Abstract
CNS trauma generates a proteolytic imbalance contributing to secondary injury, including axonopathy and neuron degeneration. Kallikrein 6 (Klk6) is a serine protease implicated in neurodegeneration and here we investigate the role of protease activated receptors 1 (PAR1) and PAR2 in mediating these effects. First we demonstrate Klk6 and the prototypical activator of PAR1, thrombin, as well as PAR1 and PAR2, are each elevated in murine experimental traumatic spinal cord injury (SCI) at acute or subacute time points. Recombinant Klk6 triggered ERK1/2 signaling in cerebellar granule neurons and in the NSC34 spinal cord motoneuron cell line, in a PI3K and MEK-dependent fashion. Importantly, lipopeptide inhibitors of PAR1 or PAR2, and PAR1 genetic deletion, each reduced Klk6-ERK1/2 activation. In addition, Klk6 and thrombin promoted degeneration of cerebellar neurons and exacerbated glutamate neurotoxicity. Moreover, genetic deletion of PAR1 blocked thrombin-mediated cerebellar neurotoxicity and reduced the neurotoxic effects of Klk6. Klk6 also increased glutamate-mediated Bim signaling, PARP cleavage and lactate dehydrogenase (LDH) release in NSC34 motoneurons and these effects were blocked by PAR1 and PAR2 lipopeptide inhibitors. Taken together these data point to a novel Klk6-signaling axis in CNS neurons that is mediated by PAR1 and PAR2 and is positioned to contribute to neurodegeneration.
Keywords: Spinal cord injury, Thrombin, Axon, Neurodegeneration, Trauma
Introduction
Deregulation of serine proteases, including members of the thrombolytic, fibrinolytic and kallikrein cascades is emerging as an important contributor to CNS pathophysiology (Cunningham et al. 1993, Tsirka et al. 1997, Scarisbrick et al. 1997, Gingrich & Traynelis 2000, Scarisbrick et al. 2002, Suo et al. 2002, Junge et al. 2003, Scarisbrick et al. 2008, Chen et al. 2012). Kallikrein 6 (Klk6) is one of the most abundant serine proteases in the adult CNS (Scarisbrick et al. 1997, Scarisbrick et al. 2001, Scarisbrick et al. 2006a), and altered levels have been associated with serious CNS disorders, including Alzheimers (Zarghooni et al. 2002, Mitsui et al. 2002, Ashby et al. 2011), Parkinsons (Ogawa et al. 2000, Iwata et al. 2003, Kasai et al. 2008), stroke (Uchida et al. 2004), spinal cord injury (SCI) (Scarisbrick et al. 1997, Terayama et al. 2004, Scarisbrick et al. 2006b), and multiple sclerosis (Scarisbrick et al. 2002, Scarisbrick et al. 2008). Despite the potential roles of Klk6 in a wide range of neurological conditions its mechanism of action remains poorly understood.
Thrombin is an abundant serum serine protease with well-defined roles in neurotoxicity in cases of CNS hemorrhage that are mediated in part by proteolytic activation of a seven transmembrane G-protein coupled receptor referred to as protease activated receptor 1 (PAR1) (Vu et al. 1991). Three other PARs (PAR2-4) have also been identified and like PAR1 are activated by proteolytic cleavage in their extracellular N-terminal domain, creating a tethered ligand that binds intramolecularly to elicit signaling. Thrombin has high affinity for PAR1 while trypsin and mast cell tryptase activate PAR2 (Ramachandran et al. 2012). As cell surface receptors, PARs endow the cell with the ability to respond, or over respond, to the rapidly changing proteolytic microenvironment such as that occurring at sites of CNS trauma. All four PARs are expressed in the CNS (Junge et al. 2004, Vandell et al. 2008), although relatively little is known regarding their CNS-specific roles or regulation in cases of injury or disease.
Currently, Klk6 is best known for its activities in hydrolysis of extracellular matrix proteins, including, laminin, fibronectin (Bernett et al. 2002, Blaber et al. 2002) and aggrecan (Scarisbrick et al. 2006b), α-synuclein (Tatebe et al. 2010) and myelin proteins (Scarisbrick et al. 2002, Blaber et al. 2004). In addition, we previously showed that Klk6 activates CNS PARs, mediating Ca2+ flux in neural cell lines (Vandell et al. 2008). Klk6 was also recently shown to trigger astrogliosis, promoting activation of ERK1/2, secretion of IL-6 and cellular stellation, in part by proteolytic activation of PAR1 (Scarisbrick et al. 2012a). Elevated levels of Klk6 have also been shown to promote degeneration of murine cortical neurons in vitro (Scarisbrick et al. 2008), which we hypothesize occurs in part by its ability to directly activate neuronal PARs.
Since Klk6 and thrombin are emerging as important regulators of neural pathophysiology, we examined their potential significance to traumatic CNS injury by determining their expression in a contusion-compression model of murine experimental SCI and assessed their neurotoxic properties toward primary cerebellar granule neurons and the NSC34 spinal cord motoneuron cell line in vitro, including the involvement of PARs. Both proteases were shown to be neurotoxic, but key differences in their abundance, regulation in SCI and in the subset of PARs they utilize to promote signaling and neural injury were identified. These studies shed new light on the complex proteolytic microenvironment present at sites of CNS injury and identify both PAR1 and PAR2 as important targets for the development of neuroprotective strategies.
Materials and Methods
Murine Clip Compression Model of Traumatic SCI
Experimental compression injury of the spinal cord was generated in twelve-week old female (22–25 g) C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) using a modified aneurysm clip (FEJOTA mouse clip, 8g closing force generating “moderate injury”) (Joshi & Fehlings 2002a). This model of SCI includes not only an initial impact, but also a persistent compression phase that results in microcystic cavitation, degenerating axons, and robust astrogliosis, all hallmarks of human traumatic SCI (Joshi & Fehlings 2002a, Joshi & Fehlings 2002b, Wells et al. 2003). Prior to compression, mice were deeply anesthetized with Xylazine (0.125 mg/kg, Akom, Inc., Decatur, IL) and Ketaset (1 mg/kg, Fort Dodge Animal Health, Fort Dodge, IA). A laminectomy was performed at T8 to T10. Injury was induced at the level of T9 by extradural application of the FEJOTA clip for a period of exactly 1 min, which produces mechanical contusion injury to both dorsal and ventral cord. Pain was minimized by administration of Buprenorphine (0.05 mg/kg, Hospira, Lake Forest, IL) subcutaneously every 12 h for 96 h post-surgery. To avoid infection, Baytril (10 mg/kg, Bayer Health Care, Shawnee Mission, KS) was administered intraperitoneally in a prophylactic fashion prior to surgery and for the first 48 h post-surgery. Bladders were manually voided twice daily even after spontaneous recovery of function until the endpoint of each experiment. All animal experiments were performed with strict adherence to NIH Guidelines for animal care and safety and were approved by the Mayo Clinic Institutional Animal Care and Use Committee.
To determine acute (3d) and more chronic (30d) compression related changes in Klk6 protein expression, groups of three experimental mice were allowed to recover to each endpoint. At each time point, 5 mm of spinal cord at the level of injury (epicenter), as well as 5 mm above and 5 mm below, were collected into individual tubes. For analysis of RNA expression levels, separate experimental mice were prepared and the injury epicenter harvested at 3 (acute), 14 (subacute) or 30 (chronic) days post-injury (dpi). In these experiments, mice were also evaluated for the extent of locomotor recovery using the Basso Mouse Scale (BMS) (Basso et al. 2006). In each case, age and gender matched uninjured mice served as controls and all samples were snap frozen until the time of protein or RNA isolation.
Primary cerebellar granule neuron and NSC34 spinal cord motoneuron cell culture
Primary cerebellar granule neurons are a commonly used model to study mechanisms of neural signaling and survival (Contestabile 2002) and were utilized in the current studies to dissect the roles of PAR1 and PAR2 in Klk6 and thrombin-mediated signaling and neural injury. Cerebellar granule neurons were isolated from postnatal day 4 C57/BL6J mice (Jackson Laboratories), stripped of meninges, and minced. Unless otherwise indicated, all cell culture reagents were obtained from Invitrogen (Carlsbad, CA, USA) and all cells were maintained at 37°C in 95% air and 5% CO2. Isolated cerebellum was dissociated by digestion in 0.25% trypsin without EDTA for 10 min 37°C. Cells were plated at density of 1.5–2.0 × 105 cells/well on 10μg/mL poly-D-lysine (Sigma) coated glass cover slips or at 1 × 106 cells in 6-well plates. Cerebellar neurons were plated in Neurobasal A medium with 10% fetal calf serum (FCS), 2% B27 supplement, 1% N2 supplement, 50 U/mL penicillin-streptomycin, 0.45% glucose, 2mM glutamax, 1mM sodium pyruvate, and 50 μM β-mercaptoethanol (Sigma). As it is relevant to the interpretation of glutamate toxicity, we note that the media contained 1.8 mM Ca2+ and 0.81 mM Mg2+. After 24hr, media was replaced with defined, serum free media containing all components just listed except serum and N2 and neurons allowed to differentiate for an additional 72 hr prior to being subject to experimental conditions.
We have previously demonstrated that Klk6 elicits Ca2+ and MAPK signaling in NSC34 spinal cord motoneurons (Vandell et al. 2008) and here we dissect the involvement of PAR1 and/or PAR2 in mediating these effects. As a murine motoneuron cell line that extends neurite like processes (Scarisbrick et al. 2006b, Benavente et al. 2012), produces acetylcholine (Cashman et al. 1992, Johann et al. 2011), expresses glutamate receptor proteins (Eggett et al. 2000) and generates action potentials (Cashman et al. 1992), NSC34 neurons have been widely used to study mechanisms of neuron signaling and neuron degeneration (Eggett et al. 2000, Mercer et al. 2000, He et al. 2002, Raimondi et al. 2006, Prause et al. 2013). NSC34 spinal cord motoneurons were expanded on tissue culture treated plastic in high glucose Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum, 50u/mL penicillin-streptomycin and 2mM Glutamax. After plating onto laminin (10 μg/ml, Sigma) coated 6-well tissue culture plates for signaling experiments, the media was replaced with media to induce differentiation which we previously described in detail (Scarisbrick et al. 2006b, Vandell et al. 2008) and which is identical to that used for differentiation of cerebellar granule neurons.
Quantification of Klk6, thrombin and PAR RNA in SCI
To determine changes in expression of RNA encoding Klk6, thrombin, neurofilament, TNF-α, PAR1 or PAR2 in response to traumatic SCI, total RNA was isolated from intact uninjured adult mouse spinal cord, or at 3 or 30d following clip compression injury using STAT-60 (Tel-Test Inc., Friendswood, TX). The level of RNA encoding each was determined in 0.5 μg of total RNA using the iScript one-step RT-PCR kit with SYBR® Green and the iCycler iQ5 system (BioRad, Hercules, CA). In each case, RNA copy number was determined using a standard curve prepared by parallel amplification of cDNA clones diluted to a known copy number as previously described in detail (Christophi et al. 2004, Vandell et al. 2008, Scarisbrick et al. 2012b). Primers used to determine transcriptional changes included those for murine Klk6 (NM_011177.2), (F) 5′-CCTACCCTGGCAAGATCAC- 3′ and (R) 5′-GGATCCATCTGATATGAGTGC-3′; murine thrombin (NM_010168.2), (F) 5′-GTGAACCTGCCCATTGTA-3′ and (R) 5′-TTCACAAGCATCTCCTCG-3′; murine PAR1 (NM_010169.3), (F) 5′-CTTGCTGATCGTCGCCC-3′ and (R) 5′-TTCACCGTAGCATCTGTCCT-3′; murine PAR2 (NM_007974.3), (F) 5′-CCGGACCGAGAACCTTG-3′ and (R) 5′-CGGAAGAAAGACAGTGGTCAG-3′; murine neurofilament H (NM010904.3) (F) 5′-CATTGAGATTGCCGCTTACAG-3′ and (F) 5′-TTATGTGCGTGGATATGGAGG-3′; and TNF-α (NM_013693.2) hybridization probe (Mm00443258_m1, Applied Biosystems). Amplification of the housekeeping gene glyceraldehydes phosphate 3-dehydrogenase (GAPDH) (NM_008084.2) was used to verify equal loading (F: 5′-ACCACCATGGAGAAGGC - 3′ and R: 5′-GGCATGGACTGTGGTCATGA - 3′). Given the low amount of RNA isolated from the SCI epicenter, quantification of RNA expression at each time point was determined by combining RNA isolated from three independent animals and this was examined in triplicate. Changes in RNA expression in response to injury were expressed as percent change relative to uninjured control mice. Absolute copy number for each gene in uninjured spinal cord is also provided.
Quantification of Klk6 protein in traumatic-SCI
Western blots to quantify Klk6 protein levels in the uninjured spinal cord and any changes that occur in response to traumatic SCI were performed essentially as previously described (Vandell et al. 2008, Scarisbrick et al. 2012b). The SCI epicenter, or an equivalent region of spinal cord from uninjured controls, was harvested from three individual mice at each time point. Samples from each time point were then collectively homogenized in radio-immunoprecipitation assay buffer. Protein samples (50 μg) were resolved by electrophoresis on 10% to 12.5% SDS-PAGE gels (Bio-Rad Laboratories, Hercules, CA) and electroblotted. A standard curve to estimate the absolute abundance of Klk6 in each case was created by including samples of recombinant Klk6 protein (90 to 900 ng/ml) on each gel. Changes in Klk6 protein in response to compression injury were evaluated using a rabbit polyclonal antibody (Rb008) as previously described (Blaber et al. 2002, Scarisbrick et al. 2012b). All Westerns were re-probed with an antibody recognizing GAPDH (Abcam, Cambridge, MA), or β-actin (Novus Biologicals, Littleton, CO), to control for equal loading. In each case, signal was detected on film using species appropriate horseradish peroxidase conjugated secondary antibodies (GE Healthcare, Buckinghamshier, UK) and standard chemiluminescent techniques (Pierce, Rockford, IL). Films were scanned and images quantified using Image Lab 2.0 software (Bio-Rad). After establishing equal loading, the amount of Klk6 protein in each sample was determined relative to the Klk6 standard curve and expressed as the mean and s.e. of readings from 3 separate Western blots.
Expression of PAR1 and PAR2 by neurons
The relative abundance of PAR1 and PAR2 in primary cerebellar granule neurons and in the NSC34 spinal cord motoneuron cell line was evaluated by real-time RT-PCR using the primers and methods described above (see also (Vandell et al. 2008)). Expression of each gene was determined in triplicate cultures and expressed as mean copy number. In addition, neurons grown on glass cover slips were stained for PAR1 or PAR2 using standard immunofluorescence techniques. PAR1 was localized using a goat polyclonal antibody SC-8202 and PAR2 using a goat polyclonal antibody SC-8205 (Santa Cruz Biotechnology, Santa Cruz, CA). Primary antibody binding was visualized using an affinity-purified, FITC fluorochrome-conjugated secondary antibody (Jackson Immunoresearch Laboratories, Westgrove, PA). Immunostained cells were visualized using a Zeiss LSM 510 Meta confocal microscope (Carl Zeiss, Jena, Germany).
Klk6- and thrombin-mediated signaling in neurons
To evaluate changes in neuron signaling in response to Klk6 (5 μg/ml, 150 nM), or thrombin (5 μg/ml, 135 nM), primary cerebellar granule neurons or NSC34 spinal cord motoneurons were plated on 6 well plates, allowed to mature for 72 h and then treated with protease for periods of 5 or 30 min. Klk6 was expressed as recombinant protein from an insect cell/baculovirus expression system, activated and purified as we have previously described in detail (Blaber et al. 2004, Blaber et al. 2002, Scarisbrick et al. 2012a). Thrombin isolated from human plasma was obtained from enzyme research laboratories (South Bend, IN). Harvested proteins (50 μg) were electroblotted and membranes probed with primary antibodies recognizing phospho-extracellular signal regulated kinase (ERK1/2) and total ERK1/2, phospho-protein kinase B (AKT) and total AKT, Bim EL (Cell Signaling Technology, Danvers, MA) or poly-ADP-ribose polymerase (PARP antibody was a gift from Dr. S. Kauffman (Mayo Clinic, Rochester MN) (Kaufmann et al. 1993). Signal in each case was detected as described above and relative changes determined by normalizing optical density measurements to β-Actin or GAPDH.
To determine the dependence of Klk6 or thrombin-mediated signaling in cerebellar granule neurons on PAR1, signaling experiments were repeated using neurons derived from either PAR1+/+ or PAR1−/− mice (B6.129S4-F2rtm1Ajc, Jackson Laboratories, Bar Harbor, ME). PAR1−/− mice have been back crossed for more than 25 generations to C57BL6/J. The role of PAR1 and PAR2 in protease-mediated signaling was determined in primary cerebellar granule neurons and in NSC34 spinal cord motoneurons by pre-treating cultures for 3 h with vehicle alone or with 30 μM of either a PAR1 (P1pal-7, palmitate-KKSRALF-NH2), or a PAR2 (P2pal-18S, palmitate-RSSAMDENSEKKRKSAIK-NH2) lipopeptide inhibitor (Cisowski et al. 2011, Sevigny et al. 2011), alone or in combination. Palmitoylated peptides (lipopeptides), referred to as Pepducins, were synthesized by standard Fmoc solid-phase synthetic methods with C-terminal amides and the composition of each conjugated peptide confirmed by mass spectrometry (Mayo Clinic, Peptide Core). The involvement of PI3K, MEK or PKC in Klk6-mediated ERK1/2 signaling was established by determining the impact of pre-treating cultures with either Wartmannin (200 nM), U0126 (150 nM) or Go 6983 (120nM, Tocris Bioscience, Minneapolis, MN), for 30 min prior to application of recombinant Klk6 (5 μg/ml (150 nM)). Density measurements for signaling experiments were expressed as a percent of the maximal response observed and each experiment was repeated independently three times and the mean and s.e. of these triplicates used for statistical analysis and in the histograms shown.
Neurotoxic effects of Klk6 and thrombin
The potential neurotoxic effects of excess Klk6 or thrombin were evaluated in primary cultures of cerebellar granule neurons grown at a density of 1.5–2.0 × 105 cells/mm2 on poly-L-lysine coated glass cover slips. Neurons were plated and allowed to mature for 72 h in defined media prior to application of 10 μg/ml of either Klk6 (300 nM) or thrombin (270 nM). This concentration of Klk6 represents approximately 5-fold higher levels than those known to be present in human cerebrospinal fluid (Diamandis et al. 2000, Shaw & Diamandis 2007) and mirror the elevated levels of Klk6 protein quantified in the spinal cord injury epicenter and above at 3 dpi (Fig. 1b). Twenty-four h after treatment, cells were fixed with 2% paraformalydehyde and stained with Coomassie Brilliant blue R-250 (Bio-Rad). Alternatively, cultures were stained for neurofilament H protein (rabbit polyclonal, AHP245 Serotec, Raleigh, NC) using standard avidin-biotin histochemistry (Scarisbrick et al. 2008). The impact of excess glutamate on Klk6 or thrombin-elicited neurotoxicity was evaluated in parallel cultures that were additionally treated with 250 μM L-glutamate (Sigma) (Regan & Choi 1991, Eggett et al. 2000, Biasini et al. 2013) or vehicle alone.
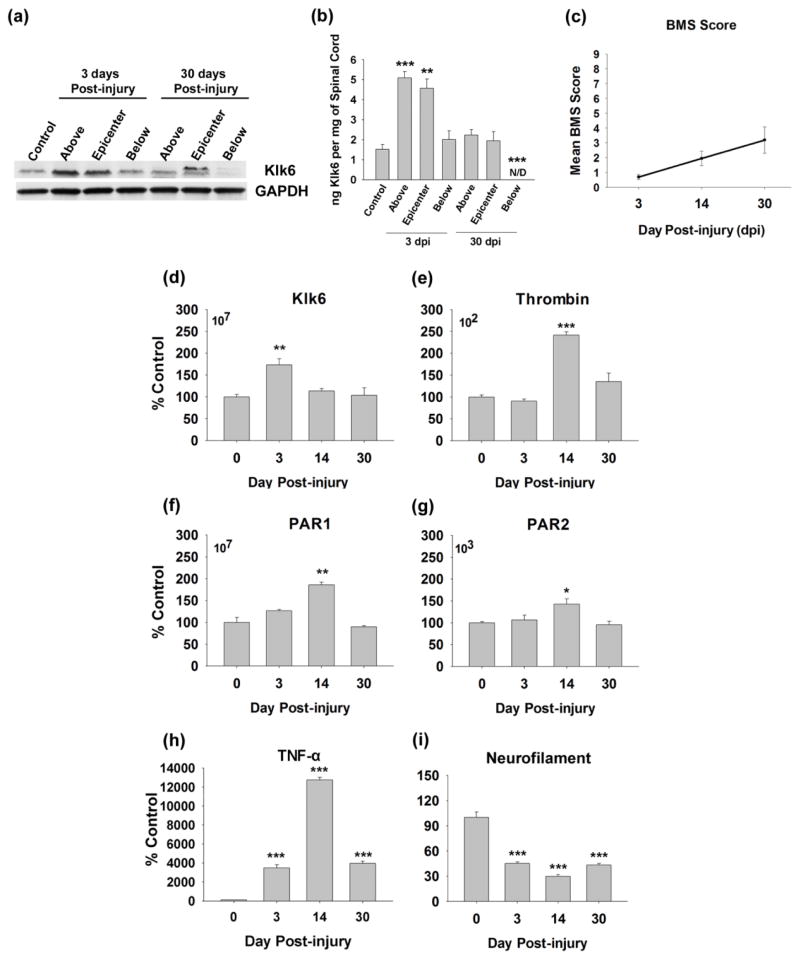
Fig. 1. Klk6, thrombin, PAR1 and PAR2 are differentially regulated in the spinal cord in response to clip compression injury.
Western blot (a) and corresponding histogram (b) show differential expression of Klk6 protein in the uninjured spinal cord (control) and at the level of the injury epicenter, as well as above and below at 3 and 30 days post-clip compression injury (GAPDH was used to control for loading). At 3 dpi, Klk6 protein levels (ng/mg of spinal cord) were elevated by 3-fold at the injury epicenter (p = 0.004) and above (p ≤ 0.001, Student’s t-test). At 30 dpi, Klk6 protein levels were significantly reduced below the epicenter (p = 0.001, Student’s t-test). (c) Graph shows the mean BMS score at acute (3d), subacute (14d) and chronic (30d) time points post-injury. Histograms (d) to (i), show changes in RNA expression for Klk6, thrombin, PAR1, PAR2, TNF-α and Neurofilament H at 3, 14 and 30 dpi relative to levels detected in the uninjured control (0 dpi) spinal cord. Klk6 RNA levels were significantly elevated at acute stages, while thrombin, PAR1 and PAR2 RNA levels were elevated at subacute time points (*p = 0.02, **p ≤ 0.008, ***p ≤ 0.001, Student’s t-test). TNF-α RNA expression was elevated by 3d, peaked at 14d and remained elevated out to 30 dpi. By contrast, neurofilament H RNA was significantly decreased from 3 to 30 dpi. The mean copy number of each gene RNA transcript amplified in 0.25 μg of RNA from uninjured spinal cord is also provided (upper left corner), indicating that Klk6 is expressed at approximately 5-log higher levels than thrombin, while PAR1 is expressed at approximately 4-log higher levels relative to PAR2.
The impact of either Klk6 or thrombin on neuron number and neurite density was quantified from 5 digitally captured 40X pre-determined microscopic fields encompassing the center and 4 poles of each cover slip taken without knowledge of the treatment groups(Regan & Choi 1991, Park et al. 1999, Scarisbrick et al. 2002, Scarisbrick et al. 2006b, Koshimizu et al. 2009). Digital images were then used to make counts of intact neurons. A mean of 170 ± 10.9 neurons were counted per coverslip. Neurite number was evaluated by superimposing a grid consisting of 522.5 μm2 squares on to each digital image and counting processes which overlapped grid lines (Scarisbrick et al. 2002). Histograms shown represent the mean and standard error of counts across 3 independent cover slips treated in parallel within a given experiment and results were reproducible across 3 experiments utilizing separate murine cerebellar granule neuron preparations.
To determine the role of PAR1 in Klk6- or thrombin-mediated neurotoxicity, experiments were repeated examining the effects of each protease toward PAR1+/+ or PAR1−/− cerebellar granule neurons grown in parallel. In these experiments, Klk6 was applied at either 150 or 300 nM and thrombin at either 135 or 270 nM. After a 24 h treatment period, neurons were stained with Coomassie and both neuron and neurite number quantified as described.
To evaluate the ability of Klk6 to exacerbate glutamate neurotoxicity, we examined its effects on Bim protein levels and cleavage of a downstream indicator of apoptosis that is PARP cleavage, in NSC34 spinal cord motoneurons. NSC34 motoneurons were treated with glutamate alone (300 μM), or with glutamate in addition to 0.5, 1 or 3 μg/ml Klk6 (15, 30 or 90 nM). The possible role of PAR1 and PAR2 in mediating Klk6-glutamate elicited Bim signaling or PARP cleavage was then evaluated by pre-treatment of cultures for 3hr with 30 μM of the PAR1- (P1pal-7) and PAR2-specific (P2pal-18s) lipopeptide inhibitors, prior to application of Klk6, or Klk6 in addition to glutamate. Protein was harvested 24 h after treatment and the level of Bim protein and cleaved PARP analyzed by Western blot. At the time of protein harvest, the media was also collected and snap frozen at −70 C for later examination of lactate dehydrogenase (LDH) levels (Clontech, Mountain View, CA).
Statistical analysis
Statistical differences in the case of pair wise comparisons were made using unpaired Student’s t-tests. When comparisons were made between multiple groups, One Way ANOVA with the Student Newman Keul’s (SNK) post hoc test was applied. In all cases, p < 0.05 was considered to be statistically significant.
Results
Regulated expression of Klk6, thrombin and their receptors in a clip compression model of traumatic SCI
To investigate the relevance of Klk6 to the SCI microenvironment, we quantified changes in Klk6 protein at 0, 3 and 30 dpi (Fig. 1a, b). Levels of Klk6 were quantified using Western blot at the level of the injury epicenter, as well as in the spinal segments for 5 mm above and 5 mm below. At 3dpi, Klk6 protein levels increased by approximately 3-fold at the injury epicenter from 1.5 ng/mg ± 0.2 to 4.6 ng/mg ± 0.4 (p = 0.004, Students t-test). Equivalent increases in Klk6 protein were also observed in the 5 mm of spinal cord tissue examined above the site of compression at 3 dpi (p ≤ 0.001, Students t-test), while Klk6 protein was unchanged in the 5 mm of spinal cord tissue below the lesion epicenter. By 30 dpi, overall Klk6 levels at the site of compression and above had returned to uninjured control levels, while below the lesion, Klk6 levels were substantially reduced (p = 0.001, Students t-test). At 30 dpi, the Klk6 antibody detected two bands, including a band of 27 kDa detected in each of the other samples, in addition to a band approximately 2 kDa greater. De novo detection of the upper band may reflect an increase in the amount of the zymogen precursor for Klk6 or differential glycosylation at 30 dpi.
To evaluate the potential for Klk6 to mediate its effects by signaling through PAR1 and/or PAR2 in traumatic SCI, we evaluated transcriptional changes in the expression of each gene at acute (3 dpi), subacute (14 dpi) and more chronic (30 dpi) time points post-injury (Fig. 1d–g). Since thrombin is the prototypical activator of PAR1 (Vu et al. 1991), and known to mediate neurotoxicity (Smirnova et al. 1998), possible transcriptional changes in this serine protease were also examined in parallel, although possibly the greatest source of thrombin in acute SCI will be extravasation. The mean BMS score of mice examined was 0.7 ± 0.2 (3 dpi), 1.9 ± 0.5 (14 dpi), and 3.2 ± 0.9 (30 dpi) respectively, paralleling paralysis and the extent of recovery seen in prior studies using the Ohio state impactor contusion injury model (Basso et al. 2006) (Fig. 1c). As expected, Klk6 RNA was detected at high levels in the uninjured spinal cord (approximately 107 copies in 0.5 μg of RNA) and elevated by approximately 1.8-fold within the injury epicenter at 3 dpi (p < 0.008, Students t-test) (Christophi et al. 2004, Scarisbrick et al. 2012b). Thrombin RNA by contrast was far less abundant in the uninjured spinal cord, (approximately 102 copies in 0.5 μg of RNA) and elevated by 2.5-fold at the site of injury by 14 dpi (p ≤ 0.001, Students t-test). Like Klk6, PAR1 RNA was expressed at high levels in the uninjured spinal cord (approximately 107 copies in 0.5 μg of RNA), while PAR2 RNA was expressed at approximately 4-log lower levels. RNA encoding each PAR was significantly elevated (1.5 to 2-fold) at the site of injury by 14 dpi (p < 0.008, Students t-test). Validating the injury model, levels of RNA encoding the pro-inflammatory cytokine TNF-α were significantly elevated, while neurofilament H RNA levels were significantly decreased over the same period post injury (Fig. 1h and 1i).
Klk6 elicits ERK1/2 signaling in neurons by activation of PAR1 and PAR2
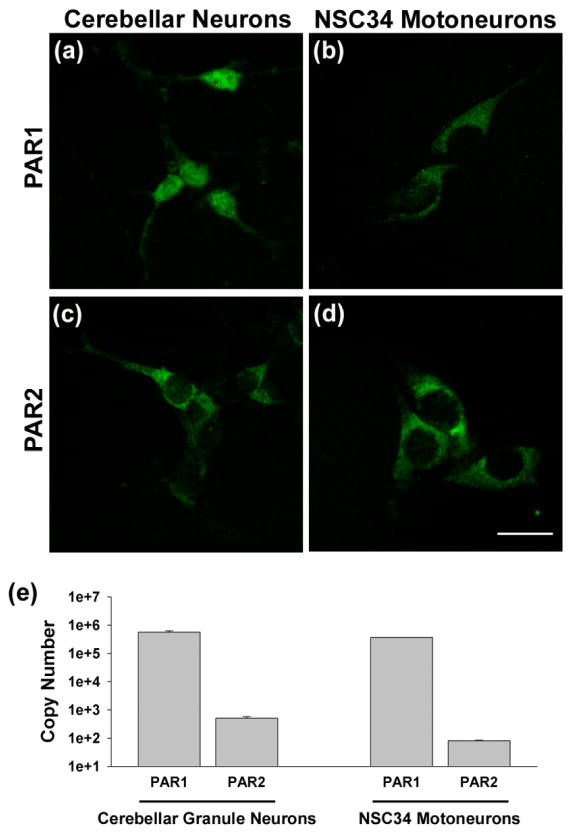
Primary murine cerebellar granule neurons and the murine NSC34 spinal cord motoneuron cell line were used to evaluate the potential neurotoxic effects mediated by Klk6 and thrombin as well as the involvement of PAR1 and PAR2 (Figs. 2–6). First, we used quantitative RT-PCR to demonstrate that PAR1 RNA was abundant in each neuron type being expressed at 5.7×105 ± 5.8 ×104 copies in 0.5 μg of cerebellar granule neuron RNA and at 3.7×105 ± 2.0 ×103 copies in an equal amount of NSC34 motoneuron RNA (Fig. 2e). By contrast, and in parallel to the relative distribution of PAR1 and PAR2 in the murine spinal cord (Fig. 1f–g) and in murine cortical neurons (Vandell et al., 2008), PAR2 RNA levels were detected at approximately 3-log lower levels, with 5.1×102 ± 6.9 ×101 and 8.1×101 ± 4.3 detected in 0.5 μg of RNA purified from cerebellar granule neurons or NSC34 motoneurons, respectively. Punctuate staining for each receptor was also visualized in each neuron type using PAR1- and PAR2-specific antibodies (Fig. 2a–d).
Fig. 2. PAR1 and PAR2 are differentially expressed in primary cerebellar granule neurons and in the NSC34 motoneuron cell line.
Photomicrographs show punctate immunofluorescence staining for PAR1 and PAR2 in (a, c) primary cerebellar granule neurons and (b, d) NSC34 motoneurons. (e) Histograms show quantitative PCR evaluation of PAR1 and PAR2 RNA in 0.5 μg of RNA isolated from cerebellar granule neurons or NSC34 motoneurons matured for 72 h in vitro. PAR1 was significantly more abundant than PAR2 in each neuron type. (Scale bar = 20 μm)
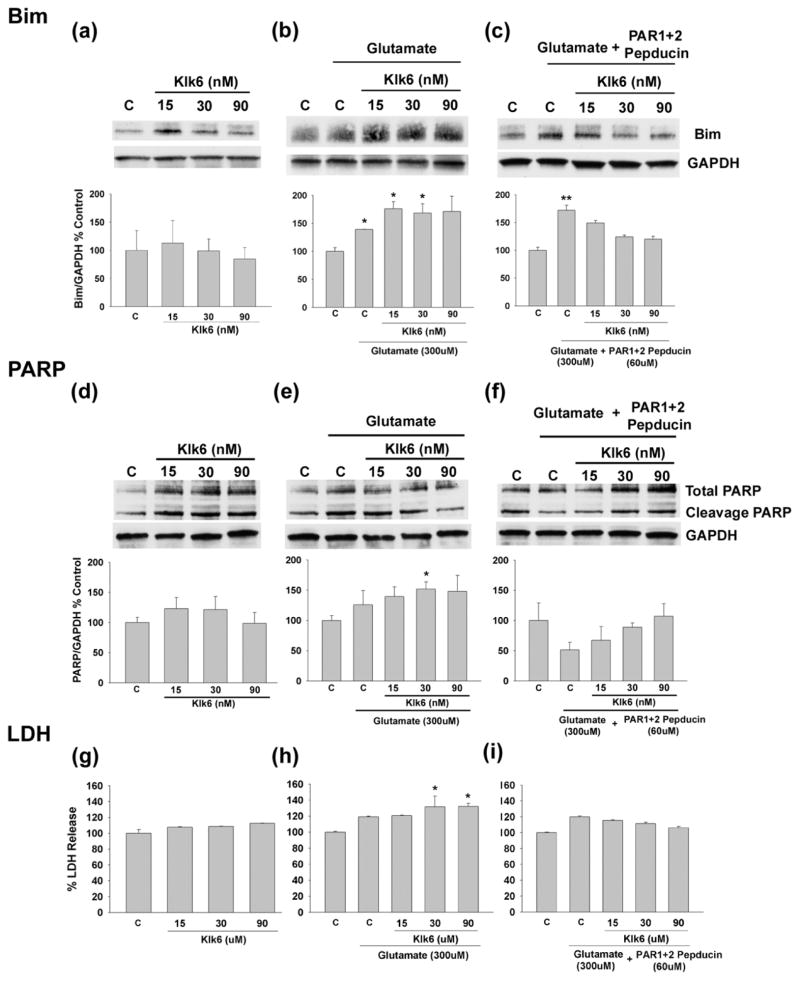
Fig. 6. Klk6 signaling through PAR1 and PAR2 enhances glutamate-mediated expression of Bim and PARP cleavage.
Western blots and corresponding histograms show that treatment of NSC34 neurons with glutamate (300 μM) for 24 hr increases expression of Bim (a to c, Bim EL isoform shown). The level of glutamate elicited Bim expression was further increased by co-application of Klk6 (0.5 μg/ml (15 nM) or 1 μg/ml (30 nM), b). In combination, Klk6 and glutamate also significantly elevated levels of cleaved PARP (full length 116kDa, cleaved 89kDa, e) and release of LDH (h). Pre-treatment of NSC34 motoneurons with lipopeptide inhibitors of PAR1 and PAR2, blocked the ability of Klk6 to enhance glutamate-mediated Bim expression, PARP signaling and LDH release (C, vehicle control; *p ≤ 0.05, **p = 0.008 Student’s t-test).
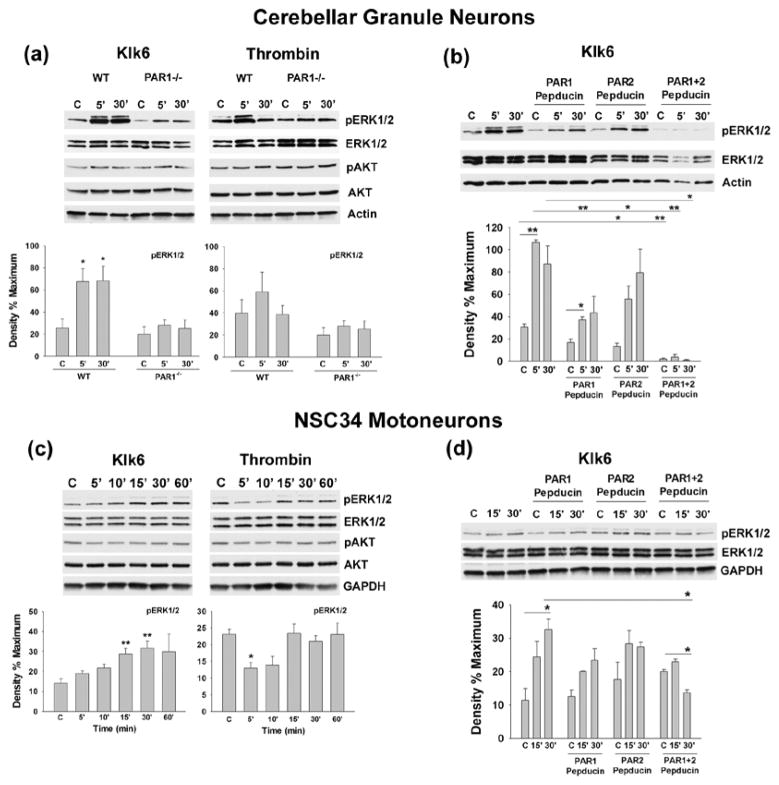
In both primary cerebellar granule neurons and NSC34 spinal cord motoneurons, Klk6 elicited robust activation of ERK1/2, although the timing of activation in each neuron type differed slightly (Fig. 3). In primary neurons, Klk6 triggered ERK1/2 activation as early as 5 min after treatment and this remained significantly elevated out to the 30 min endpoint examined (p < 0.03, Students t-test) (Fig. 3a). In NSC34 motoneurons, Klk6 elicited significant ERK1/2 activation at 15 and 30 min treatment intervals (Fig. 3c). By contrast, the thrombin elicited elevations in ERK1/2 activation seen in cerebellar granule neurons (Fig. 3a) did not reach the level of statistical significance across three independent experiments. In NSC34 motoneurons, thrombin reduced ERK1/2 signaling (Fig. 3c, p ≤ 0.05, Students t-test). Neither Klk6 nor thrombin significantly altered signaling in the AKT pathway in these neuron types under the conditions of this study.
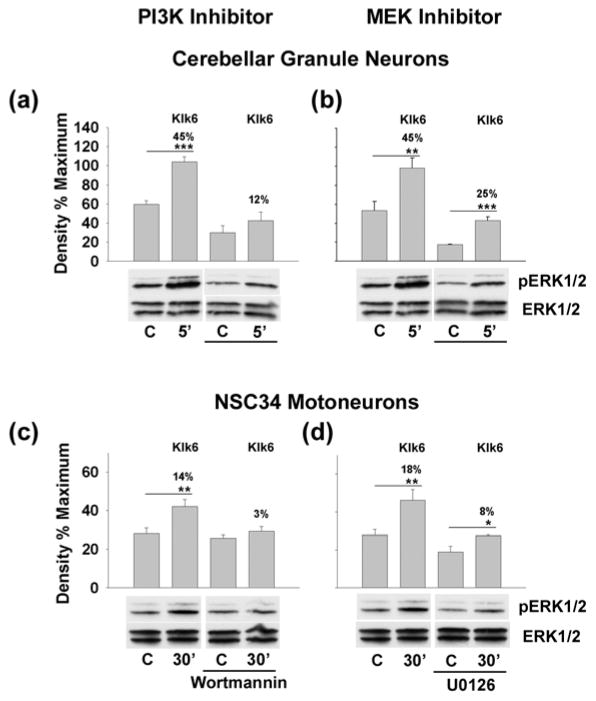
Fig. 3. Klk6-mediated ERK1/2 signaling occurs by activation of PAR1 and PAR2.
(a) Western blots and histograms show Klk6 (150 nM) and thrombin (135 nM) elicit activation of ERK1/2 in cerebellar granule neurons derived from WT, but not PAR1−/− mice. (b) and (d) show that Klk6-mediated ERK1/2 phosphorylation in cerebellar neurons can be blocked using novel lipopeptide inhibitors specific for PAR1 (P1pal-7) or PAR2 (P1pal-18s), when applied alone, or in combination. (c) Klk6 also promoted ERK1/2 activation in NSC34 motoneurons, which was blocked by co-application of the PAR1- and PAR2-specific lipopeptide inhibitors (d) (C, vehicle control; *p ≤ 0.05, **p ≤ 0.008, Student’s t-test). Klk6 or thrombin elicited changes in AKT activation did not reach the level of statistical significance. Actin and GAPDH were used to control for loading in primary granule neurons and NSC34 motoneurons, respectively.
Using a combination of primary cerebellar granule neurons derived from PAR1 deficient or WT mice, NSC34 spinal cord motoneurons and novel lipopeptide inhibitors of PAR1 and PAR2, we demonstrate that both PARs are likely to play an important role in Klk6-mediated ERK1/2 signaling (Fig. 3a–d). Genetic deletion of PAR1 in cerebellar granule neurons blocked the ability of Klk6 to elicit significant ERK1/2 activation (Fig. 3a, p ≤ 0.05, Students t-test). Pre-incubation of primary cerebellar granule neurons or NSC34 motoneurons with a lipopeptide inhibitor of PAR1 Gi3 (P1pal-7), also significantly reduced (5 min time point), or eliminated (30 min time point), the ability of Klk6 to elicit ERK1/2 signaling (Fig. 3b, p ≤ 0.05, Students t-test). Interestingly, a lipopeptide targeting PAR2 Gi3 (P1pal-18s) suppressed baseline ERK1/2 signaling in primary neurons and reduced the ability of Klk6 to elicit ERK1/2 activation at the 5 min time point (p ≤ 0.05, Students t-test). Importantly, when both PAR1- and PAR2-lipopeptides were applied to primary cerebellar granule neurons, baseline ERK1/2 activation was significantly reduced and Klk6 was unable to elicit any significant signaling at either the 5 or 30 min time points examined (Fig. 3b, p ≤ 0.008, Students t-test). In NSC34 spinal cord motoneurons, the PAR1- and PAR2-lipopeptide inhibitors also blocked Klk6-mediated ERK1/2 signaling when applied alone or in combination (Fig. 3d, p ≤ 0.05, Students t-test). The lipopeptide inhibitors applied in these studies target ERK1/2 signaling mediated by PAR1 or PAR2, and correspondingly, no significant effect on baseline AKT signaling was observed (data not shown).
To further investigate the intracellular signaling cascades leading to Klk6-mediated ERK1/2 signaling, we evaluated the ability of inhibitors of PI3K, MEK or PKC to block the signaling observed (Fig. 4). In primary cerebellar and NSC34 spinal cord motoneurons the PI3K inhibitor Wartmannin completely blocked Klk6-mediated ERK1/2 activation (Fig. 4a and c). The MEK inhibitor, U0126, also significantly suppressed the ability of Klk6 to elicit ERK1/2 signaling (Fig. 4b and d). By contrast, an inhibitor of PKC (Go 6983) had no significant effect on Klk6-mediated ERK1/2 activation in primary neurons (data not shown).
Fig. 4. Klk6 triggers ERK1/2 signaling through PI3K and MEK.
Histograms show Klk6 (150 nM)-mediated ERK1/2 signaling in cerebellar neurons (a, b) or NSC34 motoneurons (c, d) was significantly reduced by pre-application of either an inhibitor of PI3K (a, c, Wortmannin, 200 nM) or MEK (b, d, U0126, 150 nM). (C, vehicle control; *p = 0.05, ** p ≤ 0.04 and ***p = 0.003, Student’s t-test). Non-phosphorylated ERK1/2 was used to control for loading in each case.
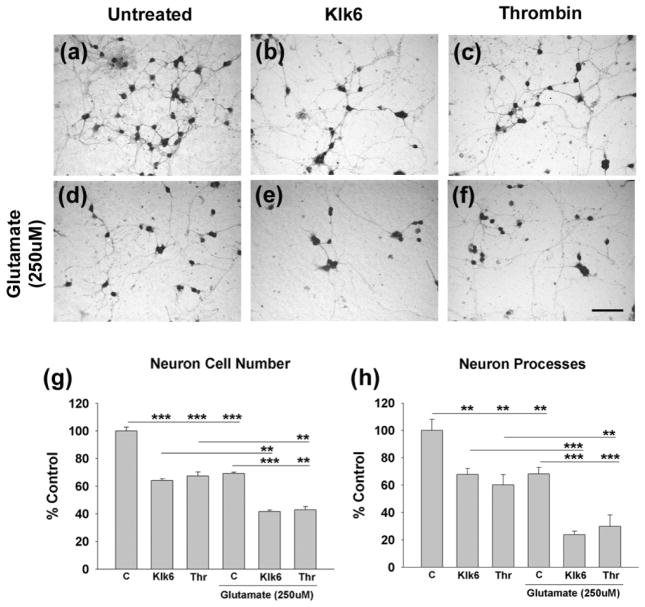
Klk6 and thrombin elicit cerebellar granule neuron toxicity in vitro and exacerbate glutamate-mediated neurotoxicity
Recombinant Klk6 triggers a significant reduction in cerebellar granule neuron numbers and neuronal processes after 24 h treatment in vitro (Fig. 5a). Parallel concentrations of thrombin elicited equivalent neurotoxic effects. Since glutamate is a well-recognized mediator of neurotoxicity, we investigated whether Klk6 or thrombin exacerbate glutamate’s effects. 250 μM glutamate, or 10 μg/ml of Klk6 (300 nM) or thrombin (270 nM), each elicited a similar level of neurotoxicity, with approximately 40 to 50% of neurons and neuronal processes being lost after 24 h treatment in vitro (Fig. 5a–f, p ≤ 0.008, SNK). When Klk6 or thrombin were applied in combination with glutamate, an increased level of neuron loss and process damage was observed relative to the effects of either agent alone (approximately a 20% increase in neuron degeneration and 30% increase in process loss (p ≤ 0.003, SNK).
Fig. 5. Klk6-mediated neurotoxicity is exacerbated by excess glutamate.
(a to f), Photomicrographs show cerebellar granule neurons either (a) untreated, or (b, c) treated with 300 nM of either Klk6 or 270 nM thrombin alone, or in combination with glutamate (250 μM, e, f) for 24 hr. Paraformalydehyde fixed cultures were stained by Coomassie and neuron and process number quantified (g, h). Klk6 and thrombin each reduced neuron and neural process number relative to vehicle treated control cultures paralleling the neurotoxic effects generated by 250 μM glutamate alone (d). The combination of glutamate in addition to Klk6, or thrombin, generated greater neuron and neural process loss than did any of these treatments alone (C, vehicle control; *p ≤ 0.05, **p ≤ 0.008; ***p ≤ 0.002, SNK). (Scale bar = 50 μm).
To investigate the possible signaling mechanism involved in Klk6-glutamate-mediated neural injury, and whether this could be altered by targeting PAR1 and PAR2 using novel lipopeptide inhibitors, we examined signaling of the pro-apoptotic protein Bim using the NSC34 spinal cord motoneuron cell line as a model. Treatment of NSC34 motoneurons with glutamate elicited a significant increase in Bim (Fig. 6a–c, p ≤ 0.05, Students t-test). While Klk6 did not elevate Bim signaling on its own, it did significantly elevate that elicited by glutamate (p ≤ 0.05, Students t-test). The co-application of Klk6 and glutamate also increased levels of cleaved PARP (Fig. 6e) and release of LDH into the cell culture supernatant (Fig. 6h). The ability of Klk6 and glutamate co-treatment to elevate Bim signaling or to increase cleaved PARP and LDH release were all blocked by pre-incubation of NSC34 motoneurons with lipopeptide inhibitors of PAR1 and PAR2 (Fig. 6c, f and i). Notably, the PAR lipopeptide inhibitors did not affect elevations in Bim seen in the presence of glutamate alone.
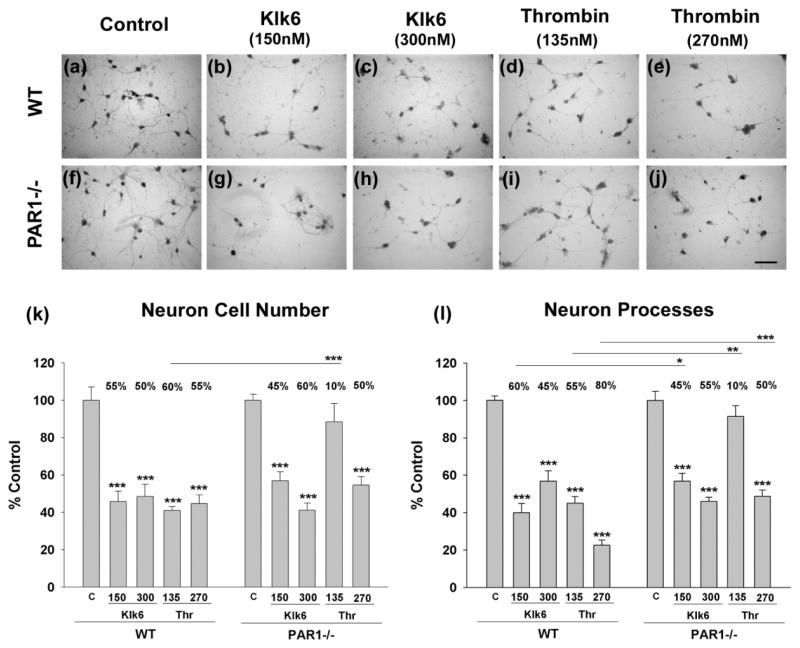
Klk6- and thrombin-induced neurite retraction are reduced with the absence of PAR1
To further evaluate the role of PAR1 in Klk6-mediated neurotoxicity we examined whether cerebellar granule neurons derived from PAR1 deficient mice show reduced levels of protease-elicited toxicity relative to neurons derived from wild type littermates (Fig. 7). In the absence of PAR1, there was a 15% reduction in the loss of neuronal processes elicited by 5 μg/ml Klk6 (150 nM, 15% reduction, p < 0.03), or by an equivalent amount of thrombin (135 nM, 45% reduction, p < 0.004). The absence of PAR1 also reduced loss of neuron processes in response to higher concentrations of thrombin (270 nM, 30% reduction, p ≤ 0.001), but not in response to the higher concentrations of Klk6 examined (300 nM). The absence of PAR1 did not significantly alter Klk6-mediated loss of neurons, but did significantly reduce that triggered by thrombin, at least at the lower concentrations (135 nM) examined (approximate 50% reduction, p ≤ 0.001, SNK).
Fig. 7. PAR1-deficiency reduces the ability of Klk6 to promote neurotoxicity.
Photomicrographs (a to j), show WT or PAR1−/− primary cerebellar granule neurons treated with 150 or 300 nM of Klk6 or 135 or 270 nM thrombin for 24 h (Coomassie stain). (k), Histogram shows quantification of neuron number in each condition and demonstrates that the absence of PAR1 blocks the ability of 135 nM thrombin to promote significant loss. (l), The absence of PAR1 also blocked or reduced the ability of thrombin to promote neurite loss in the case of both 150 and 300 nM treatment conditions. The absence of PAR1 also reduced the ability of Klk6 to promote neurite loss at the lower concentration examined (*p = 0.03, **p = 0.004, ***p ≤ 0.001, SNK). (Scale bar = 50 μm)
Discussion
In this study we identify Klk6, like thrombin, as an important mediator of CNS neurotoxicity by a mechanism involving activation of PAR1. First, we show that Klk6 and thrombin, as well as PAR1 and PAR2, are each elevated in a murine model of experimental compression SCI, positioning each as a key player in the cascade of events that drive secondary injury. Using genetic and novel lipopeptide antagonists of PARs, we show Klk6 elicits robust ERK1/2 signaling in primary cerebellar neurons and in a spinal cord motoneuron cell line by a mechanism that involves activation of both PAR1 and PAR2. Further, both Klk6 and thrombin were shown to potentiate glutamate- neurotoxicity, with the effects of Klk6 involving a PAR1- and PAR2-dependent Bim signaling cascade. Moreover, both Klk6 and thrombin mediated neurotoxicity in primary neurons in vitro was significantly reduced by genetic deletion of PAR1. Taken together these studies identify the Klk6- and thrombin-PAR signaling axes as potentially important players in the response of CNS neurons to injury and as targets for the development of neuroprotective strategies.
Regulated expression of kallikrein 6 and thrombin in traumatic SCI
Due to their participation in regulatory cascades, serine protease activity in all organ systems, including the CNS, is tightly regulated at the level of expression and proteolytic activation/inactivation. Deregulated levels of thrombin and the plasminogen activators are known to promote neuropathophysiological changes, including neuron injury and gliopathy (Tsirka et al. 1995, Gingrich & Traynelis 2000, Striggow et al. 2000, Friedmann et al. 2001, Choi et al. 2003, Nicole et al. 2005, Bennur et al. 2007). Kallikreins represent the largest contiguous cluster of serine proteases in the human genome with each found in serum and 11 of the 15 family members expressed in the CNS (Scarisbrick et al. 2006a, Shaw & Diamandis 2007). Kallikreins are therefore an important family of serine proteases positioned to mediate CNS injury and disease, particularly in cases in which the blood brain barrier is compromised such as stroke, SCI and multiple sclerosis.
Kallikrein 6 is of particular interest with respect to CNS physiology and pathophysiology since it is expressed at high levels in the brain and spinal cord and expression includes both neurons and oligodendroglia (Scarisbrick et al. 1997, Scarisbrick et al. 2000, Scarisbrick et al. 2002). In addition, Klk6 expression is induced/elevated in reactive astroglia and infiltrating immune cells in all CNS pathologies examined to date, including SCI, multiple sclerosis and glioblastoma multiforme (Scarisbrick et al. 2006b, Scarisbrick et al. 2012a). Further establishing the significance of Klk6 to CNS trauma, in this study we demonstrate that Klk6 RNA and protein are elevated acutely (3 dpi) in murine experimental SCI. Importantly, elevations in Klk6 protein were seen at the injury epicenter and in spinal segments immediately above. Interestingly, in spinal segments immediately below the epicenter, Klk6 levels were unchanged acutely and significantly reduced at more chronic stages. We previously showed Klk6 to be elevated in reactive astrocytes and monocytes/microglia in rat contusion injury and in post-mortem cases of human SCI (Scarisbrick et al. 2006b, Scarisbrick et al. 2012a) and it is therefore likely that elevations seen here reflect similar cellular changes. Importantly, immunohistochemical analysis of Klk6 in the rat contusion model also revealed peak elevations at acute stages (Scarisbrick et al. 2006b), pointing to this period as a potentially key therapeutic window. Elevations in Klk6 in response to intraperitoneal injection of the AMPA/kainite glutamate receptor agonist, kainic acid, also peaked at 3d (Scarisbrick et al. 1997). The significant reductions in Klk6 below the site of compression injury at 30 dpi may reflect a loss of activity caudal to the injury site and/or the degeneration of Klk6 producing cells.
Using quantitative real-time PCR we demonstrate that thrombin RNA is also expressed in the parenchyma of the adult spinal cord, albeit at 5-log lower levels than Klk6. In general agreement with conventional PCR results in rat contusion injury (Citron et al. 2000), we observed thrombin RNA to increase more than 2-fold subacutely. At least low levels of thrombin RNA have also been detected in rodent brain (Dihanich et al. 1991) and increases documented in other pathophysiological conditions, including ischemia and Alzheimers (Riek-Burchardt et al. 2002, Arai et al. 2006, Chen et al. 2012). There is considerable evidence that elevated levels of thrombin mediate neurotoxicity in a PAR1-dependent fashion (Smirnova et al. 1998, Turgeon et al. 1998, Choi et al. 2005, Fujimoto et al. 2006, Han et al. 2011) and this was confirmed here using primary cerebellar granule neurons as a model. Thrombin potentiates NMDA receptor function (Gingrich et al. 2000, Han et al. 2011) and promotes apoptosis of cultured neurons and astrocytes (Donovan et al. 1997). Thrombin can also have direct effects on blood brain barrier tight junctions (Kondo et al. 2009), astrogliosis (Wang et al. 2002, Nicole et al. 2005) and induces inflammatory responses, including microglial activation (Suo et al. 2002, Choi et al. 2005, Henrich-Noack et al. 2006), each of which can further exacerbate neural injury (Nishino et al. 1993, Keep et al. 2005). In cases of SCI therefore, in which the BBB is compromised, thrombin proteolytic cascades may become deregulated due to entry from blood where it is found at micromolar concentrations (Putnam 1975) and as the present studies show, as a result of transcriptional elevations within the parenchyma of the spinal cord.
Kallikrein 6 as a signaling molecule in the intact and injured CNS
Klk6 elicited robust ERK1/2 signaling in primary cerebellar granule neurons and in the NSC34 spinal cord motoneuron cell line. PAR1 was shown to be an essential mediator of this signaling, since genetic loss of the receptor in cerebellar neurons blocked Klk6-ERK1/2-activation. We also present evidence that Klk6 additionally activates PAR2 in cerebellar granule neurons and the NSC34 motoneuron cell line, since either a PAR1 or a PAR2 lipopeptide inhibitor significantly reduced Klk6-mediated ERK1/2 signaling. Importantly, simultaneous application of the PAR1 and PAR2 lipopeptide inhibitors completely blocked Klk6-ERK1/2-activation in both neuron types. Also, in each case, ERK1/2 signaling was dependent on activation of PI3K and Mek. Future studies will be needed to determine the generalizability of these findings to other neuron populations, but given the widespread expression of PARs in the CNS(Striggow et al. 2001), including cortical neurons (Vandell et al. 2008), hippocampal neurons (Gorbacheva et al. 2006) and cerebellar granule neurons as shown herein, we believe the current findings point to a new signaling axis involving Klk6 activation of PAR1 and PAR2. Importantly, by way of activation of ERK1/2 the Klk6-PAR signaling axis would be capable of affecting fundamental properties governing neuron physiology and the response to injury (Waltereit & Weller 2003).
Since we show both PAR1 and PAR2 RNA are constitutively expressed in the adult spinal cord, and are elevated at subacute time points after traumatic SCI, these G-protein coupled receptors are poised to mediate the hormone-like actions of proteases such as Klk6 under physiological and pathophysiological conditions. PAR1 was previously shown to be elevated in the contused rat spinal cord (Citron et al. 2000). Prior studies have also shown PAR1 to be associated with CNS astrocytes and a subpopulation of neurons (Junge et al. 2004, Scarisbrick et al. 2008, Han et al. 2011) and here we demonstrate abundant expression at an RNA level by cerebellar granule neurons in vitro. In cases of CNS injury, elevations in PAR1 have been specifically associated with astrocytes in Parkinsons (Ishida et al. 2006), in human immunodeficiency virus (HIV) encephalitis (Boven et al. 2003) and in rodent models of Alzheimers (Pompili et al. 2004). Elevations in PAR2 have been reported in association with neurons in HIV-1 associated dementia (Noorbakhsh et al. 2005). Interestingly, quantitative PCR in the present studies indicates PAR1 RNA is expressed at approximately 4-log higher levels in spinal cord and more than 2-log higher levels in cerebellar granule neurons relative to PAR2. Similarly, in a prior report we demonstrated significantly higher expression levels of PAR1 in murine cortical neurons, in murine whole brain and in the NSC34 spinal cord motoneuron cell line relative to PAR2 (Vandell et al. 2008). Taken together these studies point to a particularly prominent role for PAR1 in the CNS.
Involvement of kallikrein 6-PAR signaling in neurotoxicity including that mediated by glutamate
A direct role for PAR1 in mediating Klk6 and thrombin generated toxicity in CNS neurons was established in the present studies using cerebellar granule neurons derived from wild type or PAR1 deficient mice. The absence of PAR1 completely blocked thrombin-mediated degeneration of cerebellar neurons and neural processes at the lower concentrations examined. In parallel, the absence of PAR1 also significantly reduced Klk6-mediated loss of neural processes, although neurotoxicity was not blocked. Given our findings that both PAR1 and PAR2 play a role in Klk6-medited ERK1/2 signaling, it is possible that PAR2 may also play a role in Klk6-neurotoxicity and additional studies to determine the effects of blocking both PAR1 and PAR2 will be an important future direction. To further delineate the scope of action of Klk6 and thrombin in neural injury it will also be important to examine a broader range of protease concentrations and neuron cell types in future studies.
Excess glutamate signaling is a well-recognized mediator of secondary injury in cases of CNS trauma and disease by a mechanism that involves excitotoxicity (Springer et al. 1997, Lin et al. 1998, Rossi et al. 2000) or by the induction of oxidative stress (Bridges et al. 2012). Prior studies demonstrating Klk6 is up regulated in the rat spinal cord in response to activation of the AMPA/kainite glutamate receptor (Scarisbrick et al. 1997) position this secreted serine protease to play an integral role in proteolytic injury in the context of glutamate receptor activation and therefore we examined the possibility of combinatorial effects. In the present study, we demonstrate that both Klk6 and thrombin-mediated neurotoxicity toward cerebellar neurons is exacerbated by glutamate. In the case of the complete media conditions used in this study, Klk6 (300 nM), thrombin (270 nM) and glutamate (250 μM) each elicited similar levels of neural injury in primary neurons, suggesting that in excess; each can serve as a neurotoxic agent on its own. Klk6 was also shown to augment glutamate-mediated signaling through the pro-apoptotic protein Bim and well as glutamate-mediated PARP cleavage and LDH release in NSC34 spinal cord motoneurons. These Klk6-mediated pro-injury effects were further shown to be PAR-dependent since each was blocked by lipopeptide inhibitors of PAR1 and PAR2. These data taken with findings that the harmful actions of PAR1 in ischemia require NMDA receptor function (Hamill et al. 2009) suggest that PAR/glutamate receptor co-activation is an important mediator of neurotoxicity. Since glutamate was evaluated in the present studies, rather than agonists of individual receptor subtypes, additional studies will be needed to determine which specific glutamate receptor(s) may be involved. Also, determination of which mechanism of glutamate neurotoxicity, albeit excitotoxicity and/or oxidative stress due to disruption of the cystine-glutamate exchanger (Murphy et al. 1990, Ratan et al. 1994, Himi et al. 2003) could be addressed as this line of research unfolds.
Together, the results presented indicate that Klk6, like thrombin (Chen et al. 2012), is an important proteolytic mediator of signaling in CNS neurons and involved in neurotoxicity, including an exacerbation of that generated by glutamate. Of interest, we previously demonstrated that Klk6 activates PAR1 on astrocytes (Scarisbrick et al. 2012a) and activation of astrocytic PAR1 is known to promote glutamate release (Nicole et al. 2005, Hermann et al. 2009). These findings taken with those of the present study point to a PAR-agonist-mediated neurotoxicity cascade whereby Klk6 and thrombin signal directly through neuronal-PAR, or indirectly through astrocyte-PAR, to promote neuron injury. Therefore, Klk6 and the PARs it activates across neurons and glia represent novel targets for the development of neuroprotective strategies.
Acknowledgments
These studies were supported by R01NS052741, The Christopher and Dana Reeve Paralysis Foundation, RG3367, a Collaborative MS Research Award CA1060A11 and PP2009 from the National Multiple Sclerosis Society and the Craig H. Neilsen Foundation (IAS).
Abbreviations
- CNS
central nervous system
- Klk
Kallikrein
- PAR
protease-activated receptor
- ERK1/2
extracellular signal-regulated kinases
- AKT
protein kinase B
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- SCI
spinal cord injury
- BMS
Basso Mouse Scale
- dpi
days post-injury
Footnotes
The authors declare no competing financial interests.
References
- Arai T, Miklossy J, Klegeris A, Guo JP, McGeer PL. Thrombin and prothrombin are expressed by neurons and glial cells and accumulate in neurofibrillary tangles in Alzheimer disease brain. Journal of neuropathology and experimental neurology. 2006;65:19–25. doi: 10.1097/01.jnen.0000196133.74087.cb. [DOI] [PubMed] [Google Scholar]
- Ashby EL, Kehoe PG, Love S. Kallikrein-related peptidase 6 in Alzheimer’s disease and vascular dementia. Brain research. 2011;1363:1–10. doi: 10.1016/j.brainres.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Benavente F, Pinto C, Parada M, Henriquez JP, Osses N. Bone morphogenetic protein 2 inhibits neurite outgrowth of motor neuron-like NSC-34 cells and up-regulates its type II receptor. Journal of neurochemistry. 2012;122:594–604. doi: 10.1111/j.1471-4159.2012.07795.x. [DOI] [PubMed] [Google Scholar]
- Bennur S, Shankaranarayana Rao BS, Pawlak R, Strickland S, McEwen BS, Chattarji S. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience. 2007;144:8–16. doi: 10.1016/j.neuroscience.2006.08.075. [DOI] [PubMed] [Google Scholar]
- Bernett MJ, Blaber SI, Scarisbrick IA, Dhanarajan P, Thompson SM, Blaber M. Crystal structure and biochemical characterization of human kallikrein 6 reveals that a trypsin-like kallikrein is expressed in the central nervous system. J Biol Chem. 2002;277:24562–24570. doi: 10.1074/jbc.M202392200. [DOI] [PubMed] [Google Scholar]
- Biasini E, Unterberger U, Solomon IH, et al. A mutant prion protein sensitizes neurons to glutamate-induced excitotoxicity. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:2408–2418. doi: 10.1523/JNEUROSCI.3406-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaber SI, Ciric B, Christophi GP, Bernett MJ, Blaber M, Rodriguez M, Scarisbrick IA. Targeting kallikrein 6-proteolysis attenuates CNS inflammatory disease. FASEB J. 2004;19:920–922. doi: 10.1096/fj.03-1212fje. [DOI] [PubMed] [Google Scholar]
- Blaber SI, Scarisbrick IA, Bernett MJ, Dhanarajan P, Seavy MA, Jin Y, Schwartz MA, Rodriguez M, Blaber M. Enzymatic properties of rat myelencephalon-specific protease. Biochemistry. 2002;41:1165–1173. doi: 10.1021/bi015781a. [DOI] [PubMed] [Google Scholar]
- Boven LA, Vergnolle N, Henry SD, Silva C, Imai Y, Holden J, Warren K, Hollenberg MD, Power C. Up-regulation of proteinase-activated receptor 1 expression in astrocytes during HIV encephalitis. J Immunol. 2003;170:2638–2646. doi: 10.4049/jimmunol.170.5.2638. [DOI] [PubMed] [Google Scholar]
- Bridges R, Lutgen V, Lobner D, Baker DA. Thinking outside the cleft to understand synaptic activity: contribution of the cystine-glutamate antiporter (System xc-) to normal and pathological glutamatergic signaling. Pharmacol Rev. 2012;64:780–802. doi: 10.1124/pr.110.003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashman NR, Durham HD, Blusztajn JK, Oda K, Tabira T, Shaw IT, Dahrouge S, Antel JP. Neuroblastoma spinal cord (NSC) hybrid cell lines resemble developing motor neurons. Dev Dyn. 1992;194:209–221. doi: 10.1002/aja.1001940306. [DOI] [PubMed] [Google Scholar]
- Chen B, Friedman B, Whitney MA, et al. Thrombin activity associated with neuronal damage during acute focal ischemia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:7622–7631. doi: 10.1523/JNEUROSCI.0369-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Joe EH, Kim SU, Jin BK. Thrombin-induced microglial activation produces degeneration of nigral dopaminergic neurons in vivo. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:5877–5886. doi: 10.1523/JNEUROSCI.23-13-05877.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Lee DY, Kim SU, Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: role of microglial NADPH oxidase. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:4082–4090. doi: 10.1523/JNEUROSCI.4306-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophi GP, Isackson PJ, Blaber SI, Blaber M, Rodriguez M, Scarisbrick IA. Distinct promoters regulate tissue-specific and differential expression of kallikrein 6 in CNS demyelinating disease. J Neurochem. 2004;91:1439–1449. doi: 10.1111/j.1471-4159.2004.02826.x. [DOI] [PubMed] [Google Scholar]
- Cisowski J, O’Callaghan K, Kuliopulos A, et al. Targeting protease-activated receptor-1 with cell-penetrating pepducins in lung cancer. Am J Pathol. 2011;179:513–523. doi: 10.1016/j.ajpath.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citron BA, Smirnova IV, Arnold PM, Festoff BW. Upregulation of neurotoxic serine proteases, prothrombin, and protease- activated receptor 1 early after spinal cord injury. Journal of neurotrauma. 2000;17:1191–1203. doi: 10.1089/neu.2000.17.1191. [DOI] [PubMed] [Google Scholar]
- Contestabile A. Cerebellar granule cells as a model to study mechanisms of neuronal apoptosis or survival in vivo and in vitro. Cerebellum. 2002;1:41–55. doi: 10.1080/147342202753203087. [DOI] [PubMed] [Google Scholar]
- Cunningham DD, Pulliam L, Vaughan PJ. Protease nexin-1 and thrombin: injury-related processes in the brain. Thromb Haemost. 1993;70:168–171. [PubMed] [Google Scholar]
- Diamandis EP, Yousef GM, Soosaipillai AR, Bunting P. Human kallikrein 6 (zyme/protease M/neurosin): a new serum biomarker of ovarian carcinoma. Clin Biochem. 2000;33:579–583. doi: 10.1016/s0009-9120(00)00182-x. [DOI] [PubMed] [Google Scholar]
- Dihanich M, Kaser M, Reinhard E, Cunningham D, Monard D. Prothrombin mRNA is expressed by cells of the nervous system. Neuron. 1991;6:575–581. doi: 10.1016/0896-6273(91)90060-d. [DOI] [PubMed] [Google Scholar]
- Donovan FM, Pike CJ, Cotman CW, Cunningham DD. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:5316–5326. doi: 10.1523/JNEUROSCI.17-14-05316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggett CJ, Crosier S, Manning P, Cookson MR, Menzies FM, McNeil CJ, Shaw PJ. Development and characterisation of a glutamate-sensitive motor neurone cell line. Journal of neurochemistry. 2000;74:1895–1902. doi: 10.1046/j.1471-4159.2000.0741895.x. [DOI] [PubMed] [Google Scholar]
- Friedmann I, Yoles E, Schwartz M. Thrombin attenuation is neuroprotective in the injured rat optic nerve. Journal of neurochemistry. 2001;76:641–649. doi: 10.1046/j.1471-4159.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- Fujimoto S, Katsuki H, Kume T, Akaike A. Thrombin-induced delayed injury involves multiple and distinct signaling pathways in the cerebral cortex and the striatum in organotypic slice cultures. Neurobiol Dis. 2006;22:130–142. doi: 10.1016/j.nbd.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Gingrich MB, Junge CE, Lyuboslavsky P, Traynelis SF. Potentiation of NMDA receptor function by the serine protease thrombin. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2000;20:4582–4595. doi: 10.1523/JNEUROSCI.20-12-04582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich MB, Traynelis SF. Serine proteases and brain damage - is there a link? Trends in Neuroscience. 2000;23:399–407. doi: 10.1016/s0166-2236(00)01617-9. [DOI] [PubMed] [Google Scholar]
- Gorbacheva LR, Storozhevykh TP, Pinelis VG, Ishiwata S, Strukova SM. Modulation of hippocampal neuron survival by thrombin and factor Xa. Biochemistry (Mosc) 2006;71:1082–1089. doi: 10.1134/s000629790610004x. [DOI] [PubMed] [Google Scholar]
- Hamill CE, Mannaioni G, Lyuboslavsky P, Sastre AA, Traynelis SF. Protease-activated receptor 1-dependent neuronal damage involves NMDA receptor function. Exp Neurol. 2009;217:136–146. doi: 10.1016/j.expneurol.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KS, Mannaioni G, Hamill CE, Lee J, Junge CE, Lee CJ, Traynelis SF. Activation of protease activated receptor 1 increases the excitability of the dentate granule neurons of hippocampus. Mol Brain. 2011;4:32. doi: 10.1186/1756-6606-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BP, Wen W, Strong MJ. Activated microglia (BV-2) facilitation of TNF-alpha-mediated motor neuron death in vitro. J Neuroimmunol. 2002;128:31–38. doi: 10.1016/s0165-5728(02)00141-8. [DOI] [PubMed] [Google Scholar]
- Henrich-Noack P, Riek-Burchardt M, Bakdauf K, Reiser G, Reymann KG. Focal ischemia induces expression of protease-activated receptor1 (PAR1) and PAR3 on microglia and enhances PAR4 labeling in the penumbra. Brain Res. 2006;1070:232–241. doi: 10.1016/j.brainres.2005.10.100. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Van Meter MJ, Rood JC, Rogers RC. Proteinase-activated receptors in the nucleus of the solitary tract: evidence for glial-neural interactions in autonomic control of the stomach. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:9292–9300. doi: 10.1523/JNEUROSCI.6063-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himi T, Ikeda M, Yasuhara T, Murota SI. Oxidative neuronal death caused by glutamate uptake inhibition in cultured hippocampal neurons. J Neurosci Res. 2003;71:679–688. doi: 10.1002/jnr.10510. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Nagai A, Kobayashi S, Kim SU. Upregulation of protease-activated receptor-1 in astrocytes in Parkinson disease: astrocyte-mediated neuroprotection through increased levels of glutathione peroxidase. Journal of neuropathology and experimental neurology. 2006;65:66–77. doi: 10.1097/01.jnen.0000195941.48033.eb. [DOI] [PubMed] [Google Scholar]
- Iwata A, Maruyama M, Akagi T, Hashikawa T, Kanazawa I, Tsuji S, Nukina N. Alpha-synuclein degradation by serine protease neurosin: implication for pathogenesis of synucleinopathies. Hum Mol Genet. 2003;12:2625–2635. doi: 10.1093/hmg/ddg283. [DOI] [PubMed] [Google Scholar]
- Johann S, Dahm M, Kipp M, Zahn U, Beyer C. Regulation of choline acetyltransferase expression by 17 beta-oestradiol in NSC-34 cells and in the spinal cord. J Neuroendocrinol. 2011;23:839–848. doi: 10.1111/j.1365-2826.2011.02192.x. [DOI] [PubMed] [Google Scholar]
- Joshi M, Fehlings M. Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 1. Clip design, behavioral outcomes, and histopathology. J Neurotrauma. 2002a;19:175–190. doi: 10.1089/08977150252806947. [DOI] [PubMed] [Google Scholar]
- Joshi M, Fehlings M. Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 2. Quantitative neuroanatomical assessment and analysis of the relationships between axonal tracts, residual tissue, and locomotor recovery. J Neurotrauma. 2002b;19:191–203. doi: 10.1089/08977150252806956. [DOI] [PubMed] [Google Scholar]
- Junge CE, Lee CJ, Hubbard KB, Ahoabin A, Olson JJ, Hepler JR, Brat DJ, Traynelis SF. Protease-activated receptor-1 in human brain: localization and functional expression in astrocytes. Exp Neurol. 2004;1888:94–103. doi: 10.1016/j.expneurol.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Junge CE, Sugawara T, Mannaioni G, Alagarsamy S, Conn PJ, Brat DJ, Chan PH, Traynelis SF. The contribution of protease-activated receptor 1 to neuronal damage caused by transient focal cerebral ischemia. Proc Natl Acad Sci U S A. 2003;100:13019–13024. doi: 10.1073/pnas.2235594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T, Tokuda T, Yamaguchi N, Watanabe Y, Kametani F, Nakagawa M, Mizuno T. Cleavage of normal and pathological forms of alpha-synuclein by neurosin in vitro. Neurosci Lett. 2008;436:52–56. doi: 10.1016/j.neulet.2008.02.057. [DOI] [PubMed] [Google Scholar]
- Kaufmann S, Desnoyers S, Ottaviano Y, Davidson N, Poirier G. Specific proteolytic cleavage of poly(ADP-ribose) polymerase: an early marker of chemotherapy-induced apoptosis. Cancer Res. 1993;53:3976–3985. [PubMed] [Google Scholar]
- Keep RF, Xi G, Hua Y, Hoff JT. The deleterious or beneficial effects of different agents in intracerebral hemorrhage: think big, think small, or is hematoma size important? Stroke. 2005;36:1594–1596. doi: 10.1161/01.STR.0000170701.41507.e1. [DOI] [PubMed] [Google Scholar]
- Kondo N, Ogawa M, Wada H, Nishikawa S. Thrombin induces rapid disassembly of claudin-5 from the tight junction of endothelial cells. Exp Cell Res. 2009;315:2879–2887. doi: 10.1016/j.yexcr.2009.07.031. [DOI] [PubMed] [Google Scholar]
- Koshimizu H, Kiyosue K, Hara T, et al. Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol Brain. 2009;2:27. doi: 10.1186/1756-6606-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CL, Bristol LA, Jin L, Dykes-Hoberg M, Crawford T, Clawson L, Rothstein JD. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron. 1998;20:589–602. doi: 10.1016/s0896-6273(00)80997-6. [DOI] [PubMed] [Google Scholar]
- Mercer EA, Korhonen L, Skoglosa Y, Olsson PA, Kukkonen JP, Lindholm D. NAIP interacts with hippocalcin and protects neurons against calcium-induced cell death through caspase-3-dependent and -independent pathways. Embo J. 2000;19:3597–3607. doi: 10.1093/emboj/19.14.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui S, Okui A, Uemura H, Mizuno T, Yamada T, Yamamura Y, Yamaguchi N. Decreased cerebrospinal fluid levels of neurosin (KLK6), an aging-related protease, as a possible new risk factor for Alzheimer’s disease. Ann N Y Acad Sci. 2002;977:216–223. doi: 10.1111/j.1749-6632.2002.tb04818.x. [DOI] [PubMed] [Google Scholar]
- Murphy TH, Schnaar RL, Coyle JT. Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cystine uptake. Faseb J. 1990;4:1624–1633. [PubMed] [Google Scholar]
- Nicole O, Goldshmidt A, Hamill CE, Sorensen SD, Sastre A, Lyuboslavsky P, Hepler JR, McKeon RJ, Traynelis SF. Activation of protease-activated receptor-1 triggers astrogliosis after brain injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:4319–4329. doi: 10.1523/JNEUROSCI.5200-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino A, Suzuki M, Ohtani H, Motohashi O, Umezawa K, Nagura H, Yoshimoto T. Thrombin may contribute to the pathophysiology of central nervous system injury. J Neurotrauma. 1993;10:167–179. doi: 10.1089/neu.1993.10.167. [DOI] [PubMed] [Google Scholar]
- Noorbakhsh F, Vergnolle N, McArthur JC, Silva C, Vodjgani M, Andrade-Gordon P, Hollenberg MD, Power C. Proteinase-activated receptor-2 induction by neuroinflammation prevents neuronal death during HIV infection. J Immunol. 2005;174:7320–7329. doi: 10.4049/jimmunol.174.11.7320. [DOI] [PubMed] [Google Scholar]
- Ogawa K, Yamada T, Tsujioka Y, et al. Localization of a novel type trypsin-like serine protease, neurosin, in brain tissues of Alzheimer’s disease and Parkinson’s disease. Psychiatry Clin Neurosci. 2000;54:419–426. doi: 10.1046/j.1440-1819.2000.00731.x. [DOI] [PubMed] [Google Scholar]
- Park JK, Williams BP, Alberta JA, Stiles CD. Bipotent cortical progenitor cells process conflicting cues for neurons and glia in a hierarchical manner. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1999;19:10383–10389. doi: 10.1523/JNEUROSCI.19-23-10383.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompili E, Nori SL, Geloso MC, Guadagni E, Corvino V, Michetti F, Fumagalli L. Trimethyltin-induced differential expression of PAR subtypes in reactive astrocytes of the rat hippocampus. Brain Res Mol Brain Res. 2004;122:93–98. doi: 10.1016/j.molbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Prause J, Goswami A, Katona I, et al. Altered localization, abnormal modification and loss of function of Sigma receptor-1 in amyotrophic lateral sclerosis. Hum Mol Genet. 2013;22:1581–1600. doi: 10.1093/hmg/ddt008. [DOI] [PubMed] [Google Scholar]
- Putnam F. The Plasma Proteins. Academic Press; New York: 1975. [Google Scholar]
- Raimondi A, Mangolini A, Rizzardini M, et al. Cell culture models to investigate the selective vulnerability of motoneuronal mitochondria to familial ALS-linked G93ASOD1. Eur J Neurosci. 2006;24:387–399. doi: 10.1111/j.1460-9568.2006.04922.x. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Noorbakhsh F, Defea K, Hollenberg MD. Targeting proteinase-activated receptors: therapeutic potential and challenges. Nat Rev Drug Discov. 2012;11:69–86. doi: 10.1038/nrd3615. [DOI] [PubMed] [Google Scholar]
- Ratan RR, Murphy TH, Baraban JM. Macromolecular synthesis inhibitors prevent oxidative stress-induced apoptosis in embryonic cortical neurons by shunting cysteine from protein synthesis to glutathione. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1994;14:4385–4392. doi: 10.1523/JNEUROSCI.14-07-04385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan RF, Choi DW. Glutamate neurotoxicity in spinal cord cell culture. Neuroscience. 1991;43:585–591. doi: 10.1016/0306-4522(91)90317-h. [DOI] [PubMed] [Google Scholar]
- Riek-Burchardt M, Striggow F, Henrich-Noack P, Reiser G, Reymann KG. Increase of prothrombin-mRNA after global cerebral ischemia in rats, with constant expression of protease nexin-1 and protease-activated receptors. Neurosci Lett. 2002;329:181–184. doi: 10.1016/s0304-3940(02)00645-6. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Asakura K, Blaber S, Blaber M, Isackson PJ, Beito T, Rodriguez M, Windebank AJ. Preferential expression of myelencephalon specific protease by oligodendrocytes of the adult rat spinal cord white matter. Glia. 2000;30:219–230. doi: 10.1002/(sici)1098-1136(200005)30:3<219::aid-glia2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Blaber SI, Lucchinetti CF, Genain CP, Blaber M, Rodriguez M. Activity of a newly identified serine protease in CNS demyelination. Brain. 2002;125:1283–1296. doi: 10.1093/brain/awf142. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Blaber SI, Tingling JT, Rodriguez M, Blaber M, Christophi GP. Potential scope of action of tissue kallikreins in CNS immune-mediated disease. J Neuroimmunology. 2006a;178:167–176. doi: 10.1016/j.jneuroim.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Isackson PJ, Ciric B, Windebank AJ, Rodriguez M. MSP, a trypsin-like serine protease, is abundantly expressed in the human nervous system. J Comp Neurol. 2001;431:347–361. [PubMed] [Google Scholar]
- Scarisbrick IA, Linbo R, Vandell AG, et al. Kallikreins are associated with secondary progressive multiple sclerosis and promote neurodegeneration. Biol Chem. 2008;389:739–745. doi: 10.1515/BC.2008.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarisbrick IA, Radulovic M, Burda JE, Larson N, Blaber SI, Giannini C, Blaber M, Vandell AG. Kallikrein 6 is a novel molecular trigger of reactive astrogliosis. Biological Chemistry. 2012a;393:355–367. doi: 10.1515/hsz-2011-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarisbrick IA, Sabharwal P, Cruz H, et al. Dynamic role of kallikrein 6 in traumatic spinal cord injury. Eur J Neuroscience. 2006b;24:1457–1469. doi: 10.1111/j.1460-9568.2006.05021.x. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Towner MD, Isackson PJ. Nervous system specific expression of a novel serine protease: regulation in the adult rat spinal cord by excitotoxic injury. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:8156–8168. doi: 10.1523/JNEUROSCI.17-21-08156.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarisbrick IA, Yoon H, Panos M, Larson N, Blaber SI, Blaber M, Rodriguez M. Kallikrein 6 regulates early CNS demyelination in a viral model of multiple sclerosis. Brain pathology (Zurich, Switzerland) 2012b;22:709–722. doi: 10.1111/j.1750-3639.2012.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny LM, Zhang P, Bohm A, Lazarides K, Perides G, Covic L, Kuliopulos A. Interdicting protease-activated receptor-2-driven inflammation with cell-penetrating pepducins. Proc Natl Acad Sci U S A. 2011;108:8491–8496. doi: 10.1073/pnas.1017091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JL, Diamandis EP. Distribution of 15 human kallikreins in tissues and biological fluids. Clin Chem. 2007;53:1423–1432. doi: 10.1373/clinchem.2007.088104. [DOI] [PubMed] [Google Scholar]
- Smirnova IV, Zhang SX, Citron BA, Arnold PM, Festoff BW. Thrombin is an extracellular signal that activates intracellular death protease pathways inducing apoptosis in model motor neurons. Journal of neurobiology. 1998;36:64–80. [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Kennedy SE, George J, Geddes JW. Rapid calpain I activation and cytoskeletal protein degradation following traumatic spinal cord injury: attenuation with riluzole pretreatment. J Neurochem. 1997;69:1592–1600. doi: 10.1046/j.1471-4159.1997.69041592.x. [DOI] [PubMed] [Google Scholar]
- Striggow F, Riek-Burchardt M, Kiesel A, Schmidt W, Henrich-Noack P, Breder J, Krug M, Reymann KG, Reiser G. Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia. Eur J Neurosci. 2001;14:595–608. doi: 10.1046/j.0953-816x.2001.01676.x. [DOI] [PubMed] [Google Scholar]
- Striggow F, Riek M, Breder J, Henrich-Noack P, Reymann KG, Reiser G. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proc Natl Acad Sci U S A. 2000;97:2264–2269. doi: 10.1073/pnas.040552897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo Z, Wu M, Ameenuddin S, Anderson HE, Zoloty JE, Citron BA, Andrade-Gordon P, Festoff BW. Participation of protease-activated receptor-1 in thrombin-induced microglial activation. Journal of neurochemistry. 2002;80:655–666. doi: 10.1046/j.0022-3042.2001.00745.x. [DOI] [PubMed] [Google Scholar]
- Tatebe H, Watanabe Y, Kasai T, Mizuno T, Nakagawa M, Tanaka M, Tokuda T. Extracellular neurosin degrades alpha-synuclein in cultured cells. Neurosci Res. 2010;67:341–346. doi: 10.1016/j.neures.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Terayama R, Bando Y, Takahashi T, Yoshida S. Differential expression of neuropsin and protease M/neurosin in oligodendrocytes after injury to the spinal cord. Glia. 2004;48:91–101. doi: 10.1002/glia.20058. [DOI] [PubMed] [Google Scholar]
- Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377:340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- Tsirka SE, Rogove AD, Bugge TH, Degan JL, Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;15:543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon VL, Lloyd ED, Wang S, Festoff BW, Houenou LJ. Thrombin perturbs neurite outgrowth and induces apoptotic cell death in enriched chick spinal motoneuron cultures through caspase activation. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:6882–6891. doi: 10.1523/JNEUROSCI.18-17-06882.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida A, Oka Y, Aoyama M, et al. Expression of myelencephalon-specific protease in transient middle cerebral artery occlusion model of rat brain. Brain Res Mol Brain Res. 2004;126:129–136. doi: 10.1016/j.molbrainres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Vandell AG, Larson N, Laxmikanthan G, Panos M, Blaber SI, Blaber M, Scarisbrick IA. Protease Activated Receptor Dependent and Independent Signaling by Kallikreins 1 and 6 in CNS Neuron and Astroglial Cell Lines. Journal of neurochemistry. 2008;107:855–870. doi: 10.1111/j.1471-4159.2008.05658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TK, Wheaton VI, Hung DT, Charo I, Coughlin SR. Domains specifying thrombin-receptor interaction. Nature. 1991;353:674–677. doi: 10.1038/353674a0. [DOI] [PubMed] [Google Scholar]
- Waltereit R, Weller M. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol Neurobiol. 2003;27:99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- Wang H, Ubl JJ, Stricker R, Reiser G. Thrombin (PAR-1)-induced proliferation in astrocytes via MAPK involves multiple signaling pathways. Am J Physiol Cell Physiol. 2002;283:C1351–1364. doi: 10.1152/ajpcell.00001.2002. [DOI] [PubMed] [Google Scholar]
- Wells JE, Rice TK, Nuttall RK, Edwards DR, Zekki H, Rivest S, Yong VW. An adverse role of matrix metalloproteinase 12 after spinal cord injury in mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:10107–10115. doi: 10.1523/JNEUROSCI.23-31-10107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarghooni M, Soosaipillai A, Grass L, Scorilas A, Mirazimi N, Diamandis EP. Decreased concentration of human kallikrein 6 in brain extracts of Alzheimer’s disease patients. Clin Biochem. 2002;35:225–231. doi: 10.1016/s0009-9120(02)00292-8. [DOI] [PubMed] [Google Scholar]