Abstract
Non-DNA targeted effects of ionizing radiation, which include genomic instability, and a variety of bystander effects including abscopal effects and bystander mediated adaptive response, have raised concerns about the magnitude of low-dose radiation risk. Genomic instability, bystander effects and adaptive responses are powered by fundamental, but not clearly understood systems that maintain tissue homeostasis. Despite excellent research in this field by various groups, there are still gaps in our understanding of the likely mechanisms associated with non-DNA targeted effects, particularly with respect to systemic (human health) consequences at low and intermediate doses of ionizing radiation. Other outstanding questions include links between the different non-targeted responses and the variations in response observed between individuals and cell lines, possibly a function of genetic background. Furthermore, it is still not known what the initial target and early interactions in cells are that give rise to non-targeted responses in neighbouring or descendant cells. This paper provides a commentary on the current state of the field as a result of the Non-targeted effects of ionizing radiation (NOTE) Integrated Project funded by the European Union. Here we critically examine the evidence for non-targeted effects, discuss apparently contradictory results and consider implications for low-dose radiation health effects.
Keywords: Ionizing radiation, low dose, non-targeted effects, non-cancer disease, health risk
1.1 Introduction
Non-DNA targeted effects (NTE) of ionizing radiation, which include genomic instability (GI), and a variety of bystander effects (BE) including abscopal effects and bystander mediated adaptive response, have raised concerns about human risk at low doses.
In this paper we provide a brief overview of the conventional framework of biological effects of radiation exposure and explore the background to the development of the concept of NTE with respect to key radiobiological mechanisms. We shall critically examine the evidence for non-targeted effects in the radiobiology research literature, some apparently contradictory results in the field and consider the implications for low-dose health effects. Although it has been argued that NTE have implications for cancer radiotherapy (RT) (as recently reviewed [1–3]), here we limit discussion to normal tissue responses and effects of low doses, i.e., those typically encountered from occupational, environmental and medical diagnostic exposures. Other more selective reviews of the current work in this area have been published recently, as for example by Salomaa et al. [4].
1.2 Classical radiation paradigm: Target theory
For clarity, throughout this paper the following definitions will apply:
Very high – doses above 15 Gy
High – doses of 5–15 Gy
Medium – doses of 0.5–5 Gy
Low – doses of 0.05–0.5 Gy
Very low – doses below 0.05 Gy
Risks associated with ionizing radiation have been known for almost as long as ionizing radiation itself. Within a year of the discovery of X-rays by Röntgen skin burns had been reported by Stevens [5] and Gilchrist [6] and within 7 years a case of skin cancer was observed by Frieben [7], in all cases associated with high dose X-ray exposure. In general, risks associated with ionizing radiation can be divided into those defined as stochastic effects (genetic risks in offspring, somatic effects (cancer) in directly exposed population), and those termed tissue-reaction (formerly deterministic) effects. The probability of occurrence of stochastic effects but not their severity are assumed to be a function of dose, without a threshold. For the class of tissue-reaction effects defined by the International Commission on Radiological Protection (ICRP) [8, 9], it is assumed that there is a threshold dose, below which there is no effect, and the severity of effect increases with increasing dose above that point. Tissue-reaction effects are assumed to ensue when a sufficiently large number of cells are damaged within a certain critical time period such that the body cannot replace them; biologically it is therefore much more likely that there is a threshold for tissue reaction effects than for stochastic effects [10].
As outlined by Harris [11] and also United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) [12], there are biological data to suggest that cancer arises from a failure of cell differentiation, and that it may be largely unicellular in origin. Canonically, cancer is thought to result from mutagenic damage to a single cell, via direct DNA damage, which in principle could be caused by a single radiation track [12].
Conventionally, radiation effects have been explained using target theory [13]. According to this, deleterious effects of ionizing radiation, such as mutation and carcinogenesis, are attributed to damage to a cellular target, usually identified as nuclear DNA via direct absorption of radiation energy, the consequences of which are expressed in the surviving irradiated cells [12]. Therefore the progeny of a single irradiated cell would be expected to show radiation-induced genetic changes in all descendant cells, i.e., the change would be clonal.
The classic framework for radiobiology has been generally well validated by numerous interlinked experimental and theoretical studies. Although confirmation of some key assumptions remains elusive, in particular the link between the initial damage and cancer, it forms a logical basis for the standard set of models describing risk of cancer and heritable effects, and has been widely used to establish international rules and standards of radiation protection by the ICRP [8]. Although partly based on human epidemiological data for health effects, in particular those derived from the cancer incidence and mortality follow-up of the Japanese atomic bomb survivors Life Span Study (LSS) cohort [14, 15], the regulatory framework derived by the ICRP [8] relies on a number of biological assumptions and models to extrapolate to the low dose and low dose-rate regime of most interest for radiological protection. In particular, the main assumptions made by ICRP [8] and other bodies in relation to estimating stochastic effects are: 1) of targeted damage to nuclear DNA, the yield of which increases linearly with dose, i.e., proportional to the numbers of radiation tracks; 2) consequential damage to and alterations in the cell nucleus and other parts of the cell and its descendents; 3) of a linear increase with dose of the probability (but not the severity) of mutagenic cellular damage at low doses (and more rapid increase at higher doses and dose-rates), with probabilities of particular mutations and chromosome aberrations depending on DNA target sizes and relative efficiencies of repair and mis-repair [16]. These assumptions are described collectively as the linear no-threshold (LNT) hypothesis. Although only ever intended as a regulatory framework for estimating a conservative upper bound on risk, there is a considerable body of experimental and epidemiological evidence to support the fact that risks derived from medium or high dose studies such as the LSS can be extrapolated to low dose and low dose rate, as documented by Little et al. [17], although this is still subject to dispute [18]. As above, ICRP [8, 9] also describe a class of tissue-reaction effects, which are assumed to result from injury to a population of cells, and are characterised by a threshold dose and an increase in the severity of the reaction as the dose increases above the threshold. Among these tissue-reaction effects are circulatory disease, cataract, sterility (temporary and permanent), central nervous system damage, skin burns and gastro-intestinal syndrome [8–10].
The classical target theory framework has persisted despite accumulating evidence of discrepant radiobiological data. These include: 1) difficulty in accounting for cellular repair in clonogenic survival experiments and delayed reproductive death [19, 20]; 2) the appearance of clastogenic factors, i.e., molecules produced by irradiated cultures and from the serum of exposed individuals that induced breaks in the chromosomes of un-irradiated cells [21]; 3) observations of abscopal (out-of-field) effects with radiotherapy [22]; and 4) evidence of non-cancer disease, in particular cataract and circulatory disease at low and medium radiation doses, in particular among groups of radiation workers [23–28].
1.3 Non-targeted effects of ionizing radiation – challenging the conventional radiobiological framework and models
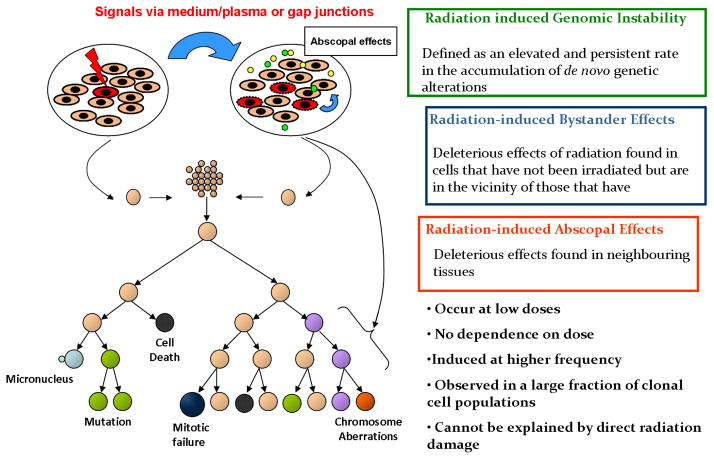
NTE are characterised by cellular responses that occur in cells in which energy from ionizing radiation has not been deposited. The distinct classes of effects include GI, and BE, which latter includes abscopal effects and bystander-mediated adaptive response (AR) (figure 1). These can also arise non-clonally in the progeny of irradiated somatic or germ cells, resulting in radiation-induced GI and transgenerational GI respectively [29, 30]. Substantial evidence for NTE emerged in the early 1990s [4, 31–33].
FIGURE 1.
Schematic of non-targeted effects of exposure to ionizing radiation.
2. Types of non-targeted effect
While radiation-induced BE and GI are often grouped together, and notwithstanding the demonstrated links between them [29], it is possible that the underlying mechanisms, which are not fully understood, may differ. In particular, GI has been described both in the descendants of directly irradiated cells [34] and among descendants of cells exposed to bystander factors [35]. The factors influencing the induction of NTE, as well as their relative contributions to an endpoint under study, are not fully understood but they are sufficiently complex that results from different laboratories may appear to conflict, depending on a number of variables, as we discuss below. There is evidence that agents other than ionizing radiation, in particular metals, induce BE or GI [36]. In addition, induction of BE by chemicals such as mitomycin C [37], phleomycin [38], chloroethylnitrosurea [39] and actinomycin D [40] as well as induction of GI by dioxins [41] have also been documented. While the underlying mechanisms have been characterised in some in vitro systems [42–45] and also in vivo [42, 43, 46] we judge that their practical relevance to low-dose cancer risk assessment and radiological protection is as yet unclear, as we further discuss in section 3. However, the situation may be different for non-malignant disease. As we discuss in section 3, there is now reasonably strong evidence of radiation-induced excess circulatory disease after low dose and low dose-rate exposure [23–25], in particular with little indication of a threshold. As we discuss later, if we interpret these associations causally the total low dose detriment would be predicted to double [25].
2.1 Radiation-induced Genomic Instability (GI)
GI is a complex phenotype observed during the development of some but not all cancers and also induced effectively by ionizing radiation. Radiation induced GI (RIGI) is observed in the progeny of irradiated cells as a delayed and stochastic appearance of de novo chromosomal aberrations, gene mutations and reproductive cell death [31, 32, 47]. There is considerable, if not complete, overlap between RIGI and the GI observed in some (non-radiation-induced) cancers; for example, in the gross chromosomal changes, so called chromosomal instability, observed in colon cancer [48] (see below and section 2.2.). A distinct type of GI, so-called microsatellite instability [49], also observed in colon cancer [48] (see section 2.2 below), is not thought to be associated with radiation-induced cancer in humans [50, 51] although it has been linked with radiation-induced cancers in vivo [52] and in vitro [53]. However, the radiation-induced gaps [54, 55] or breaks [29, 34] that are considered by some as a type of RIGI are unlikely to figure in (non-radiation-induced) GI, since gaps do not correspond to any known phenotype and breaks are generally lethal events for a cell. Many of the characteristics of the GI phenotype are unique and do not conform to the expectations of the conventional models of radiation effects. These characteristics are discussed in the paragraphs below.
High expression rate points to an epigenetic origin
The expression of GI is observed in a much larger proportion of cells irradiated with low to medium doses (about 10–20%) than for mutations from targeted effects, which are typically <10−4 per cell per Gy. Rather than a genetic mutation, instability might arise through epigenetic mechanisms, for example induced expression of a mutator gene [56, 57]. The observed high frequency of instability responses, as well as the lack of evidence of the involvement of DNA double-strand breaks per se in the initiation of instability, has led Baverstock to speculate that alterations in gene expression that disrupt cellular homeostasis may underlie induced instability [58]. There is also evidence of possible involvement of indirect, untargeted interactions between cells and complex cytokine-like signal transduction processes [42, 59–61]. Rugo et al [62] demonstrated the role of methylation in the transmission of GI in embryonic stem cells by showing that the elimination of DNA cytosine methylation genes, Dmmt1 and Dmmt3a, completely eliminated transmission of GI. Ilnytskyy and Kovalchuk [63] presented further evidence on the role of methylation and miRNA- short RNA molecules in eukaryotic cells that regulate gene expression [64] in radiation-induced GI and BE.
Non-clonal damage could not be predicted by target theory
Instability is manifested by multiple end-points; all are heterogeneous (non-clonal) in expression though the relationship between them is not yet clear. These biologically damaging responses do not conform to expectations for the conventional models of radiation effects which are, by definition, clonal in expression [34].
Dose-dependence deviates from linearity
Another notable feature is the dose-dependence of induction of instability. At low doses there is a tendency for the instability to plateau [65, 66] and in some cases even decrease at high doses, which contrasts to the generally steadily increasing responses that are conventionally observed. Radiation-induced GI therefore profoundly impacts interpretation of dose-response relationships in two ways, first by increasing the level of the response at low doses in comparison to what might be expected by extrapolation from higher doses, and secondly by the uncertainties associated with the consequences of delayed non-clonal expression of damage.
In vivo evidence
The existence of clastogenic factors in the plasma of radiation-exposed human subjects has been described in numerous studies, reviewed by Lindholm et al. [67]. Radiation-induced chromosomal instability (CIN) has been observed in vivo [68], and in vitro both in mouse and in human haematopoietic stem cells [34, 69]. However, there are some inconsistencies in results reported by these studies, reviewed by Morgan and Sowa [70]. Some studies showed that post-irradiation, GI is not universally expressed in mammalian cells in vivo or in vitro [71–73] and in other studies, whether animal or human, its expression has been reported to depend on the genotype of the irradiated cell [69, 74, 75], with considerable inter-individual variation even in those genotypes that may express high levels of instability [68]. These conflicting issues and observations may be attributed to cell type, to genotype or to other, as yet, poorly understood factors. These findings clearly indicate a need for caution in drawing generalised conclusions from limited data from individual studies or, indeed, with current knowledge, attempting to extract a simple coherent picture from the literature in general.
CIN in bone marrow has been demonstrated in vivo after 1 or 3 Gy total body low- linear energy transfer [62] irradiation. However, at lower doses of 0–0.5 Gy there was no evidence of CIN [76]. In contrast to these findings for sparsely ionizing low-LET radiation, earlier studies of bone marrow cells irradiated with a low dose of densely ionizing high-LET alpha-particles (with a mean of one particle per traversed cell) resulted in significant expression of CIN in vitro and in vivo [34, 68]. Like the studies of bone marrow cells irradiated with a low dose of alpha-particles, many studies of non-targeted effects relate to cells exposed to low fluences of alpha-particles or microbeam-generated charged particles. In these situations those cells that are traversed by a single particle receive a dose of ~ 0.3–0.5 Gy and sustain damage that is much greater and more complex than is the case for cells irradiated with very low doses of sparsely ionizing low-LET X- or gamma-rays, where a single radiation track would deliver a dose of the order of 1 to a few mGy to the irradiated cell. Also there will, for the same dose, be significant differences in the proportions of irradiated and non-irradiated bystander cells depending on the radiation quality. Obviously, it is important to be clear as to what is meant by a low and high dose to the individual cell or to population of cells in this context.
2.2 Transgenerational Genomic Instability
The phenomenon of transgenerational GI can be defined as a persistently increased rate of mutation observed in the non-exposed offspring of irradiated parents, as reviewed by Dubrova [77]. Transgenerational studies initiated by the discovery of persistently elevated mutation rates in the non-exposed progeny of irradiated cells, reviewed by Morgan [31], are designed to test the hypothesis that radiation-induced instability in the germline of irradiated parents could manifest in the offspring, affecting their mutation rates, cancer predisposition and other characteristics. There is equivocal evidence for such effects in human populations. In particular, analysis of a Belarusian population exposed as a result of the Chernobyl nuclear accident suggested excess minisatellite mutations [78, 79]. A similar magnitude of excess risk was suggested in a population exposed as a result of the Kazakhstan nuclear weapons tests [80]. However, no excess of mini- or microsatellite mutations were observed in offspring of the Japanese atomic bomb survivors [81–84] or in offspring of various groups of Chernobyl-exposed liquidators [85–88].
The results of a number of transgenerational studies show that mutation rates in the first- and second-generation offspring of irradiated male mice are significantly increased across multiple tissues [30, 89–92]. It has also been reported that in the offspring of irradiated male mice and rats transgenerational instability affects the frequency of chromosome aberrations [93], as well as mutations at protein-coding genes [90, 94] and tandem repeat DNA loci [89–92]. Taken together, these data imply that the transgenerational effects in the offspring of irradiated male mice may be explained by a genome-wide destabilization which manifests in many, or possibly all tissues. Furthermore, as transgenerational effects show non-Mendelian segregation (all offspring in both the F1 and F2 generations inherit the instability phenotype), it has been suggested that this phenomenon is attributed to an epigenetic signal arising in the paternal germline that is transmitted to their offspring [80, 89, 90].
Although the results of above mentioned studies have provided some evidence for the effects of paternal (but not maternal) irradiation on destabilization of the offspring’s genome, the mechanisms underlying the phenomenon of transgenerational GI remain poorly understood. One of the key issues regarding the mechanisms of radiation-induced GI is to investigate the initial cellular events triggering an instability signal in directly exposed cells. Given that exposure to ionizing radiation results in a plethora of DNA lesions, including double- and single-strand DNA breaks, base damage etc. [95], it appears plausible that a specific set of lesions may constitute the signal that initiates the onset of GI. It has therefore been hypothesised that radiation-induced complex double-strand DNA breaks may represent such triggering events [96]. To establish whether GI is attributed to the induction of a specific subset of DNA lesions, studies by Dubrova et al. have analysed the transgenerational effects of paternal treatment by a number of mutagens, exposure to which mostly causes alkylation of DNA and other types of DNA damage [97, 98]. The results of these studies clearly show that paternal exposure to the alkylating agent ethylnitrosourea, as well as to three anticancer drugs cyclophosphamide, mitomycin C and procarbazine can destabilise the offspring’s genome in a similar manner to that following paternal irradiation. These data therefore suggest that transgenerational GI is not attributable to a specific sub-set of DNA lesions, such as double-strand DNA breaks (DSB), but can be triggered by generalised DNA damage in the male germ cells.
The available human data suggest that maternal irradiation does not result in transgenerational instability [99], and this is supported by experimental findings. In particular, there was no evidence of excess minisatellite mutation in the germline and somatic tissues of first-generation offspring of female mice irradiated either in utero [92] or during adulthood [100]. The inability of exposed females, regardless of the stage of their irradiation, to propagate genomic instability to their offspring may be attributed to early post-fertilization events, which could somehow erase the radiation-induced de novo epigenetic signals.
Genomic instability and human cancer
There is a growing body of evidence that implicates non-radiation-induced GI as a key feature in the accumulation of the large number of mutational events required for various malignancies [101–103].
A number of human diseases are associated with CIN, and are also associated with a high risk of malignancy, as reviewed by Little [104] (see table 1). Additional clues as to the importance of instability in carcinogenesis arise from the study of certain rare genetic disorders characterised by CIN and a heritable predisposition to the development of cancer [84,85]. We discuss below the specific role played by GI in colon cancer.
Table 1.
Examples of some genetic disorders characterised by genomic instability and predisposition to cancer (reproduced from Little [104])
| Clinical disorder | Gene | Function | Major cellular abnormalities | Cancer types |
|---|---|---|---|---|
| Ataxia telangiectasia | ATM | DNA damage sensor | chromosomal instability, radiosensitivity, cell cycle abnormalities | Primarily leukaemia and lymphoma, some solid tumours |
| Nijmegen breakage syndrome | NBS1 | recombinational DNA repair | chromosomal instability, radiosensitivity, cell cycle abnormalities | Lymphoma and leukaemia |
| Bloom’s syndrome | BS | helicase (DNA replication) | chromosomal instability, elevated sister chromatid exchanges | Multiple cancers of all types |
| Fanconi’s anaemia | FAa | DNA damage sensing and repair | chromosomal instability, sensitivity to DNA crosslinking agents | Leukaemia and solid tumours |
| Familial breast cancer | BRCA1, BRCA2 | recombi national DNA repair | chromosomal instability, radiosensitivity | Breast and ovarian cancer |
| Hereditary non-polyposis colon cancer | MMRb | mismatch DNA repair | microsatellite instability, mutational instability | Colon and certain other solid tumours |
There are seven interacting FA genes.
Mismatch repair. There are several different MMR genes, inactivation of any one of which will give rise to the disorder.
It is widely accepted that colorectal carcinogenesis is characterised by two forms of instability. CIN is the predominant form of GI in colon cancer, and refers to the phenomenon whereby, relatively frequently, cells gain or lose whole chromosomes or large portions of individual chromosomes. The consequences of such alterations are imbalance of the chromosome number in the genome or structural damage on certain chromosomes. In human cancer CIN is known to be associated with alterations in the mitotic spindle checkpoint genes hBUB1 and hsMAD2 [105, 106], as also with the genes BRCA1 and BRCA2 which encode proteins implicated in DNA repair and recombination, checkpoint control of the cell cycle and transcription [107, 108], and with hCDC4, which is thought to be involved in the G1-S cell-cycle checkpoint [109]. In addition, 13% of colon cancers, as well as a small portion of other solid cancers, exhibit microsatellite instability (MIN), a less prevalent form of GI found in human cancer cell lines. MIN is caused by defects in the mismatch repair (MMR) mechanism, which contributes to replication fidelity by correcting incorrectly inserted DNA bases [49]. Defects in the MMR pathway lead to frequent insertions and deletions of repetitive short sequences, so-called microsatellites, across the genome.
However, it is still controversial whether transmissible GI is an initiating event for colon cancer. Loeb [101, 102] proposed that an early step in colon carcinogenesis is mutation in a gene controlling GI. Consequently, models have been developed recently incorporating CIN [110–115], although few have been rigorously fitted to data [113–115]. Tomlinson and Bodmer [116] argued that cancer is an evolutionary process, and that the observed accumulation of chromosomal and other damage in colon cancers may simply be the result of selection for cells with growth advantage, with mutations “piggybacking” on this process of selection. The fact that models that allow for single [113] or multiple types of destabilizations [115] yielded not much better fits to Surveillance Epidemiology and End Results (SEER) colon cancer data than that of a model not involving GI [117], suggests that, based on the fit of these models to these population-based data, there is little evidence for or against the involvement of GI in colon cancer.
In a European cohort study of more than 20,000 persons, higher rate of chromosomal aberrations in lymphocytes was shown to predict future cancer risk [118], suggesting (weakly) that induction of GI may be attributed to genetic factors.. Several mechanisms have been proposed for induction of GI by ionizing radiation [70]; these include, for example, deficiencies in components of DNA repair pathways in those mice sensitive to the induction of CIN [119]. The genetic predisposition to GI response observed by many different investigators with different cell systems is consistent with a considerable variation in the capacity to produce and/or respond to clastogenic factors, which are produced via superoxide. Furthermore, in vivo studies by Coates et al [120] have demonstrated genotype-dependent macrophage activity in irradiated mice. The authors suggest that these data are consistent with genetic influences on pro- and anti-inflammatory activation states being a major determinant of delayed cytogenetic damage post-irradiation in vivo [120, 121].
Finally, the mechanism of propagation of instability has not yet been clearly identified. Nor has there been identification of the initial radiation targets, nor the types of initial molecular damage that trigger the subsequent trans-acting and delayed consequences of instability.
2.3 Radiation Induced Bystander Effects (BE)
Many of the characteristics of BE do not conform to conventional target-theory. In the following paragraphs, we briefly highlight characteristic factors that influence the induction of BE, discuss the discrepancies that are reported in the field of BE, and the impact these issues have on radiation risk assessment and radiotherapy.
Biological effects are observed in non-irradiated cells as a consequence of cellular communication
Radiation-induced BE is characterised by biological responses that are observed in un-irradiated cells. This is assumed to occur as a result of cells receiving signals from irradiated cells through gap junction communications or culture medium from irradiated cells via diffusible factors. BE has been observed in a range of cell types and for several biological end points. It is also observed both for external beam and radionuclide exposures [122, 123]; the in vitro and in vivo evidence, and proposed mechanisms, have been extensively reviewed [1, 31, 32, 63, 124–126]. Possibly related to these in vitro observations of BE are abscopal or out-of-field effects observed experimentally in vivo [127], and in patients treated with radiotherapy [22]. The interrelationship between these “long-range” BE and short range cellular effects have not yet been clearly defined, but they are probably manifestations of an integrated stress response to localised irradiation. A variety of cytokines and other signalling molecules play a role in the transduction of BE responses [45, 128–131].
High frequency and non-clonality
BE is seen for most end-points including DNA damage, cell killing, chromosome aberrations, mutations and transformation [31–33]. Although these types of biological damage overlap with effects that are well-known to occur in directly-irradiated cells, they do not conform to expectations for the conventional class of radiation effects. For example, the DNA damage response in bystander cells is known to be different from that observed in directly-irradiated cells, involving ATR dependent signalling from stalled replication forks, instead of direct ATM-mediated signalling [132].
Also, like those observed in GI, BE occur at very high frequency and are heterogeneous (non-clonal) in their expression [133].
Dose-dependence is highly non-linear
As with GI, BE has been observed following a variety of radiation types and exposure protocols, especially at low-dose exposure. BE has been shown to be induced at very low doses and fluences (in the mGy range for low LET and single high-LET particles delivered to an individual cell), and increasing dose further did not increase the effect [134]; however, this does not necessarily imply non-linearity in the underlying biophysical dose-response relationships, simply intercommunicating cell populations that can be in one of four states [135]. Somewhat supporting this theoretical model, experimental findings suggest that following microbeam exposure there is a binary (on–off) response, with the probability of effect increasing with radiation dose [136].
Modifying factors
As with GI, and as noted above, BE is seen both in vitro and in vivo [31, 32]. Of particular interest is a recent study that provides evidence for bystander induction of cancer in a Ptch1+/− mouse model of brain cancer [137] after medium to high dose (3, 8.3 Gy) partial-body radiation exposure. This could be due to abscopal, or out-of-field, effects [138]. The relevance of this work to low-dose risk in humans is unclear, but suggests, as with some others [31, 32], that bystander effects can act to increase rather than decrease risk. It has been reported that not all cell types produce bystander signals and not all cell types respond [139–141]. This variable expression of BE could be due to the presence of multiple pathways involved in the various bystander phenomena. A study of peripheral blood lymphocytes irradiated with a microbeam yielded evidence of genotype-dependent differences in the magnitude of response to radiation exposure and bystander signal [142]. Additionally, activation times following exposure to the signals, as well as a variety of genetic and epigenetic factors have been shown to modify these processes. For example, it was found that medium from irradiated human epithelial cells, but not human fibroblasts, could reduce the clonogenic survival of a range of un-irradiated cells [139]. However, the effect was dependent on: 1) cell density, from which the medium was obtained, at the time of irradiation; 2) time of analysis, for example, excess calcium fluxes (very likely reflecting the effect of receipt of the signal molecule from the conditioned medium) were measured within 30 seconds, whilst reactive oxygen species (ROS) levels and apoptosis, the longer term consequences of processing of the signal by the cell, were measured 6 hours and 2 days, respectively, after medium transfer. Similarly, delayed-induction of reproductive cell death was measured in the un-irradiated bystander cells 9 days post-media transfer [143]. These studies, and others, confirm that the biological/experimental system, radiation type and dose, biological endpoints, system of analysis, and time of analysis post-irradiation should all be considered in comparing results of different experiments.
A variety of biological effects are induced as a result of cellular communication
BE have been observed for a variety of biological end points including: damage-inducible stress responses; sister chromatid exchange; micronucleus formation; apoptosis; gene mutation; CIN; and in vitro cell transformation [31–33]. Several signalling molecules have been identified that mediate communication between irradiated and bystander populations [125]. These include: transmissible signals that are generated by irradiated cells such as ROS [144]; nitric oxide species (NOS) [145]; and cytokines [146] that can be transmitted to the bystander non-irradiated cells either through gap junctions [147, 148] or through media [149, 150]. Several mechanistic studies have shown an important role for the NF-κB-dependent gene expression of interleukin 8, interleukin 6, cyclooxygenase-2, tumour necrosis factor and interleukin 33 in bystander cells. These studies suggest that directly irradiated cells produce cytokines and prostaglandin E2 by means of autocrine/paracrine mechanisms, which in turn further activate signalling pathways. [3]. From gene expression and gene ontology analysis, NF-κB forms a major transcriptional hub in bystander cells in contrast to directly irradiated cells where p53 drives transcriptional responses in response to a direct DNA damage signal [151].
From these results and others, it is reasonable to propose that the biological consequences of such a wide range of responses can be both damaging (i.e., sister chromatid exchanges, gene mutation, and transformation in vitro, and in vivo induction of medullablastoma through the transmission of bystander signals from the back of the irradiated mice to the cerebellum) [137] and protective (i.e., apoptotic cell death in vivo and in vitro, as well as the enhancement of cell differentiation with loss of proliferation in tissue in vivo with either high- or low-LET radiation) [141, 152, 153].
Bystander effect and genomic instability are interlinked
BE and GI share some important phenotypes such as multiple end points, e.g., micronuclei formation, increased mutation and induced chromosomal rearrangements, and up-regulation of oxidative stress; these are expressed at high frequency and there is generally an absence of conventional dose – response. Some studies have suggested they are inter-related [29, 68] (Figure 1). In particular, there is evidence for a link between BE and GI in vitro, reviewed by Lorimore et al. [154]. In all cases cellular communication between irradiated and un-irradiated cells either through secreted soluble factors or gap junction intercellular communication (GJIC) led to induction of GI. Grid shielding experiments, where the majority of unshielded cells died, showed that levels of CIN remained the same irrespective of the grid being in place or not [29]. This demonstrated that most of the cells demonstrating CIN were not irradiated, so that induction of damage was caused by intercellular communication, i.e., BE. Both co-culture and media-transfer experiments were seen to induce GI early in the communication process.
2.4 Adaptive Response (AR)
Currently AR is considered by some to be a component of NTE. AR is characterised by biological systems that are exposed to a small “priming” radiation dose (or to some other mild stress, e.g., heat) that exhibit a reduced detrimental effect when subsequently given another radiation dose, the “challenge” dose [155]. The observed detriment is generally below that of the priming + challenge dose given together, or that of the challenge dose alone. Although there have been reports of links between AR and BE, e.g., [156, 157], AR has few characteristics that overlap with GI and BE. In a substantial proportion of systems no AR is seen [158–161] and in some cases there is even an inverse AR, i.e., radio-sensitisation [159]. AR generally requires priming doses of at least 5 mGy [162] and the effect of the conditioning dose rarely lasts more than a day [163], so that it is arguably irrelevant to very low-dose or low dose-rate risk. AR exhibits some similarity to low dose hyper-radiosensitivity [164, 165], which is characterised by an elevated cell killing sensitivity in vitro to very low doses (<0.1 Gy) of radiation. Even assuming that it is of relevance in vivo, it is not clear what the health impact of a modestly elevated killing of cells in a tissue would be, although it is likely to be beneficial. As such, arguably the mechanisms underlying AR are unlikely to be related to those of GI or BE. Most AR experiments lack bystander cells (i.e., all cells are irradiated with both conditioning and challenging doses) and generally relatively high doses are generally used for the challenge doses. Although there are some experiments in which AR are induced by bystander processes (e.g., medium transfer), that does not make AR an intrinsic part of the BE mechanism per se, any more than it would all the many other possible consequences of bystander signals (e.g., DNA damage, effects on other DNA response pathways, cell killing, mutations, aberrations, transformation, apoptosis, etc.).
To conclude, our perception of the cellular response to low-level ionizing radiation has radically changed with the discoveries of GI and BE. These processes also complicate attempts at risk assessment. However, the mechanisms and inter-relationships amongst these processes need to be better understood before they can be incorporated into risk assessment with confidence. Further research is essential to address gaps in our understanding of non-targeted effects, in particular to confirm, or negate, the relevance of such processes for human health, specifically radiation risk assessment and cancer therapy.
3 Implications for low dose risk
Critical issues for radiological protection are the health consequences of dose rates of the order of a few mGy per year. A low LET dose of 1 mGy corresponds to an average of about one electron track hitting each cell nucleus [12, 166]. As Brenner et al. [167] point out; this means that at very low doses (10 mGy or less over a year) it is unlikely that temporally and spatially separate electron tracks could cooperatively produce nuclear DNA damage. Brenner et al. [167] surmise from this that in this very low dose region nuclear DNA damage at a cellular level would be proportional to dose.
Cancer
It has been known for some time that the efficiency of cellular repair processes varies with dose and dose rate [12], and this may be the reason for the curvature in cancer dose response and dose rate effects observed in epidemiological and animal data [12, 168]. Repair of double strand breaks (DSBs) relies on a number of pathways, even the most accurate of which, homologous recombination, is prone to errors [166]; other repair pathways, e.g., non-homologous end joining, single-strand annealing, are intrinsically much more error prone [166, 169]. The variation in efficacy of repair that undoubtedly occurs will affect the magnitude of unrepaired and misrepaired damage and, whereas unrepaired damage is likely to result in cell death, misrepaired damage will invariably result in mutation.
Non-targeted effects imply that radiation may affect targets other than the directly irradiated cellular nuclear DNA. In fact there is now strong evidence that a DNA damage response is elicited in cells not directly irradiated. Because of this substantial enlargement of the effective target size implied by the bystander effect, this would imply non-linearity of dose response even at very low dose-rates (<10 mGy/year). Observations of bystander responses in in vitro systems have led to suggestions that at low doses these non-targeted effects could contribute to substantial elevations in low dose risk [170], although this interpretation has been challenged [171]. On the other hand, it has been suggested that such bystander effects could be part of a sensitive response system and thus be protective [152, 172–174].
Until recently there has been little epidemiological data to suggest elevated cancer risk at very low doses [168]. Perhaps the most compelling evidence comes from studies of in utero exposure, which suggest that there are elevated risks of leukaemia and most other cancer types following 10–20 mGy diagnostic exposure [175–179]. The radiation risks (per unit dose) implied by these studies are similar in magnitude to those following much higher dose exposure in early life in the Japanese atomic bomb survivors [178]. Although a causal interpretation of the risks in these diagnostic studies is still controversial [180], the recent publication of a report demonstrating excess solid cancer risks among the in utero exposed atomic bomb survivors, that are also very similar to those following early childhood exposure in this cohort, provides strongly supportive evidence [181], and suggest that the elevated cancer risks observed in the diagnostic in utero studies are causally associated with radiation exposure. A recent study of leukaemia and brain cancer relation to computerised tomography (CT) in a paediatrically exposed UK cohort suggested significant excess risks of both endpoints at very low doses (< 50 mGy), consistent with what would be expected by extrapolation from high dose studies [182]. A recent large UK case-control study of childhood leukaemia in relation to natural background radiation exposure also suggested significant excess leukaemia risks at even lower levels of dose (~5 mGy), again at a level consistent with those that would be expected by linear extrapolation from high dose studies [183]. All these studies are supportive of low dose linearity in the dose response, i.e., LNT.
Circulatory disease
For some time it has been known that high doses of radiation to the heart or coronary arteries result in long term increased risk of various types of circulatory disease [184]. At high radiation doses, such as would be received by patients treated with radiotherapy (RT), a variety of other (so-called deterministic or tissue reaction) effects are observed, presumably resulting from inactivation of large numbers of cells and associated functional impairment of the affected tissue. Among such effects are direct damage to the structures of the heart – including marked diffuse fibrotic damage, especially of the pericardium and myocardium, pericardial adhesions, microvascular damage and stenosis of the valves – and to the coronary arteries; these sorts of damage occur both in patients receiving RT and in experimental animals [184]. There are plausible, if not completely understood, mechanisms by which high doses of radiation affect the blood circulatory system [185].
However, there is emerging evidence of excess risk of cardiovascular disease at much lower radiation doses and occurring over much longer intervals after radiation exposure in the Japanese atomic bomb survivor Life Span Study cohort [15, 186, 187] and in some occupationally-exposed groups [25, 188–194] although not in all [195].
As above, the mechanisms of radiation-induced vascular disease induction, even at high dose, are far from being understood. While they cannot be absolutely ruled out, somatic mutational mechanisms are unlikely to play a role in the aetiology of cardiovascular disease [196–198]. Systematic review of these data and biological mechanisms for such low dose effects indicated that the most likely causative effect of radiation is damage to endothelial cells and subsequent induction of an inflammatory response, although it seems unlikely, assuming conventional DNA-damage mechanisms, that this would extend to low dose and low dose-rate exposure [23, 199]. There is much evidence to support this within the Life Span Study cohort. For example, elevated levels of the pro-inflammatory cytokines IL-6, CRP, TNF-α and INF-γ, but also increased levels of the (generally) anti-inflammatory cytokine IL-10, have been observed in the Japanese atomic bomb survivors [200, 201]. There was also dose-related elevation in erythrocyte sedimentation rate and in levels of IgG, IgA and total immunoglobulins in this cohort, all markers of systemic inflammation [200]. Given the possible role of infections in cardiovascular disease [202, 203], it is of interest that certain T-cell and B-cell population numbers are known to vary with radiation dose among the Japanese atomic bomb survivors [204]. The atomic bomb survivors also demonstrate dose-dependent decreases in levels of CD4+ helper T-cells [201]; decreased levels of helper T-cells have also been found in blood samples from Japanese atomic bomb survivors with myocardial infarction [205].
Supporting evidence is available from many experimental in vivo studies at high dose. In particular, recent experimental studies by [206] demonstrate that high dose exposure to the cardiovascular system is associated with an earlier onset and accelerated development of macrophage-rich, inflammatory atherosclerotic lesions prone to intra-plaque haemorrhage and may also cause a decrease in myocardial perfusion. Both macro-vascular and micro-vascular radiation effects involve the endothelium and pro-inflammatory signalling cascades. Whether or not inflammation plays a role in the radiation induction of atherosclerosis, it is likely to be a major co-contributor to the development of atherosclerotic lesions [207]. If inflammation is arguably the most likely cause of radiation-induced atherosclerotic disease, the major question is how low doses and low dose rates of radiation, in particular a single electron track, can initiate an inflammatory cascade. At sub mGy doses of low LET radiation cells that are hit by electron tracks will be spatially and temporally isolated – for example a 0.1 mGy dose will only result in one cell nucleus in 10 being hit [12]. Without non-DNA-targeted effects to in some way enlarge the effective target size, that such an event could initiate an on-going inflammatory reaction is improbable. As such, if radiation can modify (whether positively or negatively) the atherosclerotic disease process, it is likely that non-DNA-targeted effects are implicated. Set against this, a recent paper has postulated a novel mechanism, based on monocyte cell killing in the intima and subsequent upregulation of monocyte chemo-attractant protein 1 (MCP-1) resulting in initiation of an inflammatory cascade [208]. This mechanism predicts excess cardiovascular risk at low doses and low dose rates, and is quantitatively consistent with the magnitude of excess risk observed in occupational groups [25, 208]. However, the detailed assumptions made by the model have yet to be experimentally tested.
Taken together, based on the present state of experimental research into radiation-induced cardiovascular disease one can conclude that radiation may cause both types of cardiovascular disease, that is to say micro-vascular disease, which is characterised by a decrease of capillary density causing chronic ischaemic heart disease and focal myocardial degeneration, and macro-vascular disease, through the faster development of age-related atherosclerosis in the coronary arteries [185]. On the other hand, the epidemiological studies do not give unequivocal information on the types of cardiovascular diseases that are induced by radiation. It is assumed that this might depend on dose, dose distribution and other risk factors present in different animal strains and humans. Moreover, both types may show different latency periods at different dose levels. Both macro-vascular and micro-vascular radiation effects involve the endothelium and pro-inflammatory signalling cascades. A substantial number of studies have investigated radiation effects on endothelial cells in vitro, and a few studies have confirmed some of these observations in vivo, summarised by Schultz-Hector and Trott [185]. While there is a suggestive role for NTE in enlarging the effective target size in induction of inflammation in radiation-induced cardiovascular disease, conventional cell-killing mechanisms cannot be excluded [25, 208].
A recent systematic review and meta-analysis of medium and low dose studies documented significant radiation-associated excess risk overall and in two out of four subtypes of circulatory disease [25]. Risk coefficients from the meta-analysis were used to estimate population risks for nine major developed countries. Risks for these are shown in Table 2. Estimated excess population risks for all circulatory diseases combined ranged from 2.5% per Sv (95% CI 0.8 to 4.2) for France to 8.5% per Sv (95% CI 4.0 to 13.2) for Russia. The analysis was limited by heterogeneity among studies (particularly for non-cardiac endpoints), the possibility of uncontrolled confounding in some occupational groups by lifestyle factors, and higher dose groups (>0.5 Sv) generally driving the observed trends. If confirmed, the findings suggest that overall radiation-related mortality is about twice that currently estimated based on estimates for cancer endpoints alone, which range from 4.2% to 5.6% per Sv for these populations (Table 2) [25].
Table 2.
Estimated Excess Risk of Radiation-Exposure-Induced Death for Various Subtypes of Circulatory Disease, by Country, taken from Little et al. [25]. All calculations assume a single acutely delivered test dose of 0.01 Sv, and are calculated assuming a random-effects model.
| Country (year at which underlying mortality rates were determined) | Baseline proportion of deaths due to circulatory disease | Radiation-Exposure-Induced Death, × 10−2 Sv (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| Ischaemic heart disease (ICD10 I20–I25) | Other (non- ischaemic) heart disease (ICD10 I26–I52) | Cerebrovascular disease (stroke) (ICD10 I60–I69) | Other circulatory disease (ICD10 I00–I19, I53–I59, I70–I99) | All circulatory disease (ICD10 I00–I99) | UNSCEAR risks [168] | |||
| All solid cancer (ICD10C00–C80) | Leukaemia excluding CLL (ICD10 C91–C95 – C91.1) | |||||||
| China (2000) | 42.1% | 0.92 (0.41, 1.42) | 0.11 (−0.16, 0.37) | 4.31 (0.48, 8.14) | 1.43 (−0.01, 2.86) | 6.76 (2.63, 10.89) | 3.95 3.89 |
0.27 0.42 |
| France (2007) | 20.8% | 0.50 (0.22, 0.78) | 0.54 (−0.85, 1.94) | 0.92 (0.10, 1.74) | 0.53 (0.00, 1.05) | 2.50 (0.77, 4.22) | - | - |
| Germany (2006) | 48.7% | 1.71 (0.76, 2.65) | 0.97 (−1.52, 3.46) | 1.69 (0.19, 3.19) | 1.38 (−0.01, 2.76) | 5.75 (2.39, 9.10) | - | - |
| Japan (2009) | 31.1% | 0.57 (0.25, 0.88) | 0.80 (−1.25, 2.85) | 2.19 (0.24, 4.14) | 0.45 (0.00, 0.91) | 4.01 (1.13, 6.89) | 4.65 4.90 |
0.32 0.43 |
| Russia (2006) | 64.4% | 2.82 (1.26, 4.39) | 0.31 (−0.49, 1.11) | 4.59 (0.51, 8.66) | 0.79 (0.00, 1.57) | 8.51 (4.00, 13.02) | - | - |
| Spain (2005) | 35.8% | 0.91 (0.41, 1.42) | 0.82 (−1.28, 2.52) | 1.91 (0.21, 3.60) | 0.81 (0.00, 1.63) | 4.45 (1.73, 7.17) | ||
| Ukraine (2008) | 69.2% | 4.14 (1.85, 6.43) | 0.20 (−0.31, 0.70) | 2.85 (0.31, 5.39) | 0.93 (0.00, 1.85) | 8.11 (4.53, 11.69) | ||
| UK (2003) | 39.9% | 1.70 (0.76, 2.64) | 0.37 (−0.58, 1.32) | 2.24 (0.25, 4.22) | 0.76 (0.00, 1.53) | 5.07 (2.55, 7.58) | 5.15 4.40 |
0.38 0.43 |
| USA (2005) | 39.3% | 1.82 (0.81, 2.82) | 0.57 (−0.89, 2.03) | 1.29 (0.14, 2.44) | 0.80 (0.00, 1.61) | 4.48 (2.22, 6.74) | 4.74 4.41 |
0.47 0.42 |
Cataract
For some time it has been known that high radiation doses of 1 Gy or more could induce posterior subcapsular cataract (PSC) [10]. There is accumulating evidence from the Japanese atomic bomb survivors [27, 209–211], Chernobyl liquidators [26], US astronauts [212] and various other exposed groups [28, 213] to suggest that cortical cataracts can also be induced by low and medium doses of ionizing radiation, although there is little evidence that nuclear cataracts are radiogenic; the main epidemiological datasets are summarised in Table 3. The atomic bomb survivor data do not suggest any threshold for cataract. For example Nakashima et al. [210] found a best estimate of 0.6 Sv (90% CI <0, 1.2) for cortical cataract and 0.7 (90% CI <0, 2.8) for posterior subcapsular cataract (PSC). Likewise, a threshold estimate for all surgically removed cataracts in this cohort of 0.1 Sv (95% CI <0, 0.8) was observed [27]. However, estimated thresholds for various cataract endpoints in a cohort of Chernobyl liquidators [26] were in the range 0.34–0.50 Gy, with lower 95% CI in the range 0.17–0.19 Gy, and upper 95% CI in the range 0.51–0.69 Gy.
Table 3.
Cataract risk estimates. Excess odds ratio (/Gy) (+95% CI).
| Cohort | Ascertainment | Endpoint | Excess odds ratio (EOR) Gy−1 (95% CI) |
|---|---|---|---|
| Swedish skin haemangioma [227] | LOCSI | Cortical | 0.50(0.15, 0.95) |
| Posterior subcapsular | 0.49(0.07, 1.08) | ||
| A-bomb AHS [210] | LOCS II | Cortical | 0.30(0.10, 0.53)a |
| Posterior subcapsular | 0.44(0.19, 0.73)a | ||
| Nuclear opacity | 0.07 (−0.11, 0.30)a | ||
| Nuclear colour | 0.01 (−0.17, 0.24)a | ||
| A-bomb AHS cataract surgery [211] | Surgical removal | All cataract removal | 0.32 (0.20, 0.47) |
| Icelandic airline pilots [228] | WHO | Nuclear | 20 (0, 30) |
| Cortical | <0(<0, >0) | ||
| Posterior subcapsular | <0(<0, >0) | ||
| Chernobyl recovery worker [26] | Merriam-Focht | Non-nuclear stage 1–5 | 0.65 (0.18, 1.30) |
| Posterior subcapsular stage 1 | 0.42 (0.01, 1.00) | ||
| Nuclear | 0.07 (−0.44, 1.04) | ||
| All cataract stage 1–5 | 0.70 (0.22, 1.38) | ||
| US Radiologic technologist [229] | Self-reported removal | All cataract removal | 2.0 (−0.7, 4.7) |
| Finnish interventional radiologists [230] | LOCS II | Cortical or posterior excluding nuclear | −2 (−16, 13) |
| All opacity | 13 (−2, 28) |
EOR Sv−1.
The mechanisms of radiation-induced cataractogenesis are still uncertain. Cataract is thought to result from disruption to the eye lens fibres, which are continuously replaced throughout life. At a microscopic level, radiation damage to a single lens epithelial or fibre cells probably results in small localised changes in lens transparency and is therefore a stochastic event. Support for this hypothesis is provided by the linear relationship between radiation dose and the number of small, discrete dots in the posterior lens cortex of animals exposed to either low or high-LET radiation. Di Paola et al. [214] suggested that accumulation and coalescence of these micro-opacities results in populations of damaged lens fibre cells forming larger lens defects, eventually resulting in a clinical opacity. Chylack et al used a similar approach to score PSC “centres” and suggested a relationship between galactic cosmic radiation exposure and PSC size [212]. Using this approach, if a minimum number of damaged cells were required before a lens opacity were clinically observed, that would suggest a requirement for a threshold radiation dose and therefore radiation cataract could be classified as a tissue reaction (deterministic) response.
Other non-cancer effects
The atomic bomb survivor data suggest that there is significant excess risk for non-malignant digestive disease [15, 215]. However, this is not generally observed in other exposed groups [216].
Many studies of childhood cancer survivors (principally of leukaemia) document cognitive impairment associated with high dose cranial irradiation [217]. Slightly surprisingly, Hall et al. [218] observed cognitive impairment in a Swedish group treated for haemangioma in infancy with much lower doses, with ~50% reduction in high school attendance associated with >100 mGy exposure; there were similar dose-related reductions in cognitive test performance. In utero exposed Japanese atomic bomb survivor data also suggest cognitive impairment at high dose, but there is no cognitive impairment (e.g., reduction in IQ) in the 0–100 mGy dose range [219]. It is not clear the extent to which these two datasets are statistically compatible given the different metrics used; even if they were not, the obvious differences between the exposure-age range make meaningful comparisons difficult.
4 Frameworks of radiation biology, radiation risk and radiation protection
The frameworks of radiation biology, radiation risk and radiation protection are linked and evolve in parallel. The first guidelines for radiation protection issued in 1928 were based on protection from acute deterministic effects (limit based on skin erythema dose). After the atomic bombings in Hiroshima and Nagasaki it became apparent that radiation also induces cancer, and from then on the basis for radiation protection was prevention of tissue reaction (deterministic) effects and limitation of stochastic effects (cancer and hereditary effects). The system of protection has the advantages of being relatively simple, quantitative, covering a wide variety of exposure scenarios and radiation types. The system of protection relies on a number of assumptions and approximations that are deemed to be acceptable for prospective planning purposes in radiation protection. These assumptions need to be tested against new evidence from radiobiology and epidemiology. From the societal point of view, there should be added value of any changes introduced, such as better protection or simplification of the system.
Until recently there has been little direct epidemiological evidence on cancer risk at very low doses [17, 18, 220], and this gap in knowledge was filled with the LNT assumption based on the radiobiological DNA target framework [8]. Strong epidemiological evidence to support LNT has recently emerged [182, 183]. Although NTE may modify cancer risk at low doses, alongside direct DNA damage, we judge that it is not yet clear whether the overall impact of such effects would lead to increased or decreased risk, although it is very likely that the dose response will be non-linear. However, the epidemiological data now place reasonably strict limits on the extent to which linear extrapolation from the medium and high dose data will over- or under-estimate cancer risks [17, 183, 221]. For hereditary effects, there is no sound epidemiological evidence and risk estimates are mainly based on radiobiological (animal) experiments.
Set against that, there is accumulating evidence of radiogenic excess risk of various categories of non-malignant disease, in particular circulatory disease [23–25] and cataract [28, 213] at medium or low doses. Although classified by the ICRP as tissue-reaction (deterministic) effects [8], they apparently violate the ICRP assumption of a dose threshold, below which the effects do not occur; there is evidence that these occur in occupationally-exposed groups. In that respect these late-arising non-cancer diseases have more of the characteristics of stochastic rather than tissue-reaction (deterministic) effects.
Although the precise mechanisms are not known, it is reasonably clear that DNA-damage is not the relevant initiating lesion for radiation-induced circulatory disease [23, 208]. The signalling nature of the disease also makes it very likely that NTE are implicated. As discussed above, if we interpret these associations causally the total low dose detriment would be predicted to double [25].
As with circulatory disease, DNA-targeted effects also cannot readily explain the mechanism of induction of cataract. We believe that the change in the radiation biological paradigm will lead to better understanding of radiation risk related to the late-arising non-cancer effects. The science in this area is at its infancy and more radiobiological and epidemiological research is needed in this area. From the radiation protection point of view, a key question is whether these effects show a threshold and whether the detriment would be large enough to warrant a change in dose limits and/or to be taken into account in tissue weighting factors.
There have been several attempts to model non-targeted effects, in particular models of atherosclerosis-related circulatory disease of Little et al. [208], GI in relation to cancer of Little et al. [113–115] and Nowak et al. [222], and of BE by Brenner et al. [170, 223], Little et al. [135, 171, 224], Nikjoo and Khvostunov [225], Stewart et al. [226] and many others. However, there is still no universally accepted biological model for most categories of NTE. Therefore, work needs to continue in order to formalise such a model.
5 Future research needs
Elucidating the mechanisms of NTE calls for further research; moving away from the conventional DNA targeted effect framework, future studies should concentrate on exploring more complex experimental systems instead of single cells and cell cultures. The resulting data may need evaluation by a systems biological approach to allow the study of cellular communication and 3-D tissue systems. There also needs to be further development of more relevant in vivo models. Given the evidence that agents other than ionizing radiation, in particular metals [36] and certain chemicals [37–41], can initiate NTE, there is a need for research to investigate similarities to and differences from radiation-induced NTE and compare the risk of health effects caused by these agents. If the underlying mechanisms are the same and if NTE contribute significantly to human health effects following exposure to these different agents, then one may expect to see a common pattern in risk.
Even though the initial events in certain sorts of NTE induction may be similar (calcium flux followed by reactive oxygen and nitrogen species), the downstream effects, i.e., cytokine concentrations, and changes in cellular physiology are likely to be tissue dependent and model systems should be chosen accordingly. There is growing evidence for the role of epigenetic mechanisms in the transmission of GI as well as in the formation of BE-induced DNA breaks. The rapid development of high-throughput epigenetic screening technologies opens new avenues for the understanding of the interaction of genome and environment, including the effects of ionizing radiation. A key question for evaluation of low dose radiation induced cancer risk is the relative contribution of DNA-targeted and non-targeted effects at low and high doses.
There should be a clear evaluation of how genetic sensitivity and epigenetic changes induced by high, medium and low doses of radiation influence risk. Greater attention should also be given to exploring the mechanisms of non-cancer effects as opposed to low dose cancer risk, as the former impacts on a wider range of tissues, and is likely to have at least as large an impact on detriment at low doses.
It is essential that the heterogeneity in response of non-targeted effects of radiation be addressed, as well as mechanistic pathways. The mechanisms involved with initiation and perpetuation of GI should be investigated for all radiation types and bystander effects, particularly for low doses of low LET and low-fluence high-LET particles as well as the influence of genetic factors in its induction. A priority must be to confirm, or negate, the relevance of such processes to human health risks.
Supplementary Material
Acknowledgments
This work was partially supported by the European Commission under the contract FP6–036465 (NOTE). This work was also supported by the Intramural Research Program of the National Institutes of Health, the National Cancer Institute, Division of Cancer Epidemiology and Genetics. The authors would like to thank Miss Kim Chapman, from Professor Kadhim’s group, for formatting assistance.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Reference List
- 1.Prise KM, O’Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer. 2009;9:351–360. doi: 10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sjostedt S, Bezak E. Non-targeted effects of ionising radiation and radiotherapy. Australas Phys Eng Sci Med. 2010;33:219–231. doi: 10.1007/s13246-010-0030-8. [DOI] [PubMed] [Google Scholar]
- 3.Hei TK, Zhou H, Chai Y, Ponnaiya B, Ivanov VN. Radiation induced non-targeted response: mechanism and potential clinical implications. Curr Mol Pharmacol. 2011;4:96–105. doi: 10.2174/1874467211104020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salomaa SI, Wright EG, Hildebrandt G, Kadhim MA, Little MP, Prise KM, Belyakov OV. Editorial-Non-DNA targeted effects. Mutat Res. 2010;687:1–2. doi: 10.1016/j.mrfmmm.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 5.Stevens LG. Injurious effects on the skin. BMJ. 1896;1:998. [Google Scholar]
- 6.Gilchrist TC. A case of dermatitis due to the x rays. Bulletin Johns Hopkins Hospital. 1897;8(71):17–22. [Google Scholar]
- 7.Frieben A. Demonstration eines Cancroids des rechten Handrückens, das sich nach langdauernder Einwirkung von Röntgenstrahlen bei einem 33 jährigen Mann entwickelt hatte. Fortschr Röntgenstr. 1902;6:106. [Google Scholar]
- 8.International Commission on Radiological Protection. ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs - threshold doses for tissue reactions in a radiation protection context. ICRP publication 118, Ann ICRP. 2012;41:1–322. doi: 10.1016/j.icrp.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 9.International Commission on Radiological Protection. ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs - threshold doses for tissue reactions in a radiation protection context. ICRP publication 118, Ann ICRP. 2012;41:1–322. doi: 10.1016/j.icrp.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Edwards AA, Lloyd DC. Risks from ionising radiation: deterministic effects. J Radiol Prot. 1998;18:175–183. doi: 10.1088/0952-4746/18/3/004. [DOI] [PubMed] [Google Scholar]
- 11.Harris H. A long view of fashions in cancer research. Bioessays. 2005;27:833–838. doi: 10.1002/bies.20263. [DOI] [PubMed] [Google Scholar]
- 12.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) UNSCEAR 1993 report to the General Assembly, with scientific annexes. New York: United Nations; 1993. Sources and effects of ionizing radiation; pp. 1–922. E.94.IX.2. [Google Scholar]
- 13.Lea DE. Actions of radiations on living cells. Cambridge: Cambridge University Press; 1946. [Google Scholar]
- 14.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 15.Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K. Studies of the mortality of atomic bomb survivors, report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177:229–243. doi: 10.1667/rr2629.1. [DOI] [PubMed] [Google Scholar]
- 16.Hall EJ, Giaccia A. Radiobiology for the radiologist. 7. Philadelphia, PA: Lippincott, Williams & Wilkins; 2012. pp. 1–576. [Google Scholar]
- 17.Little MP, Wakeford R, Tawn EJ, Bouffler SD, Berrington de GA. Risks associated with low doses and low dose rates of ionizing radiation: why linearity may be (almost) the best we can do. Radiology. 2009;251:6–12. doi: 10.1148/radiol.2511081686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tubiana M, Feinendegen LE, Yang C, Kaminski JM. The linear no-threshold relationship is inconsistent with radiation biologic and experimental data. Radiology. 2009;251:13–22. doi: 10.1148/radiol.2511080671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puck TT, MARCUS PI. Action of x-rays on mammalian cells. J Exp Med. 1956;103:653–666. doi: 10.1084/jem.103.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorgojo L, Little JB. Expression of lethal mutations in progeny of irradiated mammalian cells. Int J Radiat Biol. 1989;55:619–630. doi: 10.1080/09553008914550661. [DOI] [PubMed] [Google Scholar]
- 21.Emerit I. Reactive oxygen species, chromosome mutation, and cancer: possible role of clastogenic factors in carcinogenesis. Free Radic Biol Med. 1994;16:99–109. doi: 10.1016/0891-5849(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 22.Kaminski JM, Shinohara E, Summers JB, Niermann KJ, Morimoto A, Brousal J. The controversial abscopal effect. Cancer Treat Rev. 2005;31:159–172. doi: 10.1016/j.ctrv.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Little MP, Tawn EJ, Tzoulaki I, Wakeford R, Hildebrandt G, Paris F, Tapio S, Elliott P. A systematic review of epidemiological associations between low and moderate doses of ionizing radiation and late cardiovascular effects, and their possible mechanisms. Radiat Res. 2008;169:99–109. doi: 10.1667/RR1070.1. [DOI] [PubMed] [Google Scholar]
- 24.Little MP, Tawn EJ, Tzoulaki I, Wakeford R, Hildebrandt G, Paris F, Tapio S, Elliott P. Review and meta-analysis of epidemiological associations between low/moderate doses of ionizing radiation and circulatory disease risks, and their possible mechanisms. Radiat Environ Biophys. 2010;49:139–153. doi: 10.1007/s00411-009-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little MP, Azizova TV, Bazyka D, Bouffler SD, Cardis E, Chekin S, Chumak VV, Cucinotta FA, de VF, Hall P, Harrison JD, Hildebrandt G, Ivanov V, Kashcheev VV, Klymenko SV, Kreuzer M, Laurent O, Ozasa K, Schneider T, Tapio S, Taylor AM, Tzoulaki I, Vandoolaeghe WL, Wakeford R, Zablotska LB, Zhang W, Lipshultz SE. Systematic Review and Meta-Analysis of Circulatory Disease from Exposure to Low-Level Ionizing Radiation and Estimates of Potential Population Mortality Risks, Environ. Health Perspect. 2012 doi: 10.1289/ehp.1204982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Worgul BV, Kundiyev YI, Sergiyenko NM, Chumak VV, Vitte PM, Medvedovsky C, Bakhanova EV, Junk AK, Kyrychenko OY, Musijachenko NV, Shylo SA, Vitte OP, Xu S, Xue X, Shore RE. Cataracts among Chernobyl clean-up workers: implications regarding permissible eye exposures. Radiat Res. 2007;167:233–243. doi: 10.1667/rr0298.1. [DOI] [PubMed] [Google Scholar]
- 27.Neriishi K, Nakashima E, Minamoto A, Fujiwara S, Akahoshi M, Mishima HK, Kitaoka T, Shore RE. Postoperative cataract cases among atomic bomb survivors: radiation dose response and threshold. Radiat Res. 2007;168:404–408. doi: 10.1667/RR0928.1. [DOI] [PubMed] [Google Scholar]
- 28.Ainsbury EA, Bouffler SD, Dorr W, Graw J, Muirhead CR, Edwards AA, Cooper J. Radiation cataractogenesis: a review of recent studies. Radiat Res. 2009;172:1–9. doi: 10.1667/RR1688.1. [DOI] [PubMed] [Google Scholar]
- 29.Lorimore SA, Kadhim MA, Pocock DA, Papworth D, Stevens DL, Goodhead DT, Wright EG. Chromosomal instability in the descendants of unirradiated surviving cells after alpha-particle irradiation. Proc Natl Acad Sci U S A. 1998;95:5730–5733. doi: 10.1073/pnas.95.10.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubrova YE, Plumb M, Gutierrez B, Boulton E, Jeffreys AJ. Transgenerational mutation by radiation. Nature. 2000;405:37. doi: 10.1038/35011135. [DOI] [PubMed] [Google Scholar]
- 31.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: I. Radiation-induced genomic instability and bystander effects in vitro. Radiat Res. 2003;159:567–580. doi: 10.1667/0033-7587(2003)159[0567:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Morgan WF. Non-targeted and delayed effects of exposure to ionizing radiation: II. Radiation-induced genomic instability and bystander effects in vivo, clastogenic factors and transgenerational effects. Radiat Res. 2003;159:581–596. doi: 10.1667/0033-7587(2003)159[0581:nadeoe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) Non-Targeted and Delayed Effects of Exposure to Ionizing Radiation. New York: United Nations; 2009. UNSCEAR 2006 Report. Annex C; pp. 1–79. [Google Scholar]
- 34.Kadhim MA, Macdonald DA, Goodhead DT, Lorimore SA, Marsden SJ, Wright EG. Transmission of chromosomal instability after plutonium alpha-particle irradiation. Nature. 1992;355:738–740. doi: 10.1038/355738a0. [DOI] [PubMed] [Google Scholar]
- 35.Bowler DA, Moore SR, Macdonald DA, Smyth SH, Clapham P, Kadhim MA. Bystander-mediated genomic instability after high LET radiation in murine primary haemopoietic stem cells. Mutat Res. 2006;597:50–61. doi: 10.1016/j.mrfmmm.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 36.Glaviano A, Mothersill C, Case CP, Rubio MA, Newson R, Lyng F. Effects of hTERT on genomic instability caused by either metal or radiation or combined exposure. Mutagenesis. 2009;24:25–33. doi: 10.1093/mutage/gen048. [DOI] [PubMed] [Google Scholar]
- 37.Rugo RE, Almeida KH, Hendricks CA, Jonnalagadda VS, Engelward BP. A single acute exposure to a chemotherapeutic agent induces hyper-recombination in distantly descendant cells and in their neighbors. Oncogene. 2005;24:5016–5025. doi: 10.1038/sj.onc.1208690. [DOI] [PubMed] [Google Scholar]
- 38.Asur RS, Thomas RA, Tucker JD. Chemical induction of the bystander effect in normal human lymphoblastoid cells. Mutat Res. 2009;676:11–16. doi: 10.1016/j.mrgentox.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Demidem A, Morvan D, Madelmont JC. Bystander effects are induced by CENU treatment and associated with altered protein secretory activity of treated tumor cells: a relay for chemotherapy? Int J Cancer. 2006;119:992–1004. doi: 10.1002/ijc.21761. [DOI] [PubMed] [Google Scholar]
- 40.Jin C, Wu S, Lu X, Liu Q, Qi M, Lu S, Xi Q, Cai Y. Induction of the bystander effect in Chinese hamster V79 cells by actinomycin D. Toxicol Lett. 2011;202:178–185. doi: 10.1016/j.toxlet.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Korkalainen M, Huumonen K, Naarala J, Viluksela M, Juutilainen J. Dioxin induces genomic instability in mouse embryonic fibroblasts. PLoS One. 2012;7:e37895. doi: 10.1371/journal.pone.0037895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lorimore SA, Chrystal JA, Robinson JI, Coates PJ, Wright EG. Chromosomal instability in unirradiated hemaopoietic cells induced by macrophages exposed in vivo to ionizing radiation. Cancer Res. 2008;68:8122–8126. doi: 10.1158/0008-5472.CAN-08-0698. [DOI] [PubMed] [Google Scholar]
- 43.Bogdandi EN, Balogh A, Felgyinszki N, Szatmari T, Persa E, Hildebrandt G, Safrany G, Lumniczky K. Effects of low-dose radiation on the immune system of mice after total-body irradiation. Radiat Res. 2010;174:480–489. doi: 10.1667/RR2160.1. [DOI] [PubMed] [Google Scholar]
- 44.Buonanno M, de Toledo SM, Pain D, Azzam EI. Long-term consequences of radiation-induced bystander effects depend on radiation quality and dose and correlate with oxidative stress. Radiat Res. 2011;175:405–415. doi: 10.1667/RR2461.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lyng FM, Howe OL, McClean B. Reactive oxygen species-induced release of signalling factors in irradiated cells triggers membrane signalling and calcium influx in bystander cells. Int J Radiat Biol. 2011;87:683–695. doi: 10.3109/09553002.2010.549533. [DOI] [PubMed] [Google Scholar]
- 46.Jain MR, Li M, Chen W, Liu T, de Toledo SM, Pandey BN, Li H, Rabin BM, Azzam EI. In vivo space radiation-induced non-targeted responses: late effects on molecular signaling in mitochondria. Curr Mol Pharmacol. 2011;4:106–114. doi: 10.2174/1874467211104020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kadhim MA, Moore SR, Goodwin EH. Interrelationships amongst radiation-induced genomic instability, bystander effects, and the adaptive response. Mutat Res. 2004;568:21–32. doi: 10.1016/j.mrfmmm.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 48.Stoler DL, Chen N, Basik M, Kahlenberg MS, Rodriguez-Bigas MA, Petrelli NJ, Anderson GR. The onset and extent of genomic instability in sporadic colorectal tumor progression. Proc Natl Acad Sci U S A. 1999;96:15121–15126. doi: 10.1073/pnas.96.26.15121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 50.Nikiforov YE, Nikiforova M, Fagin JA. Prevalence of minisatellite and microsatellite instability in radiation-induced post-Chernobyl pediatric thyroid carcinomas. Oncogene. 1998;17:1983–1988. doi: 10.1038/sj.onc.1202120. [DOI] [PubMed] [Google Scholar]
- 51.Richter HE, Lohrer HD, Hieber L, Kellerer AM, Lengfelder E, Bauchinger M. Microsatellite instability and loss of heterozygosity in radiation-associated thyroid carcinomas of Belarussian children and adults. Carcinogenesis. 1999;20:2247–2252. doi: 10.1093/carcin/20.12.2247. [DOI] [PubMed] [Google Scholar]
- 52.Haines J, Bacher J, Coster M, Huiskamp R, Meijne E, Mancuso M, Pazzaglia S, Bouffler S. Microsatellite instability in radiation-induced murine tumours; influence of tumour type and radiation quality. Int J Radiat Biol. 2010;86:555–568. doi: 10.3109/09553001003734600. [DOI] [PubMed] [Google Scholar]
- 53.Piao CQ, Hei TK. Gene amplification and microsatellite instability induced in tumorigenic human bronchial epithelial cells by alpha particles and heavy ions. Radiat Res. 2001;155:263–267. doi: 10.1667/0033-7587(2001)155[0263:gaamii]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 54.Selvanayagam CS, Davis CM, Cornforth MN, Ullrich RL. Latent expression of p53 mutations and radiation-induced mammary cancer. Cancer Res. 1995;55:3310–3317. [PubMed] [Google Scholar]
- 55.Ullrich RL, Ponnaiya B. Radiation-induced instability and its relation to radiation carcinogenesis. Int J Radiat Biol. 1998;74:747–754. doi: 10.1080/095530098141023. [DOI] [PubMed] [Google Scholar]
- 56.Little JB. Radiation-induced genomic instability. Int J Radiat Biol. 1998;74:663–671. doi: 10.1080/095530098140925. [DOI] [PubMed] [Google Scholar]
- 57.Kovalchuk O, Baulch JE. Epigenetic changes and nontargeted radiation effects--is there a link? Environ Mol Mutagen. 2008;49:16–25. doi: 10.1002/em.20361. [DOI] [PubMed] [Google Scholar]
- 58.Baverstock K. Radiation-induced genomic instability: a paradigm-breaking phenomenon and its relevance to environmentally induced cancer. Mutat Res. 2000;454:89–109. doi: 10.1016/s0027-5107(00)00100-7. [DOI] [PubMed] [Google Scholar]
- 59.Moore SR, Marsden S, MacDonald D, Mitchell S, Folkard M, Michael B, Goodhead DT, Prise KM, Kadhim MA. Genomic instability in human lymphocytes irradiated with individual charged particles: involvement of tumor necrosis factor alpha in irradiated cells but not bystander cells. Radiat Res. 2005;163:183–190. doi: 10.1667/rr3298. [DOI] [PubMed] [Google Scholar]
- 60.Natarajan M, Gibbons CF, Mohan S, Moore S, Kadhim MA. Oxidative stress signalling: a potential mediator of tumour necrosis factor alpha-induced genomic instability in primary vascular endothelial cells. Br J Radiol. 2007;80(Spec No 1):S13–S22. doi: 10.1259/bjr/15316848. [DOI] [PubMed] [Google Scholar]
- 61.Burr KL, Robinson JI, Rastogi S, Boylan MT, Coates PJ, Lorimore SA, Wright EG. Radiation-induced delayed bystander-type effects mediated by hemopoietic cells. Radiat Res. 2010;173:760–768. doi: 10.1667/RR1937.1. [DOI] [PubMed] [Google Scholar]
- 62.Rugo RE, Mutamba JT, Mohan KN, Yee T, Chaillet JR, Greenberger JS, Engelward BP. Methyltransferases mediate cell memory of a genotoxic insult. Oncogene. 2011;30:751–756. doi: 10.1038/onc.2010.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ilnytskyy Y, Kovalchuk O. Non-targeted radiation effects-an epigenetic connection. Mutat Res. 2011;714:113–125. doi: 10.1016/j.mrfmmm.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 64.Landau DA, Slack FJ. MicroRNAs in mutagenesis, genomic instability, and DNA repair. Semin Oncol. 2011;38:743–751. doi: 10.1053/j.seminoncol.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trott KR, Jamali M, Manti L, Teibe A. Manifestations and mechanisms of radiation-induced genomic instability in V-79 Chinese hamster cells. Int J Radiat Biol. 1998;74:787–791. doi: 10.1080/095530098141078. [DOI] [PubMed] [Google Scholar]
- 66.Prise KM. New advances in radiation biology. Occup Med (Lond) 2006;56:156–161. doi: 10.1093/occmed/kql010. [DOI] [PubMed] [Google Scholar]
- 67.Lindholm C, Acheva A, Salomaa S. Clastogenic plasma factors: a short overview. Radiat Environ Biophys. 2010;49:133–138. doi: 10.1007/s00411-009-0259-3. [DOI] [PubMed] [Google Scholar]
- 68.Watson GE, Pocock DA, Papworth D, Lorimore SA, Wright EG. In vivo chromosomal instability and transmissible aberrations in the progeny of haemopoietic stem cells induced by high- and low-LET radiations. Int J Radiat Biol. 2001;77:409–417. doi: 10.1080/09553000010028476. [DOI] [PubMed] [Google Scholar]
- 69.Kadhim MA, Lorimore SA, Hepburn MD, Goodhead DT, Buckle VJ, Wright EG. Alpha-particle-induced chromosomal instability in human bone marrow cells. Lancet. 1994;344:987–988. doi: 10.1016/s0140-6736(94)91643-8. [DOI] [PubMed] [Google Scholar]
- 70.Morgan WF, Sowa MB. Non-targeted bystander effects induced by ionizing radiation. Mutat Res. 2007;616:159–164. doi: 10.1016/j.mrfmmm.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 71.Bouffler SD, Blasco MA, Cox R, Smith PJ. Telomeric sequences, radiation sensitivity and genomic instability. Int J Radiat Biol. 2001;77:995–1005. doi: 10.1080/09553000110069326. [DOI] [PubMed] [Google Scholar]
- 72.Whitehouse CA, Tawn EJ. No evidence for chromosomal instability in radiation workers with in vivo exposure to plutonium. Radiat Res. 2001;156:467–475. doi: 10.1667/0033-7587(2001)156[0467:nefcii]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 73.Dugan LC, Bedford JS. Are chromosomal instabilities induced by exposure of cultured normal human cells to low- or high-LET radiation? Radiat Res. 2003;159:301–311. doi: 10.1667/0033-7587(2003)159[0301:aciibe]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 74.Ponnaiya B, Cornforth MN, Ullrich RL. Radiation-induced chromosomal instability in BALB/c and C57BL/6 mice: the difference is as clear as black and white. Radiat Res. 1997;147:121–125. [PubMed] [Google Scholar]
- 75.Watson GE, Lorimore SA, Clutton SM, Kadhim MA, Wright EG. Genetic factors influencing alpha-particle-induced chromosomal instability. Int J Radiat Biol. 1997;71:497–503. doi: 10.1080/095530097143824. [DOI] [PubMed] [Google Scholar]
- 76.Zyuzikov NA, Coates PJ, Parry JM, Lorimore SA, Wright EG. Lack of nontargeted effects in murine bone marrow after low-dose in vivo X irradiation. Radiat Res. 2011;175:322–327. doi: 10.1667/RR2386.1. [DOI] [PubMed] [Google Scholar]
- 77.Dubrova YE. Radiation-induced transgenerational instability. Oncogene. 2003;22:7087–7093. doi: 10.1038/sj.onc.1206993. [DOI] [PubMed] [Google Scholar]
- 78.Dubrova YE, Nesterov VN, Krouchinsky NG, Ostapenko VA, Neumann R, Neil DL, Jeffreys AJ. Human minisatellite mutation rate after the Chernobyl accident. Nature. 1996;380:683–686. doi: 10.1038/380683a0. [DOI] [PubMed] [Google Scholar]
- 79.Dubrova YE, Nesterov VN, Krouchinsky NG, Ostapenko VA, Vergnaud G, Giraudeau F, Buard J, Jeffreys AJ. Further evidence for elevated human minisatellite mutation rate in Belarus eight years after the Chernobyl accident. Mutat Res. 1997;381:267–278. doi: 10.1016/s0027-5107(97)00212-1. [DOI] [PubMed] [Google Scholar]
- 80.Dubrova YE, Bersimbaev RI, Djansugurova LB, Tankimanova MK, Mamyrbaeva ZZ, Mustonen R, Lindholm C, Hulten M, Salomaa S. Nuclear weapons tests and human germline mutation rate. Science. 2002;295:1037. doi: 10.1126/science.1068102. [DOI] [PubMed] [Google Scholar]
- 81.Kodaira M, Satoh C, Hiyama K, Toyama K. Lack of effects of atomic bomb radiation on genetic instability of tandem-repetitive elements in human germ cells. Am J Hum Genet. 1995;57:1275–1283. [PMC free article] [PubMed] [Google Scholar]
- 82.Satoh C, Takahashi N, Asakawa J, Kodaira M, Kuick R, Hanash SM, Neel JV. Genetic analysis of children of atomic bomb survivors. Environ Health Perspect. 1996;104(Suppl 3):511–519. doi: 10.1289/ehp.96104s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kodaira M, Izumi S, Takahashi N, Nakamura N. No evidence of radiation effect on mutation rates at hypervariable minisatellite loci in the germ cells of atomic bomb survivors. Radiat Res. 2004;162:350–356. doi: 10.1667/rr3243. [DOI] [PubMed] [Google Scholar]
- 84.Kodaira M, Ryo H, Kamada N, Furukawa K, Takahashi N, Nakajima H, Nomura T, Nakamura N. No evidence of increased mutation rates at microsatellite loci in offspring of A-bomb survivors. Radiat Res. 2010;173:205–213. doi: 10.1667/RR1991.1. [DOI] [PubMed] [Google Scholar]
- 85.Kiuru A, Auvinen A, Luokkamaki M, Makkonen K, Veidebaum T, Tekkel M, Rahu M, Hakulinen T, Servomaa K, Rytomaa T, Mustonen R. Hereditary minisatellite mutations among the offspring of Estonian Chernobyl cleanup workers. Radiat Res. 2003;159:651–655. doi: 10.1667/0033-7587(2003)159[0651:hmmato]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 86.Slebos RJ, Little RE, Umbach DM, Antipkin Y, Zadaorozhnaja TD, Mendel NA, Sommer CA, Conway K, Parrish E, Gulino S, Taylor JA. Mini-and microsatellite mutations in children from Chernobyl accident cleanup workers. Mutat Res. 2004;559:143–151. doi: 10.1016/j.mrgentox.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 87.Furitsu K, Ryo H, Yeliseeva KG, Thuyle TT, Kawabata H, Krupnova EV, Trusova VD, Rzheutsky VA, Nakajima H, Kartel N, Nomura T. Microsatellite mutations show no increases in the children of the Chernobyl liquidators. Mutat Res. 2005;581:69–82. doi: 10.1016/j.mrgentox.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 88.Livshits LA, Malyarchuk SG, Kravchenko SA, Matsuka GH, Lukyanova EM, Antipkin YG, Arabskaya LP, Petit E, Giraudeau F, Gourmelon P, Vergnaud G, Le GB. Children of chernobyl cleanup workers do not show elevated rates of mutations in minisatellite alleles. Radiat Res. 2001;155:74–80. doi: 10.1667/0033-7587(2001)155[0074:coccwd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 89.Barber R, Plumb MA, Boulton E, Roux I, Dubrova YE. Elevated mutation rates in the germ line of first- and second-generation offspring of irradiated male mice. Proc Natl Acad Sci U S A. 2002;99:6877–6882. doi: 10.1073/pnas.102015399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barber RC, Hickenbotham P, Hatch T, Kelly D, Topchiy N, Almeida GM, Jones GD, Johnson GE, Parry JM, Rothkamm K, Dubrova YE. Radiation-induced transgenerational alterations in genome stability and DNA damage. Oncogene. 2006;25:7336–7342. doi: 10.1038/sj.onc.1209723. [DOI] [PubMed] [Google Scholar]
- 91.Hatch T, Derijck AA, Black PD, van der Heijden GW, de BP, Dubrova YE. Maternal effects of the scid mutation on radiation-induced transgenerational instability in mice. Oncogene. 2007;26:4720–4724. doi: 10.1038/sj.onc.1210253. [DOI] [PubMed] [Google Scholar]
- 92.Barber RC, Hardwick RJ, Shanks ME, Glen CD, Mughal SK, Voutounou M, Dubrova YE. The effects of in utero irradiation on mutation induction and transgenerational instability in mice. Mutat Res. 2009;664:6–12. doi: 10.1016/j.mrfmmm.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 93.Vorobtsova IE. Irradiation of male rats increases the chromosomal sensitivity of progeny to genotoxic agents. Mutagenesis. 2000;15:33–38. doi: 10.1093/mutage/15.1.33. [DOI] [PubMed] [Google Scholar]
- 94.Shiraishi K, Shimura T, Taga M, Uematsu N, Gondo Y, Ohtaki M, Kominami R, Niwa O. Persistent induction of somatic reversions of the pink-eyed unstable mutation in F1 mice born to fathers irradiated at the spermatozoa stage. Radiat Res. 2002;157:661–667. doi: 10.1667/0033-7587(2002)157[0661:piosro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 95.Frankenberg-Schwager M. Induction, repair and biological relevance of radiation-induced DNA lesions in eukaryotic cells. Radiat Environ Biophys. 1990;29:273–292. doi: 10.1007/BF01210408. [DOI] [PubMed] [Google Scholar]
- 96.Limoli CL, Kaplan MI, Phillips JW, Adair GM, Morgan WF. Differential induction of chromosomal instability by DNA strand-breaking agents. Cancer Res. 1997;57:4048–4056. [PubMed] [Google Scholar]
- 97.Dubrova YE, Hickenbotham P, Glen CD, Monger K, Wong HP, Barber RC. Paternal exposure to ethylnitrosourea results in transgenerational genomic instability in mice. Environ Mol Mutagen. 2008;49:308–311. doi: 10.1002/em.20385. [DOI] [PubMed] [Google Scholar]
- 98.Glen CD, Dubrova YE. Exposure to anticancer drugs can result in transgenerational genomic instability in mice. Proc Natl Acad Sci U S A. 2012;109:2984–2988. doi: 10.1073/pnas.1119396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dubrova YE, Ploshchanskaya OG, Kozionova OS, Akleyev AV. Minisatellite germline mutation rate in the Techa River population. Mutat Res. 2006;602:74–82. doi: 10.1016/j.mrfmmm.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 100.Abouzeid Ali HE, Barber RC, Dubrova YE. The effects of maternal irradiation during adulthood on mutation induction and transgenerational instability in mice. Mutat Res. 2012;732:21–25. doi: 10.1016/j.mrfmmm.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 101.Loeb LA. Mutator phenotype may be required for multistage carcinogenesis. Cancer Res. 1991;51:3075–3079. [PubMed] [Google Scholar]
- 102.Loeb LA. A mutator phenotype in cancer. Cancer Res. 2001;61:3230–3239. [PubMed] [Google Scholar]
- 103.Little JB. Radiation carcinogenesis. Carcinogenesis. 2000;21:397–404. doi: 10.1093/carcin/21.3.397. [DOI] [PubMed] [Google Scholar]
- 104.Little JB. Genomic instability and radiation. J Radiol Prot. 2003;23:173–181. doi: 10.1088/0952-4746/23/2/304. [DOI] [PubMed] [Google Scholar]
- 105.Li Y, Benezra R. Identification of a human mitotic checkpoint gene: hsMAD2. Science. 1996;274:246–248. doi: 10.1126/science.274.5285.246. [DOI] [PubMed] [Google Scholar]
- 106.Cahill DP, Lengauer C, Yu J, Riggins GJ, Willson JK, Markowitz SD, Kinzler KW, Vogelstein B. Mutations of mitotic checkpoint genes in human cancers. Nature. 1998;392:300–303. doi: 10.1038/32688. [DOI] [PubMed] [Google Scholar]
- 107.Milner J, Ponder B, Hughes-Davies L, Seltmann M, Kouzarides T. Transcriptional activation functions in BRCA2. Nature. 1997;386:772–773. doi: 10.1038/386772a0. [DOI] [PubMed] [Google Scholar]
- 108.Yarden RI, Pardo-Reoyo S, Sgagias M, Cowan KH, Brody LC. BRCA1 regulates the G2/M checkpoint by activating Chk1 kinase upon DNA damage. Nat Genet. 2002;30:285–289. doi: 10.1038/ng837. [DOI] [PubMed] [Google Scholar]
- 109.Rajagopalan H, Jallepalli PV, Rago C, Velculescu VE, Kinzler KW, Vogelstein B, Lengauer C. Inactivation of hCDC4 can cause chromosomal instability. Nature. 2004;428:77–81. doi: 10.1038/nature02313. [DOI] [PubMed] [Google Scholar]
- 110.Komarova NL, Sengupta A, Nowak MA. Mutation-selection networks of cancer initiation: tumor suppressor genes and chromosomal instability. J Theor Biol. 2003;223:433–450. doi: 10.1016/s0022-5193(03)00120-6. [DOI] [PubMed] [Google Scholar]
- 111.Michor F, Iwasa Y, Rajagopalan H, Lengauer C, Nowak MA. Linear model of colon cancer initiation. Cell Cycle. 2004;3:358–362. [PubMed] [Google Scholar]
- 112.Nowak MA, Komarova NL, Sengupta A, Jallepalli PV, Shih I, Vogelstein B, Lengauer C. The role of chromosomal instability in tumor initiation. Proc Natl Acad Sci U S A. 2002;99:16226–16231. doi: 10.1073/pnas.202617399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Little MP, Wright EG. A stochastic carcinogenesis model incorporating genomic instability fitted to colon cancer data. Math Biosci. 2003;183:111–134. doi: 10.1016/s0025-5564(03)00040-3. [DOI] [PubMed] [Google Scholar]
- 114.Little MP, Li G. Stochastic modelling of colon cancer: is there a role for genomic instability? Carcinogenesis. 2007;28:479–487. doi: 10.1093/carcin/bgl173. [DOI] [PubMed] [Google Scholar]
- 115.Little MP, Vineis P, Li G. A stochastic carcinogenesis model incorporating multiple types of genomic instability fitted to colon cancer data. J Theor Biol. 2008;254:229–238. doi: 10.1016/j.jtbi.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 116.Tomlinson I, Bodmer W. Selection, the mutation rate and cancer: ensuring that the tail does not wag the dog. Nat Med. 1999;5:11–12. doi: 10.1038/4687. [DOI] [PubMed] [Google Scholar]
- 117.Luebeck EG, Moolgavkar SH. Multistage carcinogenesis and the incidence of colorectal cancer. Proc Natl Acad Sci U S A. 2002;99:15095–15100. doi: 10.1073/pnas.222118199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bonassi S, Norppa H, Ceppi M, Stromberg U, Vermeulen R, Znaor A, Cebulska-Wasilewska A, Fabianova E, Fucic A, Gundy S, Hansteen IL, Knudsen LE, Lazutka J, Rossner P, Sram RJ, Boffetta P. Chromosomal aberration frequency in lymphocytes predicts the risk of cancer: results from a pooled cohort study of 22 358 subjects in 11 countries. Carcinogenesis. 2008;29:1178–1183. doi: 10.1093/carcin/bgn075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Okayasu R, Suetomi K, Yu Y, Silver A, Bedford JS, Cox R, Ullrich RL. A deficiency in DNA repair and DNA-PKcs expression in the radiosensitive BALB/c mouse. Cancer Res. 2000;60:4342–4345. [PubMed] [Google Scholar]
- 120.Coates PJ, Rundle JK, Lorimore SA, Wright EG. Indirect macrophage responses to ionizing radiation: implications for genotype-dependent bystander signaling. Cancer Res. 2008;68:450–456. doi: 10.1158/0008-5472.CAN-07-3050. [DOI] [PubMed] [Google Scholar]
- 121.Boyd M, Mairs RJ, Keith WN, Ross SC, Welsh P, Akabani G, Owens J, Vaidyanathan G, Carruthers R, Dorrens J, Zalutsky MR. An efficient targeted radiotherapy/gene therapy strategy utilising human telomerase promoters and radioastatine and harnessing radiation-mediated bystander effects. J Gene Med. 2004;6:937–947. doi: 10.1002/jgm.578. [DOI] [PubMed] [Google Scholar]
- 122.Butterworth KT, McGarry CK, Trainor C, O’Sullivan JM, Hounsell AR, Prise KM. Out-of-field cell survival following exposure to intensity-modulated radiation fields. Int J Radiat Oncol Biol Phys. 2011;79:1516–1522. doi: 10.1016/j.ijrobp.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Boyd M, Ross SC, Dorrens J, Fullerton NE, Tan KW, Zalutsky MR, Mairs RJ. Radiation-induced biologic bystander effect elicited in vitro by targeted radiopharmaceuticals labeled with alpha-, beta-, and auger electron-emitting radionuclides. J Nucl Med. 2006;47:1007–1015. [PubMed] [Google Scholar]
- 124.Mothersill C, Seymour C. Radiation-induced bystander effects: evidence for an adaptive response to low dose exposures? Dose Response. 2006;4:283–290. doi: 10.2203/dose-response.06-111.Mothersill. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hei TK, Zhou H, Ivanov VN, Hong M, Lieberman HB, Brenner DJ, Amundson SA, Geard CR. Mechanism of radiation-induced bystander effects: a unifying model. J Pharm Pharmacol. 2008;60:943–950. doi: 10.1211/jpp.60.8.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Blyth BJ, Sykes PJ. Radiation-induced bystander effects: what are they, and how relevant are they to human radiation exposures? Radiat Res. 2011;176:139–157. doi: 10.1667/rr2548.1. [DOI] [PubMed] [Google Scholar]
- 127.Camphausen K, Moses MA, Menard C, Sproull M, Beecken WD, Folkman J, O’Reilly MS. Radiation abscopal antitumor effect is mediated through p53. Cancer Res. 2003;63:1990–1993. [PubMed] [Google Scholar]
- 128.Shao C, Folkard M, Prise KM. Role of TGF-beta1 and nitric oxide in the bystander response of irradiated glioma cells. Oncogene. 2008;27:434–440. doi: 10.1038/sj.onc.1210653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dieriks B, De Vos WH, Derradji H, Baatout S, Van OP. Medium-mediated DNA repair response after ionizing radiation is correlated with the increase of specific cytokines in human fibroblasts. Mutat Res. 2010;687:40–48. doi: 10.1016/j.mrfmmm.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 130.Gow MD, Seymour CB, Ryan LA, Mothersill CE. Induction of bystander response in human glioma cells using high-energy electrons: a role for TGF-beta1. Radiat Res. 2010;173:769–778. doi: 10.1667/RR1895.1. [DOI] [PubMed] [Google Scholar]
- 131.Rodel F, Frey B, Capalbo G, Gaipl U, Keilholz L, Voll R, Hildebrandt G, Rodel C. Discontinuous induction of X-linked inhibitor of apoptosis in EA.hy.926 endothelial cells is linked to NF-kappaB activation and mediates the anti-inflammatory properties of low-dose ionising-radiation. Radiother Oncol. 2010;97:346–351. doi: 10.1016/j.radonc.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 132.Burdak-Rothkamm S, Short SC, Folkard M, Rothkamm K, Prise KM. ATR-dependent radiation-induced gamma H2AX foci in bystander primary human astrocytes and glioma cells. Oncogene. 2007;26:993–1002. doi: 10.1038/sj.onc.1209863. [DOI] [PubMed] [Google Scholar]
- 133.Chapman KL, Kelly JW, Lee R, Goodwin EH, Kadhim MA. Tracking genomic instability within irradiated and bystander populations. J Pharm Pharmacol. 2008;60:959–968. doi: 10.1211/jpp.60.8.0003. [DOI] [PubMed] [Google Scholar]
- 134.Belyakov OV, Malcolmson AM, Folkard M, Prise KM, Michael BD. Direct evidence for a bystander effect of ionizing radiation in primary human fibroblasts. Br J Cancer. 2001;84:674–679. doi: 10.1054/bjoc.2000.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Little MP, Filipe JA, Prise KM, Folkard M, Belyakov OV. A model for radiation-induced bystander effects, with allowance for spatial position and the effects of cell turnover. J Theor Biol. 2005;232:329–338. doi: 10.1016/j.jtbi.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 136.Schettino G, Folkard M, Michael BD, Prise KM. Low-dose binary behavior of bystander cell killing after microbeam irradiation of a single cell with focused c(k) x rays. Radiat Res. 2005;163:332–336. doi: 10.1667/rr3319. [DOI] [PubMed] [Google Scholar]
- 137.Mancuso M, Pasquali E, Leonardi S, Tanori M, Rebessi S, di M, Pazzaglia VS, Toni MP, Pimpinella M, Covelli V, Saran A. Oncogenic bystander radiation effects in Patched heterozygous mouse cerebellum. Proc Natl Acad Sci U S A. 2008;105:12445–12450. doi: 10.1073/pnas.0804186105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sham RL. The abscopal effect and chronic lymphocytic leukemia. Am J Med. 1995;98:307–308. doi: 10.1016/S0002-9343(99)80380-5. [DOI] [PubMed] [Google Scholar]
- 139.Mothersill C, Seymour C. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. Int J Radiat Biol. 1997;71:421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- 140.Nagar S, Smith LE, Morgan WF. Mechanisms of cell death associated with death-inducing factors from genomically unstable cell lines. Mutagenesis. 2003;18:549–560. doi: 10.1093/mutage/geg033. [DOI] [PubMed] [Google Scholar]
- 141.Hagelstrom RT, Askin KF, Williams AJ, Ramaiah L, Desaintes C, Goodwin EH, Ullrich RL, Bailey SM. DNA-PKcs and ATM influence generation of ionizing radiation-induced bystander signals. Oncogene. 2008;27:6761–6769. doi: 10.1038/onc.2008.276. [DOI] [PubMed] [Google Scholar]
- 142.Kadhim MA, Lee R, Moore SR, Macdonald DA, Chapman KL, Patel G, Prise KM. Genomic instability after targeted irradiation of human lymphocytes: evidence for inter-individual differences under bystander conditions. Mutat Res. 2010;688:91–94. doi: 10.1016/j.mrfmmm.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lyng FM, Seymour CB, Mothersill C. Production of a signal by irradiated cells which leads to a response in unirradiated cells characteristic of initiation of apoptosis. Br J Cancer. 2000;83:1223–1230. doi: 10.1054/bjoc.2000.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kashino G, Prise KM, Suzuki K, Matsuda N, Kodama S, Suzuki M, Nagata K, Kinashi Y, Masunaga S, Ono K, Watanabe M. Effective suppression of bystander effects by DMSO treatment of irradiated CHO cells. J Radiat Res. 2007;48:327–333. doi: 10.1269/jrr.07008. [DOI] [PubMed] [Google Scholar]
- 145.Shao C, Lyng FM, Folkard M, Prise KM. Calcium fluxes modulate the radiation-induced bystander responses in targeted glioma and fibroblast cells. Radiat Res. 2006;166:479–487. doi: 10.1667/RR3600.1. [DOI] [PubMed] [Google Scholar]
- 146.Banaz-Yasar F, Lennartz K, Winterhager E, Gellhaus A. Radiation-induced bystander effects in malignant trophoblast cells are independent from gap junctional communication. J Cell Biochem. 2008;103:149–161. doi: 10.1002/jcb.21395. [DOI] [PubMed] [Google Scholar]
- 147.Azzam EI, de Toledo SM, Gooding T, Little JB. Intercellular communication is involved in the bystander regulation of gene expression in human cells exposed to very low fluences of alpha particles. Radiat Res. 1998;150:497–504. [PubMed] [Google Scholar]
- 148.Azzam EI, de Toledo SM, Little JB. Direct evidence for the participation of gap junction-mediated intercellular communication in the transmission of damage signals from alpha -particle irradiated to nonirradiated cells. Proc Natl Acad Sci U S A. 2001;98:473–478. doi: 10.1073/pnas.011417098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lehnert BE, Goodwin EH, Deshpande A. Extracellular factor(s) following exposure to alpha particles can cause sister chromatid exchanges in normal human cells. Cancer Res. 1997;57:2164–2171. [PubMed] [Google Scholar]
- 150.Mothersill C, Seymour CB. Cell-cell contact during gamma irradiation is not required to induce a bystander effect in normal human keratinocytes: evidence for release during irradiation of a signal controlling survival into the medium. Radiat Res. 1998;149:256–262. [PubMed] [Google Scholar]
- 151.Ghandhi SA, Yaghoubian B, Amundson SA. Global gene expression analyses of bystander and alpha particle irradiated normal human lung fibroblasts: synchronous and differential responses. BMC Med Genomics. 2008;1:63. doi: 10.1186/1755-8794-1-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vines AM, Lyng FM, McClean B, Seymour C, Mothersill CE. Bystander effect induced changes in apoptosis related proteins and terminal differentiation in in vitro murine bladder cultures. Int J Radiat Biol. 2009;85:48–56. doi: 10.1080/09553000802635047. [DOI] [PubMed] [Google Scholar]
- 153.Belyakov OV, Folkard M, Mothersill C, Prise KM, Michael BD. Bystander-induced differentiation: a major response to targeted irradiation of a urothelial explant model. Mutat Res. 2006;597:43–49. doi: 10.1016/j.mrfmmm.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 154.Lorimore SA, Coates PJ, Wright EG. Radiation-induced genomic instability and bystander effects: inter-related nontargeted effects of exposure to ionizing radiation. Oncogene. 2003;22:7058–7069. doi: 10.1038/sj.onc.1207044. [DOI] [PubMed] [Google Scholar]
- 155.Calabrese EJ, Baldwin LA. Hormesis: a generalizable and unifying hypothesis. Crit Rev Toxicol. 2001;31:353–424. doi: 10.1080/20014091111730. [DOI] [PubMed] [Google Scholar]
- 156.Klammer H, Kadhim M, Iliakis G. Evidence of an adaptive response targeting DNA nonhomologous end joining and its transmission to bystander cells. Cancer Res. 2010;70:8498–8506. doi: 10.1158/0008-5472.CAN-10-1181. [DOI] [PubMed] [Google Scholar]
- 157.Pinto M, Azzam EI, Howell RW. Investigation of adaptive responses in bystander cells in 3D cultures containing tritium-labeled and unlabeled normal human fibroblasts. Radiat Res. 2010;174:216–227. doi: 10.1667/RR1866.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ullrich RL. Tumor induction in BALB/c mice after fractionated or protracted exposures to fission-spectrum neutrons. Radiat Res. 1984;97:587–597. [PubMed] [Google Scholar]
- 159.Maisin JR, Wambersie A, Gerber GB, Mattelin G, Lambiet-Collier M, Gueulette J. The effects of a fractionated gamma irradiation on life shortening and disease incidence in BALB/c mice. Radiat Res. 1983;94:359–373. [PubMed] [Google Scholar]
- 160.Maisin JR, Wambersie A, Gerber GB, Mattelin G, Lambiet-Collier M, De CB, Gueulette J. Life-shortening and disease incidence in C57Bl mice after single and fractionated gamma and high-energy neutron exposure. Radiat Res. 1988;113:300–317. [PubMed] [Google Scholar]
- 161.Maisin JR, Wambersie A, Gerber GB, Mattelin G, Lambiet-Collier M, De CB, Gueulette J. Life-shortening and disease incidence in mice after exposure to gamma rays or high-energy neutrons. Radiat Res. 1991;128:S117–S123. [PubMed] [Google Scholar]
- 162.Shadley JD, Wiencke JK. Induction of the adaptive response by X-rays is dependent on radiation intensity. Int J Radiat Biol. 1989;56:107–118. doi: 10.1080/09553008914551231. [DOI] [PubMed] [Google Scholar]
- 163.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) UNSCEAR 1994 report to the General Assembly, with scientific annexes. New York: United Nations; 1994. Sources and effects of ionizing radiation; pp. 1–272. E.94.IX.11. [Google Scholar]
- 164.Joiner MC, Lambin P, Malaise EP, Robson T, Arrand JE, Skov KA, Marples B. Hypersensitivity to very-low single radiation doses: its relationship to the adaptive response and induced radioresistance. Mutat Res. 1996;358:171–183. doi: 10.1016/s0027-5107(96)00118-2. [DOI] [PubMed] [Google Scholar]
- 165.Joiner MC, Marples B, Lambin P, Short SC, Turesson I. Low-dose hypersensitivity: current status and possible mechanisms. Int J Radiat Oncol Biol Phys. 2001;49:379–389. doi: 10.1016/s0360-3016(00)01471-1. [DOI] [PubMed] [Google Scholar]
- 166.National Council on Radiation Protection and Measurements (NCRP) Report No. 136. Evaluation of the linear-nonthreshold dose-response model for ionizing radiation. 2001. pp. 1–287. [Google Scholar]
- 167.Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS, Ron E, Sachs RK, Samet JM, Setlow RB, Zaider M. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci U S A. 2003;100:13761–13766. doi: 10.1073/pnas.2235592100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) Epidemiological Studies of Radiation and Cancer. New York: United Nations; 2008. UNSCEAR 2006 Report. Annex A; pp. 13–322. [Google Scholar]
- 169.International Commission on Radiological Protection. Low-dose extrapolation of radiation-related cancer risk. Ann ICRP. 2005;35:1–140. doi: 10.1016/j.icrp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 170.Brenner DJ, Little JB, Sachs RK. The bystander effect in radiation oncogenesis: II. A quantitative model. Radiat Res. 2001;155:402–408. doi: 10.1667/0033-7587(2001)155[0402:tbeiro]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 171.Little MP, Wakeford R. The bystander effect in C3H 10T1/2 cells and radon-induced lung cancer. Radiation Research. 2001;156:695–699. doi: 10.1667/0033-7587(2001)156[0695:tbeicc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 172.Limoli CL, Corcoran JJ, Jordan R, Morgan WF, Schwartz JL. A role for chromosomal instability in the development of and selection for radioresistant cell variants. Br J Cancer. 2001;84:489–492. doi: 10.1054/bjoc.2000.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Belyakov OV, Folkard M, Mothersill C, Prise KM, Michael BD. A proliferation-dependent bystander effect in primary porcine and human urothelial explants in response to targeted irradiation. Br J Cancer. 2003;88:767–774. doi: 10.1038/sj.bjc.6600804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mothersill C, Smith RW, Agnihotri N, Seymour CB. Characterization of a radiation-induced stress response communicated in vivo between zebrafish. Environ Sci Technol. 2007;41:3382–3387. doi: 10.1021/es062978n. [DOI] [PubMed] [Google Scholar]
- 175.Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol. 1997;70:130–139. doi: 10.1259/bjr.70.830.9135438. [DOI] [PubMed] [Google Scholar]
- 176.Harvey EB, Boice JD, Jr, Honeyman M, Flannery JT. Prenatal x-ray exposure and childhood cancer in twins. N Engl J Med. 1985;312:541–545. doi: 10.1056/NEJM198502283120903. [DOI] [PubMed] [Google Scholar]
- 177.MacMahon B. Prenatal x-ray exposure and childhood cancer. J Natl Cancer Inst. 1962;28:1173–1191. [PubMed] [Google Scholar]
- 178.Wakeford R, Little MP. Risk coefficients for childhood cancer after intrauterine irradiation: a review. Int J Radiat Biol. 2003;79:293–309. doi: 10.1080/0955300031000114729. [DOI] [PubMed] [Google Scholar]
- 179.STEWART A, WEBB J, Hewitt D. A survey of childhood malignancies. Br Med J. 1958;1:1495–1508. doi: 10.1136/bmj.1.5086.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.International Commission on Radiological Protection. Biological effects after prenatal irradiation (embryo and fetus). A report of the International Commission on Radiological Protection. Ann ICRP. 2003;33:5–206. [PubMed] [Google Scholar]
- 181.Preston DL, Cullings H, Suyama A, Funamoto S, Nishi N, Soda M, Mabuchi K, Kodama K, Kasagi F, Shore RE. Solid cancer incidence in atomic bomb survivors exposed in utero or as young children. J Natl Cancer Inst. 2008;100:428–436. doi: 10.1093/jnci/djn045. [DOI] [PubMed] [Google Scholar]
- 182.Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Sir Craft AW, Parker L, Berrington de GA. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kendall GM, Little MP, Wakeford R, Bunch KJ, Miles JC, Vincent TJ, Meara JR, Murphy MF. A record-based case-control study of natural background radiation and the incidence of childhood leukaemia and other cancers in Great Britain during 1980–2006. Leukemia. 2012 doi: 10.1038/leu.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Adams MJ, Hardenbergh PH, Constine LS, Lipshultz SE. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol. 2003;45:55–75. doi: 10.1016/s1040-8428(01)00227-x. [DOI] [PubMed] [Google Scholar]
- 185.Schultz-Hector S, Trott KR. Radiation-induced cardiovascular diseases: is the epidemiologic evidence compatible with the radiobiologic data? Int J Radiat Oncol Biol Phys. 2007;67:10–18. doi: 10.1016/j.ijrobp.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 186.Shimizu Y, Kodama K, Nishi N, Kasagi F, Suyama A, Soda M, Grant EJ, Sugiyama H, Sakata R, Moriwaki H, Hayashi M, Konda M, Shore RE. Radiation exposure and circulatory disease risk: Hiroshima and Nagasaki atomic bomb survivor data, 1950–2003. BMJ. 2010;340:b5349. doi: 10.1136/bmj.b5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yamada M, Wong FL, Fujiwara S, Akahoshi M, Suzuki G. Noncancer disease incidence in atomic bomb survivors, 1958–1998. Radiat Res. 2004;161:622–632. doi: 10.1667/rr3183. [DOI] [PubMed] [Google Scholar]
- 188.Howe GR, Zablotska LB, Fix JJ, Egel J, Buchanan J. Analysis of the mortality experience amongst U.S. nuclear power industry workers after chronic low-dose exposure to ionizing radiation. Radiat Res. 2004;162:517–526. doi: 10.1667/rr3258. [DOI] [PubMed] [Google Scholar]
- 189.Ivanov VK, Maksioutov MA, Chekin SY, Petrov AV, Biryukov AP, Kruglova ZG, Matyash VA, Tsyb AF, Manton KG, Kravchenko JS. The risk of radiation-induced cerebrovascular disease in Chernobyl emergency workers. Health Phys. 2006;90:199–207. doi: 10.1097/01.HP.0000175835.31663.ea. [DOI] [PubMed] [Google Scholar]
- 190.McGeoghegan D, Binks K, Gillies M, Jones S, Whaley S. The non-cancer mortality experience of male workers at British Nuclear Fuels plc, 1946–2005. Int J Epidemiol. 2008;37:506–518. doi: 10.1093/ije/dyn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Muirhead CR, O’Hagan JA, Haylock RG, Phillipson MA, Willcock T, Berridge GL, Zhang W. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer. 2009;100:206–212. doi: 10.1038/sj.bjc.6604825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Laurent O, Metz-Flamant C, Rogel A, Hubert D, Riedel A, Garcier Y, Laurier D. Relationship between occupational exposure to ionizing radiation and mortality at the French electricity company, period 1961–2003. Int Arch Occup Environ Health. 2010;83:935–944. doi: 10.1007/s00420-010-0509-3. [DOI] [PubMed] [Google Scholar]
- 193.Azizova TV, Muirhead CR, Druzhinina MB, Grigoryeva ES, Vlasenko EV, Sumina MV, O’Hagan JA, Zhang W, Haylock RG, Hunter N. Cardiovascular diseases in the cohort of workers first employed at Mayak PA in 1948–1958. Radiat Res. 2010;174:155–168. doi: 10.1667/RR1789.1. [DOI] [PubMed] [Google Scholar]
- 194.Azizova TV, Muirhead CR, Druzhinina MB, Grigoryeva ES, Vlasenko EV, Sumina MV, O’Hagan JA, Zhang W, Haylock RG, Hunter N. Cerebrovascular Diseases in the Cohort of Workers First Employed at Mayak PA in 1948–1958. Radiat Res. 2010;174:851–864. doi: 10.1667/RR1928.1. [DOI] [PubMed] [Google Scholar]
- 195.Vrijheid M, Cardis E, Ashmore P, Auvinen A, Bae JM, Engels H, Gilbert E, Gulis G, Habib R, Howe G, Kurtinaitis J, Malker H, Muirhead C, Richardson D, Rodriguez-Artalejo F, Rogel A, Schubauer-Berigan M, Tardy H, Telle-Lamberton M, Usel M, Veress K. Mortality from diseases other than cancer following low doses of ionizing radiation: results from the 15-Country Study of nuclear industry workers. Int J Epidemiol. 2007;36:1126–1135. doi: 10.1093/ije/dym138. [DOI] [PubMed] [Google Scholar]
- 196.Clarke MC, Littlewood TD, Figg N, Maguire JJ, Davenport AP, Goddard M, Bennett MR. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ Res. 2008;102:1529–1538. doi: 10.1161/CIRCRESAHA.108.175976. [DOI] [PubMed] [Google Scholar]
- 197.Kavurma MM, Figg N, Bennett MR, Mercer J, Khachigian LM, Littlewood TD. Oxidative stress regulates IGF1R expression in vascular smooth-muscle cells via p53 and HDAC recruitment. Biochem J. 2007;407:79–87. doi: 10.1042/BJ20070380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Schwartz SM, Murry CE. Proliferation and the monoclonal origins of atherosclerotic lesions. Annu Rev Med. 1998;49:437–460. doi: 10.1146/annurev.med.49.1.437. [DOI] [PubMed] [Google Scholar]
- 199.Little MP. Do non-targeted effects increase or decrease low dose risk in relation to the linear-non-threshold (LNT) model? Mutat Res. 2010;687:17–27. doi: 10.1016/j.mrfmmm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hayashi T, Morishita Y, Kubo Y, Kusunoki Y, Hayashi I, Kasagi F, Hakoda M, Kyoizumi S, Nakachi K. Long-term effects of radiation dose on inflammatory markers in atomic bomb survivors. Am J Med. 2005;118:83–86. doi: 10.1016/j.amjmed.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 201.Hayashi T, Kusunoki Y, Hakoda M, Morishita Y, Kubo Y, Maki M, Kasagi F, Kodama K, MacPhee DG, Kyoizumi S. Radiation dose-dependent increases in inflammatory response markers in A-bomb survivors. Int J Radiat Biol. 2003;79:129–136. [PubMed] [Google Scholar]
- 202.Ridker PM. Inflammation, infection, and cardiovascular risk: how good is the clinical evidence? Circulation. 1998;97:1671–1674. doi: 10.1161/01.cir.97.17.1671. [DOI] [PubMed] [Google Scholar]
- 203.Gura T. Infections: a cause of artery-clogging plaques? Science. 1998;281:35, 37. doi: 10.1126/science.281.5373.35. [DOI] [PubMed] [Google Scholar]
- 204.Kusunoki Y, Kyoizumi S, Hirai Y, Suzuki T, Nakashima E, Kodama K, Seyama T. Flow cytometry measurements of subsets of T, B and NK cells in peripheral blood lymphocytes of atomic bomb survivors. Radiat Res. 1998;150:227–236. [PubMed] [Google Scholar]
- 205.Kusunoki Y, Kyoizumi S, Yamaoka M, Kasagi F, Kodama K, Seyama T. Decreased proportion of CD4 T cells in the blood of atomic bomb survivors with myocardial infarction. Radiat Res. 1999;152:539–543. [PubMed] [Google Scholar]
- 206.Stewart FA, Heeneman S, Te PJ, Kruse J, Russell NS, Gijbels M, Daemen M. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE-/- mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol. 2006;168:649–658. doi: 10.2353/ajpath.2006.050409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 208.Little MP, Gola A, Tzoulaki I. A model of cardiovascular disease giving a plausible mechanism for the effect of fractionated low-dose ionizing radiation exposure. PLoS Comput Biol. 2009;5:e1000539. doi: 10.1371/journal.pcbi.1000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Minamoto A, Taniguchi H, Yoshitani N, Mukai S, Yokoyama T, Kumagami T, Tsuda Y, Mishima HK, Amemiya T, Nakashima E, Neriishi K, Hida A, Fujiwara S, Suzuki G, Akahoshi M. Cataract in atomic bomb survivors. Int J Radiat Biol. 2004;80:339–345. doi: 10.1080/09553000410001680332. [DOI] [PubMed] [Google Scholar]
- 210.Nakashima E, Neriishi K, Minamoto A. A reanalysis of atomic-bomb cataract data, 2000–2002: a threshold analysis. Health Phys. 2006;90:154–160. doi: 10.1097/01.hp.0000175442.03596.63. [DOI] [PubMed] [Google Scholar]
- 211.Neriishi K, Nakashima E, Akahoshi M, Hida A, Grant EJ, Masunari N, Funamoto S, Minamoto A, Fujiwara S, Shore RE. Radiation dose and cataract surgery incidence in atomic bomb survivors, 1986–2005. Radiology. 2012;265:167–174. doi: 10.1148/radiol.12111947. [DOI] [PubMed] [Google Scholar]
- 212.Chylack LT, Jr, Peterson LE, Feiveson AH, Wear ML, Manuel FK, Tung WH, Hardy DS, Marak LJ, Cucinotta FA. NASA study of cataract in astronauts (NASCA). Report 1: Cross-sectional study of the relationship of exposure to space radiation and risk of lens opacity. Radiat Res. 2009;172:10–20. doi: 10.1667/RR1580.1. [DOI] [PubMed] [Google Scholar]
- 213.Blakely EA, Kleiman NJ, Neriishi K, Chodick G, Chylack LT, Cucinotta FA, Minamoto A, Nakashima E, Kumagami T, Kitaoka T, Kanamoto T, Kiuchi Y, Chang P, Fujii N, Shore RE. Radiation cataractogenesis: epidemiology and biology. Radiat Res. 2010;173:709–717. doi: 10.1667/RRXX19.1. [DOI] [PubMed] [Google Scholar]
- 214.Di PM, Coppola M, Baarli T, Bianchi M, Sullivan AH. Biological responses to various neutron energies from 1 to 600 MeV. A II. Lens opacification in mice. Radiat Res. 1980;84:453–461. [PubMed] [Google Scholar]
- 215.Preston DL, Shimizu Y, Pierce DA, Suyama A, Mabuchi K. Studies of mortality of atomic bomb survivors. Report 13: Solid cancer and noncancer disease mortality: 1950–1997. Radiat Res. 2003;160:381–407. doi: 10.1667/rr3049. [DOI] [PubMed] [Google Scholar]
- 216.United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) Epidemiological Evaluation of Cardiovascular Disease and other Non-Cancer Diseases following Radiation Exposure. New York: United Nations; 2008. UNSCEAR 2006 Report. Annex B; pp. 325–383. [Google Scholar]
- 217.Syndikus I, Tait D, Ashley S, Jannoun L. Long-term follow-up of young children with brain tumors after irradiation. Int J Radiat Oncol Biol Phys. 1994;30:781–787. doi: 10.1016/0360-3016(94)90349-2. [DOI] [PubMed] [Google Scholar]
- 218.Hall P, Adami HO, Trichopoulos D, Pedersen NL, Lagiou P, Ekbom A, Ingvar M, Lundell M, Granath F. Effect of low doses of ionising radiation in infancy on cognitive function in adulthood: Swedish population based cohort study. BMJ. 2004;328:19. doi: 10.1136/bmj.328.7430.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schull WJ, Otake M. Cognitive function and prenatal exposure to ionizing radiation. Teratology. 1999;59:222–226. doi: 10.1002/(SICI)1096-9926(199904)59:4<222::AID-TERA6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 220.Little MP. Cancer and non-cancer effects in Japanese atomic bomb survivors. J Radiol Prot. 2009;29:A43–A59. doi: 10.1088/0952-4746/29/2A/S04. [DOI] [PubMed] [Google Scholar]
- 221.Pearce MS, Salotti JA, Little MP, McHugh K, Lee C, Kim KP, Howe NL, Ronckers CM, Rajaraman P, Craft AW, Parker L, de Gonzalez AB. Lancet. 2012. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Nowak MA, Komarova NL, Sengupta A, Jallepalli PV, Shih IM, Vogelstein B, Lengauer C. The role of chromosomal instability in tumor initiation. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16226–16231. doi: 10.1073/pnas.202617399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Brenner DJ, Sachs RK. Do low dose-rate bystander effects influence domestic radon risks? Int J Radiat Biol. 2002;78:593–604. doi: 10.1080/09553000210121740. [DOI] [PubMed] [Google Scholar]
- 224.Little MP. The bystander effect model of Brenner and Sachs fitted to lung cancer data in 11 cohorts of underground miners, and equivalence of fit of a linear relative risk model with adjustment for attained age and age at exposure. J Radiol Prot. 2004;24:243–255. doi: 10.1088/0952-4746/24/3/003. [DOI] [PubMed] [Google Scholar]
- 225.Nikjoo H, Khvostunov IK. Biophysical model of the radiation-induced bystander effect. Int J Radiat Biol. 2003;79:43–52. [PubMed] [Google Scholar]
- 226.Stewart RD, Ratnayake RK, Jennings K. Microdosimetric model for the induction of cell killing through medium-borne signals. Radiat Res. 2006;165:460–469. doi: 10.1667/rr3520.1. [DOI] [PubMed] [Google Scholar]
- 227.Hall P, Granath F, Lundell M, Olsson K, Holm LE. Lenticular opacities in individuals exposed to ionizing radiation in infancy. Radiat Res. 1999;152:190–195. [PubMed] [Google Scholar]
- 228.Rafnsson V, Olafsdottir E, Hrafnkelsson J, Sasaki H, Arnarsson A, Jonasson F. Cosmic radiation increases the risk of nuclear cataract in airline pilots: a population-based case-control study. Arch Ophthalmol. 2005;123:1102–1105. doi: 10.1001/archopht.123.8.1102. [DOI] [PubMed] [Google Scholar]
- 229.Chodick G, Bekiroglu N, Hauptmann M, Alexander BH, Freedman DM, Doody MM, Cheung LC, Simon SL, Weinstock RM, Bouville A, Sigurdson AJ. Risk of cataract after exposure to low doses of ionizing radiation: a 20-year prospective cohort study among US radiologic technologists. Am J Epidemiol. 2008;168:620–631. doi: 10.1093/aje/kwn171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mrena S, Kivela T, Kurttio P, Auvinen A. Lens opacities among physicians occupationally exposed to ionizing radiation--a pilot study in Finland. Scand J Work Environ Health. 2011;37:237–243. doi: 10.5271/sjweh.3152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.