Abstract
Antiretroviral therapy (ART) is able to suppress HIV-1 replication indefinitely in individuals who have access to these medications, are able to tolerate these drugs, and are motivated to take them daily for life. However, ART is not curative. HIV-1 persists indefinitely during ART as quiescent integrated DNA within memory CD4+ T cells and perhaps other long-lived cellular reservoirs. In this review, we discuss the role of the immune system on the establishment and maintenance of this “latent” HIV-1 reservoir. A detailed understanding of how the host immune system shapes the size and distribution of the viral reservoir should lead to the development of a new generation of immune-based therapeutics, which might eventually contribute to a curative intervention.
HIV-1 is a retrovirus that integrates into the host genome, primarily in memory CD4+ T cells that can harbor latent, replication-competent HIV-1 DNA for years (1–3). This latent HIV-1 reservoir is thought to be established during acute infection (4–6), although precisely when, where, and how the reservoir is seeded remains to be determined. The reservoir has a remarkably long half-life and is resistant to ART, resulting in lifelong infection and viral rebound in the vast majority of HIV-1-infected individuals when ART is discontinued (6, 7). Major research efforts are currently underway to understand the biology of the viral reservoir, the mechanism of viral latency, and the potential of various therapeutic approaches to target the reservoir (Fig. 1). Recent data indicate that the size of the viral reservoir may in fact be substantially larger than previously anticipated, suggesting the profound scope of this challenge (8).
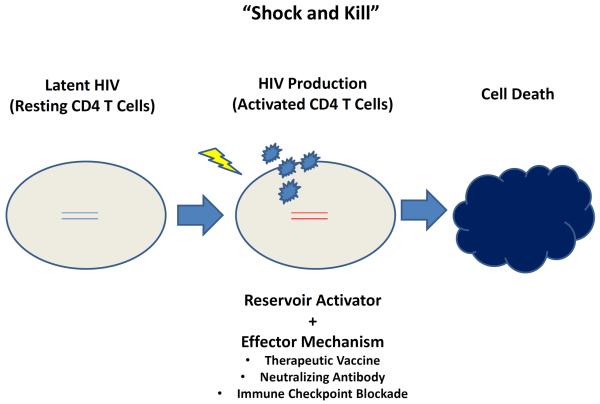
Figure 1. “Shock and Kill” Strategies.
Latent HIV-1 reservoirs in resting CD4+ T cells can be activated (“shocked”), which might make them more susceptible to be eliminated (“killed”) by immunologic effector mechanisms.
Role of the Immune System in Shaping and Maintaining the Viral Reservoir
HIV-1 infection causes profound and often irreversible changes to the adaptive and innate immune system. In the absence of ART, CD4+ T cells are progressively depleted, CD8+ T cells are often expanded, and much of the immune system is chronically activated. Some of these abnormalities improve during long-term ART, but the immune system rarely returns to normal, and chronic inflammation persists during ART. This state of heightened inflammation is driven by multiple factors, including HIV-1 production, irreversible loss of the mucosal integrity and exposure to gut microbes, and an excess burden of other pathogens such as cytomegalovirus (CMV). The implications of this chronic inflammatory state on overall health and the HIV-1 reservoir are the focus of intense investigation (9).
HIV-1 preferentially infects activated memory CD4+ T cells that express the chemokine receptor CCR5, although resting CD4+ T cells, naïve CD4+ T cells, and macrophages can also be infected. The majority of infected and activated CD4+ T cells die quickly (10), but a small fraction revert to a resting state and persistent indefinitely as the latent reservoir. Because ART blocks all or nearly all new infection events, the reservoir that exists at the time ART is initiated becomes the reservoir that persists for the life of the individual. This viral reservoir is maintained during ART by the long half-life of infected memory T cells, homeostatic proliferation of these cells (11, 12), and perhaps by low levels of cell-to-cell virus transfer (“cryptic replication”) (13). Recent studies suggest that the HIV-1 genome is often found integrated in host genes associated with cell growth (14), indicating that HIV-1 may promote its own persistence in part by promoting the continual expansion of latently infected cells (15, 16).
The viral reservoir in peripheral blood exists predominantly in memory CD4+ T cells endowed with regenerative potential, including memory stem cells and central memory cells (11, 17). The reservoir also persists in potentially shorter-lived CD4+ T effector cell populations, but whether these cells represent a stable reservoir or one that is constantly being regenerated via proliferation and differentiation is unknown. The distribution of the viral reservoir differs in tissues than blood, with the frequency of infection generally higher on a per cell basis in lymphocyte-rich tissues such as peripheral lymph nodes, the ileum and perhaps the spleen (18, 19). The higher frequency of target cell infection in these tissues may reflect cell-to-cell virus spread (20) and/or the presence of other immune cells that contribute to the maintenance of latency (21).
In the absence of therapy, the frequency of activated T cells is associated with the level of viremia. During long-term suppressive ART, a similar albeit less consistent association exists, with the frequency of activated CD4+ T cells expressing HLA-DR, CCR5, and PD-1 correlated with the frequency of cells containing HIV-1 RNA or DNA (22, 23). The mechanism for this association is not known, but likely to be bidirectional and multifactorial. Low levels of virus replication and/or production may cause T cell activation. When stable ART is “intensified” with the addition of a potent HIV-1 integrase inhibitor, markers of low-level HIV-1 replication and inflammation decline (at least in some individuals), indicating that replication persists during ART and causes an inflammatory response (13, 24).
Alternatively, an inflammatory immune environment may contribute to the persistence of the viral reservoir by a number of mechanisms (25). T cell activation promotes cell-to-cell virus transfer as activated cells are both more likely to produce virus and more likely to become infected. TCR engagement by cognate antigen or cytokines (e.g., IL-7) stimulates CD4+ T cell proliferation and the expansion of the infected cell population (12). CD4+ T cell proliferation may in fact be the most important mechanism leading to the stability of the reservoir (11). A chronic inflammatory environment would also be expected to prevent the generation of optimal HIV-1-specific immune responses. Chronic inflammation stimulates potent and sustained immunoregulatory responses, including expansion of T regulatory cells and the upregulation of PD-1 and other negative regulators on effector cells (11, 26). HIV-1-associated inflammation also stimulates the deposition of collagen in secondary lymphoid organs, which in turn causes tissue fibrosis and persistent immunodeficiency and poor host clearance mechanisms (27).
Understanding the complex virus-host interactions that lead to the establishment and maintenance of the latent HIV-1 reservoir will require advances in our fundamental understanding of T cell biology. How are memory CD4+ T cells generated and maintained? What are the life spans of the various CD4+ T cell subsets? How are the properties of T cells in the blood different from those in lymphoid tissues? How do the fates of CD4+ T cells activated via cognate antigen differ from those stimulated to proliferate by homeostatic mechanisms? How do myeloid cells, including dendritic cells, contribute to the generation and maintenance of T cell memory and latency? How will inhibitors of the pathways that regulate T cell survival and function change the life span of individual cell types?
There are also a number of critical unanswered questions related to the immunology of HIV-1 persistence. Because the size, distribution, and stability of the viral reservoir during ART is determined in part by the host immune system, factors that influence immune function, including gender, ethnicity, chronic inflammatory diseases, chronic infectious diseases, obesity and substance abuse are all likely to have important but unknown effects on the viral reservoir. The age at which the virus is acquired and ART is initiated are also likely to be important considerations. ART administered at 30 hours of age in a perinatally infected infant resulted in sustained remission and a possible cure (28), and the administration of ART in the first three months of life was associated with a sustained decay in the reservoir (29). Given that the development of memory CD4+ T cell responses generally occurs after birth, perhaps limited by immunoregulatory responses (30), it is possible that the establishment of a latent viral reservoir may be less efficient in infants (31). This remains a largely unexplored area of HIV-1 cure research.
The Berlin and Boston Patients
Much of the enthusiasm for HIV-1 cure research is derived from the case of the “Berlin Patient” (32). In 2007, an HIV-1-infected adult living in Berlin developed acute myelogenous leukemia and received an allogeneic hematopoietic stem cell transplant from a donor that carried a homozygous deletion CCR5, which renders cells highly resistant to HIV-1 infection. The procedure worked, and the patient now appears to be free of both cancer and HIV-1 for over five years (33). Despite intense efforts this outcome has not yet been repeated. Finding suitable HLA-matched donors homozyogous for the CCR5 deletion is challenging, and the few transplants that have been performed have failed due to recurrence of the underlying cancer or emergence of CXCR4-tropic viruses. Nevertheless, this case has galvanized gene therapy efforts to modify CD4+ T cells (34) and potentially also stem cells (35, 36) to render them resistant to HIV-1 infection.
A related approach is to utilize ART to protect transplanted donor cells until full chimerism occurs. This strategy is illustrated by two cases of HIV-1-infected adults in Boston who received hematopoietic stem cell transplants for treatment of refractory lymphoma (37). Under the coverage of ART, full donor chimerism was apparently achieved, with the donor immune cells eventually replacing the original immune cells over a period of years. As that occurred, HIV-1 DNA gradually declined to undetectable levels. Therapy was then interrupted in both individuals. Virus failed to rebound within the first few weeks, leading to initial optimism that these individuals may have been cured. Unfortunately, virus dramatically rebounded at weeks 12 and 32 after treatment interruption, indicating that the transplant caused a profound but incomplete elimination of the viral reservoir. This failure highlights the need either to eradicate all replication-competent virus or to enhance the capacity of the host immune system to contain the limited residual virus that may persist after a curative intervention. These observations also illustrate the limitation of our current biomarkers for HIV-1 persistence. The development and validation of a highly sensitive biomarker that can reliably detect and preferably quantify the total body burden of replication-competent HIV-1 during ART is one of the highest current priorities of the field.
Comparing the outcomes of the Berlin and Boston cases is informative. GVHD occurred in all three cases and likely contributed to reductions of the viral reservoirs. As has been observed with beneficial graft-versus-tumor effects, alloreactive donor cells targeting hematopoietic cells likely reduced the number of recipient CD4+ T cells harbouring latent HIV-1. These cases have led to increased interest in modifying host responses with immune-based therapeutics as part of a curative strategy (38). Assuming that the Berlin Patient achieved a complete sterilizing cure, and that ART was fully effective in the Boston cases, why was the virus eradicated only in the former case? One key difference in the transplant protocols was the extent of pre-transplant myeloablation, which would be expected to reduce the size of the reservoir. The Berlin Patient received an aggressive regimen of chemotherapy and total body irradiation, whereas a milder approach was used in the Boston cases. The Berlin Patient also received more aggressive immunosuppressive therapies to GVHD, and as outlined below, suppressing T cell activation/proliferation might result in further control of the reservoir.
“Shock and Kill”
Hematopoietic stem cell transplantation is too risky and too complex for the majority of HIV-1-infected individuals worldwide. Other approaches that have generated considerable enthusiasm include pharmacologic strategies to induce latently infected cells to produce virus (“shock”) together with interventions that would enhance the ability of the host to clear these virus-producing cells (“kill”) (Fig. 1). Histone deacetylase (HDAC) inhibitors have been shown to increase production of HIV-1 RNA and to a lesser degree virus particles from the viral reservoir in vivo (39). However, the magnitude of the effect of HDAC inhibitors has to date been modest, and this class of drugs has not yet demonstrated a consistent effect on the frequency of cells that harbor replication-competent HIV-1 (40–42). Other classes of anti-latency drugs and immunomodulators are therefore being explored for their capacity to stimulate the viral reservoir (40, 43, 44).
To augment the capacity of the host to eliminate reservoir cells following activation, several immunologic strategies are being explored. These strategies include therapeutic vaccines, monoclonal antibodies, and immune checkpoint inhibitors (Fig. 2).
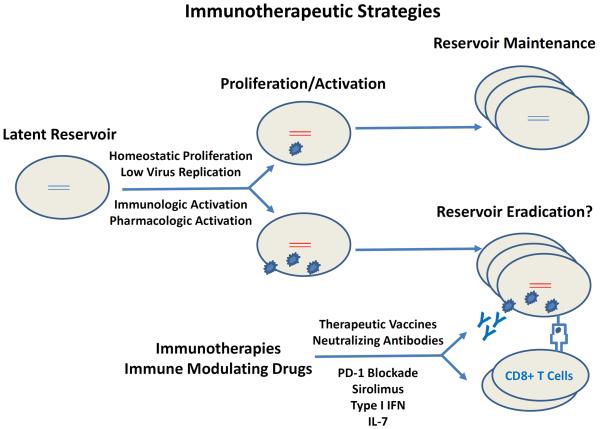
Figure 2. Immunotherapeutic Strategies.
Homeostatic proliferation and low levels of virus production likely contribute to the long-term maintenance of the viral reservoir. When the viral reservoir is subject to sufficient immunologic or pharmacologic stimulation, these cells may be eliminated by immunotherapies such as therapeutic vaccines and broadly neutralizing monoclonal antibodies, and may be modulated by immune modulating drugs such as PD-1 blockade, sirolimus, type I interferon, and IL-7.
Therapeutic Vaccines
It is likely that a latency reversing agent will need to be coupled with an immunologic strategy to clear virus-producing cells. One approach is to expand HIV-1-specific CD8+ T lymphocyte responses (45). As has been well-established in natural control of HIV-1 (i.e., “elite” control), potent CD8+ T lymphocytes can control HIV-1 by selectively killing virus-producing cells (46). This concept has led to a renewed interest in therapeutic vaccines, namely the administration of candidate HIV-1 vaccines to HIV-1-infected individuals typically on suppressive ART, with the goal of augmenting virus-specific immune responses and either accelerating the decay of the reservoir during ART or improving the control of viral rebound after interruption of ART. Several therapeutic vaccines have been evaluated in HIV-1-infected subjects, including vector-based vaccines that express HIV-1 antigens from harmless or attenuated viruses such as canarypox (ALVAC) or adenovirus serotype 5 (Ad5), as well as plasmid DNA vaccines (47–52). Although these vaccines proved immunogenic, most therapeutic vaccine studies have failed to show virologic or clinical benefit, and a few have suggested harm, potentially as a result of increasing immune activation. Infusion of antigen-pulsed autologous dendritic cells has shown potentially greater promise but requires confirmation (53).
Most of these early generation therapeutic vaccines involved relatively weak or suboptimal immunogens. Moreover, as was demonstrated in a study of DNA priming followed by Ad5 boosting, these therapeutic vaccines typically expand pre-existing T cell clones, which by definition had already failed to control virus replication prior to ART (54), either because they were dysfunctional or because they selected for a virus population containing CD8+ T cell escape mutations. A successful therapeutic vaccine would ideally induce functional CD8+ T cells specific for novel HIV-1 epitopes. The next generation of therapeutic vaccines will also likely be combined with reservoir activating agents.
Several novel therapeutic vaccines will likely be evaluated in clinical trials over the next several years. Such strategies include cytomegalovirus (CMV) vectors; adenovirus serotype 26 (Ad26) prime, modified vaccinia Ankara (MVA) boost regimens; and lymph node targeted amphiphilic peptide vaccines. Prophylactic vaccination with CMV vectors has been shown to result in the induction of broad cellular immune responses against novel epitopes and apparent clearance of the stringent challenge virus SIVmac251 in approximately half of vaccinated rhesus monkeys (55–57). The persistent replicative nature of CMV vectors is likely critical for the highly functional effector memory T cells that appear to persist indefinitely. Moreover, the ability of CMV vectors to induce unconventional class II-restricted CD8+ T cell responses provides a potentially important benefit as they target novel epitopes that likely were not previously subject to immune selection pressure (57). Clinical development of CMV vectors will presumably require attenuated vectors, and manufacturing and regulatory issues for CMV vectors remain to be determined.
Another therapeutic vaccine platform that will likely be evaluated in clinical trials involves Ad26 vectors. Prophylactic vaccination with Ad26-based prime-boost vaccine regimens, such as priming with Ad26 and boosting with the poxvirus MVA, has proven highly immunogenic and has afforded partial protection against acquisition of infection as well as reduced setpoint viral loads following stringent SIVmac251 challenges in rhesus monkeys (58, 59). Ad26 has several advantages over Ad5 as a vaccine vector, including the induction of different innate immune profiles that may reduce undesirable inflammatory responses and result in more functional T cell phenotypes. Moreover, Ad26-based prime-boost regimens have demonstrated partial protective efficacy in the stringent rhesus monkey challenge viral models in which Ad5-based regimens have failed (58–60), suggesting their potential for greater clinical utility.
Another therapeutic vaccine concept utilizes amphiphilic peptides, which have been shown to target antigen to lymph nodes and aim to induce cellular immune responses simultaneously to two or more regions of immunologic vulnerability of HIV-1 (61, 62). The extent to which these and other next generation vaccine candidates will afford therapeutic efficacy in preclinical and clinical studies remains to be determined.
Broadly Neutralizing Monoclonal Antibodies
Potent broadly neutralizing HIV-1-specific monoclonal antibodies (mAbs) are another potential HIV-1 eradication strategy under exploration. Prior studies using the earlier generation of neutralizing mAbs were generally unsuccessful in both preclinical and clinical studies (63–65), but the identification of a new generation of mAbs with improved potency and breadth has led to a resurgence of interest in this approach. In particular, two studies in rhesus monkeys demonstrated that infusion of mAb cocktails as well as certain individual mAbs in chronically SHIV-infected rhesus monkeys resulted in substantial, albeit transient, suppression of viremia (66, 67), building on previous studies in humanized mice (68). Moreover, a subset of animals with the lowest starting viral loads did not exhibit viral rebound even after mAb titers declined to undetectable levels, suggesting the potential for a durable therapeutic effect in certain circumstances (66). Infusion of one particular mAb, PGT121, resulted in not only rapid and profound suppression of plasma viral RNA but also substantial reductions of proviral DNA in peripheral blood, lymph nodes, and gastrointestinal mucosa (66). These data suggest that certain mAbs may be able to target virus-infected cells in tissues, although it remains to be determined whether these mAbs can impact the viral reservoir.
A growing number of potent and broadly neutralizing mAbs now exist that target various epitopes on the HIV-1 envelope protein. However, a central question for HIV-1 eradication strategies is whether mAbs will be able to target the viral reservoir and clear virally infected cells, potentially via Fc-mediated mechanisms such as antibody-dependent cellular cytotoxicity or antibody-dependent cell-mediated virus inhibition. Another possibility is that direct neutralization of free virions will reduce chronic antigen stimulation and augment the functionality of host virus-specific T cell responses, resulting in improved virologic control. Supporting this latter possibility is the observation that following PGT121 infusion in SHIV-infected rhesus monkeys, Gag-specific T cells exhibited decreased activation and exhaustion and demonstrated improved capacity to suppress virus replication in vitro (66). Another caveat regarding broadly neutralizing mAbs is their limited accessibility to certain anatomic reservoir sites, such as the central nervous system.
Immune Checkpoint Blockade
Another potential strategy to augment host immune control involves the blockade of immune regulators. Activated T cells express a series of receptors that when engaged by their ligands result in suppression of function and return to a non-activated state. The best characterized immune regulator is PD-1, which is a marker of functional T cell exhaustion. The ligands for PD-1, PDL-1 and PDL-2, are widely expressed in tissues. Inhibitors of the PD-1 pathway restore T cell function and have shown efficacy in the cancer field (69). These inhibitors may also enhance the capacity of the host immune system to clear chronic viral infections. Indeed, a single dose of an anti-PD-1 antibody appeared to cure HCV infection in a small subset of individuals (70). It is thought that inhibitors of other receptors that keep T cells in a suppressed state (such as CTLA-4, LAG-3, TIM-3, TIGIT, and 2B4) may also prove effective.
PD-1 inhibitors and other checkpoint blockers may also have direct effects on the establishment and maintenance of the viral reservoir (11). Because activated cells are more likely to become infected than resting cells, previously activated cells—including those expressing PD-1—might be expected to be enriched for HIV-1 (11). Indeed, the size of the reservoir is positively correlated with the frequency of PD-1 expressing cells (11, 22), and HIV-1 is enriched in PD-1 expressing memory cells (11). Clinical trials of PD-1 inhibitors in treated HIV-1 disease have been initiated.
Immune Modulating Drugs
After years of suppressive ART, the vast majority of the residual population of replication-competent virus resides in long-lived memory CD4+ T cells. The estimated size of the reservoir during ART is directly associated with the frequency of activated and proliferating cells (11, 12, 22, 23, 25), and in some studies activated and proliferating cells also contain more HIV-1 (11, 71). Therapeutic interventions that target T cell activation or proliferation may therefore impact the viral reservoir (Fig. 2).
T cell activation and proliferation are controlled by a number of signalling pathways, including those involving the mammalian target of rapamycin (mTOR), signal transducer and activator of transcription 5a (STAT5a) and forkhead box O3a (FOXO3a) (72). Specific inhibitors of these pathways might therefore reduce the size of the latent viral reservoir. For example, sirolimus (rapamycin) is a naturally occurring macrolide that inhibits mTOR, and as a consequence it blocks cell cycle progression from G1 to S phase in activated T cells and reduces CCR5 expression (73). Sirolimus also enhances memory T cell formation in response to vaccines in preclinical studies (74). We performed a retrospective analysis of ART-treated HIV-1-infected kidney transplant recipients who received sirolimus or non-mTOR-targeting drugs to prevent graft rejection. Only treatment with sirolimus was temporally associated with lower levels of cell-associated HIV-1 DNA (75), suggesting that mTOR inhibition may prove useful in a combination eradication strategy. A prospective clinical trial is being developed to evaluate this concept further. Other drugs that would limit T cell activation, including those that inhibit the JAK/STAT pathway (76), are also being developed. As chronic inflammation may contribute to excess morbidity in ART-suppressed, HIV-1-infected individuals (9), a number of therapies that target the inflammatory pathways are being developed as adjuncts to therapy (e.g., methotrexate, anti-fibrotic drugs, anti-CMV therapy, and a number of agents aimed at gut mucosa and microbial translocation). Measures of the viral reservoir are increasingly being incorporated into these studies.
The role of the type I interferon family of cytokines in the context of chronic viral infections is currently a focus of intense investigation and debate. An acute viral infection triggers immediate and potent type I interferon production by a variety of cells, leading to upregulation of hundreds of genes (the “interferon-stimulated genes”) (77). In the context of chronic HIV-1 infection, persistent interferon signaling has been associated with increased CCR5 and PD-1 expression, upregulation of indoleamine 2,3-dioxygenase and other inflammatory pathways, reduced thymopoieses, the generation of dysfunctional T cells and altered T cell homeostasis (78–81). Higher levels of interferon signaling are also correlated with poor immune reconstitution (81, 82). In murine models of chronic lymphocytic choriomeningitis virus (LCMV) infection, inhibition of type I interferon leads to decreased immune activation, decreased PD-1/PD-L1 activity, decreased amounts of the immunomodulatory cytokine interleukin (IL)-10, restored lymphoid architecture, improved CD4+ T cell responses, and ultimately enhanced viral clearance (83, 84). Although chronic type I interferon stimulation might be harmful, supra-therapeutic doses of type I interferon have also been shown to have sustained anti-HIV-1 effects in untreated chronic infection (85) and may impact reservoir size in treated disease (86). Proof-of-concept studies involving interferon-alpha and inhibitors of interferon-alpha are both planned.
As a result of the correlation between markers of T cell activation/dysfunction and the size of the viral reservoir, it is possible that immunosuppressive interventions may also contribute to a cure. It should be noted, however, that the most direct way to “shock” HIV-1 out of a state of latency may be nonspecific and potent activation of infected CD4+ T cells (87–89). The first generation of HIV-1 eradication studies performed over a decade ago involved the use of anti-CD3 antibodies (90), which trigger T cell activation, but the inflammatory response proved too toxic to pursue. An ideal immune modifying regimen would reverse HIV-1 latency in a specific manner while preventing the negative consequences of T cell activation and T cell proliferation. Given the inherent complexity of the immune response and the fact that any immune modifying therapeutic agent will invariably lead to complex and difficult-to-predict counter-regulatory responses, it is difficult to predict how such interventions will impact the viral reservoir. The complexity of attempting to use an activating agent to reverse latency is illustrated by recent studies with interleukin-7 (IL-7). Pre-clinical studies suggested that this approach would reverse latency (91). Although proof-of-concept clinical study found that IL-7 caused production of virions in vivo (92), the overall impact of the approach was an increase in the estimated reservoir size, presumably due to proliferation and expansion of infected cells (12). Experiments in improved preclinical models of ART-suppressed virus infection and proof-of-concept clinical trials will hopefully provide clarity on these and other issues.
The PrEP, PEP, and Cure Continuum
Treating HIV-1 with ART shortly after infection may also contribute to HIV-1 eradication. ART during acute HIV-1 infection reduces the size of the reservoir (93, 94), limits the generation of escape mutants, and preserves immune function. For these reasons it is thought that HIV-1-infected individuals who initiate ART during acute infection have the best chance for HIV-1 eradication, although this group represents only a small fraction of total HIV-1-infected individuals. It is also possible that very early ART may even be curative, as illustrated by the case of an HIV-1-infected infant who exhibited HIV-1 viremia and was started on ART at 30 hours of life. Therapy was discontinued after 18 months and virus remained undetectable (through at least month 30), suggesting that very early initiation of ART may prevent establishment of a long-lived reservoir (28). The applicability of these findings to sexual HIV-1 transmission in adults, however, remains uncertain.
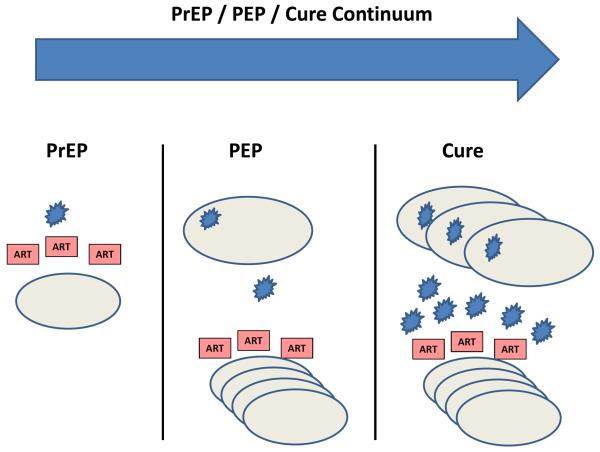
The potentially curative role of ART when administered within days of infection blurs the traditional distinction between pre-exposure prophylaxis (PrEP), post-exposure prophylaxis (PEP), and a cure (Fig. 3). It is well established that treating adults with ART within hours of HIV-1 exposure (e.g., a needlestick injury in a healthcare worker) substantially reduces the risk of acquiring HIV-1. However, antiretroviral drugs inhibit active virus replication in host cells, and thus the clinical success of PEP strategies indicates that the first HIV-1-infected cells following viral exposure can be eradicated. Indeed, if intensive virologic monitoring were employed in these individuals, then it is possible that some might exhibit transient low levels of virus in blood or tissues and thus would similarly be considered “cured”. From this perspective, the use of very early ART to eradicate HIV-1 infection may not be uncommon.
Figure 3. PrEP/PEP/Cure Continuum.
ART initiated prior to exposure is termed pre-exposure prophylaxis (PrEP), whereas ART initiated shortly after exposure is post-exposure prophylaxis (PEP) and forms a continuum with efforts aimed at virus eradication (cure). Even if early ART is not curative, it may reduce the size of the viral reservoir and preserve immune function.
Early ART that fails to block the establishment of the viral reservoir might still prevent some of the immunologic damage that typically occurs during acute HIV-1 infection, thus augmenting the capacity of the host immune system to control viral replication. Such an ART-induced shift towards a more effective host immune response and a smaller viral reservoir may in rare cases lead to sustained control of the virus (a “functional cure”). Among a cohort of adults in France who started ART during acute HIV-1 infection and then discontinued therapy after several years (the VISCONTI cohort), approximately 10–15% of subjects did not exhibit detectable viral rebound, although replication-competent virus still persisted in these individuals and they lacked protective HLA haplotypes (95). This observation has not yet been confirmed, and the mechanism for the “post-treatment controller” phenotype remains to be determined. As compared to elite controllers who naturally control HIV-1 infection without ART, the post-treatment controllers demonstrate remarkably small viral reservoirs and low levels of T cell activation, both of which may have contributed to the lack of rebound viremia when therapy was discontinued.
Conclusions and Perspectives
Advances over the past several years have suggested that HIV-1 might be eradicated or controlled under specific conditions. Thus, the increasing enthusiasm for HIV-1 eradication research, and the growing public and private investment in the HIV-1 cure agenda, is justifiable. However, as described in detail elsewhere (96, 97), major scientific challenges remain. A more detailed understanding of the biology of the latent viral reservoir and the partially effective virus-specific immune responses is critical. As the Boston patients demonstrate, improved assays are needed to quantify the reservoir, and predictive biomarkers for viral rebound are required. Reliable and predictive animal models are also needed to evaluate multiple concepts and to inform clinical research strategies.
The establishment and maintenance of the viral reservoir appears to be impacted at least in part by the immune system, and particularly by memory CD4 T cells. Immunotherapy approaches will therefore likely have an increasing role in HIV-1 eradication strategies in the future. However, the optimal strategies to stimulate viral release from latency, to augment host immune responses, and to limit negative inflammatory responses remain to be determined. Although a small number of case reports suggest that it might be possible to eradicate HIV-1 infection in unusual circumstances, no proof-of-concept yet exists that chronic HIV-1 infection can be cured by a safe and scalable intervention. Over the next few years, multiple novel and promising HIV-1 eradication concepts will be evaluated. It is likely that progress will be steady but unpredictable, and reaching the final goal may take many years. Regardless, substantial expansion of rigorous basic research, preclinical studies, and clinical trials in this field will undoubtedly lead to important advances in our understanding of the biology of the HIV-1 reservoir and the challenges that face HIV-1 eradication strategies.
ACKNOWLEDGEMENTS
The authors acknowledge support from the National Institutes of Health (AI078526, AI084794, AI095985, AI096040, AI096109 (Delaney AIDS Research Enterprise), AI100663, OD011170); the Bill and Melinda Gates Foundation (OPP1033091, OPP1040741, OPP1083689); the Ragon Institute of MGH, MIT, and Harvard; the Henry M. Jackson Foundation; and the American Foundation for AIDS Research. The authors thank B. Walker, N. Michael, J. Kim, M. Robb, R. Geleziunas, M. McCune, R. Sekaly, N. Chomont and S. Lewin for helpful discussions.
REFERENCES
- 1.Finzi D, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997 Nov 14;278:1295. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 2.Persaud D, Zhou Y, Siliciano JM, Siliciano RF. Latency in human immunodeficiency virus type 1 infection: no easy answers. J Virol. 2003 Feb;77:1659. doi: 10.1128/JVI.77.3.1659-1665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chun TW, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997 Nov 25;94:13193. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chun TW, et al. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proceedings of the National Academy of Sciences of the United States of America. 1998 Jul 21;95:8869. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun TW, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997 May 8;387:183. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 6.Chun TW, Davey RT, Jr., Engel D, Lane HC, Fauci AS. Re-emergence of HIV after stopping therapy. Nature. 1999 Oct 28;401:874. doi: 10.1038/44755. [DOI] [PubMed] [Google Scholar]
- 7.Finzi D, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999 May;5:512. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 8.Ho YC, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013 Oct 24;155:540. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deeks SG, Tracy R, Douek DC. Systemic Effects of Inflammation on Health during Chronic HIV Infection. Immunity. 2013 Oct 17;39:633. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doitsh G, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2013 Dec 19; doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chomont N, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009 Aug;15:893. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandergeeten C, et al. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood. 2013 May 23;121:4321. doi: 10.1182/blood-2012-11-465625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buzon MJ, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010 Apr;16:460. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 14.Cohen J. HIV/AIDS. Cancer genes help HIV persist, complicating cure efforts. Science. 2014 Mar 14;343:1188. doi: 10.1126/science.343.6176.1188. [DOI] [PubMed] [Google Scholar]
- 15.Josefsson L, et al. The HIV-1 reservoir in eight patients on long-term suppressive antiretroviral therapy is stable with few genetic changes over time. Proc Natl Acad Sci U S A. 2013 Nov 25; doi: 10.1073/pnas.1308313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher CV, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A. 2014 Feb 11;111:2307. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buzon MJ, et al. HIV-1 persistence in CD4+ T cells with stem cell-like properties. Nat Med. 2014 Feb;20:139. doi: 10.1038/nm.3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yukl SA, et al. The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV-positive patients on ART: implications for viral persistence. J Infect Dis. 2013 Oct 15;208:1212. doi: 10.1093/infdis/jit308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.North TW, et al. Viral sanctuaries during highly active antiretroviral therapy in a nonhuman primate model for AIDS. J Virol. 2010 Mar;84:2913. doi: 10.1128/JVI.02356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigal A, et al. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011 Sep 1;477:95. doi: 10.1038/nature10347. [DOI] [PubMed] [Google Scholar]
- 21.Evans VA, et al. Myeloid dendritic cells induce HIV-1 latency in non-proliferating CD4+ T cells. PLoS Pathog. 2013 Dec;9:e1003799. doi: 10.1371/journal.ppat.1003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hatano H, et al. Cell-Based Measures of Viral Persistence Are Associated With Immune Activation and Programmed Cell Death Protein 1 (PD-1)-Expressing CD4+ T cells. J Infect Dis. 2012 Nov 12; doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray JM, et al. HIV DNA subspecies persist in both activated and resting memory CD4+ T cells during antiretroviral therapy. J Virol. 2014 Mar;88:3516. doi: 10.1128/JVI.03331-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatano H, et al. Increase in 2-Long Terminal Repeat Circles and Decrease in D-dimer After Raltegravir Intensification in Patients With Treated HIV Infection: A Randomized, Placebo-Controlled Trial. J Infect Dis. 2013 Nov;208:1436. doi: 10.1093/infdis/jit453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klatt NR, Chomont N, Douek DC, Deeks SG. Immune activation and HIV persistence: implications for curative approaches to HIV infection. Immunol Rev. 2013 Jul;254:326. doi: 10.1111/imr.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood. 2013 Jan 3;121:29. doi: 10.1182/blood-2012-07-409755. [DOI] [PubMed] [Google Scholar]
- 27.Zeng M, et al. Lymphoid tissue damage in HIV-1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012 Jan;8:e1002437. doi: 10.1371/journal.ppat.1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Persaud D, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013 Nov 7;369:1828. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luzuriaga K, et al. Reduced HIV Reservoirs After Early Treatment HIV-1 Proviral Reservoirs Decay Continously Under Sustained Virologic Control in Early-Treated HIV-1- Infected Children. J Infect Dis. 2014 May 21; doi: 10.1093/infdis/jiu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mold JE, et al. Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science. 2010 Dec 17;330:1695. doi: 10.1126/science.1196509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siliciano RF. Opening Fronts in HIV Vaccine Development: Targeting reservoirs to clear and cure. Nat Med. 2014 May 7;20:480. doi: 10.1038/nm.3550. [DOI] [PubMed] [Google Scholar]
- 32.Hutter G, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009 Feb 12;360:692. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 33.Yukl SA, et al. Challenges in Detecting HIV Persistence during Potentially Curative Interventions: A Study of the Berlin Patient. PLoS Pathog. 2013 May;9:e1003347. doi: 10.1371/journal.ppat.1003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tebas P, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014 Mar 6;370:901. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holt N, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010 Jul 2; doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Younan PM, et al. Positive selection of mC46-expressing CD4+ T cells and maintenance of virus specific immunity in a primate AIDS model. Blood. 2013 Jul 11;122:179. doi: 10.1182/blood-2013-01-482224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henrich TJ, et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis. 2013 Jun 1;207:1694. doi: 10.1093/infdis/jit086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cannon PM, Kohn DB, Kiem HP. HIV eradication-from Berlin to Boston. Nat Biotechnol. 2014 Apr 8;32:315. doi: 10.1038/nbt.2868. [DOI] [PubMed] [Google Scholar]
- 39.Archin NM, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012 Jul 26;487:482. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med. 2014 Apr;20:425. doi: 10.1038/nm.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blazkova J, et al. Effect of histone deacetylase inhibitors on HIV production in latently infected, resting CD4(+) T cells from infected individuals receiving effective antiretroviral therapy. J Infect Dis. 2012 Sep 1;206:765. doi: 10.1093/infdis/jis412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spivak AM, et al. A Pilot Study Assessing the Safety and Latency-Reversing Activity of Disulfiram in HIV-1-Infected Adults on Antiretroviral Therapy. Clin Infect Dis. 2014 Jan 9; doi: 10.1093/cid/cit813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spivak AM, et al. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin Infect Dis. 2014 Mar;58:883. doi: 10.1093/cid/cit813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katlama C, et al. Barriers to a cure for HIV: new ways to target and eradicate HIV-1 reservoirs. Lancet. 2013 Jun 15;381:2109. doi: 10.1016/S0140-6736(13)60104-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shan L, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012 Mar 23;36:491. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Migueles SA, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008 Dec 19;29:1009. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg ES, et al. Safety and immunogenicity of therapeutic DNA vaccination in individuals treated with antiretroviral therapy during acute/early HIV-1 infection. PLoS ONE. 2010;5:e10555. doi: 10.1371/journal.pone.0010555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schooley RT, et al. AIDS clinical trials group 5197: a placebo-controlled trial of immunization of HIV-1-infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J Infect Dis. 2010 Sep 1;202:705. doi: 10.1086/655468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Angel JB, et al. A randomized controlled trial of HIV therapeutic vaccination using ALVAC with or without Remune. Aids. 2011 Mar 27;25:731. doi: 10.1097/QAD.0b013e328344cea5. [DOI] [PubMed] [Google Scholar]
- 50.Gandhi RT, et al. A randomized therapeutic vaccine trial of canarypox-HIV-pulsed dendritic cells vs. canarypox-HIV alone in HIV-1-infected patients on antiretroviral therapy. Vaccine. 2009 Oct 9;27:6088. doi: 10.1016/j.vaccine.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Autran B, et al. Greater viral rebound and reduced time to resume antiretroviral therapy after therapeutic immunization with the ALVAC-HIV vaccine (vCP1452) Aids. 2008 Jul 11;22:1313. doi: 10.1097/QAD.0b013e3282fdce94. [DOI] [PubMed] [Google Scholar]
- 52.Kilby JM, et al. A randomized, partially blinded phase 2 trial of antiretroviral therapy, HIV-specific immunizations, and interleukin-2 cycles to promote efficient control of viral replication (ACTG A5024) J Infect Dis. 2006 Dec 15;194:1672. doi: 10.1086/509508. [DOI] [PubMed] [Google Scholar]
- 53.Garcia F, et al. A Dendritic Cell-Based Vaccine Elicits T Cell Responses Associated with Control of HIV-1 Replication. Sci Transl Med. 2013 Jan 2;5:166ra2. doi: 10.1126/scitranslmed.3004682. [DOI] [PubMed] [Google Scholar]
- 54.Casazza JP, et al. Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire. J Infect Dis. 2013 Jun 15;207:1829. doi: 10.1093/infdis/jit098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hansen SG, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011 May 26;473:523. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hansen SG, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013 Oct 3;502:100. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hansen SG, et al. Cytomegalovirus vectors violate CD8+ T cell epitope recognition paradigms. Science. 2013 May 24;340:1237874. doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barouch DH, et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell. 2013 Oct 24;155:531. doi: 10.1016/j.cell.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barouch DH, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012 Feb 2;482:89. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Letvin NL, et al. Immune and Genetic Correlates of Vaccine Protection Against Mucosal Infection by SIV in Monkeys. Sci Transl Med. 2011 May 4;3:81ra36. doi: 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu H, et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014 Mar 27;507:519. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dahirel V, et al. Coordinate linkage of HIV evolution reveals regions of immunological vulnerability. Proc Natl Acad Sci U S A. 2011 Jul 12;108:11530. doi: 10.1073/pnas.1105315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poignard P, et al. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity. 1999 Apr;10:431. doi: 10.1016/s1074-7613(00)80043-6. [DOI] [PubMed] [Google Scholar]
- 64.Trkola A, et al. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med. 2005 Jun;11:615. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 65.Mehandru S, et al. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J Virol. 2007 Oct;81:11016. doi: 10.1128/JVI.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barouch DH, et al. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature. 2013 Nov 14;503:224. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shingai M, et al. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013 Nov 14;503:277. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein F, et al. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature. 2012 Dec 6;492:118. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Topalian SL, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med. 2012 Jun 2; doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gardiner D, et al. A randomized, double-blind, placebo-controlled assessment of BMS-936558, a fully human monoclonal antibody to programmed death-1 (PD-1), in patients with chronic hepatitis C virus infection. PLoS One. 2013;8:e63818. doi: 10.1371/journal.pone.0063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chun TW, et al. HIV-infected individuals receiving effective antiviral therapy for extended periods of time continually replenish their viral reservoir. J Clin Invest. 2005 Nov 1;115:3250. doi: 10.1172/JCI26197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riou C, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J Exp Med. 2007 Jan 22;204:79. doi: 10.1084/jem.20061681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heredia A, et al. Rapamycin causes down-regulation of CCR5 and accumulation of anti-HIV beta-chemokines: an approach to suppress R5 strains of HIV-1. Proc Natl Acad Sci U S A. 2003 Sep 2;100:10411. doi: 10.1073/pnas.1834278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009 Jul 2;460:108. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stock PG, et al. Reduction of HIV Persistence Following Transplantation in HIV-Infected Kidney Transplant Recipients. Am J Transplant. 2014 Apr 3; doi: 10.1111/ajt.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gavegnano C, et al. Ruxolitinib and Tofacitinib Are Potent and Selective Inhibitors of HIV-1 Replication and Virus Reactivation In Vitro. Antimicrob Agents Chemother. 2014 Apr;58:1977. doi: 10.1128/AAC.02496-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Veer MJ, et al. Functional classification of interferon-stimulated genes identified using microarrays. Journal of leukocyte biology. 2001 Jun;69:912. [PubMed] [Google Scholar]
- 78.Stoddart CA, Keir ME, McCune JM. IFN-alpha-induced upregulation of CCR5 leads to expanded HIV tropism in vivo. PLoS Pathog. 2010 Feb;6:e1000766. doi: 10.1371/journal.ppat.1000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Favre D, et al. HIV disease progression correlates with the generation of dysfunctional naive CD8(low) T cells. Blood. 2011 Feb 17;117:2189. doi: 10.1182/blood-2010-06-288035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Favre D, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010 May 19;2:32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Le Saout C, et al. Chronic exposure to type-I IFN under lymphopenic conditions alters CD4 T cell homeostasis. PLoS Pathog. 2014 Mar;10:e1003976. doi: 10.1371/journal.ppat.1003976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fernandez S, et al. CD4+ T-cell deficiency in HIV patients responding to antiretroviral therapy is associated with increased expression of interferon-stimulated genes in CD4+ T cells. J Infect Dis. 2011 Dec 15;204:1927. doi: 10.1093/infdis/jir659. [DOI] [PubMed] [Google Scholar]
- 83.Wilson EB, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013 Apr 12;340:202. doi: 10.1126/science.1235208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teijaro JR, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013 Apr 12;340:207. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pillai SK, et al. Role of retroviral restriction factors in the interferon-alpha-mediated suppression of HIV-1 in vivo. Proc Natl Acad Sci U S A. 2012 Feb 21;109:3035. doi: 10.1073/pnas.1111573109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Azzoni L, et al. Pegylated Interferon-alpha2A mono-therapy results in suppression of HIV-1 replication and decreased cell-associated HIV DNA integration. J Infect Dis. 2012 Oct 26; doi: 10.1093/infdis/jis663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bosque A, Famiglietti M, Weyrich AS, Goulston C, Planelles V. Homeostatic proliferation fails to efficiently reactivate HIV-1 latently infected central memory CD4+ T cells. PLoS Pathog. 2011 Oct;7:e1002288. doi: 10.1371/journal.ppat.1002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med. 2014 Apr;20:425. doi: 10.1038/nm.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cillo AR, et al. Quantification of HIV-1 latency reversal in resting CD4+ T cells from patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2014 Mar 31; doi: 10.1073/pnas.1402873111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kulkosky J, et al. Intensification and stimulation therapy for human immunodeficiency virus type 1 reservoirs in infected persons receiving virally suppressive highly active antiretroviral therapy. J Infect Dis. 2002 Nov 15;186:1403. doi: 10.1086/344357. [DOI] [PubMed] [Google Scholar]
- 91.Wang FX, et al. IL-7 is a potent and proviral strain-specific inducer of latent HIV-1 cellular reservoirs of infected individuals on virally suppressive HAART. J Clin Invest. 2005 Jan;115:128. doi: 10.1172/JCI22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sereti I, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009 Jun 18;113:6304. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Archin NM, et al. Immediate antiviral therapy appears to restrict resting CD4+ cell HIV-1 infection without accelerating the decay of latent infection. Proc Natl Acad Sci U S A. 2012 Jun 12;109:9523. doi: 10.1073/pnas.1120248109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ananworanich J, et al. Impact of multi-targeted antiretroviral treatment on gut T cell depletion and HIV reservoir seeding during acute HIV infection. PLoS ONE. 2012;7:e33948. doi: 10.1371/journal.pone.0033948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saez-Cirion A, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013 Mar;9:e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Richman DD, et al. The challenge of finding a cure for HIV infection. Science. 2009 Mar 6;323:1304. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 97.Deeks SG, et al. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012 Jul 20; doi: 10.1038/nri3262. [DOI] [PMC free article] [PubMed] [Google Scholar]