Abstract
Maternal cytokines may play instructive roles in development of the neonatal immune system. However, cytokines in colostrum and milk and their transfer from mothers to neonates have not been well documented, except for TGF-β. Swine provide a unique model to study lactogenic cytokines because the sow's impermeable placenta prohibits transplacental passage. We investigated IL-6 and TNF-α (pro-inflammatory), IFN-γ and IL-12, (Th1), IL-10 and IL-4 (Th2) and TGF-β1 (Th3) concentrations in sow serum and colostrum/milk and serum of their suckling and weaned piglets and in age-matched colostrum-deprived gnotobiotic piglets. All cytokines were detected in colostrum/milk and correlated with concentrations in sow serum except for mammary-derived TNF-α and TGF-β1. Detection of IL-12 and TGF-β1 in pre-suckling and colostrum-deprived gnotobiotic piglet serum suggests constitutive production: other cytokines were undetectable confirming absence of transplacental transfer. Peak median cytokine concentrations in suckling piglet serum occurred at post-partum days 1-2 (IL-4>IL-6>IFN-γ>IL-10). The effects in vitro of physiologically relevant concentrations of the two predominant lactogenic cytokines (TGF-β1 and IL-4) on porcine naive B cell responses to lipopolysaccharide (LPS) and rotavirus (RV) were investigated. High (10ng/ml) TGF-β1 suppressed immunoglobulin secreting cell responses to LPS and rotavirus; low concentrations (0.1ng/ml) promoted isotype switching to IgA antibody. Interleukin-4 induced inverse dose-dependent (0.1>10ng/ml) isotype switching to IgA and enhanced IgM secreting cell responses to LPS and rotavirus. In summary, we documented the transfer and persistence of maternal cytokines from colostrum/milk to neonates and their potential role in Th-2 biased IgA responses and reduced immunologic responsiveness of neonates.
Keywords: Cytokines, Milk, Neonate, Swine, Th2 bias
1. Introduction
Neonates depend on transfer of immune factors from their mothers via the placenta and/or breast feeding to be protected from pathogens until the maturation of their immune system. The maternal immune factors transferred to the neonates include soluble molecules (i.e. antibodies, growth factors, cytokines, etc.) and lymphoid and non-lymphoid cells. A few studies, mainly of humans, of the transfer of cytokines via the placenta and the components of colostrum/milk have suggested possible roles for maternal cytokines such as TGF-β, IL-1, IL-6, TNF-α, etc in immunologic protection of neonates and in modulating neonatal immune development during colonization by commensal bacteria (Bocci et al., 1993), but most of these studies did not assess multiple types (Th1, Th2, Th3 and T regulatory type 1 [Tr1]) of cytokines or their profiles over time in infants.
Cytokines have effects on and are produced by different T helper (Th) CD4+ cells that are classified into Th1, Th2, Th3 and Tr1 types based on studies in humans and mice. Interferon (IFN)-γ and interleukin (IL)-12 are produced by Th1 cells and they promote inflammatory and cytotoxic T lymphocyte responses. IL-4 and IL-5 are produced by Th2 cells and they promote B cell responses. T cell growth factor (TGF)-β is produced mainly by Th3 cells that have immunoregulatory functions. IL-10 is produced by Th2 and Tr1 cells and plays an important role in antibody production and anti-inflammatory responses (Foussat et al., 2003). The presence of these cytokines in mammary secretions likely influences development of the neonatal immune system. Of the maternal cytokines, TGF-β has been studied the most experimentally in humans, mice and rats. In mammals, three isoforms of TGF-β (β1, β2 and β3) have been identified of which TGF-β1 is the most abundant form in tissues whereas TGF-β2 is more abundant in body fluids (Miyazono et al., 1993). However, TGF-β1 is of particular interest as it has been reported to play an immunoregulatory role during pregnancy and at birth in humans as well as a role in the Th2 bias of neonatal immune responses (Laouar et al., 2005; Power et al., 2002). Both IL-10 and TGF-β1 were detected in the maternal and fetal circulation in humans (Power et al., 2002). The latter cytokine could favor Th2 memory responses by suppression of memory Th1 cells in the fetus predisposing neonates to the observed Th2 bias (Ludviksson et al., 2000; Wegmann et al., 1993). TGF-β1 supplied to the fetus by injection into the mother's circulation during gestation or to the neonate via milk during suckling was shown to rescue TGF-β1 -/- newborn pups from severe cardiac abnormalities (Letterio et al., 1994). In suckling rats, feeding of formula lacking TGF-β2 led to inflammatory responses to food antigens including accumulation of IL-18 and recruitment of high numbers of activated dendritic cells, eosinophils and mast cells to the intestine (Penttila et al., 2003). These inflammatory responses in suckling rat pups could be alleviated by addition of TGF-β2 to the feeding. In humans, TGF-β1 and TGF-β2 in colostrum correlated with increased serum IgA concentrations in infants during the first month of life (Ogawa et al., 2004). IL-4 is an important Th2 cytokine that antagonizes Th1 related IFN-γ production, and may contribute to Th2 biased immune responses in neonatal pigs, similar to the responses observed in human infants and neonatal mice (Adkins et al., 2001; Early and Reen, 1996). The presence of early pro-inflammatory cytokines such as IL-6 and TNF-α has also been documented in human milk, but the effects of these cytokines on the neonatal immune system were not well studied (Rudloff et al., 1992; Rudloff et al., 1993).
In swine, the Th1/Th2/Th3/Tr1 functions of these cytokines have not been clearly defined. There is limited information concerning the level and function of cytokines transferred from milk to suckling neonates or their impact on neonatal immune development in swine or other species (Wagstrom et al., 2000). Porcine milk contains abundant TGF-β, which has been suggested to play a role in regulating the intestinal immune system in neonatal pigs (Xu et al., 1999). The presence or transfer of other cytokines has not been studied in porcine colostrum/milk. There is also a lack of information for humans and animals about the persistence or function of these maternal cytokines in neonates after transfer by suckling.
The goals of this study were to investigate the transfer efficiency of various cytokines representing proinflammatory, Th1, Th2, Th3 and Tr1 cytokines (according to the classification in humans and mice) from the colostrum and milk of the sow to the serum of neonatal piglets, to determine the persistence of the passive cytokines in the serum of neonatal piglets, and to provide a basis for comparison of milk cytokine components between pigs and humans to establish their universal roles in neonatal immune development. In addition, we investigated the effects in vitro of various physiologically relevant concentrations of the two predominate and immunomodulating cytokines (TGF-β1 and IL-4) transferred via colostrum into piglet sera on porcine naive B cell responses to lipopolysaccharide (LPS) and rotavirus (RV). Our findings contribute new information to support a previously unrecognized role for maternal cytokines in the possible immunomodulation of the neonatal immune system of swine.
2. Materials and methods
2.1. Experimental design and sample collection
Near-term sows (n=10) were closely monitored and allowed to farrow naturally. The animal use protocols employed in this study were reviewed and approved by the Agricultural Animal Care and Use Committee, The Ohio State University.
In the suckling experiments, piglets (n=47) from 5 sows were allowed to suckle the sows for 14 days. Blood from piglets was collected pre-suckling (post partum day [PPD] 0) at farrowing and at PPD1, 2, 3, 5, 7, 9, 11 and 13. For PPD1-3, blood was collected from different piglets in the suckling experiments on alternative days. Pre-suckling piglets (PPD0) were also euthanized for the collection of small intestinal contents (SIC).
In the weaning experiment, a group of piglets (n=23) derived from 2 sows suckled the sows for the first two days. At PPD3, a subset of piglets (n=15) were removed from the sows and weaned onto infant formula (Similac, Abbott laboratories, Columbus, Ohio) with added soy protein (Nowfoods, Bloomingdale, Illinois) from PPD3 to 13 (post weaning day [PWD] 0 to 10), whereas the rest of the litter remained on the sow. Weaned piglets were housed together but separately from the sows and suckling piglets in the same facility and were provided a liquid diet ad libitum via a gravity flow feeding system. During the entire study period, sows and piglets were healthy and no signs of mastitis or infections were observed. Blood was collected in the weaned piglets at PPD3,5,7,9,11 and 13 (equivalent to PWD0,2,4,6,8 and 10). On PPD1-3, blood was collected from different piglets on alternative days.
As controls to assess endogenous cytokine production over time, piglets (n=20) derived asceptically by hysterectomy from 4 sows were colostrum-deprived and maintained under strict gnotobiotic conditions (lack microbial flora or extraneous microbes) in isolator units for up to 33 days of age. Blood was also collected from these gnotobiotic piglets through 33 days post-derivation. Then, the gnotobiotic piglets were euthanized for the collection of blood and SIC. Blood was collected from the sows (n=7) at PPD2,7,11 and 3 days (on average) pre-partum (PPD-3). Colostrum/milk samples were collected at PPD0,1,2,3,5,7,9,11 and 13 after farrowing.
Serum (1-2ml/piglet, 3-5ml/sows) was collected and stored at −20°C until tested. The SIC were diluted 1:2 (v/v) with 1% bovine serum albumin fraction V in phosphate buffered saline (0.5mM, pH7.2) with 250 μg/ml trypsin inhibitor and 50 μg/ml leupeptin (Sigma, St. Louis, Missouri) to inhibit proteolytic enzymes. The SIC were then clarified by centrifugation and supernatants were stored frozen at −20°C. After collection, colostrum/milk samples (15ml) were immediately centrifuged at 1200xg for 30min to remove cells and debris. Colostrum/milk supernatants were collected and stored at −20°C.
2.2. Enzyme-linked immunosorbent assay (ELISA) for porcine cytokines
Concentrations of IL-6 and TNF-α (pro-inflammatory), IFN-γ and IL-12 (Th1), IL-4 (Th2), IL-10 (Th2 and Tr1) and TGF-β1 (Th3) were measured using capture sandwich ELISA following procedures developed in our laboratory (Azevedo, 2006). The detection concentration for the TNF-α and TGF-β1 assay was 15.6 pg/ml. The detection concentrations for the other cytokines were 7.8 pg/ml. Samples below these detection concentrations (7.8 pg/ml or 15.6 pg/ml) were assigned a concentration of 4 or 8 pg/ml, respectively for calculation of the mean and for statistical analysis.
2.3. Analysis of cytokine concentration data
Standard curves for each cytokine were generated on a 4-parameter plot for each assay, and the cytokine concentrations for each serum sample was calculated from the corresponding curve fitting equation. Each sample was tested in duplicate, and the mean values were calculated and reported. The cytokine concentrations between different days in sow colostrum/milk samples and in piglet sera were compared by the Wilcoxon rank-sum test (SAS 9.1, SAS institute, NC). The cytokine concentrations in serum at PPD3-5 of unsuckled piglets derived by hysterectomy and those at PPD0 in pre-suckling piglets after natural birth were also compared (Wilcoxon rank-sum test). The cytokine concentrations in serum of suckling piglets at PPD1 were compared with the corresponding cytokine concentrations in serum of colostrum-deprived gnotobiotic pigs at derivation and through 33 days of age (Wilcoxon rank-sum test). Significant differences were considered as p<0.05 unless indicated. The cytokine concentrations between serum and colostrum/milk of the same sow at PPD2,7 and 11 were compared using the binomial proportion test (SAS 9.1, SAS institute, NC). The mean cytokine concentrations in serum and colostrum/milk samples of the corresponding sows were evaluated for correlation using Spearman coefficient (r) with p values.
2.4. Induction of immunoglobulin secreting cells (IgSC) by in vitro stimulation of porcine mononuclear cells with LPS and rotavirus in the presence of exogenous recombinant porcine TGF-β1 and IL-4
Mononuclear cells (MNC) were purified from spleens of 5 gnotobiotic piglets (19-33 days of age) using a previously published procedure (Yuan et al., 1996), and stored in 90%FBS/10% dimethylsulfoxide (DMSO) in liquid N2 until use. For in vitro stimulation, MNC were thawed quickly and washed to remove traces of DMSO. In each culture, 2x106 cells were stimulated with LPS (20 μg/ml), semi-purified Group A RV (Wa strain human rotavirus) (50 μg/ml) or mock stimulated with diluent for 6 days. The amount of each antigen used was optimized to yield the highest numbers of IgSC in the ELISPOT assay in preliminary studies. The cytokines TGF-β1 (10, 1 and 0.1 ng/ml) or IL-4 (10, 1 and 0.1ng/ml) were added to the cell cultures within the concentration ranges detected for maternally-derived cytokines transferred to the suckling piglets in our study. The MNC cultured with IL-2 (10 ng/ml) and stimulated with each antigen were included as baseline controls. The MNC cultured in the absence of cytokines and antigens were also included as controls. Each culture was performed in duplicate. After 6 days of incubation, cells were harvested, washed and loaded as undiluted, 10 and 100-fold dilutions onto microtiter plates (Nunc maxisorp, Roskilde, Denmark) coated with anti-porcine IgM (μ chain; 25 μg/ml), anti-porcine IgG (H+L chain; 2 μg/ml) (KPL Gaithersburg, Maryland) or anti-porcine IgA (α chain; 30 μg/ml) (Bethyl laboratories, Montgomery, Texas) to enumerate the total IgM, IgA and IgG secreting cells by an enzyme-linked immunospot (ELISPOT) assay previously developed in our laboratory (Yuan et al., 1996). The plates were incubated for 12h at 37°C and then washed to remove cells and incubated with peroxidase labeled anti-porcine IgM (μ) (0.1 μg/ml), IgA (α) (1 μg/ml) or IgG (γ) (1 μg/ml) conjugates (KPL) at RT for 1h. The spots were developed by TMB/H2O2 3-component substrate system (KPL) and counted microscopically with each spot representing a single IgSC.
2.5. Statistical analysis
The mean IgSC numbers detected in the presence of different concentrations of TGF-β1 and IL-4 were compared with the IgSC numbers detected in the presence of IL-2 for each pig by using the Sign test for matched pairs with significance selected at p<0.063.
3. Results
3.1. Cytokines in colostrum/milk and correlation with the concentrations in sow serum
Various levels of Th1 (IFN-γ and IL-12), Th2 (IL-4 and IL-10), Th3 (TGF-β1), Tr1 (IL-10) and pro-inflammatory (IL-6) cytokines were present in colostrum/milk. The peak concentrations of cytokines in colostrum/milk (IL-4>TGF-β1> IL-6=IFN-γ >IL-12>IL-10=TNF-α) were generally correlated with their respective concentrations in sow serum except for the much higher TGF-β1 in colostrum/milk and the absence of TNF-α in sow serum. The concentrations of all cytokines decreased at different rates in the transition from colostrum to milk (PPD0-3 versus PPD3-13).
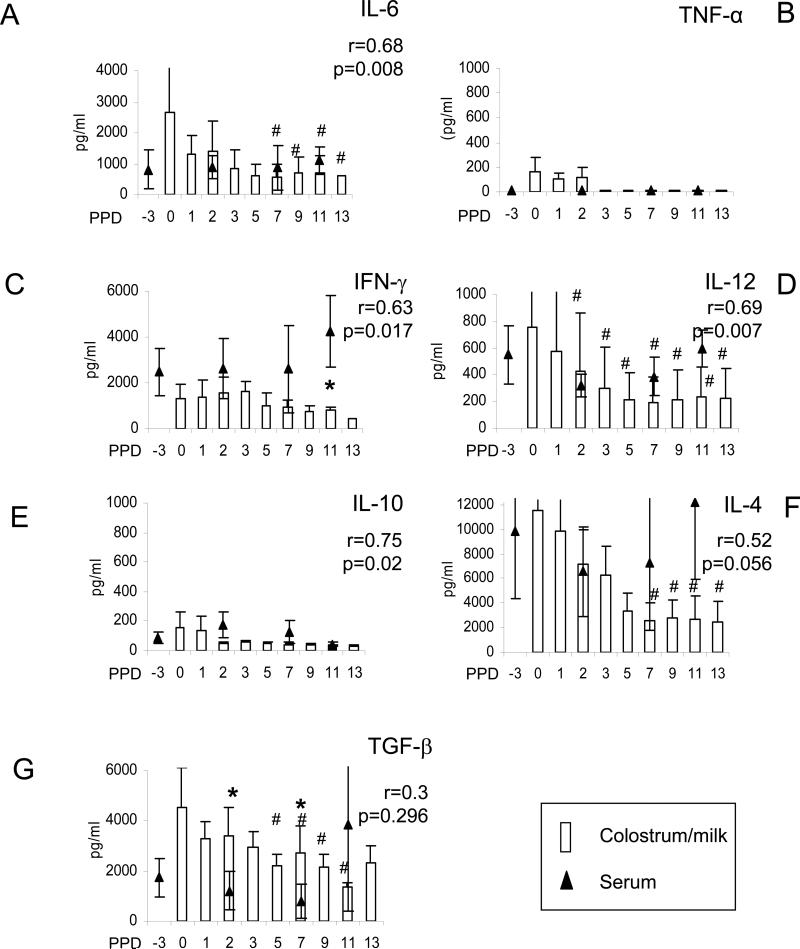
In assessing pro-inflammatory cytokines, the median IL-6 concentrations in colostrum/milk ranged from 2.7 ng/ml at PPD0 to 550 pg/ml at PPD7 (Fig.1A). The IL-6 concentrations in sow serum were generally lower than the concentrations in colostrum/early milk (PPD0 and 2) but higher than the concentrations in later milk (PPD7 and 11). The TNF-α was only detectable in colostrum at PPD0-2 (24-500 pg/ml), then was present in early milk of one sow at PPD3 and decreased to undetectable levels after PPD5 (Fig.1B). No TNF-α was detected in sow serum at any time point, suggesting the local production of this cytokine in the mammary gland.
Fig. 1.
Cytokine concentrations in colostrum/milk and the relationship with the cytokine concentrations in sow serum. Sows were bled at 3 days (on average) pre-partum (PPD-3) and at PPD2, 7, 11. Colostrum/milk samples were collected at days 0, 1, 2, 3, 5, 7, 9, 11 and 13 after farrowing. Open bars represent the mean cytokine concentrations in sow colostrum/milk; triangles represent the mean cytokine concentrations in sow sera. Standard error bars are indicted. The symbol “#” indicates significant differences in cytokine concentrations in sow milk at different days compared to the colostrum concentration at PPD1 (Wilcoxon rank-sum test, p<0.05). Single asterisks indicate significant differences in the cytokine concentrations between the sow serum and colostrum/milk at the same time point (binomial proportion test, p<0.05). Note the differences in vertical scales among the different cytokines. Five to six samples were tested for each time point. Spearman correlation coefficients between the cytokine concentrations in colostrum/milk and serum were calculated with p values <0.05 as significant.
For the Th1 cytokines, moderate correlations in the IFN-γ and IL-12 concentrations were found between sow serum and colostrum/milk (r=0.63 and 0.69, respectively, p<0.05) (Fig. 1C and 1D). The IFN-γ concentration in sow serum was higher than the concentrations in colostrum and milk throughout lactation (significantly higher at PPD11).
For the Th2 or Tr1 cytokines, the mean concentrations of IL-10 in colostrum/milk were low, ranging from 157 pg/ml (PPD0) to 30 pg/ml (PPD13). (Fig 1E). The IL-4 peak mean concentrations were highest among all the cytokines (2.5 ng/ml-11.5 ng/ml), decreased significantly in milk from PPD7-13 compared to PPD1 (Fig 1F). The IL-10 concentrations in colostrum/milk exhibited a significant correlation with the serum concentrations (r=0.75, p=0.002), whereas the correlation between the serum and colostrum/milk IL-4 was low (r=0.52, p=0.056). Thus based on these significant correlations in levels in serum versus colostrum/milk, IFN-γ, IL-12 and IL-10 in sow serum likely contribute substantially to the levels in mammary secretions.
For the Th3 cytokine, TGF-β1, the mean concentrations in colostrum/milk were also high (2.3-4.5 ng/ml) and they were significantly higher (3- to 8-fold) than those in sow serum (except for PPD11), suggesting local production of this cytokine in the mammary glands and secretion into colostrum/milk (Fig.1G). Consistent with this observation, there was no correlation between the TGF-β1 concentrations in colostrum/milk and in sow serum. The TGF-β1 concentration decreased significantly by PPD5 compared to PPD0.
3.2. Cytokine concentrations in piglet sera
No cytokines except for IL-12 and TGF-β1 were detectable in the hysterectomy-derived, colostrum-deprived naïve gnotobiotic pigs throughout the 33 days tested (data not shown), confirming the absence of endogenous production of the other cytokines examined. The concentrations of TGF-β1 and IL-12 in the gnotobiotic pigs remained steady during the entire test period (Table 1). In contrast, all cytokines detected in the colostrum/milk were also present in the sera of the suckling piglets except for TNF-α (Fig. 2). The mean peak cytokine concentrations (IL-4≥IL6>IFN-γ>IL-10) in piglet sera were detected at PPD1-2 [time of gut closure (Tizard, 2004)] in low but proportional concentrations to those in colostrum/milk. The concentrations of these cytokines in suckling piglets at PPD1 were significantly higher than those in gnotobiotic pigs at all times tested, confirming that these cytokines in suckling piglets at PPD1-2 are mainly colostrum-derived.
Table 1.
Concentrations (pg/ml) of IL-12 and TGF-β1 in serum and small intestinal contents (SIC) of neonatal piglets derived by hysterectomy or natural birth
| Delivery method | Samples | N | IL-12 | TGF-β1 |
|---|---|---|---|---|
| Hysterectomya | Serum | 20 | 84c±17 | 112c±18 |
| Natural birthb | Serum | 12 | 113±7 | 754±83*d |
| Hysterectomya | SIC | 4 | 4±0 | 15±4 |
| Natural birthb | SIC | 3 | 7±3 | 50±21 |
Serum and SIC samples were collected from piglets of 4 different sows, at 3-5 days after piglet derivation by hysterectomy and piglets were maintained under gnotobiotic and colostrum-deprived conditions
Serum and SIC samples were collected from pre-suckling piglets born naturally from 3 different sows
Mean cytokine concentration ± standard error of the mean. The concentrations of IL-6, TNF-α, IFN-γ, IL-10 and IL-4 in serum and SIC of piglets derived by hysterectomy (at 3-5 days after derivation) or by natural birth (before suckling) are not shown because they were below the detection concentrations (4-8 pg/ml). Samples below detection concentrations of the ELISA were assigned a concentration of 4 pg/ml (IL-12) or 8 pg/ml (TGF-β).
Single asterisk denotes significant difference in the concentration of TGF-β1 between piglets derived by hysterectomy and piglets born naturally (Wilcoxon rank-sum test, p<0.0001)
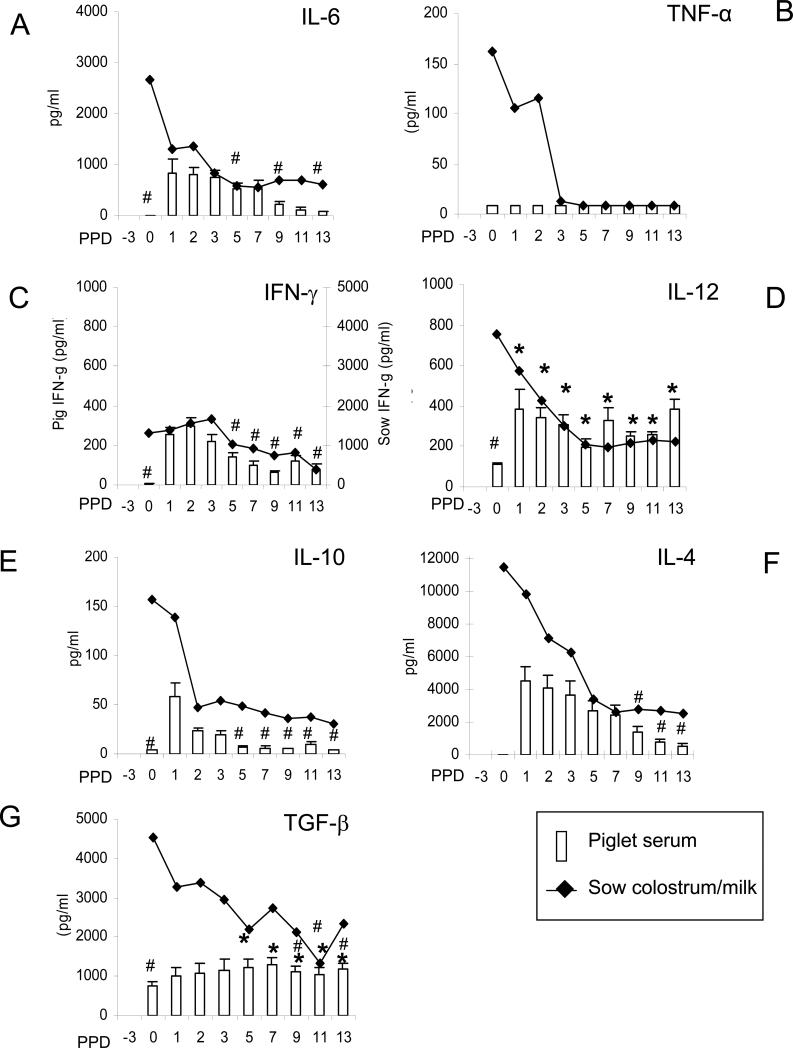
Fig. 2.
Cytokines in sera of suckling piglets. Piglets from 5 sows were allowed to suckle the sows for 14 days. Blood from piglets was collected pre-suckling (PPD0), and at PPD1, 2, 3, 5, 7, 9, 11 and 13. Open bars represent the mean cytokine concentrations in piglet sera; lines represent the mean cytokine concentrations in sow colostrum/milk. Standard error bars are included. The “#” indicates significant difference in cytokine concentrations in piglet sera at different days compared to the concentration at PPD1 (IL-10) and PPD1 and 2 for other cytokines (Wilcoxon rank-sum test, p<0.05). Asterisks indicate significant difference in TGF-β and IL-12 concentrations in piglet sera at different days compared to pre-suckling concentration (PPD0). Secondary axis was used to depict IFN-γ concentrations in sow serum and colostrum/milk. Note the difference in Y scales between cytokines. Samples below detection concentrations of the ELISA were assigned a concentration of 4 pg/ml (IL-6, IFN-γ, IL-12, IL-4 and IL-10) or 8 pg/ml (TNF-α and TGF-β). Twelve to 30 samples were tested at each time point.
The pro-inflammatory cytokine IL-6 peaked at PPD1-3, decreased significantly by PPD9 and was estimated to have a half life of 5-6 days (Fig.2A). The half life of each cytokine was estimated based on the average numbers of days for the cytokine concentration in the piglet sera to decrease to half that of the peak concentration at PID1-2. In contrast, there was no detectable TNF-α in piglet serum at any time pre-suckling and throughout the suckling period, suggesting its instability or reflecting its low concentration in sow colostrum/milk (Fig.2B).
Comparing Th1 cytokines, IFN-γ peaked at PPD1-2 and decreased significantly from the peak concentrations by PPD5 and thereafter. The estimated half life of IFN-γ was about 2-4 days (Fig.2C). Although serum IL-12 was present at birth, it increased significantly after suckling by PPD1-2, indicating that pigs acquire IL-12 passively from colostrum. The IL-12 concentrations then increased at PPD7 and thereafter indicating active production (Fig.2D). For the Th2 or Tr1 cytokines, the serum IL-10 mean concentration was low even at the peak at PPD1 (mean of 56 pg/ml), and it quickly decreased after PPD2 (Fig.2E). In contrast, the serum IL-4 concentration was high at PPD1 (mean of 4.5 ng/ml), and decreased at a slower rate than IL-10, indicative of a longer half life or active production (Fig.2F). Only by PPD9 was the serum IL-4 mean concentration significantly lower than the peak mean concentration at PPD1 and was still present at relatively high mean concentrations (mean=547 pg/ml) in piglet sera at PPD13. The estimated half life for IL-4 was 5-6 days.
For the Th3 cytokine TGF-β1, substantial mean concentrations were present (mean =754 pg/ml) in pre-suckling sera of all piglets at birth (Fig.2G). The TGF-β1 mean serum concentrations increased only ~2-fold from PPD1-13 compared to the concentration at birth, yet the increase was significant at PPD5-13, indicating active production. There was no correlation between the TGF-β1 concentrations in piglet sera and the concentration of TGF-β1 in colostrum/milk.
3.3. Comparison between serum cytokine concentrations in pre-suckling piglets born by natural birth and in piglets derived by hysterectomy
As previously mentioned, IL-6, TNF-α, IFN-γ, IL-10 and IL-4 were not detectable in the sera of piglets born by natural birth, before suckling, similar to colostrum-deprived piglets derived by hysterectomy. In addition, the serum IL-12 and TGF-β1 concentrations remained constant in the hysterectomy-derived piglets maintained under the gnotobiotic conditions throughout the 33 days tested, supporting the hypothesis that these cytokines are constitutively produced and not derived transplacentally. Similar mean concentrations of IL-12 were detected in sera of piglets derived by both methods (113 vs. 84 pg/ml). Interestingly, however, at birth an approximately 7-fold higher mean concentration of TGF-β1 was present in sera of piglets born naturally (754 pg/ml) compared to piglets derived by hysterectomy (112 pg/ml), suggesting that maternal TGF-β1 may be acquired by the piglet during the process of natural birth because low concentrations of TGF-β1 were also detected in the SIC of those piglets (Table 1). On the other hand, the process of natural birth may induce active production of TGF-β1 by piglets.
3.4. Serum cytokine concentrations in weaned piglets at various times post-weaning (PWD2-10) compared to those in suckling piglets
To study the persistence of passive cytokines in piglet serum, a subset of piglets from two sows (n=15) were weaned after suckling at PPD3 and the serum cytokine concentrations at PPD5 (PWD2), PPD7-9 (PWD4-6) and PPD11-13 (PWD8-10) were measured (Fig. 3). Suckling piglets from the same sows were included for comparison (n=8). The serum cytokine concentrations in weaned piglets at PPD7 and PPD9 or PPD11 and PPD13 were combined because no significant differences were observed between each of the two combined time points.
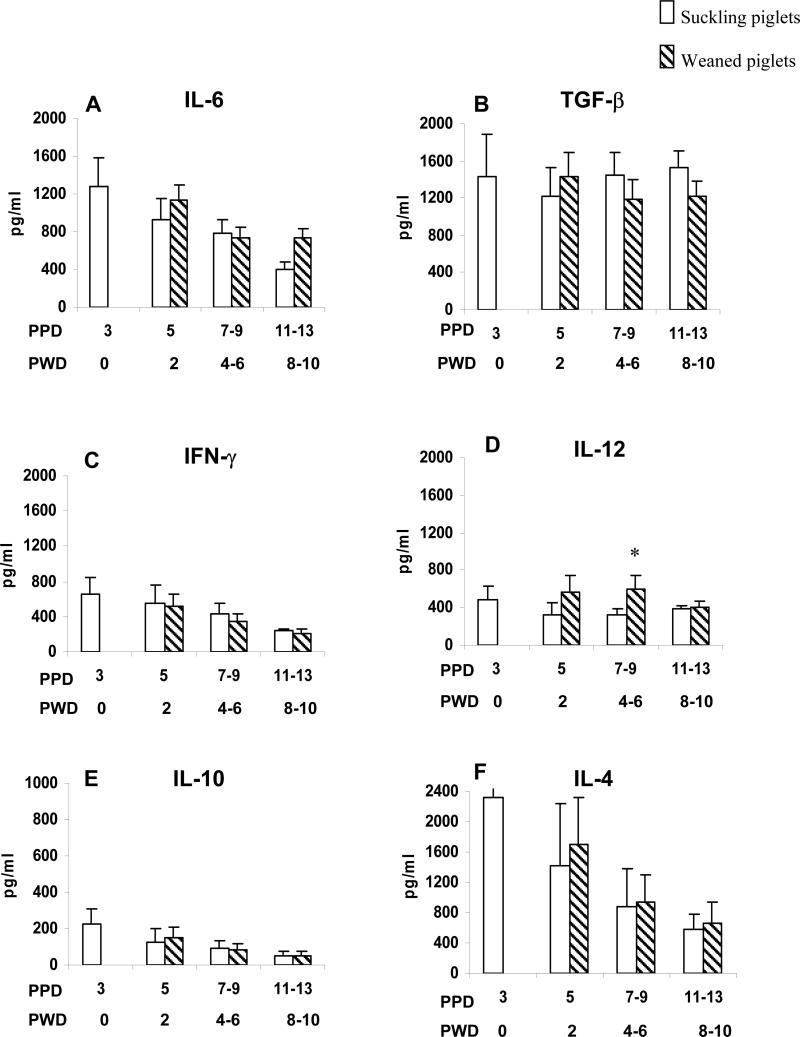
Fig. 3.
Comparison of cytokine concentrations in sera of weaned and suckling piglets. A group of piglets was allowed to suckle the sows for 14 days (suckling piglets). Another group of piglets was weaned at PPD3 through PPD13 (weaning piglets). Blood from these pigs was collected at PPD3, 5, 7, 9, 11 and 13 and the serum cytokine concentrations in the suckling piglets were compared to those in the weaned piglets at the same days. Open bars represent mean serum cytokine concentrations in suckling piglets. Striped bars represent mean serum cytokine concentrations in weaned piglets. Standard error bars are included. Asterisks represent the significant difference in cytokine concentrations of weaned piglets compared to the concentrations in suckling piglets of the same day (Wilcoxon rank-sum test, p<0.05). Note the difference in Y scales between cytokines. The X-axis indicates the PPD and the respective PWD. Six to 19 samples were available at each time point.
From PPD5-13 (PWD2-10), concentrations of IFN-γ, IL-10, IL-4 and TGF-β1 did not differ significantly between suckling and weaned piglets. These results suggest that there was no significant absorption of these cytokines from milk into piglet serum after gut closure (PPD2). The TNF-α was not detectable in serum of weaned or suckling piglets at any time (data not shown). At PPD7-9 (PWD4-6), the IL-12 concentration was significantly higher in the weaned piglets compared to the suckling piglets. The IL-6 concentration was also higher, but not significantly higher, in the weaned piglets at PPD5 (PWD 2) and PPD11-13 (PWD 8-10), suggesting that weaning (acclimation to altered commensal microflora after loss of sow milk diet) stimulates production of pro-Th1 (IL-12) and pro-inflammatory (IL-6) cytokines.
3.5. Effects of exogenous TGF-β1 and IL-4 on B cell responses to different antigens in vitro
Because TGF-β1 and IL-4 were present at the highest concentrations in sow colostrum/milk and they persisted at high concentrations in suckling piglets, we postulated that these two immunomodulating cytokines might play a role in the reduced responsiveness (maternal suppression) or Th2 bias (promoting IgA antibody responses) of neonatal pigs. To address this question, we examined the effects of these cytokines individually on MNC responses of naïve gnotobiotic pigs to LPS (a B cell mitogen) or RV (a neonatal enteric pathogen) in vitro.
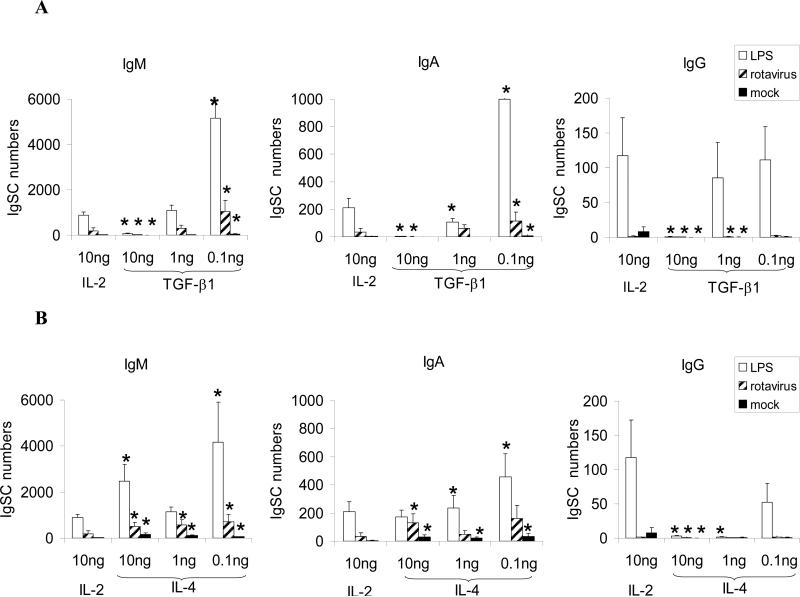
In MNC stimulated with LPS, high concentrations of TGF-β1 (10ng/ml), significantly reduced the total IgSC responses of all isotypes compared to the responses in the presence of IL-2 (Fig. 4a). In comparison, low concentrations of TGF-β1 (0.1ng/ml) enhanced IgG SC numbers and significantly enhanced IgM and IgA SC numbers, indicating that at low concentrations, TGF-β1 promoted isotypte switching to IgA and IgG SC in response to LPS. The IgSC responses of naïve B cells to RV stimulation were much lower than those to LPS, yet the impact of different TGF-β1 concentrations was similar. High concentrations of TGF-β1 (10ng/ml) also significantly reduced IgSC responses to RV compared to that obtained in the culture with IL-2. Lower TGF-β1 concentrations (0.1ng/ml) induced isotype switching to IgA but not to IgG (low number or no IgG SC were detected) in response to RV. In the absence of antigen stimulation, there was a low level of spontaneous immunoglobulin secretion in the MNC cultures with IL-2. The high concentrations of TGF-β1 (10ng/ml) also decreased survival or proliferation of B cells as indicated by the reduced IgSC numbers in mock-stimulated cultures in the presence of TGF-β1 compared to IL-2. The IgSC numbers in the MNC cultures without cytokines or antigens were absent or low and mainly of IgM isotypes (only 0-6 IgSC per culture).
Fig. 4.
Mean numbers of immunoglobulin (IgM, IgA and IgG) secreting cells induced by LPS (open bars), rotavirus (striped bars) or mock (solid bars). Mononuclear cells purified from the spleens of naïve gnotobiotic pigs (n=5) were stimulated with LPS, RV or mock in the presence of IL-2 or different concentrations of TGF-β1 or IL-4 for 6 days, and the IgM, IgA and IgG antibody secreting cells were enumerated after the incubation period. The single asterisks denote significant differences from the responses obtained in IL-2 cultures (Sign test for matched pair, p<0.063). Vertical bars represent standard error of the mean. Note the changes in the scales for the IgSC numbers (y-axis) for different antibody isotypes.
Similar to TGF-β1, IL-4 induced an inverse dose-dependent (0.1ng>10ng) isotype switching to IgA and IgG in response to LPS and isotype switching to IgA in response to RV (Fig. 4b). Unlike the effect of TGFβ-1, all concentrations of IL-4 tested enhanced the numbers of IgM SC. In the control cultures, in the absence of antigen stimulation, addition of IL-4 significantly enhanced the numbers of IgM and IgA SC compared to MNC cultures without cytokines or antigens, suggesting an effect of IL-4 on enhancing B cell proliferation and survival.
4. Discussion
In this study we determined the concentrations of various pro-inflammatory (IL-6 and TNF-α), Th1 (IFN-γ and IL-12), Th2 (IL-4 and IL-10) and Th3 (TGF-β) cytokines in sow serum and colostrum/milk, enumerated the cells secreting the Th1 and Th2 cytokines and examined the functions of porcine TGF-β and IL-4 in vitro. Our study provides new data because the limited number of previous studies of colostral cytokines in sows explored only growth factors including TGF-β (Jaeger et al., 1987; Wagstrom et al., 2000; Xu et al., 1999). Similarly, to our knowledge there have been no reports of the transfer and persistence of maternal cytokines in neonates.
Various concentrations of pro-inflammatory, Th1, Th2 and Th3 cytokines were present in colostrum/milk. The moderate correlations observed between sow blood and colostrum/milk for all cytokines except for TGF-β1 and TNF-α suggest that all but these latter cytokines are transudated or transported from the circulation to the mammary secretions, whereas TGF-β1 and TNF-α were produced locally in the mammary gland, consistent with previous reports in humans and cows (Hagiwara et al., 2000; Rudloff et al., 1992). By comparison, human milk contains high concentrations of TGF-β, TNF-α, IL-1, IL-6, IL-8 and IFN-γ (Goldman et al., 1996). In sows, in addition to TGF-β, IL-6 and IFN-γ in colostrum and milk, we also found a high concentration of IL-4, moderate concentrations of IL-12 and low concentrations of TNF-α and IL-10.
The finding that the cytokine pattern differs in piglets born by natural birth compared to that from hysterectomy-derived piglets is not unique to swine. Differences in cytokine profiles were observed in cord blood between infants born vaginally and those delivered by cesarean section. The concentrations of IFN-γ, TNF-α, IL-1β and IL-6 in human umbilical cord blood were significantly higher in cases of vaginal delivery than in cases of cesarean section (Malamitsi-Puchner et al., 2005). Elevation of TGF-β concentrations in the maternal and fetal circulation from pregnancy to birth has been reported in humans (Power et al., 2002). In our study, TGF-β1 was higher in the piglets born naturally compared to piglets derived by hysterectomy. This elevation of TGF-β1 in piglets during natural birth might be induced by labor as was seen in human infants (Malamitsi-Puchner et al., 2005), or after ingestion of amniotic fluid before or during birth as was suggested by the low concentrations of TGFβ-1 in SIC of these piglets.
The highest absorption of cytokines from colostrum into the piglets’ circulation (except for TNF-α) occurred at PPD1-2, coinciding with the gut closure period (Tizard, 2004). The high cytokine concentrations in piglet sera can be attributed to the absorption of cytokines and possibly, but to a lower extent, the colostral cells transferred to the piglet across the intestinal epithelium with the latter demonstrated in neonatal piglets (Le Jan, 1996; Tuboly et al., 1988; Williams, 1993). The piglets were estimated to ingest an average of 500-700 million colostral cells/day. The absence of TNF-α in piglets’ serum was probably due to the short half-life of this cytokine. Human TNF injected into mouse blood has a half-life of 20 min (Pietersz et al., 1998), in comparison to the 4 h half-life of IFN-α (Shechter et al., 2001) or the 100 min half-life of latent TGF-β1 (the high molecular form of TGF-β1 that needs activation for biological activity, usually by low pH, heating or urea) (Wakefield et al., 1990). The absence of TNF-α in serum from both sows and piglets suggests the existence of control mechanisms to avoid prolonged inflammatory responses which could lead to tissue damage.
To further confirm the maternal origin of these cytokines in our suckling piglets, it would have been desirable to include a group of colostrum-deprived conventional piglets as controls for the suckling and early weaned piglets. However, because of the low survival rate, and frequency of viral and bacterial infections in colostrum-deprived piglets or the necessity to treat them continuously with antibiotics, all of which can contribute to altered cytokine profiles in the colostrum-deprived piglets compared to colostrum-fed piglets raised under conventional conditions, we were unable to evaluate this control group. Therefore we included a group of colostrum-deprived gnotobiotic piglets as the controls. These piglets showed no detectable endogenous production of IL-6, TNF-α, INF-γ, IL-4 and IL-10 at birth and through 33 days of age when maintained under gnotobiotic conditions. The IL-12 and TGF-β1 were present at birth and remained unchanged through 33 days of age in these piglets. We also compared the serum cytokine levels in colostrum-deprived gnotobiotic pigs with or without colonization by commensal bacteria (lactobacillus acidophilus and L. reuteri) (Yuan and Saif, et al., unpublished 2007. Separate manuscript in preparation) and demonstrated that IL-6, TNF-α, INF-γ, IL-4 and IL-10 levels were similarly low or absent in these two groups. The concentrations of each of these cytokines in conventional suckling piglets at PPD1 were significantly higher that those in the age-matched colostrum-deprived gnotobiotic pigs (undetectable) confirming their maternal transfer post-suckling. Furthermore there were no symptomatic infections of the sows or suckling piglets during the study period. Consequently the cytokines in conventional piglet sera at PPD1 were mainly derived from sow colostrum/milk.
The impact of different concentrations of TGF-β1 and IL-4 on neonatal B cell responses was clearly demonstrated in our in vitro study. At high concentrations of TGF-β1 (10ng/ml), equivalent to the piglet serum cytokine concentrations in the early suckling period, the B cell responses were suppressed which might explain the reduced immunoresponsiveness of neonates. On the other hand, low concentrations (0.1ng/ml) of TGF-β1 increased the numbers of IgM and IgA SC in response to both LPS and RV antigens. Similarly, murine TGF-β1 (0.1-10ng/ml) acts as a bifunctional immune regulator for IgA antibody responses, enhances both early and late phases of IgA responses, yet exerts immunosuppressive effects on IL-5-induced IgA synthesis (Chen and Li, 1990). Additionally, maternal cytokines such as TGF-β, IL-6 and IL-10 present in the intestine post-suckling could also contribute to the differentiation of intestinal IgA producing cells and maturation of the neonatal intestinal immune system (Bottcher et al., 2000; Donnet-Hughes et al., 2000). The function of porcine IL-4 in promoting B cell survival and proliferation and IgA (Th2) responses is similar to that of the murine counterpart (Morris et al., 2002). However it is of future interest to know how the enhancing effects of high concentrations of IL-4 on B cell responses counterbalance the suppressive effects of the high concentrations of TGF-β in terms of the overall impact on the development of immune responses in neonates. In addition, it is also of future interest to study the role of another important milk component, soluble CD14, which is present at high concentrations (1-10μg/ml) in human milk (Labeta et al., 2000). Soluble CD14 in human milk has been shown to induce human and murine B cell growth and differentiation (Filipp et al., 2001) and plays an important role in innate immune responses (Vidal et al., 2001).
Previous studies of the functions of porcine cytokines in contributing to Th1 and Th2 responses have been unclear compared to similar studies of mice. Using B cells from conventional pigs, Crawley et al (Crawley et al., 2003) showed that Th1 cytokines (IFN-γ and IL-12) enhanced total IgG2 antibodies in some pigs, but total IgG1 antibodies in others. Similarly, recombinant porcine IL-10 upregulated IgG2 antibodies in some individuals but not in others. Raymond et al (Raymond and Wilkie, 2004) confirmed that porcine T cells exposed to hen egg white lysozyme treated dendritic cells expressed Th2 biased cytokine responses, whereas those exposed to M. tuberculosis treated DCs exhibited a Th1 bias. These findings were inconclusive possibly because of the highly variable individual genotypes of outbred pigs and their interaction with environmental factors. Our study offered the advantage of using MNC from naive gnotobiotic pigs whose B cell responses were not influenced by previous antigen encounters or the presence of heterogenous microbial flora. Although desirable to use neonatal pigs within the first week of life, the lack of sufficient MNC from these pigs hindered an investigation of the effect of different cytokine concentrations on such early neonatal immune responses. However, the continued lack of production of most endogenous cytokines by gnotobiotic pigs suggests that even the slightly older naïve gnotobiotic pigs as used in our study still accurately mimic the responses of the younger neonates.
The presence of high concentrations of Th2 cytokines or immunoregulatory cytokines such as TGF-β1 in neonates might play a critical role in allowing acquisition of the normal commensal microflora in the intestine. Recombinant swine IL-4 suppressed-LPS induced secretion of pro-inflammatory cytokines by alveolar macrophages (Nuntaprasert et al., 2005). Feeding TGF-β containing formulas to children with Crohn's disease led to reduced pro-inflammatory cytokines in the intestines (Fell, 2005). The TGF-β1 also down-regulates immune activation of intestinal epithelial cells and lamina propria immune cells (Mennechet et al., 2004). These reduced immune and inflammatory responses are likely important to permit the initial colonization of the neonatal intestine by the commensal microflora. Of interest, transient increased TGF-β expression in the pig small intestine has also been associated with weaning (Mei and Xu, 2005). In addition, as shown in humans, mice and in the present study of pigs, IL-4 and TGF-β play a role in isotype switching to IgA and the production of secretory IgA antibodies which function in immune exclusion of bacterial and viral pathogens (Iijima et al., 2001) and by their coating of commensals may limit the penetration of commensals beyond the intestinal epithelium (Macpherson and Harris, 2004). Thus the high concentrations of TGF-β1 and IL-4 in the colostrum/milk may play an important immunoregulatory role in the intestinal mucosa of the neonates, e. g. for establishment and maintenance of the initial commensal microflora in the intestine and promotion of secretory IgA responses needed to maintain gut homeostasis (Iijima et al., 2001).
In summary, this is the first study to comprehensively assess the various cytokines present in sow colostrum and milk and to document their transfer from colostrum/milk of mothers to neonates. Our study also demonstrated the immunoregulatory roles of porcine TGF-β1 and IL-4 on B cell responses of naive piglets. Based on our findings, future studies of the roles of other cytokines and the combined effect of different cytokines on naïve neonatal B cells are important to pursue. Our findings also allow a comparison of cytokine components in milk between pigs and humans to facilitate a better understanding of species similarities and differences using pigs as models for human diseases.
Acknowledgements
We thank Dr Juliette Hanson, Greg Myers, Todd Roots, Richard McCommick, Peggy Lewis, Terry Meek, Marcela Azevedo and Stacie Shafer for their technical assistance. We also thank Hong Liu for help with statistical analysis.
This work was supported by a grant from the National Institutes of Health, NIAID (RO1AI37111-05). Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center (OARDC) of The Ohio State University.
Abbreviations
- ELISPOT
enzyme-linked immunospot
- IgSC
immunoglobulin secreting cells
- LPS
lipopolysaccharide
- MNC
mononuclear cells
- PPD
post partum day
- PWD
post weaning day
- RV
rotavirus
- SIC
small intestinal contents
- TMB
tetramethylbenzidine
- Tr1
T regulatory type 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adkins B, Bu Y, Guevara P. The generation of Th memory in neonates versus adults: prolonged primary Th2 effector function and impaired development of Th1 memory effector function in murine neonates. J. Immunol. 2001;166:918–925. doi: 10.4049/jimmunol.166.2.918. [DOI] [PubMed] [Google Scholar]
- Azevedo MSP, Yuan L, Pouly S, Gonzalez AM, Jeong KI, Nguyen TV, Saif LJ. Cytokine responses in gnotobiotic pigs after infection with virulent or attenuated human rotavirus (HRV). J. Virol. 2006;80:372–382. doi: 10.1128/JVI.80.1.372-382.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocci V, von Bremen K, Corradeschi F, Franchi F, Luzzi E, Paulesu L. Presence of interferon-gamma and interleukin-6 in colostrum of normal women. Lymphokine Cytokine. Res. 1993;12:21–24. [PubMed] [Google Scholar]
- Bottcher MF, Jenmalm MC, Garofalo RP, Bjorksten B. Cytokines in breast milk from allergic and nonallergic mothers. Pediatr. Res. 2000;47:157–162. doi: 10.1203/00006450-200001000-00026. [DOI] [PubMed] [Google Scholar]
- Chen SS, Li Q. Transforming growth factor-beta 1 (TGF-beta 1) is a bifunctional immune regulator for mucosal IgA responses. Cell Immunol. 1990;128:353–361. doi: 10.1016/0008-8749(90)90032-m. [DOI] [PubMed] [Google Scholar]
- Crawley A, Raymond C, Wilkie BN. Control of immunoglobulin isotype production by porcine B-cells cultured with cytokines. Vet. Immunol. Immunopathol. 2003;91:141–154. doi: 10.1016/s0165-2427(02)00293-3. [DOI] [PubMed] [Google Scholar]
- Donnet-Hughes A, Duc N, Serrant P, Vidal K, Schiffrin EJ. Bioactive molecules in milk and their role in health and disease: the role of transforming growth factor-beta. Immunol Cell Biol. 2000;78:74–79. doi: 10.1046/j.1440-1711.2000.00882.x. [DOI] [PubMed] [Google Scholar]
- Early EM, Reen DJ. Antigen-independent responsiveness to interleukin-4 demonstrates differential regulation of newborn human T cells. Eur. J. Immunol. 1996;26:2885–2889. doi: 10.1002/eji.1830261212. [DOI] [PubMed] [Google Scholar]
- Fell JM. Control of systemic and local inflammation with transforming growth factor beta containing formulas. J. Parenter. Enteral. Nutr. 2005;29:S126–128. doi: 10.1177/01486071050290S4S126. discussion S129-133, S184-128. [DOI] [PubMed] [Google Scholar]
- Filipp D, Alizadeh-Khiavi K, Richardson C, Palma A, Paredes N, Takeuchi O, Akira S, Julius M. Soluble CD14 enriched in colostrum and milk induces B cell growth and differentiation. Proc. Natl. Acad. Sci. U S A. 2001;98:603–608. doi: 10.1073/pnas.98.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foussat A, Cottrez F, Brun V, Fournier N, Breittmayer JP, Groux H. A comparative study between T regulatory type 1 and CD4+CD25+ T cells in the control of inflammation. J. Immunol. 2003;171:5018–5026. doi: 10.4049/jimmunol.171.10.5018. [DOI] [PubMed] [Google Scholar]
- Goldman AS, Chheda S, Garofalo R, Schmalstieg FC. Cytokines in human milk: properties and potential effects upon the mammary gland and the neonate. J. Mammary Gland Biol. Neoplasia. 1996;1:251–258. doi: 10.1007/BF02018078. [DOI] [PubMed] [Google Scholar]
- Hagiwara K, Kataoka S, Yamanaka H, Kirisawa R, Iwai H. Detection of cytokines in bovine colostrum. Vet. Immunol. Immunopathol. 2000;76:183–190. doi: 10.1016/s0165-2427(00)00213-0. [DOI] [PubMed] [Google Scholar]
- Iijima H, Takahashi I, Kiyono H. Mucosal immune network in the gut for the control of infectious diseases. Rev. Med. Virol. 2001;11:117–133. doi: 10.1002/rmv.307. [DOI] [PubMed] [Google Scholar]
- Jaeger LA, Lamar CH, Bottoms GD, Cline TR. Growth-stimulating substances in porcine milk. Am. J. Vet. Res. 1987;48:1531–1533. [PubMed] [Google Scholar]
- Labeta MO, Vidal K, Nores JE, Arias M, Vita N, Morgan BP, Guillemot JC, Loyaux D, Ferrara P, Schmid D, Affolter M, Borysiewicz LK, Donnet-Hughes A, Schiffrin EJ. Innate recognition of bacteria in human milk is mediated by a milk-derived highly expressed pattern recognition receptor, soluble CD14. J. Exp. Med. 2000;191:1807–1812. doi: 10.1084/jem.191.10.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat. Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- Le Jan C. Cellular components of mammary secretions and neonatal immunity: a review. Vet. Res. 1996;27:403–417. [PubMed] [Google Scholar]
- Letterio JJ, Geiser AG, Kulkarni AB, Roche NS, Sporn MB, Roberts AB. Maternal rescue of transforming growth factor-beta 1 null mice. Science. 1994;264:1936–1938. doi: 10.1126/science.8009224. [DOI] [PubMed] [Google Scholar]
- Ludviksson BR, Seegers D, Resnick AS, Strober W. The effect of TGF-beta1 on immune responses of naive versus memory CD4+ Th1/Th2 T cells. Eur. J. Immunol. 2000;30:2101–2111. doi: 10.1002/1521-4141(200007)30:7<2101::AID-IMMU2101>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- Malamitsi-Puchner A, Protonotariou E, Boutsikou T, Makrakis E, Sarandakou A, Creatsas G. The influence of the mode of delivery on circulating cytokine concentrations in the perinatal period. Early Hum. Dev. 2005;81:387–392. doi: 10.1016/j.earlhumdev.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Mei J, Xu RJ. Transient changes of transforming growth factor-beta expression in the small intestine of the pig in association with weaning. Br. J. Nutr. 2005;93:37–45. doi: 10.1079/bjn20041302. [DOI] [PubMed] [Google Scholar]
- Mennechet FJ, Kasper LH, Rachinel N, Minns LA, Luangsay S, Vandewalle A, Buzoni-Gatel D. Intestinal intraepithelial lymphocytes prevent pathogen-driven inflammation and regulate the Smad/T-bet pathway of lamina propria CD4+ T cells. Eur. J. Immunol. 2004;34:1059–1067. doi: 10.1002/eji.200324416. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Ichijo H, Heldin CH. Transforming growth factor-beta: latent forms, binding proteins and receptors. Growth Factors. 1993;8:11–22. doi: 10.3109/08977199309029130. [DOI] [PubMed] [Google Scholar]
- Morris SC, Dragula NL, Finkelman FD. IL-4 promotes Stat6-dependent survival of autoreactive B cells in vivo without inducing autoantibody production. J. Immunol. 2002;169:1696–1704. doi: 10.4049/jimmunol.169.4.1696. [DOI] [PubMed] [Google Scholar]
- Nuntaprasert A, Mori Y, Muneta Y, Yoshihara K, Tsukiyama-Kohara K, Kai C. The effect of recombinant swine interleukin-4 on swine immune cells and on pro-inflammatory cytokine productions in pigs. Comp Immunol Microbiol Infect. Dis. 2005;28:83–101. doi: 10.1016/j.cimid.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Ogawa J, Sasahara A, Yoshida T, Sira MM, Futatani T, Kanegane H, Miyawaki T. Role of transforming growth factor-beta in breast milk for initiation of IgA production in newborn infants. Early Hum. Dev. 2004;77:67–75. doi: 10.1016/j.earlhumdev.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Penttila IA, Flesch IE, McCue AL, Powell BC, Zhou FH, Read LC, Zola H. Maternal milk regulation of cell infiltration and interleukin 18 in the intestine of suckling rat pups. Gut. 2003;52:1579–1586. doi: 10.1136/gut.52.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietersz GA, Toohey B, McKenzie IF. In vitro and in vivo evaluation of human tumor necrosis factor-alpha (hTNFalpha) chemically conjugated to monoclonal antibody. J. Drug Target. 1998;5:109–120. doi: 10.3109/10611869808995864. [DOI] [PubMed] [Google Scholar]
- Power LL, Popplewell EJ, Holloway JA, Diaper ND, Warner JO, Jones CA. Immunoregulatory molecules during pregnancy and at birth. J. Reprod. Immunol. 2002;56:19–28. doi: 10.1016/s0165-0378(01)00146-2. [DOI] [PubMed] [Google Scholar]
- Raymond CR, Wilkie BN. Th-1/Th-2 type cytokine profiles of pig T-cells cultured with antigen-treated monocyte-derived dendritic cells. Vaccine. 2004;22:1016–1023. doi: 10.1016/j.vaccine.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Rudloff HE, Schmalstieg FC, Jr., Mushtaha AA, Palkowetz KH, Liu SK, Goldman AS. Tumor necrosis factor-alpha in human milk. Pediatr. Res. 1992;31:29–33. doi: 10.1203/00006450-199201000-00005. [DOI] [PubMed] [Google Scholar]
- Rudloff HE, Schmalstieg FC, Jr., Palkowetz KH, Paszkiewicz EJ, Goldman AS. Interleukin-6 in human milk. J. Reprod. Immunol. 1993;23:13–20. doi: 10.1016/0165-0378(93)90023-b. [DOI] [PubMed] [Google Scholar]
- Shechter Y, Preciado-Patt L, Schreiber G, Fridkin M. Prolonging the half-life of human interferon-alpha 2 in circulation: Design, preparation, and analysis of (2-sulfo-9-fluorenylmethoxycarbonyl)7- interferon-alpha 2. Proc. Natl. Acad. Sci. U S A. 2001;98:1212–1217. doi: 10.1073/pnas.98.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizard IR. Veterinary Immunology: An Introduction. Saunders; Philadelphia: 2004. [Google Scholar]
- Tuboly S, Bernath S, Glavits R, Medveczky I. Intestinal absorption of colostral lymphoid cells in newborn piglets. Vet. Immunol. Immunopathol. 1988;20:75–85. doi: 10.1016/0165-2427(88)90027-x. [DOI] [PubMed] [Google Scholar]
- Vidal K, Labeta MO, Schiffrin EJ, Donnet-Hughes A. Soluble CD14 in human breast milk and its role in innate immune responses. Acta. Odontol. Scand. 2001;59:330–334. doi: 10.1080/000163501750541219. [DOI] [PubMed] [Google Scholar]
- Wagstrom EA, Yoon KJ, Zimmerman JJ. Immune components in porcine mammary secretions. Viral Immunol. 2000;13:383–397. doi: 10.1089/08828240050144699. [DOI] [PubMed] [Google Scholar]
- Wakefield LM, Winokur TS, Hollands RS, Christopherson K, Levinson AD, Sporn MB. Recombinant latent transforming growth factor beta 1 has a longer plasma half-life in rats than active transforming growth factor beta 1, and a different tissue distribution. J. Clin. Invest. 1990;86:1976–1984. doi: 10.1172/JCI114932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol. Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- Williams PP. Immunomodulating effects of intestinal absorbed maternal colostral leukocytes by neonatal pigs. Can. J. Vet. Res. 1993;57:1–8. [PMC free article] [PubMed] [Google Scholar]
- Xu R, Doan QC, Regester GO. Detection and characterisation of transforming growth factor-beta in porcine colostrum. Biol. Neonate. 1999;75:59–64. doi: 10.1159/000014078. [DOI] [PubMed] [Google Scholar]
- Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J. Virol. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]