Abstract
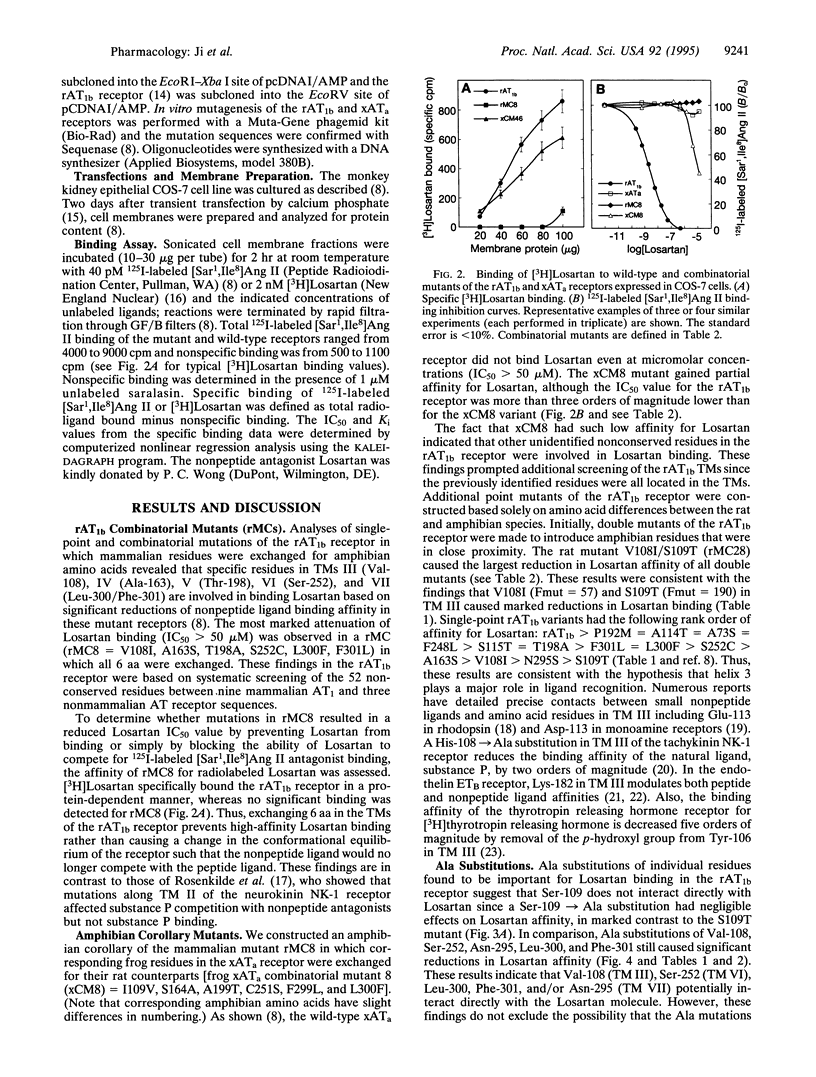
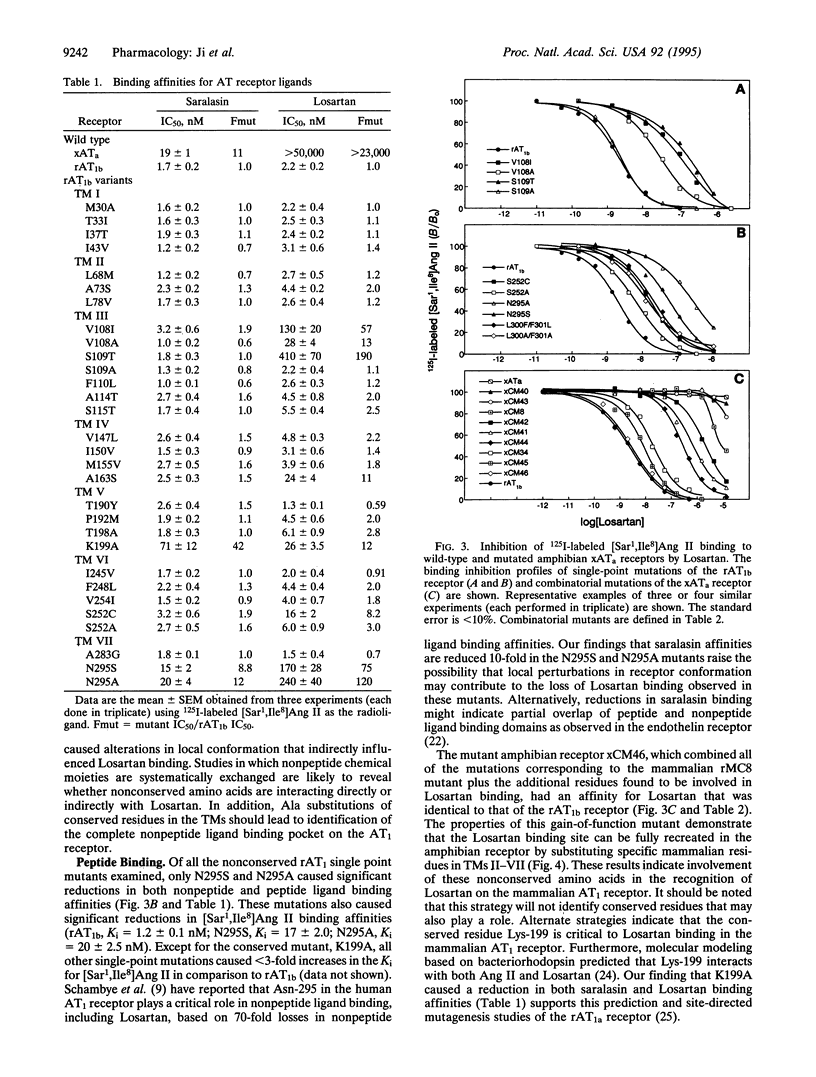
Mutational analysis based on the pharmacological differences between mammalian and amphibian angiotensin II receptors (AT receptors) previously identified 7 aa residues located in transmembrane domains (TMs) III (Val-108), IV (Ala-163), V (Pro-192, Thr-198), VI (Ser-252), and VII (Leu-300, Phe-301) of the rat AT receptor type 1b (rAT1b receptor) that significantly influenced binding of the nonpeptide antagonist Losartan. Further studies have shown that an additional 6 residues in the rAT1b receptor TMs II (Ala-73), III (Ser-109, Ala-114, Ser-115), VI (Phe-248), and VII (Asn-295) are important in Losartan binding. The 13 residues required for Losartan binding in the mammalian receptor were exchanged for the corresponding amino acids in the Xenopus AT receptor type a (xATa receptor) to generate a mutant amphibian receptor that bound Losartan with the same affinity as the rAT1b receptor (Losartan IC50 values: rAT1b, 2.2 +/- 0.2 nM: xATa, > 50 microM; mutant, 2.0 +/- 0.1 nM). To our knowledge, this is the first report of a gain-of-function mutant in which the residues crucial to formation of a ligand binding site in a mammalian peptide hormone receptor were transferred to a previously unresponsive receptor by site-directed mutagenesis. Ala substitutions and comparison of mammalian and amphibian combinatorial mutants indicated that TM III in the rAT1b receptor plays a key role in Losartan binding. Identification of residues involved in nonpeptide ligand binding will facilitate studies aimed at elucidating the chemical basis for ligand recognition in the AT receptor and peptide hormone receptors in general.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elling C. E., Nielsen S. M., Schwartz T. W. Conversion of antagonist-binding site to metal-ion site in the tachykinin NK-1 receptor. Nature. 1995 Mar 2;374(6517):74–77. doi: 10.1038/374074a0. [DOI] [PubMed] [Google Scholar]
- Fong T. M., Huang R. R., Strader C. D. Localization of agonist and antagonist binding domains of the human neurokinin-1 receptor. J Biol Chem. 1992 Dec 25;267(36):25664–25667. [PubMed] [Google Scholar]
- Gerszten R. E., Chen J., Ishii M., Ishii K., Wang L., Nanevicz T., Turck C. W., Vu T. K., Coughlin S. R. Specificity of the thrombin receptor for agonist peptide is defined by its extracellular surface. Nature. 1994 Apr 14;368(6472):648–651. doi: 10.1038/368648a0. [DOI] [PubMed] [Google Scholar]
- Gether U., Johansen T. E., Snider R. M., Lowe J. A., 3rd, Nakanishi S., Schwartz T. W. Different binding epitopes on the NK1 receptor for substance P and non-peptide antagonist. Nature. 1993 Mar 25;362(6418):345–348. doi: 10.1038/362345a0. [DOI] [PubMed] [Google Scholar]
- Groblewski T., Maigret B., Nouet S., Larguier R., Lombard C., Bonnafous J. C., Marie J. Amino acids of the third transmembrane domain of the AT1A angiotensin II receptor are involved in the differential recognition of peptide and nonpeptide ligands. Biochem Biophys Res Commun. 1995 Apr 6;209(1):153–160. doi: 10.1006/bbrc.1995.1483. [DOI] [PubMed] [Google Scholar]
- Hjorth S. A., Schambye H. T., Greenlee W. J., Schwartz T. W. Identification of peptide binding residues in the extracellular domains of the AT1 receptor. J Biol Chem. 1994 Dec 9;269(49):30953–30959. [PubMed] [Google Scholar]
- Ji H., Leung M., Zhang Y., Catt K. J., Sandberg K. Differential structural requirements for specific binding of nonpeptide and peptide antagonists to the AT1 angiotensin receptor. Identification of amino acid residues that determine binding of the antihypertensive drug losartan. J Biol Chem. 1994 Jun 17;269(24):16533–16536. [PubMed] [Google Scholar]
- Ji H., Sandberg K., Catt K. J. Novel angiotensin II antagonists distinguish amphibian from mammalian angiotensin II receptors expressed in Xenopus laevis oocytes. Mol Pharmacol. 1991 Feb;39(2):120–123. [PubMed] [Google Scholar]
- Ji H., Sandberg K., Zhang Y., Catt K. J. Molecular cloning, sequencing and functional expression of an amphibian angiotensin II receptor. Biochem Biophys Res Commun. 1993 Jul 30;194(2):756–762. doi: 10.1006/bbrc.1993.1886. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Brinkmann J. A., Longton E. D., Peishoff C. E., Lago M. A., Leber J. D., Cousins R. D., Gao A., Stadel J. M., Kumar C. S. Lysine 182 of endothelin B receptor modulates agonist selectivity and antagonist affinity: evidence for the overlap of peptide and non-peptide ligand binding sites. Biochemistry. 1994 Dec 6;33(48):14543–14549. doi: 10.1021/bi00252a022. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Brinkmann J. A., Longton E. D., Peishoff C. E., Lago M. A., Leber J. D., Cousins R. D., Gao A., Stadel J. M., Kumar C. S. Lysine 182 of endothelin B receptor modulates agonist selectivity and antagonist affinity: evidence for the overlap of peptide and non-peptide ligand binding sites. Biochemistry. 1994 Dec 6;33(48):14543–14549. doi: 10.1021/bi00252a022. [DOI] [PubMed] [Google Scholar]
- Murphy T. J., Nakamura Y., Takeuchi K., Alexander R. W. A cloned angiotensin receptor isoform from the turkey adrenal gland is pharmacologically distinct from mammalian angiotensin receptors. Mol Pharmacol. 1993 Jul;44(1):1–7. [PubMed] [Google Scholar]
- Noda K., Saad Y., Kinoshita A., Boyle T. P., Graham R. M., Husain A., Karnik S. S. Tetrazole and carboxylate groups of angiotensin receptor antagonists bind to the same subsite by different mechanisms. J Biol Chem. 1995 Feb 3;270(5):2284–2289. doi: 10.1074/jbc.270.5.2284. [DOI] [PubMed] [Google Scholar]
- Nouet S., Dodey P., Renaut P., Marie J., Pruneau D., Larguier R., Lombard C., Bonnafous J. C. Properties of [3H]LF 7-0156, a new nonpeptide antagonist radioligand for the type 1 angiotensin II receptor. Mol Pharmacol. 1994 Oct;46(4):693–701. [PubMed] [Google Scholar]
- Perlman J. H., Thaw C. N., Laakkonen L., Bowers C. Y., Osman R., Gershengorn M. C. Hydrogen bonding interaction of thyrotropin-releasing hormone (TRH) with transmembrane tyrosine 106 of the TRH receptor. J Biol Chem. 1994 Jan 21;269(3):1610–1613. [PubMed] [Google Scholar]
- Rosenkilde M. M., Cahir M., Gether U., Hjorth S. A., Schwartz T. W. Mutations along transmembrane segment II of the NK-1 receptor affect substance P competition with non-peptide antagonists but not substance P binding. J Biol Chem. 1994 Nov 11;269(45):28160–28164. [PubMed] [Google Scholar]
- Sachais B. S., Snider R. M., Lowe J. A., 3rd, Krause J. E. Molecular basis for the species selectivity of the substance P antagonist CP-96,345. J Biol Chem. 1993 Feb 5;268(4):2319–2323. [PubMed] [Google Scholar]
- Sandberg K., Ji H., Clark A. J., Shapira H., Catt K. J. Cloning and expression of a novel angiotensin II receptor subtype. J Biol Chem. 1992 May 15;267(14):9455–9458. [PubMed] [Google Scholar]
- Sandberg K., Ji H., Millan M. A., Catt K. J. Amphibian myocardial angiotensin II receptors are distinct from mammalian AT1 and AT2 receptor subtypes. FEBS Lett. 1991 Jun 24;284(2):281–284. doi: 10.1016/0014-5793(91)80704-7. [DOI] [PubMed] [Google Scholar]
- Savarese T. M., Fraser C. M. In vitro mutagenesis and the search for structure-function relationships among G protein-coupled receptors. Biochem J. 1992 Apr 1;283(Pt 1):1–19. doi: 10.1042/bj2830001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambye H. T., Hjorth S. A., Bergsma D. J., Sathe G., Schwartz T. W. Differentiation between binding sites for angiotensin II and nonpeptide antagonists on the angiotensin II type 1 receptors. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7046–7050. doi: 10.1073/pnas.91.15.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz T. W. Locating ligand-binding sites in 7TM receptors by protein engineering. Curr Opin Biotechnol. 1994 Aug;5(4):434–444. doi: 10.1016/0958-1669(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Fong T. M., Tota M. R., Underwood D., Dixon R. A. Structure and function of G protein-coupled receptors. Annu Rev Biochem. 1994;63:101–132. doi: 10.1146/annurev.bi.63.070194.000533. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Dixon R. A. Structural basis of beta-adrenergic receptor function. FASEB J. 1989 May;3(7):1825–1832. doi: 10.1096/fasebj.3.7.2541037. [DOI] [PubMed] [Google Scholar]
- Timmermans P. B., Wong P. C., Chiu A. T., Herblin W. F., Benfield P., Carini D. J., Lee R. J., Wexler R. R., Saye J. A., Smith R. D. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993 Jun;45(2):205–251. [PubMed] [Google Scholar]
- Underwood D. J., Strader C. D., Rivero R., Patchett A. A., Greenlee W., Prendergast K. Structural model of antagonist and agonist binding to the angiotensin II, AT1 subtype, G protein coupled receptor. Chem Biol. 1994 Dec;1(4):211–221. doi: 10.1016/1074-5521(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Zhu G., Wu L. H., Mauzy C., Egloff A. M., Mirzadegan T., Chung F. Z. Replacement of lysine-181 by aspartic acid in the third transmembrane region of endothelin type B receptor reduces its affinity to endothelin peptides and sarafotoxin 6c without affecting G protein coupling. J Cell Biochem. 1992 Oct;50(2):159–164. doi: 10.1002/jcb.240500206. [DOI] [PubMed] [Google Scholar]



