Abstract
The persistence of airway hyperresponsiveness (AHR) and serotonergic enhancement of airway smooth muscle (ASM) contraction induced by ozone (O3) plus allergen has not been evaluated. If this mechanism persists after a prolonged recovery, it would indicate that early-life exposure to O3 plus allergen induces functional changes predisposing allergic individuals to asthma-related symptoms throughout life, even in the absence of environmental insult. A persistent serotonergic mechanism in asthma exacerbations may offer a novel therapeutic target, widening treatment options for patients with asthma. The objective of this study was to determine if previously documented AHR and serotonin-enhanced ASM contraction in allergic monkeys exposed to O3 plus house dust mite allergen (HDMA) persist after prolonged recovery. Infant rhesus monkeys sensitized to HDMA were exposed to filtered air (FA) (n = 6) or HDMA plus O3 (n = 6) for 5 months. Monkeys were then housed in a FA environment for 30 months. At 3 years, airway responsiveness was assessed. Airway rings were then harvested, and ASM contraction was evaluated using electrical field stimulation with and without exogenous serotonin and serotonin-subtype receptor antagonists. Animals exposed to O3 plus HDMA exhibited persistent AHR. Serotonin exacerbated the ASM contraction in the exposure group but not in the FA group. Serotonin subtype receptors 2, 3, and 4 appear to drive the response. Our study shows that AHR and serotonin-dependent exacerbation of cholinergic-mediated ASM contraction induced by early-life exposure to O3 plus allergen persist for at least 2.5 years and may contribute to a persistent asthma phenotype.
Keywords: serotonin, ozone, antigens, hyperresponsiveness, Macaca mulatta
Clinical Relevance
Airway hyperresponsiveness and serotonin-dependent exacerbation of cholinergic-mediated airway smooth muscle contraction induced by early-life exposure to ozone plus allergen persist for at least 2.5 years and may contribute to a persistent asthma phenotype. These findings substantiate the need to minimize exposure of young individuals to known environmental contributors to asthma during critical periods of lung maturation because damage inflicted during these times can contribute to prolonged asthma symptoms. The identification of a persistent 5-HT–mediated enhanced airway smooth muscle contraction may identify novel therapeutic targets for pharmacological intervention in the treatment of childhood asthma.
Asthma is one of the most common chronic childhood conditions in the United States. In 2011, ∼ 7 million children suffered from asthma (1). The most frequent reason for school absences is asthma, accounting for one third of school days missed, and the severity of symptoms is negatively correlated with achievement (2).
The link between ozone (O3) and house dust mite allergen (HDMA) exposure and childhood asthma has been supported by a wealth of research (3–9). In the latest State of the Air report, almost half of United States citizens—over 148 million people—live in areas with unhealthy O3 levels (6).
Although the mechanisms responsible for asthma symptoms are debatable, research shows that O3 and HDMA exposure leads to functional, structural, neural, vascular, and immunological alterations in airways (10–16). This airway remodeling indicates that environmental insults early in life can have life-long deleterious effects on lung function and may lead to chronic asthma symptoms.
Airway hyperresponsiveness (AHR), a functional hallmark of asthma, is assessed with a bronchoprovocation test (17). The presence of AHR after O3 and HDMA exposure indicates that airway function has been compromised. In humans, nonhuman primates, and other models of asthma, O3 and HDMA exposure has been shown to increase AHR (18–21).
Recent literature suggests that serotonin (5-HT) plays a role in the asthma response (22–24). Animal studies show that 5-HT increases airway resistance and O3 + HDMA exposure of infant monkeys results in 5-HT–positive cells within airway epithelia (25–27). Patients with asthma have higher 5-HT plasma levels, which are inversely correlated with lung function, and drug treatments that lower plasma 5-HT decrease symptom severity and improve lung function (6, 28, 29). Our lab has shown that exposure of O3 + HDMA induces AHR and exacerbates 5-HT–mediated airway smooth muscle (ASM) contraction in a model of childhood asthma (18) and that the combined exposure of O3 + HDMA results in alterations in 9 of 10 immune, structural, and functional end points, with six of the end points demonstrating greater than additive effects of O3 or HDMA exposure alone (10). Studies have epidemiologically confirmed asthma persistence from childhood through adulthood, linking persistent symptoms to atopy, smoking, air pollution (including O3), early age at onset of asthma, and airway remodeling (30–34).
To date, no study has examined the persistence of AHR and 5-HT enhancement of ASM contraction in a controlled setting using a model of childhood asthma. The aims of this project are (1) to determine if AHR and 5-HT–enhanced ASM contraction induced by O3 + HDMA exposure persist after a prolonged recovery period in a filtered air (FA) environment and (2) to identify which 5-HT subtype receptors are responsible for driving the 5-HT response. Confirming the persistence of AHR caused by early-life exposure to O3 + HDMA will help guide environmental policy and substantiate the need to mitigate exposure, especially in young populations. Associating a 5-HT–mediated mechanism with persistent AHR offers a novel therapeutic target for asthma treatment.
Rhesus monkeys were used because they have similar lung cellular morphology, airway architecture, and immunology and undergo a similar extensive period of postnatal development compared with humans (35–39). In addition to possessing all of the components of the intrapulmonary conducting airways that are altered in humans with asthma, rhesus monkeys display a similar progression of asthma pathophysiology and symptoms (11, 40). The sensitization protocol used induces the functional, immunologic, histological, and clinical characteristics that are used to diagnose allergic asthma (40).
Materials and Methods
Care of animals complied with the Institute of Laboratory Animal Resources and the American Association for Accreditation of Laboratory Animal Care (AAALAC). Procedures were approved by the University of California - Davis Institutional Animal Care and Use Committee (41). The University of California - Davis and the California National Primate Research Center are accredited by AAALAC.
General Protocol
Twelve 30-day-old, captive-born rhesus monkeys were randomly assigned to one of two groups: FA or O3 plus HDMA (O3 + HDMA). All animals were sensitized to HDMA and exposed to 11 episodes of FA or O3 + HDMA as previously described (10, 18) (Figure 1). Exposures had a HDMA mass concentration averaging 7.05 ± 0.73 mg/m3 and a mean O3 concentration of 0.500 ± 0.005 ppm. Monkeys were killed with sodium pentobarbital (15 ml/kg). A distal tracheal portion was harvested and placed in modified Kreb’s solution.
Figure 1.
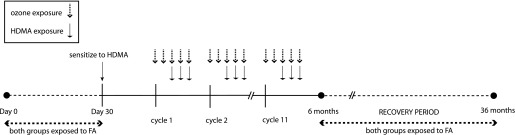
Timeline of exposure protocol. FA, filtered air; HDMA, house dust mite allergen.
Airway Responsiveness Testing
At 3 years of age, airway resistance (Raw) was measured during a histamine challenge and expressed as the concentration of histamine causing a 200% increase in Raw (EC200Raw) (40).
Electrical Field Stimulation
Airway rings were suspended between platinum wire electrodes in tissue baths (Myobath; WPI Inc., Sarasota, FL) as previously described (18). Tension was measured via Fort 10 g transducers (WPI Inc.) and recorded with Powerlab Chart 5.1 software (ADInstruments, Colorado Springs, CO). Monophasic square-wave impulses (50 V, 4 Hz, 0.5 ms) were delivered for 30 seconds every 4 minutes until three consecutive stable responses were observed. Pulses were induced via S88 Stimulators (Grass Technologies, West Warwick, RI).
5-HT Concentration-Response Curves
Six rings from each animal were used to perform 5-HT concentration-response curves during electric field stimulation (EFS)-induced contractions.
Antagonist Concentration-Response Curves
5-HT concentration-response curves were performed in the presence of antagonists (Table 1).
Table 1.
Summary of Antagonists
The Effect of 5-HT1AR Activation
Previous research identified an inhibitory pathway mediated through 5-HT1A receptors (18). These analyses were reproduced.
Baseline Responses
Acetylcholine (ACh) concentration-response curves were performed (one control and one preincubated with 10 μM 5-HT). Voltage-response curves and frequency-response curves were performed on tracheal rings.
Concluding Experiments
Airway rings were exposed to 10 mM ACh to compare tension with the initial ACh concentration-response curves. The effect of atropine (1 μM) was evaluated to ensure muscarinic-mediated contractions. To verify that the responses were neurogenic, tissue was incubated in 3 μM tetrodotoxin before EFS. All drugs were purchased from Sigma-Aldrich Co. (St. Louis, MO).
Statistical Analysis
Results are expressed as mean ± SEM. Airway responsiveness data; between-group EC50, EV50, EF50; and 5-HT direct effect values were analyzed using Student’s t tests. Within-group 5-HT direct effects and the direct effect of 8-OH-DPAT were analyzed with paired-samples t tests. Concentration-responses were compared using repeated-measures ANOVAs. Tukey post hoc testing was used to identify the source of significance. The α level was set at 0.05. Significance was based on the adjusted P value. A one-way t test was used to assess the direct effect of 5-HT on ASM contraction based on previous findings that 5-HT constricts ASM.
Results
Airway Responsiveness
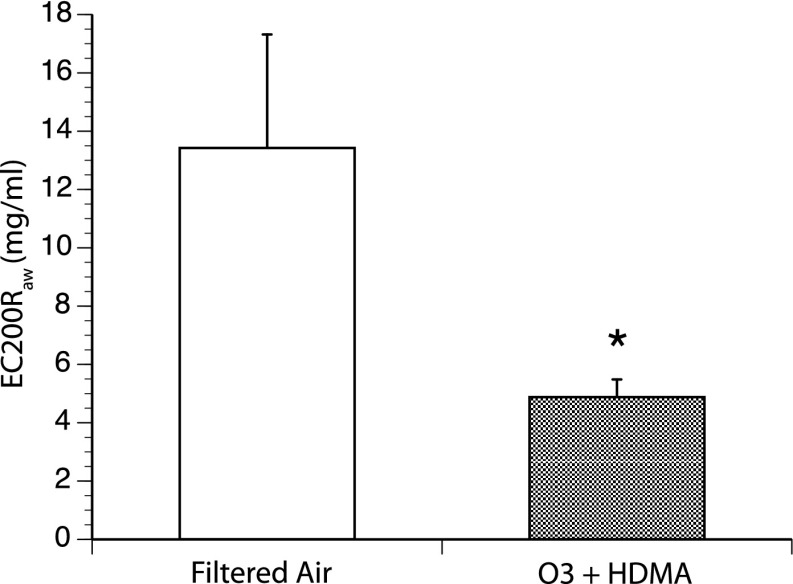
O3 + HDMA exposure induced a significant increase in airway responsiveness when compared with FA controls, even with a prolonged 2.5-year recovery period in FA (EC200Raw FA = 14.43 ± 3.89 mg/ml; O3 + HDMA = 4.88 ± 0.60 mg/ml; P = 0.04) (Figure 2).
Figure 2.
Airway responsiveness during histamine challenge. *The O3 + HDMA group exhibited a significant increase in airway responsiveness when compared with FA (P = 0.04; n = 6). EC200Raw is the effective concentration of histamine needed to induce a 200% increase in airway resistance. The lower the effective concentration, the more responsive the airway.
5-HT Concentration Response
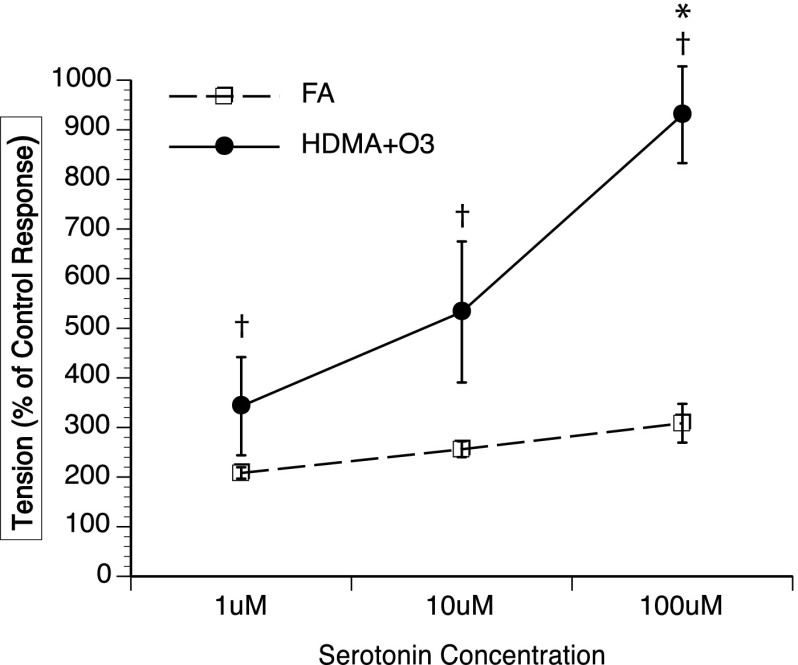
O3 + HDMA exposure resulted in enhanced airway contractility in the presence of 5-HT, indicated by significantly increased EFS-induced ASM tension production when compared with FA animals at 100 μM 5-HT (FA = 308.5 ± 39.0%; O3 + HDMA = 930.3 ± 97.4%; P = 0.008). All 5-HT concentrations in the O3 + HDMA group produced significantly greater tension than the EFS-induced tension produced during their control response (EFS-induced contraction in the absence of 5-HT; P < 0.05), which was not the case in the FA group. In the FA group, none of the 5-HT concentrations elicited a contraction significantly greater than its control response (P > 0.05) (Figure 3). There was also an overall group effect, with the O3 + HDMA group producing a significantly higher mean tension (as % of control EFS response) than the FA group (O3 + HDMA = 378.0%; FA = 214.3%; P < 0.006).
Figure 3.
Effect of serotonin (5-HT) concentration on airway contractility during electric field stimulation (EFS). *Tension production in O3 + HDMA group is significantly greater than in the FA group at 100 μM 5-HT. †In the O3 + HDMA group, tension production at each 5-HT concentration was greater than the control response. Control response is defined as the amount of tension produced via EFS before addition of 5-HT. There was no within-group effect seen in the FA group (P > 0.05; n = 6).
Antagonist Concentration Response
In the O3 + HDMA group, incubation with increasing concentrations of 5-HT2A (ketanserin), 5-HT3 (ondansetron), or 5-HT4 (GR 113808) subtype receptor antagonists attenuated the tension induced by EFS at all 5-HT concentrations, indicating that these three receptors are involved in the ASM response to 5-HT (Figure 4).
Figure 4.
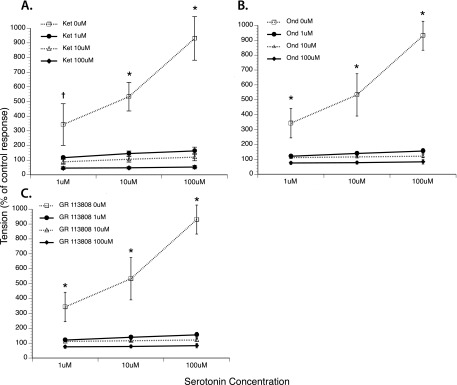
Effect of 5-HT concentration on airway contractility during EFS in the presence of 5-HT subtype receptor antagonists. (A) The 5-HT2A receptor antagonist ketanserin (Ket). (B) The 5-HT3 receptor antagonist ondansetron (Ond). (C) The 5-HT4 receptor antagonist, GR 113808. *All concentrations of the receptor antagonist induced a significant reduction in tension at each 5-HT concentration when compared with concentration-response curve with 5-HT alone. †Administration of 10 and 100 μM of antagonist significantly reduced tension compared with the concentration-response curve with 5-HT alone. “% of control” response is defined as the amount of tension produced via EFS before addition of 5-HT or 5-HT receptor antagonist (P < 0.05; n = 6).
The Effect of 5-HT1A Receptor Activation
Addition of the 5-HT1A receptor agonist 8-OH-DPAT significantly attenuated EFS-induced ASM contraction in a concentration-dependent manner (Figure 5A). This effect was seen in both the FA and O3 + HDMA groups, indicating that exposure had no effect. The direct effect of 5-HT1A receptor activation on ASM tension induced by 100 μM exogenous ACh was also evaluated. Concentrations of 10 and 100 μM 8-OH-DPAT significantly attenuated ACh-induced tension in both the FA and O3 + HDMA groups. There was no between-group difference in the response, indicating that exposure had no effect on 5-HT1A receptor activation (Figure 5B).
Figure 5.
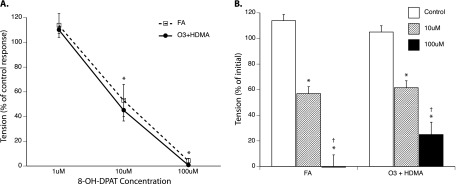
(A) Effect of the 5-HT1A agonist 8-OH-DPAT on airway contractility during EFS. *EFS-induced tension is significantly less than within-group EFS control contraction before agonist addition. “% of control response” is defined as the amount of tension produced via EFS before addition of 5-HT or 5-HT receptor agonist. There was no difference in response between groups. (B) Direct effect of 8-OH-DPAT on airway smooth muscle (ASM) precontracted with 100 μM ACh. *ASM tension is significantly less than within-group control after 10 minutes of incubation with agonist. †Tension at 100 μM 5-HT is significantly less than within-group tension at 10 μM 5-HT. There was no difference in ASM tension between groups (P > 0.05; n = 6).
Direct Effect of 5-HT on ASM Tension
The addition of 10 μM 5-HT produced a small, but consistent, increase in ASM tension in the FA and O3 + HDMA groups. In the FA group, tension increased from 0.992 ± 0.007 g to 1.204 ± 0.229 g (P = 0.037). This increase was just over 6% of the maximal response to ACh. In the O3 + HDMA group, 10 μM 5-HT caused a tension increase amounting to 12.75% of the maximal ACh response (Figure 6A). When comparing the change in absolute tension between the FA and O3 + HDMA groups, O3 + HDMA exposure produced a significantly greater 5-HT–induced tension increase compared with the FA group (0.549 ± 0.146 g versus 0.211 ± 0.094 g) (Figure 6B). This indicates that 5-HT directly induces ASM contraction and that O3 + HDMA exposure exacerbates this response.
Figure 6.
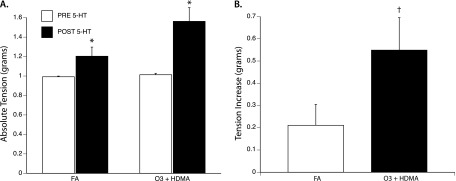
Direct effect of 5-HT on ASM tension. (A) *Addition of 10 μM 5-HT induced a significant increase in ASM compared with the within-group baseline tension. PRE 5-HT, tissue at baseline tension of 1.0 g before addition of 5-HT; POST 5-HT, tissue tension after addition of 10 μM 5-HT. (B) †5-HT–induced tension increase was significantly greater in the O3 + HDMA group than in the FA group (P < 0.05; n = 6).
Baseline ASM Response
Before 5-HT addition, frequency-response and voltage-response curves were performed. There was no difference in the voltage needed to induce 50% of maximum EFS tension (EV50) between groups (EV50, FA = 41.6 ± 2.5 V; O3 + HDMA = 42.5 ± 4.4 V; P > 0.05). There was no significant difference in the frequency necessary to induce 50% of maximum EFS tension (EF50) between groups (EV50, FA = 9.6 ± 2.4 Hz; O3 + HDMA = 7.1 ± 0.7 Hz; P > 0.05). This indicates that without exogenous 5-HT added to the tissue baths, the airway rings from the O3 + HDMA animals and the FA animals responded similarly to EFS.
Concluding Experiments
Tissue response to 10 mM ACh at the conclusion of the experiment produced over 93% (1.442 g versus 1.541 g) of the tension seen at the beginning of the protocol, indicating adequate tissue viability throughout the testing. Atropine and tetrodotoxin completely attenuated EFS response, confirming that EFS-induced contractions were neurogenic of origin and induced by activation of cholinergic receptors on the ASM.
Discussion
Although persistent asthma symptoms have been linked to environmental O3 and allergen exposure, no study to date has examined the persistent effect of O3 + HDMA exposure in a model of childhood asthma. Our previous research showed that cyclical exposure to O3 + HDMA from 1 to 6 months of life in allergic rhesus monkeys results in a hyperresponsive airway and in a 5-HT–mediated enhancement of ASM contraction (18). With the wealth of epidemiologic data supporting the negative effects of early-life exposure to O3 and allergens, it makes sense to question whether or not the functional decrements seen with O3 + HDMA exposure in our model of childhood asthma would persist if the animals were allowed a prolonged recovery in a FA environment (6, 42–44). This study confirms that O3 + HDMA exposure induces persistent AHR and exacerbated 5-HT–mediated ASM contraction, even after a prolonged recovery period, in a model of childhood asthma.
Airway Responsiveness
AHR is a functional indicator of asthma. When comparing EC200Raw between the O3 + HDMA exposure and FA groups, the exposure group required a significantly lower dose (Figure 2). The AHR seen in the O3 + HDMA group closely resembles that of our previous work using the same exposure protocol that did not allow for a prolonged recovery (18). This signifies that the functional decrement induced by O3 + HDMA exposure seen after 5 months of exposure persists even after 2.5 years of recovery in a FA environment. The persistence of AHR after a prolonged recovery period underscores the deleterious effects of early-life exposure to O3 + HDMA and that such exposure not only leads to acute pulmonary dysfunction in allergic individuals but induces chronic changes in airway function that remain after a long recovery period even when the environmental insult is no longer present.
Unlike previous studies (25, 45, 46) in which sensitivity to HDMA was maintained in the O3 + HDMA group during recovery, persistent functional alterations were found in this study even though there was no attempt to ensure maintained HDMA sensitivity and the recovery period was extended from 6 to 30 months. The impact of this observation is even more significant when one considers that the exposure occurred during a period of rapid postnatal lung development and that the detrimental effects were still present at an age equivalent to preadolescence in humans. This reinforces the need to minimize children’s exposures to air pollution and allergens during the extended postnatal maturation of the lungs, otherwise risking decrements in lung function lasting into adulthood, regardless of the presence of environmental insults.
5-HT Concentration-Response Curves
Not only did the pulmonary functional decrements induced by O3 + HDMA exposure persist after the prolonged recovery period, but the 5-HT–mediated exacerbation of ASM contraction did as well (Figure 3). The persistence of a 5-HT mechanism with the functional decrements indicates that altered serotonergic signaling at the postganglionic nerve innervating ASM may play a role in the persistent AHR induced by O3 + HDMA exposure. 5-HT has been implicated in asthma from clinical, inflammatory, immunologic, and neurogenic points of view (23, 28, 29, 47–52). It is well substantiated that 5-HT can enhance the neuronal release of ACh at nerve endings (53). Mechanistically, it is possible that an increase in the presence of 5-HT or an up-regulation of 5-HT receptors at the postganglionic nerve could induce exacerbated 5-HT–mediated ASM contraction, leading to AHR and contributing to chronic asthma symptoms. 5-HT has been shown to up-regulate thromboxane release, leading to AHR (22). Exposure to O3 + HDMA induces the proliferation of 5-HT–containing cells in the airway epithelia (25), and 5-HT levels are increased in the bronchoalveolar lavage fluid of patients with asthma after allergen challenge (23). In mice, allergen challenge induces 5-HT release from platelets (23). These studies indicate a plausible mechanism for 5-HT contributing to AHR and offer possible sources of 5-HT in the asthmatic airway.
Antagonist Concentration-Response Curves
To identify the specific 5-HT subtype receptors involved in the 5-HT response in the O3 + HDMA group, 5-HT concentration response curves where conducted in the presence of 5-HT2A, 5-HT3, and 5-HT4 subtype receptor antagonists. These receptors were targeted due to previous experiments run by this group and an extensive review of literature (18, 27, 49, 54, 55). Separate incubation with 5-HT2A, 5-HT3, and 5-HT4 subtype receptor antagonists significantly attenuated the ASM contractile response to 5-HT, indicating that these receptors play a prominent role in the O3 + HDMA–induced enhancement of ASM contraction.
The Effect of 5-HT1A Receptor Activation
As noted in our previous exposure study, a counterbalancing inhibitory 5-HT effect was seen, mediated through 5-HT1A receptors (18). The 5-HT1A receptor agonist 8-OH-DPAT attenuated any tissue response to EFS and was able to diminish the tension produced by exogenous ACh, indicating that these receptors exert their effect at the ASM, as opposed to inducing postganglionic neural inhibition. This inhibitory effect was seen in both the FA and O3 + HDMA groups, with exposure having no effect. Although dysregulation of an inhibitory pathway could lead to AHR and enhanced ASM contraction, these results suggest that increased ASM contraction with 5-HT is due to up-regulation of an excitatory pathway rather than the down-regulation of an inhibitory pathway.
Direct Effect of 5-HT on ASM Tension
O3 + HDMA exposure enhanced the direct effect of 5-HT on ASM (Figure 6). 5-HT has been shown to directly contract ASM in multiple species (56, 56–58). The enhancement of 5-HT’s ability to contract ASM is consistent with recent research identifying that a similar exposure protocol in rhesus monkeys induces an up-regulation of 5-HT receptor expression on ASM (59).
Although a functional study of this nature using EFS on whole excised airway tissue allows insight into the role of 5-HT in ASM function and AHR in a model of childhood allergic asthma, the limitations of such an experimental preparation must be acknowledged. Pharmacologic identification of receptor subtypes is common practice, but one must be careful when drawing conclusions. We attempted to use the most selective antagonists available, but, due to the variance of published receptor affinities, quantitative rank-order comparison of the contributions of each receptor subtype could not be established. Therefore, it was deemed imprudent to make assumptions regarding the relative contribution of each identified 5-HT receptor subtype (2–4) that was shown to be involved in the serotonergic enhancement of ASM contraction in the exposed animals. Current immunohistochemical studies are underway to identify which receptor subtypes are present at the ganglia and terminal axon of cholinergic nerves in FA and exposed monkeys.
This study focused on a conducting airway site located in the middle to lower trachea. Previous research shows that vascular remodeling in HDMA-exposed airways is generation specific (60). It is possible that O3 + HDMA exposure may have differential effects along segments of the tracheobronchial tree. Studies evaluating ASM function at alternate airway generations could assess whether exposure effects are widespread and consistent throughout conducting airways. Also, studies examining the intensity and longevity of the persistence of AHR and enhanced ASM contraction at different time points of exposure and recovery could help identify a critical window during postnatal lung development in which the airway is most susceptible to environmental toxicant damage.
This study did not use strategies to differentiate between responses due to HDMA-associated immune responses or reactive oxygen species formation via O3 exposure. Previous research has shown a synergistic effect of O3 + HDMA exposure, with O3 amplifying the allergic, structural, and neural remodeling effects of HDMA sensitization and inhalation (10, 14, 25). Also, previous work has shown that immediately after 6 months of exposure to HDMA, O3, or O3 + HDMA, similar 5-HT–induced increases in neurally mediated ASM contractions were seen in all the groups (18). The common factor in all of these exposures is inflammation, whether it was induced via an HDMA-driven immune response or reactive oxygen species production via O3 exposure. This experimental design did not allow us to differentiate between immune versus oxidant-induced inflammation, but this is a viable avenue for future research.
Further evaluation linking histological and structural changes to alterations in airway function and 5-HT handling will bridge a critical gap in our proposed model. Future research could also evaluate the effectiveness of 5-HT receptor antagonists at reversing AHR in whole animal studies. This study focused on the persistence of the combined effect of O3 + HDMA exposure on AHR and 5-HT enhancement of ASM contraction and did not investigate the effect of exposure to each environmental insult separately, so the individual contributions of O3 or HDMA to persistence cannot be addressed.
In conclusion, this study verifies for the first time that combined exposure to two recognized environmental contributors to asthma, O3 and HDMA, induce prolonged decrements in lung function and lead to a 5-HT–mediated exacerbation of ASM contraction in a model of childhood asthma. These hallmarks of asthma—AHR and enhanced ASM contraction—persisted even after exposure to O3 + HDMA had been discontinued for 2.5 years. This study also identified three 5-HT subtype receptors that contribute to the enhanced ASM contractile response (5-HT2A, 5-HT3, and 5-HT4). These findings substantiate the need to minimize exposure of young individuals to known environmental contributors to asthma during critical periods of lung maturation because damage inflicted during these times can contribute to prolonged asthma symptoms. The identification of a persistent 5-HT–mediated enhanced ASM contraction may identify novel therapeutic targets for pharmacological intervention in the treatment of childhood asthma.
Footnotes
This research was supported by a National Institute of Health Sciences Program Project grant P01ES000628–33.
Author Contributions: Conception and design: D.H., L.M., E.S., B.M. Analysis and interpretation: E.S., B.M., E.W. Drafting the manuscript for important intellectual content: B.M., E.S.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0387OC on January 31, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bloom BCR, Freeman G. Hyattsville, MD: Department of Health and Human Services; 2012. Summary health statistics for U.S. children: national health interview survey, 2011. [PubMed] [Google Scholar]
- 2.Krenitsky-Korn S. High school students with asthma: attitudes about school health, absenteeism, and its impact on academic achievement. Pediatr Nurs. 2011;37:61–68. [PubMed] [Google Scholar]
- 3.YoussefAgha AH, Jayawardene WP, Lohrmann DK, El Afandi GS. Air pollution indicators predict outbreaks of asthma exacerbations among elementary school children: integration of daily environmental and school health surveillance systems in Pennsylvania. J Environ Monit. 2012;14:3202–3210. doi: 10.1039/c2em30430a. [DOI] [PubMed] [Google Scholar]
- 4.Rosenlund M, Forastiere F, Porta D, De Sario M, Badaloni C, Perucci CA. Traffic-related air pollution in relation to respiratory symptoms, allergic sensitisation and lung function in schoolchildren. Thorax. 2009;64:573–580. doi: 10.1136/thx.2007.094953. [DOI] [PubMed] [Google Scholar]
- 5.Gielen MH, van der Zee SC, van Wijnen JH, van Steen CJ, Brunekreef B. Acute effects of summer air pollution on respiratory health of asthmatic children. Am J Respir Crit Care Med. 1997;155:2105–2108. doi: 10.1164/ajrccm.155.6.9196122. [DOI] [PubMed] [Google Scholar]
- 6.Bernstein DI. Traffic-related pollutants and wheezing in children. J Asthma. 2012;49:5–7. doi: 10.3109/02770903.2011.641049. [DOI] [PubMed] [Google Scholar]
- 7.Schelegle ES, Morales CA, Walby WF, Marion S, Allen RP. 6.6-hour inhalation of ozone concentrations from 60 to 87 parts per billion in healthy humans. Am J Respir Crit Care Med. 2009;180:265–272. doi: 10.1164/rccm.200809-1484OC. [DOI] [PubMed] [Google Scholar]
- 8.Strickland MJ, Darrow LA, Klein M, Flanders WD, Sarnat JA, Waller LA, Sarnat SE, Mulholland JA, Tolbert PE. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med. 2010;182:307–316. doi: 10.1164/rccm.200908-1201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, Avol E, Margolis HG, Peters JM. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002;359:386–391. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- 10.Schelegle ES, Miller LA, Gershwin LJ, Fanucchi MV, Van Winkle LS, Gerriets JE, Walby WF, Mitchell V, Tarkington BK, Wong VJ, et al. Repeated episodes of ozone inhalation amplifies the effects of allergen sensitization and inhalation on airway immune and structural development in Rhesus monkeys. Toxicol Appl Pharmacol. 2003;191:74–85. doi: 10.1016/s0041-008x(03)00218-7. [DOI] [PubMed] [Google Scholar]
- 11.Evans MJ, Fanucchi MV, Baker GL, Van Winkle LS, Pantle LM, Nishio SJ, Schelegle ES, Gershwin LJ, Miller LA, Hyde DM, et al. Atypical development of the tracheal basement membrane zone of infant rhesus monkeys exposed to ozone and allergen. Am J Physiol Lung Cell Mol Physiol. 2003;285:L931–L939. doi: 10.1152/ajplung.00175.2003. [DOI] [PubMed] [Google Scholar]
- 12.Fanucchi MV, Plopper CG, Evans MJ, Hyde DM, Van Winkle LS, Gershwin LJ, Schelegle ES. Cyclic exposure to ozone alters distal airway development in infant rhesus monkeys. Am J Physiol Lung Cell Mol Physiol. 2006;291:L644–L650. doi: 10.1152/ajplung.00027.2006. [DOI] [PubMed] [Google Scholar]
- 13.Joad JP, Kott KS, Bric JM, Peake JL, Plopper CG, Schelegle ES, Gershwin LJ, Pinkerton KE. Structural and functional localization of airway effects from episodic exposure of infant monkeys to allergen and/or ozone. Toxicol Appl Pharmacol. 2006;214:237–243. doi: 10.1016/j.taap.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Larson SD, Schelegle ES, Walby WF, Gershwin LJ, Fanuccihi MV, Evans MJ, Joad JP, Tarkington BK, Hyde DM, Plopper CG. Postnatal remodeling of the neural components of the epithelial-mesenchymal trophic unit in the proximal airways of infant rhesus monkeys exposed to ozone and allergen. Toxicol Appl Pharmacol. 2004;194:211–220. doi: 10.1016/j.taap.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 15.Pichyangkul S, Tongtawe P, Kum-Arb U, Yongvanitchit K, Gettayacamin M, Hollingdale MR, Limsalakpetch A, Stewart VA, Lanar DE, Dutta S, et al. Evaluation of the safety and immunogenicity of Plasmodium falciparum apical membrane antigen 1, merozoite surface protein 1 or RTS,S vaccines with adjuvant system AS02A administered alone or concurrently in rhesus monkeys. Vaccine. 2009;28:452–462. doi: 10.1016/j.vaccine.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Avdalovic MV, Tyler NK, Putney L, Nishio SJ, Quesenberry S, Singh PJ, Miller LA, Schelegle ES, Plopper CG, Vu T, et al. Ozone exposure during the early postnatal period alters the timing and pattern of alveolar growth and development in nonhuman primates. Anat Rec (Hoboken) 2012;295:1707–1716. doi: 10.1002/ar.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backer V, Groth S, Dirksen A, Bach-Mortensen N, Hansen KK, Laursen EM, Wendelboe D. Sensitivity and specificity of the histamine challenge test for the diagnosis of asthma in an unselected sample of children and adolescents. Eur Respir J. 1991;4:1093–1100. [PubMed] [Google Scholar]
- 18.Moore BD, Hyde D, Miller L, Wong E, Frelinger J, Schelegle ES. Allergen and ozone exacerbate serotonin-induced increases in airway smooth muscle contraction in a model of childhood asthma. Respiration. 2012;3:529–542. doi: 10.1159/000336835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulze J, Voss S, Zissler U, Rose MA, Zielen S, Schubert R. Airway responses and inflammation in subjects with asthma after four days of repeated high-single-dose allergen challenge. Respir Res. 2012;13:78. doi: 10.1186/1465-9921-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bao A, Liang L, Li F, Zhang M, Zhou X. Effects of acute ozone exposure on lung peak allergic inflammation of mice. Front Biosci (Landmark Ed) 2013;18:838–851. doi: 10.2741/4147. [DOI] [PubMed] [Google Scholar]
- 21.Scannell C, Chen L, Aris RM, Tager I, Christian D, Ferrando R, Welch B, Kelly T, Balmes JR. Greater ozone-induced inflammatory responses in subjects with asthma. Am J Respir Crit Care Med. 1996;154:24–29. doi: 10.1164/ajrccm.154.1.8680687. [DOI] [PubMed] [Google Scholar]
- 22.Montano LM, Carbajal V, Vargas MH, Garcia-Hernandez LM, Diaz-Hernandez V, Checa M, Barajas-Lopez C. Histamine, carbachol, and serotonin induce hyperresponsiveness to atp in guinea pig tracheas: Involvement of cox-2 pathway. Pflugers Arch. 2013;465:1171–1179. doi: 10.1007/s00424-013-1253-9. [DOI] [PubMed] [Google Scholar]
- 23.Dürk T, Duerschmied D, Müller T, Grimm M, Reuter S, Vieira RP, Ayata K, Cicko S, Sorichter S, Walther DJ, et al. Production of serotonin by tryptophan hydroxylase 1 and release via platelets contribute to allergic airway inflammation. Am J Respir Crit Care Med. 2013;187:476–485. doi: 10.1164/rccm.201208-1440OC. [DOI] [PubMed] [Google Scholar]
- 24.Cooper PR, Zhang J, Damera G, Hoshi T, Zopf DA, Panettieri RA., Jr C-027 inhibits ige-mediated passive sensitization bronchoconstriction and acts as a histamine and serotonin antagonist in human airways. Allergy Asthma Proc. 2011;32:359–365. doi: 10.2500/aap.2011.32.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kajekar R, Pieczarka EM, Smiley-Jewell SM, Schelegle ES, Fanucchi MV, Plopper CG. Early postnatal exposure to allergen and ozone leads to hyperinnervation of the pulmonary epithelium. Respir Physiol Neurobiol. 2007;155:55–63. doi: 10.1016/j.resp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Aizawa H, Takata S, Shigyo M, Matsumoto K, Inoue H, Hara N. N-omega-nitro-l-arginine methyl ester increases airway responsiveness to serotonin but not to acetylcholine in cats in vivo. Respiration. 2001;68:286–291. doi: 10.1159/000050512. [DOI] [PubMed] [Google Scholar]
- 27.Segura P, Vargas MH, Cordoba-Rodriguez G, Chavez J, Arreola JL, Campos-Bedolla P, Ruiz V, Garcia-Hernandez LM, Mendez C, Montano LM. Role of 5-ht2a, 5-ht4 and 5-ht7 receptors in the antigen-induced airway hyperresponsiveness in guinea-pigs. Clin Exp Allergy. 2010;40:327–338. doi: 10.1111/j.1365-2222.2009.03412.x. [DOI] [PubMed] [Google Scholar]
- 28.Lechin F, van der Dijs B, Orozco B, Lechin M, Lechin AE. Increased levels of free serotonin in plasma of symptomatic asthmatic patients. Ann Allergy Asthma Immunol. 1996;77:245–253. doi: 10.1016/S1081-1206(10)63263-2. [DOI] [PubMed] [Google Scholar]
- 29.Lechin F, van der Dijs B, Orozco B, Jara H, Rada I, Lechin ME, Lechin AE. Neuropharmacologic treatment of bronchial asthma with the antidepressant tianeptine: a double-blind, crossover placebo-controlled study. Clin Pharmacol Ther. 1998;64:223–232. doi: 10.1016/S0009-9236(98)90156-4. [DOI] [PubMed] [Google Scholar]
- 30.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen F, Taylor DR, Flannery EM, Cowan JO, Greene JM, Herbison GP, Sears MR. Risk factors for airway remodeling in asthma manifested by a low postbronchodilator FEV1/vital capacity ratio: a longitudinal population study from childhood to adulthood. Am J Respir Crit Care Med. 2002;165:1480–1488. doi: 10.1164/rccm.2108009. [DOI] [PubMed] [Google Scholar]
- 32.Phelan PD, Robertson CF, Olinsky A. The melbourne asthma study: 1964–1999. J Allergy Clin Immunol. 2002;109:189–194. doi: 10.1067/mai.2002.120951. [DOI] [PubMed] [Google Scholar]
- 33.Wright RJ, Brunst KJ. Programming of respiratory health in childhood: influence of outdoor air pollution. Curr Opin Pediatr. 2013;25:232–239. doi: 10.1097/MOP.0b013e32835e78cc. [DOI] [PubMed] [Google Scholar]
- 34.Al-Hegelan M, Tighe RM, Castillo C, Hollingsworth JW. Ambient ozone and pulmonary innate immunity. Immunol Res. 2011;49:173–191. doi: 10.1007/s12026-010-8180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans MJ, Fanucchi MV, Plopper CG, Hyde DM. Postnatal development of the lamina reticularis in primate airways. Anat Rec (Hoboken) 2010;293:947–954. doi: 10.1002/ar.20824. [DOI] [PubMed] [Google Scholar]
- 36.Plopper CG, Heidsiek JG, Weir AJ, George JA, Hyde DM. Tracheobronchial epithelium in the adult rhesus monkey: a quantitative histochemical and ultrastructural study. Am J Anat. 1989;184:31–40. doi: 10.1002/aja.1001840104. [DOI] [PubMed] [Google Scholar]
- 37.Plopper CG, Alley JL, Weir AJ. Differentiation of tracheal epithelium during fetal lung maturation in the rhesus monkey Macaca mulatta. Am J Anat. 1986;175:59–71. doi: 10.1002/aja.1001750107. [DOI] [PubMed] [Google Scholar]
- 38.Plopper C, St George J, Cardoso W, Wu R, Pinkerton K, Buckpitt A. Development of airway epithelium. Patterns of expression for markers of differentiation. Chest. 1992;101(Suppl):2S–5S. [PubMed] [Google Scholar]
- 39.Plopper CG, Weir AJ, Nishio SJ, Cranz DL, St George JA. Tracheal submucosal gland development in the rhesus monkey, Macaca mulatta: ultrastructure and histochemistry. Anat Embryol (Berl) 1986;174:167–178. doi: 10.1007/BF00824332. [DOI] [PubMed] [Google Scholar]
- 40.Schelegle ES, Gershwin LJ, Miller LAC, Fanucchi MV, Van Winkle LS, Gerriets JP, Walby WF, Omlor AM, Buckpitt AR, Tarkington BK, et al. Allergic asthma induced in rhesus monkeys by house dust mite (Dermatophagoides farinae) Am J Pathol. 2001;158:333–341. doi: 10.1016/S0002-9440(10)63973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academies Press; 2011. Guide for the care and use of laboratory animals. [Google Scholar]
- 42.Brunst KJ, Ryan PH, Lockey JE, Bernstein DI, McKay RT, Khurana Hershey GK, Villareal M, Biagini Myers JM, Levin L, Burkle J, Evans S, Lemasters GK. Unraveling the relationship between aeroallergen sensitization, gender, second-hand smoke exposure, and impaired lung function. Pediatr Allergy Immunol. 2012;23:479–487. doi: 10.1111/j.1399-3038.2012.01292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Celedón JC, Milton DK, Ramsey CD, Litonjua AA, Ryan L, Platts-Mills TA, Gold DR. Exposure to dust mite allergen and endotoxin in early life and asthma and atopy in childhood. J Allergy Clin Immunol. 2007;120:144–149. doi: 10.1016/j.jaci.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bateson TF, Schwartz J. Children’s response to air pollutants. J Toxicol Environ Health A. 2008;71:238–243. doi: 10.1080/15287390701598234. [DOI] [PubMed] [Google Scholar]
- 45.Evans MJ, Fanucchi MV, Baker GL, Van Winkle LS, Pantle LM, Nishio SJ, Schelegle ES, Gershwin LJ, Miller LA, Hyde DM, et al. The remodelled tracheal basement membrane zone of infant rhesus monkeys after 6 months of recovery. Clin Exp Allergy. 2004;34:1131–1136. doi: 10.1111/j.1365-2222.2004.02004.x. [DOI] [PubMed] [Google Scholar]
- 46.Plopper CG, Smiley-Jewell SM, Miller LA, Fanucchi MV, Evans MJ, Buckpitt AR, Avdalovic M, Gershwin LJ, Joad JP, Kajekar R, et al. Asthma/allergic airways disease: does postnatal exposure to environmental toxicants promote airway pathobiology? Toxicol Pathol. 2007;35:97–110. doi: 10.1080/01926230601132030. [DOI] [PubMed] [Google Scholar]
- 47.Idzko M, Panther E, Stratz C, Müller T, Bayer H, Zissel G, Dürk T, Sorichter S, Di Virgilio F, Geissler M, et al. The serotoninergic receptors of human dendritic cells: identification and coupling to cytokine release. J Immunol. 2004;172:6011–6019. doi: 10.4049/jimmunol.172.10.6011. [DOI] [PubMed] [Google Scholar]
- 48.Dupont L, Pype J, Demedts M, De Leyn P, Deneffe G, Verleden G. The effects of 5-ht on cholinergic contraction in human airways in vitro. Eur Respir J. 1999;14:642–649. doi: 10.1034/j.1399-3003.1999.14c26.x. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi T, Ward JK, Tadjkarimi S, Yacoub MH, Barnes PJ, Belvisi MG. 5-Hydroxytryptamine facilitates cholinergic bronchoconstriction in human and guinea pig airways. Am J Respir Crit Care Med. 1995;152:377–380. doi: 10.1164/ajrccm.152.1.7599849. [DOI] [PubMed] [Google Scholar]
- 50.Szarek JL, Zhang JZ, Gruetter CA. Mechanisms of 5-hydroxytryptamine-induced contraction of isolated rat intrapulmonary bronchi. Pulm Pharmacol. 1995;8:273–281. doi: 10.1006/pulp.1995.1037. [DOI] [PubMed] [Google Scholar]
- 51.Cutz E, Yeger H, Pan J. Pulmonary neuroendocrine cell system in pediatric lung disease-recent advances. Pediatr Dev Pathol. 2007;10:419–435. doi: 10.2350/07-04-0267.1. [DOI] [PubMed] [Google Scholar]
- 52.Kushnir-Sukhov NM, Brown JM, Wu Y, Kirshenbaum A, Metcalfe DD. Human mast cells are capable of serotonin synthesis and release. J Allergy Clin Immunol. 2007;119:498–499. doi: 10.1016/j.jaci.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Fink KB, Göthert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev. 2007;59:360–417. doi: 10.1124/pr.107.07103. [DOI] [PubMed] [Google Scholar]
- 54.Cazzola M, Assogna G, Lucchetti G, Cicchitto G, D’Amato G. Effect of ketanserin, a new blocking agent of the 5-HT2 receptor, on airway responsiveness in asthma. Allergy. 1990;45:151–153. doi: 10.1111/j.1398-9995.1990.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 55.Matera MG, De Santis D, D’Agostino B, Pallotta M, Vacca C, Cazzola M, Rossi F. Role of 5-hydroxytryptamine in mediating adenosine-induced airway contraction. Immunopharmacology. 1995;29:73–78. doi: 10.1016/0162-3109(95)00046-v. [DOI] [PubMed] [Google Scholar]
- 56.Buckner CK, Dea D, Liberati N, Krell RD. Guinea pig pulmonary responses to serotonin (5-HT) and related agonists. Ann N Y Acad Sci. 1991;629:392–393. doi: 10.1111/j.1749-6632.1991.tb37993.x. [DOI] [PubMed] [Google Scholar]
- 57.Doucet MY, Jones TR, Ford-Hutchinson AW. Responses of equine trachealis and lung parenchyma to methacholine, histamine, serotonin, prostanoids, and leukotrienes in vitro. Can J Physiol Pharmacol. 1990;68:379–383. doi: 10.1139/y90-053. [DOI] [PubMed] [Google Scholar]
- 58.Seehase S, Schlepütz M, Switalla S, Mätz-Rensing K, Kaup FJ, Zöller M, Schlumbohm C, Fuchs E, Lauenstein HD, Winkler C, et al. Bronchoconstriction in nonhuman primates: a species comparison. J Appl Physiol (1985) 2011;111:791–798. doi: 10.1152/japplphysiol.00162.2011. [DOI] [PubMed] [Google Scholar]
- 59.Murphy SR, Schelegle ES, Miller LA, Hyde DM, Van Winkle LS. Ozone exposure alters serotonin and serotonin receptor expression in the developing lung. Toxicol Sci. 2013;134:168–179. doi: 10.1093/toxsci/kft090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Avdalovic MV, Putney LF, Schelegle ES, Miller L, Usachenko JL, Tyler NK, Plopper CG, Gershwin LJ, Hyde DM. Vascular remodeling is airway generation-specific in a primate model of chronic asthma. Am J Respir Crit Care Med. 2006;174:1069–1076. doi: 10.1164/rccm.200506-848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frishman WH, Grewall P. Serotonin and the heart. Ann Med. 2000;32:195–209. doi: 10.3109/07853890008998827. [DOI] [PubMed] [Google Scholar]
- 62.Ju JM, Hwang JH, Piao LH, Park HW, Park JS, Shin DH, Cho JG, Kim KK, Kim JH. Ketanserin, a 5-HT2 antagonist, directly inhibits the ATP-sensitive potassium channel in mouse ventricular myocytes. J Cardiovasc Pharmacol. 2006;47:96–102. doi: 10.1097/01.fjc.0000196238.51018.e9. [DOI] [PubMed] [Google Scholar]
- 63.Ye JH, Ponnudurai R, Schaefer R. Ondansetron: a selective 5-HT(3) receptor antagonist and its applications in CNS-related disorders. CNS Drug Rev. 2001;7:199–213. doi: 10.1111/j.1527-3458.2001.tb00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Youssefyeh RD, Campbell HF, Klein S, Airey JE, Darkes P, Powers M, Schnapper M, Neuenschwander K, Fitzpatrick LR, Pendley CE, et al. Development of high-affinity 5-HT3 receptor antagonists: 1. Initial structure-activity relationship of novel benzamides. J Med Chem. 1992;35:895–903. doi: 10.1021/jm00083a014. [DOI] [PubMed] [Google Scholar]
- 65.Gale JD, Grossman CJ, Whitehead JW, Oxford AW, Bunce KT, Humphrey PP. GR113808: a novel, selective antagonist with high affinity at the 5-HT4 receptor. Br J Pharmacol. 1994;111:332–338. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pindon A, van Hecke G, van Gompel P, Lesage AS, Leysen JE, Jurzak M. Differences in signal transduction of two 5-HT4 receptor splice variants: compound specificity and dual coupling with Galphas- and Galphai/o-proteins. Mol Pharmacol. 2002;61:85–96. doi: 10.1124/mol.61.1.85. [DOI] [PubMed] [Google Scholar]