Abstract
A double-blind, randomized clinical trial was conducted to determine the effects of prevention of zinc deficiency on cognitive and sensorimotor development during infancy. At 6 mo of age, infants were randomly assigned to be administered a daily liquid supplement containing 10 mg/d of zinc (zinc sulfate), 10 mg/d of iron (ferrous sulfate), and 0.5 mg/d of copper (copper oxide), or an identical daily liquid supplement containing only 10 mg/d of iron and 0.5 mg/d of copper. Various controls were implemented to ensure adherence to the supplement protocol. A battery of developmental assessments was administered from 6 to 18 mo of age that included a visual habituation/recognition memory task augmented with heart rate at 6, 9, and 12 mo of age; the Bayley Scales of Infant Development, 2nd edition (BSID2) at 6, 12, and 18 mo; the A-not-B error task at 9 and 12 mo; and free-play attention tasks at 12 and 18 mo. Only infants supplemented with zinc had the normative decline in look duration from 6 to 12 mo during habituation and a normative decline in shifting between objects on free-play multiple-object attention tasks from 12 to 18 mo of age. The 2 groups did not differ on any of the psychophysiologic indices, the BSID2, or the A-not-B error task. The findings are consistent with zinc supplementation supporting a profile of normative information processing and active attentional profiles during the first 2 y of life. This trial was registered at clinicaltrials.gov as NCT00589264.
Introduction
Zinc is a trace mineral essential to all forms of life because of its fundamental role in gene expression, cell development, and replication, but millions of people throughout the world have an inadequate concentration of zinc in the diet because of limited access to zinc-rich foods and the abundance of zinc inhibitors common in cereal-based diets (1). Two periods appear to increase the risk of zinc deficiency in the young child: gestation and older infancy. Fetal accumulation of zinc during pregnancy is a function of maternal zinc status, and thus, newborn zinc deficiency (low serum zinc concentration) is likely in populations with inadequate dietary zinc intakes during pregnancy (2). This deficiency is likely transitory for all but the most vulnerable (preterm or low-birth-weight infants) because the zinc content of colostrum is high, and some zinc becomes available to the infant as part of the hematologic changes accompanying the transition to the extra-uterine environment (3). Beginning at around 6 mo of age, however, breast milk intakes no longer provide sufficient zinc to meet requirements (4), making zinc-rich complementary foods necessary (5). If zinc-rich sources are not available on a routine basis, zinc deficiency develops over time, and concern regarding the potential adverse effects of inadequate zinc intakes on growth and development will continue as long as dietary intakes remain inadequate.
Multiple lines of evidence suggest that zinc nutriture influences child development (6, 7). Zinc is a critical nutrient for central nervous system development; it is necessary for enzymes involved in brain growth, for proteins that provide brain structure for neurotransmission, and for neurotransmitters involved in brain memory function. Zinc is also involved in steroid hormone transport, receptor binding and metabolism, and neurotransmitter precursor production, all of which ultimately affect brain function (6). In the brain, zinc is found predominantly in the areas that modulate cognitive, behavioral, and affective responses to stimuli, including the hippocampus, the cerebellum (8), and the prefrontal cortex (9). In addition, zinc is concentrated in neurons found in the cortex and the limbic system, particularly in regions providing cortico-cortico, cortico-limbic, and thalamocortic connections (10, 11), where it functions as an important neuromodulator (12).
It was postulated that zinc deficiency affects cognitive development during infancy by influencing motor development, activity, and attention (7). Evidence for an effect of zinc on sensorimotor development and activity is mixed; some supplementation trials reported positive effects (13–18), whereas others have yielded null effects (19–22), and one study reported a negative effect (23). Studies showing positive outcomes often include vulnerable infants (ill, low- or very-low-birth weight, or those who were currently malnourished) who were provided 5–10 mg/d of supplemental zinc, and effects were detected with general tests of sensorimotor development, such as the Bayley (24) and Griffiths scales (25).
However, research linking attentional measures with zinc nutriture has previously been performed only with adults and older children. Penland (26) found that adult men consuming diets low in zinc performed worse on sensorimotor, attention, perception, memory, and spatial tasks. Similar findings were apparent in a study of adult women (27) in a crossover design examining the effects of both zinc depletion and repletion. The results from zinc supplementation trials in children are mixed; a trial with stunted school-aged children in Canada found no differences in attention by using the Detroit Test of Learning Abilities (28), but trials involving school-aged children in China and Mexican-American children in Texas found positive effects (29–31). Studies directly examining the role of zinc on measures of neurodevelopment and cognition have not been conducted in infants, despite the availability of measures to assess such outcomes. This paper reports the results of a controlled trial with a population in Peru that historically was deficient in both iron and zinc during early childhood. Infants from this population were provided a supplement containing either copper and iron (FC8 condition) or iron plus copper and zinc (FCZ condition) from 6 to 18 mo of age, and were assessed on measures of attention, memory/inhibition, and overall sensorimotor function across that time period with the aim of determining whether zinc supplementation might sustain normative neurodevelopmental function. In keeping with the recommendation of tracking developmental trajectories in the conduct of nutritional supplement studies (32), we assessed measures in these infants repeatedly across the age range of the study.
Participants and Methods
Study population and recruitment
The study was conducted at the Centro de Salud Materno Infantil San Jose in Villa El Salvador, a large shantytown with over 300,000 inhabitants of varying socioeconomic status within the metropolitan area of Lima, Peru. Most homes have electricity and access to safe water and a toilet (although not always within the home). Housing varies from brick (55%) to wood (15%) to cardboard (25%). Micronutrient intake in the diet of children from this population is particularly inadequate for calcium, iron, and zinc (33). The protocol was approved by the institutional review boards at the Instituto de Investigación Nutricional, the University of Kansas, and The Johns Hopkins University Bloomberg School of Public Health.
Potential participants were identified through a field survey of infants 0–6 mo of age and pregnant women. Field workers explained the study to mothers of infants 4–6 mo of age and made an appointment for them at the study clinic. Once there, they learned more about the study and provided signed consent, and we determined their final eligibility for the study. Inclusion criteria were the following: birth weight >2500 g, gestational age >37 wk completed, free from major malformations, genetic abnormalities, or health problems associated with developmental delays, no known vision or hearing problems, and no plans to move from the hospital catchment area during the study duration. To avoid potential confounding effects of pre-existing anemia, we took a blood sample via finger prick. Infants with hemoglobin concentrations >103 g/L were considered eligible for enrollment (34); those with concentrations ≤103 g/L were treated with supplemental iron and followed up separately.
Supplementation
Random assignment to supplement type was carried out in blocks of 2 within strata based on sex. The FCZ group was administered a liquid supplement containing 10 mg/d of zinc (as zinc sulfate), 10 mg/d of iron (as ferrous sulfate), and 0.5 mg/d of copper (as copper oxide), whereas the FC group was administered an identical liquid supplement containing only 10 mg/d of iron and 0.5 mg/d of copper. The Peruvian Ministry of Health guidelines for preventing iron deficiency anemia in infancy indicate universal supplementation beginning at age 6 mo with 10 mg/d of iron. The dosage of zinc was chosen because it was the most common dose used in prior supplementation studies in this age group. To ensure adequate copper status, given the dosages of iron and zinc, we chose a dosage of copper about twice the 2001 U.S. RDA for 12- to 18-mo-old infants (35). The supplements were prepared by IQFARMA, a certified national laboratory. The supplement bottles and liquids themselves were indistinguishable, and each had a spoon metered to deliver 2.5 mL of supplement. At enrollment, the infants were assigned a unique identification number, which corresponded to the correct supplement type to be taken by the next infant recruited within that stratum. The correspondence between ID number and supplement type was sealed in a document and kept with the manufacturer and the director of the Instituto de Investigación Nutricional. The investigators, the families of study participants, and data analysts had no knowledge of treatment groups until data analyses were complete.
Data collection
At enrollment, women were interviewed to gather information on socioeconomic status and family characteristics. All infants were evaluated by a physician and anthropometric measures of weight and length were taken following standard protocols; for analyses, these were expressed as Z-scores (14, 36). Mothers were then provided an initial allocation of the supplement and advised to give their infant 1 daily dose. During subsequent monthly pediatric evaluations, the nurse collected the used supplement bottle and provided them with a new one. Each bottle was weighed prior to distribution, and the nurse weighed and then recorded the amount of liquid remaining in each returned bottle. Fieldworkers visited participants’ homes weekly to inquire about illnesses since the last visit and supplement administration. Specifically, they asked the mother to recall the days she had given the supplement to her infant since their last visit. Blood samples were taken at 6 (baseline), 12, and 18 mo of age to assess iron, copper, and zinc status. A detailed report on compliance with supplementation and the biochemical findings on infant iron, zinc, and copper status was published (37). Overall median compliance was high (81% of dose delivered) and did not differ over time or by supplement type. At baseline, the mean plasma zinc concentrations in the 2 groups did not vary by supplement type (11.0 ± 2.1 and 11.0 ± 2.0 μmol/L for the FC and FCZ groups, respectively), with the proportion of children with zinc deficiency (plasma zinc concentrations <9.9 μmol/L) being 30–31% in both groups. Plasma zinc concentration and prevalences of zinc deficiency were maintained in the FCZ group over time, whereas the mean concentration in the FC group declined, with prevalences of zinc deficiency of 47.4% and 39.2% at 12 and 18 mo, respectively. Hemoglobin and plasma copper concentrations increased in both groups from 6 to 12 mo and were then maintained through 18 mo.
A set of assessments designed to capture various aspects of cognitive development, including attention, memory, and learning from 6 to 18 mo of age, and a global measure of developmental status, the Bayley Scales of Infant Development, 2nd edition (BSID2), was administered. The assessments were performed by 2 Peruvian psychologists trained in each measure by J.C. and K.N.K. The following sections briefly describe each assessment.
Visual habituation
At 6, 9, and 12 mo of age, infants were administered a visual habituation task augmented with simultaneous heart rate (HR) recording (38). The stimuli used were 6 color slides of women’s faces (all matched for size, representing a mix of ethnicities, with a neutral expression and a constant background) obtained from online facial databases. Major studies on the psychometrics (39), predictive validity (38), and developmental functions (40) for habituation were all derived from work by using faces. Stimulus pairs were arranged so that infants saw different faces at each visit, with presentation of each stimulus pair balanced across ages.
Habituation protocol.
Infants were seated in a darkened clinic room in their mother’s lap. All stimuli were presented on a laptop computer (0.42-m screen). The visual angle of the habituation and paired-comparison stimuli was ∼20° during the paired-comparison trials (see below), paired stimuli were separated by 18°. An observer unaware of stimulus identity coded the infants’ looking from a video image of the infants’ faces from behind the laptop. Records of infant looking were synchronized with HR recording for later analysis.
Habituation (a decline in look duration to repetitive stimulus presentations) is a widely used measure of visual learning in infants (41). An infant-controlled sequence (39) using a floating-point calculation for habituation criterion (38) and common variables for definitions of valid looks and interstimulus intervals (42) was employed. Two observers were trained to reliability prior to the start of the study, and reliability was calculated from checks on DVD recordings of the sessions; inter-rater correlations ranged from 0.94 to 0.99 for individual sessions.
The critical measures from this paradigm include look duration (39) and HR-defined phases of attention (43) that reflect initial orienting to, sustained attention (SA) to, and disengagement/attention termination (AT) from the presented stimulus. Of these 3 phases, SA (characterized by HR deceleration during looking) represents the infant’s active engagement with and processing of the visual stimulus under inspection (44). Habituation measures have been shown to be sensitive to nutritional manipulations during infancy (45) and have been shown to be modestly predictive of later cognitive outcomes (38, 46); infants showing more robust declines in look duration over the course of the first year and overall higher levels of sustained attention have been reported to account for 5–15% of the variance in later vocabulary and standardized IQ scores (47). Across the first year, look duration declines, and the decline was consistently associated with more rapid processing of stimuli (48, 49) and better ability to disengage attention from visual stimuli (50, 51).
Paired-comparison recognition test.
Following habituation, infants were tested for recognition memory of the habituation stimulus by pairing the habituation face with a novel one in 2 5-s trials, across which lateral positions of the novel and familiar targets were balanced. Successful recognition memory is generally indicated by preference for the novel stimulus (48). Observers were also trained to reliability for novelty preferences, and reliability checks were conducted for habituation data. Reliability for novelty preference was very high (0.95).
Measurement and reduction of HR.
HR was measured with shielded Ag-AgCl electrodes placed on the infant’s abdomen. The electrocardiogram was digitized at 250 Hz sample rate (BioPac) and synchronized with stimulus events and fixations. Custom software parsed infants’ looking into categories of orienting, SA, and AT (52); details on the calculation and measurement of these phases appear elsewhere (38, 43). The primary variable was the proportion of time spent looking in these 3 phases.
Free-play tasks and measures
At 12 and 18 mo of age, infants participated in single-object (SO) and multiple-object (MO) free-play tasks to assess endogenous (controlled) attention (53). During the SO task, infants were presented with 1 complex electronic toy to manipulate and explore for 5 min. During the MO task, infants were presented with 6 small toys (e.g., vehicles, blocks, small infant toys) to manipulate and explore for 5 min. Each task was video recorded, and observers coded the duration of looking to and looking away (inattention) from the toys. Looks to the toys and episodes of inattention were defined as being 1 s or longer in duration; looks and episodes of inattention that were separated by disruptions of <1 s were combined. Coders recorded looking durations through frame-by-frame analyses of recordings. For the MO task, coders also recorded the number of looks to the toys and how often infants shifted their attention from one toy to another. Reliabilities for coding ranged from 0.99 to 0.73, and percentage agreement for the number of looks to the toy averaged 91% across both ages.
BSID2
The BSID2 is a standardized instrument for measuring sensorimotor development during infancy; the scale is used to assess the attainment of various milestones and simple tasks or abilities from birth to 30 mo (24). It yields a Mental Development Index (MDI) thought to assess cognitive abilities and a Psychomotor Development Index (PDI), which reflects fine and gross motor development.
A-not-B task
The A-not-B task measures reflect inhibitory and memory processes mediated by frontal brain areas (54). Performance on this task was shown to be sensitive to metabolic disorders that are relevant to cognitive function in infancy (55). In this task, a toy is repeatedly hidden under a cup at 1 of 2 locations to the left and to the right of the infant’s midline. After the infant successfully finds the toy at one location (A), it is hidden at the second location (B). After a delay imposed before allowing the infant to search, infants often search at the incorrect location (A) rather than the correct one (B). We examined infants’ performance in searching while systematically increasing delays (0, 3, 5, 7, 10, 12, 15, and 19 s) during the session. The primary metric was the length of the delay infants could tolerate before searching incorrectly (i.e., at A). The task was administered at 9 and 12 mo of age; in U.S. samples, 9-mo-old infants tolerate delays in the range of 3–9 s, and 12-mo-old infants tolerate delays from 8–12 s (56).
Statistical analyses
We applied t tests to continuous variables and χ2 tests to categorical variables to compare maternal and infant characteristics of the FCZ and FC groups at enrollment. Trial outcome data were subjected to mixed-model analyses, which are well-suited to longitudinal data with missing data because they use all available longitudinal data. Supplement (FC vs. FCZ), “visit” (i.e., the age at which the instrument was administered), and “sex” (where appropriate; see below) were entered as fixed factors, and “subjects” as random factors. Covariance was left unstructured in these analyses. We followed an intention-to-treat approach in the analyses of these data. We evaluated whether compliance with supplementation affected results, but because it did not change the outcome of any observed findings, those results are not shown. Significant interactions were decomposed with ANOVA or t tests. Novelty preferences were tested against chance values (0.50) by using one-sample t tests. All data analyses were performed by using IBM PASW Statistics (SPSS). Data reported are means ± SEMs; α level was set at P = 0.05.
Results
Final sample sizes and sample characteristics
The trial was designed to enroll 300 infants with an anticipated loss to follow-up of 10%, with power calculations made for the child development outcomes of the study. However, because of funding constraints, the sample size was reduced. A total of 275 infants were evaluated for eligibility and 251 were randomly assigned, 122 to the FC group and 129 to the FCZ group. Overall, 13% infants left the study, leaving 108 in the FC group and 101 in the FCZ group. A consort diagram of the study is shown elsewhere (37). Of the 251 infants enrolled for the study, 227 (90.4%) were seen for habituation at 6 mo, with 220 successful completions. At 9 mo, 214 (85.4%) infants were seen, with 194 successful completions. At 12 mo, 204 (81.3%) infants were seen, with 183 successful completions; 191 completed the SO free-play task, and 163 completed the MO task. A total of 179 (71.3%) infants had complete habituation data at all 3 assessments. At 18 mo, 175 infants completed the SO task, and 173 infants completed the MO task. Given available sample sizes (with power = 0.80; α = 0.05), the minimal detectable difference in the outcomes by supplement type was 0.35 SD.
Demographic data were available on 248 of the 251 children and families enrolled in the study; those data are shown in Table 1. The only difference between the 2 supplemented groups was that the percentage of single mothers was slightly higher in the FC group (P = 0.05). However, when entered as a covariate in analyses, this variable did not affect any of the outcomes and did not change any of the conclusions or interpretations.
TABLE 1.
Sample demographics1
| Variable | Overall (n = 248) | FC group (n = 121) | FCZ group (n = 127) |
| Mother’s age, y | 27.2 ± 5.84 | 27.3 ± 5.76 | 27.0 ± 5.94 |
| Mother’s education, y | 11.2 ± 2.65 | 11.3 ± 2.97 | 11.1 ± 2.30 |
| Number of siblings | 0.85 ± 0.95 | 0.83 ± 0.93 | 0.86 ± 0.97 |
| Mother’s occupation2 | 3.23 ± 3.98 | 3.60 ± 4.21 | 2.87 ± 3.74 |
| Single mother | 8 | 12* | 5 |
| Family owns car | 5 | 7 | 4 |
| Family owns radio | 86 | 83 | 83 |
| Family owns television | 97 | 96 | 98 |
| Family owns telephone | 75 | 79 | 72 |
| Family owns refrigerator | 60 | 63 | 57 |
Values for continuous variables are means ± SDs or percentages. *Different from FCZ, P < 0.05. FC, supplement containing copper and iron; FCZ, supplement containing iron plus copper and zinc; n refers to the sample size represented by means in the respective columns.
Mother's occupation was rated on a scale from 1 to 15, with 1 being the poorest/least prestigious occupation, and 15 being the highest.
At baseline, the mean weight-for-age Z-score (WAZ) and length-for-age Z-score (LAZ) did not differ by supplement type [comparing the FCZ and FC groups, respectively (WAZ: 0.5 ± 1.0 vs. 0.4 ± 0.7; LAZ: −0.5 ± 0.9 vs. −0.6 ± 0.8)] (37). Anthropometric status was maintained over the study period, and there remained no differences by supplement type [comparing the FCZ and FC groups, respectively (WAZ: 0.1 ± 0.9 vs. 0.1 ± 0.8; LAZ: −0.5 ± 1.0 vs. −0.6 ± 0.9)].
A detailed report on compliance with supplementation and the biochemical findings on iron, zinc, and copper status was published (37). Overall median compliance was high (81% of doses delivered), and did not differ over time or by supplement type. At baseline, the mean plasma zinc concentrations in the 2 groups did not vary by supplement type (11.0 ± 2.1 and 11.0 ± 2.0 μmol/L for the FC and FCZ groups, respectively). Plasma zinc concentration was maintained in the FCZ group over time, whereas that in the FC group declined, with mean differences in concentration of 1.00 ± 0.30 μmol/L at 12 mo and 0.69 ± 0.32 μmol/L at 18 mo. Hemoglobin and plasma copper concentrations increased in both groups from 6 to 12 mo and were then maintained through 18 mo.
Habituation
Preliminary analyses indicated no differences as a function of sex, and so it was excluded as a factor in analyses.
Look-duration variables.
Three summary look-duration variables were derived from the entire habituation session: total looking (the sum of look durations during habituation), mean look duration (the mean of all look durations during habituation), and the duration of the longest (peak) look. Because duration-based variables generate skewed distributions, we square-root transformed all variables to adjust for normality.
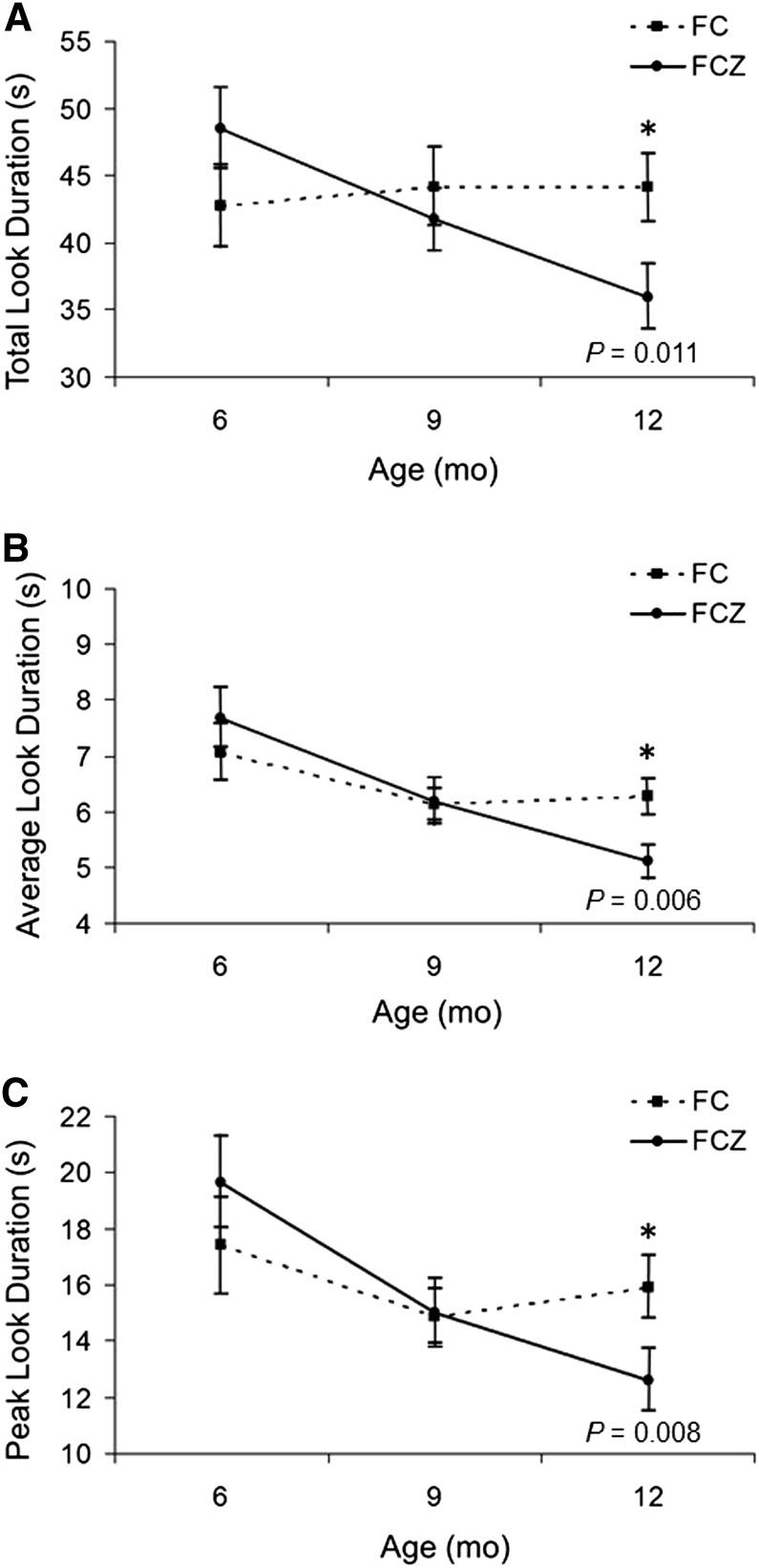
Analyses of total looking yielded a significant age × supplement interaction (P = 0.015), which was probed with separate Age analyses for each group; the FCZ group had a significant (P = 0.006) decline from 6 to 12 mo, whereas the FC group had no change. Analyses of mean look duration had the same pattern, with a significant main effect for age (P = 0.001) attributable to older infants looking for shorter durations than younger infants. A significant age × supplement interaction (P = 0.036) was attributable to the FCZ group showing a significant decline (P < 0.001) from 6 to 12 mo, and the FC group showing no change. Finally, the results for peak look duration yielded a significant main effect for age (P = 0.009) and a significant age × supplement interaction (P = 0.022), with the FCZ group showing a significant decline (P < 0.001) from 6 to 12 mo, and the FC group showing no change.
The results of the duration-based variables are shown in Figure 1. In each case, there were no differences between the 2 supplement groups at baseline (6 mo of age) and at 9 mo, but differences between the groups were statistically significant (respective Ps for total, mean, and peak look duration were 0.011, 0.006, and 0.008) at 12 mo. In all cases, the normative developmental decline expected for these variables was seen only for the FCZ group.
FIGURE 1.
Developmental course for look-duration variables as a function of age and FC/FCZ assignment. Results from total look duration from the habituation procedure (A); mean look duration (B); and longest or peak look duration (C). Analyses were performed on square-root transformed variables; raw durations are presented below. Data plotted are means ± SEMs. The respective sample sizes are FC = 111 and FCZ = 114 at 6 mo; FC = 109 and FCZ = 104 at 9 mo; and FC = 104 and FCZ = 101 at 12 mo. *Different from FCZ at that time, P < 0.05. FC, supplement containing copper and iron; FCZ, supplement containing iron plus copper and zinc.
HR and HR-defined phases of attention.
Analyses conducted on infants’ HR during each period of the habituation session, and during each of the HR-defined phases of attention, yielded significant (P < 0.001) effects of age at each point, but no main effects or interactions involving supplement approached significance. Mixed-model analyses of the proportion of time spent in orienting, SA, and AT did not yield any significant main effects or interactions involving supplementation. In keeping with other reports (45, 57), the proportion of orienting increased with age (P = 0.009), and SA decreased with age (P = 0.008); no significant age effects emerged for AT. Figure 2 shows the typical HR response during various periods of a habituation trial and shows that the 2 supplementation conditions did not vary from one another in any meaningful way.
FIGURE 2.
Infants’ mean HR data averaged across all habituation trials as a function of age and FC/FCZ assignment. The shaded area represents when the stimulus was available (Stimulus On), and the solid line box represents the period during which the infant was looking (Infant Looking). Data are presented separately for each age (labeled) and for the 2 supplementation groups. The respective sample sizes are FC = 111 and FCZ = 114 at 6 mo; FC = 101 and FCZ = 92 at 9 mo; and FC = 97 and FCZ = 95 at 12 mo. AT, attention termination; bpm, beats per minute; FC, supplement containing copper and iron; FCZ, supplement containing iron plus copper and zinc; HR, heart rate; Latency, the period after stimulus presentation but prior to looking onset; OR, orienting; PreStim, 2-s period prior to stimulus presentation; PostLook, the period after look termination but prior to stimulus withdrawal; SA, sustained attention.
Novelty preference
At 6 mo of age, 191 infants successfully completed the paired-comparison phase, 180 at 9 mo, and 176 at 12 mo. Infants recognized the habituation face at each age, as novelty preferences significantly exceeded chance values (0.50) at 6 (P < 0.001), 9 (P < 0.01), and 12 mo (P < 0.01). A mixed-model analysis of this variable confirmed that the overall magnitude of the novelty preference decreased significantly with age (P = 0.039), an effect seen in previous research (38). No significant effects emerged involving supplement.
SO and MO free-play analyses
The analyses on the SO task measures had no significant main effects or interactions involving supplement, revealing only significant main effects of age (all P < 0.001). Infants had more total looking at 12 mo (270.5 s ± 1.4) than 18-mo-old infants (250.0 s ± 1.8), longer mean look lengths at 12 mo (31.5 s ± 1.7) than at 18 mo (19.8 s ± 0.9), and fewer episodes of inattention at 12 mo (8.7 s ± 0.4) than at 18 mo (12.9 s ± 0.4).
The analyses on the MO task yielded significant main effects of age (all P < 0.001). We observed less looking at 12 mo (233.6 s ± 2.3) than at 18 mo (249.9 s ± 2.3), shorter mean look lengths at 12 mo (16.0 s ± 0.7) than at 18 mo (22.3 s ± 1.2), more episodes of inattention at 12 mo (12.4 s ± 0.4) than at 18 mo (8.5 s ± 0.4), and more shifts in attention at 12 mo (74.5 s ± 1.2) than at 18 mo (65.5 s ± 1.2). The analysis of the number of shifts revealed an age × supplement interaction (P < 0.05). Both groups had a decline across ages 12–18 mo, but the FCZ group had a significantly steeper decline (P < 0.001) across age than did the FC group, whose pattern of change across age was only marginally significant (P = 0.06). At 12 mo, the FCZ group had significantly more shifts (P = 0.031) than the FC group, but there were no differences between the groups at 18 mo (Fig. 3).
FIGURE 3.
Number of fixation shifts among objects presented during the MO free-play session, as a function of age and FC/FCZ assignment. Data are presented separately for each age (labeled) and for the 2 supplementation conditions; data plotted are means ± SEMs. The respective sample sizes are FC = 81 and FCZ = 81 at 12 mo, and FC = 87 and FCZ = 86 at 18 mo. *Different from FCZ at that time, P < 0.05. FC, supplement containing copper and iron; FCZ, supplement containing iron plus copper and zinc; MO, multiple object.
BSID2
The BSID2 was successfully administered to 251 infants at 6 mo, 212 at 12 mo, and 207 at 18 mo. Preliminary analyses of the BSID2 indicated significant effects of sex, and so this was included in final analyses of this variable. Mixed-model analyses of the MDI yielded main effects for age (P < 0.001) and sex (P = 0.002). Infants’ MDI scores increased (P < 0.05) from 6 mo (101.8 ± 0.6) to 12 mo (103.3 ± 0.6) and then declined at 18 mo (94.7 ± 0.6). Females (100.8 ± 0.4) scored higher on the MDI than males (98.9 ± 0.5) collapsed across all 3 ages. No effects involving supplement were significant. Analysis of the PDI yielded only a main effect for age (P < 0.001) because scores at 18 mo (104.0 ± 0.4) significantly (P < 0.05) exceeded scores at 6 mo (100.9 ± 0.5) and 12 mo (100.8 ± 0.5). Again, analyses yielded no significant effects involving supplement.
A-not-B task
A total of 93 infants completed this task at 9 mo, and 217 completed the task at 12 mo. A considerable number of infants [41 infants (44.1%) at 9 mo; 86 infants (39.6%) at 12 mo] never searched successfully at the new (correct) location, but these totals were equally divided between the 2 supplement groups. Mixed-model analyses involving factors of age (2), sex (2), and supplement (2) were conducted on infants who did pass the initial stages of the test, but these analyses yielded only a significant effect of age (P < 0.001); the 12-mo-old infants were able to tolerate slightly longer delays (4.4 s ± 0.3) than the 9-mo-old infants (3.17 s ± 0.3). The delays tolerated by the 9-mo-old infants were within the normative realm of those reported for U.S. samples, but the performance of 12-mo-old infants fell below that previously reported for that age group in the United States. No main effect or interaction involving the supplement group approached statistical significance.
Discussion
The major finding from the early assessments in this clinical trial is that supplementing infants with iron, copper, and zinc (compared with supplementation with iron and copper only) resulted in normal developmental trajectories in attentional variables from habituation and a MO free-play task. Within the habituation procedure, numerous studies have documented the decline in look duration as the normal course of development (40, 47), and infants who show this decline tend to score higher on standardized tests of cognitive and language development in later childhood (38, 46, 47). The habituation task yields measures of attention at both behavioral and psychophysiologic indices of attention; the effect for zinc was observed only for behavioral measures, and although the overall attentional profile for zinc-supplemented infants was manifest in terms of a decline in looking time, the differences between the groups did not attain statistical significance until 12 mo of age, 6 mo after supplementation began. Biochemical evidence from this sample (37) indicated that supplementation maintained zinc status in the treatment group, whereas the control group had a decline. The preservation of zinc status therefore may have allowed the FCZ group to progress in age-associated changes in the attentional variables.
Zinc supplementation did not affect the psychophysiologic variables of attention, such as HR or HR-defined phases of attention (orienting, SA, and AT). The finding may be viewed as consistent with zinc affecting more motor-based aspects of attention, which would be in keeping with other findings in the literature linking zinc status and supplementation to motor development in infancy (16, 17, 21).
Analyses of free-play tasks indicated good performance levels. At both 12 and 18 mo, infants were attentive to the stimuli presented in both the SO (90.2% at 12 mo, 83.34% at 18 mo) and MO task (77.85% at 12 mo, 83.29% at 18 mo). We observed robust effects of age on both tasks that (in some cases) favored females. The normative overall decline in shifting during the MO task, which was observed for the overall group from 12 to 18 mo, was significantly more robust for infants administered zinc supplementation than infants who were not administered zinc. The decline in shifting seen on this task from 12 to 18 mo was largely driven by supplemented infants’ higher rate of shifting at 12 mo; this higher rate may reflect a more active pattern of attention, which would be consistent with more rapid information processing as suggested by shorter looks at 12 mo on habituation.
No significant effects of supplementation were observed on the BSID2, A-not-B task, or SO attention task. In this population, after significant gains in BSID2 MDI standard scores observed from 6 to 12 mo, infants fell behind norms at 18 mo; PDI scores had gains from 12 to 18 mo. Performance on the A-not-B task at 9 mo was roughly equivalent to that seen in U.S. samples, but at 12 mo the entire sample fell behind previously reported norms.
It is difficult to compare our findings with the extant literature because of population and methodologic and reporting differences. Siegel et al. (58) did use the A-not-B task and the Fagan Test of Infant Intelligence to examine information processing and novelty preference in Nepali infants who were part of a larger supplementation trial. The infants were more undernourished than our sample and >25% were anemic at enrollment. They were enrolled at ∼7–9 mo of age, supplemented for variable lengths of time (3–5 mo), and assessed at 39 and/or 52 wk of age. There were multiple supplements provided, and the findings for the supplement contrast similar to ours (5 mg/d of zinc with or without 6.25 mg/d of iron and 250 μg/d of folic acid) were not reported. The authors reported no supplementation differences (compared with placebo) for fixation duration or novelty preference at 39 wk, but lower novelty preference in those supplemented with zinc, iron, and folic acid compared with placebo at 52 wk. At 39 wk, the zinc, iron, and folic acid group was more likely to be accurate in the A-not-B task (compared with placebo), but this was reversed at 52 wk. The authors noted that the A-not-B task was a challenge for the infants to perform and concluded that interventions that are broader in scope and of longer duration are needed to supplement child development in this setting.
In conclusion, supplementation with zinc maintained normative developmental trajectories for selected measures of attention during the first 18 mo of life. The fact that supplementation did not affect BSID2 performance may be attributable to the attenuated sensitivity of global measures of developmental status (59). However, this possibility alone does not account for the lack of supplementation effects on other measures in the trials (the A-not-B task, the physiologically based indices of attention, or the SO attentional tasks). It may be that the effects of zinc supplementation are more specific, in that they maintain normative developmental improvements in processing speed or in more motoric- or activation-based systems of attention (60, 61). Differences were not seen between the groups at 18 mo, but transient effects are not uncommon in studies of nutrition in early development (62) and, as other studies have shown (63), may not necessarily suggest that the supplementation did not have long-term consequences. Although the effects of zinc seen here may not necessarily reflect the amelioration of an existing developmental delay (e.g., the scores of nonsupplemented children on global developmental assessments were in the normal range), they are consistent with reduction of long-term risk in this sample, given the association between early measures of attention and later cognitive and language outcomes.
Further research is needed in this area to confirm or replicate these findings, which are unique in suggesting that preventive zinc supplementation helped to sustain normative neurodevelopment among infants consuming a diet low in zinc. Studies are needed with additional tools to further understand the neurophysiologic or behavioral pathways leading to the differences observed here. Studies using the current methods in populations with more severe zinc deficiency would also be informative.
Acknowledgments
L.E.C., J.C., and K.N.K. designed the research; J.C. and K.N.K. trained staff on the developmental outcomes; N.Z., F.L., and C.A. collected the data and conducted the research; L.E.C., J.C., K.N.K., and L.L.K. analyzed the data; and L.E.C., J.C., and K.N.K. wrote the paper and had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AT, attention termination; BSID2, Bayley Scales of Infant Development, 2nd edition; FC, supplement containing copper and iron; FCZ, supplement containing iron plus copper and zinc; HR, heart rate; LAZ, length-for-age Z-score; MDI, Mental Development Index; MO, multiple object; PDI, Psychomotor Development Index; SA, sustained attention; SO, single object; WAZ, weight-for-age Z-score.
References
- 1.Sandstead HH. Zinc deficiency: a public health problem. Am J Dis Child 1991;145:853–9. [DOI] [PubMed] [Google Scholar]
- 2.Caulfield LE, Zavaleta N, Figueroa A. Adding zinc to prenatal iron and folate supplements improves maternal and neonatal zinc status in a Peruvian population. Am J Clin Nutr 1999;69:1257–63. [DOI] [PubMed] [Google Scholar]
- 3.WHO. WHO: trace elements in human nutrition and health. Geneva, Switzerland: WHO; 1996. [Google Scholar]
- 4.Krebs NF. Dietary zinc and iron sources, physical growth and cognitive development of breastfed infants. J Nutr 2000;130:358S–60S. [DOI] [PubMed] [Google Scholar]
- 5.Brown KH, Dewey KG, Allen LH, Brown KH, Dewey KG, Allen LH. Complementary feeding of youngchildren in developing countries: a review of current scientific knowledge. Geneva, Switzerland: WHO; 1998.
- 6.Golub MS, Keen CL, Gershwin ME, Hendrickx AG. Developmental zinc deficiency and behavior. J Nutr 1995;125:2263S–71S. [DOI] [PubMed] [Google Scholar]
- 7.Black MM. Zinc deficiency and child development. Am J Clin Nutr 1998;68:464S–9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akshoomoff NA, Courchesne E. A new role for the cerebellum in cognitive operations. Behav Neurosci 1992;106:731–8. [DOI] [PubMed] [Google Scholar]
- 9.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci 2000;23:475–83. [DOI] [PubMed] [Google Scholar]
- 10.Frederickson CJ, Suh SW, Silva D, Thompson RB. Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr 2000;130:1471S–83S. [DOI] [PubMed] [Google Scholar]
- 11.Long Y, Frederickson CJ. A zinc-containing fiber system of thalamic origin. Neuroreport 1994;5:2026–8. [DOI] [PubMed] [Google Scholar]
- 12.Smart TG, Xie XM, Krishek BJ. Modulation of inhibitory and excitatory amino-acid receptor-ion channels by zinc. Prog Neurobiol 1994;42:393–441. [DOI] [PubMed] [Google Scholar]
- 13.Friel JK, Andrews WL, Matthew JD, Long DR, Cornel AM, Cox M, McKim E, Zerbe GO. Zinc supplementation in very low birthweight infants. J Pediatr Gastroenterol Nutr 1993;17:97–104. [DOI] [PubMed] [Google Scholar]
- 14.Black MM, Sazawal S, Khosla S, Kumar J, Anand S, Black RE. Motor development among small-for-gestational-age infants: effects of zinc supplementation and home environment. FASEB J 1999;13:A879–879. [Google Scholar]
- 15.Ashworth A, Morris SS, Lira PIC, Grantham-McGregor SM. Zinc supplementation, mental development and behaviour in low birth weight term infants in northeast Brazil. Eur J Clin Nutr 1998;52:223–7. [DOI] [PubMed] [Google Scholar]
- 16.Bentley ME, Caulfield LE, Ram M, Santizo MC, Hurtado E, Rivera JA, Ruel MT, Brown KH. Zinc supplementation affects the activity patterns of rural Guatemalan infants. J Nutr 1997;127:1333–8. [DOI] [PubMed] [Google Scholar]
- 17.Sazawal S, Bentley M, Black RE, Dhingra P, George S, Bhan MK. Effect of zinc supplementation on observed activity in low socioeconomic Indian preschool children. Pediatrics 1996;98:1132–7. [PubMed] [Google Scholar]
- 18.Castillo-Durán C, Perales CG, Hertrampf ED, Marin VB, Rivera FA, Icaza G. Effect of zinc supplementation on development and growth of Chilean infants. J Pediatr 2001;138:229–35. [DOI] [PubMed] [Google Scholar]
- 19.Taneja S, Bhandari N, Bahl R, Bhan MK. Impact of zinc supplementation on mental and psychomotor scores of children aged 12 to 18 months: a randomized, double-blind trial. J Pediatr 2005;146:506–11. [DOI] [PubMed] [Google Scholar]
- 20.Lind T, Lonnerdal B, Stenlund H, Gamayanti IL, Ismail D, Seswandhana R, Persson LA. A community-based randomized controlled trial of iron and zinc supplementation in Indonesian infants: effects on growth and development. Am J Clin Nutr 2004;80:729–36. [DOI] [PubMed] [Google Scholar]
- 21.Black MM, Sazawal S, Black RE, Khosla S, Kumar J, Menon V. Cognitive and motor development among small-for-gestational-age infants: impact of zinc supplementation, birth weight, and caregiving practices. Pediatrics 2004;113:1297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olney DK, Pollitt E, Kariger PK, Khalfan SS, Ali NS, Tielsch JM, Sazawal S, Black R, Allen LH, Stoltzfus RJ. Combined iron and folic acid supplementation with or without zinc reduces time to walking unassisted among Zanzibari infants 5-to 11-mo old. J Nutr 2006;136:2427–34. [DOI] [PubMed] [Google Scholar]
- 23.Hamadani JD, Fuchs GJ, Osendarp SJM, Khatun F, Huda SN, Grantham-McGregor SM. Randomized controlled trial of the effect of zinc supplementation on the mental development of Bangladeshi infants. Am J Clin Nutr 2001;74:381–6. [DOI] [PubMed] [Google Scholar]
- 24.Bayley N. Bayley scales of infant development. 2nd ed. San Antonio, TX: The Psychological Corporation; 1970.
- 25.Griffiths R. The abilities of young children. London: Child Development Research Centre; 1970.
- 26.Penland JG. Cognitive performance effects of low zinc (ZN) intakes in healthy adult men. FASEB J 1991;5:A938–938. [Google Scholar]
- 27.Darnell LS, Sandstead HH. Iron, zinc, and cognition of women. Clin Res 1991;39:A644–644. [Google Scholar]
- 28.Gibson RS, Vanderkooy PDS, Macdonald AC, Goldman A, Ryan BA, Berry M. A growth-limiting, mild zinc deficiency syndrome in some southern Ontario bows with low height percentiles. Am J Clin Nutr 1989;49:1266–73. [DOI] [PubMed] [Google Scholar]
- 29.Penland JG, Sandstead HH, Alcock NW, Dayal HH, Chen XC, Li JS, Zhao FJ, Yang JJ. A preliminary report: effects of zinc and micronutrient repletion on growth and neuropsychological function of urban Chinese children. J Am Coll Nutr 1997;16:268–72. [DOI] [PubMed] [Google Scholar]
- 30.Sandstead HH, Penland JG, Alcock NW, Hari H, Xue D, Chen C, Li JS, Zhao F, Yang JJ. Effects of repletion with zinc and other micronutrients on neuropsychologic performance and growth of Chinese children. Am J Clin Nutr 1998;68:470S–5S. [DOI] [PubMed] [Google Scholar]
- 31.Penland J, Sandstead H, Egger N, Dayal H, Alcock N, Plotkin R, Rocco C, Zavaleta A. Zinc, iron and micronutrient supplementation effects on cognitive and psychomotor function of Mexican-American school children. FASEB J 1999;13:A921–921. [Google Scholar]
- 32.Colombo J. Recent advances in infant cognition: implications for long-chain polyunsaturated fatty acid supplementation studies. Lipids 2001;36:919–26. [DOI] [PubMed] [Google Scholar]
- 33.Sacco LM, Caulfield LE, Zavaleta N, Retamozo L. Dietary pattern and usual nutrient intakes of Peruvian women during pregnancy. Eur J Clin Nutr 2003;57:1492–7. [DOI] [PubMed] [Google Scholar]
- 34.Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics 2003;112:846–54. [PubMed] [Google Scholar]
- 35.Food and Nutrition Board IoM. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington: National Academy Press; 2001. [PubMed] [Google Scholar]
- 36.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva, Switzerland: WHO; 2006. [Google Scholar]
- 37.Caulfield LE, Zavaleta N, Chen P, Colombo J, Kannass K. Mineral status of non-anemic Peruvian infants taking an iron and copper syrup with or without zinc from 6 to 18 months of age: a randomized controlled trial. Nutrition 2013;29:1336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colombo J, Shaddy DJ, Richman WA, Maikranz JM, Blaga OM. The developmental course of habituation in infancy and preschool outcome. Infancy 2004;5:1–38. [Google Scholar]
- 39.Colombo J, Mitchell DW, O'Brien M, Horowitz FD. The stability of visual habituation during the first year of life. Child Dev 1987;58:474–87. [PubMed] [Google Scholar]
- 40.Colombo J, Mitchell DW. Individual differences in early visual attention: fixation time and information processing. In: Colombo J, Fagen JW, editors. Individual differences in infancy: reliability, stability, prediction. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1990. p. 193–227.
- 41.Sokolov EN. Perception and the conditioned reflex. Oxford, England: Publishing House Moscow Univer; 1958. [Google Scholar]
- 42.Colombo J, Horowitz FD. A parametric study of the infant control procedure. Infant Behav Dev 1985;8:117–21. [Google Scholar]
- 43.Colombo J, Richman WA, Shaddy DJ, Greenhoot AF, Maikranz JM. Heart rate-defined phases of attention, look duration, and infant performance in the paired-comparison paradigm. Child Dev 2001;72:1605–16. [DOI] [PubMed] [Google Scholar]
- 44.Richards JE. Development and stability in visual sustained attention in 14, 20, and 26 week old infants. Psychophysiology 1989;26:422–30. [DOI] [PubMed] [Google Scholar]
- 45.Colombo J, Kannass KN, Shaddy DJ, Kundurthi S, Maikranz JM, Anderson CJ, Blaga OM, Carlson SE. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev 2004;75:1254–67. [DOI] [PubMed] [Google Scholar]
- 46.Colombo J, Shaddy DJ, Blaga OM, Anderson CJ, Kannass KN, Richman WA. Early attentional predictors of vocabulary in childhood. In: Colombo J, McCardle P, Freund L, editors. Infant pathways to language: methods, models, and research directions. New York: Psychology Press; 2009. p. 143–67.
- 47.Colombo J.Infant cognition: predicting later intellectual functioning. Newbury Park, CA: Sage Publications; 1993.
- 48.Colombo J, Mitchell DW, Horowitz FD. Infant visual attention in the paired-comparison paradigm: test-retest and attention-performance relations. Child Dev 1988;59:1198–210. [DOI] [PubMed] [Google Scholar]
- 49.Colombo J, Mitchell DW, Coldren JT, Freeseman LJ. Individual differences in infant visual attention: are short lookers faster processors or feature processors? Child Dev 1991;62:1247–57. [PubMed] [Google Scholar]
- 50.Frick JE, Colombo J, Saxon TF. Individual and developmental differences in disengagement of fixation in early infancy. Child Dev 1999;70:537–48. [DOI] [PubMed] [Google Scholar]
- 51.Blaga OM, Colombo J. Visual processing and infant ocular latencies in the overlap paradigm. Dev Psychol 2006;42:1069–76. [DOI] [PubMed] [Google Scholar]
- 52.Richards JE. The development of sustained visual-attention in infants from 14 to 26 weeks of age. Psychophysiology 1985;22:409–16. [DOI] [PubMed] [Google Scholar]
- 53.Kannass KN, Oakes LM. The development of attention and its relations to language in infancy and toddlerhood. J Cogn Dev 2008;9:222–46. [Google Scholar]
- 54.Diamond A, Amso D. Contributions of neuroscience to our understanding of cognitive development. Curr Dir Psychol Sci 2008;17:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diamond A, Prevor MB, Callender G, Druin DP. Prefrontal cortex cognitive deficits in children treated early and continuously for PKU. Monogr Soc Res Child Dev 1997;62:1–208. [PubMed] [Google Scholar]
- 56.Diamond A. Understanding the A-not-B error: working memory vs. reinforced response, or active trace vs. latent trace. Dev Sci 1998;1:185–9. [Google Scholar]
- 57.Colombo J, Shaddy DJ, Anderson CJ, Gibson LJ, Blaga OM, Kannass KN. What habituates in infant visual habituation? A psychophysiological analysis. Infancy 2010;15:107–24. [DOI] [PubMed] [Google Scholar]
- 58.Siegel EH, Kordas K, Stoltzfus RJ, Katz J, Khatry SK, LeClerq SC, Tielsch JM. Inconsistent effects of iron-folic acid and/or zinc supplementation on the cognitive development of infants. J Health Popul Nutr 2011;29:593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colombo J, Carlson SE. Is the measure the message: the BSID and nutritional interventions. Pediatrics 2012;129:1166–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pribram KH, McGuinness D. Arousal, activation, and effort in the control of attention. Psychol Rev 1975;82:116–49. [DOI] [PubMed] [Google Scholar]
- 61.Cohen RA. Spatial determinants of attention. In: The neuropsychology of attention. New York: Plenum Press; 1993. p. 393–407.
- 62.Wainwright PE, Colombo J. Nutrition and the development of cognitive functions: interpretation of behavioral studies in animals and human infants. Am J Clin Nutr 2006;84:961–70. [DOI] [PubMed] [Google Scholar]
- 63.Colombo J, Carlson SE, Cheatham CL, Shaddy DJ, Kerling EH, Thodosoff JM, Gustafson KM, Brez C. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am J Clin Nutr 2013;98:403–12. [DOI] [PMC free article] [PubMed] [Google Scholar]