Abstract
In order to investigate the unique contribution of individual wine grape (Vitis vinifera) berry tissues and water-deficit to wine quality traits, a survey of tissue-specific differences in protein and selected metabolites was conducted using pericarp (skin and pulp) and seeds of berries from vines grown under well watered and water-deficit stress conditions. Of 1,047 proteins surveyed from pericarp by 2D-PAGE, 90 identified proteins showed differential expression between the skin and pulp. Of 695 proteins surveyed from seed tissue, 163 were identified and revealed that the seed and pericarp proteomes were nearly completely distinct from one another. Water-deficit stress altered the abundance of approximately 7% of pericarp proteins, but had little effect on seed protein expression. Comparison of protein and available mRNA expression patterns showed that 32% pericarp and 69% seed proteins exhibited similar quantitative expression patterns indicating that protein accumulation patterns are strongly influenced by post-transcriptional processes. About half of the 32 metabolites surveyed showed tissue-specific differences in abundance with water-deficit stress affecting the accumulation seven of these compounds. These results provide novel insights into the likely tissue-specific origins and the influence of water deficit stress on the accumulation of key flavor and aroma compounds in wine.
Keywords: Vitis vinifera L, Water deficit stress, Tissue-specific proteins, Metabolites, Two-dimensional gel electrophoresis
1 Introduction
The berries of grape vine (Vitis vinifera L.) and related species are one of the most widely grown and economically most import fruit crops in the world. Since its initial domestication more than 7,000 years ago [1, 2], berries have been used for wine production, as well as grape juice, table grapes, raisins, and more recently for leaf, seed, and skin extracts by the nutraceutical and cosmetic industries [3, 4]. The genetic diversity of grapevine have been narrowed considerably by the selection of only a few familiar cultivars (e.g., Chardonnay, Cabernet Sauvignon, Syrah (Shiraz) and Merlot) now grown worldwide [1]. Quality traits, which are generally linked to a specific tissue, such as skin color due to the production of anthocyanins and proanthocyanidins, are controlled by relatively few genes [5, 6]. However, traits considered as desirable for one product could be undesirable for another. For example, seedlessness is a highly desirable trait in table grapes, however, seeds contain a high concentration of condensed tannins (i.e., proanthocyanidins), which are considered indispensable for conferring astringency and color stability to red wines [7]. The skin, pulp, and seed tissues of grape berries each confer unique properties to wine. The skin confers color, aroma, and other organoleptic properties of wine. The pulp contributes the majority of sugars, which are transformed into alcohol during the fermentation process. Skin and pulp tissues are the main source of volatile aroma compounds, such as terpenes, norisoprenoids, and thiols stored as sugar or amino acid conjugates [8]. The seed contains flavan-3-ol monomers and procyanidins (seed tannins), which contribute important organoleptic properties to wine [7].
Analysis of the protein composition of grape berries and must has been used to examine varietal and developmental differences as well as to analyze chemical and environmental effects in grape. Polyacrylamide gel electrophoresis (PAGE) analysis of must proteins has provided a means to readily identify different grape varieties [9]. Electrospray ionization-mass spectrometry (ESI-MS) has also been used to differentiate varieties by identifying different classes of pathogenesis-related (PR) proteins in grape juice [10]. In contrast, other researchers have concluded that one- and two-dimensional PAGE analysis of PR proteins was inadequate to readily differentiate varieties [11].
Protein extraction methods for mature grape berry clusters have been optimized with phenol-based methods being superior to TCA/acetone methods [12]. Proteomic comparison of ripe berry mesocarp from six different Vitis cultivars revealed that most 2D-PAGE profiles were ~70% similar to one another with the exception of a few proteins, such as alcohol dehydrogenase (ADH), which displayed large polymorphisms among the different cultivars [13]. High light and CO2 concentrations apparently stabilized RuBisCO in grapevine plantlets as monitored by 1D- and 2D-PAGE and immunoblotting [14]. Herbicide stress on grapevine shoots, root, and leaves induced antioxidant and photorespiratory enzymes, as well as a set of pathogenesis-related (PR) proteins [15]. Chronic salinity and water-deficit stress of grapevine shoots revealed distinct differences in protein expression patterns in cv. Chardonnay and cv. Cabernet Sauvignon [16]. Vitis leaves, in which alcohol dehydrogenase was over- or under-expressed, revealed abundance changes in carbon metabolism-associated proteins [17]. Analysis of grape berry skin proteins from cv. Cabernet Sauvignon at the beginning and end of véraision and in mature, harvest stage berries showed ripening-related protein abundance increases associated with anthocyanin biosynthesis and pathogen defense [18]. A similar, yet more comprehensive, analysis of berry ripening in V. vinifera cv. Nebbiolo Lampia showed that more than 100 proteins were differentially expressed during berry development [19]. More recently, analysis of changes in the expression of 67 grape skin proteins were monitored from véraison to fully ripe berries of V. vinifera cv. Barbera showing that many proteins with (a)biotic stress responses were developmentally regulated [20].
In order to better understand the complex transcriptional regulatory hierarchy controlling tissue-specific gene expression patterns, several studies have investigated the steady-state transcript abundance in discrete berry tissues. Large-scale expressed sequence tag (EST) sampling has been used to identify differences in expression associated with different organ and tissue types and developmental stages [21-23]. Large-scale mRNA expression profiling studies have investigated expression in flowers and developing berries of V. vinifera [24-27], in a fleshless berry mutant (cv. Ugni Blanc) [28], and in the skin of ripening berries of seven different V. vinifera cultivars [29]. More recently, large-scale mRNA expression profiles within skin, pulp, and seed tissues of well-watered and water-deficit stressed vines of Cabernet Sauvignon were surveyed using the GeneChip® V. vinifera (Grape) Genome Array [30]. However, no proteomic studies have been performed to investigate protein expression differences among different berry tissues.
In order to obtain information on protein expression changes in grape berry tissues in response to well watered and water-deficit stress conditions, a comparative 2D-PAGE analysis was performed using discrete tissue from the pericarp tissues (skin and pulp) and seed. Approximately 7% of the more than 1,000 skin and pulp proteins surveyed showed a two-fold or greater change in abundance in response to water deficit stress indicating that water-deficit stress can have a major impact on protein expression profiles in grape pericarp tissues. From the 695 seed proteins surveyed in the seed, seed protein expression patterns were completely distinct from those in the skin and pulp tissues, mainly due to high concentrations of seed storage proteins. Skin abundant proteins were associated mainly with the phenylpropanoid pathway, pathogenesis-related (PR) proteins, heat shock proteins, and polyphenol oxidase, whereas pulp abundant proteins included those involved in primary energy metabolism. Water deficit stress led to tissue-specific changes in protein expression. The skin showed increased abundance of proteosome, reactive oxygen detoxification enzymes, and selected enzymes involved in flavonoid biosynthesis, whereas pulp tissues showed increased in glutamate decarboxylase, PR proteins, and methionine synthase. Changes in the abundance of selected metabolites were also monitored in parallel with protein expression analysis. Tissue-specific and water status-dependent differences in metabolite profiles were also evident.
2 Material and methods
2.1 Plant material and growth conditions
Vitis vinifera L. cv. Cabernet Sauvignon berries were sampled on September 29, 2005, at which time the berries were fully ripe and corresponded to stage 38 (berry harvest) of the modified E-L system [31] from 20-year-old vines in the Shenandoah Vineyard (Amador County, CA, USA), stored on ice for 3 hours, frozen in liquid nitrogen and stored at −80° C. Therefore, it is possible that changes in the proteome and metabolome may have occurred during the time the berries were stored on ice. Pulp, skin and seeds were then separated without allowing berries to thaw. Skin was peeled off the pulp using a scalpel. The pulp was then cut into two halves from which the seeds were carefully removed. As complete removal of pulp cells from the skin or seed tissues was not possible, the observed differences in tissue-specific patterns reported here may show some residual contamination from pulp tissues. For the well-watered plants, irrigation was performed from E-L stage 27 [31]. Water deficit treated vines were never irrigated. Berry clusters were harvested from the sunny (Southern) side of the vine. Six biological replicates were collected and analyzed from both well watered and water-deficit treated vines.
2.2 Stem xylem water potential
Fully mature leaves were selected for stem water potential measurements [32]. A single leaf per plant was tightly zipped in a plastic bag to eliminate transpiration. Aluminum foil was then placed around the bag, deflecting light and heat. After two hours of equilibration time, the excised leaf was placed in a 3005 Plant Water Status Console pressure chamber (Soilmoisture Equipment Corp., Santa Barbara, CA, USA). The foil was removed before sealing the bagged leaf in the chamber. The balancing pressure required to visibly push stem xylem sap to the cut surface was recorded.
2.3 Brix assay
The Brix (total soluble solids) was assayed from juice crushed from harvested berries with a refractometer (BRIX30, Leica, Bannockburn, IL, USA).
2.4 Protein extraction
Protein extractions were performed in sets of 4 random samples, with the constraint that 2 biological replicates were never processed within the same set. Five g of skin or pulp tissue or 1 g of seed were ground to a fine powder in liquid nitrogen with mortar and pestle. Extraction was adapted from the phenol extraction protocol 4 as described [12], which was adapted from previously described protocols [33, 34]. Powder was vortexed in 10 mL of Sucrose Buffer 4 (0.7 M sucrose, 0.5 M Tris-HCl pH = 7.5, 50 mM EDTA, 0.1 M potassium chloride, 2 mM PMSF, 2% ß-ME, 1 Complete™ protease inhibitor cocktail tablet (Roche Diagnostics, Indianapolis, IN, USA), and 1% PVPP) and incubated for 10 min at 4° C. After incubation, an equal volume of 1 M Tris-saturated phenol (pH = 7.9) was added. The mixture was stored at −20° C for 30 min with vortexing every 10 min. The phases were separated by centrifugation (30 min at 0° C at 3,210 x g). The upper phenol phase was collected and re-extracted with an equal volume of Sucrose Buffer 4. Five volumes of 0.1 M ammonium acetate in cold MeOH were added to the phenol phase to precipitate proteins, followed by incubation at −20° C overnight. The pellet was washed with 5 mL of cold 0.1 M ammonium acetate/MeOH 50:50 w/v, two times with 5 mL of cold acetone and once in 2 ml of cold acetone/ethanol 50:50 v/v. The pellet was then vacuum-dried 5 min and resolubilized in 1.5 ml of Rehydration Buffer (7 M urea, 2 M thiourea, 4% CHAPS, 10 mM DTT, 1% carrier ampholyte, pH = 5–7, and 1% carrier ampholyte, pH = 3–10). PVPP (50 mg) was added to each sample, then each sample was vortexed, and centrifuged (15 min at −4° C at 10,000 x g) and the supernatant was stored at −80° C.
2.5 Protein assays
Protein concentrations were determined using an EZQ™ Protein Quantitation Kit (Invitrogen, Carlsbad, CA, USA) with ovalbumin as a standard, according manufacturer's instructions. Concentration ranged from 3.6 mg/ml to 10.6 mg/ml.
2.6 2-DE and gel staining
In order to reduce technical variation, no more than 2 of the 6 replicates were processed within the same set of 2-D SDS-PAGE gels. The 2-D SDS-PAGE protocol was adapted from O’Farrell (1975) [35]. IEF was carried out using immobilized pH gradient (IPG) strips (24 cm, pH = 4–7, Immobiline™ DryStrip, GE Healthcare, Piscataway, NJ, USA). The loading volume used was 440 μL of protein extract, corresponding to a protein amount of 1.2 mg per strip. Protein IEF was performed using a Protean® IEF Cell (Bio-Rad, Hercules, CA, USA) at 20° C as follows: active rehydration at 50 V for 12 h, 200 V for 30 min with a linear increase in voltage, 500 V for 30 min with a linear increase in voltage, 1000 V for 1 h with a linear increase in voltage, and 10,000 V with a rapid increase in voltage until a total of 85,000 Vh had been reached. Strips were then stored at −20° C until further use. Once thawed, the strips were washed for 30 min in Equilibration Buffer (6 M urea, 30% glycerol, 2 M Tris-HCl pH 8.8, and 2% SDS) containing 1% w/v DTT followed by washing with Equilibration Buffer containing 2.5% w/v iodoacetamide for 30 min. SDS-PAGE was performed using non-commercial 12% polyacrylamide gels (18 cm × 20 cm × 1 mm) and run at 40 V for 2 h and 120 V for 15 h in a Bio-Rad Protean® II XL 2-D Multi-Cell. A Coomassie Brilliant Blue (CBB) G-250 procedure was used to stain the 2-D gels [36]. The gels were washed twice in 50% EtOH/2% phosphoric acid/deionized water (diH2O) v/v/v for 1 h, then transferred to 2% phosphoric acid for 60 min, and finally allowed to shake for 3 days in 15% EtOH/17% ammonium sulfate/2% phosphoric acid/0.2% CBB G-250/dH2O v/w/v/w/v. The 2-D gels were imaged using a VersaDoc® Imaging System Model 1000 (Bio-Rad).
2.7 Protein identification
Spot excision was performed using the ProteomeWorks™ spot cutter (Bio-Rad); then trypsin digested according to [37] using the Investigator™ ProPrep™ (Genomic Solutions, Ann Arbor, MI, USA). The tryptic fragments were analyzed using an ABI 4700 Proteomics Analyzer (Applied Biosystems, Foster City, CA, USA) MALDI TOF/TOF™ mass spectrometer (MS). A 0.5 mL aliquot of a matrix solution containing 10 mg/mL alpha-cyano-4-hydroxycinnamic acid (Sigma-Aldrich, Inc., St. Louis, MO, USA) and 10 mM ammonium phosphate (Sigma-Aldrich) in 70% acetonitrile was co-spotted with 0.5 mL of sample [38]. The data were acquired in reflector mode from a mass range of 700 to 4,000 Da, and 2,500 laser shots were averaged for each mass spectrum. Each sample was internally calibrated if both the 842.51 and 2211.10 ions from trypsin autolysis were present. When both ions were not found, the instrument used the default calibration. The eight most intense ions from the MS analysis, not present on the exclusion list, were subjected to MS/MS analysis. To this end, the mass range was 70 to precursor ion with a precursor window of 21–3 Da with an average of 5,000 laser shots for each spectrum. The resulting file was then searched by using automated MASCOT software (http://www.matrixscience.com/) through the IDQuest (Bio-Rad) interface was used for searching the NCBI nonredundant database (ver. 22_05_2007; 4,970,641 sequences), or the Contigs from Vitis Gene Index ver. 5.0 (ver. 18_9_2006, 23,871 sequences). Peptide tolerance was 20 ppm; 1 missed cleavage was allowed; MS/MS tolerance was 0.8 Da. The possibility of matching multiple translated isoforms was examined by manual analysis of peptides covering the sequences.
2.8 Statistical analysis
Results from 6 different gels were compared for well-watered and water-deficit-stressed vines for each tissue and the results of 12 different gels were compared between skin and pulp. Differences in spot abundance were statistically evaluated using the ANOVA method with geneANOVA software [39]. The number of detected spots showing differences with a P-value of ≤0.05 was then determined. The spots were counted as valuable if their normalized intensity was higher than 0.01% of the total spot intensity. However, for non-detected spots a background value was used in the gels where they did not appear in order to limit the rate of false positives. Average CV was calculated for each experiment with and without background values. Spots were identified and then curated manually with respect to spot quality (e.g., sharpness, resolution) and the quality of spot matching to reduce false positives.
For protein and mRNA abundance comparisons, Log2 values of the protein and mRNA ratios between pulp and skin values were plotted and the regression curve was determined using Excel. The mRNA expression values determined by microarray expression profiling were obtained from [30]. The proteome analysis reported here was performed using berries harvested one year later from the same vines at the same harvest date as were used for mRNA profiling.
2.9 Metabolite extraction and derivatization protocol
Polar metabolites were extracted and derivatized with a water/chloroform protocol according to previously described procedures [40]. Freeze-dried berry tissue (6 mg) was placed in a standard screw-cap-threaded, glass vial. Samples were incubated in HPLC grade chloroform for 1 hour at 50°C in an oven. A volume of Millipore NANOpure™ water containing 12.5 mg L−1 of ribitol as an internal standard was added to each sample, and then incubated for an additional hour at 50°C. Finally, vials were allowed to cool to room temperature and then spun at 2,900 x g for 30 min. One mL of the polar phase was dried down in a vacuum concentrator overnight. Polar samples were derivatized by the addition of 120 μL of 15 mg mL−1 of methoxyamine HCl in pyridine, sonicated for 30 min, and incubated at 50° C for 1 h. 120 μL of MSTFA + 1% TMCS were added, incubated at 50° C for 1 h, and analyzed immediately with a PolarisQ™ 230 GC-MS (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Derivatized samples (120 μL) were transferred to a 200 μL silanized vial insert and run at an injection split of 200:1 to bring the large peaks to a concentration within the range of the detector and 10:1 for detection of lower peaks. The inlet and transfer lines were held at 240° C and 320° C, respectively. Separation was achieved with a temperature program of 80° C for 3 min, then ramped at 5° C min−1 to 315° C and held for 17 min, using a 60 m DB-5MS column (J&W Scientific, 0.25 mm ID, 0.25 μm film thickness) and a constant flow of 1.0 ml min−1. All organic acids, sugars and amino acids were verified with standards purchased from Sigma-Aldrich, Inc.
2.10 Metabolite data processing
Metabolites were identified in the chromatograms using two different software packages: AMDIS (ver. 2.64, United States Department of Defense, USA) and Xcalibur (ver. 1.3; Thermo Fisher Scientific, Inc.). The software matched the mass spectrum in each peak against three different metabolite libraries : NIST ver. 2.0 library (http://www.nist.gov/srd/), T_MSRI_ID library of the Golm Metabolome Database [41], and a local database containing more than 50 standards. Quantification of the area of the chromatogram peaks was determined using Xcalibur and normalized as a ratio of the area of the peak of the ribitol internal standard.
3 Results
3.1 Physiological data
Fully ripe berry samples were harvested from E-L stage 38 berries [31]. This harvest date corresponded to the time of commercial harvest of the vineyard. Stem water potential differences were monitored for well-watered and water-deficit treated vines as a comprehensive indicator of water-deficit in the vines [42]. Stem water potentials were significantly more negative for water-deficit treated vines than for well-watered vines at the time of harvest (Table 1). Brix values, an approximate measure of the mass ratio of dissolved solids to water in fruit juices, were also significantly different between berries harvested from well-watered and water-deficit-stress treated vines. These values are close to the generally recommended value (23° Brix) for harvest of cv. Cabernet Sauvignon in central California. However, no significant differences in berry diameter were observed (Table 1).
Table 1.
Physiological data for berries harvested from vines grown under well watered and water-deficit stress conditions.
| Sample | Stem water potential (MPa) | Berry refractive index (°Brix) | Berry size (mm) |
|---|---|---|---|
| Well watered vine | −0.58 (±0.07)a | 19.78 (±1.02)a | 11.23 (±0.33)a |
| Water-deficit vine | −0.86 (±0.11)a,b | 21.73 (±0.74)a,b | 11.27 (±0.30)a |
n = 6.
Difference between well watered and water-deficit were determined by to be significant (p-value < 0.01) by the student’s t-test. Standard errors are indicated in parentheses.
3.2 Comparative 2D-PAGE analyses of berry tissue proteins
Three different berry tissues (i.e., skin, pulp, and seed) were dissected manually as a starting point for 2D-PAGE analysis. A relatively large number of biological sample replicates (six) for each tissue type and water status treatment were performed to obtain a statistically robust assessment of the differences in protein expression patterns. Each replicate was considered a biological replicate because it was collected from a different vine. Replicate samples were extracted and analyzed such that no more than 2 gels from the same tissue/condition were processed at the same time within the same set of samples. In total, 1,047 spots were detected in skin and pulp from vines subjected to either well watered or water-deficit stress conditions (Table 2). For these two tissues, an average of 854 spots per gel with an intensity value greater than 0.01% of the total average spot intensity was detected. In contrast, seeds presented a totally different profile that was not directly comparable to the skin and pulp tissue profiles. Therefore, 2D-PAGE gels for this tissue were processed independently until final spot matching. In seeds, a total of 695 spots was detected in seeds from vines subjected to either well watered or water-deficit stress conditions (Table 2). An average of 605 spots per gel with intensities higher than 0.01% of the total spots intensity was detected. To maximize the number of proteins identified in this study, such as transcription factor or hormone metabolism-related proteins that typically are of low abundance, faint spots were included, not only leading to a relatively high number of spots per gels (Table 2), but also a relatively high average coefficient of variation (CV). However, these CV values were within a range that was consistent with previously reported average or mean values for other plant proteomic analyses (0.26-0.31 [43]; 0.47-0.75 [44]; and 0.24 [45]). The decision to retain background values tended also to increase CV values.
Table 2.
Average numbers of spots and coefficients of variation (CV) for each berry tissue and water treatment condition. The spots were counted regardless of their intensity (I) or according to CV values greater than 0.01% or 0.05% of the total intensity of all spots.
| SkinWW | SkinWD | PulpWW | PulpWD | SeedWW | SeedWD | |
|---|---|---|---|---|---|---|
| Total spots | 1046 | 1046 | 1046 | 1046 | 695 | 695 |
| Average CV (total spots) | 0.84 | 0.74 | 0.82 | 0.81 | 0.74 | 0.76 |
| Spots (I>0.01%) | 835±59 | 870±30 | 855±30 | 855±68 | 608±41 | 602±23 |
| Average CV (I>0.01%) | 0.65 | 0.58 | 0.64 | 0.63 | 0.59 | 0.60 |
| Spots (I>0.05%) | 462±55 | 509±20 | 457±37 | 482±48 | 365±109 | 366±58 |
| Average CV (I>0.05%) | 0.55 | 0.56 | 0.56 | 0.55 | 0.53 | 0.54 |
WW = well watered; WD = water-deficit treated. n = 6.
In order to identify differentially expressed proteins among the three different berry tissues, 2D-PAGE gels were compared. Spots that displayed differential abundance after ANOVA (p<0.05) and a two-fold ratio or greater difference were identified and then curated manually with respect to spot quality (e.g., sharpness, resolution) and the quality of spot-matching to reduced false positives. Analysis of pericarp proteins revealed 90 spots that displayed differential abundance after ANOVA (p<0.05) and a two-fold ratio or greater difference: 54 were more abundant in the skin (Fig. 1 and Table 3) and 36 were more abundant in the pulp (Fig. 2 and Table 3). A majority of proteins (217 in total) showed a relatively constant abundance between the skin and pulp (see Additional Figure 1 and Additional Table 5).
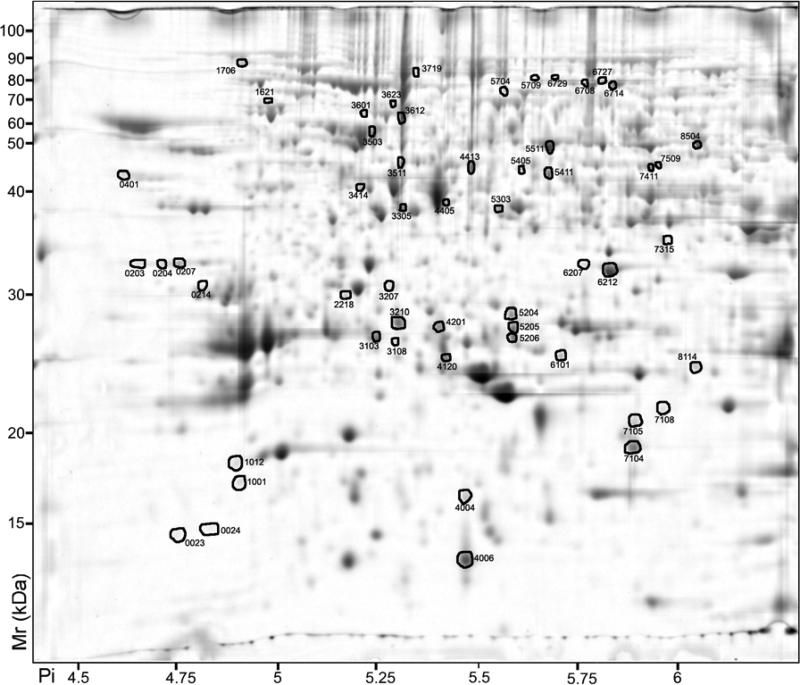
Figure 1.
2D-PAGE analysis of Vitis vinifera cv. Cabernet Sauvignon berry skin proteins. Proteins that exhibited a significant (p < 0.05) two-fold or greater change between the skin and pulp are indicated by circles and standard spot numbers on a representative gel. See Table 3 for detailed listing of proteins.
Table 3.
Proteins whose abundance was significantly different between pulp and skin. SSP, standard spot number; P/K, normalized spot volume in the pulp divided by the normalized spot volume in the skin, from 12 different plants; Pval, P value; VvGI5, match from the translated Vitis vinifera gene index Release 5; ThMr, theoretical molecular mass; Exp Mr, experimental molecular mass; ThPi, theoretical isoelectric point (Pi); Exp Pi, experimental isoelectric point (Pi); Pep, number of peptides mass and number of MS/MS ions matching the query; Mowse score; % Cov, percentage of coverage; Annotation, description of protein identity; Uniprot, Uniprot ID of the most closely related Unigene from VvGI5.
| SSP | P/K | Pval | VvGI5 | ThMr | Exp Mr | ThPi | Exp Pi | Pep | Mowse score | % Cov | Annotation | Uniprot |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenylpropanoid pathway | ||||||||||||
| 6101 | 0.04 | .000 | TC69505 | 24222 | 24983 | 5.62 | 5.73 | 13+6 | 395 | 42 | Glutathione S-transferase | Q56AY1 . |
| 5411 | 0.04 | .000 | TC69652 | 40167 | 42968 | 5.63 | 5.70 | 35+6 | 539 | 75 | Leucoanthocyanidin dioxgenase | Q8LP73 |
| 3103 | 0.10 | .000 | TC58036 | 25629 | 26375 | 5.37 | 5.26 | 7+2 | 129 | 12 | Glutathione S-transferase | Q9M6R4 |
| 3210 | 0.11 | .000 | TC55034 | 26764 | 27699 | 5.61 | 5.31 | 9+4 | 313 | 30 | Chalcone isomerase | P51117 |
| 5206 | 0.12 | .000 | TC70299 | 25341 | 26382 | 5.45 | 5.6 | 7+2 | 155 | 44 | Caffeoyl-CoA O-methyltransferase | Q2YHM9 |
| 7411 | 0.28 | .000 | AF000371 | 50121 | 43883 | 5.98 | 5.96 | 13+5 | 335 | 28 | UDP-glucose:flavonoid 3-O-glc transferase | Q9AR43 |
| 4405 | 0.32 | .023 | TC70298 | 40848 | 39433 | 5.44 | 5.44 | 17+7 | 526 | 41 | Flavonone-3-hydroxylase | P41090 |
| 8302 | 3.33 | .021 | TC52848 | 33830 | 34548 | 6.16 | 6.05 | 6+4 | 220 | 18 | Isoflavone reductase | O81355 |
| Amino acid metabolism | ||||||||||||
| 6714 | 0.30 | .002 | TC55403 | 77154 | 78904 | 6.04 | 5.86 | 12+2 | 84.3 | 31 | Glycyl tRNA synthetase | O23627 |
| 4413 | 0.32 | .000 | TC51748 | 42930 | 42888 | 5.5 | 5.5 | 30+7 | 539 | 57 | S-adenosylmethionine synthetase 2 | Q96552 |
| 6708 | 0.32 | .025 | TC55403 | 77154 | 78220 | 6.04 | 5.79 | 8+2 | 167 | 22 | Glycyl tRNA synthetase | O23627 |
| 5204 | 0.35 | .002 | TC58487 | 27173 | 28285 | 5.64 | 5.60 | 14+4 | 369 | 31 | Aluminum-induced protein | Q9FG81 |
| 5303 | 0.41 | .021 | TC64127 | 41725 | 38816 | 5.7 | 5.57 | 17+7 | 213 | 31 | Glutamine synthetase cytosolic isozyme 2 | P51119 |
| 7509 | 0.41 | .018 | TC67021/TC51696 | 51280 | 44216 | 6.44 | 5.97 | 15+2/11+2 | 78.4/61.6 | 29/27 | Ornithine aminotransferase/UDP-glucose:flavonoid 3-O-glc transferase | Q9FVJ2 /Q9AR43 |
| 3305 | 0.44 | .003 | TC60125 | 38931 | 38861 | 5.4 | 5.33 | 18+6 | 421 | 44 | Glutamine synthetase | Q93XJ6 |
| 4318 | 4.15 | .015 | TC55907 | 35827 | 35829 | 5.24 | 5.48 | 9+2 | 65.8 | 31 | Homocysteine S-methyltransferase | Q8LAX0 |
| Energy | ||||||||||||
| 1621 | 0.14 | .000 | TC67963 | 61983 | 67575 | 5.2 | 5.02 | 8+2 | 66.6 | 9 | RuBisCO subunit binding-protein alpha | Q2PEP1 |
| 8504 | 0.20 | .000 | TC57584 | 52518 | 50702 | 6.33 | 6.08 | 30+8 | 398 | 21 | RuBisCO, large subunit | Q9MVF6 |
| 203 | 0.25 | .001 | TC51789 | 24720 | 32916 | 4.6 | 4.66 | 12+2 | 101 | 37 | Cytochrome c oxidase subunit 6b-1 | Q8LHA3 |
| 4120 | 0.30 | .007 | TC62932 | 23416 | 24839 | 5.37 | 5.44 | 11+5 | 164 | 37 | Chlorophyll a-b binding protein 8 | P27522 |
| 3207 | 0.32 | .000 | TC54765 | 35289 | 30799 | 6.08 | 5.3 | 3+3 | 156 | 7 | Oxygen evolving enhancer protein 1 | Q9LRC4 |
| 204 | 0.33 | .003 | TC51789/TC52065 | 20911 | 32849 | 4.53 | 4.72 | 9+5/7+1 | 249/148 | 33/28 | Cytochrome-c oxidase/Pru2 protein precursor | F86357 /Q43608 |
| 6729 | 0.40 | .007 | TC52655 | 50682 | 80938 | 6.47 | 5.72 | 20+4 | 174 | 31 | Transketolase 1 | O78327 |
| 2218 | 0.41 | .006 | TC53930 | 35091 | 29895 | 7.54 | 5.18 | 15+7 | 433 | 44 | Oxygen evolving enhancer protein 1 | Q9LRC4 |
| 6727 | 0.43 | .011 | TC52655 | 50682 | 80895 | 6.47 | 5.84 | 24+2 | 117 | 40 | Transketolase 1 | O78327 |
| 5511 | 0.44 | .003 | TC60581 | 47873 | 48953 | 5.67 | 5.70 | 25+8 | 896 | 51 | Enolase 1 | Q9LEJ0 |
| 3503 | 0.49 | .015 | TC63054a | 59179 | 55592 | 5.9 | 5.25 | 9+3 | 124 | 41 | ATP synthase beta chain, mitochondrial | P17614 |
| 3114 | 2.03 | .012 | TC56801 | 25524 | 26379 | 5.64 | 5.39 | 15+5 | 229 | 41 | Soluble inorganic pyrophosphatase | Q6YVH9 |
| 8303 | 4.28 | .006 | TC52261 | 42948 | 36911 | 8.13 | 6.06 | 15+7 | 661 | 34 | Plastidic fructose-bisphosphate aldolase | Q8LL68 |
| Biotic and Abiotic Stress | ||||||||||||
| 7104 | 0.03 | .000 | TC68949 | 17128 | 19044 | 5.96 | 5.92 | 8+3 | 189 | 24 | Pathogenesis-related protein 10 | Q9FS43 |
| 7105 | 0.07 | .026 | TC68949 | 17128 | 20473 | 5.96 | 5.92 | 7+6 | 223 | 24 | Pathogenesis-related protein 10 | Q9FS43 |
| 7108 | 0.27 | .001 | TC55027 | 22871 | 21286 | 4.71 | 5.99 | 2+2 | 143 | 7 | Ripening-related protein grip22 | Q9M4H4 |
| 1012 | 0.32 | .046 | TC51691 | 68396 | 18178 | 6.47 | 4.90 | 10+2 | 63.1 | 15 | Polyphenol oxidase | P43311 |
| 1706 | 0.34 | .000 | TC54161 | 75365 | 78785 | 5.15 | 4.96 | 18+4 | 450 | 30 | Heat shock protein 70 | Q39641 |
| 4006 | 0.41 | .000 | TC58333 | 15716 | 13591 | 5.56 | 5.49 | 3+1 | 125 | 24 | PR-4 type protein D | O81228 |
| 3719 | 0.44 | .034 | TC53932 | 47429 | 85072 | 5.11 | 5.36 | 4+3 | 88.7 | 8 | Molecular chaperone Hsp90-1 | Q6UJX6 |
| 6212 | 0.46 | .037 | TC61082 | 13359 | 32142 | 6.11 | 5.85 | 4+2 | 221 | 18 | Beta 1-3 glucanase | Q9M3U4 |
| 1104 | 2.22 | .023 | TC52579 | 17262 | 19493 | 5.15 | 4.94 | 4+1 | 61.2 | 16 | Peroxiredoxin | Q8S3L0 |
| 7720 | 2.27 | .044 | TC65221 | 65479 | 77910 | 6.04 | 5.99 | 18+0 | 47.4 | 31 | Stress-induced protein sti1 | Q9STH1 |
| 3111 | 2.79 | .002 | TC55027 | 22871 | 21329 | 4.71 | 5.36 | 3+3 | 86.9 | 11 | Ripening-related protein grip22 | Q9M4H4 |
| 2518 | 3.28 | .005 | TC68410 | 23681 | 41567 | 5.21 | 5.13 | 10+4 | 172 | 26 | Dehydrin | Q41111 |
| 1009 | 3.97 | .035 | TC65039 | 17444 | 17639 | 4.97 | 5.05 | 2+2 | 165 | 12 | Major cherry allergen Pru av1.0201 | Q6QHU2 |
| 1403 | 4.49 | .025 | TC68410 | 23681 | 41193 | 5.21 | 4.93 | 18+6 | 397 | 34 | Dehydrin | Q41111 |
| Other metabolism | ||||||||||||
| 5405 | 0.20 | .002 | TC59251 | 15662 | 43636 | 5.48 | 5.63 | 4+3 | 66 | 23 | GDP-mannose | Q8W4J5 |
| pyrophosphorylase | ||||||||||||
| 7315 | 0.24 | .022 | TC55889 | 34731 | 35180 | 6.07 | 6 | 8+2 | 63.3 | 19 | NADPH-dependent mannose 6-P reductase | Q9FVN7 |
| 5205 | 0.26 | .000 | TC63232 | 12438 | 27094 | 5.51 | 5.61 | 7+4 | 340 | 22 | 3-beta hydroxysteroid dehydrogenase | Q65XW4 |
| 5704 | 0.46 | .036 | TC51756 | 35016 | 76362 | 6.09 | 5.59 | 8+2 | 80 | 24 | Succinate dehydrogenase | O82663 |
| 6416 | 2.22 | .028 | TC69306 | 43514 | 40639 | 6.55 | 5.83 | 29+7 | 546 | 60 | Alcohol dehydrogenase 2 | Q9FZ01 |
| 4313 | 2.58 | .008 | TC65374 | 41001 | 38628 | 5.66 | 5.54 | 9+1 | 56 | 18 | Alpha-1 4-glucan-protein synthase | Q9SC19 |
| 5721 | 3.21 | .006 | TC56029 | 62272 | 70321 | 6.09 | 5.66 | 14+4 | 119 | 30 | Pyruvate decarboxylase 1 | Q9FVE1 |
| Other proteins | ||||||||||||
| 8114 | 0.09 | .003 | TC67872 | 24602 | 24225 | 6.3 | 6.07 | 7+6 | 335 | 10 | 20S proteasome alpha subunit F | O82531 |
| 214 | 0.14 | .007 | TC66224 | 29540 | 30609 | 4.74 | 4.85 | 16+5 | 264 | 43 | 14-3-3 protein | Q9LKL0 |
| 3601 | 0.15 | .001 | TC55206 | 64597 | 65725 | 5.8 | 5.22 | 6+3 | 152 | 11 | Chaperonin-60 beta subunit precursor | P93570 |
| 207 | 0.18 | .000 | TC52065 | 24886 | 32846 | 4.5 | 4.75 | 10+4 | 268 | 41 | 11S globulin-like protein | Q8W1C2 |
| 3414 | 0.31 | .013 | TC60835 | 41725 | 41140 | 5.31 | 5.21 | 8+6 | 512 | 16 | Actin | Q8H6A3 |
| 401 | 0.35 | .013 | TC53110a | 38041 | 42988 | 4.62 | 4.61 | 11+6 | 402 | 25 | Ankyrin-repeat protein | Q6TKQ6 |
| 3511 | 0.36 | .009 | TC53890 | 46766 | 44200 | 5.38 | 5.38 | 38+5 | 258 | 49 | Eukaryotic initiation factor 4A-9 | Q40471 |
| 3612 | 0.40 | .005 | TC55206 | 60597 | 64438 | 5.8 | 5.73 | 21+5 | 137 | 33 | Chaperonin-60 beta subunit precursor | P93570 |
| 3009 | 2.20 | .003 | TC57670a | 15352 | 17695 | 6.34 | 5.36 | 12+5 | 538 | 47 | Actin-depolymerizing factor 2 | Q9FVI1 |
| 3308 | 2.59 | .004 | TC52033 | 28429 | 36906 | 6.4 | 5.35 | 8+1 | 82 | 22 | Hypothetical protein | Q5AS50 |
| 504 | 3.72 | .007 | TC57144 | 42852 | 40976 | 4.71 | 4.68 | 6+1 | 103 | 23 | Hypothetical protein (Putative RNA-binding protein) | Q7XXQ8 |
| 4504 | 3.74 | .000 | TC62003a | 43130 | 48804 | 6.14 | 5.43 | 19+5 | 197 | 30 | Unknown | Q9SUU6 |
| 1324 | 4.74 | .024 | TC56411 | 34871 | 36102 | 4.89 | 4.99 | 18+4 | 224 | 39 | Ser/Thr protein phosphatase | Q42912 |
| Unidentified proteins | ||||||||||||
| SSP | P/K | Pval | Exp Mr | Exp Pi | SSP | P/K | Pval | Exp Mr | Exp Pi | SSP | P/K | Pval | Exp Mr | Exp Pi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 21 | 9.65 | .033 | 16187 | 4.86 | 2016 | 3.63 | .012 | 16132 | 5.15 | 5418 | 3.26 | .030 | 39312 | 5.68 |
| 23 | 0.14 | .000 | 14686 | 4.76 | 3108 | 0.34 | .006 | 26011 | 5.31 | 5709 | 0.38 | .040 | 81132 | 5.67 |
| 24 | 0.08 | .020 | 14931 | 4.84 | 3623 | 0.43 | .042 | 70437 | 5.3 | 5915 | 5.32 | .030 | 112502 | 5.69 |
| 125 | 2.84 | .036 | 23615 | 4.74 | 4004 | 0.39 | .032 | 16403 | 5.49 | 6004 | 4.80 | .016 | 16530 | 5.83 |
| 127 | 10.67 | .024 | 21760 | 4.64 | 4201 | 0.30 | .001 | 27331 | 5.42 | 6007 | 4.85 | .031 | 16569 | 5.87 |
| 131 | 5.16 | 042 | 19973 | 4.66 | 5316 | 3.93 | .010 | 34531 | 5.62 | 6008 | 7.73 | .003 | 16701 | 5.77 |
| 227 | 7.76 | .002 | 28074 | 4.65 | 5317 | 3.69 | .017 | 34412 | 5.65 | 6207 | 0.25 | .015 | 32782 | 5.79 |
| 1001 | 0.18 | .019 | 17157 | 4.91 | 5318 | 5.86 | .004 | 34545 | 5.69 | 7316 | 3.66 | .027 | 34091 | 5.93 |
| 1316 | 4.84 | .022 | 33761 | 4.89 | 5412 | 2.30 | .022 | 40654 | 5.71 | 7820 | 2.57 | .011 | 90326 | 5.95 |
Protein spot could be related to other isoforms.
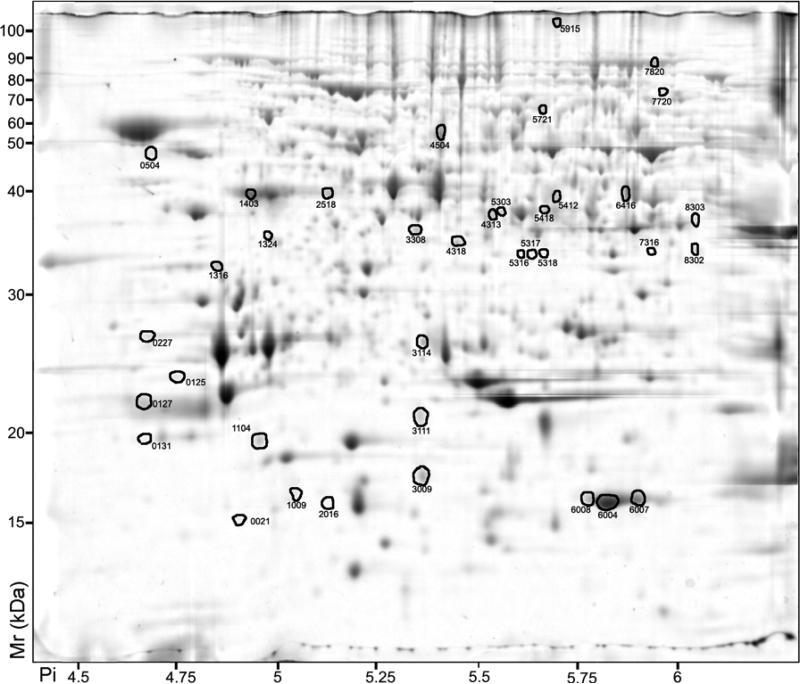
Figure 2.
2D-PAGE analysis of Vitis vinifera cv. Cabernet Sauvignon berry pulp proteins. Proteins that exhibited a significant (p < 0.05) two-fold or greater change between the skin and pulp are indicated by circles and standard spot numbers on a representative gel. See Table 3 for detailed listing of proteins.
Because of the low number of proteins that could be matched between seed and the pericarp tissues, no statistical analysis could be performed. Therefore, proteins were considered to be specific to the seed if their abundance was: 1) higher than 0.1% of the total spot intensity and 2) two-fold or greater more abundant than the spots localized at the same position on pericarp gels. Using these criteria, 163 seed protein spots were identified (Fig. 3 and Table 4), including 19 spots that matched protein spots present within pericarp tissues. A reciprocal comparative analysis could not be performed because of the relative over abundance of storage proteins in seed compared with pericarp tissues, which significantly reduced the relative abundance of other proteins.
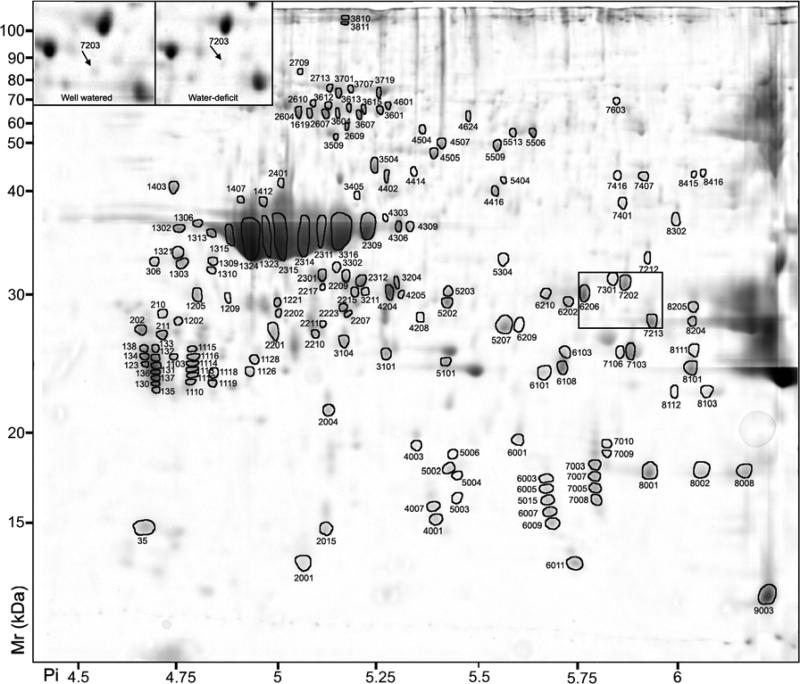
Figure 3.
2D-PAGE analysis of Vitis vinifera cv. Cabernet Sauvignon berry seed proteins. Proteins that exhibited a significant (p < 0.05) two-fold or greater change between the seed and pericarp tissues are indicated by circles and standard spot numbers on a representative gel. See Table 4 for detailed listing of proteins. Inset: spot 7203 (arrow), which was more abundant in seed of well watered than water-deficit treated berries.
Table 4.
Proteins expressed predominantly in seed tissues. SSP, standard spot number; Diff abun, normalized spot volume in the seed and difference in the seed versus pericarp if detected (ratio is indicated in parenthesis) from 12 different plants; VvGI5, match from the translated Vitis vinifera gene index Release 5; ThMr, theoretical molecular mass; Exp Mr, experimental molecular mass; ThPi, theoretical isoelectric point (Pi); Exp Pi, experimental isoelectric point (Pi); Pep, number of peptides mass and number of MS/MS ions matching the query; Mowse score; % Cov, percentage of coverage; Annotation, description of protein identity; Uniprot, Uniprot ID of the most closely related Unigene from VvGI5.
| SSP | Diff abun | VvGI5 | Th Mr | Exp Mr | Th Pi | Exp Pi | Pep | Mowse score | % Cov | Annotation | Uniprot |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Globulin TC51863/TC52006 group | |||||||||||
| 1126 | 0.10 | TC52006 | 25000 | 23850 | 9.99 | 4.91 | 6+6 | 390 | 16 | 11S globulin-like protein | Q8W1C2 |
| 1128 | 0.10 | TC52006 | 25000 | 24797 | 9.99 | 4.93 | 3+3 | 391 | 9 | 11S globulin-like protein | Q8W1C2 |
| 1202 | 0.10 | TC52006 | 25000 | 27802 | 9.99 | 4.73 | 4+3 | 279 | 14 | 11S globulin-like protein | Q8W1C2 |
| 4303 | 0.10 | TC51863 | 57516 | 37758 | 8.37 | 5.26 | 6+5 | 303 | 42 | 11S globulin-like protein | Q8W1C2 |
| 4504 | 0.10 | TC51863 | 57516 | 57885 | 8.37 | 5.35 | 8+2 | 286 | 56 | 11S globulin-like protein | Q8W1C2 |
| 7009 | 0.10 | TC51863 | 57516 | 18702 | 8.37 | 5.82 | 4+3 | 201 | 24 | 11S globulin-like protein | Q8W1C2 |
| 1112 | 0.11 | TC52006 | 25000 | 23712 | 9.99 | 4.77 | 6+3 | 209 | 10 | 11S globulin-like protein | Q8W1C2 |
| 1118 | 0.11 | TC52006 | 25000 | 23804 | 9.99 | 4.82 | 6+4 | 330 | 11 | 11S globulin-like protein | Q8W1C2 |
| 3810 | 0.11 | TC52006 | 25000 | 111772 | 9.99 | 5.16 | 10+8 | 772 | 20 | 11S globulin-like protein | Q8W1C2 |
| 1110 | 0.11 | TC52006 | 25000 | 23203 | 9.99 | 4.76 | 5+5 | 286 | 14 | 11S globulin-like protein | Q8W1C2 |
| 2401 | 0.11 | TC52006 | 25000 | 41967 | 9.99 | 4.99 | 7+5 | 287 | 16 | 11S globulin-like protein | Q8W1C2 |
| 1119 | 0.12 | TC52006 | 25000 | 23192 | 9.99 | 4.82 | 5+4 | 363 | 10 | 11S globulin-like protein | Q8W1C2 |
| 3405 | 0.12 | TC52006 | 25000 | 40414 | 9.99 | 5.19 | 6+4 | 196 | 13 | 11S globulin-like protein | Q8W1C2 |
| 3811 | 0.12 | TC52006 | 25000 | 109517 | 9.99 | 5.15 | 9+7 | 532 | 19 | 11S globulin-like protein | Q8W1C2 |
| 1113 | 0.13 | TC52006 | 25000 | 24021 | 9.99 | 4.77 | 5+4 | 403 | 14 | 11S globulin-like protein | Q8W1C2 |
| 6101 | 0.13 | TC52006 | 25000 | 23825 | 9.99 | 5.66 | 5+1 | 67.1 | 9 | 11S globulin-like protein | Q8W1C2 |
| 7010 | 0.13 | TC51863 | 57516 | 19229 | 8.37 | 5.82 | 5+5 | 280 | 24 | 11S globulin-like protein | Q8W1C2 |
| 130 | 0.15 | TC51863 | 57516 | 23927 | 8.37 | 4.67 | 5+3 | 224 | 10 | 11S globulin-like protein | Q8W1C2 |
| 5002 | 0.16 | TC51863 | 57516 | 17865 | 8.37 | 5.42 | 5+5 | 244 | 24 | 11S globulin-like protein | Q8W1C2 |
| 3204 | 0.17 (3.1) | TC51863 | 57516 | 30952 | 8.37 | 5.29 | 6+5 | 489 | 39 | 11S globulin-like protein | Q8W1C2 |
| 4306 | 0.17 | TC52006 | 25000 | 36715 | 9.99 | 5.28 | 7+5 | 331 | 16 | 11S globulin-like protein | Q8W1C2 |
| 7007 | 0.17 | TC51863 | 57516 | 17515 | 8.37 | 5.80 | 6+6 | 310 | 25 | 11S globulin-like protein | Q8W1C2 |
| 7003 | 0.18 | TC51863 | 57516 | 18100 | 8.37 | 5.80 | 5+5 | 233 | 24 | 11S globulin-like protein | Q8W1C2 |
| 136 | 0.19 | TC52006 | 25000 | 23079 | 9.99 | 4.67 | 5+4 | 326 | 11 | 11S globulin-like protein | Q8W1C2 |
| 3211 | 0.20 | TC51863 | 57516 | 30306 | 8.37 | 5.21 | 8+6 | 456 | 42 | 11S globulin-like protein | Q8W1C2 |
| 3601 | 0.20 (9.6) | TC52006 | 25000 | 67559 | 9.99 | 5.24 | 11+7 | 663 | 21 | 11S globulin-like protein | Q8W1C2 |
| 5004 | 0.21 | TC51863 | 57516 | 17524 | 8.37 | 5.45 | 3+3 | 91.7 | 16 | 11S globulin-like protein | Q8W1C2 |
| 2607 | 0.21 | TC52006 | 25000 | 66685 | 9.99 | 5.11 | 8+5 | 323 | 19 | 11S globulin-like protein | Q8W1C2 |
| 2610 | 0.21 | TC52006 | 25000 | 70653 | 9.99 | 5.08 | 10+8 | 683 | 20 | 11S globulin-like protein | Q8W1C2 |
| 3607 | 0.22 | TC52006 | 25000 | 65389 | 9.99 | 5.19 | 6+4 | 256 | 13 | 11S globulin-like protein | Q8W1C2 |
| 7008 | 0.23 | TC51863 | 57516 | 16276 | 8.37 | 5.80 | 6+6 | 292 | 25 | 11S globulin-like protein | Q8W1C2 |
| 7005 | 0.25 | TC51863 | 57516 | 16886 | 8.37 | 5.80 | 5+5 | 219 | 24 | 11S globulin-like protein | Q8W1C2 |
| 135 | 0.26 | TC52006 | 25000 | 23545 | 9.99 | 4.67 | 7+4 | 319 | 16 | 11S globulin-like protein | Q8W1C2 |
| 3615 | 0.26 | TC51863 | 57516 | 67900 | 8.37 | 5.21 | 8+7 | 657 | 42 | 11S globulin-like protein | Q8W1C2 |
| 4205 | 0.26 | TC51863 | 57516 | 30014 | 8.37 | 5.30 | 8+7 | 505 | 42 | 11S globulin-like protein | Q8W1C2 |
| 3612 | 0.27 | TC52006 | 25000 | 69277 | 9.99 | 5.12 | 9+8 | 679 | 19 | 11S globulin-like protein | Q8W1C2 |
| 123 | 0.31 | TC51863 | 57516 | 25400 | 8.37 | 4.67 | 1+1 | 77.9 | 8 | 11S globulin-like protein | Q8W1C2 |
| 2312 | 0.31 (5.4) | TC51863 | 57516 | 31200 | 8.37 | 5.20 | 5+3 | 143 | 34 | 11S globulin-like protein | Q8W1C2 |
| 131 | 0.33 | TC52006 | 25000 | 24412 | 9.99 | 4.67 | 5+3 | 302 | 14 | 11S globulin-like protein | Q8W1C2 |
| 3604 | 0.33 | TC52006 | 25000 | 68058 | 9.99 | 5.17 | 8+7 | 583 | 19 | 11S globulin-like protein | Q8W1C2 |
| 2301 | 0.34 (12) | TC51863 | 57516 | 31649 | 8.37 | 5.09 | 4+2 | 108 | 24 | 11S globulin-like protein | Q8W1C2 |
| 4204 | 0.62 | TC51863 | 57516 | 29952 | 8.37 | 5.27 | 8+5 | 330 | 42 | 11S globulin-like protein | Q8W1C2 |
| 5202 | 0.64 | TC51863 | 57516 | 29326 | 8.37 | 5.42 | 8+6 | 605 | 42 | 11S globulin-like protein | Q8W1C2 |
| 1407 | 0.65 | TC52006 | 25000 | 39898 | 9.99 | 4.89 | 10+8 | 774 | 20 | 11S globulin-like protein | Q8W1C2 |
| 1412 | 1.45 | TC52006 | 25000 | 39664 | 9.99 | 4.94 | 6+5 | 459 | 13 | 11S globulin-like protein | Q8W1C2 |
| 2309 | 2.35 (122)TC52006 | 25000 | 37096 | 9.99 | 5.22 | 9+7 | 709 | 19 | 11S globulin-like protein | Q8W1C2 | |
| 2311 | 2.57 | TC52006 | 25000 | 36848 | 9.99 | 5.08 | 9+7 | 716 | 19 | 11S globulin-like protein | Q8W1C2 |
| 3316 | 5.64 | TC52006 | 25000 | 36947 | 9.99 | 5.14 | 7+5 | 382 | 17 | 11S globulin-like protein | Q8W1C2 |
| Globulin TC51818/TC52065 group | |||||||||||
| 202 | 0.10 (2.5) | TC52065 | 34951 | 27034 | 10.26 | 4.64 | 7+4 | 257 | 30 | 11S globulin isoform 3 | Q8W1C2 |
| 1619 | 0.10 (4.2) | TC52065 | 34951 | 66206 | 10.26 | 5.07 | 5+4 | 303 | 23 | 11S globulin isoform 3 | Q8W1C2 |
| 2210 | 0.10 | TC52065 | 28618 | 26633 | 4.98 | 5.08 | 6+5 | 399 | 29 | 11S globulin isoform 3 | Q8W1C2 |
| 4007 | 0.10 | TC52065 | 28618 | 15977 | 4.98 | 5.38 | 4+5 | 205 | 17 | 11S globulin isoform 3 | Q8W1C2 |
| 2217 | 0.12 | TC51818 | 40942 | 30739 | 5.72 | 5.10 | 2+2 | 184 | 13 | 11S globulin-like protein | Q8W1C2 |
| 5006 | 0.12 | TC52065 | 34951 | 18701 | 10.26 | 5.43 | 3+1 | 72 | 17 | 11S globulin isoform 3 | Q8W1C2 |
| 133 | 0.13 | TC52065 | 34951 | 24976 | 10.26 | 4.65 | 8+3 | 354 | 21 | 11S globulin isoform 3 | Q8W1C2 |
| 134 | 0.13 | TC52065 | 34951 | 24446 | 10.26 | 4.65 | 6+4 | 412 | 22 | 11S globulin isoform 3 | Q8W1C2 |
| 4001 | 0.13 (7) | TC52065 | 28618 | 15399 | 4.98 | 5.39 | 6+5 | 269 | 23 | 11S globulin isoform 3 | Q8W1C2 |
| 6003 | 0.13 | TC51818 | 40942 | 17367 | 5.72 | 5.67 | 6+4 | 172 | 11 | 11S globulin-like protein | Q8W1C2 |
| 1116 | 0.15 | TC52065 | 34951 | 24938 | 10.26 | 4.77 | 4+4 | 339 | 20 | 11S globulin isoform 3 | Q8W1C2 |
| 2207 | 0.15 (5.4) | TC52065 | 34951 | 28357 | 10.26 | 5.17 | 6+5 | 470 | 25 | 11S globulin isoform 3 | Q8W1C2 |
| 6005 | 0.15 | TC51818 | 40942 | 16840 | 5.72 | 5.67 | 6+4 | 324 | 18 | 11S globulin-like protein | Q8W1C2 |
| 137 | 0.16 | TC52065 | 34951 | 22698 | 10.26 | 4.67 | 5+4 | 499 | 21 | 11S globulin isoform 3 | Q8W1C2 |
| 138 | 0.16 | TC52065 | 34951 | 25524 | 10.26 | 4.64 | 2+2 | 152 | 8 | 11S globulin isoform 3 | Q8W1C2 |
| 1302 | 0.16 | TC52065 | 28618 | 36607 | 4.98 | 4.73 | 9+4 | 336 | 35 | 11S globulin isoform 3 | Q8W1C2 |
| 1221 | 0.16 (2.2) | TC52065 / TC62049 | 34951 / 29316 | 29301 | 10.26/4.99 4.94 | 10+5/7+3 | 511 /256 | 39 /21 | 11S globulin isoform 3 / Polyneuridine-aldehyde esterase | Q8W1C2/Q9SE93 | |
| 5015 | 0.17 (4.4) | TC51818 | 40942 | 16285 | 5.72 | 5.67 | 7+4 | 309 | 18 | 11S globulin-like protein | Q8W1C2 |
| 211 | 0.19 | TC52065 | 34951 | 26649 | 10.26 | 4.69 | 6+4 | 325 | 16 | 11S globulin isoform 3 | Q8W1C2 |
| 1306 | 0.19 | TC51818 | 40942 | 37198 | 5.72 | 4.79 | 8+6 | 543 | 30 | 11S globulin-like protein | Q8W1C2 |
| 3104 | 0.19 | TC52065 | 34951 | 26000 | 10.26 | 5.16 | 10+7 | 515 | 39 | 11S globulin isoform 3 | Q8W1C2 |
| 2604 | 0.23 | TC52065 | 34951 | 66091 | 10.26 | 5.04 | 5+4 | 212 | 26 | 11S globulin isoform 3 | Q8W1C2 |
| 6007 | 0.25 | TC51818 | 40942 | 15740 | 5.72 | 5.68 | 8+3 | 237 | 21 | 11S globulin-like protein | Q8W1C2 |
| 6009 | 0.26 | TC51818 | 40942 | 15204 | 5.72 | 5.68 | 5+4 | 228 | 13 | 11S globulin-like protein | Q8W1C2 |
| 2223 | 0.33 (5) | TC52065 | 34951 | 28973 | 10.26 | 5.15 | 5+4 | 291 | 15 | 11S globulin isoform 3 | Q8W1C2 |
| 1313 | 0.57 | TC52065 | 34951 | 36959 | 10.26 | 4.82 | 3+3 | 137 | 17 | 11S globulin isoform 3 | Q8W1C2 |
| 1315 | 0.65 | TC52065 | 34951 | 35753 | 10.26 | 4.86 | 11+7 | 579 | 43 | 11S globulin isoform 3 | Q8W1C2 |
| 1323 | 2.37 | TC52065 | 28618 | 36799 | 4.98 | 4.96 | 11+7 | 703 | 48 | 11S globulin isoform 3 | Q8W1C2 |
| 1324 | 5.54 | TC52065 | 28618 | 36066 | 4.98 | 4.91 | 11+4 | 489 | 44 | 11S globulin isoform 3 | Q8W1C2 |
| 2314 | 5.86 | TC51818 | 40942 | 36651 | 5.72 | 5.05 | 8+6 | 602 | 23 | 11S globulin-like protein | |
| Other globulins | |||||||||||
| 1103 | 0.10 | CB34791231578 | 24720 | 8.69 | 4.72 | 7+5 | 346 | 33 | 11S globulin-like protein | Q8W1C2 | |
| 1114 | 0.17 | CB34791231578 | 24330 | 8.69 | 4.77 | 11+5 | 319 | 42 | 11S globulin-like protein | Q8W1C2 | |
| 2211 | 0.24 | TC58877 | 43842 | 27202 | 5.42 | 5.10 | 5+2 | 96.2 | 9 | 11S globulin seed storage protein | Q38712 |
| Other seed storage proteins | |||||||||||
| 8111 | 0.12 | TC52776 | 48518 | 25484 | 6.74 | 6.05 | 10+6 | 355 | 18 | PreproMP27-MP32 | Q39651 |
| 4003 | 0.13 | TC54826 | 18424 | 19150 | 6.38 | 5.34 | 7+5 | 278 | 27 | Seed maturation protein PM31 | Q9XET1 |
| 2001 | 0.20 (5.4) | TC65957 | 22331 | 135176 9.03 | 5.05 | 1+2 | 129 | 17 | 2S albumin | Q84NG9 | |
| 2201 | 0.21 | TC52776 | 48518 | 26798 | 6.74 | 4.97 | 6+4 | 139 | 14 | PreproMP27-MP32 | Q39651 |
| 6103 | 0.23 | TC52776 | 48518 | 25173 | 6.74 | 5.72 | 5+3 | 224 | 15 | PreproMP27-MP32 | Q39651 |
| 8204 | 0.38 | TC51747 | 35463 | 27799 | 8.74 | 6.04 | 28+5 | 558 | 31 | 48-kDa glycoprotein | Q8S4P9 |
| 8205 | 0.52 | TC51747 | 35463 | 28949 | 8.74 | 6.04 | 15+6 | 551 | 15 | 48-kDa glycoprotein | Q8S4P9 |
| 7213 | 0.53 (4.5) | TC51747 | 23770 | 27758 | 6.53 | 5.94 | 17+6 | 392 | 21 | 48-kDa glycoprotein | Q8S4P9 |
| 8101 | 1.02 | TC52776 | 48518 | 24053 | 6.74 | 6.04 | 11+4 | 310 | 22 | PreproMP27-MP32 | Q39651 |
| Carbohydrate metabolism | |||||||||||
| 7401 | 0.12 | TC63671 | 13988 | 39394 | 8.85 | 5.86 | 3+2 | 125 | 20 | Steroleosin-B | Q8LKV5 |
| 7603 | 0.12 | TC62850 | 49786 | 73376 | 5.54 | 5.85 | 11+4 | 130 | 17 | Glucose-6-phosphate 1-dehydrogenase, cyt | P37830 |
| 8415 | 0.14 | TC63091 | 41054 | 43306 | 6.2 | 6.04 | 20+7 | 645 | 38 | Alcohol dehydrogenase 1 | Q43690 |
| 7416 | 0.16 | TC69306 | 43715 | 43030 | 6.56 | 5.85 | 7+4 | 223 | 17 | Alcohol dehydrogenase 2 | Q9FZ01 |
| 4624 | 0.17 (9.7) | TC53291 | 54243 | 64648 | 5.56 | 5.47 | 14+7 | 578 | 41 | Galactokinase | Q9SEE5 |
| 5506 | 0.18 (13) | TC60581 | 49790 | 56351 | 5.8 | 5.64 | 24+7 | 650 | 48 | Enolase 1 (2-phosphoglycerate dehydratase) | Q9LEJ0 |
| 7407 | 0.27 | TC52072 | 42422 | 42803 | 6.29 | 5.92 | 19+7 | 553 | 40 | Phosphoglycerate kinase, cytosolic | Q42962 |
| Other metabolism | |||||||||||
| 5404 | 0.10 | TC61960 | 39231 | 42269 | 5.94 | 5.56 | 11+5 | 340 | 30 | AX110P-like protein | Q9SZ83 |
| 4309 | 0.11 | TC51797 | 33806 | 36825 | 5.39 | 5.32 | 8+7 | 360 | 21 | Allergenic isoflavone reductase | Q9FUW6 |
| 5513 | 0.13 | TC66898 | 56548 | 56616 | 7.46 | 5.59 | 18+7 | 445 | 28 | Succinate-semialdehyde dehydrogenase | Q2T3E1 |
| 9003 | 0.35 | TC59490 | 11945 | 12270 | 8.46 | 6.23 | 4+2 | 137 | 20 | Pru p 1 | Q9LED1 |
| Energy | |||||||||||
| 1209 | 0.10 | TC56895 | 29382 | 29127 | 5.4 | 4.86 | 8+7 | 333 | 35 | Chlorophyll A/B binding protein | Q32291 |
| 2609 | 0.10 (2.6) | TC63054 | 63793 | 60032 | 6.52 | 5.16 | 11+4 | 408 | 35 | ATP synthase beta chain, mitochondrial | P17614 |
| 3302 | 0.17 | TC54765 | 35289 | 32396 | 6.08 | 5.13 | 11+8 | 509 | 35 | Oxygen evolving enhancer protein 1 | Q9LRC4 |
| Protein fate | |||||||||||
| 4601 | 0.12 | TC69987 | 61344 | 69410 | 5.85 | 5.27 | 18+3 | 136 | 51 | Chaperonin CPN60-2, mitochondrial | Q05046 |
| 4416 | 0.13 (2.6) | TC56234 | 41388 | 40990 | 5.47 | 5.54 | 7+3 | 224 | 22 | Protein disulfide isomerase | Q6I685 |
| 3509 | 0.14 | TC55000 | 41492 | 551881 | 5.1 | 5.13 | 10+3 | 139 | 23 | Mitochondrial processing peptidase alpha subunit | P29677 |
| 1321 | 0.16 | TC60801/TC57433 | 55662/29344 | 33888 | 4.99/4.68 | 4.73 | 9+5/8+3 | 291/174 | 17/22 | Aspartic proteinase 3/ 14-3-3 | Q9FRW7/P46266 |
| 1303 | 0.29 | TC60801 | 55662 | 33313 | 4.99 | 4.74 | 8+6 | 456 | 16 | Aspartic proteinase 3 | Q9FRW7 |
| Biotic and Abiotic Stress | |||||||||||
| 6209 | 0.10 | TC52173 | 56548 | 27267 | 7.46 | 5.60 | 15+7 | 348 | 28 | Glutathione-S-transferase | O49235 |
| 6210 | 0.10 | TC51718 | 27557 | 30390 | 5.71 | 5.68 | 12+2 | 132 | 26 | Cytosolic ascorbate peroxidase | Q41772 |
| 1205 | 0.11 | TC60929 | 27270 | 30162 | 5.38 | 4.78 | 3+2 | 234 | 8 | Class IV chitinase | Q7XAU6 |
| 1403 | 0.11 | TC54145 | 34679 | 41372 | 4.66 | 4.72 | 14+6 | 394 | 37 | Salt tolerance protein | Q5PXN9 |
| 2713 | 0.11 (3) | TC70328 | 71342 | 77942 | 5.14 | 5.12 | 4+3 | 172 | 11 | Hsc70 protein, | Q40151 |
| 3707 | 0.11 | TC53154 | 48727 | 774092 | 5.02 | 5.18 | 12+4 | 94 | 14 | Heat shock protein 70 | Q40693 |
| 7106 | 0.15 | TC51764 | 25284 | 25328 | 6.8 | 5.86 | 5+3 | 90.1 | 12 | Superoxide dismutase [Mn], mitochondrial | P11796 |
| 3701 | 0.18 | TC53231 | 71171 | 75498 | 5.17 | 5.14 | 28+6 | 633 | 34 | Heat shock cognate protein 70 | Q8GSN4 |
| 6202 | 0.24 (3.8) | TC51718 | 27557 | 29355 | 5.71 | 5.72 | 3+2 | 112 | 11 | Cytosolic ascorbate peroxidase | Q41772 |
| 7103 | 0.34 | TC51764 | 28111 | 25065 | 6.6 | 5.89 | 15+6 | 420 | 30 | Superoxide dismutase [Mn], mitochondrial | P11796 |
| 8103 | 0.34 | TC67773 | 27081 | 22470 | 9.23 | 6.10 | 11+3 | 213 | 24 | P-lip hydroperoxide glutathione peroxidase | O48646 |
| Other proteins | |||||||||||
| 306 | 0.11 | TC60052 | 26349 | 33136 | 4.77 | 4.67 | 12+6 | 582 | 52 | Late embryogenesis abundant protein D-34 | P09444 |
| 4507 | 0.12 | TC56794 | 36730 | 50787 | 9.88 | 5.40 | 8+3 | 269 | 23 | Late embryogenesis abundant protein | CAB86908 |
| 5509 | 0.12 | TC56794 | 36730 | 50215 | 9.88 | 5.54 | 2+1 | 93 | 9 | Late embryogenesis abundant protein | CAB86908 |
| 1309 | 0.14 | TC55192/TC60801 | 28739/55662 | 33228 | 4.79/4.99 | 4.86 | 13+6/7+2 | 244/102 | 27/16 | 14-3-3 protein 7/Aspartic proteinase 3 | P93212/Q9FRW7 |
| 3504 | 0.29 | TC60835 | 41726 | 44013 | 5.31 | 5.23 | 19+6 | 499 | 27 | Actin | Q8H6A3 |
| Unknown proteins | |||||||||||
| 8112 | 0.10 | CB978962 31781 | 22379 | 7.9 | 6 | 8+6 | 362 | 30 | Unknown protein | 10178125 | |
| 4414 | 0.11 | TC54532 | 40334 | 43120 | 5.77 | 5.33 | 14+7 | 399 | 25 | Unknown | CAB02653 |
| 7301 | 0.13 | TC57576 | 21011 | 31315 | 5.24 | 5.84 | 5+2 | 60.8 | 12 | Unknown | Q941A4 |
| 6206 | 0.56 (7.6) | TC57394 | 26532 | 30143 | 5.94 | 5.76 | 4+1 | 107 | 15 | Embryo-specific protein | Q9ZNS9 |
| Water-status regulated protein WW/DS | |||||||||||
| 7203 | (4.17) | TC58896 | 26032 | 28706 | 6.24 | 5.88 | 8+3 | 123 | 29 | Vacuolar H+-ATPase, subunit E | Q8SA35 |
| Unidentified proteins | |||||||||||
| SSP | Diff abun | Exp Mr | Exp Pi | SSP | Diff abun | Exp Mr | Exp Pi | SSP | Diff abun | Exp Mr | Exp Pi |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 35 | 0.10 (9.9) | 15007 | 4.65 | 132 | 0.20 | 24876 | 4.67 | 210 | 0.10 | 28391 | 4.69 |
| 1115 | 0.10 | 25561 | 4.77 | 1310 | 0.14 | 32449 | 4.82 | 2004 | 0.18 | 21266 | 5.11 |
| 2015 | 0.10 (8.7) | 14939 | 5.11 | 2202 | 0.15 | 28412 | 4.99 | 2209 | 0.26 (5.9) | 31507 | 5.16 |
| 2215 | 0.14 (5.9) | 30182 | 5.18 | 2315 | 8.11 | 36997 | 5 | 2709 | 0.15 | 85731 | 5.04 |
| 3101 | 0.23 (4.7) | 25174 | 5.26 | 3613 | 0.17 | 67407 | 5.14 | 3719 | 0.38 | 75761 | 5.24 |
| 4208 | 0.10 | 28045 | 5.35 | 4402 | 0.14 | 42494 | 5.26 | 4505 | 0.15 | 46339 | 5.38 |
| 5003 | 0.10 | 16352 | 5.44 | 5101 | 0.28 | 24614 | 5.41 | 5203 | 0.10 | 30143 | 5.42 |
| 5207 | 0.34 | 27279 | 5.56 | 5304 | 0.11 | 33362 | 5.56 | 6001 | 0.20 | 19465 | 5.60 |
| 6011 | 0.17 | 13507 | 5.74 | 6108 | 0.33 (2.4) | 24272 | 5.71 | 7202 | 0.49 (3.6) | 31176 | 5.87 |
| 7212 | 0.12 (2.3) | 33452 | 5.93 | 8001 | 0.46 | 17691 | 5.93 | 8002 | 0.23 | 17713 | 6.06 |
| 8008 | 0.28 | 17738 | 6.18 | 8302 | 0.19 | 37726 | 6 | 8416 | 0.12 | 43382 | 6.07 |
3.3 Identification of differentially expressed proteins among tissues
Protein spots that displayed differential abundance after ANOVA (p<0.05) and a two-fold or greater ratio threshold filtering were eluted from representative 2-D gels, digested with trypsin and analyzed by MALDI TOF/TOF tandem mass spectrometry. Within pericarp tissues comparison, 47 of 54 protein spots that were more abundant in skin and 18 of 36 protein spots more abundant in pulp were identified (Table 3). Proteins expressed in the skin with functions related to phenylpropanoid and amino acid biosynthesis, light and dark reactions of photosynthesis, biotic stress responses (e.g., pathogenesis-related (PR) proteins) and heat shock proteins, were much more abundant relative to pulp proteins. In contrast, pulp tissues showed high abundance of proteins with functions in reactive oxygen scavenging (ROS), ripening-related proteins (e.g., grip22), abiotic stress response (e.g., stress-induced proteins and dehydrins), and several unknown or hypothetical proteins (Table 3).
In seeds, a majority of spots (130/163 or 80%) spots analyzed as being abundant (>0.1% of the total spot intensity) and two-fold or greater more abundant than in pericarp tissues could be assigned functions based on MS data (Table 4). Of those proteins assigned a function, a majority (94/130 or 72%) was identified as globular or other seed storage proteins, seed maturation or late embryogenesis abundant proteins. Other classes of proteins included those with functions in carbohydrate or energy metabolism, protein fate, and biotic or abiotic stress responses (Table 4).
3.4 Comparative 2D-PAGE analyses of berry proteins in response to water-deficit stress
Water deficit irrigation treatment is known to alter the composition of grapevine berries [46] and reduce leaf area and photosynthesis within the canopy [47]. Water deficit stress can also have profound effects on the mRNA abundance within different berry tissues [30]. In order to determine if water deficit causes corresponding changes in protein composition within the berry, we analyzed the protein profiles of berry tissues collected from grapevines that were well watered or water-deficit stressed. Spots that displayed differential abundance after ANOVA (p<0.05) and a two-fold ratio or greater difference were identified and then curated manually to reduce false positives. Analysis of skin proteins revealed 31 spots that showed significantly different abundance upon water stress treatment (14 in well-watered; 17 in water-deficit treated). After manual curation, 18 spots were identified: 9 were more abundant in the well-watered berries (Fig. 4A and Table 5) and 9 were more abundant in water-deficit-stressed berries (Fig. 4B and Table 5). Analysis of pulp tissue identified 28 spots with significantly different abundance upon water stress treatment (18 in well-watered; 10 in water-deficit treated). After manual curation, 12 spots were identified: 7 were more abundant in the well-watered berries (Fig. 4C and Table 6) and 5 were more abundant in water-deficit-stressed berries (Fig. 4D and Table 6). Analysis of seed proteins revealed only 6 spots that showed significantly different abundance in the seed upon water stress treatment (5 in well-watered; 1 in water-deficit treated). After manual curation, only 1 spot (SSP:7203, TC58896), which encoded a vacuolar H+-ATPase subunit E, was identified that was more abundant in the well-watered berries (Fig. 3 inset, Table 4).
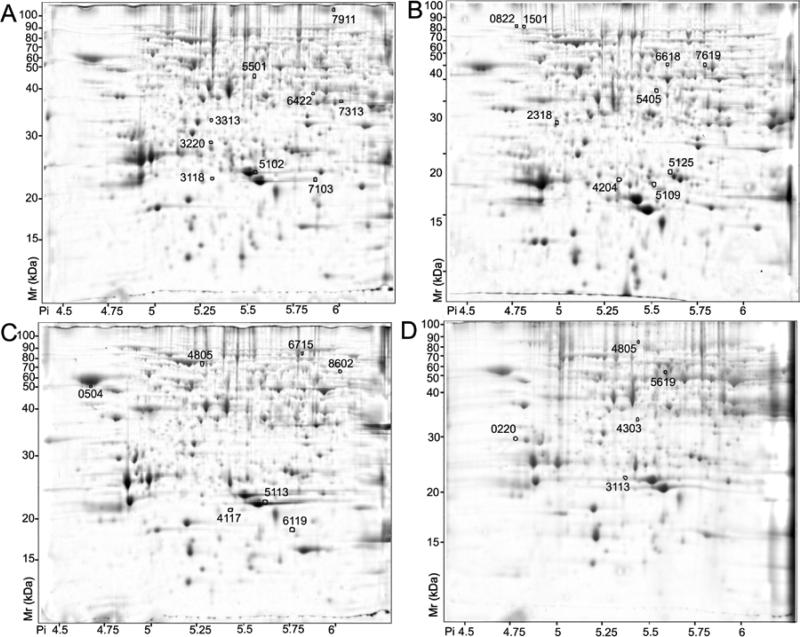
Figure 4.
Spots whose abundance differed significantly (p < 0.05; two-fold or greater change) with water status. A) Pulp proteins more abundant under well-watered conditions. B) Pulp proteins more abundant under water-deficit conditions. C) Skin proteins more abundant under well watered conditions. D) Skin proteins more abundant under water-deficit conditions are indicated by circles and standard spot numbers on a representative gel. See Tables 5 and 6 for detailed listing of pulp and skin proteins, respectively.
Table 5.
Proteins whose abundance was significantly different between well watered and water-deficit in skin. SSP, standard spot number; WW/WD, normalized spot volume in the well-watered skin divided by the normalized spot volume in the water-deficit skin, from 12 different plants; Pval, P value; VvGI5, match from the translated Vitis vinifera gene index Release 5; ThMr, theoretical molecular mass; Exp Mr, experimental molecular mass; ThPi, theoretical isoelectric point (Pi); Exp Pi, experimental isoelectric point (Pi); Pep, number of peptides mass and number of MS/MS ions matching the query; Mowse score; % Cov, percentage of coverage; Annotation, description of protein identity; Uniprot, Uniprot ID of the most closely related Unigene from VvGI5.
| SSP | WW/WD | Pval | Vvgi5 | Th Mr | Exp Mr | Th Pi | Exp Pi | Pep | Mows score | % cov | Annotation | Uniprot |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2318 | 0.11 | .037 | TC51818 | 40942 | 35857 | 5.72 | 5.07 | 11+5 | 263 | 34 | 11S globulin-like protein | Q8W1C2 .000 |
| 822 | 0.25 | .042 | TC60724 | 39345 | 93831 | 9.14 | 4.85 | 22+4 | 143 | 26 | Patellin 1 | Q2Q0V7 |
| 4204 | 0.29 | .040 | TC54618 | 25584 | 26482 | 5.51 | 5.44 | 14+4 | 179 | 34 | Proteasome subunit alpha type 2-B | Q8L4A7 |
| 5405 | 0.29 | .016 | TC42731 | 15662 | 43635 | 5.48 | 5.63 | 4+3 | 66 | 23 | GDP-mannose pyrophosphorylase | Q8W4J5 |
| 5215 | 0.32 | .005 | TC51718 | 27557 | 27714 | 5.71 | 5.71 | 18+6 | 447 | 46 | Cytosolic ascorbate peroxidase | Q41772 |
| 6618 | 0.34 | .018 | TC65305 | 54992 | 61368 | 5.80 | 5.69 | 18+3 | 238 | 45 | Neutral leucine aminopeptidase | Q8GZD8 |
| 1801 | 0.42 | .003 | TC60724 | 67754 | 92542 | 4.72 | 4.89 | 14+7 | 553 | 18 | Patellin 1 | Q2Q0V7 |
| 7619 | 0.44 | .008 | TC54806 | 58481 | 59979 | 6.42 | 5.89 | 33+5 | 372 | 52 | Mitochondrial processing peptidase beta sb | Q9AXQ2 |
| 5109 | 0.45 | .032 | TC68818 | 29626 | 25805 | 6.71 | 5.62 | 7+6 | 322 | 21 | 20S proteasome beta subunit PBB2 | O81152 |
| 7313 | 2.07 | .024 | TC57431 | 35488 | 37683 | 6.18 | 6.03 | 22+3 | 182 | 53 | Cytosolic malate dehydrogenase | Q9FT00 |
| 3220 | 2.36 | .049 | TC55034 | 25124 | 28931 | 5.26 | 5.31 | 11+7 | 561 | 36 | Chalcone isomerase | P51117 |
| 5102 | 2.57 | .041 | TC51878 | 27794 | 23592 | 8.33 | 5.56 | 13+6 | 386 | 36 | Oxygen-evolving enhancer 2 chloroplast | Q9SLQ8 |
| 5501 | 3.10 | .033 | TC69652 | 40193 | 44173 | 5.63 | 5.55 | 20+1 | 62 | 57 | Leucoanthocyanidindioxgenase | P51093 |
| 3313 | 7.36 | .008 | TC45122 | 29869 | 33325 | 5.15 | 5.23 | 4+3 | 130 | 13 | Cyclase | Q2I313 |
| Unidentified proteins | ||||||||||||
| SSP | WW/DS | Pval | Exp Mr | Exp Pi | SSP | WW/DS | Pval | Exp Mr | Exp Pi | SSP | WW/DS | Pval | Exp Mr | Exp Pi |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3118 | 4.990 | .024 | 22455 | 5.32 | 6422 | 2.392 | .041 | 39246 | 5.88 | 7103 | 3.246 | .020 | 22627 | 5.90 |
| 7911 | 3.996 | .003 | 111063 | 5.99 |
Table 6.
Proteins whose abundance was significantly different between well watered and water-deficit in pulp. SSP, standard spot number; WW/WD, normalized spot volume in the well-watered pulp divided by the normalized spot volume in the water-deficit pulp, from 12 different plants; Pval, P value; VvGI5, match from the translated Vitis vinifera gene index Release 5; ThMr, theoretical molecular mass; Exp Mr, experimental molecular mass; ThPi, theoretical isoelectric point (Pi); Exp Pi, experimental isoelectric point (Pi); Pep, number of peptides mass and number of MS/MS ions matching the query; Mowse score; % Cov, percentage of coverage; Annotation, description of protein identity; Uniprot, Uniprot ID of the most closely related Unigene from VvGI5.
| SSP | WW/WD | Pval | VVGI5C | ThMr | Exp Mr | ThPi | Exp Pi | Pep | Mowse score | % cov | Annotation | Uniprot |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4303 | 0.26 | .017 | TC55139/TC51797 | 33809 | 35211 | 5.39 | 5.43 | 9+3 / 7+3 | 139 /131 | 22 /20 | Glyoxalase I, partial (92%) / Allergenic isoflavone reductase Bet v6.0102 | Q9ZWJ2/Q9FUW6 |
| 5619 | 0.29 | .038 | TC54345 | 57126 | 64508 | 5.51 | 5.72 | 22+5 | 168 | 32 | Glutamate decarboxylase | P54767 |
| 220 | 0.42 | .027 | TC57433 | 29344 | 29904 | 4.68 | 4.77 | 15+4 | 331 | 39 | 14-3-3 protein | P46266 |
| 3113 | 0.43 | .048 | TC68794 | 27224 | 24074 | 5.38 | 5.37 | 2+2 | 244 | 11 | Class IV endochitinase | Q7XAU6 |
| 8602 | 2.26 | .004 | TC57645 | 54492 | 60444 | 7.04 | 6.05 | 36+7 | 549 | 59 | UDP-glucose pyrophosphorylase | Q8W557 |
| 6119 | 2.27 | .025 | TC63472 | 18147 | 18729 | 6.17 | 5.81 | 10+4 | 146 | 20 | 18.6 kDa heat-shock protein | Q39929 |
| 504 | 2.89 | .016 | TC57144 | 42853 | 40976 | 4.71 | 4.68 | 6+1 | 103 | 23 | Latex abundant protein | Q6XNP6 |
| 3613 | 2.93 | .023 | TC56912 | 30947 | 65458 | 5.92 | 5.35 | 17+3 / 16+3 | 174 | 42 / 66 | Vacuolar H+-ATPase A / UDP-glucose pyrophosphorylase | Q9MB47/17026394 |
| 6715 | 4.51 | .016 | TC52137 | 84537 | 85179 | 6.09 | 5.86 | 7+3 | 144 | 15 | Methionine synthase | Q42662 |
| Unidentified proteins | ||||||||||||
| SSP | WW/WD | Exp Mr | Exp Pi | SSP | WW/DS | Exp Mr | Exp Pi | SSP | WW/DS | Exp Mr | Exp Pi | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4117 | 3.37 | .023 | 21273 | 5.46 | 4805 | 0.30 | .046 | 88278 | 5.46 | 5113 | 2.9 | .035 | 22663 | 5.65 |
3.5 Identification of differentially expressed proteins in response to water-deficit stress
Protein spots that displayed differential abundance after ANOVA (p<0.05) and a two-fold or greater ratio threshold filtering were eluted and analyzed as described above. Within skin tissue, 9 of 9 protein spots that were more abundant under water deficit stress and 5 of 9 protein spots more abundant under well-watered conditions were identified successfully (Table 5). Within pulp tissue, 4 of 5 protein spots that were more abundant under water deficit stress and 5 of 7 protein spots more abundant under well-watered conditions were identified successfully (Table 6). The constellation of water-deficit stress-induced proteins was completely distinct for each of these two tissues. However, the skin displayed a notable increase in the relative abundance of proteosome subunits and peptidases and the ROS scavenging enzyme, cytosolic ascorbate peroxidase, whereas the pulp showed increases in isoflavone reductase, glutamate decarboxylase and an endochitinase. In contrast, water-deficit had little effect on the expression of seed proteins.
3.6 Correlation of mRNA and protein expression patterns
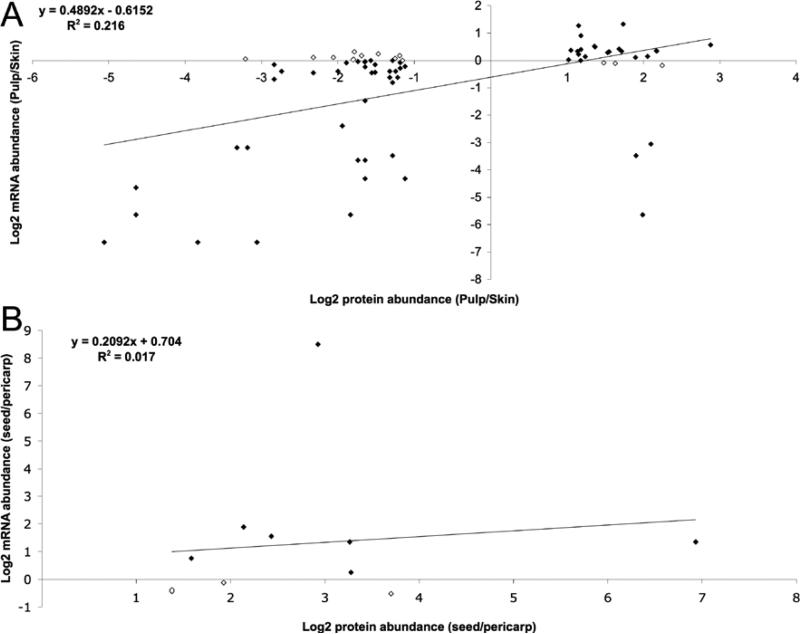
Protein abundance changes within tissues and in response to water-deficit stress were compared with mRNA changes within the same berry tissues [30]. MALDI TOF/TOF MS/MS identification results were analyzed carefully in order to match accurately a protein identity with a specific member of a gene family within a multigene family. From the 65 identified proteins in the skin/pulp comparison, the possibility of matching to more than one mRNA could not be ruled out for four proteins spots. For eight proteins, comparison with mRNA could not be performed because corresponding probe sets for mRNA expression data were not present on the GeneChip® Vitis Genome Array (Affymetrix®) (see Additional Table 1). For identified proteins exhibiting tissue-specific expression patterns, 47 of 57 (82%) had a corresponding mRNAs expression pattern that was preferentially expressed in the same tissue as their protein counterpart. These data indicate a rather good qualitative correlation between mRNA and protein expression patterns in general. However, only 18 of 57 (32%) of these proteins exhibited a corresponding two-fold or greater ratio of differential expression at both the mRNA and protein levels. The Pearson correlation coefficient between quantitative protein and mRNA abundance log2 difference ratios for the entire pericarp data set was 0.216 (Fig. 5A). The Pearson correlation coefficient between protein and mRNA abundance log2 difference ratios for the seed vs. pericarp was only 0.017 (Fig. 5B). These data indicate a poor overall correlation between mRNA and protein expression patterns for genes expressed preferentially within a specific tissue.
Figure 5.
Correlation of protein and mRNA abundance in different berry tissues. Values are expressed as log2 ratio of abundance: A) in pulp vs. skin B) in seed vs. pericarp. Solid diamonds represent individual gene products following similar trends for both mRNA and protein expression.
In the water status comparisons of skin and pulp tissues, 2 of the 23 identified spots did not have a mRNA counterpart on the microarray chips, and 11/21 (52%) of proteins showed agreement with the general trend of mRNA expression in response to water-deficit stress treatment (see Additional Tables 2 and 3). However, none of the transcripts displayed a two-fold or greater ratio difference in expression.
In the seed/pericarp comparison, of the 132 proteins identified, corresponding mRNA expression data were available for 77 proteins. Of these, 62 of 77 (81%) mRNA were expressed preferentially in the seed and a majority, 53 of 77 (69%) presented a twofold or greater ratio difference in both protein and mRNA (see Additional Table 4).
3.7 Metabolite analysis
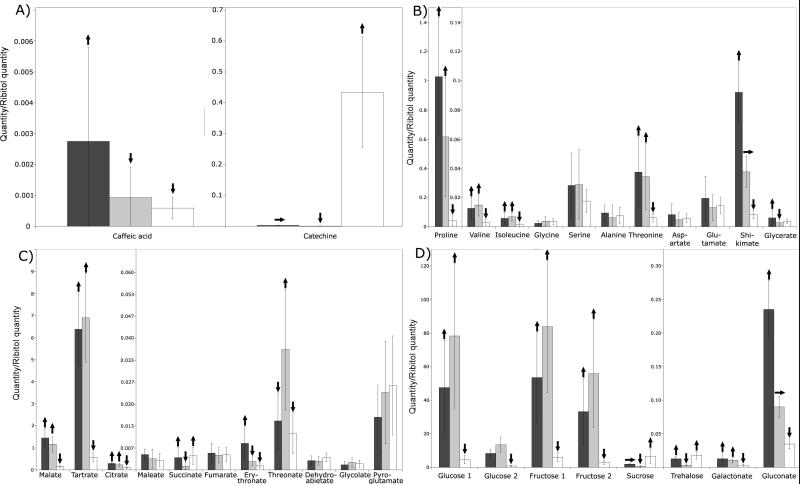
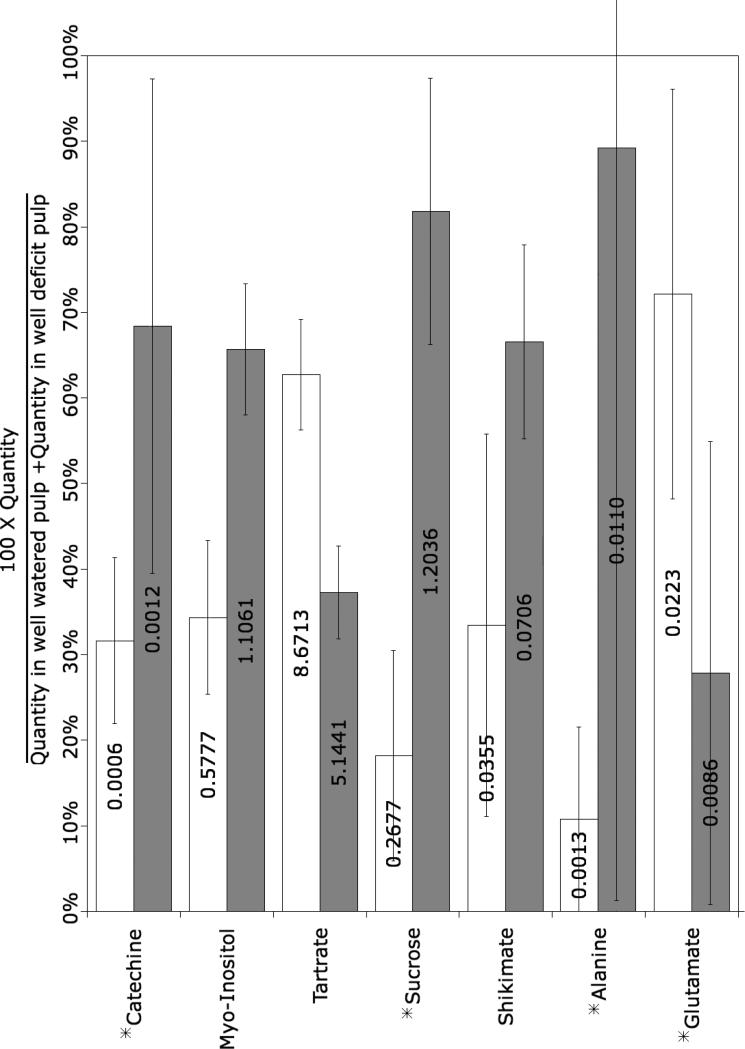
In order to explore possible relationships between protein abundance and metabolite accumulation in different berry tissue, the relative abundance of polar metabolites was analyzed by GC-MS. Large differences in the relative abundance of selected metabolites were found among the different tissues. For example, 6/13 amino acids, 6/13 organic acids and 6/6 sugars analyzed shown significant differences in abundance among skin, pulp and seed tissues (Fig. 6). The effect of water deficit on the accumulation of selected metabolites was also determined. Catechin, sucrose and alanine were more abundant in the pulp of berries from water-deficit treated vines, whereas glutamate and tartrate were more abundant in the pulp of berries from well-watered vines (Figure 7).
Figure 6.
Identification and quantitative differences of selected metabolites within different tissues: A) phenylpropanoids, B) amino acids, C) organics acids, and D) sugars. Skin (black bars), pulp (gray bars), seed (white bars). Up arrows indicate that the metabolite was significantly (ANOVA p<0.005, two-fold change or greater) more abundant than in tissues with a horizontal or arrow down. Horizontal arrows indicate that the metabolite was significantly (p<0.005, two-fold change or greater) less abundant than in tissues with an up arrow and more abundant than in tissues with a down arrow. Down arrows indicate that the metabolite was significantly (p<0.005, two-fold ratio change or greater) less abundant than in tissues with a horizontal or up arrow. Error bars represent standard deviation of the mean, n = 5.
Figure 7.
Metabolites that differed significantly (ANOVA p< 0.05) between pulp derived from well watered and water-deficit treated vines. Asterisk (*) indicates metabolites that exhibited a two-fold or greater change in abundance. Error bars represent standard deviation of the mean, n = 5. Values within bars are the ratio relative to the internal standard (ribitol).
4 Discussion
We have performed the first survey of tissue-specific protein expression patterns within pericarp tissues (skin and pulp) and seed tissues of grape berries from grapevines subjected to well watered and water-deficit stress conditions. Although previous studies have reported proteomic analysis of grape berry skin proteins [18, 20] or whole berries at various times during berry development [19], none have investigated tissue-specific protein expression patterns. Our survey of a total of 1,047 spots (average of 854) from pericarp tissues (skin and pulp) revealed that while most proteins showed a relatively constant expression between skin and pulp, 90 (8.6%) spots exhibited a two-fold ratio or greater difference in tissue expression (Fig. 1 and 2 and Table 3). Furthermore, our survey of a total of 695 spots (average of 605) from seed tissues indicated that seeds presented a proteome that was completely distinct from that of the pericarp, mainly due to high concentrations of seed storage proteins (Fig. 3 and Table 4). A comparison of our results with recent reports of skin-specific [18, 20] or pericarp [19] proteomic analyses of wine grape berries indicated generally strong agreement skin-specific protein expression patterns with only a few exceptions (e.g., isoflavone reductase and methionine synthase) (Additional Table 6). In most cases, tissue-specific enzyme localization was in general agreement with substrate localization as might be expected.
4.1 Phenylpropanoid pathway
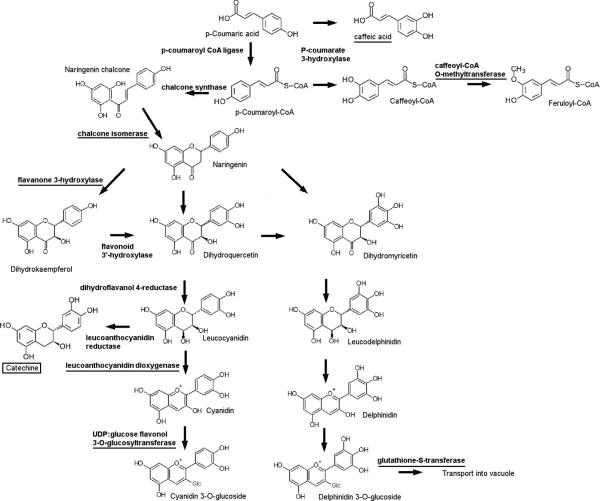
All identified proteins with functions in the phenylpropanoid pathway (Table 3) showed expression specific to the skin consistent with their mRNA expression profiles [30, 48]. Phenolic compounds are major wine constituents responsible for organoleptic properties such as color and astringency. Moreover, a majority of phenolic compounds in wine are derived from flavonoids (e.g., tannins, anthocyanins). For red grapes, roughly 30-40% of the total phenolic content is located in the skins and 60-70% in the seeds [49]. All of the identified spots in this functional group, with the exception of caffeoyl-CoA O-methyltransferase (SSP:5206, TC70299), correspond to enzymes with functions in the latter steps of anthocyanin biosynthesis (Figure 8). This caffeoyl-CoA O-methyltransferase is likely to be involved in anthocyanin methylation as a closely related methyltransferase from Mesembryanthemum crystallinum was specific for flavonoids in addition to caffeoyl-CoA [50]. The corresponding mRNA for this enzyme is also preferentially expressed in the skin [30] or pericarp of red wine grape varieties [48]. The expression of chalcone isomerase (SSP:3210, TC55034), which is a key enzyme of the anthocyanin biosynthetic pathway, was also mainly in the skin, consistent with its mRNA expression pattern [30]. Similarly, the anthocyanin biosynthetic enzymes, flavonone-3-hydroxylase (SSP:4405, TC70298) and leucoanthocyanidin dioxygenase (SSP:5411; TC69652), exhibited skin-specific mRNA and protein expression patterns (Table 3). Both of these enzymes showed increased protein abundance following véraison in cv. Cabernet Sauvignon [18]. UDP-glucose:flavonol-3-O-glucosyltransferasae (UFGT, SSP:7411, AF000371) mRNA was also expressed specifically in the skin [51]. This enzyme plays a critical role in anthocyanin accumulation as retrotransposon-induced mutation of the MYB transcription factor (VvmybA1) gene [52] leading to its expression impairs anthocyanin accumulation in red grape varieties [53] with this mutation being common to many white grape varieties [5]. The enzymatic activity of recombinant UFGT has been documented in vitro [54]. Two isoforms of UFGT were found to accumulate preferentially following véraision in cv. Cabernet Sauvignon [18]. Two isozymes of glutathione-S-transferase (GST) (SSP:3103, TC58036 and SSP:6101, TC69505), which also showed skin-specific mRNA and protein expression, play a critical role in the final step of anthocyanin accumulation by conjugating glutathione to cyanidin glucoside, which is then transported into the vacuole [55]. mRNA encoding GST was more abundant in red versus white grape skin [48]. The 5’ flanking promoter regions of both UFGT and GST genes share MYB cis-element binding domain sites [56], indicating that the relative mRNA and protein expression of both genes may be coordinately regulated. Lastly, an isoflavone reductase (SSP:8302, TC52848), which is involved in isoflavonoid phytoalexin biosynthesis [57], was more abundantly expressed in the pulp than in the skin, which is also in accordance to its mRNA profile [30]. Overall, most flavonoid biosynthetic enzymes identified were found to be localized to the skin (Figure 8), consistent with known expression patterns and previous proteomic analysis of berry skins (Additional Table 6).
Figure 8. Enzymes and metabolites differentially expressed across tissues within a simplified flavonoid biosynthetic pathway.
Underlined names indicate the enzymes or metabolites more abundant in skin; Boxed name indicates the metabolite more abundant in the seed.
Metabolite analyses revealed tissue-specific differences in the relative abundance of selected compounds. For example, caffeic acid exhibited specific expression in the skin (Figure 6A). This precursor metabolite is a possible substrate of caffeoyl-CoA O-methyltransferase (SSP:5206), whose mRNA [30] and protein (Table 3) are also expressed preferentially in the skin. Alternatively, this enzyme may be specific for flavonoid as mentioned above. Catechine, a flavonoid, was significantly more abundant in seed (Figure 6A) and in water-deficit stressed berries (Figure 7), consistent with the mRNA expression pattern of leucoanthocyanin reductase in these tissues [30], however, this biosynthetic enzyme was not identified in this study. The tissue-specific expression patterns of key phenylpropanoid enzymes established here will be critical to rational manipulation of the expression of these enzymes in the future.
4.2 Amino acid metabolism
Nitrogen is mainly incorporated into the berry through glutamine synthetase/glutamate synthase. Two isoforms of glutamine synthetase (SSP:3305, TC60125 and SSP:5303, 64127) were identified as homologues of cytosolic glutamine synthetase, which catalyze glutamine synthesis using glutamate, a key nitrogen reserve in plants. Cytosolic isoforms of this enzyme have been detected in vascular tissues of grape berry pulp [58] and in berry skins where the protein was found to be 3.5 times more abundant prevéraison than postvéraison [18]. This phloem-specific expression pattern indicates that the cytosolic glutamine synthetase isoenzyme functions to generate glutamine for intercellular nitrogen transport [59]. The higher abundance of both enzymes in the skin (Table 3) probably reflects the higher percentage of vascular tissues within berry skin. Glutamate also appeared to be slightly more abundant in the skin (Fig. 6), although this difference was not significant and significantly less abundant in pulp following water-deficit stress (Figure 7).
Homocysteine S-methyltransferase (SSP:4318; TC55907), which catalyzes the last step of methionine biosynthesis, was highly abundant in the pulp. This pattern of enzyme expression would predict that methionine content would be higher in the pulp than in the skin, although no direct metabolite data are available to confirm this prediction. Methionine is metabolized during malolactic fermentation into various sulfur compounds [60]. Methionine content is well correlated with the abundance of the volatile compound methionol (3-(methylthio)-propanol), which has a raw-potato odor, in fermented must [61]. In contrast, S-adenosylmethionine (SAM) synthetase (SSP:4413, TC51748), which catalyzes the first step of methionine degradation, was highly abundant in the skin. SAM synthetase may participate in the production of aromatic compounds requiring methylation, such as 2-methoxy-3-alkylpyrazine or 2-hydroxy-3-alkylpyrazine, in the skin [62]. Alternatively, SAM synthetase may be required for lignin biosynthesis, as caffeoyl-CoA O-methyltransferase (SSP:5206), which was also more abundant in the skin, requires SAM as a co-factor.
Proline is the most abundant amino acid in grape berry, specifically in cv. Cabernet Sauvignon, and accumulates to high levels during the later stages of fruit ripening. Proline is synthesized from glutamate by delta 1-pyrroline-5-carboxylate synthetase (P5CS), which is localized mainly in pericarp tissue [63]. Proline was found to accumulate predominantly in skin and pulp tissues (Fig. 6B), consistent with the subcellular localization of P5CS. Like proline, threonine is clearly more abundant in pericarp rather than seed tissues. Threonine levels in must are strongly correlated with the accumulation of odorants related to fatty acid synthesis including, ethyl acetate, ethyl butyrate, and hexanoic and octanoic acids and longer chain alcohols such as isoamyl alcohol and β-phenylethanol [61].
Shikimic acid, which is an important precursor of aromatic amino acids, was most abundant in skin, less abundant in the pulp, and least abundant in the seed (Fig. 6B). Shikimic acid is thought to serve as the major source of precursors for two groups of important wine aromatic compounds that include volatile phenols and vanillin [64]. These results provide novel insights into the probable tissue-specific origins of key flavor and aroma compounds in wine.
4.3 Energy
Two proteins with energy-related functions were expressed preferentially in the pulp: a cytosolic inorganic pyrophosphatase (SSP:3114, TC56801) and a plastic fructosebisphosphate aldolase (SSP:8303, TC52261). Inorganic pyrophosphatase catalyzes the hydrolysis of pyrophosphate (PPi), which is formed principally as the product of the many biosynthetic reactions that utilize ATP. In the pulp, its function is probably related to regulation of inorganic pyrophosphate in the cytosol [65]. Fructose-bisphosphate aldolase is a glycolytic enzyme that catalyses the reversible aldol cleavage or condensation of fructose-1,6-bisphosphate into dihydroxyacetone-phosphate and glyceraldehyde 3-phosphate and is abundant in the pulp, but not the seeds of ripe berries [58].
The remaining proteins with functions related to energy production and photosynthesis were preferentially expressed in berry skin. These included ATP synthase beta subunit (SSP:3503, TC63054) and cytochrome c oxidase (COX), subunit 6b-1 (SSP:203, 204; TC51789), the terminal enzyme of the respiratory chain that oxidizes cytochrome c and transfers electrons to molecular oxygen to form molecular water. During berry development, transcripts encoding proteins with photosynthesis-related functions are most strongly expressed at flowering and two-weeks after flowering and then decline steadily in abundance throughout berry development [24, 25]. However, the skin of ripe berries still contained detectable amounts of proteins with functions related to photosynthesis and carbon assimilation. For example, several light harvesting components (Chlorophyll a-b binding protein (SSP:4120, TC62932), photosystem II components (Oxygen evolving enhancer protein 1 (SSP:2218, TC53930; SSP:3207, TC54765) and enzymes involved in carbon fixation including the large subunit of RuBisCO (ribulose-1,5-bisphosphate carboxylase/oxygenase) (SSP:8504, TC57584); RuBisCO subunit binding-protein alpha (SSP:1621, TC67963) and transketolase 1 (SSP:6727, SSP:6729; TC52655) were more abundant in the skin than in the pulp (Table 3). RuBisCO has been detected in skin [18], but was reported to be in very low abundance in the pulp of mature berries [58]. Transketolase has also been reportedly expressed in berry skin and shown to increase in abundance late in berry ripening [20] (Additional Table 6), possibly to supply carbon skeletons for biosynthetic pathways operating in this tissue during ripening. The expression pattern of photosystem and carbon assimilation proteins indicates that the skin might retain a functional photosynthetic apparatus or its remnants undergoing degradation in mature berries.
4.4 Biotic and abiotic stress responses
Grape ripening-related proteins (GRIPs) are abundant in postvéraison ripening berries [66]. Grip22-related proteins, which have been identified to be allergens in Kiwi [67], were found to be expressed in either the pulp (SSP:3111, TC55027) or the skin (SSP:7108, TC55027). However, the corresponding transcript for this protein showed no tissue-specific expression (Table 3). These two Grip22 proteins did differ in their experimentally determined pI, suggesting tissue-specific posttranslational modification. GRIP homologs in Kiwi are known to undergo glycosylation, which may be critical for the allergenic potential of these proteins [68, 69]. A second allergenic protein (SSP:1009, TC65039), which was more abundant in the pulp, and identified as a major allergen in cherry [70], is actually a pathogenesis-related (PR) protein (Table 3). Two skin-abundant PR proteins (SSP:7104, SSP:7105; TC68949) were also identified as PR10 proteins and are probably different products encoded by the same gene as judged by their relative proximity within the gel. PR10 has been observed previously to be expressed in mature berry skin [18] and increased in abundance during the later stages of ripening and are thought to play a role in berry protection [20]. SSP:4006 (TC58333) corresponds to a PR4 protein, which known to have antifungal activity [71], and thus may also contribute to berry protection. Another PR protein, β-1,3 glucanase (SSP:6212, TC61082), which is induced by fungal pathogen treatment [72], was found to be most abundant in skin tissue (Table 3) as was the corresponding transcript encoding this protein [30]. β-1,3 glucanases have also been found to increase in activity [18] and protein abundance during berry ripening in skin tissue [19, 20] (Additional Table 6).
A number of heat shock proteins (HSPs) were also found in the skin. SSP1706 (TC54161) was found to be a close homologue of a watermelon HSP70 [73]. Unfortunately, no peptides corresponded with N-terminal transit peptide of the grape homologue, so the subcellular location of this protein cannot be predicted. A second HSP (SSP:3719, TC53932) corresponds to a tobacco HSP90, which has been shown to play a key role in the conformational regulation of R protein recognition complexes and viral resistance [74]. Various other classes of HSPs, including multiple HSP70 isoforms have been described in grape berries [19] (Additional Table 6).
Preventing oxidative browning of grapes caused by polyphenol oxidase (PPO) is a major concern for the dry fruit industry and the production of white wine [75]. SSP:1012 (TC51691), which corresponds to a polyphenol oxidase gene described previously [76], was most abundant in the skin consistent with an earlier report that confirmed that grape berry skins contain high amounts of PPO activity [77]. However, more abundant isoforms (SSP:1004, SSP:1007; TC51691) of PPO were identified (see Additional Table 5), but these did not show differential abundance among berry tissues. Several PPO isoforms are abundantly expressed in berry skins at véraison [20] and then their abundance declines towards ripening [19, 20] (Additional Table 6). Members of another class of stress proteins, the late embryogenesis abundant (LEA) proteins (SSP:1403, SSP:2518), exhibited pulp-specific expression patterns. These proteins belong to the SKn group of acidic dehydrins [78]. The apparent molecular weight of these two proteins is higher than predicted consistent with previous observations for this group of proteins [79]. A late embryogenesis protein was also reported previously to be expressed in berry skin [20].
4.5 Organic acids
Tartrate is one of the most abundant organic acids in grape berries. The enzyme catalyzing the formation of tartrate (L-idonate dehydrogenase) in berries has been recently identified and belongs to the ascorbic acid degradation pathway [80]. The innermost region of the berry pulp surrounding the seed has been shown to contain the highest tartrate concentrations, but seeds were not assayed directly by these authors [81]. We observed that tartrate is significantly less abundant in the seed than in surrounding tissues (Figure 6C), despite the observation that transcripts for L-idonate dehydrogenase are most abundant in seeds [30]. More detailed analysis of the expression of the genes and corresponding enzymes that participate in tartrate biosynthesis is needed. The detection of high concentrations of threonate in the pulp of berries (Figure 6C) also reinforces the hypothesis that ascorbate is metabolized into oxalate and threonate in grape although the catalyzing enzyme remains unknown [82]. Malate, like tartrate, is among the most abundant organic acids and the major determinant of titratable acidity in berries. Malate was more abundant in the pericarp than in the seed consistent with previous results [81] and the relative transcript abundance for malate biosynthetic enzymes [30]. Succinate was also more abundant in the skin than in pulp, as was succinate dehydrogenase (SSP:5704, TC51756)) (Table 3). Succinate has not been detected previously in grape berry juice [83], likely due to the difference in detection methods employed and the low relative abundance of succinate, which is about 100-fold less abundant than the major organic acids.
4.6 Carbohydrates
The major forms of stored carbohydrates in berries are glucose and fructose, which are derived mainly from sucrose exported from the leaf and transported in the phloem to the berry cluster [84] or derived from starch reserves within the berry itself [27]. This study confirmed these previous observations as pulp and skin accumulated much larger amounts of fructose and glucose than sucrose (Figure 6D). In contrast, seed tissues accumulated more sucrose than glucose and fructose (Figure 6D) consistent with earlier observations [85]. Gluconate, which can be metabolized via the oxidative pentose phosphate pathway in plants [86], is more abundant in skin than in pulp and seed (Figure 6D). High concentrations of gluconate can cause severe problems in winemaking processes including the control of alcoholic fermentation, biological aging, and stability [87]. Trehalose was found predominantly in the seed and skin (Figure 6D) where it may function as an osmoprotectant in these tissues or play roles in cell wall structure, cell division, glycolysis and starch accumulation [88]. In grape berry, trehalose phosphate synthase (1608440_at; TC48283) tissue-specific mRNA accumulation showed a similar pattern with trehalose itself [30].
4.7 Water-deficit stress responses
Approximately 7% of all skin and pulp proteins showed a two-fold or greater change in abundance in response to water deficit stress (Tables 5 and 6; Additional Tables 2 and 3) indicating that water-deficit stress can have a major impact on protein expression profiles in grape pericarp tissues. In skin, there was an apparent increase in the abundance of proteosome alpha (SSP4204, TC54618) and beta (SSP:5109, TC68818) subunits and several peptidases (Table 5), indicating that water-deficit stress is likely to increase protein turnover in the berry. Induction of multiple proteosome subunits in response to water-deficit stress has been observed previously in grapevine leaves [16]. Skin tissue also exhibited an increased abundance of cytosolic ascorbate peroxidase (SSP:5215, TC51718). Water-deficit stress is known to increase the mRNA expression of cytosolic ascorbate peroxidase in various plant species, including cowpea, where it is responsible for H2O2 detoxification and protection against oxidative stress [89].
In contrast, some proteins appeared more abundant in skin of berries from well-watered vines. For example, two isozymes involved in flavonoid biosynthesis, chalcone isomerase (SSP:3220, TC55034) and leucoanthocyanin dioxygenase (SSP:5501, TC69652) were significantly more abundance in well-watered vines (Table 5). Interestingly, a second, skin-specific isozyme of chalcone isomerase (SSP:3210) and leucoanthocyanin dioxygenase (SSP:5411), respectively, showed no significant change in abundance following water deficit stress. Water deficit causes an increase in the mRNA of most flavonoid biosynthetic enzymes [30, 90, 91] and an increase in flavonoid content of both berries and wine [46, 90, 91]. These secondary isozymes are likely to fulfill flavonoid biosynthesis demands under water-deficit conditions demonstrating the existence of a division of labor between these different isozymes depending on water status.
In contrast, pulp tissues exhibited a completely different set of proteins with differential expression to water-deficit stress (Table 6). For example glutamate decarboxylase (SSP:5619; TC54345), which catalyzes the decarboxylation of L-glutamate to form gamma-aminobutyrate (GABA) and CO2 [92], was more abundant and glutamate accumulation declined significantly under water-deficit stress (Figure 7). Interestingly, the product of this reaction, GABA, has been observed to be more abundant under water-deficit tissues of green coffee beans [93] and tomato suspension cells which led to increased rates of glutamate decarboxylation into alanine and GABA [94]. The observed reduction of glutamate abundance and increase in alanine under water stress (Figure 7) in grape berry pulp indicates that glutamate decarboxylase is also involved in metabolic adjustments under water stress in grape berry pulp. In contrast, no change in glutamate abundance was observed in the skin following water-deficit stress. In addition, the large increases in myo-inositol and sucrose observed under water deficit stress (Figure 7) probably reflect their respective roles as osmoprotectants and precursors for the formation of raffinose series sugars, which are critical to enhanced water-deficit stress tolerance [95]. A class IV endochitinase (SSP:3113, TC68794) was also induced by water-deficit stress as is commonly observed for many other PR proteins. This class of protein was shown previously to accumulate during the later stages of berry ripening to high concentrations [96]. The observed increase in shikimate in pulp under water-deficit stress may be related to an increase of shikimate derived volatile compounds as well as tannins, flavonoids, and lignans [97].
Several other pulp proteins were significantly enhanced in their expression under well-watered conditions including methionine synthase (SSP:6715, TC52137), which catalyzes the transfer of a methyl group from 5-methyltetrahydrofolate to homocysteine resulting in methionine formation. UDP-glucose pyrophosphorylase (UGP, SSP:8602, TC57645) abundance was significantly higher in pulp from well-watered vines. UGP catalyzes the formation of UDP-glucose from the phosphorylation of glucose-1-phosphate and participates in the biosynthesis of carbohydrates and cellulose in sink tissues [98]. In grape leaves, increased UGP abundance was correlated with a decrease of sucrose abundance [17], as was observed here.
4.8 mRNA and protein expression correlation
Comparison of mRNA and protein expression patterns between the skin and pulp revealed that most proteins (82%) showed the same tissue-preferential expression pattern, as did their corresponding mRNAs with a Pearson correlation coefficient of 0.46. These data indicate only a moderate correlation between mRNA and protein expression patterns in these tissues. However, if the magnitude of tissue-specific expression were considered at the two-fold or greater ratio of differential expression for both mRNA and protein levels, then only 32% of proteins showed similar patterns of expression between these two tissues with a Pearson correlation coefficient between protein and mRNA abundance log2 difference ratios of 0.216 (Fig. 5A).
Comparison of mRNA and protein expression patterns between the seed and pericarp also revealed that most proteins (81%) showed the same tissue-preferential expression patterns. In contrast to pericarp tissues, however, a majority of seed proteins (69%) presented a two-fold or greater ratio difference in both protein and mRNA relative abundance. However, the Pearson correlation coefficient between protein and mRNA abundance log2 difference ratios for the seed vs. pericarp was only 0.017 (Fig. 5B). These data indicate a rather poor correlation between mRNA and protein expression patterns for genes expressed preferentially within a specific tissue. About half (52%) of pericarp proteins showed the same water-status-dependent trend in expression as did their corresponding mRNAs, however, none of the transcripts/protein comparisons displayed a two-fold or greater difference in expression.
Other studies comparing relative protein abundance with relative transcript abundance estimated by EST counting, northern blotting, or microarray expression profiling generally showed a moderate to weak correlation (r = 0.50 or less) depending on the study [99-102]. Our results and results from these studies indicate that more than half of documented protein accumulation differences are determined after transcription via protein modifications, differential rates of translation, or differences in protein stability and turnover.
5 Concluding remarks
This large proteomic study has for the first time surveyed the tissue-specific differences in protein expression of mature wine grape (Vitis vinifera) berries. More than 1,700 protein spots were mapped by 2D PAGE and more than 250 proteins were identified by MALDI-MS/MS that showed tissue-specific expression among skin, pulp, and seed tissues. About 90 identified proteins showed differential expression between the skin and pulp and 163 identified proteins showed seed-specific expression. The skin was significantly enriched in proteins of the phenylpropanoid and amino acid biosynthesis pathways and photosynthesis and pathogenesis-related response proteins. In contrast, the seed proteome was completely distinct from that of the pericarp and was comprised largely of seed storage, maturation, or late embryogenesis abundant proteins. Comparison of tissue-specific protein expression patterns with available mRNA expression patterns revealed that a majority (81%-82%) of proteins showed qualitative, tissue-preferential expression pattern consistent with their corresponding mRNAs. However, relatively fewer pericarp (32%) or seed (69%) proteins showed similar patterns of quantitative expression among tissues. These results clearly indicate that protein accumulation patterns are strongly influenced by post-transcriptional processes in agreement with other large-scale proteome studies in plants.
The impact of water-deficit stress was also studied and found to have a profound influence on tissue-specific protein expression patterns resulting in significant changes in the expression of approximately 7% of pericarp proteins. Skin responses to water deficit stress were diverse with an obvious increase in the abundance of proteosome subunit proteins and proteases, reactive oxygen detoxification enzymes, and selected enzymes involved in flavonoid biosynthesis, whereas the pulp showed increases in isoflavone reductase, glutamate decarboxylase and an endochitinase. In contrast, water-deficit caused little effect on the expression of seed proteins as might be expected as the seeds in mature berries would be at the end of their developmentally programmed preparative phase for desiccation tolerance present in most orthodox seeds.
Finally, a limited survey of 32 metabolites by GC-MS showed that 18 exhibited tissue-specific differences in abundance with skins accumulating high concentrations of caffeic acid, proline, shikimate, and gluconate. Water-deficit stress caused increases in alanine, catechine, myo-inositol, shikimate and sucrose, but decreases in glutamate and tartrate in pulp tissue. Overall, these results provide many novel insights into the likely tissue-specific origins and the influence of water deficit stress on the tissue-specific accumulation of enzymes that catalyze the formation of key flavor and aroma compounds in wine including organic acids, specific sugars, phenylpropanoids, proanthocyanins, and volatile compounds.
Supplementary Material
Acknowledgments
This work was support by grants from the National Science Foundation Plant Genome Program (DBI-0217653) to GRC and JCC, and the University of Nevada Agricultural Experiment Station, publication No. 03087111. Support for the Nevada Proteomics Center was made possible by NIH Grant Number P20 RR-016464 from the INBRE-BRIN Program of the National Center for Research Resources and the NIH IDeA Network of Biomedical Research Excellence (INBRE, RR-03-008). The authors thank Kathy Schegg and David Quilici of the Nevada Proteomics Center for support and for performing MS analyses and Rebecca Albion and Kitty Spreeman for invaluable technical support. The authors would like to especially thank Leon Sobon of Sobon Estate and Shenandoah Vineyards, Amador County, California (http://www.sobonwine.com/) for allowing us to collect the berry samples used in this study.
Abbreviations
- CA
carrier ampholyte
- MSTFA
N-Methyl-N-trimethylsilyltrifluoroacetamide
- PR
pathogenesis-related
- PMSF
phenylmethanesulphonylfluoride
- PVPP
polyvinylpyrrolidone
- RuBisCO
ribulose 1,5-bisphosphate carboxylase/oxygenase
- TMCS
Trimethylchlorosilane
Footnotes
9 Supporting information
Additional Figure 1: 2D-PAGE analysis of Vitis vinifera cv. Cabernet Sauvignon berry pericarp proteins constant relative abundance in pulp and skin. Proteins that exhibited a non-significant (p < 0.05) change between the skin and pulp are indicated by circles and standard spot numbers on a representative gel. See Additional Table 5 for detailed listing of proteins.
Additional Table 1: Proteins whose abundance was significantly different between pulp and skin with corresponding mRNA expression.
Additional Table 2: Proteins whose abundance was significantly different between well watered and water-deficit in pulp with corresponding mRNA expression values.
Additional Table 3: Proteins whose abundance was significantly different between well watered and water-deficit in skin with corresponding mRNA expression values.
Additional Table 4: Proteins predominantly presented in seed with their corresponding mRNA expression values.
Additional Table 5: Proteins with constant relative abundance in pulp and skin.
Additional Table 6: Proteins also identified in Deytieux et al., 2007 [18], Giribaldi et al., 2007 [19], and Negri et al. 2008 [20].
References
- 1.This P, Lacombe T, Thomas M. Historical origins and genetic diversity of wine grapes. Trends Genet. 2006;22:511–519. doi: 10.1016/j.tig.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Arroyo-Garcia R, Ruiz-Garcia L, Bolling L, Ocete R, et al. Multiple origins of cultivated grapevine (Vitis vinifera L. ssp. sativa) based on chloroplast DNA polymorphisms. Molec. Ecol. 2006;15:3707–3714. doi: 10.1111/j.1365-294X.2006.03049.x. [DOI] [PubMed] [Google Scholar]
- 3.Iriti M, Faoro F. Grape phytochemicals: a bouquet of old and new nutraceuticals for human health. Medical Hypotheses. 2006;67:833–838. doi: 10.1016/j.mehy.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 4.Monagas M, Hernandez-Ledesma B, Gomez-Cordoves C, Bartolome B. Commercial dietary ingredients from Vitis vinifera L. leaves and grape skins: antioxidant and chemical characterization J. Agric. Food Chem. 2006;54:319–327. doi: 10.1021/jf051807j. [DOI] [PubMed] [Google Scholar]
- 5.This P, Lacombe T, Cadle-Davidson M, Owens C. Wine grape (Vitis vinifera L.) color associates with allelic variation in the domestication gene VvmybA1. Theor. Appl. Genet. 2007;114:723–730. doi: 10.1007/s00122-006-0472-2. [DOI] [PubMed] [Google Scholar]
- 6.Bogs J, Jaffe F, Takos A, Walker A, Robinson S. The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development. Plant Physiol. 2007;143:1347–1361. doi: 10.1104/pp.106.093203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez K, Kennedy J, Agosin E. Characterization of Vitis vinifera L. Cv. Carmenere grape and wine proanthocyanidins. J. Agric. Food Chem. 2007;55:3675–3680. doi: 10.1021/jf063232b. [DOI] [PubMed] [Google Scholar]
- 8.Lund S, Bohlmann J. The molecular basis for wine grape quality--a volatile subject. Science. 2006;311:804–805. doi: 10.1126/science.1118962. [DOI] [PubMed] [Google Scholar]
- 9.Moreno-Arribas M, Cabello F, Polo M, Martin-Alvarez P, Pueyo E. Assessment of the native electrophoretic analysis of total grape must proteins for the characterization of Vitis vinifera L. cultivars. J. Agric. Food Chem. 1999;47:114–120. doi: 10.1021/jf980483e. [DOI] [PubMed] [Google Scholar]
- 10.Hayasaka Y, Adams K, Pocock K, Baldock G, et al. Use of electrospray mass spectrometry for mass determination of grape (Vitis vinifera) juice pathogenesis-related proteins: a potential tool for varietal differentiation. J. Agric. Food Chem. 2001;49:1830–1839. doi: 10.1021/jf001163+. [DOI] [PubMed] [Google Scholar]
- 11.Monteiro S, Picarra-Pereira M, Teixeira A, Loureiro V, Ferreira R. Environmental conditions during vegetative growth determine the major proteins that accumulate in mature grapes. J. Agric. Food Chem. 2003;51:4046–4053. doi: 10.1021/jf020456v. [DOI] [PubMed] [Google Scholar]
- 12.Vincent D, Wheatley M, Cramer G. Optimization of protein extraction and solubilization for mature grape berry clusters. Electrophoresis. 2006;27:1853–1865. doi: 10.1002/elps.200500698. [DOI] [PubMed] [Google Scholar]
- 13.Sarry J-E, Sommerer N, Sauvage F-X, Bergoin A, et al. Grape berry biochemistry revisited upon proteomic analysis of the mesocarp. Proteomics. 2004;4:201–215. doi: 10.1002/pmic.200300499. [DOI] [PubMed] [Google Scholar]
- 14.Carvalho L, Esquivel M, Martins I, Ricardo C, Amancio S. Monitoring the stability of Rubisco in micropropagated grapevine (Vitis vinifera L.) by two-dimensional electrophoresis. J. Plant Physiol. 2005;162:365–374. doi: 10.1016/j.jplph.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Castro A, Carapito C, Zorn N, Magne C, et al. Proteomic analysis of grapevine (Vitis vinifera L.) tissues subjected to herbicide stress. J. Exp. Bot. 2005;56:2783–2795. doi: 10.1093/jxb/eri271. [DOI] [PubMed] [Google Scholar]
- 16.Vincent D, Ergul A, Bohlman M, Tattersall E, et al. Proteomic analysis reveals differences between Vitis vinifera L. cv. Chardonnay and cv. Cabernet Sauvignon and their responses to water deficit and salinity. J. Exp. Bot. 2007;58:1873–1892. doi: 10.1093/jxb/erm012. [DOI] [PubMed] [Google Scholar]
- 17.Sauvage F-X, Pradal M, Chatelet P, Tesniere C. Proteome changes in leaves from grapevine (Vitis vinifera L.) transformed for alcohol dehydrogenase activity. J. Agric. Food Chem. 2007;55:2597–2603. doi: 10.1021/jf063723w. [DOI] [PubMed] [Google Scholar]
- 18.Deytieux C, Geny L, Lapaillerie D, Claverol S, et al. Proteome analysis of grape skins during ripening. J. Exp. Bot. 2007;58:851–1862. doi: 10.1093/jxb/erm049. [DOI] [PubMed] [Google Scholar]
- 19.Giribaldi M, Perugini I, Sauvage F, Schubert A. Analysis of protein changes during grape berry ripening by 2-DE and MALDI-TOF. Proteomics. 2007;7:3154–3170. doi: 10.1002/pmic.200600974. [DOI] [PubMed] [Google Scholar]
- 20.Negri A, Prinsi B, Rossoni M, Failla O, et al. Proteome changes in the skin of the grape cultivar Barbara among different stages of ripening. BMC Genomics. 2008;9:378. doi: 10.1186/1471-2164-9-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moser C, Segala C, Fontana P, Salakhudtinov I, et al. Comparative analysis of expressed sequence tags from different organs of Vitis vinifera L. Funct. Integ. Genomics. 2005;5:208–217. doi: 10.1007/s10142-005-0143-4. [DOI] [PubMed] [Google Scholar]
- 22.da Silva F, Iandolino A, Al-Kayal F, Bohlmann M, et al. Characterizing the grape transcriptome. Analysis of expressed sequence tags from multiple Vitis species and development of a compendium of gene expression during berry development. Plant Physiol. 2005;139:574–597. doi: 10.1104/pp.105.065748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng F, Reid K, Liao N, Schlosser J, et al. Generation of ESTs in Vitis vinifera wine grape (Cabernet Sauvignon) and table grape (Muscat Hamburg) and discovery of new candidate genes with potential roles in berry development. Gene. 2007;402:40–50. doi: 10.1016/j.gene.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Waters L, Holton T, Ablett E, Lee L, Henry R. cDNA microarrays analysis of developing grape (Vitis vinifera cv. Shiraz) berry skin. Funct. Integ. Genomics. 2005;5:40–58. doi: 10.1007/s10142-004-0124-z. [DOI] [PubMed] [Google Scholar]
- 25.Terrier N, Glissant D, Grimplet J, Barrieu F, et al. Isogene specific oligo arrays reveal multifaceted changes in gene expression during grape berry (Vitis vinifera L.) development. Planta. 2005;222:832–847. doi: 10.1007/s00425-005-0017-y. [DOI] [PubMed] [Google Scholar]
- 26.Pilati S, Perazzolli M, Malossini A, Cestaro A, et al. Genome-wide transcriptional analysis of grapevine berry ripening reveals a set of genes similarly modulated during three seasons and the occurrence of an oxidative burst at vèraison. BMC Genomics. 2007;8:428. doi: 10.1186/1471-2164-8-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deluc L, Grimplet J, Wheatley M, Tillett R, et al. Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genomics. 2007;8:429. doi: 10.1186/1471-2164-8-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandez L, Torregrosa L, Terrier N, Sreekantan L, et al. Identification of genes associated with flesh morphogenesis during grapevine fruit development. Plant Molec. Biol. 2007;63:307–323. doi: 10.1007/s11103-006-9090-2. [DOI] [PubMed] [Google Scholar]
- 29.Waters D, Holton T, Ablett E, Slade L, Henry R. The ripening wine grape berry skin transcriptome. Plant Sci. 2006;171:132–138. [Google Scholar]
- 30.Grimplet J, Deluc L, Tillett R, Wheatley M, et al. Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genomics. 2007;8:187. doi: 10.1186/1471-2164-8-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coombe B. Adoption of a system for identifying grapevine growth stages. Aust. J. Grape. Wine. Res. 1995;1:104–110. [Google Scholar]
- 32.McCutchan J, Shackel K. Stem-water potential as a sensitive indicator of water stress in prune trees (Prunus domestica L. cv. French). J. Amer. Soc. Hort. Sci. 1992;117:607–611. [Google Scholar]
- 33.Saravanan R, Rose J. A critical evaluation of sample extraction techniques for enhanced proteomic analysis of recalcitrant plant tissues. Proteomics. 2004;4:2522–2532. doi: 10.1002/pmic.200300789. [DOI] [PubMed] [Google Scholar]
- 34.Hurkman W, Tanaka C. Solubilization of plant membrane proteins for analysis bytwo-dimensional gel electrophoresis. Plant Physiol. 1986;81:802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Farrell P. High resolution two-dimensional electrophoresis of proteins. J. Biol. Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 36.Neuhoff V, Arold N, Taube D, Ehrhardt W. Improved staining of proteins in polyacrylamide gels including isoelectric focusing gels with clear background at nanogram sensitivity using Coomassie Brilliant Blue G-250 and R-250. Electrophoresis. 1988;9:255–262. doi: 10.1002/elps.1150090603. [DOI] [PubMed] [Google Scholar]
- 37.Rosenfeld J, Capdevielle J, Guillemot J, Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal. Biochem. 1992;203:173–179. doi: 10.1016/0003-2697(92)90061-b. [DOI] [PubMed] [Google Scholar]
- 38.Zhu X, Papayannopoulos I. Improvement in the detection of low concentration protein digests on a MALDI TOF/TOF workstation by reducing alpha-cyano-4-hydroxycinnamic acid adductions. J. Biomolec.Techniq. 2003;14:298–307. [PMC free article] [PubMed] [Google Scholar]
- 39.Didier G, Brezellec P, Remy E, Henaut A. GeneANOVA-gene expression analysis of variance. Bioinform. 2002;18:490–491. doi: 10.1093/bioinformatics/18.3.490. [DOI] [PubMed] [Google Scholar]
- 40.Broeckling C, Huhman D, Farag M, Smith J, et al. Metabolic profiling of Medicago truncatula cell cultures reveals the effects of biotic and abiotic elicitors on metabolism. J. Exp. Bot. 2005;56:323–336. doi: 10.1093/jxb/eri058. [DOI] [PubMed] [Google Scholar]
- 41.Kopka J, Schauer N, Krueger S, Birkemeyer C, et al. GMD@CSB.DB: the Golm Metabolome Database. Bioinform. 2005;21:1635–1638. doi: 10.1093/bioinformatics/bti236. [DOI] [PubMed] [Google Scholar]
- 42.Chone X, Van Leeuwen C, Dubourdieu D, Gaudillere J. Stem water potential is a sensitive indicator of grapevine water status. Ann. Bot. (Lond) 2001;87:477–483. [Google Scholar]
- 43.Parker R, Flowers T, Moore A, Harpham N. An accurate and reproducible method for proteome profiling of the effects of salt stress in the rice leaf lamina. J. Exp. Bot. 2006;57:1109–1118. doi: 10.1093/jxb/erj134. [DOI] [PubMed] [Google Scholar]
- 44.Hajduch M, Casteel J, Hurrelmeyer K, Song Z, et al. Proteomic analysis of seed filling in Brassica napus. Developmental characterization of metabolic isozymes using high-resolution two-dimensional gel electrophoresis. Plant Physiol. 2006;141:32–46. doi: 10.1104/pp.105.075390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asirvatham V, Watson B, Sumner L. Analytical and biological variances associated with proteomic studies of Medicago truncatula by two-dimensional polyacrylamide gel electrophoresis. Proteomics. 2002;2:960–968. doi: 10.1002/1615-9861(200208)2:8<960::AID-PROT960>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 46.Grimplet J, Deluc L, Cramer G, Cushman J, Matthew A, Jenks PMH, Jain SM, editors. Advances in molecular-breeding toward drought and salt tolerant crops. Springer; Dordrecht, The Netherlands: 2007. [Google Scholar]
- 47.Escalona J, Flexas J, Bota J, Medrano H. Distribution of leaf photosynthesis and transpiration within grapevine canopies under different drought conditions. Vitis. 2003;42:57–64. [Google Scholar]
- 48.Ageorges A, Fernandez L, Vialet S, Merdinoglu D, et al. Four specific isogenes of the anthocyanin metabolic pathway are systematically co-expressed with the red colour of grape berries. Plant Sci. 2006;170:372–383. [Google Scholar]
- 49.Shi J, Yu J, Pohorly J, Kakuda Y. Polyphenolics in grape seeds-biochemistry and functionality. J. Medic. Food. 2003;6:291–299. doi: 10.1089/109662003772519831. [DOI] [PubMed] [Google Scholar]
- 50.Ibdah M, Zhang X, Schmidt J, Vogt T. A novel Mg(2+)-dependent O-methyltransferase in the phenylpropanoid metabolism of Mesembryanthemum crystallinum. J. Biol. Chem. 2003;278:43961–43972. doi: 10.1074/jbc.M304932200. [DOI] [PubMed] [Google Scholar]
- 51.Castellarin S, Di Gaspero G, Marconi R, Nonis A, et al. Colour variation in red grapevines (Vitis vinifera L.): genomic organisation, expression of flavonoid 3′-hydroxylase, flavonoid 3′,5′-hydroxylase genes and related metabolite profiling of red cyanidin-/blue delphinidin-based anthocyanins in berry skin. BMC Genomics. 2006;7:12. doi: 10.1186/1471-2164-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kobayashi S, Ishimaru M, Hiraoka K, Honda C. Myb-related genes of the Kyoho grape (Vitis labruscana) regulate anthocyanin biosynthesis. Planta. 2002;215:924–933. doi: 10.1007/s00425-002-0830-5. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi S, Goto-Yamamoto N, Hirochika H. Retrotransposon-induced mutations in grape skin color. Science. 2004;304:982. doi: 10.1126/science.1095011. [DOI] [PubMed] [Google Scholar]
- 54.Ford C, Boss P, Hoj P. Cloning and characterization of Vitis vinifera UDP-glucose:flavonoid 3-O-glucosyltransferase, a homologue of the enzyme encoded by the maize Bronze-1 locus that may primarily serve to glucosylate anthocyanidins in vivo. J. Biol. Chem. 1998;273:9224–9233. doi: 10.1074/jbc.273.15.9224. [DOI] [PubMed] [Google Scholar]
- 55.Alfenito M, Souer E, Goodman C, Buell R, et al. Functional complementation of anthocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell. 1998;10:1135–1149. doi: 10.1105/tpc.10.7.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kobayashi S, Ishimaru M, Ding C, Yakushiji H, Goto N. Comparison of UDP-glucose:flavonoid 3-O-glucosyltransferase (UFGT) gene sequences between white grapes (Vitis vinifera) and their sports with red skin. Plant Sci. 2001;160:543–550. doi: 10.1016/s0168-9452(00)00425-8. [DOI] [PubMed] [Google Scholar]
- 57.Wang X, He X, Lin J, Shao H, et al. Crystal structure of isoflavone reductase from Alfalfa (Medicago sativa L.). J. Molec. Biol. 2006;358:1341–1352. doi: 10.1016/j.jmb.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 58.Famiani F, Walker R, Tecsi L, Chen Z, et al. An immunohistochemical study of the compartmentation of metabolism during the development of grape (Vitis vinifera L.) berries. J. Exp. Bot. 2000;51:675–683. [PubMed] [Google Scholar]
- 59.Edwards J, Walker E, Coruzzi G. Cell-specific expression in transgenic plants reveals nonoverlapping roles for chloroplast and cytosolic glutamine synthetase. Proc. Natl. Acad. Sci. USA. 1990;87:3459–3463. doi: 10.1073/pnas.87.9.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pripis-Nicolau L, de Revel G, Bertrand A, Lonvaud-Funel A. Methionine catabolism and production of volatile sulphur compounds by Oenococcus oeni. J. Appl. Microbiol. 2004;96:1176–1184. doi: 10.1111/j.1365-2672.2004.02257.x. [DOI] [PubMed] [Google Scholar]
- 61.Hernandez-Orte P, Cacho J, Ferreira V. Relationship between varietal amino acid profile of grapes and wine aromatic composition. Experiments with model solutions and chemometric study. J. Agric. Food Chem. 2002;50:2891–2899. doi: 10.1021/jf011395o. [DOI] [PubMed] [Google Scholar]
- 62.Hashizume K, Tozawa K, Endo M, Aramaki I. S-Adenosyl-L-methionine-dependent O-methylation of 2-hydroxy-3-alkylpyrazine in wine grapes: a putative final step of methoxypyrazine biosynthesis. Biosci. Biotechnol. Biochem. 2001;65:795–801. doi: 10.1271/bbb.65.795. [DOI] [PubMed] [Google Scholar]
- 63.Stines A, Naylor D, Hoj P, van Heeswijck R. Proline accumulation in developing grapevine fruit occurs independently of changes in the levels of delta1-pyrroline-5-carboxylate synthetase mRNA or protein. Plant Physiol. 1999;120:923–931. doi: 10.1104/pp.120.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ibarz M, Ferreira V, Hernández-Orte P, Loscos N, Cacho J. Optimization and evaluation of a procedure for the gas chromatographic-mass spectrometric analysis of the aromas generated by fast acid hydrolysis of flavor precursors extracted from grapes. J. Chromatogr. A. 2006;1116:217–229. doi: 10.1016/j.chroma.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 65.Rojas-Beltran J, Dubois F, Mortiaux F, Portetelle D, et al. Identification of cytosolic Mg2+-dependent soluble inorganic pyrophosphatases in potato and phylogenetic analysis. Plant Mol. Biol. 1999;39:449–461. doi: 10.1023/a:1006136624210. [DOI] [PubMed] [Google Scholar]
- 66.Davies C, Robinson S. Differential screening indicates a dramatic change in mRNA profiles during grape berry ripening. Cloning and characterization of cDNAs encoding putative cell wall and stress response proteins. Plant Physiol. 2000;122:803–812. doi: 10.1104/pp.122.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tamburrini M, Cerasuolo I, Carratore V, Stanziola A, et al. Kiwellin, a novel protein from kiwi fruit. Purification, biochemical characterization and identification as an allergen. Protein J. 2005;24:423–429. doi: 10.1007/s10930-005-7638-7. [DOI] [PubMed] [Google Scholar]
- 68.Fahlbusch B, Rudeschko O, Schumann C, Steurich F, et al. Further characterization of IgE-binding antigens in kiwi, with particular emphasis on glycoprotein allergens. J. Investig. Allergol. Clin. Immunol. 1998;8:325–332. [PubMed] [Google Scholar]
- 69.Huby R, Dearman R, Kimber I. Why are some proteins allergens? Toxicol Sci. 2000;55:235–246. doi: 10.1093/toxsci/55.2.235. [DOI] [PubMed] [Google Scholar]
- 70.Reuter A, Fortunato D, Garoffo L, Napolitano L, et al. Novel isoforms of Pru av 1 with diverging immunoglobulin E binding properties identified by a synergistic combination of molecular biology and proteomics. Proteomics. 2005;5:282–289. doi: 10.1002/pmic.200400874. [DOI] [PubMed] [Google Scholar]
- 71.Caporale C, Facchiano A, Bertini L, Leonardi L, et al. Comparing the modeled structures of PR-4 proteins from wheat. J. Mol. Model. 2003;9:9–15. doi: 10.1007/s00894-002-0103-z. [DOI] [PubMed] [Google Scholar]
- 72.Aziz A, Heyraud A, Lambert B. Oligogalacturonide signal transduction, induction of defense-related responses and protection of grapevine against Botrytis cinerea. Planta. 2004;218:767–774. doi: 10.1007/s00425-003-1153-x. [DOI] [PubMed] [Google Scholar]
- 73.Wimmer B, Lottspeich F, van der Klei I, Veenhuis M, Gietl C. The glyoxysomal and plastid molecular chaperones (70-kDa heat shock protein) of watermelon cotyledons are encoded by a single gene. Proc. Natl. Acad. Sci. USA. 1997;94:13624–13629. doi: 10.1073/pnas.94.25.13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y, Burch-Smith T, Schiff M, Feng S, Dinesh-Kumar S. Molecular chaperone Hsp90 associates with resistance protein N and its signaling proteins SGT1 and Rar1 to modulate an innate immune response in plants. J. Biol. Chem. 2004;279:2101–2108. doi: 10.1074/jbc.M310029200. [DOI] [PubMed] [Google Scholar]
- 75.Boulton R, Singleton V, Bisson L, Kunkee R. Principles and Practices of Winemaking Aspen Publishers. New York: 1998. [Google Scholar]
- 76.Dry I, Robinson S. Molecular cloning and characterisation of grape berry polyphenol oxidase. Plant Mol. Biol. 1994;26:495–502. doi: 10.1007/BF00039560. [DOI] [PubMed] [Google Scholar]
- 77.Rathjen H, Robinson S. Characterisation of a variegated grapevine mutant ahowing reduced polyphenol oxidase activity. Funct. Plant Biol. 1992;19:43–54. [Google Scholar]
- 78.Battaglia M, Olvera-Carrillo Y, Garciarrubio A, Campos F, Covarrubias A. The enigmatic LEA proteins and other hydrophilins. Plant Physiol. 2008;148:6–24. doi: 10.1104/pp.108.120725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soulages J, Kim K, Arrese E, Walters C, Cushman J. Conformation of a group 2 late embryogenesis abundant protein from soybean. Evidence of poly (L-proline)-type II structure. Plant Physiol. 2003;131:963–975. doi: 10.1104/pp.015891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DeBolt S, Cook D, Ford C. L-tartaric acid synthesis from vitamin C in higher plants. Proc. Natl. Acad. Sci. USA. 2006;103:5608–5613. doi: 10.1073/pnas.0510864103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Possner D, Kliewer W. The localization of acids, sugars, potassium and calcium in developing grape berries. Vitis. 1985;24:229–240. [Google Scholar]
- 82.Debolt S, Melino V, Ford C. Ascorbate as a biosynthetic precursor in plants. Ann. Bot. (Lond) 2007;99:3–8. doi: 10.1093/aob/mcl236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mato I, Suarez-Luque S, Huidobro J. Simple determination of main organic acids in grape juice and wine by using capillary zone electrophoresis with direct UV detection. Food Chem. 2007;102:104–112. [Google Scholar]
- 84.Conde C, Agasse A, Glissant D, Tavares R, et al. Pathways of glucose regulation of monosaccharide transport in grape cells. Plant Physiol. 2006;141:1563–1577. doi: 10.1104/pp.106.080804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hawker JS, Ruffner H, Walker R. The sucrose content of some australian grapes. Amer. J. Enol. Vitic. 1976;27:125–129. [Google Scholar]
- 86.Garlick A, Moore C, Kruger N. Monitoring flux through the oxidative pentose phosphate pathway using [1-14C]gluconate. Planta. 2002;216:265–272. doi: 10.1007/s00425-002-0842-1. [DOI] [PubMed] [Google Scholar]
- 87.Peinado R, Moreno J, Medina M, Mauricio J. Potential application of a glucose-transport-deficient mutant of Schizosaccharomyces pombe for removing gluconic acid from grape must. J. Agric. Food Chem. 2005;53:1017–1021. doi: 10.1021/jf048764b. [DOI] [PubMed] [Google Scholar]
- 88.Gomez L, Baud S, Graham I. The role of trehalose-6-phosphate synthase in Arabidopsis embryo development. Biochem. Soc. Trans. 2005;33:280–282. doi: 10.1042/BST0330280. [DOI] [PubMed] [Google Scholar]
- 89.D'Arcy-Lameta A, Ferrari-Iliou R, Contour-Ansel D, Pham-Thi A, Zuily-Fodil Y. Isolation and characterization of four ascorbate peroxidase cDNAs responsive to water deficit in cowpea leaves. Ann. Bot. (Lond) 2006;97:133–140. doi: 10.1093/aob/mcj010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Castellarin S, Pfeiffer A, Sivilotti P, Degan M, et al. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell. Environ. 2007;30:1381–1399. doi: 10.1111/j.1365-3040.2007.01716.x. [DOI] [PubMed] [Google Scholar]
- 91.Castellarin S, Matthews M, Di Gaspero G, Gambetta G. Water deficits accelerate ripening and induce changes in gene expression regulating flavonoid biosynthesis in grape berries. Planta. 2007;227:101–112. doi: 10.1007/s00425-007-0598-8. [DOI] [PubMed] [Google Scholar]
- 92.Fait A, Fromm H, Walter D, Galili G, Fernie A. Highway or byway: the metabolic role of the GABA shunt in plants. Trends Plant Sci. 2007;13:14–19. doi: 10.1016/j.tplants.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 93.Bytof G, Knopp S-E, Schieberle P, Teutsch I, Selmar D. Influence of processing on the generation of gamma-aminobutyric acid in green coffee beans. Euro. Food Res. Tech. 2005;220:245–250. [Google Scholar]
- 94.Rhodes D, Handa S, Bressan R. Metabolic changes associated with adaptation of plant cells to water stress. Plant Physiol. 1986;82:890–903. doi: 10.1104/pp.82.4.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taji T, Ohsumi C, Iuchi S, Seki M, et al. Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J. 2002;29:417–426. doi: 10.1046/j.0960-7412.2001.01227.x. [DOI] [PubMed] [Google Scholar]
- 96.Robinson S, Jacobs A, Dry I. A class IV chitinase is highly expressed in grape berries during ripening. Plant Physiol. 1997;114:771–778. doi: 10.1104/pp.114.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tesnière C, Torregrosa L, Pradal M, Souquet J, et al. Effects of genetic manipulation of alcohol dehydrogenase levels on the response to stress and the synthesis of secondary metabolites in grapevine leaves. J. Exp. Bot. 2006;57:91–99. doi: 10.1093/jxb/erj007. [DOI] [PubMed] [Google Scholar]
- 98.Coleman H, Ellis D, Gilbert M, Mansfield S. Up-regulation of sucrose synthase and UDP-glucose pyrophosphorylase impacts plant growth and metabolism. Plant Biotechnol. J. 2006;4:87–101. doi: 10.1111/j.1467-7652.2005.00160.x. [DOI] [PubMed] [Google Scholar]
- 99.Watson B, Asirvatham V, Wang L, Sumner W. Mapping the proteome of barrel medic (Medicago truncatula). Plant Physiol. 2003;131:1104–1123. doi: 10.1104/pp.102.019034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu Y, Lamkemeyer T, Jakob A, Mi G, et al. Comparative proteome analyses of maize (Zea mays L.) primary roots prior to lateral root initiation reveal differential protein expression in the lateral root initiation mutant rum1. Proteomics. 2006;6:4300–4308. doi: 10.1002/pmic.200600145. [DOI] [PubMed] [Google Scholar]
- 101.Faurobert M, Mihr C, Bertin N, Pawlowski T, et al. Major proteome variations associated with cherry tomato pericarp development and ripening. Plant Physiol. 2007;143:1327–1346. doi: 10.1104/pp.106.092817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gion J-M, Lalanne C, Le Provost G, Ferry-Dumazet H, et al. The proteome of maritime pine wood forming tissue. Proteomics. 2005;5:3731–3751. doi: 10.1002/pmic.200401197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.