Abstract
Nonclassical MHC class Ib (class Ib) genes are a family of highly diverse and rapidly evolving genes wherein gene numbers, organization and expression markedly differ even among closely related species rendering class Ib phylogeny difficult to establish. Whereas among mammals there are few unambiguous class Ib gene orthologs, different amphibian species belonging to the anuran subfamily Xenopodinae exhibit an unusually high degree of conservation among multiple class Ib gene lineages. Comparative genomic analysis of class Ib gene loci of two divergent (~65 million years) Xenopodinae subfamily members X. laevis (allotetraploid) and X. tropicalis (diploid) shows that both species possess a large cluster of class Ib genes denoted as Xenopus/Silurana nonclassical (XNC/SNC). Our study reveals two distinct phylogenetic patterns among these genes: some gene lineages display a high degree of flexibility, as demonstrated by species-specific expansion and contractions, whereas other class Ib gene lineages have been maintained as monogenic subfamilies with very few changes in their nucleotide sequence across divergent species. In this second category, we further investigated the XNC/SNC10 gene lineage that in X. laevis is required for the development of a distinct semi-invariant T cell population. We report compelling evidence of the remarkable high degree of conservation of this gene lineage that is present in all 12 species of the Xenopodinae examined, including species with different degrees of ploidy ranging from 2, 4, 8 to 12N. This suggests that the critical role of XNC10 during early T cell development is conserved in amphibians.
Keywords: Amphibians, Xenopus, XNC/SNC, class I-like, MHC evolution, XNC10
Introduction
Major histocompatibility complex (MHC) class I genes constitute key components of the vertebrate immune system, encoding molecules that play integral roles in both adaptive and innate immune responses. MHC class I genes are further subdivided into classical MHC class Ia (class Ia) and non-classical MHC class Ib (class Ib) genes, which are structurally similar but functionally disparate. Class Ia genes have been described in all groups of jawed vertebrates (Gnathostomes) and encode highly polymorphic molecules essential for αβCD8+ T cell differentiation and function (Kasahara et al. 1995; Flajnik and Kasahara 2001). In addition, Gnathostomes have variable numbers of heterogeneous, typically non-polymorphic class Ib genes involved in a variety of functions including both immune and non-immune related roles (Adams and Luoma 2013; Rodgers and Cook 2005). While class Ia genes play a central role in the adaptive immune response, class Ib molecules are generally considered as regulators of innate immune responses, particularly as NK and innate-like T cell receptor ligands (Adams and Luoma 2013). In particular, recent studies in mammals have elucidated the roles of the class Ib molecules CD1d and MR1 in the differentiation of distinct semi-invariant T cell subsets, which are involved in regulating the immune responses to a variety of different microbes (Le Bourhis et al. 2010; Matsuda and Gapin 2005; Skold and Behar 2003; Treiner et al. 2003) and reviewed in (Kronenberg and Gapin 2002; Le Bourhis et al. 2011). However, despite these recent advances the functional roles of many class Ib molecules are still not fully elucidated.
Among challenges in studying the functional roles of class Ib molecules is the rapid evolution undergone by these genes, culminating in extensive variation in their gene numbers and genomic organization even among closely related species (Nei et al. 1997; Nei and Rooney 2005; Piontkivska and Nei 2003)(Adams and Parham 2001). Furthermore, distinct class Ib genes appear to have been subjected to variable selective pressures resulting in multiple species-specific specializations including expansions and/or contractions of individual gene families (Adams and Luoma 2013; Adams and Parham 2001; Flajnik and Kasahara 2001). Combined these factors contribute to the difficulty in establishing evolutionary relationships and class Ib phylogeny across different species. As such, although comparative analyses have revealed functional similarities between certain class Ib molecules of evolutionary divergent species, few unambiguous class Ib orthologs or even homologs have been described.
Homologs of CD1, one of the most conserved class Ib gene families described to date, are present in both mammals and birds (Baker and Miller 2007; Miller et al. 2005) albeit, depending on the species, both the presence of different isoforms and the total number of CD1 genes in the genome varies (Dascher 2007). CD1 proteins bind and present lipid antigens to both conventional and unconventional T cells providing a way for the immune system to recognize and respond to both self and non-self (exogenous as well as endogenous) lipid and glycolipid antigens. Similarly, homologs of the MHC-1-like related protein 1 (MR1) gene have been described in a number of different mammalian species including placentals and marsupials (Tsukamoto et al. 2013). Monomorphic MHC class I-like molecule structurally similar to MR1 termed YF1*7.1 has also been described in chickens (Hee et al. 2010; Kjer-Nielsen et al. 2012). In mouse, MR1 binds and presents microbial vitamin B metabolites (Kjer-Nielsen et al. 2012), thus representing an additional nonclassical dependent recognition for a specific set of antigens. Despite the presence of multiple class Ib genes in ectothermic vertebrates, to date no CD1 or MR1 homolog have been identified in these species. Moreover, although amphibians as well as cartilaginous and bony fish posses a number of distinct and divergent class Ib genes very little is known about the biological relevance and phylogenetic relationships of these ectothermic vertebrate class Ib genes.
Amphibians represent a key phylogenetic position connecting mammals with vertebrates of more ancient origin such as bony and cartilaginous fish, (reviewed in (Robert and Ohta 2009)). In addition, amphibians belonging to the anuran subfamily Xenopodinae is one of few extant vertebrate groups containing natural polyploid species ranging from diploid (2N) to dodecaploid (12N) (Evans 2008). Representative species from two separate genera belonging to the Xenopodinae subfamily X. laevis and X. (Silurana) tropicalis (Bewick et al. 2012) both posses a large number of class Ib genes (Flajnik et al. 1993; Goyos et al. 2011). These class Ib genes, like their mammalian counterparts, are heterogeneous, less polymorphic, and less ubiquitously expressed compared to class Ia. However, in contrast to most mammalian class Ib genes, we have observed an unusually high degree of conservation of distinct class Ib gene subfamilies between X. laevis and X. tropicalis (Goyos et al. 2011). Notably, this high degree of conservation is apparent despite the fact that these species are estimated to have diverged from the last common ancestor more then 65 million years ago, roughly corresponding to the split between rodent and primate ancestors (Evans 2008; Evans et al. 2004). Class Ib genes isolated from X. laevis and X. tropicalis have been grouped into multiple subfamilies based on sequence similarities in the α1 and α2 domains (Flajnik et al. 1993; Goyos et al. 2011). The nomenclature of these amphibian class Ib genes was based on a three-letter code whereby the first letter refers to the species genus and thus XNC for Xenopus non-classical and SNC for those genes coming from X. tropicalis (originally named Silurana tropicalis) non-classical, and were numbered based on the order of discovery and subsequently according to homology and/or orthology with previously described class Ib gene subfamilies. Furthermore, we have recently demonstrated that the X. laevis class Ib gene, XNC10 is required for the development and function of a population of semi-invariant T (iT) cells (Edholm et al. 2013). Notably, XNC10 and its unequivocal X. tropicalis ortholog represent a unique class Ib lineage, divergent from other XNC/SNC genes, and it has been suggested that this gene is conserved in other Xenopus species (Goyos et al. 2009; Goyos et al. 2011). To further delineate the evolutionary relationships among all XNC/SNC class Ib genes and XNC/SNC10 in particular, we have identified and characterized all individual subfamilies of X. laevis class Ib genes and conducted a comprehensive genomic and phylogenetic study of class Ib genes between X. laevis and X. tropicalis. Furthermore, we demonstrate that the XNC/SNC10 gene lineage is highly conserved among divergent amphibian species of the Xenopodinae subfamily regardless of genome ploidy.
Material and Methods
Experimental animals
Outbred strain of X. laevis, X. borealis and X. (Silurana) tropicalis were from the Xenopus laevis Research Resource for Immunology at the University of Rochester (http://www.urmc.rochester.edu/smd/mbi/xenopus/index.htm. All animals were handled under strict laboratory and UCAR regulations (100577/2003-151), and discomfort was minimized at all times.
Identification of XNC genes
Putative XNC genes where identified and annotated using the BLAST-like alignment tool (BLAT) algorithm with nucleotide sequences of all known XNC and SNC α1, α2 and α3 domains as queries against the X. laevis genome using both the USSC genome bioinformatics Xenopus genomes hosted at NIMR (http://genomes.nimr.mrc.ac.uk, Nov, 2012 assembly and the Xenopus laevis Genome project http://xenopus.lab.nig.ac.jp, XenVis 2.0 assembly. The nucleotide and amino acid sequences of known genes were retrieved from GenBank using ENTREZ at http://www.ncbi.nlm.nih.gov.
Identification of XNC/SNC10 homologs
XNC/SNC10 homologs were isolated from genomic DNA using either degenerate primers designed to recognize consensus XNC/SNC10 motifs in the α1 and α2 encoding exons or sub-lineage specific primer (sTable 1). Genomic DNA from X. laevis, X. borealis and X. tropicalis was purified from liver using Trizol reagent (Invitrogen, CA) following the manufacturers protocol. Genomic DNA samples from X. gilli, X. mulleri, X. clivii, X. andreii, X. amietti, X. vestitus, X. ruwenzoriensis, X. longipes, X. tropicalis and X. paratropicalis were kindly provided by Ben Evans (McMaster University, Hamilton, ON, Canada). A total of 50 ng of genomic DNA was used for PCR. Typical parameters were 5 min at 94°C, followed by 30 cycles of 94°C for 30 sec, 58°C to 61°C (depending on the primer pair) for 30 sec, and 72°C for 1 min, with a final extension cycle of 72°C for 10 min. All RT-PCR products where cloned into the pGEM-T easy vector (Promega, WI) and verified by sequencing.
Sequence analysis
Nucleotide and amino acid pairwise alignments were made using CLUSTALW (Thompson et al. 1997) and neighbor-joining trees were drawn using MEGA v5.2.2 (Tamura et al. 2011). Genetic distances were calculated by estimating the number of amino acid substitutions using the p-distance method employing pairwise deletion of gaps. Numbers on nodes represent percentages above 50 of 10,000 bootstrap replicates supporting each partition. Putative ligand binding residues were determined based on alignment with human HLA-A. Accession numbers of species used are as follows: Hs MR1: AAC72900; Oa MR1: ACJ60632, Mm MR1: NP_032235, Ss MR1: XP_003130402, Me MR1: BAM66417, Md MR1: BAM66416, Hs HLA-E: AAA52655, Hs HLA-G: NP_002118, Mm Qa-1: XP_003945787, Mm Qa-2: NP_001188389, Pp MHC-R9: AAF03410, MHC-R6: AAF03409, Amcr-AU: AC668408, Xt MHC: NP_001106387, Xl MHC: AAA16064, Hs HLA-A2: EAX03243, Gg CD1d.1: BAE19763; Gg Cd1d.2: AAQ75347, Hs CD1a: CAA28049, Hs CD1b: BAJ20663, Hs CD1c: CAG33361, Hs CD1d: AAA51935, CD1e: CAB93156, Mm CD1d-1: NP_031666, Mm CD1d-2: ACM45456, Dn CD1: XP_004447409, Ss CD1: XP_005663293, Cs CD1: XP_004443514, Ec CD1: AEO72063.
Quantitative PCR
RNA from developmental stage 53 X. laevis tadpole tissues was prepared using Trizol reagent (Invitrogen) and treated with DNase (Ambion, Life technologies), according to the manufacturer’s protocol. 500 ng total RNA was transcribed into cDNA using oligo-dT and iScript reverse transcriptase (Bio-Rad). qPCR was performed with specific primers for XNC10.1 and XNC10.2 and GAPDH was used as a template control (sTable I). Quantitative PCR gene expression was performed using the delta^delta CT method using the ABI 7300 real-time PCR system and PerfeCta® SYBR Green FastMix ROX (Quanta Bioscience Inc). Thermocycling parameters were 2 min at 95°C, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Expression analysis of XNC10.1 and XNC10.2 were normalized to the GAPDH endodgenous control and against the lowest observed tissue expression. Expression analysis was performed using the ABI sequence detection system software (SDS) and all primers were validated prior to use.
Results
3.1 Genomic organization of the XNC/SNC loci
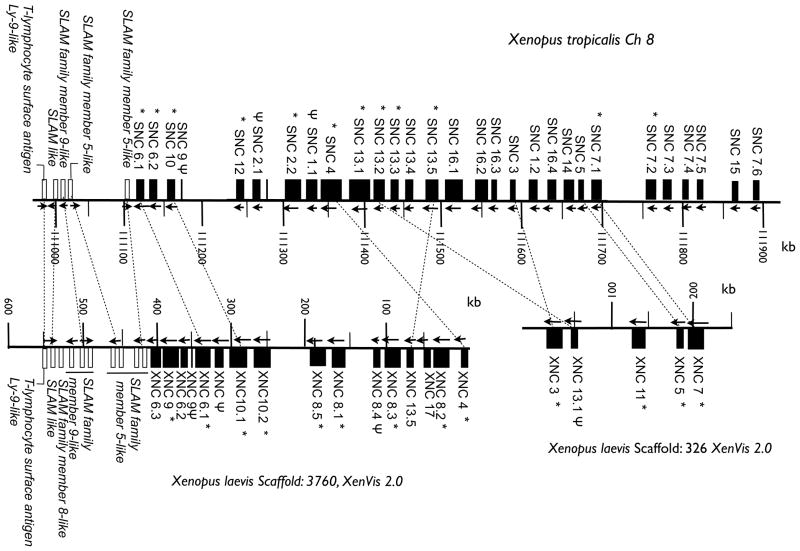
In silico analysis of the X. tropicalis genome have revealed the existence of at least 29 SNC genes (including pseudogenes) that based on sequence similarities in the α1/α2 domains where grouped into 14 subfamilies (Goyos et al. 2011). Based on Southern blot analysis, X. laevis was estimated to have approximately 20 XNC genes per haplotype and to date XNC gene transcripts have been grouped into 11 different subfamilies (Flajnik et al. 1993; Goyos et al. 2009). However, in the absence of genomic sequence information, the exact number of XNC genes and subfamilies was unknown. Recently, in addition to the genome of the only diploid frog in the Xenopodinae subfamily, X. tropicalis (Hellsten et al. 2010), the genome of the allotetraploid frog, X. laevis has been fully sequenced and annotated using a male of the MHC homozygous J inbred strain (http://genomes.nimr.mrc.ac.uk and http://xenopus.lab.nig.ac.jp). To define the genomic organization of XNC genes and determine the level of gene synteny between X. laevis and X. tropicalis XNC/SNC loci, we annotated the XNC gene models and performed a comparative analysis with the X. tropicalis class Ib loci. Our previous findings based on the partially annotated X. tropicalis genome (version 4.1) indicated the presence of two separate scaffolds each containing multiple SNC genes (Goyos et al. 2011). By mapping these two scaffolds to the now fully assembled X. tropicalis genome we determined that they are in fact linked and located at the end of chromosome 8, approximately 65 Mbp distant from the X. tropicalis classical MHC class Ia gene (Fig. 1).
Fig 1. Genomic organization of X. tropicalis and X. laevis nonclassical MHC genes (SNC and XNC).
Organization of the 29 SNC and 21 XNC genes including pseudogenes, indicated by Ψ. SNC genes are located on chromosome 8 and XNC genes are located on two different scaffolds encompassing a total of 0.8 Mbp (drawn to scale). Scaffold numbers for X. laevis are based on the Xenopus laevis Genome project http://xenopus.lab.nig.ac.jp, XenVis 2.0 assembly and chromosome number and gene location for X. tropicalis are based on the USSC genome bioinformatics Xenopus genomes hosted at NIMR (http://genomes.nimr.mrc.ac.uk, July, 2013 assembly. Expressed genes, as supported by EST identifications and/or RT-PCR, are indicated by * and arrows indicate transcriptional orientation. Flanking non-class Ib genes are shown as white boxes. Orthologous relationships defined by multiple sequence alignments and phylogenetic analysis between XNC and SNC genes are indicated by dashed lines.
To characterize the genomic organization of XNC genes, we searched the recently available X. laevis genomic databases. Because the X. laevis genome assembly is still in progress, we found a few orphan scaffolds with XNC sequences including XNC1 and XNC2 that mapped on scaffolds 214755 and 54732 of 110 and 36 kbp, respectively. In addition to XNC2, scaffold 54732 contained another putative XNC gene (XNC6.4) and a partial XNC gene (XNC14). Only XNC1 was found on scaffold 214755. All the remaining XNC genes mapped to one of two scaffolds, 3760 and 326 (http://xenopus.lab.nig.ac.jp, version XenVis 2.0). Combined these two scaffolds encompass 0.83 Mbp that contain a total of 21 predicted XNC genes and a number of class Ib-like gene fragments (Fig. 1 and Table 1). Our search resulted in the identification of all the previously defined XNC genes (Flajnik et al. 1993; Goyos et al. 2009) plus 12 additional XNC genes. Out of these 12 new genes, seven displayed clear homology to previously defined XNC subfamilies, including four genes classified as members of the XNC8 subfamily (XNC8.2, XNC8.3, XNC8.4 and XNC8.5) and three as belonging to the XNC6 (XNC6.2, XNC6.3 and XNC6.4) subfamily. This suggests that some of the identified XNC genes are members of distinct subfamilies including more than one gene.
Table 1.
Class Ib residues at conserved positions involved in MHC class Ia terminal peptide anchoring.
| Class Ib: XNC/SNC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HLA-A2 | CD1d | Xl Class Ia | MR1 | CLUSTER 1a | CLUSTER 2a | CLUSTER 3a | CLUSTER 4a | CLUSTER 5a | CLUSTER 6a | CLUSTER 7a | |
| A pocket | Tyr7 | Cys | - | - | - | -/Phe (Leu/Val) | - (Phe) | - | -/Leu | - (Phe) | - |
| Tyr59 | Gln | - | His | Leu/His/Asp/Asn | Met (Ile) | His(Glu) | His | His (Leu) | His (-) | Ala | |
| Tyr159 | Leu | - | Trp | - | -/Phe | Phe (Leu/Ser) | - | - (Asn) | Phe/- | - | |
| Trp167 | Phe | Gly | - | His (Gly) | Arg | His | His (Tyr) | His/Gln (Asn/Tyr) | His (Asn) | Asp | |
| Tyr171 | Leu | - | Phe | - (His) | - (Phe) | - (Phe) | - | - | - | - | |
| F pocket | Arg/Tyr84 | Met | - | His | Ser | Ile/Leu | Leu/(Ser) | Phe | Phe/Tyr | Val/Phe | Met |
| Thr143 | Ala, Pro | - | Thr/Ile | Ala (Val) | Val (Ile/Ala) | Leu | Leu/Met | Met (Val/Ile) | Val (Leu/Met) | Val | |
| Lys146 | Val | - | Ala | Arg/- | Ile (Val/Leu) | GAP | Ser/Arg/Glu/Gln | Gln (leu) | Leu | Gln | |
| Trp147 | Leu | - | Trp | - (Arg) | - | - | - | - | - | Cys | |
| Ligand | peptides | lipids | vitamin B metabolites | ||||||||
Putative ligand binding residues of Xenopus/Siluriana class Ia and class Ib where predicted based on alignment with human HLA-A.
Cluster refers to phylogenetically related families of XNC/SNC sequences.
- conservation of residue with MHC class Ia; GAP, gap in the sequence such that no equivalent residue is determined.
Bold, unique residue among the differnet XNC/SNC molecules. x/x, equal distribution of two different amino acids (x) amino acid only detected in 1–2 of sequences in the group.
Grey shade, hydrophobic amino acids
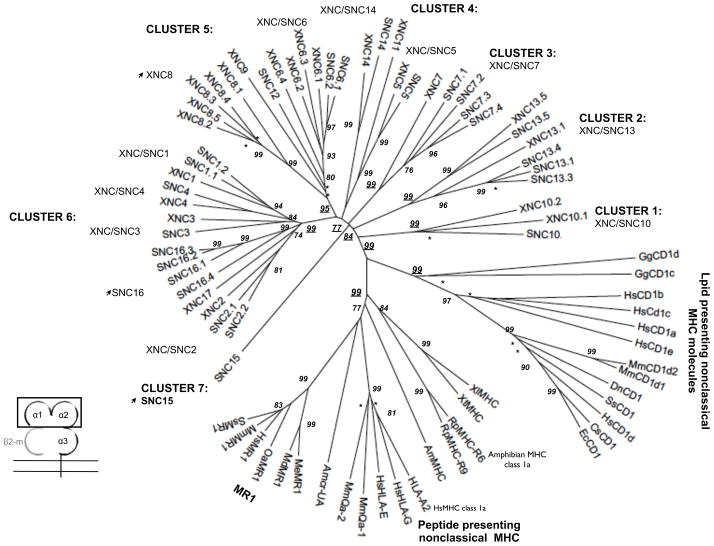
To infer orthologous relationships among X. laevis and X. tropicalis class Ib genes, multiple amino acid alignments of XNC and SNC α1/α2 domains were used to generate a neighbor-joining phylogenetic tree. As previously described, orthologous subfamilies were defined as any clade with a bootstrap value of 80 or higher (Goyos et al. 2011) (Fig. 2). Of the 12 new unassigned XNC sequences three (XNC14, XNC13.1 and XNC13.5) clearly cluster with distinct SNC gene subfamilies. One XNC gene designated as a new XNC subfamily, XNC17, showed similar levels of homology to XNC1, 2 and 3 genes with 62–66% amino acid identity in the α1/α2 domains. This XNC gene, while clustering together with the XNC/SNC subfamily lineages 1, 2, 3, 4 and 16, did not have a clear subfamily relationship among any of the described XNC/SNC genes.
Fig 2. The phylogenetic relationships of XNC and SNC α1/α2 domains compared to selected vertebrate classical and nonclassical MHC genes.
The neighbor-joining tree was constructed from amino acid alignments of the alpha 1 and alpha 2 domains using pairwise gap deletions and the p-distance method to estimate evolutionary distance. The tree was drawn using MEGA 5.2. and confidence values were measured using 10,000 bootstrap replications with the values indicated at key nodes and * indicating values < 50. Arrows indicate non-classical subfamilies within the Xenopodinae without clear orthologous relationships between X. laevis and X.tropicalis. Species abbreviations are: Xl, X. laevis, Xt, X. tropicalis; Hs, human; Mm, mouse; Ss, pig; Gg, chicken; Me, Tammar wallaby; Wd, short-tailed opossum, Oa, sheep; Ec, horse; Cs, rhinoceros; Dn, nine-banded armadillo; Rp, northern leopard frog and Amcr, Galapagos marine iguana.
Additionally, using the SNC10 α1 deduced amino acid sequence as search criteria; we identified a new XNC gene adjacent to the previously described XNC10 gene. This gene showed the highest degree of similarity to the XNC/SNC10 lineage with a α1 amino acid identity of 66% and 50% to SNC10 and XNC10 respectively. Moreover, as suggested by phylogenetic analysis supported by high bootstrap values, this XNC gene clustered within the XNC/SNC10 lineage that formed a branch separate from both the classical MHC class Ia genes and the other class Ib genes (Fig 2). Hence, we designated this gene as XNC10.2 to indicate that it is a distinct member of the XNC/SNC10 lineage.
Comparative analysis of the deduced XNC and SNC loci shows that although the number of genes in a given subfamily and the organization of individual genes vary between the two Xenopus species, there is an overall high degree of genetic synteny (Fig 1). The majority of class Ib genes of both X. laevis and X. tropicalis are clustered together in the same transcriptional orientation, with only a few other genes interspersed. In addition to the expressed class Ib genes (indicated by a * in Fig. 1), and typical to MHC gene loci, both X. laevis and X. tropicalis have multiple class Ib pseudogenes and/or gene fragments at varying stages of disintegration, ranging from nearly complete genes to a single exon. Finally, both XNC and SNC genes are flanked by a number of SLAM-family member-like genes as well as a T-lymphocyte surface antigen Ly-9-like gene that shows clear locus conservation between the two Xenopus species.
3.2 Evolution of distinct XNC/SNC Gene lineages: examples of both conservation and divergence
Despite the relatively long evolutionary time (~ 65 million years) separating Xenopus and Silurana genera, multiple class Ib genes within the representative species X. tropicalis (Silurana) and X. laevis (Xenopus) exhibit an unusual degree of conservation (Goyos et al. 2011), which is further supported by the conserved gene synteny. Aside from this overall high degree of evolutionary preservation, we also found examples of species-specific adaptations, such as expansions and contractions of specific XNC/SNC subfamilies.
Examples of evolutionary conservation include the three monogenetic subfamilies XNC/SNC3, 4 and 5. Among these, the XNC/SNC5 genes show the highest degree of conservation (83% and 84% amino acid identity in the α1 and the α2 domain, respectively). In contrast, examples of species-specific divergences include genes that are present and diversified or expanded in one species but not in the other species. In this category, the XNC8 subfamily is expanded in X. laevis, while no corresponding gene was found in X. tropicalis. The XNC8 subfamily is comprised of five genes that (with the exception of XNC8.2) are organized in consecutive order (Fig. 1). Using RT-PCR, all XNC8 gene-specific transcripts, with the exception of XNC8.4, were isolated and confirmed by sequencing. XNC8.3 was predominantly expressed in the thymus, whereas XNC8.1, 8.2 and 8.5 were ubiquitously expressed (data not shown). Conversely, in X. tropicalis the SNC16 subfamily was expanded while no corresponding ortholog was found in X. laevis. A second set of species-specific specializations is constituted by gene families present in both X. laevis and X. tropicalis but with varying degrees of expansion such as XNC/SNC6, XNC/SNC7 and XNC/SNC13. Finally, putative homologs of functional class Ib genes such as XNC9 are present in one species but are only detected as pseudogenes and/or gene fragments in another species suggesting that this gene is being silenced and lost in a species-specific manner. In summary, while the majority of class Ib gene lineages are preserved in these two divergent amphibian species, the extent of gene duplications and mutations within individual lineages vary.
3.3 Identification of amphibian class Ib gene lineage subgroups
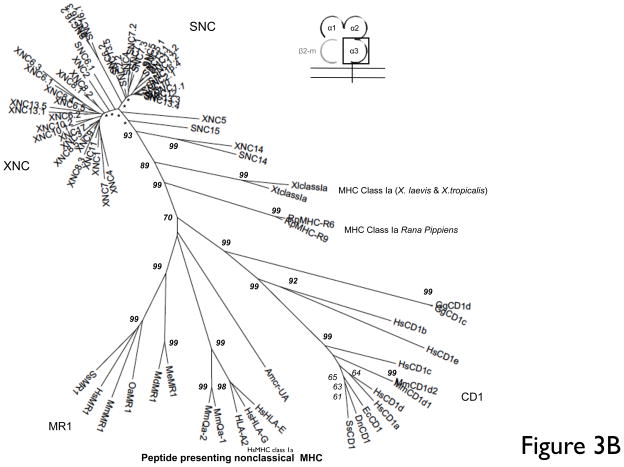
Phylogenetic analysis based on nucleotide and deduced amino acid multiple alignments of the α1/α2 domains shows that XNC/SNC genes form a monophyletic clade with respect to other vertebrate class Ia and class Ib genes. This supports the hypothesis that these genes evolved from a common ancestor. However, despite the overall high level of evolutionary conservation between class Ib gene in the two species, it is also apparent from the phylogenetic analysis that individual class Ib gene subfamilies have undergone further species-specific lineage expansions and contractions, likely reflecting differences in selective pressures thought to influence class Ib evolution. Indeed, the different class Ib gene subfamilies are divergent, sharing within a species between 30–68% amino acid identity in the α1/α2 domains. To gain further insight into individual relationships of different class Ib genes, we performed a detailed comparisons of all class Ib genes thus far identified in X. laevis and X. tropicalis. Focusing on either individual α1, α2 (data not shown) or combined α1/α2 domains that together form the putative peptide binding region, the Xenopus/Silurana class Ib genes segregate into seven clusters, supported by high bootstrap values (Fig. 2 and sFig1). With the exception of SNC15, which cluster within the class Ib gene clade but forms a separate branch distinct from other class Ib genes, all the other clusters contain both XNC and SNC genes.
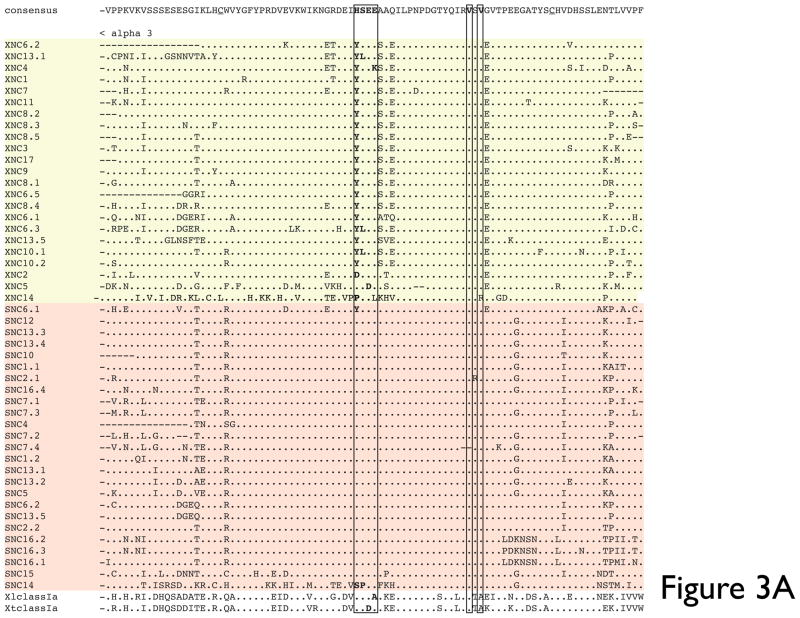
In contrast to this loci-based clustering of the α1/α2 domains, the α3 domains of XNC and SNC genes cluster according to species and show a relatively high degree of conservation (67–94% amino acid identity) (Fig. 3A, B). One of the evolutionary conserved MHC class I signature features is the putative CD8α-binding site (QDTE-(x)16-AxxV), in particular residues Q226 and D227. We examined the presence of this motif in the XNC and SNC α3 domains. As previously reported for X. laevis class I sequences, this motif is poorly conserved in XNCs and class Ia sequences (Flajnik et al. 1993). Instead, the majority of XNC sequences are characterized by a YSEE-(x)16-VxxV motif. Comparably, we found that all SNC sequences, with the exception of SNC6 and SNC14, are characterized by a HSEE-(x)16-VxxV motif (Fig 3A). Similarly, specific residues in CD8α, which in mammals have been shown to be important for class I interaction (i.e., Arg4, Lys21 and Leu25), are not conserved in either X. laevis or X. tropicalis CD8α sequences (Chida et al. 2011) which suggests a species-specific co-evolution of MHC class Ia and class Ib molecules with their putative cognate T cell co-receptor. Within the XNC/SNC α3 cluster the XNC/SNC14 subfamily is the most divergent, and, as supported by high bootstrap values, forms a separate branch away from the main clade. This suggests that the XNC/SNC14 gene lineage was one of the first to diverge from the Xenopodinae class Ia.
Fig 3. Multiple deduced amino acid sequence alignment and phylogenetic relationships of the α3 domains of X. tropicalis and X. laevis nonclassical MHC genes (SNC and XNC).
(A) Deduced amino acid alignment of XNC and SNC α3 domains with X. laevis and X. tropicalis MHC class Ia. A consensus sequence is shown at the top and dots indicate amino acids identical to this sequence; (-) represent gaps in the alignment and conserved cysteines are in bold and underlined. The MHC class I CD8 binding site is boxed and indicated in bold. (B) The neighbor-joining tree was constructed from amino acid alignments of the alpha 3 domains using pairwise gap deletions and the p-distance method to estimate evolutionary distance. The tree was drawn using MEGA 5.2. and confidence values were measured using 10,000 bootstrap replications with the values indicated at key nodes with * indicating values <50. Species abbreviations are: Xl, X. laevis, St, S. tropicalis; Hs, human; Mm, mouse; Ss, pig; Gg, chicken; Me, tammar wallaby; Wd, short-tailed opossum, Oa, sheep; Ec, horse; Cs, rhinoceros; Dn, nine-banded armadillo; Rp,northern leopard frog and Amcr, Galapagos marine iguana.
To obtain further evidence of class Ib gene lineage relationships, we analyzed the molecular features and potential conservation of the putative putative ligand binding regions of aligned sequences within each cluster (Table 2 and sFig1). In mammals, classical MHC class Ia peptide binding involves side chain-independent recognition of the peptide main chain via nine invariant, highly evolutionary conserved amino acid residues clustered in two shallow pockets, A and F, located at either ends of the antigen binding groove. As previously described (Flajnik et al. 1993; Robert and Ohta 2009) these specific residues can be identified based on deduced amino acid alignments with human class Ia HLA-A invariant residues and are highly conserved in the X. laevis class Ia sequences (Bjorkman et al. 1987; Saper et al. 1991). In contrast, these residues are not conserved in the mammalian non-peptide binding nonclassical MHC molecules CD1d (Beckman et al. 1994; Brigl and Brenner 2004) and MR1 (Kjer-Nielsen et al. 2012; Patel et al. 2013). Similarly, for each XNC/SNC cluster, with the exception of a conserved Trp at position 147 in the F pocket, and two conserved Tyr at positions 7 and 171 respectively in the A pocket, the canonical class Ia peptide binding residues within each cluster were distinct from each other as well as from those of human HLA-A and X. laevis/X. tropicalis class Ia sequences. Thus the lack of full conservation of peptide anchoring tyrosine residues in the A and F pocket of XNC/SNC sequences suggests that these molecules bind non-peptide based antigens. However, a notable feature is that among gene members within each XNC/SNC cluster, these putative ligand binding residues are conserved suggesting that genes within a specific cluster might have a similar specialization, possibly through interaction with a conserved antigenic ligand motif (Table 2). For example, the XNC/SNC10 gene lineage (cluster 1), exhibits five residues distinct from class Ia and other XNC/SNC proteins. In the A pocket, a notable substitution (Try167 to Glu/His167) results in the loss of Trp 167, which in class Ia blocks the amino-terminal extension of peptides (Hansen et al. 2007). Although it is difficult to decipher the nature of specific class Ib ligands by sequence analysis alone, it is likely that these different gene lineages encode proteins that bind distinct antigens. Collectively these data suggest that the large family of XNC/SNC class Ib genes can be subdivided into different groups with presumably functionally divergent roles.
Table 2.
Putative class Ib residues at conserved positions involved in terminal peptide anchoring.
| Class Ib: XNC/SNC | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HLA-A2 | CD1d | Xl Class Ia | MR1 | CLUSTER 1a | CLUSTER 2a | CLUSTER 3a | CLUSTER 4a | CLUSTER 5a | CLUSTER 6a | CLUSTER 7a | |
| A pocket | Tyr7 | Cys | - | - | - | -/Phe (Leu/Val) | - (Phe) | - | -/Leu | - (Phe) | - |
| Tyr59 | Gln | - | His | Leu/His/Asp/Asn | Met (Ile) | His(Glu) | His | His (Leu) | His (-) | Ala | |
| Tyr159 | Leu | - | Trp | - | -/Phe | Phe (Leu/Ser) | - | - (Asn) | Phe/- | - | |
| Trp167 | Phe | Gly | - | His (Gly) | Arg | His | His (Tyr) | His/Gln (Asn/Tyr) | His (Asn) | Asp | |
| Tyr171 | Leu | - | Phe | - (His) | - (Phe) | - (Phe) | - | - | - | - | |
| F pocket | Arg/Tyr84 | Met | - | His | Ser | Ile/Leu | Leu/(Ser) | Phe | Phe/Tyr | Val/Phe | Met |
| Thr143 | Ala, Pro | - | Thr/Ile | Ala (Val) | Val (Ile/Ala) | Leu | Leu/Met | Met (Val/Ile) | Val (Leu/Met) | Val | |
| Lys146 | Val | - | Ala | Arg/- | Ile (Val/Leu) | GAP | Ser/Arg/Glu/Gln | Gln (leu) | Leu | Gln | |
| Trp147 | Leu | - | Trp | - (Arg) | - | - | - | - | - | Cys | |
| Ligand | peptides | lipids | vitamin metabolites | ||||||||
Putative peptide binding residues of Xenopus/Siluriana class Ia and class Ib where predicted based on alignment with human HLA-A.
Cluster refers to phylogenetically related families of XNC/SNC sequences.
- conservation of residue with MHC class Ia; GAP, gap in the sequence such that no equivalent residue is determined.
Bold, unique residue among the differnet XNC/SNC molecules. x/x, equal distribution of two different amino acids (x) amino acid only detected in 1–2 of sequences in the group.
Grey shade, hydrophobic amino acids
3.4 The XNC/SNC10 gene lineage is highly conserved in multiple polyploid species of the Xenopodinae subfamily
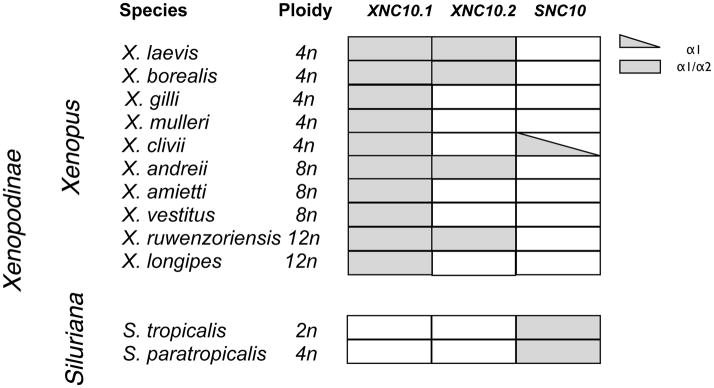
Recently, we demonstrated that the expression of XNC10.1 is essential for the development of a distinct subset of semi-invariant T cells (Edholm et al. 2013). Although the equivalent to these class Ib-restricted iT cells have not yet been identified in X. tropicalis, the high degree of conservation of primary sequence and differential gene expression profiles between XNC10.1 and SNC10 suggest that SNC10 performs a similar function in X. tropicalis. Based on Southern blot analysis it has been suggested that the XNC10.1 and SNC10 genes have been differentially retained across Xenopodinae species. To further characterize the evolutionary relationships of these genes, we investigated whether XNC/SNC10.1 and 10.2 gene orthologs were present among Xenopodinae species. We focused our investigation on exon 2 (encoding the α1 domain) and exon 3 (encoding the α2 domain) as these have the highest degree of divergence. As a template for PCR we used genomic DNA isolated from 10 species belonging to the Xenopus genus with varying degrees of ploidy ranging from 2N to 12N, as well as 2 species belonging to the Silurana genus (Table 3). Using degenerate primers targeting conserved XNC/SNC10 sequence motifs we obtained XNC/SNC10-related sequences of exon 2 and 3 from all species, thus confirming the preservation of XNC/SNC gene in all Xenopodinae species tested. We then designed gene-specific primers to exon 2 and 3 of XNC10.1, XNC10.2 and SNC10. We obtained XNC10.1-related sequences from all species sampled from Xenopus genus, but none from the Silurana genus. Similarly, we isolated SNC10-related sequences from the two Silurana species (X. tropicalis and X. paratropicalis). In addition, using primers specific for the SNC10 exon 2 we obtained a gene fragment from X. gilli that upon sequencing was confirmed as SNC10-like. However, despite numerous efforts, we were unable to isolate an SNC10-like exon 3 from X. gilli. Comparably, XNC10.2-related sequences were only isolated from four species belonging to the Xenopus genus (X. laevis, X. borealis, X. andreii and X. ruwenzorienzis) suggesting that the XNC10.2 gene has, in the majority of species tested, either been lost or diverged beyond the typing system employed here. Notably, one of the XNC10.2 variants isolated from X. ruwenzorienzis has a four-nucleotide insertion in exon 2, resulting in a frameshift and premature termination of the α1 sequence.
Table 3.
Species distribution of XNC/SNC10-related exons from polyploid species of the Xenopodinae subfamily.
|
Grey shading indicate presence of XNC10.1 / XNC10.2 and/or SNC10 α1 and α2 encoding exons
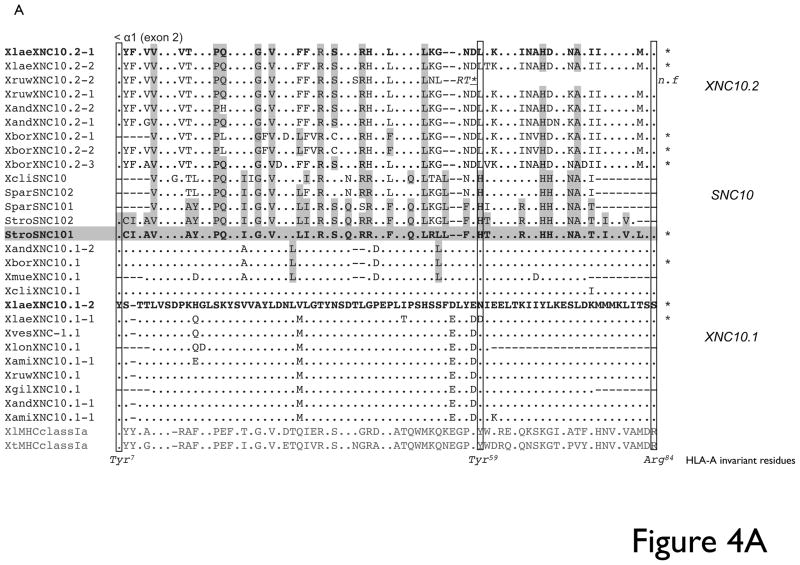
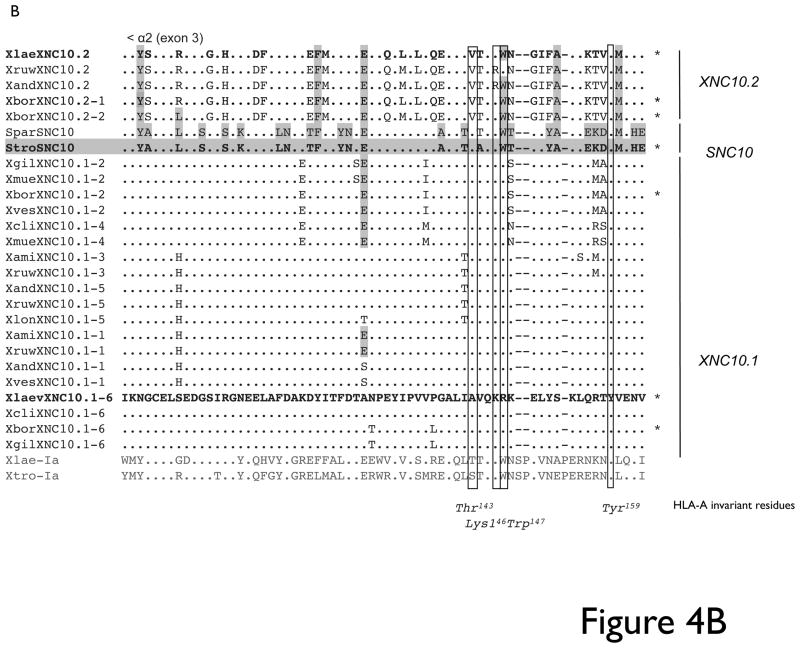
From the 10 Xenopus species tested, we identified three predominant XNC10.1 exon 2 sequences (Fig 4A) and six XNC10.1 exon 3 sequences (Fig 4B), which overall showed a remarkably high degree of sequence conservation. Notably, for each species regardless of ploidy, we isolated a maximum of three different sequences. Similarly, for XNC10.2 three different sequences were isolated from X. borealis (4N), while for the remaining species (X. laevis/2N, X. andreii/8N and X. ruwenzorienzis/12N) two different sequences with either one or two nucleotide differences were isolated. From the two representative species of the Silurana genus, two different SNC10 exon 2 and a single exon 3 sequence were identified. Overall, the three genes within the XNC/SNC10 gene lineage display a high degree of sequence conservation and with the exception of positions 146 and 147 in the α2 domain of XNC10.2, the putative ligand binding residues are absolutely conserved in all sequences isolated for a specific gene (Table 4). Notably, all the SNC/XNC10 sequences display an overall conservation of putative invariant anchoring residues in the F pocket, while displaying more variability in the putative A pocket residues consistent with a potentially conserved C-terminus Ag docking moiety.
Fig 4. XNC/SNC10 sequence analysis.
Deduced amino acid alignment of (A) alpha1/exon 2 and (B) alpha 2/exon 3 of XNC/SNC10 genes from different Xenopodinae species. Dots indicates amino acid identical to X. laevis XNC10.1 and grey shading indicate amino acids identical to X. tropicalis SNC10 while (-) represents gaps in the alignment. Sequences from the different species grouping as either XNC10.1, XNC10.2 or SNC10 are indicated on the right and * indicate that the gene is expressed. Putative peptide anchoring residues, based on alignment with human HLA-A, are boxed and indicated at the bottom.
Table 4.
Putative class Ib residues at conserved positions involved in terminal peptide anchoring.
| residuea | domain | XNC10.1 | XNC10.2 | SNC10 |
|---|---|---|---|---|
| Tyr7 | α1 | Tyr 7/7b | Tyr 4/4 | Tyr 1/3 nd 2/3 |
| Tyr59 | α1 | Asn 7/7 | Leu 4/4 | His 3/3 |
| Tyr159 | α2 | Tyr 7/7 | Tyr 4/4 | Tyr 2/2 |
| Trp167 | α2 | Glu 7/7 | His 5/5 | His 1/2 nd 1/2 |
| Tyr171 | α2 | His 7/7 | Tyr 5/5 | Tyr 1/2 nd 1/2 |
| Tyr/Arg84 | α1 | Ser 7/7 | Ser 4/4 | Ser 1/3 nd 2/3 |
| Thr143 | α2 | Ala 7/7 | Val 5/5 | Ala 2/2 |
| Lys146 | α2 | Lys 7/7 | Lys 2/5 ; Arg 3/5 | Lys 2/2 |
| Trp147 | α2 | Arg 7/7 | Arg 2/5 ; Trp 3/5 | Trp 2/2 |
putative PBR of Xenopodinae XNC/SNC10 gene lineage were predicted by alignment with human HLA-A
Numbering based on HLA-A2
Indicate the number of species that have conserved amino acids at the indicated position/ total number of species analyzed
nd= not determined
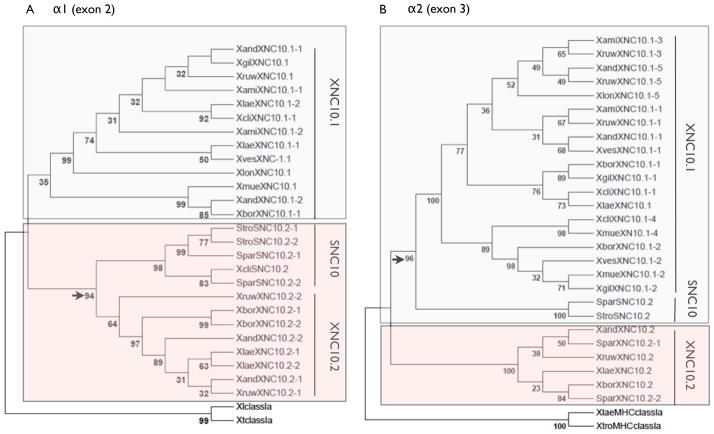
To elucidate the evolutionary history of the XNC10/SNC10 lineage, we performed phylogenetic analysis (Fig. 5) revealing that both exon 2 and 3 of the different SNC10 and XNC10.1/2 sequences represent three clearly distinctive groups. Furthermore, when comparing exon 2 sequences, the SNC10 sequences cluster with the XNC10.2 sequences with a high bootstrap value. Conversely, for exon 3, the SNC10 clusters with the XNC10.1 sequences, while the XNC10.2 sequences supported by high bootstrap values form a distinct cluster. Collectively these data suggests that the three XNC/SNC10 genes share a common ancestor. It is also interesting to note that within each main branch the different sequence variants cluster together in a non-species distinctive pattern.
Fig 5. Phylogenetic relationships between XNC/SNC10 gene lineage within multiple species of the Xenopodinae subfamily.
Neighbor-joining trees were constructed based on amino acid alignments of (A) alpha1/exon 2 and (B) alpha 2/exon 3 of XNC/SNC10 sequences from different Xenopodinae species. Trees were rooted with X. laevis and X. tropicalis MHC class Ia genes, accession numbers: ABA43373.1 and AAP36728.1 respectively. The trees were drawn using MEGA 5.2. using pairwise gap deletions and the p-distance method to estimate evolutionary distance and confidence values were measured using 10,000 bootstrap replications with the values indicated at key nodes. Trees generated based on nucleotide alignments resulted in similar topology (data not shown).
3.5 Differential expression pattern of the XNC/SNC gene lineage
Both X. laevis XNC10.1 and S. tropicalis SNC10 are primarily expressed in the adult and larval thymi and are detected on thymocytes from early onset of thymic organogenesis (3 days post fertilization; developmental stage 39; (Goyos et al. 2009; Goyos et al. 2011). We were therefore interested in determining the expression patterns of the XNC10 sub-lineage i.e XNC10.1 and XNC10.2 in X. laevis. Accordingly, we performed qPCR gene expression using XNC10.1 and XNC10.2 specific primers. In contrast to the predominant thymic expression of the XNC10.1 gene, the XNC10.2 gene was predominantly expressed in the gill, kidney and liver (Fig 6).
Fig 6. Differential expression of XNC10.1 and XNC10.2.
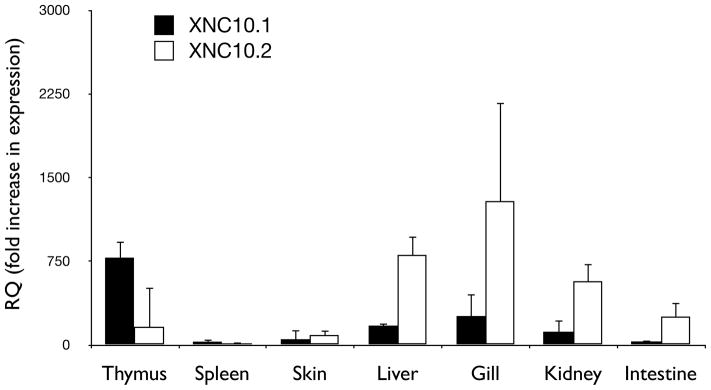
Expression of XNC10.1 and XNC10.2 in various tissues of X. laevis and developmental stage 53/54 tadpoles. Results are normalized to an endogenous control (GAPDH) and expressed as fold change compared to expression of XNC10.2 in spleen. All results are presented as mean ± s.e.m, n = 5 for each group.
Discussion
Although class Ib genes have been identified across jawed vertebrates, from elasmobranch to mammals, (reviewed in (Flajnik and Kasahara 2001)), they are very heterogeneous in sequences, gene number, organization and expression profiles. Moreover, the dynamic evolution of class Ib genes has resulted in extensive diversification of their molecular structure and function. This evolutionary diversification has resulted in multiple species-specific adaptations and, even between closely related species, there are few unambiguous class Ib orthologs (Adams and Luoma 2013; Kulski et al. 2002). The work presented here, markedly extending previous findings (Goyos et al. 2011), highlights the unusual high degree of class Ib conservation across divergent amphibian species and provides new insight into the evolution of class Ib genes.
We conducted an extensive comparative analysis of Xenopodinae class Ib genes, including class Ib sequences from closely related species (within either the Xenopus or Silurana genus) and more divergent species (between representatives of the Xenopus and Silurana genus). This study reveals two major phylogenetic patterns. On the one hand, some gene lineages have been maintained as monogenetic subfamilies with remarkably few changes in their nucleotide sequence across divergent species, consistent with a strict functional conservation. On the other hand, other class Ib gene lineages display a higher degree of flexibility, as demonstrated by species-specific adaptations including expansion and contractions of specific gene family, which attests to the distinct selective pressures thought to influence class Ib evolution resulting in functional divergence. In addition to the variable selective pressures exerted on the different XNC/SNC gene families, phylogenetic analyses also confirm our initial report (Goyos et al. 2011) suggesting that in Xenopodinae spp the α3 domain has undergone strong evolutionary constraints different from those exerted on the α1/α2 domains. This phylogenetic relationship, whereby the α3 domain remains more conserved and clustered according to species, while the α1/α2 part of the same molecule are more divergent is reminiscent of the family of receptors recognizing CD94, which includes the human HLA-E, mouse H2-Qa1 and rat RT-BM (Joly and Rouillon 2006). This supports the idea that concerted evolution takes place among class Ib genes within a species. However, juxtaposed to this more divergent α1/α2 domain, there is also a strong conservation of the α1/α2 putative ligand binding residues within each phylogenetically defined XNC/SNC cluster suggesting that these specific residues are under negative selection, possibly to ensure that a distinct function (such as antigen presentation) is maintained.
Both X. laevis and X. tropicalis have a large number of class Ib genes, their genomes contain at minimum of 25 XNC and 29 SNC genes, respectively, and the majority if not all of these genes are linked and located distally from the class Ia gene. Out of the 17 distinct class Ib gene lineages identified to date, 11 display orthologous relationships between X. laevis and X. tropicalis. This degree of conservation of class Ib genes, with the exception of CD1 and MR1, is in contrast to that observed across mammals and indicative of a different pattern of class Ib evolution in amphibians. This implies that various XNC/SNC gene lineages have evolved to perform distinct functions. In mammals both CD1 and MR1 are located outside the MHC region (Adams and Luoma 2013) and have been shown to function as restricting element for distinct populations of semi-invariant T cells. These include the CD1d restricted iNKT cells (Bendelac et al. 1995; Bendelac et al. 2007) and the MR1 restricted mucosal associated invariant T (MAIT) cells (Treiner et al. 2003). CD1 and MR1 are less heterogeneous than class Ia and most class Ib genes, and both CD1 and MR1 orthologous genes have been described in most mammals. CD1 orthologs have also been identified in chickens suggesting that this gene was present prior to the mammalian/avian split corresponding to ~310 million years of evolution (Miller et al. 2005). Notably, none of the class Ib genes of X. laevis or X. tropicalis identified to date share significant sequence identity with either CD1 or MR1. Additionally, by close range synteny analysis (Y. Ohta, personal communication), neither CD1 nor MR1 like genes were found in the class Ib genomic loci of either X. tropicalis or X. laevis.
The fact that class Ib genes are present in all taxa of jawed vertebrates attest to their evolutionary primordial origins However, the evolutionary history of class Ib genes in lower vertebrates remains largely unknown and the phylogenetic relationships of these genes are difficult to outline. For example, a large number of divergent class Ib genes have been identified in the urodele amphibian, Ambystoma mexicanium, although none of these genes are orthologous to any of the XNC/SNC genes identified to date (Sammut et al. 1999). Furthermore, in contrast to Xenopus where all class Ib genes identified to date form a single linkage group outside the MHC, Southern blot analysis suggest that in A. mexicanium class Ib, class Ia as well as MHC class II genes are linked (Sammut et al. 1999) Elasmobranchs also have variable numbers of class Ib genes including highly divergent species-specific genes (Bartl et al. 1997; Wang et al. 2003). Similarly, teleosts encode multiple class I genes that based on evolutionary relationships have been grouped into distinct lineages (U-, Z/ZE-, L- and S-lineage) (Lukacs et al. 2010). These teleost class I lineages are differentially distributed in divergent species, with some lineages like the U-lineage, containing both class Ia and class Ib like genes being broadly represented. In contrast, genes belonging to the L-lineage are highly divergent class Ib genes and have so far only been identified in salmonids and cyprinids (Dijkstra et al. 2007). Notably, Atlantic cod (Gadhus Morhua) has lost MHC class II genes coinciding with a large expansion (more than 100 genes) of their U-lineage class I genes. Based on phylogenetic analysis, these genes segregate into two distinct clades that display distinct ratios of non-synonymous to synonymous mutation and differences in the average nucleotide diversity per site, suggesting possible neofunctionalization for this MHC class I lineage (Star et al. 2011). Interestingly, in contrast to other vertebrate groups, the MHC class I and II regions of teleosts are located on different linkage groups, which might have facilitated independent evolution of these two systems (Lukacs et al. 2010). However, despite the presence of class Ib genes in all ectothermic vertebrates, to date no conserved class Ib gene ortholog have been identified across distant species.
A unique feature of the Xenopus immune system not found in mammals is the drastic physiological transition between larval and adult stages that occurs during metamorphosis. In particular, while class Ia transcripts are detected in tadpoles (Goyos and Robert, unpublished), there is no consistent class Ia surface protein expression until the onset of metamorphosis (Flajnik et al. 1986; Rollins-Smith et al. 1997; Salter-Cid et al. 1998). Irrespective of this, tadpoles are immunocompetent and have circulating CD8+ T cells (Barlow and Cohen 1983). This could either be due to low levels (below antibody detection) of class Ia protein expression capable of supporting T cell development and function. Alternatively, it has been suggested that during this life stage, class Ib molecules may compensates for the low class Ia expression. In support of this we have shown that XNC10.1, in a manner similar to CD1d and MR1, is required for the development and function of a distinct semi-invariant T cell population (Edholm et al. 2013). Also, we recently showed that in the spleen of early developmental tadpoles (before optimal class Ia surface expression), there is a preponderance of six unique invariant TCRα rearrangements in the CD8− and CD8dim T cell populations (Edholm et al. 2013). In light of this, it is tempting to speculate that there are additional class Ib-restricted iT cell populations in the tadpole and that this pool of innate-like T cells are capable of facilitating immune effector functions. Considering the wide expansion of class Ib genes in many ectothermic vertebrate species (Xenopus, A. mexicanium, G. Morhua), it is possible that ectothermic vertebrates relies heavily on class Ib-dependent innate-like invariant T cells. This type of immunity may provide selective advantage for organisms that undergo rapid external development by maximizing the use of a small number of lymphocytes and minimizing negative selection in the thymus.
Our present findings indicate that despite the otherwise high variability across the genomes of these species including varying levels of polyploidy the XNC/SNC10 gene lineage is conserved in all Xenopodinae species examined to date. Notably, XNC10.1 and SNC10 share the same expression pattern with a predominant and developmentally early thymocyte expression, from the onset of thymic organogenesis suggesting that these molecules have similar conserved functions. Phylogenetic analysis confirms that XNC10.1, XNC10.2 and SNC10 represent three distinct genes and that each species tested has at least one and sometimes two of these genes in their genome. Whereas XNC10.1 is represented in all species tested of the Xenopus genus, SNC10 sequences, with the exception of an SNC10-like α1 sequence isolated from X. clivii, are exclusively found in the Silurana genus. Comparably, XNC10.2 is found sporadically throughout the Xenopus genus suggesting that this gene has either been lost or undergone extensive modifications in the majority of Xenopus species. Therefore, although the XNC10.2 gene is found in X. laevis, X. borealis, X. andreii and X. ruwenzoriensis, there may be a combinatorial effect of lower XNC10 gene dosage in polyploidy species (X. borealis and X. laevis are tetraploid, X. andreii is octotetraploid and X. ruwenzoriensis is dodecaploid) and XNC10.2 locus degradation, which can preclude their identification by PCR. Interestingly, in X. ruwenzoriensis (12N) close to half of the α1 sequences isolated were nonfunctional, suggesting that this gene is approaching non-functionality. Nevertheless, gene expression studies in both X. laevis and X. borealis show that at least in these two species both XNC10.1 and 10.2 genes are expressed at the mRNA level. Moreover, in X. laevis XNC10.1 and 10.2 display distinct non-overlapping expression patterns suggesting that these molecules have distinct functional roles.
Collectively, these results provide strong supportive evidence that class Ib genes represent an evolutionary ancient gene family that perform functional roles distinct from those of class Ia. Indeed, the high degree of genetic conservation of multiple nonclassical MHC class Ib lineages observed in the anuran subfamily Xenopodinae imply preservation for biologically important functions.
Supplementary Material
Acknowledgments
We would like to thank Tina Martin for the expert animal husbandry. We also gratefully thank Drs. Martin Flajnik and Leon Grayfer, as well as Nikesha Haynes-Gilmore for helpful discussions and critical reading of the manuscript. This research was supported by grants R24-AI-059830 from National Institute of Allergy and Infectious Diseases (NIH/NIAID).
Bibliography
- Adams EJ, Luoma AM. The adaptable major histocompatibility complex (MHC) fold: structure and function of nonclassical and MHC class I-like molecules. Annu Rev Immunol. 2013;31:529–61. doi: 10.1146/annurev-immunol-032712-095912. [DOI] [PubMed] [Google Scholar]
- Adams EJ, Parham P. Species-specific evolution of MHC class I genes in the higher primates. Immunol Rev. 2001;183:41–64. doi: 10.1034/j.1600-065x.2001.1830104.x. [DOI] [PubMed] [Google Scholar]
- Baker ML, Miller RD. Evolution of mammalian CD1: marsupial CD1 is not orthologous to the eutherian isoforms and is a pseudogene in the opossum Monodelphis domestica. Immunology. 2007;121:113–21. doi: 10.1111/j.1365-2567.2007.02545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow EH, Cohen N. The thymus dependency of transplantation allotolerance in the metamorphosing frog Xenopus laevis. Transplantation. 1983;35:612–9. doi: 10.1097/00007890-198306000-00018. [DOI] [PubMed] [Google Scholar]
- Bartl S, Baish MA, Flajnik MF, Ohta Y. Identification of class I genes in cartilaginous fish, the most ancient group of vertebrates displaying an adaptive immune response. J Immunol. 1997;159:6097–104. [PubMed] [Google Scholar]
- Beckman EM, Porcelli SA, Morita CT, Behar SM, Furlong ST, Brenner MB. Recognition of a lipid antigen by CD1-restricted alpha beta+ T cells. Nature. 1994;372:691–4. doi: 10.1038/372691a0. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–5. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Bewick AJ, Chain FJ, Heled J, Evans BJ. The pipid root. Syst Biol. 2012;61:913–26. doi: 10.1093/sysbio/sys039. [DOI] [PubMed] [Google Scholar]
- Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987;329:506–12. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–90. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- Chida AS, Goyos A, Robert J. Phylogenetic and developmental study of CD4, CD8 alpha and beta T cell co-receptor homologs in two amphibian species, Xenopus tropicalis and Xenopus laevis. Dev Comp Immunol. 2011;35:366–77. doi: 10.1016/j.dci.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher CC. Evolutionary biology of CD1. Curr Top Microbiol Immunol. 2007;314:3–26. doi: 10.1007/978-3-540-69511-0_1. [DOI] [PubMed] [Google Scholar]
- Dijkstra JM, Katagiri T, Hosomichi K, Yanagiya K, Inoko H, Ototake M, Aoki T, Hashimoto K, Shiina T. A third broad lineage of major histocompatibility complex (MHC) class I in teleost fish; MHC class II linkage and processed genes. Immunogenetics. 2007;59:305–21. doi: 10.1007/s00251-007-0198-6. [DOI] [PubMed] [Google Scholar]
- Edholm ES, Albertorio Saez LM, Gill AL, Gill SR, Grayfer L, Haynes N, Myers JR, Robert J. Nonclassical MHC class I-dependent invariant T cells are evolutionarily conserved and prominent from early development in amphibians. Proc Natl Acad Sci U S A. 2013;110:14342–7. doi: 10.1073/pnas.1309840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BJ. Genome evolution and speciation genetics of clawed frogs (Xenopus and Silurana) Front Biosci. 2008;13:4687–706. doi: 10.2741/3033. [DOI] [PubMed] [Google Scholar]
- Evans BJ, Kelley DB, Tinsley RC, Melnick DJ, Cannatella DC. A mitochondrial DNA phylogeny of African clawed frogs: phylogeography and implications for polyploid evolution. Mol Phylogenet Evol. 2004;33:197–213. doi: 10.1016/j.ympev.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Kasahara M. Comparative genomics of the MHC: glimpses into the evolution of the adaptive immune system. Immunity. 2001;15:351–62. doi: 10.1016/s1074-7613(01)00198-4. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Kasahara M, Shum BP, Salter-Cid L, Taylor E, Du Pasquier L. A novel type of class I gene organization in vertebrates: a large family of non-MHC-linked class I genes is expressed at the RNA level in the amphibian Xenopus. EMBO J. 1993;12:4385–96. doi: 10.1002/j.1460-2075.1993.tb06123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik MF, Kaufman JF, Hsu E, Manes M, Parisot R, Du Pasquier L. Major histocompatibility complex-encoded class I molecules are absent in immunologically competent Xenopus before metamorphosis. J Immunol. 1986;137:3891–9. [PubMed] [Google Scholar]
- Goyos A, Ohta Y, Guselnikov S, Robert J. Novel nonclassical MHC class Ib genes associated with CD8 T cell development and thymic tumors. Mol Immunol. 2009;46:1775–86. doi: 10.1016/j.molimm.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyos A, Sowa J, Ohta Y, Robert J. Remarkable conservation of distinct nonclassical MHC class I lineages in divergent amphibian species. J Immunol. 2011;186:372–81. doi: 10.4049/jimmunol.1001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TH, Huang S, Arnold PL, Fremont DH. Patterns of nonclassical MHC antigen presentation. Nat Immunol. 2007;8:563–8. doi: 10.1038/ni1475. [DOI] [PubMed] [Google Scholar]
- Hellsten U, Harland RM, Gilchrist MJ, Hendrix D, Jurka J, Kapitonov V, Ovcharenko I, Putnam NH, Shu S, Taher L, Blitz IL, Blumberg B, Dichmann DS, Dubchak I, Amaya E, Detter JC, Fletcher R, Gerhard DS, Goodstein D, Graves T, Grigoriev IV, Grimwood J, Kawashima T, Lindquist E, Lucas SM, Mead PE, Mitros T, Ogino H, Ohta Y, Poliakov AV, Pollet N, Robert J, Salamov A, Sater AK, Schmutz J, Terry A, Vize PD, Warren WC, Wells D, Wills A, Wilson RK, Zimmerman LB, Zorn AM, Grainger R, Grammer T, Khokha MK, Richardson PM, Rokhsar DS. The genome of the Western clawed frog Xenopus tropicalis. Science. 2010;328:633–6. doi: 10.1126/science.1183670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly E, Rouillon V. The orthology of HLA-E and H2-Qa1 is hidden by their concerted evolution with other MHC class I molecules. Biol Direct. 2006;1:2. doi: 10.1186/1745-6150-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M, Flajnik MF, Ishibashi T, Natori T. Evolution of the major histocompatibility complex: a current overview. Transpl Immunol. 1995;3:1–20. doi: 10.1016/0966-3274(95)80001-8. [DOI] [PubMed] [Google Scholar]
- Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–68. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- Kulski JK, Shiina T, Anzai T, Kohara S, Inoko H. Comparative genomic analysis of the MHC: the evolution of class I duplication blocks, diversity and complexity from shark to man. Immunol Rev. 2002;190:95–122. doi: 10.1034/j.1600-065x.2002.19008.x. [DOI] [PubMed] [Google Scholar]
- Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, Lantz O. Mucosal-associated invariant T cells: unconventional development and function. Trends Immunol. 2011;32:212–8. doi: 10.1016/j.it.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Le Bourhis L, Martin E, Peguillet I, Guihot A, Froux N, Core M, Levy E, Dusseaux M, Meyssonnier V, Premel V, Ngo C, Riteau B, Duban L, Robert D, Huang S, Rottman M, Soudais C, Lantz O. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11:701–8. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- Lukacs MF, Harstad H, Bakke HG, Beetz-Sargent M, McKinnel L, Lubieniecki KP, Koop BF, Grimholt U. Comprehensive analysis of MHC class I genes from the U-, S-, and Z-lineages in Atlantic salmon. BMC Genomics. 2010;11:154. doi: 10.1186/1471-2164-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda JL, Gapin L. Developmental program of mouse Valpha14i NKT cells. Curr Opin Immunol. 2005;17:122–30. doi: 10.1016/j.coi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Miller MM, Wang C, Parisini E, Coletta RD, Goto RM, Lee SY, Barral DC, Townes M, Roura-Mir C, Ford HL, Brenner MB, Dascher CC. Characterization of two avian MHC-like genes reveals an ancient origin of the CD1 family. Proc Natl Acad Sci U S A. 2005;102:8674–9. doi: 10.1073/pnas.0500105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Gu X, Sitnikova T. Evolution by the birth-and-death process in multigene families of the vertebrate immune system. Proc Natl Acad Sci U S A. 1997;94:7799–806. doi: 10.1073/pnas.94.15.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Rooney AP. Concerted and birth-and-death evolution of multigene families. Annu Rev Genet. 2005;39:121–52. doi: 10.1146/annurev.genet.39.073003.112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel O, Kjer-Nielsen L, Le Nours J, Eckle SB, Birkinshaw R, Beddoe T, Corbett AJ, Liu L, Miles JJ, Meehan B, Reantragoon R, Sandoval-Romero ML, Sullivan LC, Brooks AG, Chen Z, Fairlie DP, McCluskey J, Rossjohn J. Recognition of vitamin B metabolites by mucosal-associated invariant T cells. Nat Commun. 2013;4:2142. doi: 10.1038/ncomms3142. [DOI] [PubMed] [Google Scholar]
- Piontkivska H, Nei M. Birth-and-death evolution in primate MHC class I genes: divergence time estimates. Mol Biol Evol. 2003;20:601–9. doi: 10.1093/molbev/msg064. [DOI] [PubMed] [Google Scholar]
- Robert J, Ohta Y. Comparative and developmental study of the immune system in Xenopus. Dev Dyn. 2009;238:1249–70. doi: 10.1002/dvdy.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JR, Cook RG. MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol. 2005;5:459–71. doi: 10.1038/nri1635. [DOI] [PubMed] [Google Scholar]
- Rollins-Smith LA, Barker KS, Davis AT. Involvement of glucocorticoids in the reorganization of the amphibian immune system at metamorphosis. Dev Immunol. 1997;5:145–52. doi: 10.1155/1997/84841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter-Cid L, Nonaka M, Flajnik MF. Expression of MHC class Ia and class Ib during ontogeny: high expression in epithelia and coregulation of class Ia and lmp7 genes. J Immunol. 1998;160:2853–61. [PubMed] [Google Scholar]
- Sammut B, Du Pasquier L, Ducoroy P, Laurens V, Marcuz A, Tournefier A. Axolotl MHC architecture and polymorphism. Eur J Immunol. 1999;29:2897–907. doi: 10.1002/(SICI)1521-4141(199909)29:09<2897::AID-IMMU2897>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Saper MA, Bjorkman PJ, Wiley DC. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 A resolution. J Mol Biol. 1991;219:277–319. doi: 10.1016/0022-2836(91)90567-p. [DOI] [PubMed] [Google Scholar]
- Skold M, Behar SM. Role of CD1d-restricted NKT cells in microbial immunity. Infect Immun. 2003;71:5447–55. doi: 10.1128/IAI.71.10.5447-5455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Star B, Nederbragt AJ, Jentoft S, Grimholt U, Malmstrom M, Gregers TF, Rounge TB, Paulsen J, Solbakken MH, Sharma A, Wetten OF, Lanzen A, Winer R, Knight J, Vogel JH, Aken B, Andersen O, Lagesen K, Tooming-Klunderud A, Edvardsen RB, Tina KG, Espelund M, Nepal C, Previti C, Karlsen BO, Moum T, Skage M, Berg PR, Gjoen T, Kuhl H, Thorsen J, Malde K, Reinhardt R, Du L, Johansen SD, Searle S, Lien S, Nilsen F, Jonassen I, Omholt SW, Stenseth NC, Jakobsen KS. The genome sequence of Atlantic cod reveals a unique immune system. Nature. 2011;477:207–10. doi: 10.1038/nature10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–9. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–82. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, Affaticati P, Gilfillan S, Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–9. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K, Deakin JE, Graves JA, Hashimoto K. Exceptionally high conservation of the MHC class I-related gene, MR1, among mammals. Immunogenetics. 2013;65:115–24. doi: 10.1007/s00251-012-0666-5. [DOI] [PubMed] [Google Scholar]
- Wang C, Perera TV, Ford HL, Dascher CC. Characterization of a divergent non-classical MHC class I gene in sharks. Immunogenetics. 2003;55:57–61. doi: 10.1007/s00251-003-0542-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.