Abstract
Ca2+ entry is essential for regulating vital physiological functions in all neuronal cells. Although neurons are engaged in multiple modes of Ca2+ entry that regulates variety of neuronal functions, we will only discuss a subset of specialized Ca2+-permeable non-selective Transient Receptor Potential Canonical (TRPC) channels and summarize their physiological and pathological role in these excitable cells. Depletion of endoplasmic reticulum (ER) Ca2+ stores, due to G-protein coupled receptor activation, has been shown to activate TRPC channels in both excitable and non-excitable cells. While all seven members of TRPC channels are predominately expressed in neuronal cells, the ion channel properties, mode of activation, and their physiological responses are quite distinct. Moreover, many of these TRPC channels have also been suggested to be associated with neuronal development, proliferation and differentiation. In addition, TRPCs also regulate neurosecretion, long-term potentiation and synaptic plasticity. Similarly, perturbations in Ca2+ entry via the TRPC channels have been also suggested in a spectrum of neuropathological conditions. Hence, understanding the precise involvement of TRPCs in neuronal function and in neurodegenerative conditions would presumably unveil avenues for plausible therapeutic interventions for these devastating neuronal diseases.
Keywords: Ca2+, TRPC channels, neuronal function, neurodegenerative diseases
1. Introduction
In neurons, Ca2+ is essential for a variety of physiological processes that regulate functions, such as gene transcription to neuronal growth, survival and even differentiation [1]. Ca2+ homeostasis is a tightly regulated process throughout the nervous system in order to maintain the tight Ca2+ balance between the intra- and extra-cellular fluids of the neuronal cells. Too much or too little Ca2+ can be deadly to these neurons, so the Ca2+ levels are carefully controlled in and outside of the cell. Such disturbances in Ca2+ homeostasis have been involved in neurodegenerative diseases, such as Parkinson’s, Alzheimer’s and Huntington’s [2,3], which is mainly due to the high dependence of Ca2+ signaling in various neuronal cells essential for their function [4]. Ca2+ mobilization in neuronal cells is tightly regulated by different Ca2+ channels and pumps in the plasma membrane and in the organelle membranes. An increase of intracellular Ca2+, specifically due to Ca2+ release from intracellular ER stores, as well as Ca2+ entry across the plasma membrane via various ion channels, including the endogenous store-operated Ca2+ entry (SOCE) channels, has gained much attention in recent years and will be the focus of this review.
Although several mechanisms are known to control Ca2+ influx across the plasma membrane, Ca2+ influx could be more directly controlled either by store-depletion per se (through SOCE), or by the activation of the G-protein coupled receptors, or by the alterations in the membrane potential (through the activation of the voltage-gated Ca2+ channels). Since Ca2+ regulate such diverse processes, it is hard to pin-point a physiological function to one particular Ca2+ channel, and factors, such as amplitude, the amount of cytosolic Ca2+, the spatial distribution of individual Ca2+ channels and their modulators, may indeed be critical for regulating these diverse neuronal processes [5]. Although the significance of voltage-gated Ca2+ channels in neuronal cells is quite apparent, recent evidence have been gaining momentum to suggest an equally important role of SOCE channels. Ca2+ influx through SOCE mechanism is not only essential for the refilling of the ER Ca2+ stores, but is also critical for maintaining [Ca2+]i that regulates neuronal functions, such as neurosecretion, sensation, long-term potentiation, synaptic plasticity, gene regulation, as well as neuronal growth and differentiation. SOCE channels also maintain a prolonged increase in cytosolic Ca2+ upon stimulation that is not only essential for the refilling of the ER Ca2+ stores, but can also activate many of the Ca2+-dependent processes that regulates neuronal functions. Two major families of proteins, Transient Receptor Potential Canonical (TRPC) and ORAI channels, have been identified in various cells including neurons, which have been shown to be critical for SOCE. Although the molecular components of SOCE have not been conclusively identified, especially in neuronal cells, ample evidence suggests the involvement of TRPCs in this process. Available data indicate that TRPC proteins initiate Ca2+ entry pathways and are essential in maintaining cytosolic, ER and mitochondrial Ca2+ levels. A number of biological functions have also been assigned to these TRPC proteins and are thus presented in this review [6,7,8,9].
2. TRPC Channels Properties and Their Mode of Activation
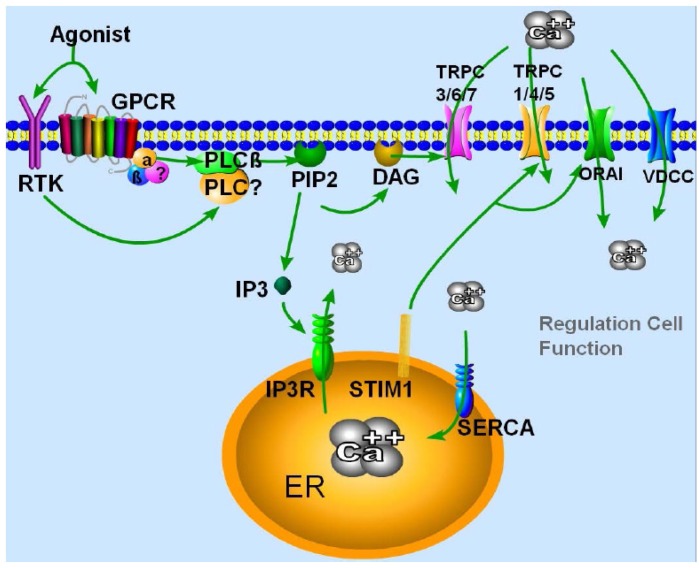
Transient receptor potential (TRP) channels constitute a family of ion channels that are divided into TRPC (canonical/classical), TRPV (vanilloid) and TRPM (melastatin) sub-families. Importantly, all members of the TRP family are moderately conserved and share some homology among them, especially among each group member [10]. TRPC channels are an important Ca2+ influx pathway that is activated by store depletion per se and is present in most cell types. Generally, the binding of a hormone or a growth factor to its receptor is localized at the plasma membrane (PM). Activation of the G-protein (Gq/11) leads to PIP2 hydrolysis, which generates IP3 and DAG [11] (Figure 1). IP3 binds to the IP3R (IP3 receptor) and initiates Ca2+ release from the ER stores, which empties the Ca2+ pool and initiates Ca2+ dissociation from the EF hand domain of the stromal interaction molecule-1 (STIM1) protein [12] (Figure 1). Importantly, STIM1 has been identified as the molecular link between ER Ca2+ store depletion and SOCE activation. Oligomerization of STIM1 occurs, followed by STIM1 translocation to the ER-PM junction, where it interacts with TRPCs and with some voltage-gated Ca2+ channels to modulate Ca2+ influx [13,14,15,16].
Figure 1.
Activation and regulation of TRPC channels. Binding of an agonist to RTKs or GPCR, initiate a signaling cascade, causing PLC-mediated hydrolysis of phosphatidylinositol (4,5) bisphosphate (PIP2) to inositol (1,4,5)-triphosphate (IP3) and diacylglycerol (DAG). DAG could directly activate certain TRPC channels. IP3 binds to IP3R, a ligand-gated ion channel, which leads to the release of Ca2+ from the internal ER stores. Depletion of Ca2+ from the internal stores, in turn, allows STIM1 to aggregate, followed by the activation of the TRPC or ORAI channels in the plasma membrane, which allows Ca2+ to enter the cell that orchestrates cellular functions. It also could depolarize the membrane and activate the voltage-dependent Ca2+ channel (VDCC). SERCA pumps are shown to work concertedly to maintain steady-state levels of intracellular Ca2+.
Although TRPC family contains 7 members (C1–C7) (Table 1) a unique property of these channels is that they all function as non-selective Ca2+ entry channels, with somewhat distinct mode of activation (Figure 1). However, based on their similarities with regard to the structure-function relationships, TRPC family can be further divided into two sub-groups. The first group consists of TRPC1/TRPC4/TRPC5 channels that can be regulated by receptor stimulation as well as by store-depletion, of which the latter requires the channel association with STIM1 and/or Orai1 proteins, thus are suggested as components of the SOCE channels [17,18]. On the other hand the second group comprises of TRPC3/TRPC6/TRPC7 that are activated by receptor stimulation [19]. TRPC2 is a pseudogene in humans, but has a role in rodent behavior and pheromone sensing [20]. It is however important to note that, although one can pharmacologically separate these channels in vitro, their activation in a physiological context is always linked to PLC mediated signaling following stimulation of membrane G-protein coupled receptors (GPCRs) or receptor tyrosine kinases (RTKs). Since TRPC proteins are capable of forming functional channels by heteromeric interactions [21], the receptor- or store-dependent activation thus outlines a common feature in channel activation [22]. Furthermore, regardless, as how these TRPC channels are regulated, they all contribute towards Ca2+ entry, which is fundamental for various neuronal functions.
Table 1.
Expression of the TRPC channel and its properties in neuronal cells.
| Subfamily | Cellular expression in Neurons | Ion channel properties | References |
|---|---|---|---|
| TRPC1 | Brain, retina, peripheral axons and the mechanosensory terminals | Non selective, 16 pS current conductance, non-rectifying or mildly inward rectifying with a reverse potential of about +10 mV | [27,28,29,30] |
| TRPC2 | Dendritic tips of the vomeronasal sensory neurons and spermatozoa (mouse) | Partially selective with a Pca/PNa ratio of 2.7, 42 pS current conductance, non-rectifying | [31,32] |
| TRPC3 | Central nervous system (CNS) | Non selective, 60–66 pS current conductance, slightly dual (inward and outward) rectifying with a reverse potential of +5 mV | [33,34,35,36] |
| TRPC4 | CNS, retina | Non selective, 30–42 pS current conductance, dual (inward and outward) rectifying with a reverse potential of about +10 mV | [37,38,39,40] |
| TRPC5 | Brain, especially in fetal brain | Partially selective with a Pca/PNa ratio of 9, 47–66 pS current conductance, dual (inward and outward) rectifying as a homomer, outwardly rectifying when expressed with TRPC1 or TRPC4 | [30,38,40,41,42,43] |
| TRPC6 | Brain, retina | Partially selective with a Pca/PNa ratio of 5, 28–37 pS current conductance, dual (inward and outward) rectifying or inward rectifying | [33,35,44,45,46] |
| TRPC7 | CNS (human); weak in CNS (mouse) | Partially selective with a Pca/PNa ratio of 5.9, 25–50 pS current conductance, slightly outward rectifying | [35,44,47,48] |
Importantly, in neuronal cells, Ca2+ entry via the G-protein coupled mechanism has also been implicated in the shaping of action potentials, synaptic transmission and sensory transduction [23,24]. Additionally, changes in [Ca2+]i are also known to regulate the motility of many cellular structures, including the axonal growth cones [25] and the dendritic filopodia of developing neurons [26]. Thus, it can be anticipated that TRPC channels may have a significant role in regulating these fundamental neuronal processes.
3. Physiological Function of TRPCs in Neuronal Cells
Ca2+ plays a vital role in regulating various neuronal functions, like, neuronal survival, growth and differentiation. Ca2+ also acts as a ubiquitous secondary messenger to modulate neuronal functions and also plays an important role in the relay of information via membrane depolarization [4,49,50]. TRPCs are predominantly expressed in neuronal cells and, thus, could be prime contributors in regulating fundamental neuronal functions via regulating the Ca2+ flux in these neuronal cells [50]. Recent studies have shown an association of TRPC channels with neuronal development, synaptic functions and neuronal differentiation [51], and thus, they could be critical for neuronal function, as described below.
3.1. TRPC1
During neuronal development, every stage is involved in the compartmentalization of distinct neuron that utilizes Ca2+ and its downstream signaling molecules to perform varied complex processes that are essential for their development. Intracellular Ca2+ has diverse effects in shaping the axons and dendritic processes. Importantly, it has been shown that TRPC1 is highly expressed in embryonic CNS in mammals, compared with adults, indicating that TRPC1 could be involved in early development and the proliferation of neurons [52]. However, TRPC1 knockout mice are born healthy and survive in a control environment, but not in stressed conditions, without any moderate neuronal defects, suggesting that in the absence of TRPC1, perhaps other TRPC channels might compensate for its function [53]. In addition, there are slight discrepancies with regard to the involvement of TRPC channels in neuronal proliferation vs. regeneration, since TRPCs shows different levels of expression in neuronal populations that control cell proliferation, but not regeneration. Furthermore, the differences could be attributed to different neuronal populations that could have different expressions of TRPC isoforms, which could form heteromultimers and could therefore function differently in different tissues [43,53,54]. Stimulation of TRPC1 via different agonists could very well also have a different physiological response that could be attributed to the spatial temporal resolution of Ca2+ signaling in neurons. Interestingly, TRPC1, TRPC2 and TRPC4 have been shown to exhibit higher expression, whereas TRPC3 and TRPC6 expressions were decreased in neuronal stem cell (NSC) populations [55,56]. These results suggest that perhaps in NSC cells, TRPC1 could associate with TRPC4 rather than TRPC3 or TRPC5 and, thus, could bring about a different physiological function (probably regeneration). Interestingly, in most of the cases, the survival and proliferation induced by TRPC1 appears to be dependent on its Ca2+ entry; however, it remains to be seen if TRPC1 could potentially regulate other proteins, independent of its Ca2+ influx ability. TRPC1 has also been shown to be expressed in perisynaptic regions of the synapse and was physically associated with mGluR1 [57]. Furthermore, expression of a dominant-negative TRPC1-pore mutant (F561A) in cerebellar Purkinje neurons resulted in a 49% reduction of mGluR-evoked slow excitatory postsynaptic currents (EPSCs), whereas fast transmission mediated by AMPA-type glutamate receptors remained unaffected, indicating that mGluR1 receptor activation is essential for the gating of TRPC1. However, another report showed no localization of TRPC1 at the synapse, and no functional activity of TRPC1 was observed in these regions [43]. These discrepancies can again be due to several factors, and recent reports have shown that membrane targeting and regulation of TRPC1 was dependent on its association with lipid rafts and its functional interaction with STIM1 and caveolin1 [13]; however, this has not been confirmed in neuronal cells.
3.2. TRPC2
TRPC2 is a unique member of the TRPC sub-family, since its expression in mammals is lost and is considered a pseudogene in higher mammals [58,59]. Relatively little is known about its physiological significance and interaction with other Ca2+ regulating signaling molecules [60]. In rodents, the loss of TRPC2 expression results in the reduction in their ability to detect pheromones, leading to gender-specific behaviors [60,61]. TRPC2 is present in the vomeronasal organs (VNO), which are responsible for detecting water-soluble pheromones. Liman et al. showed for the first time the role of TRPC2 in vomeronasal sensory neurons, and ultrastructural analysis revealed that VNO sensory receptor cells express TRPC2 [32]. TRPC2 knockout mice had a phenotype in which the sensory response to pheromones in urine was abolished, and the male failed to recognize the difference between male and female [32,62,63]. TRPC2 knockout male mice started to show sexual behavior towards other male mice [63]. The female TRPC2 knockout mice started showing male characteristic behaviors, such as mounting, pelvic thrust and ultra-sonic vocalizations [60,64]. Although humans do not express TRPC2 channels and do not have similar VNO neurons (they are degenerated), they have a functional olfactory system, and it could be suggested that perhaps another TRPC isoform could decode olfactory cues and could be responsible for normal and abnormal social behavior in humans [65]. Thus, it would be interesting to probe this research further and establish if other TRPC isoforms are involved in abnormal human behaviors [66,67].
3.3. TRPC3
The expression of TRPC3 is highest in the brain, but the importance of TRPC3 in the nervous system is not completely understood. Initial studies by Li et al. [68] revealed a role for TRPC3 channels in BDNF signaling. Moreover, it was shown that in pontine neurons, Trk receptors and TRPC3 are expressed during the same developmental stages of the brain, and the BDNF-stimulated non-selective cationic current (IBDNF) was mediated by TRPC3. In another study using mouse cerebellar Purkinje cells, it was established that TRPC3 is required for the slow synaptic potentials and inward currents evoked by group I metabotropic receptor (mGluR1) synaptic signaling [60]. Similarly, in cerebellar granule neurons (CGNs), BDNF-induced elevation of Ca2+ contributes to axonal growth cone guidance [69]. The specific involvement of TRPC3 in BDNF-induced growth cone plasticity was further demonstrated by silencing endogenous TRPC3 or by expressing a dominant-negative TRPC3 that inhibited growth cone formation. Furthermore, in the pyramidal neurons of rat hippocampus an involvement of TRPC3 channels in BDNF-induced dendritic spine formation has also been reported [70]. In H19-7 rat hippocampal neuronal cells, TRPC3 along with TRPC1 was significantly increased under differentiating conditions, whereas the expression of TRPC4 and TRPC7 were decreased [52]. Similarly, overexpression of a dominant-negative form of TRPC3 or TRPC6 affects the nerve-growth-cone guidance by BDNF [71]. Thus, this reciprocal regulation of TRPC channel expression in these neurons suggests their developmentally important function.
Ca2+ entry via the G-protein-coupled mechanism has also been implicated in the shaping of action potentials, synaptic transmission and sensory transduction [72,73,74]. In mice lacking TRPC3, but not TRPC1, TRPC4 or TRPC6, the mGluR1-mediated slow synaptic potentials were completely absent. This abnormality in the glutamate neurotransmission in the postsynaptic neurons has resulted in impaired walking behavior, thus establishing a fundamental role for TRPC3 channels in motor coordination [75,76,77]. In yet another study, to identify crucial gene products implicated in cerebral ataxia, a phenotype-driven dominant mutagenesis screen was performed, and an ataxic mouse mutant by the name of moonwalker (Mwk) mice was identified. These mice exhibited a gain-of-function mutation (T635A) and maintained sustained activation of TRPC3 channels, perhaps due to the lack of negative feedback regulation by PKCγ-mediated phosphorylation of TRPC3. As a result, diminished dendritic barbarization and progressive loss of Purkinje neurons were observed. Although these studies contradict one another, they still suggest that TRPC3 could have a pivotal role in Purkinje neurons, and future studies are needed to confirm the role of TRPC3 in ataxia. Another possibility could be that in both of these conditions, Ca2+ signaling is altered, and as a tight balance of Ca2+ signaling is essential for neuronal function, they might have different effects. Corroborating these results is another study with a TRPC3 knockout mouse model, where the TRPC3 promoter region was disrupted. These mouse exhibit atrophy and progressive paralysis, suggesting an obligatory role of TRPC3 channels in neuronal signaling, differentiation and development [78]. Importantly, TRPC3 has been shown to associate with SNARE complex proteins [79], indicating that TRPC3 could be important for neurosecretion. Furthermore, the receptor for activated C-kinase-1 (RACK1), a multifunctional scaffolding protein known to be a key regulator of various signaling cascades in the CNS, have been shown to interact with TRPC3 in cultured rat hippocampal neurons [80]. However, the functional implications of such an interaction are yet to be defined. Interestingly, TRPC3 in astrocyte contributes to the critical homeostatic functions necessary for the metabolic and trophic support of neurons in response to neuronal injury and inflammatory activation of glia [81].
3.4. TRPC4
TRPC4 has also been shown to be highly expressed in excitable cells and is involved in the response to neural injury, the regulation of neurite outgrowth and regulating neuronal exocytosis [82]. TRPC4 has also been shown to be co-expressed with TRPC5 in CA1 pyramidal neurons of the hippocampus, which play an important role in neuronal Ca2+ homeostasis [71]. In gastrointestinal pacemaker cells and in mouse visceral smooth muscle cells, TRPC4 is also important for the control of muscarinic stimulation [83]. ATP induces an increased expression of TRPC4, which requires cAMP response element-binding protein (CREB) phosphorylation [84]. Studies by Huang et al. 2007 have further shown that TRPC4 expression is restricted to granule and their precursor cell in rat cerebellum, suggesting that TRPC4 is perhaps important for proper granule cell development; however, its function in vivo is still not well defined. TRPC4 is also shown to have a role in acute and delayed neuronal injury in focal cerebral ischemia [85], suggesting that TRPC4 could be a viable target, and its inhibition could protect against cerebral ischemia.
3.5. TRPC5
TRPC5 also plays an important role in the CNS and is important in neuronal function. TRPC5 is mainly involved in the regulation of hippocampal neurite length and growth cone morphology in young rat hippocampal neurons [60,86]. TRPC5 also regulates neurite outgrowth [87]. In neurons, BDNF triggers Ca2+ release from the internal stores via activation of the PLC-γ-IP3 pathway, which, in turn, activates the TRPC channel and allows the enhanced elevation of Ca2+, which is required to trigger the attractive turning of the neurons. In cultured hippocampal neurons, the expression of DN-TRPC5 elevates neurite extension, whereas overexpression of wild-type TRPC5 inhibits neurite growth [86]. Hence, TRPC5-containing channels may allow a larger Ca2+ influx than TRPC3/TRPC6 channels, leading to neurite growth inhibition [36,86], whereas a modest level of Ca2+ influx through TRPC3/TRPC6 channels is sufficient to trigger growth-cone tuning. Thus, by allowing different patterns of Ca2+ influx, diverse TRPC channels may thus carry out distinct functions at the growth cone. Axon formation via CAMPKK activation of CAMPK was suppressed with TRPC5 knockdown [88]. Studies by Wu G. et al. identified TRPC5 protein on the cell bodies of CGNs and demonstrated TRPC5 channels as a critical modulator of this PLC-pathway-mediated neurite outgrowth [89]. C5 knockout mice were shown to exhibit diminished innate fear levels in response to innately aversive stimuli. It has been reasoned that the lack of TRPC5 channel potentiation by Group I mGluRs and/or CCK2 receptors and the subsequent lack of membrane depolarization prevents the transmission of information to output neurons of the innate fear circuitry, thus resulting in the above-mentioned fear-related behavior [90]. Yan et al. in 2009 showed that TRPC5 channels have a role in generating Ca2+-activated slow after depolarization (sADP) currents signaled by muscarinic receptors [91].
3.6. TRPC6
TRPC6 is widely expressed in the cardiac neurons [92], in retinal ganglion cells [93], in the neurons of olfactory epithelium [94] and in parts of the brain, such as cortex, substantia nigra, hippocampus and cerebellum [95,96]. TRPC6 transgenic mice (that overexpress a mutant TRPC6) showed enhancement in spine formation and spatial learning and memory in Morris water maze [97,98]. Activation of Neurontin receptors by substance-P in the noradrenergic A7 neurons has been shown to bring about a TRPC6-specific non-selective cationic conductance, thus providing evidence for the involvement of TRPC6 channels in nociception [99]. TRPC6 in neurons has been shown to co-operate with other TRP channels, in a heteromeric assembly, to regulate critical neuronal functions. The significance of heteromeric TRPC6 channels in brain development is underscored by the finding that, in embryonic rat brain, TRPC6 physically associates with TRPC1, TRPC4 and TRPC5, respectively [43]. In dorsal root ganglion (DRG) neurons, TRPC6 co-operates with TRPC1 and TRPV4 to regulate nociception [53]. TRPC6, in conjunction with TRPC3, is involved in growth-cone guidance [36]. In rat cerebellar granule neurons, BDNF-induced activation of TrkB receptors resulted in a Ca2+-dependent growth-cone turning. BDNF-induced Ca2+ influx was shown to be mediated via TRPC3/TRPC6 channels. In the primary mid-brain neurons of rat, TRPC5 and TRPC6 co-localized with PDGF-βR and were found to regulate TRPC6 silencing, also abolishing the effect of BDNF on spine formation. This effect of TRPC6 channels on dendritic spine formation was largely mediated by the activation of the CaMKIV-CREB pathway [98].
3.7. TRPC7
TRPC7 is expressed in the nervous system (dorsal root ganglion cells), keratinocytes, uterine myometrium and in leukemia cells [60]. During pregnancy, in the uterus [100], and during development in dorsal root ganglion cells, the TRPC7 expression changes [101]. TRPC7 in rodents via a calcineurin-dependent pathway mediates the angiotensin II-induced myocardial apoptosis [102]. PGE2-induced apoptosis in K562 human leukemia cells is regulated by TRPC7 [103]. Ablating the expression of both TRPC6 and TRPC7 eliminated the intrinsic light response of the M1-subtype of melanopsin-expressing, intrinsically-photosensitive retinal ganglion cells [104].
4. TRPC Channels in Neurodegenerative Diseases
Changes in the intracellular Ca2+ concentration stimulate a number of intracellular events and could either trigger or inhibit cell death process, leading to neuronal injury. Furthermore, neuronal injury is a consequence of both the increase and decrease of cytosolic Ca2+ [5,105], and disturbances in Ca2+ homeostasis have been implicated in neurodegenerative diseases, such as, PD, AD and HD [106,107,108,109,110,111]. Although it is not surprising that disturbances in Ca2+ signaling pathways underlie neuronal loss, the cellular mechanism(s) underlying neurodegeneration remains to be elucidated [112]. However, as many factors involved in neuronal function are dependent on Ca2+ signaling, it could be anticipated that the loss of these critical functions could make these neurons vulnerable [5,105].
Several factors that contribute towards neuronal injury, including the generation of free radicals, the impairment of mitochondrial function, ER stress and apoptosis, have been proposed to be regulated by alterations in cytosolic Ca2+. Additionally, some of these factors have also been shown to be involved in the activation of TRPC channels and their regulator, STIM1 [113,114,115,116]. TRPC1 has been shown to be activated by STIM1, and recently, STIM1 has been shown to be activated by ROS [117,118]. Furthermore, as neurons are heavily dependent on mitochondrial function and have a high requirement for ATP, they could be of high importance, and Ca2+ entry has been shown to be vital for ATP synthesis. Similarly, as a byproduct of increased mitochondrial function, the generation of reactive oxygen species (ROS) is increased, which may further advance these neurons towards degeneration. Furthermore, postsynaptic scaffolding proteins have been shown to be associated with TPRC channels that are associated with the inhibition of intracellular Ca2+ overload-mediated ROS generation by TRPC channels in neurotoxin-induced neuronal injury [119]. Although at present, the consequence of ROS-mediated activation of STIM1-TRPC1 with regard to neuronal degeneration is not yet identified, it could be plausible that this could be one of the mechanisms that could lead to neuronal loss. Moreover, increased cytosolic Ca2+ leads to inappropriate activation of Ca2+-dependent processes, which stay inactive at low Ca2+ levels, causing metabolic derangements, leading to neuronal death [5,105,112]. Additionally, this could also lead to the loss of mitochondrial membrane potential, depletion of ATP and increased free radical production, creating oxidative stress within the cell [120]. The free radicals thus released from the mitochondria disturb the intracellular Ca2+ homeostasis, possibly by compromising the function of the Ca2+ signaling components of the ER and PM [121]. With regard to ER store-depletion, the cell has its own way of replenishing the stores through the activation of SOCE channels. Additionally, neurotoxins that mimic the degeneration of dopaminergic neurons drastically decrease TRPC1 expression [122]. As a result, the ER fails to maintain the normal Ca2+ levels required for its proper functioning, eventually leading to ER stress [123,124]. The mitochondrion, on the other hand, takes up the excess Ca2+ present in the cytoplasm (released from the ER) and loses its membrane potential due to Ca2+ overload. This, in turn, exacerbates the free radical production, thus creating a vicious cycle of oxidative stress, which initiates an intrinsic apoptotic cascade. Our findings also suggest that the overexpression of TRPC1 combats some of the negative effects of MPP+-induced oxidative stress and mitochondrial dysfunction, thus providing the cells an opportunity to recover from the stressed state.
Another mechanism that could also affect neuronal viability is ER stress. ER Ca2+ is essential for the proper folding and synthesis of neuronal proteins, and a decrease in ER Ca2+ can induce ER stress, which can activate cell death cascades [125]. In addition, in all of these neurodegenerative processes, abnormal protein folding is the key, suggesting that controlled Ca2+ influx from external media is critical for neuronal functioning and survival. Since TRPCs are essential for replenishing and for maintaining ER Ca2+, the chronic depletion of ER Ca2+, as would occur in the absence of TRPC function, could influence ER-dependent processes, such as protein folding and trafficking, the ER stress response and apoptosis. Accumulating evidence over the last decade has shown a strong link between the pathogenesis of AD and impaired Ca2+ homeostasis [112]. Furthermore, TRPC1 has been shown to be essential for Huntington disease [126], and mutations in TRPC1 will affect the risk of late-onset AD [127]. However, the mechanisms of it need further clarification. Interestingly, presenilins are also suggested to function as ER Ca2+ leak channels [128] and have been shown to be mutated in some AD cases. Importantly, mutant PS1 and PS2 transgenic mice, as well as neurons expressing presenilin mutations have been shown to have larger Ca2+ stores and an increased dynamic flow of Ca2+ into the cytosol [129,130]. The proposed mechanism for this effect is reasoned to be due to the disruption of the Ca2+ leak function of mutant presenilin, creating an imbalance between Ca2+ leak and Ca2+ reuptake. Contrary to this ‘Ca2+ overload’ hypothesis, there is strong evidence showing no increase in Ca2+ stores, but rather, Ca2+ stores in PS1 and PS2 mutant phenotypes are reduced [131,132,133]. It has also been shown that FAD PS1 and PS2 mutants interact with IP3R, rendering it sensitive to lower IP3 concentrations, thus affecting the receptor’s gating and resulting in exaggerated [Ca2+]cyt [133]. These discrepancies could be attributed to the type of mutations on the presenilin and the cell type used, which would dictate the eventual phenotypic disparity. However, recently, it has been identified that PS2 and ORAI2 prevented the overload of ER Ca2+, which may regulate PIP2 levels to control Ca2+ extrusion via a feedback mechanism [134].
Another possibility is that SOCE could provide a mild conditioning stress that activates adaptive cellular stress responses, resulting in the upregulation of genes encoding neuroprotective proteins, such as neurotrophic factors, protein chaperones and antioxidant enzymes [135]. Consistent with this mechanism, transient Ca2+ influx is known to be required for the neurotrophic effects of glutamate receptor activation, and TRPC1 channels have been shown to mediate the actions of the brain-derived neurotrophic factor (BDNF) at some synapses [136]. Ca2+ may also mediate the cell survival-promoting function of glial cell line-derived neurotrophic factor (GDNF) in substantia nigra dopaminergic neurons [137]. The involvement of neurotrophic factor signaling upstream or downstream of TRPC1 channel activation may be part of a previously unknown adaptive neuronal stress response pathway [138]. Changes in the content of Ca2+ stores directly affect SOCE, and it has been shown that SOCE was abrogated with most of the presenilin mutations. Consistent with this, the knockdown of PS1 showed a marked potentiation of SOCE, suggesting that PS1 in general negatively regulates SOCE, and their mutations create a gain-of-function phenotype, which would further augment the inhibition. Although, at present, the direct identification of any TRPC channels is missing, a recent study showed that mouse embryonic fibroblast lacking presenilins had increased levels of STIM1 and decreased levels of STIM2 expression with a marked potentiation of SOCE. Consist with this, STIM2 has been shown to mediate neuronal SOCE, which continuously activated the Ca2+/calmodulin-dependent protein kinase II, which was decreased in mouse models of AD [139]. However, in another report, expression of FAD mutants in these cells attenuated SOCE without altering the STIM expression [140]. PS2 has been shown to influence TRPC6-mediated Ca2+ entry [131]. Coexpression of PS2 or PS2 mutants with TRPC6 completely abrogates agonist-induced Ca2+ entry through TRPC6; however, these studies were performed in non-neuronal cells, and it is not clear if a similar mechanism occurs in neurons. In spite of the mounting evidence for the role of Ca2+ in general and SOCE specifically in the pathogenicity of AD, mechanistic insight in this regard is lacking. Thus, in this context, the question of how the PS1 mutants regulate the TRPC channels needs further clarification.
5. Conclusions
In conclusion, we have discussed the involvement of TRPC channels in neuronal function; however, as seven TRPCs are expressed in neuronal cells, it is likely that they all could compensate for the loss of a particular TRPC channel. Nevertheless, accumulating evidence indicates that TRPC channels mediate physiological function in neuronal cells. Therefore, establishing their impact on pathophysiology and neuronal diseases is critical. Furthermore, increasing knowledge regarding the link between TRPC channels and various neuronal diseases should also lead to a fruitful development of innovative drugs or therapies to combat these deliberating diseases, as currently, no viable therapies are available for these patients. In addition, as some of these TRPC have redundant functions, it would be essential to develop KO mice with various TRPC deletions, as that could provide a much better scenario and establish their function in particular diseases. Furthermore, for clinical and translation aspect new areas of research such as: does SNPs, epigenetics modulations, and mutations regulate TRPCs expression and function; how predictive/useful will the animal models be in order to test drugs that can modulate TRPC channel function; can TRPC modulators identified using animal models, be safe and effective in combating neuronal diseases are still needed.
Acknowledgments
We duly acknowledge the grant support from the National Institutes of Health (DE017102).
Abbreviations
- TRPC
transient receptor potential canonical cation channels
- STIM
stromal interaction molecule
- PKC
protein kinase C
- ATP
adenosine triphosphate
- PLC
phospholipase C
- IP3R
inositol trisphosphate receptor
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- ER
endoplasmic reticulum
- PM
plasma membrane
- DAG
diacylglycerol
- ORAI
calcium release-activated calcium channel protein
- SOCE
store-operated calcium entry
- GPCR
G-protein coupled receptor
- BDNF
brain-derived neurotrophic factor
- CNS
central nervous system
- PD
Parkinson’s disease
- AD
Alzheimer’s disease
- HD
Huntington’s disease
- RTKs
Receptor Tyrosine Kinases
- Pca/PNa
Possible ratio.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Selvaraj S., Sun Y., Singh B.B. TRPC channels and their implication in neurological diseases. CNS Neurol. Disord. Drug Targets. 2010;9:94–104. doi: 10.2174/187152710790966650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamamoto S., Wajima T., Hara Y., Nishida M., Mori Y. Transient receptor potential channels in Alzheimer’s disease. Biochim. Biophys. Acta. 2007;1772:958–967. doi: 10.1016/j.bbadis.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Reboreda A., Jimenez-Diaz L., Navarro-Lopez J.D. TRP channels and neural persistent activity. Adv. Exp. Med. Biol. 2011;704:595–613. doi: 10.1007/978-94-007-0265-3_32. [DOI] [PubMed] [Google Scholar]
- 5.Berridge M.J., Lipp P., Bootman M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 6.Putney J.W. The physiological function of store-operated calcium entry. Neurochem. Res. 2011;36:1157–1165. doi: 10.1007/s11064-010-0383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smyth J.T., Hwang S.Y., Tomita T., DeHaven W.I., Mercer J.C., Putney J.W. Activation and regulation of store-operated calcium entry. J. Cell. Mol. Med. 2010;14:2337–2349. doi: 10.1111/j.1582-4934.2010.01168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu X., Cheng K.T., Bandyopadhyay B.C., Pani B., Dietrich A., Paria B.C., Swaim W.D., Beech D., Yildrim E., Singh B.B., et al. Attenuation of store-operated Ca2+ current impairs salivary gland fluid secretion in TRPC1(–/–) mice. Proc. Natl. Acad. Sci. USA. 2007;104:17542–17547. doi: 10.1073/pnas.0701254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao Y., Plummer N.W., George M.D., Abramowitz J., Zhu M.X., Birnbaumer L. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc. Natl. Acad. Sci. USA. 2009;106:3202–3206. doi: 10.1073/pnas.0813346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan Z., Yang H., Reinach P.S. Transient receptor potential (TRP) gene superfamily encoding cation channels. Hum. Genomics. 2011;5:108–116. doi: 10.1186/1479-7364-5-2-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Putney J.W. Physiological mechanisms of TRPC activation. Pflugers Archiv. 2005;451:29–34. doi: 10.1007/s00424-005-1416-4. [DOI] [PubMed] [Google Scholar]
- 12.Hao B., Lu Y., Wang Q., Guo W., Cheung K.H., Yue J. Role of STIM1 in survival and neural differentiation of mouse embryonic stem cells independent of Orai1-mediated Ca entry. Stem Cell Res. 2013;12:452–466. doi: 10.1016/j.scr.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 13.Pani B., Ong H.L., Liu X., Rauser K., Ambudkar I.S., Singh B.B. Lipid rafts determine clustering of STIM1 in endoplasmic reticulum-plasma membrane junctions and regulation of store-operated Ca2+ entry (SOCE) J. Biol. Chem. 2008;283:17333–17340. doi: 10.1074/jbc.M800107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pani B., Bollimuntha S., Singh B.B. The TR (i)P to Ca(2)(+) signaling just got STIMy: An update on STIM1 activated TRPC channels. Front. Biosci. 2012;17:805–823. doi: 10.2741/3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park C.Y., Shcheglovitov A., Dolmetsch R. The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science. 2010;330:101–105. doi: 10.1126/science.1191027. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Deng X., Mancarella S., Hendron E., Eguchi S., Soboloff J., Tang X.D., Gill D.L. The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science. 2010;330:105–109. doi: 10.1126/science.1191086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X., Ong H.L., Pani B., Johnson K., Swaim W.B., Singh B., Ambudkar I. Effect of cell swelling on ER/PM junctional interactions and channel assembly involved in SOCE. Cell Calcium. 2010;47:491–499. doi: 10.1016/j.ceca.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ong H.L., Cheng K.T., Liu X., Bandyopadhyay B.C., Paria B.C., Soboloff J., Pani B., Gwack Y., Srikanth S., Singh B.B., et al. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J. Biol. Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minke B., Cook B. TRP channel proteins and signal transduction. Physiol. Rev. 2002;82:429–472. doi: 10.1152/physrev.00001.2002. [DOI] [PubMed] [Google Scholar]
- 20.Kiselyov K., van Rossum D.B., Patterson R.L. TRPC channels in pheromone sensing. Vitam. Horm. 2010;83:197–213. doi: 10.1016/S0083-6729(10)83008-0. [DOI] [PubMed] [Google Scholar]
- 21.Bandyopadhyay B.C., Swaim W.D., Liu X., Redman R.S., Patterson R.L., Ambudkar I.S. Apical localization of a functional TRPC3/TRPC6-Ca2+-signaling complex in polarized epithelial cells. Role in apical Ca2+ influx. J. Biol. Chem. 2005;280:12908–12916. doi: 10.1074/jbc.M410013200. [DOI] [PubMed] [Google Scholar]
- 22.Liu X., Bandyopadhyay B.C., Singh B.B., Groschner K., Ambudkar I.S. Molecular analysis of a store-operated and 2-acetyl-sn-glycerol-sensitive non-selective cation channel. Heteromeric assembly of TRPC1-TRPC3. J. Biol. Chem. 2005;280:21600–21606. doi: 10.1074/jbc.C400492200. [DOI] [PubMed] [Google Scholar]
- 23.Andrade-Talavera Y., Duque-Feria P., Sihra T.S., Rodriguez-Moreno A. Pre-synaptic kainate receptor-mediated facilitation of glutamate release involves PKA and Ca(2+) -calmodulin at thalamocortical synapses. J. Neurochem. 2013;126:565–578. doi: 10.1111/jnc.12310. [DOI] [PubMed] [Google Scholar]
- 24.Wong A.C., Birnbaumer L., Housley G.D. Canonical transient receptor potential channel subtype 3-mediated hair cell Ca(2+) entry regulates sound transduction and auditory neurotransmission. Eur. J. Neurosci. 2013;37:1478–1486. doi: 10.1111/ejn.12158. [DOI] [PubMed] [Google Scholar]
- 25.Kerstein P.C., Jacques-Fricke B.T., Rengifo J., Mogen B.J., Williams J.C., Gottlieb P.A., Sachs F., Gomez T.M. Mechanosensitive TRPC1 channels promote calpain proteolysis of talin to regulate spinal axon outgrowth. J. Neurosci. 2013;33:273–285. doi: 10.1523/JNEUROSCI.2142-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohmann C., Bonhoeffer T. A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron. 2008;59:253–260. doi: 10.1016/j.neuron.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Zitt C., Zobel A., Obukhov A.G., Harteneck C., Kalkbrenner F., Luckhoff A., Schultz G. Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron. 1996;16:1189–1196. doi: 10.1016/S0896-6273(00)80145-2. [DOI] [PubMed] [Google Scholar]
- 28.Wes P.D., Chevesich J., Jeromin A., Rosenberg C., Stetten G., Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl. Acad. Sci. USA. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu X., Wang W., Singh B.B., Lockwich T., Jadlowiec J., O’Connell B., Wellner R., Zhu M.X., Ambudkar I.S. Trp1, a candidate protein for the store-operated Ca(2+) influx mechanism in salivary gland cells. J. Biol. Chem. 2000;275:3403–3411. doi: 10.1074/jbc.275.5.3403. [DOI] [PubMed] [Google Scholar]
- 30.Strubing C., Krapivinsky G., Krapivinsky L., Clapham D.E. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/S0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 31.Lucas P., Ukhanov K., Leinders-Zufall T., Zufall F. A diacylglycerol-gated cation channel in vomeronasal neuron dendrites is impaired in TRPC2 mutant mice: Mechanism of pheromone transduction. Neuron. 2003;40:551–561. doi: 10.1016/S0896-6273(03)00675-5. [DOI] [PubMed] [Google Scholar]
- 32.Liman E.R., Corey D.P., Dulac C. TRP2: A candidate transduction channel for mammalian pheromone sensory signaling. Proc. Natl. Acad. Sci. USA. 1999;96:5791–5796. doi: 10.1073/pnas.96.10.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofmann T., Obukhov A.G., Schaefer M., Harteneck C., Gudermann T., Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- 34.Clapham D.E., Julius D., Montell C., Schultz G. International Union of Pharmacology. XLIX. Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol. Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- 35.Lemonnier L., Trebak M., Putney J.W., Jr. Complex regulation of the TRPC3, 6 and 7 channel subfamily by diacylglycerol and phosphatidylinositol-4,5-bisphosphate. Cell Calcium. 2008;43:506–514. doi: 10.1016/j.ceca.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Jia Y.C., Cui K., Li N., Zheng Z.Y., Wang Y.Z., Yuan X.B. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature. 2005;434:894–898. doi: 10.1038/nature03477. [DOI] [PubMed] [Google Scholar]
- 37.Philipp S., Cavalie A., Freichel M., Wissenbach U., Zimmer S., Trost C., Marquart A., Murakami M., Flockerzi V. A mammalian capacitative calcium entry channel homologous to Drosophila TRP and TRPL. EMBO J. 1996;15:6166–6171. [PMC free article] [PubMed] [Google Scholar]
- 38.Schaefer M., Plant T.D., Obukhov A.G., Hofmann T., Gudermann T., Schultz G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J. Biol. Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- 39.Warnat J., Philipp S., Zimmer S., Flockerzi V., Cavalie A. Phenotype of a recombinant store-operated channel: Highly selective permeation of Ca2+ J. Physiol. 1999;518:631–638. doi: 10.1111/j.1469-7793.1999.0631p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philipp S., Hambrecht J., Braslavski L., Schroth G., Freichel M., Murakami M., Cavalie A., Flockerzi V. A novel capacitative calcium entry channel expressed in excitable cells. EMBO J. 1998;17:4274–4282. doi: 10.1093/emboj/17.15.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okada T., Shimizu S., Wakamori M., Maeda A., Kurosaki T., Takada N., Imoto K., Mori Y. Molecular cloning and functional characterization of a novel receptor-activated TRP Ca2+ channel from mouse brain. J. Biol. Chem. 1998;273:10279–10287. doi: 10.1074/jbc.273.17.10279. [DOI] [PubMed] [Google Scholar]
- 42.Yamada H., Wakamori M., Hara Y., Takahashi Y., Konishi K., Imoto K., Mori Y. Spontaneous single-channel activity of neuronal TRP5 channel recombinantly expressed in HEK293 cells. Neurosci. Lett. 2000;285:111–114. doi: 10.1016/s0304-3940(00)01033-8. [DOI] [PubMed] [Google Scholar]
- 43.Strubing C., Krapivinsky G., Krapivinsky L., Clapham D.E. Formation of novel TRPC channels by complex subunit interactions in embryonic brain. J. Biol. Chem. 2003;278:39014–39019. doi: 10.1074/jbc.M306705200. [DOI] [PubMed] [Google Scholar]
- 44.Shi J., Mori E., Mori Y., Mori M., Li J., Ito Y., Inoue R. Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J. Physiol. 2004;561:415–432. doi: 10.1113/jphysiol.2004.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang W.C., Young J.S., Glitsch M.D. Changes in TRPC channel expression during postnatal development of cerebellar neurons. Cell Calcium. 2007;42:1–10. doi: 10.1016/j.ceca.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Riccio A., Medhurst A.D., Mattei C., Kelsell R.E., Calver A.R., Randall A.D., Benham C.D., Pangalos M.N. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Brain Res. Mol. Brain Res. 2002;109:95–104. doi: 10.1016/S0169-328X(02)00527-2. [DOI] [PubMed] [Google Scholar]
- 47.Okada T., Inoue R., Yamazaki K., Maeda A., Kurosaki T., Yamakuni T., Tanaka I., Shimizu S., Ikenaka K., Imoto K., et al. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. Ca(2+)-permeable cation channel that is constitutively activated and enhanced by stimulation of G protein-coupled receptor. J. Biol. Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- 48.Riccio A., Mattei C., Kelsell R.E., Medhurst A.D., Calver A.R., Randall A.D., Davis J.B., Benham C.D., Pangalos M.N. Cloning and functional expression of human short TRP7, a candidate protein for store-operated Ca2+ influx. J. Biol. Chem. 2002;277:12302–12309. doi: 10.1074/jbc.M112313200. [DOI] [PubMed] [Google Scholar]
- 49.Bezprozvanny I., Hiesinger P.R. The synaptic maintenance problem: Membrane recycling, Ca2+ homeostasis and late onset degeneration. Mol. Neurodegener. 2013;8 doi: 10.1186/1750-1326-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bollimuntha S., Selvaraj S., Singh B.B. Emerging roles of canonical TRP channels in neuronal function. Adv. Exp. Med. Biol. 2011;704:573–593. doi: 10.1007/978-94-007-0265-3_31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu X., Zagranichnaya T.K., Gurda G.T., Eves E.M., Villereal M.L. A TRPC1/TRPC3-mediated increase in store-operated calcium entry is required for differentiation of H19-7 hippocampal neuronal cells. J. Biol. Chem. 2004;279:43392–43402. doi: 10.1074/jbc.M408959200. [DOI] [PubMed] [Google Scholar]
- 52.Boudes M., Uvin P., Pinto S., Freichel M., Birnbaumer L., Voets T., de Ridder D., Vennekens R. Crucial role of TRPC1 and TRPC4 in cystitis-induced neuronal sprouting and bladder overactivity. PLoS One. 2013;8:e69550. doi: 10.1371/journal.pone.0069550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alessandri-Haber N., Dina O.A., Chen X., Levine J.D. TRPC1 and TRPC6 channels cooperate with TRPV4 to mediate mechanical hyperalgesia and nociceptor sensitization. J. Neurosci. 2009;29:6217–6228. doi: 10.1523/JNEUROSCI.0893-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phelan K.D., Shwe U.T., Abramowitz J., Wu H., Rhee S.W., Howell M.D., Gottschall P.E., Freichel M., Flockerzi V., Birnbaumer L., et al. Canonical transient receptor channel 5 (TRPC5) and TRPC1/4 contribute to seizure and excitotoxicity by distinct cellular mechanisms. Mol. Pharmacol. 2013;83:429–438. doi: 10.1124/mol.112.082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ariano P., Dalmazzo S., Owsianik G., Nilius B., Lovisolo D. TRPC channels are involved in calcium-dependent migration and proliferation in immortalized GnRH neurons. Cell Calcium. 2011;49:387–394. doi: 10.1016/j.ceca.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Fiorio Pla A., Maric D., Brazer S.-C., Giacobini P., Liu X., Chang Y.H., Ambudkar I.S., Barker J.L. Canonical transient receptor potential 1 plays a role in basic fibroblast growth factor (bFGF)/FGF receptor-1-induced Ca2+ entry and embryonic rat neural stem cell proliferation. J. Neurosci. 2005;25:2687–2701. doi: 10.1523/JNEUROSCI.0951-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim S.J., Kim Y.S., Yuan J.P., Petralia R.S., Worley P.F., Linden D.J. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- 58.Liman E.R. Regulation by voltage and adenine nucleotides of a Ca2+-activated cation channel from hamster vomeronasal sensory neurons. J. Physiol. 2003;548:777–787. doi: 10.1113/jphysiol.2002.037119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang J., Webb D.M. Evolutionary deterioration of the vomeronasal pheromone transduction pathway in catarrhine primates. Proc. Natl. Acad. Sci. USA. 2003;100:8337–8341. doi: 10.1073/pnas.1331721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abramowitz J., Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297–328. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yildirim E., Birnbaumer L. TRPC2: Molecular biology and functional importance. Handb. Exp. Pharmacol. 2007;179:53–75. doi: 10.1007/978-3-540-34891-7_3. [DOI] [PubMed] [Google Scholar]
- 62.Leypold B.G., Yu C.R., Leinders-Zufall T., Kim M.M., Zufall F., Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc. Natl. Acad. Sci. USA. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stowers L., Holy T.E., Meister M., Dulac C., Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 64.Kimchi T., Xu J., Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- 65.Wu M.V., Manoli D.S., Fraser E.J., Coats J.K., Tollkuhn J., Honda S.-I., Harada N., Shah N.M. Estrogen masculinizes neural pathways and sex-specific behaviors. Cell. 2009;139:61–72. doi: 10.1016/j.cell.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jungnickel M.K., Marrero H., Birnbaumer L., Lémos J.R., Florman H.M. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat. Cell Biol. 2001;3:499–502. doi: 10.1038/35074570. [DOI] [PubMed] [Google Scholar]
- 67.Sutton K.A., Jungnickel M.K., Wang Y., Cullen K., Lambert S., Florman H.M. Enkurin is a novel calmodulin and TRPC channel binding protein in sperm. Dev. Biol. 2004;274:426–435. doi: 10.1016/j.ydbio.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 68.Li H.S., Xu X.Z., Montell C. Activation of a TRPC3-dependent cation current through the neurotrophin BDNF. Neuron. 1999;24:261–273. doi: 10.1016/S0896-6273(00)80838-7. [DOI] [PubMed] [Google Scholar]
- 69.Amaral M.D., Pozzo-Miller L. TRPC3 channels are necessary for brain-derived neurotrophic factor to activate a nonselective cationic current and to induce dendritic spine formation. J. Neurosci. 2007;27:5179–5189. doi: 10.1523/JNEUROSCI.5499-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amaral M.D., Pozzo-Miller L. BDNF induces calcium elevations associated with ibdnf, a nonselective cationic current mediated by TRPC channels. J. Neurophysiol. 2007;98:2476–2482. doi: 10.1152/jn.00797.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freichel M., Vennekens R., Olausson J., Stolz S., Philipp S.E., Weissgerber P., Flockerzi V. Functional role of TRPC proteins in native systems: Implications from knockout and knock-down studies. J. Physiol. 2005;567:59–66. doi: 10.1113/jphysiol.2005.092999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Congar P., Leinekugel X., Ben-Ari Y., Crépel V. A long-lasting calcium-activated nonselective cationic current is generated by synaptic stimulation or exogenous activation of group I metabotropic glutamate receptors in CA1 pyramidal neurons. J. Neurosci. 1997;17:5366–5379. doi: 10.1523/JNEUROSCI.17-14-05366.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Linden R. The survival of developing neurons: A review of afferent control. Neuroscience. 1994;58:671–682. doi: 10.1016/0306-4522(94)90447-2. [DOI] [PubMed] [Google Scholar]
- 74.Ruat M., Traiffort E. Roles of the calcium sensing receptor in the central nervous system. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:429–442. doi: 10.1016/j.beem.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 75.Harteneck C., Gollasch M. Pharmacological modulation of diacylglycerol-sensitive TRPC3/6/7 channels. Curr. Pharm. Biotechnol. 2011;12:35–41. doi: 10.2174/138920111793937943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hartmann J., Dragicevic E., Adelsberger H., Henning H.A., Sumser M., Abramowitz J., Blum R., Dietrich A., Freichel M., Flockerzi V., et al. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su B., Ji Y.-S., Sun X.-L., Liu X.-H., Chen Z.-Y. Brain-derived neurotrophic factor (BDNF)-induced mitochondrial motility arrest and presynaptic docking contribute to BDNF-enhanced synaptic transmission. J. Biol. Chem. 2014;289:1213–1226. doi: 10.1074/jbc.M113.526129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodríguez-Santiago M., Mendoza-Torres M., Jiménez-Bremont J.F., López-Revilla R. Knockout of the trcp3 gene causes a recessive neuromotor disease in mice. Biochem. Biophys. Res. Commun. 2007;360:874–879. doi: 10.1016/j.bbrc.2007.06.150. [DOI] [PubMed] [Google Scholar]
- 79.Singh B.B., Lockwich T.P., Bandyopadhyay B.C., Liu X., Bollimuntha S., Brazer S.-C., Combs C., Das S., Leenders A.G.M., Sheng Z.-H., et al. VAMP2-dependent exocytosis regulates plasma membrane insertion of TRPC3 channels and contributes to agonist-stimulated Ca2+ influx. Mol. Cell. 2004;15:635–646. doi: 10.1016/j.molcel.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 80.Bandyopadhyay B.C., Ong H.L., Lockwich T.P., Liu X., Paria B.C., Singh B.B., Ambudkar I.S. TRPC3 controls agonist-stimulated intracellular Ca2+ release by mediating the interaction between inositol 1,4,5-trisphosphate receptor and RACK1. J. Biol. Chem. 2008;283:32821–32830. doi: 10.1074/jbc.M805382200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Streifel K.M., Miller J., Mouneimne R., Tjalkens R.B. Manganese inhibits ATP-induced calcium entry through the transient receptor potential channel TRPC3 in astrocytes. Neurotoxicology. 2013;34:160–166. doi: 10.1016/j.neuro.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Obukhov A.G., Nowycky M.C. TRPC4 can be activated by G-protein-coupled receptors and provides sufficient Ca(2+) to trigger exocytosis in neuroendocrine cells. J. Biol. Chem. 2002;277:16172–16178. doi: 10.1074/jbc.M111664200. [DOI] [PubMed] [Google Scholar]
- 83.Lee K.P., Jun J.Y., Chang I.-Y., Suh S.-H., So I., Kim K.W. TRPC4 is an essential component of the nonselective cation channel activated by muscarinic stimulation in mouse visceral smooth muscle cells. Mol. Cells. 2005;20:435–441. [PubMed] [Google Scholar]
- 84.Zhang S., Remillard C.V., Fantozzi I., Yuan J.X.J. ATP-induced mitogenesis is mediated by cyclic AMP response element-binding protein-enhanced TRPC4 expression and activity in human pulmonary artery smooth muscle cells. Am. J. Physiol. Cell Physiol. 2004;287:C1192–C1201. doi: 10.1152/ajpcell.00158.2004. [DOI] [PubMed] [Google Scholar]
- 85.Gao Y.-Q., Gao H., Zhou Z.-Y., Lu S.-D., Sun F.-Y. Expression of transient receptor potential channel 4 in striatum and hippocampus of rats is increased after focal cerebral ischemia. Sheng Li Xue Bao. 2004;56:153–157. [PubMed] [Google Scholar]
- 86.Greka A., Navarro B., Oancea E., Duggan A., Clapham D.E. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat. Neurosci. 2003;6:837–845. doi: 10.1038/nn1092. [DOI] [PubMed] [Google Scholar]
- 87.Hui H., McHugh D., Hannan M., Zeng F., Xu S.-Z., Khan S.-U.-H., Levenson R., Beech D.J., Weiss J.L. Calcium-sensing mechanism in TRPC5 channels contributing to retardation of neurite outgrowth. J. Physiol. 2006;572:165–172. doi: 10.1113/jphysiol.2005.102889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Davare M.A., Fortin D.A., Saneyoshi T., Nygaard S., Kaech S., Banker G., Soderling T.R., Wayman G.A. Transient receptor potential canonical 5 channels activate Ca2+/calmodulin kinase Igamma to promote axon formation in hippocampal neurons. J.Neurosci. 2009;29:9794–9808. doi: 10.1523/JNEUROSCI.1544-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu G., Lu Z.-H., Obukhov A.G., Nowycky M.C., Ledeen R.W. Induction of calcium influx through TRPC5 channels by cross-linking of GM1 ganglioside associated with alpha5beta1 integrin initiates neurite outgrowth. J. Neurosci. 2007;27:7447–7458. doi: 10.1523/JNEUROSCI.4266-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Riccio A., Li Y., Moon J., Kim K.-S., Smith K.S., Rudolph U., Gapon S., Yao G.L., Tsvetkov E., Rodig S.J., et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell. 2009;137:761–772. doi: 10.1016/j.cell.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yan H.-D., Villalobos C., Andrade R. TRPC channels mediate a muscarinic receptor-induced afterdepolarization in cerebral cortex. J. Neurosci. 2009;29:10038–10046. doi: 10.1523/JNEUROSCI.1042-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Calupca M.A., Locknar S.A., Parsons R.L. TRPC6 immunoreactivity is colocalized with neuronal nitric oxide synthase in extrinsic fibers innervating guinea pig intrinsic cardiac ganglia. J. Comp. Neurol. 2002;450:283–291. doi: 10.1002/cne.10322. [DOI] [PubMed] [Google Scholar]
- 93.Warren E.J., Allen C.N., Brown R.L., Robinson D.W. The light-activated signaling pathway in SCN-projecting rat retinal ganglion cells. Eur. J. Neurosci. 2006;23:2477–2487. doi: 10.1111/j.1460-9568.2006.04777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Elsaesser R., Montani G., Tirindelli R., Paysan J. Phosphatidyl-inositide signalling proteins in a novel class of sensory cells in the mammalian olfactory epithelium. Eur. J. Neurosci. 2005;21:2692–2700. doi: 10.1111/j.1460-9568.2005.04108.x. [DOI] [PubMed] [Google Scholar]
- 95.Chung Y.H., Sun Ahn H., Kim D., Hoon Shin D., Su Kim S., Yong Kim K., Bok Lee W., Ik Cha C. Immunohistochemical study on the distribution of TRPC channels in the rat hippocampus. Brain Res. 2006;1085:132–137. doi: 10.1016/j.brainres.2006.02.087. [DOI] [PubMed] [Google Scholar]
- 96.Giampà C., DeMarch Z., Patassini S., Bernardi G., Fusco F.R. Immunohistochemical localization of TRPC6 in the rat substantia nigra. Neurosci. Lett. 2007;424:170–174. doi: 10.1016/j.neulet.2007.07.049. [DOI] [PubMed] [Google Scholar]
- 97.Tai Y., Feng S., Ge R., Du W., Zhang X., He Z., Wang Y. TRPC6 channels promote dendritic growth via the CaMKIV-CREB pathway. J. Cell. Sci. 2008;121:2301–2307. doi: 10.1242/jcs.026906. [DOI] [PubMed] [Google Scholar]
- 98.Zhou J., Du W., Zhou K., Tai Y., Yao H., Jia Y., Ding Y., Wang Y. Critical role of TRPC6 channels in the formation of excitatory synapses. Nat. Neurosci. 2008;11:741–743. doi: 10.1038/nn.2127. [DOI] [PubMed] [Google Scholar]
- 99.Min M.-Y., Shih P.-Y., Wu Y.-W., Lu H.-W., Lee M.-L., Yang H.-W. Neurokinin 1 receptor activates transient receptor potential-like currents in noradrenergic A7 neurons in rats. Mol. Cell. Neurosci. 2009;42:56–65. doi: 10.1016/j.mcn.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 100.Babich L.G., Ku C.-Y., Young H.W.J., Huang H., Blackburn M.R., Sanborn B.M. Expression of capacitative calcium TrpC proteins in rat myometrium during pregnancy. Biol. Reprod. 2004;70:919–924. doi: 10.1095/biolreprod.103.023325. [DOI] [PubMed] [Google Scholar]
- 101.Elg S., Marmigere F., Mattsson J.P., Ernfors P. Cellular subtype distribution and developmental regulation of TRPC channel members in the mouse dorsal root ganglion. J. Comp. Neurol. 2007;503:35–46. doi: 10.1002/cne.21351. [DOI] [PubMed] [Google Scholar]
- 102.Satoh S., Tanaka H., Ueda Y., Oyama J.-I., Sugano M., Sumimoto H., Mori Y., Makino N. Transient receptor potential (TRP) protein 7 acts as a G protein-activated Ca2+ channel mediating angiotensin II-induced myocardial apoptosis. Mol. Cell. Biochem. 2007;294:205–215. doi: 10.1007/s11010-006-9261-0. [DOI] [PubMed] [Google Scholar]
- 103.Föller M., Kasinathan R.S., Duranton C., Wieder T., Huber S.M., Lang F. PGE2-induced apoptotic cell death in K562 human leukaemia cells. Cell. Physiol. Biochem. 2006;17:201–210. doi: 10.1159/000094125. [DOI] [PubMed] [Google Scholar]
- 104.Xue T., Do M.T., Riccio A., Jiang Z., Hsieh J., Wang H.C., Merbs S.L., Welsbie D.S., Yoshioka T., Weissgerber P., et al. Melanopsin signalling in mammalian iris and retina. Nature. 2011;479:67–73. doi: 10.1038/nature10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Putney J.W., Jr. Capacitative calcium entry in the nervous system. Cell Calcium. 2003;34:339–344. doi: 10.1016/S0143-4160(03)00143-X. [DOI] [PubMed] [Google Scholar]
- 106.Chen B.T., Rice M.E. Novel Ca2+ dependence and time course of somatodendritic dopamine release: Substantia nigra vs. striatum. J. Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Patel J.C., Witkovsky P., Avshalumov M.V., Rice M.E. Mobilization of calcium from intracellular stores facilitates somatodendritic dopamine release. J. Neurosci. 2009;29:6568–6579. doi: 10.1523/JNEUROSCI.0181-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Small D.H. Dysregulation of calcium homeostasis in Alzheimer’s disease. Neurochem. Res. 2009;34:1824–1829. doi: 10.1007/s11064-009-9960-5. [DOI] [PubMed] [Google Scholar]
- 109.Yu J.T., Chang R.C., Tan L. Calcium dysregulation in Alzheimer’s disease: From mechanisms to therapeutic opportunities. Progr. Neurobiol. 2009;89:240–255. doi: 10.1016/j.pneurobio.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 110.Giacomello M., Oliveros J.C., Naranjo J.R., Carafoli E. Neuronal Ca(2+) dyshomeostasis in Huntington disease. Prion. 2013;7:76–84. doi: 10.4161/pri.23581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suzuki M., Nagai Y., Wada K., Koike T. Calcium leak through ryanodine receptor is involved in neuronal death induced by mutant huntingtin. Biochem. Biophys. Res. Commun. 2012;429:18–23. doi: 10.1016/j.bbrc.2012.10.107. [DOI] [PubMed] [Google Scholar]
- 112.Bezprozvanny I., Mattson M.P. Neuronal calcium mishandling and the pathogenesis of Alzheimer’s disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Selvaraj S., Sun Y., Watt J.A., Wang S., Lei S., Birnbaumer L., Singh B.B. Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J. Clin. Investig. 2012;122:1354–1367. doi: 10.1172/JCI61332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Henke N., Albrecht P., Pfeiffer A., Toutzaris D., Zanger K., Methner A. Stromal interaction molecule 1 (STIM1) is involved in the regulation of mitochondrial shape and bioenergetics and plays a role in oxidative stress. J. Biol. Chem. 2012;287:42042–42052. doi: 10.1074/jbc.M112.417212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Feng S., Li H., Tai Y., Huang J., Su Y., Abramowitz J., Zhu M.X., Birnbaumer L., Wang Y. Canonical transient receptor potential 3 channels regulate mitochondrial calcium uptake. Proc. Natl. Acad. Sci. USA. 2013;110:11011–11016. doi: 10.1073/pnas.1309531110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Timmons J.A., Rao J.N., Turner D.J., Zou T., Liu L., Xiao L., Wang P.Y., Wang J.Y. Induced expression of STIM1 sensitizes intestinal epithelial cells to apoptosis by modulating store-operated Ca2+ influx. J. Gastrointest. Surg. 2012;16:1397–1405. doi: 10.1007/s11605-012-1876-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Song M.Y., Makino A., Yuan J.X. Role of reactive oxygen species and redox in regulating the function of transient receptor potential channels. Antioxidants Redox Signal. 2011;15:1549–1565. doi: 10.1089/ars.2010.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miller B.A., Zhang W. TRP channels as mediators of oxidative stress. Adv. Exp. Med. Biol. 2011;704:531–544. doi: 10.1007/978-94-007-0265-3_29. [DOI] [PubMed] [Google Scholar]
- 119.Chen T., Yang Y.F., Luo P., Liu W., Dai S.H., Zheng X.R., Fei Z., Jiang X.F. Homer1 knockdown protects dopamine neurons through regulating calcium homeostasis in an in vitro model of Parkinson’s disease. Cell. Signalling. 2013;25:2863–2870. doi: 10.1016/j.cellsig.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 120.Przedborski S., Tieu K., Perier C., Vila M. MPTP as a mitochondrial neurotoxic model of Parkinson’s disease. J. Bioenerget. Biomembr. 2004;36:375–379. doi: 10.1023/B:JOBB.0000041771.66775.d5. [DOI] [PubMed] [Google Scholar]
- 121.Mattson M.P. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- 122.Zeng X., Pan Z.G., Shao Y., Wu X.N., Liu S.X., Li N.L., Wang W.M. SKF-96365 attenuates toxin-induced neuronal injury through opposite regulatory effects on Homer1a and Homer1b/c in cultured rat mesencephalic cells. Neurosci. Lett. 2013;543:183–188. doi: 10.1016/j.neulet.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 123.Bollimuntha S., Singh B.B., Shavali S., Sharma S.K., Ebadi M. TRPC1-mediated inhibition of 1-methyl-4-phenylpyridinium ion neurotoxicity in human SH-SY5Y neuroblastoma cells. J. Biol. Chem. 2005;280:2132–2140. doi: 10.1074/jbc.M407384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Selvaraj S., Watt J.A., Singh B.B. TRPC1 inhibits apoptotic cell degeneration induced by dopaminergic neurotoxin MPTP/MPP(+) Cell Calcium. 2009;46:209–218. doi: 10.1016/j.ceca.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ermak G., Davies K.J. Calcium and oxidative stress: From cell signaling to cell death. Mol. Immunol. 2002;38:713–721. doi: 10.1016/S0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- 126.Wu J., Shih H.P., Vigont V., Hrdlicka L., Diggins L., Singh C., Mahoney M., Chesworth R., Shapiro G., Zimina O., et al. Neuronal store-operated calcium entry pathway as a novel therapeutic target for Huntington’s disease treatment. Chem. Biol. 2011;18:777–793. doi: 10.1016/j.chembiol.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Briones N., Dinu V. Data mining of high density genomic variant data for prediction of Alzheimer’s disease risk. BMC Med. Genet. 2012;13:7. doi: 10.1186/1471-2350-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tu H., Nelson O., Bezprozvanny A., Wang Z., Lee S.F., Hao Y.H., Serneels L., de Strooper B., Yu G., Bezprozvanny I. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stutzmann G.E., Smith I., Caccamo A., Oddo S., Laferla F.M., Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J. Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.LaFerla F.M. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat. Rev. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 131.Lessard C.B., Lussier M.P., Cayouette S., Bourque G., Boulay G. The overexpression of presenilin2 and Alzheimer’s-disease-linked presenilin2 variants influences TRPC6-enhanced Ca2+ entry into HEK293 cells. Cell. Signalling. 2005;17:437–445. doi: 10.1016/j.cellsig.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 132.Giacomello M., Barbiero L., Zatti G., Squitti R., Binetti G., Pozzan T., Fasolato C., Ghidoni R., Pizzo P. Reduction of Ca2+ stores and capacitative Ca2+ entry is associated with the familial Alzheimer’s disease presenilin-2 T122R mutation and anticipates the onset of dementia. Neurobiol. Dis. 2005;18:638–648. doi: 10.1016/j.nbd.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 133.Cheung K.H., Shineman D., Muller M., Cardenas C., Mei L., Yang J., Tomita T., Iwatsubo T., Lee V.M., Foskett J.K. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bandara S., Malmersjo S., Meyer T. Regulators of calcium homeostasis identified by inference of kinetic model parameters from live single cells perturbed by siRNA. Sci. Signal. 2013;6:ra56. doi: 10.1126/scisignal.2003649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stranahan A.M., Mattson M.P. Recruiting adaptive cellular stress responses for successful brain ageing. Nat. Rev. Neurosci. 2012;13:209–216. doi: 10.1038/nrn3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McGurk J.S., Shim S., Kim J.Y., Wen Z., Song H., Ming G.L. Postsynaptic TRPC1 function contributes to BDNF-induced synaptic potentiation at the developing neuromuscular junction. J. Neurosci. 2011;31:14754–14762. doi: 10.1523/JNEUROSCI.3599-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang H.J., Cao J.P., Yu J.K., Zhang L.C., Jiang Z.J., Gao D.S. Calbindin-D28K expression induced by glial cell line-derived neurotrophic factor in substantia nigra neurons dependent on PI3K/Akt/NF-kappaB signaling pathway. Eur. J. Pharmacol. 2008;595:7–12. doi: 10.1016/j.ejphar.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 138.Mattson M.P. Parkinson’s disease: Don’t mess with calcium. J. Clin. Investig. 2012;122:1195–1198. doi: 10.1172/JCI62835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sun S., Zhang H., Liu J., Popugaeva E., Xu N.J., Feske S., White C.L., 3rd, Bezprozvanny I. Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron. 2014;82:79–93. doi: 10.1016/j.neuron.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bojarski L., Pomorski P., Szybinska A., Drab M., Skibinska-Kijek A., Gruszczynska-Biegala J., Kuznicki J. Presenilin-dependent expression of STIM proteins and dysregulation of capacitative Ca2+ entry in familial Alzheimer’s disease. Biochim. Biophys. Acta. 2009;1793:1050–1057. doi: 10.1016/j.bbamcr.2008.11.008. [DOI] [PubMed] [Google Scholar]