Abstract
Objective
Dopaminergic neuronal death in Parkinson’s disease (PD) is accompanied by oxidative stress and preceded by glutathione depletion. The development of disease-modifying therapies for PD has been hindered by a paucity of animal models that mimic these features and demonstrate an age-related progression. The EAAC1−/− mouse may be useful in this regard, because EAAC1−/− mouse neurons have impaired neuronal cysteine uptake, resulting in reduced neuronal glutathione content and chronic oxidative stress. Here we aimed to (1) characterize the age-related changes in nigral dopaminergic neurons in the EAAC1−/− mouse, and (2) use the EAAC1−/− mouse to evaluate N-acetylcysteine, a membrane-permeable cysteine pro-drug, as a potential disease-modifying intervention for PD.
Methods
Wild-type mice, EAAC1−/− mice, and EAAC1−/− mice chronically treated with N-acetylcysteine were evaluated at serial time points for evidence of oxidative stress, dopaminergic cell death, and motor abnormalities.
Results
EAAC1−/− mice showed age-dependent loss of dopaminergic neurons in the substantia nigra pars compacta, with more than 40% of these neurons lost by age 12 months. This neuronal loss was accompanied by increased nitrotyrosine formation, nitrosylated α-synuclein, and microglial activation. These changes were substantially reduced in mice that received N-acetylcysteine.
Interpretation
These findings suggest that the EAAC1−/− mouse may be a useful model of the chronic neuronal oxidative stress that occurs in PD. The salutary effects of N-acetylcysteine in this mouse model provide an impetus for clinical evaluation of glutathione repletion in PD.
Parkinson’s disease (PD) leads to cell death in several neuronal populations, particularly the dopaminergic neurons of the substantia nigra pars compacta (SNc). Between 5% and 10% of PD can now be attributed to heritable genetic mutations, but the cause of the more common, sporadic PD remains elusive. Several lines of evidence suggest that oxidative stress and depletion of glutathione, a major endogenous antioxidant, contributes to neuronal death in both hereditary and sporadic PD. Oxidative stress and dopaminergic neuronal death are produced by chemical agents epidemiologically associated with PD, such as paraquat and rotenone.1,2 Postmortem studies of PD patients show increased lipid and protein oxidation products in the SNc and markedly reduced levels of glutathione.3,4 Moreover, the glutathione depletion precedes dopaminergic neuronal death in PD5 and does not occur in other neurodegenerative disorders affecting the SNc,6 thus suggesting a specific and causal role for glutathione depletion in PD pathogenesis.
Development of interventions to slow or prevent neuronal death in PD has been hindered by a paucity of animal models of the chronic, sustained neuronal oxidative stress observed in human PD.7,8 6-Hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), and other toxins used to generate animal models of PD produce massive, acute oxidative stress in the targeted neuronal populations, leading to rapid cell death and motor abnormalities. These models generate a phenotype resembling human PD, but they do not recapitulate the chronic oxidative stress and slow neurodegeneration that occurs over decades in human PD. The EAAC1−/− mouse may be a more useful model in this respect because this mouse strain exhibits chronic, neuron-specific oxidative stress9. EAAC1 (also termed EAAT3 and SLC1A1) is an excitatory amino acid transporter expressed selectively by eurons in the central nervous system (CNS).10,11 It is the primary route for neuronal uptake of cysteine,9,12,13 the rate-limiting substrate in glutathione synthesis.14 Mice lacking EAAC1 have decreased neuronal glutathione content, increased markers of neuronal oxidative stress, and a slow, age-dependent reduction in overall brain size9 ; however, the effect of EAAC1 deficiency on SNc dopaminergic neurons has not previously been reported.
The striking depletion of glutathione in dopaminergic neurons in PD, coupled with the importance of glutathione for neuronal survival, has led several authors to suggest that preventing this glutathione depletion may be neuroprotective in PD.15,16 Glutathione is involved in the elimination of peroxides and nitrosylated proteins in both the cytosol and mitochondria. N-acetylcysteine (NAC) is a cell membrane–permeable form of cysteine that can cross the blood-brain barrier and replete neuroal glutathione content.9,17,18 NAC is well-tolerated and currently in clinical use for other indications. Here, using the EAAC1−/− mouse as a model of chronic neuronal oxidative stress, we show an age-dependent loss of SNc dopaminergic neurons in the EAAC1−/− mouse, and further show that this neuronal loss is reduced by oral administration of NAC. These results provide a rationale for evaluating glutathione repletion as a disease-modifying therapy for PD.
Subjects and Methods
Animal and human studies were performed in accordance with protocols approved by institutional review committees at the San Francisco Veterans Affairs Medical Center. Reagents were obtained from Sigma-Aldrich except where noted.
EAAC1−/− Mice
The EAAC1−/− mice have exon 1 of the EAAC1 gene disrupted by a neomycin resistance (NEO) cassette.20 EAAC1−/− mice were obtained from Miltenyi Biotec GmbH (Bergisch Gladbach, Germany) and subsequently outbred to wild-type (WT) CD-1 mice for more than 10 generations, as described previously.9 WT mice were maintained using the offspring from the latter outcrosses. Breeding stock from the WT and EAAC1−/− mice were subsequently intercrossed at least once every 8 generations to prevent genetic drift, in accordance with the Banbury Conference recommendations.21 Genotypes were confirmed by polymerase chain reaction (PCR) of tail DNA, using 3 primers that together amplify sequences unique to the WT and allele and to the inserted NEO cassette of the disrupted allele. The forward primer (5’-ACGAGCTCGG GATGTGACT-3’) was common to both sequences. The WT reverse primer (5’-CAGGGTGGAGAGCA-GCAG-3’) produces a 63-bp PCR product, and the NEO reverse primer (5’-GCTCTTCGTCCAGATCATCC-3’) produces a 1,295-bp PCR product (Supporting Information Fig S1). Genotyping gels were run on 3% agarose gels using a 100-bp ladder (Promega) for size determinations.
Administration of N-Acetylcysteine
One cohort of mice was fed NAC in the drinking water beginning 1 week after weaning. The water contained 4mg/ml NAC for the first 6 months, then 2mg/ml for the remainder of the study. The NAC solution was pH-adjusted to 6.0 to match the pH of the water normally given to the animals in the animal facility, and the solution was replaced twice weekly to limit autoxidation. For studies of NAC’s effect on neuronal glutathione content, 4-month-old EAAC1−/− mice were given 2mg/ml NAC in drinking water for 7 days.
Immunohistochemistry and Confocal Imaging
Mice were perfusion-fixed with 4% formaldehyde. The brains were postfixed in 4% formaldehyde at 4°C, followed by cryoprotection in 20% sucrose and freezing. The frozen brains were coronally sectioned and immunostained using the following antibodies: rabbit anti-EAAC1 (1:200, gift from Dr. J Rothstein, Johns Hopkins University); sheep anti-tyrosine hydroxylase (1:400, Chemicon, AB1542); mouse anti-NeuN (1:250, Chemicon, MAB377); rabbit anti-nitrotyrosine (anti-nTyr; Chemicon, 1:100, AB5411); and rabbit anti–ionized calcium binding adaptor molecule 1 (Iba1; 1:400, Wako, 019–19741). Antibody binding was detected using fluorescent donkey anti-rabbit, anti-mouse, and anti-sheep immunoglobulin G (IgG) (all 1:250; Invitrogen) as previously described.9,22 Confocal images were captured using a Zeiss LSM 510 Meta confocal microscope using sequential scans for double-stained and triple-stained samples. Control sections prepared with no primary or no secondary antibodies showed no detectable signal. Controls also established that signal attributed to each of the fluorophores was completely absent when omitted from the staining procedure.
Neuronal Cell Counts
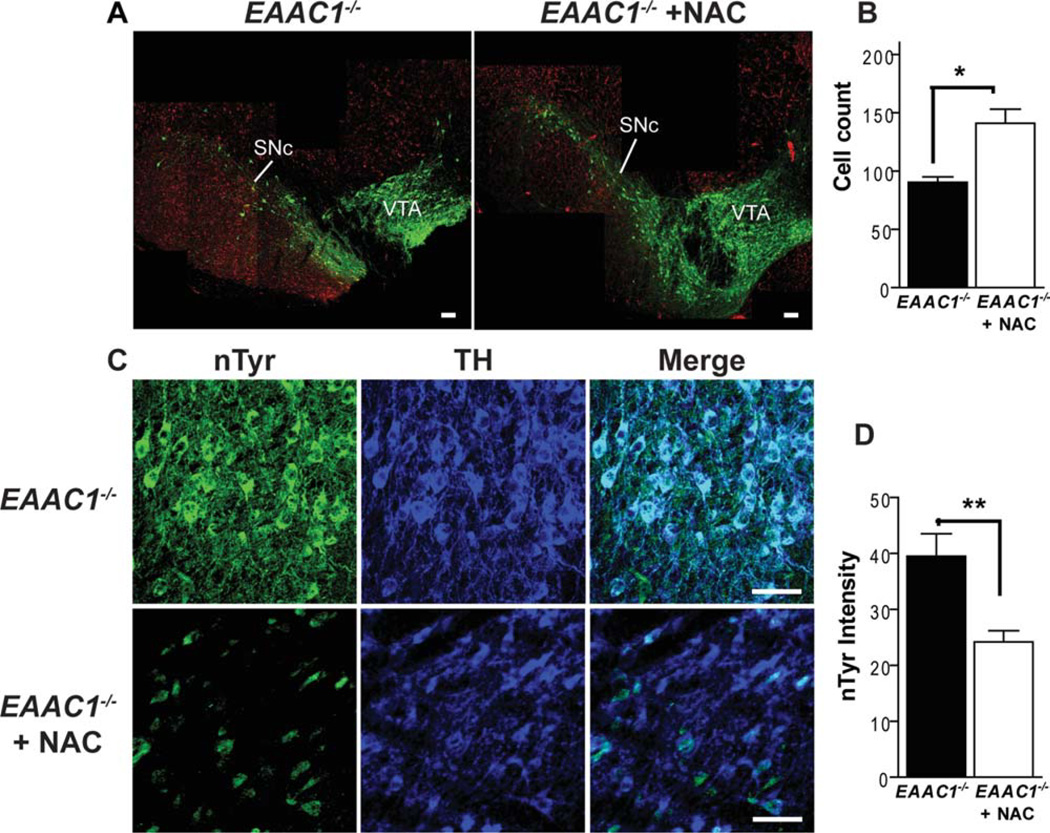
Quantification of SNc dopaminergic neurons was performed using a stereological approach.23 Sequential 30-µm cryostat sections were obtained spanning the rostral-caudal extent of the SNc. Cell counts were performed on 4 evenly-spaced sections from each brain, with the most caudal section analyzed at Bregma −3.60 mm, and the most rostral section at Bregma −3.00 mm24. The sections were stained for tyrosine hydroxylase (TH) and photographed with a confocal microscope using an optical width of 12.4 µm and a z-step of 6.1 µm. The SNc was identified by TH staining and standard landmarks. 24 Cell counting was performed on an optical z-section 18–19 lm from the upper surface of each physical section to prevent bias due to cell loss from the cut surfaces. Cells were counted throughout the entire SNc on the optical sections, rather than on random samples of the sections, to avoid estimation errors introduced by sampling. TH-positive neurons were counted only if the nucleus was fully contained in the optical section, as evidenced by a circumscribed lacune in TH staining of the cell soma. This served to exclude any bias in cell counts that could otherwise result from changes in neuronal soma size. Counts from each section were summed for each animal and expressed as the mean number of cells per bilateral midbrain section. Cell counts were performed twice, by 2 individuals blinded to the mouse treatment group, and the independently obtained cell counts were averaged to produce a single value for each animal. The cell counts obtained by the different observers were within 9.1 ± 7.4% (mean ± SD) of one another.
Quantification of nTyr and Reactive Thiols
Evaluations were performed on a coronal section from each mouse corresponding to Bregma − 3.4 mm.24 sections were immunostained together to minimize differences introduced by the staining procedure. nTyr staining of dopaminergic neurons was measured by first using the TH staining to define regions of interest on confocal photomicrographs of each section, and then measuring nTyr staining intensity selectively in these regions of interest. The intensity of nTyr staining over all TH-positive SNc neurons was averaged to generate a single value for each brain.
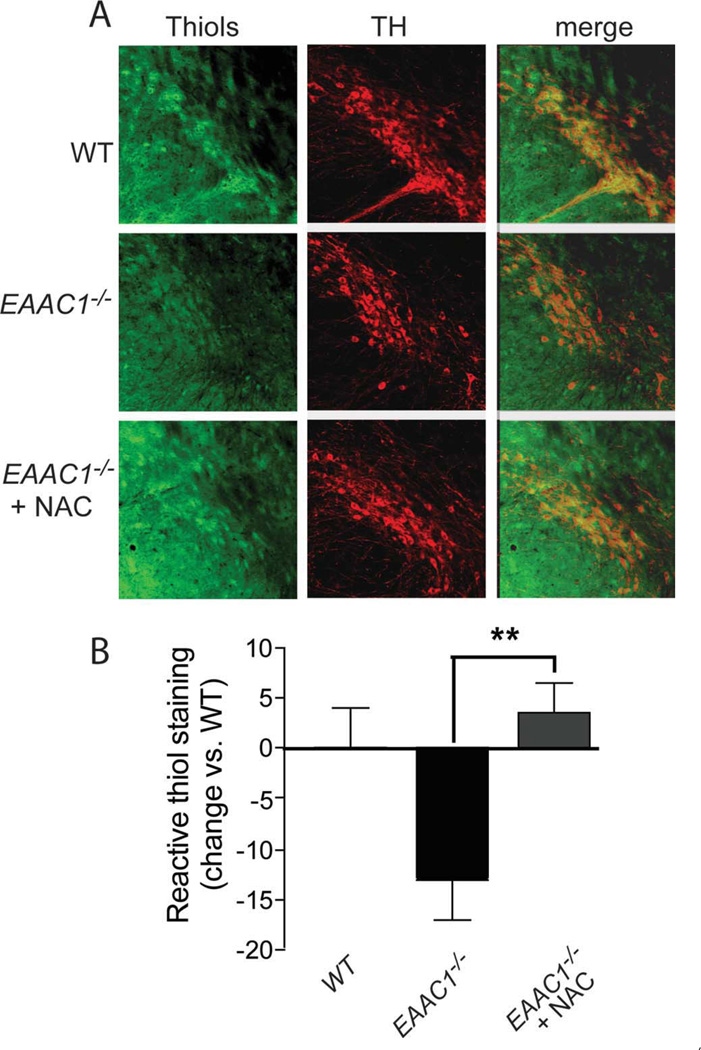
Reactive thiols in TH-positive neurons were analyzed by a similar method.9 Fixed sections were first incubated for 24 hours at 4°C in a serum-free solution of 2.5 µM Alexa Fluor 488 C5-maleimide (Invitrogen). C5-maleimide binds to reactive thiols, of which glutathione is the dominant species. The sections were subsequently immunostained for TH to identify dopaminergic neurons. The TH staining was used to define regions of interest on confocal photomicrographs of each section, and then the intensity of C5-maleimide staining was measured within these regions of interest. The intensity of C5-maleimide staining over all TH-positive SNc neurons was averaged on 4 evenly spaced sections spanning the SNc to generate a single value for each brain.
Measurements of Biogenic Amines
Analyses were performed on striata isolated from WT and EAAC1−/− mice age 9–11 months. The striata were isolated on a chilled glass plate and homogenized in 0.1 M perchloric acid. Dopamine and related metabolites were measured by the CMN/KC Neurochemistry Core Lab at Vanderbilt University, using high-performance liquid chromatography with internal standards and electrochemical detection. Results were normalized to protein content.
Microglial Activation
Evaluations were performed on the coronal section from each mouse corresponding to Bregma −3.20mm, which is near the rostral-caudal midpoint of the SNc.24 Sections from each brain were immunostained simultaneously for TH and Iba1. To optimize imaging of microglial processes, a z-stack of 20 overlapping 2-µm-thick confocal images was acquired through each section and then compressed to generate a single image. Microglia morphology was evaluated as described previously,22,25 using the criteria described and illustrated in Supporting Information Figure S2.
Western Blots
Western blots were performed as described previously.9,22 In brief, whole-brain lysates were homogenized in ice-cold radioimmuno-precipitation assay buffer and centrifuged at 5,000 rpm. The supernatant was added to sample buffer containing β-mercaptoethanol (final concentration 7.5%), electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, transferred to polyvinylidene fluoride membranes, and incubated with antibody to nitrated α-synuclein (nSyn14; Chemicon, 1:5,000). After membrane stripping, the membranes were reprobed with antibody to EAAC1 (Alpha Diagnostics, 1:1,000) to confirm the genotype. Comparable loading was confirmed by staining for β-actin (Sigma, 1:6,000). Antibody binding was visualized with the chemiluminescence detection (ECL Plus, GE Healthcare).
Behavioral Testing
Studies of motor function were conducted in a quiet, dimly-lit room. The open field test of spontaneous locomotor activity26 was performed by placing mice in a Plexiglas enclosure equipped with 2 rows of infrared photocell panels interfaced with a computer. The lower row detected horizontal motion, and the higher row detected vertical motion (rearing). On each of 3 consecutive days, open field activity was recorded for 10 minutes after an initial 1-minute adaptation period. Rotarod testing26,27 was performed by placing mice onto a rotating dowel, with the speed of rotation increasing at a constant rate over the course of the trial. The time each animal was able to stay on the dowel was measured on 3 trials per day for 3 consecutive days. Mice were allowed a minimum of 1 minute recovery between each trial. For the pole test of agility26,28 mice were placed facing upward near the top of a cloth-covered vertical pole, 30cm high and 1.5cm in diameter. The time required for the animals to turn around and climb to the bottom was recorded. Tests were performed twice in succession on 3 consecutive days, and the mean time recorded after discard of the furthest outlying time point. Trials in which the animal was unable to successfully turn around and reach the bottom were terminated at 3 minutes.
Human Tissues
Formalin-fixed, paraffin-embedded human midbrain samples from 4 PD brains and 4 control brains were acquired from the Harvard Brain Tissue Resource Center. All samples were from individuals ranging in age from 72 to 79 years old, and were obtained after postmortem intervals of 15–28 hours. The PD brains all showed Lewy bodies and moderate to severe cell loss in the SNc. Sections (5lm) through the SNc and the red nucleus were prepared on a sliding microtome. Paraffin was removed by xylene immersion, and rehydration was performed in a graded ethanol wash series. The samples were then boiled for 10 minutes in citrate buffer to achieve heat-induced epitope retrieval29. Immunostaining for TH and EAAC1 were performed as described for the mouse tissues.
Statistical Analysis
Microglial activation data were analyzed with a Student t test. Cell counts, biogenic amine, C5-maleimide staining, nTyr, and behavioral data were analyzed by one-way analysis of variance (ANOVA) followed by the Bonferroni post-hoc test for comparisons between multiple groups. Where cell count and nTyr data are displayed in more than 1 figure, statistical analyses accounted for all multiple comparisons.
Results
Progressive Loss of SNc Dopaminergic Neurons in EAAC1−/− Mice
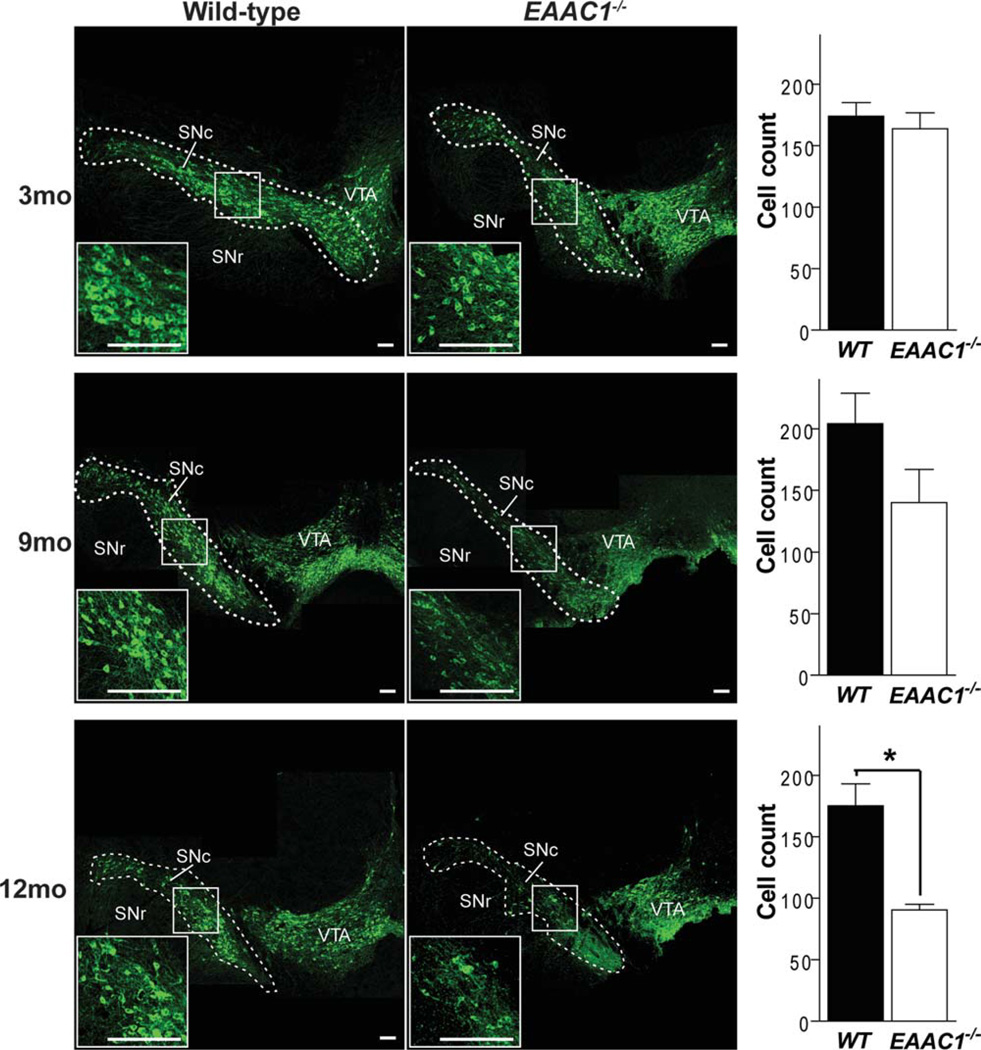
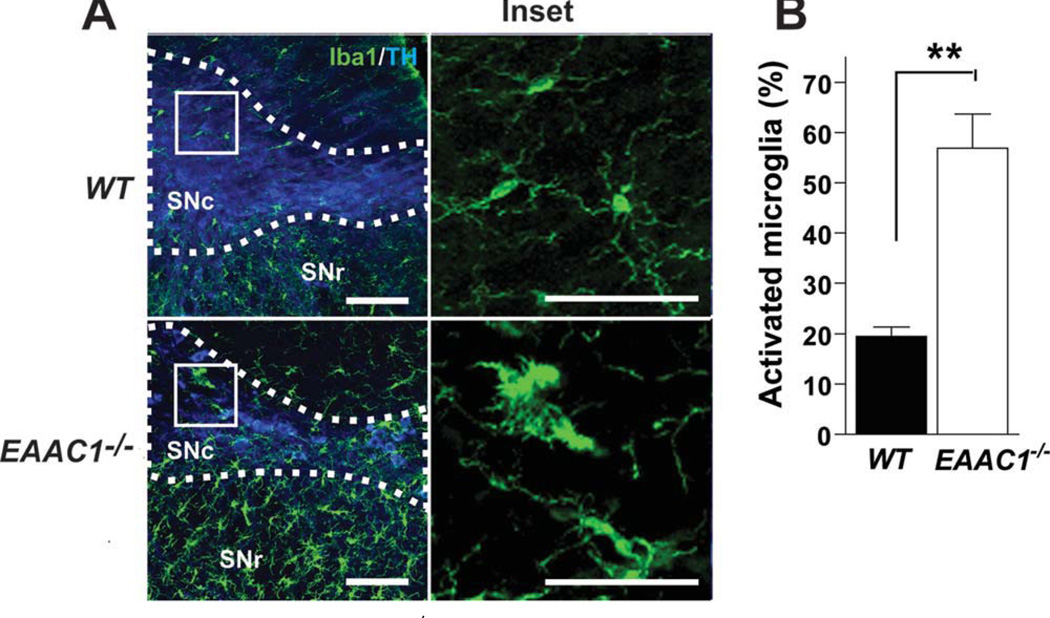
The number of dopaminergic neurons in the SNc was evaluated in WT and EAAC1−/− mice at 3, 9, and 12 months of age (Fig 1). No differences were found at 3 months, but a significant, 41 ± 10% decrease in the EAAC1−/− brains was apparent by age 12 months (see Fig 1). Microglia assume an activated morphology in the presence of injured and dying neurons, and activated microglia are present in the SNc in PD.30 To complement the studies of neuronal loss, brain sections were stained for the microglia marker, Iba1, to assess microglial morphology in 12-month old EAAC1−/− and WT mice. The EAAC1−/− mice showed increased Iba1 im-munoreactivity and activated morphology in the SNc and surrounding area (Fig 2).
FIGURE 1.
Progressive loss of SNc dopaminergic neurons in EAAC1−/− mice. Confocal images of the dorsal midbrain of WT and EAAC1−/− mice, with dopaminergic neurons stained green (anti-TH). Insets show magnified view of boxed areas. Graphs show quantification of dopaminergic neurons in the SNc at 3, 9, and 12 months of age. Bar = 200 µm. **p < 0.01; n = 3–7. SNc = substantia nigra pars compacta; SNr = substantia nigra pars reticulata; VTA = ventral tegmental area; WT = wild-type.
FIGURE 2.
Microglial activation in the SNc of EAAC1−/− mice. (A) Confocal images of the dorsal midbrain of WT and EAAC1−/− mice, with microglia stained green (anti-Iba1). Dopaminergic neurons were stained blue (anti-TH) to delineate the SNc. Right-hand figures show magnified view of microglia in the inset boxed areas. Bar = 100 µm in low-power view, 50µm in high-power view. (B) Quantification of microglial activation in the SNc. *p < 0.01; n = 3. SNc = substantia nigra pars compacta; SNr = substantia nigra pars reticulata; VTA = ventral tegmental area; WT = wild-type.
Despite these changes in the SNc, measurements of dopamine and related biogenic amines in the striatum showed only nonsignificant reductions in the EAAC1−/−mice (Supporting Information Fig S3). Immunostaining for tyrosine hydroxylase in the striatum similarly showed no significant reduction (not shown), presumably because of the compensatory sprouting from surviving SNc dopaminergic neurons that occurs with chronic lesions 31,32.
Increased Oxidative Stress in EAAC1−/− SNc Dopaminergic Neurons
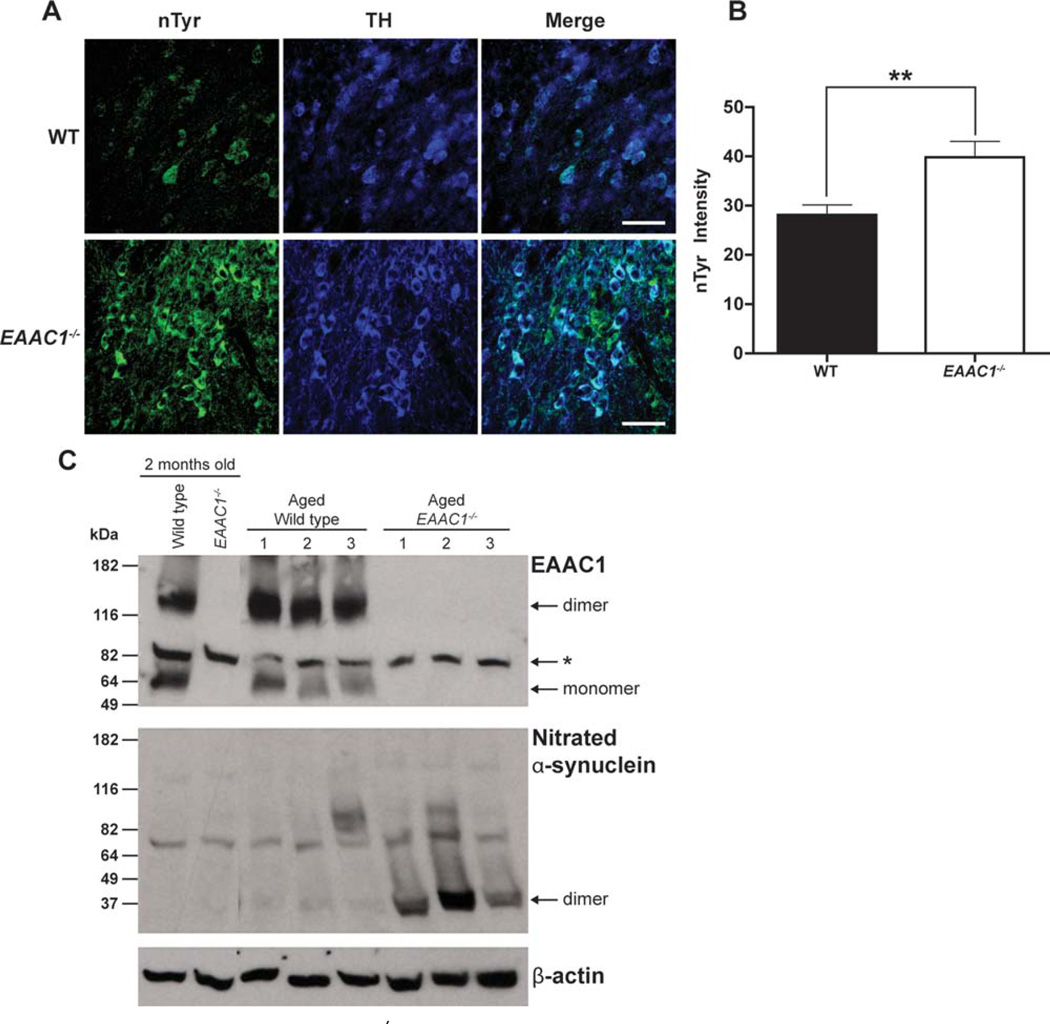
Glutathione is important for metabolism of peroxides, nitric oxide, and other oxidant species. EAAC1−/− mice have reduced neuronal glutathione content due to the lack of EAAC1-mediated neuronal cysteine uptake.9,13,33,34 To assess the level of oxidative stress in EAAC1−/− midbrain dopaminergic neurons, brain sections were immuno-stained for the presence of nTyr-modified proteins.35 SNc dopaminergic neurons in the EAAC1−/− mice showed an increase in nTyr immunoreactivity (Fig 3A, B). As a second measure of oxidative stress, nitrosylated α-synuclein was measured in WT and EAAC1−/− brains. Aggregated α-synuclein fibrils are the primary component of Lewy bodies found in PD, and the formation of these aggregates is accelerated by nitrosylation.36 Nitration and oxidation of α-synuclein produce cross-linked a-synuclein oligomers that are stable to sodium dodecyl sulfate.37 Western blots of brain tissue from WT and EAAC1−/− mice confirmed a striking increase in nitrosylated α-synuclein, primarily as dimers and higher-order aggregates, in the aged EAAC1−/− mice (Fig 3C).
FIGURE 3.
Increased oxidative stress in EAAC1−/− SNc dopaminergic neurons. (A) Confocal images of SNc from WT and EAAC1−/− mice. Sections are stained for nitrotyrosine (nTyr, green) as a marker of oxidative stress, and for tyrosine hydroxylase (TH, blue) to identify dopaminergic neurons. Bar = 50 µm. (B) Quantified data show that the nTyr signal localized to dopaminergic neurons is increased in EAAC1−/− mice. **p < 0.01; n = 7. (C) Western blots prepared from WT and EAAC1−/− mouse brain show accumulation of nitrated α-synuclein in 12-month-old EAAC1−/− brains but not in 12-month-old WT brains. The 2 left-hand lanes were prepared on a different gel than the other lanes. *Denotes a nonspecific band recognized by the antibody to EAAC1. WT = wild-type.
EAAC1 Expression in Mouse and Human SNc Dopaminergic Neurons
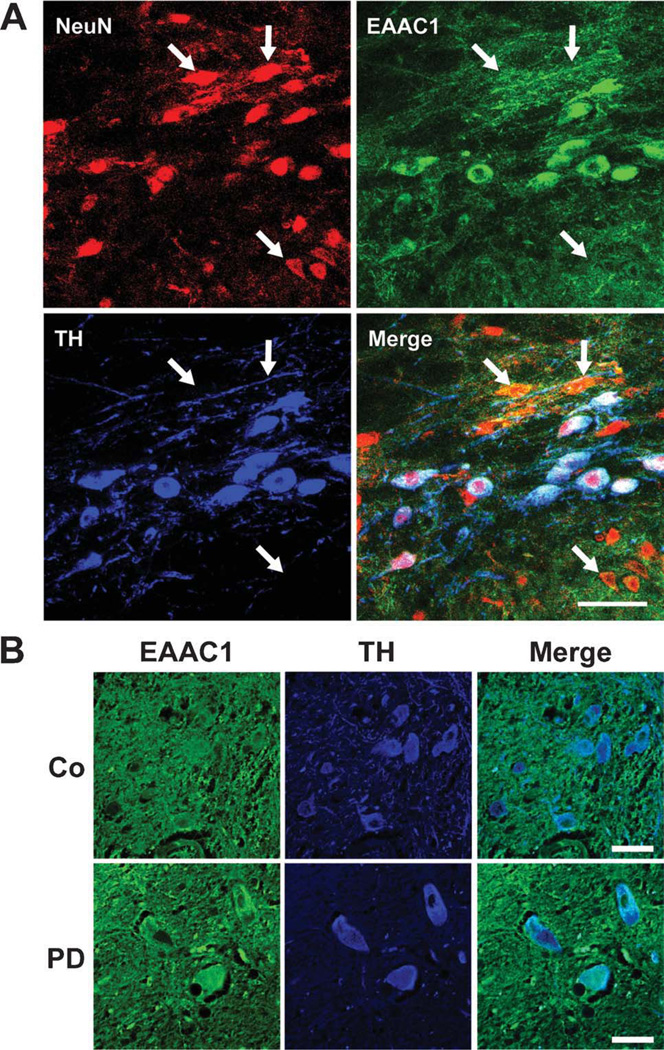
The distribution of EAAC1 expression in the mouse midbrain was evaluated by immunostaining, with neuronal nuclei identified by NeuN expression and dopaminergic neurons identified by tyrosine hydroxylase (TH) expression. EAAC1 was diffusely distributed in the midbrain, consistent with its ubiquitous expression over neuronal cell bodies and processes.10,11 However, there was higher EAAC1 expression over dopaminergic (TH-positive) neurons (Fig 4A), in agreement with previous reports.11,33 Human postmortem midbrain sections were similarly stained for EAAC1 and TH, but for technical reasons the anti-NeuN antibody could not be used with the postmortem tissue. As in the mouse brain, normal human brain showed increased EAAC1 expression in dopaminergic (TH-positive) neurons (see Fig 4B). The EAAC1 signal in the surviving dopaminergic neurons of the PD brain sections was at least as great as in the normal brain sections, but variability in the immunostaining quality in the postmortem tissue precluded a quantifiable comparison.
FIGURE 4.
EAAC1 expression in mouse and human SNc dopaminergic neurons. (A) Sections through mouse SNc immunostained for NeuN (red) to identify neuronal nuclei; for EAAC1 (green); and for tyrosine hydroxylase (TH, blue) to identify dopaminergic neurons. Confocal images show dopaminergic neurons to be densely stained for EAAC1, and nondopaminergic neurons (some denoted by arrows) to be less densely stained. Bar = 50µm; representative of n = 4. (B) Sections through human normal and PD brains immunostained for EAAC1 (green), and for TH (blue) to identify dopaminergic neurons. The TH-positive dopaminergic neurons show coexpression of EAAC1 in both PD and control brains. Bar = 50µm; representative of n = 4 control and 4 PD brains.
Oral NAC Restores Reactive Thiol Content in EAAC1−/− SNc Dopaminergic Neurons
NAC is a membrane-permeable form of cysteine that has previously been shown to facilitate neuronal glutathione synthesis in neuron cultures and in mouse hippocampal neurons in situ.9,18 To confirm that oral NAC could replete glutathione in dopaminergic SNc neurons, EAAC1−/− mice were fed NAC in drinking water for 7 days. The content of reactive thiols, of which glutathione is the major species, was evaluated by histochemical staining with C5-maleimide on SNc brain sections prepared from these mice. Immunostaining for tyrosine hydroxylase identified dopaminergic neurons and processes on the same sections. Dopaminergic neurons from the EAAC1−/− mice showed significantly less C5-maleimide staining than neurons from WT mice, and this reduction was reversed in EAAC1−/− mice fed NAC (Fig 5).
FIGURE 5.
Oral N-acetylcysteine restores reactive thiol content of SNc dopaminergic neurons in EAAC1−/− mice. (A) Representative confocal sections through mouse SNc labeled with C5-maleimide (green) to label reactive thiols and immunostained for tyrosine hydroxylase (red) to identify dopaminergic neurons. Bar = 50µm. (B) Quantification of C5-maeimide fluorescence in TH-positive neurons indicates that reactive thiol content of dopaminergic neurons is lower in EAAC1−/− mice than in WT mice, but normalized in EAAC1−/− mice treated with oral NAC. *p < 0.05; n = 4.
NAC Reduces SNc Dopaminergic Cell Loss in EAAC1−/− Mice
A cohort of EAAC1−/− mice was therefore given NAC in the drinking water from weaning through 12 months of age to determine whether long-term administration could prevent neuronal death resulting from chronic oxidative stress. Brains evaluated at 12 months of age showed less dopaminergic neuronal loss in the SNc of EAAC1 mice treated with NAC (Fig 6A). These neurons also showed less oxidative stress, as indicated by nTyr immunoreactivity, than neurons in untreated EAAC1 −/− mice (see Fig 6B).
FIGURE 6.
N-acetylcysteine improves survival of SNc dopaminergic neurons in EAAC1−/− mice. (A) Images prepared as in Figure 1, with dopaminergic neurons stained green (anti-TH) and neuronal nuclei stained red (anti-NeuN). Neuronal loss in the SNc is reduced in mice treated with N-acetylcysteine (NAC). Bars = 200µm. (B) Cell counts of SNc dopaminergic neurons in EAAC1−/− mice with and without NAC treatment. **p < 0.01; n = 5–6. Note that the cell count data for EAAC1−/− mice without NAC treatment are the same as in Figure 1, reshown here to facilitate comparisons. (C) Sections are stained for nitrotyrosine (nTyr, green) as a marker of oxidative stress, and for tyrosine hydroxylase (TH, blue) to identify dopaminergic neurons. Bars = 50µm. (D) Quantified data show that the nTyr signal localized to dopaminergic neurons is reduced in the EAAC1−/− mice treated with NAC. **p < 0.01; n = 5–7.
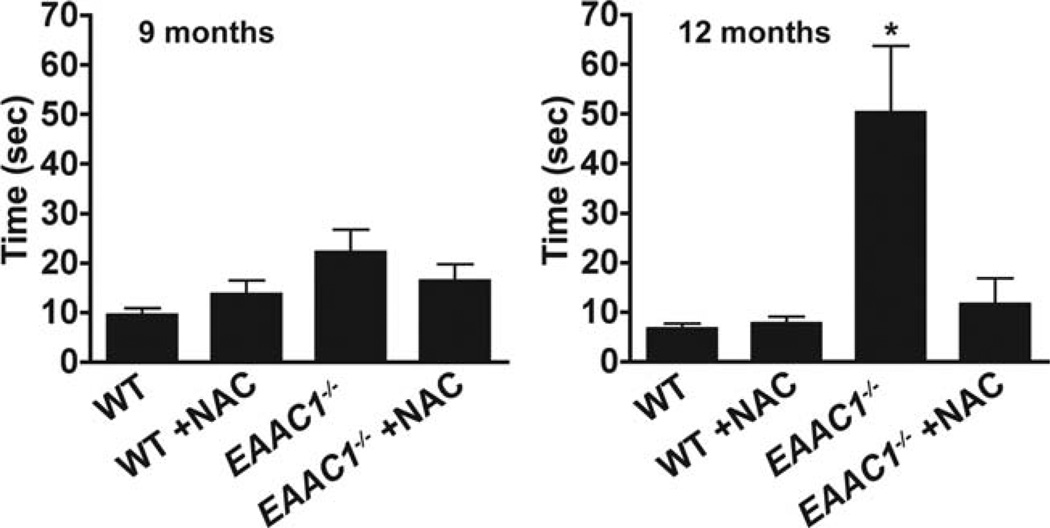
NAC Improves Pole Test Performance in EAAC1−/− Mice
Mice with chronic bilateral loss of dopaminergic neurons generally display little motor dysfunction unless the loss is very extensive.26 Here we compared W mice, untreated EAACr−/− mice, and NAC-treated EAACr−/− mice using a battery of tests of designed to detect abnormalities in the mouse nigrostriatal system: the open field test for spontaneous activity, the rotarod test of limb dexterity, and the pole test of balance and coordination.26–28 The open field test and rotarod tests showed no differences between the treatment groups (data not shown). On the pole test, however, the untreated EAAC1−/− mice performed significantly worse than the WT mice at age 12 months, and the EAAC1−/− mice treated with NAC performed significantly better than the untreated EAAC1−/−mice (Fig 7).
FIGURE 7.
N-acetyl-cysteine preserves motor function in EAAC1−/− mice. EAAC1−/− and wild-type (WT) mice were continuously treated with NAC-supplemented water (NAC) or normal water, and motor agility was evaluated by the pole test at ages 9 and 12 months. Y-axis shows the mean time required for mice to invert position and climb down the vertical pole. *p < 0.05; n = 7–10.
Discussion
Neurons do not take up extracellular glutathione directly, but instead rely primarily on glial-derived cysteine as a precursor for glutathione synthesis.14 The EAAC1−/−mouse has impaired neuronal cysteine uptake, resulting in chronic neuronal oxidative stress and age-dependent brain atrophy9. Results of the present studies show that dopaminergic neurons of the SNc are particularly affected in the EAAC1−/− mouse, with more than 40% lost by age 12 months. This neuronal loss is accompanied by increased markers of oxidative stress and by increased microglial activation. These changes were largely prevented by long-term oral administration of NAC.
Although EAAC1 is expressed by all CNS neurons,10,11 results presented here and previously indicate that EAAC1 expression is especially dense on SNc dopaminergic neurons.11,33 This increased expression may reflect a high basal requirement for glutathione synthesis in these neurons in response to an intrinsically elevated rate of oxidant production.8,38 Consistent with this idea, pharmacological inhibition of EAAC1 has been reported to produce glutathione loss and subsequent cell death selectively in the dopaminergic neurons of rat and mouse midbrain.33 Similarly, a transgenic mouse constructed by Chinta and colleagues,39 in which glutathione synthesis is impaired in catecholaminergic neurons, exhibits increased protein nitrosylation, reduced mitochondrial complex 1 activity, and a modest degree of dopaminergic cell loss. Together, these findings suggest a key role for EAAC1 in dopaminergic neuronal glutathione metabolism and a contributory role for glutathione depletion in dopaminergic neuronal death.
Many animal models used to replicate histological features of PD, such as the 6-hydroxydopamine and MPTP models, generate massive oxidative stress and cell death over a few days time.38,40,41 By contrast, the oxidative stress associated with human PD is low-grade and chronic, extending over decades. The ultimate cause of neuronal death in PD remains uncertain, but evidence suggests that it may result from accumulated nuclear and mitochondrial DNA mutations, some of which lead to additional oxidant production.8,42 The EAAC1−/− mouse provides a model of chronic neuronal oxidative stress, and could thereby provide insights into cell death mechanisms and therapeutic approaches relevant to chronic oxidative stress. The EAAC1−/− mouse does not, however, provide a good model of the motor deficits in PD; deficits were found only with the pole test, and only at 12 months of age. The paucity of motor findings may reflect the relatively modest degree of dopaminergic neuronal loss (<50%), which permits sprouting from remaining neurons and other compensatory changes.26,31,32 Moreover, oxidative stress and neuronal loss in EAAC1−/− mice also occurs to some extent in neuronal populations not significantly affected by PD,9 a factor that further complicates interpretation of motor and functional abnormalities. EAAC1 is also capable of transporting glutamate, albeit with lower affinity than cysteine.43 EAAC1 uptake of glutamate does not contribute to bulk glutamate clearance from the extracellular space, but loss of glutamate transport into neurons could have additional effects on neuronal metabolism and motor function, unrelated to glutathione homeostasis.44
It is not known whether changes in EAAC1 expression or activity occur in PD. Like many transporters, the activity of EAAC1 is highly regulated by translocation to and from the cell surface, with only a fraction of available EAAC1 being present on the cell surface at any one time.45 Several signaling pathways have been identified as having significant influence on EAAC1 cell-surface expression, including, protein kinase C, platelet-derived growth factor, syntaxin 1A, cholesterol, and the glutamate transporter associated protein 3–18 (GTRAP3–18).45–48 Of note, GTRAP3–18 has also been shown to modulate neuronal glutathione concentrations,34 suggesting that perturbations of this or other signaling pathways that influence EAAC1 cell-surface expression could, in principle, contribute to the neuronal glutathione depletion observed in PD.
Unlike cysteine, NAC can cross the blood-brain barrier and passively cross cell membranes to provide substrate for glutathione synthesis.17,18 Glutathione is the dominant reactive thiol in cells, and it is in equilibrium with other reactive thiols.49 The C5-maleimide method was used here to show that oral NAC can restore reactive thiol content in EAAC1−/− dopaminergic SNc neurons. Prior studies show that the effect of NAC on neuronal C5-maleimide staining requires glutathione synthesis, further indicating that glutathione is a major contributor to this signal.
A key finding here is that long-term oral administration of NAC prevents the neuronal death, oxidative stress, and motor abnormalities otherwise observed in the aged EAAC1−/− mice. This finding suggests that NAC or similar compounds could be used to treat disorders in which neuronal glutathione synthesis does not meet demand. NAC is currently approved for use in the United States as a mucolytic, for treatment of acetaminophen overdose, and to prevent contrast nephropathy. The drug is also currently under study for treatment of schizophrenia, amyotrophic lateral sclerosis, and other disorders.19 In addition, an extensive literature documents salutary effects of NAC in other settings involving oxidative stress.19 It remains to be established whether glutathione depletion is a primary cause of oxidative stress in PD, a result of oxidative stress, or both, but the evidence that the glutathione depletion can promote dopaminergic neuronal death, coupled with the ability of NAC to promote glutathione synthesis, provides a scientific rationale for evaluating glutathione repletion as a treatment approach for PD.
Supplementary Material
Acknowledgments
This research was supported by grants from the U.S. Department of Veterans Affairs (to R.A.S.) and the Michael J. Fox Foundation for Parkinson’s Research (to R.A.S.).
We thank Colleen Hefner for expert technical assistance, and Drs. William Marks and Graham A. Glass for their critical reviews of the manuscript.
Footnotes
Potential Conflict of Interest
Nothing to report.
References
- 1.Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson’s disease. NeuroRx. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betarbet R, Sherer TB, MacKenzie G, et al. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 3.Yoritaka A, Hattori N, Uchida K, et al. Immunohistochemical detection of 4-hydroxynonenal protein adducts in Parkinson disease. Proc Natl Acad Sci U S A. 1996;93:2696–2701. doi: 10.1073/pnas.93.7.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Floor E, Wetzel MG. Increased protein oxidation in human substantia nigra pars compacta in comparison with basal ganglia and prefrontal cortex measured with an improved dinitrophenylhydrazine assay. J Neurochem. 1998;70:268–275. doi: 10.1046/j.1471-4159.1998.70010268.x. [DOI] [PubMed] [Google Scholar]
- 5.Dexter DT, Sian J, Rose S, et al. Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease. Ann Neurol. 1994;35:38–44. doi: 10.1002/ana.410350107. [DOI] [PubMed] [Google Scholar]
- 6.Sian J, Dexter DT, Lees AJ, et al. Alterations in glutathione levels in Parkinson’s disease and other neurodegenerative disorders affecting basal ganglia. Ann Neurol. 1994;36:348–355. doi: 10.1002/ana.410360305. [DOI] [PubMed] [Google Scholar]
- 7.Schober A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- 8.Thomas B, Beal MF. Parkinson’s disease. Hum Mol Genet. 2007;16:R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 9.Aoyama K, Suh SW, Hamby AM, et al. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- 10.Rothstein JD, Martin L, Levey AI, et al. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 11.Shashidharan P, Huntley GW, Murray JM, et al. Immunohistochemical localization of the neuron-specific glutamate transporter EAAC1 (EAAT3) in rat brain and spinal cord revealed by a novel monoclonal antibody. Brain Res. 1997;773:139–148. doi: 10.1016/s0006-8993(97)00921-9. [DOI] [PubMed] [Google Scholar]
- 12.Watabe M, Aoyama K, Nakaki T. Regulation of glutathione synthesis via interaction between glutamate transport-associated protein 3–18 (GTRAP3–18) and excitatory amino acid carrier-1 (EAAC1) at plasma membrane. Mol Pharmacol. 2007;72:1103–1110. doi: 10.1124/mol.107.039461. [DOI] [PubMed] [Google Scholar]
- 13.Himi T, Ikeda M, Yasuhara T, et al. Role of neuronal glutamate transporter in the cysteine uptake and intracellular glutathione levels in cultured cortical neurons. J Neural Transm. 2003;110:1337–1348. doi: 10.1007/s00702-003-0049-z. [DOI] [PubMed] [Google Scholar]
- 14.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 15.Zeevalk GD, Razmpour R, Bernard LP. Glutathione and Parkinson’s disease: is this the elephant in the room. Biomed Pharmacother. 2008;62:236–249. doi: 10.1016/j.biopha.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 16.Offen D, Ziv I, Sternin H, et al. Prevention of dopamine-induced cell death by thiol antioxidants: possible implications for treatment of Parkinson’s disease. Exp Neurol. 1996;141:32–39. doi: 10.1006/exnr.1996.0136. [DOI] [PubMed] [Google Scholar]
- 17.Farr SA, Poon HF, Dogrukol-Ak D, et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84:1173–1183. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- 18.Dringen R, Hamprecht B. N-acetylcysteine, but not methionine or 2-oxothiazolidine-4-carboxylate, serves as cysteine donor for the synthesis of glutathione in cultured neurons derived from embryonal rat brain. Neurosci Lett. 1999;259:79–82. doi: 10.1016/s0304-3940(98)00894-5. [DOI] [PubMed] [Google Scholar]
- 19.Dodd S, Dean O, Copolov DL, et al. N-acetylcysteine for antioxi-dant therapy: pharmacology and clinical utility. Expert Opin Biol Ther. 2008;8:1955–1962. doi: 10.1517/14728220802517901. [DOI] [PubMed] [Google Scholar]
- 20.Peghini P, Janzen J, Stoffel W. Glutamate transporter EAAC-1-deficient mice develop dicarboxylic aminoaciduria and behavioral abnormalities but no neurodegeneration. EMBO J. 1997;16:3822–3832. doi: 10.1093/emboj/16.13.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mutant mice and neuroscience,: recommendations concerning background. Banbury Conference on genetic background in mice. Neuron. 1997;19:755–759. doi: 10.1016/s0896-6273(00)80958-7. [DOI] [PubMed] [Google Scholar]
- 22.Kauppinen TM, Higashi Y, Suh SW, et al. Zinc triggers microglial activation. J Neurosci. 2008;28:5827–5835. doi: 10.1523/JNEUROSCI.1236-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- 24.Hof PR, Young WG, Bloom FE, et al. Comparative cytoarchitectonic atlas of the C57BL/6 and 129/Sv mouse brains. 1st. Amsterdam: Elsevier Science B.V; 2000. p. 275. [Google Scholar]
- 25.Kauppinen TM, Suh SW, Berman AE, et al. Inhibition of poly(ADP-ribose) polymerase suppresses inflammation and promotes recovery after ischemic injury. J Cereb Blood Flow Metab. 2009;29:820–829. doi: 10.1038/jcbfm.2009.9. [DOI] [PubMed] [Google Scholar]
- 26.Meredith GE, Kang UJ. Behavioral models of Parkinson’s disease in rodents: a new look at an old problem. Mov Disord. 2006;21:1595–1606. doi: 10.1002/mds.21010. [DOI] [PubMed] [Google Scholar]
- 27.Rozas G, Lopez-Martin E, Guerra MJ, Labandeira-Garcia JL. The overall rod performance test in the MPTP-treated-mouse model of Parkinsonism. J Neurosci Methods. 1998;83:165–175. doi: 10.1016/s0165-0270(98)00078-8. [DOI] [PubMed] [Google Scholar]
- 28.Matsuura K, Kabuto H, Makino H, Ogawa N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods. 1997;73:45–48. doi: 10.1016/s0165-0270(96)02211-x. [DOI] [PubMed] [Google Scholar]
- 29.Shi SR, Key ME, Kalra KL. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohisto-chemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 30.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 31.Bezard E, Dovero S, Imbert C, et al. Spontaneous long-term compensatory dopaminergic sprouting in MPTP-treated mice. Synapse. 2000;38:363–368. doi: 10.1002/1098-2396(20001201)38:3<363::AID-SYN16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 32.Iravani MM, Syed E, Jackson MJ, et al. A modified MPTP treatment regime produces reproducible partial nigrostriatal lesions in common marmosets. Eur J Neurosci. 2005;21:841–854. doi: 10.1111/j.1460-9568.2005.03915.x. [DOI] [PubMed] [Google Scholar]
- 33.Nafia I, Re DB, Masmejean F, et al. Preferential vulnerability of mesencephalic dopamine neurons to glutamate transporter dysfunction. J Neurochem. 2008;105:484–496. doi: 10.1111/j.1471-4159.2007.05146.x. [DOI] [PubMed] [Google Scholar]
- 34.Watabe M, Aoyama K, Nakaki T. A dominant role of GTRAP3-18 in neuronal glutathione synthesis. J Neurosci. 2008;28:9404–9413. doi: 10.1523/JNEUROSCI.3351-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 36.Hodara R, Norris EH, Giasson BI, et al. Functional consequences of alpha-synuclein tyrosine nitration: diminished binding to lipid vesicles and increased fibril formation. J Biol Chem. 2004;279:47746–47753. doi: 10.1074/jbc.M408906200. [DOI] [PubMed] [Google Scholar]
- 37.Duda JE, Lee VM, Trojanowski JQ. Neuropathology of synuclein aggregates. J Neurosci Res. 2000;61:121–127. doi: 10.1002/1097-4547(20000715)61:2<121::AID-JNR1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 38.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence. Nat Med. 2004;10(suppl):S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 39.Chinta SJ, Kumar MJ, Hsu M, et al. Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J Neurosci. 2007;27:13997–14006. doi: 10.1523/JNEUROSCI.3885-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melrose HL, Lincoln SJ, Tyndall GM, Farrer MJ. Parkinson’s disease: a rethink of rodent models. Exp Brain Res. 2006;173:196–204. doi: 10.1007/s00221-006-0461-3. [DOI] [PubMed] [Google Scholar]
- 41.Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson’s disease. Bioessays. 2002;24:308–318. doi: 10.1002/bies.10067. [DOI] [PubMed] [Google Scholar]
- 42.Bender A, Krishnan KJ, Morris CM, et al. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- 43.Zerangue N, Kavanaugh MP. Interaction of L-cysteine with a human excitatory amino acid transporter. J Physiol. 1996;493:419–423. doi: 10.1113/jphysiol.1996.sp021393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sepkuty JP, Cohen AS, Eccles C, et al. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J Neurosci. 2002;22:6372–6379. doi: 10.1523/JNEUROSCI.22-15-06372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fournier KM, Gonzalez MI, Robinson MB. Rapid trafficking of the neuronal glutamate transporter, EAAC1: evidence for distinct trafficking pathways differentially regulated by protein kinase C and platelet-derived growth factor. J Biol Chem. 2004;279:34505–34513. doi: 10.1074/jbc.M404032200. [DOI] [PubMed] [Google Scholar]
- 46.Yu YX, Shen L, Xia P, et al. Syntaxin 1A promotes the endocytic sorting of EAAC1 leading to inhibition of glutamate transport. J Cell Sci. 2006;119:3776–3787. doi: 10.1242/jcs.03151. [DOI] [PubMed] [Google Scholar]
- 47.Canolle B, Masmejean F, Melon C, et al. Glial soluble factors regulate the activity and expression of the neuronal glutamate transporter EAAC1: implication of cholesterol. J Neurochem. 2004;88:1521–1532. doi: 10.1046/j.1471-4159.2003.02301.x. [DOI] [PubMed] [Google Scholar]
- 48.Lin CI, Orlov I, Ruggiero AM, et al. Modulation of the neuronal glutamate transporter EAAC1 by the interacting protein GTRAP3-18. Nature. 2001;410:84–88. doi: 10.1038/35065084. [DOI] [PubMed] [Google Scholar]
- 49.Jones DP, Go YM, Anderson CL, et al. Cysteine/cystine couple is a newly recognized node in the circuitry for biologic redox signaling and control. FASEB J. 2004;18:1246–1248. doi: 10.1096/fj.03-0971fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.