Abstract
Based on evidence of an increased risk of death, drug agencies issued safety warnings about the use of second generation antipsychotics (SGAs) in the elderly with dementia in 2004. This warning was extended to all antipsychotics in 2008. Little is known about the impact of these warnings on use.
We conducted a quasi-experimental study (interrupted time-series) in France, for 2003–2011, including subjects aged ≥65 with dementia and subjects aged ≥65 without dementia in the EGB database (1/97th representative random sample of claims from the main Health Insurance scheme). Outcomes were monthly rates of use of antipsychotics (by class and agent) and of 5 comparison drug classes (antidepressants, benzodiazepines, dermatologicals, antidiabetics, antiasthmatics). Trends were analyzed by joinpoint regression, impact of warnings by linear segmented regression.
In patients with dementia (n=7169), there was a 40% reduction in antipsychotic use from 14.2% in 2003 to 10.2% in 2011. The reduction began before 2004 and was unaffected by the warnings. Use of first generation antipsychotics declined over the period, while use of SGAs increased and leveled off from 2007. Use of the 5 comparison drug classes increased on the period. In subjects without dementia (n=91942), rates of overall antipsychotic use decreased from 2.3% in 2003 to 1.8% in 2011 with no effect of the warnings. Meanwhile, use of SGAs continuously increased from 0.37% to 0.64%.
Antipsychotic use decreased in the elderly between 2003 and 2011, especially in dementia. The timing of the decrease, however, did not coincide with safety warnings.
Keywords: Drug utilization/Trends, Elderly, Dementia, Alzheimer’s disease, Antipsychotics
1. Introduction
Most patients suffering from dementia experience behavioral and psychological symptoms (BPSD) (Chan et al., 2003; Lyketsos et al., 2002; Lyketsos et al., 2000; Mega et al., 1996; Sink et al., 2004), many of which are particularly disruptive for the patient and her caregivers such as agitation, aggression, oppositional behavior, delusions or hallucinations. BPSDs are associated with increased hospital lengths of stay (Wancata et al., 2003), institutionalization (Steele et al., 1990; Stern et al., 1997; Yaffe et al., 2002), and caregiver distress and depression (Black and Almeida, 2004; Kaufer et al., 1998). Antipsychotics have been commonly used to treat aggression or psychotic symptoms occurring in dementia in spite of only modest evidence of their efficacy (Ballard and Waite, 2006; Schneider et al., 2006; Sink et al., 2005), and lack of regulatory approval for use in dementia. In fact, risperidone is the only drug labeled for BPSD and only in some countries (i.e. approved in July 2008 in France).
The reassessment of clinical trials data showed an increased risk of death with some second generation antipsychotics (SGAs) prompting some countries to issue safety warnings. The first safety reports were published in 2002 in Canada (Wooltorton, 2002). In 2003, the US MedWatch issued a warning about an increased risk of cerebrovascular adverse events with risperidone (US Food and Drug Administration, 2003) and further articles reporting the higher risk of death were published in 2005 (Gill et al., 2005; Schneider et al., 2005). National drug agencies in the U.S., UK, Italy, Spain, etc. issued warnings about the increased risk of mortality associated with SGAs between 2004 and 2005. Afterwards, articles have compared the mortality risk between SGAs and first generation antipsychotics (FGAs) and concluded that FGAs shared that increased risk (Gill et al., 2007; Schneeweiss et al., 2007; Wang et al., 2005). Numerous drug agencies then issued warnings for FGAs in 2008.
The French drug agency (Agence Nationale de Sécurité du Médicament, formerly known as Agence Française de Sécurité Sanitaire des Produits de Santé AFSSaPS) issued three safety warnings on antipsychotics in dementia the first one on March 9th 2004 concerned the use of two SGAs (olanzapine and risperidone), the second on February 3rd 2005 concerned another SGA (aripiprazole; as this drug was not available at the time of the first warning) and the last one on December 9th 2008 extended the warning to all antipsychotics. Warnings were spread through “Dear health care provider” letters sent to physicians and pharmacists by firms. The new safety information was added to the drugs’ monographs and patient information leaflets found in every drug package. No study has so far assessed the impact of these warnings in France.
Few studies have investigated the impact of safety warnings on antipsychotic use in dementia patients abroad and only one provided information about the 2008 warning in the community or about all warnings. A study showed a modest impact of the warnings issued by Health Canada in Ontario (Valiyeva et al., 2008). Conflicting results have been published for the US. A first paper reported a huge decrease in antipsychotic use following the first US Food and Drug Administration warning (Dorsey et al., 2010), while another group reported only a small decline (Desai et al., 2012; Kales et al., 2011). In the region of Valencia in Spain, a decrease in doses of olanzapine and risperidone used by pensioners was seen following the 2004 safety warnings and 2005 prior authorization requirement (Sanfelix-Gimeno et al., 2009), and similar reductions have been reported in Scotland (Guthrie et al., 2013). Additionally, studies suggested a reduction in antipsychotic use in the US (Desai et al., 2012), and Italy (Franchi et al., 2012) but not in Germany (Schulze et al., 2013) following the 2004 warning and an audit found a reduction in antipsychotic use in elderly patients with dementia between 2006 and 2010 in England (Health and Social Care Information Centre, 2012), but these studies did not provide time series methodology. Last, one study found an impact of the 2008 warning on the use of antipsychotics in one teaching hospital in England (Thomas et al., 2013) and another one in 87 general practices in Scotland (Guthrie et al., 2013).
The aims of this study were to describe trends in the use of antipsychotics in elderly patients with dementia in France between 2003 and 2011 and to assess the impact of the French drug agency warnings on use. To take into account secular trends, we described trends in the use of other drug classes in dementia and of antipsychotics in elderly patients without dementia.
2. Experimental procedures
2.1. Design
We conducted a quasi-experimental study using interrupted time-series design for the period January 2003–July 2011.
2.2. Source of Data
The EGB (Echantillon Généraliste de Bénéficiaires) database contains claims for a 1/97th random sample of the population living in France (Tuppin et al., 2010). From 2003, it contains claims for visits to physicians and other health care providers, drugs dispensed in retail pharmacies and submitted for reimbursement, and from 2005 onward it contains information about hospital stays in medicine and surgery wards (no information is available for psychiatric wards, rehabilitation centers and long-term care facilities). Some medical information (with International classification of diseases (ICD) codes) is also available through two means: 1- a registry tracking patients with one or more of 30 chronic conditions which entitle patients to full coverage for care related to this chronic condition and 2- diagnoses recorded during hospital stays.
We restricted the database to individuals insured by the main health insurance scheme (all individuals but farmers, independent workers and public servants or retired people from these occupations, about 77% of the population (Tuppin et al., 2010)) because data for other schemes were only available after 2009.
2.3. Population
We were mainly interested in patients with dementia but we also identified a “control group” of elderly subjects without dementia to control for secular trends in utilization of antipsychotics for other uses.
2.3.1. Subjects with dementia
Dementia was defined by at least one of these 3 criteria:
the use of antidementia drugs (donepezil, rivastigmine, galantamine, memantine) defined as at least 2 reimbursements in a 12-month period, or
the registration with the chronic condition ‘Alzheimer’s disease or related diseases’, or
a hospitalization with a diagnosis code (main diagnosis, related diagnosis or associated diagnoses) for Alzheimer’s disease or related dementias (ICD10 codes: F00, F01, F02, F03, G30).
The first date for any of these criteria was the index date.
We excluded: 1-patients identified as suffering from a ‘chronic psychiatric illness’ (other than dementia) before the index date as antipsychotics were likely to be prescribed for another indication than BPSD, 2-patients younger than 65 years old at the index date and 3-patients present in the database for less than 31 days.
2.3.2. Subjects without dementia
To assess trends of antipsychotic use in a similar group of elderly patients without dementia, we identified patients 65 years of age and older with no criteria for dementia over the studied period and present in the database for 31 days and longer.
2.4. Duration of observation
Utilization patterns were measured till death, loss-to-follow-up (i.e. alive but with no pharmaceutical consumption for at least 3 months) or till July 31st 2011, whichever came first.
2.5 Drugs
Antipsychotics were identified as the N05A class (except lithium, veralipride and chlorproethazine as these drugs are not used in psychosis or agitation) in the anatomical therapeutic and chemical (ATC) classification (WHO Collaborating Centre for Drug Statistics Methodology, 2011). Amisulpride, clozapine, olanzapine, risperidone, aripiprazole (available from June 2004) were recorded as SGAs. Quetiapine was not available in France at that time.
In order to assess the natural trend of drug use in elderly patients with dementia, we selected 5 other drug classes as comparison classes that were not affected by any safety advisories during the study period (even though some publications have suggested some safety concerns for some of these classes) and are commonly used in this population: 1- selective serotonin reuptake inhibitors (SSRIs) (ATC code: N06AB), 2- benzodiazepine and benzodiazepine-like drugs (N05BA, N05CD, N03AE01), 3- drugs used in diabetes (A10), 4- drugs for obstructive airways diseases (R03) and 5- dermatologicals (D). In particular, benzodiazepines trends were interesting to study since these agents may have been substituted for antipsychotics following the antipsychotic warnings. We did not expect trends to have changed over the period for the other classes.
We recorded all drug dispensations in retail pharmacies and therefore captured the total use for these drug classes apart from use during hospital stays and in some nursing homes (with an internal pharmacy), which are not captured in the EGB database. There is no other source of financing for outpatient drugs in France
2.6. Outcomes
Between January 2003 and July 2011, we calculated the monthly prevalence of antipsychotic use and monthly mean daily doses for antipsychotics (any antipsychotic, any FGA, any SGA, risperidone, olanzapine) and five other drug classes.
Monthly prevalence of use was calculated as the number of users divided by the number of patients with dementia present in the database in that specific month. Persistent use of antipsychotics was identified as at least two dispensations of any antipsychotic over three consecutive months.
Mean daily doses were calculated each month as the mean defined daily dose (DDD) (WHO Collaborating Centre for Drug Statistics Methodology, 2011) used by the antipsychotic users. As no official DDD was available for cyamemazine, droperidol and carpipramine, we allocated to these drugs a DDD of 100mg, 20mg and 100mg respectively, using the usual dose recommended in France in psychotic disorders (Dictionnaire Vidal, 2011).
2.7. Analysis
2.7.1. Trends
We first inspected trends for outliers. An important drop in the number of patients with dementia using antipsychotics was seen in August 2003 (65 patients stopped using antipsychotics that month) which coincided with an exceptional heat wave in France (Michenot et al., 2006) (it is likely that these patients did not stop using antipsychotics but were hospitalized). We replaced this outlying value by the mean of values for July 2003 and September 2003. Also, in order to focus on trends, we used 3-month moving average series to smooth monthly fluctuations.
We used joinpoint regression models to identify possible breaks in trends. The main advantage of this technique is its flexibility; we did not have to specify dates when trends were expected to change a priori. These models assume a linear trend between joinpoints (or breaks in trends) and continuity at the joinpoints. We specified the range of the number of joinpoints to be tested between 0 and 2 (since there were 2 warnings). First, all the possible locations for joinpoints were identified using a grid search method. The best possible fits were determined by testing the sum square of errors at each location. Then, the optimal model (i.e. the number of joinpoints) was determined using a series of permutation tests based on F-statistics by a Monte Carlo simulation approach (Kim et al., 2000). These two steps were automatically performed using the Joinpoint Regression Program V3.5 (Statistical Methodology and Applications Branch and Data Modeling Branch, Surveillance Research Program, National Cancer Institute).
2.7.2. Impact of safety warnings on the use of antipsychotics in elderly patients with dementia
Given the very limited use of aripiprazole in dementia (it accounted for 0.07% of antipsychotic use in 2005 and 0.89% in 2011 in subjects with dementia) we chose to dismiss the 2005 warning about aripiprazole and rather studied the impact of the 2 main warnings on antipsychotic use.
Therefore, we conducted segmented regression analyses (Wagner et al., 2002) on 3 segments based on the time of the 2 main warnings: 1- pre-SGA warning segment (January 1 2003 – February 29 2004), 2- post-SGA warning and pre-FGA warning segment (April 1 2004 – November 31 2008) and 3- post-warning for all antipsychotics segment (January 1 2009 – July 30 2011). The models assume a linear trend on each segment and quantify the immediate changes in level of use between the last month of one segment and the first month of the next. In order to account for autocorrelation in our series, we used the stepwise autoregression method implemented in SAS software (determination of a high-order model with many autoregressive lags and then sequential removal of autoregressive parameters until all remaining autoregressive parameters have significant t tests). The significant autocorrelation parameters are included in the segmented regression models. We checked for the absence of heteroskedasticity using autoregressive conditional heteroskedasticity (ARCH) tests and residual autocorrelation for the chosen models using Ljung-Box tests. In order to account for a delayed impact of the warnings, we introduced various lags in the segmented regression analyses (3, 6, 12 months) after the dates of the warnings in the segmented regression analyses. The results did not substantially change and are not reported herein. Analyses were conducted using SAS V9.3 (SAS Institute, Cary, NC, USA).
2.7.3. Sensitivity analyses
We inspected trends on subsamples to assess the robustness of our findings. First, to check that the way we had defined dementia did not affect our findings, we used 4 other definitions: 1- at least 2 dispensations of antidementia drugs over a 12-month period, 2- at least 2 dispensations of cholinesterase inhibitors (donepezil, galantamine, rivastigmine) over a 12-month period, 3- registration with the chronic condition “Alzheimer’s disease and related disorders”, 4- hospital diagnoses for dementia. Second, to assess the impact of the warnings on a population of patients newly diagnosed with dementia, we excluded patients with a prevalent dementia and included only incident cases (i.e. with at least a 6-month window before the index date).
2.8 Ethics
Ethical approval was not required for this study since the analysis was carried out on an anonymised database.
3. Results
3.1 Patients
We identified 7169 patients with dementia (table 1). They were mainly women (70.4%), aged on average of 82.4 years (standard deviation (sd)= 6.9) at the index date. On average patients received 16.1 (sd= 9.4) different drugs during the year following the index date, and in particular, 56.9% used antidementia drugs. Median follow-up was 2.1 years (mean= 2.7, sd= 2.3) and 25% of patients were present in the database for more than 4 years.
Table 1.
Characteristics of patients with dementia at index date
| Variable | N=7169 | % |
|---|---|---|
| Female sex | 5048 | 70.4 |
| Age, years | ||
| 65–74 | 1062 | 14.8 |
| 75–84 | 3580 | 49.9 |
| ≥85 | 2527 | 35.3 |
| Drug prescriptionsa | ||
| Antidementia drugs | 3022 | 56.9 |
| Donepezil | 1875 | 26.8 |
| Rivastigmine | 947 | 13.5 |
| Galantamine | 943 | 13.5 |
| Memantineb | 981 | 14.0 |
| Antipsychotics | 1739 | 24.8 |
| First generation antipsychotics | 1159 | 16.5 |
| Second generation antipsychotics | 805 | 11.5 |
| Antidepressants | 3163 | 45.2 |
| Selective serotonin reuptake inhibitors | 2123 | 30.3 |
| Antiepileptics | 828 | 11.8 |
| Anxiolytics-hypnotics | 3223 | 46.0 |
| Cardiovascular drugs | 5528 | 78.9 |
| Cardiac therapy | 2261 | 32.3 |
| Central antihypertensives | 407 | 5.8 |
| Diuretics | 2148 | 30.7 |
| Peripheral vasodilators | 639 | 9.1 |
| Vasoprotectives | 887 | 12.7 |
| Beta blocking agents | 1549 | 22.1 |
| Calcium channel blockers | 1563 | 22.3 |
| Agents acting on the renin-angiotensin system | 2676 | 38.2 |
| Lipid modifying agents | 1931 | 27.6 |
| Drugs used in diabetes | 989 | 14.2 |
| Drugs for obstructive airway diseases | 932 | 13.3 |
| Dermatologicals | 3400 | 48.5 |
| Comorbidities (registration with chronic condition) | ||
| Stroke | 325 | 4.5 |
| Severe cardiac disorder (heart failure, arrhythmia, valvulopathy, congenital heart disease) | 588 | 8.2 |
| Coronary disease | 563 | 7.8 |
| Diabetes mellitus | 797 | 11.1 |
| Parkinson disease | 246 | 3.4 |
| Cancer | 794 | 11.1 |
during the 12 months following index date (n=7006)
Memantine was available on the French market in August 2003 (approval in May 2002).
Drugs were defined in the ATC classification: Antidementia drugs (N06D excluding N06DX02), Antipsychotics (N05A excluding N05AN01, N05AL06, N05AB04), First generation antipsychotics (Antipsychotics excluding second generation antipsychotics), Second generation antipsychotics (N05AL05, N05AH02, N05AH03, N05AX08, N05AX12), Antidepressants (N06A), Selective serotonin reuptake inhibitors (N06AB), Antiepileptics (N03), Anxiolytics-hypnotics (N05B, N05C), Cardiovascular drugs (C), Cardiac therapy (C01), Central antihypertensives (C02), Diuretics (C03), Peripheral vasodilators (C04), Vasoprotectives (C05), Beta blocking agents (C07), Calcium channel blockers (C08), Agents acting on the renin-angiotensin system (C09), Drugs used in diabetes (A10), Drugs for obstructive airway diseases (R03), Dermatologicals (D).
Overall, 2714 patients (37.9%; 95%CI [36.7–39.0]) had any antipsychotic use at any point between 2003 and 2011. The majority of users (on average 91.2%) consumed antipsychotics on a persistent basis. This proportion remained steady over the study period (data not shown). Between 2003 and 2011, 1251 (46.1%) patients had used FGAs, 784 (28.9%) SGAs, and 679 (25.0%) both FGAs and SGAs. Overall, risperidone and olanzapine accounted for 79.3% and 15.0% of SGA use, respectively.
3.2 Trends
3.2.1 Prevalence of antipsychotic use
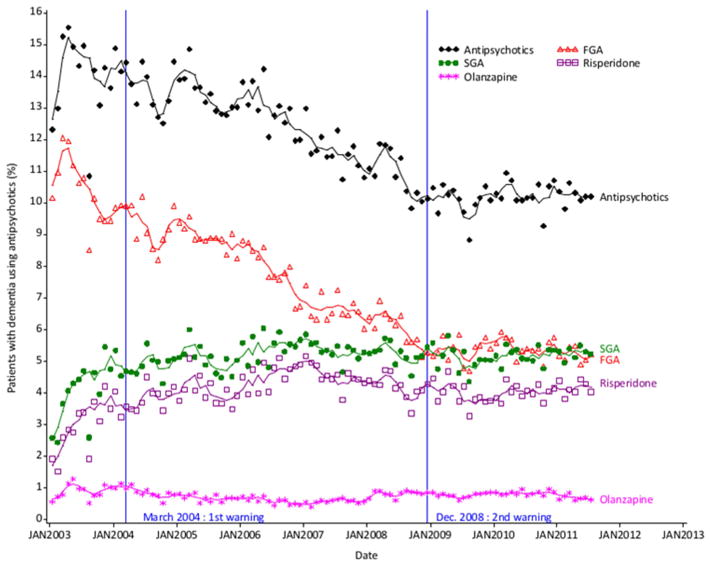
Figure 1 shows trends in antipsychotic rates of use. The joinpoint analysis identified one break in trend for overall antipsychotics in September 2003. After an initial growing trend until the end of 2003, mean monthly rates of antipsychotic use gradually decreased from 14.2% in 2003 to 10.2% in 2011 (slopes: 0.15 from January 2003 to September 2003 and −0.06 afterwards).
Fig. 1.
Trends in monthly prevalence of overall antipsychotic, FGA, SGA, risperidone and olanzapine use in patients with dementia
Symbols represent observations. Lines represent 3-month moving average series.
Abbreviations: FGA: first generation antipsychotic, SGA: second generation antipsychotic.
There was no breakpoint in the trend for FGAs: the use of FGAs gradually decreased over the period (slope: −0.06). In contrast, the use of SGAs increased sharply until December 2003, then increased moderately between December 2003 and January 2007 and finally leveled off to about 50% of overall antipsychotic use (slopes: 0.23 from January 2003 to December 2003, 0.01 from January 2004 to January 2007 and −0.01 afterwards).
Divergent trends were seen for olanzapine and risperidone. The share of olanzapine in SGA use declined from 21.9% in 2003 to 13.3% in 2011, while the share of risperidone increased from 73.4% to 78.8% on the same period.
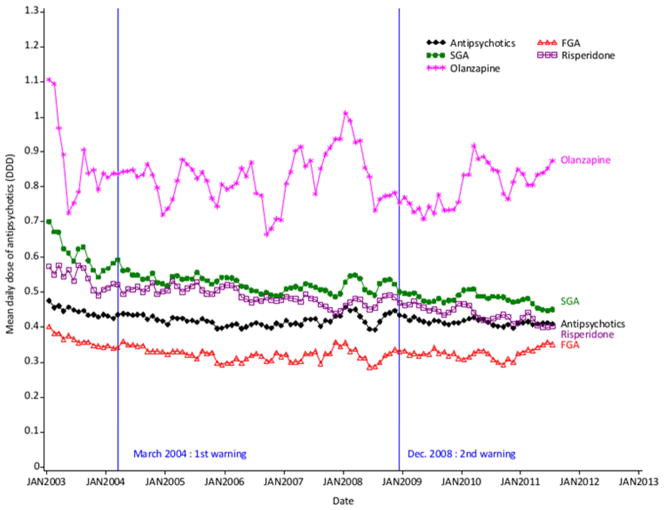
3.2.2 Doses
Mean daily doses of SGAs were consistently higher than for FGAs (0.52 vs. 0.33 DDDs, p<0.001) over the period. FGA doses remained constant, whereas SGA mean doses decreased from 0.61 DDDs in 2003 to 0.46 in 2011, with divergent trends for risperidone and olanzapine (figure 2).
Fig. 2.
Trends in mean daily dose of antipsychotics, FGAs and SGAs, risperidone and olanzapine among users with dementia (3-month moving average series)
Abbreviations: DDD: defined daily dose, FGA: first generation antipsychotic, SGA: second generation antipsychotic.
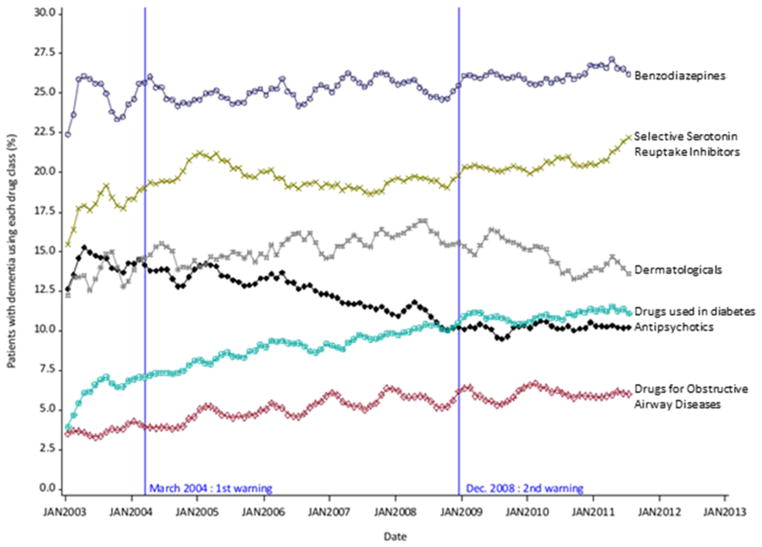
3.2.3. Use of other drugs
Among the 5 other drug classes studied, all exhibited a growing trend over 2003–2011 in patients with dementia (figure 3). No changes in trend were seen in relation with the antipsychotics warning (in particular for benzodiazepines).
Fig. 3.
Trends in monthly prevalence of use for overall antipsychotics and other drug classes in patients with dementia (3 month-moving average series)
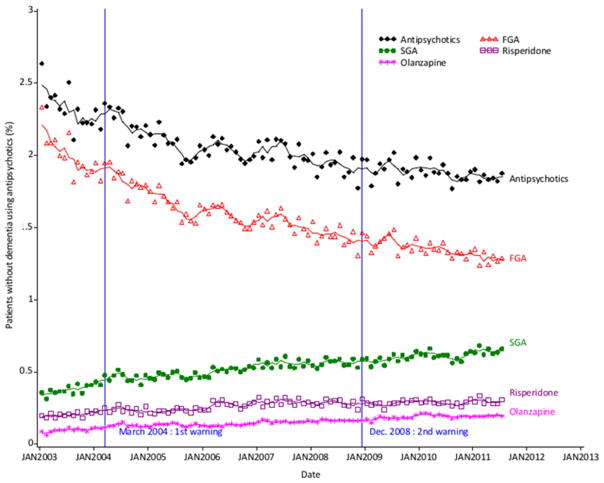
3.2.4. Antipsychotic use in elderly people without dementia
Among 91,942 elderly subjects without dementia (mean age=71.0 years (sd=7.2), 57% of women, 3.6% registered with a chronic psychiatric condition), mean monthly rate of antipsychotic use was 2.0% on the period. The trend in use of overall antipsychotics followed a similar pattern to that in patients with dementia (decreasing from 2.3% in 2003 to 1.8% in 2011), while the use of SGAs continuously increased from 0.37% in 2003 to 0.64% in 2011 (figure 4). There were no abrupt changes following the warnings in the use of antipsychotics.
Fig. 4.
Trends in monthly prevalence of overall antipsychotic, FGA, SGA use in patients without dementia
Symbols represent observations. Lines represent 3-month moving average series.
Abbreviations: FGA: first generation antipsychotic, SGA: second generation antipsychotic.
3.3. Impact of warnings
3.3.1. 1st warning
Compared to the pre warning period, the slope of the second segment (post-SGA warning and pre-FGA warning) was significantly lower for overall antipsychotics (0.073 vs. −0.067, p<10−4) and SGAs (0.133 vs. 0.004, p<10−4) but not for FGAs (−0.037 vs. −0.078, p=0.108) (segmented regressions). However, the use of SGAs (risperidone, but not olanzapine) kept increasing until January 2007 and then leveled off till 2011 as shown by the joinpoint analysis.
3.3.2. 2nd warning
No decrease was associated with the second warning on any of the studied series. Moreover, the gradual decline that was seen for overall antipsychotics and FGAs since 2003 came to an end and the use of FGAs leveled off in 2009–2011 (figure 1).
3.4. Sensitivity analyses
3.4.1. Different definitions of dementia
Whatever the criteria used to define dementia, at least 2 dispensations of antidementia drugs over a 12-month period (n=4216), at least 2 dispensations of cholinesterase inhibitors over a 12-month period (n=3572), registration with the chronic condition “Alzheimer’s disease and related disorders” (n=4090), hospital diagnoses for dementia (n=4754), we observed similar trends in antipsychotic use to the ones reported above (supplementary figure 1).
3.4.2. Patients with incident dementia
Due to the short period available to us before the first warning, there were too few people newly diagnosed with dementia before the first warning (i.e. n=23 in August 2003 and n=244 in February 2004) to provide an accurate assessment of the impact of the first warning in incident patients. We plotted trends for antipsychotic use in this subgroup of patients and observed somewhat similar trends with a lower level of overall use and a higher use of SGAs (supplementary figure 2).
4. Discussion
The use of antipsychotics in the elderly decreased in France between 2003 and 2011. This was particularly the case for patients with dementia. Divergent trends were seen between classes with a decline of FGAs and an increase in SGA use. If overall use decreased, it did not coincide with safety warnings published by the French drug agency in 2004 and 2008. Additionally, we did not observe a decrease in mean dose, persistent use, or an impact in patients newly diagnosed with dementia. However, the comparison of SGA trends in patients with and without dementia suggests a specific change of practice in dementia where SGA use leveled off from January 2007.
Comparison with previous publications that assessed the impact of warnings on antipsychotic use is difficult for four main reasons. First for analytical reasons since some studies did not report detailed enough data (e.g. mean use for 2-year periods (Desai et al., 2012) or annual use (Franchi et al., 2012; Schulze et al., 2013)) or did not use standardized data (e.g. no denominator (Dorsey et al., 2010; Sanfelix-Gimeno et al., 2009)) or used the elderly population as a denominator (Valiyeva et al., 2008)). Second, the only study that used similar standardization methods was conducted in the US Veterans Affairs’ health care system and thus included only 3% of women (Kales et al., 2011), which raises some generalizability issues. Third, safety warnings were combined with prior authorization requirements for the use of antipsychotics in the elderly in some of the settings (Sanfelix-Gimeno et al., 2009). Last, patterns of use of antipsychotics in France are very different from what is observed in North America where SGAs took over most of the market by 1999 (Gallini et al., 2013). The decreasing trend in overall antipsychotic use observed before the first warning in our study was only found in some previous publications (Franchi et al., 2012; Kales et al., 2011; Schulze et al., 2013).
Response to safety warnings is mixed depending on the drug, condition it treats, the nature of the risks and the treatment setting (Dusetzina et al., 2012; Reber et al., 2013). Prior analyses would have predicted the antipsychotic warning to have a significant effect on prescribing because antipsychotics are mainly prescribed by general practitioners in France (Lecadet et al., 2003; Martin et al., 2004), who tend to be more responsive to warnings, and because there was an increased risk of death (Reber et al., 2013). Yet, like suggested in Germany (Schulze et al., 2013), the safety warnings in France did not seem to have an impact on antipsychotic prescribing. Some factors specific to our study setting may explain the negative results we report. It is likely that the pre-warning decreasing trend in overall antipsychotic use and the lower use of antipsychotics in dementia observed in our study than in the US (Kales et al., 2011) may have prevented us from showing any impact of the warning. Besides, content of the warnings may not have been optimal as they did not inform physicians with guidelines and alternatives for the management of BPSD. The lack of providing alternatives is a factor of poor efficacy for advisories (Bahri, 2010; Dusetzina et al., 2012). Indeed, recommendations for the management of BPSDs were only published by a separate agency (French Health Authority) in May 2009 and in between mixed signals have been sent to prescribers since risperidone was approved for BPSD in July 2008.
Our study has some limitations. First, time series analysis is one of the strongest designs for the analysis of the longitudinal effects of interventions (Wagner et al., 2002). However it does not allow us to disentangle the effect of the warnings from concurrent events. In particular, some important events occurred after the 1st warning: the release of new information about SGA effectiveness in 2006 (Schneider et al., 2006), the approval of risperidone for BPSD in July 2008, the publication of the “dementia antipsychotic withdrawal trial” in January 2009 (Ballard et al., 2009), the recommendations for management of BPSD by the French Health Authority in May 2009 (Haute Autorité de Santé, 2009). Also, we were unable to correlate trends in antipsychotic use with trends in industry promotion which is recognized as a key factor driving prescribing (Wazana, 2000). Second, the dementia diagnoses of the patients were not ascertained and some patients with undiagnosed dementia may have been included in the group without dementia. But according to a recent publication, dementia is currently fairly well recognized in the health insurance database (Bertrand et al., 2013). Using two of the three criteria we used to define dementia, the authors estimated they had identified 75% of patients diagnosed with dementia. We also had no information about the appropriateness of antipsychotic prescribing since we had no access to clinical data. Third, besides the excluded insurance schemes (farmers, independent workers), an unknown proportion of nursing home patients (who are more likely to use antipsychotics (Alzheimer Cooperative Valuation in Europe: The European Joint Action on Dementia, 2013)) were not included because drug consumption of residents living in nursing homes with an internal pharmacy is not recorded in the EGB database. However, we did not only include community-dwelling people and were unable to know the patients’ living arrangements and thus to identify nursing home patients. Therefore, we mainly provided an assessment of the impact of safety warnings for patients living in the community, and it is possible that other trends may be found in other settings like nursing homes or hospitals. Last, the patterns of use of APs are not perfectly captured by claims data. Especially, if elderly people use low dose of antipsychotics (they may have treatment leftovers that are carried on for months), drug dispensing may not be a good proxy for drug intake. We used smoothed series (3-month moving average) to minimise this limitation.
5. Conclusion
This study, using a national representative sample for an 8.5-year period, is the first to assess the impact of the 2008 safety warning on antipsychotic use in dementia. Despite the apparent lack of impact of the safety warnings published by the drug agency, antipsychotic use clearly decreased in patients with dementia between 2003 and 2011 in France..
Supplementary Material
Acknowledgments
This research was supported by two unconditional grants from the Direction de la recherche, des études, de l’évaluation et des statistiques, Ministry for Health, France and the Ministry of Higher Education and Research (Plan Alzheimer 2008–2012), France.
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
The authors would like to thank Prof. JL Montastruc, Dr A Piau, Dr H Bagheri, Dr J Micaleff-Roll, Dr F Montastruc for their valuable comments for the interpretation of the data and preparation of the manuscript and the Fondation Plan Alzheimer for their support.
List of abbreviations
- ATC
anatomical, therapeutic and chemical (classification)
- BPSD
behavioral and psychological symptoms of dementia
- DDD
defined daily dose
- EGB
échantillon généraliste de bénéficiaires
- FGA
first generation antipsychotics
- ICD
international classification of diseases
- SGA
second generation antipsychotics
Footnotes
The authors have no conflict of interest relevant to the content of this study.
References
- Synthesis report. 2013. Alzheimer Cooperative Valuation in Europe: The European Joint Action on Dementia. [Google Scholar]
- Bahri P. Public pharmacovigilance communication: a process calling for evidence-based, objective-driven strategies. Drug Saf. 2010;33:1065–1079. doi: 10.2165/11539040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Ballard C, Hanney ML, Theodoulou M, Douglas S, McShane R, Kossakowski K, Gill R, Juszczak E, Yu LM, Jacoby R. The dementia antipsychotic withdrawal trial (DART-AD): long-term follow-up of a randomised placebo-controlled trial. Lancet Neurol. 2009;8:151–157. doi: 10.1016/S1474-4422(08)70295-3. [DOI] [PubMed] [Google Scholar]
- Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006:CD003476. doi: 10.1002/14651858.CD003476.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand M, Tzourio C, Alperovitch A. Trends in recognition and treatment of dementia in france analysis of the 2004 to 2010 database of the national health insurance plan. Alzheimer Dis Assoc Disord. 2013;27:213–217. doi: 10.1097/WAD.0b013e3182695a3b. [DOI] [PubMed] [Google Scholar]
- Black W, Almeida OP. A systematic review of the association between the Behavioral and Psychological Symptoms of Dementia and burden of care. Int Psychogeriatr. 2004;16:295–315. doi: 10.1017/s1041610204000468. [DOI] [PubMed] [Google Scholar]
- Chan DC, Kasper JD, Black BS, Rabins PV. Prevalence and correlates of behavioral and psychiatric symptoms in community-dwelling elders with dementia or mild cognitive impairment: the Memory and Medical Care Study. Int J Geriatr Psychiatry. 2003;18:174–182. doi: 10.1002/gps.781. [DOI] [PubMed] [Google Scholar]
- Desai VC, Heaton PC, Kelton CM. Impact of the Food and Drug Administration’s antipsychotic black box warning on psychotropic drug prescribing in elderly patients with dementia in outpatient and office-based settings. Alzheimers Dement. 2012;8:453–457. doi: 10.1016/j.jalz.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Dictionnaire Vidal. 2011 [Google Scholar]
- Dorsey ER, Rabbani A, Gallagher SA, Conti RM, Alexander GC. Impact of FDA black box advisory on antipsychotic medication use. Arch Intern Med. 2010;170:96–103. doi: 10.1001/archinternmed.2009.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusetzina SB, Higashi AS, Dorsey ER, Conti R, Huskamp HA, Zhu S, Garfield CF, Alexander GC. Impact of FDA drug risk communications on health care utilization and health behaviors: a systematic review. Med Care. 2012;50:466–478. doi: 10.1097/MLR.0b013e318245a160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi C, Tettamanti M, Marengoni A, Bonometti F, Pasina L, Cortesi L, Fortino I, Bortolotti A, Merlino L, Lucca U, Riva E, Nobili A. Changes in trend of antipsychotics prescription in patients treated with cholinesterase inhibitors after warnings from Italian Medicines Agency. Results from the EPIFARM-Elderly Project. Eur Neuropsychopharmacol. 2012;22:569–577. doi: 10.1016/j.euroneuro.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Gallini A, Donohue JM, Huskamp HA. Diffusion of Antipsychotics in the U.S. and French Markets, 1998–2008. Psychiatr Serv. 2013 doi: 10.1176/appi.ps.004662012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Bronskill SE, Normand SL, Anderson GM, Sykora K, Lam K, Bell CM, Lee PE, Fischer HD, Herrmann N, Gurwitz JH, Rochon PA. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146:775–786. doi: 10.7326/0003-4819-146-11-200706050-00006. [DOI] [PubMed] [Google Scholar]
- Gill SS, Rochon PA, Herrmann N, Lee PE, Sykora K, Gunraj N, Normand SL, Gurwitz JH, Marras C, Wodchis WP, Mamdani M. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ. 2005;330:445. doi: 10.1136/bmj.38330.470486.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie B, Clark SA, Reynish EL, McCowan C, Morales DR. Differential impact of two risk communications on antipsychotic prescribing to people with dementia in Scotland: segmented regression time series analysis 2001–2011. PLoS One. 2013;8:e68976. doi: 10.1371/journal.pone.0068976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haute Autorité de Santé. Recommendations of practice. 2009. Alzheimer’s disease and related diseases: mangement of perturbating behavioral disorders. [Google Scholar]
- Health and Social Care Information Centre. National dementia and antipsychotic prescribing audit. 2012. [Google Scholar]
- Kales HC, Zivin K, Kim HM, Valenstein M, Chiang C, Ignacio RV, Ganoczy D, Cunningham F, Schneider LS, Blow FC. Trends in antipsychotic use in dementia 1999–2007. Arch Gen Psychiatry. 2011;68:190–197. doi: 10.1001/archgenpsychiatry.2010.200. [DOI] [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Christine D, Bray T, Castellon S, Masterman D, MacMillan A, Ketchel P, DeKosky ST. Assessing the impact of neuropsychiatric symptoms in Alzheimer’s disease: the Neuropsychiatric Inventory Caregiver Distress Scale. J Am Geriatr Soc. 1998;46:210–215. doi: 10.1111/j.1532-5415.1998.tb02542.x. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Lecadet J, Vidal P, Baris B, Vallier N, Fender P, Allemand H, groupe Medipath Psychotropic medications: prescriptions and use in metropolitan France. I. National data for 2000. Rev Med Ass Maladie. 2003;34:75–84. [Google Scholar]
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Steinberg M, Tschanz JT, Norton MC, Steffens DC, Breitner JC. Mental and behavioral disturbances in dementia: findings from the Cache County Study on Memory in Aging. Am J Psychiatry. 2000;157:708–714. doi: 10.1176/appi.ajp.157.5.708. [DOI] [PubMed] [Google Scholar]
- Martin K, Begaud B, Verdoux H, Lechevallier N, Latry P, Moore N. Patterns of risperidone prescription: a utilization study in south-west France. Acta Psychiatr Scand. 2004;109:202–206. doi: 10.1046/j.0001-690x.2003.00238.x. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996;46:130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- Michenot F, Sommet A, Bagheri H, Lapeyre-Mestre M, Montastruc JL. Adverse drug reactions in patients older than 70 years during the heat wave occurred in France in summer 2003: A study from the French PharmacoVigilance Database. Pharmacoepidemiol Drug Saf. 2006;15:735–740. doi: 10.1002/pds.1284. [DOI] [PubMed] [Google Scholar]
- Reber KC, Piening S, Wieringa JE, Straus SM, Raine JM, de Graeff PA, Haaijer-Ruskamp FM, Mol PG. When direct health-care professional communications have an impact on inappropriate and unsafe use of medicines. Clin Pharmacol Ther. 2013;93:360–365. doi: 10.1038/clpt.2012.262. [DOI] [PubMed] [Google Scholar]
- Sanfelix-Gimeno G, Cervera-Casino P, Peiro S, Lopez-Valcarcel BG, Blazquez A, Barbera T. Effectiveness of safety warnings in atypical antipsychotic drugs: an interrupted time-series analysis in Spain. Drug Saf. 2009;32:1075–1087. doi: 10.2165/11316520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Schneeweiss S, Setoguchi S, Brookhart A, Dormuth C, Wang PS. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176:627–632. doi: 10.1503/cmaj.061250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- Schneider LS, Tariot PN, Dagerman KS, Davis SM, Hsiao JK, Ismail MS, Lebowitz BD, Lyketsos CG, Ryan JM, Stroup TS, Sultzer DL, Weintraub D, Lieberman JA. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355:1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- Schulze J, van den Bussche H, Glaeske G, Kaduszkiewicz H, Wiese B, Hoffmann F. Impact of safety warnings on antipsychotic prescriptions in dementia: Nothing has changed but the years and the substances. Eur Neuropsychopharmacol. 2013 doi: 10.1016/j.euroneuro.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Sink KM, Covinsky KE, Newcomer R, Yaffe K. Ethnic differences in the prevalence and pattern of dementia-related behaviors. J Am Geriatr Soc. 2004;52:1277–1283. doi: 10.1111/j.1532-5415.2004.52356.x. [DOI] [PubMed] [Google Scholar]
- Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293:596–608. doi: 10.1001/jama.293.5.596. [DOI] [PubMed] [Google Scholar]
- Steele C, Rovner B, Chase GA, Folstein M. Psychiatric symptoms and nursing home placement of patients with Alzheimer’s disease. Am J Psychiatry. 1990;147:1049–1051. doi: 10.1176/ajp.147.8.1049. [DOI] [PubMed] [Google Scholar]
- Stern Y, Tang MX, Albert MS, Brandt J, Jacobs DM, Bell K, Marder K, Sano M, Devanand D, Albert SM, Bylsma F, Tsai WY. Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA. 1997;277:806–812. [PubMed] [Google Scholar]
- Thomas SK, Hodson J, McIlroy G, Dhami A, Coleman JJ. The Impact of Direct Healthcare Professional Communication on Prescribing Practice in the UK Hospital Setting: An Interrupted Time Series Analysis. Drug Saf. 2013 doi: 10.1007/s40264-013-0057-3. [DOI] [PubMed] [Google Scholar]
- Tuppin P, de Roquefeuil L, Weill A, Ricordeau P, Merliere Y. French national health insurance information system and the permanent beneficiaries sample. Rev Epidemiol Sante Publique. 2010;58:286–290. doi: 10.1016/j.respe.2010.04.005. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration. 2003 [Google Scholar]
- Valiyeva E, Herrmann N, Rochon PA, Gill SS, Anderson GM. Effect of regulatory warnings on antipsychotic prescription rates among elderly patients with dementia: a population-based time-series analysis. CMAJ. 2008;179:438–446. doi: 10.1503/cmaj.071540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- Wancata J, Windhaber J, Krautgartner M, Alexandrowicz R. The consequences of non-cognitive symptoms of dementia in medical hospital departments. Int J Psychiatry Med. 2003;33:257–271. doi: 10.2190/ABXK-FMWG-98YP-D1CU. [DOI] [PubMed] [Google Scholar]
- Wang PS, Schneeweiss S, Avorn J, Fischer MA, Mogun H, Solomon DH, Brookhart MA. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335–2341. doi: 10.1056/NEJMoa052827. [DOI] [PubMed] [Google Scholar]
- Wazana A. Physicians and the pharmaceutical industry: is a gift ever just a gift? JAMA. 2000;283:373–380. doi: 10.1001/jama.283.3.373. [DOI] [PubMed] [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD index. 2011. [Google Scholar]
- Wooltorton E. Risperidone (Risperdal): increased rate of cerebrovascular events in dementia trials. CMAJ. 2002;167:1269–1270. [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Fox P, Newcomer R, Sands L, Lindquist K, Dane K, Covinsky KE. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287:2090–2097. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.