Abstract
Experiments performed in actively proliferating plant cells both in space and simulated microgravity have evidenced a common effect: cell proliferation appears enhanced whereas cell growth is depleted. Coordination of cell growth and proliferation, called meristematic competence, is a major feature of meristematic cells and its disruption may lead to important alterations in the developmental pattern of the plant. Auxin is known to be a mediator of the transduction of the gravitropic signal and a regulator of the rates of growth and proliferation in meristematic cells, as well as of their further differentiation. Therefore, gravity sensing, gravitropism, auxin levels, and meristematic competence are mutually interrelated. However, our experiments in simulated microgravity, using both mechanical and magnetic levitation technologies, have revealed that this interdependence is neither strict nor univocal and may include additional factors and mechanisms. Available data indicate that altered gravity may affect cell growth and proliferation by mechanisms alternative to the transduction of the gravitropic signal perceived by columella cells in the root tip. These mechanisms would include gravity sensing independent from statolith displacement and transduction mediators other than polar auxin transport.
Keywords: cell cycle, ribosome biogenesis, nucleolus, graviperception, simulated microgravity, Arabidopsis
The study of the effect of gravity on biological functions and mechanisms, e.g., those related to plant development, is largely facilitated by avoiding the persistent effect of Earth gravity. For this purpose, it is necessary to perform real microgravity experiments in orbiting space facilities such as the International Space Station (ISS), since this is the only way to obtain durable good quality microgravity.1 However, access to the ISS is compromised in quantity and quality of the experimental approaches that can be developed in each scientific mission. Alternatively, Ground Based Facilities (GBF) can be used on ground to achieve simulated microgravity conditions using both mechanical/inertial technologies such as the one used in 2D-clinostat and random positioning machines (RPM),2 or magnetic levitation facilities,3 but risking that the observed effect is the sum of the microgravity effects and the artifacts or side-effects of the simulation technology involved.1
A previous experiment was designed in our laboratory to be performed in real microgravity conditions (during the ISS Cervantes mission in 2003), in parallel with a simulated microgravity experiment (RPM) and with a ground 1g control. Cell proliferation and growth in root meristematic cells were analyzed in the different samples. In both real and simulated microgravity, a similar enhanced rate of cell proliferation was revealed, accompanied by a reduction of ribosome biogenesis per cell, compared with 1g controls4; ribosome biogenesis is generally recognized as a reliable indicator of cell growth in highly proliferative meristematic cells.5,6 These were relevant findings, since the alteration of cell growth and proliferation in the root meristem (the so-called “meristematic competence”7) may have consequences at the level of development and shaping of the whole plant.8 Furthermore, auxin regulation may link these cellular alterations in the meristem with the gravitropic signal perceived by columella cells in the root tip. When the environmental gravity conditions change, a transduction cascade results in the modification of the levels and distribution of auxin throughout the root.9,10 Auxin is a phytohormone that influences multiple aspects of growth and differentiation in plants, among which the coordination between cell growth and cell division. Low levels of auxin induce cell elongation, enlargement, and differentiation; whereas high levels of auxin stimulate cell proliferation and cell cycle progression.8,11 As a consequence, the role of auxin as a mediator of the maintenance of meristematic competence under normal gravity conditions would be modified in response to the change in the environmental gravity conditions.9
The strict link between cell growth and cell proliferation is uncoupled by the synergistic contribution of both altered gravity and high energy magnetic fields required for levitation
The results of a magnetic levitation experiment with a similar design to the Cervantes Mission one, but involving some more complexity, have recently been published.12 The novel aspects were the use of the transgenic line CYCB1::GUS, allowing the in situ detection of the expression of the cyclin B1 gene, and the sequential character of the study, comprising 2 sampling points in the seedling development, namely at two and 4 days after seed hydration. Apart from this, the magnet architecture and the properties of the applied magnetic field allowed us the use of 3 different positions for placing the samples, each one characterized by a different level of effective gravity (g*). The gravity levels used were 0g*, 1g* and 2g*. For each position and for each sampling time, the analysis consisted of biometrical estimations of the seedling and root length, quantitative measurements at the cellular level, including number of cells per millimeter in specific cell files, in order to get an estimate of the cell proliferation rate, quantitative densitometric estimations of the expression of cyclin B1 gene, and morphometric, ultrastructural, and immunocytochemical study of the nucleolus, in order to know the rate of ribosome biogenesis, which, as previously indicated, is a fully reliable marker of cell growth in the root meristem. All these results were in agreement with the previous findings in spaceflight,4 thus validating the magnetic levitation technology as a microgravity simulation facility. Certainly, the high magnetic field responsible for the gravity alteration perceived by samples was capable of partially masking some of the levitation effects. In addition, parallel reports analyzing cell growth and proliferation in seedlings exposed to simulated microgravity and hypergravity conditions obtained by mechanical means have been released13 with similar conclusions to those obtained from spaceflight and magnetically levitated samples.
Magnetic levitation, polar auxin transport, and gravitropism
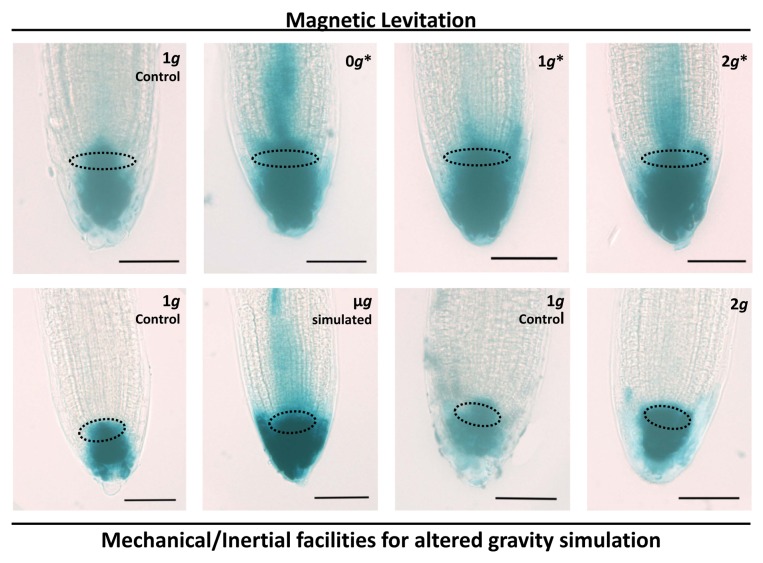
An additional methodological novelty of the magnetic levitation experiment, in comparison to previous studies, was the use of the DR5::GUS strain to reveal the auxin distribution pattern in the root tip in the different conditions existing inside and outside the magnet. In samples exposed to magnetic levitation, auxin distribution always appeared abnormal at all 3 levels of effective gravity,12 with a pattern resembling the distribution observed after drug-induced inhibition of polar transport14 (Fig. 1, first row). The same staining pattern was observed under RPM-simulated microgravity, but not during mechanical hypergravity exposure to 2g13 (Fig. 1, second row).
Figure 1. Auxin distribution in root tips revealed by GUS staining. The use of the reporter gene line DR5::GUS allowed the microscopical visualization of the auxin distribution. Whole mount preparation of roots were stained and observed by light microscopy. In the upper row, microscopical images of DR5::GUS-stained root meristems from seedlings grown for 4 d in the magnetic levitation facilities;12 From left to right: samples from the 1g external control, 0g*, 1g*, and 2g* positions in the magnet. In the lower row, the same approach was used in experiments using mechanical/inertial facilities for altered gravity simulation. From left to right: samples from the 1g external control and samples grown under simulated microgravity in the Random Positioning Machine (RPM), followed by the 1g external control and samples grown under hypergravity (2g) in the long-diameter centrifuge (LDC).13 The GUS staining shows the distribution of auxin in the root tip. The area limited by dotted lines in each image corresponds to the quiescent center. Two patterns of staining can be distinguished in the images: the first one comprises the quiescent center and the columella and can be found in images corresponding to 1g controls and in the 2g LDC-grown sample. The second pattern shows the same stained areas, but the staining extends to the whole root tip, including at least a part of the root meristem, with a faint extension toward the central cylinder of the root. These patterns have been described in the literature as corresponding, respectively, to normal and drug-inhibited polar auxin transport.14 Bars indicate 50 µm.
To understand the different effects of the mechanical and magnetic methods of altering gravity on the polar auxin transport, we should consider 2 important limitations of magnetic levitation, when biological materials are exposed to it: first, this technology requires magnetic fields of very high magnitude (around 12 Tesla) concentrated in a small area to maximize magnetic field gradient. This magnetic field itself leads to effects on cell components, as observed in the 1g* samples in which gravity is not compensated, but the high energy magnetic fields are still present. The result is that polar auxin transport is affected by this high energy magnetic field. However, as shown in Figure 1, the effect is maximized in the 0g* position, associated with other alterations in cell proliferation and cell growth parameters; in this position, samples are supporting a synergy between magnetic field and microgravity simulation.12 Second, diamagnetic levitation acts at the molecular level and depends on diamagnetic properties and density of each material; since the setting of the 0g* position within the magnet corresponds to the diamagnetic levitation of water, there will be different materials in the cells which will not levitate in these conditions. Specifically, this is the case of starch granules which are the main component of statoliths, the starch-based organelles in the columella cells responsible of the gravitropism.15 The consequences of this fact are multiple: in general, the different susceptibility to the magnetic field of various organelles may lead to an overall cellular readjustment, affecting differentially to the various cellular components; in particular, if statoliths do not levitate under water levitation conditions, seedlings will show conventional gravitropic responses at the 0g* position in the magnet. This means that disruption of meristematic competence in root meristematic cells resulting from magnetic levitation-induced effective microgravity is independent of statolith movements in columella cells and of gravitropic alterations in the root growth, but it is associated with auxin delocalization in the root tip.
An additional interesting consideration that can be extracted from this result is that the behavior of statoliths in the 0g* position in the magnet could resemble the response of these organelles to a scenario of partial (fractional) gravity. The magnetic field exerts a force on statoliths in the opposite sense to the gravity force, which is not capable of totally counteracting the Earth gravity. More investigation is needed to know exactly the resulting g* received by statoliths in these conditions and to compare the situation in the magnet with a real or simulated environmental scenario including a similar gravity level.
The complex mechanism of transduction of gravity mechanosignals: different systems and different mediators
In a previous paper in this journal, published as an addendum to the article reporting the results of our first spaceflight experiment, we stressed the crucial role played by auxin as the key mediator between the altered gravitational mechanosignal and the response of root meristematic cells involving the disruption of meristematic competence.9 Now, the main lesson learned from our experiment on magnetic levitation, apart from the confirmation of the effect of gravity alteration on meristematic cells, is that the mechanisms of sensing and transduction of this signal in the root are more complex than initially considered and may involve different players in addition to auxin.
In any case, this novel assumption, experimentally supported, should not lead us to minimize the central role played by auxin. As discussed in our previous paper, polar auxin transport is an unequivocal target of the transduction of the gravitropic signals originated in the root cap cells when they are converted from mechanical into chemical16,17; moreover, auxin controls the continuous activity of growth and proliferation in meristematic cells, and its distribution in roots sets up distinct zones for cell division, cell expansion or elongation, and cell differentiation and determines the balance between them.18,19 However, in our magnetic levitation experiment, we have shown that it is possible to find alterations in cellular functions induced by a gravity change which has not been perceived by the statolith-containing columella cells of the root cap. Actually, in both plant and animal cells it has been reported the perception of mechanical signals by cells not apparently specialized in gravity sensing20-22 and, in several laboratories, different research groups, including us, have reported genomic and proteomic effects of an altered gravity environment (even of spaceflight) on Arabidopsis callus cell cultures.23-27
In the case of the magnetic levitation, the response at the meristematic cell level is associated to an altered polar auxin transport; this means that there should be an intermediate factor capable of linking the signal sensed in the cell by the diamagnetic levitation of water and the alteration of the polar auxin transport. In turn, cell cultures lack both, gravitropic signals induced by statolith movements and polar auxin transport However, gravity alteration is sensed by cells and results in cellular and molecular effects, also including disruption of meristematic competence (unpublished results). Therefore, in this case, a factor (single or multiple) should be found capable of linking the gravity alteration signal and the regulation of cell growth and proliferation, without any involvement of any change of the auxin levels.
The conclusion of this analysis is that gravity sensing may or may not involve statolith movement and, consequently it may or may not produce gravitropic effects; furthermore, the transduction of the signal may or may not affect the polar auxin transport, in order to induce in meristematic cells the alterations in growth and proliferation capable of disrupting meristematic competence. It is conceivable that different mechanisms of gravity sensing and signal transduction (within a cell or throughout cells) involving different molecular and cellular players and mediators may exist in different biological systems and even co-exist in a biological model (plants or cell cultures; real or simulated microgravity; mechanical or magnetic simulation, see Fig. 2). In fact, it has been proposed that cells outside the cap root area are capable of producing a partial gravitropic response in maize.29,30 Consequently, sensing of altered gravity and its downstream effects involve multiple cell types and several different intra cellular mechanisms.
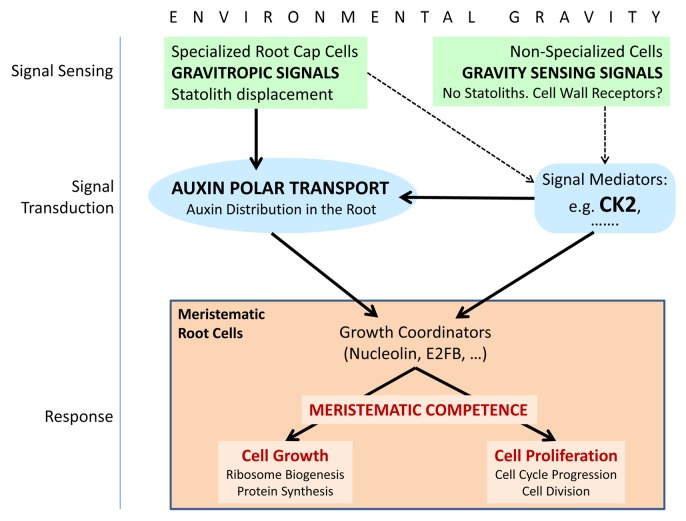
Figure 2. Schematic model of the main factors and functional processes playing a role in the regulation of the functionality of meristematic cells by environmental gravity. Solid arrows represent experimentally supported connections, whereas dashed arrows indicate suitable processes, compatible with experimental data, but still pending of further investigation for their demonstration. The scheme is based on a previously published model.9 Sensing of the parameters of the gravity vector (magnitude, direction) may occur in different cellular types of the root by different mechanisms. In cells of the columella, in the root cap, gravity induces displacement (sedimentation) of statoliths, which is a requisite for establishing the gravitropic growth of the root. In fact, those environmental alterations which do not change the statolith position do not result in gravitropic changes. Gravitropic signals are transduced from the root cap to other regions of the root, resulting in alterations of the polar auxin transport. The mechanism of transduction of this signal is not totally understood and not all the mediators of this process have been experimentally identified. However, it is well known that the levels of auxin in root meristematic cells regulate the rates of cell growth and proliferation and establish the close coordination of these functions, that is, meristematic competence. In root cells other than columella cells, gravity can be sensed by mechanisms different from the statolith sedimentation. This alternative mechanism of gravity sensing can also be functional in proliferating in vitro cultured cells. Interestingly, in absence of statolith displacements, the effects of gravity alteration on cell growth and proliferation also produce the disruption of meristematic competence. Furthermore, this effect may occur in absence of any alteration of auxin levels, as it is the case of cells in culture. Whereas cell wall has been proposed as a gravity receptor,28 mediators of the transduction of gravity mechanosignal sensed in this way are experimentally unknown. The protein kinase CK2 is proposed as a candidate to be part of this scheme in view of the experimental findings that put it in close relationship with some physiological and cellular processes involved, such as polar auxin transport, ribosome biogenesis, and cell cycle.
An interesting example of a molecular factor that could be inserted in this complex model is casein kinase 2 (CK2). This highly conserved ubiquitous protein kinase plays critical roles in a large variety of processes of animals, plants, and yeast. In plants, it has been shown to participate in the regulation of different developmental pathways, as well as in mechanisms of stress response.31 Recently, a role of CK2 in the regulation of polar auxin transport has been reported,32,33 resulting in an enhanced gravitropic response of mutant plants depleted in CK2 activity, which re-orient their root growth after rotation toward the new gravity vector faster than wild-type plants.33 Independent of this auxin-related function, CK2 is known to play a major role in the regulation of the cell cycle at different levels, specifically in the G2/M transition34 and also in ribosome biogenesis, since phosphorylation of nucleolin by CK2 is necessary for a normal processing of pre-ribosomal precursors.35,36 Therefore, although there is no direct evidence of the involvement of CK2 in the response to gravity alteration, the known functions of this essential protein kinase appoint it as a suitable candidate for this functional role. The use of the CK2 negative mutant in experiments of real or simulated microgravity could help in discerning this problem. In any case, the functions of CK2 in auxin transport, cell cycle, and ribosome biogenesis are a good example of the suitability of a model involving different mechanisms of gravity sensing and different signal transduction pathways to produce the same final result, namely the disruption in the cell proliferation and cell growth (Fig. 2).
Future prospects
The use of different altered gravity conditions to better understand the mechanisms of the disruption of meristematic competence has been successful, but providing more questions that need to be answered with more experiments. Apart from the specific experiment mentioned above, in order to test the effective involvement of CK2 in the process, we would need, in general, to keep exploring altered gravity effects in cell cultures, trying to uncover how many particular mechanisms of the alteration of meristematic competence are acting, and their dependence (or not) on auxin and/or on gravitropism-specialized organelles. On the other hand, further spaceflight missions are required to confirm these findings by using different mutants affecting auxin-responsive elements as well as cell proliferation and cell growth markers, e.g., nucleolin mutants. In the balance between ground-based facilities and spaceflight experiments in the International Space Station we expect to obtain further developments to be extrapolated to sustainable agriculture in suboptimal environmental conditions including life support systems for space exploration in the forthcoming years.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Work performed in the authors’ laboratory was supported by grants of the Spanish National Plan for Research and Development, Ref. Nos. AYA2010-11834-E, and AYA2012-33982 and by ESA Access to GBFs contract numbers 4200022650 and 4000105761. M.A.V. was supported by the Spanish FPI Program (Ref. BES-2010-035741) and K.Y. by the Spanish CSIC JAE-PreDoc Program (Ref. JAEPre_2010_01894).
References
- 1.Herranz R, Anken R, Boonstra J, Braun M, Christianen PCM, Geest MD, Hauslage J, Hilbig R, Hill RJA, Lebert M, Medina FJ, Vagt N, Ullrich O, Loon JJWAv, Hemmersbach R. . Ground-based facilities for simulation of microgravity, including terminology and organism-specific recommendations for their use. Astrobiology 2013; 13:1 - 17; http://dx.doi.org/ 10.1089/ast.2012.0876; PMID: 23252378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Loon JJWA. . Some history and use of the Random Positioning Machine, RPM, in gravity related research. Adv Space Res 2007; 39:1161 - 5; http://dx.doi.org/ 10.1016/j.asr.2007.02.016 [DOI] [Google Scholar]
- 3.Valles JM Jr., Guevorkian K. . Low gravity on earth by magnetic levitation of biological material. J Gravit Physiol 2002; 9:11 - 4; PMID: 14703664 [PubMed] [Google Scholar]
- 4.Matía I, González-Camacho F, Herranz R, Kiss JZ, Gasset G, van Loon JJ, Marco R, Javier Medina F. . Plant cell proliferation and growth are altered by microgravity conditions in spaceflight. J Plant Physiol 2010; 167:184 - 93; http://dx.doi.org/ 10.1016/j.jplph.2009.08.012; PMID: 19864040 [DOI] [PubMed] [Google Scholar]
- 5.Baserga R. . Is cell size important?. Cell Cycle 2007; 6:814 - 6; http://dx.doi.org/ 10.4161/cc.6.7.4049; PMID: 17404503 [DOI] [PubMed] [Google Scholar]
- 6.Srivastava M, Pollard HB. . Molecular dissection of nucleolin’s role in growth and cell proliferation: new insights. FASEB J 1999; 13:1911 - 22; PMID: 10544174 [PubMed] [Google Scholar]
- 7.Mizukami Y. . A matter of size: developmental control of organ size in plants. Curr Opin Plant Biol 2001; 4:533 - 9; http://dx.doi.org/ 10.1016/S1369-5266(00)00212-0; PMID: 11641070 [DOI] [PubMed] [Google Scholar]
- 8.Perrot-Rechenmann C. . Cellular responses to auxin: division versus expansion. Cold Spring Harb Perspect Biol 2010; 2:a001446; http://dx.doi.org/ 10.1101/cshperspect.a001446; PMID: 20452959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medina FJ, Herranz R. . Microgravity environment uncouples cell growth and cell proliferation in root meristematic cells: the mediator role of auxin. Plant Signal Behav 2010; 5:176 - 9; http://dx.doi.org/ 10.4161/psb.5.2.10966; PMID: 20173415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muday GK. . Auxins and tropisms. J Plant Growth Regul 2001; 20:226 - 43; http://dx.doi.org/ 10.1007/s003440010027; PMID: 12033223 [DOI] [PubMed] [Google Scholar]
- 11.Magyar Z, De Veylder L, Atanassova A, Bakó L, Inzé D, Bögre L. . The role of the Arabidopsis E2FB transcription factor in regulating auxin-dependent cell division. Plant Cell 2005; 17:2527 - 41; http://dx.doi.org/ 10.1105/tpc.105.033761; PMID: 16055635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manzano AI, Larkin OJ, Dijkstra CE, Anthony P, Davey MR, Eaves L, Hill RJ, Herranz R, Medina FJ. . Meristematic cell proliferation and ribosome biogenesis are decoupled in diamagnetically levitated Arabidopsis seedlings. BMC Plant Biol 2013; 13:124; http://dx.doi.org/ 10.1186/1471-2229-13-124; PMID: 24006876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manzano AI, Herranz R, Van Loon J, Medina FJ. . Cell growth and cell proliferation decoupling under hypergravity environments induced by centrifugation. Microgravity Sci Technol 2012; 24:373 - 81; http://dx.doi.org/ 10.1007/s12217-012-9301-1 [DOI] [Google Scholar]
- 14.Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, et al. . Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 2001; 13:843 - 52; PMID: 11283340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuznetsov OA, Hasenstein KH. . Intracellular magnetophoresis of amyloplasts and induction of root curvature. Planta 1996; 198:87 - 94; http://dx.doi.org/ 10.1007/BF00197590; PMID: 8580774 [DOI] [PubMed] [Google Scholar]
- 16.Boonsirichai K, Guan C, Chen R, Masson PH. . Root gravitropism: an experimental tool to investigate basic cellular and molecular processes underlying mechanosensing and signal transmission in plants. Annu Rev Plant Biol 2002; 53:421 - 47; http://dx.doi.org/ 10.1146/annurev.arplant.53.100301.135158; PMID: 12221983 [DOI] [PubMed] [Google Scholar]
- 17.Kiss JZ. . Mechanisms of the early phases of plant gravitropism. CRC Crit Rev Plant Sci 2000; 19:551 - 73; http://dx.doi.org/ 10.1016/S0735-2689(01)80008-3; PMID: 11806421 [DOI] [PubMed] [Google Scholar]
- 18.Bhalerao RP, Bennett MJ. . The case for morphogens in plants. Nat Cell Biol 2003; 5:939 - 43; http://dx.doi.org/ 10.1038/ncb1103-939; PMID: 14593411 [DOI] [PubMed] [Google Scholar]
- 19.Teale WD, Paponov IA, Palme K. . Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 2006; 7:847 - 59; http://dx.doi.org/ 10.1038/nrm2020; PMID: 16990790 [DOI] [PubMed] [Google Scholar]
- 20.Kordyum EL. . Biology of plant cells in microgravity and under clinostating. Int Rev Cytol 1997; 171:1 - 78; http://dx.doi.org/ 10.1016/S0074-7696(08)62585-1; PMID: 9066125 [DOI] [PubMed] [Google Scholar]
- 21.Dai ZQ, Wang R, Ling SK, Wan YM, Li YH. . Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells. Cell Prolif 2007; 40:671 - 84; http://dx.doi.org/ 10.1111/j.1365-2184.2007.00461.x; PMID: 17877609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cogoli A, Cogoli-Greuter M. . Activation and proliferation of lymphocytes and other mammalian cells in microgravity. Adv Space Biol Med 1997; 6:33 - 79; http://dx.doi.org/ 10.1016/S1569-2574(08)60077-5; PMID: 9048133 [DOI] [PubMed] [Google Scholar]
- 23.Herranz R, Manzano AI, van Loon JJ, Christianen PC, Medina FJ. . Proteomic signature of Arabidopsis cell cultures exposed to magnetically induced hyper- and microgravity environments. Astrobiology 2013; 13:217 - 24; http://dx.doi.org/ 10.1089/ast.2012.0883; PMID: 23510084 [DOI] [PubMed] [Google Scholar]
- 24.Manzano AI, van Loon JJWA, Christianen PC, Gonzalez-Rubio JM, Medina FJ, Herranz R. . Gravitational and magnetic field variations synergize to cause subtle variations in the global transcriptional state of Arabidopsis in vitro callus cultures. BMC Genomics 2012; 13:105; http://dx.doi.org/ 10.1186/1471-2164-13-105; PMID: 22435851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul AL, Ferl RJ, Meisel MW. . High magnetic field induced changes of gene expression in arabidopsis. Biomagn Res Technol 2006; 4:7; http://dx.doi.org/ 10.1186/1477-044X-4-7; PMID: 17187667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul A-L, Zupanska AK, Ostrow DT, Zhang Y, Sun Y, Li J-L, Shanker S, Farmerie WG, Amalfitano CE, Ferl RJ. . Spaceflight transcriptomes: unique responses to a novel environment. Astrobiology 2012; 12:40 - 56; http://dx.doi.org/ 10.1089/ast.2011.0696; PMID: 22221117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barjaktarović Z, Nordheim A, Lamkemeyer T, Fladerer C, Madlung J, Hampp R. . Time-course of changes in amounts of specific proteins upon exposure to hyper-g, 2-D clinorotation, and 3-D random positioning of Arabidopsis cell cultures. J Exp Bot 2007; 58:4357 - 63; http://dx.doi.org/ 10.1093/jxb/erm302; PMID: 18182437 [DOI] [PubMed] [Google Scholar]
- 28.Hoson T, Saito Y, Soga K, Wakabayashi K. . Signal perception, transduction, and response in gravity resistance. Another graviresponse in plants. Adv Space Res 2005; 36:1196 - 202; http://dx.doi.org/ 10.1016/j.asr.2005.04.095 [DOI] [Google Scholar]
- 29.Mancuso S, Barlow PW, Volkmann D, Baluska F. . Actin turnover-mediated gravity response in maize root apices: gravitropism of decapped roots implicates gravisensing outside of the root cap. Plant Signal Behav 2006; 1:52 - 8; http://dx.doi.org/ 10.4161/psb.1.2.2432; PMID: 19521476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolverton C, Mullen JL, Ishikawa H, Evans ML. . Root gravitropism in response to a signal originating outside of the cap. Planta 2002; 215:153 - 7; http://dx.doi.org/ 10.1007/s00425-001-0726-9; PMID: 12012252 [DOI] [PubMed] [Google Scholar]
- 31.Mulekar JJ, Bu Q, Chen F, Huq E. . Casein kinase II α subunits affect multiple developmental and stress-responsive pathways in Arabidopsis. Plant J 2012; 69:343 - 54; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04794.x; PMID: 21950772 [DOI] [PubMed] [Google Scholar]
- 32.Marquès-Bueno MM, Moreno-Romero J, Abas L, de Michele R, Martínez MC. . Linking protein kinase CK2 and auxin transport. Plant Signal Behav 2011; 6:1603 - 5; http://dx.doi.org/ 10.4161/psb.6.10.17136; PMID: 21918377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marquès-Bueno MM, Moreno-Romero J, Abas L, De Michele R, Martínez MC. . A dominant negative mutant of protein kinase CK2 exhibits altered auxin responses in Arabidopsis. Plant J 2011; 67:169 - 80; http://dx.doi.org/ 10.1111/j.1365-313X.2011.04585.x; PMID: 21435053 [DOI] [PubMed] [Google Scholar]
- 34.Espunya MC, Combettes B, Dot J, Chaubet-Gigot N, Martínez MC. . Cell-cycle modulation of CK2 activity in tobacco BY-2 cells. Plant J 1999; 19:655 - 66; http://dx.doi.org/ 10.1046/j.1365-313x.1999.00563.x; PMID: 10571851 [DOI] [PubMed] [Google Scholar]
- 35.Bögre L, Jonak C, Mink M, Meskiene I, Traas J, Ha DTC, Swoboda I, Plank C, Wagner E, Heberle-Bors E, et al. . Developmental and cell cycle regulation of alfalfa nucMs1, a plant homolog of the yeast Nsr1 and mammalian nucleolin. Plant Cell 1996; 8:417 - 28; PMID: 8721748 [PMC free article] [PubMed] [Google Scholar]
- 36.Caizergues-Ferrer M, Belenguer P, Lapeyre B, Amalric F, Wallace MO, Olson MOJ. . Phosphorylation of nucleolin by a nucleolar type NII protein kinase. Biochemistry 1987; 26:7876 - 83; http://dx.doi.org/ 10.1021/bi00398a051; PMID: 3427111 [DOI] [PubMed] [Google Scholar]