Abstract
Background: Evidence indicates that men and African Americans may be more susceptible to weight gain resulting from sleep loss than women and whites, respectively. Increased daily caloric intake is a major behavioral mechanism that underlies the relation between sleep loss and weight gain.
Objective: We sought to assess sex and race differences in caloric intake, macronutrient intake, and meal timing during sleep restriction.
Design: Forty-four healthy adults aged 21–50 y (mean ± SD: 32.7 ± 8.7 y; n = 21 women, n = 16 whites) completed an in-laboratory protocol that included 2 consecutive baseline nights [10 or 12 h time in bed (TIB)/night; 2200–0800 or 2200–1000] followed by 5 consecutive sleep-restriction nights (4 h TIB/night; 0400–0800). Caloric intake and meal-timing data were collected during the 2 d after baseline sleep and the first 3 d after sleep restriction.
Results: During sleep restriction, subjects increased daily caloric intake (P < 0.001) and fat intake (P = 0.024), including obtaining more calories from condiments, desserts, and salty snacks (Ps < 0.05) and consumed 532.6 ± 295.6 cal during late-night hours (2200–0359). Relative to women, men consumed more daily calories during baseline and sleep restriction, exhibited a greater increase in caloric intake during sleep restriction (d = 0.62), and consumed a higher percentage of daily calories during late-night hours (d = 0.78, Ps < 0.05). African Americans and whites did not significantly differ in daily caloric intake, increased caloric intake during sleep restriction, or meal timing. However, African Americans consumed more carbohydrates, less protein, and more caffeine-free soda and juice than whites did during the study (Ps < 0.05).
Conclusions: Men may be more susceptible to weight gain during sleep loss than women due to a larger increase in daily caloric intake, particularly during late-night hours. These findings are relevant to the promotion of public health awareness by highlighting nutritional risk factors and modifiable behaviors for weight gain related to sleep-wake timing. This trial was registered at clinicaltrials.gov as NCT02128737 and NCT02130791.
INTRODUCTION
A growing body of epidemiologic and laboratory evidence suggests that short sleep duration may be a risk factor for weight gain and obesity. Cross-sectional and prospective cohort studies have shown that short sleep duration is associated with higher BMI (1, 2) and increased risk of obesity (3, 4) and is a predictor of greater weight gain (5–7). Two recent laboratory studies showed that sleep restriction (SR) (4–5 h time in bed [TIB]/night for 5 consecutive nights) led to weight gain in healthy adults (8, 9).
Increased daily caloric intake is a major behavioral mechanism that underlies the relation between short sleep duration and weight gain. SR leads to an increase in daily caloric intake (8–13) and greater caloric consumption from snacking (14). It remains unclear whether this increased caloric intake is due to the overconsumption of a specific macronutrient; 2 studies showed that SR increased carbohydrate consumption (8, 14), whereas another study reported increased fat consumption (12). Delayed meal timing has also been identified as a significant contributor to weight gain (15–18). SR promotes the consumption of additional calories during evening and late-night hours (8, 9).
Several epidemiologic studies have shown a stronger association between shorter sleep duration and higher BMI in men than women (19–21). This pattern has also been observed in adolescents (22, 23) and children (24, 25). Using self-report surveys, 2 recent population studies showed that sleep duration was inversely associated with BMI in men only, whereas poor sleep quality was positively associated with BMI in women only (20, 21). In a prospective cohort study, short sleep duration was associated with weight gain and the development of obesity at 1 y follow-up in men but not women (26). We showed that men gained more weight than women during a controlled sleep-restriction experiment (9).
Race differences also exist in the relation between sleep duration and weight. African Americans are more likely to be shorter sleepers than are whites (3, 27). Two epidemiologic studies showed that the association between short sleep duration and increased odds for obesity was stronger in African Americans than whites (28, 29). We showed that African Americans gained more weight than whites did during a controlled sleep-restriction experiment (9).
Collectively, these lines of evidence suggest that sleep loss potentiates weight gain by promoting an increase in daily caloric intake and that there are sex and race differences in the propensity for such weight gain. However, it is unknown whether sex or race differences exist in the increased caloric intake response to SR. To address this deficiency, we experimentally tested the hypothesis that men and African Americans would exhibit a greater increase in caloric intake during SR than would women and whites, respectively. We also explored sex and race differences in macronutrient intake and meal timing.
SUBJECTS AND METHODS
Subjects
Healthy individuals, aged 21–50 y, were recruited in response to study advertisements. The subjects reported habitual nightly sleep durations between 6.5 and 8.5 h, habitual bedtimes between 2200 and 0000, and habitual morning awakenings between 0600 and 0900; these reports were confirmed objectively by using actigraphy. Subjects had no evidence of habitual napping, no sleep disturbances (ie, no complaints of insomnia, daytime sleepiness, or other sleep-wake disturbances), and neither extreme morningness nor extreme eveningness as assessed by questionnaire (30). Subjects were free of acute and chronic medical and psychological conditions as established by interviews, clinical histories, questionnaires, physical examinations, and blood (including a fasting blood glucose test) and urine tests. Subjects were nonsmokers and did not participate in shift work, transmeridian travel, or irregular sleep-wake routines in the 60 d before the study. Enrolled subjects were monitored at home by using actigraphy, sleep-wake diaries, and time-stamped call-ins to assess bedtime and wake time during the week before the in-laboratory phase and the week after the laboratory phase. Subjects were not permitted to use caffeine, alcohol, tobacco, and medications (except oral contraceptives) in the week before the laboratory experiment as verified by urine screenings. Sleep disorders were excluded by a night of laboratory polysomnography and oximetry measurements.
Research protocols were approved by the Institutional Review Board of the University of Pennsylvania. All subjects provided written informed consent before enrollment and were compensated for their participation.
Experimental design
Subjects participated in 1 of 2 protocols in the Sleep and Chronobiology Laboratory at the Hospital of the University of Pennsylvania and were studied for 14 or 18 consecutive days continuously with daily clinical checks of vital signs and symptoms by nurses (with an independent physician on call). Both protocols consisted of 2 initial baseline nights of 10 or 12 h TIB/night (2200–0800 or 2200–1000) followed by 5 nights of sleep restricted to 4 h TIB/night (0400–0800). The daily schedule for sleep and wake, neurobehavioral and physiologic testing, and times available for eating and drinking were nearly identical for both protocols. Data collected during the first 5 d of both protocols were used for analyses in this study. Five consecutive nights of sleep restricted to 4 h/night produces cumulative neurobehavioral deficits in most healthy adults (31) and is within the range of sleep loss that occurs as a result of lifestyle factors (32).
During the in-laboratory phase of the study, subjects were not permitted to leave the laboratory. Subjects were ambulatory and permitted to watch television, read, play video or board games, and perform other sedentary activities between test bouts (which were completed while sitting at a computer) but were not allowed to exercise. Subjects wore a wrist actigraph throughout the in-laboratory protocol. On certain protocol days, subjects wore ambulatory electroencephalography and electrocardiography recording equipment for 24-h intervals. Light levels in the laboratory were held constant at <50 lux during scheduled wakefulness and <1 lux during scheduled sleep periods. The ambient temperature was maintained between 22°C and 24°C. Subjects were behaviorally monitored by trained staff continuously throughout the protocol to ensure adherence.
Measures
Subjects selected their meals and snacks by choosing from various menu options, selecting additional food and drink available in the kitchen within the laboratory suite (which included a refrigerator, microwave, and toaster oven), and making requests to the study staff. To ensure subjects were provided sufficient time to eat each day, three 30- to 45-min eating opportunities were specified in the protocol during days with a 2200 bedtime (0900, 1235, and 1830) and one additional 30-min opportunity to eat was specified in the protocol during days with a 0400 bedtime (0030). In addition to these specified meal times, subjects were allowed to consume food and drink at any time during the protocol other than when they were completing neurobehavioral tests or sleeping. During a typical day, in addition to the meal times specified in the protocol, subjects could consume food and drink from 0945 to 1000, 1105 to 1200, 1310 to 1400, 1430 to 1600, 1630 to 1645, 1730 to 1800, 1920 to 2000, 2030 to 2200, 2230 to 0000, 0115 to 0200, and 0230 to 0350. Subjects could eat what they preordered through menus or could select from other foods available in the laboratory kitchen and could eat as much (or as little) as they wanted. Subjects retrieved their own food and drink from the laboratory kitchen when they wanted to eat or drink and could eat at a table in the common area or privately in their bedrooms.
All food was weighed and recorded before being provided to subjects. To enhance the measurement accuracy of each food item's weight, items were served in individual containers. Each day, a detailed description of items, amounts consumed, and intake times were recorded by trained monitors. In addition, any food or drink that was left over after each meal was weighed and recorded. Intake data were entered into The Food Processor SQL program (version 10.11; ESHA Research), which is a validated (33) professional nutrition-analysis software and database program that provides components of food and drink intake including calories and macronutrients.
Caloric intake from wake time (0800 or 1000) to 2200 during the 2 d after baseline sleep was averaged and defined as baseline caloric intake. Caloric intake from wake time (0800) to 0359 during the first 3 d after SR was averaged and defined as SR caloric intake. Caloric intake was also measured during the fourth and fifth days after SR; however, these data were not included in the current analyses because some of the subjects (n = 18) fasted during the fourth day of SR as part of a separate experiment.
Statistical analyses
Repeated-measures ANOVAs were used to compare caloric intake, macronutrient intake, and meal timing between baseline and SR in all subjects. Mixed-model ANOVAs, with age as a covariate, the baseline compared with SR as the repeated-measures variable (sleep condition), and race and sex as between-subjects factors, were used to compare sex and race differences in the change in daily caloric intake (including calories consumed from specific food and drink categories), macronutrient intake, and meal timing. Between-subjects ANOVAs, with age as a covariate, were used to examine differences in caloric intake during baseline and SR, increased caloric intake (caloric intake during SR minus caloric intake during baseline), food and drink categories, and late-night eating (calories consumed between 2200 and 0359) between sex and race groups. Statistical analyses were conducted with IBM SPSS Statistics for Windows (version 20.0; IBM). Effect sizes were calculated by using Cohen's d (34). The false discovery rate (35) was used when differences related to food and drink categories were examined to account for multiple comparisons.
RESULTS
Subject characteristics
Forty-four of 47 enrolled subjects completed the study. The 3 noncompleters were either withdrawn because of protocol noncompliance (n = 1) or withdrew because of health or personal issues unrelated to the protocol (n = 2). Men (n = 23) were taller (mean ± SD height: 1.78 ± 0.08 m) and weighed more (82.4 ± 14.62 kg) than women did (n = 21; 1.63 ± 0.07 m; 65.30 ± 9.86 kg; Ps < 0.001). Men and women did not differ in age (P = 0.28), BMI (P = 0.29), chronotype (P = 0.24), or percentages of African Americans and whites (P = 0.82; Table 1). African Americans (n = 28) and whites (n = 16) did not differ in height (P = 0.10), weight (P = 0.34), age (P = 0.14), BMI (P = 0.96), chronotype (P = 0.52), or percentages of men and women (P = 0.82; Table 1). Sleep duration and timing during the week before the in-laboratory study were assessed by using wrist actigraphy; there were no sex or race differences in prestudy sleep durations, onsets, offsets, or midpoints (Ps > 0.10; Table 1).
TABLE 1.
Subject characteristics1
| n | Age | BMI | Men | African American | Chronotype2 | Sleep duration3 | Sleep midpoint3 | |
| y | 2 | % | % | h | Time ± min | |||
| All subjects | 44 | 32.7 ± 8.74 | 25.2 ± 3.5 | 52.2 | 63.6 | 40.76 ± 5.89 | 8.04 ± 0.34 | 03:39 ± 46.8 |
| Men | 23 | 34.1 ± 7.9 | 25.7 ± 3.3 | — | 65.2 (15)5 | 42.05 ± 5.71 | 8.02 ± 0.55 | 03:24 ± 41.4 |
| Women | 21 | 31.2 ± 9.6 | 24.6 ± 3.7 | — | 61.9 (13) | 39.48 ± 5.92 | 8.06 ± 0.35 | 03:54 ± 48.6 |
| African Americans | 28 | 31.3 ± 8.0 | 25.2 ± 3.2 | 53.6 (15) | — | 41.31 ± 5.63 | 7.97 ± 0.48 | 03:37 ± 49.2 |
| Whites | 16 | 35.3 ± 9.6 | 25.2 ± 4.1 | 50.0 (8) | — | 39.88 ± 6.38 | 8.16 ± 0.41 | 03:40 ± 43.8 |
There were no significant differences between sex or race groups (t tests or Mann-Whitney U test), Ps > 0.10.
Morningness-eveningness composite scale.
Sleep duration and sleep midpoint were measured by using wrist actigraphy supplemented by a sleep diary for 1 wk before study entry.
Mean ± SD (all such values).
n in parentheses (all such values).
Caloric intake
Caloric intake was significantly greater during SR than baseline (F[1,43] = 54.94, P < 0.001). When we calculated caloric intake as a percentage of caloric need (estimated by using the Harris-Benedict equation for resting metabolic rate × 1.4), subjects consumed 109.28 ± 29.54% of caloric need during baseline and 133.04 ± 36.65% of caloric need during SR. In both conditions, subjects consumed significantly more calories than the amount needed to fulfill 100% of this estimation of caloric need (baseline: t43 = 2.08, P = 0.043; SR: t43 = 5.98, P < 0.001); however, intake during SR was significantly greater than during baseline (t43 = 7.57, P < 0.001).
A mixed-model ANOVA revealed a significant sex × sleep condition interaction (F[1,39] = 6.99, P = 0.012) and a main effect of sex (F[1,39] = 8.15, P = 0.004). Men consumed more calories during baseline (F[1,39] = 4.60, P = 0.038) and SR (F[1,39] = 10.15, P = 0.003) and exhibited a greater increase in caloric intake during SR (SR minus baseline; F[1, 39] = 6.99, P = 0.012, d = 0.92; Figure 1A) than did women. When we controlled for differences in baseline caloric intake, men also exhibited a greater percentage increase in caloric intake than did women (SR divided by baseline: men, 28.5%; women, 16.9%; F[1,42] = 4.27, P = 0.045, d = 0.62; Figure 1A). By contrast, the race × sleep condition interaction was not significant (P = 0.83), and there was no main effect of race (P = 0.53) (Figure 1B).
FIGURE 1.
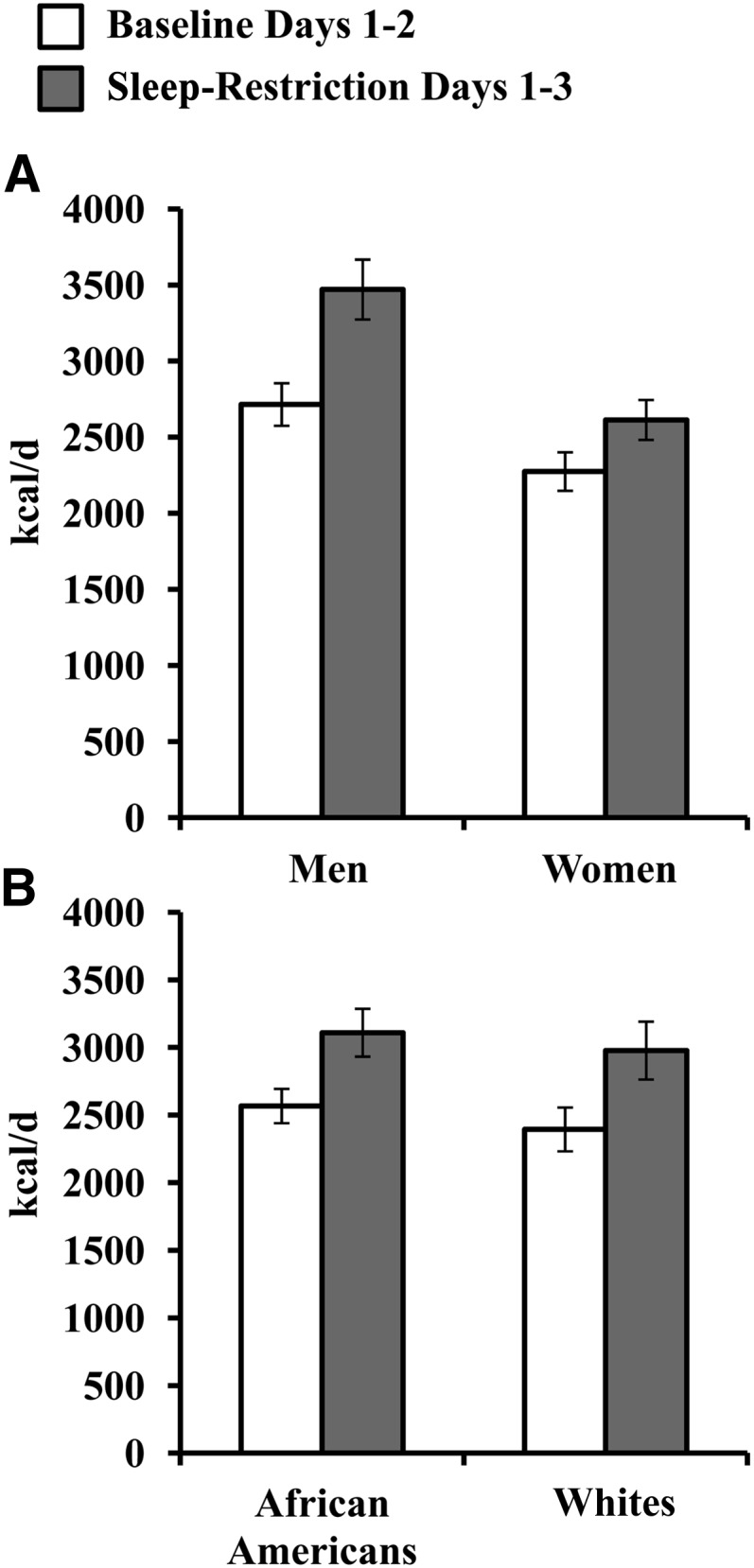
Mean (±SEM) daily caloric intake during baseline and sleep restriction. Subjects consumed more calories during sleep restriction than baseline (P < 0.001; repeated-measures ANOVA). A: Daily caloric intake showed a significant sex × sleep condition interaction (P = 0.012) and a significant main effect of sex (P = 0.004). Men (n = 23) consumed more calories during baseline (P = 0.038) and sleep restriction (P = 0.003) and exhibited a greater increase in caloric intake during sleep loss (sleep restriction − baseline; P = 0.012, d = 0.92) than did women (n = 21). When differences in baseline caloric intake were controlled for, men also exhibited a greater percentage increase in caloric intake than did women (sleep restriction÷baseline; 28.5% compared with 16.9%; P = 0.045, d = 0.62). B: The race × sleep condition interaction for daily caloric intake was not significant (P = 0.83), and there was no main effect of race (P = 0.53).
Macronutrient intake
The percentage of calories derived from protein was significantly lower (F[1,43] = 14.73, P < 0.001), and the percentage of calories derived from fat was significantly higher (F[1,43] = 5.50, P = 0.024) during SR than baseline (Table 2). The percentage of calories derived from carbohydrates did not differ between baseline and SR (P = 0.97). There were no significant sex × sleep condition interactions for the percentage of calories derived from protein, carbohydrates, or fat (Ps > 0.63), and there were no significant main effects of sex for macronutrient intake (Ps > 0.75). There was a significant race × sleep condition interaction for the percentage of calories derived from protein (F[1,39] = 5.58, P = 0.023) but not for the percentage of calories derived from carbohydrates (P = 0.42) or fat (P = 0.09). There were significant main effects of race for the percentage of calories derived from protein and carbohydrates but not from fat (P = 0.41), whereby African Americans consumed a lower percentage of calories from protein (F[1,39] = 19.47, P < 0.001, d = 1.50) and a higher percentage of calories from carbohydrates (F[1,39] = 6.06, P = 0.018, d = 0.87) than did whites.
TABLE 2.
Macronutrient intake during baseline and sleep restriction1
| Baseline days 1–2 |
Sleep-restriction days 1–3 |
|||||
| Protein | Carbohydrates | Fat | Protein | Carbohydrates | Fat | |
| All subjects (n = 44) | 14.9 ± 2.7 | 58.0 ± 5.6 | 28.9 ± 5.2 | 13.3 ± 2.2a | 58.0 ± 4.5 | 30.6 ± 4.6a |
| Men (n = 23) | 15.1 ± 2.4 | 58.1 ± 5.6 | 28.6 ± 5.4 | 13.3 ± 2.3 | 58.0 ± 4.8 | 30.5 ± 5.0 |
| Women (n = 21) | 14.6 ± 3.0 | 57.8 ± 5.9 | 29.2 ± 5.2 | 13.3 ± 2.2 | 58.0 ± 4.1 | 30.6 ± 4.2 |
| African Americans (n = 28) | 13.5 ± 2.0b | 59.6 ± 4.8b | 28.3 ± 4.8 | 12.8 ± 2.0b | 59.0 ± 4.5b | 30.1 ± 4.8 |
| Whites (n = 16) | 17.2 ± 2.2 | 55.3 ± 6.1 | 29.9 ± 5.9 | 14.3 ± 2.3 | 56.1 ± 3.9 | 31.3 ± 4.3 |
All values are means ± SD percentages. Each macronutrient is presented as calories derived from protein, carbohydrates, or fat divided by daily caloric intake. There were no significant sex × sleep condition interactions or main effects of sex for the percentage of calories derived from each macronutrient (Ps > 0.63). There was a significant race × sleep condition interaction for the percentage of calories derived from protein (P = 0.023) but not for the percentage of calories derived from carbohydrates or fat (Ps > 0.41). aSignificantly different from baseline, Ps < 0.05 (repeated-measures ANOVA); bsignificant main effects of race for the percentage of calories derived from protein (P < 0.001) and carbohydrates (P = 0.018) (mixed-model ANOVA).
To determine the source of observed macronutrient intake changes, we assessed calories consumed from the following food and drink categories during baseline and SR: 1) meat, eggs, and fish; 2) fruit, vegetables, and salad; 3) bread, cereal, plain rice, and pasta; 4) condiments (ketchup, mustard, mayonnaise, peanut butter, syrup, and jelly); 5) desserts; 6) salty snacks (chips, pretzels, crackers, and popcorn); 7) caffeine-free soda and juice; and 8) milk (36). During SR, subjects consumed more calories from bread, cereal, plain rice ,and pasta (F[1,43] = 5.21, P = 0.028), condiments (F[1,43] = 7.41, P = 0.009), desserts (F[1,43] = 13.36, P = 0.001), salty snacks (F[1,43] = 8.29, P = 0.006), and caffeine-free soda and juice (F[1,43] = 14.93, P < 0.001) than during baseline (all comparisons remained significant after the false discovery rate correction). Calories consumed from meat, eggs, and fish (P = 0.086), fruit, vegetables, and salad (P = 0.66), and milk (P = 0.061) did not differ between baseline and SR (Table 3).
TABLE 3.
Caloric intake by food and drink category in all subjects (n = 44)1
| Food and drink category | Baseline days 1–2 | Sleep-restriction days 1–3 |
| kcal | kcal | |
| Meat, eggs, and fish | 381.55 ± 128.92 | 419.77 ± 149.36 |
| Fruit, vegetables, and salad | 177.65 ± 149.61 | 170.66 ± 138.02 |
| Bread, cereal, plain rice, and pasta | 572.27 ± 192.71 | 659.51 ± 259.88a |
| Condiments | 231.69 ± 139.55 | 276.44 ± 181.81a |
| Desserts | 343.38 ± 286.83 | 544.34 ± 284.23a |
| Chips, pretzels, crackers, and popcorn | 78.88 ± 123.72 | 150.91 ± 202.57a |
| Caffeine-free soda and juice | 272.64 ± 160.91 | 346.18 ± 219.36a |
| Milk | 84.16 ± 98.45 | 119.78 ± 152.89 |
All values are means ± SDs. aSignificantly higher than baseline, P < 0.05 (repeated-measures ANOVA).
Consistent with the null findings related to sex differences in macronutrient intake, sex × sleep condition interactions were not significant for each food and drink category (Ps > 0.05); however, there was a main effect of sex for the bread, cereal, plain rice, and pasta category whereby men consumed more calories from foods in this category than did women (F[1,39] = 12.27, P < 0.05). There was a significant race × sleep condition interaction for fruit, vegetables, and salad (F[1,39] = 9.48, P = 0.004) and a trend for meats, eggs, and fish (F[1,39] = 4.38, P = 0.043; not significant after the false discovery rate correction). African Americans consumed fewer calories from fruit, vegetables, and salad during baseline (F[1,39] = 11.08, P = 0.002, d = 0.88) but did not differ from whites during SR (P = 0.29). There was also a main effect of race for calories consumed from caffeine-free soda and juice, whereby African Americans consumed more calories from caffeine-free soda and juice than whites did (F[1,39] = 14.02, P < 0.01, d = 1.25).
Meal timing
Daily caloric intake was calculated for the following 3 time intervals: 0800–1459, 1500–2159, and 2200–0359; the first 2 time intervals were created by dividing common waking hours from baseline and SR protocol days into 2 equal 7-h intervals, and the third time interval equaled the 6 h of wakefulness that occurred only during SR (9). Caloric intake during each time interval was also calculated as a percentage of daily caloric intake.
During SR, subjects consumed fewer calories (F[1,43] = 7.72, P = 0.008) and a lower percentage of daily caloric intake (F[1,43] = 84.64, P < 0.001) from 0800 to 1459 than during baseline. Also during SR, subjects consumed more calories from 1500 to 2159 (F[1,43] = 12.12, P = 0.001) than during baseline; however, the percentage of daily caloric intake consumed during this time period did not differ between conditions (P = 0.32). During late-night hours of additional wakefulness during SR (2200–0359), subjects consumed 532.6 ± 295.6 calories, which accounted for 16.37 ± 6.60% of daily caloric intake.
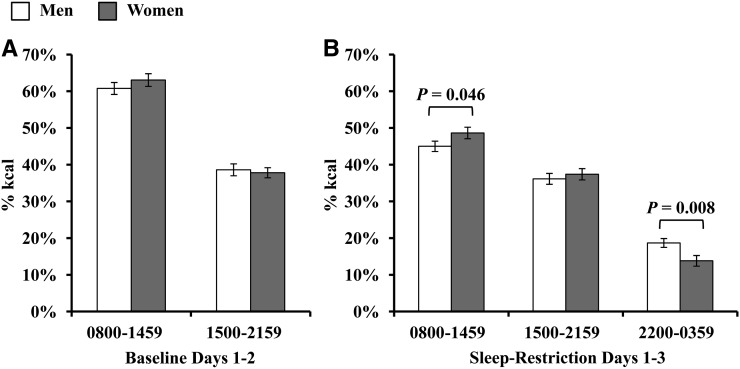
There were no significant sex × sleep condition interactions for calories consumed between 0800 and 1459 or 1500 and 2159 (Ps > 0.29). There was a main effect of sex for calories consumed between 1500 and 2159 with men having consumed more calories than women did (F[1,39] = 8.40, P = 0.006); a similar pattern was observed for calories consumed between 0800 and 1459, although this main effect was not significant (P = 0.078). Men consumed significantly more calories between 2200 and 0359 than women did (men: 674.11 ± 298.06 kcal; women: 377.71 ± 204.82 kcal; F[1,39] = 13.64, P = 0.001, d = 1.16). When sex differences in daily caloric intake were controlled for, men did not differ from women in the percentage of daily caloric intake consumed between 0800 and 1459 or between 1500 and 2159 during baseline (Ps > 0.21; Figure 2A). However, during SR, men consumed a lower percentage of daily calories between 0800 and 1459h (F[1,39] = 4.26, P = 0.046, d = 0.52), a similar percentage of daily calories between 1500 and 2159 (P = 0.68), and a higher percentage of daily calories between 2200 and 0359 (F[1,39\ = 7.89, P = 0.008, d = 0.78; Figure 2B) than did women.
FIGURE 2.
Sex differences in meal timing during baseline and sleep restriction. A: Men did not differ from women in the mean (±SEM) percentage of daily caloric intake consumed between 0800 and 1459 or between 1500 and 2159 during baseline (Ps > 0.21). B: During sleep restriction, men consumed a lower percentage of daily calories between 0800 and 1459 (P = 0.046, d = 0.52), a similar percentage of daily calories between 1500 and 2159 (P = 0.68), and a higher percentage of daily calories from 2200 to 0359 (P = 0.008, d = 0.78) than did women.
There were no significant race × sleep condition interactions or main effects of race for calories consumed between 0800 and 1459 and 1500 and 2159 (Ps > 0.23). In addition, African Americans did not differ from whites in the amount of calories consumed from 2200 to 0359 (P = 0.40).
DISCUSSION
In the first study to systematically examine sex and race differences in caloric intake during experimental sleep loss, we showed that men exhibited a greater increase in daily caloric intake during SR as a result of consuming more calories during late-night hours than women did. African Americans and whites exhibited similar increases in caloric intake and late-night eating during SR. There were significant differences between African Americans and whites in macronutrient intake; African Americans consumed more calories from carbohydrates (including more calories from caffeine-free soda and juice) and fewer calories from protein than did whites. These findings promote public health awareness by highlighting nutritional risk factors and modifiable behaviors for weight gain in men and African Americans related to sleep-wake timing.
Our data showing large sex differences in caloric intake are consistent with population evidence suggesting that men are more susceptible to weight gain resulting from sleep loss (19–21) and with our experimental findings that men gained more weight than women did during an SR protocol (9). As a result of their larger mass, men eat more than women do (37); indeed, we showed that men consumed more calories during baseline and SR than did women. Although caloric intake increased during SR in both sexes, men exhibited a larger increase in caloric intake even after baseline intake differences were controlled for.
Sex hormones may underlie sex differences in eating behaviors. In women, food-intake changes across the menstrual cycle, in part because of changes in estrogen concentrations (37). The effect of sleep loss on the menstrual cycle and estrogen concentrations in humans is unknown; however, in rats, paradoxical (ie, rapid eye movement) sleep deprivation led to a disruption of the estrous cycle and lower estrogen concentrations (38). We did not systematically assess the menstrual cycle phase during the protocol, and women were allowed to use oral contraceptives. Given the sex differences we observed, and that estradiol administration has an anorexigenic effect in rodents (37), future studies should assess how SR affects the menstrual cycle and estrogen concentrations and examine if this relates to changes in food intake. Sleep also influences testosterone concentrations; plasma concentrations peak during sleep (a sleep-dependent effect), and experimental sleep deprivation decreases testosterone concentrations in men (39–41). Decreased testosterone concentrations have been associated with increased adiposity, but to our knowledge, no studies have examined the effect of androgens on food intake in humans (37).
The effect of SR on peripheral controls of eating may also underlie the observed sex differences. There are sex and menstrual cycle differences in gastric emptying rates and in the effect of gastric mechanoreception on food intake (37). Although there has been evidence that gastric emptying is delayed during sleep (42), the effect of sleep loss on gastric emptying is unknown. Insulin, ghrelin, and leptin signaling may also play a role. There is mounting evidence that sleep loss is associated with decreased insulin sensitivity (43, 44), and intranasal insulin administration decreases food intake in men but not women (45). SR increases the orexigenic hormone ghrelin in men but not women (46). In rats, ghrelin administration increased food intake to a greater degree in males than in females (37). Finally, SR may increase the anorexigenic hormone leptin to a greater degree in women than men (47). In rats, females are more sensitive to the satiating effects of leptin administration than are males (48). Future studies with large sample sizes are needed to confirm these sex differences in peripheral controls of eating and assess how sleep loss affects these signals differently in men and women.
Sex differences in eating behaviors and attitudes about food are also important to consider. Women scored higher in dietary restraint and in concern over weight control than men did (49), and in one study that induced overfeeding, women reduced subsequent caloric intake to a greater extent than men did, suggesting that women are more sensitive to signals of a positive energy balance (50). Chaput et al (51) posited that the association between short sleep duration and weight gain depends on the degree of disinhibited eating in adults. Future research is needed to further elucidate the effect of various eating behaviors (eg, restraint and disinhibition) on increased caloric intake and weight gain because of SR and to assess whether sex differences in eating attitudes and behaviors relate to these differences.
We showed previously that sleep-restricted African Americans gained more weight than sleep-restricted whites gained; however, in the current study, we did not find differences between African Americans and whites in terms of increased caloric intake or late-night eating during SR. Energy expenditure was not assessed in this study and may explain weight gain differences in the absence of caloric intake differences between African Americans and whites. Although activity levels were relatively low in the current study, African Americans may have been less active than were whites. Physical activity is decreased during the day after SR, and studies have shown that African American adults are less active than white adults are under non–sleep-deprived and nonlaboratory conditions (52–54). Future studies should examine if there are race differences in the effect of sleep loss on physical activity levels. Sleep loss can also lead to changes in resting metabolic rate. The extended wakefulness associated with sleep loss leads to an increase in energy expenditure because resting metabolic rate is higher than sleeping metabolic rate (8, 55, 56), and some studies have shown that resting metabolic rate is lower during the day after sleep loss (57, 58). Compared with whites, African Americans may exhibit a smaller increase in energy expenditure during extended wakefulness and/or a greater reduction in resting metabolic rate after sleep loss. Because African Americans have a lower sleeping metabolic rate (59) and resting metabolic rate (60) than whites do under non–sleep-deprived conditions, future studies should examine if there are race differences in the effect of sleep loss on metabolic rate. Finally, African Americans may exhibit a higher respiratory quotient (lower fat oxidation rate) than whites do during SR. African Americans have a higher respiratory quotient than whites do during non–sleep-deprived conditions (59), and a higher respiratory quotient is associated with greater weight gain (61).
Notably, our subjects consumed a higher percentage of calories from fat and a lower percentage of calories from protein during SR than baseline in concordance with studies that showed an association between sleep loss and increased fat intake (12, 62) and with data from our previous study showing that subjects consumed a greater percentage of calories from fat during late-night caloric intake (9) (although in that study, we showed that macronutrient intake did not vary across protocol days). In the current study, subjects consumed more calories from specific food categories including bread, cereal, plain rice, and pasta; condiments; desserts; and salty snacks (chips, pretzels, and popcorn) during SR. This finding is consistent with a study reporting that adolescents consumed more foods with a high glycemic index, particularly desserts and sweets, when sleep restricted (36) and a study that reported an increased craving for salty and sweet foods in sleep-restricted men (63).
We also observed significant race differences in macronutrient intake with African Americans having consumed a lower percentage of calories from protein and a higher percentage of calories from carbohydrates than did whites. Additional examination revealed that African Americans consumed more calories from caffeine-free soda and juice than did whites. This finding is consistent with epidemiologic studies that showed greater intake of sugar-sweetened beverages in African American children and adults (64, 65). Although it is unlikely that these differences were exclusively related to SR, increased intake of carbohydrates, specifically caffeine-free soda and juice, may have contributed to the greater weight gain observed in African Americans in our previous study (9), as calories from sugar-sweetened beverages contribute to weight gain (66).
In conclusion, epidemiologic and laboratory studies have indicated that men and African Americans may be more vulnerable to the weight-gain effect of sleep loss than women and whites, respectively. To our knowledge, this is the first study to find that men exhibited a greater increase in caloric intake and consumed more calories during late-night hours than did women during SR. Although there were no race differences in daily caloric intake, African Americans consumed more calories from caffeine-free soda and juice than did whites, which may relate to weight gain. More research is needed, particularly focused on components of energy expenditure, to understand other sources of susceptibility to weight gain during sleep loss in African Americans. The current findings extend previous research by identifying increased caloric intake and late-night eating as behavioral mechanisms underlying sex differences in weight gain because of sleep loss. Moreover, our results promote public health awareness in men and African Americans by underscoring key risk factors and modifiable behaviors for weight gain related to sleep loss.
Acknowledgments
We thank the subjects who participated in the experiments and faculty and staff of the Division of Sleep and Chronobiology who helped acquire data.
The authors’ responsibilities were as follows—AMS: conducted the research, analyzed data, and had primary responsibility for the final content of the manuscript; and all authors: designed the research, wrote the manuscript, and read and approved the final manuscript. DFD is compensated for serving on a scientific advisory council for Mars Inc. AMS and NG had no conflicts of interest.
REFERENCES
- 1.Ford ES, Li C, Wheaton AG, Chapman DP, Perry GS, Croft JB. Sleep duration and body mass index and waist circumference among US adults. Obesity (Silver Spring) 2014;22:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moraes W, Poyares D, Zalcman I, de Mello MT, Bittencourt LR, Santos-Silva R, Tufik S. Association between body mass index and sleep duration assessed by objective methods in a representative sample of the adult population. Sleep Med 2013;14:312–8. [DOI] [PubMed] [Google Scholar]
- 3.Singh M, Drake CL, Roehrs T, Hudgel DW, Roth T. The association between obesity and short sleep duration: a population-based study. J Clin Sleep Med 2005;1:357–63. [PubMed] [Google Scholar]
- 4.Di Milia L, Vandelanotte C, Duncan MJ. The association between short sleep and obesity after controlling for demographic, lifestyle, work and health related factors. Sleep Med 2013;14:319–23. [DOI] [PubMed] [Google Scholar]
- 5.Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, Rössler W, Angst J. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep 2004;27:661–6. [DOI] [PubMed] [Google Scholar]
- 6.Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep 2008;31:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi D, Takahashi O, Deshpande GA, Shimbo T, Fukui T. Association between weight gain, obesity, and sleep duration: a large-scale 3-year cohort study. Sleep Breath 2012;16:829–33. [DOI] [PubMed] [Google Scholar]
- 8.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP., Jr Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci USA 2013;110:5695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep 2013;36:981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, Hitze B, Later W, Wilms B, Settler U, Peters A, Kiosz D, Muller MJ. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts 2008;1:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr 2010;91:1550–9. [DOI] [PubMed] [Google Scholar]
- 12.St-Onge MP, Roberts AL, Chen J, Kelleman M, O'Keeffe M, RoyChoudhury A, Jones PJ. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr 2011;94:410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calvin AD, Carter RE, Adachi T, Macedo PG, Albuquerque FN, van der Walt C, Bukartyk J, Davison DE, Levine JA, Somers VK. Effects of experimental sleep restriction on caloric intake and activity energy expenditure. Chest 2013;144:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 2009;89:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011;19:1374–81. [DOI] [PubMed] [Google Scholar]
- 16.Garaulet M, Gomez-Abellan P, Alburquerque-Bejar JJ, Lee YC, Ordovas JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 2013;37:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allison KC, Goel N, Ahima RS. Delayed timing of eating: impact on weight and metabolism. Curr Obes Rep 2014;3:91–100. [DOI] [PubMed] [Google Scholar]
- 18.Baron KG, Reid KJ, Horn LV, Zee PC. Contribution of evening macronutrient intake to total caloric intake and body mass index. Appetite 2013;60:246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko GT, Chan JC, Chan AW, Wong PT, Hui SS, Tong SD, Ng SM, Chow F, Chan CL. Association between sleeping hours, working hours and obesity in Hong Kong Chinese: the 'better health for better Hong Kong’ health promotion campaign. Int J Obes (Lond) 2007;31:254–60. [DOI] [PubMed] [Google Scholar]
- 20.Meyer KA, Wall MM, Larson NI, Laska MN, Neumark-Sztainer D. Sleep duration and BMI in a sample of young adults. Obesity (Silver Spring) 2012;20:1279–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang TC, Matthews SA, Chen VY. Stochastic variability in stress, sleep duration, and sleep quality across the distribution of body mass index: insights from quantile regression. Int J Behav Med 2014;21:282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knutson KL. Sex differences in the association between sleep and body mass index in adolescents. J Pediatr 2005;147:830–4. [DOI] [PubMed] [Google Scholar]
- 23.Araújo J, Severo M, Ramos E. Sleep duration and adiposity during adolescence. Pediatrics 2012;130:e1146–54. [DOI] [PubMed] [Google Scholar]
- 24.Shi Z, Taylor AW, Gill TK, Tuckerman J, Adams R, Martin J. Short sleep duration and obesity among Australian children. BMC Public Health 2010;10:609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tatone-Tokuda F, Dubois L, Ramsay T, Girard M, Touchette E, Petit D, Montplaisir JY. Sex differences in the association between sleep duration, diet and body mass index: a birth cohort study. J Sleep Res 2012;21:448–60. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep 2010;33:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hale L, Do DP. Racial differences in self-reports of sleep duration in a population-based study. Sleep 2007;30:1096–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donat M, Brown C, Williams N, Pandey A, Racine C, McFarlane SI, Jean-Louis G. Linking sleep duration and obesity among black and white US adults. Clin Prac (Lond) 2013;10:661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grandner MA, Chakravorty S, Perlis ML, Oliver L, Gurubhagavatula I. Habitual sleep duration associated with self-reported and objectively determined cardiometabolic risk factors. Sleep Med 2014;15:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol 1989;74:728–38. [DOI] [PubMed] [Google Scholar]
- 31.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003;26:117–26. [DOI] [PubMed] [Google Scholar]
- 32.Luckhaupt SE, Tak S, Calvert GM. The prevalence of short sleep duration by industry and occupation in the National Health Interview Survey. Sleep 2010;33:149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hise ME, Sullivan DK, Jacobsen DJ, Johnson SL, Donnelly JE. Validation of energy intake measurements determined from observer-recorded food records and recall methods compared with the doubly labeled water method in overweight and obese individuals. Am J Clin Nutr 2002;75:263–7. [DOI] [PubMed] [Google Scholar]
- 34.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates, 1988. [Google Scholar]
- 35.Storey JD. A direct approach to false discovery rates. J R Stat Soc B 2002;64:479–98. [Google Scholar]
- 36.Beebe DW, Simon S, Summer S, Hemmer S, Strotman D, Dolan LM. Dietary intake following experimentally restricted sleep in adolescents. Sleep 2013;36:827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol 2013;305:R1215–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antunes IB, Andersen ML, Baracat EC, Tufik S. The effects of paradoxical sleep deprivation on estrous cycles of the female rats. Horm Behav 2006;49:433–40. [DOI] [PubMed] [Google Scholar]
- 39.Cote KA, McCormick CM, Geniole SN, Renn RP, MacAulay SD. Sleep deprivation lowers reactive aggression and testosterone in men. Biol Psychol 2013;92:249–56. [DOI] [PubMed] [Google Scholar]
- 40.Carter JR, Durocher JJ, Larson RA, DellaValla JP, Yang H. Sympathetic neural responses to 24-hour sleep deprivation in humans: sex differences. Am J Physiol Heart Circ Physiol 2012;302:H1991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leproult R, Van Cauter E. Effect of 1 week of sleep restriction on testosterone levels in young healthy men. JAMA 2011;305:2173–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pasricha PJ. Effect of sleep on gastroesophageal physiology and airway protective mechanisms. Am J Med 2003;115(Suppl 3A):114S–8S. [DOI] [PubMed] [Google Scholar]
- 43.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 2010;59:2126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen KW, Corssmit EP, Romijn JA. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab 2010;95:2963–8. [DOI] [PubMed] [Google Scholar]
- 45.Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab 2008;93:1339–44. [DOI] [PubMed] [Google Scholar]
- 46.St-Onge MP, O'Keeffe M, Roberts AL, Roychoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep 2012;35:1503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simpson NS, Banks S, Dinges DF. Sleep restriction is associated with increased morning plasma leptin concentrations, especially in women. Biol Res Nurs 2010;12:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol 2010;122:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rolls BJ, Fedoroff IC, Guthrie JF. Sex differences in eating behavior and body weight regulation. Health Psychol 1991;10:133–42. [DOI] [PubMed] [Google Scholar]
- 50.Cornier MA, Grunwald GK, Johnson SL, Bessesen DH. Effects of short-term overfeeding on hunger, satiety, and energy intake in thin and reduced-obese individuals. Appetite 2004;43:253–9. [DOI] [PubMed] [Google Scholar]
- 51.Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between short sleep duration and weight gain is dependent on disinhibited eating behavior in adults. Sleep 2011;34:1291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, Born J, Schultes B. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr 2009;90:1476–82. [DOI] [PubMed] [Google Scholar]
- 53.Bromley LE, Booth JN, 3rd, Kilkus JM, Imperial JG, Penev PD. Sleep restriction decreases the physical activity of adults at risk for type 2 diabetes. Sleep 2012;35:977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vásquez E, Shaw BA, Gensburg L, Okorodudu D, Corsino L. Racial and ethnic differences in physical activity and bone density: National Health and Nutrition Examination Survey, 2007-2008. Prev Chronic Dis 2013;10:E216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jung CM, Melanson EL, Frydendall EJ, Perreault L, Eckel RH, Wright KP. Energy expenditure during sleep, sleep deprivation and sleep following sleep deprivation in adult humans. J Physiol 2011;589:235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shechter A, Rising R, Albu JB, St-Onge MP. Experimental sleep curtailment causes wake-dependent increases in 24-h energy expenditure as measured by whole-room indirect calorimetry. Am J Clin Nutr 2013;98:1433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Benedict C, Hallschmid M, Lassen A, Mahnke C, Schultes B, Schiöth HB, Born J, Lange T. Acute sleep deprivation reduces energy expenditure in healthy men. Am J Clin Nutr 2011;93:1229–36. [DOI] [PubMed] [Google Scholar]
- 58.Buxton OM, Cain SW, O'Connor SP, Porter JH, Duffy JF, Wang W, Czeisler CA, Shea SA. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med 2012;4:129ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weyer C, Snitker S, Bogardus C, Ravussin E. Energy metabolism in African Americans: potential risk factors for obesity. Am J Clin Nutr 1999;70:13–20. [DOI] [PubMed] [Google Scholar]
- 60.Gannon B, DiPietro L, Poehlman ET. Do African Americans have lower energy expenditure than Caucasians? Int J Obes Relat Metab Disord 2000;24:4–13. [DOI] [PubMed] [Google Scholar]
- 61.Zurlo F, Lillioja S, Esposito-Del Puente A, Nyomba BL, Raz I, Saad MF, Swinburn BA, Knowler WC, Bogardus C, Ravussin E. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol 1990;259:E650–7. [DOI] [PubMed] [Google Scholar]
- 62.Weiss A, Xu F, Storfer-Isser A, Thomas A, Ievers-Landis CE, Redline S. The association of sleep duration with adolescents’ fat and carbohydrate consumption. Sleep 2010;33:1201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Ann Intern Med 2004;141:846–50. [DOI] [PubMed] [Google Scholar]
- 64.Bleich SN, Wang YC, Wang Y, Gortmaker SL. Increasing consumption of sugar-sweetened beverages among US adults: 1988-1994 to 1999-2004. Am J Clin Nutr 2009;89:372–81. [DOI] [PubMed] [Google Scholar]
- 65.Taveras EM, Gillman MW, Kleinman K, Rich-Edwards JW, Rifas-Shiman SL. Racial/ethnic differences in early-life risk factors for childhood obesity. Pediatrics 2010;125:686–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caprio S. Calories from soft drinks–do they matter? N Engl J Med 2012;367:1462–3. [DOI] [PubMed] [Google Scholar]