Abstract
Mitochondrial decay plays a central role in the aging process. Although certainly multifactorial in nature, defective operation of the electron transport chain (ETC) constitutes a key mechanism involved in the age-associated loss of mitochondrial energy metabolism. Primarily, mitochondrial dysfunction affects the aging animal by limiting bioenergetic reserve capacity and/or increasing oxidative stress via enhanced electron leakage from the ETC. Even though the important aging characteristics of mitochondrial decay are known, the molecular events underlying inefficient electron flux that ultimately leads to higher superoxide appearance and impaired respiration are not completely understood. This review focuses on the potential role(s) that age-associated destabilization of the macromolecular organization of the ETC (i.e. supercomplexes) may be important for development of the mitochondrial aging phenotype, particularly in post-mitotic tissues.
Keywords: aging, mitochondrial dysfunction, electron transport supercomplexes.
1. Introduction
Alterations of the components of the mitochondrial energy transduction system represent an important part of the etiology of mitochondrial decay and cellular dysfunction in aging tissues [1]. In particular, accumulation of age-related changes to both the structure and function of the electron transport chain (ETC) [2-5] and alterations of the lipid milieu of the inner mitochondrial membrane (IMM) have been noted [6-11]. Moreover, these changes to the IMM may contribute to decreased aerobic metabolism, increased generation of reactive oxygen species (ROS) and oxidative damage (for reviews see [12-15]), and higher appearance of proapoptotic factors in the cytosol [16-18]. Nevertheless, the relationship between IMM structural alterations with age and their precise consequence to mitochondrial decay is only poorly understood. This is particularly true for the protein complexes of the ETC, whose overall structural organization is currently being revised from that of individual electron transport complexes in the IMM to large “supercomplexes” composed of differing stoichiometries [19-21]. Assembly and stability of supercomplexes appears to be highly important for regulation of mitochondrial bioenergetics as even slight losses in their formation, as shown in Barth syndrome, correlate with severe cellular dysfunction [22]. Furthermore, supercomplex destabilization is the main underlying factor involved in the loss of mitochondrial bioenergetics in a canine model of acute heart failure [23, 24]. Thus, there is growing evidence that supercomplex destabilization plays a critical role in the progression of pathophysiologies where mitochondrial dysfunction has been detected. By analogy, a rationale exists that disintegration of supercomplexes is one of the characteristic features of age-related mitochondrial decay in post-mitotic tissues [25-27]. The focus of this paper is thus two-fold, namely, to review the critical factors associated with mitochondrial decay and how alterations to supercomplex ultrastructure may be an important underlying facet leading to the mitochondrial aging phenotype.
2. Mitochondrial dysfunction in the aging process
2.1. General aspects regarding the etiology of mitochondrial decay in aging
Mitochondrial decay plays a central role in the aging process [1]. Although the age-associated loss of mitochondrial function is undoubtedly multifactorial, several lines of evidence indicate that certain molecular and cellular alterations are significantly involved in the progression of changes that ultimately lead to impaired mitochondrial energy metabolism. Age-related oxidative damage and deletions of mitochondrial DNA (mtDNA) that correlate with lower respiratory activity have been reported in liver [9] and cardiac muscle [28] of rats, liver [29] and skeletal muscle [30] of primates, and skeletal [31] and cardiac muscle [28, 32] of humans. Moreover, mtDNA deletions accumulate during the progression of atrial fibrillation in human aging [32]. On the other hand, impaired autophagy that regulates mitochondrial homeostasis also appears to contribute to the age-related accumulation of respiratory defects in tissues [33-35]. In this regard, Terman et al. showed that inhibition of autophagy in neonatal rat cardiomyocytes leads to morphological alterations of mitochondria and changes in membrane potential (Δψm), similar to that of senescent cells [36]. This group further concluded that abnormal mitochondria accumulate in post-mitotic tissues as a consequence of a defective autophagy process [36, 37].
It has been hypothesized that the aforementioned characteristics of mitochondrial decay are symptomatic of a vicious downward spiral where defective ETC complexes contribute to enhanced ROS generation by mitochondria, which in turn increases mtDNA damage and mutations, which eventually reciprocally affects structure of the ETC components (for recent reviews see [12, 13]). Ultimately, higher rates of ROS appearance increase oxidative modification of membrane lipids and proteins to the point where accumulation of molecular defects overcomes the capacity to maintain mitochondrial homeostasis through autophagy [13, 33, 36].
2.2. Consequences of mitochondrial decay on cellular function
An immediate consequence of the age-related impairment of mitochondrial function would be decreased aerobic energy transduction. A consistent picture has emerged from examination of mitochondrial bioenergetics in intact cells from young and old animals of many species. Here, mitochondrial membrane potential (Δψm) and cellular energy status progressively decline with age in both freshly isolated rat hepatocytes [9] and in human skin fibroblasts taken from very young (fetal material) versus very old (103 years) human donors [38]. This work is now buttressed by reports connecting mitochondrial decay to organ decline in vivo. Using 31P magnetic resonance spectroscopy and optical approaches to simultaneously monitor ATP synthesis and O2 uptake, Marcinek et al. showed that working skeletal muscle of aged C57Bl/6 mice experienced a 50% decline in the mitochondrial P/O ratio, an indicator of the efficiency of ATP synthesis coupled to respiration, and a consequent loss in energy charge versus young animals [39]. This is in agreement with Kostler et al. who observed that high-energy phosphates in the human heart declined significantly in vivo with age, suggesting energy reserve capacity in this organ is highly attenuated [40]. Furthermore, using positron emission tomography for analyzing cardiac function in humans, Kates et al. found that aging significantly decreases fatty acid oxidation rates, with respect to myocardial oxygen consumption rates [41].
In addition, aging reportedly diminishes State 3 respiration rates during oxidation of NADH-associated substrates in rat mitochondria isolated from hippocampus and brain cortex [42, 43], skeletal muscle [44], liver [45], kidney [46], and heart [3, 4, 17, 44], as well as in human skeletal muscle mitochondria [47]. Nevertheless, it is less clear whether aging modifies respiratory control (RCR) and P/O ratios when using in vitro models. Experiments on isolated rat heart mitochondria showed that even though State 3 respiration declines with age, the P/O ratio and RCR remain unaffected [4, 44, 48]. In line with these findings, O'Toole et al. reported a lack of age-related changes in the P/O and RCR in rat kidney mitochondria [46]. In contrast, other reports reveal that RCR values decline with age in rat liver mitochondria [45] and also in mitochondria from rat hippocampus [43]. To summarize, aging decreases mitochondrial ADP-stimulated respiration and cellular aerobic metabolism, but the degree of this decline is controversial. In vitro studies may not precisely reflect the magnitude that age-related mitochondrial decay impairs cellular energy metabolism in vivo.
Analysis of mitochondrial calcium handling, a key aspect of overall mitochondrial function, shows that aging induces higher vulnerability to calcium overload and propensity to permeability transition in mitochondria in brains and livers from B6D2F1 mice [18] and in interfibrillar mitochondria from Fischer 344 [17] and Fischer 344 × Brown Norway rats [16]. Furthermore, Hofer et al. showed that the age-related loss of calcium-accumulating capacity in mitochondria from Fischer 344 × Brown Norway rats correlated with higher concentrations of cytosolic cytochrome c and resultant higher caspase 3 activity [16]. In further support for these observations, it was recently observed that human cardiomyocytes display higher expression of proapoptotic proteins (e.g. Bax) with age, which correlate with increased cytosolic levels of cytochrome c and caspase 9 [49].
Lastly, a strong relationship has emerged between mitochondrial decay and oxidative damage to mitochondrial and extramitochondrial cellular components (for reviews see [12-15]). Consistent with this view are studies, including our own, showing higher levels of mtDNA deletions and oxidative damage (see Section 2.1.), oxidative DNA damage [49, 50], lipid peroxidation [50-53], and lipid adduction to proteins [54-56] with age. Cardiolipin, a mitochondrial specific phospholipid, may be particularly prone to oxidative damage and loss (see Section 3.2. and also [57]). This is because of the highly unsaturated nature of its acyl side-chains and its proximity to ROS emanating from the ETC [58-61]. Additionally, most amino acid residues can be oxidized by ROS, which lead to formation of disulfide bonds and carbonyl derivatives [49, 62-66]. Proteins in proximity to decaying mitochondria may be particularly susceptible to oxidative damage and dysfunction. Thus, mitochondrial-driven oxidative damage may not only influence progression of mitochondrial decay but also adversely affect cell survival and overall organ function.
Even though the aforementioned characteristics of mitochondria in aged tissues show varying degrees of alterations, the available evidence nevertheless supports the view that that electron flux efficiency through the ETC declines while oxidant leak is enhanced with age. Both of these age-associated deficits emanate from an altered IMM. The following section will provide evidence for the functional consequences of mitochondrial decay, especially with respect to ETC function.
3. Age-associated changes of the mitochondrial energy transduction system
3.1. Catalytic and structural alterations of the respiratory chain complexes in aging
Dysfunction of the components of the ETC strongly correlates to the age-related mitochondrial decline in bioenergetic reserve observed in different organs [2-5]. In both rodents and humans, complex I catalytic activity declines with age in liver [5, 67], brain [43, 68], and heart [5, 49, 69, 70]. In addition, several alterations of the complex III holo-protein result in its lower activity, particularly in post-mitotic tissues of aging rodents [2, 4, 69, 71] and primates [72]. These results agree with other studies showing that defects of complex IV correlate with lower mitochondrial oxidative capacity in post-mitotic tissues of elder humans [73, 74], primates [72, 75], and rodents [3, 43, 76-79].
Age-associated alterations of the ETC components promote inefficient electron transport (i.e. higher electron leakage) and increased ROS appearance in mitochondria [70, 71, 80, 81]. In turn, higher ROS generation contributes to ETC dysfunction by initiating oxidative modifications of ETC proteins [55, 69, 76] and mtDNA [30, 82] (see also Section 2.2.). However, the mechanism(s) of superoxide (O2•−) generation under physiological circumstances are not completely understood, and the role that the ETC plays in increased O2•− with age is not yet clear. In this regard, experimental observations by Moghaddas et al. indicate that oxidative modification of the Qo binding site in complex III is the main factor involved in higher O2•− generation in the aged heart [71]. On the other hand, complex I may also be an important source of O2•− generation that could contribute to enhanced O2•− with age [70, 81, 83, 84].
Regardless of the specific site of production, increased ROS from an impaired ETC correlates with a decline in mitochondrial antioxidant status with age. The mono-thiol antioxidant, glutathione (GSH), is particularly diminished in mitochondria from aged tissue. We showed that aging leads to deficits in both mitochondrial GSH levels and its redox ratio (GSH/GSSG) in the brain and heart of rats [85]. Moreover, we observed both an age-related loss of GSH and ascorbate in rat hepatocytes [86] and isolated rat heart mitochondria [76]. The aging rat heart also displayed significantly lower ascorbate concentrations [50]. Thus, mitochondria from aged tissues of a variety of species display increased ROS output, lower antioxidant defenses, and greater oxidative damage.
3.2. Age-related changes in the lipid composition of the inner membrane
Aging leads to alterations of the lipid composition of the IMM that also contribute to impaired bioenergetic capacity of organs and tissues. An age-related decline in coenzyme Q, a key isoprene-derivative that mediates electron transfer between several IMM protein complexes, has been observed in plasma [87] and also in cardiac muscle from human subjects [88, 89]. In addition, tissue levels of coenzyme Q appear to decline in heart, kidney, and skeletal muscle from aged rats [90]. This loss appears to be more pronounced when coenzyme Q levels are measured in isolated mitochondria versus whole tissues [10, 11]. Additionally, multiple studies suggest that aging leads to lower cardiolipin levels and/or its acyl side-chain composition. Cardiolipin is a phospholipid that is almost exclusively located to mitochondria and acts as an important cofactor of several IMM proteins [58, 59]. Studies using brain [68], liver [91], and cardiac mitochondria [6-8, 92-94], and our own work with isolated rat hepatocytes [86, 95] indicate that cardiolipin significantly declines with age. However, the extent of cardiolipin loss and/or whether such declines are functionally consequential is controversial. Hoppel and colleagues provided evidence that interfibrillar mitochondria of the aging rat heart have no changes in cardiolipin levels or composition, in contrast to earlier reports [96]. The reason for the discrepancy between this work and the aforementioned studies is not clear, although a partial answer may lie in the different methods used to extract lipids from mitochondrial fractions (e.g. one-phase organic systems versus two-phase mixtures) and the various techniques employed for separation and analysis of cardiolipin.
Despite the controversial degree of general cardiolipin loss, there is a growing consensus that significant remodeling of cardiolipin acyl side-chains occurs with age. Helmy et al. observed an age-related increase in the ratio of monolyso-cardiolipin to mature cardiolipin in the guinea pig kidney [97], an indicator of defective incorporation of acyl chain units into cardiolipin during the remodeling cycle [98]. In addition, tetralinoleoyl-cardiolipin ([18:2]4-cardiolipin), the predominant species in the heart [61, 99], appears to be the most adversely affected on an age basis [100]. We also recently observed that aging leads to a significant loss of (18:2)4-cardiolipin in rat heart interfibrillar mitochondria, although total cardiolipin levels were unaffected (unpublished results). These results partially agree with the observations made by Hoppel and coworkers [96]. Alteration of acyl side-chains, as evident in aging tissues, would be expected to adversely affect ETC electron movement because of protein conformational changes. Thus, cardiolipin molecular composition is markedly affected with age, possibly due to defects in remodeling of mature cardiolipin.
Although aging does not significantly affect the levels of major phospholipids (e.g. phosphatidylethanolamine) [6-8, 91], the overall IMM composition and its fluidity are significantly changed with age. Cholesterol accumulates in the IMM, alters membrane fluidity, and may promote greater H+ leakage [7, 91]. In a similar vein, using LC-MS/MS analysis, we recently demonstrated that aged cardiac mitochondria accumulate ceramide, a pro-apoptotic sphingolipid, which results in inhibition of complex IV activity [79]. Furthermore, three ceramide isoforms in the IMM (e.g. those with 16, 18, and 24:1 acyl chains) caused the increase in overall ceramide levels [79]. Thus, extensive age-related alterations of the lipid milieu of the IMM occur, which adversely affect membrane structural organization and contribute to limiting the biological function of the ETC complexes.
4. Supercomplex destabilization as a new underlying factor of mitochondrial decay in aging
4.1. Supercomplex organization of the electron transport chain
The ETC comprises four large protein complexes, which along with the F1FO-ATP synthase (complex V) and mobile electron carriers (e.g. cytochrome c and coenzyme Q), constitute the machinery for converting metabolic energy transiently stored as reduced coenzymes into ATP. Until recently, the prevailing view was that the components of the ETC were distinct entities where rapid, random collisions allowed electron transfer between complexes [101-104]. However, with the advent of Blue Native-PAGE (BN-PAGE) technology [19-21], there is a growing awareness that the individual components of the ETC may actually exist as large macromolecular assemblies, or so-called supercomplexes. Evidence accumulated from functional and structural studies now supports the existence of these supramolecular assemblies, which includes oxygen consumption characteristics [105], metabolic flux control analysis [106-111], and three-dimensional structures of the I1III2IV11 supercomplex from bovine heart mitochondria at relatively good resolution (~ 20 Å) [112, 113].
Although there was initial skepticism whether supercomplexes were artifacts from the use of a non-ionic detergent (e.g. digitonin) in their isolation, further extensive characterization of supercomplexes from mitochondria of different sources (Table 1) appears to have mitigated most concerns regarding their biological existence. This characterization includes identification of striking variations in stoichiometries of components comprising supercomplexes in tissues from the same species [114] or from cells obtained from different human tissues (Table 1). Moreover, metabolic flux control analysis measuring the kinetic behavior of the mitochondrial ETC and the ubiquinone pool further provide supporting evidence for supercomplex organization of the ETC [106-108, 110, 111]. Briefly, complexes I and III appear to be kinetically linked [106, 107, 111], while there is no association of complex II with any other complex. This latter result is in good agreement with BN-PAGE analysis using cardiac mitochondria [19, 25, 114, 115], which indicates that supercomplexes are comprised of varying stoichiometries of complexes I, III, and IV. Finally, cryo-electron microscopic and tomographic studies independently show the existence of I1III2IV1 supercomplexes [112, 116]. These studies show that the distance between binding sites of coenzyme Q at complexes I and III is only ~13 nm [112, 116] and that the distance between binding sites of cytochrome c at complexes III and IV is ~10 nm [112, 116]. Such distances are much shorter than the minimum effective lengths determined for diffusion of coenzyme Q (37.9 nm) and cytochrome c (24.8 nm) during electron transfer by a random collision mechanism [102]. This suggests that supercomplexes offer a catalytic advantage of faster and more efficient electron transfer by limiting the overall distance between redox cofactors.
Table 1.
Tissue distribution of cardiolipins and identified mitochondrial electron transport supercomplexes.
| Source of mitochondria | Symmetric cardiolipin a (% of total) | Refs. | Supercomplexes |
Refs. | |
|---|---|---|---|---|---|
| I1III2 b | I1III2IVN c | ||||
| Heart | |||||
| Bovine | (18:2)4 (70-73) | [142] | + | + | [19, 136] |
| Rat | (18:2)4 (77) | [99, 124] | + | + | [25, 115] |
| Dog | (18:2)4 (77-79) | [23, 99] | + | + | [24] |
| Liver | |||||
| Rat | (18:2)4 (57) | [142, 143] | + | — | [114] |
| Mouse | n.d. | +(V1) d | + (II1) | [105] | |
| Skeletal muscle | |||||
| Rat | (18:2)4 (73) | [99] | + | + | [27, 114] |
| Human | (18:2)4 (79-81) | [124, 144] | + | + | [118] |
| Cell line | |||||
| C2C12 | n.d. | + (V1) | + (II1) | [132] | |
| HeLa | n.d. | + | + | [129] | |
| HEK-293 | n.d. | + | + | [128] | |
| HL-60 | n.d. | — | + | [141] | |
| PBMC (human) | n.d. | — | + | [141] | |
| Lymphoblasts (human) | (18:1)4 (32) | [61, 145] | + | + | [22] |
| Skin fibroblasts | |||||
| Mouse | n.d. | + | + | [130] | |
| Human | (18:2)4 (20-30) | [146, 147] | — | + | [149] |
| Lung fibroblasts (mouse) | (18:1)4 (n.d.) | [148] | — | + | [148] |
| Osteosarcomacybrids (human) | n.d. | — | + | [120] | |
| TPC-1 (human) | n.d. | + | — | [107] | |
| Other sources | |||||
| Brain cortex (rat) | n.d. | + | + | [26] | |
| Kidney (rat) | (18:2)4 (50) | [124] | + | + | [46, 114] |
| Human placenta | (18:2)4 (20) | [124] | + | + | [150] |
| S. cerevisiae | (18:1)4 (31) | [61] | III2IV1e | III2IV2 | [19, 122] |
| N. crassa | (18:2)4 (29) | [143] | + | + | [151] |
| C. elegans | n.d. | + | + | [131] | |
| P. anserina | n.d. | + | + | [152, 153] | |
| P. denitrificans | n.d. | — | I1III4IV4 | [135] | |
| Plants | |||||
| Potato | n.d. | + | + | [154-156] | |
| Spinach green | n.d. | + | + | [157] | |
| leaves | |||||
| A. maculatum | n.d. | + | + | [158] | |
| Arabidopsis | n.d. | + | — | [159] | |
| Bamboo | n.d. | + (V1) | — | [160] | |
| Maize | n.d. | + | [161] | ||
Molecular species are presented as the acyl chains (parenthesis) and their corresponding stoichiometries (subscript).
I1III2 denotes a supercomplex comprising a single copy of complex I and dimeric complex III.
I1III2IVN denotes supercomplexes comprising a single copy of complex I, dimeric complex III, and variable (N=1 - 4) copies of complex IV.
Roman numeral in parenthesis denotes additional OXPHOS complexes also reported as part of supercomplexes.
Alternative mitochondrial supercomplexes, different than I1III2 and/or I1III2IVN-type assemblies.
n.d. = not determined
Finally, and in relation to the role that supercomplex destabilization plays in mitochondrial aging (see Section 4.2.), supercomplexes may be necessary for general stability of the ETC. Moreno-Lastres et al. demonstrated that complexes III and IV are required for full assembly of complex I in mitochondria from human osteosarcoma cybrids [117]. Other studies show that removing complex III results in loss of the I1III2 supercomplex in human mitochondria from skeletal muscle [118], skin fibroblasts [119], and osteosarcoma cybrid cells [120]. Therefore, it is reasonable to theorize that not only does supercomplex organization mediate respiratory activity, but these macromolecular assemblies also regulate stability of individual components of the ETC.
In summary, there is growing evidence that the ETC is actually assembled as a solid-state macromolecular assembly where defective supercomplex organization results in pathologies as varied as heart failure [23, 24], Barth syndrome [22], and Leigh syndrome [118, 119]. Both the theoretical and experimental evidence suggesting that supercomplex destabilization is part of mitochondrial aging will now be discussed.
4.2. Destabilization of supercomplexes and its implications on mitochondrial decay in aging
4.2.1. Evidence of supercomplex destabilization in aged tissues
While only a limited number of studies have examined how aging affects supercomplexes, the information gleaned so far strongly suggests that the size and complexity of these assemblies diminish on an age basis. To our knowledge, we were the first to show supercomplex deterioration in cardiac mitochondria from old rats [25]. Primarily, supercomplexes comprising the highest molecular weight assemblies declined to the greatest extent with age, although most of the electron transport supercomplexes showed some degree of loss [25]. However, it is notable that supercomplex disintegration was not from age-associated decrements in a particular ETC component [25]. In support of our work in the heart, Frenzel et al. also reported supercomplex destabilization in cortical tissue of aging rat brains [26]. This study also noted that I1III2IVN supercomplexes are particularly adversely affected [26]. However in contrast to these studies, Lombardi et al. noted an age-associated increase in I1III2IVN supercomplexes in rat skeletal muscle [27], which was hypothesized to be a compensation for the significant loss of the smaller I1III2 supercomplex. Finally, a recent report showed no age-associated alterations in supercomplex organization at all in rat kidney mitochondria [46], even though loss of State 3 respiration was detected [46]. Taken together, there appears to be varying degrees of age-dependent supercomplex disorganization where brain and heart mitochondria are the most adversely affected, and other tissues (e.g. skeletal muscle and kidney) have lesser or no supercomplex decrements. The reason(s) for this variability are not presently clear but could stem from tissue-specific factors related to supercomplex stabilization and/or levels of individual ETC components available for supercomplex formation. However, it is quite conceivable that part of the seeming variability for supercomplex destabilization may stem from the small number of studies performed to date and differences in analytical protocols and quantitative analysis used. Nevertheless, the identification of severe age-associated alterations in supercomplex levels, especially in post-mitotic tissue, warrants further analysis of both the causes and the consequences to mitochondrial function (see also Section 4.2.2).
4.2.2. Major factors that alter supercomplex stability in aging
The precise mechanism(s) involved in supercomplex destabilization have yet to be elucidated. However, genetic studies using yeast [121-123] as well as cryo-electron microscopy [112] are beginning to elucidate a role for lipid-protein interactions as a partial mechanism for this deterioration. For example, experiments on yeast mitochondria indicate that cardiolipin lowers electrostatic repulsion at the interface between complexes III and IV [122]. Moreover, the ETC complexes assembled as the I1III2IV1 supercomplex are not in close contact with each other (2-5 nm apart) within the IMM [112], which indicates that lipid-protein interactions are key to maintaining supercomplex stability. In addition, there is a strong correlation between the levels of I1III2IVN supercomplexes and mitochondria from tissues displaying relatively high content (~70-80%) of cardiolipins with (18:2)4-acyl side-chains (Table 1). This association fits with observations that “symmetrical” cardiolipins containing similar acyl chains promote protein-protein interactions and ETC function [61, 99, 124-126]. Indeed, the aforementioned study by Frenzel et al. suggested that the age-related destabilization of brain cortical supercomplexes was mediated by altered cardiolipin acyl chain content [26]. Thus, it is tempting to hypothesize that the oxidative damage of unsaturated cardiolipin side-chains that has been observed in the heart and brain from aged animals [68, 70] leads to supercomplex deterioration by altering binding of cardiolipin to ETC proteins. In support of this hypothesis, Diaz et al. recently reported that increased ROS generation destabilizes supercomplexes in mouse lung fibroblasts [127]. In this study, lower supercomplex levels correlated with decreased stability of complex I in an antimycin A-supported model of ROS generation. Nevertheless, it is not clear whether oxidatively damaged membrane lipids also contributed to supercomplex destabilization under the same conditions [127]. These studies provide a framework for further work on the role that IMM lipid composition (see section 3.2) plays in both supercomplex formation and destabilization with age.
In addition to lipid involvement, disintegration of supercomplexes may also stem from alterations to proteins of the IMM. In particular, complex IV is a key player in supercomplex formation or, alternatively, supercomplex destabilization with age. In fact, we and others showed that complex IV activity declines in aged rat heart interfibrillar mitochondria [3, 76, 79], which correlates with extensive oxidative modification of this complex [76]. Moreover, alterations of several complex IV subunits are associated with defective assembly of I1III2IVN supercomplexes in mitochondria from human cells [118, 120, 128, 129], rodents [105, 130], and C. elegans [131]. For example, phosphorylation of complex IV results in the loss of respirasomes in a canine model of acute heart failure [23]. Therefore, oxidative or posttranslational modifications of complex IV may regulate supercomplex stability in cardiac mitochondria [23]. Finally, studies from multiple laboratories have supplied exciting new information showing that specific protein factors are involved in supercomplex assembly [132-134]. Rcf1 (Respiratory Supercomplex Factor 1) associates with complex IV and is required for supercomplex stabilization in yeast mitochondria. Moreover, Chen et al. showed that defective organization of I1III2IVN supercomplexes results from siRNA-mediated knock down of the rcf1 mammalian homologue, HIG2A (hypoxia inducible gene 1, family member 2A), in C2C12 myoblasts [132]. Thus, HIG2A or other as yet unidentified proteins may also regulate supercomplex stability, particularly in post-mitotic tissues (e.g. the heart and brain) that show the most severe supercomplex disintegration with age.
In summary, both lipid factor(s) and proteins associated with the IMM may be involved in the assembly and maintenance of mitochondrial supercomplexes (Fig. 1). While little evidence currently exists for a precise role of any one factor in the loss of supercomplexes with age, nevertheless, it is noteworthy that both cardiolipin structural alterations and protein oxidation markedly increases on an age basis, particularly in mitochondria where supercomplexes deteriorate most appreciably. Identification of the specific biological molecules involved in supercomplex assembly will be necessary to further identify both tissue specific variability of supercomplex assemblies and their age-associated decline.
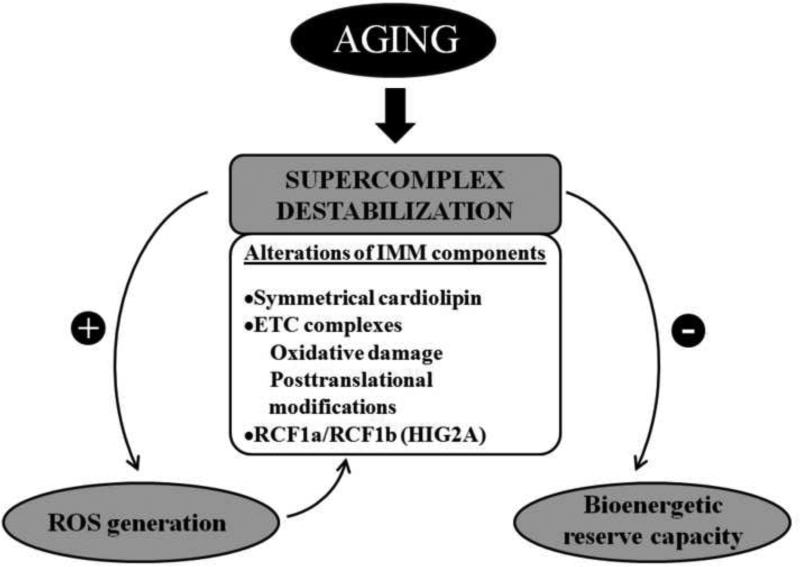
Figure 1.
Supercomplex destabilization and mitochondrial decay in aging. Age-related alterations of the IMM components are presented as important factors affecting stability of supercomplex organization. Potential consequences of supercomplex destabilization on mitochondrial function include higher generation of O2•−, which may affect supercomplexes by increasing oxidative damage of IMM lipids and proteins, and diminished bioenergetic reserve capacity. RCF1a and RCF1b: human homologues of the yeast respiratory supercomplex factor (Rcf1). HIG2A: hypoxia inducible gene 1, domain family member 2A.
4.2.3. Supercomplex destabilization and impaired mitochondrial bioenergetics in aging
Since their elucidation by Schägger and colleagues [19, 135, 136], it has been hypothesized that the biological reason for the existence of supercomplexes is to efficiently pass electrons through the ETC to O2. Conversely, age-associated decrements in supercomplex assembly may theoretically result in inefficient ETC electron flux, adversely affecting energy reserve capacity. Experimental support for a role of supercomplex assembly in efficient electron transport comes from studies showing stability of the I1III2 supercomplex correlates with higher NADH-cytochrome c oxidoreductase activity [107, 108, 110]. Moreover, pioneering work by Zhang et al. [121] showed that supercomplex destabilization in a yeast strain lacking the cardiolipin synthase gene (i.e. crd1Δ mutants) was associated with abnormal growth on nonfermentable substrates [121]. In addition, Greenberg et al. showed that crd1Δ yeast displayed defective mitochondrial respiration and oxidative phosphorylation characteristics but only under extreme conditions, such as elevated temperature or osmotic shock [137-139]. Thus, it appears that supercomplexes are not required for maintaining basal respiratory activity but for supporting bioenergetic reserve capacity. Taken together, these observations provide support that supercomplex destabilization diminishes State 3 respiration and also limits bioenergetic reserve capacity in vivo. This phenotype parallels characteristics of mitochondrial decay and at least in part explains the metabolic limitations of aging in aerobically active tissues (see Section 2.2.). Significantly more work will be needed in order to fully elucidate the true implications of supercomplex destabilization on the loss of mitochondrial bioenergetics.
4.2.4. Supercomplex destabilization and ROS generation in aging
An obvious advantage for the ETC integrated as a supercomplex would be for efficient electron flux from reduced coenzymes to molecular oxygen [19]. In this regard, Panov et al. hypothesized that supercomplexes prevent superoxide formation even during maximal electron flux by maintaining all the electron carriers (i.e. Fe-S clusters and ubiquinone in complexes I and III) involved in O2•− generation in a permanently oxidized state [140]. Thus, supercomplexes would represent an evolutionary adaptation to prevent excessive ROS formation [140]. Alternatively, lack of supercomplexes has been implicated in high basal mitochondrial O2•− production in human neutrophils [141]. One study showed that mitochondria from mononuclear leukocytes and human leukemia cells (HL-60) contain I1III2IVN supercomplexes, but these particular macromolecular assemblies disappear when HL-60 cells differentiate into neutrophils [141]. While the respiratory supercomplexes were not necessary to maintain membrane potential (Δψm), supercomplex loss in differentiated cells resulted in significantly higher rates of O2•− generation [141]. Furthermore, Lenaz et al. showed that mouse fibroblasts expressing the activated form of the k-ras oncogene lacked supercomplexes, which correlated with a higher rate of ROS appearance with respect to wild type fibroblasts [108]. Finally, new insights as to the role of supercomplexes in preventing ROS formation have been provided by the recent discovery of the Rcf1 subunit in yeast mitochondria [132, 133]. In these studies, rcf1Δ yeast displayed significantly higher rates of ROS appearance and increased oxidative damage versus wild type cells [132, 133].
In summary, supercomplex disassembly in aged post-mitotic tissues correlates with a wealth of information showing higher superoxide leak from the ETC with age. Taken together with the aforementioned studies on supercomplexes and their influence on O2•− generation, it is enticing to speculate a cause-and-effect relationship between higher ROS and supercomplex deterioration. Figure 1 shows a schematic representation of this hypothesis. Nevertheless, there is still a dearth of experimental support for a functional role of supercomplexes in preventing higher rates of ROS appearance in post-mitotic tissues.
5. Conclusions and perspectives
Structural and functional alterations of the IMM components are associated with mitochondrial decay that adversely affects aerobic energy metabolism in aging tissues. Loss of catalytic function of the ETC complexes is recognized as an underlying factor of the age-related decrease in bioenergetic reserve capacity. Herein, we have presented theoretical and current experimental evidence that disintegration of ETC supercomplex ultrastructure may lead to many aspects of the mitochondrial aging phenotype, especially in post-mitotic tissues like the heart and brain. As discussed, supercomplex deterioration may be multifactorial where both IMM lipid and proteins may be involved. The precise elucidation of the factor(s) that integrate ETC components as supercomplexes should also shed light on age-related decline in supercomplex assembly and, by analogy, provide molecular target(s) for therapeutic intervention to maintain ETC function. Moreover, equal efforts should be directed toward defining the consequences of supercomplex disassembly with respect to known characteristics of mitochondrial decay. It should be noted that even small losses of supercomplex levels correlate with severe consequences to organ function (cf Barth Syndrome). However, studies with yeast suggest that basal bioenergetics is not affected with supercomplex disassembly. It is likely that supercomplex deterioration would mainly limit electron transport efficiency, leading to enhanced ROS/oxidative damage, as well as limitations in energy reserve capacity. Reciprocally, increased ROS generation would cause supercomplex destabilization by oxidatively altering the structural organization of lipids (e.g. cardiolipin) and proteins involved in the assembly of mitochondrial supercomplexes. Thus, one could envision that aging leads to a vicious downward spiral of oxidative damage to IMM constituents, supercomplex disintegration and increasing oxidative damage, which is followed by more severe supercomplex destruction. This scenario could be extended to age-accelerating syndromes where chronic oxidative stress is evident. For example, it would be interesting to determine whether mitochondria from diabetic subjects also display loss of supercomplexes similar to normal aging. Further studies are needed to elucidate whether defective supercomplex organization limits energy supply and aerobic metabolism in the old animal.
Highlights.
The inner mitochondrial membrane decays with age, affecting energy metabolism.
Supercomplex destabilization is discussed as a novel aspect of mitochondrial aging.
Discovery of supercomplex loss opens new possibilities to decline in bioenergetics.
Acknowledgements
This work was funded by grants from the National Institute on Aging (2R01AG017141-06A2) and the National Center for Complementary and Alternative Medicine (P01AT002034). We also acknowledge the facilities service core of the Environmental Health Science Center (NIEHS ES00240). The authors would like to thank Stephen Lawson for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supercomplex assemblies are presented as the Roman numeral which represents the particular electron transport complex, along with a subscript indicating its stoichiometry relative to other electron transport chain components.
References
- 1.Bratic I, Trifunovic A. Mitochondrial energy metabolism and ageing. Biochim Biophys Acta. 2010;1797:961–7. doi: 10.1016/j.bbabio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Sandhu SK, Kaur G. Mitochondrial electron transport chain complexes in aging rat brain and lymphocytes. Biogerontology. 2003;4:19–29. doi: 10.1023/a:1022473219044. [DOI] [PubMed] [Google Scholar]
- 3.Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- 4.Lesnefsky EJ, Gudz TI, Moghaddas S, Migita CT, Ikeda-Saito M, Turkaly PJ, et al. Aging decreases electron transport complex III activity in heart interfibrillar mitochondria by alteration of the cytochrome c binding site. J Mol Cell Cardiol. 2001;33:37–47. doi: 10.1006/jmcc.2000.1273. [DOI] [PubMed] [Google Scholar]
- 5.Lenaz G, Bovina C, Castelluccio C, Fato R, Formiggini G, Genova ML, et al. Mitochondrial complex I defects in aging. Mol Cell Biochem. 1997;174:329–33. [PubMed] [Google Scholar]
- 6.Nohl H, Kramer R. Molecular basis of age-dependent changes in the activity of adenine nucleotide translocase. Mech Ageing Dev. 1980;14:137–44. doi: 10.1016/0047-6374(80)90112-8. [DOI] [PubMed] [Google Scholar]
- 7.Lewin MB, Timiras PS. Lipid changes with aging in cardiac mitochondrial membranes. Mech Ageing Dev. 1984;24:343–51. doi: 10.1016/0047-6374(84)90119-2. [DOI] [PubMed] [Google Scholar]
- 8.McMillin JB, Taffet GE, Taegtmeyer H, Hudson EK, Tate CA. Mitochondrial metabolism and substrate competition in the aging Fischer rat heart. Cardiovasc Res. 1993;27:2222–8. doi: 10.1093/cvr/27.12.2222. [DOI] [PubMed] [Google Scholar]
- 9.Hagen TM, Yowe DL, Bartholomew JC, Wehr CM, Do KL, Park JY, et al. Mitochondrial decay in hepatocytes from old rats: membrane potential declines, heterogeneity and oxidants increase. Proc Natl Acad Sci USA. 1997;94:3064–9. doi: 10.1073/pnas.94.7.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamzalov S, Sohal RS. Effect of age and caloric restriction on coenzyme Q and alpha-tocopherol levels in the rat. Exp Gerontol. 2004;39:1199–205. doi: 10.1016/j.exger.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Sohal RS, Forster MJ. Coenzyme Q, oxidative stress and aging. Mitochondrion. 2007;7(Suppl):S103–11. doi: 10.1016/j.mito.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct. 2012;2012:646354. doi: 10.1155/2012/646354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M, et al. Mitochondria ros crosstalk in the control of cell death and aging. J Signal Transduct. 2012;2012:329635. doi: 10.1155/2012/329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–22. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 15.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–72. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofer T, Servais S, Seo AY, Marzetti E, Hiona A, Upadhyay SJ, et al. Bioenergetics and permeability transition pore opening in heart subsarcolemmal and interfibrillar mitochondria: effects of aging and lifelong calorie restriction. Mech Ageing Dev. 2009;130:297–307. doi: 10.1016/j.mad.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahangir A, Ozcan C, Holmuhamedov EL, Terzic A. Increased calcium vulnerability of senescent cardiac mitochondria: protective role for a mitochondrial potassium channel opener. Mech Ageing Dev. 2001;122:1073–86. doi: 10.1016/s0047-6374(01)00242-1. [DOI] [PubMed] [Google Scholar]
- 18.Mather M, Rottenberg H. Aging enhances the activation of the permeability transition pore in mitochondria. Biochem Biophys Res Commun. 2000;273:603–8. doi: 10.1006/bbrc.2000.2994. [DOI] [PubMed] [Google Scholar]
- 19.Schagger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19:1777–83. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wittig I, Carrozzo R, Santorelli FM, Schagger H. Supercomplexes and subcomplexes of mitochondrial oxidative phosphorylation. Biochim Biophys Acta. 2006;1757:1066–72. doi: 10.1016/j.bbabio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Wittig I, Schagger H. Supramolecular organization of ATP synthase and respiratory chain in mitochondrial membranes. Biochim Biophys Acta. 2009;1787:672–80. doi: 10.1016/j.bbabio.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 22.McKenzie M, Lazarou M, Thorburn DR, Ryan MT. Mitochondrial respiratory chain supercomplexes are destabilized in Barth Syndrome patients. J Mol Biol. 2006;361:462–9. doi: 10.1016/j.jmb.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 23.Rosca M, Minkler P, Hoppel CL. Cardiac mitochondria in heart failure: normal cardiolipin profile and increased threonine phosphorylation of complex IV. Biochim Biophys Acta. 2011;1807:1373–82. doi: 10.1016/j.bbabio.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Rosca MG, Vazquez EJ, Kerner J, Parland W, Chandler MP, Stanley W, et al. Cardiac mitochondria in heart failure: decrease in respirasomes and oxidative phosphorylation. Cardiovasc Res. 2008;80:30–9. doi: 10.1093/cvr/cvn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez LA, Monette JS, Chavez JD, Maier CS, Hagen TM. Supercomplexes of the mitochondrial electron transport chain decline in the aging rat heart. Arch Biochem Biophys. 2009;490:30–5. doi: 10.1016/j.abb.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frenzel M, Rommelspacher H, Sugawa MD, Dencher NA. Ageing alters the supramolecular architecture of OxPhos complexes in rat brain cortex. Exp Gerontol. 2010;45:563–72. doi: 10.1016/j.exger.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Lombardi A, Silvestri E, Cioffi F, Senese R, Lanni A, Goglia F, et al. Defining the transcriptomic and proteomic profiles of rat ageing skeletal muscle by the use of a cDNA array, 2D- and Blue native-PAGE approach. J Proteomics. 2009;72:708–21. doi: 10.1016/j.jprot.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Hayakawa M, Sugiyama S, Hattori K, Takasawa M, Ozawa T. Age-associated damage in mitochondrial DNA in human hearts. Mol Cell Biochem. 1993;119:95–103. doi: 10.1007/BF00926859. [DOI] [PubMed] [Google Scholar]
- 29.Castro Mdel R, Suarez E, Kraiselburd E, Isidro A, Paz J, Ferder L, et al. Aging increases mitochondrial DNA damage and oxidative stress in liver of rhesus monkeys. Exp Gerontol. 2012;47:29–37. doi: 10.1016/j.exger.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pak JW, Herbst A, Bua E, Gokey N, McKenzie D, Aiken JM. Mitochondrial DNA mutations as a fundamental mechanism in physiological declines associated with aging. Aging cell. 2003;2:1–7. doi: 10.1046/j.1474-9728.2003.00034.x. [DOI] [PubMed] [Google Scholar]
- 31.Cooper JM, Mann VM, Schapira AH. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: effect of ageing. J Neurol Sci. 1992;113:91–8. doi: 10.1016/0022-510x(92)90270-u. [DOI] [PubMed] [Google Scholar]
- 32.Lai LP, Tsai CC, Su MJ, Lin JL, Chen YS, Tseng YZ, et al. Atrial fibrillation is associated with accumulation of aging-related common type mitochondrial DNA deletion mutation in human atrial tissue. Chest. 2003;123:539–44. doi: 10.1378/chest.123.2.539. [DOI] [PubMed] [Google Scholar]
- 33.Brunk UT, Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002;269:1996–2002. doi: 10.1046/j.1432-1033.2002.02869.x. [DOI] [PubMed] [Google Scholar]
- 34.Muster B, Kohl W, Wittig I, Strecker V, Joos F, Haase W, et al. Respiratory chain complexes in dynamic mitochondria display a patchy distribution in life cells. PLoS One. 2010;5:e11910. doi: 10.1371/journal.pone.0011910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weber TA, Reichert AS. Impaired quality control of mitochondria: aging from a new perspective. Exp Gerontol. 2010;45:503–11. doi: 10.1016/j.exger.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Terman A, Dalen H, Eaton JW, Neuzil J, Brunk UT. Mitochondrial recycling and aging of cardiac myocytes: the role of autophagocytosis. Exp Gerontol. 2003;38:863–76. doi: 10.1016/s0531-5565(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 37.Terman A, Brunk UT. Myocyte aging and mitochondrial turnover. Exp Gerontol. 2004;39:701–5. doi: 10.1016/j.exger.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Greco M, Villani G, Mazzucchelli F, Bresolin N, Papa S, Attardi G. Marked aging-related decline in efficiency of oxidative phosphorylation in human skin fibroblasts. FASEB J. 2003;17:1706–8. doi: 10.1096/fj.02-1009fje. [DOI] [PubMed] [Google Scholar]
- 39.Marcinek DJ, Schenkman KA, Ciesielski WA, Lee D, Conley KE. Reduced mitochondrial coupling in vivo alters cellular energetics in aged mouse skeletal muscle. J Physiol. 2005;569:467–73. doi: 10.1113/jphysiol.2005.097782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kostler H, Landschutz W, Koeppe S, Seyfarth T, Lipke C, Sandstede J, et al. Age and gender dependence of human cardiac phosphorus metabolites determined by SLOOP 31P MR spectroscopy. Magn Reson Med. 2006;56:907–11. doi: 10.1002/mrm.21027. [DOI] [PubMed] [Google Scholar]
- 41.Kates AM, Herrero P, Dence C, Soto P, Srinivasan M, Delano DG, et al. Impact of aging on substrate metabolism by the human heart. J Am Coll Cardiol. 2003;41:293–9. doi: 10.1016/s0735-1097(02)02714-6. [DOI] [PubMed] [Google Scholar]
- 42.Boveris A, Navarro A. Brain mitochondrial dysfunction in aging. IUBMB Life. 2008;60:308–14. doi: 10.1002/iub.46. [DOI] [PubMed] [Google Scholar]
- 43.Navarro A, Lopez-Cepero JM, Bandez MJ, Sanchez-Pino MJ, Gomez C, Cadenas E, et al. Hippocampal mitochondrial dysfunction in rat aging. Am J Physiol Regul Integr Comp Physiol. 2008;294:R501–9. doi: 10.1152/ajpregu.00492.2007. [DOI] [PubMed] [Google Scholar]
- 44.Chen JC, Warshaw JB, Sanadi DR. Regulation of mitochondrial respiration in senescence. J Cell Physiol. 1972;80:141–8. doi: 10.1002/jcp.1040800115. [DOI] [PubMed] [Google Scholar]
- 45.Kim JH, Woldgiorgis G, Elson CE, Shrago E. Age-related changes in respiration coupled to phosphorylation. I. Hepatic mitochondria. Mech Ageing Dev. 1988;46:263–77. doi: 10.1016/0047-6374(88)90129-7. [DOI] [PubMed] [Google Scholar]
- 46.O'Toole JF, Patel HV, Naples CJ, Fujioka H, Hoppel CL. Decreased cytochrome c mediates an age-related decline of oxidative phosphorylation in rat kidney mitochondria. Biochem J. 2010;427:105–12. doi: 10.1042/BJ20091373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Short KR, Bigelow ML, Kahl J, Singh R, Coenen-Schimke J, Raghavakaimal S, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–23. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paradies G, Ruggiero FM. Age-related changes in the activity of the pyruvate carrier and in the lipid composition in rat-heart mitochondria. Biochim Biophys Acta. 1990;1016:207–12. doi: 10.1016/0005-2728(90)90060-h. [DOI] [PubMed] [Google Scholar]
- 49.Niemann B, Chen Y, Teschner M, Li L, Silber RE, Rohrbach S. Obesity induces signs of premature cardiac aging in younger patients: the role of mitochondria. J Am Coll Cardiol. 2011;57:577–85. doi: 10.1016/j.jacc.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 50.Suh JH, Shigeno ET, Morrow JD, Cox B, Rocha AE, Frei B, et al. Oxidative stress in the aging rat heart is reversed by dietary supplementation with (R)-(alpha)-lipoic acid. FASEB J. 2001;15:700–6. doi: 10.1096/fj.00-0176com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lesnefsky EJ, Hoppel CL. Cardiolipin as an oxidative target in cardiac mitochondria in the aged rat. Biochim Biophys Acta. 2008;1777:1020–7. doi: 10.1016/j.bbabio.2008.05.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lesnefsky EJ, Minkler P, Hoppel CL. Enhanced modification of cardiolipin during ischemia in the aged heart. J Mol Cell Cardiol. 2009;46:1008–15. doi: 10.1016/j.yjmcc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Miro O, Casademont J, Casals E, Perea M, Urbano-Marquez A, Rustin P, et al. Aging is associated with increased lipid peroxidation in human hearts, but not with mitochondrial respiratory chain enzyme defects. Cardiovasc Res. 2000;47:624–31. doi: 10.1016/s0008-6363(00)00122-x. [DOI] [PubMed] [Google Scholar]
- 54.Judge S, Leeuwenburgh C. Cardiac mitochondrial bioenergetics, oxidative stress, and aging. Am J Physiol Cell Physiol. 2007;292:C1983–92. doi: 10.1152/ajpcell.00285.2006. [DOI] [PubMed] [Google Scholar]
- 55.Choksi KB, Boylston WH, Rabek JP, Widger WR, Papaconstantinou J. Oxidatively damaged proteins of heart mitochondrial electron transport complexes. Biochim Biophys Acta. 2004;1688:95–101. doi: 10.1016/j.bbadis.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 56.Moreau R, Heath SH, Doneanu CE, Lindsay JG, Hagen TM. Age-related increase in 4-hydroxynonenal adduction to rat heart alpha-ketoglutarate dehydrogenase does not cause loss of its catalytic activity. Antioxid Redox Signal. 2003;5:517–27. doi: 10.1089/152308603770310167. [DOI] [PubMed] [Google Scholar]
- 57.Paradies G, Petrosillo G, Paradies V, Ruggiero FM. Oxidative stress, mitochondrial bioenergetics, and cardiolipin in aging. Free Radic Biol Med. 2010;48:1286–95. doi: 10.1016/j.freeradbiomed.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 58.Houtkooper RH, Vaz FM. Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci. 2008;65:2493–506. doi: 10.1007/s00018-008-8030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res. 2000;39:257–88. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- 60.Schlame M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J Lipid Res. 2008;49:1607–20. doi: 10.1194/jlr.R700018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schlame M, Ren M, Xu Y, Greenberg ML, Haller I. Molecular symmetry in mitochondrial cardiolipins. Chem Phys Lipids. 2005;138:38–49. doi: 10.1016/j.chemphyslip.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 62.Tatarkova Z, Kuka S, Racay P, Lehotsky J, Dobrota D, Mistuna D, et al. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol Res. 2011;60:281–9. doi: 10.33549/physiolres.932019. [DOI] [PubMed] [Google Scholar]
- 63.Li SY, Du M, Dolence EK, Fang CX, Mayer GE, Ceylan-Isik AF, et al. Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glycation endproducts and protein modification. Aging cell. 2005;4:57–64. doi: 10.1111/j.1474-9728.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- 64.Kanski J, Behring A, Pelling J, Schoneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: effects of biological aging. Am J Physiol Heart Circ Physiol. 2005;288:H371–81. doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- 65.Musicco C, Capelli V, Pesce V, Timperio AM, Calvani M, Mosconi L, et al. Accumulation of overoxidized Peroxiredoxin III in aged rat liver mitochondria. Biochim Biophys Acta. 2009;1787:890–6. doi: 10.1016/j.bbabio.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 66.Bakala H, Delaval E, Hamelin M, Bismuth J, Borot-Laloi C, Corman B, et al. Changes in rat liver mitochondria with aging. Lon protease-like reactivity and N(epsilon)-carboxymethyllysine accumulation in the matrix. Eur J Biochem. 2003;270:2295–302. doi: 10.1046/j.1432-1033.2003.03598.x. [DOI] [PubMed] [Google Scholar]
- 67.Lenaz G, D'Aurelio M, Merlo Pich M, Genova ML, Ventura B, Bovina C, et al. Mitochondrial bioenergetics in aging. Biochim Biophys Acta. 2000;1459:397–404. doi: 10.1016/s0005-2728(00)00177-8. [DOI] [PubMed] [Google Scholar]
- 68.Petrosillo G, Matera M, Casanova G, Ruggiero FM, Paradies G. Mitochondrial dysfunction in rat brain with aging Involvement of complex I, reactive oxygen species and cardiolipin. Neurochemistry international. 2008;53:126–31. doi: 10.1016/j.neuint.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Choksi KB, Papaconstantinou J. Age-related alterations in oxidatively damaged proteins of mouse heart mitochondrial electron transport chain complexes. Free Radic Biol Med. 2008;44:1795–805. doi: 10.1016/j.freeradbiomed.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petrosillo G, Matera M, Moro N, Ruggiero FM, Paradies G. Mitochondrial complex I dysfunction in rat heart with aging: critical role of reactive oxygen species and cardiolipin. Free Radic Biol Med. 2009;46:88–94. doi: 10.1016/j.freeradbiomed.2008.09.031. [DOI] [PubMed] [Google Scholar]
- 71.Moghaddas S, Hoppel CL, Lesnefsky EJ. Aging defect at the QO site of complex III augments oxyradical production in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 2003;414:59–66. doi: 10.1016/s0003-9861(03)00166-8. [DOI] [PubMed] [Google Scholar]
- 72.Muller-Hocker J, Schafer S, Link TA, Possekel S, Hammer C. Defects of the respiratory chain in various tissues of old monkeys: a cytochemical-immunocytochemical study. Mech Ageing Dev. 1996;86:197–213. doi: 10.1016/0047-6374(95)01692-9. [DOI] [PubMed] [Google Scholar]
- 73.Muller-Hocker J. Cytochrome-c-oxidase deficient cardiomyocytes in the human heart--an age-related phenomenon. A histochemical ultracytochemical study. Am J Pathol. 1989;134:1167–73. [PMC free article] [PubMed] [Google Scholar]
- 74.Ojaimi J, Masters CL, Opeskin K, McKelvie P, Byrne E. Mitochondrial respiratory chain activity in the human brain as a function of age. Mech Ageing Dev. 1999;111:39–47. doi: 10.1016/s0047-6374(99)00071-8. [DOI] [PubMed] [Google Scholar]
- 75.Lopez ME, Van Zeeland NL, Dahl DB, Weindruch R, Aiken JM. Cellular phenotypes of age-associated skeletal muscle mitochondrial abnormalities in rhesus monkeys. Mutat Res. 2000;452:123–38. doi: 10.1016/s0027-5107(00)00059-2. [DOI] [PubMed] [Google Scholar]
- 76.Suh JH, Heath SH, Hagen TM. Two subpopulations of mitochondria in the aging rat heart display heterogenous levels of oxidative stress. Free Radic Biol Med. 2003;35:1064–72. doi: 10.1016/s0891-5849(03)00468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hansford RG, Castro F. Age-linked changes in the activity of enzymes of the tricarboxylate cycle and lipid oxidation, and of carnitine content, in muscles of the rat. Mech Ageing Dev. 1982;19:191–200. doi: 10.1016/0047-6374(82)90010-0. [DOI] [PubMed] [Google Scholar]
- 78.Paradies G, Ruggiero FM, Petrosillo G, Gadaleta MN, Quagliariello E. Effect of aging and acetyl-L-carnitine on the activity of cytochrome oxidase and adenine nucleotide translocase in rat heart mitochondria. FEBS Lett. 1994;350:213–5. doi: 10.1016/0014-5793(94)00763-2. [DOI] [PubMed] [Google Scholar]
- 79.Monette JS, Gomez LA, Moreau RF, Dunn KC, Butler JA, Finlay LA, et al. (R)-alpha-Lipoic acid treatment restores ceramide balance in aging rat cardiac mitochondria. Pharmacol Res. 2011;63:23–9. doi: 10.1016/j.phrs.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nohl H, Hegner D. Do mitochondria produce oxygen radicals in vivo? Eur J Biochem. 1978;82:563–7. doi: 10.1111/j.1432-1033.1978.tb12051.x. [DOI] [PubMed] [Google Scholar]
- 81.Barja G. Mitochondrial oxygen radical generation and leak: sites of production in states 4 and 3, organ specificity, and relation to aging and longevity. J Bioenerg Biomembr. 1999;31:347–66. doi: 10.1023/a:1005427919188. [DOI] [PubMed] [Google Scholar]
- 82.Wanagat J, Wolff MR, Aiken JM. Age-associated changes in function, structure and mitochondrial genetic and enzymatic abnormalities in the Fischer 344 x Brown Norway F(1) hybrid rat heart. J Mol Cell Cardiol. 2002;34:17–28. doi: 10.1006/jmcc.2001.1483. [DOI] [PubMed] [Google Scholar]
- 83.Ohnishi ST, Ohnishi T, Muranaka S, Fujita H, Kimura H, Uemura K, et al. A possible site of superoxide generation in the complex I segment of rat heart mitochondria. J Bioenerg Biomembr. 2005;37:1–15. doi: 10.1007/s10863-005-4117-y. [DOI] [PubMed] [Google Scholar]
- 84.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suh JH, Wang H, Liu RM, Liu J, Hagen TM. (R)-alpha-lipoic acid reverses the age-related loss in GSH redox status in post-mitotic tissues: evidence for increased cysteine requirement for GSH synthesis. Arch Biochem Biophys. 2004;423:126–35. doi: 10.1016/j.abb.2003.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hagen TM, Ingersoll RT, Wehr CM, Lykkesfeldt J, Vinarsky V, Bartholomew JC, et al. Acetyl-L-carnitine fed to old rats partially restores mitochondrial function and ambulatory activity. Proc Natl Acad Sci USA. 1998;95:9562–6. doi: 10.1073/pnas.95.16.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miles MV, Horn PS, Tang PH, Morrison JA, Miles L, DeGrauw T, et al. Age-related changes in plasma coenzyme Q10 concentrations and redox state in apparently healthy children and adults. Clin Chim Acta. 2004;347:139–44. doi: 10.1016/j.cccn.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 88.Rosenfeldt FL, Pepe S, Linnane A, Nagley P, Rowland M, Ou R, et al. Coenzyme Q10 protects the aging heart against stress: studies in rats, human tissues, and patients. Ann N Y Acad Sci. 2002;959:355–9. doi: 10.1111/j.1749-6632.2002.tb02106.x. discussion 463-5. [DOI] [PubMed] [Google Scholar]
- 89.Kalen A, Appelkvist EL, Dallner G. Age-related changes in the lipid compositions of rat and human tissues. Lipids. 1989;24:579–84. doi: 10.1007/BF02535072. [DOI] [PubMed] [Google Scholar]
- 90.Beyer RE, Burnett BA, Cartwright KJ, Edington DW, Falzon MJ, Kreitman KR, et al. Tissue coenzyme Q (ubiquinone) and protein concentrations over the life span of the laboratory rat. Mech Ageing Dev. 1985;32:267–81. doi: 10.1016/0047-6374(85)90085-5. [DOI] [PubMed] [Google Scholar]
- 91.Vorbeck ML, Martin AP, Long JW, Jr., Smith JM, Orr RR., Jr. Aging-dependent modification of lipid composition and lipid structural order parameter of hepatic mitochondria. Arch Biochem Biophys. 1982;217:351–61. doi: 10.1016/0003-9861(82)90511-2. [DOI] [PubMed] [Google Scholar]
- 92.Paradies G, Petrosillo G, Gadaleta MN, Ruggiero FM. The effect of aging and acetyl-L-carnitine on the pyruvate transport and oxidation in rat heart mitochondria. FEBS Lett. 1999;454:207–9. doi: 10.1016/s0014-5793(99)00809-1. [DOI] [PubMed] [Google Scholar]
- 93.Paradies G, Ruggiero FM, Petrosillo G, Gadaleta MN, Quagliariello E. Carnitine acylcarnitine translocase activity in cardiac mitochondria from aged rats: the effect of acetyl-L-carnitine. Mech Ageing Dev. 1995;84:103–12. doi: 10.1016/0047-6374(95)01636-8. [DOI] [PubMed] [Google Scholar]
- 94.Paradies G, Ruggiero FM, Petrosillo G, Quagliariello E. Age-dependent decline in the cytochrome c oxidase activity in rat heart mitochondria: role of cardiolipin. FEBS Lett. 1997;406:136–8. doi: 10.1016/s0014-5793(97)00264-0. [DOI] [PubMed] [Google Scholar]
- 95.Hagen TM, Wehr CM, Ames BN. Mitochondrial decay in aging. Reversal through supplementation of acetyl-L-carnitine and N-tert-butyl-alpha-phenyl-nitrone. Ann NY Acad Sci. 1998;854:214–23. doi: 10.1111/j.1749-6632.1998.tb09904.x. [DOI] [PubMed] [Google Scholar]
- 96.Moghaddas S, Stoll MS, Minkler PE, Salomon RG, Hoppel CL, Lesnefsky EJ. Preservation of cardiolipin content during aging in rat heart interfibrillar mitochondria. J Gerontol A Biol Sci Med Sci. 2002;57:B22–8. doi: 10.1093/gerona/57.1.b22. [DOI] [PubMed] [Google Scholar]
- 97.Helmy FM, Hack MH, Juracka A. Age-related changes of the endogenous cardiolipin and plasmalogens of guinea pig kidney and their in vitro hydrolysis by endogenous phospholipases: a thin layer chromatographic analysis in conjunction with densitometric measurement. Cell Biochem Funct. 2003;21:337–44. doi: 10.1002/cbf.1035. [DOI] [PubMed] [Google Scholar]
- 98.Malhotra A, Xu Y, Ren M, Schlame M. Formation of molecular species of mitochondrial cardiolipin. 1. A novel transacylation mechanism to shuttle fatty acids between sn-1 and sn-2 positions of multiple phospholipid species. Biochim Biophys Acta. 2009;1791:314–20. doi: 10.1016/j.bbalip.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schlame M, Shanske S, Doty S, Konig T, Sculco T, DiMauro S, et al. Microanalysis of cardiolipin in small biopsies including skeletal muscle from patients with mitochondrial disease. J Lipid Res. 1999;40:1585–92. [PubMed] [Google Scholar]
- 100.Lee HJ, Mayette J, Rapoport SI, Bazinet RP. Selective remodeling of cardiolipin fatty acids in the aged rat heart. Lipids in health and disease. 2006;5:2. doi: 10.1186/1476-511X-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hackenbrock CR, Chazotte B, Gupte SS. The random collision model and a critical assessment of diffusion and collision in mitochondrial electron transport. J Bioenerg Biomembr. 1986;18:331–68. doi: 10.1007/BF00743010. [DOI] [PubMed] [Google Scholar]
- 102.Chazotte B, Hackenbrock CR. The multicollisional, obstructed, long-range diffusional nature of mitochondrial electron transport. J Biol Chem. 1988;263:14359–67. [PubMed] [Google Scholar]
- 103.Gupte SS, Hackenbrock CR. The role of cytochrome c diffusion in mitochondrial electron transport. J Biol Chem. 1988;263:5248–53. [PubMed] [Google Scholar]
- 104.Gupte SS, Hackenbrock CR. Multidimensional diffusion modes and collision frequencies of cytochrome c with its redox partners. J Biol Chem. 1988;263:5241–7. [PubMed] [Google Scholar]
- 105.Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA. Respiratory active mitochondrial supercomplexes. Molecular cell. 2008;32:529–39. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 106.Bianchi C, Genova ML, Parenti Castelli G, Lenaz G. The mitochondrial respiratory chain is partially organized in a supercomplex assembly: kinetic evidence using flux control analysis. J Biol Chem. 2004;279:36562–9. doi: 10.1074/jbc.M405135200. [DOI] [PubMed] [Google Scholar]
- 107.Genova ML, Baracca A, Biondi A, Casalena G, Faccioli M, Falasca AI, et al. Is supercomplex organization of the respiratory chain required for optimal electron transfer activity? Biochim Biophys Acta. 2008;1777:740–6. doi: 10.1016/j.bbabio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 108.Lenaz G, Baracca A, Barbero G, Bergamini C, Dalmonte ME, Del Sole M, et al. Mitochondrial respiratory chain super-complex I-III in physiology and pathology. Biochim Biophys Acta. 2010;1797:633–40. doi: 10.1016/j.bbabio.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 109.Lenaz G, Genova ML. Kinetics of integrated electron transfer in the mitochondrial respiratory chain: random collisions vs. solid state electron channeling. Am J Physiol Cell Physiol. 2007;292:C1221–39. doi: 10.1152/ajpcell.00263.2006. [DOI] [PubMed] [Google Scholar]
- 110.Lenaz G, Genova ML. Structural and functional organization of the mitochondrial respiratory chain: a dynamic super-assembly. Int J Biochem Cell Biol. 2009;41:1750–72. doi: 10.1016/j.biocel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 111.Lenaz G, Genova ML. Mobility and function of coenzyme Q (ubiquinone) in the mitochondrial respiratory chain. Biochim Biophys Acta. 2009;1787:563–73. doi: 10.1016/j.bbabio.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 112.Althoff T, Mills DJ, Popot JL, Kuhlbrandt W. Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. EMBO J. 2011;30:4652–64. doi: 10.1038/emboj.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dranka BP, Benavides GA, Diers AR, Giordano S, Zelickson BR, Reily C, et al. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic Biol Med. 2011;51:1621–35. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reifschneider NH, Goto S, Nakamoto H, Takahashi R, Sugawa M, Dencher NA, et al. Defining the mitochondrial proteomes from five rat organs in a physiologically significant context using 2D blue-native/SDS-PAGE. Journal of proteome research. 2006;5:1117–32. doi: 10.1021/pr0504440. [DOI] [PubMed] [Google Scholar]
- 115.Wittig I, Schagger H. Advantages and limitations of clear-native PAGE. Proteomics. 2005;5:4338–46. doi: 10.1002/pmic.200500081. [DOI] [PubMed] [Google Scholar]
- 116.Dudkina NV, Kudryashev M, Stahlberg H, Boekema EJ. Interaction of complexes I, III, and IV within the bovine respirasome by single particle cryoelectron tomography. Proc Natl Acad Sci U S A. 2011;108:15196–200. doi: 10.1073/pnas.1107819108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moreno-Lastres D, Fontanesi F, Garcia-Consuegra I, Martin MA, Arenas J, Barrientos A, et al. Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metab. 2012;15:324–35. doi: 10.1016/j.cmet.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schagger H, de Coo R, Bauer MF, Hofmann S, Godinot C, Brandt U. Significance of respirasomes for the assembly/stability of human respiratory chain complex I. J Biol Chem. 2004;279:36349–53. doi: 10.1074/jbc.M404033200. [DOI] [PubMed] [Google Scholar]
- 119.McKenzie M, Lazarou M, Thorburn DR, Ryan MT. Analysis of mitochondrial subunit assembly into respiratory chain complexes using Blue Native polyacrylamide gel electrophoresis. Anal Biochem. 2007;364:128–37. doi: 10.1016/j.ab.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 120.D'Aurelio M, Gajewski CD, Lenaz G, Manfredi G. Respiratory chain supercomplexes set the threshold for respiration defects in human mtDNA mutant cybrids. Human molecular genetics. 2006;15:2157–69. doi: 10.1093/hmg/ddl141. [DOI] [PubMed] [Google Scholar]
- 121.Zhang M, Mileykovskaya E, Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem. 2002;277:43553–6. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- 122.Wenz T, Hielscher R, Hellwig P, Schagger H, Richers S, Hunte C. Role of phospholipids in respiratory cytochrome bc(1) complex catalysis and supercomplex formation. Biochim Biophys Acta. 2009;1787:609–16. doi: 10.1016/j.bbabio.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 123.Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, et al. Cardiolipin stabilizes respiratory chain supercomplexes. J Biol Chem. 2003;278:52873–80. doi: 10.1074/jbc.M308366200. [DOI] [PubMed] [Google Scholar]
- 124.Schlame M, Towbin JA, Heerdt PM, Jehle R, DiMauro S, Blanck TJ. Deficiency of tetralinoleoyl-cardiolipin in Barth syndrome. Ann Neurol. 2002;51:634–7. doi: 10.1002/ana.10176. [DOI] [PubMed] [Google Scholar]
- 125.Schlame M, Ren M. The role of cardiolipin in the structural organization of mitochondrial membranes. Biochim Biophys Acta. 2009;1788:2080–3. doi: 10.1016/j.bbamem.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schlame M, Horvath L, Vigh L. Relationship between lipid saturation and lipid-protein interaction in liver mitochondria modified by catalytic hydrogenation with reference to cardiolipin molecular species. Biochem J. 1990;265:79–85. doi: 10.1042/bj2650079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Diaz F, Enriquez JA, Moraes CT. Cells Lacking Rieske Iron-Sulfur Protein Have a Reactive Oxygen Species-Associated Decrease in Respiratory Complexes I and IV. Mol Cell Biol. 2012;32:415–29. doi: 10.1128/MCB.06051-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fornuskova D, Stiburek L, Wenchich L, Vinsova K, Hansikova H, Zeman J. Novel insights into the assembly and function of human nuclear-encoded cytochrome c oxidase subunits 4, 5a, 6a, 7a and 7b. Biochem J. 2010;428:363–74. doi: 10.1042/BJ20091714. [DOI] [PubMed] [Google Scholar]
- 129.Oswald C, Krause-Buchholz U, Rodel G. Knockdown of human COX17 affects assembly and supramolecular organization of cytochrome c oxidase. J Mol Biol. 2009;389:470–9. doi: 10.1016/j.jmb.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 130.Diaz F, Fukui H, Garcia S, Moraes CT. Cytochrome c oxidase is required for the assembly/stability of respiratory complex I in mouse fibroblasts. Mol Cell Biol. 2006;26:4872–81. doi: 10.1128/MCB.01767-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Suthammarak W, Yang YY, Morgan PG, Sedensky MM. Complex I function is defective in complex IV-deficient Caenorhabditis elegans. J Biol Chem. 2009;284:6425–35. doi: 10.1074/jbc.M805733200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chen YC, Taylor EB, Dephoure N, Heo JM, Tonhato A, Papandreou I, et al. Identification of a protein mediating respiratory supercomplex stability. Cell Metab. 2012;15:348–60. doi: 10.1016/j.cmet.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vukotic M, Oeljeklaus S, Wiese S, Vogtle FN, Meisinger C, Meyer HE, et al. Rcf1 mediates cytochrome oxidase assembly and respirasome formation, revealing heterogeneity of the enzyme complex. Cell Metab. 2012;15:336–47. doi: 10.1016/j.cmet.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 134.Strogolova V, Furness A, Robb-McGrath M, Garlich J, Stuart RA. Rcf1 and Rcf2, Members of the Hypoxia-Induced Gene 1 Protein Family, Are Critical Components of the Mitochondrial Cytochrome bc1-Cytochrome c Oxidase Supercomplex. Mol Cell Biol. 2012;32:1363–73. doi: 10.1128/MCB.06369-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schagger H. Respiratory chain supercomplexes of mitochondria and bacteria. Biochim Biophys Acta. 2002;1555:154–9. doi: 10.1016/s0005-2728(02)00271-2. [DOI] [PubMed] [Google Scholar]
- 136.Schagger H, Pfeiffer K. The ratio of oxidative phosphorylation complexes I-V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. J Biol Chem. 2001;276:37861–7. doi: 10.1074/jbc.M106474200. [DOI] [PubMed] [Google Scholar]
- 137.Koshkin V, Greenberg ML. Oxidative phosphorylation in cardiolipin-lacking yeast mitochondria. Biochem J. 2000;347(Pt 3):687–91. [PMC free article] [PubMed] [Google Scholar]
- 138.Koshkin V, Greenberg ML. Cardiolipin prevents rate-dependent uncoupling and provides osmotic stability in yeast mitochondria. Biochem J. 2002;364:317–22. doi: 10.1042/bj3640317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jiang F, Rizavi HS, Greenberg ML. Cardiolipin is not essential for the growth of Saccharomyces cerevisiae on fermentable or non-fermentable carbon sources. Mol Microbiol. 1997;26:481–91. doi: 10.1046/j.1365-2958.1997.5841950.x. [DOI] [PubMed] [Google Scholar]
- 140.Panov A, Dikalov S, Shalbuyeva N, Hemendinger R, Greenamyre JT, Rosenfeld J. Species- and tissue-specific relationships between mitochondrial permeability transition and generation of ROS in brain and liver mitochondria of rats and mice. Am J Physiol Cell Physiol. 2007;292:C708–18. doi: 10.1152/ajpcell.00202.2006. [DOI] [PubMed] [Google Scholar]
- 141.van Raam BJ, Sluiter W, de Wit E, Roos D, Verhoeven AJ, Kuijpers TW. Mitochondrial membrane potential in human neutrophils is maintained by complex III activity in the absence of supercomplex organisation. PLoS One. 2008;3:e2013. doi: 10.1371/journal.pone.0002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Minkler PE, Hoppel CL. Separation and characterization of cardiolipin molecular species by reverse-phase ion pair high-performance liquid chromatography-mass spectrometry. J Lipid Res. 2010;51:856–65. doi: 10.1194/jlr.D002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schlame M, Brody S, Hostetler KY. Mitochondrial cardiolipin in diverse eukaryotes. Comparison of biosynthetic reactions and molecular acyl species. Eur J Biochem. 1993;212:727–35. doi: 10.1111/j.1432-1033.1993.tb17711.x. [DOI] [PubMed] [Google Scholar]
- 144.Ritov VB, Menshikova EV, Kelley DE. Analysis of cardiolipin in human muscle biopsy. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;831:63–71. doi: 10.1016/j.jchromb.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 145.Valianpour F, Mitsakos V, Schlemmer D, Towbin JA, Taylor JM, Ekert PG, et al. Monolysocardiolipins accumulate in Barth syndrome but do not lead to enhanced apoptosis. J Lipid Res. 2005;46:1182–95. doi: 10.1194/jlr.M500056-JLR200. [DOI] [PubMed] [Google Scholar]
- 146.Valianpour F, Wanders RJ, Overmars H, Vreken P, Van Gennip AH, Baas F, et al. Cardiolipin deficiency in X-linked cardioskeletal myopathy and neutropenia (Barth syndrome, MIM 302060): a study in cultured skin fibroblasts. J Pediatr. 2002;141:729–33. doi: 10.1067/mpd.2002.129174. [DOI] [PubMed] [Google Scholar]
- 147.van Werkhoven MA, Thorburn DR, Gedeon AK, Pitt JJ. Monolysocardiolipin in cultured fibroblasts is a sensitive and specific marker for Barth Syndrome. J Lipid Res. 2006;47:2346–51. doi: 10.1194/jlr.D600024-JLR200. [DOI] [PubMed] [Google Scholar]
- 148.Vempati UD, Han X, Moraes CT. Lack of cytochrome c in mouse fibroblasts disrupts assembly/stability of respiratory complexes I and IV. J Biol Chem. 2009;284:4383–91. doi: 10.1074/jbc.M805972200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lazarou M, Smith SM, Thorburn DR, Ryan MT, McKenzie M. Assembly of nuclear DNA-encoded subunits into mitochondrial complex IV, and their preferential integration into supercomplex forms in patient mitochondria. FEBS J. 2009;276:6701–13. doi: 10.1111/j.1742-4658.2009.07384.x. [DOI] [PubMed] [Google Scholar]
- 150.De los Rios Castillo D, Zarco-Zavala M, Olvera-Sanchez S, Pardo JP, Juarez O, Martinez F, et al. Atypical cristae morphology of human syncytiotrophoblast mitochondria: role for complex V. J Biol Chem. 2011;286:23911–9. doi: 10.1074/jbc.M111.252056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Marques I, Dencher NA, Videira A, Krause F. Supramolecular organization of the respiratory chain in Neurospora crassa mitochondria. Eukaryotic cell. 2007;6:2391–405. doi: 10.1128/EC.00149-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maas MF, Krause F, Dencher NA, Sainsard-Chanet A. Respiratory complexes III and IV are not essential for the assembly/stability of complex I in fungi. J Mol Biol. 2009;387:259–69. doi: 10.1016/j.jmb.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 153.Krause F, Scheckhuber CQ, Werner A, Rexroth S, Reifschneider NH, Dencher NA, et al. OXPHOS Supercomplexes: respiration and life-span control in the aging model Podospora anserina. Ann N Y Acad Sci. 2006;1067:106–15. doi: 10.1196/annals.1354.013. [DOI] [PubMed] [Google Scholar]
- 154.Bultema JB, Braun HP, Boekema EJ, Kouril R. Megacomplex organization of the oxidative phosphorylation system by structural analysis of respiratory supercomplexes from potato. Biochim Biophys Acta. 2009;1787:60–7. doi: 10.1016/j.bbabio.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 155.Ramirez-Aguilar SJ, Keuthe M, Rocha M, Fedyaev VV, Kramp K, Gupta KJ, et al. The composition of plant mitochondrial supercomplexes changes with oxygen availability. J Biol Chem. 2011;286:43045–53. doi: 10.1074/jbc.M111.252544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eubel H, Heinemeyer J, Braun HP. Identification and characterization of respirasomes in potato mitochondria. Plant Physiol. 2004;134:1450–9. doi: 10.1104/pp.103.038018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Krause F, Reifschneider NH, Vocke D, Seelert H, Rexroth S, Dencher NA. “Respirasome”-like supercomplexes in green leaf mitochondria of spinach. J Biol Chem. 2004;279:48369–75. doi: 10.1074/jbc.M406085200. [DOI] [PubMed] [Google Scholar]
- 158.Sunderhaus S, Klodmann J, Lenz C, Braun HP. Supramolecular structure of the OXPHOS system in highly thermogenic tissue of Arum maculatum. Plant Physiol Biochem. 2010;48:265–72. doi: 10.1016/j.plaphy.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 159.Eubel H, Jansch L, Braun HP. New insights into the respiratory chain of plant mitochondria. Supercomplexes and a unique composition of complex II. Plant Physiol. 2003;133:274–86. doi: 10.1104/pp.103.024620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chien LF, Wu YC, Chen HP. Mitochondrial energy metabolism in young bamboo rhizomes from Bambusa oldhamii and Phyllostachys edulis during shooting stage. Plant Physiol Biochem. 2011;49:449–57. doi: 10.1016/j.plaphy.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 161.Peters K, Dudkina NV, Jansch L, Braun HP, Boekema EJ. A structural investigation of complex I and I+III2 supercomplex from Zea mays at 11-13 A resolution: assignment of the carbonic anhydrase domain and evidence for structural heterogeneity within complex I. Biochim Biophys Acta. 2008;1777:84–93. doi: 10.1016/j.bbabio.2007.10.012. [DOI] [PubMed] [Google Scholar]