Abstract
Prostate cancer is the most commonly diagnosed cancer in men and is the second leading cause of cancer-related deaths in men each year. Androgen-deprivation therapy is and has been the gold standard of care for advanced or metastatic prostate cancer for decades. While this treatment strategy initially shows benefit, eventually tumors recur as castration-resistant prostate cancer (CRPC) for which there are limited treatment options with only modest survival benefit. Upregulation of the insulin-like growth factor receptor type I (IGF-IR) signaling axis has been shown to drive the survival of prostate cancer cells in many studies. As many IGF-IR blockades have been developed, few have been tested pre-clinically and even fewer have entered clinical trials for prostate cancer therapy. In this review, we will update the most recent pre-clinical and clinical studies of IGF-IR therapy for prostate cancer. We will also discuss the challenges for IGF-IR targeted therapies to achieve clinical benefit for prostate cancer.
Keywords: IGF-IR, Prostate Cancer, Metastasis, Mechanisms, Therapy
1 Introduction
Prostate cancer is still the most commonly diagnosed cancer in men and remains as the second leading cause of cancer-related deaths in men each year, with an estimate of over 29,000 deaths in 2013 (1). Androgen-deprivation therapy, either medical or surgical, remains the backbone for advanced or metastatic prostate cancer treatment. While this treatment strategy initially has benefit, eventually tumors become resistant to castration, necessitating additional therapeutic interventions. Improved understanding of mechanisms driving castration-resistance has led to development of multiple new therapies and improved outcomes. However, survival is still limited, and targeting of other dysregulated signaling pathways that promote prostate cancer cell proliferation, invasion, and survival are being explored. This review will focus on one of these pathways, specifically type I insulin-like growth factor receptor (IGF-IR) signaling and its role in prostate cancer, as well as early pre-clinical and clinical studies to inhibit this pathway with goal of anti-tumor effect.
IGF-IR activation and signaling are mainly stimulated by binding to its ligands. IGF-IR displays a hierarchical binding preference to its ligands, IGF-I > IGF-II > Insulin (2, 3). IGF-IR signaling is essential for development, survival, and proliferation of many cell types (4, 5). IGF-IR is expressed in the entire body and has important roles in cell cycle, anaerobic breathing, growth (in children), and aging (4–7). Deregulation of IGF-IR signaling, including dysregulation in IGF-IR expression itself and the ligands (IGF), has been suggested to significantly impact cancer development and progression (8). In addition, IGF-IR signaling is known to be a critical determinant of response to cancer therapy, such as radiation and chemotherapy (9–11). Increased serum levels of ligand IGF-I have been associated with increased risk and mortality of many cancer types (8, 12, 13). The expression and prognostic value of IGF-IR in malignant progression, however, has been controversial and seems to be cancer type-dependent. However, in most cancer types, such as colorectal, gastric, endometrial, and breast cancers, overexpression of IGF-IR has been associated with an aggressive phenotype, tumor progression and therapy-resistance and poor clinical outcome. In particular, targeting IGF-IR as a therapy has been extensively studied and previously reviewed in breast cancer (14).
Data from epidemiological and clinical pathological studies have generally agreed that IGF-IR signaling is elevated in prostate cancer in comparison to benign prostate tissue (15–19). However, IGF-IR expression throughout prostate cancer progression, especially with metastasis, is controversial. Analysis of clinical specimens show elevated IGF-IR expression in primary prostate cancer versus benign prostate epithelium, and the trend is persistent up through metastatic diseases (20). A majority of human prostate xenograft studies also show increased IGF-IR expression in metastatic and AI diseases over primary tumors (21, 22). Other studies with different prostate cancer models have shown conflicting results that decrease in IGF-IR expression is necessarily for progression to AI and metastatic diseases (23, 24).
Paradoxically, reports on the individual value of IGF-IR expression and serum levels of IGF in relation to prostate cancer etiology and progression are inconsistent and somewhat controversial. A large amount of clinical studies resulting from prospective and case-controlled investigation suggest a significant correlation of elevated serum levels of IGF-I with increased prostate cancer risk (13, 25–32). On the contrary, a number of other studies did not support this correlation (33, 34). A more recent large nested case-control study with 630 cases of prostate cancer and control subjects concluded that high serum levels of IGF-I only had a trend of increased risk of prostate cancer, but was not statistically significant. Instead, the study found a significant correlation of high serum IGF-I with risk of more progressive diseases (35). More provocatively, transgenic mice with prostate-specific deletion of IGF-IR in the context of compromised function of tumor suppressor gene p53 developed more aggressive prostate carcinoma than wild-type counterparts (36).
These controversial reports in regards to IGF-IR expression and relationship with prostate cancer risk and progression may arise from the large amount of heterogeneity within prostate cancers, sampling bias, methods of evaluation, and/or the unique experimental system being studied. Also, an individual evaluation of IGF-IR expression or serum levels of IGF may not be sufficient to assess the association with cancer risk or progression. A more comprehensive evaluation of IGF-IR signaling pathways is needed. Nevertheless, these paradoxical observations suggest that subject-based intrinsic dysregulation of IGF-IR expression may be necessary for development of more aggressive phenotype of prostate cancer after initial transformation. Inevitably, this complexity of IGF-IR expression and signaling during prostate cancer disease progression emphasizes the dilemma and potential difficulty in effectively targeting IGF-IR for prostate cancer therapy in clinical practice. In this review, we will focus on the current understanding of IGF-IR signaling in prostate cancer and potential complexity in clinical targeting. We will also summarize recent and ongoing pre-clinical and clinical studies in targeting IGF-IR for prostate cancer therapy and strengthen the concept that co-targeting IGF-IR along with other important pathologic processes may achieve maximum therapeutic benefit for prostate cancer. Key questions to yet be answered in effectively targeting IGF-IR for prostate cancer therapy will also be addressed.
2 IGF-IR structure and signaling
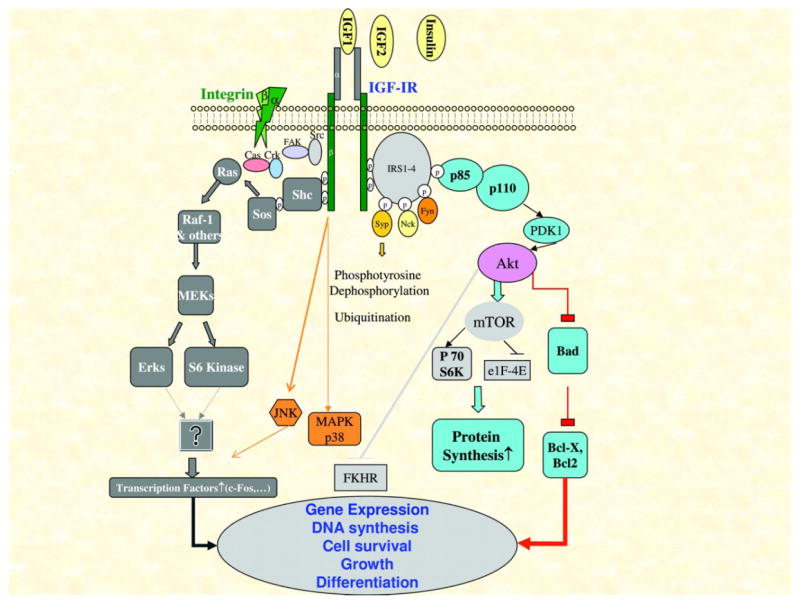
The IGF-IR is a tetrameric transmembrane receptor tyrosine kinase composed of two alpha and two beta subunits linked by disulfide bond (Fig 1). The alpha subunit is extracellular and responsible for ligand binding, whereas the beta subunit composed of the transmembrane and cytoplasmic region is critical for intracellular signaling. The fully functional IGF-IR can be activated by three ligands, IGF-I, IGF-II, and insulin. Among which, IGF-I has the highest affinity and has been mostly studied in association with cancer risk and progression. Insulin has the lowest affinity for IGF-IR and has not been extensively studies in association with cancer progression (6, 37, 38). IGF-IR has 70% homology to the insulin receptor (IR), with which it shares some of the signaling pathways (38). Upon ligand binding to the alpha subunit, the intrinsic tyrosine kinase activity autophosphorylates tyrosine residues in the beta subunit and thus initiates a cascade of down-stream signaling events to activate IRS-1/PI-3 Kinase and PKB/Grb2/sos/Ras/MapK pathways (6). Activation of these pathways has been shown to be important in cell proliferation, survival, and transformation. It has also been documented as a mechanism in cancer metastasis and resistance to therapy (9–11, 39, 40).
Figure 1.
IGF-IR signal transduction cascade upon ligand activation. A schematic representation of the major signaling pathways and the biological out resulted from IGF-IR activation. [Adopted from Samani A A et al. Endocrine Reviews 2007;28: 20–47.]
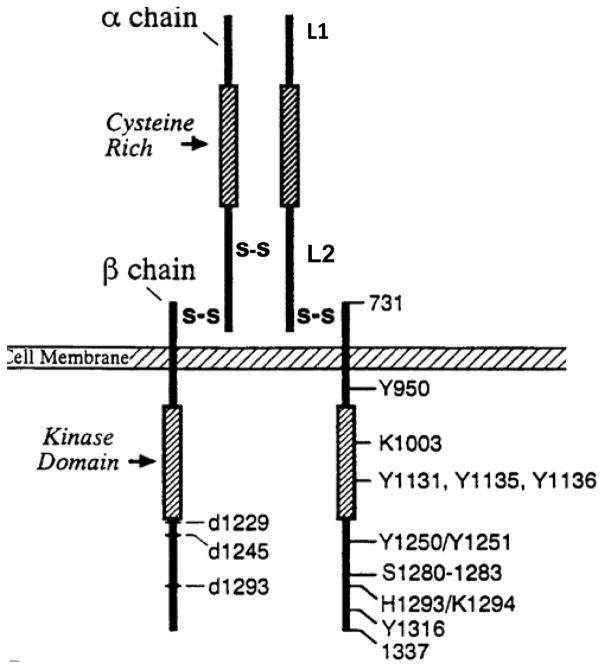
The beta-subunit is critical for full activation of IGF-IR. The structure-functional relation of IGF-IR beta subunit has been extensively studies by Beserga’s group using a variety of beta-subunit mutant receptors (Fig 2). A mutation in tyrosine 950 (Y950) or the C-terminus of the IGF-IR (roughly the last 100 amino-acids) was shown to be dispensable for mitogenesis and protection from apoptosis, but is required for differentiation and the transformation of cells in vitro and in vivo (41, 42). A mutation at the cytoplasmic lysine 1003 (the ATP-binding site) resulted in complete loss of receptor function (41). A triple mutation in the tyrosine kinase domain (Y1131, Y1135 and Y1136) resulted in defective receptor, which failed to transmit a mitogenic signal (43, 44). Based on the understanding of the functional consequences of IGF-IR, targeting the IGF-IR kinase domain has been proposed for cancer therapy (6).
Figure 2.
Structure and Function of IGF-IR. The IGF-IR ectodomain contains two homologous domains (L1 and L2), separated by a Cys-rich region (Cys152 to Cys298) containing 22 cysteine residues. Intracellularly, each IGF-IR monomer contains a tyrosine kinase catalytic domain (residues 973–1229) flanked by two regulatory regions: a juxtamembrane region, residues 930–972, and the C-tail, residues 1230–1337 that contain the phosphotyrosine binding sites for signaling molecules.
3 Regulation of IGF-IR signaling in prostate cancers
The molecular mechanism of how IGF-IR signaling is differentially regulated during prostate cancer development and progression is not well defined and remains to be an active investigation focus. To date studies are largely focusing on regulation of IGF-IR expression in prostate cancer cells at the transcriptional level although sporadic studies have reported the regulation at a post-transcriptional level. Similar to studies in many other cancer types, regulation of IGF-IR transcripts in prostate cancer cells has been reported to be mainly mediated by defective tumor suppressor genes, such as BRCA1 and transcriptional factor Kruppel-like factor 6 (KLF6) (45–47), epigenetic changes such as methylation of master regulators (48), or IGF-IR autoregulation through translocating to the nucleus (49). In this review, we will focus on tumor-suppressor-mediated regulation. We will also summarize studies on androgen-mediated regulation of IGF-IR signaling in prostate cancer.
The promoter region of IGF-IR lacks the transcriptional regulatory elements TATA or CAAT box (50, 51). Like many genes that lack these regulatory elements, the proximal 5′-flanking region of the IGF-IR promoter region is highly GC-rich and contains multiple binding sites for zinc finger transcriptional factors (52). KLF6, a ubiquitous transcriptional factor and a tumor suppressor gene, has been shown to transactivate IGF-IR gene transcription through interaction with the zinc finger protein Sp1 and tumor suppressor p53 (45). The KLF6 gene is located at the chromosomal region 10p that is deleted in most sporadic prostate cancers (53). A large percentage of prostate tumors displayed loss of heterozygosity (LOH) at the KLF6 locus and mutations in the KLF6 alleles (54). Forced expression of tumor-associated mutated KLF6 led to the defect in its ability to transactivate IGF-IR transcription (55). Liu et al. discovered spliced variants of KLF6 in human prostate tumors using microdissection and array analyses and further demonstrated that androgen-dependent LnCaP cells with forced expression of KLF6 loss-of-function splicing variants displayed a survival advantage in the culture when androgen was withdrawn (55). Thus, the loss-of-function mutation of KLF6 has been implicated in prostate cancer progression to androgen-independence. Given the evidence that decrease in IGF-IR expression is associated with development of more aggressive phenotype of prostate cancer, these studies suggest that dysregulation of IGF-IR expression through KLF6 loss-of-function may be an intrinsic mechanism for prostate cancer progression to hormone independence.
In prostate cancer cells, the tumor suppressor BRCA1 is shown to interact with androgen receptor (AR) and regulate IGF-IR expression in an AR-dependent fashion (51). BRCA1 was originally identified as the familial breast and ovarian cancer susceptibility gene-1 that encodes a 220kDa phosphorylated transcriptional factor with tumor suppressor activity (56). BRCA1 mutation was initially found to be associated with the risk of breast and ovarian cancer at very young age and with the etiology of sporadic type of cancers (56–58). BRCA1 is normally targeted to the nucleus and participates in regulation of transcription and DNA damage repair pathways (19, 59). BRCA1 is expressed at a low level in normal prostate epithelium and is upregulated in prostate carcinoma (51). In AR-negative prostate cancer cell lines, an inverse correlation of BRCA1 expression and IGF-IR expression has been found. In subsequent studies, BRCA1 can suppress IGF-IR promoter activity in AR-negative M12 prostate cancer cell lines. In AR-positive prostate cancer LuCaP and C4-2 cell lines, BRCA1 has been shown to enhance AR transcriptional activity and enhance IGF-IR expression at the transcriptional level (46).
Beyond these commonly recognized regulatory mechanisms, in prostate cancers, androgen has also been consistently shown to upregulate IGF-IR expression although the mechanisms are somewhat controversial by different studies, potentially due to the discrepancy in different cell lines. Pandini et al showed that engagement of AR by androgen upregulates IGF-IR expression in AR+ LuCap cells and PC-3 cells overexpressing AR (60). They also showed AR mutants that lost DNA binding and transcriptional activity were still able to upregulate IGF-IR expression in HEK293 kidney cells in response to androgen. Based on these observations, Pandini et al concluded that AR regulates IGF-IR expression through non-genomic pathways. Recently, Schayek et al. (2010) consistently showed that re-expression of wild-type androgen-receptor (AR) in an AR-negative metastatic prostate cancer cell line M12 led to significant increase in IGF-IR expression (61). However, when they expressed two AR mutants that were compromised in the ability to bind to androgens or co-regulators, no effect on IGF-IR expression was seen. Schayek et al. thus concluded that androgens regulate IGF-IR expression through a genomic pathway. To further support this conclusion, they further showed that AR can directly bind to IGF-IR promoter region by CHIP assay.
4 IGF-IR signaling in Prostate Cancer Progression to Androgen-independence
The vast majority of patients with recurrent prostate cancer are responsive to standard androgen deprivation therapy. However, the disease eventually progresses to castration-resistance. Multiple mechanisms have been proposed to convey the progression (62, 63), including; 1) epigenetic mutations of androgen receptor to allow androgen-independent activation; 2) alternative splicing of AR to delete ligand-binding domain thus and allow constitutive activation of AR; 3) transactivation of AR by growth factors and cytokines; 4) sustained intratumoral androgen level through intracrine steroidogenic pathways within the prostate tumor microenvironment (64, 65). IGF-IR signaling to activate PI3K/AKT pathway has been proposed to be one of the mechanisms to transactivate AR in the absence of androgen and progression to AI diseases (66–69). Increased AKT activity has been shown to be associated with prostate cancer progression to castration-resistance through multiple pathways (70–74), including direct phosphorylation of AR, activation of wnt/GSK3 pathway, activation of NF-kB pathway, and forkhead box-O (FOXO) family of transcriptional factors. These studies suggest that co-targeting IGF-IR pathways and androgen deprivation may offer synergistic therapeutic benefits for prostate cancer.
5 IGF-IR signaling in prostate cancer metastasis
The mechanism of IGF-IR signaling in mediating prostate cancer metastasis is a complex and largely site-specific. In addition, prostate cancer cells can achieve metastatic potential through IGF-IR signaling to modify cell adhesion and mobility, independent of tumor cell growth potential (75). These complexities must be taken into when considering evaluation of IGF-IR targeting therapy for metastatic prostate cancer.
IGF-IR was shown to regulate cancer lymphatic metastasis through facilitating angiogenesis and lymphangiogenesis, by induction of VEGF-C expression (76). This has been confirmed with high level of VEGF-C detected in prostate cancer patients with lymph node metastasis (77). Li et al further showed that androgen-deprivation can upregulate VEGF-C expression through down-regulation of the IGF-IR pathway and activate the forkhead transcriptional factor FOXO-1 (74). In bone metastasis, bone-derived IGF-I can bridge the crosstalk between bone and metastasized cancer cells via activation of the IGF-IR/Akt/NF-κB pathway (78). Therefore, disruption of IGF-IR and NK-kB pathways may represent a promising therapeutic intervention for bone metastasis, whereas co-targeting IGF-IR and VEGF may be more effective to treat lymphatic metastasis. In liver metastasis, IGF-IR-mediated mechanism of cancer cell survival is critical for metastatic colonization (79). Normal liver cells do not express IGF-IR but secret large amount of IGF-I. When circulating cancer cells were drained to the liver, the IGF-I rich environment allows the colonization and growth of circulating tumor cells to establish metastasis. Thus, the mechanism of liver metastasis has no association with overexpression of IGF-IR on primary tumors and is more related to a hospitable liver microenvironment. In this instance, specific targeting IGF-IR signaling pathways may effectively inhibit metastatic tumor growth in the liver.
Transactivation of IGF-IR also plays a significant role in mediating inflammation-associated prostate cancer metastasis. We recently showed that chronic exposure of benign non-tumorigenic prostate epithelium cell line p69 to exogenous IL-6 can induce epithelium-mesenchymal transition and facilitate metastatic potential through transactivation of IGF-IR signaling and IL-6 autocrine pathways (80). Blocking IGF-IR signaling with an antibody IMC-A12 (cixutumumab) abolishes the effect of IL-6 and abrogates IL-6 signaling in these cells. Whether inhibition of IGF-IR can suppress IL-6-mediated metastasis in vivo and what-specific IL-6 signaling pathways could be co-targeted to suppress inflammation-mediated metastasis would be logical areas of exploration.
6 Pre-clinical studies of targeting IGF-IR for cancer therapy
IGF-IR inhibitory reagents, including a large variety of human monoclonal antibodies and few small molecules, have been developed for potential cancer therapy with distinct targeting mechanisms: target IGF-IR ligands, blocking of ligand binding, inhibits IGF-IR tyrosine kinase activity, or down regulation of IGF-IR expression through receptor internalization and degradation. These reagents have been tested in multiple pre-clinical cancer models and some are in clinical trial for multiple cancer types (81–83). Among these reagents, only few have been tested therapeutically with pre-clinical human prostate cancer models (Table 1). All the studies suggested that monotherapy with an IGF-IR antagonist can only achieve limited effect and that combined therapy of an IGF-IR antagonist with androgen deprivation therapy or cytotoxic chemotherapeutics deliver more desirable outcomes. However, all of these studies were limited to evaluation of primary tumor growth.
Table 1.
Preclinical Targeting IGF-IR signaling in Prostate Cancer
| Antagonist | Property | Model | Therapy Effect | Ref |
|---|---|---|---|---|
| IMC-A12 (Cixutumumab) | Full human mAb induce IGF-IR internalization | LuCaP35-AD and LuCaP35v-AI | Inhibits AD and AI tumor growth by inducing cell cycle arrest Enhance castration effect on AD tumor growth Combined therapy with Docetaxel enhanced treatment effect on AI LuCaP35V and LuCaP 23.1 in comparison to monotherapy |
(86–88) |
| AMG479 (Genitumab) | Full human mAb, Inhibits IGF binds to IGF-IR | VCaP and CWR22Rv1 | Treatment alone retard VCaP growth with enhanced therapeutic effect when combined with castration. No effect on AI CWR- 22RV1 | (91, 92) |
| ATL 1101 | Antisense oligonucleotide, suppress IGF-IR expression | LNCaP and PC-3 | Monotherapy suppress PC-3 tumor growth and delays LNCaP progression to CRPC | (93) |
Cixutumumab (formerly IMC-A12), a fully human IgG1 monoclonal antibody, was shown to induce internalization of IGF-IR (84, 85). Cixutumumab was tested extensively for prostate cancer therapy with preclinical androgen-dependent (AD), androgen-independent (AI), and osseous human prostate cancer models (86–88). Alone, cixutumumab can induce cell cycle arrest of both AD and AI tumor cells and the effect of castration can be enhanced by delay of progression to AI tumors (86, 88). Cixutumumab can also enhance the effect of docetaxel as combined therapy through enhanced negative regulation of genes associated with cell cycle progression, survival and therapeutic resistance (87). Goel et al recently showed that the VEGF/VEGF receptor neuropilin-2 (NRP2) signaling can repress the expression of IGF-IR at transcriptional level and thus IGF-IR signaling in prostate cancer cells (89). Combined therapy of co-targeting NRP2 with shRNA and IGF-IR with cixutumumab resulted in synergistic effect and complete inhibition of PC-3 tumor growth in vivo, whereas mono-therapy only showed minimal or moderate effect (89). Because of the compensatory relation between NRP2 and IGF-IR expression, NRP2 is proposed to be a robust biomarker for predicting responses to IGF-IR therapy (89).
Ganitumab (formerly AMG 479), a fully human antibody that inhibits binding of IGF-I and IGF-II to IGF-IR (90), is a newly developed IGF-IR blockade that has been tested for prostate cancer therapy in preclinical models. Ganitumab was shown to inhibit ligand-induced phosphorylation of IGF-IR and the downstream effector AKT resulting in reduced proliferation of multiple androgen-dependent and castration-resistant human prostate cancer cell lines in vitro (91). It was shown that ganitumab treatment alone retarded androgen-dependent VCaP prostate tumor growth and blocked the growth of castration-resistant VCaP xenografts for over 11.5 weeks of treatment. When combined with castration, ganitumab showed a potent therapeutic effect and achieved a long period suppression of VCaP xenograft growth (91). Ganitumab alone did not have appreciable therapeutic effect in established castration-resistant CWR-22Rv1 xenograft tumor. However, very recent study by Galet et al showed that when combined with calorie restricted diet, Ganitumab showed significant inhibition of tumor growth of CWR-22Rv1 xenografts (92).
To date the only small molecule targeting human IGF-IR for prostate cancer therapy was ATL1101, a 2′-MOE-modified antisense oligonucleotide. In vitro study showed that ATL1101 suppressed proliferation and increased apoptosis in PC-3 and LNCaP cells in androgen-deprived conditions. ATL1101 also showed in vivo suppression of PC-3 tumor growth and delaying castration-resistant progression of LNCaP xenografts (93).
7 Perspectives and challenges in clinical translation of targeting IGF-IR
Knowledge surrounding IGF-IR signaling in prostate cancer cell survival and the inhibitory effect of IGF-IR blocking antibodies or antagonists on the growth of human prostate cancer xenografts in pre-clinical animal models imply that IGF-IR may be an effective target for prostate cancer treatment, in particular in combination with other current standard care therapeutics. However, translation into clinical application for effectively treating prostate cancer patients may face various challenges in selection of optimal subject as well as evaluating clinical outcomes. First, as we have mentioned earlier in the introduction, reports to-date pertaining whether IGF-IR expression is persistent during prostate cancer progression are inconsistent and controversial to some extent. This ambiguity raises the question whether targeting IGF-IR can only be selectively effective at certain disease states. If reducing IGF-IR signaling is necessary for prostate cancer progression to metastasis as have been shown by some studies (23, 24, 36), targeting IGF-IR at the inappropriate stage may result in an unfavorable clinical outcome. Second, as activation of IGF-IR is ligand dependent, valid serological or tissue markers, such as serum levels of IGF or tissue levels of activation of IGF-IR pathway, would be beneficial to distinguish potential responders versus non-responders. This issue has not been sufficiently addressed in pre-clinical studies to date. Third, as IGF-IR signaling regulates cancer cell metastasis through modulating cell mobility, independent of cell growth (75–77), evaluating whether inhibition of IGF-IR indeed withholds or delays disease progression to metastasis is clinically difficult, as prior studies in this prostate cancer disease state have necessitated extremely large studies with many patient screen failures with modest to no success (94–98). These critical challenges likely need to be resolved to achieve positive clinical outcomes with IGF-IR targeting therapy; we suggest better understanding of IGF-IR expression through metastatic biopsy studies and development/refinement of blood-based biomarkers for patient selection.
8 Clinical experience with IGF-IR inhibition for cancer therapy
Although there are many approaches to inhibition of IGF-IR, the most frequently tested approach in the clinic has been the utilization of monoclonal antibodies directed towards the external binding domain of the receptor. Although, multiple monoclonal antibodies have entered the clinical trial domain (see Table 2), only two have seen extensive testing in prostate cancer.
Table 2.
Agents targeting IGF-IR in clinical trials (derived from clinicaltrials.gov on June 20, 2013)
| Drug | Mechanism | Phase | Malignancies |
|---|---|---|---|
| Cixutumumab (IMC- A12) | IgG1 monoclonal antibody | II | Prostate, breast, non-small cell lung, neuroendocrine, mesothelioma, head & neck squamous, esophageal, colon, hepatocellular, thymic, sarcoma, adrenocorticol |
| Figitumumab (CP-751,871) | IgG2 monoclonal antibody | II | Prostate, breast, non-small cell lung, small cell lung, colon, myeloma |
| Ganitumab (AMG 479) | IgG1 monoclonal antibody | II | Breast, non-small cell lung, small cell lung, colon, pancreatic, PNET/carcinoid, ovarian, Ewing’s sarcoma |
| Dalotuzumab (MK-0646) | Monoclonal antibody | II | Breast, non-small cell lung, small cell lung, colon, NET, myeloma |
| RG1507 | Monoclonal antibody | II | Breast, non-small cell lung, sarcoma |
| AVE 1642 | Monoclonal antibody | I | Breast, hepatocellular, myeloma |
| Linsitinib (OSI 906) | Monoclonal antibody with dual IGF-IR and insulin receptor inhibition | III | Prostate, non-small cell lung, small cell lung, head & neck squamous, colon, hepatocellular, pancreatic, myeloma, adrenocorticol |
| Picropodophyllin (AXL 1717) | Small molecule | II | Non-small cell lung, astrocytoma |
| BI 836845 | IGF ligand neutralizing antibody | I | Non-specific solid tumors |
Cixutumumab was initially tested in a phase 2 monotherapy trial where it was dosed intravenously at 10 mg/kg every 2 weeks in men with metastatic, asymptomatic castration-resistant prostate cancer (CRPC) resulted in disease stabilization for ≥6 months in 9 of 31 (29%) patients with hyperglycemia noted in 6 (19.4%), none of which required treatment discontinuation (99). In a 10 patient dosing-schedule expansion, patients were treated with 20 mg/kg every 3 weeks, with 3 (30%) experiencing disease stabilization for ≥6 months (100). Since these were non-randomized trials, it is difficult to know the true antitumor efficacy of cixutumumab versus patient selection in the castration-resistant setting.
A targeted approach to maximize selection of patients with tumor biology driven by the IGF-IR axis in CRPC may be relevant. An ongoing phase 1–2 trial for metastatic CRPC combining cixutumumab with temsirolimus, a mTOR inhibitor, aims to address the paradoxical activation of IGF/AKT signaling seen with inhibition of PI3K/AKT/mTOR signaling (101). Since single agent mTOR inhibition has not been incredibly successful against prostate cancer, the approach to inhibit IGF-IR in combination could address a potential resistance mechanism.
With pre-clinical data supporting both apoptosis and G1 cell arrest in castration-sensitive C42B xenografts and only G2 arrest in castration-resistant murine models, it also makes sense to shift the treatment paradigm with IGF-IR inhibitors to a castration-sensitive disease state (88). In a neoadjuvant trial with cixutumumab combined with goserelin and bicalutamide, serum levels of growth hormone, IGF-1, IGF-II, IGFBP-3, c-peptide and insulin increased while IGFBP-1 levels decreased significantly, without change in glucose when compared to control samples from patients in a concurrent clinical trial of neoadjuvant ADT alone (102). This showed pharmacodynamic effect, although no clear correlation could be drawn with PSA levels. A recently accrued large randomized, phase 2 trial (SWOG 0925) has treated over 200 men with newly diagnosed castration-sensitive metastatic prostate cancer either with combined ADT and cixutumumab or with combined ADT alone (NCT01120236). The primary endpoint will be evaluation of the previously described 7-month absolute PSA value as a surrogate for survival (103). Since this is the only randomized trial with IGF-IR inhibitors, we may be able to gain a sense of clinically-relevant efficacy from this cooperative group trial, especially since cixutumumab is administered in combination with ADT in the newly metastatic, previously untreated disease state. Should this trial show an improvement in the undetectable PSA endpoint at 7 months in the cixutumumab treated patients, an additional, large randomized phase 3 trial with a survival endpoint will likely be necessary for IGF-IR targeting therapy to achieve regulatory approval. However, should this trial ultimately be negative, multiple blood-based biomarkers are being evaluated to include circulating tumor cells, microRNA profiles and insulin, IGF-1, free IGF-1, growth hormone and IGFBP-3; these biomarkers may shed light on patient populations apt to have improved outcomes with cixutumumab.
Figitumumab is a highly specific IgG2 monoclonal antibody specific to IGF-IR that may have additional efficacy when added to docetaxel in men with mCRPC; however, it is difficult to ascertain the additional efficacy of IGF-IR inhibition in an open label, phase Ib dose escalation trial (104). Yet, in a small 14 patient phase II trial of preoperative figitumumab in the castration-sensitive setting, not only was pharmacodynamic activity shown by decrease in IGF-IR immunohistochemistry expression, but androgen receptor expression was also concurrently decreased (105). Most impressive is the fact that treatment with single-agent figitumumab induced ≥25% and ≥50% PSA declines from baseline in 94% and 31% of patients, respectively. This trial not only proves pharmacodynamic effect of figitumumab inhibition of IGF-IR in castration-sensitive prostate cancer tissue, but also highlights effect on clinically meaningful endpoints.
In addition to the above monoclonal antibodies, there are many other agents undergoing clinical trial testing that inhibit IGF-IR. Multiple other mechanistic approaches have involved small molecule inhibition of IGF-IR, dual monoclonal antibody inhibition of IGF-IR with the insulin receptor and IGF ligand neutralization. The agents and specific diseases they are being tested in are listed in Table 2.
9 Conclusions
The landscape for prostate cancer is changing quickly with the introduction of multiple new agents that prolong survival, all with unique mechanisms of action. However, resistance eventually develops and our understanding of the mechanisms driving resistance is limited. Additionally, most new agents are being used in patients with very advanced castration-resistant disease, and we will likely have to better understand biologically rationale combinations to effect overall cure rates. Therefore, new agents that address alternative pathways are critical.
IGF-IR is one of the pathways with accumulating epidemiologic, pre-clinical and early clinical evidence to support this approach. Although results in the castration-resistant setting have been modest at best, pre-clinical evidence offers more hope in an earlier setting when the disease is still castration-sensitive, and clinical trials are ongoing to confirm pre-clinical findings. Our assessment, however, is that the approach to IGF-IR inhibition is probably best achieved in combination, either early with the initiation of ADT or perhaps with more extensive study with cytotoxic chemotherapy. However, whenever combination therapy is attempted, it is difficult to recognize clear efficacy in a single-arm trial without a comparator. For that reason, results from the fully-accrued SWOG 0925 trial will be critical, since it will provide randomized data from a castration-sensitive patient population and will be accompanied by many biomarker studies. We recommend only proceeding in the future with IGF-IR-targeted therapies in combination with rational agents in randomized trials supported by clear pre-clinical evidence of biologic synergism or at least additive effect. These future trials should be bolstered with supportive tissue and blood-based biomarkers to aid interpretation of overall result and inform on likely biologic heterogeneity present in regards to the IGF-IR axis in patients with prostate cancer.
10 Key unanswered questions.
The controversial role of IGF-IR expression and signaling in prostate cancer progression is needed to understand the true relationship with IGF-IR.
Better understanding of IGF-IR regulation is necessary to understand potential other targets or co-targets to affect IGF-IR activity.
What is the best and most clinically meaningful approach to inhibit IGF-IR?
What is the best agent to combine IGF-IR inhibition with to induce the greatest clinical activity?
Do pre-clinical models of IGF-IR inhibition predict what we will see in human clinical trials of analogous settings?
What are valid biomarkers that might predict clinical response to IGF-IR inhibition?
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Gennigens C, Menetrier-Caux C, Droz JP. Insulin-Like Growth Factor (IGF) family and prostate cancer. Critical reviews in oncology/hematology. 2006;58:124–45. doi: 10.1016/j.critrevonc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Kojima S, Inahara M, Suzuki H, Ichikawa T, Furuya Y. Implications of insulin-like growth factor-I for prostate cancer therapies. International journal of urology: official journal of the Japanese Urological Association. 2009;16:161–7. doi: 10.1111/j.1442-2042.2008.02224.x. [DOI] [PubMed] [Google Scholar]
- 4.Baserga R, Porcu P, Rubini M, Sell C. Cell cycle control by the IGF-1 receptor and its ligands. Advances in experimental medicine and biology. 1993;343:105–12. doi: 10.1007/978-1-4615-2988-0_11. [DOI] [PubMed] [Google Scholar]
- 5.Adams TE, Epa VC, Garrett TP, Ward CW. Structure and function of the type 1 insulin-like growth factor receptor. Cellular and molecular life sciences: CMLS. 2000;57:1050–93. doi: 10.1007/PL00000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. International journal of cancer. Journal international du cancer. 2003;107:873–7. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- 7.Cruickshank J, Grossman DI, Peng RK, Famula TR, Oberbauer AM. Spatial distribution of growth hormone receptor, insulin-like growth factor-I receptor and apoptotic chondrocytes during growth plate development. The Journal of endocrinology. 2005;184:543–53. doi: 10.1677/joe.1.05947. [DOI] [PubMed] [Google Scholar]
- 8.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 9.DeAngelis T, Wu K, Pestell R, Baserga R. The type 1 insulin-like growth factor receptor and resistance to DACH1. Cell Cycle. 2011;10:1956–9. doi: 10.4161/cc.10.12.15800. [DOI] [PubMed] [Google Scholar]
- 10.Goel HL, Sayeed A, Breen M, Zarif MJ, Garlick DS, Leav I, Davis RJ, Fitzgerald TJ, Morrione A, Hsieh CC, Liu Q, Dicker AP, Altieri DC, Languino LR. beta1 integrins mediate resistance to ionizing radiation in vivo by inhibiting c-Jun amino terminal kinase1. J Cell Physiol. 2013 doi: 10.1002/jcp.24323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casa AJ, Dearth RK, Litzenburger BC, Lee AV, Cui X. The type I insulin-like growth factor receptor pathway: a key player in cancer therapeutic resistance. Front Biosci. 2008;13:3273–87. doi: 10.2741/2925. [DOI] [PubMed] [Google Scholar]
- 12.Helle SI. The insulin-like growth factor system in advanced breast cancer. Best practice & research. Clinical endocrinology & metabolism. 2004;18:67–79. doi: 10.1016/s1521-690x(03)00045-9. [DOI] [PubMed] [Google Scholar]
- 13.Pollak M, Beamer W, Zhang JC. Insulin-like growth factors and prostate cancer. Cancer metastasis reviews. 1998;17:383–90. doi: 10.1023/a:1006154108619. [DOI] [PubMed] [Google Scholar]
- 14.Gross JM, Yee D. The type-1 insulin-like growth factor receptor tyrosine kinase and breast cancer: biology and therapeutic relevance. Cancer metastasis reviews. 2003;22:327–36. doi: 10.1023/a:1023720928680. [DOI] [PubMed] [Google Scholar]
- 15.Polychronakos C, Janthly U, Lehoux JG, Koutsilieris M. Mitogenic effects of insulin and insulin-like growth factors on PA-III rat prostate adenocarcinoma cells: characterization of the receptors involved. Prostate. 1991;19:313–21. doi: 10.1002/pros.2990190405. [DOI] [PubMed] [Google Scholar]
- 16.Werner H, Maor S. The insulin-like growth factor-I receptor gene: a downstream target for oncogene and tumor suppressor action. Trends in endocrinology and metabolism: TEM. 2006;17:236–42. doi: 10.1016/j.tem.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Khandwala HM, McCutcheon IE, Flyvbjerg A, Friend KE. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocrine reviews. 2000;21:215–44. doi: 10.1210/edrv.21.3.0399. [DOI] [PubMed] [Google Scholar]
- 18.DiGiovanni J, Kiguchi K, Frijhoff A, Wilker E, Bol DK, Beltran L, Moats S, Ramirez A, Jorcano J, Conti C. Deregulated expression of insulin-like growth factor 1 in prostate epithelium leads to neoplasia in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:3455–60. doi: 10.1073/pnas.97.7.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CF, Li S, Chen Y, Chen PL, Sharp ZD, Lee WH. The nuclear localization sequences of the BRCA1 protein interact with the importin-alpha subunit of the nuclear transport signal receptor. The Journal of biological chemistry. 1996;271:32863–8. doi: 10.1074/jbc.271.51.32863. [DOI] [PubMed] [Google Scholar]
- 20.Hellawell GO, Turner GD, Davies DR, Poulsom R, Brewster SF, Macaulay VM. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res. 2002;62:2942–50. [PubMed] [Google Scholar]
- 21.Krueckl SL, Sikes RA, Edlund NM, Bell RH, Hurtado-Coll A, Fazli L, Gleave ME, Cox ME. Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer research. 2004;64:8620–9. doi: 10.1158/0008-5472.CAN-04-2446. [DOI] [PubMed] [Google Scholar]
- 22.Nickerson T, Chang F, Lorimer D, Smeekens SP, Sawyers CL, Pollak M. In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR) Cancer Res. 2001;61:6276–80. [PubMed] [Google Scholar]
- 23.Plymate SR, Bae VL, Maddison L, Quinn LS, Ware JL. Reexpression of the type 1 insulin-like growth factor receptor inhibits the malignant phenotype of simian virus 40 T antigen immortalized human prostate epithelial cells. Endocrinology. 1997;138:1728–35. doi: 10.1210/endo.138.4.5071. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan PJ, Mohan S, Cohen P, Foster BA, Greenberg NM. The insulin-like growth factor axis and prostate cancer: lessons from the transgenic adenocarcinoma of mouse prostate (TRAMP) model. Cancer research. 1999;59:2203–9. [PubMed] [Google Scholar]
- 25.Stattin P, Rinaldi S, Biessy C, Stenman UH, Hallmans G, Kaaks R. High levels of circulating insulin-like growth factor-I increase prostate cancer risk: a prospective study in a population-based nonscreened cohort. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22:3104–12. doi: 10.1200/JCO.2004.10.105. [DOI] [PubMed] [Google Scholar]
- 26.Mantzoros CS, Tzonou A, Signorello LB, Stampfer M, Trichopoulos D, Adami HO. Insulin-like growth factor 1 in relation to prostate cancer and benign prostatic hyperplasia. British journal of cancer. 1997;76:1115–8. doi: 10.1038/bjc.1997.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–6. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 28.Wolk A, Mantzoros CS, Andersson SO, Bergstrom R, Signorello LB, Lagiou P, Adami HO, Trichopoulos D. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. Journal of the National Cancer Institute. 1998;90:911–5. doi: 10.1093/jnci/90.12.911. [DOI] [PubMed] [Google Scholar]
- 29.Harman SM, Metter EJ, Blackman MR, Landis PK, Carter HB. Serum levels of insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-3, and prostate-specific antigen as predictors of clinical prostate cancer. The Journal of clinical endocrinology and metabolism. 2000;85:4258–65. doi: 10.1210/jcem.85.11.6990. [DOI] [PubMed] [Google Scholar]
- 30.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–53. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 31.Chokkalingam AP, Pollak M, Fillmore CM, Gao YT, Stanczyk FZ, Deng J, Sesterhenn IA, Mostofi FK, Fears TR, Madigan MP, Ziegler RG, Fraumeni JF, Jr, Hsing AW. Insulin-like growth factors and prostate cancer: a population-based case-control study in China. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10:421–7. [PubMed] [Google Scholar]
- 32.Platz EA, Pollak MN, Leitzmann MF, Stampfer MJ, Willett WC, Giovannucci E. Plasma insulin-like growth factor-1 and binding protein-3 and subsequent risk of prostate cancer in the PSA era. Cancer causes & control: CCC. 2005;16:255–62. doi: 10.1007/s10552-004-3484-8. [DOI] [PubMed] [Google Scholar]
- 33.Woodson K, Tangrea JA, Pollak M, Copeland TD, Taylor PR, Virtamo J, Albanes D. Serum insulin-like growth factor I: tumor marker or etiologic factor? A prospective study of prostate cancer among Finnish men. Cancer research. 2003;63:3991–4. [PubMed] [Google Scholar]
- 34.Chen C, Lewis SK, Voigt L, Fitzpatrick A, Plymate SR, Weiss NS. Prostate carcinoma incidence in relation to prediagnostic circulating levels of insulin-like growth factor I, insulin-like growth factor binding protein 3, and insulin. Cancer. 2005;103:76–84. doi: 10.1002/cncr.20727. [DOI] [PubMed] [Google Scholar]
- 35.Allen NE, Key TJ, Appleby PN, Travis RC, Roddam AW, Rinaldi S, Egevad L, Rohrmann S, Linseisen J, Pischon T, Boeing H, Johnsen NF, Tjonneland A, Gronbaek H, Overvad K, Kiemeney L, Bueno-de-Mesquita HB, Bingham S, Khaw KT, Tumino R, Berrino F, Mattiello A, Sacerdote C, Palli D, Quiros JR, Ardanaz E, Navarro C, Larranaga N, Gonzalez C, Sanchez MJ, Trichopoulou A, Travezea C, Trichopoulos D, Jenab M, Ferrari P, Riboli E, Kaaks R. Serum insulin-like growth factor (IGF)-I and IGF-binding protein-3 concentrations and prostate cancer risk: results from the European Prospective Investigation into Cancer and Nutrition. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:1121–7. doi: 10.1158/1055-9965.EPI-06-1062. [DOI] [PubMed] [Google Scholar]
- 36.Sutherland BW, Knoblaugh SE, Kaplan-Lefko PJ, Wang F, Holzenberger M, Greenberg NM. Conditional deletion of insulin-like growth factor-I receptor in prostate epithelium. Cancer Res. 2008;68:3495–504. doi: 10.1158/0008-5472.CAN-07-6531. [DOI] [PubMed] [Google Scholar]
- 37.LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127–37. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- 38.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocrine reviews. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 39.Dallas NA, Xia L, Fan F, Gray MJ, Gaur P, van Buren G, 2nd, Samuel S, Kim MP, Lim SJ, Ellis LM. Chemoresistant colorectal cancer cells, the cancer stem cell phenotype, and increased sensitivity to insulin-like growth factor-I receptor inhibition. Cancer research. 2009;69:1951–7. doi: 10.1158/0008-5472.CAN-08-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He Y, Zhang J, Zheng J, Du W, Xiao H, Liu W, Li X, Chen X, Yang L, Huang S. The insulin-like growth factor-1 receptor kinase inhibitor, NVP-ADW742, suppresses survival and resistance to chemotherapy in acute myeloid leukemia cells. Oncology research. 2010;19:35–43. doi: 10.3727/096504010x12828372551821. [DOI] [PubMed] [Google Scholar]
- 41.Morrione A, Romano G, Navarro M, Reiss K, Valentinis B, Dews M, Eves E, Rosner MR, Baserga R. Insulin-like growth factor I receptor signaling in differentiation of neuronal H19-7 cells. Cancer research. 2000;60:2263–72. [PubMed] [Google Scholar]
- 42.Valentinis B, Romano G, Peruzzi F, Morrione A, Prisco M, Soddu S, Cristofanelli B, Sacchi A, Baserga R. Growth and differentiation signals by the insulin-like growth factor 1 receptor in hemopoietic cells are mediated through different pathways. The Journal of biological chemistry. 1999;274:12423–30. doi: 10.1074/jbc.274.18.12423. [DOI] [PubMed] [Google Scholar]
- 43.Gronborg M, Wulff BS, Rasmussen JS, Kjeldsen T, Gammeltoft S. Structure-function relationship of the insulin-like growth factor-I receptor tyrosine kinase. The Journal of biological chemistry. 1993;268:23435–40. [PubMed] [Google Scholar]
- 44.Li S, Ferber A, Miura M, Baserga R. Mitogenicity and transforming activity of the insulin-like growth factor-I receptor with mutations in the tyrosine kinase domain. The Journal of biological chemistry. 1994;269:32558–64. [PubMed] [Google Scholar]
- 45.Rubinstein M, Idelman G, Plymate SR, Narla G, Friedman SL, Werner H. Transcriptional activation of the insulin-like growth factor I receptor gene by the Kruppel-like factor 6 (KLF6) tumor suppressor protein: potential interactions between KLF6 and p53. Endocrinology. 2004;145:3769–77. doi: 10.1210/en.2004-0173. [DOI] [PubMed] [Google Scholar]
- 46.Schayek H, Haugk K, Sun S, True LD, Plymate SR, Werner H. Tumor suppressor BRCA1 is expressed in prostate cancer and controls insulin-like growth factor I receptor (IGF-IR) gene transcription in an androgen receptor-dependent manner. Clin Cancer Res. 2009;15:1558–65. doi: 10.1158/1078-0432.CCR-08-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li D, Kumaraswamy E, Harlan-Williams LM, Jensen RA. The role of BRCA1 and BRCA2 in prostate cancer. Frontiers in bioscience: a journal and virtual library. 2013;18:1445–59. doi: 10.2741/4191. [DOI] [PubMed] [Google Scholar]
- 48.Schayek H, Bentov I, Jacob-Hirsch J, Yeung C, Khanna C, Helman LJ, Plymate SR, Werner H. Global methylation analysis identifies PITX2 as an upstream regulator of the androgen receptor and IGF-I receptor genes in prostate cancer. Horm Metab Res. 2012;44:511–9. doi: 10.1055/s-0032-1311566. [DOI] [PubMed] [Google Scholar]
- 49.Thomas R, Kim MH. A HIF-1alpha-dependent autocrine feedback loop promotes survival of serum-deprived prostate cancer cells. The Prostate. 2009;69:263–75. doi: 10.1002/pros.20885. [DOI] [PubMed] [Google Scholar]
- 50.Werner H, Stannard B, Bach MA, LeRoith D, Roberts CT., Jr Cloning and characterization of the proximal promoter region of the rat insulin-like growth factor I (IGF-I) receptor gene. Biochemical and biophysical research communications. 1990;169:1021–7. doi: 10.1016/0006-291x(90)91996-6. [DOI] [PubMed] [Google Scholar]
- 51.Cooke DW, Bankert LA, Roberts CT, Jr, LeRoith D, Casella SJ. Analysis of the human type I insulin-like growth factor receptor promoter region. Biochemical and biophysical research communications. 1991;177:1113–20. doi: 10.1016/0006-291x(91)90654-p. [DOI] [PubMed] [Google Scholar]
- 52.Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. Journal of cellular physiology. 2001;188:143–60. doi: 10.1002/jcp.1111. [DOI] [PubMed] [Google Scholar]
- 53.Trybus TM, Burgess AC, Wojno KJ, Glover TW, Macoska JA. Distinct areas of allelic loss on chromosomal regions 10p and 10q in human prostate cancer. Cancer research. 1996;56:2263–7. [PubMed] [Google Scholar]
- 54.Narla G, Heath KE, Reeves HL, Li D, Giono LE, Kimmelman AC, Glucksman MJ, Narla J, Eng FJ, Chan AM, Ferrari AC, Martignetti JA, Friedman SL. KLF6, a candidate tumor suppressor gene mutated in prostate cancer. Science. 2001;294:2563–6. doi: 10.1126/science.1066326. [DOI] [PubMed] [Google Scholar]
- 55.Liu X, Gomez-Pinillos A, Loder C, Carrillo-de Santa Pau E, Qiao R, Unger PD, Kurek R, Oddoux C, Melamed J, Gallagher RE, Mandeli J, Ferrari AC. KLF6 loss of function in human prostate cancer progression is implicated in resistance to androgen deprivation. Am J Pathol. 2012;181:1007–16. doi: 10.1016/j.ajpath.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, Liu Q, Cochran C, Bennett LM, Ding W, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994;266:66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- 57.Futreal PA, Liu Q, Shattuck-Eidens D, Cochran C, Harshman K, Tavtigian S, Bennett LM, Haugen-Strano A, Swensen J, Miki Y, et al. BRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994;266:120–2. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- 58.Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, Savage K, Gillett CE, Schmitt FC, Ashworth A, Tutt AN. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26:2126–32. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y, Farmer AA, Chen CF, Jones DC, Chen PL, Lee WH. BRCA1 is a 220-kDa nuclear phosphoprotein that is expressed and phosphorylated in a cell cycle-dependent manner. Cancer research. 1996;56:3168–72. [PubMed] [Google Scholar]
- 60.Pandini G, Mineo R, Frasca F, Roberts CT, Jr, Marcelli M, Vigneri R, Belfiore A. Androgens up-regulate the insulin-like growth factor-I receptor in prostate cancer cells. Cancer research. 2005;65:1849–57. doi: 10.1158/0008-5472.CAN-04-1837. [DOI] [PubMed] [Google Scholar]
- 61.Schayek H, Seti H, Greenberg NM, Sun S, Werner H, Plymate SR. Differential regulation of insulin-like growth factor-I receptor gene expression by wild type and mutant androgen receptor in prostate cancer cells. Mol Cell Endocrinol. 2010;323:239–45. doi: 10.1016/j.mce.2010.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shafi AA, Yen AE, Weigel NL. Androgen Receptors in Hormone-Dependent and Castration-Resistant Prostate Cancer. Pharmacology & therapeutics. 2013 doi: 10.1016/j.pharmthera.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 63.Yuan X, Cai C, Chen S, Yu Z, Balk SP. Androgen receptor functions in castration-resistant prostate cancer and mechanisms of resistance to new agents targeting the androgen axis. Oncogene. 2013 doi: 10.1038/onc.2013.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11:4653–7. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 65.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer research. 2008;68:4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu JD, Haugk K, Woodke L, Nelson P, Coleman I, Plymate SR. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. Journal of cellular biochemistry. 2006;99:392–401. doi: 10.1002/jcb.20929. [DOI] [PubMed] [Google Scholar]
- 67.Lin HK, Yeh S, Kang HY, Chang C. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:7200–5. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, Hung MC. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer research. 2000;60:6841–5. [PubMed] [Google Scholar]
- 69.Manin M, Baron S, Goossens K, Beaudoin C, Jean C, Veyssiere G, Verhoeven G, Morel L. Androgen receptor expression is regulated by the phosphoinositide 3-kinase/Akt pathway in normal and tumoral epithelial cells. The Biochemical journal. 2002;366:729–36. doi: 10.1042/BJ20020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Graff JR, Konicek BW, McNulty AM, Wang Z, Houck K, Allen S, Paul JD, Hbaiu A, Goode RG, Sandusky GE, Vessella RL, Neubauer BL. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. The Journal of biological chemistry. 2000;275:24500–5. doi: 10.1074/jbc.M003145200. [DOI] [PubMed] [Google Scholar]
- 71.Murillo H, Huang H, Schmidt LJ, Smith DI, Tindall DJ. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology. 2001;142:4795–805. doi: 10.1210/endo.142.11.8467. [DOI] [PubMed] [Google Scholar]
- 72.Jiang YG, Luo Y, He DL, Li X, Zhang LL, Peng T, Li MC, Lin YH. Role of Wnt/beta-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. International journal of urology: official journal of the Japanese Urological Association. 2007;14:1034–9. doi: 10.1111/j.1442-2042.2007.01866.x. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Kreisberg JI, Ghosh PM. Cross-talk between the androgen receptor and the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer. Current cancer drug targets. 2007;7:591–604. doi: 10.2174/156800907781662248. [DOI] [PubMed] [Google Scholar]
- 74.Li J, Wang E, Rinaldo F, Datta K. Upregulation of VEGF-C by androgen depletion: the involvement of IGF-IR-FOXO pathway. Oncogene. 2005;24:5510–20. doi: 10.1038/sj.onc.1208693. [DOI] [PubMed] [Google Scholar]
- 75.Reiss K, Wang JY, Romano G, Furnari FB, Cavenee WK, Morrione A, Tu X, Baserga R. IGF-I receptor signaling in a prostatic cancer cell line with a PTEN mutation. Oncogene. 2000;19:2687–94. doi: 10.1038/sj.onc.1203587. [DOI] [PubMed] [Google Scholar]
- 76.Tang Y, Zhang D, Fallavollita L, Brodt P. Vascular endothelial growth factor C expression and lymph node metastasis are regulated by the type I insulin-like growth factor receptor. Cancer research. 2003;63:1166–71. [PubMed] [Google Scholar]
- 77.Tsurusaki T, Kanda S, Sakai H, Kanetake H, Saito Y, Alitalo K, Koji T. Vascular endothelial growth factor-C expression in human prostatic carcinoma and its relationship to lymph node metastasis. British journal of cancer. 1999;80:309–13. doi: 10.1038/sj.bjc.6690356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hiraga T, Myoui A, Hashimoto N, Sasaki A, Hata K, Morita Y, Yoshikawa H, Rosen CJ, Mundy GR, Yoneda T. Bone-derived IGF mediates crosstalk between bone and breast cancer cells in bony metastases. Cancer research. 2012;72:4238–49. doi: 10.1158/0008-5472.CAN-11-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li S, Wang N, Brodt P. Metastatic cells can escape the proapoptotic effects of TNF-alpha through increased autocrine IL-6/STAT3 signaling. Cancer research. 2012;72:865–75. doi: 10.1158/0008-5472.CAN-11-1357. [DOI] [PubMed] [Google Scholar]
- 80.Rojas A, Liu G, Coleman I, Nelson PS, Zhang M, Dash R, Fisher PB, Plymate SR, Wu JD. IL-6 promotes prostate tumorigenesis and progression through autocrine cross-activation of IGF-IR. Oncogene. 2011;30:2345–55. doi: 10.1038/onc.2010.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yap TA, Olmos D, Molife LR, de Bono JS. Targeting the insulin-like growth factor signaling pathway: figitumumab and other novel anticancer strategies. Expert opinion on investigational drugs. 2011;20:1293–304. doi: 10.1517/13543784.2011.602630. [DOI] [PubMed] [Google Scholar]
- 82.Baserga R. The decline and fall of the IGF-I receptor. Journal of cellular physiology. 2013;228:675–9. doi: 10.1002/jcp.24217. [DOI] [PubMed] [Google Scholar]
- 83.Ozkan EE. Plasma and tissue insulin-like growth factor-I receptor (IGF-IR) as a prognostic marker for prostate cancer and anti-IGF-IR agents as novel therapeutic strategy for refractory cases: a review. Molecular and cellular endocrinology. 2011;344:1–24. doi: 10.1016/j.mce.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 84.Rowinsky EK, Youssoufian H, Tonra JR, Solomon P, Burtrum D, Ludwig DL. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:5549s–55s. doi: 10.1158/1078-0432.CCR-07-1109. [DOI] [PubMed] [Google Scholar]
- 85.Burtrum D, Zhu Z, Lu D, Anderson DM, Prewett M, Pereira DS, Bassi R, Abdullah R, Hooper AT, Koo H, Jimenez X, Johnson D, Apblett R, Kussie P, Bohlen P, Witte L, Hicklin DJ, Ludwig DL. A fully human monoclonal antibody to the insulin-like growth factor I receptor blocks ligand-dependent signaling and inhibits human tumor growth in vivo. Cancer research. 2003;63:8912–21. [PubMed] [Google Scholar]
- 86.Plymate SR, Haugk K, Coleman I, Woodke L, Vessella R, Nelson P, Montgomery RB, Ludwig DL, Wu JD. An antibody targeting the type I insulin-like growth factor receptor enhances the castration-induced response in androgen-dependent prostate cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2007;13:6429–39. doi: 10.1158/1078-0432.CCR-07-0648. [DOI] [PubMed] [Google Scholar]
- 87.Wu JD, Haugk K, Coleman I, Woodke L, Vessella R, Nelson P, Montgomery RB, Ludwig DL, Plymate SR. Combined in vivo effect of A12, a type 1 insulin-like growth factor receptor antibody, and docetaxel against prostate cancer tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:6153–60. doi: 10.1158/1078-0432.CCR-06-0443. [DOI] [PubMed] [Google Scholar]
- 88.Wu JD, Odman A, Higgins LM, Haugk K, Vessella R, Ludwig DL, Plymate SR. In vivo effects of the human type I insulin-like growth factor receptor antibody A12 on androgen-dependent and androgen-independent xenograft human prostate tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11:3065–74. doi: 10.1158/1078-0432.CCR-04-1586. [DOI] [PubMed] [Google Scholar]
- 89.Goel HL, Chang C, Pursell B, Leav I, Lyle S, Xi HS, Hsieh CC, Adisetiyo H, Roy-Burman P, Coleman IM, Nelson PS, Vessella RL, Davis RJ, Plymate SR, Mercurio AM. VEGF/neuropilin-2 regulation of Bmi-1 and consequent repression of IGF-IR define a novel mechanism of aggressive prostate cancer. Cancer discovery. 2012;2:906–21. doi: 10.1158/2159-8290.CD-12-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beltran PJ, Mitchell P, Chung YA, Cajulis E, Lu J, Belmontes B, Ho J, Tsai MM, Zhu M, Vonderfecht S, Baserga R, Kendall R, Radinsky R, Calzone FJ. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Molecular cancer therapeutics. 2009;8:1095–105. doi: 10.1158/1535-7163.MCT-08-1171. [DOI] [PubMed] [Google Scholar]
- 91.Fahrenholtz CD, Beltran PJ, Burnstein KL. Targeting IGF-IR with ganitumab inhibits tumorigenesis and increases durability of response to androgen-deprivation therapy in VCaP prostate cancer xenografts. Mol Cancer Ther. 2013;12:394–404. doi: 10.1158/1535-7163.MCT-12-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Galet C, Gray A, Said JW, Castor B, Wan J, Beltran PJ, Calzone FJ, Elashoff D, Cohen P, Aronson WJ. Effects of Calorie Restriction and IGF-1 Receptor Blockade on the Progression of 22Rv1 Prostate Cancer Xenografts. Int J Mol Sci. 2013;14:13782–95. doi: 10.3390/ijms140713782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Furukawa J, Wraight CJ, Freier SM, Peralta E, Atley LM, Monia BP, Gleave ME, Cox ME. Antisense oligonucleotide targeting of insulin-like growth factor-1 receptor (IGF-1R) in prostate cancer. Prostate. 2010;70:206–18. doi: 10.1002/pros.21054. [DOI] [PubMed] [Google Scholar]
- 94.Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, Bilhartz DL, Wynne C, Murray R, Zinner NR, Schulman C, Linnartz R, Zheng M, Goessl C, Hei YJ, Small EJ, Cook R, Higano CS. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–25. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 95.Nelson JB, Love W, Chin JL, Saad F, Schulman CC, Sleep DJ, Qian J, Steinberg J, Carducci M. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer. 2008;113:2478–87. doi: 10.1002/cncr.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miller K, Moul JW, Gleave M, Fizazi K, Nelson JB, Morris T, Nathan FE, McIntosh S, Pemberton K, Higano CS. Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 16:187–92. doi: 10.1038/pcan.2013.2. [DOI] [PubMed] [Google Scholar]
- 97.Yu EY, Miller K, Nelson J, Gleave M, Fizazi K, Moul JW, Nathan FE, Higano CS. Detection of previously unidentified metastatic disease as a leading cause of screening failure in a phase III trial of zibotentan versus placebo in patients with nonmetastatic, castration resistant prostate cancer. J Urol. 188:103–9. doi: 10.1016/j.juro.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, Miller K, Sieber P, Karsh L, Damiao R, Tammela TL, Egerdie B, Van Poppel H, Chin J, Morote J, Gomez-Veiga F, Borkowski T, Ye Z, Kupic A, Dansey R, Goessl C. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 379:39–46. doi: 10.1016/S0140-6736(11)61226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Higano CS, Alumkal JJ, Ryan CJ, Yu EY, Beer TM, Chandrawansa K, Katz T, Youssoufian H, Schwartz JD. A phase II study evaluating the efficacy and safety of single agent IMC A12, a monoclonal antibody, against the insulin-like growth factor-1 receptor, as monotherapy in patients with metastatic, asymptomatic castration-resistant prostate cancer. J Clin Oncol. 2009;27:15s. (suppl; abstr 5142) [Google Scholar]
- 100.Higano CS, Alumkal JJ, Ryan CJ, Yu EY, Beer TM, Fox FE, Dontabhaktuni A, Youssoufian H, Schwartz JD. A phase II study of cixutumumab (IMC-A12), a monoclonal antibody against the insulin-like growth factor 1 receptor (IGF-IR), monotherapy in metastatic castration-resistant prostate cancer: Feasibility of every 3-week dosing and updated results. 2010 Genitourinary Cancers Symposium; 2010. p. Abstract 189. [Google Scholar]
- 101.Rathkopf DE, Danila DC, Chudow JJ, Morris MJ, Slovin SF, Fine S, Fox JJ, Larson SM, Rosen N, Scher HI. Anti-insulin-like growth factor-1 receptor (IGF-IR) monoclonal antibody cixutumumab plus mammalian target of rapamycin (mTOR) inhibitor temsirolimus in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:15s. (suppl; abstr TPS242) [Google Scholar]
- 102.Dean JP, Sprenger CC, Wan J, Haugk K, Ellis WJ, Lin DW, Corman JM, Dalkin BL, Mostaghel E, Nelson PS, Cohen P, Montgomery B, Plymate SR. Response of the Insulin-Like Growth Factor (IGF) System to IGF-IR Inhibition and Androgen Deprivation in a Neoadjuvant Prostate Cancer Trial: Effects of Obesity and Androgen Deprivation. J Clin Endocrinol Metab. 2013;98:E820–8. doi: 10.1210/jc.2012-3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hussain M, Tangen CM, Higano C, Schelhammer PF, Faulkner J, Crawford ED, Wilding G, Akdas A, Small EJ, Donnelly B, MacVicar G, Raghavan D Southwest Oncology Group T. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24:3984–90. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 104.Molife LR, Fong PC, Paccagnella L, Reid AH, Shaw HM, Vidal L, Arkenau HT, Karavasilis V, Yap TA, Olmos D, Spicer J, Postel-Vinay S, Yin D, Lipton A, Demers L, Leitzel K, Gualberto A, de Bono JS. The insulin-like growth factor-I receptor inhibitor figitumumab (CP-751,871) in combination with docetaxel in patients with advanced solid tumours: results of a phase Ib dose-escalation, open-label study. Br J Cancer. 2010;103:332–9. doi: 10.1038/sj.bjc.6605767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chi KN, Gleave ME, Fazli L, Goldenberg SL, So A, Kollmannsberger C, Murray N, Tinker A, Pollak M. A phase II pharmacodynamic study of preoperative figitumumab in patients with localized prostate cancer. Clin Cancer Res. 2012;18:3407–13. doi: 10.1158/1078-0432.CCR-12-0482. [DOI] [PubMed] [Google Scholar]