Abstract
Cell–microenvironment interactions play a critical role in the transformation of normal cells into cancer; however, the underlying mechanisms and effects are far from being well understood. Tissue Engineering provides innovative culture tools and strategies to study tumorigenesis under pathologically relevant culture conditions. Specifically, integration of biomaterials, scaffold fabrication, and micro/nano-fabrication techniques offers great promise to reveal the dynamic role of chemical, cell–cell, cell–extracellular matrix, and mechanical interactions in the pathogenesis of cancer. Due to the central importance of blood vessel formation in tumor growth, progression, and drug response, this review will discuss specific design parameters for the development of culture microenvironments to study tumor angiogenesis. Tumor engineering approaches have the potential to revolutionize our understanding of cancer, provide new platforms for testing of anti-cancer drugs, and may ultimately result in improved treatment strategies.
Introduction and Background
Cancer remains the second leading cause of death in the United States and represents a complex disease that may be prevented or promoted by homeostatic or aberrant cell–microenvironment interactions, respectively.1,2 For example, cell–extracellular matrix (ECM) and cell–cell interactions as well as chemical and mechanical cues play an important role at each stage of cancer pathogenesis from the development and progression of a primary tumor, to metastasis, to drug responsiveness.1 Nevertheless, the functional dynamics underlying cell–microenvironment interactions remain poorly understood.
Traditional approaches to study tumorigenesis primarily involve the culture of tumor cells in Petri dishes and in vivo mouse models. Although two-dimensional (2D) culture techniques have advanced our understanding of cancer, this format poorly reflects the microenvironmental context of tumors in vivo.3,4 In vivo experiments, on the other hand, provide limited ability to control isolated aspects of cell signaling, due to the inherent complexity of living systems. To address these challenges, three-dimensional (3D) culture systems are increasingly applied to study tumorigenesis under pathologically relevant yet well-defined culture conditions. For example, in vitro assays using laminin-rich ECM (i.e., Matrigel™) have transformed our understanding of cancer as a microenvironmentally controlled disease.5,6
Tissue engineering, originally developed for tissue regeneration therapy,7 has the potential to transform cancer research by further advancing our qualitative and quantitative understanding of tumorigenesis. Specifically, tissue engineering provides biologically inspired materials, culture techniques, and analytical tools that enable the recreation of humanized 3D tumor microenvironments in vitro. Tissue-engineered tumor models not only establish pathologically relevant culture conditions,3 but also provide beneficial study conditions with regard to mechanical stability of the systems, convenience of handling, applicability in animal studies, and ability to spatiotemporally regulate signaling events conducive to tumor growth.
Given the central importance of new blood vessel recruitment (angiogenesis) for tumor growth, metastasis, and drug response,8–10 the development of tissue-engineered 3D culture models to study these events is particularly attractive. Tumors display an inherent potential to promote the activation and recruitment of endothelial cells by secreting enhanced concentrations of pro-angiogenic factors, including vascular endothelial growth factor (VEGF).11 Despite the enormous potential of anti-VEGF treatment for the prevention of cancer growth, therapies of this type have only shown modest clinical success.9,10
This article reviews specific tumor engineering approaches that may be used for the development of pathologically relevant 3D culture microenvironments. Tumor angiogenesis will be used as a prototype for the discussion of specific design parameters. We highlight the biological effects of chemical, cell–cell, cell–ECM, and mechanical interactions on tumor vascularization and discuss emerging engineering tools that may be used to isolate the underlying mechanisms under biologically inspired conditions in vitro.
Chemical Cues
Excessive tumor cell proliferation and the subsequent development of hypoxia lead to an overproduction of pro-angiogenic factors that drive tumor vascularization.8,9,12 Although VEGF has been most widely investigated for its role in tumor angiogenesis, cancer cells secrete an array of other growth factors and cytokines (e.g., basic fibroblast growth factor and interleukin-8) that collectively promote new vessel formation.13 The molecular interplay between these factors is regulated by spatiotemporal concentration profiles that arise as a function of pro-angiogenic factor production, diffusion, convection mediated by circulation as well as interstitial flow,14 consumption, and elimination. Engineered tumor models need to recapitulate these qualitative and quantitative relationships to provide biologically relevant culture conditions.
Spatiotemporal control over the presentation of chemical cues can be achieved by polymeric drug delivery vehicles. For example, alginate- or poly(ethylene glycol) (PEG)-based hydrogels can be used to recreate the growth factor binding and release characteristics of natural ECMs.15,16 These systems can be covalently modified to exhibit cell adhesion motifs (e.g., RGD) and/or proteolytically degradable cross-links to permit cell invasion and subsequent cell-demanded release of the respective molecule(s) of interest.15,17,18 Alternatively, solid polymers such as poly (alpha-hydroxy) esters (e.g., poly(lactic-co-glycolic acid) [PLG]) are frequently used for the controlled release of biomolecules due to their well-defined and controllable degradation profiles.19 In combination with appropriate scaffold fabrication techniques, these materials enable delivery of multiple morphogens in a simultaneous or sequential manner. More specifically, physical association of growth factors with the surface or bulk of porous PLG scaffolds allows for fast or slow release of biomolecules, respectively.20 This strategy may be invaluable to study changes in tumor angiogenesis that may result from sequential activation of angiogenic factor signaling in vivo.21 Additionally, PLG-based scaffolds can be designed to recreate spatially compartmentalized angiogenic factor concentrations.22 Such systems permit studies of chemotactic migration or gradient-driven differences in tumor angiogenesis. Alternatively, 3D tumor cell culture within microfluidic scaffolds (Fig. 1a) offers a promising strategy to exert spatiotemporal control over chemical factor presentation.23,24 While the 3D culture context ensures in vivo-like angiogenic factor expression by tumor cells,3 convective mass transfer via the integrated microchannels serves to control the distributions of soluble factors that likely influence tumor angiogenesis.23 Further, the microchannels can serve to integrate advanced functionalities. For example, they can be used as templates for explicit endothelia that can respond to pro-angiogenic activity.25
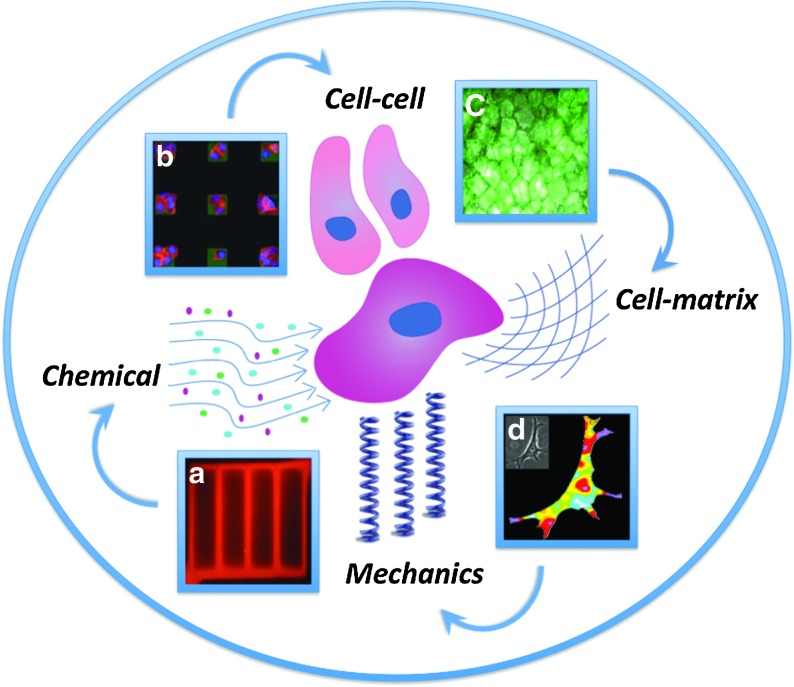
FIG. 1.
Tissue engineering provides innovative tools to recreate tumor-inherent cell–microenvironment interactions in vitro. For example, hydrogel-based microfluidic three-dimensional (3D) culture systems provide spatiotemporal control over soluble factor presentation and allow studies of the role of chemical cues in tumor angiogenesis23 (a: diffusion of rhodamine through a microfluidic collagen network [image courtesy of A.D. Stroock and N.W. Choi]). To probe the role of cell–cell interactions, micropatterning techniques allow culture of cells in the presence and absence of direct cell–cell contact30 (b: Parylene peel-arrays patterned with fibronectin [green] and seeded with tumor cells [red]). Polymer-based artificial extracellular matrices can be used to broadly recreate 3D interactions of tumor cells with their surrounding extracellular matrix3 (c: tumor cells cultured within 3D porous poly [lactic-co-glycolic acid] scaffolds and stained with the viability stain calcein). To assess the role of tumor rigidity on tumor angiogenesis, traction force microscopy permits the quantification of cell-generated forces on matrices of varying elasticity83 (d: traction force map of tumor-associated stroma cell [image courtesy of C.A. Reinhart-King and J.P. Califano]).
Cell–Cell Interactions
In addition to indirect cell–cell interactions mediated by paracrine chemical signaling, tumor angiogenesis is regulated by direct cell–cell contact. Cell–cell adhesions between normal epithelial cells are typically stabilized by E-cadherin-rich adherens junctions.26 However, during tumorigenesis these transmembrane proteins are often downregulated,27,28 which can induce a change in cell–cell interactions as well as impair tissue polarity, both of which can regulate the angiogenic capability of tumor cells.29,30 Altered microenvironmental concentrations of pro-angiogenic factors, in turn, control the formation of intercellular junctions between endothelial cells by modulating expression and signaling of VE-cadherin.31,32 These changes are directly relevant to tumor vascularization since dysfunctional VE-cadherin signaling ultimately promotes invasion angiogenesis and blood vessel permeability.33–35
Microscale engineering technologies offer great promise to investigate the role of intercellular junctions in modulating tumor angiogenesis. Traditionally, the effect of cell–cell interactions on modulating tumor angiogenesis has been investigated by plating cells in either sparse or dense monolayers. However, this approach only provides a very rough level of control over the localization and quantity of cell–cell interactions. To overcome these limitations, microcontact-printing or parylene-template-based micropatterning techniques (Fig. 1b) can be used to seed cells individually or in the presence of cell–cell contact.30,36 Alternatively, cell–cell junctions can be controlled micromechanically by using micromachined silicon culture substrates with movable and interchangeable parts.37 This 2D culture setup enables experimental control over tissue composition, spatial organization, and timeframe of exposure to cell–cell interactions, thereby resolving the direct and indirect effects of cell–cell interactions. While all of these approaches provide exquisite control over 2D cell–cell interactions, they may only partially recreate conditions influencing cell–cell interactions on the tissue level.38
Multiple strategies have been utilized to manipulate cell–cell interactions with 3D cell aggregates. For example, dielectrophoretic forces can create high-resolution 3D cellular structures of tumor or tumor stroma cells within a photopolymerizable hydrogel.39 Similarly, PEG microwell cultures or collagen-based lithographically defined tissue arrays permit control over the size, shape, and homogeneity of 3D cultures and may be used to recapitulate 3D cell–cell contact intrinsic to tumor angiogenesis.40,41 Extension of these approaches to incorporate multiple cell types—including tumor cells, endothelial cells, tumor-associated fibroblasts, and stem or progenitor cells—will further improve our understanding of tumor angiogenesis, given the importance of these poly-cellular interactions on blood vessel formation.42–44 Such systems will not only be important tools for basic research, but also provide the experimental basis for advanced drug screening.
Cell–ECM Interactions
Tumor angiogenesis relies critically on cell–ECM interactions that are mediated by integrin cell surface receptors. There is evidence that particular integrins can act as either promoters or negative regulators of pathological angiogenesis.45 Deregulation of this signaling in the presence of a tumor not only promotes the malignant phenotype and angiogenic capability of tumor cells,46–48 but also activates the adhesion, migration, and tube formation of endothelial cells.49 These differences in cell behavior are mediated by the ability of integrins to transduce bi-directional signals into and out of the cell and to undergo reciprocal interactions with other cell surface receptors (e.g., growth factor receptors).50 Alternatively, integrin-dependent changes in signaling may be induced by differential presentation of ECM ligands (e.g., ligand type,51 density52 and spacing of adhesion sites,53 and matrix topography54) or the presence of hypoxia.55
Three-dimensional culture in artificial ECMs may provide a more accurate understanding of the role of cell–ECM interactions in tumor angiogenesis. While culture within porous PLG scaffolds can be used to broadly test the effect of culture dimensionality on ECM protein expression and consequential changes in integrin signaling (Fig. 1c),3 peptide-modified alginate gels permit more specific dissection of the role of 2D versus 3D integrin engagement on the angiogenic capability of tumor cells.47 For example, these materials can be produced with varying types (e.g., RGD [fibronectin-derived] and YIGSR [laminin-derived]) and densities of adhesion peptides and can be readily tuned in their mechanical properties,56–58 allowing more defined control over cell–ECM interactions than PLG scaffolds. Similarly, functionalization of photocrosslinkable PEG hydrogels with acrylate-PEG-peptide conjugates can be used to test the effect of cell–ECM interactions in response to different individual or combined adhesion sequences.59 Simultaneous introduction of proteolytically degradable domains (cleaved, for example, by cell-released matrix-metalloproteases)60–62 and/or immobilized gradients of pro-angiogenic factors63 makes these materials particularly powerful platforms as they allow the study of tumor-dependent changes in angiogenic sprouting in a dynamic and cell-responsive manner.
Studies of cell–ECM interactions can also be performed with novel micro- and nano-fabrication techniques. For example, nanoscale patterns of adhesive ligands can be generated with nanoparticle gradients that are based on self-assembly of diblock copolymer micelles.64 This approach has the potential to reveal how cells explore positional clustering of integrins to interpret the tumor-ECM environment. To investigate the mechanisms and effects through which topographical features of the tumor-ECM modulate blood vessel formation, silicon substrates with uniform grooves and ridges can be engineered on a similar scale as that of the tumor ECM.54 Integration of these approaches with 3D matrix fabrication and culture techniques will provide greater insight into the role of cell–ECM interactions critical to tumor angiogenesis.
Mechanical Signaling
Mechanical forces also regulate tumor angiogenesis, and these forces can either be cell-generated or derived from external stimulation. As tumors are stiffer than normal tissues, tumor-residing cells are able to more strongly adhere to their surrounding matrix (Fig. 1d).65 The resulting increase in cytoskeletal tension, however, promotes the malignant transformation of cancer cells, and drives vascularization by modulating VEGF-receptor signaling in endothelial cells.65,66 Mechanical stiffness also directly affects endothelial cell network formation and angiogenesis.67,68 Additionally, tumors frequently exhibit elevated interstitial pressure,69 and the resulting strains regulate the proliferative and angiogenic capability of tumor cells.70,71 Lastly, fluid forces that originate from tumor-inherent changes in blood and interstitial fluid flow not only impact the behavior of tumor or tumor-associated cells directly,72 but may also exert a synergistic effect on pro-angiogenic signaling.14
Recreation of normal or tumorigenic tissue stiffness with artificial ECMs enables the study of tumor angiogenesis as a function of cell-generated forces. For example, the rigidity of RGD-modified polyacrylamide73 and alginate57 hydrogels is readily adjustable by photo- and ionic cross-linking, respectively. With this procedure, it is possible to investigate changes in cellular behavior as a function of altered cell mechanics rather than differences in the concentration of cell adhesion motifs. Variation of collagen or Matrigel concentration is another strategy that is frequently employed to mimic the elasticity of tumors.74 However, these approaches simultaneously change adhesion peptide concentrations, which can alter cell behavior independently of differences in matrix rigidity.75,76 Finally, matrix stiffness may be modified by altering cross-linking pH and temperature, as well as cross-linker catalyst concentration.77 It has to be noted, however, that process parameters must remain within a cell-compatible range, imposing certain limitations on these methods.
To investigate the effect of external strains on tumor angiogenesis, tissue-engineered tumor models can be exposed to tumor-mimetic compression and/or perfusion. More specifically, cyclic mechanical compression of scaffold-based 3D tumor models with various oscillation patterns could provide insights into mechano-regulated signaling pathways that may influence both the angiogenic capability of tumor cells and the invasive characteristics of endothelial cells.78,79 To study tumor angiogenesis in response to changes in interstitial fluid flow, low level fluid forces can be applied using 3D radial flow chambers with cell-incorporating artificial ECMs.80 Microfluidic 3D culture systems and polymeric honeycomb scaffolds with aligned pores, on the other hand, offer great promise to gain a better understanding of the effect of varying blood flow rates, as these systems provide temporal control over perfusion and transport phenomena via the integrated microchannels.23,25,81,82
Conclusions
Tissue engineering provides highly innovative culture tools and strategies that can be tailored to study tumorigenesis in general and tumor angiogenesis in particular. The ability to experimentally control in vitro systems not only has the potential to transform our understanding of how chemical, cell–cell, cell–ECM, and mechanical cues work in concert to propagate tumor growth, but can also enable improved drug testing. Interdisciplinary collaborations between cancer biologists and tissues engineers are paramount to further advance studies of cancer biology and may ultimately lead to improved therapies for cancer patients.
Acknowledgments
This review was composed in memory of Caroline M. Coffey (1980–2009), whose potential in the field of tissue engineering was inspirational. We thank A.D. Stroock and D.W. Infanger for their valuable comments on the article and the Cornell Nanobiotechnology Center (supported by the STC Program of the National Science Foundation under Agreement No. ECS-9876771), the Morgan Tissue Engineering Fund, NYSTEM, and NYSTAR for funding. E.M. Chandler is supported by an NSF Graduate Research Fellowship.
Disclosure Statement
No competing financial interests exist.
References
- 1.Bissell M.J. Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ingber D.E. Can cancer be reversed by engineering the tumor microenvironment? Semin Cancer Biol. 2008;18:356. doi: 10.1016/j.semcancer.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischbach C. Chen R. Matsumoto T. Schmelzle T. Brugge J.S. Polverini P.J. Mooney D.J. Engineering tumors with 3D scaffolds. Nat Methods. 2007;4:855. doi: 10.1038/nmeth1085. [DOI] [PubMed] [Google Scholar]
- 4.Pampaloni F. Reynaud E.G. Stelzer E.H. The third dimension bridges the gap between cell culture and live tissue. Nat Rev Mol Cell Biol. 2007;8:839. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 5.Lee G.Y. Kenny P.A. Lee E.H. Bissell M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods. 2007;4:359. doi: 10.1038/nmeth1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bissell M.J. Radisky D.C. Rizki A. Weaver V.M. Petersen O.W. The organizing principle: microenvironmental influences in the normal and malignant breast. Differentiation. 2002;70:537. doi: 10.1046/j.1432-0436.2002.700907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 8.Folkman J. Bach M. Rowe J.W. Davidoff F. Lambert P. Hirsch C. Goldberg A. Hiatt H.H. Glass J. Henshaw E. Tumor angiogenesis—therapeutic implications. N Engl J Med. 1971;285:1182. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 9.Kerbel R.S. Molecular origins of cancer: tumor angiogenesis. N Engl J Med. 2008;358:2039. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain R.K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D. Weinberg R.A. The hallmarks of cancer. Cell. 2000;100:57. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 12.Harris A.L. Hypoxia—a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 13.Kerbel R. Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 14.Helm C.L. Fleury M.E. Zisch A.H. Boschetti F. Swartz M.A. Synergy between interstitial flow and VEGF directs capillary morphogenesis in vitro through a gradient amplification mechanism. Proc Natl Acad Sci USA. 2005;102:15779. doi: 10.1073/pnas.0503681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva E.A. Kim E.S. Kong H.J. Mooney D.J. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc Natl Acad Sci USA. 2008;105:14347. doi: 10.1073/pnas.0803873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hern D.L. Hubbell J.A. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J Biomed Mater Res. 1998;39:266. doi: 10.1002/(sici)1097-4636(199802)39:2<266::aid-jbm14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Zisch A.H. Lutolf M.P. Ehrbar M. Raeber G.P. Rizzi S.C. Davies N. Schmokel H. Bezuidenhout D. Djonov V. Zilla P. Hubbell J.A. Cell-demanded release of VEGF from synthetic, biointeractive cell-ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17:2260. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 18.Lutolf M.P. Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 19.Tracy M.A. Ward K.L. Firouzabadian L. Wang Y. Dong N. Qian R. Zhang Y. Factors affecting the degradation rate of poly(lactide-co-glycolide) microspheres in vivo and in vitro. Biomaterials. 1999;20:1057. doi: 10.1016/s0142-9612(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 20.Richardson T.P. Peters M.C. Ennett A.B. Mooney D.J. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 21.Mizukami Y. Jo W.S. Duerr E.M. Gala M. Li J. Zhang X. Zimmer M.A. Iliopoulos O. Zukerberg L.R. Kohgo Y. Lynch M.P. Rueda B.R. Chung D.C. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11:992. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 22.Chen R.R. Silva E.A. Yuen W.W. Brock A.A. Fischbach C. Lin A.S. Guldberg R.E. Mooney D.J. Integrated approach to designing growth factor delivery systems. FASEB J. 2007;21:3896. doi: 10.1096/fj.06-7873com. [DOI] [PubMed] [Google Scholar]
- 23.Choi N.W. Cabodi M. Held B. Gleghorn J.P. Bonassar L.J. Stroock A.D. Microfluidic scaffolds for tissue engineering. Nat Mater. 2007;6:908. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- 24.Haessler U. Kalinin Y. Swartz M.A. Wu M.W. An agarose-based microfluidic platform with a gradient buffer for 3D chemotaxis studies. Biomed Microdevices. 2009;11:827. doi: 10.1007/s10544-009-9299-3. [DOI] [PubMed] [Google Scholar]
- 25.Chrobak K.M. Potter D.R. Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;71:185. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Yap A.S. Brieher W.M. Gumbiner B.M. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- 27.Umbas R. Schalken J.A. Aalders T.W. Carter B.S. Karthaus H.F. Schaafsma H.E. Debruyne F.M. Isaacs W.B. Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer Res. 1992;52:5104. [PubMed] [Google Scholar]
- 28.Zhang W. Alt-Holland A. Margulis A. Shamis Y. Fusenig N.E. Rodeck U. Garlick J.A. E-cadherin loss promotes the initiation of squamous cell carcinoma invasion through modulation of integrin-mediated adhesion. J Cell Sci. 2006;119:283. doi: 10.1242/jcs.02738. [DOI] [PubMed] [Google Scholar]
- 29.Chen A. Cuevas I. Kenny P.A. Miyake H. Mace K. Ghajar C. Boudreau A. Bissell M. Boudreau N. Endothelial cell migration and vascular endothelial growth factor expression are the result of loss of breast tissue polarity. Cancer Res. 2009;69:6721. doi: 10.1158/0008-5472.CAN-08-4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan C.P. Seo B.R. Brooks D.J. Chandler E.M. Craighead H.G. Fischbach C. Parylene peel-off arrays to probe the role of cell-cell interactions in tumour angiogenesis. Integr Biol. 2009;1:587. doi: 10.1039/b908036h. [DOI] [PubMed] [Google Scholar]
- 31.Esser S. Lampugnani M.G. Corada M. Dejana E. Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J Cell Sci. 1998;111:1853. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- 32.Cavallaro U. Liebner S. Dejana E. Endothelial cadherins and tumor angiogenesis. Exp Cell Res. 2006;312:659. doi: 10.1016/j.yexcr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Bach T.L. Barsigian C. Chalupowicz D.G. Busler D. Yaen C.H. Grant D.S. Martinez J. VE-cadherin mediates endothelial cell capillary tube formation in fibrin and collagen gels. Exp Cell Res. 1998;238:324. doi: 10.1006/excr.1997.3844. [DOI] [PubMed] [Google Scholar]
- 34.Wallez Y. Vilgrain I. Huber P. Angiogenesis: the VE-cadherin switch. Trends Cardiovasc Med. 2006;16:55. doi: 10.1016/j.tcm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Carmeliet P. Lampugnani M.G. Moons L. Breviario F. Compernolle V. Bono F. Balconi G. Spagnuolo R. Oosthuyse B. Dewerchin M. Zanetti A. Angellilo A. Mattot V. Nuyens D. Lutgens E. Clotman F. de Ruiter M.C. Gittenberger-de Groot A. Poelmann R. Lupu F. Herbert J.M. Collen D. Dejana E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 36.Nelson C.M. Chen C.S. Cell-cell signaling by direct contact increases cell proliferation via a PI3K-dependent signal. FEBS Lett. 2002;514:238. doi: 10.1016/s0014-5793(02)02370-0. [DOI] [PubMed] [Google Scholar]
- 37.Hui E.E. Bhatia S.N. Micromechanical control of cell-cell interactions. Proc Natl Acad Sci USA. 2007;104:5722. doi: 10.1073/pnas.0608660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ronnovjessen L. Petersen O.W. Koteliansky V.E. Bissell M.J. The origin of the myofibroblasts in breast-cancer—recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth. J Clin Invest. 1995;95:859. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albrecht D.R. Underhill G.H. Wassermann T.B. Sah R.L. Bhatia S.N. Probing the role of multicellular organization in three-dimensional microenvironments. Nat Methods. 2006;3:369. doi: 10.1038/nmeth873. [DOI] [PubMed] [Google Scholar]
- 40.Nelson C.M. Inman J.L. Bissell M.J. Three-dimensional lithographically defined organotypic tissue arrays for quantitative analysis of morphogenesis and neoplastic progression. Nat Protocols. 2008;3:674. doi: 10.1038/nprot.2008.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karp J.M. Yeh J. Eng G. Fukuda J. Blumling J. Suh K.Y. Cheng J. Mahdavi A. Borenstein J. Langer R. Khademhosseini A. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab Chip. 2007;7:786. doi: 10.1039/b705085m. [DOI] [PubMed] [Google Scholar]
- 42.Lyden D. Hattori K. Dias S. Costa C. Blaikie P. Butros L. Chadburn A. Heissig B. Marks W. Witte L. Wu Y. Hicklin D. Zhu Z.P. Hackett N.R. Crystal P.G. Moore M.A.S. Hajjar K.A. Manova K. Benezra R. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 43.Rafii S. Lyden D. Benezra R. Hattori K. Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 44.Gao D.C. Nolan D.J. Mellick A.S. Bambino K. McDonnell K. Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 45.Reynolds L.E. Wyder L. Lively J.C. Taverna D. Robinson S.D. Huang X.Z. Sheppard D. Hynes O. Hodivala-Dilke K.M. Enhanced pathological angiogenesis in mice lacking beta(3) integrin or beta(3) and beta(5) integrins. Nat Med. 2002;8:27. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 46.Plantefaber L.C. Hynes R.O. Changes in integrin receptors on oncogenically transformed-cells. Cell. 1989;56:281. doi: 10.1016/0092-8674(89)90902-1. [DOI] [PubMed] [Google Scholar]
- 47.Fischbach C. Kong H.J. Hsiong S.X. Evangelista M.B. Yuen W. Mooney D.J. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci USA. 2009;106:399. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weaver V.M. Petersen O.W. Wang F. Larabell C.A. Briand P. Damsky C. Bissell M.J. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garmy-Susini B. Varner J.A. Roles of integrins in tumor angiogenesis and lymphangiogenesis. Lymphat Res Biol. 2008;6:155. doi: 10.1089/lrb.2008.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miranti C.K. Brugge J.S. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 51.Serini G. Valdembri D. Bussolino F. Integrins and angiogenesis: a sticky business. Exp Cell Res. 2006;312:651. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 52.Rowley J.A. Mooney D.J. Alginate type and RGD density control myoblast phenotype. J Biomed Mater Res. 2002;60:217. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- 53.Hsiong S.X. Huebsch N. Fischbach C. Kong H.J. Mooney D.J. Integrin-adhesion ligand bond formation of preosteoblasts and stem cells in three-dimensional RGD presenting matrices. Biomacromolecules. 2008;9:1843. doi: 10.1021/bm8000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karuri N.W. Liliensiek S. Teixeira A.I. Abrams G. Campbell S. Nealey P.F. Murphy C.J. Biological length scale topography enhances cell-substratum adhesion of human corneal epithelial cells. J Cell Sci. 2004;117:3153. doi: 10.1242/jcs.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skuli N. Monferran S. Delmas C. Favre G. Bonnet J. Toulas C. Cohen-Jonathan Moyal E. Alphavbeta3/alphavbeta5 integrins-FAK-RhoB: a novel pathway for hypoxia regulation in glioblastoma. Cancer Res. 2009;69:3308. doi: 10.1158/0008-5472.CAN-08-2158. [DOI] [PubMed] [Google Scholar]
- 56.Kong H.J. Smith M.K. Mooney D.J. Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials. 2003;24:4023. doi: 10.1016/s0142-9612(03)00295-3. [DOI] [PubMed] [Google Scholar]
- 57.Genes N.G. Rowley J.A. Mooney D.J. Bonassar L.J. Effect of substrate mechanics on chondrocyte adhesion to modified alginate surfaces. Arch Biochem Biophys. 2004;422:161. doi: 10.1016/j.abb.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 58.Dhoot N.O. Tobias C.A. Fischer I. Wheatley M.A. Peptide-modified alginate surfaces as a growth permissive substrate for neurite outgrowth. J Biomed Mater Res Part A. 2004;71A:191. doi: 10.1002/jbm.a.30103. [DOI] [PubMed] [Google Scholar]
- 59.Weber L.M. Hayda K.N. Haskins K. Anseth K.S. The effects of cell-matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials. 2007;28:3004. doi: 10.1016/j.biomaterials.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 60.Salinas C.N. Anseth K.S. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials. 2008;29:2370. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rizzi S.C. Hubbell J.A. Recombinant protein-co-PEG networks as cell-adhesive and proteolytically degradable hydrogel matrixes. Part 1: development and physicochernical characteristics. Biomacromolecules. 2005;6:1226. doi: 10.1021/bm049614c. [DOI] [PubMed] [Google Scholar]
- 62.Mann B.K. Gobin A.S. Tsai A.T. Schmedlen R.H. West J.L. Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials. 2001;22:3045. doi: 10.1016/s0142-9612(01)00051-5. [DOI] [PubMed] [Google Scholar]
- 63.DeLong S.A. Moon J.J. West J.L. Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials. 2005;26:3227. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 64.Arnold M. Hirschfeld-Warneken V.C. Lohmuller T. Heil P. Blummel J. Cavalcanti-Adam E.A. Lopez-Garcia M. Walther P. Kessler H. Geiger B. Spatz J.P. Induction of cell polarization and migration by a gradient of nanoscale variations in adhesive ligand spacing. Nano Lett. 2008;8:2063. doi: 10.1021/nl801483w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paszek M.J. Zahir N. Johnson K.R. Lakins J.N. Rozenberg G.I. Gefen A. Reinhart-King C.A. Margulies S.S. Dembo M. Boettiger D. Hammer D.A. Weaver V.M. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 66.Mammoto A. Connor K.M. Mammoto T. Yung C.W. Huh D. Aderman C.M. Mostoslavksy G. Smith L.E. Ingber D.E. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ingber D.E. Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro—role of extracellular-matrix. J Cell Biol. 1989;109:317. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sieminski A.L. Hebbel R.P. Gooch K.J. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res. 2004;297:574. doi: 10.1016/j.yexcr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 69.Fukumura D. Jain R.K. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc Res. 2007;74:72. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hofmann M. Guschel M. Bernd A. Bereiter-Hahn J. Kaufmann R. Tandi C. Wiig H. Kippenberger S. Lowering of tumor interstitial fluid pressure reduces tumor cell proliferation in a xenograft tumor model. Neoplasia. 2006;8:89. doi: 10.1593/neo.05469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nathan S.S. Huvos A.G. Casas-Ganem J.E. Yang R. Linkov I. Sowers R. DiResta G.R. Gorlick R. Healey J.H. Tumor interstitial fluid pressure may regulate angiogenic factors in osteosarcoma. J Orthop Res. 2008;26:1520. doi: 10.1002/jor.20633. [DOI] [PubMed] [Google Scholar]
- 72.Boucher Y. Leunig M. Jain R.K. Tumor angiogenesis and interstitial hypertension. Cancer Res. 1996;56:4264. [PubMed] [Google Scholar]
- 73.Ulrich T.A. de Juan Pardo E.M. Kumar S. The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res. 2009;69:4167. doi: 10.1158/0008-5472.CAN-08-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zaman M.H. Trapani L.M. Sieminski A.L. Mackellar D. Gong H. Kamm R.D. Wells A. Lauffenburger D.A. Matsudaira P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci USA. 2006;103:10889. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Engler A. Bacakova L. Newman C. Hategan A. Griffin M. Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86:617. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mann B.K. Tsai A.T. Scott-Burden T. West J.L. Modification of surfaces with cell adhesion peptides alters extracellular matrix deposition. Biomaterials. 1999;20:2281. doi: 10.1016/s0142-9612(99)00158-1. [DOI] [PubMed] [Google Scholar]
- 77.Rowe S.L. Lee S. Stegemann J.P. Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomater. 2007;3:59. doi: 10.1016/j.actbio.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dumas V. Perrier A. Malaval L. Laroche N. Guignandon A. Vico L. Rattner A. The effect of dual frequency cyclic compression on matrix deposition by osteoblast-like cells grown in 3D scaffolds and on modulation of VEGF variant expression. Biomaterials. 2009;30:3279. doi: 10.1016/j.biomaterials.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 79.Wille J.J. Ambrosi C.M. Yin F.C. Comparison of the effects of cyclic stretching and compression on endothelial cell morphological responses. J Biomech Eng. 2004;126:545. doi: 10.1115/1.1798053. [DOI] [PubMed] [Google Scholar]
- 80.Helm C.L. Zisch A. Swartz M.A. Engineered blood and lymphatic capillaries in 3-D VEGF-fibrin-collagen matrices with interstitial flow. Biotechnol Bioeng. 2007;96:167. doi: 10.1002/bit.21185. [DOI] [PubMed] [Google Scholar]
- 81.Korin N. Bransky A. Dinnar U. Levenberg S. Periodic “flow-stop” perfusion microchannel bioreactors for mammalian and human embryonic stem cell long-term culture. Biomed Microdevices. 2009;11:87. doi: 10.1007/s10544-008-9212-5. [DOI] [PubMed] [Google Scholar]
- 82.Yamamoto M. James D. Li H. Butler J. Rafii S. Rabbany S.Y. Generation of stable co-cultures of vascular cells in a honeycomb alginate scaffold. Tissue Eng Part A. 2009;16:299. doi: 10.1089/ten.tea.2009.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reinhart-King C.A. Dembo M. Hammer D.A. Endothelial cell traction forces on RGD-derivatized polyacrylamide substrata. Langmuir. 2003;19:1573. [Google Scholar]