Abstract
Vitamin D is a micronutrient that is needed for optimal health throughout the whole life. Vitamin D3 (cholecalciferol) can be either synthesized in the human skin upon exposure to the UV light of the sun, or it is obtained from the diet. If the photoconversion in the skin due to reduced sun exposure (e.g., in wintertime) is insufficient, intake of adequate vitamin D from the diet is essential to health. Severe vitamin D deficiency can lead to a multitude of avoidable illnesses; among them are well-known bone diseases like osteoporosis, a number of autoimmune diseases, many different cancers, and some cardiovascular diseases like hypertension are being discussed. Vitamin D is found naturally in only very few foods. Foods containing vitamin D include some fatty fish, fish liver oils, and eggs from hens that have been fed vitamin D and some fortified foods in countries with respective regulations. Based on geographic location or food availability adequate vitamin D intake might not be sufficient on a global scale. The International Osteoporosis Foundation (IOF) has collected the 25-hydroxy-vitamin D plasma levels in populations of different countries using published data and developed a global vitamin D map. This map illustrates the parts of the world, where vitamin D did not reach adequate 25-hydroxyvitamin D plasma levels: 6.7% of the papers report 25-hydroxyvitamin D plasma levels below 25 nmol/L, which indicates vitamin D deficiency, 37.3% are below 50 nmol/Land only 11.9% found 25-hydroxyvitamin D plasma levels above 75 nmol/L target as suggested by vitamin D experts. The vitamin D map is adding further evidence to the vitamin D insufficiency pandemic debate, which is also an issue in the developed world. Besides malnutrition, a condition where the diet does not match to provide the adequate levels of nutrients including micronutrients for growth and maintenance, we obviously have a situation where enough nutrients were consumed, but lacked to reach sufficient vitamin D micronutrient levels. The latter situation is known as hidden hunger. The inadequate vitamin D status impacts on health care costs, which in turn could result in significant savings, if corrected. Since little is known about the effects on the molecular level that accompany the pandemic like epigenetic imprinting, the insufficiency-triggered gene regulations or the genetic background influence on the body to maintain metabolic resilience, future research will be needed. The nutrition community is highly interested in the molecular mechanism that underlies the vitamin D insufficiency caused effect. In recent years, novel large scale technologies have become available that allow the simultaneous acquisition of transcriptome, epigenome, proteome, or metabolome data in cells of organs. These important methods are now used for nutritional approaches summarized in emerging scientific fields of nutrigenomics, nutrigenetics, or nutriepigenetics. It is believed that with the help of these novel concepts further understanding can be generated to develop future sustainable nutrition solutions to safeguard nutrition security.
Keywords: vitamin D, 25-hydroxyvitamin D, nutrition, micronutrients, hidden hunger, nutrition security, nutritional pathways, nutrigenomics
Introduction
Vitamin D is needed to maintain calcium concentrations within a narrow physiological range. This function is vital as the calcium ion is essential for a large variety of cellular and metabolic processes in the body (Berridge, 2012). To secure the calcium supplies besides intestinal absorption, calcium is stored in the skeleton and acts as a large calcium reservoir that is mainly controlled by PTH and vitamin D (Bouillon et al., 2014). Humans produce vitamin D by exposure to sunlight that includes ultraviolet B radiation; if ultraviolet B radiation is not available in sufficient amounts, vitamin D needs to be obtained from the diet or dietary supplements (Holick, 2007). The start of the vitamin D endocrine system is believed to have been initiated before the start of vertebrates and evolved over millions of years (Bouillon and Suda, 2014). Therefore, the vitamin D micronutrient either synthesized through the sun by the skin or through dietary uptake is well-adapted to the human body. The endogenously conjugated vitamin D metabolites have taken over many important roles in the maintenance of human health, of which many still await to be discovered.
In this paper, we summarize the knowledge on vitamin D as an essential micronutrient important for human health and discuss the new nutritional research on its way to gain further knowledge on the function of vitamin D for nutrition.
Vitamin D part of nutrition and content in foods
The history of vitamin D is linked to first scientific description of the classic bone disease rickets by Whistler in 1645 (Norman, 2012). Two centuries later it was Schütte who observed the usefulness of cod liver oil in the treatment of rickets and osteomalacia in 1824. The hunt for the anti-rachitic factor ended in early twentieth century, when Mellanby could demonstrate in a series of hallmark studies (1919–1924) that a nutritional component in the diet was the anti-rachitic factor to prevent rickets (Mellanby, 1919, 1976; Platt, 1956). Shortly after, vitamin D was inaugurated without the characterization of the chemical structure. In 1919, Hudschinsky showed in parallel that UV light was able to ameliorate rickets by increasing calcification in rachitic children (Huldschinsky, 1919, 1926). Both findings of the cod liver oil and the UV light preventing rickets remained independent observations until Hess and Weinstock elegantly could demonstrate that the anti-rachitic vitamin D was produce by UV irradiation in skin (Hess and Weinstock, 1925a,b). In 1936, Windaus and colleagues determined the chemical structure of the fat-soluble seco-steroid vitamin D (Windaus et al., 1936).
The vitamin D definition comprises a group of molecules called the calciferols. The main forms present in foods are cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2), whereas the metabolite 25-hydroxycholecalciferol (25-hydroxyvitamin D3) is a natural part of the food chain by its occurrence in animal products. Vitamin D3 is unique by the fact that the same nutrient can be synthesized in the skin through the action of sunlight or being taken up by diet. This dual source of intake secures the body to maintain sufficient vitamin D levels in the body. The production in skin is usually the major vitamin D3 source for the body. However, in countries that receive insufficient sun exposure, people rely on dietary vitamin D as a major source. Exposure of the precursor 7-dehydrocholesterol in the basal and suprabasal layers of the epidermis to ultraviolet B (UVB) light with a wavelength of 290–315 nm is needed for the formation of the previtamin D3. The subsequent conversion is a non-enzymatic process that includes a thermal isomerization of the previtamin D3 to produce vitamin D3 (Collins and Norman, 2001; Holick, 2011). This vitamin D3 is rapidly converted to 25-hydroxyvitamin D3 in the liver. The vitamin D status is evaluated by measuring the circulating levels of serum 25-hydroxyvitamin D, which is the sum of cutaneous synthesis (vitamin D3) or dietary contribution (vitamin D3 and vitamin D2). The 25-hydroxyvitamin D3 needs to be further hydroxylated in the kidney (or locally in other organs Lehmann et al., 2001) to form 1,25-dihydroxyvitamin D3, the active endogenous hormone, which is responsible for most of the physiological actions of vitamin D through the binding to the vitamin D receptor (VDR). The plant-derived vitamin D2 is processed in the same way. For both vitamers, vitamin D2, and vitamin D3, the consecutive molecular action is believed to be identical, whereas only 1,25-dihydroxy vitamin D3 is the endogenous hormone, the activated vitamer 1,25-dihydroxyvitamin D2 is hormone mimetic. Therefore, it was not surprising that vitamin D3 has been reported to be superior to vitamin D2 in terms of bioavailability and maintaining the vitamin D status by the majority of studies (Trang et al., 1998; Armas et al., 2004; Romagnoli et al., 2008; Glendenning et al., 2009; Heaney et al., 2011; Lehmann et al., 2013). Only one study reported that the two vitamers were essentially equipotent (Holick et al., 2008).
The level of cutaneous vitamin D3 synthesis is mainly affected by the amount of solar UVB radiation reaching the human skin, which is a function that needs to take into account the wavelength, thickness of the ozone layer in the atmosphere and solar zenith angle. Furthermore, the geographic latitude, season of the year and time of day influence and restrict the skin-borne synthesis of vitamin D3 (Webb et al., 1988; Holick, 2011). It was described that vitamin D3 synthesis in the skin declines with age, which is due in part to a fall of 7-dehydrocholesterol and the morphological changes due to biological aging (MacLaughlin and Holick, 1985; Holick et al., 1989). Matsuoka et al. (1991) have shown that in Caucasians and Asian subjects having a lighter skin pigmentation UVB radiation produce significantly higher vitamin D3 serum levels than in African American and East Indian groups. It is not of a surprise that skin pigmentation reduces vitamin D3 formation. This skin tone dependent down regulation is easily overcome by increased sun exposures (Armas et al., 2007). Apart to darker pigmented skin, cutaneous vitamin D3 production can be reduced for many other reasons like severe air pollution in large cities, less outdoor activity as a consequence of an unhealthy lifestyle change, immobility of institutionalized elderly populations, topical application of sunscreens with a high sun protection factors or cultural dress codes (e.g., veiling). Therefore, dietary intake of vitamin D through foods or supplements plays a vital part to maintain healthy vitamin D levels.
Through nutrition, vitamin D intake is limited. There are few naturally-occurring food sources containing relevant levels of vitamin D. Table 1 summarizes the vitamin D content in selected foods. Vegetarian diets are limited to the plant vitamin D2 that is only present in some mushrooms. Commercially dark cultivated white button mushrooms contain low amounts of vitamin D2, only wild mushrooms or sun-dried mushrooms contain elevated amounts of ergocalciferol (Mattila et al., 1994, 1999b, 2001; Teichmann et al., 2007). Some commercial producers include an UVB radiation step to increase the vitamin D2 content in their products (Mau et al., 1998; Roberts et al., 2008). Vitamin D2 is formed out from ergosterol in the mushrooms. Some plants that are used as foods however can contain ergosterol, but this provitamin form is not converted to vitamin D2. Vitamin D3 is not found in food-borne plants. In plants, the occurrence of vitamin D3-related compounds is scarce. Interestingly, species belonging to the botanical Solanaceae family, like Solanum malacoxylon (Solanum glaucophyllum and Solanum glaucum), contain a glycoside of the active 1,25-dihydroxyvitamin D3 hormone (Boland, 1986; Boland et al., 2003; Japelt et al., 2013). This deciduous shrub (1.5–3.0 m stem length) is widely distributed in the provinces of Buenos Aires in Argentina and in Brazil and is responsible for the calcinotic disease in cattle and other grazing animal.
Table 1.
Vitamin D content in raw products, processed foods, and fortified foods.
| Category | Foodstuff | Range | References | |
|---|---|---|---|---|
| (μg vitamin D per 100 g) | (IU vitamin D per 100 g) | |||
| RAW PRODUCTS | ||||
| Fish | Herring | 2.2–38.0 | 88–1,520 | Kobayashi et al., 1995; Mattila et al., 1995a, 1997; Ostermeyer and Schmidt, 2006; Byrdwell et al., 2013 |
| Salmon | 4.2–34.5 | 168–1,380 | Kobayashi et al., 1995; Ostermeyer and Schmidt, 2006; Lu et al., 2007; Byrdwell et al., 2013 | |
| Halibut | 4.7–27.4 | 188–1,094 | Ostermeyer and Schmidt, 2006; Byrdwell et al., 2013 | |
| Perch | 0.3–25.2 | 12–1,012 | Mattila et al., 1995a, 1997; Ostermeyer and Schmidt, 2006; Byrdwell et al., 2013 | |
| Trout | 3.8–19.0 | 152–760 | Mattila et al., 1995a; Ostermeyer and Schmidt, 2006; Byrdwell et al., 2013 | |
| Tuna | 1.7–18.7 | 68–748 | Takeuchi et al., 1984, 1986; Kobayashi et al., 1995; Byrdwell et al., 2013 | |
| Mackerel | 0.5–15.5 | 20–620 | Egaas and Lambertsen, 1979; Aminullah Bhuiyan et al., 1993; Kobayashi et al., 1995; Ostermeyer and Schmidt, 2006; Lu et al., 2007 | |
| Cod | 0.5–6.9 | 20–276 | Kobayashi et al., 1995; Mattila et al., 1995a; Ostermeyer and Schmidt, 2006; Byrdwell et al., 2013 | |
| Mushrooms | Morel | 4.2–6.3 | 168–252 | Phillips et al., 2011 |
| Dark cultivated white bottom mushrooms | 0–0.2 | 0–8 | Mattila et al., 2001; Teichmann et al., 2007; Phillips et al., 2011 | |
| Wild grown mushrooms | 0.3–29.8 | 10–1,192 | Mattila et al., 1994, 1999b, 2001; Kobayashi et al., 1995; Teichmann et al., 2007 | |
| Animal products | Pork meat | 0.1–0.7 | 4–28 | Kobayashi et al., 1995; Bilodeau et al., 2011; Strobel et al., 2013 |
| Beef meat | 0–0.95 | 0–38 | Kobayashi et al., 1995; Montgomery et al., 2000, 2002; Bilodeau et al., 2011; Strobel et al., 2013 | |
| Chicken meat | 0–0.3 | 0–12 | Kobayashi et al., 1995; Mattila et al., 1995b; Bilodeau et al., 2011; Strobel et al., 2013 | |
| Beef liver | 0–14.1 | 0–560 | Kobayashi et al., 1995; Mattila et al., 1995b; Montgomery et al., 2000, 2002 | |
| Eggs | 0.4–12.1 | 28–480 | Mattila et al., 1992, 1999a; Kobayashi et al., 1995; Bilodeau et al., 2011; Exler et al., 2013 | |
| PROCESSED FOODS | ||||
| Fish | Tuna (skipjack) liver oil | 144,400 | 5,776,000 | Takeuchi et al., 1984 |
| Halibut liver oil | 13,400 | 536,000 | Egaas and Lambertsen, 1979 | |
| Cod liver oil | 137.5–575.0 | 5,500–23,000 | Egaas and Lambertsen, 1979; Takeuchi et al., 1984 | |
| Canned pink salmon | 12.7–43.5 | 508–1,740 | Bilodeau et al., 2011 | |
| Canned sardines | 3.2–10 | 128–400 | Mattila et al., 1995a | |
| Smoked salmon | 4.9–27.2 | 196–1,088 | Ostermeyer and Schmidt, 2006 | |
| Mushrooms | Irradiated mushrooms | 6.6–77.4 | 264–3,094 | Mau et al., 1998; Roberts et al., 2008 |
| Dairy | Butter | 0.2–2.0 | 8–80 | Kobayashi et al., 1995; Mattila et al., 1995b; Jakobsen and Saxholt, 2009 |
| Cheese | 0–0.1 | 0–4 | Mattila et al., 1995b; Wagner et al., 2008 | |
| FORTIFIED FOODS | ||||
| Cereals | Corn flakes | 2–4.7 | 87–189 | Haytowitz et al., 2009; U.S. Department of Agriculture, 2013 |
| Beverages | Orange juice | 1.1 | 44 | Wacker and Holick, 2013 |
| Malted drink mix, powder | 3 | 123 | Haytowitz et al., 2009; U.S. Department of Agriculture, 2013 | |
| Dairy | Milk | 1.1–2.0 | 42–79 | Calvo et al., 2004; Haytowitz et al., 2009; U.S. Department of Agriculture, 2013 |
| Cheese | 2.6–25.0 | 102–1,000 | Haytowitz et al., 2009; Tippetts et al., 2012; U.S. Department of Agriculture, 2013 | |
Animal food products are the main dietary source for naturally occurring vitamin D3 (Schmid and Walther, 2013). Since the discovery of vitamin D, vitamin D was associated with oily fish products. It was driven by the early observation that the amount of vitamin D in a teaspoon of cod liver oil was sufficient to prevent rickets in infants. It is still the fish liver oil that contains the highest amounts of vitamin D3. The highest reported concentration was found in skipjack liver oil 144,400 μg/100 g (Takeuchi et al., 1984). The fish liver oils besides other nutritional ingredients might contain high levels of vitamin A. The vitamin A to vitamin D ratio in the fish liver oils is species and fishing area dependent. The ratio range starts with a factor of 0.5 for skipjack liver oil and can even reach an extreme ratio of 119 (pollack liver oil) (Takeuchi et al., 1984). This wide vitamin A to vitamin D ratio range is the reason why fish liver oils often need further processing. In fresh fish products we observe a huge variation in the vitamin D3 content per 100 g wet weight (Egaas and Lambertsen, 1979; Takeuchi et al., 1984, 1986; Kobayashi et al., 1995; Mattila et al., 1995a, 1997; Ostermeyer and Schmidt, 2006; Lu et al., 2007; Byrdwell et al., 2013) (Table 1). Large variations in vitamin D3 content were found within the same species, but also between the different fish species. Fish obtain their vitamin D3 requirements through their diet (Holick, 2003). Therefore, the vitamin D3 levels in the zooplankton, the primary food source of fish, or seasonal changes in the zooplankton reservoirs in the different habitats, might be the reasons for the observed fluctuation in the fish product. Interestingly, the weight, the sex, or the age of the fish could not be correlated to the vitamin D3 content. Furthermore, no significant correlation between the tissue fat content and vitamin D levels was detected (Mattila et al., 1995a, 1997). Significant differences in vitamin D3 content were found between muscle and skin tissues and even more pronounced between muscle and liver tissues (Takeuchi et al., 1986). The 25-hydroxyvitamin D3 compound was also detected, though at low concentrations (Takeuchi et al., 1986; Mattila et al., 1995a; Ovesen et al., 2003; Bilodeau et al., 2011).
Wild and sun-dried mushrooms can be a good dietary source of vitamin D2 (Mattila et al., 1994, 1999b, 2001; Kobayashi et al., 1995; Teichmann et al., 2007; Phillips et al., 2011). However, the commercially produced mushrooms, e.g., the white button mushroom, do not contain or contain only very low amounts of vitamin D2 (Mattila et al., 2001; Teichmann et al., 2007; Phillips et al., 2011). The vitamin D2 content in commercially produced mushrooms can be increased by UVB exposure during the culturing or the postharvest process (Mau et al., 1998; Roberts et al., 2008). The concentration of vitamin D in eggs can vary from 0.4 to 12.1 μg (Parrish, 1979; Mattila et al., 1992, 1999a; Bilodeau et al., 2011; Exler et al., 2013), it is in a similar range like offal (Mattila et al., 1995b; Montgomery et al., 2000, 2002). Other animal products like pork, beef, and chicken muscle meat are low in vitamin D content (Mattila et al., 1995b; Montgomery et al., 2000, 2002; Bilodeau et al., 2011; Strobel et al., 2013). By adding vitamin D3 into the feed, the vitamin D3 content can be increased in muscle and liver of cattle, to 4.6 μg per 100 g of tissue and 99.6 μg per 100 g of tissue, respectively (Montgomery et al., 2004). Milk, unless fortified, has been shown to contain no or very little amounts of vitamin D, whereas in dairy products like butter and cheese the vitamin D content is higher, but in serving size amounts still very low (Kobayashi et al., 1995; Mattila et al., 1995b; Jakobsen and Saxholt, 2009; Trenerry et al., 2011). In general, household cooking seems to have some effect on vitamin D stability depending on the actual foodstuffs and the heating process used (Mattila et al., 1999b; Jakobsen and Knuthsen, 2014).
To meet the vitamin D needs in the countries some states fortify foods. Dairy products are ideal for vitamin D fortification. In Canada vitamin D fortification is mandatory for milk (1 μg/100 ml) and margarine (13.3 μg/100 g) (Health Canada, 2014). In other countries, like the United States, vitamin D fortification is optional for products like milk, breakfast cereals, and fruit juices (Calvo et al., 2004). In the U.S. Department of Agriculture (2013) of the US Department of Agriculture (USDA)'s Nutrient Databank System (Haytowitz et al., 2009), 5036 foods have been determined for their vitamin D content, of which only 259 food items had detectable vitamin D levels. The data showed that per serving only seven fish products had >15 μg vitamin D. All 29 foods that contained between 2.5 μg 15 μg vitamin D per serving were either fortified foods (21) or fish produce (8). Two-thirds of all vitamin D containing foods were far below the 1.0 μg level, whereas 20 percent had even negligible vitamin D content per serving (below 0.1 μg).
Despite the fact that moderate sun exposure of arms and legs in summer for 5–30 min between the hours of 10 a.m. and 3 p.m. twice a week is enough to produce sufficient vitamin D3 in the body (Holick, 2007), it is astonishing that many populations that live at these privileged latitudes fail to achieve this goal (Holick and Chen, 2008; Lips, 2010; Wahl et al., 2012; Hilger et al., 2014). During winter time, when vitamin D3 production by the sun ceased, adequate vitamin D levels can only be achieved by UVB exposure from indoor tanning units, or by a daily diet of fortified foods or a few selected food items. This restricted list of options to achieve sufficient levels is one of the reasons, why the use of dietary vitamin D supplements has become so popular. It is currently the most applied and secure option to reach adequate vitamin D intake levels (Holick, 2007).
Vitamin D map, malnutrition, hidden hunger, and nutrition security
An accepted biomarker for the vitamin D status in the general population is to measure the serum concentration of 25-hydroxyvitamin D levels, which is the major circulating form of vitamin D and reflects both dietary vitamin D intake and the endogenous vitamin D production (Lips, 2001, 2007). The serum concentration of 25-hydroxyvitamin D is linked to the serum level of the active hormone 1,25-dihydroxyvitamin D and also to the clinical relevant parathyroid hormone level. Lips has classified the 25-hydroxyvitamin D levels into four stages (Lips, 2001; Lips et al., 2013): severe deficiency (<12.5 nmol/L), deficiency (12.5–25 nmol/L), insufficiency (25–50 nmol/L), repletion (>50 nmol/L). The thresholds for severe deficiency and deficiency are undisputed; however, a controversy has arisen for defining the border between insufficiency and repletion. In 2011, the Institute of Medicine (IOM) suggested a serum level of 50 nmol/L as the value at which 97.5% of the vitamin D needs of the population would be covered (Institute of Medicine, 2011; Ross et al., 2011), whereas, the Endocrine Society (ES) defined it to be higher: 75 nmol/L (Holick et al., 2011). All deficiency levels including insufficiency, as so-called mild deficiency, must be prevented through focused supplementation.
In 2010, the Institute of Medicine (IOM) introduced new dietary reference intake (DRI) values for vitamin D after comprehensive reviewing of more than 1000 high quality research articles to renew thereby their first settings from 1997 (Institute of Medicine, 2011). The DRIs address an adequate nutritional intake of all sources. The IOM has set the dietary allowance (RDA) to 600 IU per day for the general population and at 800 IU per day for persons 70 years and older, whereas 1 IU is the biological equivalent of 0.025 μg vitamin D3. The tolerable upper intake level or UL (Upper Level of Intake), which represents the safe upper limit, was set to 4000 IU per day for vitamin D intake (Ross et al., 2011). The new RDAs reflect the scientific outcome from large dietary studies that revealed vitamin D insufficiency (Looker et al., 2002; Zadshir et al., 2005). In 2012, Troesch et al. analyzed the vitamin intake from different dietary surveys that included the German Nutritional Intake Study (Nationale Verzehrstudie II) 2008 (Max Rubner-Institut, 2008), the US National Health and Nutrition Examination Survey (NHANES) from 2003 to 2008 (Centers for Disease Control and Prevention & National Center for Health Statistics, 2009), the UK (The British National Diet and Nutrition Survey, 2003) (Henderson et al., 2003) and the Netherlands (van Rossum et al., 2011), and could confirm that vitamin D is one of the critical vitamins, which intake is below the recommendation (Troesch et al., 2012).
A gap exists between the intake and the recommendation of vitamin D. The chronic insufficient intake of micronutrients like vitamin D without seeing immediate clinical signs is called Hidden Hunger. Hidden Hunger, in particular for vitamin D, is more prevalent in the populations of the developed countries as anticipated (Biesalski, 2013). Hidden Hunger is a threat for the nutrition security for a given country. Nutrition security mandates sufficient micronutrients in an adequate food supply and is required to safeguard an optimal nutritional status of a population.
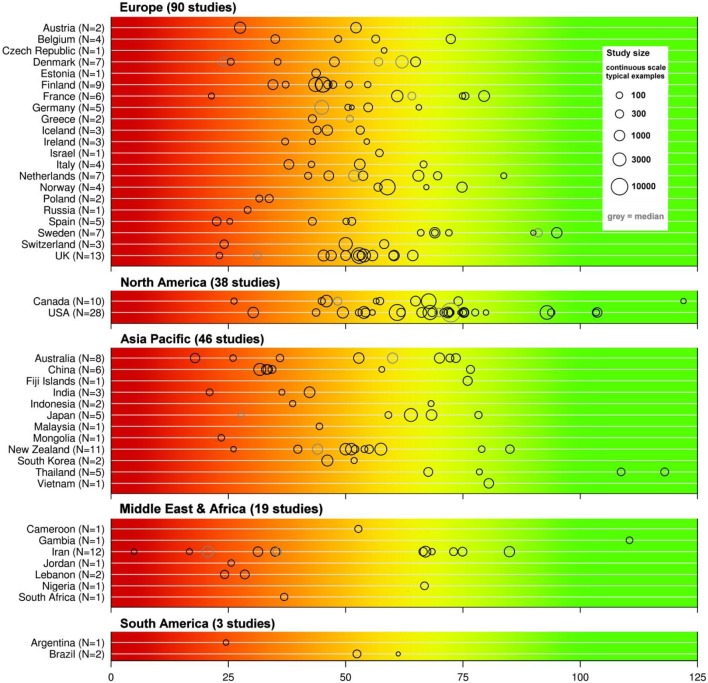
Many groups have identified vitamin D deficiency or insufficiency to become a public health problem worldwide (Holick, 2007; Holick and Chen, 2008; Mithal et al., 2009; Lips and Van Schoor, 2011; Wahl et al., 2012; Hilger et al., 2014). Mithal et al. (2009) described in their global report that most populations do not achieve a desirable vitamin D status and particular people at risk and elderly people suffer from vitamin D deficiency. In two reports, the International Osteoporosis Foundation (IOF) and its partners published the global vitamin D status map (Wahl et al., 2012; Hilger et al., 2014). The vitamin D map was based on a systematic review of the worldwide vitamin D levels, using all available publications published between 1990 and February 2011 (Hilger et al., 2014). Eligible studies include 168,389 participants from the general populations throughout the world where the mean or median serum 25-hydroxyvitamin D levels were measured. Studies included had a cross-sectional design or were based on a population based cohorts. The analysis identified nearly 200 studies from 44 countries, whereas only half of the studies were included in the global vitamin D status map as 50.2% of the studies were not representative for the target populations. Figure 1 shows the global vitamin D status map listed by countries and by continents. The largest numbers of studies were performed in Europe, followed by North America and Asia-Pacific. Available data from Latin America and even more from Africa are limited. Results of this review showed that 6.7% of the population were vitamin D deficient (mean 25-hydroxyvitamin D values <25 nmol/L), 37.3% were vitamin D insufficient according to IOM (mean values below 50 nmol/L) and 88.1% of the population showed an insufficient vitamin D status according to the ES (mean values below 75 nmol/L). No significant differences were found for gender or age, when looking at the worldwide data, but some regional differences could be identified (Hilger et al., 2014). The 25-hydroxyvitamin D serum levels were higher in Europe and the US, when compared to Middle East and Africa. This might be due to the vitamin D food fortification programs in North America (Prentice, 2008). Furthermore, the systematic analysis revealed that institutionalized elderly were more at risk to have low 25-hydroxyvitamin D levels in Europe and Asia/Pacific. The compared non-institutionalized elderly group showed higher levels, possibly due to spending more of time outdoors. The group of institutionalized elderly is therefore at high risk to become vitamin D deficient. Further research is needed to inform public health policy makers to reduce the risk for potential health consequences of low vitamin D status.
Figure 1.
Overview of published 25-hydroxyvitamin D mean/median values by countries (modified from Hilger et al., 2014). The color trend from red, yellow to green shown above the graphical diagram represents the current uncertainty around the definition of 25-hydroxyvitamin D3 serum thresholds starting from severe deficiency (red), deficiency, insufficiency to total repletion (green). The reported means are shown as black circles, studies that reported medians are given in gray circle. The study size is indicated by the circle size. Mean/median values falling within the intensely red zone are most consistent with severe vitamin D deficiency.
In the past few years the national recommendations for dietary vitamin D were adjusted in several countries; they are not harmonized across the European Union yet and vary from 200 to 800 IU. The higher recommendations for dietary vitamin D intake are increasingly being suggested in government documents, position statements and clinical practice guidelines for bone health. In 2008, the US Food and Drug Administration updated the health claim for the prevention of osteoporosis by including vitamin D to the consumption of calcium (Food and Drug Administration, 2008). In 2008, the American Academy of Pediatrics also reacted and issued an update of their guidelines for vitamin D intake and rickets prevention (Perrine et al., 2010). They doubled the recommended dose of vitamin D for children to 400 IU per day, beginning in the first few days of life and continuing throughout adolescence. In 2010, the Institute of Medicine (IOM) released the revised Dietary Reference Intakes (DRI's) for calcium and vitamin D and tripled the recommendations for vitamin D intakes to 600 IU per day for children and all adults up to age 69 years (Institute of Medicine, 2011). The IOM stated that there was insufficient evidence to make recommendations for non-skeletal benefits.
In 2012, the German, Austrian, and Swiss Nutrition Societies raised the recommended vitamin D intake to 800 IU per day, in case of absent UVB exposure, for all age groups starting from 1 year of age (German Nutrition Society, 2012). Furthermore, key opinion leaders are increasingly recommending higher daily intakes for vitamin D, between 800 and 1000 IU or even higher for people at risk or older adults. The recent statement by the IOF and the guidelines by the US ES suggest that higher vitamin D doses would be needed to achieve the desirable 25-hydroxyvitamin D serum level of 75 nmol/L for people at risk or older individuals.
Increasing the vitamin D levels in the population would also ameliorate health economics. Grant and colleagues calculated the benefit of increasing vitamin D levels to reduce the economic burden of diseases (Grant et al., 2009). A rise in the vitamin D serum level of all Europeans to 40 nmol/L would reduce the economic burden of different diseases and could save health care costs of up to 16.7%. Besides reducing the economic costs, vitamin D intake could in addition also reduce mortality rates and maintain a longer healthy life style.
Nutritional research to address and understand vitamin D insufficiency
Vitamin D deficiency is undoubtedly linked to severe consequences in the growing child by causing incomplete mineralization of the bone and in the adult accounting to wasteful osteomalacia. In the vitamin D insufficiency stage, this severity gets gradually less, but the outcome remains unchanged. Besides the established and accepted functional skeletal health relationship, more and more evidence is accumulating for falls (Pfeifer et al., 2000, 2009; Bischoff et al., 2003; Flicker et al., 2005; Broe et al., 2007; Prince et al., 2008; Bischoff-Ferrari et al., 2009) and physical performance (Bischoff-Ferrari et al., 2004; Houston et al., 2011; Ceglia et al., 2013; Redzic et al., 2013; Sohl et al., 2013; Tieland et al., 2013), which has been recognized by a health claim of the European Food and Safety Authority in 2011: “Vitamin D may reduce the risk of falling. Falling is a risk factor for bone fractures.” This health claim is targeting men and women 60 years of age and older and the dose required is a daily consumption of 800 IU vitamin D, which can come from all sources. Further emerging vitamin D health relationships include physiological parameters like improved immune response (Baeke et al., 2010; Schwalfenberg, 2011; Hewison, 2012; White, 2012), improved respiratory health(Berry et al., 2011; Charan et al., 2012; Choi et al., 2013; Hirani, 2013) possibly also relate to reduced tuberculosis incidence (Nnoaham and Clarke, 2008; Martineau et al., 2011; Mitchell et al., 2011; Coussens et al., 2012; Salahuddin et al., 2013; Huaman et al., 2014); and reduced risk to develop autoimmune diseases like multiple sclerosis (Solomon and Whitham, 2010; Cantorna, 2012; Dobson et al., 2013) or type 1 diabetes (Hypponen et al., 2001; Holick, 2003; Ramos-Lopez et al., 2006; Baeke et al., 2010; De Boer et al., 2012; Dong et al., 2013; Van Belle et al., 2013). In chronic, non-communicable diseases, vitamin D deficiency is being discussed to possibly ameliorate the incidence of some neoplastic diseases like colorectal, lung, prostate, and breast cancers (Ng et al., 2008; Rosen et al., 2012; Welsh, 2012; Cheng et al., 2013); cardiovascular diseases (CVDs) including hypertension, myocardial infarction, stroke (Forman et al., 2007; Giovannucci et al., 2008; Gardner et al., 2011; Bischoff-Ferrari et al., 2012; Tamez and Thadhani, 2012; Karakas et al., 2013; Pilz et al., 2013a; Schroten et al., 2013); life-style diseases like obesity and type 2 diabetes (Pittas et al., 2007; González-Molero et al., 2012; Khan et al., 2013; Pilz et al., 2013b; Schottker et al., 2013; Tsur et al., 2013; Van Belle et al., 2013; Bouillon et al., 2014); diseases related to the decline in sight function including age-related macular degeneration (Parekh et al., 2007; Millen et al., 2011; Lee et al., 2012); and neurological disorders including Alzheimer and Parkinson disease (Buell and Dawson-Hughes, 2008; Annweiler et al., 2012; Eyles et al., 2013; Zhao et al., 2013). One may wonder about the width of possible implications being looked at, but considering the more than 1000 genes which vitamin D is regulating through the VDR (Carlberg and Campbell, 2013), this may actually not be a surprise. To determine the potential role of vitamin D supplementation in the prevention or treatment of chronic non-skeletal diseases notwithstanding, large-scale clinical trials are demanded. In this respect for the nutrition field, four new large-scale ongoing long-term supplementation studies are expected to deliver results in near future (Table 2). The two very large studies, VITAL trial (n = 20,000) and FIND study (n = 18,000), are meant to deliver clinical evidence for the effect of vitamin D3 on cancer, CVD and diabetes outcomes. The two smaller trials, CAPS and DO-HEALTH, each having more than 2,000 participants are including cancer, infections, fractures, hypertension, cognitive function, and physical performance outcomes. In all four studies the placebo group will produce vitamin D3 in the skin and will possibly consume vitamin D through food, and therefore this will narrow the vitamin D serum level gap between the placebo and treatment groups. It remains to be seen whether the applied supplementation doses (2000 IU and 1600 IU, 3200 IU) will be sufficient to see a clear difference between the treatment and the control groups. An open likelihood will remain for the placebo group potentially obtaining sufficient vitamin D3 (600–800 IU) levels that are considered to be sufficient for skeletal effects. In such a case only an incremental increase of an additional ~1000 IU can be considered as the effective dose, for which no power calculation was available at the time before study begun. In light of such a situation, it will be of interest whether the micronutrient triage theory of Bruce Ames can be validated with vitamin D3 (Ames, 2006; McCann and Ames, 2009). The triage theory postulates, as a result of recurrent shortages of micronutrients during evolution, that the body has selected and developed a metabolic rebalancing response to shortage. These rebalancing favored micronutrient-needs for short term survival, while those only required for long-term health were starved. In the case of the micronutrient vitamin D3, calcium and bone metabolism can be considered to be secured with highest priority, therefore, it might be speculated that the 600–800 IU intake would satisfy this vitamin D3 serum level threshold. For the chronic non-skeletal diseases however, which have only secondary priority in an evolutionary perspective, higher serum vitamin D3 levels would be required. The ongoing four vitamin D3 studies that have chronic diseases as their main outcomes and use nutritionally relevant ~2000 IU are therefore well-suited to address whether the triage theory holds also true for the micronutrient vitamin D3.
Table 2.
List of ongoing large nutritional vitamin D3 supplementation trials (>2,000 subjects) using nutrition-related daily vitamin D3 doses (1,600–3,200 IU).
| Acronym | Name, clinical trial identifier | Principal investigator | Place | Participants | Dose | Duration | Main outcomes | Results expected | Web link |
|---|---|---|---|---|---|---|---|---|---|
| CAPS | Clinical Trial of Vitamin D3 to Reduce Cancer Risk in Postmenopausal Women NCT01052051 | Joan Lappe, Creighton University | USA | 2,332, healthy postmenopausal women: 55+ | 2,000 IU D3 (and 1,500 mg calcium) daily | 5 years | All cancers | 2015 | http://clinicaltrials.gov/ct2/show/NCT01052051?term=NCT01052051&rank=1 |
| VITAL | Vitamin D and Omega-3 Trial NCT01169259 | JoAnn E. Manson, Brigham and Women's Hospital | USA | 20,000, men: 50+ women: 55+ | 2,000 IU D3, daily omega-3 fatty acids | 5 years | Cancer, Cardiovascular disease | 2017 | http://clinicaltrials.gov/show/NCT01169259 |
| DO-HEALTH | Vitamin D3—Omega3—Home Exercise—Healthy Ageing and Longevity Trial NCT01745263 | Heike Bischoff-Ferrari, University Zürich | 8 European Cities | 2,152, 70+ | 2,000 IU D3 daily omega-3 fatty acids | 3 years | Infections, Fractures, Blood pressure, Cognitive function, Lower extremity function | 2017 | http://clinicaltrials.gov/ct2/show/NCT01745263?term=bischoff-ferrari&rank=1; |
| FIND | Finnish Vitamin D Trial NCT01463813 | Tomi-Pekka Tuomainen, University of Eastern Finland | Finland | 18,000 men: 60+, women: 65+ | 1,600 IU D3 daily or 3,200 IU D3 daily | 5 years | Cancer, Cardiovascular disease Diabetes | 2020 | http://clinicaltrials.gov/show/NCT01463813 |
Vitamin D3 once in the blood immediately binds to the vitamin D-binding protein (DBP) and gets transported into the liver (Holick, 2007). The first hydroxylation at position 25 generates the major circulating metabolites 25-hydroxyvitamin D3. This metabolite circulates throughout all organs and undergoes hydroxylation at position 1, which occurs mainly in the kidney, but also in other organs, to form 1,25-dihydroxyvitamin D3, the active hormone. Besides the major circulating metabolite 25-hydroxyvitamin D3 and the hormonally active metabolite 1,25-dihydroxyvitamin D3, more than 35 additional vitamin D3 metabolites are formed by the body (Bouillon et al., 1995; Norman et al., 2001). It is speculated that they might be intermediates in the catabolism of 1,25-dihydroxyvitamin D3. The human body has evolved many CYP enzymes and invests energy to form these additional 35 vitamin D3 metabolites, whether this is for the purpose to catabolize 1,25-dihydroxyvitamin D3, remains still to be answered. More appealing is the theory that these metabolites are formed to fulfill yet unknown functions of vitamin D3. This perspective could potentially also account to the pleiotropic non-skeletal health benefits reported by the many vitamin D intake studies. For some of the vitamin D3 metabolites like the 24R,25-dihydroxyvitamin D3 potential function was explored in vitro (Norman et al., 2002).
The 24R,25-dihydroxyvitamin D3 has been shown to be an essential hormone in the process of bone fracture healing. The 24R,25-dihydroxyvitamin D3 most likely initiates its biological responses via binding to the ligand binding domain of a postulated cell membrane receptor VDRmem24,25, similar to the better studied, but still not cloned cell membrane receptor for 1,25-dihydroxyvitamin D3, VDRmem1,25 (Norman et al., 2002). From the nutritional point of view, it will be of interest to investigate the function of the all vitamin D3 metabolites and relate the function to the level of vitamin D3 intake to secure the health benefit according to the triage theory.
According to the current knowledge, the vitamin D endocrine system is funneled through the biologically most active metabolite 1,25-dihydroxyvitamin D3 that is mainly produced in the kidney, but also in other organs (Bouillon et al., 2013). Mechanistically 1,25-dihydroxyvitamin D3 binds the VDR directly on a DNA sequence, the 1,25-dihydroxyvitamin D3 response element (VDRE), in the regulatory region of primary 1,25-dihydroxyvitamin D3 target genes (Carlberg and Campbell, 2013). The VDR forms together with the retinoid X receptor or putative other transcription factors a heterodimer on the VDRE, recruiting tissue-specific transcriptional co-activators and regulates through a conformational change upon 1,25-dihydroxyvitamin D3 binding the downstream gene. The VDR is widespread in more than 30 tissues (Bouillon et al., 1995) and may trigger expression of more than 1000 genes through 1,25-dihydroxyvitamin (Carlberg et al., 2013; Hossein-Nezhad et al., 2013). The regulation of tissue-specific gene expression by 1,25-dihydroxyvitamin D3 is of high interest, as it guides us toward the better understanding of the mechanistic action of vitamin D3 in the different tissues. The gained knowledge from the mechanistic studies can help to design smaller and more focused nutritional intervention RCTs to answer whether vitamin D contributes to a specific health benefit of interest. In this respect the GeneChip-based transcriptomics methodology using high-density microarrays demonstrated the expression of genes in a variety of important functions of more than 100 different pathways that could be linked to vitamin D deficiency (Bossé et al., 2007; Tarroni et al., 2012; Hossein-Nezhad et al., 2013). The development of chromatin immunoprecipitation (ChiP) methodology linked to site-specific PCR amplification of the VDR bound genomic DNA fragment, and later the methods using tiled microarrays (ChiP-chip) applying the first unbiased genome-wide approach, which then was followed by the massive parallel NGS sequencing approach of the immunoprecipitated DNA segments, opened up new avenues to investigate 1,25-dihydroxyvitamin D3 target genes in selected tissues (Ramagopalan et al., 2010; Heikkinen et al., 2011; Carlberg et al., 2012, 2013; Pike et al., 2014). In an elegant study, Carlberg et al. identified in samples of 71 pre-diabetic individuals of the VitDmet study changes in serum 25-hydroxyvitamin D3 concentrations that were associated to primary vitamin D target genes (Carlberg et al., 2013). Based on their finding the authors proposed the genes CD14 and THBD as transcriptomics biomarkers, from which the effects of a successful vitamin D3 supplementation can be evaluated. These biomarkers are potentially suitable for displaying the transcriptomics response of human tissues to vitamin D3 supplementation.
Epigenetic alterations of the genome refer to heritable and modifiable changes in gene expression that are not affecting the DNA sequence. They may be inherited as Mendelian, non-Mendelian, or environmentally caused traits. One of the 1,25-dihydroxyvitamin D3 induced epigenetic modification was shown for the hypo-methylating effect on the osteocalcin promoter (Haslberger et al., 2006). 1,25-Dihydroxyvitamin D3 was associated with the demethylation of the osteocalcin promoter and induced the osteocalcin gene expression. The activity of VDR can be modulated by epigenetic histone acetylation. The VDR alone or in concert with other transcription factors can recruit histone-modifying enzymes like histone acetyl transferases (HATs) or histone deacetylases (HDACs) and epigenically direct transcriptional expression of downstream genes (Burrell et al., 2011; Karlic and Varga, 2011; Sundar and Rahman, 2011; Hossein-Nezhad et al., 2013). The trans-generational epigenetic inheritance of vitamin D3 triggered epigenome modification is not fully explored, however maternal vitamin D deficiency has been discussed with adverse pregnancy outcomes or potential susceptibility for diseases (Burrell et al., 2011; Hossein-Nezhad and Holick, 2012). For future nutritional research it would be of great value to identify and validate epigenetic biomarkers that could serve as risk assessment tool for vitamin D insufficiency related susceptibility to develop a disease later in life.
Variations in vitamin D status have been shown to be related to inheritance. The disparity of vitamin D levels according to ethnicity given skin pigmentation is well-established (Cashman, 2014; Ng et al., 2014). Dark skinned population individuals have compared to Caucasian descendants almost one-half the serum concentrations of 25-hydroxyvitamin D (Nesby-O'dell et al., 2002). From twin studies it has been estimated that the heritability of genetic regulation of vitamin D levels to be between 23 and 80% (Dastani et al., 2013). In addition, large-scale genetic association studies using linkage disequilibrium analysis have identified genetic loci correlating with serum vitamin D level within five candidate genes (Dastani et al., 2013). The identified SNPs are within the 1alpha-hydroxylase of 25-hydroxyvitamin D (CYP27B1) gene, the 25-hydroxylase of vitamin D (CYP2R1) gene, the vitamin D carrier protein (GC) gene, the VDR gene, and the cytochrome P-450 (CYP24A1) gene coding for an enzyme that inactivates 1,25-dihydroxyvitamin D. It is important to note that replication studies in separate populations have to follow to verify the validity of the identified SNPs. The SNP information will provide the additional guidance toward a personalized nutritional advice to reach a sufficient vitamin D status.
Conclusion and future perspectives
In the recent years the knowledge about vitamin D and its implications have extended far beyond its classical role in bone health in either fields of basic research as well as in human trials. In particular, the evidence for the role of vitamin D in reducing the risk of fractures as well as decreasing the risk for falling is convincing and authorities have responded to it. Besides a health claim issued by the EFSA on the risk reduction for falling the dietary intake recommendations have been significantly increased in several countries such as the US and in Europe (Austria, Germany, Switzerland). A number of other countries around the globe are in the process of establishing new dietary intake recommendations as well. It turns out that on average a daily intake of 600–800 IU vitamin D appears to be required to meet fundamental needs of the human body, for specific applications higher daily intakes may be necessary, which will become clearer as the results of a number of ongoing clinical studies will become available.
The obvious question to answer is: do people obtain the recommended amounts of vitamin D? The diet is typically only a minor vitamin D source as only few food items contain relevant amounts of vitamin D, such as fatty sea fish. The primary vitamin D source for humans is the vitamin D synthesis in the skin from vitamin D precursors by the sunlight—provided the skin is sufficiently exposed to strong enough sun radiation. Several groups have reviewed the published results on 25-hydroxyvitamin D serum levels the established marker of the vitamin D status, showing that low 25-hydroxyvitamin D levels are found in many cohorts around the world. A recent systematic review of the global vitamin D status (Hilger et al., 2014) showed that 6.7% of the overall populations reported deficient 25-hydroxyvitamin D levels below 25 nmol/L, 37% had 25-hydroxyvitamin D levels below 50 nmol/L, and only 11% were above 75 nmol/L, which is considered an adequate status by the IOF and the ES. So a very important task ahead of us is to find efficient ways to improve the vitamin D status on the population level, be it by dietary means, food fortification, or dietary supplements.
In addition, it will be very important to gather sound and convincing evidence for the many additional implicated health benefits of vitamin D besides the ones that already reached a health claim status and to see which of them will actually hold up. This will require appropriate human studies on the one hand, and also involve the appropriate use of the novel experimental approaches like nutrigenomics, nutrigenetics, and nutriepigenetics on the other hand. In conclusion, the evidence we have for vitamin D in human health is exciting, however we have to make sure that appropriate measures are taken to improve the vitamin D status to the levels required to be beneficial for human health. In future, we will also need to further apply, exploit and invest in novel, innovative and break-through technologies in the vitamin D research to understand the underlying mechanisms by which vitamin D is exerting so many effects in the human body, which is knowledge needed to the purpose to obtain and secure optimal public health through nutrition.
Conflict of interest statement
The authors are employees of DSM Nutritional Products Ltd. and declare to have no conflict of interest.
References
- Ames B. N. (2006). Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proc. Natl. Acad. Sci. U.S.A. 103, 17589–17594 10.1073/pnas.0608757103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminullah Bhuiyan A. K. M., Ratnayake W. M. N., Ackman R. G. (1993). Nutritional composition of raw and smoked atlantic mackerel (scomber scombrus): oil- and water-soluble vitamins. J. Food Compos. Anal. 6, 172–184 10.1006/jfca.1993.1019 [DOI] [Google Scholar]
- Annweiler C., Rolland Y., Schott A. M., Blain H., Vellas B., Herrmann F. R., et al. (2012). Higher vitamin D dietary intake is associated with lower risk of Alzheimer's disease: a 7-year follow-up. J. Gerontol. A Biol. Sci. Med. Sci. 67, 1205–1211 10.1093/gerona/gls107 [DOI] [PubMed] [Google Scholar]
- Armas L. A., Dowell S., Akhter M., Duthuluru S., Huerter C., Hollis B. W., et al. (2007). Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J. Am. Acad. Dermatol. 57, 588–593 10.1016/j.jaad.2007.03.004 [DOI] [PubMed] [Google Scholar]
- Armas L. A., Hollis B. W., Heaney R. P. (2004). Vitamin D2 is much less effective than vitamin D3 in humans. J. Clin. Endocrinol. Metab. 89, 5387–5391 10.1210/jc.2004-0360 [DOI] [PubMed] [Google Scholar]
- Baeke F., Takiishi T., Korf H., Gysemans C., Mathieu C. (2010). Vitamin D: modulator of the immune system. Curr. Opin. Pharmacol. 10, 482–496 10.1016/j.coph.2010.04.001 [DOI] [PubMed] [Google Scholar]
- Berridge M. J. (2012). Calcium signalling remodelling and disease. Biochem. Soc. Trans. 40, 297–309 10.1042/BST20110766 [DOI] [PubMed] [Google Scholar]
- Berry D. J., Hesketh K., Power C., Hyppönen E. (2011). Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br. J. Nutr. 106, 1433–1440 10.1017/S0007114511001991 [DOI] [PubMed] [Google Scholar]
- Biesalski H. K. (2013). Hidden hunger in the developed world, in The Road to Good Nutrition, A Global Perspective, 1st Edn., eds Eggersdorfer M., Kraemer K., Ruel M., Van Ameringen M., Biesalski H. K., Bloem M., et al. (Basel: Karger; ), 39–50 [Google Scholar]
- Bilodeau L., Dufresne G., Deeks J., Clément G., Bertrand J., Turcotte S., et al. (2011). Determination of vitamin D3 and 25-hydroxyvitamin D3 in foodstuffs by HPLC UV-DAD and LC–MS/MS. J. Food Compos. Anal. 24, 441–448 10.1016/j.jfca.2010.08.002 [DOI] [Google Scholar]
- Bischoff H. A., Stahelin H. B., Dick W., Akos R., Knecht M., Salis C., et al. (2003). Effects of vitamin D and calcium supplementation on falls: a randomized controlled trial. J. Bone Miner. Res. 18, 343–351 10.1359/jbmr.2003.18.2.343 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari H. A., Dawson-Hughes B., Staehelin H. B., Orav J. E., Stuck A. E., Theiler R., et al. (2009). Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 339:b3692 10.1136/bmj.b3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff-Ferrari H. A., Dawson-Hughes B., Stocklin E., Sidelnikov E., Willett W. C., Edel J. O., et al. (2012). Oral supplementation with 25(OH)D3 versus vitamin D3: effects on 25(OH)D levels, lower extremity function, blood pressure, and markers of innate immunity. J. Bone Miner. Res. 27, 160–169 10.1002/jbmr.551 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari H. A., Dietrich T., Orav E. J., Hu F. B., Zhang Y., Karlson E. W., et al. (2004). Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged ≥60 y. Am. J. Clin. Nutr. 80, 752–758 [DOI] [PubMed] [Google Scholar]
- Boland R., Skliar M., Curino A., Milanesi L. (2003). Vitamin D compounds in plants. Plant Sci. 164, 357–369 10.1016/S0168-9452(02)00420-X [DOI] [Google Scholar]
- Boland R. L. (1986). Plants as a source of vitamin D3 metabolites. Nutr. Rev. 44, 1–8 10.1111/j.1753-4887.1986.tb07543.x [DOI] [PubMed] [Google Scholar]
- Bossé Y., Maghni K., Hudson T. J. (2007). 1α,25-dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol. Genomics 29, 161–168 10.1152/physiolgenomics.00134.2006 [DOI] [PubMed] [Google Scholar]
- Bouillon R., Carmeliet G., Lieben L., Watanabe M., Perino A., Auwerx J., et al. (2014). Vitamin D and energy homeostasis—Of mice and men. Nat. Rev. Endocrinol. 10, 79–87 10.1038/nrendo.2013.226 [DOI] [PubMed] [Google Scholar]
- Bouillon R., Lieben L., Mathieu C., Verstuyf A., Carmeliet G. (2013). Vitamin D action: lessons from VDR and Cyp27b1 null mice. Pediatr. Endocrinol. Rev. 10 Suppl. 2, 354–366 [PubMed] [Google Scholar]
- Bouillon R., Okamura W. H., Norman A. W. (1995). Structure-function relationships in the vitamin D endocrine system. Endocr. Rev. 16, 200–257 [DOI] [PubMed] [Google Scholar]
- Bouillon R., Suda T. (2014). Vitamin D: calcium and bone homeostasis during evolution. Bonekey Rep. 3:480 10.1038/bonekey.2013.214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broe K. E., Chen T. C., Weinberg J., Bischoff-Ferrari H. A., Holick M. F., Kiel D. P. (2007). A higher dose of vitamin D reduces the risk of falls in nursing home residents: a randomized, multiple-dose study. J. Am. Geriatr. Soc. 55, 234–239 10.1111/j.1532-5415.2007.01048.x [DOI] [PubMed] [Google Scholar]
- Buell J. S., Dawson-Hughes B. (2008). Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol. Aspects Med. 29, 415–422 10.1016/j.mam.2008.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell A. M., Handel A. E., Ramagopalan S. V., Ebers G. C., Morahan J. M. (2011). Epigenetic mechanisms in multiple sclerosis and the major histocompatibility complex (MHC). Discov. Med. 11, 187–196 [PubMed] [Google Scholar]
- Byrdwell W. C., Horst R. L., Phillips K. M., Holden J. M., Patterson K. Y., Harnly J. M., et al. (2013). Vitamin D levels in fish and shellfish determined by liquid chromatography with ultraviolet detection and mass spectrometry. J. Food Compos. Anal. 30, 109–119 10.1016/j.jfca.2013.01.005 [DOI] [Google Scholar]
- Calvo M. S., Whiting S. J., Barton C. N. (2004). Vitamin D fortification in the United States and Canada: current status and data needs. Am. J. Clin. Nutr. 80, 1710S–1716S [DOI] [PubMed] [Google Scholar]
- Cantorna M. T. (2012). Vitamin D, multiple sclerosis and inflammatory bowel disease. Arch. Biochem. Biophys. 523, 103–106 10.1016/j.abb.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg C., Campbell M. J. (2013). Vitamin D receptor signaling mechanisms: integrated actions of a well-defined transcription factor. Steroids 78, 127–136 10.1016/j.steroids.2012.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg C., Seuter S., De Mello V. D., Schwab U., Voutilainen S., Pulkki K., et al. (2013). Primary vitamin d target genes allow a categorization of possible benefits of vitamin D3 supplementation. PLoS ONE 8:e71042 10.1371/journal.pone.0071042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg C., Seuter S., Heikkinen S. (2012). The first genome-wide view of vitamin D receptor locations and their mechanistic implications. Anticancer Res. 32, 271–282 [PubMed] [Google Scholar]
- Cashman K. D. (2014). The vitamin D RDA for African American adults: higher than that for white persons? Am. J. Clin. Nutr. 99, 427–428 10.3945/ajcn.113.082271 [DOI] [PubMed] [Google Scholar]
- Ceglia L., Niramitmahapanya S., Da Silva Morais M., Rivas D. A., Harris S. S., Bischoff-Ferrari H., et al. (2013). A randomized study on the effect of vitamin D3 supplementation on skeletal muscle morphology and vitamin d receptor concentration in older women. J. Clin. Endocrinol. Metab. 98, E1927–E1935 10.1210/jc.2013-2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention & National Center for Health Statistics. (2009). NHANES 2003-2004, NHANES 2005-2006, NHANES 2007-2008. Data, Documentation, Codebooks, SAS Code. Dietary Interview. Individual Foods, Total Nutrient Intakes First and Second Day. Hyattsville, MD: U.S. Department of Health and Human Services & Centers for Disease Control and Prevention. Available online at: http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm
- Charan J., Goyal J. P., Saxena D., Yadav P. (2012). Vitamin D for prevention of respiratory tract infections: a systematic review and meta-analysis. J. Pharmacol. Pharmacother. 3, 300–303 10.4103/0976-500X.103685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T. Y., Lacroix A. Z., Beresford S. A., Goodman G. E., Thornquist M. D., Zheng Y., et al. (2013). Vitamin D intake and lung cancer risk in the Women's Health Initiative. Am. J. Clin. Nutr. 98, 1002–1011 10.3945/ajcn.112.055905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi C. J., Seo M., Choi W. S., Kim K. S., Youn S. A., Lindsey T., et al. (2013). Relationship between serum 25-hydroxyvitamin D and lung function among Korean adults in Korea National Health and Nutrition Examination Survey, 2008–2010. J. Clin. Endocrinol. Metab. 98, 1703–1710 10.1210/jc.2012-3901 [DOI] [PubMed] [Google Scholar]
- Collins E. D., Norman A. W. (2001). Vitamin D, in Handbook of Vitamins, eds Rucker R. B., Suttie J. W., Mccormick D. B., Machlin L. J. (New York, NY: Marcel Dekker Inc.), 51–113 [Google Scholar]
- Coussens A. K., Wilkinson R. J., Hanifa Y., Nikolayevskyy V., Elkington P. T., Islam K., et al. (2012). Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proc. Natl. Acad. Sci. U.S.A. 109, 15449–15454 10.1073/pnas.1200072109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastani Z., Li R., Richards B. (2013). Genetic regulation of vitamin D levels. Calcif. Tissue Int. 92, 106–117 10.1007/s00223-012-9660-z [DOI] [PubMed] [Google Scholar]
- De Boer I. H., Sachs M. C., Cleary P. A., Hoofnagle A. N., Lachin J. M., Molitch M. E., et al. (2012). Circulating Vitamin D metabolites and kidney disease in type 1 diabetes. J. Clin. Endocrinol. Metab. 97, 4780–4788 10.1210/jc.2012-2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson R., Giovannoni G., Ramagopalan S. (2013). The month of birth effect in multiple sclerosis: systematic review, meta-analysis and effect of latitude. J. Neurol. Neurosurg. Psychiatry 84, 427–432 10.1136/jnnp-2012-303934 [DOI] [PubMed] [Google Scholar]
- Dong J. Y., Zhang W. G., Chen J. J., Zhang Z. L., Han S. F., Qin L. Q. (2013). Vitamin D intake and risk of type 1 diabetes: a meta-analysis of observational studies. Nutrients 5, 3551–3562 10.3390/nu5093551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egaas E., Lambertsen G. (1979). Naturally occurring vitamin D3 in fish products analysed by HPLC, using vitamin D2 as an international standard. Int. J. Vitam. Nutr. Res. 49, 35–42 [PubMed] [Google Scholar]
- Exler J., Phillips K. M., Patterson K. Y., Holden J. M. (2013). Cholesterol and vitamin D content of eggs in the U.S. retail market. J. Food Compos. Anal. 29, 110–116 10.1016/j.jfca.2012.11.001 [DOI] [Google Scholar]
- Eyles D. W., Burne T. H. J., McGrath J. J. (2013). Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front. Neuroendocrinol. 34, 47–64 10.1016/j.yfrne.2012.07.001 [DOI] [PubMed] [Google Scholar]
- Flicker L., Macinnis R. J., Stein M. S., Scherer S. C., Mead K. E., Nowson C. A., et al. (2005). Should older people in residential care receive vitamin D to prevent falls? Results of a randomized trial. J. Am. Geriatr. Soc. 53, 1881–1888 10.1111/j.1532-5415.2005.00468.x [DOI] [PubMed] [Google Scholar]
- Food and Drug Administration. (2008). Food labeling: health claims; calcium and osteoporosis, and calcium, vitamin D, and osteoporosis. Fed. Regist. 73, 56477–56487 [PubMed] [Google Scholar]
- Forman J. P., Giovannucci E., Holmes M. D., Bischoff-Ferrari H. A., Tworoger S. S., Willett W. C., et al. (2007). Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 49, 1063–1069 10.1161/HYPERTENSIONAHA.107.087288 [DOI] [PubMed] [Google Scholar]
- Gardner D. G., Chen S., Glenn D. J., Ni W. (2011). Vitamin D and The Cardiovascular System. Amsterdam: Elsevier Inc [Google Scholar]
- German Nutrition Society. (2012). New reference values for vitamin D. Ann. Nutr. Metab. 60, 241–246 10.1159/000337547 [DOI] [PubMed] [Google Scholar]
- Giovannucci E., Liu Y., Hollis B. W., Rimm E. B. (2008). 25-hydroxyvitamin d and risk of myocardial infarction in men: a prospective study. Arch. Intern. Med. 168, 1174–1180 10.1001/archinte.168.11.1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendenning P., Chew G. T., Seymour H. M., Gillett M. J., Goldswain P. R., Inderjeeth C. A., et al. (2009). Serum 25-hydroxyvitamin D levels in vitamin D-insufficient hip fracture patients after supplementation with ergocalciferol and cholecalciferol. Bone 45, 870–875 10.1016/j.bone.2009.07.015 [DOI] [PubMed] [Google Scholar]
- González-Molero I., Rojo-Martínez G., Morcillo S., Gutiérrez-Repiso C., Rubio-Martín E., Almaraz M. C., et al. (2012). Vitamin D and incidence of diabetes: a prospective cohort study. Clin. Nutr. 31, 571–573 10.1016/j.clnu.2011.12.001 [DOI] [PubMed] [Google Scholar]
- Grant W. B., Cross H. S., Garland C. F., Gorham E. D., Moan J., Peterlik M., et al. (2009). Estimated benefit of increased vitamin D status in reducing the economic burden of disease in western Europe. Prog. Biophys. Mol. Biol. 99, 104–113 10.1016/j.pbiomolbio.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Haslberger A., Varga F., Karlic H. (2006). Recursive causality in evolution: a model for epigenetic mechanisms in cancer development. Med. Hypotheses 67, 1448–1454 10.1016/j.mehy.2006.05.047 [DOI] [PubMed] [Google Scholar]
- Haytowitz D. B., Lemar L. E., Pehrsson P. R. (2009). USDA's Nutrient Databank System -a tool for handling data from diverse sources. J. Food Compos. Anal. 22, 433–441 10.1016/j.jfca.2009.01.003 [DOI] [Google Scholar]
- Health Canada. (2014). Consolidation Food and Drugs Regulation. Ottawa, ON: The Minister of Justice Canada [Google Scholar]
- Heaney R. P., Recker R. R., Grote J., Horst R. L., Armas L. A. (2011). Vitamin D3 is more potent than vitamin D2 in humans. J. Clin. Endocrinol. Metab. 96, E447–E452 10.1210/jc.2010-2230 [DOI] [PubMed] [Google Scholar]
- Heikkinen S., Vaisanen S., Pehkonen P., Seuter S., Benes V., Carlberg C. (2011). Nuclear hormone 1alpha,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy. Nucleic Acids Res. 39, 9181–9193 10.1093/nar/gkr654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L., Irving K., Gregory J. F., Bates C. J., Prentice A., Perks J., et al. (2003). The National Diet and Nutrition Survey: Adults Aged 19–64 Years. Vol. 3: Vitamin and Mineral Intake and Urinary Analytes. London: The Stationery Office [Google Scholar]
- Hess A. F., Weinstock M. (1925a). The antirachitic value of irradiated cholesterol an phytosterol. II. Further evidence of change in biological activity. Methods Enzymol. 64, 181–191 [Google Scholar]
- Hess A. F., Weinstock M. (1925b). The antirachitic value of irradiated cholesterol an phytosterol. III. Evidence of chemical change as shown by absorption spectra. Methods Enzymol. 64, 193–201 [Google Scholar]
- Hewison M. (2012). An update on vitamin D and human immunity. Clin. Endocrinol. 76, 315–325 10.1111/j.1365-2265.2011.04261.x [DOI] [PubMed] [Google Scholar]
- Hilger J., Friedel A., Herr R., Rausch T., Roos F., Wahl D. A., et al. (2014). A systematic review of vitamin D status in populations worldwide. Br. J. Nutr. 111, 23–45 10.1017/S0007114513001840 [DOI] [PubMed] [Google Scholar]
- Hirani V. (2013). Associations between vitamin D and self-reported respiratory disease in older people from a nationally representative population survey. J. Am. Geriatr. Soc. 61, 969–973 10.1111/jgs.12254 [DOI] [PubMed] [Google Scholar]
- Holick M. F. (2003). Vitamin D: a millenium perspective. J. Cell. Biochem. 88, 296–307 10.1002/jcb.10338 [DOI] [PubMed] [Google Scholar]
- Holick M. F. (2007). Vitamin D deficiency. N. Engl J. Med 357, 266–281 10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- Holick M. F. (2011). Photobiology of Vitamin D, in Vitamin D, 3rd Edn., eds Feldman D., Pike J. W., Adams J. S. (London, GB: Elsevier; ), 13–22 [Google Scholar]
- Holick M. F., Biancuzzo R. M., Chen T. C., Klein E. K., Young A., Bibuld D., et al. (2008). Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J. Clin. Endocrinol. Metab. 93, 677–681 10.1210/jc.2007-2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M. F., Binkley N. C., Bischoff-Ferrari H. A., Gordon C. M., Hanley D. A., Heaney R. P., et al. (2011). Evaluation, treatment, and prevention of vitamin d deficiency: an endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 96, 1911–1930 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- Holick M. F., Chen T. C. (2008). Vitamin D deficiency: a worldwide problem with health consequences. Am. J. Clin. Nutr. 87, 1080S–1086S [DOI] [PubMed] [Google Scholar]
- Holick M. F., Matsuoka L. Y., Wortsman J. (1989). Age, vitamin D, and solar ultraviolet. Lancet 2, 1104–1105 10.1016/S0140-6736(89)91124-0 [DOI] [PubMed] [Google Scholar]
- Hossein-Nezhad A., Holick M. F. (2012). Optimize dietary intake of vitamin D: an epigenetic perspective. Curr. Opin. Clin. Nutr. Metab. Care 15, 567–579 10.1097/MCO.0b013e3283594978 [DOI] [PubMed] [Google Scholar]
- Hossein-Nezhad A., Spira A., Holick M. F. (2013). Influence of vitamin D status and vitamin D3 supplementation on genome wide expression of white blood cells: a randomized double-blind clinical trial. PLoS ONE 8:e58725 10.1371/journal.pone.0058725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston D. K., Tooze J. A., Hausman D. B., Johnson M. A., Nicklas B. J., Miller M. E., et al. (2011). Change in 25-hydroxyvitamin D and physical performance in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 66, 430–436 10.1093/gerona/glq235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huaman M. A., Sterling T. R., Shepherd B. E., Fiske C. T. (2014). 25-Hydroxyvitamin D levels after recovery from tuberculosis: insights into pathogenesis. Tuberculosis (Edinb) 94, 51–54 10.1016/j.tube.2013.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huldschinsky K. (1919). Heilung von Rachitis durch künstliche Höhensonne. Dtsch. Med. Wochenschr. 45, 712–713 10.1055/s-0028-1137830 [DOI] [Google Scholar]
- Huldschinsky K. (1926). The antirachitic: zone of the ultra-violet. Klinische Wochenschrift 5, 1972–1973 10.1007/BF01710213 [DOI] [Google Scholar]
- Hypponen E., Laara E., Reunanen A., Jarvelin M. R., Virtanen S. M. (2001). Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet 358, 1500–1503 10.1016/S0140-6736(01)06580-1 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine, F. A. N. B. (2011). Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; [PubMed] [Google Scholar]
- Jakobsen J., Knuthsen P. (2014). Stability of vitamin D in foodstuffs during cooking. Food Chem. 148, 170–175 10.1016/j.foodchem.2013.10.043 [DOI] [PubMed] [Google Scholar]
- Jakobsen J., Saxholt E. (2009). Vitamin D metabolites in bovine milk and butter. J. Food Compos. Anal. 22, 472–478 10.1016/j.jfca.2009.01.010 [DOI] [Google Scholar]
- Japelt R. B., Silvestro D., Smedsgaard J., Jensen P. E., Jakobsen J. (2013). Quantification of vitamin D3 and its hydroxylated metabolites in waxy leaf nightshade (Solanum glaucophyllum Desf.), tomato (Solanum lycopersicum L.) and bell pepper (Capsicum annuum L.). Food Chem. 138, 1206–1211 10.1016/j.foodchem.2012.11.064 [DOI] [PubMed] [Google Scholar]
- Karakas M., Thorand B., Zierer A., Huth C., Meisinger C., Roden M., et al. (2013). Low levels of serum 25-hydroxyvitamin d are associated with increased risk of myocardial infarction, especially in women: results from the MONICA/KORA augsburg case-cohort study. J. Clin. Endocrinol. Metab. 98, 272–280 10.1210/jc.2012-2368 [DOI] [PubMed] [Google Scholar]
- Karlic H., Varga F. (2011). Impact of vitamin D metabolism on clinical epigenetics. Clin. Epigenetics 2, 55–61 10.1007/s13148-011-0021-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan H., Kunutsor S., Franco O. H., Chowdhury R. (2013). Vitamin D, type 2 diabetes and other metabolic outcomes: a systematic review and meta-analysis of prospective studies. Proc. Nutr. Soc. 72, 89–97 10.1017/S0029665112002765 [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Takeuchi A., Okano T. (1995). Vitamin D contents in various kinds of Japanese foods, in Challenges of Modern Medicine (Rome: Ares-Serono Symposia; ), 345–350 [Google Scholar]
- Lee V., Rekhi E., Hoh Kam J., Jeffery G. (2012). Vitamin D rejuvenates aging eyes by reducing inflammation, clearing amyloid beta and improving visual function. Neurobiol. Aging 33, 2382–2389 10.1016/j.neurobiolaging.2011.12.002 [DOI] [PubMed] [Google Scholar]
- Lehmann B., Genehr T., Knuschke P., Pietzsch J., Meurer M. (2001). UVB-induced conversion of 7-dehydrocholesterol to 1alpha,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J. Invest. Dermatol. 117, 1179–1185 10.1046/j.0022-202x.2001.01538.x [DOI] [PubMed] [Google Scholar]
- Lehmann U., Hirche F., Stangl G. I., Hinz K., Westphal S., Dierkes J. (2013). Bioavailability of vitamin D2 and D3 in healthy volunteers, a randomized placebo-controlled trial. J. Clin. Endocrinol. Metab. 98, 4339–4345 10.1210/jc.2012-4287 [DOI] [PubMed] [Google Scholar]
- Lips P. (2001). Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 22, 477–501 10.1210/edrv.22.4.0437 [DOI] [PubMed] [Google Scholar]
- Lips P. (2007). Relative value of 25(OH)D and 1,25(OH)2D measurements. J. Bone Miner. Res. 22, 1668–1671 10.1359/jbmr.070716 [DOI] [PubMed] [Google Scholar]
- Lips P. (2010). Worldwide status of vitamin D nutrition. J. Steroid Biochem. Mol. Biol. 121, 297–300 10.1016/j.jsbmb.2010.02.021 [DOI] [PubMed] [Google Scholar]
- Lips P., Van Schoor N. (2011). Worldwide vitamin D status, in Vitamin D, 3rd Edn., eds Feldman D., Pike J. W., Adams J. S. (London, GB: Elsevier Inc.), 947–963 [Google Scholar]
- Lips P., Van Schoor N. M., Bravenboer N. (2013). Vitamin D-related disorders, in Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism 8th Edn., eds Clifford R. B., Rosen J., Compston J. E., Rosen V. (Singapore: John Wiley & Sons, Inc.), 613–623 [Google Scholar]
- Looker A. C., Dawson-Hughes B., Calvo M. S., Gunter E. W., Sahyoun N. R. (2002). Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone 30, 771–777 10.1016/S8756-3282(02)00692-0 [DOI] [PubMed] [Google Scholar]
- Lu Z., Chen T. C., Zhang A., Persons K. S., Kohn N., Berkowitz R., et al. (2007). An evaluation of the vitamin D3 content in fish: is the vitamin D content adequate to satisfy the dietary requirement for vitamin D? J. Steroid Biochem. Mol. Biol. 103, 642–644 10.1016/j.jsbmb.2006.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaughlin J., Holick M. F. (1985). Aging decreases the capacity of human skin to produce vitamin D3. J. Clin. Invest. 76, 1536–1538 10.1172/JCI112134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau A. R., Timms P. M., Bothamley G. H., Hanifa Y., Islam K., Claxton A. P., et al. (2011). High-dose vitamin D3 during intensive-phase antimicrobial treatment of pulmonary tuberculosis: a double-blind randomised controlled trial. Lancet 377, 242–250 10.1016/S0140-6736(10)61889-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka L. Y., Wortsman J., Haddad J. G., Kolm P., Hollis B. W. (1991). Racial pigmentation and the cutaneous synthesis of vitamin D. Arch. Dermatol. 127, 536–538 10.1001/archderm.1991.04510010104011 [DOI] [PubMed] [Google Scholar]
- Mattila P., Konko K., Eurola M., Pihlava J. M., Astola J., Vahteristo L., et al. (2001). Contents of vitamins, mineral elements, and some phenolic compounds in cultivated mushrooms. J. Agric. Food Chem. 49, 2343–2348 10.1021/jf001525d [DOI] [PubMed] [Google Scholar]
- Mattila P., Lehikoinen K., Kiiskinen T., Piironen V. (1999a). Cholecalciferol and 25-hydroxycholecalciferol content of chicken egg yolk as affected by the cholecalciferol content of feed. J. Agric. Food Chem. 47, 4089–4092 10.1021/jf990183c [DOI] [PubMed] [Google Scholar]
- Mattila P., Piironen V., Bäckman C., Asunmaa A., Uusi-Rauva E., Koivistoinen P. (1992). Determination of vitamin D3 in egg yolk by high-performance liquid chromatography with diode array detection. J. Food Compost. Anal. 5, 281–290 10.1016/0889-1575(92)90062-O [DOI] [Google Scholar]
- Mattila P., Piironen V., Haapala R., Hirvi T., Uusi-Rauva E. (1997). Possible factors responsible for the high variation in the cholecalciferol contents of fish. J. Agric. Food Chem. 45, 3891–3896 10.1021/jf970243j [DOI] [Google Scholar]
- Mattila P., Piironen V., Uusi-Rauva E., Koivistoinen P. (1995a). Cholecalciferol and 25-hydroxycholecalciferol contents in fish and fish products. J. Food Compost. Anal. 8, 232–243 10.1006/jfca.1995.1017 [DOI] [Google Scholar]
- Mattila P., Ronkainen R., Lehikoinen K., Piironen V. (1999b). Effect of Household Cooking on the Vitamin D content in Fish, Eggs, and Wild Mushrooms. J. Food Compost. Anal. 12, 153–160 10.1006/jfca.1999.0828 [DOI] [Google Scholar]
- Mattila P. H., Piironen V. I., Uusi-Rauva E. J., Koivistoinen P. E. (1994). Vitamin D contents in edible mushrooms. J. Agric. Food Chem. 42, 2449–2453 10.1021/jf00047a016 [DOI] [Google Scholar]
- Mattila P. H., Piironen V. I., Uusi-Rauva E. J., Koivistoinen P. E. (1995b). Contents of cholecalciferol, ergocalciferol, and their 25-hydroxylated metabolites in milk products and raw meat and liver as determined by HPLC. J. Agric. Food Chem. 43, 2394–2399 10.1021/jf00057a015 [DOI] [Google Scholar]
- Mau J. L., Chen P. R., Yang J. H. (1998). Ultraviolet irradiation increased vitamin D2 content in edible mushrooms. J. Agric. Food Chem. 46, 5269–5272 10.1021/jf980602q [DOI] [Google Scholar]
- Max Rubner-Institut. (2008). Nationale Verzehrsstudie II. Ergebnisbericht Teil 2. Die bundesweite Befragung zur Ernaehrung von Jugendlichen und Erwachsenen (National Food Intake Study II. Results Part 2. Countrywide Assessment of Nutrition in Adolescents and Adults). Karlsruhe: Max Rubner-Institut; Available online at: http://www.was-esse-ich.de/uploads/media/NVSII_Abschlussbericht_Teil_2.pdf [Google Scholar]
- McCann J. C., Ames B. N. (2009). Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging? Am. J. Clin. Nutr. 90, 889–907 10.3945/ajcn.2009.27930 [DOI] [PubMed] [Google Scholar]
- Mellanby E. (1919). An experimental investigation on rickets. Lancet 193, 407–412 10.1016/S0140-6736(01)25465-8 [DOI] [Google Scholar]
- Mellanby E. (1976). Nutrition classics. the lancet 1:407-12, 1919. An experimental investigation on rickets. Nutr. Rev. 34, 338–340 10.1111/j.1753-4887.1976.tb05815.x [DOI] [PubMed] [Google Scholar]
- Millen A. E., Voland R., Sondel S. A., Parekh N., Horst R. L., Wallace R. B., et al. (2011). Vitamin D status and early age-related macular degeneration in postmenopausal women. Arch. Ophthalmol. 129, 481–489 10.1001/archophthalmol.2011.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K., Griffiths C. J., Martineau A. R. (2011). Vitamin D and tuberculosis. Curr. Respir. Med. Rev. 7, 435–439 10.2174/157339811798072685 [DOI] [Google Scholar]
- Mithal A., Wahl D. A., Bonjour J. P., Burckhardt P., Dawson-Hughes B., Eisman J. A., et al. (2009). Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 20, 1807–1820 10.1007/s00198-009-0954-6 [DOI] [PubMed] [Google Scholar]
- Montgomery J. L., Carr M. A., Kerth C. R., Hilton G. G., Price B. P., Galyean M. L., et al. (2002). Effect of vitamin D3 supplementation level on the postmortem tenderization of beef from steers. J. Anim. Sci. 80, 971–981 [DOI] [PubMed] [Google Scholar]
- Montgomery J. L., King M. B., Gentry J. G., Barham A. R., Barham B. L., Hilton G. G., et al. (2004). Supplemental vitamin D3 concentration and biological type of steers. II. Tenderness, quality, and residues of beef. J. Anim. Sci. 82, 2092–2104 [DOI] [PubMed] [Google Scholar]
- Montgomery J. L., Parrish F. C., Beitz D. C., Horst R. L., Huff-Lonergan E. J., Trenkle A. H. (2000). The use of vitamin D3 to improve beef tenderness. J. Anim. Sci. 78, 2615–2621 [DOI] [PubMed] [Google Scholar]
- Nesby-O'dell S., Scanlon K. S., Cogswell M. E., Gillespie C., Hollis B. W., Looker A. C., et al. (2002). Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am. J. Clin. Nutr. 76, 187–192 [DOI] [PubMed] [Google Scholar]
- Ng K., Meyerhardt J. A., Wu K., Feskanich D., Hollis B. W., Giovannucci E. L., et al. (2008). Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J. Clin. Oncol. 26, 2984–2991 10.1200/JCO.2007.15.1027 [DOI] [PubMed] [Google Scholar]
- Ng K., Scott J. B., Drake B. F., Chan A. T., Hollis B. W., Chandler P. D., et al. (2014). Dose response to vitamin D supplementation in African Americans: results of a 4-arm, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 99, 587–598 10.3945/ajcn.113.067777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnoaham K. E., Clarke A. (2008). Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int. J. Epidemiol. 37, 113–119 10.1093/ije/dym247 [DOI] [PubMed] [Google Scholar]
- Norman A. W. (2012). The history of the discovery of vitamin D and its daughter steroid hormone. Ann. Nutr. Metab. 61, 199–206 10.1159/000343104 [DOI] [PubMed] [Google Scholar]
- Norman A. W., Ishizuka S., Okamura W. H. (2001). Ligands for the vitamin D endocrine system: different shapes function as agonists and antagonists for genomic and rapid response receptors or as a ligand for the plasma vitamin D binding protein. J. Steroid. Biochem. Mol. Biol. 76, 49–59 10.1016/S0960-0760(00)00145-X [DOI] [PubMed] [Google Scholar]
- Norman A. W., Okamura W. H., Bishop J. E., Henry H. L. (2002). Update on biological actions of 1alpha,25(OH)2-vitamin D3 (rapid effects) and 24R,25(OH)2-vitamin D3. Mol. Cell. Endocrinol. 197, 1–13 10.1016/S0303-7207(02)00273-3 [DOI] [PubMed] [Google Scholar]
- Ostermeyer U., Schmidt T. (2006). Vitamin D and provitamin D in fish: determination by HPLC with electrochemical detection. Eur. Food Res. Technol. 222, 403–413 10.1007/s00217-005-0086-y [DOI] [Google Scholar]
- Ovesen L., Brot C., Jakobsen J. (2003). Food contents and biological activity of 25-hydroxyvitamin D: a vitamin D metabolite to be reckoned with? Ann. Nutr. Metab. 47, 107–113 10.1159/000070031 [DOI] [PubMed] [Google Scholar]
- Parekh N., Chappell R. J., Millen A. E., Albert D. M., Mares J. A. (2007). Association between Vitamin D and age-related macular degeneration in the third National Health and Nutrition Examination Survey, 1988 through 1994. Arch. Ophthalmol. 125, 661–669 10.1001/archopht.125.5.661 [DOI] [PubMed] [Google Scholar]
- Parrish D. B. (1979). Determination of vitamin D in foods: a review. CRC Crit. Rev. Food Sci. Nutr. 12, 29–57 10.1080/10408397909527272 [DOI] [PubMed] [Google Scholar]
- Perrine C. G., Sharma A. J., Jefferds M. E. D., Serdula M. K., Scanlon K. S. (2010). Adherence to vitamin D recommendations among US infants. Pediatrics 125, 627–632 10.1542/peds.2009-2571 [DOI] [PubMed] [Google Scholar]
- Pfeifer M., Begerow B., Minne H. W., Abrams C., Nachtigall D., Hansen C. (2000). Effects of a short-term vitamin D and calcium supplementation on body sway and secondary hyperparathyroidism in elderly women. J. Bone Miner. Res. 15, 1113–1118 10.1359/jbmr.2000.15.6.1113 [DOI] [PubMed] [Google Scholar]
- Pfeifer M., Begerow B., Minne H. W., Suppan K., Fahrleitner-Pammer A., Dobnig H. (2009). Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos. Int. 20, 315–322 10.1007/s00198-008-0662-7 [DOI] [PubMed] [Google Scholar]
- Phillips K. M., Ruggio D. M., Horst R. L., Minor B., Simon R. R., Feeney M. J., et al. (2011). Vitamin D and sterol composition of 10 types of mushrooms from retail suppliers in the United States. J. Agric. Food Chem. 59, 7841–7853 10.1021/jf104246z [DOI] [PubMed] [Google Scholar]
- Pike J. W., Lee S. M., Meyer M. B. (2014). Regulation of gene expression by 1,25-dihydroxyvitamin D in bone cells: exploiting new approaches and defining new mechanisms. Bonekey Rep. 3, 482 10.1038/bonekey.2013.216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz S., Gaksch M., O'Hartaigh B., Tomaschitz A., März W. (2013a). The role of vitamin D deficiency in cardiovascular disease: where do we stand in 2013? Arch. Toxicol. 87, 2083–2103 10.1007/s00204-013-1152-z [DOI] [PubMed] [Google Scholar]
- Pilz S., Kienreich K., Rutters F., De Jongh R., Van Ballegooijen A. J., Grubler M., et al. (2013b). Role of vitamin D in the development of insulin resistance and type 2 diabetes. Curr. Diab. Rep. 13, 261–270 10.1007/s11892-012-0358-4 [DOI] [PubMed] [Google Scholar]
- Pittas A. G., Lau J., Hu F. B., Dawson-Hughes B. (2007). The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 92, 2017–2029 10.1210/jc.2007-0298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt B. S. (1956). Sir Edward Mellanby, 8 April 1884-30 January 1955. Br. J. Nutr. 10, 177–181 [DOI] [PubMed] [Google Scholar]
- Prentice A. (2008). Vitamin D deficiency: a global perspective. Nutr. Rev. 66, S153–S164 10.1111/j.1753-4887.2008.00100.x [DOI] [PubMed] [Google Scholar]
- Prince R. L., Austin N., Devine A., Dick I. M., Bruce D., Zhu K. (2008). Effects of ergocalciferol added to calcium on the risk of falls in elderly high-risk women. Arch. Int. Med. 168, 103–108 10.1001/archinternmed.2007.31 [DOI] [PubMed] [Google Scholar]
- Ramagopalan S. V., Heger A., Berlanga A. J., Maugeri N. J., Lincoln M. R., Burrell A., et al. (2010). A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 20, 1352–1360 10.1101/gr.107920.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Lopez E., Jansen T., Ivaskevicius V., Kahles H., Klepzig C., Oldenburg J., et al. (2006). Protection from type 1 diabetes by vitamin D receptor haplotypes. Ann. N Y. Acad. Sci. 1079, 327–334 10.1196/annals.1375.050 [DOI] [PubMed] [Google Scholar]
- Redzic M., Lewis R. M., Thomas D. T. (2013). Relationship between 25-hydoxyvitamin D, muscle strength, and incidence of injury in healthy adults: a systematic review. Nutr. Res. 33, 251–258 10.1016/j.nutres.2013.02.007 [DOI] [PubMed] [Google Scholar]
- Roberts J. S., Teichert A., McHugh T. H. (2008). Vitamin D2 formation from post-harvest UV-B treatment of mushrooms (Agaricus bisporus) and retention during storage. J. Agricultur. Food Chem. 56, 4541–4544 10.1021/jf0732511 [DOI] [PubMed] [Google Scholar]
- Romagnoli E., Mascia M. L., Cipriani C., Fassino V., Mazzei F., D'Erasmo E., et al. (2008). Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J. Clin. Endocrinol. Metab. 93, 3015–3020 10.1210/jc.2008-0350 [DOI] [PubMed] [Google Scholar]
- Rosen C. J., Adams J. S., Bikle D. D., Black D. M., Demay M. B., Manson J. E., et al. (2012). The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr. Rev. 33, 456–492 10.1210/er.2012-1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A. C., Manson J. E., Abrams S. A., Aloia J. F., Brannon P. M., Clinton S. K., et al. (2011). The 2011 report on dietary reference intakes for calcium and vitamin d from the institute of medicine: what clinicians need to know. J. Clin. Endocrinol. Metab. 96, 53–58 10.1210/jc.2010-2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin N., Ali F., Hasan Z., Rao N., Aqeel M., Mahmood F. (2013). Vitamin D accelerates clinical recovery from tuberculosis: results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis'. BMC Infect. Dis. 13:22 10.1186/1471-2334-13-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A., Walther B. (2013). Natural vitamin D content in animal products. Adv. Nutr. 4, 453–462 10.3945/an.113.003780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schottker B., Herder C., Rothenbacher D., Perna L., Muller H., Brenner H. (2013). Serum 25-hydroxyvitamin D levels and incident diabetes mellitus type 2: a competing risk analysis in a large population-based cohort of older adults. Eur. J. Epidemiol. 28, 267–275 10.1007/s10654-013-9769-z [DOI] [PubMed] [Google Scholar]
- Schroten N. F., Ruifrok W. P., Kleijn L., Dokter M. M., Sillje H. H., Lambers Heerspink H. J., et al. (2013). Short-term vitamin D3 supplementation lowers plasma renin activity in patients with stable chronic heart failure: an open-label, blinded end point, randomized prospective trial (VitD-CHF trial). Am. Heart J. 166, 357–364.e352 10.1016/j.ahj.2013.05.009 [DOI] [PubMed] [Google Scholar]
- Schwalfenberg G. K. (2011). A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 55, 96–108 10.1002/mnfr.201000174 [DOI] [PubMed] [Google Scholar]
- Sohl E., Van Schoor N. M., De Jongh R. T., Visser M., Deeg D. J., Lips P. (2013). Vitamin d status is associated with functional limitations and functional decline in older individuals. J. Clin. Endocrinol. Metab. 98, E1483–E1490 10.1210/jc.2013-1698 [DOI] [PubMed] [Google Scholar]
- Solomon A. J., Whitham R. H. (2010). Multiple sclerosis and vitamin D: a review and recommendations. Curr. Neurol. Neurosci. Rep. 10, 389–396 10.1007/s11910-010-0131-5 [DOI] [PubMed] [Google Scholar]
- Strobel N., Buddhadasa S., Adorno P., Stockham K., Greenfield H. (2013). Vitamin D and 25-hydroxyvitamin D determination in meats by LC-IT-MS. Food Chem. 138, 1042–1047 10.1016/j.foodchem.2012.08.041 [DOI] [PubMed] [Google Scholar]
- Sundar I. K., Rahman I. (2011). Vitamin D and susceptibility of chronic lung diseases: role of epigenetics. Front. Pharmacol. 2:50 10.3389/fphar.2011.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Okano T., Ayame M., Yoshikawa H., Teraoka S., Murakami Y., et al. (1984). High-performance liquid chromatographic determination of vitamin D3 in fish liver oils and eel body oils. J. Nutr. Sci. Vitaminol. 30, 421–430 10.3177/jnsv.30.421 [DOI] [PubMed] [Google Scholar]
- Takeuchi A., Okano T., Sayamoto M., Sawamura S., Kobayashi T., Motosugi M., et al. (1986). Tissue distribution of 7-dehydrocholesterol, vitamin D3 and 25-hydroxyvitamin D3 in several species of fishes. J. Nutr. Sci. Vitaminol. 32, 13–22 10.3177/jnsv.32.13 [DOI] [PubMed] [Google Scholar]
- Tamez H., Thadhani R. I. (2012). Vitamin D and hypertension: an update and review. Curr. Opin. Nephrol. Hypertens. 21, 492–499 10.1097/MNH.0b013e3283557bf0 [DOI] [PubMed] [Google Scholar]
- Tarroni P., Villa I., Mrak E., Zolezzi F., Mattioli M., Gattuso C., et al. (2012). Microarray analysis of 1,25(OH)2D3 regulated gene expression in human primary osteoblasts. J. Cell. Biochem. 113, 640–649 10.1002/jcb.23392 [DOI] [PubMed] [Google Scholar]
- Teichmann A., Dutta P. C., Staffas A., Jägerstad M. (2007). Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: effects of UV irradiation. LWT Food Sci. Technol. 40, 815–822 10.1016/j.lwt.2006.04.003 [DOI] [Google Scholar]
- Tieland M., Brouwer-Brolsma E. M., Nienaber-Rousseau C., Van Loon L. J., De Groot L. C. (2013). Low vitamin D status is associated with reduced muscle mass and impaired physical performance in frail elderly people. Eur. J. Clin. Nutr. 67, 1050–1055 10.1038/ejcn.2013.144 [DOI] [PubMed] [Google Scholar]
- Tippetts M., Martini S., Brothersen C., McMahon D. J. (2012). Fortification of cheese with vitamin D3 using dairy protein emulsions as delivery systems. J. Dairy Sci. 95, 4768–4774 10.3168/jds.2011-5134 [DOI] [PubMed] [Google Scholar]
- Trang H. M., Cole D. E., Rubin L. A., Pierratos A., Siu S., Vieth R. (1998). Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am. J. Clin. Nutr. 68, 854–858 [DOI] [PubMed] [Google Scholar]
- Trenerry V. C., Plozza T., Caridi D., Murphy S. (2011). The determination of vitamin D3 in bovine milk by liquid chromatography mass spectrometry. Food Chem. 125, 1314–1319 10.1016/j.foodchem.2010.09.097 [DOI] [Google Scholar]
- Troesch B., Hoeft B., McBurney M., Eggersdorfer M., Weber P. (2012). Dietary surveys indicate vitamin intakes below recommendations are common in representative Western countries. Br. J. Nutr. 108, 692–698 10.1017/S0007114512001808 [DOI] [PubMed] [Google Scholar]
- Tsur A., Feldman B. S., Feldhammer I., Hoshen M. B., Leibowitz G., Balicer R. D. (2013). Decreased serum concentrations of 25-hydroxycholecalciferol are associated with increased risk of progression to impaired fasting glucose and diabetes. Diabetes Care 36, 1361–1367 10.2337/dc12-1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Agriculture. (2013). USDA National Nutrient Database for Standard Reference, Release 26. Nutrient Data Laboratory Home Page. Available online at: http://www.ars.usda.gov/ba/bhnrc/ndl (Accessed February 10, 2014).
- Van Belle T. L., Gysemans C., Mathieu C. (2013). Vitamin D and diabetes: the odd couple. Trends Endocrinol. Metab. 24, 561–568 10.1016/j.tem.2013.07.002 [DOI] [PubMed] [Google Scholar]
- van Rossum C. T. M., Fransen H. P., Verkaik-Kloosterman J., Buurma-Rethans E. J. M., Ocke M. C. (2011). Dutch National Food Consumption Survey 2007–2010: Diet of Children and Adults Aged 7 to 69 Years. Bilthoven: RIVM [Google Scholar]
- Wacker M., Holick M. (2013). Vitamin D—effects on skeletal and extraskeletal health and the need for supplementation. Nutrients 5, 111–148 10.3390/nu5010111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D., Sidhom G., Whiting S. J., Rousseau D., Vieth R. (2008). The bioavailability of vitamin D from fortified cheeses and supplements is equivalent in adults. J. Nutr. 138, 1365–1371 [DOI] [PubMed] [Google Scholar]
- Wahl D. A., Cooper C., Ebeling P. R., Eggersdorfer M., Hilger J., Hoffmann K., et al. (2012). A global representation of vitamin D status in healthy populations. Arch. Osteoporos. 7, 155–172 10.1007/s11657-012-0093-0 [DOI] [PubMed] [Google Scholar]
- Webb A. R., Kline L., Holick M. F. (1988). Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in boston and edmonton will not promote vitamin D3 synthesis in human skin. J. Clin. Endocrinol. Metab. 67, 373–378 10.1210/jcem-67-2-373 [DOI] [PubMed] [Google Scholar]
- Welsh J. (2012). Cellular and molecular effects of vitamin D on carcinogenesis. Arch. Biochem. Biophys. 523, 107–114 10.1016/j.abb.2011.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. H. (2012). Vitamin D metabolism and signaling in the immune system. Rev. Endocr. Metab. Disord. 13, 21–29 10.1007/s11154-011-9195-z [DOI] [PubMed] [Google Scholar]
- Windaus A., Schenck F., Von Werder F. (1936). Über das antirachitisch wirksame Bestrahlungsprodukt aus 7-Dehydrocholesterin. Hoppe-Seyler's Zeitschrift für Physiologische Chemie 241, 100–103 10.1515/bchm2.1936.241.1-3.100 [DOI] [Google Scholar]
- Zadshir A., Tareen N., Pan D., Norris K., Martins D. (2005). The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn. Dis. 15, S5-97–S95-101 [PubMed] [Google Scholar]
- Zhao Y., Sun Y., Ji H. F., Shen L. (2013). Vitamin D levels in Alzheimer's and Parkinson's diseases: a meta-analysis. Nutrition 29, 828–832 10.1016/j.nut.2012.11.018 [DOI] [PubMed] [Google Scholar]