Abstract
Kallikreins (KLKs) are a family of 15 secreted serine proteases with emerging roles in neurological disease. To illuminate their contributions to the pathophysiology of spinal cord injury (SCI) we evaluated acute through chronic changes in the immunohistochemical appearance of six kallikreins, KLK1, KLK5, KLK6, KLK7, KLK8 and KLK9 in post-mortem human traumatic SCI cases, quantified their RNA expression levels in experimental murine SCI, and assessed the impact of recombinant forms of each enzyme toward murine cortical neurons in vitro. Temporally and spatially distinct changes in kallikrein expression were observed with partially overlapping patterns between human and murine SCI, including peak elevations (or reductions) during the acute and subacute periods. KLK9 showed the most robust changes and remained elevated chronically. Importantly, a subset of kallikreins, KLK1, KLK5, KLK6, KLK7 and KLK9 were shown to be neurotoxic toward primary neurons in vitro. Kallikrein immunoreactivity was also observed in association with swollen axons and retraction bulbs in the human SCI materials examined. Together, these findings demonstrate that elevated levels of a significant subset of kallikreins are positioned to contribute to neurodegenerative changes in cases of CNS trauma and disease and therefore represent new targets for the development of neuroprotective strategies.
Keywords: axonal injury, degeneration, inflammation, neural injury, spinal cord injury
INTRODUCTION
Kallikreins are emerging as important mediators of disease, including neurological conditions such as Alzheimers (1–5), frontotemporal dementia (6), Parkinsons (3, 7), multiple sclerosis (MS) (8, 9) and post-polio syndrome (10). Alterations in proteolytic cascades are also an important component of the response of the CNS to trauma, including SCI in which severely damaging pathophysiological events occur secondary to the primary physical injury (11). These events include a complex cascade of vascular, cellular and chemical responses such as edema, ischemia, inflammation, ionic imbalance, free radical formation, astrogliosis, demyelination, excitotoxicity and apoptosis (12–15). We and others have become particularly interested in the role of extracellular proteolysis in secondary injury since secreted proteases offer great potential for therapeutic targeting.
The inventory of proteases with likely involvement in SCI is long and growing and includes multiple members of the matrix metalloprotease family (16), cysteine proteases such as calpain (17) and serine proteases including thrombolytic enzymes (18, 19), plasminogen activators (20, 21) and tissue kallikreins (22, 23). The current effort focuses on defining the involvement of tissue kallikreins in traumatic SCI, with an emphasis on those which are expressed at significant levels in the CNS (24–26) or which have been linked to pathology in other neurological conditions (6, 8, 27–30). The classical kallikreins have been recognized for some time and include true “tissue kallikrein”, KLK1, which cleaves Lys-bradykinin to liberate kinins that have been implicated in inflammation and tumor cell invasion as well as KLK2 and KLK3 (prostate specific antigen) which are well recognized serum biomarkers of prostate cancer. Over the past decade, 12 additional kallikrein encoding genes were identified alongside these on human chromosome 19q13.4 and together these form the largest contiguous cluster of serine proteases in the human genome (31).
The urgent need to further understand the roles kallikreins play in nervous system injury and disease is highlighted by the growing list of neurological disorders in which kallikrein expression is dynamically regulated (32). For example, elevations in KLK1 occur in stroke (33) and hippocampal sclerosis (34). KLK8 is elevated in the hippocampus in Alzheimers disease (35), while KLK6 is reduced in Alzheimers plaques (1–4), CSF (36) and serum (5). KLK6 and KLK7 are also reduced in the CSF of patients with frontotemporal dementia, while KLK10 is elevated (6). KLK1 and KLK6 are each elevated in the serum of patients with progressive MS, a disease stage characterized by progressive neural injury (9). KLK6 is also localized to Lewy bodies in Parkinsons (7) and is elevated in the CSF of patients with post-polio syndrome (10). Less is known regarding the activity of kallikreins in CNS trauma, although elevations in KLK6 have been reported in human and rodent SCI (22–24) and this protease promotes neurite retraction, neuron degeneration, oligotoxicity and astrogliosis in vitro (8, 9, 28, 30). KLK8 is elevated in a crush model of murine SCI and notably KLK8 deficient mice show reductions in SCI-related axon injury and motor deficits (37).
Despite a growing recognition of the potential involvement of kallikreins in neurological disease, little is known regarding their pathophysiological actions. To begin to address this gap we made a parallel examination of the appearance of six kallikreins in human traumatic SCI and in a murine SCI model and determined the impact of the recombinant forms of each enzyme on neurite stability and neuron survival in vitro. The findings presented point to the involvement of multiple kallikreins in the response of the human spinal cord to injury at acute and chronic time points, with a subset likely to participate directly in neuron injury, including neuron and neurite loss. The results of these studies therefore point to kallikreins as targets for the development of new neuroprotective strategies relevant to traumatic SCI and other neurological disorders.
MATERIALS AND METHODS
Human Spinal Cord Injury Material
To determine the scope of action of kallikreins in human traumatic SCI we made a comprehensive analysis of the immunohistochemical appearance of six kallikreins in non-injured post-mortem human spinal cord material (n=3) and in cases of traumatic SCI ranging from 0 to 7704d post-injury (n=22). Specifically, the SCI cases included were classified as immediate acute (0 to 1d, n=3), acute (>1d to < 14d, median 4d (Range 2 to 8d), n=7), subacute (ɥ 14d to 63d, median 30d (Range 14 to 63d), n=6) and chronic (>1 yr, median 2133d (Range 413 to 7704d), n=6) time points post-traumatic injury (Table 1). These injury intervals correspond to the immediate acute (1d), acute (3, 7), subacute (14, 21, 30d) and what could be considered more chronic time points (60d) examined in murine experimental SCI. A total of 22 post-mortem cases of traumatic SCI were included in these studies with specimens obtained from the Mayo Clinic archives. All cases were confirmed as traumatic involving contusion, compression or transection of the spinal cord by examination of patient records, including autopsy reports. Cases where injury was a result of vascular events or myelopathy of non-traumatic origin were excluded. In addition, the extent of injury in each case was evaluated in hematoxylin and eosin (H&E), in addition to Luxol fast blue/periodic acid Schiff’s (LFB/PAS) stained sections, and only cases in which injury could be clearly ascertained, that is disruption of the spinal cord parenchyma, hemorrhage, inflammatory infiltrates, demyelination and/or cyst-like areas were included in subsequent studies to evaluate kallikrein expression levels. 77% of the SCI cases were male and the median age was 28.2 yr (Range 3.9 to 74 yr). 72.7% of cases were cervical, involving one or more segments C1 to C5 and the remaining cases were thoracic ranging from T1 to T7. 63% of cases were determined to be a result of motor vehicle accident, 14% falls and 14% sports-related injury (diving or go-cart), 4.5% gunshot wound and 4.5% due to unspecified trauma. Three cases with no history of CNS trauma were examined as uninjured-controls (median age 41, range 37–41; 67% male) and included death due to acute leukemia, liver disease or melanoma. The use of human materials in these studies was approved by the Mayo Clinic Institutional Review Board and all clinical follow-up was obtained by review of medical records.
TABLE 1.
Summary of the human SCI and control cases examined
| Case | Age (Yr) | Sex | Group | Injury Interval (d) |
Injury Level |
Cause of Injury |
|---|---|---|---|---|---|---|
| 1 | 29 | F | Imm. Acute | 0 | C1 | MVA |
| 2 | 19.6 | M | Imm. Acute | 0 | C3 | MVA |
| 3 | 35.8 | M | Imm. Acute | 0 | C5 | MVA |
| 4 | 4.4 | M | Acute | 2 | C1 | MVA |
| 5 | 43.8 | F | Acute‡ | 4 | C4 | MVA |
| 6 | 19.8 | M | Acute | 4 | C | MCA |
| 7 | 11.7 | M | Acute‡ | 4 | C2 | Go-cart |
| 8 | 3.9 | F | Acute | 6 | C1 | MVA |
| 9 | 18.4 | F | Acute‡ | 7 | C1–C4 | MVA |
| 10 | 36.9 | M | Acute† | 8 | T3 | MVA |
| 11 | 54.5 | M | Subacute‡ | 14 | T5 | UD |
| 12 | 77.7 | M | Subacute†‡ | 22 | T5 | MVA |
| 13 | 55.4 | M | Subacute† | 30 | T2 | GSW |
| 14 | 78.4 | M | Subacute† | 30 | T7 | Fall |
| 15 | 18.7 | M | Subacute‡ | 37 | C3 | MVA |
| 16 | 39.3 | M | Subacute | 63 | C4 | MVA |
| 17 | 9 | M | Chronic‡ | 413 | C1 | MVA |
| 18 | 65.4 | F | Chronic‡ | 1247 | C5 | Fall |
| 19 | 27.5 | M | Chronic | 1264 | C5 | MVA |
| 20 | 22.3 | M | Chronic | 3001 | C5 | Dive |
| 21 | 46.4 | M | Chronic | 5315 | T4 | Fall |
| 22 | 18.2 | M | Chronic | 7704 | C4 | Dive |
| 23 | 41 | F | Control | N/A | N/A | N/A |
| 24 | 37 | M | Control | N/A | N/A | N/A |
| 25 | 41 | M | Control | N/A | N/A | N/A |
Immediate (Imm.) Acute (0 to 1d); Acute (>1d to < 14d); Subacute (> 14d to 63d); Chronic (>413d to 2133d); MVA (motor vehicle accident); MCA (motorcycle accident); UD (cause of trauma undetermined); N/A (not applicable);
IHC available for white matter only;
IHC examined across two adjacent blocks.
Kallikrein Immunohistochemistry in Human Spinal Cord Injury
Human kallikreins were examined in 5 µm sections obtained from paraffin embedded SCI autopsy material using immunohistochemical techniques as detailed in our prior studies (8, 22, 38, 39). Endogenous peroxidase activity was quenched in de-paraffinized sections by treatment with 0.3% H2O2 in methanol and sections rehydrated through graded ethyl alcohols. Sections were then subject to steam heat-induced antigen retrieval using Antigen Retrieval Citra (BioGenex, San Ramon, CA, USA). Primary antibodies specific for each human kallikrein were applied to tissue sections overnight at 4 °C. KLK1 was detected using a mouse monoclonal antibody M01-H00003816 (Novus Biologicals, Inc. Littleton, CO, USA). KLK5 was detected with an affinity purified rabbit polyclonal antibody PA1-4238 (Thermo Fisher Scientific Inc., Rockford, IL). KLK6 was detected using either a monoclonal antibody (MSP-3-3) or a rabbit polyclonal antibody (KLK6-Rb008) each of which produced identical staining patterns as previously described (22, 28). KLK7 (6, 40) and KLK8 (41) were detected using rabbit polyclonal antibodies generated and validated as we have previously described (26). KLK9 was detected using a rabbit polyclonal antibody PAB-10236 (Orbigen, San Diego, CA, USA). Following incubation with primary antibodies, sections were washed in PBS and species appropriate AffiniPure F(ab’)2 fragment-specific biotin-conjugated secondary antibodies with minimal cross-reactivity to human serum proteins (Jackson ImmunoResearch, West Grove, PA) were applied for 1 hr at RT. Antibody binding was then visualized using HRP-conjugated streptavidin with 3’,3’-diaminobenzadine tetrahydrochloride as the substrate. Processing of tissue sections with primary antibody replaced with normal serum was used to control for non-specific binding of secondary antibodies (42). Parallel sections were stained with H&E to identify areas of tissue injury, LFB/PAS to determine areas of demyelination, Bielschowsky’s silver stain to examine axon integrity and Masson’s Trichrome to visualize collagen. The appearance of GFAP-immunoreactive (-IR) astrocytes (rabbit polyclonal, Z0334, Dako Carpenteria, CA) and CD68-IR macrophages of monocyte or microglial origin (43) (mouse monoclonal, PG-M1, Serotec, Raleigh, NC) were also evaluated in sections parallel to those used to examine kallikrein immunoreactivity.
Semi-Quantitative Assessment of Kallikrein Immunoreactivity in Human Spinal Cord Injury
The immunohistochemical appearance of kallikreins across the post-injury survival intervals examined was evaluated using a well validated semi-quantitative scale that considers both the intensity and relative abundance of immunoreactivity (44, 45). Kallikrein immunoreactivity (KLK-IR) was graded separately in the spinal cord white matter or in the gray matter without knowledge of the kallikrein being considered or the post-injury interval. In a subset of cases, only spinal cord white matter was available for assessment (see Table 1). Our analysis of KLK-IR was focused on the spinal level of injury indicated on the pathology report with tissue injury confirmed in parallel H&E stained sections. Regions of spinal cord pathology were further verified in parallel sections stained for LFB/PAS, Mason’s Trichrome, GFAP or CD68. In some cases a second adjacent block with equivalent injury was also stained and scored as indicated in Table 1. In each case, the region of spinal cord examined and KLK-IR scores were arrived at by consensus by two different observers (MR and IAS) using a multi-headed microscope. Intensity of immunoreactivity in each case was graded on a scale of 0 to 3 (no staining, weak, moderate staining, strong staining) and the percent area stained graded on a scale of 1 to 4, in 25% increments. A grade of 4 was only given if both cells and the extracellular matrix were immunoreactive. An overall immunoreactivity score was tabulated in each case as the product of intensity and percent area stained, with a maximal score of 12 as we and others have previously described (45–47). The mean and standard error of KLK-IR scores at the different post-injury intervals examined was then determined and compared to non-traumatic “uninjured” control cases using a Student’s t-test or the Rank Sum test when data were not normally distributed (47). P < 0.05 was considered statistically significant.
Murine Clip Compression Model of Traumatic Spinal Cord Injury
The potential significance of each kallikrein to traumatic SCI was further evaluated by quantitative real-time RT-PCR analysis of the expression of the murine ortholog in each case in a murine contusion-compression SCI model. Experimental SCI was generated in twelve-week old female (19–23 g) C57BL/6J mice (Jackson Laboratories) using a modified aneurysm clip (FEJOTA™ mouse clip, 8g closing force generating “moderate injury”) (48). This model of SCI includes not only an initial contusion, but also a persistent compression phase that results in microcystic cavitation, degenerating axons, and robust astrogliosis, all hallmarks of human traumatic SCI (49, 50). Prior to compression, mice were deeply anesthetized with Rompun and Ketaset (10 mg/kg) and a laminectomy performed at T9 to T11. Injury was induced at the level of T10 by extradural application of the clip for a period of exactly 1 min, which produces mechanical contusion to both dorsal and ventral aspects of the spinal cord. Before and after surgery, saline was administered subcutaneously to replace lost blood volume. Pain was minimized by administration of Buprenorphine (0.05 mg/kg, Hospira, Lake Forest, IL) subcutaneously every 12 hr for 96 hr post-surgery. To avoid infection, Baytril (10mg/kg, Bayer Health Care, Shawnee Mission, KS) was administered intraperitoneally in a prophylactic fashion for the first 72 hr post-surgery. Bladders were manually voided twice daily even after spontaneous recovery of function until the endpoint of each experiment. Any mice with signs of infection or which were moribund were immediately euthanized and not included in the study. All animal experiments were carried out with careful attention to animal comfort and in strict adherence to NIH Guidelines for animal care and safety and were approved by the Mayo Clinic Institutional Animal Care and Use Committee.
To determine immediate acute (1d), acute (3, 7d), subacute (14, 21, 30d), and more chronic (60d) compression related changes in kallikrein RNA expression, mice were allowed to recover to each of experimental endpoint (n=3 per time point, except 14d n=4). Mice receiving surgery on a given day were randomized across two or more endpoints. In each case, the level of injury, as well as one segment above and below, was collected into a single tube and snap frozen prior to RNA isolation. Mice were evaluated across seven categories of locomotor recovery using the Basso Mouse Scale the day after surgery and weekly thereafter until experimental endpoints were reached (51). Age and gender matched uninjured mice served as controls. Due to the focus of these studies on a limited amount of spinal cord tissue at the injury epicenter, samples from at least 3 mice at a given time point were pooled to facilitate RNA isolation (52). RNA was isolated using RNA STAT-60 (Tel-Test, Friendswood, TX) and stored at −80 °C until the time of analysis.
Quantitative Assessment of Murine Kallikrein Expression in Response to Compression Injury
Expression levels of the murine orthologs of human kallikreins 1, 5, 6, 7, 8 and 9 were determined by real-time RT-PCR using primers specific to each kallikrein and designed to span at least two exons (Table 2). According to the new nomenclature, non-human forms of each kallikrein are designated in lower case (Klk) (53). Accuracy of amplification was verified by analysis of melting peaks. An estimate of the overall abundance of each kallikrein RNA was made based on a standard curve created by amplification of plasmids containing the gene of interest serially diluted to known copy number in the same PCR experiment. At least 3 individual SCI epicenters at each time point were pooled (52) and 0.25 µg examined in triplicate by real time RT-PCR, with control samples and all 7 time points post-injury examined in the same PCR run. A no RNA control was included in every PCR experiment to exclude reagent contamination. The copy number of each kallikrein determined in control and injured spinal cord was normalized to the constitutively expressed gene glyceraldehyde phosphate dehydrogenase (GAPDH) amplified in parallel and the data were expressed as a percent of uninjured control (23, 25, 30, 54, 55).
TABLE 2.
Summary of primers used for quantitative real time PCR analysis of murine kallikreins (Klk), GFAP, myelin genes (MBP and PLP) and pro-inflammatory cytokines (IL-6, TNFα, IL1β).
| Accession Number |
Base Pairs |
Forward Primer | Reverse Primer |
|---|---|---|---|
| mKlk1 NM_010639 | 363–471 | CAGGAGATATGGATGGAGGCAAAGA | CCCGGCACATTGGGTTTAC |
| mKlk5 NM_026806.2 | 854–1007 | GCGGAACAGACCAGGTGTCTA | CTTCAGCATTCTCAGAGAGGG |
| mKlk6 NM_011177.1 | 749–949 | CCTACCCTGGCAAGATCA | GGATCCATCTGATATGAGTGC |
| mKlk7 NM_011872.2 | 143–245 | ACCGCTGGACAAGGAGAAA | CTCCACAGTGAAGCTGATTGC |
| mKlk8 NM_008940.2 | 1004–1236 | GTGCGGAAGTGAAAATCTAT | GGTAGTGTAGCGGCAG |
| mKlk9 NM_028660.3 | 766–884 | AGGATCTGAGCCTTGT | TCATGCTAAGATGGACAGAC |
| GFAP NM_010277.2 | 600–767 | GCAGATGAAGCCACCCTGG | GAGGTCTGGCTTGGCCAC |
| PLP NM_011123.2 | 377–537 | TCTTTGGCGACTACAAGACCAC | CACAAACTTGTCGGGATGTCCTA |
| IL-6 NM_031168.1 | Amplicon Length: 78 | The assay probe spans Exon Boundary: 2–3 (Applied Biosytems) | Assay ID: Mm00446190_m1 |
| TNFα NM_013693.2 | Amplicon Length: 81 | The assay probe spans Exon Boundary: 1–2 (Applied Biosytems) | Assay ID: Mm00443258_m1 |
| IL-1β NM_008361.3 | 110–224 | TGACAGTGATGAGAATGACCTG Probe: /56-FAM/AGT TGA CGG /ZEN/ACC CCA AAA GAT GAA GG/3IABkFQ/(IDT) | CGAGATTTGAAGCTGGATGC Assay ID: Mm.PT.51.17212823 |
| GAPDH NM_008084.2 | 351–584 | ACCACCATGGAGAAGGC | GGCATGGACTGTGGTCATGA |
Cortical Neuron Culture
To determine the potential contribution of KLK1, KLK5, KLK6, KLK7, KLK8 and KLK9 to neural injury and their likely impact on the microenvironment available for neural repair we examined the effects of the recombinant forms of each enzyme on neurite stability and neuron survival in vitro. In addition to these kallikreins already linked to the CNS (25), we also examined 3 kallikreins (KLK2, KLK3 and KLK13) not densely expressed in the CNS, but which extravasate across a damaged blood brain barrier. Murine cortical neurons were isolated from embryonic day 16 C57BL6/J mice and plated at a density of 2 × 103 cells/mm2 on poly-L-lysine (10 µg/ml) coated 12 mm2 glass cover slips. Cortical neurons were grown in defined media consisting of Neurobasal A media supplemented with 2% B27 supplement, 2mM Glutamax, 0.45% glucose and 50 U/ml penicillin-streptomycin (Invitrogen, Carlsbad, CA) (9). Neurons were allowed to mature for 96 hr in vitro and then treated with recombinant human kallikreins (10 µg/ml, 300 nM) for an additional 24 hr prior to fixation with 2% paraformalydehyde. Neurons were stained for neurofilament H protein (rabbit polyclonal, AHP245 Serotec) using standard avidin-biotin histochemistry as described above.
All human kallikreins were expressed using a baculovirus expression system and subsequently purified and activated as previously described in detail (30, 54, 56–60). 300 nM of KLK1 or KLK6 were previously shown to promote a dying back of cortical neurites in prior studies (9) and therefore, here we examined KLKs 2, 3, 5, 7, 8, 9 and 13 at parallel concentrations. This concentration is designed to be reflective of the elevated levels of kallikreins seen in SCI materials (25, 61) and is approximately 5-fold higher than the level of KLK6 detected in human CSF (26, 62).
Changes in the number of cortical neurons or neurite density in response to recombinant kallikrein were quantified from 5 digitally captured 20× microscopic fields encompassing the center and 4 poles of each cover slip, taken without knowledge of the treatment groups. Digital images were then used to make neuron counts. Neurite number was evaluated by superimposing a grid consisting of 967.5 µm2 squares on to each digital image and counting processes which overlapped grid lines (8). Histograms shown represent the mean and standard error (s.e.) of counts across three independent cover slips treated in parallel within a given experiment and results were reproducible across at least 3 experiments utilizing separate murine cortical neuron preparations. Statistical differences between groups were compared using One Way ANOVA with the Student Newman Keul’s (SNK) post hoc test, with P < 0.05 considered statistically significant.
RESULTS
Regulated Expression of Kallikreins in Human Traumatic Spinal Cord Injury
KLK Expression in the Uninjured Human Spinal Cord
In the uninjured adult human spinal cord, overall levels of kallikrein-IR were highest in the case of KLK6, followed by KLK5, KLK7, KLK9, KLK1 and KLK8 (Figs. 1, 2). Consistent with previous reports, in white matter KLK6-IR was dense in glia with the cytological appearance of oligodendroglia that is round cell bodies, a paucity of cytoplasm and processes encircling axons (Fig. 2B) (38, 39). KLK1 could also be identified in glia with a round nucleus characteristic of oligodendroglia, but was more prominent in those with a triangular shape and tapering processes resembling astrocytes in parallel GFAP-immunostained sections. KLK5, KLK7 and KLK9 were also prominent in astroglial-like cells. KLK8-IR was low when observed in white matter glia of the uninjured spinal cord.
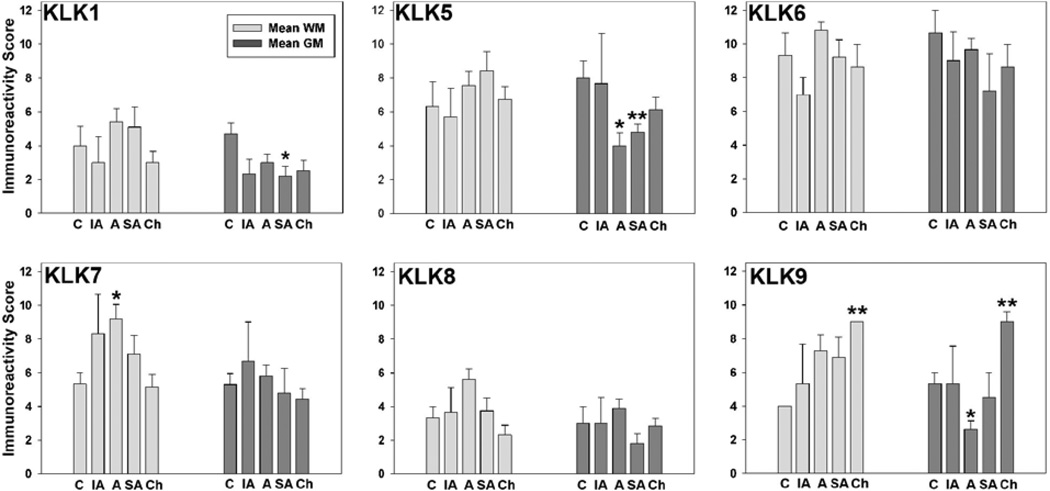
FIGURE 1. KLK immunoreactivity scores in the intact and traumatically injured human spinal cord.
Histograms show changes in kallikrein immunoreactivity (IR) scores seen in the uninjured spinal cord (C) and at immediate acute (IA, 0 to 1d), acute (A, >1d to < 14d), subacute (SA, > 14d to 63d), and chronic (Ch ≥ 413d to 2133d) time points post-injury. The most widespread changes in KLK-IR occurred during the acute and subacute periods, including significant elevations in KLK7 in white matter (*P = 0.04, Student’s t-test) and significant reductions in KLK1 (*P = 0.04, Student’s t-test), KLK5 (*P = 0.03, Rank Sum test (Acute); **P = 0.02, Student’s t-test (Subacute), and KLK9 (*P = 0.04, Rank Sum test (Acute) in gray matter. KLK9 was significantly elevated in both spinal cord white matter and gray matter at the chronic time points examined (**P = 0.01, Student’s t-test). Photomicrographs showing representative KLK-IR staining patterns in the uninjured spinal cord (Fig. 2) and at immediate acute (Fig. 3), acute (Fig. 4), subacute (Fig. 5, 6); and chronic (Fig. 7) time points are provided. Also, see Fig. 8 for comparison of changes in KLK expression in human SCI with those occurring in a murine experimental contusion-compression SCI model.
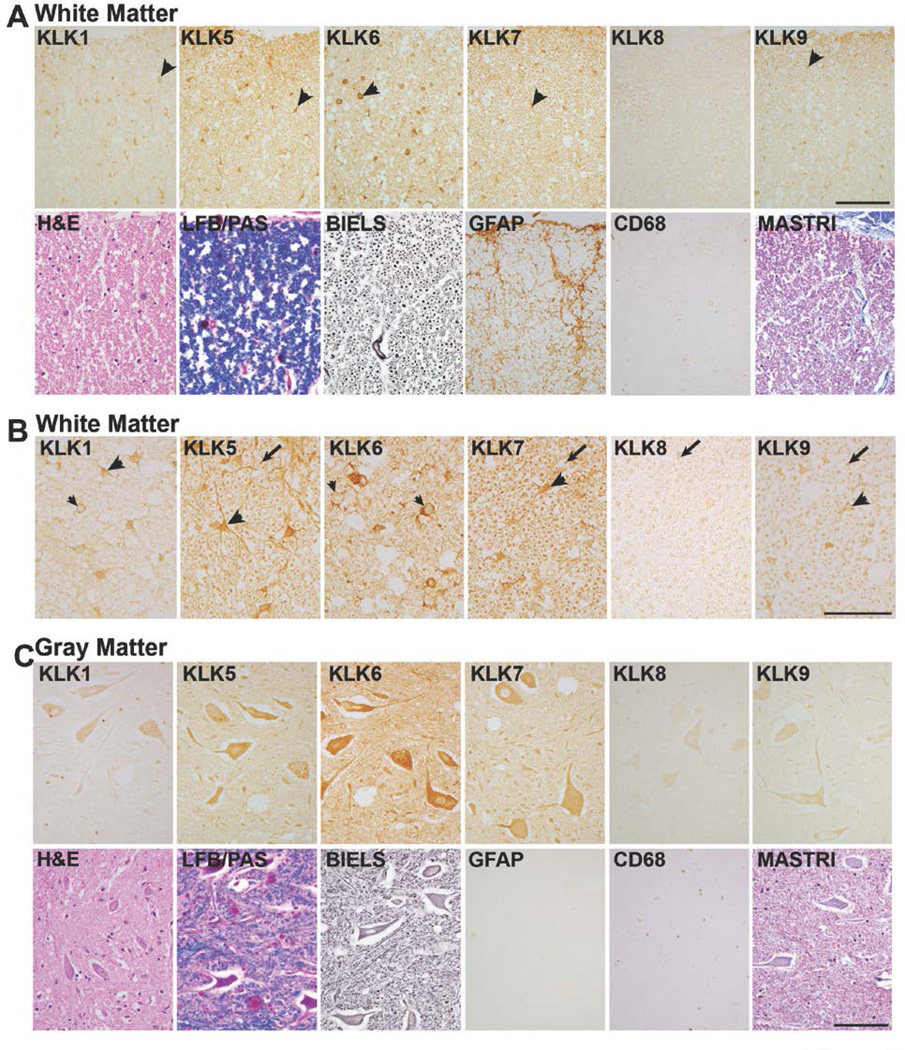
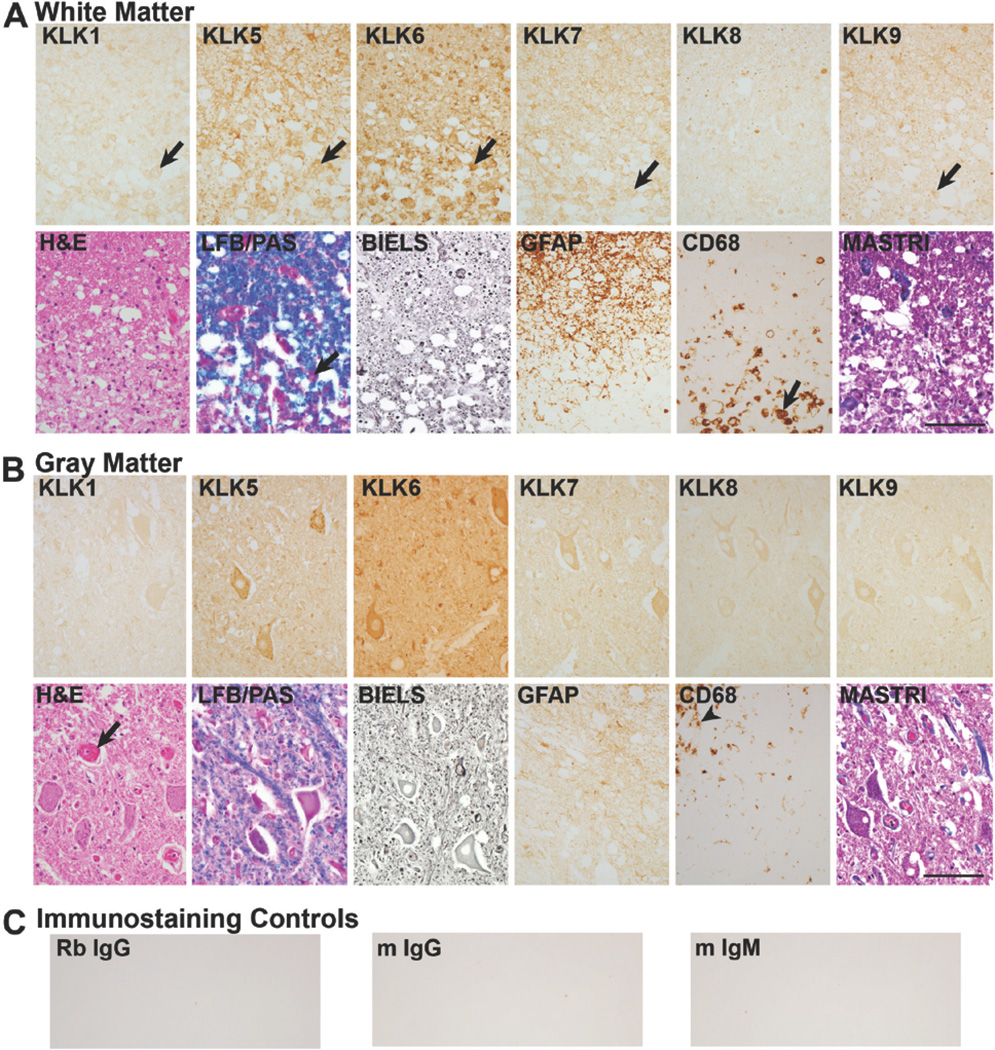
FIGURE 2. Differential expression of kallikreins in the white and gray matter of the uninjured human spinal cord.
A, In spinal cord white matter, KLKs 1, 5, 6, 7 and 9 were localized to glia (arrow heads, A). B, Higher power photomicrographs show localization of KLKs 1, 5, 7 and 9 in glia with a triangular shape resembling astrocytes (arrow heads). KLK6 predominated in glia with a round nucleus and thin rim of cytoplasm resembling oligodendroglia (small arrow heads). KLK1 was also seen a few oligodendrocyte-like cells (small arrow head). KLKs 5, 7, 8 and 9 were also localized to white matter axons (arrows). C, In spinal cord gray matter (ventral horn shown), each kallikrein was detected in neurons with highest levels of KLK6, 5 and 7 followed by KLK1 and KLK9 and finally, KLK8. Comparison of KLK-IR scores in the uninjured and injured spinal cord is provided in Fig. 1. Parallel sections were stained with H&E, LFB/PAS, Bielschowsky’ silver stain (BIELS) and Masson’s Trichrome (MASTRI), in addition to antibodies recognizing GFAP and CD68. GFAP-IR cells and processes were identified in uninjured spinal cord white matter, but were very low in uninjured gray matter. CD68-IR monocytes/microglia were very sparse or absent in both the white and gray matter of the uninjured spinal cord. The cervical spinal cord from a case in which death was due to acute leukemia is shown (Scale bar = 100 µm A and C; 50 µm B).
At least a subset of kallikreins is known to be expressed by neurons (24, 39, 63). Here we document expression of KLK6, KLK5, KLK7, KLK1, KLK9, and KLK8, listed in the relative order of abundance, in neurons across the dorsal and ventral horns of the uninjured adult spinal cord, with prominent expression in ventral horn motoneurons as seen in Fig. 2C. KLK5, KLK7, KLK9 and at least low levels of KLK8 were also readily observed in association with axons in uninjured spinal cord white matter (Fig. 2B).
KLK Expression in Human Spinal Cord White Matter after Traumatic Injury
Overall, in the human traumatic SCI cases examined there was a trend towards elevations in kallikrein expression in spinal cord white matter, although these elevations only reached the level of statistical significance in the case of KLK7 and KLK9 (Figs. 1, 3A, 4A, 5A, 6A, B, 7A). In the first 24 hr after injury (0–1d, immediate acute), KLK7- and KLK9-IR showed minor elevations in white matter, otherwise either no change, or minor decreases in kallikrein expression were observed (Fig. 1, Fig. 3). In the acutely (>1d <14d) injured spinal cord white matter, elevated levels of immunoreactivity were detected for each KLK, with elevations in KLK7-IR reaching the level of statistical significance (Student’s t-test, P=0.04, Figs. 1, 4). In the subacute (≥14d to 63d) cases examined, overall levels of immunoreactivity for KLKs 1, 5, 7 and 9 remained elevated relative to the uninjured spinal cord, although the overall changes observed did not reach the level of statistical significance (Figs. 1, 5, 6). Swollen axons and retraction bulbs (64, 65) were immunoreactive for each KLK in the later subacute cases examined, including KLK1 and KLK6 which were not apparent in association with axons of the intact spinal cord (Figs. 2B, 6B). The distribution of axons was verified in parallel sections stained by Bielschowsky's silver stain. At chronic post-injury intervals, only KLK9-IR remained significantly elevated in spinal cord white matter (Student’s t-test, P=0.02) while the other kallikreins examined were unchanged or slightly lower than that observed in the uninjured spinal cord (Figs. 1, 7). When primary antibodies were replaced with normal serum, immunoreactivity scores for either uninjured or traumatically injured spinal cord specimens were 0, reflecting the absence of staining (Fig. 5C).
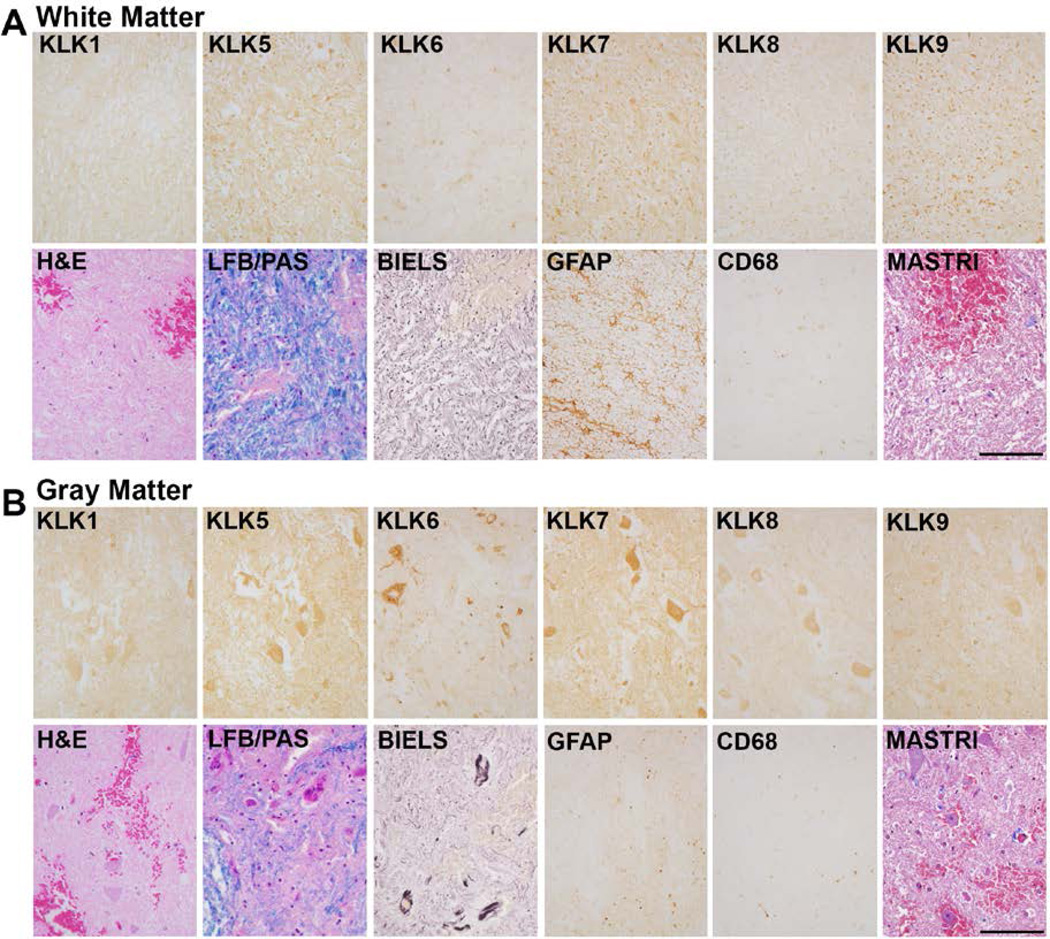
FIGURE 3. Expression of kallikreins in the immediate acute period after traumatic SCI.
A, In the immediate acute period after SCI (0–24 hr), minor elevations in KLK7 and KLK9 were seen in spinal cord white matter while levels of the other kallikreins examined were unchanged or decreased relative to those observed in the uninjured spinal cord (Fig. 1, 2). Similarly, very minor changes in kallikrein-IR were observed in spinal cord gray matter (B) in the immediate acute period (Fig. 1). Parallel sections were stained with H&E, LFB/PAS, Bielschowsky’ silver stain (BIELS) and Masson’s Trichrome (MASTRI), in addition to antibodies recognizing GFAP and CD68. Levels of GFAP-IR and CD68 in spinal cord gray and white matter in parallel sections were similar to those observed in the uninjured cases examined and patches of petechial hemorrhage were obvious in both H&E and Masson’s Trichrome (MASTRI) stained sections. LFB/PAS and Bielschowsky’s silver stain (BIELS) are also shown. Case shown is C1 fracture dislocation as a result of a motor vehicle accident (Scale bar = 100 µm).
FIGURE 4. Expression of kallikreins in the acutely injured human spinal cord white and gray matter.
A, At acute time points after traumatic injury (4d shown), elevations were observed in each of the kallikreins examined in white matter and there each was associated with glial-like cells (arrow heads, A). Elevations in white matter KLK7 were statistically significant (P = 0.04, Student’s t-test, see Fig. 1). B, Overall, KLK-IR in the gray matter at acute time points showed either no change (KLK6 and KLK7) or decreases in immunoreactivity relative to uninjured spinal cord (Fig. 2). Reductions in gray matter KLK5 and KLK9 were statistically significant (P ≤ 0.04, Rank Sum test, see Fig. 1). Cytological and histopathological signs of injury evaluated in parallel sections showed patches of petechial hemorrhage in H&E and Masson’s Trichrome (MASTRI) stained sections. Overall levels of GFAP and CD68-IR in the case shown were similar to controls although elevations were evident in other acute cases examined, defined as >1d to <14d after injury. LFB/PAS and Bielschowsky’s silver stain (BIELS) are also shown. Case shown is C4 fracture dislocation as a result of a motor vehicle accident (Scale bar = 100 µm).
FIGURE 5. Expression of kallikreins in a case of subacute traumatic SCI.
A, At subacute time points after traumatic injury (14d shown), elevations in KLK1, KLK5, KLK7 and KLK9 were observed in white matter, although these did not reach the level of statistical significance across the cases examined. Increases in CD68-IR monocytes/microglia were prevalent in white matter at subacute time points, which were either densely (KLK6), moderately (KLKs 5 and 7) or lightly (KLKs 1 and 9) KLK-IR in near adjacent sections (arrows, A). Inflammatory cells in white matter were also distinguished in LFB/PAS stained sections (arrows). Very clear increases in GFAP-IR were also observed in white matter at subacute time points. B, Overall levels of KLK1 and KLK5 were significantly reduced in spinal cord gray matter across the subacute cases examined (P ≤ 0.04, Student’s t-test, see Fig. 1). The cytoplasm of some anterior horn cells was associated with hypereosinophilia in parallel sections (arrow, B) and CD68 positive monocytes/microglia (arrow heads, B) were also observed in gray matter at subacute time points. C, Panels show the level of immunoreactivity achieved in the injured spinal cord when primary antibodies were replaced with normal serum and tissue processed with either rabbit IgG, or with mouse IgG or IgM secondary antibodies alone. Case shown is a T5 fracture dislocation, paraplegia. (Scale bar = 100 µm).
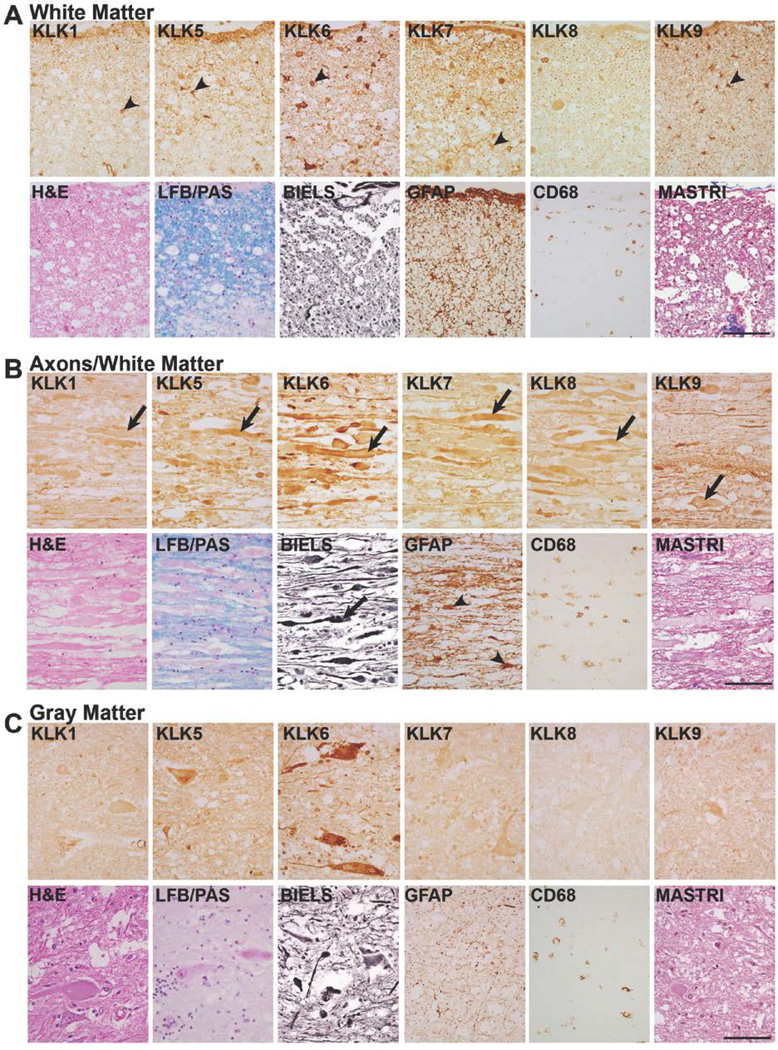
FIGURE 6. Elevated levels of kallikrein expression by axons in traumatic SCI.
A, In subacute cases (63d shown), KLKs 1, 5, 6, 7 and 9 were associated not only with white matter glia (arrow heads, A), but along with KLK8 each was also clearly identified in swollen axons and axon ovoids seen in longitudinal sections taken from the same case (arrows, B). These injured/degenerating and swollen axons were also apparent in the near adjacent Bielschowsky’s silver stained (BIELS) sections (arrows). Prominent GFAP-IR, including hypertrophic astrocytes (arrow heads, B) and CD68-IR monocytes were also seen in association with injured axons in white matter. C, Shows the overall reductions in KLK1 and KLK5 seen in gray matter during the subacute injury interval. GFAP-IR in gray matter was elevated relative to controls and CD68-IR monocytes/microglia were also evident. At this time point, numerous examples of explicit loss of LFB staining were seen both in the white and gray matter (see Fig. 1). Case shown is a C4 fracture dislocation as a result of a motor vehicle accident resulting in quadriplegia. (Scale bar = 100 µm).
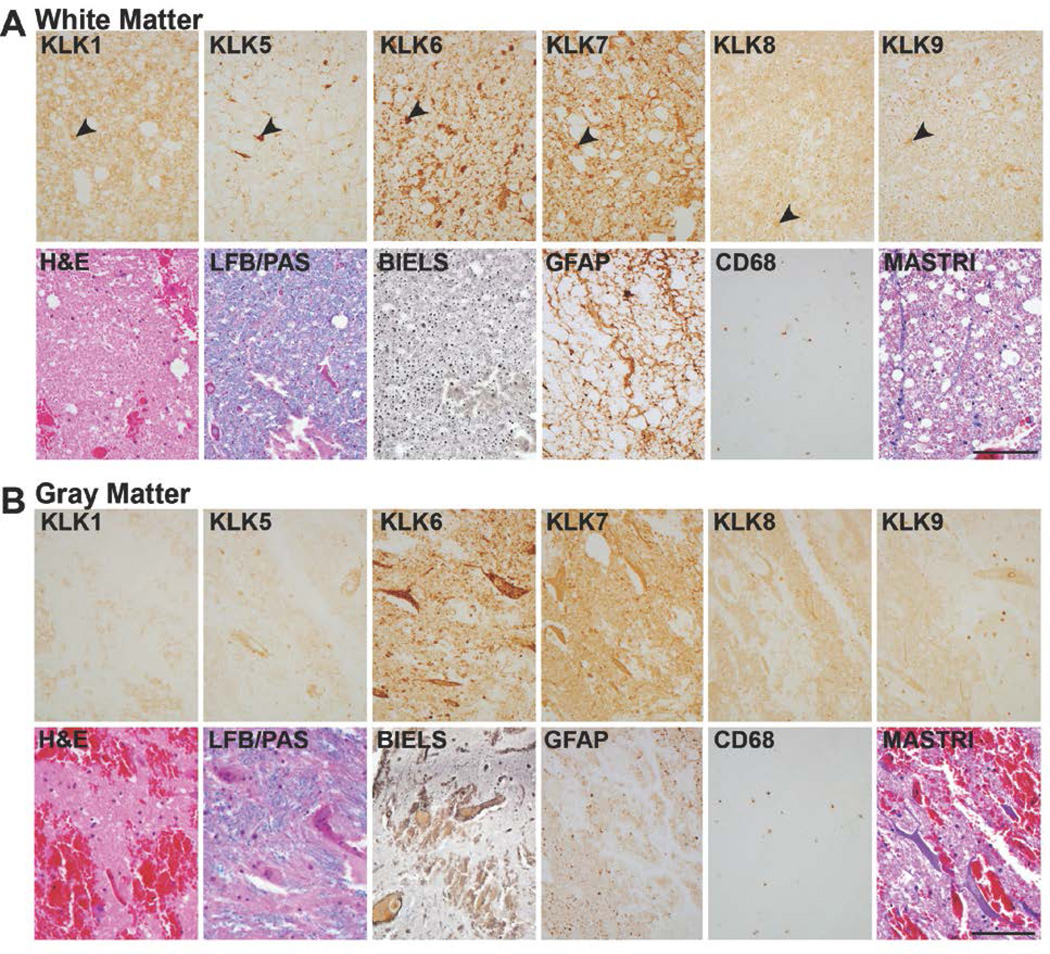
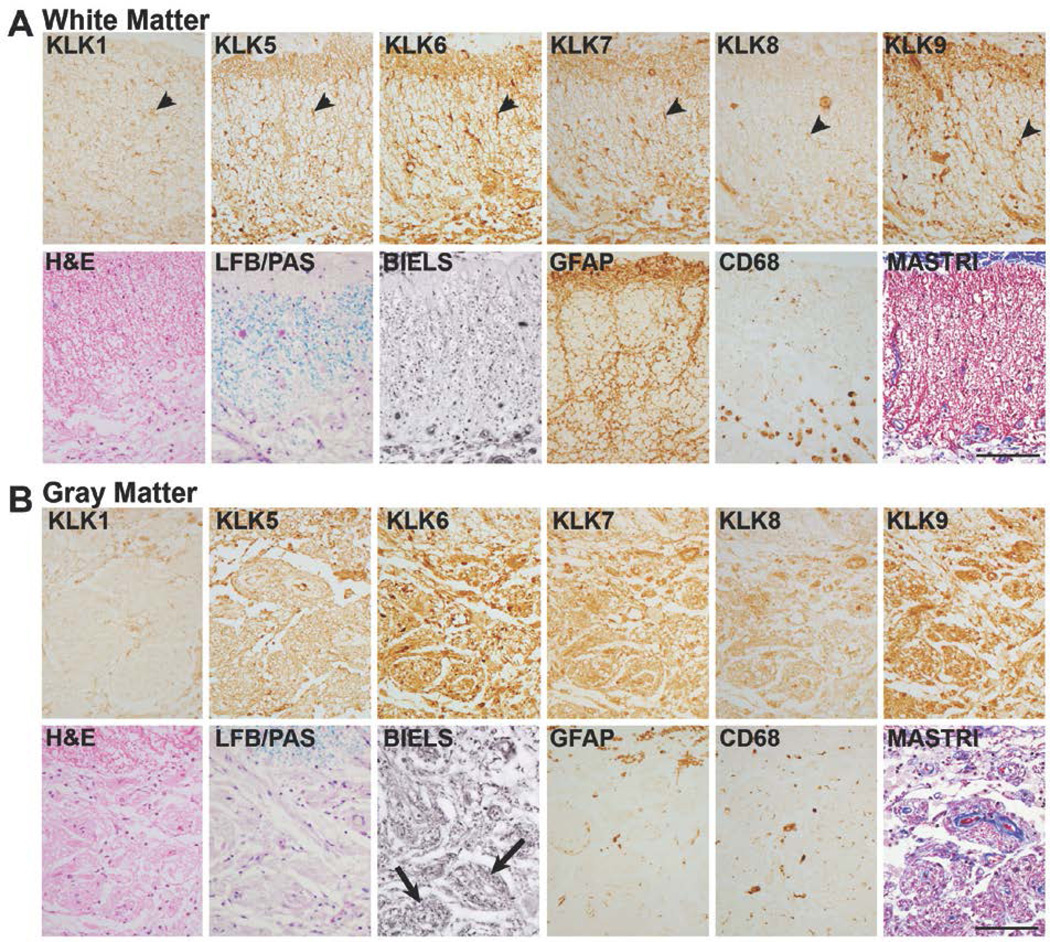
FIGURE 7. Expression of kallikreins in chronic SCI.
A, At chronic time points after injury (1247d shown), overall levels of KLK9 were significantly elevated in spinal cord white matter (P = 0.02, Rank Sum test) and in gray matter (P = 0.01, Student’s t-test), see Fig. 1. Changes in expression of the other kallikreins were also observed particularly in the cellular distribution of immunoreactivity in each case, although overall changes IR levels were not statistically significant. At this level of injury, the central core of gray matter was reorganized with some nerve fibers arranged in a fascicular fashion (arrows, B, BIELS). The density of LFB staining was reduced in both white and gray matter compared to uninjured controls (see Fig. 1). GFAP-IR and CD68-IR continued to be elevated in white matter even at very chronic stages. KLK1, 5, 6, 7 and 9 continued to be clearly associated with white matter glia, and KLK8-IR glia were also occasionally seen (arrow heads, A). Masson’s trichrome (MASTRI) was used to examine collagen. In both white and gray matter, blue stained collagen fibers were largely confined to blood vessels. Case shown is a C5 injury due to fall. (Scale bar = 100 µm).
KLK Expression in Human Spinal Cord Gray Matter after Traumatic Injury
In the gray matter of the injured spinal cord, there was an overall decrease in immunoreactivity for KLK1 and KLK5 over the time course examined with levels remaining below baseline in the most chronic cases examined (Figs. 1, 3B, 4B, 5B, 6C, 7B). Decreases in KLK1-IR in injured spinal cord gray matter were statistically significant at subacute time points (Student’s t-test, P=0.04), while decreases in KLK5-IR were statistically significant at both acute (Rank Sum test P=0.03) and subacute (Student’s t-test, P=0.02) time points. KLK9-IR was significantly reduced in the injured spinal cord gray matter at acute time points (Rank Sum test, P=0.04), but elevated by more than two-fold at chronic time points post-injury (Student’s t-test, P=0.01, Figs. 1, 7B).
KLK-Immunoreactivity in Astrocyte-Like Cells in Human SCI
Spinal cord trauma-related changes in KLK-IR observed from acute through chronic time points could be related to co-ordinate changes in the cellular hallmarks of neural injury, including the appearance of GFAP-IR astrocytes. In the uninjured cases examined, moderate levels of GFAP-IR were observed in white matter, while levels of GFAP were very low or absent in gray matter (Fig. 2). As expected (66), increases in GFAP-IR cells and processes were observed from the acute through the most chronic time points examined (Figs. 4 to 7). In the cases examined in this study, increases in GFAP-IR predominated in spinal cord white matter, although clear increases in gray matter were observed in some cases. Each KLK was elevated in astrocyte-like cells over that detected at baseline, with KLK5 being more dense than KLK7 followed by KLK9, KLK6, KLK1 and KLK8 (Figs. 2 to 7). KLK5-, KLK7- and KLK9-IR appeared in many cases to be co-extensive with that for GFAP-IR, particularly in white matter at chronic stages after injury (Figs. 6A, 7A). The most prominent staining for KLKs in astrocyte-like cells occurred in the later subacute and chronic SCI cases examined (> 63d to 7704d, Figs. 6A, 7A).
KLK-Immunoreactivity in Macrophage-Like Cells in Human SCI
Macrophages, derived from hematogenous monocytes and resident microglia are reactive for the CD68 antigen and play important roles in the response of the spinal cord to injury (43, 67–69), including involvement in cytotoxicity as well as the wound healing process. In the uninjured spinal cord and within the first 24 hr after injury (immediate acute), CD68-IR cells and processes were largely absent or extremely sparse (Figs. 2, 3) (69). Increases in CD68-IR associated with clearly defined cell bodies was seen in the acute cases examined (1d to 14d, Fig. 4), but was most prominent at subacute (≥14d) time points when staining included larger rounded macrophages as well as smaller densely CD68-IR cells with short processes, likely ramified microglia (Figs. 5 and 6). Monocytes/microglia immunoreactive for CD68 persisted in many cases out to the late chronic time points examined (Fig. 7). At subacute time points (Fig. 5), KLKs 6, 5 and 7 were prominent in rounded-macrophage-like cells prominent in the injured white matter, while KLK1 and KLK9 were seen at lower levels (Figs. 5).
Regulated Expression of Kallikreins in Murine Experimental SCI
The murine orthologs of each human kallikrein were examined in an experimental murine SCI model using quantitative real time RT-PCR. We note that antibodies specific to each of the murine kallikreins studied herein are not currently available. As expected based on our prior studies regarding the expression of Klk6 in rodent brain and spinal cord (22, 24) and kallikrein RNA and protein expression patterns documented in the human spinal cord (Fig. 1) (25, 26), of all the kallikreins examined Klk6 was by far the most abundant (Fig. 8). In the uninjured murine spinal cord, Klk6 RNA was detected at 4.2 × 107 ± 2.7 × 106 copies in 0.25 µg of RNA. Klk1 was the next most abundant of those examined, being detected at 5.2 × 103 ± 1.6 × 102 copies in a parallel amount of RNA. Significantly lower levels of each of the other kallikreins examined were observed in the uninjured spinal cord, each at approximately 102 copies in 0.25 µg of RNA with Klk9 being more abundant than Klk5, followed by Klk7 and Klk8.
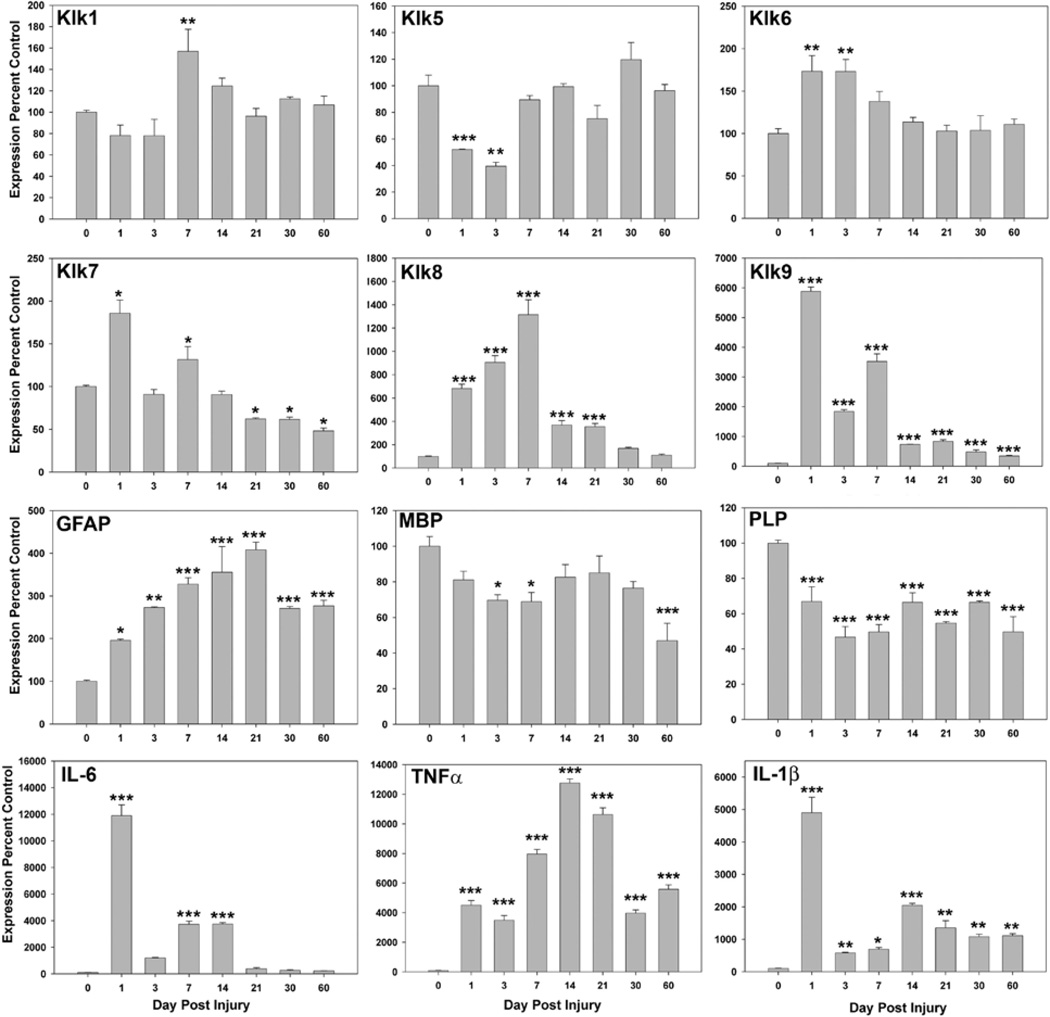
FIGURE 8. Experimental murine traumatic SCI promotes changes in kallikrein gene expression that parallel those seen at a protein level in the case of human SCI.
Histograms show the change in kallikrein RNA expression seen in response to clip-compression SCI in mice at immediate acute (1d), acute (3, 7d), subacute (14, 21, 30d) and more chronic (60d) time points after compression injury, relative to that seen in the uninjured spinal cord (n=3 at each time point, except 14d n=4). Samples at each time point were combined and examined by PCR in triplicate. Co-ordinate changes in the expression of GFAP, myelin genes (MBP and PLP) and the pro-inflammatory cytokines IL-6, TNFα and IL-1β are also provided. RNA expression levels in each case were normalized to GAPDH to control for loading and are expressed as a percent control (mean and s.e. shown, * P < 0.05, ** P ≤ 0.01, *** P ≤ 0.001, SNK).
The expression of Klk1, Klk5, Klk6, Klk7, Klk8 and Klk9 RNA were each dynamically regulated in the murine spinal cord in response to experimental compression injury over the 60d post-injury interval examined (Fig. 8). Transcriptional alterations in each kallikrein showed clear differences in direction, timing and magnitude, although examination of RNA from whole spinal cord preparations does not permit us to comment on any changes specific to gray or white mater. Overall, increased levels of expression were the predominant pattern observed. Increases in RNA expression were observed in the case of Klk1, KLlk6, Klk7, Klk8 and Klk9 (P < 0.05 SNK) and in each case this reached the highest level by 7d after injury. The magnitude of the change in RNA expression from baseline was most robust in the case of Klk8 and Klk9, each of which reached from 10- to more than 1 × 103-fold base line levels during the first 7d after injury (P ≤ 0.001, SNK). Klk8 expression remained significantly elevated through day 21 after injury, but only Klk9 continued to be elevated at the 30 and 60d endpoints examined (P ≤ 0.001, SNK). By contrast, Klk5 RNA expression showed significant decreases at 1 and 3d after injury (P < 0.002 SNK). After transient increases at 1 and 7d after injury, Klk7 also showed significant decreases in RNA expression at 21 through the 60d endpoint examined (P < 0.05 SNK).
To facilitate interpretation of the changes in kallikrein expression in terms of other experimental SCI models, we evaluated Basso Mouse Scale scores at 1d after injury and weekly thereafter. Functional deficits reflected in BMS scores induced by the 8 g Fejota clip contusion compression model were largely parallel to those seen in BL6 mice in prior studies in which a moderate injury was induced by the Infinite Horizon or Ohio State University impactor model (51). BMS scores (mean ± s.e.) at each endpoint examined were: 1d 1 ± 0.5; 3d 3 ± 0.3; 7d 2 ± 0.7; 14d 2.9 ± 1; 21d 3.7 ± 0.4; 30d 4 ± 1.1; and 60d 5 ± 1.
To put the changes in kallikrein RNA expression into the context of well characterized pathogenic events associated with SCI, levels of GFAP, MBP, PLP and the pro-inflammatory cytokines (IL-6, TNFα and IL-1β) were examined in the same RNA preparations (Fig. 8). Alterations in kallikrein expression were paralleled by significant elevations in IL-6, which showed a 12 × 103-fold increase at 1d and remained 4 × 103-fold elevated through 14d post injury (P < 0.001, SNK, Fig. 7). Another pro-inflammatory cytokine, TNFα, was also significantly elevated by 1d (4.5 × 103-fold elevated), peaked at day 14 (12.8 × 103-fold elevated) and remained elevated out to the most chronic 60d endpoint examined (5.6 × 103-fold elevated, P ≤ 0.001, SNK). Peak elevations in IL-1β were also observed at 1d 49-fold elevated P < 0.001), showed a second peak in expression at 14d 20-fold elevated, P < 0.001) and remained significantly elevated at the 60d time point 10-fold, P = 0.01). As expected, the murine contusion-compression model examined also triggered robust and sustained elevations in GFAP RNA expression, which were observed as early as 1d (2-fold) and reached a peak of 4-fold baseline levels at 21d (P ≤ 0.013, SNK). Significant elevations in GFAP RNA expression persisted through 60d (P ≤ 0.001, SNK). Across the same time points (1–60d) there was a significant decrease in PLP RNA expression, which remained at approximately 50% below baseline levels from the acute through chronic time points examined (P ≤ 0.001, SNK). In parallel, MBP RNA levels were also reduced at 3 and 7d post-injury (P ≤ 0.04, SNK), but returned to uninjured levels on days 14 through 30. By 60d however, MBP RNA levels were again significantly reduced reaching approximately 50% of that observed at baseline (P ≤ 0.001, SNK).
Kallikrein-Elicited Neural Injury In Vitro
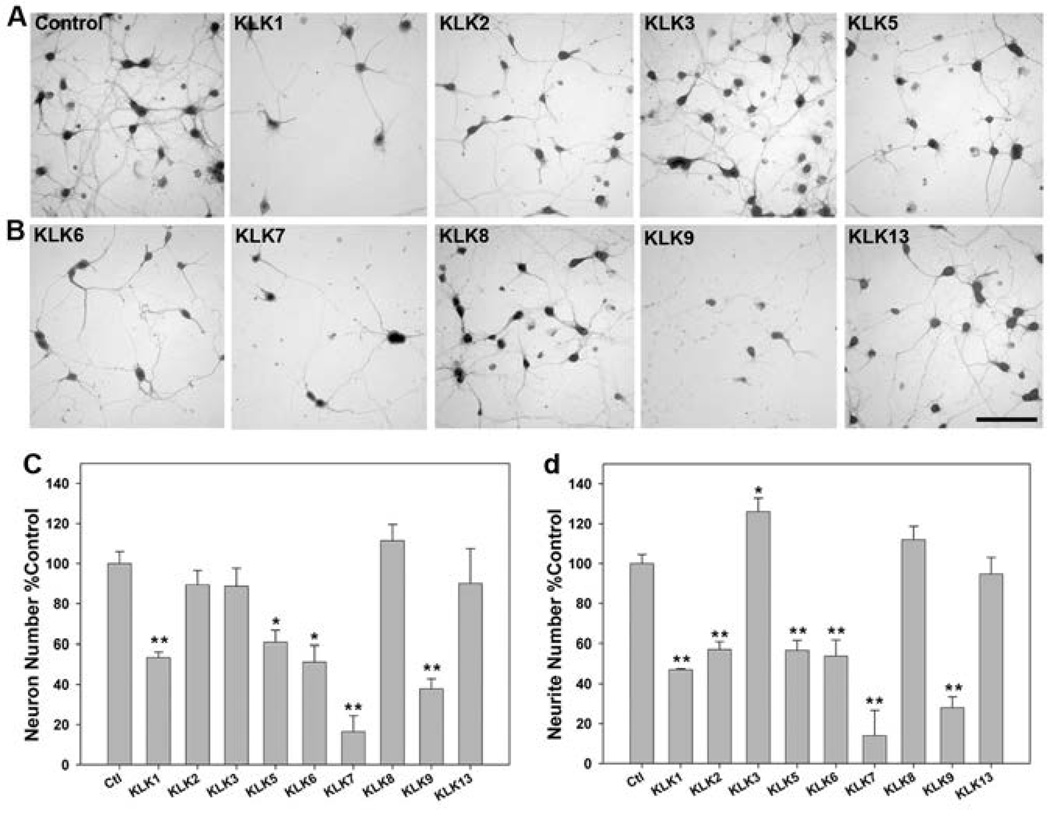
Given the abundance of several kallikreins across the spinal cord white and gray matter, differential regulation in cases of traumatic SCI (Figs. 1–8), and potential to be involved in kallikrein-specific activation cascades (57, 59) we examined the effects of elevated levels of the recombinant forms of each enzyme, in addition to KLKs 2, 3 and 13 on neuron number and neurite integrity of mature murine cortical neurons in vitro (Fig. 9). KLKs 1, 5, 6, 7 and 9 each promoted significant loss of neurons after 24 hr treatment in vitro (P ≤ 0.03, SNK) and these kallikreins, in addition to KLK2, significantly reduced neurite numbers (P ≤ 0.001, SNK, Fig. 8). By contrast, KLK3, KLK8 and KLK13 did not promote significant neural injury-related changes in vitro. Indeed, there was significant increase in neuron process density in KLK3 treated cultures (P = 0.04, SNK). KLK-induced neural injury was most dramatic in the case of KLK7 and KLK9, each of which reduced neuron survival by 60% or more (P < 0.001, SNK). KLK1, KLK5 and KLK6 reduced neuron survival by approximately 40% relative to control cultures (P ≤ 0.03, SNK). Each of these kallikreins, in addition to KLK2, also significantly reduced neurite density by 40% or more (P < 0.001, SNK), with KLK7 and KLK9 exerting the most vigorous effects. KLK8 and KLK13 did not alter neuron survival or neurite density at the concentrations and time points examined.
FIGURE 9. Differential activity of kallikreins in promoting neural injury in vitro.
Murine embryonic day 15 (E15) cortical neurons were cultured on cover glass coated with poly-L-lysine for 96 hr prior to treatment with recombinant kallikreins (10 µg/ml (300 nM)) for 24 hr (A, B). Neurons were immunostained for Neurofilament H and neuron number (C) and neurite density (D) quantified. KLKs 1, 5, 6, 7 and 9 promoted significant loss of neurons and their processes. KLK2 also promoted neurite loss, while KLK3 increased the number of neurites counted. (* P < 0.03, ** P ≤ 0.001, SNK). Histograms show the mean and s.e. of neuron and process counts across three independent cover slips treated in parallel and findings were reproducible across 3 experiments utilizing independent murine cortical neuron preparations. (Scale bar = 100 µm)
DISCUSSION
These studies document regulated patterns of expression of multiple members of the kallikrein family of secreted serine proteases (KLKs 1, 5, 6, 7, 8 and 9) in human spinal cord trauma. The most widespread changes in kallikrein expression occurred during the acute and subacute periods, with KLK9 remaining highly elevated even at late chronic post-mortem intervals. Importantly, despite differences in the evolution of the kallikrein locus across species (70), the same general patterns of kallikrein expression were observed in a complementary clinically relevant murine SCI model, pointing to conserved pathophysiological mechanisms. In addition, five of the six kallikreins studied in detail promoted injury to cortical neurons and their processes in vitro. Taken together, these studies define kallikreins as important regulators of the SCI microenvironment and as potential new targets for the design of therapies to reduce axon injury and create an environment favorable to repair across the acute through chronic SCI continuum.
Given the dynamic alterations documented in the expression of KLKs 1, 5, 6, 7, 8 and 9 in both human and rodent traumatic SCI, including the association of each with injured axons, we made a comprehensive assessment of the effects of the recombinant forms of these proteases, in addition to KLKs 2, 3 and 13, toward primary murine cortical neurons in vitro. Results demonstrate that elevated levels of KLKs 1, 2, 5, 6, 7 and 9 are capable of promoting a significant injury to neurons and/or their processes in vitro. These findings are consistent with prior studies pointing to the neurotoxic effects of KLK1 and KLK6 (9) and reveal the potential for KLKs 2, 5, 7 and 9 to promote neural injury at parallel concentrations for the first time. Taken with previously published reports implicating deregulated expression of multiple kallikreins in Alzheimers (1–5), fronto-temporal dementia (6), Parkinsons (3, 7), post-polio-syndrome (10), glioblastoma multiforme (47, 71) and multiple sclerosis (8, 9), it will be important in future studies to examine the consequences of a wider range of kallikrein concentrations and to define their parallel or differential effects towards multiple neural cell types.
Taken with the substantial elevations in kallikrein expression at one or more of the post-SCI intervals examined, additional efforts to fully resolve the mechanism(s) by which they alter neuron and neurite numbers and their potential utility as targets for neuroprotection and neural repair will be needed. The possibility that a subset of kallikreins exert their neurotoxic effects at least in part by activation of G-protein linked protease activated receptors (PARs) is supported by in vitro studies demonstrating that KLKs 5 and 6 activate one or more PARs in vitro (23, 28, 30, 61, 72–74). Importantly, the ability of KLK6 to elicit mitogen activated protein kinase signaling and to promote injury of cultured cerebellar granule neurons was recently shown to be dependent on both PAR1 and PAR2 (23). Whether other kallikreins activate neuronal PARs and the roles played in neural injury remains an important avenue for future investigation.
While increases in KLK8 were also observed in human and rodent SCI, recombinant KLK8 did not elicit direct loss of cortical neurons or neurites in vitro, nor did KLK3 or KLK13, at the concentrations and time points examined. Of interest, KLK3, better known as prostate specific antigen, acted in a nerve growth factor-like fashion promoting an increase in cortical neuron processes. While the increases in neurite density elicited by KLK8 in the present study did not reach statistical significance, a prior study showed KLK8 enhances neurite density in cultured hippocampal neurons (75). In this regard, it is important to highlight the substantial prior work indicating that KLK8 plays a key role in neuroplasticity. For example, KLK8 deficient mice show reduced early-LTP and have abnormal CA1 synapses (76, 77). Importantly, we show that KLKs 1, 5, 6, 7 and 9 are expressed across both neurons and glia of the intact human spinal cord and therefore future efforts to determine whether their regulated proteolytic actions may likewise be involved in neural plasticity, or other key neurophysiologic activities, is needed. While KLK2 and KLK3 are not densely expressed in the intact CNS (25), and their expression with injury has not been studied, each could enter the damaged brain or spinal cord in areas of increased vascular permeability. In this case, further study regarding the potential neurite outgrowth promoting effects of KLK3, and the ability of KLK2 to promote neural injury, will also be important future directions.
We note that only the direct effects of kallikreins toward neurons were examined and therefore these studies do not exclude a wider range of action in vivo, including modification of extracellular matrices or other CNS proteins and cell types that may indirectly influence neurite integrity and neuron viability. Several kallikreins are reported or predicted to degrade extracellular matrix (ECM) proteins, including basement membrane components, such as fibronectin, laminin and heat denatured collagen (8, 56, 78–80). In fact, the role of KLK8 in promoting neural plasticity occurs in part by its activities at the neuron-extracellular matrix interface, including cleavage of synaptic L1 (81), EphB2 (82), and Neuregulin 1 (83). Our prior studies indicate that KLK6 hydrolyzes laminin to decrease neurite outgrowth and aggrecan to promote neurite extension in vitro (22). Deregulated expression of KLK6 with CNS injury is therefore positioned to modulate the balance of permissive and inhibitory cues that regulate axon outgrowth and the potential for nerve regeneration. Also, KLK6 readily degrades myelin proteins, including MBP and myelin oligodendrocyte glycoprotein (8, 72) and therefore may contribute to axon injury and conduction block in SCI by promoting demyelination. Adding to the complexity of the potential consequences of elevated (or reduced) kallikrein expression at sites of CNS pathology, KLKs may participate in activation of metalloproteases (23, 84) or fibrinolytic enzymes (57–59, 85), which themselves may promote neurotoxic effects.
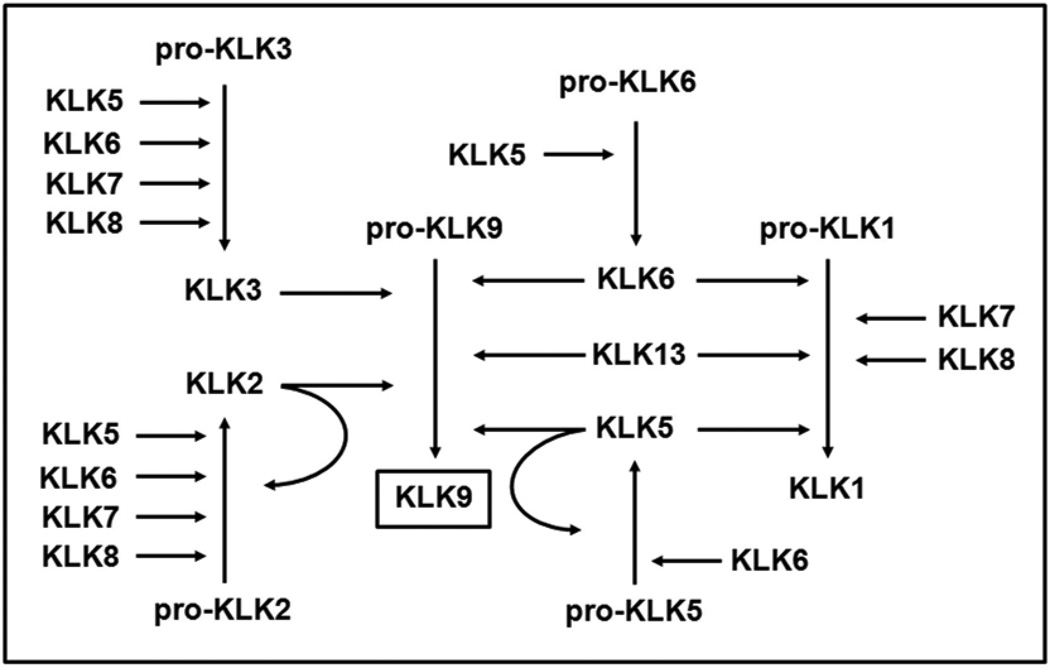
The kallikrein expression patterns described in the intact and injured spinal cord do not distinguish the relative amounts of inactive pro-kallikrein compared to that which N-terminal cleavage of a 3 to 37 amino acid pro-peptide results in the release of enzymatically active protein (57). While tools to distinguish between pro- and active kallikreins are yet to be developed, we have previously made a comprehensive analysis of activation cascades among the 15 kallikreins (57, 59). Since KLK9 not only showed the most robust and long-lasting elevations in expression across the cases of human and murine SCI examined, but was also among the most neurotoxic, we summarize the potential for the other kallikreins examined to be involved in KLK9-activation cascades (Fig. 10). For example, KLKs 2, 3, 5, 6 and 13 may directly activate pro-KLK9. In addition, KLKs 5, 6, 7 and 8 may indirectly activate pro-KLK9. While not examined here, KLKs 4, 12 and 14 also activate KLK9. Interestingly, KLK9, as well as KLK1 do not activate other kallikreins (59). Taken together these data reveal for the first time multiple avenues for kallikrein enzymatic activation in the intact and injured CNS.
Dense kallikrein staining associated with astrocyte-like cells at sites of SCI points to novel potential roles for these unique enzymes in the process of injury-induced astrogliosis. For example, KLKs 1, 5, 7 and 9 were localized to astrocyte-like cells in the intact human spinal cord and each of these, in addition to KLK6, were elevated in swollen/hypertrophic astrocytes that were prominent in the injured spinal cord from acute through chronic time points, particularly in white matter. Supporting the likely role of at least a subset of KLKs in the process of astrogliosis, KLK6 activates astrocyte PAR1 and PAR2 to promote Ca2+ flux and mitogen activated protein kinase signaling (61). In addition, KLK6 promotes robust astrocyte stellation and IL-6 secretion in vitro, in part by activation of PAR1 (28). Given the prominent association of several kallikreins with astrocyte-like cells in human SCI, a model is suggested whereby elevations in astrocyte kallikrein act in an autocrine or paracrine fashion to promote key aspects of astrogliosis, including stellation and IL-6 secretion. As this line of research proceeds, it will be necessary to determine the respective roles of individual or combinations of kallikreins in astrogliosis in order to delineate whether their effects are part of the beneficial wound healing response and/or may contribute to the formation of an astroglial nerve regeneration barrier (66, 86).
The inflammatory response in spinal cord trauma is complex and includes both cellular and chemical mediators (67, 69, 87–90). Corresponding to what has been reported, monocytes and microglia reactive for the lysosomal marker CD68 were observed from acute through the most chronic time points of human SCI examined and were most abundant from the subacute period onward (43, 69). In parallel sections, KLKs 1, 5, 6, 7 and 9 were identified in association with enlarged rounded macrophages. Notably, KLKs 1, 5, 6 and 7 have been previously implicated in inflammation (25, 91, 92) and results here support such roles in context of traumatic CNS injury. Kallikrein 6 has been previously localized to monocytes and microglia in human and rodent SCI (22) as well as in MS lesions and in viral and autoimmune MS models (8, 29, 54, 55). The association of at least low levels of KLK9 with rounded macrophages in SCI, points to potential roles for this protease in inflammatory events as well. Further supporting the involvement of a subset of kallikreins in inflammatory cascades secondary to SCI, peak elevations in KLKs 1, 6, 7, 8 and 9 seen across the acute and subacute periods in experimental murine SCI corresponded to parallel increases in the pro-inflammatory cytokines IL-6, IL-1β and TNFα, which are known to be produced by monocytes, microglia and other reactive cells, including T cells and astrocytes at sites of SCI (93, 94). The meaning of co-ordinate expression of kallikreins and cytokines is not yet known, but regulatory roles are possible based on prior studies demonstrating KLK6 promotes secretion of IL-6 from astrocytes (28) and KLK5 stimulates production of TNFα and IL-8 from keratinocytes (91). Future studies will be needed to determine how kallikreins affect the balance of cellular and chemical mediators of CNS inflammation (95–97) and whether, like the much better studied protease matrix metalloprotease 9 (50, 98), select kallikreins may serve as targets for therapeutic intervention.
Taken together, results spanning human SCI and a complementary murine experimental SCI model indicate that multiple kallikreins participate in the response of the spinal cord to trauma with possible roles in axon injury, astrogliosis and inflammatory events occurring from acute through more chronic stages. For the subset of kallikreins shown to be elevated post-injury and capable of promoting neural injury in vitro, that is KLKs 1, 5, 6, 7 and 9, additional efforts to evaluate whether they can be targeted to prevent secondary injury cascades and promote spinal cord repair and plasticity will be of particular interest.
FIGURE 10. Schematic depicts potential kallikrein activation cascades among the proteases examined in the current study.
The ability of each kallikrein to activate other members of the kallikrein family of proteases was established in our prior studies (57, 59). Here we present a theoretical scheme leading to the production of enzymatically active KLK9, which of six kallikreins examined was the most highly upregulated in the injured human and rodent spinal cord (Figs. 1 and 8) and exhibited robust neurotoxicity (Fig. 9).
Acknowledgments
Sources of Support
These studies were supported by the National Institutes of Health R01NS052741, The Christopher and Dana Reeve Paralysis Foundation, RG3367 and a Collaborative MS Research Award CA1060A11 from the National Multiple Sclerosis Society and the Craig H. Neilsen Foundation (IAS).
Footnotes
Author Contributions:
Performed Experiments: MR, NL, JW, JB, IAS
Analyzed Data: MR, NL, JW, RL, IAS
Wrote Paper: MR, HY, IAS
Provided Reagents: HY, SB, MB, EPD, MGF
REFERENCES
- 1.Little SP, Dixon EP, Norris F, et al. Zyme, a novel and potentially amyloidogenic enzyme cDNA isolated from Alzheimer's disease brain. J Biol Chem. 1997;272:25135–25142. doi: 10.1074/jbc.272.40.25135. [DOI] [PubMed] [Google Scholar]
- 2.Diamandis EP, Yousef GM, Petraki C, et al. Human kallikrein 6 as a biomarker of alzheimer's disease. Clin Biochem. 2000;33:663–667. doi: 10.1016/s0009-9120(00)00185-5. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa K, Yamada T, Tsujioka Y, et al. Localization of a novel type trypsin-like serine protease, neurosin, in brain tissues of Alzheimer's disease and Parkinson's disease. Psychiatry Clin Neurosci. 2000;54:419–426. doi: 10.1046/j.1440-1819.2000.00731.x. [DOI] [PubMed] [Google Scholar]
- 4.Zarghooni M, Soosaipillai A, Grass L, et al. Decreased concentration of human kallikrein 6 in brain extracts of Alzheimer's disease patients. Clin Biochem. 2002;35:225–231. doi: 10.1016/s0009-9120(02)00292-8. [DOI] [PubMed] [Google Scholar]
- 5.Menendez-Gonzalez M, Castro-Santos P, Calatayud MT, et al. Plasmatic level of neurosin predicts outcome of mild cognitive impairment. Int Arch Med. 2008;1:11. doi: 10.1186/1755-7682-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamandis EP, Scorilas A, Kishi T, et al. Altered kallikrein 7 and 10 concentrations in cerebrospinal fluid of patients with Alzheimer's disease and frontotemporal dementia. Clin Biochem. 2004;37:230–237. doi: 10.1016/j.clinbiochem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Iwata A, Maruyama M, Akagi T, et al. Alpha-synuclein degradation by serine protease neurosin: implication for pathogenesis of synucleinopathies. Hum Mol Genet. 2003;12:2625–2635. doi: 10.1093/hmg/ddg283. [DOI] [PubMed] [Google Scholar]
- 8.Scarisbrick IA, Blaber SI, Lucchinetti CF, et al. Activity of a newly identified serine protease in CNS demyelination. Brain. 2002;125:1283–1296. doi: 10.1093/brain/awf142. [DOI] [PubMed] [Google Scholar]
- 9.Scarisbrick IA, Linbo R, Vandell AG, et al. Kallikreins are associated with secondary progressive multiple sclerosis and promote neurodegeneration. Biol Chem. 2008;389:739–745. doi: 10.1515/BC.2008.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez H, Ottervald J, Nilsson KC, et al. Identification of novel candidate protein biomarkers for the post-polio syndrome - implications for diagnosis, neurodegeneration and neuroinflammation. J Proteomics. 2009;71:670–681. doi: 10.1016/j.jprot.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathology. 1995;5:407–413. doi: 10.1111/j.1750-3639.1995.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 12.Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- 13.Beattie MS, Hermann GE, Rogers RC, et al. Cell death in models of spinal cord injury. Prog Brain Res. 2002;137:37–47. doi: 10.1016/s0079-6123(02)37006-7. [DOI] [PubMed] [Google Scholar]
- 14.Kwon BK, Tetzlaff W, Grauer JN, et al. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004:451–464. doi: 10.1016/j.spinee.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Rice T, Larsen J, Rivest S, et al. Characterization of the early neuroinflammation after spinal cord injury in mice. Journal of neuropathology and experimental neurology. 2007;66:184–195. doi: 10.1097/01.jnen.0000248552.07338.7f. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Chang M, Hansen CN, et al. Role of matrix metalloproteinases and therapeutic benefits of their inhibition in spinal cord injury. Neurotherapeutics. 2011;8:206–220. doi: 10.1007/s13311-011-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray SK, Samantaray S, Smith JA, et al. Inhibition of cysteine proteases in acute and chronic spinal cord injury. Neurotherapeutics. 2011;8:180–186. doi: 10.1007/s13311-011-0037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Festoff BW, Ameenuddin S, Santacruz K, et al. Neuroprotective effects of recombinant thrombomodulin in controlled contusion spinal cord injury implicates thrombin signaling. J Neurotrauma. 2004;21:907–922. doi: 10.1089/0897715041526168. [DOI] [PubMed] [Google Scholar]
- 19.Taoka Y, Okajima K, Uchiba M. Antithrombin reduces compression-induced spinal cord injury in rats. J Neurotrauma. 2004;21:1818–1830. doi: 10.1089/neu.2004.21.1818. [DOI] [PubMed] [Google Scholar]
- 20.Abe Y, Nakamura H, Yoshino O, et al. Decreased neural damage after spinal cord injury in tPA-deficient mice. J Neurotrauma. 2003;20:43–57. doi: 10.1089/08977150360517173. [DOI] [PubMed] [Google Scholar]
- 21.Bukhari N, Torres L, Robinson JK, et al. Axonal regrowth after spinal cord injury via chondroitinase and the tissue plasminogen activator (tPA)/plasmin system. J Neurosci. 2012;31:14931–14943. doi: 10.1523/JNEUROSCI.3339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scarisbrick IA, Sabharwal P, Cruz H, et al. Dynamic role of kallikrein 6 in traumatic spinal cord injury. Eur J Neuroscience. 2006;24:1457–1469. doi: 10.1111/j.1460-9568.2006.05021.x. [DOI] [PubMed] [Google Scholar]
- 23.Yoon H, Radulovic M, Wu J, et al. Kallikrein 6 signals through PAR1 and PAR2 to promote neuron injury and exacerbate glutamate neurotoxicity. J Neurochemistry. 2013 doi: 10.1111/jnc.12293. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scarisbrick IA, Towner MD, Isackson PJ. Nervous system specific expression of a novel serine protease: regulation in the adult rat spinal cord by excitotoxic injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:8156–8168. doi: 10.1523/JNEUROSCI.17-21-08156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scarisbrick IA, Blaber SI, Tingling JT, et al. Potential scope of action of tissue kallikreins in CNS immune-mediated disease. J Neuroimmunology. 2006;178:167–176. doi: 10.1016/j.jneuroim.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 26.Shaw JL, Diamandis EP. Distribution of 15 human kallikreins in tissues and biological fluids. Clin Chem. 2007;53:1423–1432. doi: 10.1373/clinchem.2007.088104. [DOI] [PubMed] [Google Scholar]
- 27.Bledsoe G, Crickman S, Mao J, et al. Kallikrein/kinin protects against gentamicin-induced nephrotoxicity by inhibition of inflammation and apoptosis. Nephrol Dial Transplant. 2006;21:624–633. doi: 10.1093/ndt/gfi225. [DOI] [PubMed] [Google Scholar]
- 28.Scarisbrick IA, Radulovic M, Burda JE, et al. Kallikrein 6 is a novel molecular trigger of reactive astrogliosis. Biological Chemistry. 2012;393:355–367. doi: 10.1515/hsz-2011-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scarisbrick IA, Yoon H, Panos M, et al. Kallikrein 6 regulates early CNS demyelination in a viral model of multiple sclerosis. Brain pathology (Zurich, Switzerland) 2012;22:709–722. doi: 10.1111/j.1750-3639.2012.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burda JE, Radulovic M, Yoon H, et al. A critical role for PAR1 in KLK6-mediated oligodendrogliopathy. GLIA. 2013 doi: 10.1002/glia.22534. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lundwall A, Brattsand M. Kallikrein-related peptidases. Cell Mol Life Sci. 2008;65:2019–2038. doi: 10.1007/s00018-008-8024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scarisbrick IA. Kallikrein Activity in the Central Nervous System. In: Schmitt M, Sommerhoff C, Fritz H, Magdolen V, editors. The Kallikreins. Berlin: De Gruyter Publishing; 2012. pp. 349–372. [Google Scholar]
- 33.Chao J, Bledsoe G, Yin H, et al. The tissue kallikrein-kinin system protects against cardiovascular and renal diseases and ischemic stroke independently of blood pressure reduction. Biol Chem. 2006;387:665–675. doi: 10.1515/BC.2006.085. [DOI] [PubMed] [Google Scholar]
- 34.Simoes PS, Perosa SR, Arganaraz GA, et al. Kallikrein 1 is overexpressed by astrocytes in the hippocampus of patients with refractory temporal lobe epilepsy, associated with hippocampal sclerosis. Neurochem Int. 2011;58:477–482. doi: 10.1016/j.neuint.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu-Okabe C, Yousef GM, Diamandis EP, et al. Expression of the kallikrein gene family in normal and Alzheimer's disease brain. Neuroreport. 2001;12:2747–2751. doi: 10.1097/00001756-200108280-00031. [DOI] [PubMed] [Google Scholar]
- 36.Mitsui S, Okui A, Uemura H, et al. Decreased cerebrospinal fluid levels of neurosin (KLK6), an aging-related protease, as a possible new risk factor for Alzheimer's disease. Ann N Y Acad Sci. 2002;977:216–223. doi: 10.1111/j.1749-6632.2002.tb04818.x. [DOI] [PubMed] [Google Scholar]
- 37.Terayama R, Bando Y, Murakami K, et al. Neuropsin promotes oligodendrocyte death, demyelination and axonal degeneration after spinal cord injury. Neuroscience. 2007;148:175–187. doi: 10.1016/j.neuroscience.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 38.Scarisbrick IA, Asakura K, Blaber S, et al. Preferential expression of myelencephalon specific protease by oligodendrocytes of the adult rat spinal cord white matter. Glia. 2000;30:219–230. doi: 10.1002/(sici)1098-1136(200005)30:3<219::aid-glia2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Scarisbrick IA, Isackson PJ, Ciric B, et al. MSP, a trypsin-like serine protease, is abundantly expressed in the human nervous system. J Comp Neurol. 2001;431:347–361. [PubMed] [Google Scholar]
- 40.Petraki CD, Papanastasiou PA, Karavana VN, et al. Cellular distribution of human tissue kallikreins: immunohistochemical localization. Biological chemistry. 2006;387:653–663. doi: 10.1515/BC.2006.084. [DOI] [PubMed] [Google Scholar]
- 41.Komatsu N, Suga Y, Saijoh K, et al. Elevated human tissue kallikrein levels in the stratum corneum and serum of peeling skin syndrome-type B patients suggests an over-desquamation of corneocytes. J Invest Dermatol. 2006;126:2338–2342. doi: 10.1038/sj.jid.5700379. [DOI] [PubMed] [Google Scholar]
- 42.Burry RW. Specificity controls for immunocytochemical methods. J Histochem Cytochem. 2000;48:163–166. doi: 10.1177/002215540004800201. [DOI] [PubMed] [Google Scholar]
- 43.Yu WR, Fehlings MG. Fas/FasL-mediated apoptosis and inflammation are key features of acute human spinal cord injury: implications for translational, clinical application. Acta neuropathologica. 2011;122:747–761. doi: 10.1007/s00401-011-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von der Weid PY, Hollenberg MD, Fiorucci S, et al. Aspirin-triggered, cyclooxygenase-2-dependent lipoxin synthesis modulates vascular tone. Circulation. 2004;110:1320–1325. doi: 10.1161/01.CIR.0000140985.89766.CB. [DOI] [PubMed] [Google Scholar]
- 45.Koo CL, Kok LF, Lee MY, et al. Scoring mechanisms of p16INK4a immunohistochemistry based on either independent nucleic stain or mixed cytoplasmic with nucleic expression can significantly signal to distinguish between endocervical and endometrial adenocarcinomas in a tissue microarray study. J Transl Med. 2009;7:25. doi: 10.1186/1479-5876-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Wasielewski R, Mengel M, Wiese B, et al. Tissue array technology for testing interlaboratory and interobserver reproducibility of immunohistochemical estrogen receptor analysis in a large multicenter trial. Am J Clin Pathol. 2002;118:675–682. doi: 10.1309/URLK-6AVK-331U-0V5P. [DOI] [PubMed] [Google Scholar]
- 47.Drucker KD, Paulsen AP, Giannini C, et al. Clinical Significance and Novel Mechanism of Action of Kallikrein 6 in Glioblastoma. Neuro-Oncology. 2013;15(3):305–318. doi: 10.1093/neuonc/nos313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joshi M, Fehlings M. Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 1. Clip design, behavioral outcomes, and histopathology. J Neurotrauma. 2002;19:175–190. doi: 10.1089/08977150252806947. [DOI] [PubMed] [Google Scholar]
- 49.Joshi M, Fehlings M. Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 2. Quantitative neuroanatomical assessment and analysis of the relationships between axonal tracts, residual tissue, and locomotor recovery. J Neurotrauma. 2002;19:191–203. doi: 10.1089/08977150252806956. [DOI] [PubMed] [Google Scholar]
- 50.Wells JEA, Hurlbert RJ, Fehlings MG, et al. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628–1637. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- 51.Basso DM, Fisher LC, Anderson AJ, et al. Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- 52.Kendziorski C, Irizarry RA, Chen KS, et al. On the utility of pooling biological samples in microarray experiments. Proc Natl Acad Sci U S A. 2005;102:4252–4257. doi: 10.1073/pnas.0500607102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lundwall A, Band V, Blaber M, et al. A comprehensive nomenclature for serine proteases with homology to tissue kallikreins. Biol Chem. 2006;387:637–641. doi: 10.1515/BC.2006.082. [DOI] [PubMed] [Google Scholar]
- 54.Blaber SI, Ciric B, Christophi GP, et al. Targeting kallikrein 6-proteolysis attenuates CNS inflammatory disease. FASEB J. 2004;19:920–922. doi: 10.1096/fj.03-1212fje. [DOI] [PubMed] [Google Scholar]
- 55.Christophi GP, Isackson PJ, Blaber SI, et al. Distinct promoters regulate tissue-specific and differential expression of kallikrein 6 in CNS demyelinating disease. J Neurochem. 2004;91:1439–1449. doi: 10.1111/j.1471-4159.2004.02826.x. [DOI] [PubMed] [Google Scholar]
- 56.Blaber SI, Scarisbrick IA, Bernett MJ, et al. Enzymatic properties of rat myelencephalon-specific protease. Biochemistry. 2002;41:1165–1173. doi: 10.1021/bi015781a. [DOI] [PubMed] [Google Scholar]
- 57.Yoon H, Laxmikanthan G, Lee J, et al. Activation profiles and regulatory cascades of the human kallikrein-related peptidases. J Biol Chem. 2007;282:31852–31864. doi: 10.1074/jbc.M705190200. [DOI] [PubMed] [Google Scholar]
- 58.Yoon H, Blaber SI, Evans DM, et al. Activation Profiles of Human Kallikreinrelated Peptidases by Proteases of the Thrombostasis Axis. Protein Sci. 2008;17:1998–2007. doi: 10.1110/ps.036715.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoon H, Blaber SI, Debela M, et al. A completed KLK activome profile: investigation of activation profiles of KLK9, 10, and 15. Biol Chem. 2009;390:373–377. doi: 10.1515/BC.2009.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scarisbrick IA, Blaber M. Kallikrein-related peptidase 6. In: Barrett AJ, Rawlings ND, editors. Handbook of Proteolytic Enzymes. 2012. pp. 2780–2786. [Google Scholar]
- 61.Vandell AG, Larson N, Laxmikanthan G, et al. Protease Activated Receptor Dependent and Independent Signaling by Kallikreins 1 and 6 in CNS Neuron and Astroglial Cell Lines. Journal of neurochemistry. 2008;107:855–870. doi: 10.1111/j.1471-4159.2008.05658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diamandis EP, Yousef GM, Soosaipillai AR, et al. Human kallikrein 6 (zyme/protease M/neurosin): a new serum biomarker of ovarian carcinoma. Clin Biochem. 2000;33:579–583. doi: 10.1016/s0009-9120(00)00182-x. [DOI] [PubMed] [Google Scholar]
- 63.Chen ZL, Momota Y, Kato K, et al. Expression of neuropsin mRNA in the mouse embryo and the pregnant uterus. J Histochem Cytochem. 1998;46:313–320. doi: 10.1177/002215549804600304. [DOI] [PubMed] [Google Scholar]
- 64.Ramon y Cajal S. Cajal's Degeneration and Regeneration of the Nervous System. New York: Oxford University Press; 1991. [Google Scholar]
- 65.Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma. 1997;14:419–440. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- 66.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta neuropathologica. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 68.Popovich PG, Rooijen Nv, Hickey WF, et al. Hematogenous macrophages express CD8 and distribute to regions of lesion cavitation after spinal cord injury. Exp Neurol. 2003;182:275–287. doi: 10.1016/s0014-4886(03)00120-1. [DOI] [PubMed] [Google Scholar]
- 69.Fleming JC, Norenberg MD, Ramsay DA, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 70.Lundwall A. Old genes and new genes: The evolution of the kallikrein locus. Thromb Haemost. 2013;109 doi: 10.1160/TH12-11-0851. [DOI] [PubMed] [Google Scholar]
- 71.Talieri M, Zoma M, Devetzi M, et al. Kallikrein-related peptidase 6 (KLK6)gene expression in intracranial tumors. Tumour Biol. 2012 doi: 10.1007/s13277-012-0385-4. [DOI] [PubMed] [Google Scholar]
- 72.Angelo PF, Lima AR, Alves FM, et al. Substrate specificity of human kallikrein 6: salt and glycosaminoglycan activation effects. J Biol Chem. 2006;281:3116–3126. doi: 10.1074/jbc.M510096200. [DOI] [PubMed] [Google Scholar]
- 73.Oikonomopoulou K, Hansen KK, Saifeddine M, et al. Proteinase-activated receptors, targets for kallikrein signaling. J Biol Chem. 2006;281:32095–32112. doi: 10.1074/jbc.M513138200. [DOI] [PubMed] [Google Scholar]
- 74.Scarisbrick IA, Epstein B, Cloud BA, et al. Functional role of kallikrein 6 in regulating immune cell survival. PLoS One. 2011;6:e18376, 1–11. doi: 10.1371/journal.pone.0018376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oka T, Akisada M, Okabe A, et al. Extracellular serine protease neuropsin (KLK8) modulates neurite outgrowth and fasciculation of mouse hippocampal neurons in culture. Neurosci Lett. 2002;321:141–144. doi: 10.1016/s0304-3940(01)02470-3. [DOI] [PubMed] [Google Scholar]
- 76.Komai S, Matsuyama T, Matsumoto K, et al. Neuropsin regulates an early phase of schaffer-collateral long-term potentiation in the murine hippocampus. Eur J Neurosci. 2000;12:1479–1486. doi: 10.1046/j.1460-9568.2000.00035.x. [DOI] [PubMed] [Google Scholar]
- 77.Tamura H, Ishikawa Y, Hino N, et al. Neuropsin is essential for early processes of memory acquisition and Schaffer collateral long-term potentiation in adult mouse hippocampus in vivo. J Physiol. 2006;570:541–551. doi: 10.1113/jphysiol.2005.098715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bernett MJ, Blaber SI, Scarisbrick IA, et al. Crystal structure and biochemical characterization of human kallikrein 6 reveals that a trypsin-like kallikrein is expressed in the central nervous system. J Biol Chem. 2002;277:24562–24570. doi: 10.1074/jbc.M202392200. [DOI] [PubMed] [Google Scholar]
- 79.Borgono CA, Diamandis EP. The emerging roles of human tissue kallikreins in cancer. Nat Rev Cancer. 2004;4:876–890. doi: 10.1038/nrc1474. [DOI] [PubMed] [Google Scholar]
- 80.Sotiropoulou G, Pampalakis G, Diamandis EP. Functional roles of human kallikrein-related peptidases. J Biol Chem. 2009;284:32989–32994. doi: 10.1074/jbc.R109.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matsumoto-Miyai K, Ninomiya A, Yamasaki H, et al. NMDA-dependent proteolysis of presynaptic adhesion molecule L1 in the hippocampus by neuropsin. J Neurosci. 2003;23:7727–7736. doi: 10.1523/JNEUROSCI.23-21-07727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Attwood BK, Bourgognon JM, Patel S, et al. Neuropsin cleaves EphB2 in the amygdala to control anxiety. Nature. 2011;473:372–375. doi: 10.1038/nature09938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tamura H, Kawata M, Hamaguchi S, et al. Processing of neuregulin-1 by neuropsin regulates GABAergic neuron to control neural plasticity of the mouse hippocampus. J Neurosci. 2012;32:12657–12672. doi: 10.1523/JNEUROSCI.2542-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Menashi S, Fridman R, Desrivieres S, et al. Regulation of 92-kDa gelatinase B activity in the extracellular matrix by tissue kallikrein. Ann N Y Acad Sci. 1994;732:466–468. doi: 10.1111/j.1749-6632.1994.tb24787.x. [DOI] [PubMed] [Google Scholar]
- 85.Blaber M, Yoon H, Juliano MA, et al. Functional intersection of the kallikrein-related peptidases (KLKs) and thrombostasis axis. Biol Chem. 2010;391:311–320. doi: 10.1515/BC.2010.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grimpe B, Pressman Y, Lupa MD, et al. The role of proteoglycans in Schwann cell/astrocyte interactions and in regeneration failure at PNS/CNS interfaces. Mol Cell Neurosci. 2005;28:18–29. doi: 10.1016/j.mcn.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 87.Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal cord. 2003;41:369–378. doi: 10.1038/sj.sc.3101483. [DOI] [PubMed] [Google Scholar]
- 88.Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 2004;21:429–440. doi: 10.1089/089771504323004575. [DOI] [PubMed] [Google Scholar]
- 89.Yang L, Blumbergs PC, Jones NR, et al. Early expression and cellular localization of proinflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in human traumatic spinal cord injury. Spine (Phila Pa 1976) 2004;29:966–971. doi: 10.1097/00007632-200405010-00004. [DOI] [PubMed] [Google Scholar]
- 90.Chang HT. Subacute human spinal cord contusion: few lymphocytes and many macrophages. Spinal cord. 2007;45:174–182. doi: 10.1038/sj.sc.3101910. [DOI] [PubMed] [Google Scholar]
- 91.Briot A, Deraison C, Lacroix M, et al. Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med. 2009;206:1135–1147. doi: 10.1084/jem.20082242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chao J, Shen B, Gao L, et al. Tissue kallikrein in cardiovascular, cerebrovascular and renal diseases and skin wound healing. Biol Chem. 2010;391:345–355. doi: 10.1515/BC.2010.042. [DOI] [PubMed] [Google Scholar]
- 93.Klusman I, Schwab ME. Effects of pro-inflammatory cytokines in experimental spinal cord injury. Brain research. 1997;762:173–184. doi: 10.1016/s0006-8993(97)00381-8. [DOI] [PubMed] [Google Scholar]
- 94.Nakamura M, Okada S, Toyama Y, et al. Role of IL-6 in spinal cord injury in a mouse model. Clinical reviews in allergy & immunology. 2005;28:197–204. doi: 10.1385/CRIAI:28:3:197. [DOI] [PubMed] [Google Scholar]
- 95.Rabchevsky AG, Streit WJ. Role of microglia in postinjury repair and regeneration of the CNS. Ment Retard Dev Disabil Res Rev. 1998;4:1870192. [Google Scholar]
- 96.Schwartz M, Shaked I, Fisher J, et al. Protective autoimmunity against the enemy within: fighting glutamate toxicity. Trends in Neurosci. 2003;26:297–302. doi: 10.1016/S0166-2236(03)00126-7. [DOI] [PubMed] [Google Scholar]
- 97.Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10:235–241. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- 98.Tator CH, Hashimoto R, Raich A, et al. Translational potential of preclinical trials of neuroprotection through pharmacotherapy for spinal cord injury. Journal of neurosurgery. 2012;17:157–229. doi: 10.3171/2012.5.AOSPINE12116. [DOI] [PubMed] [Google Scholar]