Abstract
To whom should a male directs his mating? While it is a critical social interaction, little is known about molecular and cellular mechanisms controlling mammalian sexual preference. Here we report that the neurotransmitter 5-HT is required for male sexual preference. Male mice lacking central serotonergic neurons lost sexual preference but were not generally defective in olfaction. A role for 5-hydroxytryptamine (5-HT) was demonstrated by the phenotype of mice unable to synthesize 5-HT in the brain when lacking tryptophan hydroxylase 2 (Tph2). 5-hydroxytryptophan (5-HTP) injection rescued the phenotype of adult Tph2 knockout mice within 35 minutes. These results indicate that 5-HT and serotonergic neurons in the adult brain regulate mammalian sexual preference.
Interactions between members of the opposite sex are essential for sexually reproducing animals and provide fascinating subjects for biological studies. Evolutionary benefits have been hypothesized for homo- and bi-sexual traits1, 2 which exist in many animals2 from American bulls3 to Japanese rhesus monkeys4. Studies of animals with different sexual preference are essential for understanding the seemingly simple decision of a male to court a female, whose molecular and cellular mechanisms remain unclear.
Research in Drosophila has uncovered genes required for Drosophila courtship preference, but none of their homologs have been shown to affect mammalian sexual preference. Research in mammals has demonstrated that pheromone sensing in the periphery is important for sexual preference. Male mice lacking TrpC2 (TrpC2−/−), which encoded a channel expressed in the vomeronasal organ (VNO), mounted other males, emitted ultrasonic vocalization (USV) towards males, and were less aggressive towards males5, 6. Our understanding of the central mechanisms for sexual preference remains limited.
The neurotransmitter 5-hydroxytryptamine (5-HT) has been implicated in male sexual behaviors such as erection, ejaculation and orgasm in mice and humans7, 8. Depletion of 5-HT by treating animals with p-chlorophenylalanine (pCPA) or tryptophan-free diets induced male-male mounting9–11. However, pCPA treatment was thought to increase sexual activity whereas its effect on sexual preference has not been investigated. Interpretation of pCPA results was further complicated by the lack of specificity: pCPA could affect noradrenaline (NA) and dopamine (DA) at higher concentrations12.
Almost all serotonergic neurons in the brain were missing from embryogenesis to adulthood in Lmx1b conditional knockout mice in which the floxed-Lmx1b allele was deleted by ePet1-Cre13. We compared the behaviors of male mice of different genotypes: ePet1-Cre/Lmx1bfloxp/Lmx1bfloxp as homozygous mutants (Lmx1b−/−), its littermates ePet1-Cre/Lmx1bfloxp/+ as heterozygous mutants (Lmx1b+/−), and Lmx1bfloxp/Lmx1bfloxp without ePet1-Cre as the wild-type (wt) (Lmx1b+/+). We also used ePet1-Cre without Lmx1bfloxp as a control.
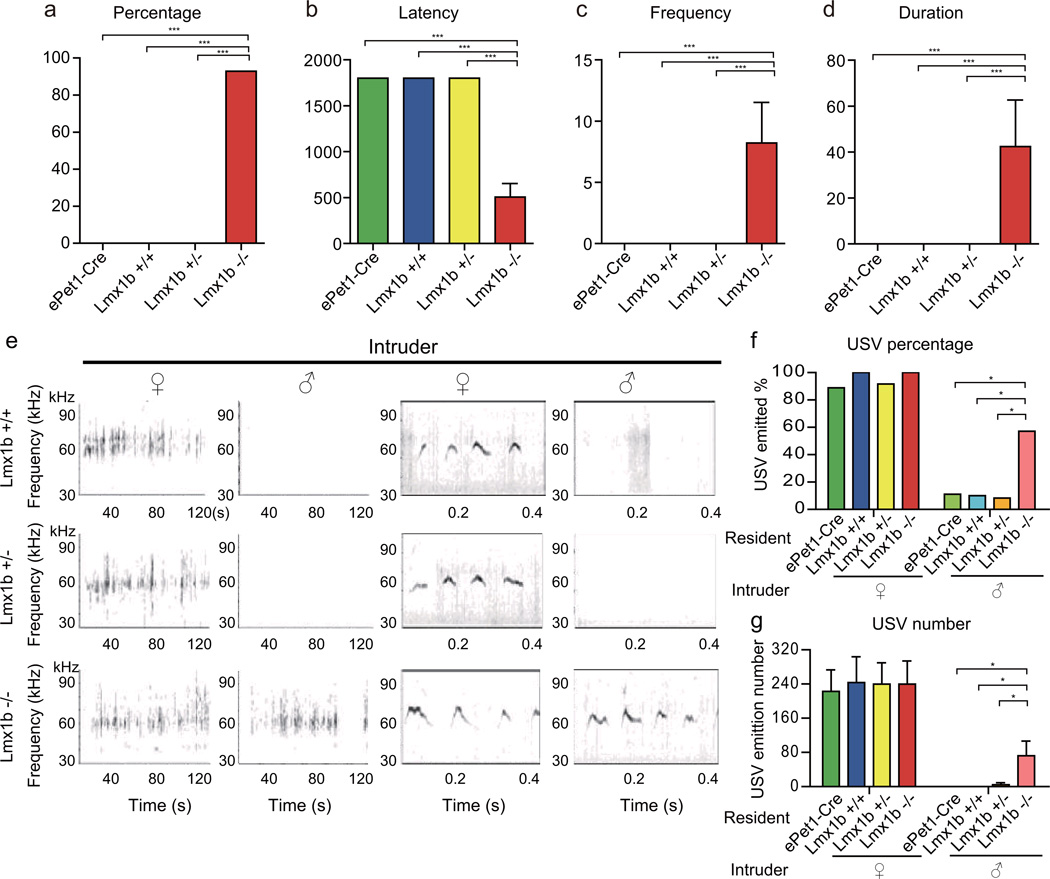
We first tested how a male responded in his home cage when a wt target C57 male was introduced. Compared to the ePet1-Cre, Lmx1b+/+ and Lmx1b+/− controls, Lmx1b−/− mice showed significantly more mounting to male intruders (Fig. 1, Supplementary Movie 1, and see Supplementary Data Set 1 for numbers of animals used and statistics for all figures). The percentage of males who mounted target males was significantly higher in Lmx1b−/− males than ePet1-Cre, Lmx1b+/− and Lmx1b+/+ males (Fig. 1a). Lmx1b−/− males mounted with a shorter latency (Fig. 1b), higher frequency (Fig. 1c), and longer duration (Fig. 1d). These results show that the absence of serotonergic neurons in the brain increased male-male mounting.
Figure 1. Male-male mounting and USV by mice lacking central serotonergic neurons.
The numbers of animals used and statistical analysis are all included in Supplementary Data 1. In all figures, * indicates p<0,05, ** p<0.01 and *** p<0.001. a–d a test male was presented in its home cage with an adult wt male and its behavior was recorded for 30 min (all data shown as mean±SEM). Compared with Lmx1b+/+, Lmx1b+/− or ePet1-Cre, Lmx1b−/− males mounted males at a higher percentage (a), lower latency (b), higher frequency (c) and longer duration (d). e, Typical USV patterns emitted by males when presented with female or male intruders. The two left panels show USVs in 2 min, while the two right panels show parts of USV graphs at higher magnifications. The X axis shows time in seconds and the Y axis shows frequency. f, Female intruders elicited USV from almost all males of ePet1-Cre, Lmx1b−/− , Lmx1b+/+ ,or Lmx1b+/−. Male intruders elicited USVs more from Lmx1b−/− males than from ePet1-Cre, Lmx1b+/+ or Lmx1b+/− males. g, The number of USVs emitted by Lmx1b−/− males towards males is higher than those by ePet1-Cre, Lmx1b+/+ or Lmx1b+/− males, whereas ePet1-Cre, Lmx1b+/+, Lmx1b+/− and Lmx1b−/− males were similar in USVs towards females.
A sexually dimorphic behavioral response of males is to emit 30–110 kHz ultrasonic vocalizations (USVs) when they encounter female mice or pheromones, which may function as love songs to facilitate female receptivity14. Lmx1b+/+, Lmx1b+/− and Lmx1b−/− males were similar in USV emission towards females (Fig. 1e–g). However, the percentage of Lmx1b−/− males emitting USV towards males was significantly higher than those by ePet1-Cre, Lmx1b+/+ or Lmx1b+/− males (Fig. 1f). Numbers of USV “syllables” emitted towards females were similar among ePet1-Cre, Lmx1b+/+, Lmx1b+/− and Lmx1b−/− males (Fig. 1g). Lmx1b−/− males emitted more USV “syllables” towards males than ePet1-Cre, Lmx1b+/+ and Lmx1b+/−. The number of USV emission by Lmx1b−/− males towards males was approximately 720 times higher than that of Lmx1b+/+ males (Fig. 1g).
While Lmx1b−/− males still emitted more USVs towards females, the preference for females over males was significantly reduced: the ratio of USVs towards females over that for males was only 3 for Lmx1b−/− males, significantly reduced from 1002 for ePet1-Cre males, 2438 for Lmx1b+/+ males and 52 for Lmx1b+/−.
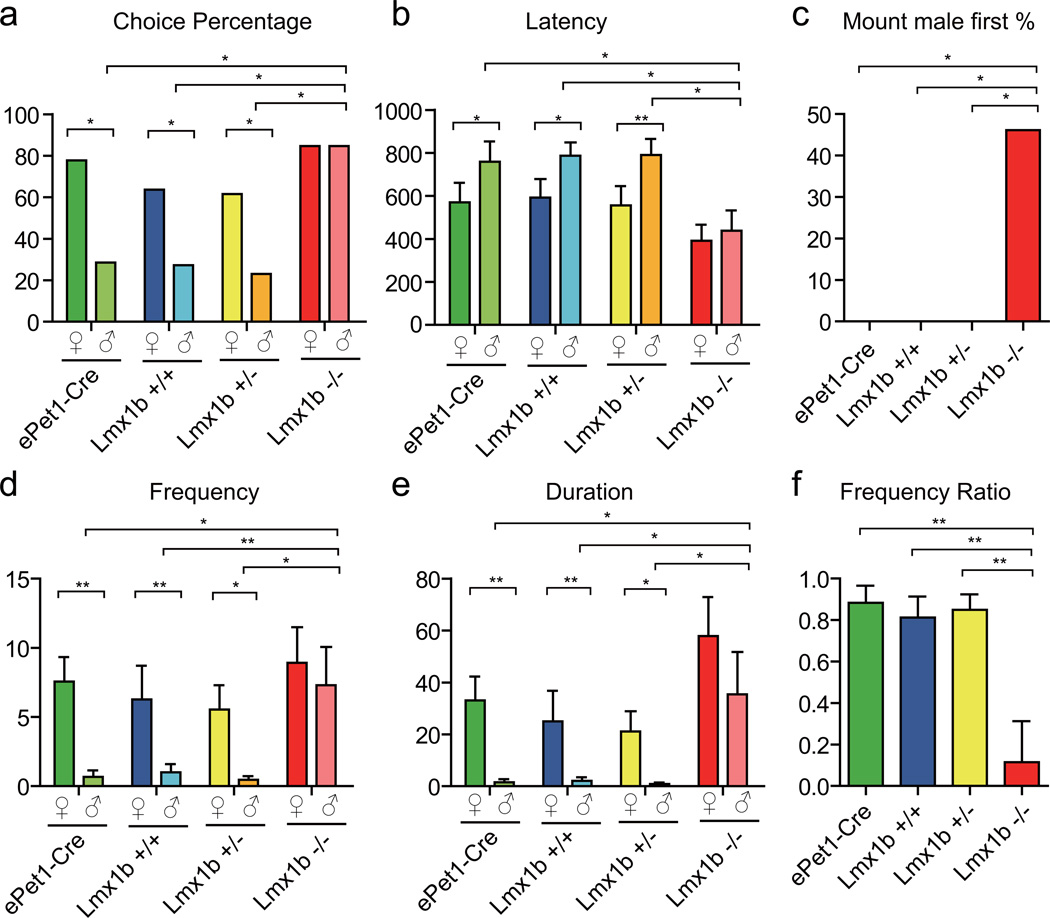
In the mating choice assay, an estrous female C57 target mouse and a sexually naïve male C57 target mouse were introduced into the home cage of a test male. Wt males preferred to mount female targets (Fig. 2a): a higher percentage of Lmx1b+/+ (or ePet1-Cre, Lmx1b+/−) males mounted female targets than male targets (Supplementary Movie 2). However, the percentage of Lmx1b−/− males mounting females was not significantly different from that mounting males. ePet1-Cre, Lmx1b+/+ and Lmx1b+/− males mounted female targets with a shorter latency, higher frequency and longer duration than male targets (Fig. 2 b, d, e), whereas Lmx1b−/− males mounted males and females with similar latencies, frequencies and durations (Supplementary Movies 2 and 3). Thus, elimination of serotonergic neurons led to a loss of sexual preference in mounting.
Figure 2. Lack of sexual preference by mice without central serotonergic neurons.
Each test male was presented with a male and an estrous female, and its mating choice was analyzed for 15 mins. a, more ePet1-Cre, Lmx1b+/+ and Lmx1b+/− males mounted female than male targets. A similar percentage of Lmx1b−/− males mounted females and males. b, ePet1-Cre, Lmx1b+/+ and Lmx1b+/− males mounted female targets faster than male targets. Mounting latencies of Lmx1b−/− males for females and males were similar. c, more than 40% Lmx1b−/− but none of the ePet1-Cre, Lmx1b+/+ or Lmx1b+/− males chose the male as their first mounting target. d, ePet1-Cre males mounted females significantly more often than males as did Lmx1b+/+ and Lmx1b+/− males. Lmx1b−/− males mounted females as often as males (p>0.05, t test). e, ePet1-Cre males spent more time mounting females than males, as did Lmx1b+/+ and Lmx1b+/− males. Lmx1b−/− males did not show differences in mounting males or females. f, The mounting frequency ratio of Lmx1b−/− was different from those of ePet1-Cre, Lmx1b+/+ and Lmx1b+/−.
Further analyses were carried out to detect a change in sexual preference separate from an increase in sexual drive: a) in the mating choice assay, all ePet1-Cre, Lmx1b+/+ and Lmx1b+/− males mounted females before males, whereas 46.2% of Lmx1b−/− mounted males first (Fig. 2c); b) the mounting frequency ratio of Lmx1b−/− males in the mating choice assay (female mounting frequency minus male mounting frequency)/(female plus male mounting) ((♀-♂)/(♂+♀)) was significantly different from ePet1-Cre, Lmx1b+/+ and Lmx1b+/− males (Fig. 2f); and c) when a test male was presented only with an estrous female target, Lmx1b−/− males were not statistically significant different from wt and heterozygous males in male-female mounting (Supplementary Fig. 1).
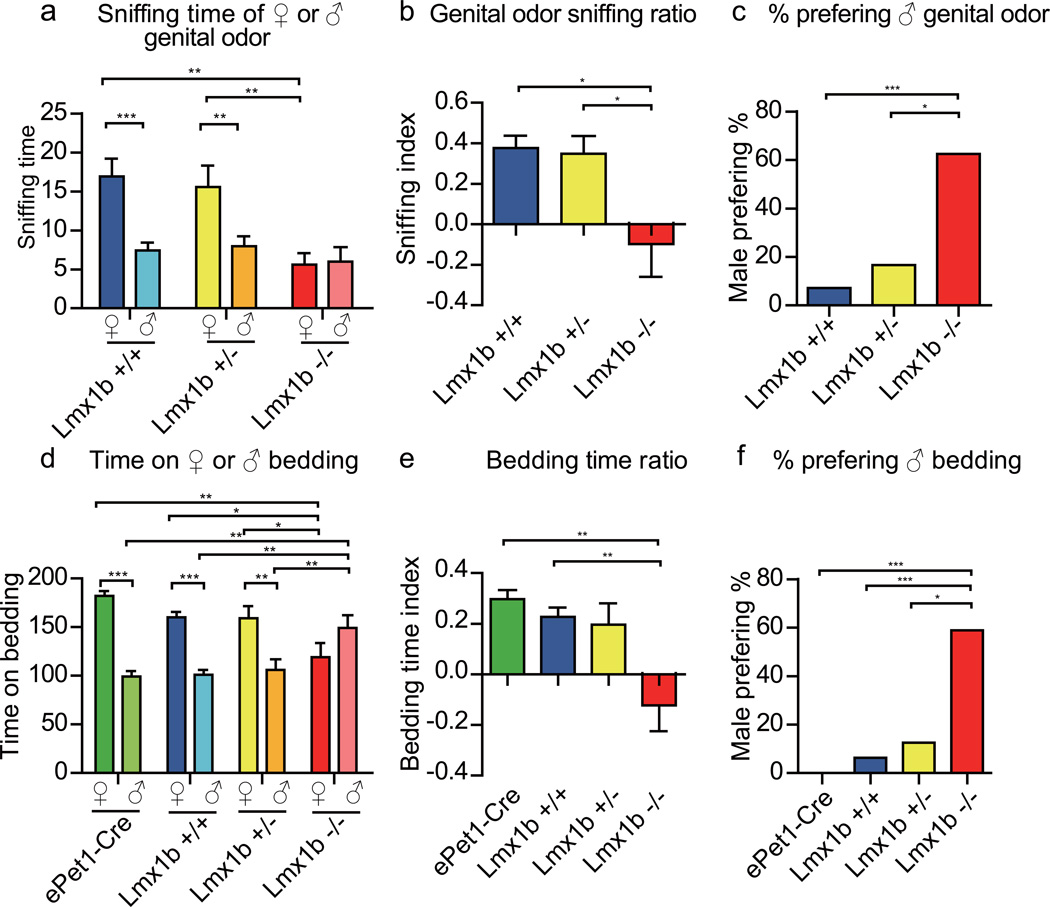
We tested male mice for their preference of pheromones present in the genitals or the bedding. In the genital odor preference assay15, a slide with one half smeared with female genitals and the other half with male genitals was presented to a test male. The total time of sniffing both halves of the slide was reduced in Lmx1b−/− males (Supplementary Fig. 2a). Lmx1b+/+ and Lmx1b+/− littermates spent significantly more time sniffing female than male genital odor, whereas Lmx1b−/− males spent equal time sniffing female and male genital odors (Fig. 3a). Lmx1b+/+, Lmx1b+/− and Lmx1b−/− were similar in sniffing male genital odor. Female genital odor sniffing time was less in Lmx1b−/− males than Lmx1b+/+ and Lmx1b+/− littermates (Fig. 3a). The genital odor preference ratio (♀-♂)/(♂+♀) of Lmx1b−/− males was significantly lower than those of Lmx1b+/+ and Lmx1b+/− males (Fig. 3b). Compared with Lmx1b+/+ and Lmx1b+/− males, a significantly higher percentage (62.5%) of Lmx1b−/− males spent more time sniffing male than female genital odor (Fig. 3c).
Figure 3. Loss of sexual preference for genital odor and bedding by males without central serotonergic neurons.
a, Lmx1b+/+ males spent more time sniffing female than male genital odor as did Lmx1b+/− males. Lmx1b−/− males spent a similar amount of time on female and male genital odor. Three groups were not significantly different in male genital odor sniffing time but Lmx1b−/− males spent less time in sniffing female genital odor than the other 2 groups. b, Sniffing ratio of Lmx1b−/− males was significantly different from Lmx1b+/+ and Lmx1b−/− males (p<0.05 for Lmx1b+/+ vs. Lmx1b−/−, p<0.05 for Lmx1b+/− vs. Lmx1b−/−, p>0.05 for Lmx1b+/+ vs. Lmx1b+/−, one-way ANOVA). c, Compared with Lmx1b+/+ and Lmx1b+/−, a higher percentage of Lmx1b−/− males spent more time sniffing male than female genital odor. d, ePet1-Cre males spent more time above female bedding than male bedding as did Lmx1b+/+ and Lmx1b+/− males. Lmx1b−/− males spent a similar amount of time above female and male bedding. Compared with ePet1-Cre,Lmx1b+/− and Lmx1b+/+, Lmx1b−/− males spent less time above female bedding but more time above male bedding. e, The bedding time ratio of Lmx1b−/− was different from ePet1-Cre and Lmx1b+/+. f, Compared with ePet1-Cre, Lmx1b+/+ and Lmx1b+/−, a significantly higher percentage of Lmx1b−/− males spent more time above male bedding.
In the bedding preference assay16, the total time spent over male and female bedding was similar among ePet1-Cre, Lmx1b+/+, Lmx1b+/− and Lmx1b−/− males (Supplementary Fig. 2b). ePet1-Cre, Lmx1b+/+ and Lmx1b+/− males spent significantly more time above female than male bedding whereas Lmx1b−/− males spent equal time above female and male beddings (Fig. 3d). Compared with ePet1-Cre, Lmx1b+/+ and Lmx1b+/− males, Lmx1b−/− males spent more time above male bedding and less time above female bedding. The bedding preference ratio of Lmx1b−/− males was significantly lower than those of ePet1-Cre, Lmx1b+/+ and Lmx1b+/− males (Fig. 3e). The percentage of males who spent more time above male bedding was significantly higher in Lmx1b−/− males (58.8%) than those in ePet1-Cre (0%), Lmx1b+/+ (6.3%) or Lmx1b+/− (12.5%) males (Fig. 3f).
Thus, in both the genital odor and bedding assays, Lmx1b−/− males have lost preference for female pheromones over male pheromones: in the genital odor preference assay, Lmx1b−/− males showed decreased sniffing time for female genital odor; in the bedding preference assay, Lmx1b−/− males showed increased time over male bedding and decreased time over female bedding.
Multiple assays involving odor or pheromone sensing were carried out to test for possible changes in olfaction. In the sesame oil preference assay17, Lmx1b+/+ and Lmx1b−/− males were indistinguishable in spending significantly more time with sesame than air (Supplementary Fig. 3a). In the fox urine avoidance assay18, Lmx1b+/+ and Lmx1b−/− males were also similar (Supplementary Fig. 3b). Thus, Lmx1b−/− males were not defective in either innate attractive or avoidance response.
In the social approach assay19. Lmx1b+/+ and Lmx1b−/− males were similar in spending more time close to a strange male than the empty chamber (Supplementary Fig. 3c).
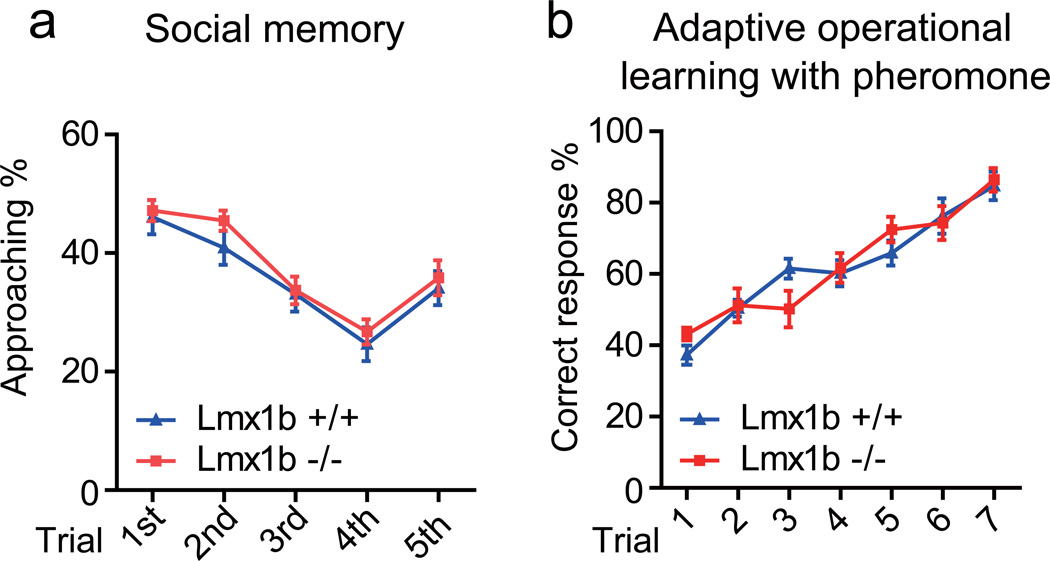
In the social recognition assay20, Lmx1b+/+ and Lmx1b−/− males spent similar time in exploring the first intruder at its initial presentation, in displaying social habituation towards the familiar intruder over the next three presentations and in displaying dishabituation when a new intruder was introduced (Fig. 4a).
Figure 4. Odor discrimination.
a, Both Lmx1b+/+ and Lmx1b−/− males showed habituation and dishabituation in sniffing time. No statistic difference was found between Lmx1b+/+ and Lmx1b−/− males at any point. b, After 7 training sessions with male and female urine, No significant difference was detected between Lmx1b+/+ and Lmx1b−/− males at any point.
An operant conditioning assay was used to test whether Lmx1b−/− males could distinguish between male and female pheromones21. Two arms of a T-maze were supplied with the odor of either female or male urine. Electroshock was applied in such a way that the test mice had to run or stay in the same arm depending on the urine. Over 3 days of training, Lmx1b+/+ and Lmx1b−/− males were similar in learning to avoid punishment (Fig. 4b). Thus, no olfactory defects for general odors or pheromones were detected in Lmx1b−/− males.
Results from Lmx1b−/− mice indicate a role for serotonergic neurons. To study the role of 5-HT, we used mice unable to synthesize 5-HT in the brain. 5-HT is synthesized in two steps: tryptophan is converted by a Tph into 5-hydroxytryptophane (5-HTP), which is converted into 5-HT by 5-hydroxytryptophan decarboxylase and aromatic L-amino acid decarboxylase.
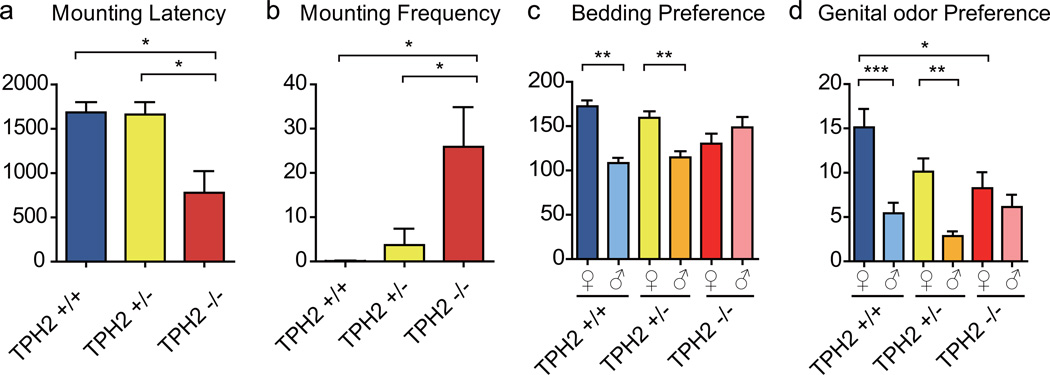
There are two Tph enzymes: Tph2 is required centrally and Tph1 peripherally. We have generated Tph2−/− mice (Kim et al, in preparation), which were viable22–24. High performance liquid chromatography (HPLC) analysis showed that 5-HT level was significantly reduced in the brains of Tph2−/− male mice (Supplementary Fig. 4a). Male-male mounting (Supplementary Movie 4) was significantly higher in Tph2−/− males than either Tph2+/+ or heterozygous Tph2+/− males: the percentage was significantly higher, duration longer, latency shorter, and frequency higher (Supplementary Fig. 4b, c; Fig. 5a, b). In the bedding preference assay, both Tph2+/+ and Tph2+/− males preferred female over male bedding, whereas Tph2−/− males showed no preference (Fig. 5c). In the genital odor preference assay, both Tph2+/+ and Tph2+/− males preferred female over male genital odor, but Tph2−/− males showed no preference (Fig. 5d).
Figure 5. Brain chemistry and behaviors of Tph2 knockout males.
Compared with Tph2+/+ and Tph2+/−, Tph2−/− males showed a shorter latency (a) and higher frequency in mounting males (b). c, Both Tph2+/+ and Tph2+/− males significantly preferred female over male bedding, whereas Tph2−/− males did not show preference between male and female bedding. d, Both Tph2+/+ and Tph2+/− males significantly preferred female over male genital odor, whereas Tph2−/− males did not show preference between male and female genital odor.
When presented with an estrous female target, male-female mounting was not significantly changed in Tph2−/− males (Supplementary Fig. 5). In mating choice, Tph2−/− males had lost preference of females over males in percentage, latency, frequency and duration (Supplementary Fig. 6a, b, d, e). No control males mounted target males before females, whereas more than 40% of Tph2−/− males mounted males first (Supplementary Fig. 6c). The mounting frequency ratio of Tph2−/− males was significantly different from both Tph2+/+ and Tph2+/− males (Supplementary Fig. 6f).
Lmx1b−/− and Tph2−/− mice lack 5-HT from embryogenesis. To study the role of 5-HT in adulthood, we took two complementary approaches: first, we depleted 5-HT from adult animals pharmacologically with pCPA25; we then attempted to rescue the phenotype of adult Tph2−/− mutants.
Adult C57BL/6J males were injected with either pCPA or saline for three consecutive days. 5-HT level was significantly reduced by pCPA (Supplementary Fig. 7). pCPA treated males showed shorter latency, higher frequency, and longer duration than control males in mounting target males (Supplementary Fig. 8 a–d), and lost bedding preference (Supplementary Fig. 8e, f).
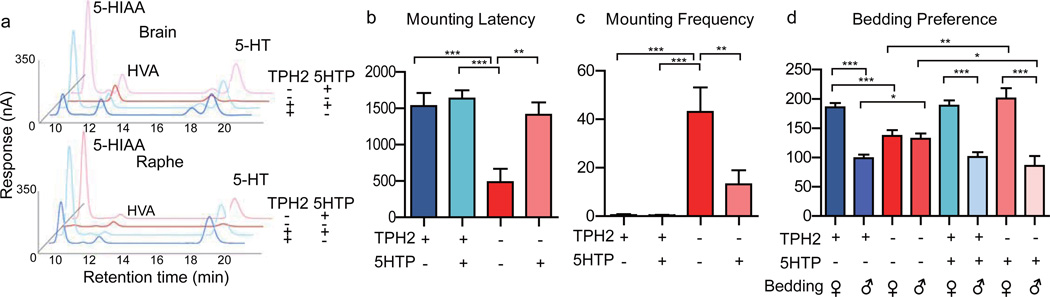
To test whether 5-HTP injection into adult animals could rescue the Tph2−/− phenotype, we first examined whether 5-HTP could rescue 5-HT synthesis in Tph2−/− males and found 5-HT levels restored 35 min after intraperitoneal injection of 5-HTP but not saline (Fig. 6a; Supplementary 9a, b).
Figure 6. 5-HTP rescue of chemical and behavioral deficits in Tph2 knockout mice.
a, Levels of 5-HT and 5-HIAA were analyzed in Tph2+/+ and Tph2−/− males 35 min after injection of either 5-HTP (40 mg/kg body weight) or control saline. b–c Male-male mounting in Tph2−/− mice was significantly rescued by 5-HTP: the latency was lengthened and frequency reduced. d, Bedding preference was monitored between 35 and 40 min after injection. 5-HTP could significantly restore the preference of female over male bedding by Tph2−/− males.
5-HTP significantly reduced male-male mounting of Tph2−/− males: the percentage was decreased, latency increased, frequency decreased and duration shortened; all returning to wt levels (Fig. 6b, 6c, Supplementary Fig. 9c, 9d). 5-HTP rescued the loss of sexual preference in mounting latency, frequency, duration in the mating choice assay (Supplementary Fig. 10 a–c) and the bedding preference of Tph2−/− males (Fig. 6d; Supplementary Fig. 9e).
When a test male was presented with a target female, Tph2−/− males similar to the wt and heterozygous in mounting percentage, latency, frequency and duration (Supplementary Figs. 5 and 11). 5-HTP injection into the Tph2−/− males did not affect male-female mounting (Supplementary Fig. 11), although 5-HTP injection into the wt males reduced male-female mounting. Because 5-HTP injection to the wt males increased the level of 5-HT beyond the wt level (Supplementary Fig. 9a, b), it indicated a dosage sensitive effect of 5-HT: 5-HT at concentrations above the wt level inhibited male-female mounting, but 5-HT concentrations between the wt and Tph2−/− levels did not affect male-female mounting.
We conclude that central serotonergic signaling is crucial for male sexual preference in mice. This is the first time that a neurotransmitter in the brain has been demonstrated to be important in mammalian sexual preference. Previous studies in mammals have implicated serotonin and dopamine in male sexual behaviors, but neither has been demonstrated to play any role in sexual preference: dopamine is thought to facilitate male sexual behaviors whereas 5HT is thought to inhibit sexual behaviors7–11, 26. Our studies have established a role for 5-HT in male sexual preference. Multiple results showed a loss in sexual preference beyond or separate from hypersexuality. 1) the ratio of male-male and male-female interactions was repeatedly measured to analyze sexual preference (Figs. 2f, 3b, 3e, 5c, 5d, 6d, Supplementary Fig. 6f, 8f, 9e, 10d); 2) Lmx1b−/− males increased USV numbers towards males, but not towards females (Fig. 1g); 3) in mating choice, the latency, frequency and duration of Lmx1b−/− males to mount males, but not those to mount females, was changed (Fig. 2 a, b, d, e); 4) in bedding preference, Lmx1b−/− (Fig. 3d) and Tph2−/− males (Fig. 5c, 6d) showed an increase of time over males but a decrease of time over females; 5) wt always mounted females before males but a significant fraction of Lmx1b−/− or Tph2−/− males mounted males first (Fig. 2c, Supplementary Fig. 6c); 6) in the genital odor preference assay, both Lmx1b−/− (Fig. 3a) and Tph2−/− (Supplementary Fig. 5d) males showed a decreased time for female genital odor, which could not be explained by hypersexuality; 7) when presented with an estrous target female, neither Lmx1b−/− males (Supplementary Fig. 1) nor Tph2−/− (Supplementary Fig. 5) were different from wt males.
An increased sexual drive was observed in males lacking 5-HT when they were tested in the presence of live target males and females (Supplementary Fig. 6). This has been noted before in mice defective for TrpC2 and VNO olfaction5, 6. TrpC2−/− males have previously been reported to have lost male-female preference in mating choice5, 6. TrpC2−/− males increased mounting towards both males and females (Fig. 2C in ref. 6). The conclusion of a loss in sexual preference in TrpC2−/− males was inferred from a relative change: TrpC2−/− males showed a 2 fold preference for females over males whereas the wt showed a 10 fold preference. The phenotypes reported here for Lmx1b−/−, Tph2−/− males and pCPA treated males were stronger than that for TrpC2−/− males in mating choice: these males did not show significant preference for females (Fig. 2, Supplementary Fig. 6).
At the present, it is not known whether 5-HT regulates the VNO pathway in pheromone sensing or further downstream in behavioral decisions. Differences have been noted between TrpC2 and Lmx1b in the brain: aggression was largely lost in TrpC2−/−, but not Lmx1b−/−, mice (data not shown). It is more likely that 5-HT regulates central decision than influencing peripheral olfaction. However, we cannot completely rule out the possibility that 5-HT regulates a specific innate olfactory pathway processing sexual information27.
In mice, it will be interesting to identify specific subsets of serotonergic neurons and serotonergic receptors involved in sexual preference.
An unavoidable question raised by our findings is whether 5-HT plays a role in sexual preference in other animals. In a positron emission tomography study of humans, the response of heterosexual men to the selective serotonin reuptake inhibitor (SSRI) fluoxetine was found to be different from that of homosexual men28. SSRIs could inhibit the compulsive sexual activity of men29. However, so far, none of these studies have investigated whether 5-HT plays a role in sexual preference. Attempts have been made to map genetic loci affecting human sexuality30, though specific genes have not been identified. Our discovery of the role for serotonergic signaling in mouse sexual preference should stimulate further studies into the role of 5-HT in sexual interactions in particular and roles of neurotransmitters in mammalian social relationships in general.
METHODS
Mouse Stocks
ePet1-Cre mice were a gift from Dr. Evan S. Deneris (Case Western Reserve University) and the floxed Lmx1b mice were a gift from Randy Johnson (M. D. Anderson Cancer Center). Tph2 knockout mice were generated by deleting exon 5 which encoded the tryptophan hydroxylase domain (for details, see Kim et al., under review). Mice were weaned at the age of 21 days. Mice were maintained on a 12L:12D schedule and housed initially in groups of five to the 10th week and then singly-housed until the end of experiments. Food and water were provided ad libitum. The room temperature was 23±1°. The humidity was 40–60%. All test mice were 12–16 weeks old. The target mice were 11–13 weeks old.
Mouse genotyping
The genomic DNA was extracted from the mice’s tail tissues at the day of weaning. The mutant mice were generated by crossing ePet1-Cre mice with Floxed Lmx1b mice and following intercross within the F1 generation mice. Littermates used in the tests were of the same sex and similar body weight as the knockout mice. The primers were: AGG CTC CAT CCA TTC TTC TC (Floxed Lmx1b1), CCA CAA TAA GCA AGA GGC AC (Floxed Lmx1b2); ATT TGC CTG CAT TAC CGG TCG (Cre1), CAG CAT TGC TGT CAC TTG GTC (Cre2).
Immunocytochemical analysis with anti-5-HT antibodies confirmed that 5-HT positive neurons were absent in Lmxb1 knockout mice (data not shown).
The Tph2 line was maintained by the crossing heterozygotes. Littermates include wt, heterozygotes and homozygous knockout mice. The primers for genotyping were: GGGCATCTCAGGACGTAGTAG, GGGCCTGCCGATAGTAACAC, GCAGCCAGTAGACGTCTCTTAC.
Measurement of 5-HT
The levels of 5-HT and its metabolites were separated by HPLC and measured by an electrochemical detector in samples from adult male mice. In 5-HTP rescue experiments, animals were injected with 40 mg/kg 5-HTP or saline (both at the volume of 5 ml/kg) They were euthanized 35 minutes later. The brain was dissected and the raphe region was isolated on ice. Samples were weighed before ultrasounication. Monoamines were extracted by perchloric acid. The sample was filtrated by 0.22 um filter before being injected into RP-HPLC (ESA). NA, DOPAC, DA, HIAA, HVA and 5-HT were measured by an electrochemical detector. Their concentrations were calculated by CoulArray software (ESA) based on standard samples. Values of amine per wet tissue weight were shown in the final figures.
Order of behavioral assays
Male mutant mice and their littermates at 12–13 weeks of age and of similar body weight were sexually naïve and group-housed with same sex mice before 10 weeks of age. After two weeks of single housing, mice were tested in the following order: bedding preference, male-male resident intruder assay, mating choice assay, sexual behaviors with an estrous female, bedding preference again (no difference was observed with results from the first bedding preference). One week of rest between each test. For Lmx1b mice, the same group of mice were used in male-male mounting, mating choice and male-female mounting. For Tph2 mice, a different group were used for male-female mounting. Sexually experienced mice were used for USV, social approach, habituation and olfactory learning assays. Sexually naïve mice were used for urine preference and olfactory tests.
Resident-intruder tests
All test mice were sexually naïve. The bedding of the test mice had not been changed for at least 4 days. Intruder mice were 11–13 weeks old, sexually naive and group-housed C57Bl/6J males. All activities within a test were recorded by an infrared camera (Sony Video Recorder, DCR-HC26C). Mounting latency, mounting frequency and total duration of mounting within 30 min were measured.
Mating choice assay
Beddings of test mice had not been changed for at least four days. Into the cage of each test male were introduced a group-housed sexually naïve 11–13 week-old C57Bl/6J male and a sexually naïve estrous 10 week-old female C57Bl/6J female. Each assay lasted 15 minutes after the target mice were introduced. All activities were recorded by an infrared camera. The latency, frequency and duration of mounting to male or female targets were analyzed.
Sexual behaviors with females
An estrous female was presented to a test male and video was recorded for 30 mins, by an infrared camera. The latency, frequency, and duration of male mounting to the female were analyzed.
Ultrasonic vocalizations
Tests were carried out with single housed adult males in the dark phase in the home cage. UltraSoundGate 116–200 system (Avisoft) was used to record the ultrasound. We recorded the background sound for 1 min before a stimulus mouse of 10–13 week old was introduced. The recording lasted for 2 mins. Recorded data was analyzed with SASLab (Avisoft)5. Sounds over the frequency range of 30 Hz-110 kHz was analyzed. Profiles of background noise created by mouse movement were very different from USVs. To confirm that the resident mouse was the source of USVs, we recorded from assays in which either the resident or the intruder mouse was devocalized. We were able to record robust USVs presented in our figures only when the intruder mouse was devocalized and not when the resident mouse was devocalized.
Genital odor preference assay
This assay was modified from a previous procedure15. The anogenital area scent from a male was rubbed on the left- or right side of a clean glass microscope slide while the anogenital area scent from a female was rubbed on the other side of the slide. 5 seconds later, the slide was hung in the middle of the cage by a clamp. The slides were ~5 cm over the bedding. Activities of the test mice were recorded for 3 min by an infrared camera and the sniff time on scent portion of either side was analyzed as were the amount of time a test male licked the slide or its nose touched the slide.
Bedding preference assay
Bedding from group-housed adult C57BJ/6J males or females were not changed for 4 days. Ten grams of male or female bedding were put in one side on the bottom of a cage in a area of 11.5 X 17 cm2. The male and female beddings were prevented from mixing by a plastic bar of 6 cm. The size of cage was 29 X 17 X 15 cm (l*w*h)16. A grid of plastic bars separated the test mice from the bedding on the bottom of the cage. The bars were 5 mm wide with 5 mm intervals. The test mouse was put into the cage to be familiarized with the cage without bedding for 5 min before the mice were taken out and the bedding and a clean grid was put into the cage. After each assay, the cage was washed with water and then alcohol to remove odor.
Olfactory learning assay
We employed a T-maze in which electric shock could be applied to either side of the horizontal chamber as described in ref. 21. Briefly, there was a door at the intersection of the horizontal and vertical chambers. The horizontal chamber of 8 × 8 × 60cm3 was divided into three parts: a left arm of 8×8×23 cm, a right arm of 8 × 8 × 23 cm and a middle zone of 8 × 8 × 14 cm. Each test mouse was introduced into the vertical chamber of the T-maze. After it entered the horizontal chamber, the door between the vertical and horizontal chambers was closed and the mouse was allowed to walk within the horizontal chamber. The mouse was not allowed to stay in the middle zone for longer than 8 s, otherwise it would be punished with electroshock. The position of the test mouse was monitored by a video recorder. Urine samples were collected from more than 20 C57BL/6J males or females and stored at −20 °. 1.5 ml urine sample was used for each test. Odor of male or female urine was puffed into the left or right arm of the horizontal chamber and expirated from the middle zone. Odor was present for 50s. We trained the test male mouse with electroshock to stay in the arm with female odor and to avoid the arm with male odor. The mouse had to make a decision to stay in or leave the arm when an odor was presented. Each training session of 18 trials lasted for 30 min. Every mouse was given 6 training sessions in 3 days before the final test. There were 10 trials in the final test. The percentages of correct choices in every training session and the final test were analyzed.
Innate behavioral responses to odors
The set-up is the same as that for the olfactory learning assay, except that no electroshock was applied. Sexually naive males (mutants or littermates) of 10 to 16 weeks old were tested for their choices of fox urine vs air, or sesame oil vs air. Fox urine was used to test the innate avoidance of predator’s odor. Fox urine was diluted at two concentrations (60X and 20X). The main air flow velocity was 250 l/h. The air flow through fox urine was 70 ml/min or 210 ml/min respectively. The time that mice spent in empty arm or fox urine arm was recorded by the Matlab software. Sesame oil was diluted by 83X was used to test the innate attraction by food. Time spent in the air arm or the sesame oil arm was recorded by Matlab software.
Social approach
Social approach experiment was tested in a modified T shaped box. A small cage separated by wire was at each end of the arms in the horizontal chamber. A test mouse was allowed to habituate for five minutes before an unfamiliar target male was randomly placed in one of the small cages. The target mouse could be seen, smelled, heard, but could not be touched. The test mouse was allowed to move in the box for 5 minutes. Its location was video recorded and analyzed by a computer.
Social memory
Single-housed adult males were tested in the dark phase and in the room where they were reared. Ovariectomized C57Bl/6J females were used as stimulus mice20. They were ovariectomized at 6 weeks old and used two weeks later. A stimulus mouse was introduced into the cage housing a test mouse for 1 minute and then was removed. After an interval of ten minutes, the same stimulus female was introduced again for 1 minute. The stimulus mouse was presented 4 times. At the fifth time, a new stimulus mouse was introduced for 1 minute. Behaviors of test mice were videotaped and time spent on body sniffing was analyzed.
5-HT depletion by pCPA treatment
Male C57Bl/6J mice of 11–13 weeks of age were used. They were injected with either 500 mg/kg of pCPA (sigma, C6506) or saline control for 3 consecutive days after 4 days of single housing. Animals were tested with adult C57 female mice. Mice that did not show mounting behavior in 15 minute were discarded. Qualified mice were then single-housed for 1 week before social behavior test and their bedding was not changed. Animals were randomly divided into pCPA or saline-treatment. pCPA was suspended in 1% Tween saline at a concentration of 50 mg/ml. The pCPA group were injected intraperitonially with pCPA (10 ml/kg, IP) at 72, 48 and 24 hours before testing. The control group received 1% Tween saline. Resident-intruder and mating choice assays were carried out. Behavioral tests were performed in the dark.
Supplementary Material
A Lmxb1−/− male mounted a wt male.
A Lmxb1+/+ male was presented with two targets, one male and one estrous female. The Lmxb1+/+ male mounted the female target.
A Lmxb1−/− male was presented with two wt targets, one male and one estrous female. The Lmxb1−/− male mounted both the male and the female targets.
A Tph2−/− male mounted a wt male.
A Tph2 +/+ male was presented with two targets, one male and one estrous female. The Tph2 +/+ male mounted the female target.
A Tph2−/− male was presented with two wt targets, one male and one estrous female. The Tph2−/− male mounted both the male and the female targets.
Acknowledgements
We are grateful to Dr Evan S. Deneris for ePet1-Cre mice; to Dr. Randy Johnson for Lmx1bfloxP mice; to Dr. Minmin Luo for discussions and the odor preference assay apparatus; to Xinhong Wang and You Wan for help with HPLC; to Jing Lang and Jun Yin for mouse breeding and genotyping; to Ping Ding, Ping Wang, Heping Lu and Xin Wang for technical assistance; to Lihua Zhao, Zhihua Qiu, Haijiang Jing for animal caring; and to the Ministry of Science and Technology (973 program 2010CB833901) and Beijing Municipal Commission on Science and Technology for grant support (to YR), and the NIH for grant support (to ZFC).
REFERENCES
- 1.Trivers RL. Parent-Offspring Conflict. Amer. Zool. 1974;14:15. [Google Scholar]
- 2.Sommer V, Vasey PL, editors. Homosexual Behaviour in Animals -- An Evolutionary Perspective. Cambridge University Press; 2006. [Google Scholar]
- 3.Price EO, Wallach SJ. Development of sexual and aggressive behaviors in Hereford bulls. J. Anim Sci. 1991;69:1019–1027. doi: 10.2527/1991.6931019x. [DOI] [PubMed] [Google Scholar]
- 4.Erwin J, Maple T. Ambisexual behavior with male-male anal penetration in male rhesus monkeys. Archives of Sexual Behavior. 1976;5:9–14. doi: 10.1007/BF01542236. [DOI] [PubMed] [Google Scholar]
- 5.Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of Sex Discrimination and Male-Male Aggression in Mice Deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- 6.Leypold BG, et al. Altered sexual and social behaviors in trp2 mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hull EM, Muschamp JW, Sato S. Dopamine and serotonin: influences on male sexual behavior. Physiology & Behavior. 2004;83:291–307. doi: 10.1016/j.physbeh.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Hull EM, Dominguez JM. Sexual behavior in male rodents. Hormones and Behavior. 2007;52:45–55. doi: 10.1016/j.yhbeh.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson J, et al. "Hypersexuality" and Behavioral Changes in Cats Caused by Administration of p-Chlorophenylalanine. Science. 1970;168:499–501. doi: 10.1126/science.168.3930.499. [DOI] [PubMed] [Google Scholar]
- 10.Malmnas C, Meyerson B. p-chlorophenylalanine and copulatory behaviour in the male rat. Nature. 1971;232:398–400. doi: 10.1038/232398a0. [DOI] [PubMed] [Google Scholar]
- 11.Salis P, Dewsbury D. p-chlorophenylalanine facilitates copulatory behaviour in male rats. Nature. 1971;232:400–401. doi: 10.1038/232400a0. [DOI] [PubMed] [Google Scholar]
- 12.Dailly E, Chenu F, Petit-Demouliere B, Bourin M. Specificity and efficacy of noradrenaline, serotonin depletion in discrete brain areas of Swiss mice by neurotoxins. Journal of Neuroscience Methods. 2006;150:111–115. doi: 10.1016/j.jneumeth.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Z-Q, et al. Lmx1b Is Required for Maintenance of Central Serotonergic Neurons and Mice Lacking Central Serotonergic System Exhibit Normal Locomotor Activity. J. Neurosci. 2006;26:12781–12788. doi: 10.1523/JNEUROSCI.4143-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Z, Holy TE. Sex selectivity of mouse ultrasonic songs. Chem Senses. 2007;32:463–473. doi: 10.1093/chemse/bjm015. [DOI] [PubMed] [Google Scholar]
- 15.Ferkin MH, Li HZ. A battery of olfactory-based screens for phenotyping the social and sexual behaviors of mice. Physiology & Behavior. 2005;85:489–499. doi: 10.1016/j.physbeh.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Moncho-Bogani J, Lanuza E, Herndez A, Novejarque A, Martez-Garc F. Attractive properties of sexual pheromones in mice: Innate or learned? Physiology & Behavior. 2002;77:167–176. doi: 10.1016/s0031-9384(02)00842-9. [DOI] [PubMed] [Google Scholar]
- 17.Burwash MD, Tobin ME, Woolhouse AD, Sullivan TP. Laboratory Evaluation of Predator Odors for Eliciting an Avoidance Response in Roof Rats (Rattus rattus) Journal of Chemical Ecology. 1998;24:49–66. [Google Scholar]
- 18.Blanchard D, et al. Failure to produce conditioning with low-dose trimethylthiazoline or cat feces as unconditioned stimuli. Behav Neurosci. 2003;117:360–368. doi: 10.1037/0735-7044.117.2.360. [DOI] [PubMed] [Google Scholar]
- 19.Nadler JJ, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes, Brain & Behavior. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson JN, et al. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 21.Yan Z, et al. Precise Circuitry Links Bilaterally Symmetric Olfactory Maps. 2008;58:613–624. doi: 10.1016/j.neuron.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Gutknecht L, et al. Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. Journal of Neural Transmission. 2008;115:1127–1132. doi: 10.1007/s00702-008-0096-6. [DOI] [PubMed] [Google Scholar]
- 23.Savelieva KV, et al. Genetic Disruption of Both Tryptophan Hydroxylase Genes Dramatically Reduces Serotonin and Affects Behavior in Models Sensitive to Antidepressants. PLoS ONE. 2008;3:e3301. doi: 10.1371/journal.pone.0003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alenina N, et al. Growth retardation and altered autonomic control in mice lacking brain serotonin. Proceedings of the National Academy of Sciences. 2009;106:10332–10337. doi: 10.1073/pnas.0810793106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koe BK, Weissman A. p-Chlorophenylalanine: a specific depletor of brain serotonin. J Pharmacol Exp Ther. 1966;154:499–516. [PubMed] [Google Scholar]
- 26.Gawienowski AM, Hodgen GD. Homosexual activity in male rats after p-Chlorophenylalanine: Effects of hypophysectomy and testosterone. Physiology & Behavior. 1971;7:551–555. doi: 10.1016/0031-9384(71)90108-9. [DOI] [PubMed] [Google Scholar]
- 27.Kobayakawa K, et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature. 2007;450:503–508. doi: 10.1038/nature06281. [DOI] [PubMed] [Google Scholar]
- 28.Kinnunen L, Moltz H, Metz J, Cooper M. Differential brain activation in exclusively homosexual and heterosexual men produced by the selective serotonin reuptake inhibitor, fluoxetine. Brain Res. 2004;1024:251–254. doi: 10.1016/j.brainres.2004.07.070. [DOI] [PubMed] [Google Scholar]
- 29.Wainberg M, et al. A double-blind study of citalopram versus placebo in the treatment of compulsive sexual behaviors in gay and bisexual men. J Clin Psychiatry. 2006;67:1968–1973. doi: 10.4088/jcp.v67n1218. [DOI] [PubMed] [Google Scholar]
- 30.Mustanski BS, et al. A genomewide scan of male sexual orientation. Hum. Genet. 2005;116:272–278. doi: 10.1007/s00439-004-1241-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A Lmxb1−/− male mounted a wt male.
A Lmxb1+/+ male was presented with two targets, one male and one estrous female. The Lmxb1+/+ male mounted the female target.
A Lmxb1−/− male was presented with two wt targets, one male and one estrous female. The Lmxb1−/− male mounted both the male and the female targets.
A Tph2−/− male mounted a wt male.
A Tph2 +/+ male was presented with two targets, one male and one estrous female. The Tph2 +/+ male mounted the female target.
A Tph2−/− male was presented with two wt targets, one male and one estrous female. The Tph2−/− male mounted both the male and the female targets.