Summary
Production of bile by the liver is crucial for the absorption of lipophilic nutrients. Dysregulation of bile acid homeostasis can lead to cholestatic liver disease and ER stress. We show using global location analysis (“ChIP-on-Chip”) and cell-type specific gene ablation that the winged helix transcription factor Foxa2 is required for normal bile acid homeostasis. As suggested by the location analysis, deletion of Foxa2 in hepatocytes in Foxa2loxP/loxPAlfp.Cre mice leads to decreased transcription of genes encoding bile acid transporters on both the basolateral and canalicular membranes, resulting in intrahepatic cholestasis. Foxa2-deficient mice are strikingly sensitive to a diet containing cholic acid, which results in toxic accumulation of hepatic bile salts, ER stress, and liver injury. In addition, we demonstrate that expression of FOXA2 is dramatically decreased in liver samples from patients with different cholestatic syndromes, suggesting that reduced FOXA2 levels could exacerbate the injury.
Introduction
The liver plays a major role in the detoxification of xenobiotics, metabolism of nutrients, and glucose homeostasis. Hepatic gene expression is regulated largely at the transcriptional level. Liver-enriched transcription factors bind cis-regulatory DNA elements to activate their target genes. The forkhead box proteins, Foxa1, Foxa2, and Foxa3, contain a highly conserved winged helix DNA binding domain1, and were initially discovered by their ability to regulate the promoters of the α1-antitrypsin and transthyretin genes2. The Foxa proteins, formerly known as HNF-3α, β, and γ, are expressed in embryonic foregut endoderm, from which the liver forms at midgestation3,4. Foxa1 and Foxa2 were shown by genetic means to be indispensable for the initiation of liver development5.
Tissue-specific transcriptional regulation is often combinatorial in nature, as promoters of target genes contain regulatory modules with multiple transcription factor binding sites5. Binding of Foxa factors to their targets is essential for several nuclear receptors to access their cis-regulatory elements. The presence of Foxa2 binding sequences near glucocorticoid (GR) response elements in the liver is necessary for maximal induction of genes activated during the response to fasting6. Foxa binding sites are also found adjacent to those for the androgen receptor (AR) in the regulatory regions of genes expressed in the epididymis and prostate7, and a nearby Foxa1 site is necessary for estrogen receptor (ER) mediated gene activation in breast cancer cells8,9. Thus, Foxa transcription factors are integral components of transcriptional regulatory networks in multiple organ systems.
Previous studies of Foxa2 overexpression in the liver have suggested that this transcription factor is important in lipid metabolism10. Because overexpression of DNA binding proteins can lead to activation or repression of non-physiological targets, we used a loss-of-function mouse model to study the role of Foxa2 in the adult liver. Using cell-type specific gene ablation, we found that Foxa2 regulates multiple genes involved in hepatic bile acid homeostasis. We demonstrate further that Foxa2 is required to prevent hepatic cholestasis, liver injury, and ER stress, when mice are fed a diet containing cholic acid. Thus, we have identified a novel role for Foxa2 in hepatic bile acid homeostasis and in the prevention of cholestatic liver injury.
Results
Global analysis of Foxa2 occupancy suggests a role for Foxa2 in bile acid metabolism
Previous loss-of-function analysis has demonstrated an essential role for Foxa2 in the integration of gluconeogenic gene expression in response to fasting6. In addition, expression profiling of multiple paradigms of Foxa2 deficiency or Foxa2 mis-expression has resulted in the identification of hundreds of genes whose mRNA levels can be influenced by changes in Foxa2 status10–12. However, expression profiling captures not only those genes that are directly dependent on Foxa2 binding to their cis-regulatory elements, but also those controlled by intermediate regulators, and even compensatory changes in response to an altered physiological state. In order to identify the subset of genes directly regulated by Foxa2 in the liver, we performed genome-wide location analysis. Chromatin immunoprecipitation (ChIP) samples from livers of wild type and Foxa2 liver-conditional null mice (Foxa2loxP/loxPAlfp.Cre)6 were hybridized to a mouse enhancer/promoter microarray with more than 36,000 elements (BCBCPromChip 5). We identified 574 enhancer and promoter regions, corresponding to 484 unique genes, as occupied by Foxa2 (Supplementary Table 1). We evaluated whether any functional categories were over-represented in the Foxa2 bound targets compared to all genes represented on the array (Supplementary Table 2). Strikingly, a cluster of categories with genes involved in lipid and steroid metabolism was identified as bound by Foxa2 in vivo.
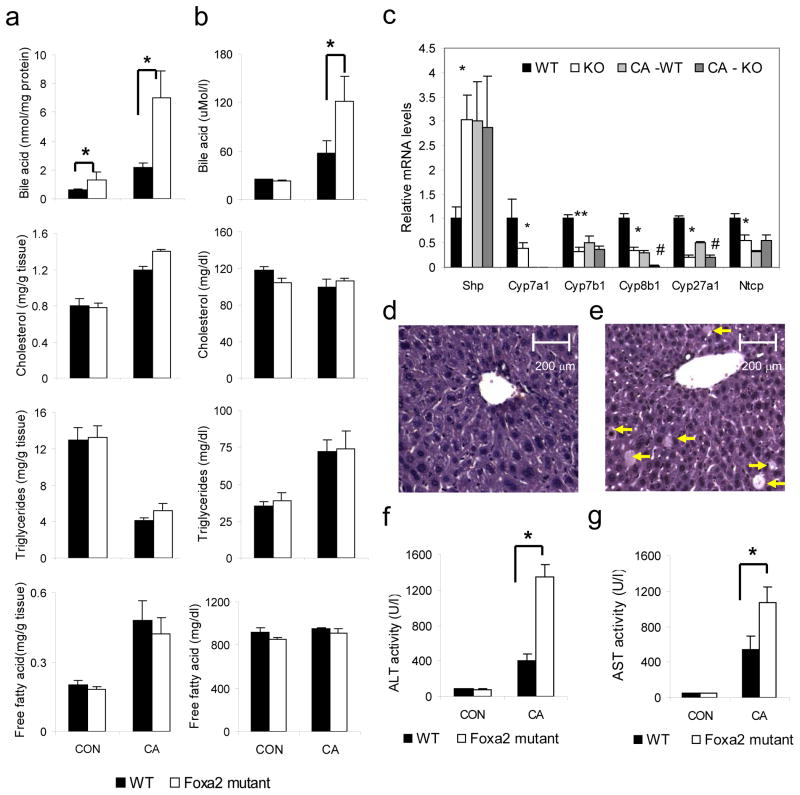
Foxa2-deficient livers accumulate bile acids
Given our findings from location analysis, we next considered whether cholesterol or bile acid homeostasis was disturbed in Foxa2 mutant mice. In the liver, a large proportion of cholesterol is eliminated by its conversion into bile acids and excretion into bile. Elevation of bile salts within hepatocytes leads to cholestatic liver disease. While hepatic bile acid levels were elevated two fold (p-value < 0.05) in Foxa2 mutants (Fig. 1a), serum bile acid concentrations were similar in Foxa2loxP/loxPAlfp.Cre mice as compared to their wild-type littermates (Fig. 1b). Since bile acid homeostasis was perturbed in Foxa2 mutants, we placed Foxa2loxP/loxPAlfp.Cre mice and their control littermates on a diet containing cholic acid. Cholic acid (CA) supplementation has been used extensively to elucidate the transcriptional control of cholesterol and bile acid metabolism by members of the nuclear hormone receptor gene family, specifically FXR, PXR and SHP13,14. Foxa2 mutants and their control littermates responded similarly to the cholic acid diet in terms of cholesterol and triglyceride metabolism (Fig. 1). However, Foxa2-deficient mice exhibited dramatically increased serum and hepatic bile acid content on the CA diet. While serum and hepatic bile acid levels increased 3-fold in control mice fed a cholic acid diet, they were elevated an additional 2-fold and 3-fold, respectively, in Foxa2-deficient mice (p-value < 0.02; Fig. 1a,b).
Figure 1.
Foxa2 controls hepatic bile acid homeostasis and protects the liver from bile acid toxicity.
(a) Hepatic bile acid levels are elevated two-fold in Foxa2 mutant mice (n = 6–7 animals per group) on regular chow, and increased an additional three-fold on bile acid diet relative to the wildtype control groups. (b) Serum bile acid concentrations are significantly increased in Foxa2loxP/loxPAlfp.Cre mice fed a bile acid diet compared with littermate controls (n = 4–5 animals per group). (c) Expression of Shp is elevated in Foxa2 mutant mice compared to the control littermates, while mRNA levels of Cyp7a1, Cyp7b1, Cyp8b1, Cyp27a1, and Ntcp are significantly reduced on standard diet (n = 7–8 animals per group). Expression of Shp increases equally for both groups on bile acid diet, while mRNA levels of Cyp7a1, Cyp7b1, Cyp8b1, Cyp27a1, and Ntcp decrease with cholic acid treatment.
Representative liver sections from cholic acid-fed wildtype (d) and Foxa2loxP/loxPAlfp.Cre mice (e) stained with hematoxylin and eosin (H&E). Cholestatic injury is apparent on histological sections as indicated by increased hepatocyte dropout (arrows) in Foxa2-deficient livers.
While serum alanine aminotransferase (ALT) (f) or aspartate aminotransferase (AST) (g) levels are not altered in Foxa2loxP/loxPAlfp.Cre mice on standard chow, the liver enzyme levels are disproportionately increased in the mutants on CA diet, significantly differing from those of their littermate controls.
Values are represented as means plus standard error. P values were determined by Student’s t test. * p-value <0.05, WT vs. KO on standard diet, ** p-value <0.01, WT vs. KO on standard diet, # p-value<0.05 WT vs KO on CA diet.
Next we investigated the consequences of the elevated hepatic bile acid levels in Foxa2 mutant mice on gene expression in the liver. Bile acids serve as ligands for the farnesoid X receptor, Fxr (Nr1h4), and in a negative feedback loop, inhibit their own synthesis. Fxr activates transcription of Shp, which inhibits expression of Cyp7a1, Cyp7b1, Cyp8b1, and Cyp27a1, critical enzymes in bile acid synthesis, and of Ntcp (Slc10a1), a transporter mediating hepatic bile acid uptake from plasma in the last step of the enterohepatic circulation. As reported previously15, Shp mRNA levels were increased approximately three-fold in livers of mice fed a cholic acid diet (Fig. 1c), an induction that is not dependent on the presence of Foxa2. Expression of Cyp7a1, the rate-limiting enzyme in bile acid synthesis, is no longer detectable after cholic acid treatment, and repression of other synthesis enzymes, such as Cyp7b1, Cyp8b1, and Cyp27a1, in livers of Foxa2-deficient mice is equivalent or more pronounced than in wild type controls on cholic acid diet. Thus, inappropriate expression of the enzymes responsible for hepatic bile acid synthesis is not the cause for cholestasis in Foxa2 mutant mice.
Strikingly, maximal activation of Shp transcription also occurs in Foxa2-deficient mice on standard chow (Fig. 1c). Consequently, expression of the bile acid synthesis enzymes and Ntcp is also reduced in Foxa2 mutants fed standard diet (Fig. 1c). The induction of the Fxr target Shp is a likely secondary consequence of the elevated levels of bile acids within the Foxa2-deficient hepatocytes (Fig. 1a). These molecular changes confirm our physiological findings of mild hepatic cholestasis in Foxa2-deficient mice even in the absence of cholic acid feeding.
Increased cholestatic liver injury in Foxa2-deficient mice
Elevated hepatic bile acid levels under cholestatic conditions lead to liver damage16. While histologically no hepatic injury was apparent in Foxa2-deficient mice fed standard chow (data not shown), Foxa2loxP/loxPAlfp.Cre mice displayed increased cholestatic injury compared to their control littermates on cholic acid diet, as evidenced by hepatocyte dropout (Fig. 1d,e). To further ascertain hepatic injury, we determined serum levels of the liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in animals on standard and cholic acid supplemented diet. While ALT or AST activity was not altered in Foxa2loxP/loxPAlfp.Cre mice on standard chow, the liver enzyme levels were disproportionately increased in the mutants on CA diet, significantly differing from those of their littermate controls, 3-fold and 2-fold, respectively (Fig. 1f,g). Together, these findings suggest that Foxa2 plays a role in protecting the liver from bile acid toxicity.
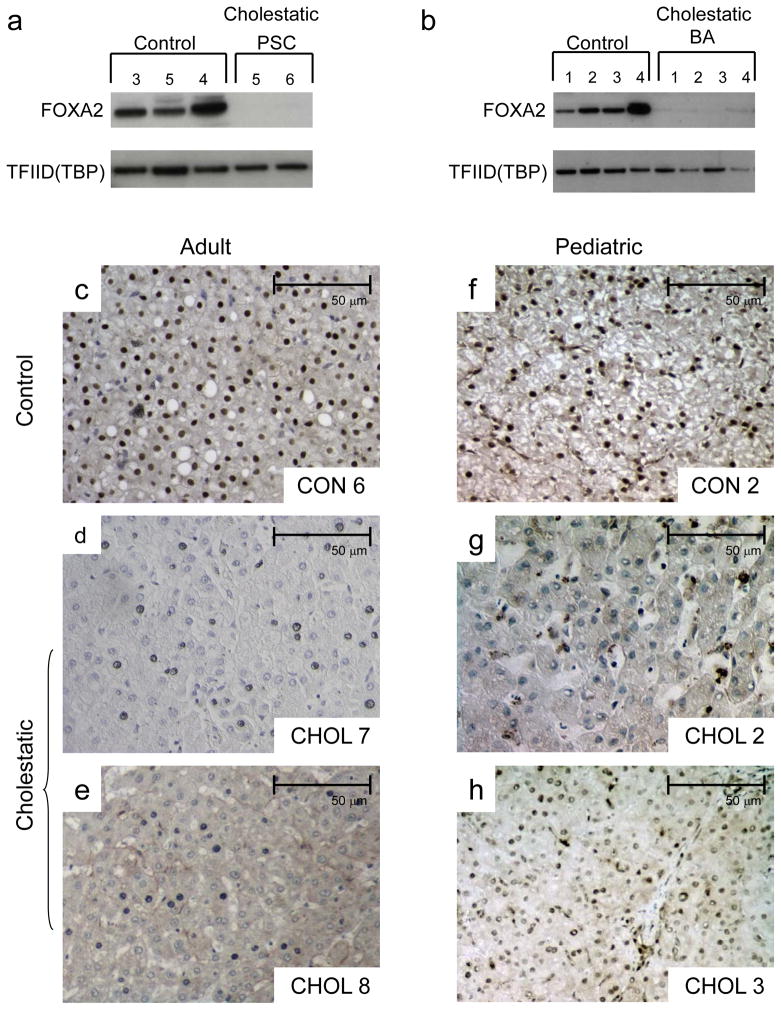
Foxa2 expression is reduced in human cholestatic livers
Since deletion of Foxa2 in hepatocytes leads to accumulation of bile acids in mice, we next considered whether expression of FOXA2 is changed under cholestatic conditions in humans. We examined hepatic FOXA2 protein levels in two sets of patients with cholestasis. FOXA2 protein levels were virtually undetectable in pediatric patients with primary sclerosing cholangitis (PSC; Fig. 2a), as well as in those with biliary atresia (BA; Fig. 2b). We also performed immunohistochemical staining of liver sections of two adult patients with primary sclerosing cholangitis (PSC), two pediatric patients with biliary atresia (BA), and control pediatric and adult patients using antibodies to FOXA2. While FOXA2 staining is clearly present in hepatocyte nuclei of controls (Fig. 2c,f), FOXA2 expression is reduced in hepatocytes of patients with PSC (Fig. 2d,e) and BA (Fig. 2g,h). Our results agree with previous reports showing a significant decrease in Foxa2 expression in rodent models of cholestasis17. Thus, cholestatic injury of differing etiologies cause downregulation of FOXA2 in humans, which together with our findings suggests that low FOXA2 levels exacerbate the injury to the liver.
Figure 2.
FOXA2 expression is reduced in human cholestatic livers
(a) Western blot analysis of protein nuclear extracts (30 μg) from three pediatric controls (corresponding to Control 3, 5, and 4 in Supplementary Table 3) and two patients with primary sclerosing cholangitis (corresponding to Cholestatic 5 and 6 in Supplementary Table 3) with antibodies against FOXA2 and TFIID (TBP) (loading control). FOXA2 protein levels were virtually undetectable in patients with primary sclerosing cholangitis.
(b) Western blot analysis of protein nuclear extracts (30 μg) from four pediatric controls (corresponding to Control 1 through 4 in Supplementary Table 3) and four patients with biliary atresia (corresponding to Cholestatic 1 through 4) with antibodies against FOXA2 and TFIID (TBP) (loading control). FOXA2 protein levels were virtually undetectable in patients with biliary atresia.
Immunohistochemical detection of FOXA2 protein in liver sections of adult (c) and pediatric (f) control and cholestatic patients (d–e, g–h). Labels in each panel correspond to patient numbers listed in Supplementary Table 3. While FOXA2 staining is clearly present in hepatocyte nuclei of the controls, FOXA2 presence is virtually absent in hepatocytes of the adult patients with primary sclerosing cholangitis (c, d) and pediatric patients with biliary atresia (g, h).
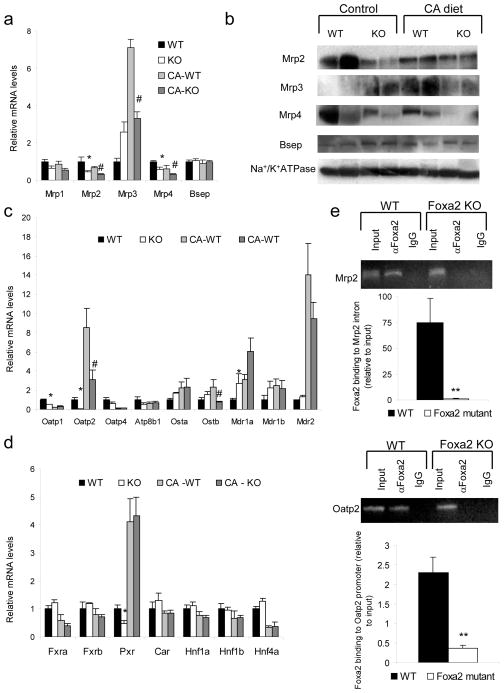
Foxa2 regulates genes involved in bile formation and hepatic bile acid transport
To determine the cause of bile acid accumulation in livers of Foxa2loxP/loxPAlfp.Cre mice, we measured hepatic mRNA levels of bile acid transporters associated with cholestasis. Transporters located at the canalicular membrane include Bsep (Abcb11), a bile salt export pump, Mrp2 (Abcc2), a multispecific organic anion carrier involved in biliary secretion, and Mdr2, essential for secreting phospholipids into bile. On the basolateral membrane Mrp3 (Abcc3), Mrp4 (Abcc4), and Ostα/Ostβ export bile acids and their conjugates from the hepatocyte back into circulation18–20, while Oatp1 (Slco1a1), Oatp2 (Slco1a4) and Oatp4 (Slco1b4) are involved in sodium-independent hepatic uptake of bile salts21. Expression of transporters in the Mrp family was altered in absence of Foxa2. Both mRNA and protein levels of Mrp2 and Mrp4 were decreased in the livers of Foxa2 mutant mice on both standard chow and cholic acid enriched feed (Fig. 3a,b). Furthermore, the induction of Mrp3 normally seen in liver of mice fed a cholic acid supplemented diet is blunted in the absence of Foxa2 (Fig. 3a,b). Expression of Bsep did not change either on mRNA or protein level in Foxa2loxP/loxPAlfp.Cre animals on either diet treatment. Previous reports have also found no change in Bsep expression by cholic acid in rodents15.
Figure 3.
Foxa2 regulates several genes involved in hepatic bile acid transport directly. (a) Quantitative RT- PCR analysis for mRNA and (b) Western blot analysis of protein membrane fractions for expression of hepatic transporters. Both mRNA and protein levels of Mrp2 and Mrp4 are significantly decreased in Foxa2 mutants on both control and CA diet, while expression of Mrp3 is increased on standard chow but reduced on bile acid treatment in livers of Foxa2-deficient animals. (c) Expression of Oatp1 and Oatp2 is significantly reduced in Foxa2 mutants compared to controls on standard diet. mRNA levels of Oatp2 and Ostβ are decreased in livers of Foxa2-deficient mice on a diet supplemented with cholic acid. (d) Expression of numerous hepatic transcription factors involved in bile acid metabolism (n = 4 animals per group). While expression of Pxr is reduced 2-fold in Foxa2-deficient mice on standard diet, transcription of this gene is activated similarly on CA diet. mRNA levels of other DNA binding proteins do not differ between Foxa2 mutants and wildtype controls on either diet treatment.
(e) Binding of Foxa2 to the Mrp2 intron and Oatp2 promoter is enriched relative to input chromatin in wildtype controls (n = 3 for each group). No promoter occupancy is seen in Foxa2-deficient liver, demonstrating the specificity of the assay for Foxa2.
Values are represented as means plus standard error. P values were determined by Student’s t test. * p-value <0.05, **, p-value< 0.01, WT vs. KO on standard diet, # p-value<0.05, ## p-value <0.01, WT vs KO on CA diet
In rodent models of Mrp2 deficiency, failure to export its endogenous substrates, which include biliribin and other organic anions as well as conjugated bile acids, hinders efflux of biliary glutathione (GSH)22–24. GSH is the principal osmotic force driving bile formation25. Thus, reduction in Mrp2 expression results in reduced bile flow23,24, and likely contributes to cholestasis in Foxa2-deficient livers. Administration of spironolactone in a rodent model of cholestasis, where Mrp2 protein levels are reduced but Bsep expression is not changed, leads to upregulation of Mrp2, normalization of bile flow, biliary excretion of glutathione, and reduction of elevated serum bile acids to physiological levels24. In addition to reduced expression of Mrp2, Foxa2loxP/loxPAlfp.Cre animals also exhibit reduced expression of basolateral export proteins, Mrp3 and Mrp4, when fed a cholic acid diet.
Expression of basolateral transporters in Oatp family, involved in sodium-independent uptake of bile salts, is also affected in Foxa2-deficient hepatocytes. mRNA levels of Oatp1 and Oatp2 are reduced 2-fold and 10-fold, respectively, on standard diet, and the induction of Oatp2 normally seen in liver of mice fed a cholic acid supplemented diet is blunted in the absence of Foxa2 (Fig. 3c). Decreased levels of OATP2 are seen in patients with inflammation-induced icteric cholestasis26 and primary sclerosing cholangitis (PSC)27. Hence, reduced expression of Oatp2 likely contributes to elevated bile acids in the serum seen in Foxa2loxP/loxPAlfp.Cre animals.
Mrp2, Mrp3, and Mrp4, are known to be controlled by the pregnane X receptor (Pxr, Nr1i2) and constitutive androstane receptor (Car, Nr1i3). In addition, the nuclear receptors Fxr, Pxr, and Car have been shown to play complementary roles in protecting the liver from bile acid toxicity28 and several other transcription factors (Hnf1α, Hnf1β, and Hnf4α) have been established to play a central role on bile acid metabolism29–31. Downregulation of Mrp2 and Mrp4 in Foxa2loxP/loxPAlfp.Cre mice on standard chow could potentially be explained by a two-fold decrease in Pxr expression (Fig. 3d). However, the effects on transporter gene expression in Foxa2 mutants on the CA diet cannot be attributed to changes to the aforementioned hepatic transcription factors, because none of them are dependent on Foxa2 under these conditions (Fig. 3d).
Next we wanted to ascertain whether the genes encoding bile acid transporters downregulated in Foxa2 mutants were direct targets of Foxa2. We performed chromatin immunoprecipitation (ChIP) with an anti-Foxa2 antibody on livers of wild-type and Foxa2loxP/loxPAlfp.Cre mice. Quantitative PCR carried out on DNA immunoprecipitated from wildtype liver chromatin revealed that Foxa2 occupies binding sites located in the 13th intron of Mrp2 gene and about 2150 bp upstream of the Oatp2 transcriptional start site (Fig. 3e), suggesting that both Mrp2 and Oatp2 are direct targets of Foxa2 in vivo. Enrichment of the intron and promoter amplicons is eliminated in chromatin samples from Foxa2-deficient livers, confirming the specificity of the immunoprecipitation assay (Fig. 3e).
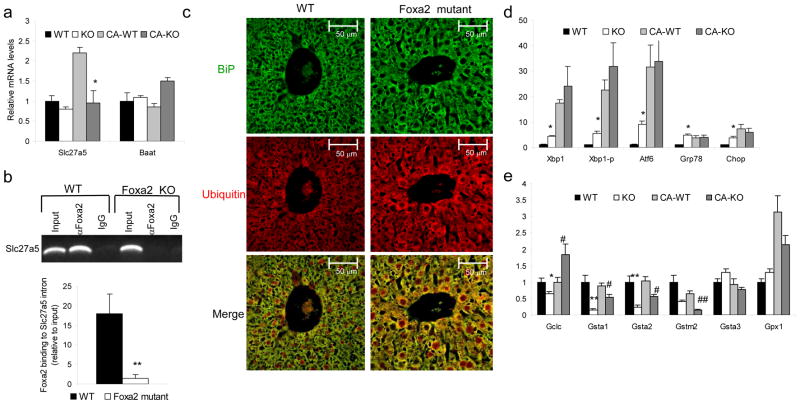
Foxa2 regulates bile acid CoA ligase (Slc27a5)
Conversion of bile acids into their amino acid derivatives, which renders them more soluble, occurs in two steps. First, bile acid CoA ligase (Slc27a5) converts bile acids to their CoA thioesters. Then, Baat catalyzes the intermediates into bile acid CoA esters which are excreted into bile32. Expression of Slc27a5 showed a downward trend on standard chow and was reduced about 2-fold (p-value <0.05) in Foxa2loxP/loxPAlfp.Cre mice on the cholic acid diet (Fig. 4a). A ChIP assay confirmed that Foxa2 occupies a regulatory region in the second exon of Slc27a5 (Fig. 4b), thus identifying bile acid CoA ligase as a direct transcriptional target of Foxa2. Incontrast, mRNA levels of Baat did not change on either diet treatment (Fig. 4a), suggesting that other transcription factors play a dominant role in the regulation of this gene. The cumulative defects in expression of bile acid CoA ligase and the transporters discussed above explain the accumulation of bile acids in livers of Foxa2-deficient mice.
Figure 4.
Foxa2 deficiency in the liver causes ER stress..
(a) Expression of Slc27a5 in liver RNA of wild-type and Foxa2loxP/loxPAlfp.Cre mice shows a 2-fold decrease in Foxa2 mutants as compared to their control littermates on cholic acid diet, while expression of Baat, or bile acid CoA amino acid N-acyltransferase, is not changed (n = 4 animals for each group).
(b) ChIP analysis of promoter occupancy in liver chromatin from wild-type or Foxa2loxP/loxPAlfp.Cre mice (n = 3 for each group). The second exon amplicon of Slc27a5 is enriched 20-fold relative to input DNA in liver chromatin from wildtype mice. This binding is completely lost in the absence of Foxa2.
(c) Immunofluorescence staining of liver sections from control and mutant mice using antibodies against BiP (top panel) and ubiquitin (middle panel). Mutant liver sections exhibit elevated levels of the chaperone protein BiP, as well as elevated cytosolic ubiquitin (middle panel), where aberrant ER proteins are translocated to be degraded by the ubiquitin-proteasome system. The merge of the two confocal images demonstrates increased co-localization of BiP and ubiquitin in livers of Foxa2loxP/loxP;Alfp.Cre mice (bottom panel).
(d) Expression of ER stress markers in liver RNA of wild-type and Foxa2loxP/loxPAlfp.Cre mice (n = 4 animals per group). (e) Q-PCR analysis for messenger RNA of hepatic glutathione enzymes (n = 4 animals per group).
Values are represented as means plus standard error. P values were determined by Student’s t test. * p-value <0.05, **, p-value< 0.01, WT vs. KO on standard diet, # p-value<0.05, ## p-value <0.01, WT vs KO on CA diet
Deletion of Foxa2 in liver leads to endoplasmic reticulum (ER) stress
A rise in bile acids in the liver is toxic and results in endoplasmic reticulum (ER) stress33. As bile acids are already elevated in livers of Foxa2loxP/loxPAlfp.Cre mice on regular diet, we examined whether hepatocytes from Foxa2loxP/loxPAlfp.Cre mice displayed signs of ER stress. We performed immunofluorescence staining of liver sections of Foxa2 deficient mice and their control littermates with antibodies to BiP, an ER chaperone that is upregulated during the unfolded protein response (UPR), and ubiquitin, a major component of the ubiquitin-proteasome system (UPS), which degrades misfolded proteins (Fig. 4c). Mutant livers displayed elevated levels of BiP and a dilated ER, consistent with ER stress34, as well as increased cytoplasmic ubiquitin. Misfolded proteins migrate from the ER to the cytosol, where they are poly-ubiquitinated and degraded in the proteasome35. Co-localization of BiP, an ER chaperone, and ubiquitinated proteins is increased in livers of Foxa2loxP/loxPAlfp.Cre mice, suggesting an accretion of misfolded proteins in the ER caused by delay in their degradation, also a sign of ER stress36. Next, we examined expression of molecular markers of ER stress. These include regulators of the unfolded protein response (UPR), Atf6, Xbp1, Xbp1-p and Chop/Gadd153, a transcription factor controlling ER stress-induced apoptosis; and BiP. Quantitative PCR was performed on RNA isolated from livers of Foxa2loxP/loxPAlfp.Cre mice and their control littermates on regular and cholic acid diet. Hepatic mRNA levels for ER stress transcription factors were elevated 2- to 9-fold, while expression of BiP increased approximately 6-fold (Fig. 4d) in Foxa2 mutants. This indicates that the mild cholestasis due to absence of Foxa2 in the liver in mice fed standard chow is sufficient to induce endoplasmic reticulum stress in the mutant animals.
Foxa2 affects glutathione conjugation and hepatic glutathione metabolism
Exposure of hepatocytes to hydrophobic bile acids during cholestasis has been linked to production of oxidative stress by-products37. The tripeptide glutathione (GSH) is the major antioxidant and redox regulator in vivo. GSH detoxifies many reactive electrophilic molecules in hepatocytes, which, when conjugated with glutathione, can be excreted into bile. The conjugation of these compounds is catalyzed by a family of glutathione S-transferase enzymes (GST), such as Gstm2, that was identified as a Foxa2 target by our location analysis. In addition, several genes classified as oxidoreductase enzymes were bound by Foxa2 in vivo. We measured mRNA levels of Gstm2 and several other glutathione S-transferase enzymes, Gsta1 and Gsta2, in the livers of control and Foxa2-deficient mice and found that these genes were downregulated significantly on both standard chow and cholic acid supplemented diet (Fig. 4e). While expression of Gsta1 and Gsta2 is activated somewhat in Foxa2loxP/loxPAlfp.Cre mice by cholic acid feeding, mRNA levels of these enzymes cannot reach those observed in wildtype animals. We propose that reduction in glutathione S-transferase enzymes in the knockout animals impacts conjugation of cholestatic by-products and their excretion into bile and contributes to the liver injury seen in Foxa2loxP/loxPAlfp.Cremice.
Foxa2 plays a role in bile acid detoxification
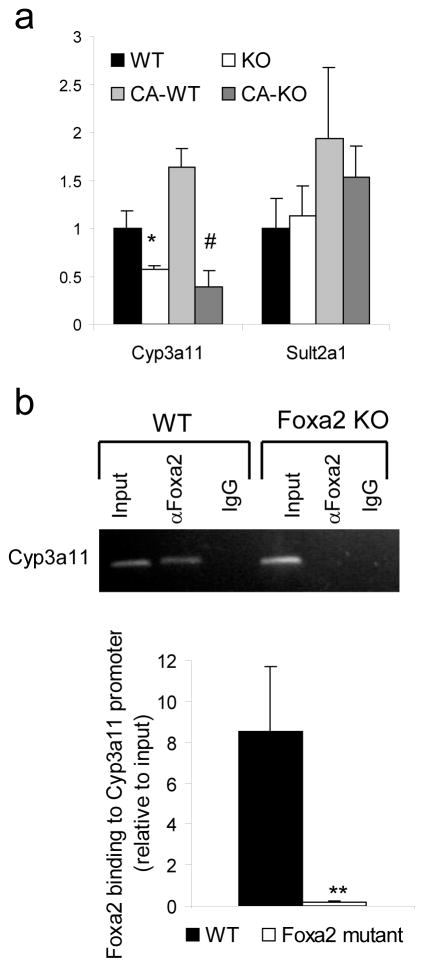
Hepatic injury during cholestasis is ameliorated by rendering bile acids more hydrophilic and less toxic by hydroxylation and conjugation with sulfate38. Initially, Cyp3a11 converts bile acids into more hydrophilic compounds that are subsequently sulfoconjugated by Sult2a1. Transcription of the genes that encode both enzymes is activated in response to cholestasis39. While expression of both Cyp3a11 and Sult2a1 increased with cholic acid treatment of control mice, mRNA levels of Cyp3a11 were significantly downregulated in Foxa2 mutants fed either standard or cholic acid enriched diet (Fig. 5a). The lack of induction of Cyp3a11 during cholestasis39,40 is associated with liver injury. Therefore, reduced expression of this enzyme in Foxa2 mutant mice is likely contributing to the liver toxicity and increased liver damage observed in this model.
Figure 5.
Foxa2 regulates the phase I detoxification enzyme, Cyp3a11.
(a) Quantitative RT-PCR analysis for expression of Cyp3a11 in liver RNA of wild-type and Foxa2loxP/loxPAlfp.Cre mice shows a reduction of 2-fold on standard diet and 5-fold decrease on cholic acid diet in the absence of Foxa2, while expression of Sult2a1, a phase II detoxification enzyme is not changed (n = 4 animals for each group). Values are represented as means plus standard error. P values were determined by Student’s t test. * p-value <0.05, WT vs. KO on standard diet, # p-value<0.05 WT vs. KO on CA diet
(b) ChIP analysis of promoter occupancy evaluated by conventional and quantitative PCR in liver chromatin from wild-type or Foxa2loxP/loxPAlfp.Cre mice (n = 3 for each group). The Cyp3a11 promoter amplicon is enriched 8-fold relative to input DNA in liver chromatin from wildtype mice. This binding is completely lost in the absence of Foxa2. Values are represented as means plus standard error. P values were determined by Student’s t test. **, p -value< 0.01.
Cyp3a11 is a direct target of Foxa2 as demonstrated by ChIP using primers specific for sites positioned about 180 and 80 bp upstream of the Cyp3a11 transcriptional start site (Fig. 5b). This binding is absent in Foxa2 deficient liver, confirming the specificity of the assay. These data extend previous findings that had identified a binding site for FOXA in the human orthologue of Cyp3a11, termed CYP3A4, in vitro41.
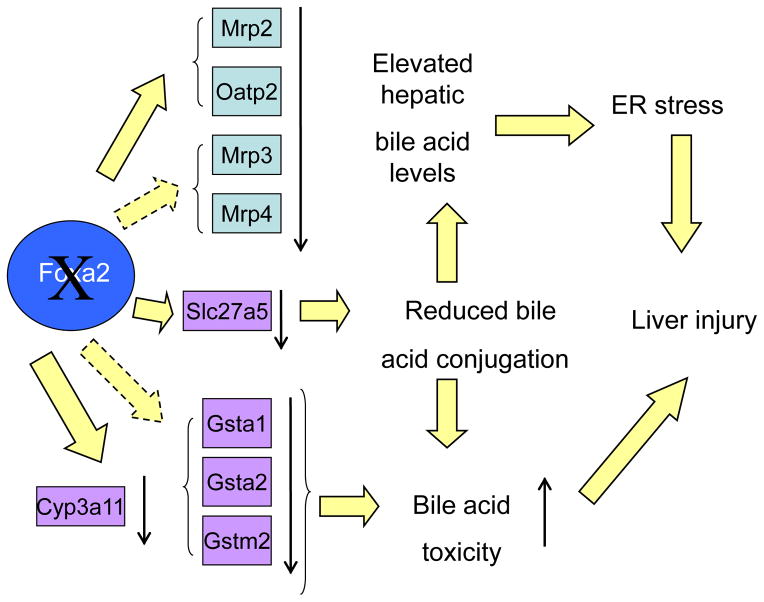
In summary (schematized in Figure 6), deletion of Foxa2 in hepatocytes causes a decrease in expression of bile acid transporters, both on the basolateral and canalicular membranes, resulting in mild cholestasis. Challenging Foxa2 mutants with a cholic acid diet leads to severe intrahepatic cholestasis and a disproportionate rise in serum bile acids. Accumulation of hepatic bile acids is exacerbated by reduced expression of the glutathione S-transferase, conjugation, and hydroxylation enzymes, and leads to endoplasmic reticulum stress and significant liver injury in Foxa2loxP/loxPAlfp.Cremice.
Figure 6.
A model for the regulation of hepatic bile acid homeostasis by Foxa2.
Foxa2 regulates the expression of Mrp2 and Oatp2 directly and that of Mrp3 and Mrp4 indirectly. Mrp3 and Mrp4 facilitate export of bile acids from hepatocytes into circulation, the organic anion carrier Oatp2 is involved in sodium-independent hepatic uptake of bile salts, while Mrp2 is involved in secretion of bile acids and their anionic conjugates into bile. Expression of conjugation enzymes Slc27a5, Gsta1, Gsta2, and Gstm2 also depends on Foxa2. Reduced conjugation of bile acids coupled with decreases in mRNA levels of the transporters due to absence of Foxa2 leads to elevated hepatic bile acids, which in turn cause ER stress. Expression of Cyp3a11 is directly controlled by Foxa2 as well. Cyp3a11 is important in hydroxylation of the hydrophobic bile acids, protecting the liver from bile acid toxicity. Together, ER stress and increased bile acid toxicity in Foxa2loxP/loxPAlfp.Cremice lead to liver injury.
Discussion
The role of Foxa2 in hepatic transcriptional regulation has been studied previously with several paradigms of overexpression10,12 which produced conflicting results. Overexpression of a DNA binding protein can lead to artificial effects, as binding sites with a weak consensus, which are not normally targets, will be occupied by the exogenous protein present at supra-physiological levels. To overcome these limitations, we employed tissue-specific gene ablation to derive mice deficient for Foxa2 in hepatocytes. Our previous study of Foxa2 in the liver42 focused on glucose metabolism because majority of the Foxa targets known ten years ago were genes involved in this process. In addition, we employed a genome-wide enhancer/promoter microarray to identify the set of genes directly bound by Foxa2 in vivo, allowing us to identify numerous novel Foxa2 targets in the liver. Using these combined approaches, we have shown that Foxa2 controls the expression of multiple genes involved in bile acid metabolism, which together explain the cholestatic phenotype of Foxa2 mutant mice.
In a study by Rausa and colleagues, transgenic mice overexpressing rat Foxa2 (Hnf3β) in the liver exhibited elevated bile acids in serum, up to 50-fold by 6 weeks of age12. The phenotype was attributed to reduced expression of Ntcp, a sinusoidal transporter importing bile acids into the hepatocyte from circulation, a defect that preceded the physiological changes. However, the endogenous expression of mouse Foxa2, as well as Foxa1, was completely abolished in this model, while that of Foxa3 and Hnf6 was reduced about 3-fold. Hence, changes in gene expression in this transgenic model could be the consequence of altered levels of other transcription factors rather than that of direct regulation by Foxa2. In addition, we observed a reduction in hepatic mRNA levels of Ntcp in Foxa2loxP/loxP;Alfp.Cre mice, opposite to what would have been predicted from the findings in the transgene model. Reduction of Ntcp expression is expected in our mouse model since elevated bile acid levels activate Shp, which inhibits Ntcp transcription in a mechanism to reduce bile acid import, protecting the hepatocyte from bile acid-mediated damage in cholestatic conditions43 . Downregulation of Ntcp, as is seen in obstructive cholestasis, is occasionally associated with an increase in serumbile acid levels44, but the increase in serum bile acid levels depends on the load from the intestine and the capacity of the basolateral bile acid transporters. The reduction in Ntcp expression we see in Foxa2 mutant mice on normal diet is not severe enough to be a limiting factor. However, when these mice are fed a cholic acid containing diet, the bile salt load from the intestine increases dramatically and serum bile salt levels become elevated. Furthermore, hepatocyte damage observed in the study by Rausa and colleagues was attributed to diminished expression of Mdr2, a canalicular phospholipid transporter. Expression of Mdr2 is not changed in our loss of function model.
Wolfrum and colleagues10 performed an expression profile comparing wildtype mice expressing constitutively acting adenoviral Foxa2T156A with controls infected with adenoviral GFP. Overexpression of Foxa2 by this technique also raised expression of Foxa1, Foxa3, Hnf4α, and Pparγ, other transcription factors central to hepatic metabolism. A small subset of targets in this study and ours overlap. Expression of several genes, including Cyp7b1 and Oatp2 is elevated in the overexpression model and reduced in our loss-of-function model, supporting previous findings that Foxa2 is a transcriptional activator45. Although the study by Wolfrum and colleagues had implicated Foxa2 in lipid metabolism, it did not connect Foxa2 to bile acid metabolism in particular, nor identify expression changes for the bile acid transporter genes Mrp2 and Mrp4, the bile acid CoA ligase Slc27a5, or the hydroxylation enzyme Cyp3a11, all downregulated in our loss-of-function model and essential to explaining the cholestatic phenotype of Foxa2loxP/loxP;Alfp.Cre mice. The differences between the two gene lists could be explained by changes in other DNA binding proteins in the overexpression model, and the possibility of contacting low-affinity binding sites in this system.
Deletion of Foxa2 in the liver leads to cholestasis, which is exacerbated when the feed is supplemented with cholic acid. Expression of Bsep, the major bile salt export pump thought to be responsible for most of bile salt excretion, is not changed in the knockout animals on either mRNA or protein level. Instead, accumulation of hepatic bile acids can be explained by lowered hepatic mRNA and protein levels of several transporters of bile acids and their conjugates, including Mrp2, Mrp4, and Oatp2 on both standard and cholic acid diets, and Mrp3, whose induction normally seen in livers of mice fed a cholic acid supplemented feed is blunted in the absence of Foxa2.
While mutations in Bsep have been associated with Progressive Intrahepatic Cholestasis Type2 (PFIC2) in humans, the phenotype of Bsep knockout mice is much less severe46. Bsep null mice show substantial bile acid secretion and are protected from severe cholestatic injury by hydroxylation of bile acids and existence of an alternative export system for bile acids, Mdr1. Mdr1 levels are increased in Bsep mutant animals47, as they are in Foxa2 knockout mice. In addition, genetic variation in BSEP has been excluded in etiology of several cholestatic conditions in humans, including primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC)48. Thus, intrahepatic cholestasis can occur in the absence of changes in BSEP in humans, while genetic studies in mice have shown that Bsep is not the crucial player in bile salt export in rodents.
We also document that expression of the bile acid conjugation enzyme bile acid CoA ligase (Slc27a5) is directly dependent on Foxa2. Bile acid CoA ligase knockout animals, while exhibiting a severe conjugation defect, fail to develop cholestasis because they express normal or even elevated levels of bile acid and bile acid conjugate transporters. Expression of Bsep and Ntcp is upregulated in Slc27a5-deficient mice32. In contrast, reduced expression of conjugation enzymes in the Foxa2 knockout animals is coupled with a transporter defect, resulting in hepatic cholestasis.
Treatment with bile acids has been shown to induce ER stress33, which has been implicated in the pathogenesis of cholestatic liver disease. While cholic acid diet also induces ER stress in wildtype mice, liver injury is exacerbated in Foxa2-deficient livers due to downregulation of Cyp3a11, a key hydroxylation enzyme that protects the liver from cholestatic injury. CYP3A4, the human homolog of Cyp3a11, is the most abundant cytochrome P450 enzyme expressed in the liver. We provide the first in vivo evidence that Foxa2 prevents hepatic ER stress and protects the liver from bile acid toxicity by activating Cyp3a11 expression.
While previous reports have shown reduced nuclear Foxa2 expression in rodent models of reduced biliary secretion and biliary obstruction17,23, we demonstrate that expression of FOXA2 is reduced in human cholestatic livers of patients of various ages and with different etiologies. This is the first study connecting FOXA2 to human cholestatic liver disease, and, together with our findings in the mouse model suggests that strategies to maintain FOXA2 expression in cholestatic patients might be a novel therapeutic goal.
Experimental procedures
Animals
The derivation of the Foxa2loxP/loxP;Alfp.Cre mouse model has been described previously6. All our mice have been backcrossed to the CD1 mouse strain. Mice were genotyped by PCR of tail DNA. Two to three months old male mice were used in all studies. The bile acid feeding study was performed with experimental diet consisting of a standard rodent diet (5010, PMI Laboratory autoclavable rodent diet) supplemented with 0.5% cholic acid (CA). Mice were placed on the CA supplemented diet for 7 days. At the end of the study, the mice were fasted overnight, anesthetized, and blood collected from the vena cava was processed to serum.
Human Liver Samples
The human pediatric liver samples were obtained from the Children Hospital of Philadelphia’s (CHOP) Liver and Bile Specimen Repository, IRB protocol #1999-7-1371. The specimens were collected at the time of liver transplantation and immediately frozen in liquid nitrogen. Adult sections of livers were fixed in 10% neutral buffered formalin and paraffin embedded. Five micron sections were obtained for immunohistochemical staining.
Immunofluorescence and immunohistochemistry
Indirect immunofluorescence and immunohistochemistry were performed as described previously 6. Slides subjected to immunohistochemistry were counterstained with heamotoxylin. The following antibodies were used: rabbit anti-ubiquitin (1:200 dilution, DAKO), goat anti-Grp78 (1:200 dilution, SC-1050; Santa Cruz Biotechnology), indocarbocyanine-conjugated donkey rabbit IgG (1:600 dilution; Jackson ImmunoResearch Laboratories Inc.), carbocyanine-conjugated donkey anti-goat (1:200 dilution; Jackson ImmunoResearch Laboratories Inc.), anti-Foxa249 (rabbit polyclonal at 1:1000 dilution), and biotinylated goat anti-rabbit (1:200 dilution, Vector).
RNA reverse transcription and real-time PCR
Liver RNA was isolated from Foxa2loxP/loxP;Alfp.Cre and control littermates, and quantitative reverse transcription-PCR were performed as described 6 .
Chromatin immunoprecipitation (ChIP)
ChIP and the following real time PCR reactions were performed as described 6 Snap-frozen mouse liver (100 mg) from wild type and Foxa2 mutant mice (n = 3 animals per group) was used to prepare chromatin. Immunoprecipitation was performed with rabbit anti-Foxa2 serum49 or control pre-immune rabbit IgG (Upstate).
Western blot analysis
For nuclear extracts, snap-frozen human liver (100 mg) was homogenized in 1 ml of Resuspension Solution (10 mM Hepes pH 7.9, 1 mM EDTA, 60 mM KCl, 0.5% NP-40, 1 mM DTT, 1 mM PMSF) containing protease inhibitors. The homogenate was centrifuged at 1500 rpm for 5 minutes at 4°C. The remaining pellet was resuspended in 250 μl of Lysis Buffer (50mM Tris pH 7.5, 150 mM NaCl, 1% NP-40, 0.5% Na deoxycholic acid) with protease inhibitors. The solution was sonicated for 8 minutes using Bioruptor (Diogenode SA), and centrifuged at 13,000 rpm for 1 minute. Western blot was completed as described previously50. The primary antibodies used were rabbit anti-Foxa249 (1:5000) and rabbit anti-TFIID(TBP) (1:100, Santa Cruz, sc-273). Membrane fractions for protein expression of transporters were prepared using Compartmental Protein Extraction Kit (K3012010, Biochain Institute, Inc.). The primary antibodies used were rabbit anti-Abcb11 (1:100, Abgent, AP6110a), rabbit anti-Mrp2 (1:100, Kamiya Biomedical Company, MC-267), goat anti-Mrp3 (1:100, Santa Cruz, sc-5775), rat anti-Mrp4 (1:100, Santa Cruz, sc-59615) and mouse anti-Na/K-Atpase (1:2000, Santa Cruz, sc-48345).
Mouse enhancer/promoter microarray analysis
Amplification and labeling of immunoprecipitated chromatin was performed as described previously50. Labeled DNA samples were hybridized to two arrays, the Mouse PromoterChip BCBC-5A and the Mouse PromoterChip BCBC-5B. Statistical analysis of the microarray data was performed using the significance of microarrays (SAM)51 package with a false discovery rate (FDR) of 10% and fold change cutoff of 1.4. The data have been deposited into ArrayExpress (accession number XXXX).
Serum and Tissue Chemistry
Serum chemistry was analyzed by AniLytics Incorporated (Gaithersburg, MD). For tissue chemistry, snap-frozen mouse liver (100 mg) was homogenized in 0.5 ml of ice-cold PBS containing protease inhibitors (Complete, Roche) using a Kontes glass homogenizer (2mL). The homogenizer was rinsed with additional 0.5 ml of PBS solution, and the resulting 1 ml mixture was centrifuged at 600g @ 4°C for 10 min. The supernatant was diluted 5x with PBS and centrifuged further at 105,000g for 90 min. Total bile acid content of the 105K supernatant, containing the cytosolic fraction, was quantified using the Bile Acid L3K Assay kit (Diagnostic Chemicals). The protein concentration was measured using the Bradford assay. Hepatic cholesterol (Wako # 439-17501), non-esterified fatty acids (Wako # 994-75409), and triglycerides (Wako # 998-40391) were determined using enzymatic assay kits.
Sequence Analysis
The Matrix Search sequence analysis tool (http://rulai.cshl.edu/cgi-bin/TRED/tred.cgi?process=analysisMatrixForm) was used to scan regulatory regions of prospective Foxa2 targets from enhancer/promoter microarrays for Foxa2 binding sites. MSCAN algorithm (http://mscan.cgb.ki.se/cgi-bin/MSCAN) was utilized to detect potential clusters of Foxa2 binding sites in genomic sequences. Conservation of Foxa2 binding sites was determined with CONREAL software (http://conreal.niob.knaw.nl/) and visualized with Vertebrate Multiz Alignment and Conservation track of the UCSC Genome Browser (http://genome.ucsc.edu/).
Supplementary Material
Acknowledgments
The authors thank Dr. David D. Moore for advice on the cholic acid diet, Dr. Jeff Whittsett for providing antibodies, Drs. Ke Ma and Linda E. Greenbaum for critical reading of the manuscript, Dr. Diana Ye, Athansios Arsenlis, Geetu Tuteja, and Dr. Nan Gao for contributions to this project, and Elizabeth Rand, M.D. and the Fred and Suzanne Biesecker Pediatric Liver Center for providing pediatric liver samples. We are grateful to Sophia Hammani and Elizabeth Helmbrecht for care of the animals. Our studies were assisted by the University of Pennsylvania Diabetes Center (P30DK19525). This work was supported by grants DK-049210 and DK-056947 to KHK. IMB was supported by training grant T32-HG000046 and Penn Genomics Institute Graduate Fellowship.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Author Contributions
I.M.B. developed the project, performed most of the experiments and data analysis and wrote the draft of the manuscript. N.E.R and P.W. performed some of the experiments and data analysis. E.E.F and J.R.F. contributed to the human tissue studies. K.H.K. directed the project and reviewed and edited the manuscript.
References
- 1.Kaestner KH. The hepatocyte nuclear factor 3 (HNF3 or FOXA) family in metabolism. Trends Endocrinol Metab. 2000;11:281–5. doi: 10.1016/s1043-2760(00)00271-x. [DOI] [PubMed] [Google Scholar]
- 2.Costa RH, Grayson DR, Darnell JE., Jr Multiple hepatocyte -enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell Biol. 1989;9:1415–25. doi: 10.1128/mcb.9.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang SL, et al. The formation and maintenance of the definitive endoderm lineage in the mouse: involvement of HNF3/forkhead proteins. Development. 1993;119:1301–15. doi: 10.1242/dev.119.4.1301. [DOI] [PubMed] [Google Scholar]
- 4.Monaghan AP, Kaestner KH, Grau E, Schutz G. Postimplantation expression patterns indicate a role for the mouse forkhead/HNF-3 alpha, beta and gamma genes in determination of the definitive endoderm, chordamesoderm and neuroectoderm. Development. 1993;119:567–78. doi: 10.1242/dev.119.3.567. [DOI] [PubMed] [Google Scholar]
- 5.Lee CS, Friedman JR, Fulmer JT, Kaestner KH. The initiation of liver development is dependent on Foxa transcription factors. Nature. 2005;435:944–7. doi: 10.1038/nature03649. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Rubins NE, Ahima RS, Greenbaum LE, Kaestner KH. Foxa2 integrates the transcriptional response of the hepatocyte to fasting. Cell Metab. 2005;2:141–8. doi: 10.1016/j.cmet.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki K, et al. Epididymis-specific promoter-driven gene targeting: a transcription factor which regulates epididymis-specific gene expression. Mol Cell Endocrinol. 2006;250:184–9. doi: 10.1016/j.mce.2005.12.043. [DOI] [PubMed] [Google Scholar]
- 8.Carroll JS, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Laganiere J, et al. From the Cover: Location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc Natl Acad Sci U S A. 2005;102:11651–6. doi: 10.1073/pnas.0505575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432:1027–32. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- 11.Lantz KA, et al. Foxa2 regulates multiple pathways of insulin secretion. J Clin Invest. 2004;114:512–20. doi: 10.1172/JCI21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rausa FM, et al. Elevated levels of hepatocyte nuclear factor 3beta in mouse hepatocytes influence expression of genes involved in bile acid and glucose homeostasis. Mol Cell Biol. 2000;20:8264–82. doi: 10.1128/mcb.20.21.8264-8282.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodwin B, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–26. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 14.Lu TT, et al. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507–15. doi: 10.1016/s1097-2765(00)00050-2. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, et al. Redundant pathways for negative feedback regulation of bile acid production. Dev Cell. 2002;2:721–31. doi: 10.1016/s1534-5807(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 16.Fischer S, Beuers U, Spengler U, Zwiebel FM, Koebe HG. Hepatic levels of bile acids in end-stage chronic cholestatic liver disease. Clin Chim Acta. 1996;251:173–86. doi: 10.1016/0009-8981(96)06305-x. [DOI] [PubMed] [Google Scholar]
- 17.Zollner G, et al. Role of nuclear receptors and hepatocyte-enriched transcription factors for Ntcp repression in biliary obstruction in mouse liver. Am J Physiol Gastrointest Liver Physiol. 2005;289:G798–805. doi: 10.1152/ajpgi.00319.2004. [DOI] [PubMed] [Google Scholar]
- 18.Kullak-Ublick GA, Stieger B, Meier PJ. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology. 2004;126:322–42. doi: 10.1053/j.gastro.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Eloranta JJ, Kullak-Ublick GA. Coordinate transcriptional regulation of bile acid homeostasis and drug metabolism. Arch Biochem Biophys. 2005;433:397–412. doi: 10.1016/j.abb.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 20.Mennone A, et al. Mrp4−/− mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology. 2006;43:1013–21. doi: 10.1002/hep.21158. [DOI] [PubMed] [Google Scholar]
- 21.Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609:1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez-Checa JC, Takikawa H, Horie T, Ookhtens M, Kaplowitz N. Canalicular transport of reduced glutathione in normal and mutant Eisai hyperbilirubinemic rats. J Biol Chem. 1992;267:1667–73. [PubMed] [Google Scholar]
- 23.Geier A, et al. Characterization of organic anion transporter regulation, glutathione metabolism and bile formation in the obese Zucker rat. J Hepatol. 2005;43:1021–30. doi: 10.1016/j.jhep.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz ML, et al. Beneficial effect of spironolactone administration on ethynylestradiol-induced cholestasis in the rat: involvement of up-regulation of multidrug resistance-associated protein 2. Drug Metab Dispos. 2007;35:2060–6. doi: 10.1124/dmd.107.016519. [DOI] [PubMed] [Google Scholar]
- 25.Ballatori N, Truong AT. Glutathione as a primary osmotic driving force in hepatic bile formation. Am J Physiol. 1992;263:G617–24. doi: 10.1152/ajpgi.1992.263.5.G617. [DOI] [PubMed] [Google Scholar]
- 26.Zollner G, et al. Hepatobiliary transporter expression in percutaneous liver biopsies of patients with cholestatic liver diseases. Hepatology. 2001;33:633–46. doi: 10.1053/jhep.2001.22646. [DOI] [PubMed] [Google Scholar]
- 27.Oswald M, Kullak-Ublick GA, Paumgartner G, Beuers U. Expression of hepatic transporters OATP-C and MRP2 in primary sclerosing cholangitis. Liver. 2001;21:247–53. doi: 10.1034/j.1600-0676.2001.021004247.x. [DOI] [PubMed] [Google Scholar]
- 28.Guo GL, et al. Complementary roles of farnesoid X receptor, pregnane X receptor, and constitutive androstane receptor in protection against bile acid toxicity. J Biol Chem. 2003;278:45062–71. doi: 10.1074/jbc.M307145200. [DOI] [PubMed] [Google Scholar]
- 29.Shih DQ, et al. Hepatocyte nuclear factor-1alpha is an essential regulator of bile acid and plasma cholesterol metabolism. Nat Genet. 2001;27:375–82. doi: 10.1038/86871. [DOI] [PubMed] [Google Scholar]
- 30.Coffinier C, et al. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1beta. Development. 2002;129:1829–38. doi: 10.1242/dev.129.8.1829. [DOI] [PubMed] [Google Scholar]
- 31.Inoue Y, Yu AM, Inoue J, Gonzalez FJ. Hepatocyte nuclear factor 4alpha is a central regulator of bile acid conjugation. J Biol Chem. 2004;279:2480–9. doi: 10.1074/jbc.M311015200. [DOI] [PubMed] [Google Scholar]
- 32.Hubbard B, et al. Mice deleted for fatty acid transport protein 5 have defective bile acid conjugation and are protected from obesity. Gastroenterology. 2006;130:1259–69. doi: 10.1053/j.gastro.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Bernstein H, et al. Activation of the promoters of genes associated with DNA damage, oxidative stress, ER stress and protein malfolding by the bile salt, deoxycholate. Toxicol Lett. 1999;108:37–46. doi: 10.1016/s0378-4274(99)00113-7. [DOI] [PubMed] [Google Scholar]
- 34.Riggs AC, et al. Mice conditionally lacking the Wolfram gene in pancreatic islet beta cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia. 2005;48:2313–21. doi: 10.1007/s00125-005-1947-4. [DOI] [PubMed] [Google Scholar]
- 35.Menendez-Benito V, Verhoef LG, Masucci MG, Dantuma NP. Endoplasmic reticulum stress compromises the ubiquitin-proteasome system. Hum Mol Genet. 2005;14:2787–99. doi: 10.1093/hmg/ddi312. [DOI] [PubMed] [Google Scholar]
- 36.Zhao L, Longo-Guess C, Harris BS, Lee JW, Ackerman SL. Protein accumulation and neurodegeneration in the woozy mutant mouse is caused by disruption of SIL1, a cochaperone of BiP. Nat Genet. 2005;37:974–9. doi: 10.1038/ng1620. [DOI] [PubMed] [Google Scholar]
- 37.Vendemiale G, Grattagliano I, Lupo L, Memeo V, Altomare E. Hepatic oxidative alterations in patients with extra-hepatic cholestasis. Effect of surgical drainage. J Hepatol. 2002;37:601–5. doi: 10.1016/s0168-8278(02)00234-9. [DOI] [PubMed] [Google Scholar]
- 38.Zollner G, Marschall HU, Wagner M, Trauner M. Role of nuclear receptors in the adaptive response to bile acids and cholestasis: pathogenetic and therapeutic considerations. Mol Pharm. 2006;3:231–51. doi: 10.1021/mp060010s. [DOI] [PubMed] [Google Scholar]
- 39.Xie W, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci U S A. 2001;98:3375–80. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staudinger JL, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98:3369–74. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bombail V, Taylor K, Gibson GG, Plant N. Role of Sp1, C/EBP alpha, HNF3, and PXR in the basal-and xenobiotic -mediated regulation of the CYP3A4 gene. Drug Metab Dispos. 2004;32:525–35. doi: 10.1124/dmd.32.5.525. [DOI] [PubMed] [Google Scholar]
- 42.Sund NJ, et al. Hepatocyte nuclear factor 3beta (Foxa2) is dispensable for maintaining the differentiated state of the adult hepatocyte. Mol Cell Biol. 2000;20:5175–83. doi: 10.1128/mcb.20.14.5175-5183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denson LA, et al. The orphan nuclear receptor, shp, mediates bile acid-induced inhibition of the rat bile acid transporter, ntcp. Gastroenterology. 2001;121:140–7. doi: 10.1053/gast.2001.25503. [DOI] [PubMed] [Google Scholar]
- 44.Zollner G, et al. Induction of short heterodimer partner 1 precedes downregulation of Ntcp in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2002;282:G184–91. doi: 10.1152/ajpgi.00215.2001. [DOI] [PubMed] [Google Scholar]
- 45.Lai E, Prezioso VR, Tao WF, Chen WS, Darnell JE., Jr Hepatocyte nuclear factor 3 alpha belongs to a gene family in mammals that is homologous to the Drosophila homeotic gene fork head. Genes Dev. 1991;5:416–27. doi: 10.1101/gad.5.3.416. [DOI] [PubMed] [Google Scholar]
- 46.Wang R, et al. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci U S A. 2001;98:2011–6. doi: 10.1073/pnas.031465498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang R, et al. Severe cholestasis induced by cholic acid feeding in knockout mice of sister of P-glycoprotein. Hepatology. 2003;38:1489–99. doi: 10.1016/j.hep.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 48.Pauli-Magnus C, et al. BSEP and MDR3 haplotype structure in healthy Caucasians, primary biliary cirrhosis and primary sclerosing cholangitis. Hepatology. 2004;39:779–91. doi: 10.1002/hep.20159. [DOI] [PubMed] [Google Scholar]
- 49.Besnard V, Wert SE, Hull WM, Whitsett JA. Immunohistochemical localization of Foxa1 and Foxa2 in mouse embryos and adult tissues. Gene Expr Patterns. 2004;5:193–208. doi: 10.1016/j.modgep.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Rubins NE, et al. Transcriptional networks in the liver: hepatocyte nuclear factor 6 function is largely independent of Foxa2. Mol Cell Biol. 2005;25:7069–77. doi: 10.1128/MCB.25.16.7069-7077.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–21. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.