are represented in light pink, catalytic residues in yellow, DNA in blue sticks, peptide in green sticks and both sticks colored by element (N, blue; O, red; P, orange; Vanadate, grey). For TDP2, magnesium is represented as the green sphere. (E) and (F) Detailed contacts between substrates and TDP residues in the catalytic site of TDP1 (E) and TDP2 (F). Catalytic residues are represented as yellow sticks; residues involved in polar interactions are in cyan sticks; residues involved in hydrophobic interactions in magenta sticks. All sticks are colored by element (N, blue; O, red; P, orange; Vanadate, grey). Dashed lines highlight polar interactions.
3.3. TDP1 catalytic mechanism
TDP1 does not require nucleotide cofactor or metal. Yet, its catalytic mechanism is relatively complex, as TDP1 processes its substrates in two steps with a transient covalent intermediate [73] (Fig. 5). The first step consists in a nucleophilic attack of the Top1-DNA phosphotyrosyl bond by H263 residue from the N-terminal HKN motif. The H493 residue from the opposite HKN motif acts as a general acid and donates a proton to the tyrosine-containing peptide-leaving group (Fig. 5A). A transient covalent phosphoamide bond is formed between H263 and the 3′-end of the substrate (Fig. 5B). The opposite H493 residue acts as a general base and hydrolyzes this covalent intermediate via an activated water molecule (Fig. 5C) [74]. This generates a product with a 3′-phosphate end (Fig. 5D), which needs to be further processes by polynucleotide kinase phosphatase (PNKP) [78]. Mutation of one of the catalytic histidines to an arginine residue at position 493 (H493R) results in the accumulation of covalent TDP1-DNA intermediates (Fig. 5E) [48] ultimately leading to a rare autosomal recessive neurodegenerative disease called spinocerebellar ataxia with axonal neuropathy (SCAN1) [79]. As discussed above, wild-type TDP1 can process this phosphoamide intermediate [58], which explains why SCAN1 patients have the H493R homozygous mutation [79].
Fig. 5.
TDP1 catalytic cycle. (A) Nucleophilic attack of the phosphodiester backbone by the imidazole N2 atom of H263. H493 donates a proton to the tyrosyl moiety of the leaving group. (B) Phosphohistidine covalent intermediate. (C) Second nucleophilic attack via an activated water molecule by H493. (D): Generation of a final 3′-phosphate product and free TDP1. (E) The SCAN-1 mutations (H493R) leads to an accumulation of the Tdp1-DNA intermediate and a defect in TDP1 turn- over rate.
3.4. TDP1 regulation, post-translational modifications and association with various repair pathways
Post-translational modifications by phosphorylation, poly(ADP-ribosyl)ation, SUMOylation and ubiquitylation are common regulators for the recruitment, modulation of enzymatic activity and stability of DNA damage response (DDR) proteins. The primary DDR transducers are the nuclear serine-threonine kinases ataxia-telangiectasia mutated (ATM) [80–82], DNA-dependent protein kinase (DNA-PK), and ataxia telangiectasia and Rad3-related (ATR) [83]. Upon rapid activation in response to DSBs [82,84], ATM phosphorylates a plethora of key players in DDR pathways [80,85]. DNA-PK is also activated by DSBs but is primarily involved in non-homologous end-joining (NHEJ) [86] and apoptotic pathways [87]. Following the initial hypothesis that TDP1 was a target for DDR kinases [63], ATM and DNA-PK were both found to activate TDP1 by phosphorylation at serine 81 (S81, Fig. 4A) [88]. TDP1 phosphorylation at S81 increases the stability and recruitment of TDP1 to DNA damage sites rather than by directly affecting its catalytic activity [88,89]. TDP1-S81 phosphorylation also promotes interactions of TDP1 with XRCC1 and ligase IIIα [88,89], which then recruit PNKP [78,90].
Although XRCC1 is classically implicated in single-strand breaks rejoining in the BER pathway [91], it has also been proposed to play a role in DSB repair in an alternative end-joining pathway [92,93]. XRCC1-deficient cells display a significant defect in rejoining radiation-induced DSB [94], and XRCC1 depletion sensitizes cells to the DSB-inducing agent bleomycin [95]. XRCC1-deficient cells are hypersensitive to camptothecin [78,96–98]. A link between the ATM-Chk2 pathway and XRCC1 phosphorylation has recently been proposed [99]. Furthermore, DNA-PK has been shown to interact with and to phosphorylate XRCC1 at serine 371 [100,101]. Camptothecin-induced XRCC1 foci co-localize with γH2AX and pS81-TDP1 foci formed at DSBs [88]. These sites most likely correspond to the small fraction of the Top1cc that are converted into irreversible Top1-DNA lesions by collision with replication [102–104] and transcription machineries [32]. Thus, it is plausible that XRCC1 is critical for the repair of lesions associated with Top1-linked DSBs. Phosphorylated TDP1-S81 protects cells against camptothecin- and IR-induced DNA damage. Yet, it is still unclear whether this phosphorylation impacts single-strand break as well as DSB repair, since the phosphorylation appears to be driven by DSB formation [88,89].
TDP1-SUMOylation at lysine 111 (K111, Fig. 4A) promotes DNA repair and TDP1 accumulation at Top1cc-induced and UV-laser-induced DNA damage sites [105]. SUMOylation is catalyzed in a three-step process by E1, E2, and E3 enzymes followed by desumoylation by proteases specifically recognizing SUMO conjugates [106,107]. Unlike TDP1 phosphorylation (see above), which is induced by DNA damage, TDP1-SUMOylation is independent of DNA damage. Moreover, mutating the SUMOylation site (K111R) of TDP1 does not inhibit TDP1-ligase III association [105]. SUMOylation of TDP1 has been implicated in the recruitment of TDP1 at transcription-dependent Top1cc sites [49], which is consistent with the role of TDP1 in protecting post-mitotic neurons against Top1cc damage [105]. Notably, Top1 is also SUMOylated in human and yeast cells [108,109]. SUMO conjugation regulates Top1 subcellular localization, and initiate the repair signal by ubiquitin-dependent proteasomal degradation of Top1cc [108–111], allowing subsequent access of DNA repair enzymes including TDP1 to the Top1 active site tyrosyl-DNA linkage [59,96]. In fission yeast, the SUMO E3 ligase (Nse2) and the structure specific endonuclease Rad16-Swi10 work together to repair Top1cc in a TDP1 independent pathway [112].
The implication of poly(ADP-ribose)polymerase-1 (PARP1) in the repair of Top1cc stems from several studies showing that PARP1 knockout cells are hypersensitive to camptothecin [27,96,113]; that PAR accumulates in the nucleus of camptothecin-treated cells [29,114,115]; and that PARP inhibitors enhance the activity of camptothecin and its clinical derivatives (topotecan and irinotecan) by inhibiting the repair of Top1-induced DNA lesions [29,96,114,115], by inhibiting the release of Top1 from stalled replication complexes [116,117] and by suppressing the restart of replication forks reversed by Top1cc [118]. Moreover, PARP1 knockout cells have less TDP1 activity [96] and the PARP inhibitor ABT-888 (veliparib) fails to sensitize Top1 inhibitors in TDP1-deficient cells [29,113–115]. PARP1 catalyzes the nicotinamide adenine dinucleotide (NAD+)-dependent addition of ADP-ribose polymers (PAR) onto itself and chromatin proteins including Top1, XRCC1, ligase III and histones [119,120]. Recently, the N-terminus regulatory domain of TDP1 has been shown to directly bind the catalytic domain of PARP1. PARP1 PARylates TDP1 without interfering with its phosphodiesterase activity [113]. PARylation stabilizes TDP1 in response to Top1cc-induced DNA damage, and recruits both TDP1 and XRCC1 to Top1cc-induced DNA damage sites, which is in keeping with the role of PARP1 in Top1cc repair. Therefore, both PARylation and SUMOylation recruit TDP1 to DNA damage sites [105,113]. TDP1-SUMOylation may be important for repairing transcription-associated irreversible Top1cc in post-mitotic cells like neurons [105], whereas PARylation may regulate TDP1 stability and recruitment in actively proliferating cells [88,113]. Thus, the emerging view is that the N-terminal domain (NTD, 1–148 amino acids) of human TDP1, which is dispensable for catalytic activity, acts as a molecular switch that determine whether Top1cc are repaired by TDP1 when PARP1 is activated or by the alternative endonuclease pathways (Fig. 2) [113].
4. Tyrosyl-DNA phosphodiesterase 2 (TDP2)
4.1. More than a simple DNA repair enzyme: Roles in picornavirus infections and signal transduction: TDP2 TTRAP EAPII VPg unlinkase
The biology of TDP2 extends beyond topoisomerases and DNA repair. By contrast to TDP1, there is no obvious TDP2 ortholog in yeast, and human TDP2 appears as a multitask protein. Before being named TDP2 by Caldecott and coworkers [121], TDP2 was known as TTRAP (TRAF and TNF receptor-associated protein) and/or ETS1-associated protein 2 (EAPII). TTRAP (TDP2) was identified as an intracellular tumor necrosis factor (TNF) receptor binding protein [122] that increased MAPK/JNK/p38 activation while inhibiting the activation of NFkB. TTRAP (TDP2) was shown to interact with the TNF receptor-associated factors TRAF 2, 3, 5 and 6, with the highest affinity for TRAF6. Soon after the identification of TTRAP, peptide sequence analyses suggested the DNA enzymatic functions of TTRAP (TDP2) and its similarity with APE1 and the superfamily of Mg2+/Mn2+-dependent phosphodiesterases [123]. Yet, it took almost 10 years to demonstrate the enzymatic function of TDP2 for Top2 repair [121], and 3 more years to demonstrate that TDP2 was the VPg unlinkase for picornaviruses [124].
TTRAP/TDP2 was independently discovered and named EAPII (ETS1-associated protein 2) following a yeast two-hybrid screen for novel ETS1-interacting proteins [125]. EAPII (TDP2) was defined as a negative transcription regulator for ETS1 and AP1 [125]. Studies on the roles of TDP2 in signal transduction have continued after the identification of TTRAP/EAPII/TDP2 enzymatic functions. Recently, TDP2/EAPII has been shown to promote cell death induced by TGF-β, suggesting a potential role as modulator between pro-survival and pro-apoptotic signaling by TGF-β [126]. In a study aimed at understanding lung cancer development, EAPII/TDP2 was found elevated in lung cancer tissues, promoting cancer cell proliferation in culture and xenografts, activating the Raf–MEK–ERK cascade, leading to upregulation of MYC and cyclin D1, accelerated G1/S transition, increased cell proliferation and tumor growth [127]. Another recent study confirmed the overexpression of TDP2 in human lung cancers, together with p53 mutations, suggesting the TDP2 could be upregulated by mutant p53 in lung cancers [128]. Mutant p53 forms a complex with ETS2, as well as ETS1, which could activate TDP2 transcription [128].
4.2. TDP2 substrates
Although TDP2 was discovered by screening yeast S. cerevisiae for camptothecin hypersensitivity in strains lacking tdp1 and rad1 [129], the physiological activity of TDP2 appears limited to the removal of trapped topoisomerases with 5′-tyrosyl DNA termini [129,130] (Table 3). Cells deficient for TDP2 are selectively hypersensitive to Top2 inhibitors including etoposide, doxorubicin and the catalytic inhibitor ICRF-193 [129–132], and TDP2-deficient mice treated with low doses of etoposide show intestinal and lymphoid toxicities [132]. TDP2 and TDP1 have minor overlapping activities as TDP1 has weak activity for 5′-phosphotyrosyl bonds and TDP2 has weak activity for 3′-phosphotyrosyl bonds [45,60,129,133]. Contrary to the broad specificity of TDP1, which cleaves a spectrum of phosphodiester bonds in addition to phosphotyrosyl residues (see Fig. 3), the phosphodiesterase activity of TDP2 is specific for phosphotyrosyl bonds (compare Figs. 6 and 3) [129,134]. For instance, TDP2 can readily hydrolyze a 5′-digoxigenin-DNA adduct only when it includes a tyrosyl linker (see Fig. 3B in [134]) (Fig. 3C). Regarding the DNA substrate, TDP2 preferentially cleaves 5′-tyrosyl bond at the end of single-stranded substrates, as well as DNA substrates with a 5′-overhang, mimicking Top2cc (see Fig. 1E). TDP2 does not require a long DNA segment; substrates as short as five nucleotides can indeed be processed [134]. Unlike TDP1, TDP2 shows no nucleosidase activity.
Fig. 6.
TDP2 substrates all include a 5′-phenolic ring. (A) Physiological substrates include 5′-tyrosyl covalent bonds with Top2α and Top2β (left) [121] and the replicative picornavirus protein VPg [124] (2nd from left). Top3α and β are plausible candidates as well. (B) Top1 covalent complexes are processed much less efficiently [121,133]. (C): Substrate used for biochemical experiments and drug screening: T5PNP [153], NPPP [154] and digoxygenin [134].
Unexpectedly, TDP2 was recently identified as the host VPg unlinkase for picornaviruses, a large family of viruses including poliovirus, coxackieviruses, rhinoviruses and the classical foot-and-mouth disease virus [124]. TDP2 activity is vital for viral replication as the 5′-end of the viral genomic RNA of picornavirus is covalently linked to a small (>20 residues) viral protein (VPg) cap via a phosphotyrosyl bond [135]. Upon infection, VPg is removed from the 5′-end of RNA by the 5′-phosphotyrosyl activity of TDP2, thus allowing translation of virus-encoded proteins [124]. Picornavirus family comprises many pathogens responsible for diseases in human and animals, such as polio, common cold, and foot-and-mouth disease. Consequently, inhibitor of TDP2 may serve as treatments for vast populations exposed to these diseases.
In addition to Top2, Top3 also forms cleavage complexes via 5′-phosphotyrosyl covalent intermediates (see Fig. 1 and beginning sections of this review). As covalent complexes of both Top3α-DNA and Top3β-RNA can potentially arise in cells, it is plausible that TDP2 plays a role in rescuing trapped Top3cc (Table 3).
4.3. TDP2 structure
Human TDP2 is smaller than TDP1 with a molecular mass of 41 kDa (362 amino acid residues) (Table 3). Like TDP1, TDP2 is a two-domain protein with the catalytic domain in the C-terminus (pink in Fig. 4B) while its N-terminal domain bears an ubiquitin-associated (UBA) domain, which probably plays a regulatory role [136]. TDP2 belongs to the EEP-domain nucleases that cleave DNA and RNA backbones [136]. Sequence alignment shows that TDP2 contains four conserved catalytic motifs (TWN, LQE, GDXN and SDH) shared by Mg2+/Mn2+-dependent nucleases including DNase I and AP endonuclease APE1 [129,134]. Most residues in all four motifs have been confirmed to be critical for TDP2 phosphodiesterase activity [129,134]. Sequence comparison shows similarity with APE1, and biochemical studies show that these two enzymes share catalytic mechanisms [134].
The crystal structures of TDP2 proteins from mouse, worm (Caenorhabditis elegans) and zebra fish have recently been reported, revealing a monomeric organization with a catalytic site formed by four residues chelating one magnesium ion [136,137]. The truncated (delta-120) zebra fish TDP2 crystal structure is presented in Fig. 4D and F. It shows a narrow single stranded DNA binding channel leading to the catalytic site formed by residues N129, E161, D271 and H320 (residues N120, E152, D262 and H351 in human TDP2) that coordinate one magnesium ion (Fig. 4D–E). The catalytic tetrad arrangement of TDP2 shares similarities with those of DNase I and APE1 [137]. However, in contrast to TDP2, DNase I and APE1 have a shallow DNA binding channel compatible with double-stranded DNA binding. Both the mouse and the zebra fish TDP2 structures reveal that a minimum of two unpaired nucleotides (single-stranded) is necessary to access the catalytic site [136,137] (Fig. 4D). Similarly to TDP1, the DNA substrate stabilization in the TDP2 catalytic site is not sequence -specific. The DNA phosphodiester backbone is positioned by several H-bonds between the oxygen atoms of the phosphate groups of the three terminal nucleotides and the nitrogen residues of K240, R275, R303 and S320 (Fig. 4F). Some of these H-bonds may be water-mediated. In addition, residue Y318, which is part of one of the sidewalls forming the DNA-binding groove, stacks against the base of the penultimate nucleotide (G-2, Fig. 4F).
4.4. TDP2 catalytic mechanism
Unlike TDP1, TDP2 requires divalent metals but does not form a transient covalent catalytic intermediate (Table 3) [129,134,138]. Mg2+, Mn2+, Co2+ are much more efficient than Ca2+ or Zn2+) for catalysis [134]. Biochemical titration studies suggest that TDP2 requires two metals for catalysis [134], although only one metal is detected in crystallographic analyses [136,137]. Figure 7 summarizes the 2-metal model. The first metal coordinated by residues D262, H351 and N264 (see also Fig. 4F) also coordinates a deprotonated water molecule for the nucleophilic attack of the phosphate group (Fig. 7B). The second metal is coordinated by the carboxylic functions of D122 and E152. Residue N120 bridges both metal binding sites by hydrogen bonding with residues E152 and D262 (Fig. 7, see also Fig. 4F). TDP2 generates 5′-phosphate nucleic acid ends (Fig. 7C), which, unlike the 3′-phosphate TDP1 products, can be readily processed by ligases (see below and Table 3).
4.5. TDP2 regulation and associated repair pathways
The repair pathways associated with TDP2 are different from those of TDP1 (see Fig. 2). Processing of a Top2cc (see Fig. 2B) by TDP2 generates a DSB with 4 base overhangs and with 5′-phosphate and 3′-hydroxyl ends, which are direct substrates for end joining by the non-homologous end joining (NHEJ) proteins Ku and ligase IV [137,139]. Accordingly, a recent study using isogenic DT40 cells showed an epistatic relationship between TDP2 and Ku for the repair of Top2cc induced by etoposide [132]. Notably, Ku and ligase IV knockout cells are more sensitive to etoposide than TDP2 knockout cells [131,132], indicating that Ku exhibits additional functions for repairing irreversible Top2cc. Whether Ku and other components of the NHEJ pathway bind to TDP2 remains to be established.
Homologous recombination (HR) is also important for the repair of Top2-induced DNA damage, as demonstrated genetically both in yeast and DT40 models [131,139,140]. The two pathways probably work in parallel with the HR pathway downstream from the endonuclease pathway(s) (Fig. 2B). A recent study showing increased Rad51 foci and increased sister chromatid exchanges in TDP2-deficient cells treated with etoposide, suggests a possible synthetic lethality between the HR and TDP2 pathways [132].
It has been suggested that the initial step for recognizing and removing topoisomerase-DNA adducts is mediated by ubiquitylation of DNA topoisomerases, leading to their proteosomal degradation [141]. Thus, it will be important to determine how TDP2 activities are coupled with ubiquitin ligases and the proteasome, which is a particularly compelling question due to the presence of an UBA domain in the C-terminus of TDP2 (Fig. 4B). By contrast to TDP1, to our knowledge, nothing is known regarding TDP2 post-translation modifications.
5. TDP inhibitors
TDP1 inhibitors have recently been reviewed [28] and the following section will focus on the principles and rationale for the development of TDP1 and TDP2 inhibitors. DNA repair inhibitors such as PARP inhibitors have reached the forefront of cancer chemotherapy, and led to the principle of “synthetic lethality” [142–144], which is based on the selective cytotoxic activity of an inhibitor in cancer cells lacking parallel pathways that are present in normal cells (exemplary case of BRCA-deficient tumors for PARP inhibitors).
The rational for targeting TDP1 and TDP2 in combination with Top1 and Top2 inhibitors is rooted in the hypersensitivity of TDP1-and TDP2-deficient vertebrate cells to topoisomerase inhibitors [45,47,49,51,130–133]. Because TDP1 or TDP2 knockout vertebrate cells show normal cell growth and knockout mice are viable without obvious phenotype [50–52,132], it is expected that TDP1 or TDP2 inhibitors will be well tolerated.
A recent study using a panel of ~50 DNA repair mutant DT40 cell lines enabled a comparison of the differential contribution of repair genes to drug sensitivity and demonstrated the interplay of multiple pathways for the repair of Top1- and Top2-induced cellular damages [131]. This panel confirmed that TDP1 inactivation resulted in Top1 inhibitors sensitivity, as well as PARP1 and homologous recombination genes, and that TDP2-deficient cells were among the most sensitized cells to Top2 inhibitors, as well as cells deficient for NHEJ (Ku and ligase IV). On the other hand, homologous recombination (HR) genes and ATM genes were involved in the repair of damage induced by both Top1 and Top2 inhibitors [131]. Because tumor cells are commonly deficient for parallel DNA repair pathways [145], under the principle of synthetic lethality TDP- and topoisomerase-targeted combination therapies should improve potency and selectivity toward cancer cells with preexisting repair and checkpoint deficiencies (see Fig. 2).
5.1. Therapeutic rationale for TDP1 inhibitors and conditional lethality
The early studies on genetic hypersensitivity of yeast S. cerevisiae to camptothecin revealed that TDP1-dependent repair was redundant with endonuclease pathways represented by Rad1-Rad10 (orthologs of XPF-ERCC1 in humans) [55,61] and Mre11 [61,146,147] (see Fig. 2A). The yeast Rad1-Rad10 results have been extended to human cells, leading to the proposal that XPF-ERCC1-deficient cancers would represent a synthetic lethal combination for both PARP inhibitors and TDP1 inhibitors [29]. The association with the PARP inhibitors derives from the recent finding that TDP1 is epistatic with PARP1 in DT40 and human cells and that PARylation is critical to localize and retain TDP1 at DNA damage sites [113] (Fig. 2A). Because other nucleases are involved in the Top1-DNA complex endonuclease pathways, including Mre11 [61,146–148] and CtIP [113,149,150] (Fig. 2A), it is plausible to propose using TDP1 (and PARP) inhibitors in combination with Top1 inhibitors in Mre11-deficient cancers. Indeed, Mre11 inactivation is relatively frequent in mismatch repair-deficient colon carcinomas [151]. At the present time, it is unclear whether CtIP-inactivating mutations are present in human cancers.
Cancer-associated cell cycle checkpoint deficiencies (in the ATM, ATR, BRCA1, BRCA2, Chk1 and Chk2 pathways) represent other synthetic lethal opportunities for combinations with TDP1 and Top1 inhibitors (Fig. 2A). Support for this possibility stems from the early studies from Nash and coworkers showing that inactivation of the master cell cycle checkpoint gene rad9 results in marked synergism toward camptothecin in tdp1-inactivated strains [41]. The molecular mechanism for such synergism could result from cross complementation between the TDP1-PARP and the HR and endonuclease pathways (see Fig. 2A). The rationale for extending these observations to human cells is that human orthologs of yeast S. cerevisiae Rad9 gene include key DDR and checkpoint proteins with BRCT domains, which are frequently altered in cancers.
Because TDP1 repairs a broad spectrum of lesions induced by anticancer agents (see Section 4.1), it is conceivable that, in addition to Top1cc inhibitors, TDP1 inhibitors will be useful in combination with a broad spectrum of existing anticancer agents including monofunctional alkylating agents (temozolomide), cytarabine, radiomimetic drugs (bleomycin) and radiotherapy.
5.2. Therapeutic rationale for TDP2 inhibitors
Synthetic lethality information is more limited for TDP2 than TDP1. One reason is that yeast does not appear to use TDP2 pathways for repairing aborted Top2cc, and its primary pathways rely on endonucleases [140] (see Fig. 2B). Nevertheless, vertebrate cells use both the endonuclease and TDP2 pathways, and contrary to Top1 inhibitors, Ku and ligase IV are most critical for Top2cc repair [131] (Fig. 2). Because identical nucleases are involved for the excision of both Top2 and Top1 covalent complexes (see Fig. 2A and B), the same principles apply to the rationale to combine TDP2 and Top2 inhibitors for Mre11-deficient tumors [151] (see above).
Another possible use for TDP2 inhibitors is as antiviral agents since TDP2 has been identified as the picornavirus VPg unlinkase [124] (see Section 4.2). Picornaviruses are among the most common human pathogens, including poliovirus, rhinoviruses, coxackieviruses, which cause a broad range of diseases (meningitis, encephalitis, common cold, hand-foot-and-mouth disease, conjunctivitis, herpangina, myositis and myocarditis) for which there are not effective treatments.
5.3. TDP inhibitor screens
The first functional assays of TDP1 and TDP2 were established using commercially synthesized DNA oligonucleotides with 3′-or 5′-phosphotyrosyl moieties, respectively. However, gel-based assays are cumbersome and unsuitable for large-scale high-throughput screening. The wide spectrum of substrates processed by TDP1 allows the use of fluorescent substrates easily amenable to high-throughput assays (Fig. 3G). Many substrates take advantage of electrochemiluminescence (BV Tag, Fig. 3G) [72], fluorescence energy resonance transfer (FRET, 6-FAM, BV Tag, Fig. 3G) [28] or Amplified Luminescent Proximity Homogeneous Assay technology (ALPHA, FITC, Fig. 3G) [152] for easy signal detection.
TDP2 recognizes specifically 5′-phosphotyrosyl moieties. Yet, attachment of various moieties can be used for high throughput screening. For instance, TDP2 efficiently processes a phosphotyrosine DNA substrate tagged with a digoxygenin (Fig. 6C) [134]. A recent study showed that TDP2 efficiently processes p-nitrophenyl-thymidine-5′-phosphate (T5PNP), a well-established substrate for snake venom phosphodiesterase (Fig. 6C) [153]. T5PNP closely resembles the structure of a 5′-tyrosyl DNA substrate (Fig. 6A) and the liberated p-nitrophenol molecule absorbs light at a different wavelength than T5PNP, allowing easy monitoring of the cleavage reaction. Another relatively simple and inexpensive substrate, 4-nitrophenyl phenylphosphonate (NPPP, Fig. 6C), is also processed by TDP2 to liberate p-nitrophenol [154]. NPPP has begun to be used in a high-throughput screen for TDP2 inhibitors [155].
A relatively large number of TDP1 inhibitors have been reported from biochemical screen. Yet, none of them has shown cellular activity. Aminoglycoside antibiotics and ribosome inhibitors were the first TDP1 inhibitors reported with IC50 values in the millimolar range [156]. Furamidine, a diamidine compound in phase III clinical trials against the parasitic disease trypanosomiasis, was identified by high-throughput screening (HTS) and subsequently confirmed by biochemical assays as a micromolar inhibitor of TDP1 [72]. Binding studies revealed that furamidine binds both TDP1 and its single stranded DNA substrate [72]. Its mechanism of action on TDP1 is therefore distinct from the known selective binding of furamidine to AT-rich sites in the minor groove of duplex DNA. Phosphotyrosine mimetics including methyl-3,4-dephostatin [152] and the steroid compound NSC88915 [157] were also discovered as submicromolar TDP1 inhibitors by HTS assays. These compounds bear either a hydroxyl-substituted benzyl ring or an aromatic sulfonyl ester group that simulate the phosphotyrosyl moiety present on the TDP1 DNA substrate. Recently, analogs of the two indenoisoquinolines top1 inhibitors indotecan (LMP400) and indimitecan (LMP776) have been reported to inhibit TDP1 in the low micromolar range [158,159]. Indotecan and indimitecan which are currently in Phase I clinical trials are exclusively top1-selective and do not inhibit TDP1. Chemical modifications of their indenoisoquinoline core allowed the modulation of the TDP1 activity with generation of Top1/TDP1 dual- and TDP1-selective-inhibitors [158,159]. Some of these inhibitors directly bind the enzyme and inhibit TDP1 in a competitive manner. These inhibitors also represent the first series of TDP1 inhibitors, which maintain their potency in whole cell extract-based biochemical assays with increased stringency [158,159]. Arylidene thioxothiazolidinone derivatives have been reported to inhibit TDP1 at submicromolar concentration and similarly to indenoisoquinoline derivatives, some of them also exhibit activity in biochemical assays using whole cell extract [160]. The best compound was shown to interact with TDP1 by SPR without any interaction with the DNA substrate. Despite solid potency, arylidene thioxothiazolidinone do not exhibit synergism with camptothecin in cellular assays and their chemotype is often subject to controversies regarding their potential promiscuity.
Because, TDP2 was only identified recently, only a small number of TDP2 inhibitors have been reported. Toxoflavin and deazaflavin derivatives inhibit TDP2 at nanomolar concentration and appear to be selective for TDP2 vs TDP1 [155]. However, toxoflavin derivatives suffer from redox activity in biochemical assays and deazaflavin compounds exhibit low cell permeability. It is likely that TDP2 inhibitors will emerge in the near future. Counter screening with APE1 could be useful in biochemical assays.
6. Concluding remarks
TDP1 and TDP2 are recently discovered DNA repair enzymes involved in a variety of biological functions in addition to the repair of trapped topoisomerase cleavage complexes. Biological and cellular studies are warranted to elucidate their regulation, post-translational modifications, binding partners and associated pathways. The discovery of intimate relationships between TDP1 and PARP1 and between TDP2 and Ku, and the synthetic relationship between XPF-ERCC1 and TDP1, Mre11 and TDP1 and between Mre11 and TDP2 have expanded our views on the cross talks between repair pathways.
Based on the broad biological functions of TDP1 and TDP2 and the availability of crystal structure, biochemical assays, and genetically engineered cellular systems based on TDP1 and TDP2 knockout and complemented cells, it should be relatively straightforward and highly valuable to develop TDP1 and TDP2 inhibitors.
Acknowledgments
We wish to thank the past and present members of the Laboratory of Molecular Pharmacology who have contributed profoundly to our studies and understanding of topoisomerases, topoisomerase inhibitors, and TDPs. Special thanks to Kurt W. Kohn who first hypothesized that topoisomerases were the targets of anti-cancer drugs and for his permanent support, to Thomas Dexheimer, ZeHong Miao and Smitha Antony for their contribution to our early studies on TDP1, and to Linkun An and Monica Abdelmalak for their current effort on TDP inhibitors. We also wish to thank Dr. Shunichi Takeda, Kyoto University for long-term collaboration on DT40 systems applied to TDP1, TDP2 and topoisomerase repair pathways.
Our studies are supported by the Center for Cancer Research, Intramural Program of the National Cancer Institute (Z01 BC 006150).
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References
- 1.Pommier Y, Leo E, Zhang H, Marchand C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol. 2010;17:421–433. doi: 10.1016/j.chembiol.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seol Y, Zhang H, Pommier Y, Neuman KC. A kinetic clutch governs religation by type IB topoisomerases and determines camptothecin sensitivity. Proc Nat Acad Sci USA. 2012;109:16125–16130. doi: 10.1073/pnas.1206480109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoeffler AJ, Berger JM. DNA topoisomerases: harnessing and constraining energy to govern chromosome topology. Q Rev Biophys. 2008;41:41–101. doi: 10.1017/S003358350800468X. [DOI] [PubMed] [Google Scholar]
- 4.Wu L, Davies SL, North PS, Goulaouic H, Riou JF, Turley H, Gatter KC, Hickson ID. The Bloom’s syndrome gene product interacts with topoisomerase III. J Biol Chem. 2000;275:9636–9644. doi: 10.1074/jbc.275.13.9636. [DOI] [PubMed] [Google Scholar]
- 5.Plank JL, Wu J, Hsieh TS. Topoisomerase IIIalpha and Bloom’s helicase can resolve a mobile double Holliday junction substrate through convergent branch migration. Proc Nat Acad Sci USA. 2006;103:11118–11123. doi: 10.1073/pnas.0604873103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen SH, Wu J, Hsieh TS. Essential functions of topoisomerase III alpha in the nucleus and mitochondria. In: Pommier Y, editor. DNA Topoisomerases and Cancer. Springer; New York, NY: 2012. pp. 103–117. [Google Scholar]
- 7.Stoll G, Pietilainen OP, Linder B, Suvisaari J, Brosi C, Hennah W, Leppa V, Torniainen M, Ripatti S, Ala-Mello S, Plottner O, Rehnstrom K, Tuulio-Henriksson A, Varilo T, Tallila J, Kristiansson K, Isohanni M, Kaprio J, Eriksson JG, Raitakari OT, Lehtimaki T, Jarvelin MR, Salomaa V, Hurles M, Stefansson H, Peltonen L, Sullivan PF, Paunio T, Lonnqvist J, Daly MJ, Fischer U, Freimer NB, Palotie A. Deletion of TOP3beta, a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders. Nat Neurosci. 2013;16:1228–1237. doi: 10.1038/nn.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu D, Shen W, Guo R, Xue Y, Peng W, Sima J, Yang J, Sharov A, Srikantan S, Yang J, Fox D, 3rd, Qian Y, Martindale JL, Piao Y, Machamer J, Joshi SR, Mohanty S, Shaw AC, Lloyd TE, Brown GW, Ko MS, Gorospe M, Zou S, Wang W. Top3beta is an RNA topoisomerase that works with fragile X syndrome protein to promote synapse formation. Nat Neurosci. 2013;16:1238–1247. doi: 10.1038/nn.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang HS, Allen JA, Mabb AM, King IF, Miriyala J, Taylor-Blake B, Sciaky N, Dutton JW, Jr, Lee HM, Chen X, Jin J, Bridges AS, Zylka MJ, Roth BL, Philpot BD. Topoisomerase inhibitors unsilence the dormant allele of Ube3a in neurons. Nature. 2012;481:185–189. doi: 10.1038/nature10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell WT, Coulson RL, Gonzales ML, Crary FK, Wong SS, Adams S, Ach RA, Tsang P, Yamada NA, Yasui DH, Chedin F, Lasalle JM. R-loop formation at Snord116 mediates topotecan inhibition of Ube3a-antisense and allele-specific chromatin decondensation. Proc Nat Acad Sci USA. 2013;110:13938–13943. doi: 10.1073/pnas.1305426110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindahl T, Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972;11:3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- 12.Boiteux S, Jinks-Robertson S. DNA repair mechanisms and the bypass of DNA damage in Saccharomyces cerevisiae. Genetics. 2013;193:1025–1064. doi: 10.1534/genetics.112.145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim N, Huang SY, Williams JS, Li YC, Clark AB, Cho JE, Kunkel TA, Pommier Y, Jinks-Robertson S. Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I. Science. 2011;332:1561–1564. doi: 10.1126/science.1205016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sekiguchi J, Shuman S. Site-specific ribonuclease activity of eukaryotic DNA topoisomerase I. Mol Cell. 1997;1:89–97. doi: 10.1016/s1097-2765(00)80010-6. [DOI] [PubMed] [Google Scholar]
- 15.Sparks JL, Chon H, Cerritelli SM, Kunkel TA, Johansson E, Crouch RJ, Burgers PM. RNase H2-initiated ribonucleotide excision repair. Mol Cell. 2012;47:980–986. doi: 10.1016/j.molcel.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pourquier P, Pilon A, Kohlhagen G, Mazumder A, Sharma A, Pommier Y. Trapping of mammalian topoisomerase I and recombinations induced by damaged DNA containing nicks or gaps: importance of DNA end phosphorylation and camptothecin effects. J Biol Chem. 1997;272:26441–26447. doi: 10.1074/jbc.272.42.26441. [DOI] [PubMed] [Google Scholar]
- 17.Pommier Y, Marchand C. Interfacial inhibitors: targeting macromolecular complexes. Nat Rev Drug Discovery. 2012;11:25–36. doi: 10.1038/nrd3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nitiss J, Wang JC. DNA topoisomerase-targeting antitumor drugs can be studied in yeast. Proc Nat Acad Sci USA. 1988;85:7501–7505. doi: 10.1073/pnas.85.20.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjornsti MA, Benedetti P, Viglianti GA, Wang JC. Expression of human DNA topoisomerase I in Yeast cells lacking yeast DNA topoisomerase I: restoration of sensitivity of the cells to the antitumor drug camptothecin. Cancer Res. 1989;49:6318–6323. [PubMed] [Google Scholar]
- 20.Miao ZH, Player A, Shankavaram U, Wang YH, Zimonjic DB, Lorenzi PL, Liao ZY, Liu H, Shimura T, Zhang HL, Meng LH, Zhang YW, Kawasaki ES, Popescu NC, Aladjem MI, Goldstein DJ, Weinstein JN, Pommier Y. Non-classic functions of human topoisomerase I: genome-wide and pharmacologic analyses. Cancer Res. 2007;67:8752–8761. doi: 10.1158/0008-5472.CAN-06-4554. [DOI] [PubMed] [Google Scholar]
- 21.Jarvinen TA, Liu ET. Simultaneous amplification of HER-2 (ERBB2) and topoisomerase IIalpha (TOP2A) genes—molecular basis for combination chemotherapy in cancer. Curr Cancer Drug Targets. 2006;6:579–602. doi: 10.2174/156800906778742497. [DOI] [PubMed] [Google Scholar]
- 22.Pommier Y, Pourquier P, Urasaki Y, Wu J, Laco G. Topoisomerase I inhibitors: selectivity and cellular resistance. Drug Resist Update. 1999;2:307–318. doi: 10.1054/drup.1999.0102. [DOI] [PubMed] [Google Scholar]
- 23.Murai J, Huang S-yN, Das BB, Renaud A, Zhang Y, Doroshow JH, Ji J, Takeda S, Pommier Y. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72:5588–5599. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 25.Holm C, Covey JM, Kerrigan D, Pommier Y. Differential requirement of DNA replication for the cytotoxicity of DNA topoisomerase I and II inhibitors in Chinese hamster DC3F cells. Cancer Res. 1989;49:6365–6368. [PubMed] [Google Scholar]
- 26.Hsiang YH, Liu LF, Wall ME, Wani MC, Nicholas AW, Manikumar G, Kirschenbaum S, Silber R, Potmesil M. DNA topoisomerase I-mediated DNA cleavage and cytotoxicity of camptothecin analogs. Cancer Res. 1989;49:4385–4389. [PubMed] [Google Scholar]
- 27.Maede Y, Shimizu H, Fukushima T, Kogame T, Nakamura T, Miki T, Takeda S, Pommier Y, Murai J. Differential and common DNA repair pathways for topoisomerase I- and II-targeted drugs in a genetic DT40 repair cell screen panel. Mol Cancer Ther. 2014;13:214–220. doi: 10.1158/1535-7163.MCT-13-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang SY, Pommier Y, Marchand C. Tyrosyl-DNA phosphodiesterase 1 (Tdp1) inhibitors. Expert Opin Ther Pat. 2011;21:1285–1292. doi: 10.1517/13543776.2011.604314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YW, Regairaz M, Seiler JA, Agama KK, Doroshow JH, Pommier Y. Poly(ADP-ribose) polymerase and XPF-ERCC1 participate in distinct pathways for the repair of topoisomerase I-induced DNA damage in mammalian cells. Nucleic Acids Res. 2011;39:3607–3620. doi: 10.1093/nar/gkq1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, Liu LF. Processing of topoisomerase I cleavable complexes into DNA damage by transcription. Nucleic Acids Res. 1997;25:4181–4186. doi: 10.1093/nar/25.21.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koster DA, Palle K, Bot ES, Bjornsti MA, Dekker NH. Antitumour drugs impede DNA uncoiling by topoisomerase I. Nature. 2007;448:213–217. doi: 10.1038/nature05938. [DOI] [PubMed] [Google Scholar]
- 32.Sordet O, Redon CE, Guirouilh-Barbat J, Smith S, Solier S, Douarre C, Conti C, Nakamura AJ, Das BB, Nicolas E, Kohn KW, Bonner WM, Pommier Y. Ataxia telangiectasia mutated activation by transcription- and topoisomerase I-induced DNA double-strand breaks. EMBO Rep. 2009;10:887–893. doi: 10.1038/embor.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pommier Y, Zwelling LA, Mattern MR, Erickson LC, Kerrigan D, Schwartz R, Kohn KW. Effects of dimethyl sulfoxide and thiourea upon intercalator-induced DNA single-strand breaks in mouse leukemia (L1210) cells. Cancer Res. 1983;43:5718–5724. [PubMed] [Google Scholar]
- 34.Long BH, Musial ST, Brattain MG. Comparison of cytotoxicity and DNA breakage activity of congeners of podophyllotoxin including VP16-213 and VM26: a quantitative structure-activity relationship. Biochemistry. 1984;23:1183–1188. doi: 10.1021/bi00301a024. [DOI] [PubMed] [Google Scholar]
- 35.Ban Y, Ho CW, Lin RK, Lyu YL, Liu LF. Activation of a novel ubiquitin-independent proteasome pathway when RNA polymerase II encounters a protein roadblock. Mol Cell Biol. 2013;33:4008–4016. doi: 10.1128/MCB.00403-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Long BH, Musial ST, Brattain MG. Single- and double-strand DNA breakage and repair in human lung adenocarcinoma cells exposed to etoposide and teniposide. Cancer Res. 1985;45:3106–3112. [PubMed] [Google Scholar]
- 37.Pommier Y. Drugging topoisomerases: lessons and challenges. ACS Chem Biol. 2013;8:82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwelling LA, Michaels S, Erickson LC, Ungerleider RS, Nichols M, Kohn KW. Protein-associated deoxyribonucleic acid strand breaks in L1210 cells treated with the deoxyribonucleic acid intercalating agents 4′-(9-acridinylamino) methanesulfon-m-anisidide and adriamycin. Biochemistry. 1981;20:6553–6563. doi: 10.1021/bi00526a006. [DOI] [PubMed] [Google Scholar]
- 39.Yang SW, Burgin AB, Huizenga BN, Robertson CA, Yao KC, Nash HA. A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type I topoisomerases. Proc Nat Acad Sci USA. 1996;93:11534–11539. doi: 10.1073/pnas.93.21.11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pouliot JJ, Yao KC, Robertson CA, Nash HA. Yeast gene for a Tyr-DNA phosphodiesterase that repairs topoisomerase I complexes. Science. 1999;286:552–555. doi: 10.1126/science.286.5439.552. [DOI] [PubMed] [Google Scholar]
- 41.Pouliot JJ, Robertson CA, Nash HA. Pathways for repair of topoisome-rase I covalent complexes in Saccharomyces cerevisiae. Genes Cells. 2001;6:677–687. doi: 10.1046/j.1365-2443.2001.00452.x. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Barcelo JM, Lee B, Kohlhagen G, Zimonjic DB, Popescu NC, Pommier Y. Human mitochondrial topoisomerase I. Proc Nat Acad Sci USA. 2001;98:10608–10613. doi: 10.1073/pnas.191321998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das BB, Dexheimer TS, Maddali K, Pommier Y. Role of tyrosyl-DNA phosphodiesterase (TDP1) in mitochondria. Proc Nat Acad Sci USA. 2010;107:19790–19795. doi: 10.1073/pnas.1009814107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Khamisy SF. To live or to die: a matter of processing damaged DNA termini in neurons. EMBO Mol Med. 2011;3:78–88. doi: 10.1002/emmm.201000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murai J, Huang SY, Das BB, Dexheimer TS, Takeda S, Pommier Y. Tyrosyl-DNA phosphodiesterase 1 (TDP1) repairs DNA damage induced by topoisomerases I and II and base alkylation in vertebrate cells. J Biol Chem. 2012;287:12848–12857. doi: 10.1074/jbc.M111.333963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banerjee B, Roy A, Sen N, Majumder HK. A tyrosyl DNA phosphodieste-rase 1 from kinetoplastid parasite Leishmania donovani (LdTdp1) capable of removing topo I-DNA covalent complexes. Mol Microbiol. 2010;78:119–137. doi: 10.1111/j.1365-2958.2010.07318.x. [DOI] [PubMed] [Google Scholar]
- 47.El-Khamisy SF, Saifi GM, Weinfeld M, Johansson F, Helleday T, Lupski JR, Caldecott KW. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature. 2005;434:108–113. doi: 10.1038/nature03314. [DOI] [PubMed] [Google Scholar]
- 48.Interthal H, Chen HJ, Kehl-Fie TE, Zotzmann J, Leppard JB, Champoux JJ. SCAN1 mutant Tdp1 accumulates the enzyme–DNA intermediate and causes camptothecin hypersensitivity. EMBO J. 2005;24:2224–2233. doi: 10.1038/sj.emboj.7600694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miao ZH, Agama K, Sordet O, Povirk L, Kohn KW, Pommier Y. Hereditary ataxia SCAN1 cells are defective for the repair of transcription-dependent topoisomerase I cleavage complexes. DNA Repair (Amst) 2006;5:1489–1494. doi: 10.1016/j.dnarep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Gao R, Das BB, Chatterjee R, Abaan OD, Agama K, Matuo R, Vinson C, Meltzer PS, Pommier Y. Epigenetic and genetic inactivation of tyrosyl-DNA-phosphodiesterase 1 (TDP1) in human lung cancer cells from the NCI-60 panel. DNA Repair (Amst) 2014;13:1–9. doi: 10.1016/j.dnarep.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Katyal S, el-Khamisy SF, Russell HR, Li Y, Ju L, Caldecott KW, McKinnon PJ. TDP1 facilitates chromosomal single-strand break repair in neurons and is neuroprotective in vivo. EMBO J. 2007;26:4720–4731. doi: 10.1038/sj.emboj.7601869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hirano R, Interthal H, Huang C, Nakamura T, Deguchi K, Choi K, Bhattacharjee MB, Arimura K, Umehara F, Izumo S, Northrop JL, Salih MA, Inoue K, Armstrong DL, Champoux JJ, Boerkoel CF. Spinocerebellar ataxia with axonal neuropathy: consequence of a Tdp1 recessive neomorphic mutation? EMBO J. 2007;26:4732–4743. doi: 10.1038/sj.emboj.7601885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alagoz M, Wells OS, El-Khamisy SF. TDP1 deficiency sensitizes human cells to base damage via distinct topoisomerase I and PARP mechanisms with potential applications for cancer therapy. Nucleic Acids Res. 2014;42:3089–3103. doi: 10.1093/nar/gkt1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nilsen L, Forstrom RJ, Bjoras M, Alseth I. AP endonuclease independent repair of abasic sites in Schizosaccharomyces pombe. Nucleic Acids Res. 2012;40:2000–2009. doi: 10.1093/nar/gkr933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vance JR, Wilson TE. Yeast Tdp1 and Rad1-Rad10 function as redundant pathways for repairing Top1 replicative damage. Proc Nat Acad Sci USA. 2002;99:13669–13674. doi: 10.1073/pnas.202242599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Debéthune L, Kohlhagen G, Grandas A, Pommier Y. Processing of nucleopep-tides mimicking the topoisomerase I-DNA covalent complex by tyrosyl-DNA phosphodiesterase. Nucleic Acids Res. 2002;30:1198–1204. doi: 10.1093/nar/30.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raymond AC, Staker BL, Burgin AB. Substrate specificity of tyrosyl-DNA phosphodiesterase I (Tdp1) J Biol Chem. 2005;280:22029–22035. doi: 10.1074/jbc.M502148200. [DOI] [PubMed] [Google Scholar]
- 58.Interthal H, Chen HJ, Champoux JJ. Human Tdp1 cleaves a broad spectrum of substrates, including phosphoamide linkages. J Biol Chem. 2005;280:36518–36528. doi: 10.1074/jbc.M508898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Interthal H, Champoux JJ. Effects of DNA and protein size on substrate cleavage by human tyrosyl-DNA phosphodiesterase 1. Biochem J. 2011;436:559–566. doi: 10.1042/BJ20101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nitiss KC, Malik M, He X, White SW, Nitiss JL. Tyrosyl-DNA phosphodie-sterase (Tdp1) participates in the repair of Top2-mediated DNA damage. Proc Nat Acad Sci USA. 2006;103:8953–8958. doi: 10.1073/pnas.0603455103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu C, Pouliot JJ, Nash HA. Repair of topoisomerase I covalent complexes in the absence of the tyrosyl-DNA phosphodiesterase Tdp1. Proc Nat Acad Sci USA. 2002;99:14970–14975. doi: 10.1073/pnas.182557199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Inamdar KV, Pouliot JJ, Zhou T, Lees-Miller SP, Rasouli-Nia A, Povirk LF. Conversion of phosphoglycolate to phosphate termini on 3′ overhangs of DNA double strand breaks by the human tyrosyl-DNA phosphodiesterase hTdp1. J Biol Chem. 2002;277:27162–27168. doi: 10.1074/jbc.M204688200. [DOI] [PubMed] [Google Scholar]
- 63.Zhou T, Lee JW, Tatavarthi H, Lupski JR, Valerie K, Povirk LF. Deficiency in 3′-phosphoglycolate processing in human cells with a hereditary mutation in tyrosyl-DNA phosphodiesterase (TDP1) Nucleic Acids Res. 2005;33:289–297. doi: 10.1093/nar/gki170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou T, Akopiants K, Mohapatra S, Lin PS, Valerie K, Ramsden DA, Lees-Miller SP, Povirk LF. Tyrosyl-DNA phosphodiesterase and the repair of 3′-phosphoglycolate-terminated DNA double-strand breaks. DNA Repair (Amst) 2009;8:901–911. doi: 10.1016/j.dnarep.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lebedeva NA, Rechkunova NI, Lavrik OI. AP-site cleavage activity of tyrosyl-DNA phosphodiesterase 1. FEBS Lett. 2011;585:683–686. doi: 10.1016/j.febslet.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 66.El-Khamisy SF, Katyal S, Patel P, Ju L, McKinnon PJ, Caldecott KW. Synergistic decrease of DNA single-strand break repair rates in mouse neural cells lacking both Tdp1 and aprataxin. DNA Repair (Amst) 2009;8:760–766. doi: 10.1016/j.dnarep.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ben Hassine S, Arcangioli B. Tdp1 protects against oxidative DNA damage in non-dividing fission yeast. EMBO J. 2009;28:632–640. doi: 10.1038/emboj.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dexheimer TS, Stephen AG, Fivash MJ, Fisher RJ, Pommier Y. The DNA binding and 3′-end preferential activity of human tyrosyl-DNA phosphodie-sterase. Nucleic Acids Res. 2010;38:2444–2452. doi: 10.1093/nar/gkp1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang SY, Murai J, Dalla Rosa I, Dexheimer TS, Naumova A, Gmeiner WH, Pommier Y. TDP1 repairs nuclear and mitochondrial DNA damage induced by chain-terminating anticancer and antiviral nucleoside analogs. Nucleic Acids Res. 2013;41:7793–7803. doi: 10.1093/nar/gkt483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raymond AC, Rideout MC, Staker B, Hjerrild K, Burgin AB. Analysis of human tyrosyl-DNA phosphodiesterase I catalytic residues. J Mol Biol. 2004;338:895–906. doi: 10.1016/j.jmb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 71.Rideout MC, Raymond AC, Burgin AB. Design and synthesis of fluorescent substrates for human tyrosyl-DNA phosphodiesterase I. Nucleic Acids Res. 2004;32:4657–4664. doi: 10.1093/nar/gkh796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Antony S, Marchand C, Stephen AG, Thibaut L, Agama KK, Fisher RJ, Pommier Y. Novel high-throughput electrochemiluminescent assay for identification of human tyrosyl-DNA phosphodiesterase (Tdp1) inhibitors and characterization of furamidine (NSC 305831) as an inhibitor of Tdp1. Nucleic Acids Res. 2007;35:4474–4484. doi: 10.1093/nar/gkm463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Interthal H, Pouliot JJ, Champoux JJ. The tyrosyl-DNA phosphodiesterase Tdp1 is a member of the phospholipase D superfamily. Proc Nat Acad Sci USA. 2001;98:12009–12014. doi: 10.1073/pnas.211429198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davies DR, Interthal H, Champoux JJ, Hol WG. The crystal structure of human tyrosyl-DNA phosphodiesterase, Tdp1. Structure. 2002;10:237–248. doi: 10.1016/s0969-2126(02)00707-4. [DOI] [PubMed] [Google Scholar]
- 75.Davies DR, Interthal H, Champoux JJ, Hol WG. Crystal structure of a transition state mimic for Tdp1 assembled from vanadate, DNA, and a topo-isomerase I-derived peptide. Chem Biol. 2003;10:139–147. doi: 10.1016/s1074-5521(03)00021-8. [DOI] [PubMed] [Google Scholar]
- 76.Porter SE, Champoux JJ. Mapping in vivo topoisomerase I sites on simian virus 40 DNA: asymmetric distribution of sites on replicating molecules. Mol Cell Biol. 1989;9:541–550. doi: 10.1128/mcb.9.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jaxel C, Capranico G, Kerrigan D, Kohn KW, Pommier Y. Effect of local DNA sequence on topoisomerase I cleavage in the presence or absence of camptothecin. J Biol Chem. 1991;266:20418–20423. [PubMed] [Google Scholar]
- 78.Plo I, Liao ZY, Barcelo JM, Kohlhagen G, Caldecott KW, Weinfeld M, Pommier Y. Association of XRCC1 and tyrosyl DNA phosphodiesterase (Tdp1) for the repair of topoisomerase I-mediated DNA lesions. DNA Repair (Amst) 2003;2:1087–1100. doi: 10.1016/s1568-7864(03)00116-2. [DOI] [PubMed] [Google Scholar]
- 79.Takashima H, Boerkoel CF, John J, Saifi GM, Salih MA, Armstrong D, Mao Y, Quiocho FA, Roa BB, Nakagawa M, Stockton DW, Lupski JR. Mutation of TDP1, encoding a topoisomerase I-dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nat Genet. 2002;32:267–272. doi: 10.1038/ng987. [DOI] [PubMed] [Google Scholar]
- 80.Shiloh Y. The ATM-mediated DNA-damage response: taking shape. Trends Biochem Sci. 2006;31:402–410. doi: 10.1016/j.tibs.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 81.Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol. 2013;14:197–210. [PubMed] [Google Scholar]
- 82.Lee JH, Paull TT. Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene. 2007;26:7741–7748. doi: 10.1038/sj.onc.1210872. [DOI] [PubMed] [Google Scholar]
- 83.Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature. 2003;421:499–506. doi: 10.1038/nature01368. [DOI] [PubMed] [Google Scholar]
- 85.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 86.Weterings E, Chen DJ. DNA-dependent protein kinase in nonhomologous end joining: a lock with multiple keys? J Cell Biol. 2007;179:183–186. doi: 10.1083/jcb.200705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Solier S, Kohn KW, Scroggins B, Xu W, Trepel J, Neckers L, Pommier Y. Feature article: heat shock protein 90alpha (HSP90alpha), a substrate and chaperone of DNA-PK necessary for the apoptotic response. Proc Nat Acad Sci USA. 2012;109:12866–12872. doi: 10.1073/pnas.1203617109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Das BB, Antony S, Gupta S, Dexheimer TS, Redon CE, Garfield S, Shiloh Y, Pommier Y. Optimal function of the DNA repair enzyme TDP1 requires its phosphorylation by ATM and/or DNA-PK. EMBO J. 2009;28:3667–3680. doi: 10.1038/emboj.2009.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chiang SC, Carroll J, El-Khamisy SF. TDP1 serine 81 promotes interaction with DNA ligase IIIalpha and facilitates cell survival following DNA damage. Cell Cycle. 2010;9:588–595. doi: 10.4161/cc.9.3.10598. [DOI] [PubMed] [Google Scholar]
- 90.Mani RS, Fanta M, Karimi-Busheri F, Silver E, Virgen CA, Caldecott KW, Cass CE, Weinfeld M. XRCC1 stimulates polynucleotide kinase by enhancing its damage discrimination and displacement from DNA repair intermediates. J Biol Chem. 2007;282:28004–28013. doi: 10.1074/jbc.M704867200. [DOI] [PubMed] [Google Scholar]
- 91.Caldecott KW. Single-strand break repair and genetic disease. Nat Rev Genet. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 92.Audebert M, Salles B, Calsou P. Involvement of poly(ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J Biol Chem. 2004;279:55117–55126. doi: 10.1074/jbc.M404524200. [DOI] [PubMed] [Google Scholar]
- 93.Rosidi B, Wang M, Wu W, Sharma A, Wang H, Iliakis G. Histone H1 functions as a stimulatory factor in backup pathways of NHEJ. Nucleic Acids Res. 2008;36:1610–1623. doi: 10.1093/nar/gkn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nocentini S. Rejoining kinetics of DNA single- and double-strand breaks in normal and DNA ligase-deficient cells after exposure to ultraviolet C and gamma radiation: an evaluation of ligating activities involved in different DNA repair processes. Radiat Res. 1999;151:423–432. [PubMed] [Google Scholar]
- 95.Rosell R, Skrzypski M, Jassem E, Taron M, Bartolucci R, Sanchez JJ, Mendez P, Chaib I, Perez-Roca L, Szymanowska A, Rzyman W, Puma F, Kobierska-Gulida G, Farabi R, Jassem J. BRCA1: a novel prognostic factor in resected non-small-cell lung cancer. PLoS One. 2007;2:e1129. doi: 10.1371/journal.pone.0001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pommier Y, Barcelo JM, Rao VA, Sordet O, Jobson AG, Thibaut L, Miao ZH, Seiler JA, Zhang H, Marchand C, Agama K, Nitiss JL, Redon C. Repair of topoisomerase I-mediated DNA damage. Prog Nucleic Acid Res Mol Biol. 2006;81:179–229. doi: 10.1016/S0079-6603(06)81005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.D’Onofrio G, Tramontano F, Dorio AS, Muzi A, Maselli V, Fulgione D, Graziani G, Malanga M, Quesada P. Poly(Adp-ribose) polymerase signaling of topoisomerase 1-dependent DNA damage in carcinoma cells. Biochem Pharmacol. 2010 doi: 10.1016/j.bcp.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 98.Horton JK, Watson M, Stefanick DF, Shaughnessy DT, Taylor JA, Wilson SH. XRCC1 and DNA polymerase beta in cellular protection against cytotoxic DNA single-strand breaks. Cell Res. 2008;18:48–63. doi: 10.1038/cr.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chou WC, Wang HC, Wong FH, Ding SL, Wu PE, Shieh SY, Shen CY. Chk2-dependent phosphorylation of XRCC1 in the DNA damage response promotes base excision repair. EMBO J. 2008;27:3140–3150. doi: 10.1038/emboj.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Levy N, Martz A, Bresson A, Spenlehauer C, de Murcia G, Menissierde Murcia J. XRCC1 is phosphorylated by DNA-dependent protein kinase in response to DNA damage. Nucleic Acids Res. 2006;34:32–41. doi: 10.1093/nar/gkj409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Toulany M, Dittmann K, Fehrenbacher B, Schaller M, Baumann M, Rodemann HP. PI3K-Akt signaling regulates basal, but MAP-kinase signaling regulates radiation-induced XRCC1 expression in human tumor cells in vitro. DNA Repair (Amst) 2008;7:1746–1756. doi: 10.1016/j.dnarep.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 102.Strumberg D, Pilon AA, Smith M, Hickey R, Malkas L, Pommier Y. Conversion of topoisomerase I cleavage complexes on the leading strand of ribosomal DNA into 5′-phosphorylated DNA double-strand breaks by replication runoff. Mol Cell Biol. 2000;20:3977–3987. doi: 10.1128/mcb.20.11.3977-3987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Furuta T, Takemura H, Liao ZY, Aune GJ, Redon C, Sedelnikova OA, Pilch DR, Rogakou EP, Celeste A, Chen HT, Nussenzweig A, Aladjem MI, Bonner WM, Pommier Y. Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J Biol Chem. 2003;278:20303–20312. doi: 10.1074/jbc.M300198200. [DOI] [PubMed] [Google Scholar]
- 104.Seiler JA, Conti C, Syed A, Aladjem MI, Pommier Y. The intra-S-phase checkpoint affects both DNA replication initiation and elongation: single-cell and -DNA fiber analyses. Mol Cell Biol. 2007;27:5806–5818. doi: 10.1128/MCB.02278-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hudson JJ, Chiang SC, Wells OS, Rookyard C, El-Khamisy SF. SUMO modification of the neuroprotective protein TDP1 facilitates chromosomal single-strand break repair. Nat Commun. 2012;3:733. doi: 10.1038/ncomms1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Flotho A, Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem. 2013;82:357–385. doi: 10.1146/annurev-biochem-061909-093311. [DOI] [PubMed] [Google Scholar]
- 107.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- 108.Mao Y, Sun M, Desai SD, Liu LF. SUMO-1 conjugation to topoisomerase I: a possible repair response to topoisomerase-mediated DNA damage. Proc Nat Acad Sci USA. 2000;97:4046–4051. doi: 10.1073/pnas.080536597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Horie K, Tomida A, Sugimoto Y, Yasugi T, Yoshikawa H, Taketani Y, Tsuruo T. SUMO-1 conjugation to intact DNA topoisomerase I amplifies cleavable complex formation induced by camptothecin. Oncogene. 2002;21:7913–7922. doi: 10.1038/sj.onc.1205917. [DOI] [PubMed] [Google Scholar]
- 110.Lin CP, Ban Y, Lyu YL, Desai SD, Liu LF. A ubiquitin-proteasome pathway for the repair of topoisomerase I-DNA covalent complexes. J Biol Chem. 2008;283:21074–21083. doi: 10.1074/jbc.M803493200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Desai SD, Li TK, Rodriguez-Bauman A, Rubin EH, Liu LF. Ubiquitin/26S proteasome-mediated degradation of topoisomerase I as a resistance mechanism to camptothecin in tumor cells. Cancer Res. 2001;61:5926–5932. [PubMed] [Google Scholar]
- 112.Heideker J, Prudden J, Perry JJ, Tainer JA, Boddy MN. SUMO-targeted ubiquitin ligase, Rad60, and Nse2 SUMO ligase suppress spontaneous Top1-mediated DNA damage and genome instability. PLos Genet. 2011;7:e1001320. doi: 10.1371/journal.pgen.1001320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Das BB, Huang SN, Murai J, Rehman I, Amé JC, Sengupta S, Das SK, Majumdar P, Zhang H, Biard D, Majumder HK, Schreiber V, Pommier Y. PARP1-TDP1 coupling for the repair of topoisomerase I-induced DNA damage. Nucl Acids Res. 2014;42:4435–4449. doi: 10.1093/nar/gku088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Patel AG, Flatten KS, Schneider PA, Dai NT, McDonald JS, Poirier GG, Kaufmann SH. Enhanced killing of cancer cells by poly(ADP-ribose) polymerase inhibitors and topoisomerase I inhibitors reflects poisoning of both enzymes. J Biol Chem. 2012;287:4198–4210. doi: 10.1074/jbc.M111.296475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bowman KJ, Newell DR, Calvert AH, Curtin NJ. Differential effects of the poly (ADP-ribose) polymerase (PARP) inhibitor NU1025 on topoisomerase I and II inhibitor cytotoxicity in L1210 cells in vitro. Br J Cancer. 2001;84:106–112. doi: 10.1054/bjoc.2000.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ray Chaudhuri A, Hashimoto Y, Herrador R, Neelsen KJ, Fachinetti D, Bermejo R, Cocito A, Costanzo V, Lopes M. Topoisomerase I poisoning results in PARP-mediated replication fork reversal. Nat Struct Mol Biol. 2012;19:419–423. doi: 10.1038/nsmb.2258. [DOI] [PubMed] [Google Scholar]
- 117.Sugimura K, Takebayashi S, Taguchi H, Takeda S, Okumura K. PARP-1 ensures regulation of replication fork progression by homologous recombination on damaged DNA. J Cell Biol. 2008;183:1203–1212. doi: 10.1083/jcb.200806068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berti M, Chaudhuri AR, Thangavel S, Gomathinayagam S, Kenig S, Vujanovic M, Odreman F, Glatter T, Graziano S, Mendoza-Maldonado R, Marino F, Lucic B, Biasin V, Gstaiger M, Aebersold R, Sidorova JM, Monnat RJ, Jr, Lopes M, Vindigni A. Human RECQ1 promotes restart of replication forks reversed by DNA topoisomerase I inhibition. Nat Struct Mol Biol. 2013;20:347–354. doi: 10.1038/nsmb.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–528. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 120.Hottiger MO, Hassa PO, Luscher B, Schuler H, Koch-Nolte F. Toward a uni-fied nomenclature for mammalian ADP-ribosyltransferases. Trends Biochem Sci. 2010;35:208–219. doi: 10.1016/j.tibs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 121.Ledesma FC, El Khamisy SF, Zuma MC, Osborn K, Caldecott KW. A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature. 2009;461:674–678. doi: 10.1038/nature08444. [DOI] [PubMed] [Google Scholar]
- 122.Pype S, Declercq W, Ibrahimi A, Michiels C, Van Rietschoten JG, Dewulf N, de Boer M, Vandenabeele P, Huylebroeck D, Remacle JE. TTRAP, a novel protein that associates with CD40, tumor necrosis factor (TNF) receptor-75 and TNF receptor-associated factors (TRAFs), and that inhibits nuclear factor-kappa B activation. J Biol Chem. 2000;275:18586–18593. doi: 10.1074/jbc.M000531200. [DOI] [PubMed] [Google Scholar]
- 123.Rodrigues-Lima F, Josephs M, Katan M, Cassinat B. Sequence analysis iden-tifies TTRAP, a protein that associates with CD40 and TNF receptor-associated factors, as a member of a superfamily of divalent cation-dependent phospho-diesterases. Biochem Biophys Res Commun. 2001;285:1274–1279. doi: 10.1006/bbrc.2001.5328. [DOI] [PubMed] [Google Scholar]
- 124.Virgen-Slane R, Rozovics JM, Fitzgerald KD, Ngo T, Chou W, van der Heden van Noort GJ, Filippov DV, Gershon PD, Semler BL. An RNA virus hijacks an incognito function of a DNA repair enzyme. Proc Nat Acad Sci USA. 2012;109:14634–14639. doi: 10.1073/pnas.1208096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pei H, Yordy JS, Leng Q, Zhao Q, Watson DK, Li R. EAPII interacts with ETS1 and modulates its transcriptional function. Oncogene. 2003;22:2699–2709. doi: 10.1038/sj.onc.1206374. [DOI] [PubMed] [Google Scholar]
- 126.Varady G, Sarkadi B, Fatyol K. TTRAP is a novel component of the non-canonical TRAF6-TAK1 TGF-beta signaling pathway. PLoS One. 2011;6:e25548. doi: 10.1371/journal.pone.0025548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li C, Fan S, Owonikoko TK, Khuri FR, Sun SY, Li R. Oncogenic role of EAPII in lung cancer development and its activation of the MAPK-ERK pathway. Oncogene. 2011;30:3802–3812. doi: 10.1038/onc.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Do PM, Varanasi L, Fan S, Li C, Kubacka I, Newman V, Chauhan K, Daniels SR, Boccetta M, Garrett MR, Li R, Martinez LA. Mutant p53 cooperates with ETS2 to promote etoposide resistance. Genes Dev. 2012;26:830–845. doi: 10.1101/gad.181685.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cortes Ledesma F, El Khamisy SF, Zuma MC, Osborn K, Caldecott KW. A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature. 2009;461:674–678. doi: 10.1038/nature08444. [DOI] [PubMed] [Google Scholar]
- 130.Zeng Z, Cortes-Ledesma F, El-Khamisy SF, Caldecott KW. TDP2/TTRAP is the major 5′-tyrosyl DNA phosphodiesterase activity in vertebrate cells and is critical for cellular resistance to topoisomerase II-induced DNA damage. J Biol Chem. 2010;286:403–409. doi: 10.1074/jbc.M110.181016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gomez-Herreros F, Schuurs-Hoeijmakers JH, McCormack M, Greally MT, Rulten S, Romero-Granados R, Counihan TJ, Chaila E, Conroy J, Ennis S, Delanty N, Cortes-Ledesma F, de Brouwer AP, Cavalleri GL, El-Khamisy SF, de Vries BB, Caldecott KW. TDP2 protects transcription from abortive topo-isomerase activity and is required for normal neural function. Nat Genet. 2014;46:516–521. doi: 10.1038/ng.2929. [DOI] [PubMed] [Google Scholar]
- 132.Gomez-Herreros F, Romero-Granados R, Zeng Z, Alvarez-Quilon A, Quintero C, Ju L, Umans L, Vermeire L, Huylebroeck D, Caldecott KW, Cortes-Ledesma F. TDP2-dependent non-homologous end-joining protects against topoisomerase II-induced DNA breaks and genome instability in cells and in vivo. PLoS Genet. 2013;9:e1003226. doi: 10.1371/journal.pgen.1003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zeng Z, Sharma A, Ju L, Murai J, Umans L, Vermeire L, Pommier Y, Takeda S, Huylebroeck D, Caldecott KW, El-Khamisy SF. TDP2 promotes repair of topoisomerase I-mediated DNA damage in the absence of TDP1. Nucleic Acids Res. 2012;40:8371–8380. doi: 10.1093/nar/gks622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gao R, Huang SY, Marchand C, Pommier Y. Biochemical characterization of human tyrosyl-DNA phosphodiesterase 2 (TDP2/TTRAP): a Mg2+/Mn2+-dependent phosphodiesterase specific for the repair of topoisomerase cleavage complexes. J Biol Chem. 2012;287:30842–30852. doi: 10.1074/jbc.M112.393983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ambros V, Baltimore D. Protein is linked to the 5′ end of poliovirus RNA by a phosphodiester linkage to tyrosine. J Biol Chem. 1978;253:5263–5266. [PubMed] [Google Scholar]
- 136.Schellenberg MJ, Appel CD, Adhikari S, Robertson PD, Ramsden DA, Williams RS. Mechanism of repair of 5′-topoisomerase II-DNA adducts by mammalian tyrosyl-DNA phosphodiesterase 2. Nat Struct Mol Biol. 2012;19:1363–1371. doi: 10.1038/nsmb.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shi K, Kurahashi K, Gao R, Tsutakawa SE, Tainer JA, Pommier Y, Aihara H. Structural basis for recognition of 5′-phosphotyrosine adducts by Tdp2. Nat Struct Mol Biol. 2012;19:1372–1377. doi: 10.1038/nsmb.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Adhikari S, Karmahapatra S, Karve T, Bandyopadhyay S, Woodrick J, Manthena P, Glasgow E, Byers S, Saha T, Uren A. Characterization of magnesium requirement of human 5′-tyrosyl DNA phosphodiesterase mediated reaction. BMC Res (Notes) 2012;5:134. doi: 10.1186/1756-0500-5-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nitiss JL, Nitiss KC. Tdp2: a means to fixing the ends. PLoS Genet. 2013;9:e1003370. doi: 10.1371/journal.pgen.1003370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nitiss J, Soans E, Berk J, Seth A, Mishina M, Nitiss KC. Repair of topoisomerase II-mediated DNA damage: fixing DNA damage arising from a protein covalently trapped on DNA. In: Pommier Y, editor. DNA Topoisomerases and Cancer. Springer Humana Press; New York, NY: 2012. pp. 381–407. [Google Scholar]
- 141.Mao Y, Desai SD, Ting CY, Hwang JL, Liu LF. 26 S proteasome-mediated degradation of topoisomerase II cleavable complexes. J Biol Chem. 2001;276:40652–40658. doi: 10.1074/jbc.M104009200. [DOI] [PubMed] [Google Scholar]
- 142.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 143.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 144.Turner NC, Lord CJ, Iorns E, Brough R, Swift S, Elliott R, Rayter S, Tutt AN, Ashworth A. A synthetic lethal siRNA screen identifying genes mediating sensitivity to a PARP inhibitor. EMBO J. 2008;27:1368–1377. doi: 10.1038/emboj.2008.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nat Rev Cancer. 2012;12:801–817. doi: 10.1038/nrc3399. [DOI] [PubMed] [Google Scholar]
- 146.Malik M, Nitiss JL. DNA repair functions that control sensitivity to topoisomerase-targeting drugs. Eukaryotic Cell. 2004;3:82–90. doi: 10.1128/EC.3.1.82-90.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Deng C, Brown JA, You D, Brown JM. Multiple endonucleases function to repair covalent topoisomerase I complexes in Saccharomyces cerevisiae. Genetics. 2005;170:591–600. doi: 10.1534/genetics.104.028795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sacho EJ, Maizels N. DNA repair factor MRE11/RAD50 cleaves 3′-phosphotyrosyl bonds and resects DNA to repair damage caused by topoisomerase 1 poisons. J Biol Chem. 2011;286:44945–44951. doi: 10.1074/jbc.M111.299347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nakamura K, Kogame T, Oshiumi H, Shinohara A, Sumitomo Y, Agama K, Pommier Y, Tsutsui KM, Tsutsui K, Hartsuiker E, Ogi T, Takeda S, Taniguchi Y. Collaborative action of Brca1 and CtIP in elimination of covalent modifications from double-strand breaks to facilitate subsequent break repair. PLoS Genet. 2010;6:e1000828. doi: 10.1371/journal.pgen.1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sartori AA, Lukas C, Coates J, Mistrik M, Fu S, Bartek J, Baer R, Lukas J, Jackson SP. Human CtIP promotes DNA end resection. Nature. 2007;450:509–514. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Giannini G, Rinaldi C, Ristori E, Ambrosini MI, Cerignoli F, Viel A, Bidoli E, Berni S, D’Amati G, Scambia G, Frati L, Screpanti I, Gulino A. Mutations of an intronic repeat induce impaired MRE11 expression in primary human cancer with microsatellite instability. Oncogene. 2004;23:2640–2647. doi: 10.1038/sj.onc.1207409. [DOI] [PubMed] [Google Scholar]
- 152.Marchand C, Lea WA, Jadhav A, Dexheimer TS, Austin CP, Inglese J, Pommier Y, Simeonov A. Identification of phosphotyrosine mimetic inhibitors of human tyrosyl-DNA phosphodiesterase I by a novel AlphaScreen high-throughput assay. Mol Cancer Ther. 2009;8:240–248. doi: 10.1158/1535-7163.MCT-08-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Adhikari S, Karmahapatra SK, Elias H, Dhopeshwarkar P, Williams RS, Byers S, Uren A, Roy R. Development of a novel assay for human tyrosyl DNA phosphodiesterase 2. Anal Biochem. 2011;416:112–116. doi: 10.1016/j.ab.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Thomson G, Watson A, Caldecott K, Denneny O, Depledge P, Hamilton N, Hopkins G, Jordan A, Morrow C, Raoof A, Waddell I, Ogilvie D. Generation of assays and antibodies to facilitate the study of human 5′-tyrosyl DNA phosphodiesterase. Anal Biochem. 2013;436:145–150. doi: 10.1016/j.ab.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 155.Raoof A, Depledge P, Hamilton NM, Hamilton NS, Hitchin JR, Hopkins GV, Jordan AM, Maguire LA, McGonagle AE, Mould DP, Rushbrooke M, Small HF, Smith KM, Thomson GJ, Turlais F, Waddell ID, Waszkowycz B, Watson AJ, Ogilvie DJ. Toxoflavins and deazaflavins as the first reported selective small molecule inhibitors of tyrosyl-DNA phosphodiesterase II. J Med Chem. 2013;56:6352–6370. doi: 10.1021/jm400568p. [DOI] [PubMed] [Google Scholar]
- 156.Liao Z, Thibaut L, Jobson A, Pommier Y. Inhibition of human tyrosyl-DNA phosphodiesterase by aminoglycoside antibiotics and ribosome inhibitors. Mol Pharmacol. 2006;70:366–372. doi: 10.1124/mol.105.021865. [DOI] [PubMed] [Google Scholar]
- 157.Dexheimer TS, Gediya LK, Stephen AG, Weidlich I, Antony S, Marchand C, Interthal H, Nicklaus M, Fisher RJ, Njar VC, Pommier Y. 4-Pregnen-21-ol-3,20-dione-21-(4-bromobenzenesulfonate) (NSC 88915) and related novel steroid derivatives as tyrosyl-DNA phosphodiesterase (Tdp1) inhibitors. J Med Chem. 2009;52:7122–7131. doi: 10.1021/jm901061s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Conda-Sheridan M, Reddy PV, Morrell A, Cobb BT, Marchand C, Agama K, Chergui A, Renaud A, Stephen AG, Bindu LK, Pommier Y, Cushman M. Synthesis and biological evaluation of indenoisoquinolines that inhibit both tyrosyl-DNA phosphodiesterase I (Tdp1) and topoisomerase I (Top1) J Med Chem. 2013;56:182–200. doi: 10.1021/jm3014458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nguyen TX, Morrell A, Conda-Sheridan M, Marchand C, Agama K, Bermingham A, Stephen AG, Chergui A, Naumova A, Fisher R, O’Keefe BR, Pommier Y, Cushman M. Synthesis and biological evaluation of the first dual tyrosyl-DNA phosphodiesterase I (Tdp1)-topoisomerase I (Top1) inhibitors. J Med Chem. 2012;55:4457–4478. doi: 10.1021/jm300335n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sirivolu VR, Vernekar SK, Marchand C, Naumova A, Chergui A, Renaud A, Stephen AG, Chen F, Sham YY, Pommier Y, Wang Z. 5-Arylidenethioxothiazolidinones as inhibitors of tyrosyl-DNA phosphodie-sterase I. J Med Chem. 2012;55:8671–8684. doi: 10.1021/jm3008773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim Y, Spitz GS, Veturi U, Lach FP, Auerbach AD, Smogorzewska A. Regulation of multiple DNA repair pathways by the Fanconi anemia protein SLX4. Blood. 2013;121:54–63. doi: 10.1182/blood-2012-07-441212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hartsuiker E, Neale MJ, Carr AM. Distinct requirements for the Rad32(Mre11) nuclease and Ctp1(CtIP) in the removal of covalently bound topoisomerase I and II from DNA. Mol Cell. 2009;33:117–123. doi: 10.1016/j.molcel.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pourquier P, Pommier Y. Topoisomerase I-mediated DNA damage. Adv Cancer Res. 2001;80:189–216. doi: 10.1016/s0065-230x(01)80016-6. [DOI] [PubMed] [Google Scholar]
- 164.Sordet O, Khan QA, Pommier Y. Apoptotic topoisomerase I-DNA complexes induced by oxygen radicals and mitochondrial dysfunction. Cell Cycle. 2004;3:1095–1097. [PubMed] [Google Scholar]
- 165.Pourquier P, Ueng LM, Kohlhagen G, Mazumder A, Gupta M, Kohn KW, Pommier Y. Effects of uracil incorporation, DNA mismatches, and abasic sites on cleavage and religation activities of mammalian topoisomerase I. J Biol Chem. 1997;272:7792–7796. doi: 10.1074/jbc.272.12.7792. [DOI] [PubMed] [Google Scholar]
- 166.Lesher DT, Pommier Y, Stewart L, Redinbo MR. 8-Oxoguanine rearranges the active site of human topoisomerase I. Proc Nat Acad Sci USA. 2002;99:12102–12107. doi: 10.1073/pnas.192282699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang X, Henningfeld KA, Hecht SM. DNA topoisomerase I-mediated formation of structurally modified DNA duplexes, effects of metal ions and topoisomerase I inhibitors. Biochemistry. 1998;37:2691–2700. doi: 10.1021/bi972707c. [DOI] [PubMed] [Google Scholar]
- 168.Leteurtre F, Kohlhagen G, Fesen MR, Tanizawa A, Kohn KW, Pommier Y. Effects of DNA methylation on topoisomerase I and II cleavage activities. J Biol Chem. 1994;269:7893–7900. [PubMed] [Google Scholar]
- 169.Antony S, Arimondo PB, Sun JS, Pommier Y. Position- and orientation-specific enhancement of topoisomerase I cleavage complexes by triplex DNA structures. Nucleic Acids Res. 2004;32:5163–5173. doi: 10.1093/nar/gkh847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sordet O, Khan Q, Kohn KW, Pommier Y. Apoptosis induced by topoisomerase inhibitors. Curr Med Chem Anticancer Agents. 2003;3:271–290. doi: 10.2174/1568011033482378. [DOI] [PubMed] [Google Scholar]
- 171.Sordet O, Khan QA, Plo I, Pourquier P, Urasaki Y, Yoshida A, Antony S, Kohlhagen G, Solary E, Saparbaev M, Laval J, Pommier Y. Apoptotic topoisome-rase I-DNA complexes induced by staurosporine-mediated oxygen radicals. J Biol Chem. 2004;279:50499–50504. doi: 10.1074/jbc.M410277200. [DOI] [PubMed] [Google Scholar]
- 172.Sordet O, Liao Z, Liu H, Antony S, Stevens EV, Kohlhagen G, Fu H, Pommier Y. Topoisomerase I-DNA complexes contribute to arsenic trioxide-induced apoptosis. J Biol Chem. 2004;279:33968–33975. doi: 10.1074/jbc.M404620200. [DOI] [PubMed] [Google Scholar]
- 173.Lanza A, Tornatelli S, Rodolfo C, Scanavini MC, Pedrini AM. Human DNA topoisomerase I-mediated cleavages stimulated by ultraviolet light-induced DNA damage. J Biol Chem. 1996;271:6978–6986. doi: 10.1074/jbc.271.12.6978. [DOI] [PubMed] [Google Scholar]
- 174.Subramanian D, Rosenstein BS, Muller MT. Ultraviolet-induced DNA damage stimulates topoisomerase I-DNA complex formation in vivo: possible relationship with DNA repair. Cancer Res. 1998;58:976–984. [PubMed] [Google Scholar]
- 175.Pourquier P, Waltman JL, Urasaki Y, Loktionova NA, Pegg AE, Nitiss JL, Pommier Y. Topoisomerase I-mediated cytotoxicity of N-methyl-N′-nitro-N-nitrosoguanidine: trapping of topoisomerase I by the O6-methylguanine. Cancer Res. 2001;61:53–58. [PubMed] [Google Scholar]
- 176.Pommier Y, Laco GS, Kohlhagen G, Sayer JM, Kroth H, Jerina DM. Position-specific trapping of topoisomerase I-DNA cleavage complexes by intercalated benzo [a]-pyrene diol epoxide adducts at the 6-amino group of adenine. Proc Nat Acad Sci USA. 2000;97:10739–10744. doi: 10.1073/pnas.190312697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Pommier Y, Kohlhagen G, Pourquier P, Sayer JM, Kroth H, Jerina DM. Benzo [a]pyrene epoxide adducts in DNA are potent inhibitors of a normal topoisomerase I cleavage site and powerful inducers of other topoisomerase I cleavages. Proc Nat Acad Sci USA. 2000;97:2040–2045. doi: 10.1073/pnas.040397497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Pommier Y, Kohlhagen G, Laco GS, Kroth H, Sayer JM, Jerina DM. Different effects on human topoisomerase I by minor groove and intercalated deoxyguanosine adducts derived from two polycyclic aromatic hydrocarbon diol epoxides at or near a normal cleavage site. J Biol Chem. 2002;277:13666–13672. doi: 10.1074/jbc.M200209200. [DOI] [PubMed] [Google Scholar]
- 179.Pourquier P, Bjornsti MA, Pommier Y. Induction of topoisomerase I cleavage complexes by the vinyl chloride adduct, 1,N6-ethenoadenine. J Biol Chem. 1998;273:27245–27249. doi: 10.1074/jbc.273.42.27245. [DOI] [PubMed] [Google Scholar]
- 180.Antony S, Theruvathu JA, Brooks PJ, Lesher DT, Redinbo M, Pommier Y. Enhancement of camptothecin-induced topoisomerase I cleavage complexes by the acetaldehyde adduct N2-ethyl-2′-deoxyguanosine. Nucleic Acids Res. 2004;32:5685–5692. doi: 10.1093/nar/gkh902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dexheimer TS, Kozekova A, Rizzo CJ, Stone MP, Pommier Y. The modulation of topoisomerase I-mediated DNA cleavage and the induction of DNA-topoisomerase I crosslinks by crotonaldehyde-derived DNA adducts. Nucleic Acids Res. 2008;36:4128–4136. doi: 10.1093/nar/gkn334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kingma PS, Osheroff N. The response of eukaryotic topoisomerases to DNA damage. Biochim Biophys Acta. 1998;1400:223–232. doi: 10.1016/s0167-4781(98)00138-9. [DOI] [PubMed] [Google Scholar]
- 183.Kingma PS, Burden DA, Osheroff N. Binding of etoposide to topoisomerase II in the absence of DNA: decreased affinity as a mechanism of drug resistance. Biochemistry. 1999;38:3457–3461. doi: 10.1021/bi982855i. [DOI] [PubMed] [Google Scholar]
- 184.Kingma PS, Greider CA, Osheroff N. Spontaneous DNA lesions poison human topoisomerase IIα and stimulate cleavage proximal to leukemic 11q23 chromosomal breakpoints. Biochemistry. 1997;36:5934–5939. doi: 10.1021/bi970507v. [DOI] [PubMed] [Google Scholar]
- 185.Sabourin M, Osheroff N. Sensitivity of human type II topoisomerases to DNA damage: stimulation of enzyme-mediated DNA cleavage by abasic, oxidized and alkylated lesions. Nucleic Acids Res. 2000;28:1947–1954. doi: 10.1093/nar/28.9.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Velez-Cruz R, Riggins JN, Daniels JS, Cai H, Guengerich FP, Marnett LJ, Osheroff N. Exocyclic DNA lesions stimulate DNA cleavage mediated by human topoisomerase II alpha in vitro and in cultured cells. Biochemistry. 2005;44:3972–3981. doi: 10.1021/bi0478289. [DOI] [PubMed] [Google Scholar]
- 187.Wilstermann AM, Osheroff N. Base excision repair intermediates as topoisomerase II poisons. J Biol Chem. 2001;276:46290–46296. doi: 10.1074/jbc.M105733200. [DOI] [PubMed] [Google Scholar]
- 188.Kingma PS, Osheroff N. Spontaneous DNA damage stimulates topoisomerase II-mediated DNA cleavage. J Biol Chem. 1997;272:7488–7493. doi: 10.1074/jbc.272.11.7488. [DOI] [PubMed] [Google Scholar]
- 189.Bigioni M, Zunino F, Capranico G. Base mutation analysis of topoisomerase II-idarubicin-DNA ternary complex formation. Evidence for enzyme subunit cooperativity in DNA cleavage. Nucleic Acids Res. 1994;22:2274–2281. doi: 10.1093/nar/22.12.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang Y, Knudsen BR, Bjergbaek L, Westergaard O, Andersen AH. Stimulated activity of human topoisomerases IIalpha and IIbeta on RNA-containing substrates. J Biol Chem. 1999;274:22839–22846. doi: 10.1074/jbc.274.32.22839. [DOI] [PubMed] [Google Scholar]
- 191.Wang Y, Thyssen A, Westergaard O, Andersen AH. Position-specific effect of ribonucleotides on the cleavage activity of human topoisomerase II. Nucleic Acids Res. 2000;28:4815–4821. doi: 10.1093/nar/28.24.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Deweese JE, Burgin AB, Osheroff N. Using 3′-bridging phosphorothiolates to isolate the forward DNA cleavage reaction of human topoisomerase IIalpha. Biochemistry. 2008;47:4129–4140. doi: 10.1021/bi702194x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Deweese JE, Osheroff N. Coordinating the two protomer active sites of human topoisomerase IIalpha: nicks as topoisomerase II poisons. Biochemistry. 2009;48:1439–1441. doi: 10.1021/bi8021679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Corbett AH, Zechiedrich EL, Lloyd RS, Osheroff N. Inhibition of eukaryotic topoisomerase II by ultraviolet-induced cyclobutane pyrimidine dimers. J Biol Chem. 1991;266:19666–19671. [PubMed] [Google Scholar]
- 195.Cline SD, Osheroff N. Cytosine arabinoside (araC) lesions are position-specific topoisomerase II poisons and stimulate DNA cleavage mediated by the human type II enzymes. J Biol Chem. 1999;274:29740–29743. doi: 10.1074/jbc.274.42.29740. [DOI] [PubMed] [Google Scholar]
- 196.Khan QA, Kohlhagen G, Marshall R, Austin CA, Kalena GP, Kroth H, Sayer JM, Jerina DM, Pommier Y. Position-specific trapping of topoisome-rase II by benzo[a]pyrene diol epoxide adducts: implications for interactions with intercalating anticancer agents. Proc Nat Acad Sci USA. 2003;100:12498–12503. doi: 10.1073/pnas.2032456100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Francks C, Paracchini S, Smith SD, Richardson AJ, Scerri TS, Cardon LR, Marlow AJ, MacPhie IL, Walter J, Pennington BF, Fisher SE, Olson RK, DeFries JC, Stein JF, Monaco AP. A 77-kilobase region of chromosome 6p22.2 is associated with dyslexia in families from the United Kingdom and from the United States. Am J Hum Genet. 2004;75:1046–1058. doi: 10.1086/426404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Paracchini S, Thomas A, Castro S, Lai C, Paramasivam M, Wang Y, Keating BJ, Taylor JM, Hacking DF, Scerri T, Francks C, Richardson AJ, Wade-Martins R, Stein JF, Knight JC, Copp AJ, Loturco J, Monaco AP. The chromosome 6p22 haplotype associated with dyslexia reduces the expression of KIAA0319, a novel gene involved in neuronal migration. Hum Mol Genet. 2006;15:1659–1666. doi: 10.1093/hmg/ddl089. [DOI] [PubMed] [Google Scholar]
- 199.Cope N, Harold D, Hill G, Moskvina V, Stevenson J, Holmans P, Owen MJ, O’Donovan MC, Williams J. Strong evidence that KIAA0319 on chromosome 6p is a susceptibility gene for developmental dyslexia. Am J Hum Genet. 2005;76:581–591. doi: 10.1086/429131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Vilotti S, Codrich M, Dal Ferro M, Pinto M, Ferrer I, Collavin L, Gustincich S, Zucchelli S. Parkinson’s disease DJ-1 L166P alters rRNA biogenesis by exclusion of TTRAP from the nucleolus and sequestration into cytoplasmic aggregates via TRAF6. PLoS One. 2012;7:e35051. doi: 10.1371/journal.pone.0035051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vilotti S, Biagioli M, Foti R, Dal Ferro M, Lavina ZS, Collavin L, Del Sal G, Zucchelli S, Gustincich S. The PML nuclear bodies-associated protein TTRAP regulates ribosome biogenesis in nucleolar cavities upon proteasome inhibition. Cell Death Differ. 2012;19:488–500. doi: 10.1038/cdd.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zucchelli S, Vilotti S, Calligaris R, Lavina ZS, Biagioli M, Foti R, De Maso L, Pinto M, Gorza M, Speretta E, Casseler C, Tell G, Del Sal G, Gustincich S. Aggresome-forming TTRAP mediates pro-apoptotic properties of Parkinson’s disease-associated DJ-1 missense mutations. Cell Death Differ. 2009;16:428–438. doi: 10.1038/cdd.2008.169. [DOI] [PubMed] [Google Scholar]
- 203.Hawkins AJ, Subler MA, Akopiants K, Wiley JL, Taylor SM, Rice AC, Windle JJ, Valerie K, Povirk LF. In vitro complementation of Tdp1 deficiency indicates a stabilized enzyme–DNA adduct from tyrosyl but not glycolate lesions as a consequence of the SCAN1 mutation. DNA Repair (Amst) 2009;8:654–663. doi: 10.1016/j.dnarep.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]