Abstract
Cytokinin has long been shown to be an essential modulator of growth and development in plants. However, its implications in plant immunity have only recently been realized. The interaction between jasmonate and salicylate pathways is regarded as a central backbone of plant immune defense. However, the effect of cytokinin on the jasmonate and salicylate mediated balance in plant immunity is still not known. Here, we analyze the impact of cytokinin on the jasmonate-salicylate antagonism in Arabidopsis immunity regarding infection with hemibiotrophic pathogen Pseudomonas syringae pv tomato DC3000 (Pst DC3000). Systems biology analysis of a refined hormone immune pathway model provides insights into the impact of cytokinin on the balance between jasmonate and salicylic acid pathways in Arabidopsis. Targeted experiments validate model simulations monitoring bacterial growth in wild type plants as well as in jasmonate pathway mutants. An integrated analysis shows that CK promotes the SA pathway of plant immunity and does not promote JA-mediated Arabidopsis susceptibility against infection with Pst DC3000. Finally, we discuss these results in the context of an emerging model of auxin-cytokinin antagonism in plant immunity.
Keywords: Modelling, cytokinin, hormone, infection, interaction, pathogen, systems biology
The antagonism between salicylic acid (SA) and jasmonic acid (JA) in immune dynamics of plants is well known.1 Plant growth hormones such as auxin have been shown to mediate Arabidopsis susceptibility against infection with biotrophic pathogens in SA inhibition dependent2 and independent3 manners. Gibberellic acid (GA) on the contrary antagonizes JA responses by degrading DELLA proteins and enhances plant immunity.4 Different types of cytokinin (CK) such as kinetin, trans-zeatin, 6-benzylaminopurine, and thidiazuron have been shown to promote plant defense against hemi-biotrophic and biotrophic pathogens.5,6 Enhanced CK levels and increased sensitivity to CK reported to protect Arabidopsis against infection with necrotrophic pathogens.7 However, also a promoting role of CK in the establishment of gall-causing pathogens and green-island generating fungal biotrophs in plants has been described.7,8 These reports point to a dual role of CK in promoting both jasmonate as well as salicylate pathways of immune defense in plants.
We focused specifically on the biological aspect of the CK based modulation and its impact on SA-JA balance in immunity for model plant Arabidopsis against infection with Pst DC3000. Plant immunity is a phenomenon of intricate hormonal interplays.1 Systems biology approaches have higher potential to resolve complex hormonal interactions occur in plant during immune defense.1,9,10 Recently, an integrated Boolean model on hormonal interactions has been shown to define the emerging role of CK in regulating immune dynamics against pathogen infection in Arabidopsis.9 We reconstituted this starting model,9 where additional unequivocal modifications were introduced with supporting references for nodes and types of interactions (Fig. S1 and Table S1 show which additional nodes are added to starting model). The extended and refined Boolean network includes additional nodes to better specify mutual interaction between pathways of jasmonate and salicylate as well as CK-mediated crosstalk to these pathways. In particular, we considered newly emerging connections within the JA pathway such as interactions between various types of JAZ proteins and MYC2 as well as in general how this sub-network is embedded and connected to the whole plant immune network (Fig. S1). Moreover, specific metabolic enzymes for hormones glycosylation and ROS (Reactive Oxygen Species) homeostasis were also included to consider their effects on hormonal regulation of plant immunity. In this regard, ABA-glucosyltransferase as well as SA and CK glycosylation enzymes are included in the model, which influence the homeostasis of active hormone circulation. Generation and scavenging of ROS plays a pivotal role in plant pathogen defense. Interactions between ROS and SA are maintained through various factors such as RbohD, peroxidases, SOD, and various types of catalases (Fig. S1).
The implemented Boolean model is capable to handle both gene expression events as well as biochemical molecular interactions such as hormone signaling in canonical pathways, protein kinase cascades (see network topology, Fig. S1). Node connections are based on the Boolean logic of activation and inhibition. All positive attributes such as overexpression, activation, stabilization, promotion, protection, and so forth are regarded as activation (YES gates). Downregulation, repression, degradation, and so forth were modeled as inhibition (NOT gates). Furthermore, we retested the network in its logical connectivity and activation states after incorporating various modifications to reconfirm the robustness and validity of the simulation results obtained. It is important to distinguish molecular interaction with signaling molecules and pre-existing proteins (gene expression products often constitutive) and interactions between nodes merely altering the gene expression level (thus controlled level of proteins). Furthermore, the case of ethylene perception and signal transduction is shown as a relay of repeated NOT gates, as the interactions shown are biochemical. Likewise, two-component system (TCS)-based CK-mediated phosphorelay are events of repeated YES gates. We distinguish between all these different types of interactions and provide detailed literature support for each and every node and edge of the network (Table S1).
Analysis of the new model as readout of the immune marker node pathogenesis related protein1 (PR1) provides insights into the role of various hormones in immune dynamics of Arabidopsis. It is worth mentioning that our simulations not only lead a path across the network to immune node (PR1); rather it takes the behavior of each and every node across the network into consideration (see Naseem et al.9 for further details). Therefore, instead of the activation of a factor such as PR1, it defines the full network effects and reactions upon a given input stimuli. We analyzed the impact of CK on the SA–JA hormonal balance in plant immunity. We performed targeted experiments to validate model simulations by monitoring bacterial growth in wild type and mutant Arabidopsis plants.
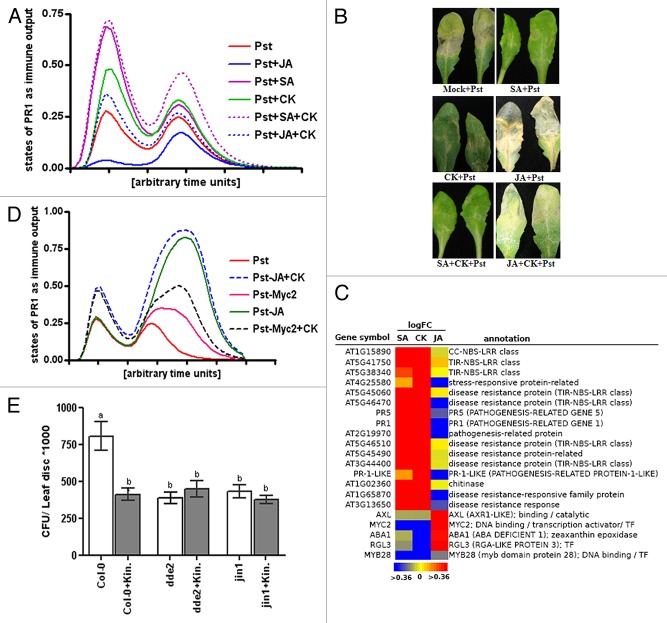
We computed immune output (Fig. S1 and Methods) by taking the node of Pst DC3000 alone as input signal to the system as well as in combination with nodes representing plant hormones such as SA, JA, and CK (Fig. 1A). Simulations of the model show an increase in immune output for Pst infection in combination with SA and CK in comparison to an infection pulse by Pst alone. However, addition of the node representing JA in combination with Pst infection as combined input causes a decline in the immune output compared with that computed for SA, CK, and Pst alone (Fig. 1A). Furthermore, combination of SA and CK to Pst infection as input further enhances immune response. The combination of JA and CK brought back the immune signal to the level of Pst alone. The signal is low in comparison to the combined output signal for SA and CK (Fig. 1A). Summarizing these different results, our model shows synergism between CKs and SA and an antagonistic interaction between JA and CKs in modulating immune dynamics of Arabidopsis against infection with Pst DC3000. To validate these model simulations, we experimentally monitored bacterial growth in 4-week- old Arabidopsis leaves with and without application of the aforementioned hormonal combinations. We noticed a significant decline in bacterial growth in Arabidopsis upon the application of SA and kinetin as well as their combination in comparison to control treatments (Fig. 1B; Fig. S2). However, external application of methyl-jasmonate (Me-JA) alone lead to significantly higher bacterial growth as compared with control, SA as well as kinetin treatments. We did not notice significant differences for bacterial multiplication between mock treatment and the combination of Me-JA and kinetin solutions (Fig. 1B; Fig. S2). We therefore conclude that by promoting SA responses, CK protects Arabidopsis against infection with Pst DC3000 and attenuates susceptibility imposed by enhanced JA responses.
Figure 1. Integrated analysis of the effect of CK on the balance between SA and JA pathways of resistance in Arabidopsis against infection by Pst DC3000. (A) Simulating the impact of CK on the interplay between SA and JA pathways of immunity. Trajectories show immune signal outputs as activation of disease marker node PR1 (y-axis) over arbitrary units of time (x-axis) for Pst DC3000 alone (red line) as well as Pst DC3000 in combination with hormonal input stimuli such as JA (blue line), SA (pink line), CK (green line), combined input stimuli of SA and CK (pink dots), and combined input stimuli of JA and CK (blue dots). Figure keys as lines and dots are shown on the right. (B) Experimental data on the growth of Pst DC3000 in Arabidopsis leaves treated with hormones. Four-week-old Col-0 Arabidopsis leaves are syringe infiltrated with Pst DC3000 (105cfu/ml). 24h before pathogen inoculation, leaves were treated with 10mM MgCl2 as control (left panel; top row), 1mM SA (right panel; top row), 10μM kinetin (left panel; top row), 1mM MeJA (right panel; middle row), combination of SA and kinetin (left panel; bottom row), and combination of MeJA and kinetin (right panel; bottom row). Symptoms were photographed 3 d after pathogen infiltration. Similar results were obtained in 3 independent experiments. See further quantification of bacterial growth in Figure S2. (C) The impact of SA, JA, and CK on the regulation of immune-related genes. Raw Arabidopsis microarray data on hormones treatment vs mock was extracted from GEO (Gene Expression Omnibus) database of NCBI. GEO2R, a GEO-based web portal was used for the identification of significantly regulated genes across SA vs mock (GEO experiment ID: GSE 3984), Zeatin vs mock (GEO experiment ID: GSE 6832), and MeJA vs mock (GEO experiment ID: GSE21762). Data are plotted as heatmap including gene symbols (left row), logFC-values for SA, CK, and JA (middle row), and a short annotation of the regulated genes (right row). See further details on expression analysis, t-values, and P-values in Table S2. (D) Simulating the impact of CK on infection by Pst DC3000 in modulated JA responses. Trajectories show immune signal outputs as activation of disease marker node PR1 (y-axis) over arbitrary units of time (x-axis) for Pst DC3000 alone (red line) as well as Pst DC3000 with (blue dots) and without (green line) CK input stimuli and deletion of node representing JA, with (black dots) and without (pink lines) CK input stimuli and deletion of node representing MYC2. Figure keys as lines and dots are shown on the right. (E) Effect of CK on infection by Pst DC3000 in JA mutants. Bacterial growth was quantified for Col-0 with kinetin, for dde2 mutants with and without CK, and for jin1 mutants with and without addition of kinetin. Arabidopsis Col-0 and mutants leaves were syringe infiltrated with a suspension of Pst DC3000 (103 colony-forming units [CFU]/mL). Statistical differences (a to b) between treatments were calculated by analysis of variance (p < 0.05, n = 5). The experiment was repeated 3 times with similar observed trends.
The synergism between pathways of SA and CK and antagonism between JA and CK was analyzed further. The interaction between SA and CK pathways for pathogen defense occurs downstream of SA biosynthesis10 and the B-type cytokinin response regulator ARR2 has been shown to interact with bZIP transcription factor (TGA3) in regulating defense marker gene PR1.7 However, promoting interaction between SA and cytokinin is not limited to the expression of an immune marker gene; genome-wide, there is broader overlay between pathways of SA and CK in regulating genes relevant to pathogen defense in plants (Fig. 1C; Table S2). We analyzed genome-wide regulation of immunity-related genes modulated by SA, CK, and Me-JA using microarray experiments which had previously been deposited to Gene Expression Omnibus (GEO; accessions, Figure 1C legend). According to this analysis, SA regulates immune genes such as the class of CC-NBS-LRRs and TIR-NBS-LRRs as well as pathogenesis-related protein genes and chitinases. These genes are also similarly regulated by CK. However, application of Me-JA has either no regulatory effect or has opposing effects on the regulation of these immune-related genes (Fig. 1C). Genes that mediate susceptibility against infection with Pst DC3000 are upregulated by JA. Examples are MYC2, ABA1, and RGL3. However, these are downregulated by SA and CKs. We also analyzed the level of JA accumulation after treating Arabidopsis leaves with kinetin. We did not find significant increase in the accumulation of JA levels after Pst DC3000 challenge with and without the application of exogenous kinetin (Fig. S3). Unlike the interaction between ARR2 and TGA,7 which represents a point of crosstalk between pathways of SA and CK, the nature of interaction between JA and CK is presently not well described.
To get further insights into the crosstalk between JA and CK and its impact on Pst DC3000 and Arabidopsis interactions, we analyzed our model again (Fig. 1D): We simulate immune output of the infection by Pst DC3000 in the absence of JA (to mimic the effect of JA biosynthesis mutant) and MYC2 (to mimic the effect of important JA-dependent immune transcriptional responses) from our network. We tested both conditions with and without the input signal of CK. According to these analyses, removals of the JA pathway from the plant hormone immune network (Fig. S1) further promotes the immune signal against infection by Pst DC3000 in Arabidopsis in comparison to the wild type situation (Fig. 1D). Moreover, activation of CK with removal of JA further enhances the immune output signal in comparison to the deletion of JA alone. Likewise, our analysis shows that deletion of MYC2 transcription factor promotes the immune signal of Arabidopsis to the infection of Pst DC3000. Moreover, addition of CK as input after deleting the MYC2 node from the network further enhances the immune signal output (Fig. 1D). It is worth mentioning that the impact of the deletion of JA synthesis is stronger than the removal of MYC2 from the network topology and thus points to MYC2 independent JA dependent responses.
To validate these modeling simulations regarding the interaction of JA and CK in plant immunity, we experimentally quantified the spread of Pst DC3000 in wild type as well as JA biosynthesis and signaling mutants with and without application of CK. In the experiments, external application of kinetin significantly reduced bacterial multiplication in Col-0 Arabidopsis plants in comparison to control treatment (Fig. 1E). A mutant of JA biosynthesis pathway enzyme allene oxide synthase (also known as dde2: DELAYED DEHISCENCE 2) manifested significantly decreased bacterial growth as compared with wild type plants (Fig. 1E). However, treatment of dde2 mutants with CK did not significantly affect bacterial growth in comparison to the treatment of these mutant plants with mock solution. Similarly, Jin1 mutant showed significant reduction in bacterial multiplication as compared with wild type plants, but treatment of CK did not significantly change the bacterial growth with the application of kinetin as compared with mock treatment (Fig. 1E). Taken together, our modeling results and experimental data establish that enhanced immune responses due to impaired JA pathway cannot be reversed into susceptibility with the application of CK.
CK has been shown to be involved in the generation of green-islands by fungal biotrophs as well as to have promoting effects on plant resistance against infection with necrotrophic pathogens, viruses, and attack by herbivores.7,8 As well, mutation of CK signaling inhibitors A type ARRs also lead to increased expression of JA pathway marker gene PDF 1.2.5 This evidence points to the possibility of CK positive crosstalk to JA mediated immune pathway in plants. However, with the above modeling and targeted experiments, we show that CK promotes the SA pathway of plant immunity and does not promote JA-mediated Arabidopsis susceptibility against infection with Pst DC3000. These results are consistent with an emerging model of antagonism between auxin and CK in plant immunity.11 According to this model, auxin promotes plant susceptibility by reinforcing JA immune pathway, while increased CK responses promote the SA pathway of resistance. CK signaling circuitry has the potential to reinforce both JA as well as SA sectors of immunity. However, for the interaction of Pst DC3000 with Arabidopsis, our data support only positive crosstalk between CK and SA. Future studies on how CK signaling switches system states in promoting the JA pathway of immunity against infection by certain pathogens will be quite intriguing.
Supplementary Material
Acknowledgments
We thank Deutsche Forschungsgemeinschaft for funding (Da 208/10–2).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
Supplementary material may be found here: http://www.landesbioscience.com/journals/psb/article/26791/
References
- 1.Pieterse CMJ, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SCM. Hormonal modulation of plant immunity. Annu Rev Cell Dev Biol. 2012;28:489–521. doi: 10.1146/annurev-cellbio-092910-154055. [DOI] [PubMed] [Google Scholar]
- 2.Robert-Seilaniantz A, MacLean D, Jikumaru Y, Hill L, Yamaguchi S, Kamiya Y, Jones JD. The microRNA miR393 re-directs secondary metabolite biosynthesis away from camalexin and towards glucosinolates. Plant J. 2011;67:218–31. doi: 10.1111/j.1365-313X.2011.04591.x. [DOI] [PubMed] [Google Scholar]
- 3.Mutka AM, Fawley S, Tsao T, Kunkel BN. Auxin promotes susceptibility to Pseudomonas syringae via a mechanism independent of suppression of salicylic acid-mediated defenses. Plant J. 2013;74:746–54. doi: 10.1111/tpj.12157. [DOI] [PubMed] [Google Scholar]
- 4.Yang D, Yao J, Mei C, Tong X, Zeng L, Li Q, et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. 2012;109 doi: 10.1073/pnas.1201616109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argueso CT, Ferreira FJ, Epple P, To JPC, Hutchison CE, Schaller GE, Dangl JL, Kieber JJ. Two-component elements mediate interactions between cytokinin and salicylic acid in plant immunity. PLoS Genet. 2012;8:e1002448. doi: 10.1371/journal.pgen.1002448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grosskinsky DK, Naseem M, Abdelmohsen UR, Plickert N, Engelke T, Griebel T, Zeier J, Novák O, Strnad M, Pfeifhofer H, et al. Cytokinins mediate resistance against Pseudomonas syringae in tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling. Plant Physiol. 2011;157:815–30. doi: 10.1104/pp.111.182931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi J, Choi D, Lee S, Ryu C-M, Hwang I. Cytokinins and plant immunity: old foes or new friends? Trends Plant Sci. 2011;16:388–94. doi: 10.1016/j.tplants.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Walters DR, McRoberts N, Fitt BDL. Are green islands red herrings? Significance of green islands in plant interactions with pathogens and pests. Biol Rev Camb Philos Soc. 2008;83:79–102. doi: 10.1111/j.1469-185X.2007.00033.x. [DOI] [PubMed] [Google Scholar]
- 9.Naseem M, Philippi N, Hussain A, Wangorsch G, Ahmed N, Dandekar T. Integrated systems view on networking by hormones in Arabidopsis immunity reveals multiple crosstalk for cytokinin. Plant Cell. 2012;24:1793–814. doi: 10.1105/tpc.112.098335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naseem M, Kunz M, Ahmed N, Dandekar T. Integration of boolean models on hormonal interactions and prospects of cytokinin-auxin crosstalk in plant immunity. Plant Signal Behav. 2013;8 doi: 10.4161/psb.23890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naseem M, Dandekar T. The role of auxin-cytokinin antagonism in plant-pathogen interactions. PLoS Pathog. 2012;8:e1003026. doi: 10.1371/journal.ppat.1003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.