Abstract
Coronary artery calcification is a well-established predictor of future cardiac events; however, it is not a predictor of unstable plaque. The intimal calcification of the atherosclerotic plaques may begin with smooth muscle cell apoptosis and/or release of matrix vesicles, and is almost always seen microscopically in pathologic intimal thickening (PIT), which appears as microcalcification (≥0.5 μm, typically <15 μm in diameter). Calcification increases with macrophage infiltration into the lipid pool in early fibroatheroma where they undergo apoptosis and/or release matrix vesicles. The confluence of calcified areas involves extracellular matrix and the necrotic core, which can be identified by radiography as speckled (≤2 mm) or fragmented (>2, <5 mm) calcification. The calcification in thin-cap fibroatheromas and plaque rupture is generally less than what is observed in stable plaques and is usually speckled or fragmented. Fragmented calcification spreads into the surrounding collagen-rich matrix forming calcified sheets, the hallmarks of fibrocalcific plaques. The calcified sheets may break into nodules with fibrin deposition, and when accompanied by luminal protrusion, it is associated with thrombosis. Calcification is highest in fibrocalcific plaques followed by healed plaque rupture and is the least in erosion and PIT. The extent of calcification is greater in men than women especially in the premenopausal period and is also greater in whites compared with blacks. The mechanisms of intimal calcification remain poorly understood in man. Calcification often occurs in the presence of apoptosis of smooth muscle cells and macrophages with matrix vesicles accompanied by expression of osteogenic markers within the vessel wall.
Introduction
Three main types of vascular calcification have been reported; medial Mönckeberg’s arterial calcification; intimal calcification associated with atherosclerosis and infantile calcification. In the current review article we will focus on (intimal) calcification related to coronary atherosclerosis, although other beds will be also mentioned. Atherosclerotic calcification has intrigued pathologists, cardiologist and researchers of lipid metabolism and inflammation for over a century; however, despite extensive research in this area, our mechanistic understanding of atherosclerotic calcification in man remains limited. An important factor contributing is the lack of good animal models of human atherosclerosis. This deficiency can be explained by the different life span of species that cannot be compensated in contemporary animal models. Human atherosclerosis progresses over decades before becoming manifest in a large majority of cases in the sixth and seventh decades, while atherosclerotic animal models typically involve breeding periods ranging from months to a few years.1 By far the most studied animal being the genetically modified mouse, with either Apo E or the LDL receptor deficient, that develop atherosclerosis via increases in serum VLDL and disruption of cellular LDL uptake, respectively, resulting in excessive accumulation of lipids in the vascular wall. In these animals, minimal calcification and no thrombosis is seen, which is significantly different from the observations made in man. Nevertheless, the mouse model has been extremely useful for the understanding of basic pathways involved in atherosclerosis.1, 2
Atherosclerosis occurs in the presence of risk factors, especially hyperlipidemia and manifests focally at branch points as a chronic inflammatory process induced by lipid deposits in the arterial wall. Calcification of the atherosclerotic plaque begins in middle age and is ubiquitously observed in older individuals. The disease is highly prevalent worldwide but relatively few suffer a clinical event.3 Clinical manifestations of the coronary disease include myocardial infarction, unstable and stable angina, and sudden coronary death (SCD); carotid disease includes stroke and transient ischemic attack, while peripheral artery disease manifestation includes claudication and critical limb ischemia. In patients dying from coronary thrombosis, the main etiology is acute plaque rupture, less frequently erosion and least often calcified nodule.4
In this review, we will concentrate on the human coronary atherosclerotic calcification with emphasis on plaque progression. The various plaque types and the degree of narrowing will be described. Furthermore, we will review the difference in its prevalence among male and females and how race may also influence the extent of calcification. Although pathological mechanisms of calcification are likely multifactorial, there is little consensus and therefore we will emphasize mainly on those that may be more applicable to man.
Atherosclerosis Plaque Progression
The natural history of atherosclerosis is considered to be a dynamic process varying from early lesion development to more advanced plaques complicated by acute thrombosis. Intimal thickening begins with an increase in smooth muscle cells (SMCs) and proteoglycan-collagenous matrix with little or no infiltration of inflammatory cells, which is classified as adaptive intimal thickening (AIT), and is related to blood flow. Atherosclerotic plaques have been described to begin as fatty streak with accumulation of lipid-laden foamy macrophages and SMCs, although this lesion is known to regress.5 To our knowledge, the earliest progressive atherosclerotic lesion is pathologic intimal thickening (PIT), which is characterized by the presence of acellular lipid pools within the intima but close to the media where there is a lack of SMCs but there is an abundance of proteoglycans and lipid deposition.4, 6 As plaques progress, there is infiltration by macrophages and T-lymphocytes close to the lumen and remote from the lipid pools.
The more advanced stage of atherosclerotic lesion is classified as fibroatheroma,4, 7 which is characterized by the presence of a lipid rich necrotic core encapsulated by fibrous tissue. The early phase of fibroatheroma (early fibroatheroma) shows infiltration of macrophages into the lipid pool and focal loss of proteoglycans and/or collagen matrix. The accumulation of free cholesterol in early fibroatheromas is not extensive, while the late fibroatheroma consists of discrete collections of acellular debris, increased free cholesterol, and near complete depletion of extracellular matrix.
Thin-cap fibroatheroma (TCFA), conceptually referred to as vulnerable plaque, consists of a large necrotic core harbored by a thin (<65 μm) fibrous cap which is heavily infiltrated by macrophages and to a lesser extent T-lymphocytes with a paucity or absence of SMCs.8 This well-characterized ominous lesion is considered to be the prelude of plaque rupture. The only difference between plaque rupture and thin cap fibroatheroma is a disrupted thin fibrous cap infiltrated by macrophages and T-cells and a larger necrotic core.
In contrast to plaque ruptures, erosions frequently occur in moderately diseased arteries and the fibrous cap remains intact. In erosion lesions the luminal surface underneath the thrombus lacks endothelium and is rich in proteoglycans and SMCs.9 The underlying plaque is composed either of PIT or fibroatheromas without an extensive necrotic core, hemorrhage, or calcification. The etiology of plaque erosions remains unknown; however, we believe it may be a consequence of vasospasm.
Healed thrombi include those occurring from healed plaque rupture and erosion.10 The provisional matrix at the site of healing typically consists of an organizing platelet-fibrin-rich thrombus that is infiltrated by SMCs and granulation tissue with accumulated proteoglycans and type III collagen, which eventually converts into a type I rich collagen-scar. Healed ruptures may be single or consecutive events resulting in multiple layers of necrotic cores interspersed by fibrous tissue. With respect to the sequence of ruptures, the earliest tear can be found in deeper intima suggestive of previous thrombotic events, which sequentially leads to increased plaque burden and luminal narrowing.10
Progression of Calcification in the Course of Atherosclerosis (Figure 1 and 2)
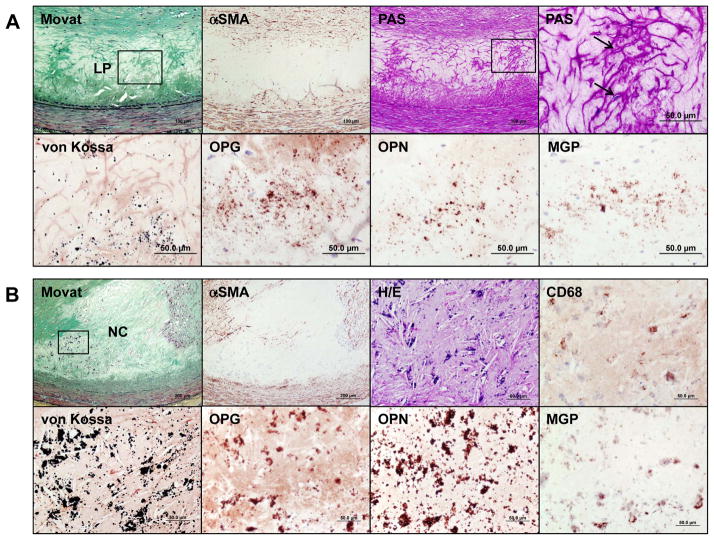
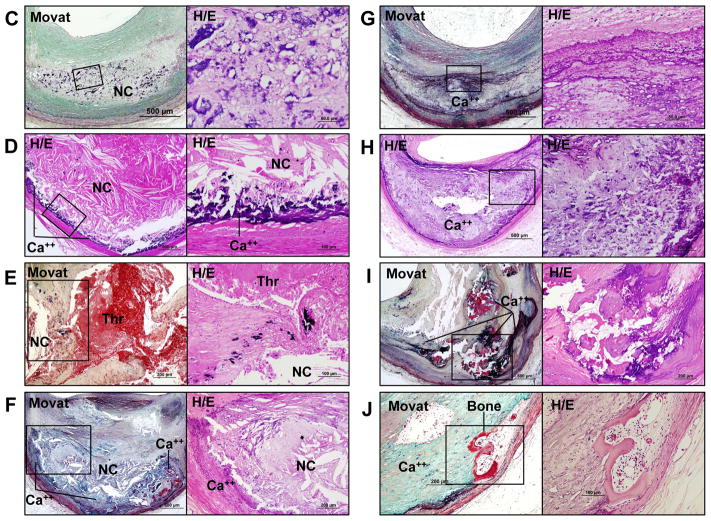
Figure 1.
Progression of coronary calcification. Non-decalcified arterial segments (A and B) and decalcified segments (C to J) were serially cut for the microscopic assessment. A shows pathologic intimal thickening (PIT) characterized by lipid pool (LP) that lacks smooth muscle cells (SMCs) (negative for α-smooth muscle actin [α-SMA]) and shows the presence of apoptotic SMCs which can be identified by prominent basement membrane which stains positive with periodic acid Schiff (PAS) and the arrows point to in the high-power image (top right corner). Early microcalcification (≥0.5 μm, typically <15 μm in diameter) likely results from SMC apoptosis and calcification is detected by von Kossa staining within the LP (corresponding with a boxed area in the Movat image) where bone related proteins such as osteoprotegerin (OPG), osteopontin (OPN), and matrix Gla protein (MGP) are detected. Early necrotic core (NC) (B) not only lacks SMCs but is infiltrated by macrophages which eventually undergo apoptosis and calcification, which is observed as punctate (≥15 μm) areas of calcification. The microcalcification in early NC show variable amounts of staining for macrophage CD68 antigen; however, von Kossa staining clearly shows relatively larger punctate areas of calcification resulting from macrophage cell death within the NC as compared to microcalcification of dying SMCs. These calcified macrophages show co-localization of bone related proteins. Substantial amount of macrophage calcification can be observed in early NC (C) but the degree of calcification in NC typically increases towards the medial wall where fragmented calcifications can be seen (D). Microcalcification resulting from macrophage or SMC deaths can also be detected within a thin-fibrous cap and may be associated with plaque rupture (E). Calcification generally progress into the surrounding area of the NC (F), which leads to the development of sheets of calcification where both collagen matrix (G) and necrotic core itself are calcified (H). Nodular calcification may occur within the plaque in the absence of luminal thrombus and is characterized by breaks in calcified plates with fragments of calcium separated by fibrin (I). Ossification may occur at the edge of an area of calcification especially in nodular calcification (J). Immunohistochemical stainings in A and B were performed with the use of antibodies for CD68 (dilution 1:800; Dako, Carpinteria, CA), α-SMA (dilution 1:400, Dako), OPG (dilution 1:50, Novus Biologicals, LLC, Littleton, CO), OPN (dilution 1:400, generously provided by Larry W. Fisher, PhD, National Institute of Health, Bethesda, MD), and MGP (dilution 1:200, Enzo Life Science, Farmingdale, NY), respectively.
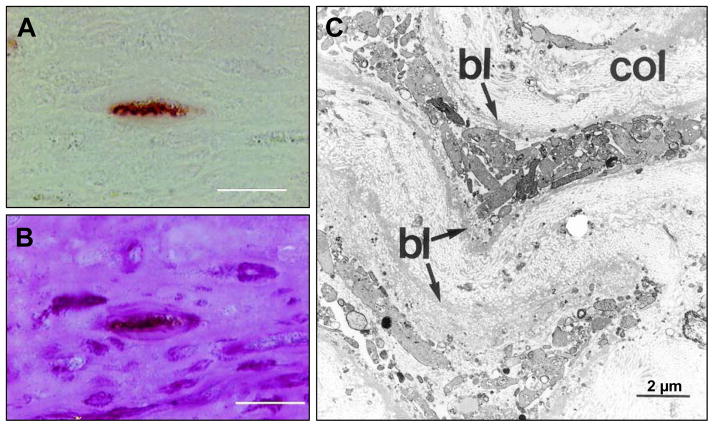
Figure 2.
Advanced human atherosclerotic plaque showing apoptotic smooth muscle cells (SMCs). A is stained by the TUNEL technique and B is the same TUNEL-labeled section subsequently stained by PAS (periodic acid-Schiff) stain (TUNEL+PAS technique). A labeled nucleus is present in a cell–poor area in the fibrous cap (A), and this TUNEL-positive nucleus belongs to a cell that is surrounded by a prominent cage of PAS-positive basal laminae (B). This points to an SMC undergoing apoptotic cell death. Most of the SMCs in this region do not express α-SMC actin. Adjacent to this cell are PAS-positive empty cages of thickened basal lamina. C is transmission electron microscopy (TEM) image of an advanced human atherosclerotic plaque. Two SMCs are demonstrated that are completely disintegrated into myriad vesicles (granulovesicular degeneration). The prominent basal laminae (bl) around these clusters of vesicles led us to conclude that the vesicles are of SMC and not of macrophage origin. (Reproduced with permission from Kockx MM, et al. Circulation. 1998;97:2307–2315.)
The progression of calcification may begin with microcalcification (≥0.5 μm, typically <15 μm),11–13 which is seen following special stains for calcification, e.g., von Kossa stain, Alizarin red, etc. (Figure 1A). Early calcification is apparent within the lipid pools as stippling by calcium stains which likely originates from SMC apoptosis or matrix vesicles and is generally 1 μm or greater in size that can be appreciated by light microscopy.11, 13, 14 Electron microscopic examination of vascular wall has revealed that the initial calcification occurs in matrix vesicles which vary in size from 100 to 700 nm in diameter.15 The loss of intimal SMCs has also been identified by the presence of prominent basement membrane which stains strongly with periodic acid-Schiff (PAS)11 and contains central areas of apoptotic bodies derived from SMCs (Figure 1A and 2). Calcification is typically located in the intima close to the internal elastic lamina.
Microcalcification may also occur from macrophage releasing matrix vesicles or from apoptosis.16 Macrophage apoptosis may result in a different morphologic appearance of calcification. While SMC apoptosis results in fine microcalcification, macrophage apoptosis exhibits large punctate, blocky appearance (Figure 1B, 1C). Microcalcification is frequently observed in TCFAs, despite the fact that its contribution to plaque vulnerability remains to be determined. These microcalcifications often coalesce into larger masses and involve both the necrotic core and the surrounding collagen-rich extracellular matrix to form speckled and fragments of calcification. This particular pattern also starts in the deeper region of the necrotic core close to the internal elastic lamina (Figure 1D and 1E). Further progression of calcification is seen as extension from the outer rim of the necrotic core (Figure 1F) into the surrounding collagenous matrix. Importantly, either the central core becomes fully calcified or may remain non-calcified at this stage. Nevertheless further progression of disease results in calcified plaque that forms calcified sheets or plates (Figure 1G and 1H). Calcified plates may fracture which results in the formation of nodular calcification that is accompanied by fibrin deposition (Figure 1I). These nodules may protrude into the lumen or into the media, when the former occurs it results in luminal protrusion and there is discontinuity of the overlying collagen and endothelium with acute thrombosis. The latter calcified nodules are rare occurring in 2 to 7% of cases with thrombosis but are more frequent (4 –14%) in the carotid plaques.17
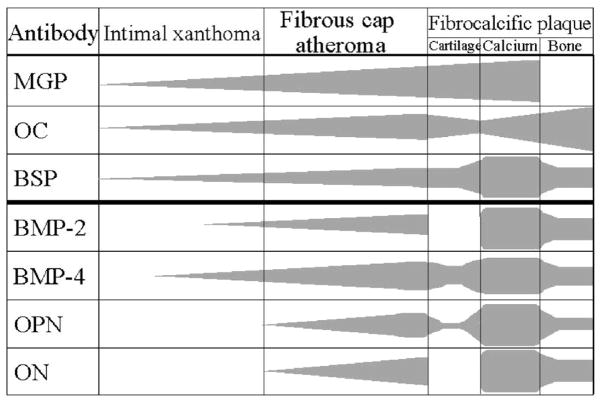
Bone formation can rarely be observed in areas of arterial calcification (Figure 1J), particularly in heavily calcified segments, which indicates osteogenesis induced at sites of arterial calcification. The incidence of bone formation has been reported to be 19% in peripheral vascular disease in patient who underwent lower limb amputation.18 However, to our knowledge the frequency of bone formation in coronary arteries has not been reported, but is uncommon. Several lines of evidence have suggested that arterial calcification shares features with skeletal bone formation and calcification, such as chondrocyte and osteoblast differentiation, mineralization, bone matrix deposition, and bone resorption. Bone related proteins including bone morphogenic protein-2 and -4 (BMP-2, BMP-4), bone sialoprotein (BSP), osteocalcin (OC), osteonectin (ON), osteiopontin (OPN), and osteoprotegerin (OPG) have been reported to be present in calcified arteries.19, 20 Dhore et al.19 evaluated immunoreactivity pattern of bone matrix proteins in human non-diseased aorta, intimal xanthoma, fibroatheroma, and fibrocalcific plaques. They found that matrix Gla protein (MGP) and BSP were highly expressed in the intima and media of non-diseased aortas including endothelial cells, SMCs and elastic fibers. BMP-4, ON, and OPN were highly expressed only in the medial SMCs. On the other hand, in advanced lesions, BSP, BMP-2, BMP-4, ON, and OPG were expressed in intimal SMCs located at the shoulder region of fibroatheroma, whereas MGP, BSP, OC, BMP-4, OPN, and ON were expressed in foam cells and in the lipid core. In lesions with fibrocalcific plaques, which are rich in collagen, with large areas of calcification and necrotic core and in those with a bone structure showing cartilage tissue, calcification and bone tissue; stains for BMP-2, BMP-4, OPN and ON were positive (Figure 3).19 Immunohistochemical studies in early coronary plaques from SCD victims showed positive staining for OPG, OPN, and MGP at the sites of microcalcification (Figure 1A, 1B). Roijers et al.20 have shown intima areas with abundant microcalcification expressing OC and BMP-2 in coronary arteries. In contrast, uncarboxylated MGP expression is negative in the AIT but is increased in fibroatheromas, whereas carboxylated MGP is highly expressed in PIT and fibroatheromas.20 Although the above proteins are present in early plaques, the mechanism of calcification/osteogensis in the arterial wall remains to be determined.
Figure 3.
Immunoreactivity pattern of bone matrix proteins in human nondiseased aorta, intimal xanthoma, fibrous cap atheroma, and fibrocalcific plaques. The table represents the immuno-histochemical pattern of the bone matrix regulatory proteins MGP, OC, BSP, BMP-2, BMP-4, OPN, and ON in human atherogenesis. Fibrocalcific plaques were divided into cartilage tissue, calcium deposits, and bone tissue, structures that were present in these lesions. MGP, OC, and BSP were present in early as well as advanced lesions, whereas BMP-2, BMP-4, OPN, and ON were only present in advanced plaques. (Reproduced with permission from Dhore CR, et al. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003.)
Calcification is common in all atherosclerotic lesions independent of location, i.e., peripheral arteries- superficial femoral, deep femoral, peroneal, popliteal, anterior and posterior tibial, dorsal pedis, and the carotid arteries. The peripheral arteries not only show calcification of intimal atherosclerosis but also medial Mönckeberg’s calcification. The peripheral artery atherosclerosis shows greater collagen deposition and calcification than other arteries like the coronary and carotid. On the other hand, the internal mammary artery is resistant to the development of atherosclerosis and calcification but the reasons are poorly understood. Carotid artery calcification has been recently studied and is reported to be present in 50% of asymptomatic individuals from the MESA study at a mean age of 57.9 years (63% women).21 Howard et al.22 have shown in symptomatic patients with carotid disease who manifest with cerebral symptoms versus those with ocular symptoms have less calcification but larger necrotic core. Similarly, most patients with asymptomatic carotid stenosis undergoing surgical carotid endarterectomy have higher incidence of calcification, 68% versus symptomatic individuals 49%.23
Calcification and Plaque Stability (Figure 4)
Figure 4.
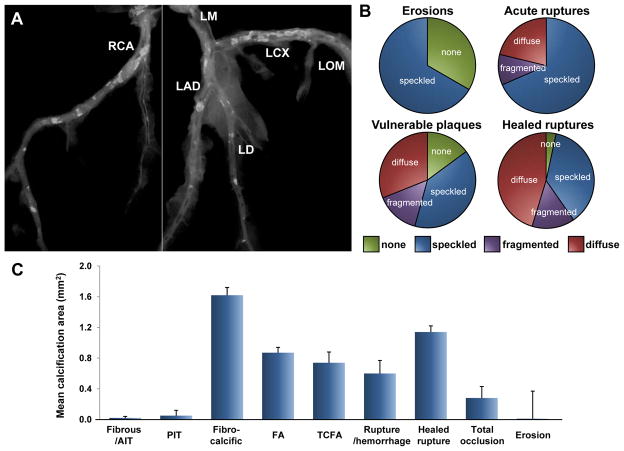
Coronary calcification and plaque morphology in humans. A is a radiograph of the coronary arteries following removal from the heart. B shows the type of radiographic calcification in different plaques. Radiographic calcification was typed according to the classification of Friedrich et al (Friedrich GJ, et al. Am Heart J 1994;128:435–41) and in brief absence of calcification, speckled, and fragmented (linear or wide, single focus of calcium >2 mm in diameter), or diffuse (≥5mm segment of continuous calcium). Bar graph in C shows mean area of calcification in different plaque morphologies in sudden coronary death victims. T-bars indicate SEM. AIT=adaptive intimal thickening; FA=fibroatheroma; LAD=left anterior descending artery; LCX=left circumflex artery; LD=left diagonal artery; LM=left main coronary artery; LOM=left obtuse marginal branch; PIT=pathologic intimal thickening; RCA=right coronary artery; TCFA=thin-cap fibroatheroma. (B and C are reproduced with permission from Burke AP, et al. Herz. 2001;26:239–244.)
It remains unclear whether coronary calcification predicts plaque instability or is merely a marker of plaque burden. Serial intravascular ultrasound (IVUS) studies have revealed that heavily calcified plaques are more resistant to change in atheroma volume as compared to less calcified plaques; whereas the presence of spotty calcification as compared to its absence is associated with greater progression of atheroma volume in patients with stable coronary artery disease.24, 25 Biomechanical models utilizing coronary plaques from human autopsy cases have suggested that calcification within the ruptured or stable plaque does not increase fibrous cap stress, while greater lipid area is associated with increased vessel wall stress.26 Autopsy studies have shown a weak but inverse correlation between calcification and macrophage areas in coronary plaques from SCD victims.27 Moreover, clinical imaging studies in patients with acute coronary syndromes as well as autopsy observations in SCD victims have demonstrated less calcification in ruptured or vulnerable plaques as compared to stable plaques (Figure 4).28, 29 These findings support the notion that calcium generally confers stability to plaques rather than the opposite. Nevertheless, as it stands, location of the calcification seems to be paramount. Vengrenyuk et al.12 have reported that microcalcification within a thin-fibrous cap (typically 10 μm in diameter) facilitates plaque rupture through local increase in stress that leads to interfacial debonding (Figure 1E). However, their more recent manuscript shows microcalcification >5 μm in diameter may be harmful and can predict plaque rupture while microcalcification <5 μm in diameter is seemingly less harmful.13
We have previously reported that maximum calcification is seen in healed plaque ruptures, followed by fibroatheroma, thin cap fibroatheroma, plaque rupture, with least calcification seen in plaque erosion and PIT.27 The degree of calcification by plaque type in SCD victims is shown in Figure 4. The mean calcified area is maximum in fibrocalcific lesion and least in AIT/fibrous plaques or erosion. Total occlusions display variable degrees of calcification, and the dimension of calcification depends on the underlying plaque morphology as well as the length of the thrombus.
Mauriello et al.30 have recently shown that calcification is maximum in individuals dying from acute myocardial infarction relative to control patients dying from non-cardiac causes but with at least one coronary artery showing >50% stenosis. However, the coronary calcification did not correlate with presence of unstable characteristics, thus suggesting that coronary calcification is a predictor of generic risk of acute events but is not useful for identifying vulnerable lesions. We have shown that age at presentation may be an important predictor of calcification among unstable and stable plaques. There seems to be an inverse relationship between calcification, plaque stability and age.31 While patients presenting in the 4th decade with ruptured/TCFA lesions (unstable plaques) showed greater calcification than those exhibiting stable plaques, no differences were observed in the fifties and sixties. In contrast, significantly greater calcification was observed in the seventies in stable than in unstable plaques.29
Results from Computed Tomography (CT)
Although the use of computed tomography (CT) for the detection of future cardiovascular events has engendered a fair amount of controversy in the past, the level of evidence has tremendously increased and has been given a level IIa recommendation for risk stratifying individuals at intermediate risk (10–20%, 10-year risk).32 Although the risk of future coronary event is higher in asymptomatic individual with a high absolute calcium score, it is the risk for an individual patient that is best predicted by comparing calcium score percentiles; patients with a calcium score >75th percentile had 19 times the risk of suffering a hard coronary event as compared to those with a score of <25th percentile, while the risk of events in patients in the upper risk factor quartile was 6.5 times greater than that of patients in the lowest quartile.33 Recent studies have demonstrated that individuals with low LDL cholesterol and no reported risk factors but with any coronary artery calcium present have a greater likelyhood of coronary heart disease as compared to those without any calcification over a median follow-up of 5.4 years.34 Similarly absence of coronary calcification imparts a very low risk of future coronary events.35
According to the literature, CT can only identify calcification areas of 1.03 to 1.37 mm2 in size i.e., 3 to 4 contiguous pixels within a plaque area of 5 mm2.36 Therefore, microcalcification seen in early plaques (PIT and early fibroatheroma) will not be identified by CT, this includes calcification of both SMCs as well as macrophages. Only once aggregation of calcium occurs in fibroatheromas which is almost always observed near the intimal medial border involving both large areas of adjoining necrotic core and/or collagen that CT can identify these lesions.
Determinants of Calcification - Gender and Race Differences (Figure 5)
Figure 5.
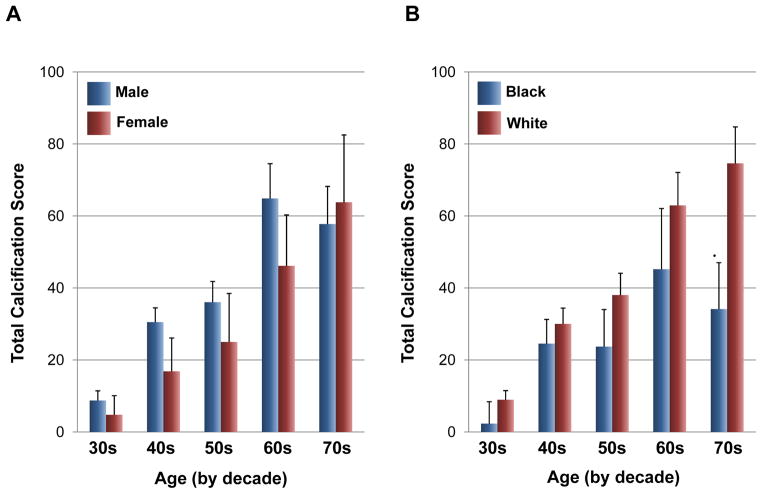
Bar graphs showing total calcification score in sudden coronary death (SCD) victims stratified by decade in male and female (A) as well as in black and white (B). Calcification was scored based on one point per mm of epicardial artery involved by calcification by visual inspection of radiographs (density was not assessed). (A is reproduced with permission from Burke AP, et al. Z Kardiol 2000;89:Suppl 2,II/49-II/53. B is reproduced from Burke AP, et al. Circulation 2002;106:II-481)
Gender differences in calcification have been appreciated for a long time. The incidence of coronary artery disease in women is delayed by 10 to 15 years as compared to men, likely due to the protective effects of estrogen.37 The effect of estrogen therapy on coronary calcification was studied in women 50 to 59 years over a seven-year period of treatment. Calcification burden was significantly less in women assigned to estrogen substitution as compared to those receiving placebo.38 However, it must be stated that a prior large study “The women’s Health Initiative (WHI) trial” of conjugated equine estrogen given in post-menopausal women who had undergone hysterectomy reported a hazard ratio of 0.95 (confidence interval 0.79 to 1.16) for nonfatal myocardial infarction plus fatal coronary heart disease in those receiving estrogen versus placebo. Nevertheless secondary analysis by age group suggested that the results were different in younger versus older women. In our registry of 108 human hearts (70 men, mean age 50.4±12.2 years, and 38 women, mean age 49.6±12.2 years) who died of SCD, the extent of coronary calcification was assessed on a semi-quantitative scale (grade 0 = no calcification; grade 1 = calcification <40 μm in diameter; grade 2 = >40 μm in diameter involving only one quadrant; grade 3 = in 2 arterial quadrants; grade 4 = in 3 arterial quadrants; grade 5 = involving the entire arterial circumference).39 When stratified by decades, the extent of calcification was greater in men as compared to women up to the sixties, whereas in the seventies, the prevalence was similar, suggesting that coronary calcification develops rapidly during the postmenopausal period (Figure 5A). Our laboratory previously reported that the degree of calcification in postmenopausal women is three times higher than in premenopausal women assessed in men and women dying suddenly.40
It has also been reported that race affects the degree of calcification. In the Multi-Ethnic Study of Atherosclerosis (MESA) in asymptomatic individual 45 to 84 years in age after adjusting for various clinical parameters, the relative risk for calcification was 0.78 (95% CI 0.74 to 0.82) in blacks, 0.85 (95% CI 0.79 to 0.91) in Hispanics, and 0.92 (95% CI 0.85 to 0.99) in Chinese relative to the white race.41 Our autopsy assessment in SCD victims showed that the total coronary calcification scores when stratified by decades was consistently greater in whites as compared to blacks in all decades (Figure 5B).42 This finding is in line with previous clinical observations showing that the prevalence and extent of calcification was substantially greater in whites as compared to blacks when assessed by CT.43 Although the mechanisms responsible for the racial difference in coronary calcification are not fully understood, several possible explanations have been proposed. Bone mineral density is less in whites than in blacks44 and bone density is inversely related to aortic calcification.45 There may be inherent genetic predisposition for coronary calcification in blacks and whites, which remains to be determined. Huang et al.46 showed that race-related genes, such as GAB2, which is expressed at a lower level in blacks and also in those with low calcification may be another explanation for the lower prevalence of calcification in blacks. Furthermore, it has been reported that soluble epoxide hydroxylase gene polymorphism is associated with coronary calcification in blacks, but not in whites.47 Although multiple variations of gene expression are likely to be involved,46 the precise genetic impact on calcification remains to be elucidated.
Calcification, Vascular Remodeling and Luminal Stenosis (Figure 6 and 7)
Figure 6.
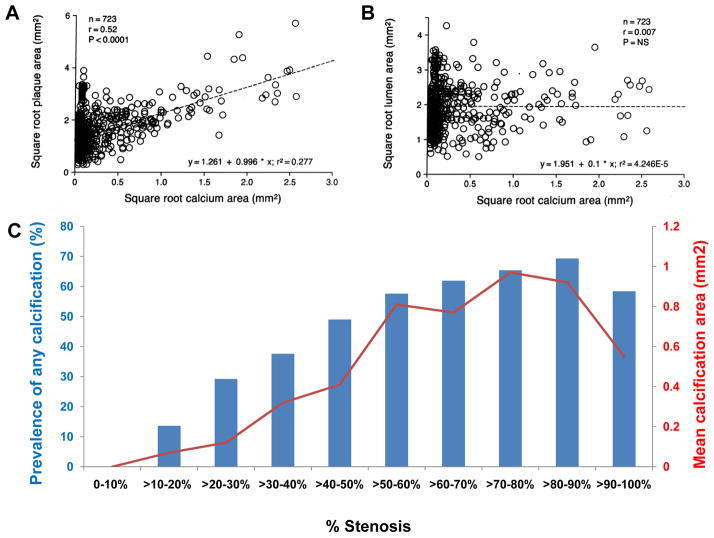
A shows correlation between square root of coronary calcium area values (mm2) detected by histopathologic and microradiographic analysis and square root of plaque area values (mm2) for each of the 723 coronary artery segments in humans. B shows square root of coronary calcium area (mm2) detected by histopathologic and microradiographic analysis versus square root of lumen area (mm2) for each of the 723 coronary artery segments where no relation was identified. C shows relationship between percent stenosis and the degree of calcification in sudden coronary death victims. Each blue bar represents prevalence of any calcification (%), whereas each red dot represents mean calcification area (mm2). (A and B are reproduced with permission from Sangiorgi G, et al. J Am Coll Cardiol. 1998;31:126–133. Data in C is stratified by decades from Burke AP, et al. Herz. 2001;26:239–244)
Figure 7.
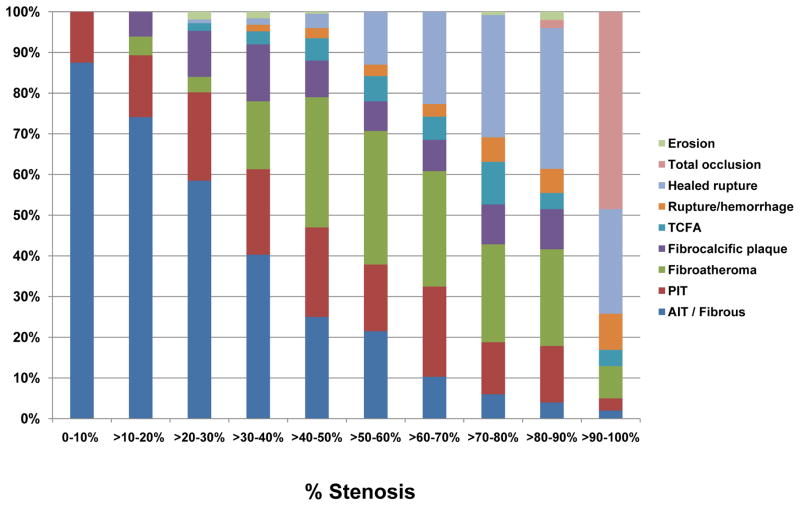
Prevalence of various coronary plaque morphologies at 10% incremental cross-sectional area narrowing in sudden coronary death victims. Abbreviations as in Figure 4. (Data presented as each 10% increase in narrowing from Burke AP, et al. Herz. 2001;26:239–244)
Sangiorgi et al.48 demonstrated that arterial calcification correlated with plaque burden, but had only a weak correlation with lumen narrowing (Figure 6A and 6B). Since coronary calcification has been shown to be a predictor of future events, it is not surprising that there is a good correlation between calcification and plaque area. The poor correlation between calcification and lumen area needs to be interpreted with caution as effective lumen area represents multifactorial processes. Conventionally, lumen area decreases as plaque burden increases beyond 40% stenosis introducing a negative correlation among these parameters. A deviation from this notion is expected when there is compensatory enlargement of the vessel, termed positive remodeling. This relationship was clearly demonstrated by Glagov et al.49 who showed that lumen area does not decrease until the lesion occupies ≥40 percent of the internal elastic lamina area. Compensatory enlargement may be the main explanation why there is no correlation between calcification and lumen area in early plaque development when calcification is progressing. The poor correlation between calcification area and lumen area can also be partially explained on the basis of plaque morphology with highly fibrotic plaques being associated with negative remodeling.50 Luminal narrowing may only occur in advanced atherosclerotic lesions after the vascular capacity of compensatory enlargement has exhausted. We investigated the association between arterial remodeling and plaque components and have shown that arterial expansion was strongly correlated with calcification, macrophage infiltration, and lipid core.50
Another intriguing finding is that there is an excellent correlation between percent stenosis and the percentage of calcification (%) as well as mean calcification area (mm2) (Figure 6C).27, 50 The positive correlation between percent stenosis and the degree of calcification may be partly explained by the relationship between plaque morphology and percent stenosis.27, 50 When percent stenosis is stratified by decades, the prevalence of complex lesions and calcification increase incrementally. In other words, complex lesions, especially healed plaque ruptures are frequently observed in higher-grade stenosis, and are likely to be accompanied by greater calcification (Figure 7).39
The Location of Coronary Calcification
Our data indicate that calcified area is greater at proximal than in distal locations in the three main epicardial arteries (LAD, LCX, RCA) in patients presenting with CAD at a mean age of 64±14 years.50 This has also been reported by Sangiorgi et al.,48 in 13 patients with only 2 having died of CAD. A recent longitudinal IVUS study showed that plaque burden was greatest in proximal and least in distal coronary arteries.51 However, calcification was similar in proximal and middle and least in the distal arteries in individuals presenting with acute coronary syndromes with a median age of 58 years.51 This observation is in keeping with the previous notion that plaque burden correlates with calcification as reported by histology, angiography, IVUS and now by cardiac CT.36, 52
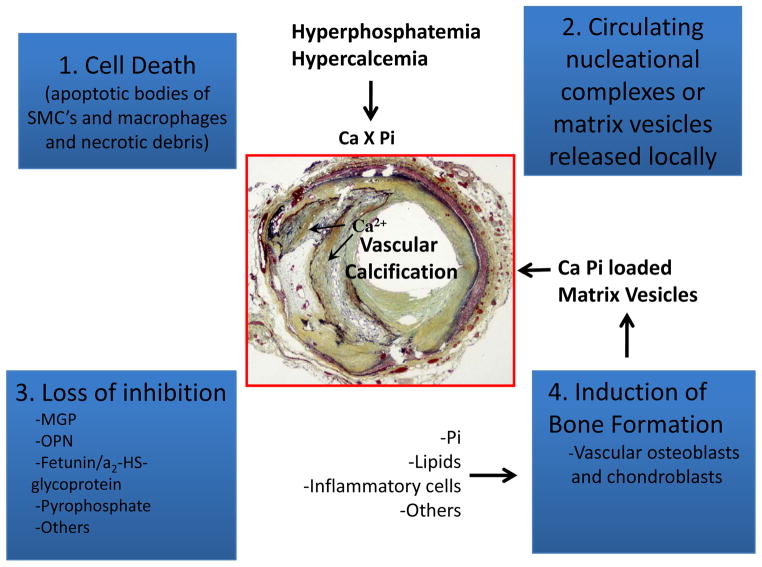
Mechanisms of Atherosclerotic Calcification (Figure 8)
Figure 8.
Schematic illustrating four, non–mutually exclusive theories for vascular calcification: (1), cell death leading to release of apoptotic bodies and/or necrotic debris that may serve to nucleate apatite at sites of injury; (2), circulating nucleational complexes released from actively remodeling bone or matrix vesicular released locally; (3) loss of inhibition as a result of deficiency of constitutively expressed tissue-derived and circulating mineralization inhibitors leads to default apatite deposition; and (4) induction of bone formation resulting from altered differentiation of vascular smooth muscle or stem cells. (Reproduced and modified with permission from Speer MY, et al. Cardiovasc Pathol 2004;13: 63–70.)
As stated above, atherosclerotic intimal calcification is different from medial Mönckeberg’s calcification and the latter occurs independently from intimal calcification. Most importantly medial calcification is not associated with lipid deposition or inflammation. Medial calcification is first appreciated within the elastic lamellae, both within the media and in the internal elastic lamina, followed by medial SMC. It is accelerated in the presence of diabetes and chronic renal failure. The SMCs lose their contractile phenotype (α-actin and SM22-α) and gain osteochondrogenic markers such as osteopontin, Runt-related transcription factor 2 (RUNX2) also known as core-binding factor subunit alpha-1 (CBF-alpha-1) (RUNX2/Cbfa1), alkaline phosphatase, and osteocalcin. It has also been shown that MGP-null mice undergo spontaneous arterial medial calcification, pointing towards the relevance of MGP in medial calcification.53, 54
The molecular mechanisms are different between intimal and medial calcification, and vascular intimal calcification is now considered an active process and that many non-mutually exclusive mechanisms exist.55, 56 There is little consensus which mechanisms of atherosclerotic intimal calcification apply best to man. There appear to be different schools of thought raising four non-mutually exclusive concepts (Figure 8), with those who foster the idea that SMCs acquire an osteogenic profile and calcification occurs akin to bone formation. It is believed that extracellular vesicles (matrix vesicles) calcify when calcium phosphates appear inside these matrix vesicles, forming hydroxyapatite crystals, akin to bone formation.57 Apoptotic cell death generated form SMCs and macrophages calcify in the extracellular environment and that nucleate hydroxyapatite mineral crystallization occurs.58 Within this group, there are those of us who believe in the concept that chronic low levels of SMC and macrophage apoptosis is the main driver of calcification in man. Another concept is related to the loss of inhibitors of calcification molecules which are normally expressed in the vessel wall such as MGP, OPN, fetuin, pyrophosphates, and others that may lead to spontaneous calcification. Fetuin, a circulating glycoprotein, is a major inhibitor of apatite found in circulation, and decrease in fetuin levels results in augmented vascular calcification and higher cardiovascular mortality in hemodialysis patients. Also, the presence of bone proteins in the vessel wall such as OPN, OC, and BMP-2, and the presence of bone and cartilage in vessel walls suggest osteogenic origin. Indeed, it has been shown in culture studies that SMCs phenotypically change to cartilage and bone and calcify under various conditions, and this view of calcification is being presented by Demer et al. in this editorial series on calcification. The cell death view along with other investigators like Bennett,58 Kockx,11 and Proudfoot59 are more in keeping with our observations in man.
Roijers et al.20 reported that microcalcification was occasionally observed in AIT and that there was abundant increase in microcalcification in AHA type II, III and IV lesions, i.e., fatty streak, PIT and fibroatheroma. They also showed that microcalcification preceded the presence of calcifying proteins like OC and BMP-2. However, when these proteins were absent, other proteins such as uncarboxylated MGP were observed, which is released as biologically inactive precursor and thought to play an important role in the inhibition of calcification. Owing to a delay to active form, i.e., its carboxylation remains blocked during the course of atherosclerosis and likely results in early calcification. Clarke et al.60 have shown in in vitro studies that vascular SMC muscle cell apoptosis accelerates calcification. Vascular SMC apoptosis physiologically occurs without consequences; however, failure to clear apoptotic cell bodies by phagocytes results in release of proinflammatory cytokines.61
Lutgens et al.62 have shown that low level of apoptosis is mostly seen in lesions of fibroatheroma, with or without surface defects (AHA type IV, V, and VI lesions). In terms of localization, apoptosis was most predominant in the lipid core in type IV lesions and equally divided between lipid core and fibrous cap in type V lesions.62 Interestingly, in ruptured plaques it was equally divided between lipid core, fibrous cap and the area underlying the rupture. Kockx et al.11 have shown that SMC apoptosis is rare in fatty streak lesions. If present, it mostly occurs in SMCs which are enclosed by a cage of thickened basement membrane, frequently within the deeper layers of the plaque (Figure 2). In keeping with this, the necrotic core of advanced atherosclerotic plaques is largely acellular indicating cell death which must have occurred earlier or enlargement of the necrotic core may be secondary to plaque hemorrhage.63 Surrounding the necrotic core, especially towards the luminal surface, there is a dense infiltration of macrophages and these also show changes of apoptosis.64 A significantly greater number of apoptotic bodies have been identified within the necrotic core as compared to other parts of the plaque.65 Low level apoptosis has been shown by Clarke et al.58 to accelerate calcification in SM22α-hDTR ApoE−/− mice promoting calcification in early and in late vulnerable plaques. It is not only apoptosis that is necessary but the calcification must occur at these very sites of necrosis. Proudfoot et al.59 showed in a culture model that SMC nodules calcify at sites of apoptosis and that this can be prevented by inhibiting apoptosis. Similarly, others have shown that calcification is enhanced after induction of hyperphosphatemia or addition of TGF-β1 in interstitial aortic valve cells.66, 67 It is clear that calcium and phosphate ions in biologic fluids are tightly regulated and any abnormality as in chronic renal failure results in higher rates of calcification in soft tissues.
Although our understanding of atherosclerosis has progressed at the molecular level through the introduction of genetically engineered mouse models in the last few decades, there remain many unanswered questions, especially regarding the origin, mechanism and purpose of calcification in human atherosclerosis. There has been tremendous progress in research related to the mechanisms of bone-formation, which strongly supports the school of thought that vascular calcification may be akin to bone formation. Also, it has been shown in the mouse model that vascular SMC undergo osteochondrogenesis and contribute to atherosclerotic intimal calcification.68 However, looking at this topic from a more observational point of view, vascular calcification is largely observed in areas of cell death. Data showing viable SMCs that do not calcify has been shown in culture studies. Reynolds et al., have shown that the matrix around the SMCs calcifies, the cells remained viable, and once the calcified matrix is removed, they proliferate, thus showing viability of the SMCs.69, 70 In addition, true lacunar bone formation including osteoblasts and osteoclasts in coronary atherosclerosis remains exceptional to our knowledge. Also, presence of cartilage has only rarely been reported71 in peripheral arteries while reports inferring the existence of cartilaginous metaplasia in coronary lesions are lacking to date. From a pathologic standpoint, vascular calcification seems to constitute a destructive event in advanced atherosclerotic lesions. Statin treatment has been shown to at least halt disease progression with respect to cholesterol accumulation in the vessel wall. Surprisingly, statin treatment has been reported to even increase calcification of atherosclerotic lesions thus suggesting that calcification may be a marker of stable, non-progressive atherosclerosis process.72 It could also be that location of calcification is the most important and that small calcifications in the thin fibrous cap may contribute to plaque rupture. It is clear that large calcification located near the intimal-medical border does not impart instability to a plaque but may even be harbingers of stability. Novel imaging modalities, both invasive and non-invasive, will likely result in better understanding of calcification dynamics in the future.
Conclusions
Despite the undoubted association between coronary atherosclerotic calcification and prediction of future cardiovascular events, there is only weak correlation between calcification-induced coronary plaque progression and luminal narrowing. Coronary calcification is reported to occur sporadically in AIT/fibrous lesions and is almost uniformly seen in PIT with or without macrophages. In PIT lesions, calcification can be observed microscopically as microcalcification which is usually 0.5 to 15 μm in size and enlarging to ≥15 μm in early fibroatheromas, when macrophages infiltrate the necrotic core and undergo apoptosis. Confluent calcified areas begin to appear in fibroatheromas and occasionally in PIT and fibrous lesions. Calcified areas are mostly located around the necrotic core close to the media. In the course of atherosclerosis progression, calcified areas enlarge to form calcified sheets, which are hallmarks of stable plaques and fibrocalcific lesions. The sheets may break and lead to nodular calcification, which is often observed as small rounded calcified fragments separated by fibrin with luminal protrusion and an overlying thrombus. Calcification is greater in men than women especially in the premenopausal period. African Americans have less calcification than Caucasians. Maximum calcification is observed in fibrocalcific plaques followed by healed plaque rupture and fibroatheroma, whereas ruptured plaques and TCFA have much less calcification. Stable plaques overall show greater calcification than unstable plaques. The mechanisms of calcification are beginning to be better understood through the development of animal models and molecular modifications.
Mainly three types of calcification have been described in the vasculature and the most prevalent being atherosclerotic, followed by medial calcification and least frequent is arterial calcification of infancy. Atherosclerotic intimal calcification has been liked to bone formation with matrix vesicle release but other factors may also play a role such as loss of calcification-inhibiting pathways and SMC and macrophage apoptotic cell death. Factors that govern calcification include alterations in calcium and phosphate balance, especially in patients with renal failure, diabetes mellitus, and lipid oxidation products. In the future, strategies to control or remove calcification may enable us to reduce the burden of atherosclerotic disease.
Significance.
Intimal atherosclerotic calcification is the most common form of calcification; begins early and is first appreciated in pathologic intimal thickening when it can only be identified by special stains. It progresses from microscopic specking to punctate lesion accompanied by apoptosis of smooth muscle cells and macrophages and/or release of matrix vesicles. These microcalcified areas coalesce with eventual formation of fragments of calcification seen in fibroatheromas. The fragmented calcified areas are located close to the medial wall and extend to involve the necrotic core and the adjacent collagen eventually forming calcified sheets. Calcification is significantly greater in men than women and correlates with plaque area but not with lumen area. It is only at this stage when calcified fragments reaches a threshold of 1.03 to 1.37 mm2 in size that computed tomography (CT) can identify calcification. CT imaging techniques have shown that individuals with a high calcium score have a higher risk of developing future cardiovascular events.
Acknowledgments
Sources of Funding
CVPath Institute Inc., Gaithersburg, Maryland, USA provided full support for this work. Dr. Otsuka is supported by a research fellowship from the Uehara Memorial Foundation, Tokyo, Japan. Dr. Sakakura is supported by a research fellowship from Banyu Life Science Foundation International.
Footnotes
Disclosures
None.
References
- 1.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 2.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 4.Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. doi: 10.1161/01.atv.20.5.1262. [DOI] [PubMed] [Google Scholar]
- 5.Velican C. A dissecting view on the role of the fatty streak in the pathogenesis of human atherosclerosis: culprit or bystander? Med Interne. 1981;19:321–337. [PubMed] [Google Scholar]
- 6.Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, Rosenfeld ME, Schaffer SA, Schwartz CJ, Wagner WD, Wissler RW. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1994;89:2462–2478. doi: 10.1161/01.cir.89.5.2462. [DOI] [PubMed] [Google Scholar]
- 7.Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 8.Kolodgie FD, Burke AP, Farb A, Gold HK, Yuan J, Narula J, Finn AV, Virmani R. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol. 2001;16:285–292. doi: 10.1097/00001573-200109000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Kolodgie FD, Burke AP, Farb A, Weber DK, Kutys R, Wight TN, Virmani R. Differential accumulation of proteoglycans and hyaluronan in culprit lesions: insights into plaque erosion. Arterioscler Thromb Vasc Biol. 2002;22:1642–1648. doi: 10.1161/01.atv.0000034021.92658.4c. [DOI] [PubMed] [Google Scholar]
- 10.Burke AP, Kolodgie FD, Farb A, Weber DK, Malcom GT, Smialek J, Virmani R. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934–940. doi: 10.1161/01.cir.103.7.934. [DOI] [PubMed] [Google Scholar]
- 11.Kockx MM, De Meyer GR, Muhring J, Jacob W, Bult H, Herman AG. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation. 1998;97:2307–2315. doi: 10.1161/01.cir.97.23.2307. [DOI] [PubMed] [Google Scholar]
- 12.Vengrenyuk Y, Carlier S, Xanthos S, Cardoso L, Ganatos P, Virmani R, Einav S, Gilchrist L, Weinbaum S. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A. 2006;103:14678–14683. doi: 10.1073/pnas.0606310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly-Arnold A, Maldonado N, Laudier D, Aikawa E, Cardoso L, Weinbaum S. Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proc Natl Acad Sci U S A. 2013;110:10741–10746. doi: 10.1073/pnas.1308814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kapustin AN, Shanahan CM. Calcium regulation of vascular smooth muscle cell-derived matrix vesicles. Trends Cardiovasc Med. 2012;22:133–137. doi: 10.1016/j.tcm.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Tanimura A, McGregor DH, Anderson HC. Calcification in atherosclerosis. I. Human studies. J Exp Pathol. 1986;2:261–273. [PubMed] [Google Scholar]
- 16.New SE, Goettsch C, Aikawa M, Marchini JF, Shibasaki M, Yabusaki K, Libby P, Shanahan CM, Croce K, Aikawa E. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res. 2013;113:72–77. doi: 10.1161/CIRCRESAHA.113.301036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauriello A, Sangiorgi GM, Virmani R, Trimarchi S, Holmes DR, Jr, Kolodgie FD, Piepgras DG, Piperno G, Liotti D, Narula J, Righini P, Ippoliti A, Spagnoli LG. A pathobiologic link between risk factors profile and morphological markers of carotid instability. Atherosclerosis. 2010;208:572–580. doi: 10.1016/j.atherosclerosis.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 18.Soor GS, Vukin I, Leong SW, Oreopoulos G, Butany J. Peripheral vascular disease: who gets it and why? A histomorphological analysis of 261 arterial segments from 58 cases. Pathology. 2008;40:385–391. doi: 10.1080/00313020802036764. [DOI] [PubMed] [Google Scholar]
- 19.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 20.Roijers RB, Debernardi N, Cleutjens JP, Schurgers LJ, Mutsaers PH, van der Vusse GJ. Microcalcifications in early intimal lesions of atherosclerotic human coronary arteries. Am J Pathol. 2011;178:2879–2887. doi: 10.1016/j.ajpath.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polak JF, Tracy R, Harrington A, Zavodni AE, O’Leary DH. Carotid artery plaque and progression of coronary artery calcium: the multi-ethnic study of atherosclerosis. J Am Soc Echocardiogr. 2013;26:548–555. doi: 10.1016/j.echo.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard DP, van Lammeren GW, Redgrave JN, Moll FL, de Vries JP, de Kleijn DP, de Borst GJ, Pasterkamp G, Rothwell PM. Histological features of carotid plaque in patients with ocular ischemia versus cerebral events. Stroke. 2013;44:734–739. doi: 10.1161/STROKEAHA.112.678672. [DOI] [PubMed] [Google Scholar]
- 23.van Lammeren GW, den Hartog AG, Pasterkamp G, Vink A, de Vries JP, Moll FL, de Borst GJ. Asymptomatic carotid artery stenosis: identification of subgroups with different underlying plaque characteristics. Eur J Vasc Endovasc Surg. 2012;43:632–636. doi: 10.1016/j.ejvs.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Kataoka Y, Wolski K, Uno K, Puri R, Tuzcu EM, Nissen SE, Nicholls SJ. Spotty calcification as a marker of accelerated progression of coronary atherosclerosis: insights from serial intravascular ultrasound. J Am Coll Cardiol. 2012;59:1592–1597. doi: 10.1016/j.jacc.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Nicholls SJ, Tuzcu EM, Wolski K, Sipahi I, Schoenhagen P, Crowe T, Kapadia SR, Hazen SL, Nissen SE. Coronary artery calcification and changes in atheroma burden in response to established medical therapies. J Am Coll Cardiol. 2007;49:263–270. doi: 10.1016/j.jacc.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 26.Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT. The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation. 2001;103:1051–1056. doi: 10.1161/01.cir.103.8.1051. [DOI] [PubMed] [Google Scholar]
- 27.Burke AP, Weber DK, Kolodgie FD, Farb A, Taylor AJ, Virmani R. Pathophysiology of calcium deposition in coronary arteries. Herz. 2001;26:239–244. doi: 10.1007/pl00002026. [DOI] [PubMed] [Google Scholar]
- 28.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–326. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 29.Otsuka F, Finn AV, Virmani R. Do vulnerable and ruptured plaques hide in heavily calcified arteries? Atherosclerosis. 2013;229:34–37. doi: 10.1016/j.atherosclerosis.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 30.Mauriello A, Servadei F, Zoccai GB, Giacobbi E, Anemona L, Bonanno E, Casella S. Coronary calcification identifies the vulnerable patient rather than the vulnerable Plaque. Atherosclerosis. 2013;229:124–129. doi: 10.1016/j.atherosclerosis.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Mintz GS, Pichard AD, Popma JJ, Kent KM, Satler LF, Bucher TA, Leon MB. Determinants and correlates of target lesion calcium in coronary artery disease: a clinical, angiographic and intravascular ultrasound study. J Am Coll Cardiol. 1997;29:268–274. doi: 10.1016/s0735-1097(96)00479-2. [DOI] [PubMed] [Google Scholar]
- 32.Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50–103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Raggi P. Prognostic implications of absolute and relative calcium scores. Herz. 2001;26:252–259. doi: 10.1007/pl00002028. [DOI] [PubMed] [Google Scholar]
- 34.Blankstein R, Budoff MJ, Shaw LJ, Goff DC, Jr, Polak JF, Lima J, Blumenthal RS, Nasir K. Predictors of coronary heart disease events among asymptomatic persons with low low-density lipoprotein cholesterol MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2011;58:364–374. doi: 10.1016/j.jacc.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 35.Joshi PH, Blaha MJ, Blumenthal RS, Blankstein R, Nasir K. What is the role of calcium scoring in the age of coronary computed tomographic angiography? J Nucl Cardiol. 2012;19:1226–1235. doi: 10.1007/s12350-012-9626-6. [DOI] [PubMed] [Google Scholar]
- 36.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 37.Williams JK, Adams MR, Klopfenstein HS. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990;81:1680–1687. doi: 10.1161/01.cir.81.5.1680. [DOI] [PubMed] [Google Scholar]
- 38.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 39.Burke AP, Taylor A, Farb A, Malcom GT, Virmani R. Coronary calcification: insights from sudden coronary death victims. Z Kardiol. 2000;89 (Suppl 2):49–53. doi: 10.1007/s003920070099. [DOI] [PubMed] [Google Scholar]
- 40.Burke AP, Farb A, Malcom G, Virmani R. Effect of menopause on plaque morphologic characteristics in coronary atherosclerosis. Am Heart J. 2001;141:S58–62. doi: 10.1067/mhj.2001.109946. [DOI] [PubMed] [Google Scholar]
- 41.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, Ouyang P, Jackson S, Saad MF. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–1320. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 42.Burke AP, Farb A, Kutys R, Zieske A, Weber D, Virmani R. Atherosclerotic coronary plaques in African Americans are less likely to calcify than coronary plaques in Caucasian Americans. Circulation. 2002;106 II-481 Abstract. [Google Scholar]
- 43.Lee TC, O’Malley PG, Feuerstein I, Taylor AJ. The prevalence and severity of coronary artery calcification on coronary artery computed tomography in black and white subjects. J Am Coll Cardiol. 2003;41:39–44. doi: 10.1016/s0735-1097(02)02618-9. [DOI] [PubMed] [Google Scholar]
- 44.Henry YM, Eastell R. Ethnic and gender differences in bone mineral density and bone turnover in young adults: effect of bone size. Osteoporos Int. 2000;11:512–517. doi: 10.1007/s001980070094. [DOI] [PubMed] [Google Scholar]
- 45.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O’Donnell CJ, Wilson PW. Bone loss and the progression of abdominal aortic calcification over a 25 year period: the Framingham Heart Study. Calcif Tissue Int. 2001;68:271–276. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- 46.Huang CC, Lloyd-Jones DM, Guo X, Rajamannan NM, Lin S, Du P, Huang Q, Hou L, Liu K. Gene expression variation between African Americans and whites is associated with coronary artery calcification: the multiethnic study of atherosclerosis. Physiol Genomics. 2011;43:836–843. doi: 10.1152/physiolgenomics.00243.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fornage M, Boerwinkle E, Doris PA, Jacobs D, Liu K, Wong ND. Polymorphism of the soluble epoxide hydrolase is associated with coronary artery calcification in African-American subjects: The Coronary Artery Risk Development in Young Adults (CARDIA) study. Circulation. 2004;109:335–339. doi: 10.1161/01.CIR.0000109487.46725.02. [DOI] [PubMed] [Google Scholar]
- 48.Sangiorgi G, Rumberger JA, Severson A, Edwards WD, Gregoire J, Fitzpatrick LA, Schwartz RS. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol. 1998;31:126–133. doi: 10.1016/s0735-1097(97)00443-9. [DOI] [PubMed] [Google Scholar]
- 49.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 50.Burke AP, Kolodgie FD, Farb A, Weber D, Virmani R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation. 2002;105:297–303. doi: 10.1161/hc0302.102610. [DOI] [PubMed] [Google Scholar]
- 51.Wykrzykowska JJ, Mintz GS, Garcia-Garcia HM, Maehara A, Fahy M, Xu K, Inguez A, Fajadet J, Lansky A, Templin B, Zhang Z, de Bruyne B, Weisz G, Serruys PW, Stone GW. Longitudinal distribution of plaque burden and necrotic core-rich plaques in nonculprit lesions of patients presenting with acute coronary syndromes. JACC Cardiovasc Imaging. 2012;5:S10–18. doi: 10.1016/j.jcmg.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Rosen BD, Fernandes V, McClelland RL, Carr JJ, Detrano R, Bluemke DA, Lima JA. Relationship between baseline coronary calcium score and demonstration of coronary artery stenoses during follow-up MESA (Multi-Ethnic Study of Atherosclerosis) JACC Cardiovasc Imaging. 2009;2:1175–1183. doi: 10.1016/j.jcmg.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pai A, Leaf EM, El-Abbadi M, Giachelli CM. Elastin degradation and vascular smooth muscle cell phenotype change precede cell loss and arterial medial calcification in a uremic mouse model of chronic kidney disease. Am J Pathol. 2011;178:764–773. doi: 10.1016/j.ajpath.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Speer MY, Giachelli CM. Regulation of cardiovascular calcification. Cardiovasc Pathol. 2004;13:63–70. doi: 10.1016/S1054-8807(03)00130-3. [DOI] [PubMed] [Google Scholar]
- 56.Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol. 2004;15:2959–2964. doi: 10.1097/01.ASN.0000145894.57533.C4. [DOI] [PubMed] [Google Scholar]
- 57.Amizuka N, Hasegawa T, Oda K, Luiz de Freitas PH, Hoshi K, Li M, Ozawa H. Histology of epiphyseal cartilage calcification and endochondral ossification. Front Biosci (Elite Ed) 2012;4:2085–2100. doi: 10.2741/e526. [DOI] [PubMed] [Google Scholar]
- 58.Clarke MC, Littlewood TD, Figg N, Maguire JJ, Davenport AP, Goddard M, Bennett MR. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ Res. 2008;102:1529–1538. doi: 10.1161/CIRCRESAHA.108.175976. [DOI] [PubMed] [Google Scholar]
- 59.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res. 2000;87:1055–1062. doi: 10.1161/01.res.87.11.1055. [DOI] [PubMed] [Google Scholar]
- 60.Clarke MC, Talib S, Figg NL, Bennett MR. Vascular smooth muscle cell apoptosis induces interleukin-1-directed inflammation: effects of hyperlipidemia-mediated inhibition of phagocytosis. Circ Res. 2010;106:363–372. doi: 10.1161/CIRCRESAHA.109.208389. [DOI] [PubMed] [Google Scholar]
- 61.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, Bennett MR. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 62.Lutgens E, de Muinck ED, Kitslaar PJ, Tordoir JH, Wellens HJ, Daemen MJ. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res. 1999;41:473–479. doi: 10.1016/s0008-6363(98)00311-3. [DOI] [PubMed] [Google Scholar]
- 63.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- 64.Kolodgie FD, Narula J, Burke AP, Haider N, Farb A, Hui-Liang Y, Smialek J, Virmani R. Localization of apoptotic macrophages at the site of plaque rupture in sudden coronary death. Am J Pathol. 2000;157:1259–1268. doi: 10.1016/S0002-9440(10)64641-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 66.Bonetti A, Della Mora A, Contin M, Tubaro F, Marchini M, Ortolani F. Ultrastructural and spectrophotometric study on the effects of putative triggers on aortic valve interstitial cells in in vitro models simulating metastatic calcification. Anat Rec (Hoboken) 2012;295:1117–1127. doi: 10.1002/ar.22494. [DOI] [PubMed] [Google Scholar]
- 67.Jian B, Narula N, Li QY, Mohler ER, 3rd, Levy RJ. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg. 2003;75:457–465. doi: 10.1016/s0003-4975(02)04312-6. discussion 465–456. [DOI] [PubMed] [Google Scholar]
- 68.Naik V, Leaf EM, Hu JH, Yang HY, Nguyen NB, Giachelli CM, Speer MY. Sources of cells that contribute to atherosclerotic intimal calcification: an in vivo genetic fate mapping study. Cardiovasc Res. 2012;94:545–554. doi: 10.1093/cvr/cvs126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karwowski W, Naumnik B, Szczepanski M, Mysliwiec M. The mechanism of vascular calcification - a systematic review. Med Sci Monit. 2012;18:RA1–11. doi: 10.12659/MSM.882181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 71.Qiao JH, Mertens RB, Fishbein MC, Geller SA. Cartilaginous metaplasia in calcified diabetic peripheral vascular disease: morphologic evidence of enchondral ossification. Hum Pathol. 2003;34:402–407. doi: 10.1053/hupa.2003.72. [DOI] [PubMed] [Google Scholar]
- 72.Saremi A, Bahn G, Reaven PD. Progression of vascular calcification is increased with statin use in the Veterans Affairs Diabetes Trial (VADT) Diabetes Care. 2012;35:2390–2392. doi: 10.2337/dc12-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]