Abstract
The decline in neuronal function during aging may result from increases in extracellular glutamate (Glu), Glu-induced neurotoxicity, and altered mitochondrial metabolism. To study metabolic responses to persistently high levels of Glu at synapses during aging, we used transgenic (Tg) mice that over-express the enzyme Glu dehydrogenase (GDH) in brain neurons and release excess Glu in synapses. Mitochondrial GDH is important in amino acid and carbohydrate metabolism and in anaplerotic reactions. We monitored changes in nineteen neurochemicals in the hippocampus and striatum of adult, middle aged, and aged Tg and wild type (wt) mice, in vivo, using proton (1H) magnetic resonance spectroscopy. Significant differences between adult Tg and wt were higher Glu, N-acetyl aspartate (NAA), and NAA + NAA−Glu (NAAG) levels, and lower lactate in the Tg hippocampus and striatum than those of wt. During aging, consistent changes in Tg and wt hippocampus and striatum included increases in myo-inositol and NAAG. The levels of glutamine (Gln), a key neurochemical in the Gln-Glu cycle between neurons and astroglia, increased during aging in both the striatum and hippocampus of Tg mice, but only in the striatum of the wt mice. Age-related increases of Glu were observed only in the striatum of the Tg mice.
Keywords: Brain metabolism, Hippocampus, Striatum, Magnetic resonance spectroscopy
Introduction
The function of glutamate (Glu) as the major excitatory neurotransmitter in the mammalian central nervous system (CNS) is well known, as are also the long-lasting effects that Glu receptor activation may have on neuronal structure and function [1, 2]. Repeated activation of Glu receptors can lead to long-lasting modification of synaptic activity [1, 3, 4], altered metabolic states in neurons [2, 5–7], and the initiation of processes that may lead to neuronal injury and death [8–13].
During aging, there is a gradual rise in extracellular Glu in the brain [14–16] and an increase in the sensitivity of certain neurons to the cytotoxic effects of Glu [17]. A significant decrease in the dendrite levels of the microtubule-associated protein 2 (MAP2), a marker of dendrite structure, have been observed in the aging brain [18–20]. Based on the strong relationship between Glu hyperactivity and decreases in MAP2 labeling in dendrites of sensitive neurons [21–25], the MAP2 decreases may be considered an index of the effects of a hyper-glutamatergic state in the aging brain. Neurodegeneration induced by high levels of extrasynaptic Glu is associated with changes in synaptic and dendritic morphology and in the levels of many synapse-associated proteins, MAP2 being simply one such dendrite and synapse-related protein [26, 27]. Most studies of neurochemical changes associated with aging have been focused on changes in the levels of the neurotransmitters acetylcholine, 5-hydroxytryptamine, dopamine, and nor-epinephrine [28–30] or on the age-related changes in glucose metabolism [31, 32]. However, only few studies have been focused on Glu metabolism by neurons and glia in brain during aging [33].
One approach for studying the neuronal responses to persistently high levels of glutamatergic activity at synapses is the use of transgenic (Tg) or null mutant animals with altered Glu metabolism and release or re-uptake. Null mutant mice for the high affinity glial Glu transporter genes have high levels of extracellular Glu and suffer extensive brain damage and embryonic lethality [34–36]. Null mutants for the gene Tsc1 that controls the expression and function of Glu transporters in the CNS, also maintain high baseline, non-stimulated extracellular Glu levels and suffer from extensive neuronal damage, intractable seizures, and have markedly shorter lifespan [37]. Thus, animals with decreased expression of Glu transporter genes in the CNS are not suitable for studying aging-related changes in brain metabolism resulting from excess Glu activity.
Alternatively, Tg mice over-expressing the gene for Glu dehydrogenase 1 (Glud1), a mitochondrial matrix enzyme, only in CNS neurons have elevated levels of Glu in brain, presumably the result of increases in intracellular Glu as baseline extracellular Glu is only modestly and non-significantly increased [40]. These mice transiently release excess Glu at synapses and exhibit age-related neuronal degeneration [38, 39]. However, the synaptic and neuronal cell losses are confined to select groups of neurons, for example, the pyramidal cells of the CA1 but not those of the CA3 region of the hippocampus, or the striatal but not the cerebellar neurons [38, 39]. Because these Glud1 Tg mice have a lifespan that is close to normal for C57BL/6 mice (Hui et al. unpublished observations), they may be a useful model for studying changes in metabolic processes, as well as molecular signaling, cell structure, synaptic activity, and electrical properties of neurons during aging.
As described by Plaitakis et al. [41], the enzyme Glu dehydrogenase (GDH) is a crucial enzyme in “linking amino acid and carbohydrate metabolism” and contributing to “Krebs cycle anaplerotic mechanisms”. Thus, over-expression of GDH in brain throughout the lifespan of an organism may have profound effects on not only glutamate metabolism, but on cellular metabolism of amino acids and carbohydrates, as well as on mitochondrial and cellular bioenergetics. Magnetic resonance spectroscopy (MRS) is a non-invasive procedure that can be used to measure metabolites in the living brain [42, 43]. In the present study, we used proton 1H MRS to obtain in vivo measurements of nineteen neurochemicals in two selected brain regions, the hippocampus and striatum. These measurements were obtained in wild type (wt) and Glud1 Tg mice during the aging process. Our goal was to determine whether the Glud1 Tg mice undergo a differential pattern of age-related changes in specific metabolites in brain that might be ascribed to GDH hyperactivity, over-stimulation of Glu receptors, or an adaptation to both excess GDH and Glu neurotransmitter activity.
Methods
Experimental Animals
The procedure for generating the Glud1 Tg mice was detailed previously [38]. Age-matched wt mice of the same genetic background (C57BL/6) were used as the controls. All animals were housed in a 12 h light/dark cycle with food and water ad libitum. All animal procedures were performed in accordance with guidelines established by the Institutional Animal Care and Use Committee (IACUC) of the University of Kansas and the University of Kansas Medical Center. The MRS data analyses of neurochemicals were obtained from three mouse groups at the following three age ranges: adult i.e., 8–12.5 month old, 18 Glud1 Tg and 15 wt mice; middle-aged, 13–17.5 month old, 8 Tg and 15 wt mice; and old, 18–22.5 month old, 15 Glud1 Tg and 12 wt mice. Among the three age groups, only three mice from the 13–17.5 month-old group were scanned longitudinally, i.e., at the 13–17.5 and the 18–22.5 month-old period.
Magnetic Resonance Spectroscopy Measurements of Neurochemicals in the Hippocampus and Striatum
The 1H MRS data were acquired from the hippocampus and striatum of Glud1 Tg and wt mice using a 9.4 T MR system (Agilent Technology, Santa Clara, CA). The 1H MRS scans were performed on these mice at the three aforementioned age ranges: adult, middle aged, and old mice from volumes of 4–6 μl using a spin echo, full-intensity acquired localized spectroscopy sequence (echo time 3 ms; repetition time 4 s) [44]. The in vivo 1H MR spectra were acquired from the voxels located in the left hippocampus and the striatum. The voxel size of the hippocampal VOI was 2.2 × 1.2 × 2.4 mm3; and hippocampal tissues were estimated to be over 80 % in a given VOI. The voxel size of the striatal VOI was 1.7 × 1.8 × 2.0 mm3 and included mainly striatal tissues (over 95 % in a given VOI). During MRS scans, the animals were anesthetized using a gas mixture of 1:1 air:oxygen with 1–1.5 % isoflurane. Their core temperature maintained at 37 °C. Magnetic field homogeneity was optimized using FASTMAP, a fast automatic shimming technique [45]. Concentrations of 19 neurochemicals were quantified using an MRS data analysis package, LCModel (linear combination of model spectra of metabolite solutions in vitro) software [46].
Statistical Analyses
Neurochemical levels were compared cross-sectionally among three age groups (8–12.5 vs. 13–17.5 months and 8–12.5 vs. 18–22.5 months) and two genotypes (only for 8–12.5 months) using two-sample t tests. The p values from the independent t tests were adjusted for multiple comparisons with the false discovery rate (FDR) correction using the fdrtool package [47] in R software [48]. Mean differences with FDR-adjusted p values of <0.05 were considered significant.
Results
In Vivo Metabolic Differences Between Glud1 Tg and wt Mouse Hippocampus and Striatum
In order to assess the effects that overexpression of Glud1 in CNS neurons may have on the biochemical state in the brains of living Glud1 Tg mice, we employed 1H MRS to measure the levels of 20 metabolites in wt and Glud1 Tg mouse hippocampus and striatum (Fig. 1a, b). These measurements included neurochemicals that may be related to Glu metabolism, such as glutamine (Gln), aspartate (Asp), γ-aminobutyric acid (GABA), N-acetylaspartate (NAA), and N-acetylaspartylglutamate (NAAG). In the present study, 1H MRS measurements were focused on equal volumes of tissue (voxels) in the hippocampus and striatum regions of wt and Glud1 Tg mice. These two regions were selected because they exhibit decreases in MAP2-labeled dendrites and dendritic spines, as well as decreases in synaptophysin-labeled nerve terminals in the Glud1 Tg compared with wt mice [38]. Furthermore, the morphological changes in the hippocampus become progressively and significantly greater as the Glud1 Tg mice age [38]. 1H MRS measurements were performed in three age ranges: adult (8–12.5 months), middle aged (13–17.5 months), and old (18–22.5 months) mice. The brain is fully developed at 8 months and the transgene-related neuronal damage in the hippocampus is clearly discernible at that age [38].
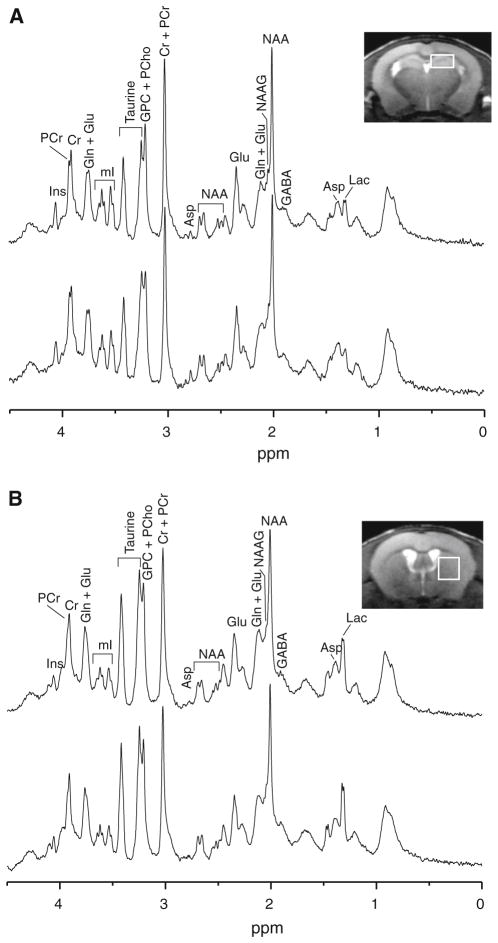
Fig. 1.
a Representative in vivo 1H MR spectra acquired from the hippocampus of Glud1 transgenic (Tg, top) and wild type (wt, bottom) mice. b Representative in vivo 1H MR spectra acquired from the striatum of Tg (top) and wt (bottom) mice. Neurochemical abbreviations are alanine (Ala), aspartate (Asp), ascorbate (Asc), choline (Cho), creatine (Cr), γ-aminobutyric acid (GABA), glucose (Glc), glutamate (Glu), glutamine (Gln), glutathione (GSH), glyceropho-sphoryl-choline (GPC), phosphorylcholine (PCho), myo-Inositol (mI), lactate (Lac), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphocreatine (PCr), phosphorylethanolamine (PE), and serine (Ser)
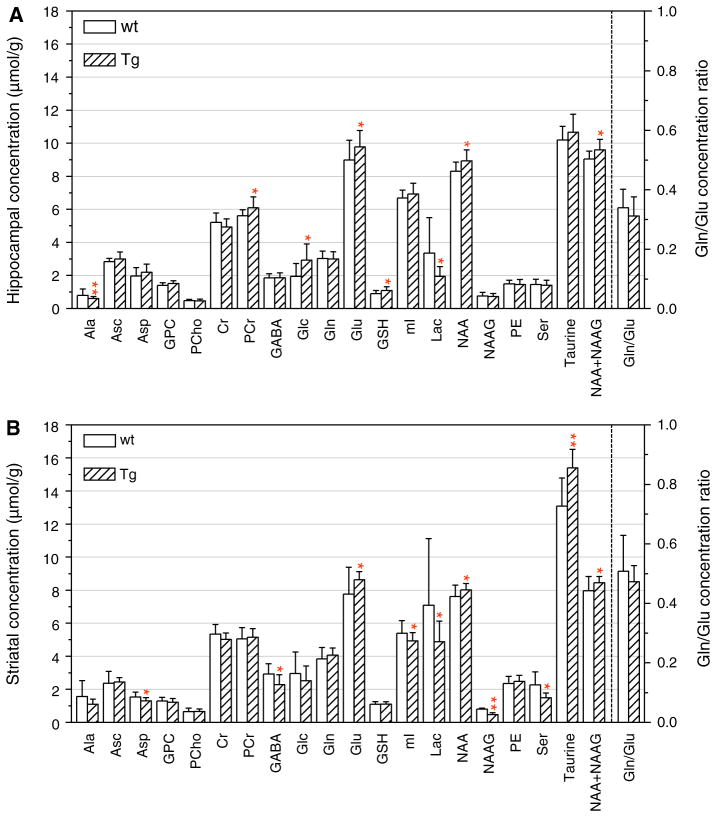
We previously reported on the differences in the concentrations of a few neurochemicals between Tg and wt mouse brain in a relatively small population of 9 month old mice [38]. Here, we first describe the results of neuro-chemical measurements obtained using a larger group of 8–12.5 month old Tg and wt mice. In this adult group, we detected five neurochemicals whose levels were significantly elevated in the hippocampus of Tg as compared with wt mice (Fig. 2a). These were Glu (p = 0.05), phosphocreatine (PCr) (p = 0.03), glucose (Glc) (p = 0.02), glutathione (GSH) (p = 0.03), and NAA (p = 0.02). The levels of the combined NAA and NAAG (NAA + NAAG) (p = 0.02) were also significantly elevated in the Tg as compared with wt hippocampus. This difference in NAA plus NAAG might have been primarily due to the elevation of NAA since the NAAG levels did not differ between wt and Tg (Fig. 2a). There was only one metabolite that was at significantly (p = 0.02) lower levels in the Tg compared with the wt hippocampus, lactic acid (Lac). The levels of the remaining metabolites did not differ significantly between Tg and wt mice.
Fig. 2.
Comparison of neurochemical profiles of adult (8–12.5 month-old) Tg and wt mice. The profiles consist of the concentration of 19 metabolites measured from the hippocampus (top) and striatum (bottom) using in vivo 1H MRS. The calculated ratio of Gln to Glu concentration in Tg and wt hippocampus and striatum is shown on the right hand side of each panel, and the numerical values for this ratio are shown on the right ordinate. The symbols (single asterisks) and (double asterisks) indicate levels of significance in the differences between Tg and wt hippocampus and striatum: *0.01 <p <0.05; **p <0.01
In the striatum of adult Tg mice, the levels of four neuro-chemicals including those of the combined NAA + NAAG, were significantly higher than those measured in wt striatum (Fig. 2b). These were: Glu (p = 0.04), NAA (p = 0.05), NAA + NAAG (p = 0.04), and taurine (Tau) (p <0.01). Thus, the levels of Glu, NAA, and NAA + NAAG were differentially elevated in both hippocampus and striatum of the Tg mice. Also in the striatum, the levels of six metabolites were significantly lower in Tg than those in wt mice. These neurochemicals were: aspartate (Asp) (p = 0.04), GABA (p = 0.02), Lac (p = 0.04), myo-inositol (mI) (p = 0.05), NAAG (p = 0.01), and serine (Ser) (p = 0.01). The only neurochemical that was consistently lower in both hippocampus and striatum was Lac.
The significantly higher levels in Tg versus wt mice of GSH, PCr, NAA, NAA + NAAG, and Glc in the hippocampus, and of NAA, NAA + NAAG, and taurine in the striatum, represent new observations, as were also the lower levels of Lac in both hippocampus and striatum, and of GABA, mI, Ser and NAAG in the striatum only in Tg mice. However, the metabolic profiles of wt and Tg mouse hippocampus and striatum at a single age represent only a snapshot of what may be a progressive, aging-related, differential change in the metabolic activity in the brains of these two genotypes of C57BL/6 mice.
Age-Related Changes in Neurochemical Levels in Glud1 Tg and wt Mouse Hippocampus and Striatum
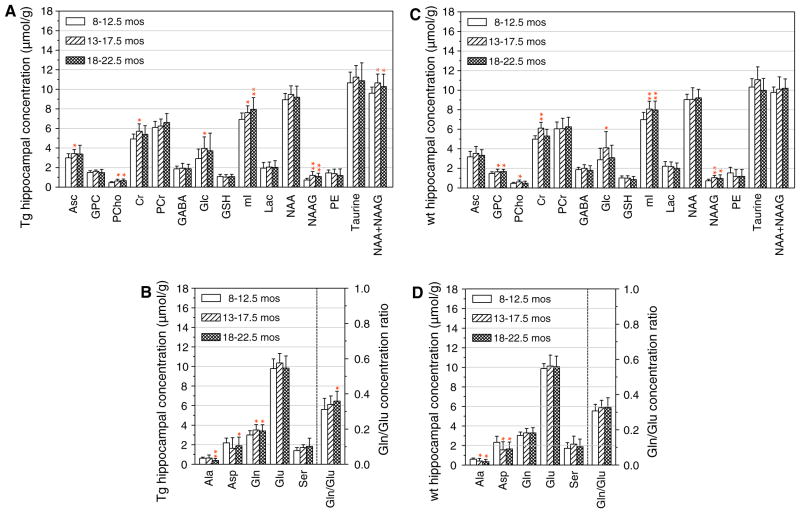
To explore possible age-associated changes in the levels of the metabolites we detected in hippocampus and striatum, we performed in vivo MRS measurements in adult, middle-aged, and old-aged Tg and wt mice. Eight neurochemicals, including NAA + NAAG, increased significantly with advancing age in the hippocampus of Tg mice and two neurochemicals, alanine (Ala) and Asp, had age-related decreases (Fig. 3a, b). In the wt mouse hippocampus, the levels of eight neurochemicals changed significantly with advancing age. Of these, the levels of six increased and those of two, Ala and Asp, decreased significantly with advancing age (Fig. 3c, d). Similarities in age-related changes in the levels of neurochemicals in the hippocampus of both Tg and wt mice included increases in creatine (Cr), phosphorylcholine, (PCho), Glc, mI, and NAAG, and decreases in Ala and Asp (Fig. 3). Particularly noteworthy differences between Tg and wt hippocampus levels during aging were those for Gln and the ratio of Gln to Glu (Gln/ Glu), both of which increased in the hippocampus of the Tg mice (p ≤ 0.023 and p ≤ 0.04, respectively). The amino acids Gln and Glu are linked in terms of metabolic inter-conversions of Glu to Gln in glial cells and of the reverse in glutamatergic neurons, thus the age-associated elevations in Gln and the Gln/Glu ratio may represent a characteristic change of the aging process in the hippocampus of Glud1 Tg mice.
Fig. 3.
Effects of the aging process on the concentration of neuro-chemicals in the hippocampus of Tg (a, b) and wt (c, d) mice. The concentrations of some of the amino acids as well as the ratio of Gln to Glu concentrations are shown in b and d. In b and d, the right hand ordinate shows the numerical values of the Gln to Glu ratios. The age groups of the Tg and wt mice were 8–12.5, 13–17.5, and 18–22.5 months old as indicated in the figures. The group size for Tg and wt mice at each age are described in the “Methods” section. Statistical comparisons were between the concentration values for the 13–17.5 or the 18–22.5 month-old mice and those of the 8–12.5 month-old mice. The symbols (single asterisks) and (double asterisks) indicate statistically significant differences with p values of 0.01 <p <0.05 and p <0.01, respectively
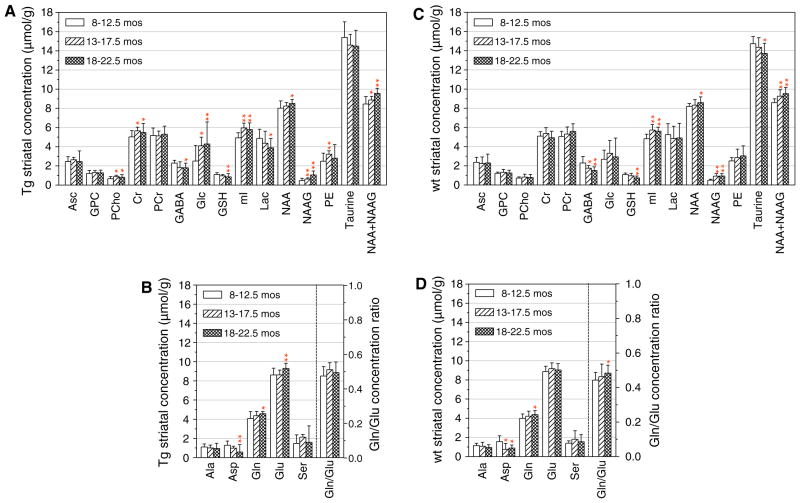
There were several common changes in the levels of neurochemicals across age in the striatum of wt and Tg mice. These included significant decreases with advancing age in three neurochemicals: Asp, GABA, and GSH, and significant increases in five neurochemicals: Gln, mI, NAA, NAAG, NAA + NAAG (Fig. 4a, b). The Glu levels in the striatum increased with age only in the Tg mice, whereas those of Gln increased in both the wt and Tg striatum (Fig. 4).
Fig. 4.
Effects of the aging process on the concentration of neuro-chemicals in the striatum of Tg (a, b) and wt (c, d) mice. The data were obtained from the same groups of mice as those shown in Fig. 3 and the symbols and statistical comparisons were the same as described in the legend of Fig. 3
Discussion
The studies described above represent a unique in vivo neurochemical investigation covering the age span from adulthood to old age in two important brain regions, the hippocampus and striatum, and in two mouse genotypes, the Glud1 Tg and the respective wt C57BL/6 cohorts. The 1H MRS assessment in two brain regions of the Tg and wt mice has identified both aging-related metabolic changes, as well as changes in brain neurochemicals resulting from glutamatergic and GDH hyperactivity in the Glud1 mouse brain. The present study of aging-related changes in the brain was initiated using adult Tg and wt mice, 8–12.5 months-old, in order to avoid possible confounding effects of early developmental influences resulting from the expression of the transgene.
In the adult mice, we replicated the previous observations of elevated Glu levels in hippocampus and striatum [38]. In terms of the overall metabolic status of the Glud1 mice as judged from the different levels of the neuro-chemicals examined in the present study, two of the differences that we detected were particularly interesting. The first was the significantly higher levels of GSH in the Tg as compared with the wt mouse hippocampus, and the second was the significantly lower levels of Lac in both hippocampus and striatum of the Tg than those of the wt mice.
A possible interpretation of the origin and relevance of these two specific neurochemical differences between wt and Tg hippocampus and striatum might be that the elevated levels of GSH and the lower levels of Lac in Tg versus wt mice were indicative of alterations in the astrocyte–neuronal shuttle of metabolites that has been described in relationship to neuronal activation and the release of Glu into the synapse [49, 50]. The release of Glu by neurons activates the synthesis of GSH and enhances the activity of the glycolytic pathway in astrocytes [49]. The former action of Glu leads to increases in GSH formation in astrocytes, while the activation of the glycolytic pathway leads to increased formation of Lac by astrocytes. Increases in GSH levels might represent an adaptive response as GSH leads to enhanced protection of neurons from excess oxidative stress initiated by Glu receptor stimulation [2, 9, 51].
The increased formation of Lac in astrocytes leads to Lac release by astrocytes and the uptake of Lac by activated neurons. The neuronal uptake of Lac averts the induction of neuronal oxidative stress that occurs when glycolysis is the sole source of energy production in neurons [49, 52]. The Lac taken up by neurons is converted to pyruvate which provides the substrate for oxidative phosphorylation in neurons with high energy demands. It is possible that the expression of the Glud1 transgene in neurons of the Tg mice leads to increases in mitochondrial metabolism and in the overall metabolic capacity of mitochondria in the Tg hippocampus and striatum. Thus, the lower Lac levels in the hippocampus and striatum of the Glud1 Tg mice might be an indication of higher rates of utilization of Lac by brain neurons because of their greater mitochondrial capacity than those of wt mice, or an indication of a Glu-induced hyper-active state of neurons in the brain of the Tg animals. The latter interpretation would be consistent with the previously reported switch from Glc-dependent glycolysis to Lac-dependent energy generation in neurons that are stimulated by excess Glu [49, 50]. We are currently exploring the possibility that there may also be a differential metabolic capacity of mitochondria isolated from three different regions of Tg and wt mouse brains.
The rationale for studying metabolite changes in two brain regions was based on our past observations that neurons in adjoining regions of the brain may differ very significantly in terms of gene expression patterns and in their vulnerability to metabolic and oxidative stress, including the stress brought about by the aging process [38, 39, 51, 54–57]. Furthermore, it has previously been shown that the levels of Glu, Gln, GABA and Asp may vary by a factor of five and up to a factor of 21 in different regions of rat brains, and that the effects of aging on the levels of these amino acids are not identical across various rat [58] or mouse brain regions [59]. Thus, we anticipated that we might observe some differences between hippocampus and striatum with regard to both baseline levels and aging-related changes in the levels of Glu, Gln, Asp and other neurochemicals measured in the hippocampus and striatum.
An interesting finding in the present study was that hippocampal Lac concentrations were consistently lower than those in the striatum of both wt and Glud1 Tg mice. This was true for both the measurements obtained with adult mice as well as those with mice at progressively older ages. Others have shown that the hippocampus has lower concentrations of Lac than either cerebral cortex or striatum [60]. One possible explanation for these observations might be that the lower Lac concentrations indicate high utilization of Lac for energy generation by hippocampal neurons, a region of the brain with a large population of glutamatergic innervation.
Another consistent observation in the present study was that of higher NAA levels in adult Tg hippocampus and striatum compared with those in wt mice. This was unexpected in light of our observation of neuronal damage in the hippocampus of the Tg mice at that age [38] and evidence that NAA levels are lower in the presence of damaged neurons, both in humans and animals [61–64]. A likely explanation for the elevations in NAA concentrations in the Tg mouse brain is that NAA concentrations are the result of increased neuronal and mitochondrial activity in the Glud1 Tg mice [65]. Mitochondrial Glu metabolism leads to the synthesis of Asp as part of transamination reactions and this feeds into the synthesis of NAA [66–68]. However, changes in the levels of Asp may differ from those of NAA as Asp is involved in cytoplasmic and intramitochondrial transamination reactions that are linked to both the tricarboxylic acid cycle and the urea cycle. Thus, changes in Asp levels are not determined only by the activation of the NAA and NAAG synthetic pathways. Nevertheless, even though it would be difficult to pinpoint the reasons for the direction of changes in Asp levels with advancing age, our observation of consistent age-related decreases in Asp levels in both wt and Tg hippocampus and striatum fits with previous observations of such aging-associated decreases in the brains of senescence accelerated mice [69].
With regard to the aging process, there were only a few consistent age-associated neurochemical changes that might be considered as possible markers of the aging process in both the hippocampus and striatum of wt and Tg mice. The decline of Asp and the increase in NAAG levels in both brain regions and in both genotypes, might be considered as in vivo neurochemical markers of the effects of the aging process in the hippocampus and striatum. The significant increases in NAAG concentrations during aging represent an intriguing new observation. It is possible that the aging process in both wt and Tg hippocampus and striatum, led to the progressive activation of the NAAG biosynthetic pathway. NAAG has been shown to function as a neurotransmitter or neuromodulator in the CNS acting pre-synaptically to decrease the release of Glu from nerve endings [61, 70]. As already pointed out in preceding sections, brain aging is associated with both higher levels of depolarization-induced release of Glu and diminished Glu re-uptake [14–16, 69]. Even though the mechanism for the increases in NAAG levels in the brain is not known at this time, the higher NAAG levels may modulate Glu release during brain aging and dampen the Glu-initiated stimulation of neurons. Thus, NAAG may play a significant role in maintaining the neurons of Tg and wt mice alive as an organism ages.
With regard to possible differential effects of the aging process on the brains of the Glud1 Tg as compared with the wt mice, there was a more consistent effect of aging on the levels of Glc, Gln, Glu, and the ratio of Gln/Glu in the Tg than in the wt mice, especially in the hippocampus region. The increases in the brain levels of Glc may be a sign of diminished Glc utilization which would be consistent with observations of reduced Glc metabolic rates in human brain during aging, especially in the frontal and temporal cortex [71, 72]. Such decreases in Glc metabolic rates may result from either cell damage or from shifts in the use of Lac in place of Glc as substrate for oxidative phosphorylation in hyper-activated neurons of the Tg mouse brains. Both possibilities are likely in the Glud1 Tg mice. In addition, the differential aging-related changes in Gln, Glu, and Gln/ Glu ratios in Tg versus wt mice, may also be signs of an acceleration of age-associated neurodegenerative processes in the Tg mouse hippocampus and striatum. We base this idea not only on our previous observations of an accelerated loss of dendrites during aging in the hippocampus of Tg as compared with wt mice [38, 39], but also on the fact that in another model of accelerated aging that occurs in the absence of a disease, the senescence accelerated mouse model, both Glu and Gln brain levels are higher in the “senescence prone” as compared with the “senescence resistant” mice [73, 74].
In the preceding paragraphs, we drew comparisons between the data we obtained using MRS measurements and some data from the literature obtained by direct biochemical or immunochemical analyses. We recognize the differences in the mode of gathering these data and of some of the limitations of the approach that was used in the present study. For example, the use of anesthesia during MR scans may present a confounding as it may alter the levels of some neurochemicals, such as those of Lac. However, the observed differences in the levels of brain neurochemicals among genotypes and age groups would still be valid as all animals were scanned under the same conditions of light anesthesia (isoflurane at 1–1.5 %). A second limitation of our studies might be related to the voxel definition of the MRS volumes of the hippocampus. It is estimated from the anatomical MR images that the hippocampal VOI contains up to about 15–20 % of non-hippocampal tissue, including white matter and cortical and deep gray matter. The inclusion of components of these regions was necessitated by the requirement of a rectangular shape for the definition of the voxels for the MRS measurements of the hippocampus, a brain region with a three-dimensional curvilinear structure.
We believe that the studies described in this report provide the background for future directed explorations into the biochemistry of the brain during aging and under the influence of excess Glu activity. Studies of the protein levels and activity of relevant enzymes, such as the two isoforms of lactate dehydrogenase, NAAG synthetase, and monocarboxylic acid transporter, as well as direct measurements of mitochondrial enzyme activities, would be informative in developing a mechanistic understanding of the neurochemical changes observed in the present study.
Acknowledgments
This work was funded by grants from the National Institutes of Health, NIA: AG12993 and AG035982; and NICHD: HD02528. The support of the Higuchi Biosciences Center and of the Hoglund Brain Imaging Center at the University of Kansas is acknowledged.
Contributor Information
In-Young Choi, Hoglund Brain Imaging Center, University of Kansas Medical Center, Kansas City, KS, USA. Department of Neurology, University of Kansas Medical Center, Kansas City, KS, USA. Department of Molecular and Integrative Physiology, University of Kansas Medical Center, Kansas City, KS, USA.
Phil Lee, Hoglund Brain Imaging Center, University of Kansas Medical Center, Kansas City, KS, USA. Department of Molecular and Integrative Physiology, University of Kansas Medical Center, Kansas City, KS, USA.
Wen-Tung Wang, Hoglund Brain Imaging Center, University of Kansas Medical Center, Kansas City, KS, USA.
Dongwei Hui, Higuchi Biosciences Center, University of Kansas, 2099 Constant Ave., Campus West, Lawrence, KS 66047, USA. Department of Pharmacology and Toxicology, University of Kansas, Lawrence, KS, USA.
Xinkun Wang, Higuchi Biosciences Center, University of Kansas, 2099 Constant Ave., Campus West, Lawrence, KS 66047, USA. Department of Pharmacology and Toxicology, University of Kansas, Lawrence, KS, USA.
William M. Brooks, Hoglund Brain Imaging Center, University of Kansas Medical Center, Kansas City, KS, USA. Department of Neurology, University of Kansas Medical Center, Kansas City, KS, USA
Elias K. Michaelis, Email: emichaelis@ku.edu, Higuchi Biosciences Center, University of Kansas, 2099 Constant Ave., Campus West, Lawrence, KS 66047, USA. Department of Pharmacology and Toxicology, University of Kansas, Lawrence, KS, USA
References
- 1.Cotman CW, Monaghan DT, Ganong AH. Excitatory amino acid neurotransmission: NMDA receptors and Hebb-type synaptic plasticity. Annu Rev Neurosci. 1988;11:61–80. doi: 10.1146/annurev.ne.11.030188.000425. [DOI] [PubMed] [Google Scholar]
- 2.Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol. 1998;54:369–415. doi: 10.1016/s0301-0082(97)00055-5. [DOI] [PubMed] [Google Scholar]
- 3.Ito M. Long-term depression. Annu Rev Neurosci. 1989;12:85–102. doi: 10.1146/annurev.ne.12.030189.000505. [DOI] [PubMed] [Google Scholar]
- 4.Bear MF, Abraham WC. Long-term depression in hippocampus. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- 5.Mattson MP. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann NY Acad Sci. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng A, Hou Y, Mattson MP. Mitochondria and neuro-plasticity. ASN Neuro. 2010;2(5):e00045. doi: 10.1042/AN20100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aarts M, Iihara K, Wei WL, Xiong ZG, Arundine M, Cerwinski W, MacDonald JF, Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 9.Lafon-Cazal M, Culcasi M, Gaven F, Pietri S, Bockaert J. Nitric oxide, superoxide and peroxynitrite: putative mediators of NMDA-induced cell death in cerebellar granule cells. Neuro-pharmacology. 1993;32:1259–1266. doi: 10.1016/0028-3908(93)90020-4. [DOI] [PubMed] [Google Scholar]
- 10.Meldrum B, Evans M, Griffiths T, Simon R. Ischaemic brain damage: the role of excitatory activity and of calcium entry. Br J Anaesth. 1985;57:44–46. doi: 10.1093/bja/57.1.44. [DOI] [PubMed] [Google Scholar]
- 11.Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- 12.Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 13.Dawson VL, Dawson TM. Nitric oxide in neurodegeneration. Prog Brain Res. 1998;118:215–229. doi: 10.1016/s0079-6123(08)63210-0. [DOI] [PubMed] [Google Scholar]
- 14.Zoia C, Cogliati T, Tagliabue E, Cavaletti G, Sala G, Galimberti G, Rivolta I, Rossi V, Frattola L, Ferrarese C. Glutamate transporters in platelets: EAAT1 decrease in aging and in Alzheimer’s disease. Neurobiol Aging. 2004;25:149–157. doi: 10.1016/s0197-4580(03)00085-x. [DOI] [PubMed] [Google Scholar]
- 15.Nickell J, Pomerleau F, Allen J, Gerhardt GA. Age-related changes in the dynamics of potassium-evoked L-glutamate release in the striatum of Fischer 344 rats. J Neural Transm. 2005;112:87–96. doi: 10.1007/s00702-004-0151-x. [DOI] [PubMed] [Google Scholar]
- 16.Zoia CP, Tagliabue E, Isella V, Begni B, Fumagalli L, Brighina L, Appollonio I, Racchi M, Ferrarese C. Fibroblast glutamate transport in aging and in AD: correlations with disease severity. Neurobiol Aging. 2005;26:825–832. doi: 10.1016/j.neurobiolaging.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Brewer GJ. Neuronal plasticity and stressor toxicity during aging. Exp Gerontol. 2000;35:1165–1183. doi: 10.1016/s0531-5565(00)00121-2. [DOI] [PubMed] [Google Scholar]
- 18.Chauhan N, Siegel G. Age-dependent organotypic expression of microtubule-associated proteins (MAP1, MAP2, and MAP5) in rat brain. Neurochem Res. 1997;22:713–719. doi: 10.1023/a:1027306227402. [DOI] [PubMed] [Google Scholar]
- 19.Di Stefano G, Casoli T, Fattoretti P, Gracciotti N, Solazzi M, Bertoni-Freddari C. Distribution of map2 in hippocampus and cerebellum of young and old rats by quantitative immunohistochemistry. J Histochem Cytochem. 2001;49:1065–1066. doi: 10.1177/002215540104900818. [DOI] [PubMed] [Google Scholar]
- 20.Di Stefano G, Casoli T, Fattoretti P, Balietti M, Grossi Y, Giorgetti B, Bertoni-Freddari C. Level and distribution of microtubule-associated protein-2 (MAP2) as an index of dendritic structural dynamics. Rejuvenation Res. 2006;9:94–98. doi: 10.1089/rej.2006.9.94. [DOI] [PubMed] [Google Scholar]
- 21.Arias C, Arrieta I, Massieu L, Tapia R. Neuronal damage and MAP2 changes induced by the glutamate transport inhibitor dihydrokainate and by kainate in rat hippocampus in vivo. Exp Brain Res. 1997;116:467–476. doi: 10.1007/pl00005774. [DOI] [PubMed] [Google Scholar]
- 22.Hoskison MM, Yanagawa Y, Obata K, Shuttleworth CW. Calcium-dependent NMDA-induced dendritic injury and MAP2 loss in acute hippocampal slices. Neuroscience. 2007;145:66–79. doi: 10.1016/j.neuroscience.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buddle M, Eberhardt E, Ciminello LH, Levin T, Wing R, DiPasquale K, Raley-Susman KM. Microtubule-associated protein 2 (MAP2) associates with the NMDA receptor and is spatially redistributed within rat hippocampal neurons after oxygen-glucose deprivation. Brain Res. 2003;978:38–50. doi: 10.1016/s0006-8993(03)02758-6. [DOI] [PubMed] [Google Scholar]
- 24.Sánchez C, Ulloa L, Montoro RJ, López-Barneo J, Avila J. NMDA-glutamate receptors regulate phosphorylation of dendritic cytoskeletal proteins in the hippocampus. Brain Res. 1997;765:141–148. doi: 10.1016/s0006-8993(97)00563-5. [DOI] [PubMed] [Google Scholar]
- 25.Quinlan EM, Halpain S. Postsynaptic mechanisms for bidirectional control of MAP2 phosphorylation by glutamate receptors. Neuron. 1996;16:357–368. doi: 10.1016/s0896-6273(00)80053-7. [DOI] [PubMed] [Google Scholar]
- 26.Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61:657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kabuto H, Yokoi I, Mori A, Murakami M, Sawada S. Neurochemical changes related to ageing in the senescence-accelerated mouse brain and the effect of chronic administration of nimodipine. Mech Ageing Dev. 1995;80:1–9. doi: 10.1016/0047-6374(94)01542-t. [DOI] [PubMed] [Google Scholar]
- 29.Gozlan H, Daval G, Verge D, Spampinato U, Fattaccini CM, Gallissot MC, El Mestikawy S, Hamon M. Aging associated changes in serotoninergic and dopaminergic pre- and post-synaptic neurochemical markers in the rat brain. Neurobiol Aging. 1990;11:437–449. doi: 10.1016/0197-4580(90)90011-n. [DOI] [PubMed] [Google Scholar]
- 30.Gottfries CG. Neurochemical aspects on aging and diseases with cognitive impairment. J Neurosci Res. 1990;27:541–547. doi: 10.1002/jnr.490270415. [DOI] [PubMed] [Google Scholar]
- 31.Murphy DG, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, Szczepanik J, Schapiro MB, Grady CL, Horwitz B, Rapoport SI. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996;53:585–594. doi: 10.1001/archpsyc.1996.01830070031007. [DOI] [PubMed] [Google Scholar]
- 32.Gage F, Kelly P, Bjorklund A. Regional changes in brain glucose metabolism reflect cognitive impairments in aged rats. J Neurosci. 1984;4:2856–2865. doi: 10.1523/JNEUROSCI.04-11-02856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boumezbeur F, Mason GF, de Graaf RA, Behar KL, Cline GW, Shulman GI, Rothman DL, Petersen KF. Altered brain mitochondrial metabolism in healthy aging as assessed by in vivo magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 2010;30:211–221. doi: 10.1038/jcbfm.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsugami TR, Tanemura K, Mieda M, Nakatomi R, Yamada K, Kondo T, Ogawa M, Obata K, Watanabe M, Hashikawa T, Tanaka K. From the cover: indispensability of the glutamate transporters GLAST and GLT1 to brain development. Proc Natl Acad Sci USA. 2006;103:12161–12166. doi: 10.1073/pnas.0509144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 37.Zeng LH, Ouyang Y, Gazit V, Cirrito JR, Jansen LA, Ess KC, Yamada KA, Wozniak DF, Holtzman DM, Gutmann DH, Wong M. Abnormal glutamate homeostasis and impaired synaptic plasticity and learning in a mouse model of tuberous sclerosis complex. Neurobiol Dis. 2007;28:184–196. doi: 10.1016/j.nbd.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bao X, Pal R, Hascup KN, Wang Y, Wang WT, Xu W, Hui D, Agbas A, Wang X, Michaelis ML, Choi IY, Belousov AB, Gerhardt GA, Michaelis EK. Transgenic expression of Glud1 (glutamate dehydrogenase 1) in neurons: in vivo model of enhanced glutamate release, altered synaptic plasticity, and selective neuronal vulnerability. J Neurosci. 2009;29:13929–13944. doi: 10.1523/JNEUROSCI.4413-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michaelis EK, Wang X, Pal R, Bao X, Hascup KN, Wang Y, Wang WT, Hui D, Agbas A, Choi IY, Belousov A, Gerhardt GA. Neuronal Glud1 (glutamate dehydrogenase 1) over-expressing mice: increased glutamate formation and synaptic release, loss of synaptic activity, and adaptive changes in genomic expression. Neurochem Int. 2011;59:473–481. doi: 10.1016/j.neuint.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hascup KN, Bao X, Hascup ER, Hui D, Xu W, Pomerleau F, Huettl P, Michaelis ML, Michaelis EK, Gerhardt GA. Differential levels of glutamate dehydrogenase 1 (GLUD1) in Balb/c and C57BL/6 mice and the effects of overexpression of the Glud1 gene on glutamate release in striatum. ASN Neuro. 2011;3(2):e00057. doi: 10.1042/AN20110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plaitakis A, Zaganas I, Spanaki C. Deregulation of glutamate dehydrogenase in human neurologic disorders. J Neurosci Res. 2013;91:1007–1017. doi: 10.1002/jnr.23176. [DOI] [PubMed] [Google Scholar]
- 42.Choi IY, Lee SP, Guilfoyle DN, Helpern JA. In vivo NMR studies of neurodegenerative diseases in transgenic and rodent models. Neurochem Res. 2003;28:987–1001. doi: 10.1023/a:1023370104289. [DOI] [PubMed] [Google Scholar]
- 43.Pfeuffer J, Tkac I, Provencher SW, Gruetter R. Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time (1)H NMR spectra of the rat brain. J Magn Reson. 1999;141:104–120. doi: 10.1006/jmre.1999.1895. [DOI] [PubMed] [Google Scholar]
- 44.Mlynarik V, Gambarota G, Frenkel H, Gruetter R. Localized short-echo-time proton MR spectroscopy with full signal-intensity acquisition. Magn Reson Med. 2006;56:965–970. doi: 10.1002/mrm.21043. [DOI] [PubMed] [Google Scholar]
- 45.Gruetter R. Automatic, localized in vivo adjustment of all first- and second-order shim coils. Magn Reson Med. 1993;29:804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- 46.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 47.Strimmer K. fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics. 2008;24:1461–1462. doi: 10.1093/bioinformatics/btn209. [DOI] [PubMed] [Google Scholar]
- 48.Team RC. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna: 2012. http://www.R-project.org/ [Google Scholar]
- 49.Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte–neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 50.Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism and their relevance to functional brain imaging. Philos Trans R Soc Lond Ser B Biol Sci. 1999;354:1155–1163. doi: 10.1098/rstb.1999.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrero-Mendez A, Almeida A, Fernandez E, Maestre C, Moncada S, Bolanos JP. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C–Cdh1. Nat Cell Biol. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 53.Boumezbeur F, Petersen KF, Cline GW, Mason GF, Behar KL, Shulman GI, Rothman DL. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci. 2010;30:13983–13991. doi: 10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, Pal R, Chen XW, Kumar KN, Kim OJ, Michaelis EK. Genome-wide transcriptome profiling of region-specific vulnerability to oxidative stress in the hippocampus. Genomics. 2007;90:201–212. doi: 10.1016/j.ygeno.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, Pal R, Chen XW, Limpeanchob N, Kumar KN, Michaelis EK. High intrinsic oxidative stress may underlie selective vulnerability of the hippocampal CA1 region. Brain Res Mol Brain Res. 2005;140:120–126. doi: 10.1016/j.molbrainres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 56.Wang X, Zaidi A, Pal R, Garrett AS, Braceras R, Chen XW, Michaelis ML, Michaelis EK. Genomic and biochemical approaches in the discovery of mechanisms for selective neuronal vulnerability to oxidative stress. BMC Neurosci. 2009;10:12. doi: 10.1186/1471-2202-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang X, Michaelis ML, Michaelis EK. Functional genomics of brain aging and Alzheimer’s disease: focus on selective neuronal vulnerability. Curr Genomics. 2010;11:618–633. doi: 10.2174/138920210793360943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banay-Schwartz M, Lajtha A, Palkovits M. Changes with aging in the levels of amino acids in rat CNS structural elements I. Glutamate and related amino acids. Neurochem Res. 1989;14:555–562. doi: 10.1007/BF00964918. [DOI] [PubMed] [Google Scholar]
- 59.Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Age-related glutamate and glutamine concentration changes in normal human brain: 1H MR spectroscopy study at 4 T. Neurobiol Aging. 2005;26:665–672. doi: 10.1016/j.neurobiolaging.2004.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Crumrine RC, LaManna JC. Regional cerebral metabolites, blood flow, plasma volume, and mean transit time in total cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1991;11:272–282. doi: 10.1038/jcbfm.1991.59. [DOI] [PubMed] [Google Scholar]
- 61.Neale JH, Bzdega T, Wroblewska B. N-acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem. 2000;75:443–452. doi: 10.1046/j.1471-4159.2000.0750443.x. [DOI] [PubMed] [Google Scholar]
- 62.Friedman SD, Brooks WM, Jung RE, Chiulli SJ, Sloan JH, Montoya BT, Hart BL, Yeo RA. Quantitative proton MRS predicts outcome after traumatic brain injury. Neurology. 1999;52:1384–1391. doi: 10.1212/wnl.52.7.1384. [DOI] [PubMed] [Google Scholar]
- 63.Brooks WM, Jung RE, Ford CC, Greinel EJ, Sibbitt WL., Jr Relationship between neurometabolite derangement and neuro-cognitive dysfunction in systemic lupus erythematosus. J Rheumatol. 1999;26:81–85. [PubMed] [Google Scholar]
- 64.Harris JL, Yeh HW, Choi IY, Lee P, Berman NE, Swerdlow RH, Craciunas SC, Brooks WM. Altered neurochemical profile after traumatic brain injury: (1)H-MRS biomarkers of pathological mechanisms. J Cereb Blood Flow Metab. 2012;32:2122–2134. doi: 10.1038/jcbfm.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel TB, Clark JB. Synthesis of N-acetyl-L-aspartate by rat brain mitochondria and its involvement in mitochondrial/ cytosolic carbon transport. Biochem J. 1979;184:539–546. doi: 10.1042/bj1840539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Satrustegui J, Contreras L, Ramos M, Marmol P, del Arco A, Saheki T, Pardo B. Role of aralar, the mitochondrial transporter of aspartate–glutamate, in brain N-acetylaspartate formation and Ca(2+) signaling in neuronal mitochondria. J Neurosci Res. 2007;85:3359–3366. doi: 10.1002/jnr.21299. [DOI] [PubMed] [Google Scholar]
- 68.Clark JF, Doepke A, Filosa JA, Wardle RL, Lu A, Meeker TJ, Pyne-Geithman GJ. N-acetylaspartate as a reservoir for glutamate. Med Hypotheses. 2006;67:506–512. doi: 10.1016/j.mehy.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 69.Saransaari P, Oja SS. Age-related changes in the uptake and release of glutamate and aspartate in the mouse brain. Mech Ageing Dev. 1995;81:61–71. doi: 10.1016/0047-6374(95)01583-l. [DOI] [PubMed] [Google Scholar]
- 70.Neale JH, Olszewski RT, Zuo D, Janczura KJ, Profaci CP, Lavin KM, Madore JC, Bzdega T. Advances in understanding the peptide neurotransmitter NAAG and appearance of a new member of the NAAG neuropeptide family. J Neurochem. 2011;118:490–498. doi: 10.1111/j.1471-4159.2011.07338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Mandel F, Alexander GE, Grady C, Pietrini P, Eidelberg D. The metabolic topography of normal aging. J Cereb Blood Flow Metab. 1996;16:385–398. doi: 10.1097/00004647-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Ançevic VI, Alavi A, Souder E, Mozley PD, Gur RE, Bénard F, Munz DL. Regional cerebral glucose metabolism in healthy volunteers determined by fluordeoxyglucose positron emission tomography: appearance and variance in the transaxial, coronal, and sagittal planes. Clin Nucl Med. 2000;25:596–602. doi: 10.1097/00003072-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 73.Okuma Y, Nomura Y. Senescence-accelerated mouse (SAM) as an animal model of senile dementia: pharmacological, neurochemical and molecular biological approach. Jpn J Pharmacol. 1998;78:399–404. doi: 10.1254/jjp.78.399. [DOI] [PubMed] [Google Scholar]
- 74.Kitamura Y, Zhao XH, Ohnuki T, Takei M, Nomura Y. Age-related changes in transmitter glutamate and NMDA receptor/channels in the brain of senescence-accelerated mouse. Neurosci Lett. 1992;137:169–172. doi: 10.1016/0304-3940(92)90396-o. [DOI] [PubMed] [Google Scholar]