Abstract
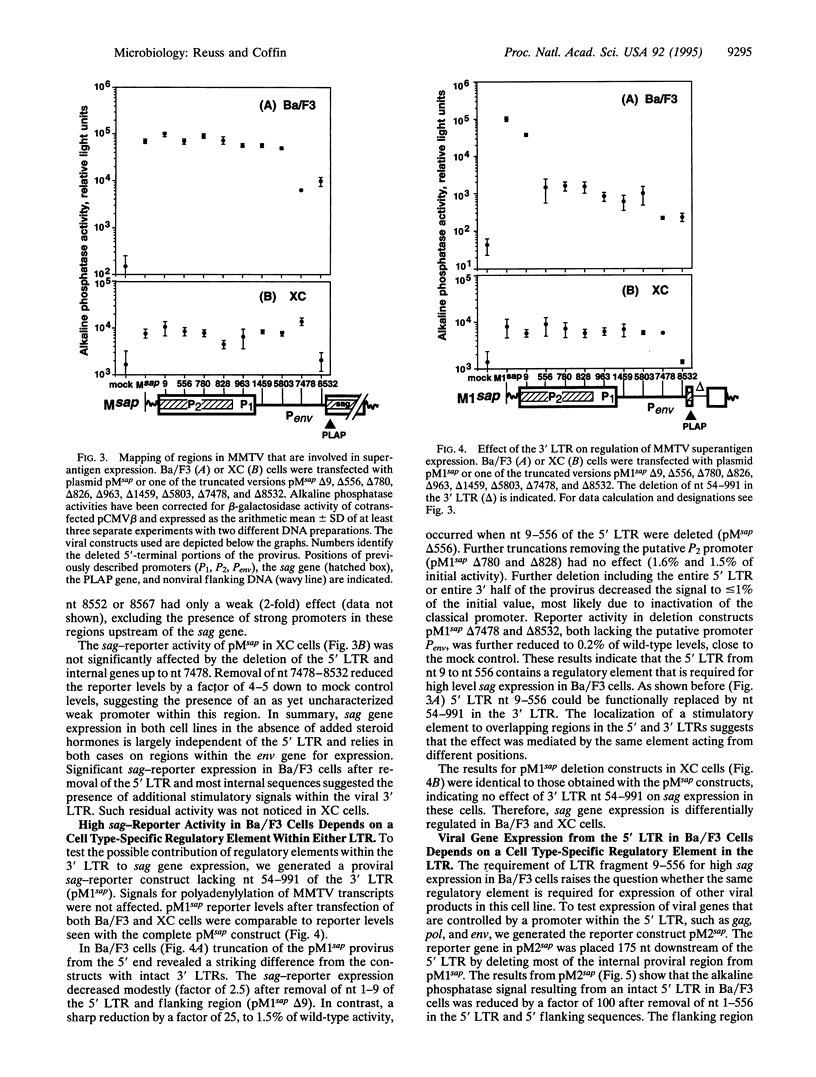
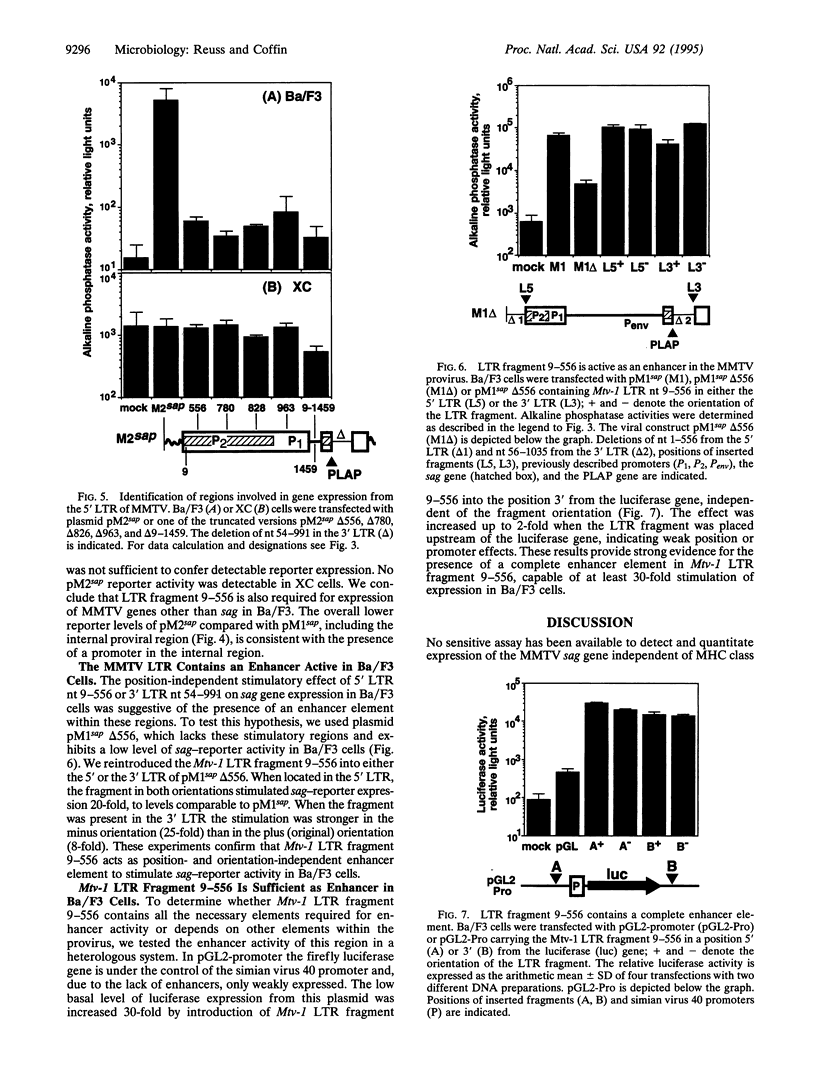
The mechanisms regulating expression of mouse mammary tumor virus (MMTV)-encoded superantigens from the viral sag gene are largely unknown, due to problems with detection and quantification of these low-abundance proteins. To study the expression and regulation of the MMTV sag gene, we have developed a sensitive and quantitative reporter gene assay based on a recombinant superantigen-human placental alkaline phosphatase fusion protein. High sag-reporter expression in Ba/F3, an early B-lymphoid cell line, depends on enhancers in either of the viral long terminal repeats (LTRs) and is largely independent of promoters in the 5' LTR. The same enhancer region is also required for general expression of MMTV genes from the 5' LTR. The enhancer was mapped to a 548-bp fragment of the MMTV LTR lying within sag and shown to be sufficient to stimulate expression from a heterologous simian virus 40 promoter. No enhancer activity of the MMTV LTR was observed in XC sarcoma cells, which are permissive for MMTV. Our results demonstrate a major role for this enhancer in MMTV gene expression in early B-lymphoid cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger J., Howard A. D., Gerber L., Cullen B. R., Udenfriend S. Expression of active, membrane-bound human placental alkaline phosphatase by transfected simian cells. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4885–4889. doi: 10.1073/pnas.84.14.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutner U., Kraus E., Kitamura D., Rajewsky K., Huber B. T. B cells are essential for murine mammary tumor virus transmission, but not for presentation of endogenous superantigens. J Exp Med. 1994 May 1;179(5):1457–1466. doi: 10.1084/jem.179.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M., Renshaw-Gegg L., Renne R., Luciw P. A. Characterization of the internal promoter of simian foamy viruses. J Virol. 1994 Aug;68(8):4811–4820. doi: 10.1128/jvi.68.8.4811-4820.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K., Ferrick D. A., Morris D. W. Structure and biological activity of the subgenomic Mtv-6 endogenous provirus. Virology. 1995 Jan 10;206(1):395–402. doi: 10.1016/s0042-6822(95)80055-7. [DOI] [PubMed] [Google Scholar]
- Choi Y., Marrack P., Kappler J. W. Structural analysis of a mouse mammary tumor virus superantigen. J Exp Med. 1992 Mar 1;175(3):847–852. doi: 10.1084/jem.175.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouse C. A., Pauley R. J. Molecular cloning and sequencing of the MTV-1 LTR: evidence for a LTR sequence alteration. Virus Res. 1989 Feb;12(2):123–137. doi: 10.1016/0168-1702(89)90059-2. [DOI] [PubMed] [Google Scholar]
- Elliott J. F., Pohajdak B., Talbot D. J., Shaw J., Paetkau V. Phorbol diester-inducible, cyclosporine-suppressible transcription from a novel promoter within the mouse mammary tumor virus env gene. J Virol. 1988 Apr;62(4):1373–1380. doi: 10.1128/jvi.62.4.1373-1380.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzburg W. H., Heinemann F., Wintersperger S., Miethke T., Wagner H., Erfle V., Salmons B. Endogenous superantigen expression controlled by a novel promoter in the MMTV long terminal repeat. Nature. 1993 Jul 8;364(6433):154–158. doi: 10.1038/364154a0. [DOI] [PubMed] [Google Scholar]
- Günzburg W. H., Salmons B. Factors controlling the expression of mouse mammary tumour virus. Biochem J. 1992 May 1;283(Pt 3):625–632. doi: 10.1042/bj2830625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Held W., Shakhov A. N., Izui S., Waanders G. A., Scarpellino L., MacDonald H. R., Acha-Orbea H. Superantigen-reactive CD4+ T cells are required to stimulate B cells after infection with mouse mammary tumor virus. J Exp Med. 1993 Feb 1;177(2):359–366. doi: 10.1084/jem.177.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L. B., Corley R. B. Lipopolysaccharide and dexamethasone induce mouse mammary tumor proviral gene expression and differentiation in B lymphocytes through distinct regulatory pathways. Mol Cell Biol. 1990 Aug;10(8):4211–4220. doi: 10.1128/mcb.10.8.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman A. J., Bourgarel P., Meo T., Rieckhof G. E. The mouse mammary tumour virus long terminal repeat encodes a type II transmembrane glycoprotein. EMBO J. 1992 May;11(5):1901–1905. doi: 10.1002/j.1460-2075.1992.tb05242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liegler T. J., Blair P. B. Direct detection of exogenous mouse mammary tumor virus sequences in lymphoid cells of BALB/cfC3H female mice. J Virol. 1986 Jul;59(1):159–162. doi: 10.1128/jvi.59.1.159-162.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund F. E., Corley R. B. Regulated expression of mouse mammary tumor proviral genes in cells of the B lineage. J Exp Med. 1991 Dec 1;174(6):1439–1450. doi: 10.1084/jem.174.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund F. E., Randall T. D., Woodland D. L., Corley R. B. MHC class II limits the functional expression of endogenous superantigens in B cells. J Immunol. 1993 Jan 1;150(1):78–86. [PubMed] [Google Scholar]
- Löchelt M., Muranyi W., Flügel R. M. Human foamy virus genome possesses an internal, Bel-1-dependent and functional promoter. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7317–7321. doi: 10.1073/pnas.90.15.7317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor G. R., Caskey C. T. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989 Mar 25;17(6):2365–2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequencing of an apparent proviral copy of env mRNA defines determinants of expression of the mouse mammary tumor virus env gene. J Virol. 1983 Sep;47(3):495–504. doi: 10.1128/jvi.47.3.495-504.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. L., Garner R., Paetkau V. An activation-dependent, T-lymphocyte-specific transcriptional activator in the mouse mammary tumor virus env gene. Mol Cell Biol. 1992 Jul;12(7):3262–3272. doi: 10.1128/mcb.12.7.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mink S., Härtig E., Jennewein P., Doppler W., Cato A. C. A mammary cell-specific enhancer in mouse mammary tumor virus DNA is composed of multiple regulatory elements including binding sites for CTF/NFI and a novel transcription factor, mammary cell-activating factor. Mol Cell Biol. 1992 Nov;12(11):4906–4918. doi: 10.1128/mcb.12.11.4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R., Dixon M., Smith R., Peters G., Dickson C. Complete nucleotide sequence of a milk-transmitted mouse mammary tumor virus: two frameshift suppression events are required for translation of gag and pol. J Virol. 1987 Feb;61(2):480–490. doi: 10.1128/jvi.61.2.480-490.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Pullen A. M., Wade T., Marrack P., Kappler J. W. Identification of the region of T cell receptor beta chain that interacts with the self-superantigen MIs-1a. Cell. 1990 Jun 29;61(7):1365–1374. doi: 10.1016/0092-8674(90)90700-o. [DOI] [PubMed] [Google Scholar]
- Salmons B., Günzburg W. H. Current perspectives in the biology of mouse mammary tumour virus. Virus Res. 1987 Aug;8(2):81–102. doi: 10.1016/0168-1702(87)90022-0. [DOI] [PubMed] [Google Scholar]
- Shackleford G. M., Varmus H. E. Construction of a clonable, infectious, and tumorigenic mouse mammary tumor virus provirus and a derivative genetic vector. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9655–9659. doi: 10.1073/pnas.85.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow G. M., Scherer M. T., Kappler J. W., Marrack P. Detection and biochemical characterization of the mouse mammary tumor virus 7 superantigen (Mls-1a). Cell. 1992 Nov 27;71(5):719–730. doi: 10.1016/0092-8674(92)90549-r. [DOI] [PubMed] [Google Scholar]
- Yanagawa S., Tanaka H., Ishimoto A. Identification of a novel mammary cell line-specific enhancer element in the long terminal repeat of mouse mammary tumor virus, which interacts with its hormone-responsive element. J Virol. 1991 Jan;65(1):526–531. doi: 10.1128/jvi.65.1.526-531.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Longmore G., Lodish H. F. Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity. Nature. 1990 Dec 13;348(6302):647–649. doi: 10.1038/348647a0. [DOI] [PubMed] [Google Scholar]
- van Ooyen A. J., Michalides R. J., Nusse R. Structural analysis of a 1.7-kilobase mouse mammary tumor virus-specific RNA. J Virol. 1983 May;46(2):362–370. doi: 10.1128/jvi.46.2.362-370.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



