Abstract
Pigment epithelium-derived factor (PEDF) has many biological activities. But it's not known whether PEDF and its functional peptides could protect against hypoxia-induced cell death and the mechanisms are still unclear. We used cultured H9c2 cells and primary cardiomyocytes to show that apoptosis and necroptosis were significantly increased after hypoxia. Both PEDF and its fuctional peptides 44mer reduced apoptosis and necroptosis rates and inhibited the expression of cleaved caspase 3 and receptor-interacting protein 3 (RIP3). Furthermore, PEDF and 44mer could up-regulate super oxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) levels, promote clearing of reactive oxygen species (ROS) and malondialdehyde (MDA). While, 34mer, another functional peptides had no effect on cell apoptosis and necroptosis. Hereby this is the first evidence that PEDF and its functional peptide 44mer protect cultured H9c2 cells and primary cardiomyocytes against apoptosis and necroptosis under hypoxic condition via the anti-oxidative mechanism.
Acute myocardial infarction (AMI) is a common cardiovascular disease with serious consequences in mortality, morbidity and cost to the society1,2. During AMI, the production of Adenosine triphosphate (ATP) is decreased because of reduced oxygen supply, which increases glycolysis and mitochondrial oxidative phosphorylation dysbolism, the main changes of myocardial cells to generate high concentration of H+, Ca2+, NADH+, and lactic acid3,4. These promote mitochondrial dysfunction and reactive oxygen species (ROS) accumulation5. Under this condition, tumor necrosis factor α/TNF receptor 1 (TNF-α/TNFR1) and receptor-interacting protein (RIP) RIP1-RIP3 compound formation prompt to activate metabolic enzymes and strengthen glutamic acid, glutamine and the oxidation of glycogen metabolism, which also increase the generation of ROS6. With the outbreak increase of ischemic myocardial intracellular ROS, multiple damage signaling pathways are activated7. Cell nucleus DNA is impaired, ATP is further consumed, which generate more ROS8. At the same time, the activity of antioxidant enzymes such as the super oxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) and so on are decreased9. Along with the progression, this situation does not only occur in the infarct myocardial, but also appears in non-infarction myocardial tissue3. Accordingly, important feature of the myocardial pathological changes during AMI is mass ROS generation and impaired function to remove ROS. Also this was confirmed in cultured myocardial cells5.
Previous studies suggested that apoptosis and necrosis are two mainly forms of AMI leading to myocardial cell death10. Recent studies showed that differ from the traditional concept of apoptosis and necrosis, a considerable number of cell necrosis does not rely on caspase-dependent kinase activation in vivo. It was activated by a particular factor with certain pathways and procedures. This kind of necrosis can cause severe inflammation and damage cascade effects. It's also known as necroptosis or program necrosis with the above features. Necroptosis is the main cause of organ failure such as AMI, cerebral hypoxia and renal hypoxia5.
Pigment epithelium-derived factor (PEDF) was originally discovered by Joyce Tombran-Tink and Lincoln Johnson in 198911. It's a secreted protein of roughly 50 kDa size and 418 amino acids in length. It is a secreted pleiotropic solitary glycoprotein that belongs to the non-inhibitory serpin family group and contains highly-conserved folding conformation. PEDF is expressed in multiple tissues and has many biological activities, such as anti angiogenesis, vascular permeability resistance, anti-inflammatory, anti-oxidation, anti-tumor, cytoprotection and neuronal protection12,13,14.
Recent studies demonstrated that segmental functional peptides of PEDF play part of the biological function similar to that of the holoprotein, such as anti-angiogenesis, vascular permeability resistance, and neuronal protective effect. Among these functional peptides, two different epitopes 34mer(Asp44-Asn77)and 44mer(Val78–Thr121) draw most attention. 34mer can induce apoptosis in endothelial cells and inhibit angiogenesis, while 44mer showed neurotrophic and cytoprotective effect15.
Our previous studies have demonstrated that PEDF showed a variety of biological effects both in the normal heart and infarcted myocardium. After the interference of local myocardial expression of PEDF, on the one hand local vascular regeneration was increased, but the vascular permeability was increased with vascular perfusion dysfunction which was not conducive to functional recovery of myocardial infarction; On the other hand increased inflammatory cell infiltration and a large number of apoptotic myocardial cell caused impaired heart function which was not conducive to infarcted myocardium angiogenesis. After overexpression of PEDF, although permeability of local regenerative vessel was reduced, angiogenesis was almost inhibited. Moreover, reduction of myocardial apoptosis significantly improved cardiac function which played a significant role in myocardial protection.
It has demonstrated that PEDF has a strong antioxidant effect. PEDF can inhibit oxidative damage-induced apoptosis and dysfunction in cultured retinal pericytes, prevent ROS production and protect the retina endothelial cells from damage in high glucose environment16,17. However, related studies of PEDF and its functional peptides on cultured cardiomyocytes in vitro are very few. Whether the effects of PEDF and its functional peptides on hypoxia myocardial cells is relative to antioxidant effect remain to be confirmed.
In this study, we use cultured H9c2 cells and myocardial cells to observe whether PEDF and its functional peptides18,19, 34mer and 44mer play a protective role in apoptosis and necroptosis induced by hypoxia and explore whether the cytoprotective effects are through antioxidant mechanisms.
Results
Apoptosis and necroptosis were increased after hypoxia in cultured H9c2 cells
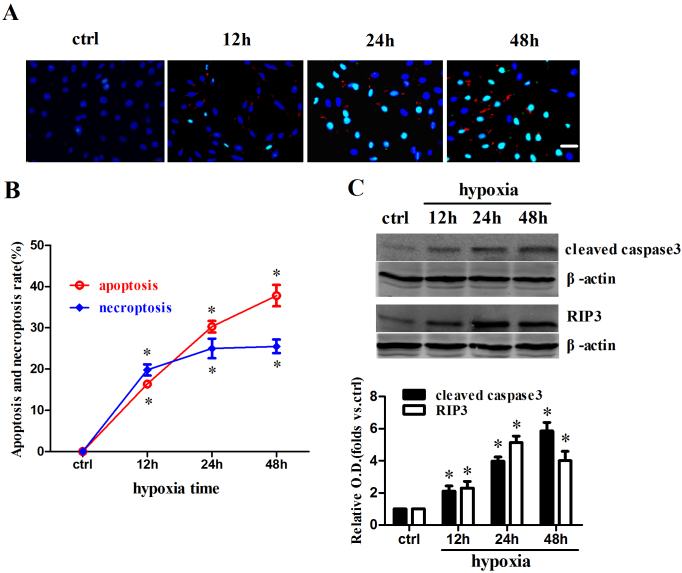
First we measured the apoptotic and necroptosis rate after hypoxia in H9c2 cells at different time point (Figure 1a). Both rates were increased significantly with the extension of hypoxic time and apoptosis rate showed a more significant time-dependent trend (Figure 1b). The levels of cleaved caspase3 and RIP3, which represent apoptosis and necroptosis respectively, were also increased after hypoxia with similar time-dependent trend (Figure 1c). These results indicate that cell death mode changes from coexistence of apoptosis and necroptosis to apoptosis dominated along with hypoxia time.
Figure 1. Apoptosis and necroptosis were increased after hypoxia in cultured H9c2 cells.
(a) Immunofluorescent images showed that apoptotic and necroptic cells were increased after hypoxia in cultured H9c2 cells. TUNEL (green) and cleaved caspase3 (red) staining were performed at various time points (0 h, 12 h, 24 h, 48 h) of hypoxia. Nuclei were stained with Hoechst (blue). Both cleaved caspase3 and TUNEL positive were apoptotic cells, while cleaved caspase3 negative but TUNEL positive were known as necroptic cells. (b) Both apoptosis and necroptosis rates were increased after hypoxia. Cells were counted in each of 10 randomly selected microscopic field (×400) (n = 10; *P < 0.05 compare to corresponding control group). (c) Cleaved caspase3 and RIP3 levels were increased after hypoxia in cultured H9c2 cells. The samples were processed using the biotin switch method followed by Western blotting. Blots were cropped and full length blots appear in the supplementary information. (n = 4; *P < 0.05 compare to corresponding control group). Scale bar: 20 μm. Data are represented as means ± S.E.M.
PEDF and 44mer protected H9c2 cells against hypoxia-induced apoptosis and necroptosis
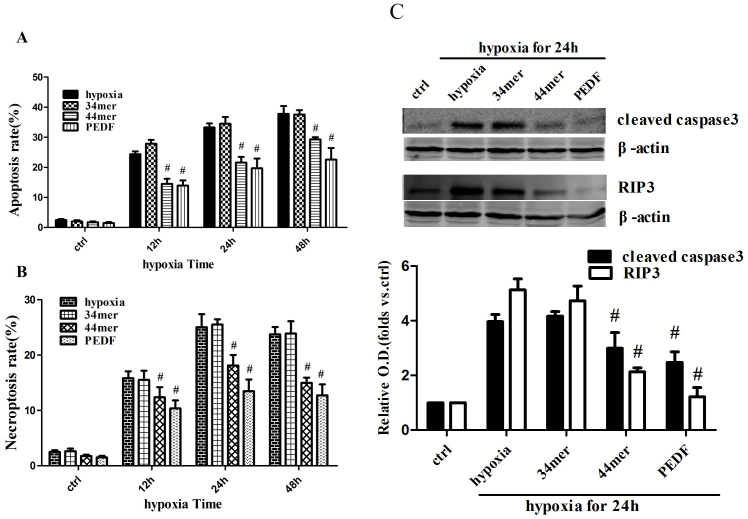
Next we investigated whether PEDF and its functional peptides had protected effects on apoptosis and necroptosis. Under normal culture conditions PEDF and its functional peptides 34mer, 44mer themselves had no significant affect on cell viability in cultured H9c2 cells (Figure 2a), while under condition of hypoxia PEDF and 44mer performed anti-apoptotic and anti-necroptosis effects (P < 0.05). 34mer had no cytoprotection effect (P > 0.05). Cleaved caspase3 and RIP3 levels were also decreased in PEDF and 44mer groups (hypoxia for 24 h) (Figure 2b). These results suggest that PEDF and 44mer inhibit the expression of cleaved caspase3 and RIP3, and then inhibit both apoptosis and necroptosis.
Figure 2. PEDF and 44mer protected against hypoxia-induced apoptosis and necroptosis in cultured H9c2 cells.
(a) PEDF (10 nM) and 44mer (10 nM) treatment decreased both apoptosis and necroptosis induced by hypoxia in cultured H9c2 cells, while 34mer (10 nM) treatment had no effect. Cells were counted in each of 10 randomly selected microscopic field (×400) (n = 10; #P < 0.05 compare to corresponding hypoxia group). (b) The levels of cleaved caspase3 and RIP3 were decreased when treated with PEDF (10 nM) or 44mer (10 nM) during 24 h hypoxia in cultured H9c2 cells, while 34mer (10 nM) treatment had no effect. The samples were processed using the biotin switch method followed by Western blotting. Blots were cropped and full length blots appear in the supplementary information. (n = 4; #P < 0.05 compare to corresponding hypoxia group). Data are represented as means ± S.E.M.
Apoptosis and necroptosis pathways could be converted to each other when one was inhibited
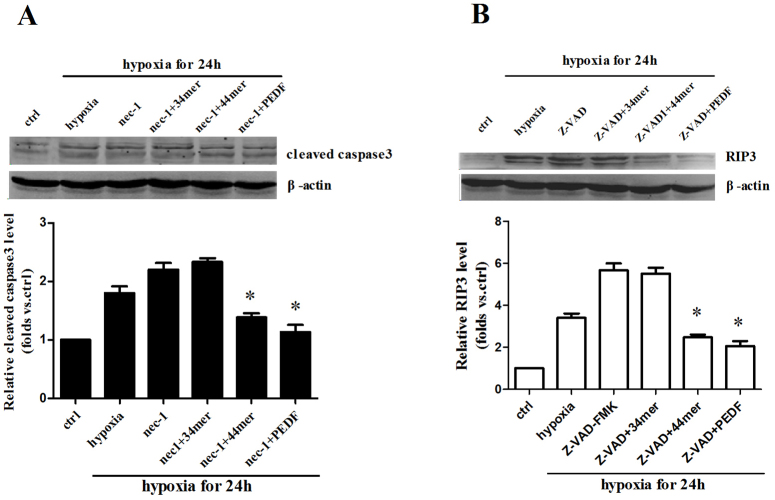
To test the relationship between apoptosis and necroptosis, we used necrostatin-1 (nec-1)20, a specific blocker of necroptosis and Z-VAD-FMK21, a caspase inhibitor. The level of cleaved caspase3 was increased in nec-1 group after 24 h hypoxia. PEDF and 44mer could decrease the level of cleaved caspase3 in nec-1 group (Figure 3a). On the other hand, Z-VAD-FMK could increase the RIP3 expression after 24 h hypoxia. PEDF and 44mer could also decrease the level of RIP3 in Z-VAD-FMK group (Figure 3b). These results indicate that apoptosis and necroptosis pathways can be converted to each other when one is inhibited. Both PEDF and 44mer inhibit cleaved caspase3 and RIP3 expression when necroptosis and apoptosis are blocked respectively.
Figure 3. Apoptosis and necroptosis pathways could be converted to each other when one was inhibited.
(a) Cellular proteins were extracted after hypoxia for 24 hours in cultured H9c2 cells. Biotin switch method followed by Western blotting showed that Necrostatin-1 (nec-1, 100 μM), a specific blocker of necroptosis increased the level of cleaved caspase3. PEDF and 44mer could decrease the level of cleaved caspase3 in nec-1 group (n = 4; *P < 0.05 compare to corresponding hypoxia group and nec-1 group). (b) Z-VAD-FMK (20 μM), a cell permeable pan caspase inhibitor, could increase the RIP3 expression after 24 h hypoxia. PEDF and 44mer could also decrease the level of RIP3 in Z-VAD-FMK group. Blots were cropped and full length blots appear in the supplementary information. (n = 4; *P < 0.05 compare to corresponding hypoxia group and Z-VAD-FMK group). Data are represented as means ± S.E.M.
PEDF and 44mer could reduce the generation of ROS and promote the clearance of ROS
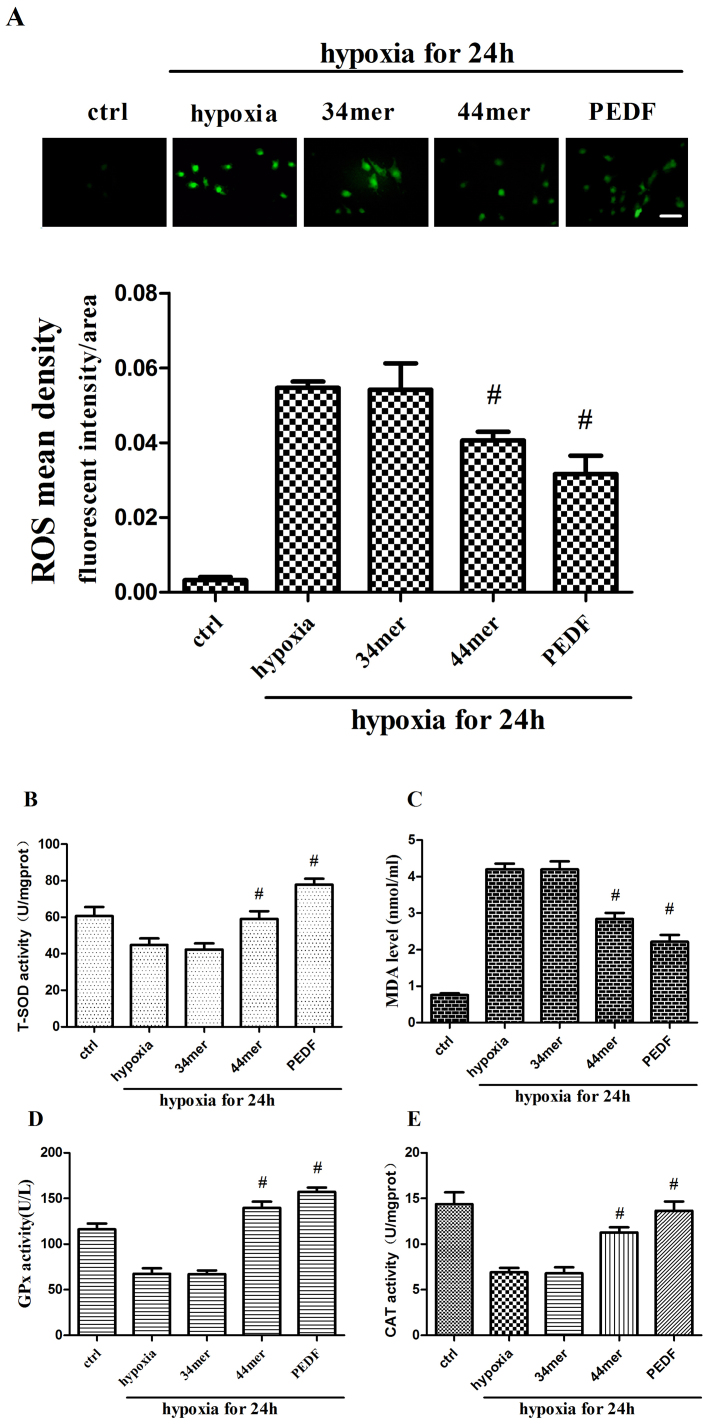
ROS is the key factor of hypoxia22. The level of ROS was increased after 24 h hypoxia in cultured H9c2 cells. PEDF and 44mer could decrease ROS level (P < 0.05), while 34mer had no effect (Figure 4a). PEDF and 44mer could also decrease MDA level which was increased after 24 h hypoxia (Figure 4c). To the contrary, T-SOD, CAT and GPx activities showed opposite trends (Figure 4b, d, and e). These results revealed that PEDF and 44mer could up-regulate SOD, GPx and CAT activities, thus promoted clearing of ROS and MDA. The results suggest that PEDF and 44mer may protect hypoxia-induced apoptosis and necroptosis via anti-oxidation effect.
Figure 4. PEDF and 44mer reduced cellular ROS and MDA levels, increased SOD, GPx and CAT activities.
(a) ROS level was measured with DCFH-DA fluorescence probe method as described before and analyzed by Image-Pro Plus. Mean fluorescent intensity of ROS were decreased in PEDF and 44mer groups. (n = 6; #P < 0.05 compare to corresponding hypoxia group). (b) T-SOD activities were examined with xanthine oxidase method. T-SOD activities were increased in PEDF and 44mer groups. (n = 6; #P < 0.05 compare to corresponding hypoxia group). (c) MDA levels were examined with TBA method. MDA levels were decreased in PEDF and 44mer groups. (n = 6; #P < 0.05 compare to corresponding hypoxia group). (d) GPx activities were examined using ultraviolet spectrophotometric method. GPx activities were increased in PEDF and 44mer groups. (n = 6; #P < 0.05 compare to corresponding hypoxia group). (e) CAT activities were tested with colorimetric method. CAT activities were increased in PEDF and 44mer groups. Scale bar, 20 μm. Data are represented as means ± S E M.
PEDF and 44mer protect against hypoxia-induced cell death in cultured neonatal rat myocardial cells
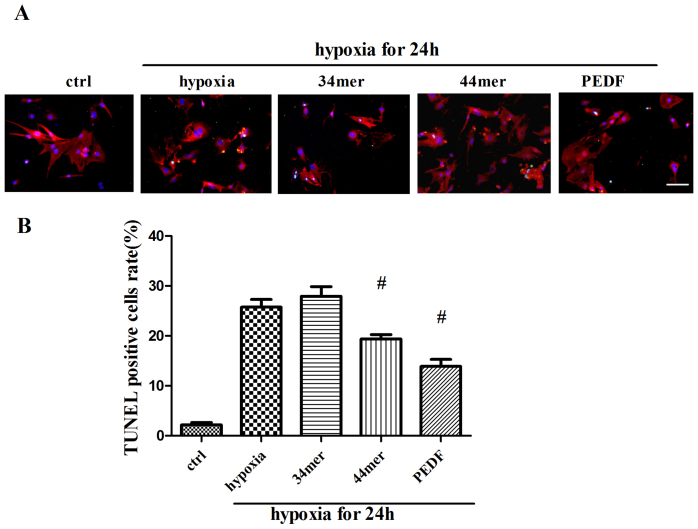
We further verified the protective effects of PEDF and 44mer on apoptosis and necroptosis in cultured neonatal rat myocardial cells. Neonatal rat myocardial cells were treated with same hypoxic condition as that of H9c2 cells. PEDF and 44mer protects neonatal rat myocardial cells against hypoxia-induced cell death, while 34mer had no effect (Figure 5).
Figure 5. PEDF and 44mer protected against hypoxia-induced cell death in cultured neonatal rat myocardial cells.
(a) The purity of neonatal myocardial cells was about 95% identified by α-sa staining. TUNEL (green) and α-sa (red) staining were performed at each group (control, 24 h hypoxia with or without 34mer, 44mer, PEDF). Nucleis were stained with Hoechst (blue). Immunofluorescent images showed that TUNEL positive cells (arrow indicated) were increased after hypoxia. PEDF and 44mer protected against TUNEL positive cells rate, while 34mer had no effect. (b) Statistic analyses from immunofluorescent images. (n = 10; #P < 0.05 compare to hypoxia group). Scale bar, 20 μm. Data are represented as means ± S.E.M.
Discussion
In this study we found for the first time that PEDF and its functional peptides 44mer could protect hypoxia–induced apoptosis and necroptosis in cultured H9c2 cells and neonatal rat myocardial cells. The protective effects are relevant to antioxidant capacity.
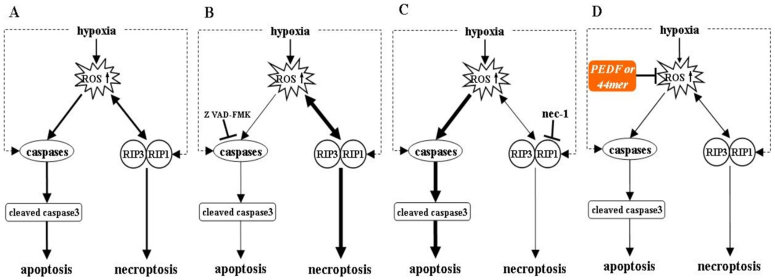
During AMI, the production of ROS plays an important role in apoptosis and necrosis23. Under hypoxic environment, influenced by external stimuli, both apoptosis and necroptosis pathways are activated (Figure 6)3. In the apoptosis pathway, caspase 3 is the most important terminal cleavage enzyme24. RIP3 can promote RIP1 phosphorylation, and then phosphorylated RIP1 and RIP3 form a more stable complex with a significant increase of intracellular ROS, which lead to cell necroptosis. With the presence of Z-VAD-FMK, a cell-permeable pan-caspase inhibitor, necroptosis becomes the main form of cell death25. On the contrary, when necroptosis pathway is blocked by nec-1, apoptosis pathway becomes the main form of cell death26. The possible protective effects of PEDF and 44mer may be relevant to the inhibition of intracellular ROS production and increased ROS clearance, thus blocking both apoptosis and necroptosis pathways, resulting in protecting cells and improving their survival under hypoxic conditions.
Figure 6. Possible mechanisms for hypoxia-induced apoptosis and necroptosis and the protective effects of PEDF and 44mer.
Under hypoxic condition, both apoptosis and necroptosis pathways are activated. Apoptosis pathway is blocked with the presence of Z-VAD-FMK due to the inhibition of caspases. Necroptosis becomes the main form of cell death. On the contrary, when necroptosis pathway is blocked with the nec-1, apoptosis pathway becomes the main form of cell death. The possible protective effects of PEDF and 44mer may be relevant to the inhibition of intracellular ROS production and increased ROS clearance, thus blocking both apoptosis and necroptosis pathways, resulting in protecting cells and improving their survival under hypoxic conditions.
Both 34mer and 44mer are functional peptides of PEDF protein. 44mer has antioxidant and cellular protection effects similar to PEDF, while 34mer had no such effects. The different function of these two peptides may be related to their binding different receptors. It has been demonstrated that there are two receptors of PEDF, laminin receptor (LR) and PEDF receptor (PEDFR) which express in a variety of cells including endothelial cells27. PEDF can bind both receptors to exert biological effects. 34mer is considered binding LR, while 44mer binding PEDFR15.
Recent research found that activation of apoptosis-promoting protein Bel-associated X protein (Bax) could mediate cell apoptosis and necrosis simultaneously. ROS outbreak increased in cardiomyocytes activates cell-intrinsic apoptotic pathway and induces activation of Bcl-2 protein, up-regulates mitochondrial membrane Bax/Bak activation25,28. Oligomerization of Bax and Bak conformation forms holes in the outer mitochondrial membrane (OMM), causing the increase of mitochondrial outer membrane permeability (MOMP), resulting in the release of cytochrome C into the cytoplasm, activating caspase-9 and caspase-3, and leading to apoptosis finally29,30. Meanwhile, with the depletion of ATP, mitochondrial permeability transition pore (mPTP) opening is increased, which is related to the intracellular mitochondrial Bax translocation. MPTP opening leads to electrochemical gradient loss of inner mitochondrial membrane (IMM), causing a large number of H2O and solute molecules into the mitochondria, resulting in mitochondrial swelling and loss of mitochondrial membrane potential, blocking ATP generation, and leading to necroptosis31. In summary, Bax activation of the mitochondrial membrane is an important internal factor that promotes apoptosis and necroptosis. Thus, under hypoxic condition, Bax activation and shift to mitochondrial is an important cross-link of apoptosis and necroptosis pathways.
So we hypothesis that inhibiting ROS generation and improving cardiac ROS scavenging ability, or activating anti-Bax protective pathway and inhibiting Bax translocation from the cytosol to the mitochondria may be an effective way to reduce myocardial apoptosis and necroptosis in myocardial cells under hypoxic conditions. Accordingly, we plan to further explore whether PEDF and 44mer play a cytoprotective effect by binding to PEDFR and anti-Bax mechanisms in anti-procedural of apoptosis and necroptosis.
Our research have showed that 44mer exhibit similar biological functions to PEDF protein, suggesting that the properties of PEDF and 44mer make them strong candidates for studying in ischemic heart disease, oxidative stress-related diseases. More importantly, derived peptides have a broader application prospects than holoprotein because of less immune response. It is crucial to understand the signal pathways of PEDF to maximize the therapeutic potential of this protein and its derivatives.
Methods
Reagents
Anti-cleaved caspase-3 (#9664), anti-β-actin (#13E5) bodies were purchased from Cell Signaling Technology, Inc. (Cell Signaling, MA). Anti-RIP 3 (#ab56164) bodies were purchased from Abcam, Inc. (Abcam, Cambridge, UK). Monoclonal anti-actin (α-sarcomeric) antibody (#A2172), necrostatin-1 (#N9037), Hoechst 33258 (#861405) and 2′, 7′-Dichlorofluorescin diacetate (DCFH-DA; #D6883) were purchased from Sigma-Aldrich Co (St. Louis, MO). In situ cell death detection kit (#11684795910) was purchased from Roche Ltd (Roche, USA). Superoxide dismutase (SOD; #A001-1), malonaldehyde (MDA; #A002-1), and catalase (CAT; #A007-1) detection kit were purchased from JIANCHENG Bioengineering Institute (Nanjing City, P.R China). Cellular glutathione peroxidase (GPx; #S0056) assay kit and caspase inhibitor Z-VAD-FMK (#C1202) were purchased from Beyotime Institute of Biotechnology (Nantong City, P.R China).
Preparations of PEDF protein and peptides
Recombinant PEDF was synthesized by CUSABIO BIOTECH CO.,Ltd. Synthetic peptides 34mer and 44mer were designed from amino acid positions 44–77 (DPFFKAPVNKLAAAVSNFGYDLYRLRSGAVSTGN) and 78–121 (ILLSPLSVATALSALSLGAEQRTESVIHRALYYDLINNPDIHST) of the rat PEDF sequence (GenBankTM accession number NM_177927), respectively, and prepared by GL Biochem (Shanghai) Ltd., followed by high pressure liquid chromatography purification (>90% purity) and amino-terminal sequence determination. The resulting peptides were soluble in aqueous solutions.
Cell culture and hypoxia
The embryonic rat heart derived H9c2 cells were cultured in DMEM (Gibco) supplemented with 10% fetal bovine serum (Gibco), antibiotics (50 U/ml penicillin and 50 μg/ml streptomycin) at 37°C in an atmosphere of 95% air and 5% CO2. Sub-confluent cells (70 ~ 80%) were subcultured 1:4. The cells were trypsinized, plated (10000 ~ 12000 cells/cm2) in 60-mm dishes for cleaved caspase3 and RIP3 activity assay or in 48-well culture plate (Corning, USA) for immunofluorescence staining and grown for 24 h before hypoxia treatments. Hypoxia was achieved by culturing the cells in D-Hank's liquid with glucose deprivation in an tri-gas incubator (Heal Force, Shanghai, China) saturated with 5% CO2/1% O2 at 37°C for the indicated time periods.
Rat primary cardiomyocytes cultures
Neonatal Sprague-Dawley (SD) rats (1–3 d old, weighing 5–7 g, means 6.1 ± 0.7 g) were obtained from the Experimental Animal Centre of Xuzhou Medical College. All experiments were performed in accordance with relevant guidelines and regulations. The study protocol was approved by the local Institutional Review Board at the authors affiliated institution30. Ventricular cardiomyocytes from 1–3-day postnatal SD-rats were isolated and cultivated as described with minor modifications. Briefly, after anesthesia with diethyl ether, neonatal rats were sterilized, then hearts were dissected, minced, and trypsinized. The dissociated cells were preplated for 1 hour in the presence of 0.1 mmol/L bromodeoxyuridine (BRDU, Sigma) to selectively enrich for cardiomyocytes. The inclusion of BRDU resulted in inhibition of the growth of cardiac fibroblasts. The resultant cell suspension (10000 ~ 12000 cells/cm2) was plated onto 48 Well Culture Plate in culture medium. More than 90% of the cells were cardio myocytes, as evaluated by indirect immunofluorescence staining with a monoclonal anti-actin (α-sarcomeric) antibody (A2172, Sigma).
Double labeling with cleaved caspase3 immunofluorescence and TUNEL staining
Cells were seeded in 48-well plates (Corning, USA). After hypoxia, cells were treated with 4% paraformaldehyde for 10 min at room temperature, and then washed three times in PBS, pH 7.4. Next cells were incubated with 1% goat serum diluted in PBS for 1 hour at room temperature, and then incubated with anti-cleaved caspase 3 antibody in diluting buffer (1% bovine serum albumin, 0.1% in PBS) overnight at 4°C. Cells were incubated with anti-mouse secondary antibody (Abcam) 1:200 in PBS for 1 hour at room temperature.Terminal dexynucleotidyl transferase(TdT)-mediated dUTP nick end labeling (TUNEL) staining was then performed using an in situ cell death detection kit (Roche, USA) according to the manufacturer's protocol. The cells were incubated with reaction buffer containing enzyme solution and label solution with an enzyme-to-label ratio of 1:9 at 37°C for 1 h33. Cells were counterstained using Hoechst stain (Sigma-Aldrich; St. Louis, MO). The cells were observed under a fluorescence microscope (Olympus, Japan).
Western blotting analysis
For western blotting analysis the cells were solubilized in lysis buffer (100 mmol/L Tris-HCl, 4% SDS, 20% glycerine, 200 mmol/L DTT and protease inhibitors, pH 6.8). Total cellular protein was denatured by boiling for 10 min with an equal volume of 2 × Tris-glycine SDS buffer. Protein was separated by 10% SDS-PAGE and transferred to nitrocellulose membrane (Millipore, USA). After blocking with 5% non-fat milk/PBS-T for 3 h at room temperature, the membranes were incubated with a goat anti-cleaved caspase 3 antibody (Cell Signaling, MA) and a goat anti-RIP 3 antibody (Abcam, UK), respectively. Then, fluorescently labeled secondary antibody (Rockland, USA) was added for 1 h and subsequently scanned by the Odyssey Infrared Imaging System (Li-Cor Biosciences, USA).
Detection of intracellular ROS generation
The generation of intracellular ROS was measured by monitoring the increasing fluorescence of 2′7′-dichlorofluorescein (DCF). 2′7′-dichlorodihydorofluorescein diacetate (DCFH-DA; Sigma, St. Louis, MO) is cell-permeant, it can enter the cell where intracellular esterases cleave off the diacetate group. The resulting DCFH is retained in the cytoplasm and oxidized to DCF by ROS. 5 × 104 H9c2 cells were seeded into each well of a 48-well plate. After 24 h hypoxia with or without PEDF and 44mer, cells were then washed once with phenol red-free medium, and incubated in 200 μl working solution of DCFH-DA (20 μM) at 37°C for 30 min. The cells were observed under a fluorescence microscope (Olympus, Japan). The fluorescence of DCF was monitored at the excitation and emission wavelengths of 485 nm and 530 nm.
Measurement of T-SOD, MDA, CAT and GPx
T-SOD, MDA, GPx and CAT activities were measured using respective detection kits according to the manufacturers' instructions. T-SOD activities were examined with xanthine oxidase method. MDA levels were examined with TBA method. GPx activities were examined using ultraviolet spectrophotometric method. CAT activities were tested with colorimetric method. Datas were observed using multi-mode microplate reader(Synergy 2, Bio-Tek, USA).
Data analysis and statistics
Values are expressed as mean ± S.E.M. Statistical analysis of the results was carried out using the Student's t-test or one-way analysis of the variance (ANOVA) followed by the Duncan's new multiple range method or Newman-Keuls test. P < 0.05 was considered significant.
Author Contributions
X.G., W.Z., T.S., X.J. and G.D.Y. performed the experiments. X.G., H.Z., T.S., Z.X.Z. and H.H.Y. analysed the data. X.G., H.Z. and T.S. designed the research. X.G. and Z.M.Z. wrote this manuscript. H.Y.D. supervised this work.
Supplementary Material
SUPPLEMENTARY INFO
Acknowledgments
This work was supported by National Natural Science Foundation of China (81270173) and Xuzhou Science and Technology Plan(XM09B098).
References
- Boersma E. et al. Acute myocardial infarction. Lancet 361, 847–858 (2003). [DOI] [PubMed] [Google Scholar]
- White H. D. & Chew D. P. Acute myocardial infarction. Lancet 372, 570–584 (2008). [DOI] [PubMed] [Google Scholar]
- Krijnen P. et al. Apoptosis in myocardial ischaemia and infarction. Am J Clin Pathol. 55, 801–811 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiong M. et al. Cardiomyocyte death: mechanisms and translational implications. Cell death dis. 2, e244 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R. S., Kaplinskiy V. & Kitsis R. N. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev of Phys. 72, 19–44 (2010). [DOI] [PubMed] [Google Scholar]
- Vandenabeele P., Declercq W., Van Herreweghe F. & Vanden Berghe T. The role of the kinases RIP1 and RIP3 in TNF-induced necrosis. Sci Signl. 3, re4 (2010). [DOI] [PubMed] [Google Scholar]
- Hwang J.-T., Kwon D. Y., Park O. J. & Kim M. S. Resveratrol protects ROS-induced cell death by activating AMPK in H9c2 cardiac muscle cells. Genes Nutrition 2, 323–326 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M., So E., Simons A., Spitz D. & Ouchi T. DNA damage induces reactive oxygen species generation through the H2AX-Nox1/Rac1 pathway. Cell Death Dis. 3, e249 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S. et al. Downregulation of CuZn-superoxide dismutase contributes to β-adrenergic receptor-mediated oxidative stress in the heart. Cardiovasc Res. 74, 445–455 (2007). [DOI] [PubMed] [Google Scholar]
- Edinger A. L. & Thompson C. B. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 16, 663–669 (2004). [DOI] [PubMed] [Google Scholar]
- Tombran-Tink J., Chader G. G. & Johnson L. V. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp Eye Res. 53, 411–414 (1991). [DOI] [PubMed] [Google Scholar]
- Filleur S., Nelius T., De Riese W. & Kennedy R. Characterization of PEDF: A multi-functional serpin family protein. J Cell. Biochem. 106, 769–775 (2009). [DOI] [PubMed] [Google Scholar]
- Wang Y., Subramanian P., Shen D. et al. Pigment epithelium-derived factor reduces apoptosis and pro-inflammatory cytokine gene expression in a murine model of focal retinal degeneration. ASN Neuro. 5(5), e00126 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian P., Locatelli-Hoops S., Kenealey J. et al. Pigment epithelium-derived factor (PEDF) prevents retinal cell death via PEDF Receptor (PEDF-R): identification of a functional ligand binding site. J Biol Chem. 288(33), 23928–42 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi T., Yamagishi S.-I. & Sata M. Structure-function relationships of PEDF. Curr Mol Med. 10, 302–311 (2010). [DOI] [PubMed] [Google Scholar]
- Amano S. et al. Pigment epithelium-derived factor inhibits oxidative stress-induced apoptosis and dysfunction of cultured retinal pericytes. Microvas Res. 69, 45–55 (2005). [DOI] [PubMed] [Google Scholar]
- Tsao Y.-P., Ho T.-C., Chen S.-L. & Cheng H.-C. Pigment epithelium-derived factor inhibits oxidative stress-induced cell death by activation of extracellular signal-regulated kinases in cultured retinal pigment epithelial cells. Life Sci. 79, 545–550 (2006). [DOI] [PubMed] [Google Scholar]
- Hescheler J. et al. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 69, 1476–1486 (1991). [DOI] [PubMed] [Google Scholar]
- Simpson P. & Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cross-striations, ultrastructure, and chronotropic response to isoproterenol. Circ Res. 50, 101–116 (1982). [DOI] [PubMed] [Google Scholar]
- Smith C. C. et al. Necrostatin: a potentially novel cardioprotective agent? Cardiovas Drugs Ther. 21, 227–233 (2007). [DOI] [PubMed] [Google Scholar]
- Ekhterae D. et al. ARC Inhibits Cytochrome c Release From Mitochondria and Protects Against Hypoxia-Induced Apoptosis in Heart-Derived H9c2 Cells. Circ Res. (1999). [DOI] [PubMed] [Google Scholar]
- Guzy R. D. et al. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 1, 401–408 (2005). [DOI] [PubMed] [Google Scholar]
- Chen G. & Goeddel D. V. TNF-R1 signaling: a beautiful pathway. Science 296, 1634–1635 (2002). [DOI] [PubMed] [Google Scholar]
- Sun N. et al. GluR6-FasL-Trx2 mediates denitrosylation and activation of procaspase-3 in cerebral ischemia/reperfusion in rats. Cell Death Dis. 4, e771 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L. & Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell 135, 1161–1163 (2008). [DOI] [PubMed] [Google Scholar]
- Han W., Xie J., Li L., Liu Z. & Hu X. Necrostatin-1 reverts shikonin-induced necroptosis to apoptosis. Apoptosis 14, 674–686 (2009). [DOI] [PubMed] [Google Scholar]
- Alberdi E., Aymerich M. S. & Becerra S. P. Binding of Pigment Epithelium-derived Factor (PEDF) to Retinoblastoma Cells and Cerebellar Granule Neurons EVIDENCE FOR A PEDF RECEPTOR. J Bio Chem. 274, 31605–31612 (1999). [DOI] [PubMed] [Google Scholar]
- Li D., Ueta E., Kimura T., Yamamoto T. & Osaki T. Reactive oxygen species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 95, 644–650 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green D. R. & Kroemer G. The pathophysiology of mitochondrial cell death. Science 305, 626–629 (2004). [DOI] [PubMed] [Google Scholar]
- Jeong S.-Y. & Seol D.-W. The role of mitochondria in apoptosis. BMB Rep. 41, 11–22 (2008). [DOI] [PubMed] [Google Scholar]
- Linkermann A. et al. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 81, 751–761 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. Sequential, timely and controlled expression of hVEGF165 and Ang-1 effectively improves functional angiogenesis and cardiac function in vivo. Gene Ther. 20, 893–900 (2013). [DOI] [PubMed] [Google Scholar]
- Hurst P. R., Mora J. M. & Fenwick M. A. Caspase-3, TUNEL and ultrastructural studies of small follicles in adult human ovarian biopsies. Hum Repd. 21, 1974–1980 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY INFO