Abstract
In the peritoneal fluid, macrophages and their secretory cytokines are essential for endometriosis, but the factors that favor their involvement in the endometriosis-associated inflammatory response are still elusive. Given the anomalous expression of indoleamine 2,3-dioxygenase-1 (IDO1) in endometrial stromal cells (ESCs) and its potentially important roles in immune modulation, we aimed to determine the effects of IDO1 in ESCs on macrophages and the mechanism of those effects. Normal ESCs and ectopic ESCs transfected with the SD11-IDO1 shRNA (short hairpin RNA) or vector-only plasmid SD11 were subsequently co-cultured with peripheral blood (PB)-derived monocytes (PBMC)-driven macrophages directly and indirectly. Cytokine production was determined by analyzing the supernatant of the co-culture unit by enzyme-linked immunosorbent assay (ELISA). The phenotypes and the phagocytic ability of the macrophages were determined by flow cytometry. Compared to normal ESCs, the PBMC-driven macrophages co-cultured with ectopic ESCs displayed lower phagocytic ability. Additionally, macrophages co-cultured with ectopic ESCs exhibited higher levels of CD163 and CD209 and lower levels of HLA-DR and CD11c. Moreover, both the intracellular expression and extracellular secretion of interleukin-10 (IL-10) and transforming growth factor-β1 (TGF-β1) were significantly increased, while that of IL-12p70 was decreased in macrophages after being co-cultured with ectopic ESCs. However, there was no significant difference in macrophage phagocytic ability, immunophenotype or cytokine secretion between the direct and indirect co-culture units. Reversely, SD11-IDO1 shRNA transfection of ectopic ESCs could abrogate the decreased phagocytic ability and alternative activation of macrophages in ectopic ESC-macrophage co-culture unit, suggesting that higher IDO1 in ectopic ESCs was indispensable for the induction of macrophage tolerance. Furthermore, the decrease in phagocytic macrophages and alternatively activated macrophages induced by IDO1 in ectopic ESCs was reversed by the addition of an IL-33 inhibitor, that is, soluble ST2 (sST2). Therefore, through the activation of IL-33, the increased expression of IDO1 in ectopic ESCs contributed to the truncated phagocytic ability of macrophages in endometriosis.
Keywords: Endometriosis; indoleamine 2,3-dioxygenase-1; macrophage; endometrial stromal cell; interleukin-33; cytokine
Introduction
Endometriosis, which causes pelvic pain and infertility in women of reproductive age, is a chronic disease characterized by the presence of endometrial tissue outside the uterine cavity. However, an in-depth understanding of the pathophysiology of endometriosis remains elusive. Inflammation of the surrounding pelvic microenvironment is thought to play an important role in both the initiation and progression of endometriosis in addition to ovarian steroid hormones [1].
Mirroring the T helper 1 - T helper 2 (Th1-Th2) polarization, two distinct states of polarized activation for macrophages have been recognized: the classically activated (M1) macrophage phenotype and the alternatively activated (M2) macrophage phenotype. M2 polarization, which is driven mainly by interleukin-4 (IL-4), IL-13, and IL-33, presents as increased phagocytic activity, high expression levels of scavenging, mannose and galactose receptors (such as CD163, CD206, CD209, CXCR4), low expression of IL-12 and high expression of IL-10 [2]. Additionally, IL-10 and transforming growth factor-β1 (TGF-β1) can give rise to a “M2-like” state, which shares some but not all of the features of the M2 cell state [3]. Macrophages are the dominant immune cells that are recruited to the peritoneal cavity to remove the retrograded endometrial debris in patients with endometriosis. Several studies have indicated that the pelvic macrophages seem to polarize to the M2 state, which displays the alternatively activated or anti-inflammatory characteristics [4,5]. Furthermore, the phenotypic and functional alterations of M2 peritoneal macrophages lead to a low phagocytic capacity, which may be closely related to the pathogenesis of endometriosis. However, despite of the important role of macrophages in endometriosis, little is known about the factors that regulate the differentiation and polarization of macrophages in endometriosis.
Indoleamine 2,3-dioxygenase (IDO) is the intracellular enzyme that catalyzes the initial and rate-limiting step of the metabolism of the essential amino acid tryptophan in the kynurenine pathway. The IDO family consists of 2 enzymes: IDO1 and IDO2, and the IDO2 gene appears to be functional only in mice, not in humans [6]. In recent decades, IDO1 has been shown in numerous studies to produce a marked tolerance effect in fetal rejection, organ transplantation, autoimmune disorders and cancer [7]. Previously, we demonstrated an increased expression of IDO1 in endometriosis derived-endometrial tissues [8]. Thus, in the present study, we aimed to determine if IDO1 in endometrial stromal cells (ESCs) could influence macrophage tolerance and furthermore be involved in the pathogenesis of endometriosis.
Materials and methods
Subjects and sample collection
Endometrial or endometriotic samples were obtained from patients (age 22-43 years) who underwent laparoscopy and additional curettage for treatment of ovary dermoid cyst (n=15; 32.6 ± 8.5 years old) or endometriosis (n=15; 30.2 ± 6.8 years old) in Obstetrics and Gynecology Hospital of Fudan University between May, 2011 and May, 2013. None of the women had taken medications or received hormonal therapy for at least 6 months prior to surgery. All patients of dermoid cyst or endometriosis were diagnosed by laparoscopic examination and pathological findings. And all of the laparoscopy procedures were performed during the luteal phase of the menstrual cycle. Each subject completed a signed, written consent form approved by the Research Ethics Committee of the Obstetrics and Gynecology Hospital, Shanghai Medical School, Fudan University.
Peripheral blood samples (15 mL) from the control subjects were taken sterilely in heparinized Hank’s buffer solution (Gibco, Gaithersburg, MD, USA) before the administration of anesthesia. The samples were immediately transported to a laboratory on ice for bead sorting of the monocytes. The endometrial or endometriotic samples tissue samples were obtained from the patients during surgery under sterile conditions and were transported to the laboratory on ice in DMEM (Dulbecco’s modified Eagle’s medium)/F-12 (Gibco, USA).
Cell culture
We purified ESCs as described previously [8]. The endometrial or endometriotic tissues from the patients were minced into 2 mm pieces and incubated in DMEM/F12 containing collagenase type IV (0.1%; Sigma, San Francisco, CA, USA) and deoxyribonuclease type I (DNase I; 3000 U; Sigma, USA) with constant agitation for 70 min at 37°C. The resulting dispersion was filtered in turn through 100 μm and 70 μm nylon strainers (Becton Dickinson, Franklin Lakes, NJ, USA). The filtrate was then centrifuged at 800×g for 15 min to further remove the leukocytes and erythrocytes and was washed with phosphate-buffered saline (PBS, Gibco, USA). The ESCs were resuspended in DMEM/F-12 containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA), plated on culture flasks, and incubated at 37°C in 5% CO2. The culture medium was replaced every 3 days. Cell viability was assessed using a Trypan Blue exclusion assay (approximately 89.7%). The purity of the ESCs was more than 95% as determined by the following conditions using immunocytochemistry: diffuse and strong immunostaining for vimentin and negative immunostaining for cytokeratin-7 (CK7).
IDO1 interference by shRNA transfection
Ectopic ESCs were plated in 24-well plates with DMEM/F-12 plus 10% FBS. When the cells had reached confluency, Lipofectamine 2000 (Invitrogen; USA), OPTI-MEM (Gibco, USA) and the SD11-IDO1 shRNA (short hairpin RNA) (GeneChem, Shanghai, China) were mixed, incubated for 20 min and added to the cells according to the manufacturer’s protocol. Cells transfected with the vector-only plasmid SD11 (GeneChem, China), were used as the negative control, and ESCs without plasmid transfection were treated as the blank control. After 6 h of incubation, these cells were incubated with macrophages in DMEM/F-12 with FBS in 5% CO2 at 37°C. The effect of plasmid transfection on IDO1 protein expression in ESCs has been shown by us previously [9].
Generation of human macrophages
Peripheral blood mononuclear cells (PBMC) were isolated from the patient blood samples by Ficoll-Hypaque density gradient centrifugation. CD14+ cells were obtained through positive selection by CD14+ micromagnetic beads according to the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). Cell population purity was checked by flow cytometry using a FITC-anti CD14 monoclonal antibody (mAb) (1 ul; BD Biosciences, San Diego, CA, USA) and was found to be >95%.
Then the monocytes were cultured with granulocyte macrophage colony-stimulating factor (GM-CSF, 5 ng/ml; Leukine, Genzyme Corporation, UK) and macrophage colony-stimulating factor (M-CSF, 20 ng/ml; R&D systems, USA) in RPMI 1640 medium (Gibco, USA) containing 10% FBS and 2 mM L-glutamine for up to 6 days. The medium that contained GM-CSF and M-CSF was changed every 3 days. After 6 days, the monocyte-derived macrophages were washed and incubated with ESCs in direct and indirect co-culture units for another 2 days.
Cell co-culture unit
The normal ESCs, ectopic ESCs and the transfected ectopic ESCs (ESCs transfected with vector-only SD11 plasmid or the SD11-IDO1 shRNA, respectively) were cultured in 24-well plates (Corning, Steuben County, NY, USA) at a density of 1×105 cells/well. In the contact co-culture unit, macrophages were subsequently added to the wells directly at the same density as the ESCs. On the other hand, the immune cells were placed in the upper compartment of the transwell chamber inserts (0.4 μm aperture, 12 mm diameter; Corning, USA) in the noncontact transwell co-culture unit. Meanwhile, ESCs or macrophages of 1×105 cells/well were cultured alone as controls. After serum starvation for 8 h, the co-culture unit was then added with recombinant IL-33 (rIL-33, 10 ng/ml; R&D Systems, Minneapolis, NM, USA) or soluble ST2 isoform (sST-2, 100 ng/ml; R&D Systems, USA); vehicle (1‰ PBS) served as the control. The total volume of medium in each well was 800 μL. Forty-eight hours later, after gently scattering any adhered immune cells, the supernatants were collected from all wells and centrifuged immediately at 400×g for 10 min. The medium was aliquoted and stored at -70°C for the cytokine assay, while the cell pellet was collected for immediate analysis by flow cytometry.
Flow cytometry
The cultured macrophages were double stained with PE/Cy7 anti-CD68 mAb (2.5 μl; Biolegend, San Diego, CA, USA) and one of the following mAb: anti-CD11c-FITC (2.5 μl; Biolegend, USA), anti-HLA-DR-PE (10 μl; Biolegend, USA), anti-CD206-PE (10 μl; Biolegend, USA), anti-CD163-APC (2.5 μl; Biolegend, USA), anti-CD209-APC (2.5 μl; Biolegend, USA), anti-IL12p35-Alexa Fluor 647 (10 μl; eBioscience, San Diego, CA, USA), anti-TGF-β1-PE (10 μl; Biolegend, USA), or anti-IL-10-APC (2.5 μl; Biolegend, USA). The cells were concurrently stained with anti-IgG2a-FITC and anti-IgG2a-PE/PE-Cy7/APC as controls. After the addition of the mAbs, the samples were allowed to react at 4°C for 30 minutes. Then, the cells were suspended in PBS and centrifugated at 400×g for 8 minutes. The cells were resuspended in 0.5 mL PBS and were analyzed by Facs Calibur BD flow cytometry (Becton Dickson, San Jose, CA, USA). The resulting data were analyzed using the LYSYS II software program (Becton Dickson, Franklin Lakes, NJ, USA).
Phagocytosis assay
Fluoresbrite carboxy NYO-labeled beads (1 μm-diameter microspheres; Polysciences, Inc., Warrington, PA, USA) were mixed with the macrophages (in a ratio of 10:1) from direct and indirect co-culture units for 30 min with shaking at 37°C. The unbound beads were washed away by PBS and then separated from the cells using density-gradient centrifugation in 2% bovine serum albumin. The ratio of macrophages that ingested fluorescent beads (% phagocytic cells) were determined using Facs Calibur BD flow cytometry (Becton Dickson, San Jose, CA, USA), and the resulting data were analyzed using the LYSYS II software program (Becton Dickson, Franklin Lakes, NJ, USA).
Cytokine assay by enzyme-linked immunosorbent assay (ELISA)
The presence of IL-4, IL-10, IL-12p70, IL-13, IL-23, TGF-β1 and IL-33 in the cultured supernatant samples was determined by ELISA using commercially available kits (IL-4, IL-10, IL-12p70, IL-13, IL-23, TGF-β1: Excell Biology, Shanghai, China; IL-33: Biolegend, USA) according to the manufacturer’s instructions. The absorbance of each well was measured by a DigiScan Microplate Reader (Assys Hitech, Kornenburg, Austria) at the wavelength of 450 nm. The cultured macrophages were homogenized to measure the total protein levels using a BCA (bicinchoninic acid) protein assay kit (Pierce; Rockford, IL, USA), and the ELISA data were standardized to the total protein of the cell lysates. The sensitivity of the kits were 2 pg/mL for IL-4; 4 pg/mL for IL-10; 30 pg/mL for IL-13, IL-12p70 and IL-23; 7 pg/mL for TGF-β1; and 4.14 pg/mL for IL-33.
Statistical analysis
The statistical analyses were performed using the SPSS statistical software package, version 15.0 (SPSS Inc.), according to One-way analysis of variance (ANOVA) with post hoc test. The data are expressed as means ± SD. The differences were considered to be statistically significant for P values <0.05.
Results
IDO1 in ectopic ESCs modulated macrophage phagocytosis and phenotypes
Previously, we demonstrated lower IDO1 expression in normal endometrial tissues compared to the endometriosis-derived eutopic and ectopic endometrial tissues [8]. To further investigate the effect of IDO1 in ESCs, we co-cultured human monocytes-derived macrophages with normal ESCs, ectopic ESCs, ectopic ESCs transfected with SD11-IDO1 shRNA or vector-only plasmid SD11 directly and indirectly. First, we aimed to determine whether IDO1 originating from ESCs is responsible for the phagocytic ability of monocyte-derived macrophages. The ectopic ESCs with higher IDO1 expression markedly inhibited the phagocytic capacity of co-cultured macrophages than normal ESCs (Figure 1; group c vs. group b, P<0.01). And IDO1 interference of ectopic ESCs could further impair the effect induced by ectopic ESCs (Figure 1; group e vs. group c, P<0.01), but not totally (Figure 1; group e vs. group b, P<0.01).
Figure 1.
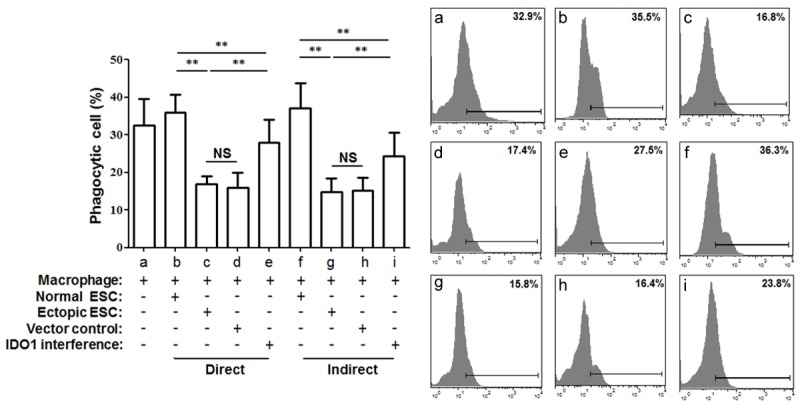
Phagocytic ability of macrophages in ESC-macrophage co-culture units. Normal ESCs from endometriosis-free women, ectopic ESCs from endometriosis women, ectopic ESCs treated with SD11-IDO1 shRNA or vector-only plasmid SD11 were co-cultured with human macrophages directly and indirectly. Human CD68+ macrophages were generated from primary human monocytes following positive immunomagnetic selection and differentiated for 6 days in the presence of both GM-CSF and M-CSF. The phagocytosis of macrophages was detected by flow cytometry, and it was significantly lower when cultured with ectopic ESCs than with normal ones. IDO1 interference of ectopic ESCs was able to reverse the decreased phagocytosis of macrophage in ectopic ESC-macrophage co-culture unit. No difference in phagocytic ability was observed between direct and indirect co-culture units. Normal ESC: ESC from endometriosis-free patients; Ectopic ESC: ESC from endometriosis-derived endometriotic tissue; Vector control: ectopic ESC treated with vector-only plasmid SD11; IDO1 interference: ectopic ESC transfected with SD11-IDO1 shRNA; Phagocytic cells (%): the ratio of macrophages that ingested fluorescent beads. Representative data are shown as well as the means ± SD of 15 different experiments. **P<0.01.
Additionally, ectopic ESCs significantly down-regulated the HLA-DR, CD11c positive percentage (Figure 2A and 2B; group c vs. group b, P<0.01) and up-regulated the CD163, CD209 positive percentage in CD68+ macrophages from the co-culture units (Figure 2C and 2E; group c vs. group b, P<0.01). However, the IDO1 interference of ectopic ESCs partially weakened the phenotypic change induced by ectopic ESCs (Figure 2A-C and 2E; group e vs. group c, P<0.05). The CD206 expression of CD68+ macrophage was not affected by ectopic ESCs in co-culture unit (Figure 2D; P>0.05). No difference in macrophage phagocytic rate and phenotype was detected between the direct and indirect units (Figures 1 and 2, P>0.05). And there was no difference in macrophage phagocytosis, phenotype or cytokine production when they were co-cultured with the non-transfected ectopic ESCs (blank controls) or the ectopic ESCs transfected with the vector-only (negative controls) (Figures 1, 2, 3 and 4; group d vs. group c, P>0.05).
Figure 2.
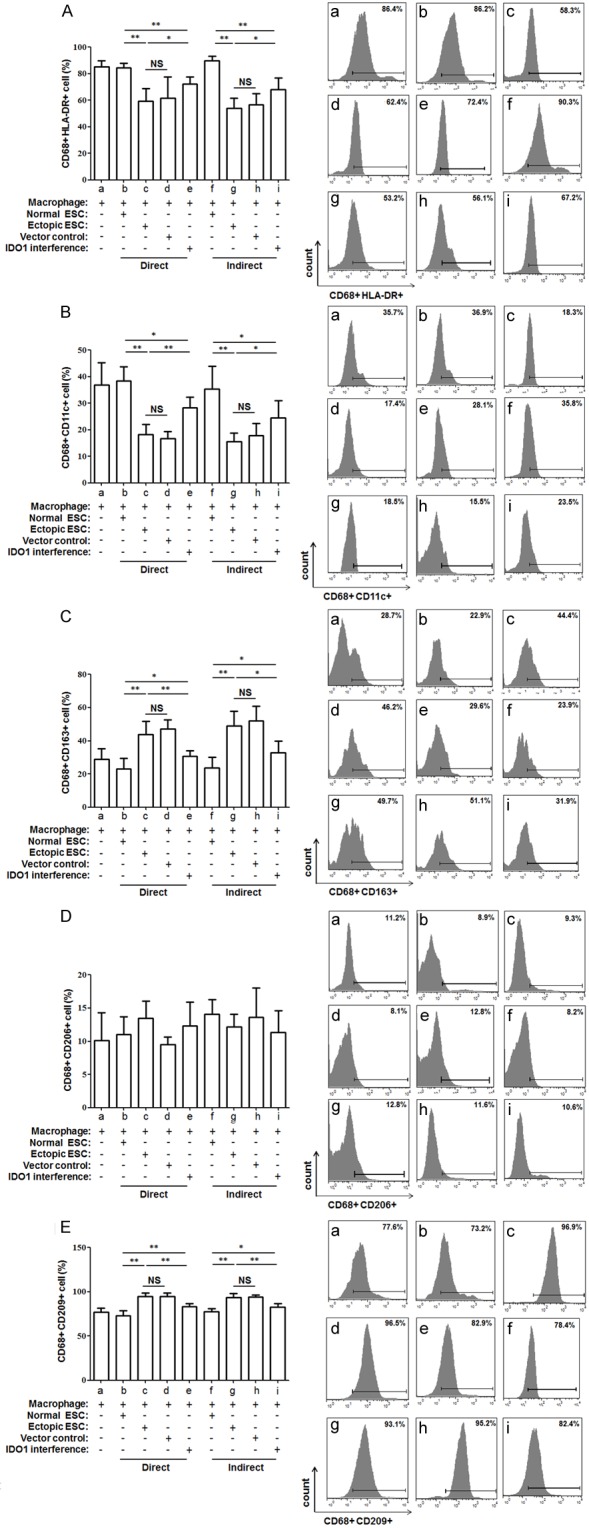
Differential flow cytometric detection of macrophage phenotypes. To detect the phenotypes of co-cultured macropahges, they were double stained with macrophage marker CD68 and HLA-DR (A), CD11c (B), CD163 (C), CD206 (D), CD209 (E) and detected by flow cytometry. HLA-DR and CD11c levels were statistically significantly higher, while CD163 and CD209 levels were decreased in ectopic ESC-macrophage units compared with normal ESC-macrophage ones. And a reverse of the above phenotypic change was observed after SD11-IDO1 shRNA transfection of ectopic ESCs. Normal ESC: ESC from endometriosis-free patients; Ectopic ESC: ESC from endometriosis-derived endometriotic tissue; Vector control: ectopic ESC treated with vector-only plasmid SD11; IDO1 interference: ectopic ESC transfected with SD11-IDO1 shRNA. Representative data are shown as well as the means ± SD of 15 different experiments. *P<0.05; **P<0.01.
Figure 3.
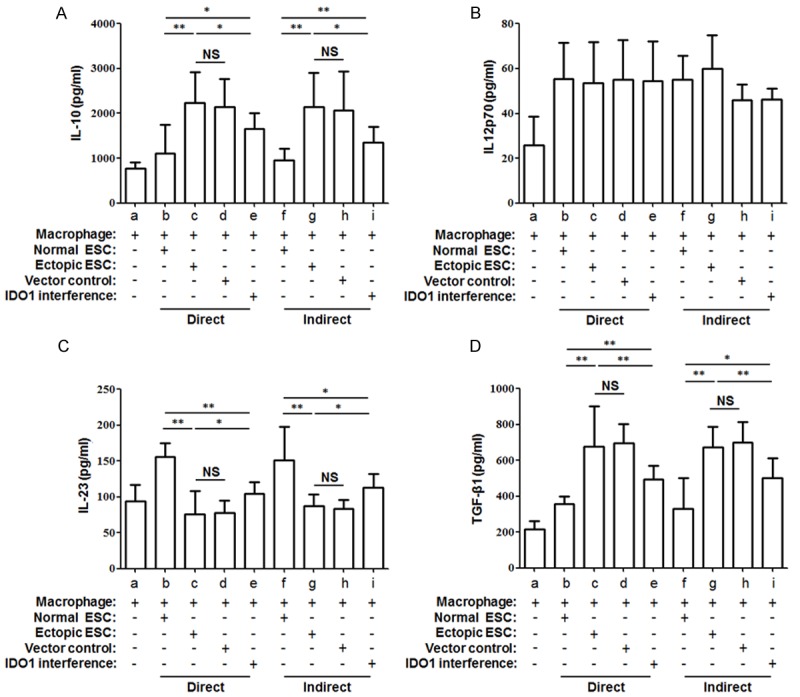
Comparison of ESC-macrophage co-culture cytokine secretion. The cytokine production of IL-10 (A), IL-12p70 (B), IL-23 (C) and TGF-β1 (D) in ESC-macrophage co-culture units were determined by ELISA. IDO1 interference of ectopic ESC reversed the markedly raised level of IL-10 (A) and TGF-β1 (D) and depressed level of IL-12p70 (B) in ectopic ESC-macrophage co-culture unit. Normal ESC: ESC from endometriosis-free patients; Ectopic ESC: ESC from endometriosis-derived endometriotic tissue; Vector control: ectopic ESC treated with vector-only plasmid SD11; IDO1 interference: ectopic ESC transfected with SD11-IDO1 shRNA. Data were shown as the means ± SD of 15 different experiments. *P<0.05; **P<0.01.
Figure 4.
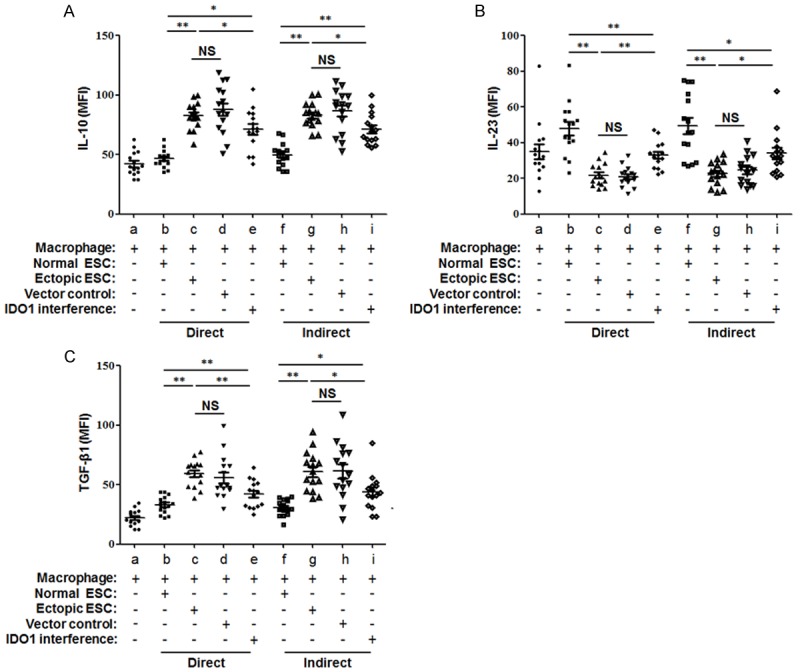
Cytokine profile analysis of macrophages in co-culture units. Normal ESCs, ectopic ESCs, ectopic ESCs transfected with SD11-IDO1 shRNA or vector-only plasmid SD11 were co-cultured with monocyte-derived macrophage directly and indirectly. MFI of IL-10 (A), IL-23 (B) and TGF-β1 (C) in CD68+ macrophages were demonstrated by flow cytometry analysis. Herein, Normal ESC: ESC from endometriosis-free patients; Ectopic ESC: ESC from endometriosis-derived endometriotic tissue; Vector control: ectopic ESC treated with vector-only plasmid SD11; IDO1 interference: ectopic ESC transfected with SD11-IDO1 shRNA; MFI: mean fluorescence intensity. Representative data are shown as well as the means ± SD of different experiments. *P<0.05; **P<0.01.
M2 cytokine bias in ectopic ESC-macrophage co-culture units
Then we aim to study the effect of IDO1 in ectopic ESCs on the macrophage cytokine profile switch. We compared the secretion levels of IL-10, IL-12p70, IL-23 and TGF-β1, which reflect macrophage polarization, in the supernatant of ESC-macrophage co-culture units. The mean level of IL-23 was reduced markedly in the direct and indirect ectopic ESC-macrophage co-culture units (Figure 3C; group c vs. group b, P<0.01). However, a significantly higher level of IL-10 and TGF-β1 in ectopic ESC-macrophage units than normal ones was noted (Figure 3A and 3D; group c vs. group b, P<0.01). The lower IL-23 and higher IL-10, TGF-β1 were partially reversed by SD11-IDO1 shRNA transfection of ectopic ESCs in co-culture units (Figure 3A, 3C and 3D; group e vs. group c, P<0.05). And the cytokine profile of IDO1 interference ectopic ESC-macrophage unit still existed significant M2 cytokine bias when compared with normal ESC-macrophage unit (Figure 3A, 3C and 3D; group e vs. group b, P<0.05). None of the cytokine production was affected by the manner of the co-culture (Figure 3A-D, P>0.05).
In addition to macrophages in co-culture units, ESCs can also produce cytokines in the co-culture units. We observed that there were no significant differences in IL-10, IL-23 or TGF-β1 production among normal ESCs, ectopic ESCs and ectopic ESCs with plasmid transfection (Supplementary Figure 1; P>0.05). It suggested that the diverse cytokine secretion of ESC-macrophage unit was mainly determined by the ESC-instructed macrophage. To further confirmed the effect of ESCs on macrophage cytokine profile, we performed FACS to evaluate the mean fluorescence intensity (MFI) of intracellular cytokine IL-10, IL-23 and TGF-β1 in CD68+ macrophages. Consistent with the results of cytokine profile detected in the co-culture supernatants by ELISA, the MFI of IL-10 and TGF-β1 in the macrophages of the co-culture systems was elevated in ectopic ESC-macrophage units, along with the down-regulation of IL-23 (Figure 4A-C; group c vs. group b, P<0.01). The intracellular M2 cytokine bias of macrophage was partially reversed by the IDO1 interference of ectopic ESCs (Figure 4A-C; group e vs. group c, P<0.01). There was no significant difference in intracellular cytokine expression between the direct or indirect co-culture manner (Figure 4; P>0.05).
IDO1 in ectopic ESCs promoted IL-33 secretion
The soluble cytokines, including II-4, IL-13 and L-33, are the approved regulators involved in human macrophage polarization. To further explore how IDO1 in ESCs induces the M2 polarization of macrophages, we detected the potential mediators II-4, IL-13 and L-33 in the supernatants of the cultured normal ESC, ectopic ESC, ectopic ESC transfected with SD11-IDO1 shRNA or vector-only plasmid SD11. We found that the IL-33 production of ectopic ESCs was 3.3-fold that of the control (Figure 5; ectopic ESC vs. normal ESC, P<0.01), and that elevation was reversed after the treatment of SD11-IDO1 shRNA (Figure 5; IDO1 interference vs. ectopic ESC, P<0.01). It suggested that IDO1-induced IL-33 secretion in ectopic ESCs might participate in the macrophage polarization of endometriosis.
Figure 5.
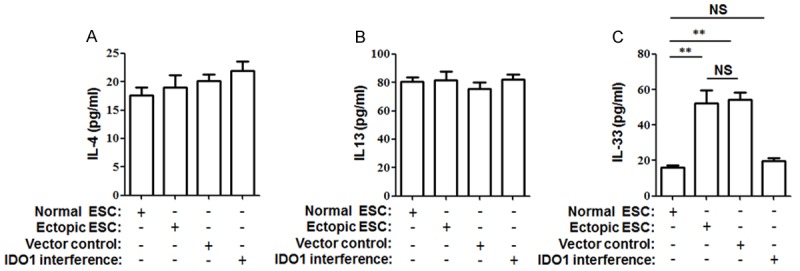
IL-33 secretion was modulated by IDO1 in ESCs. Normal ESCs, ectopic ESCs, ectopic ESCs transfected with SD11-IDO1 shRNA or vector-only plasmid SD11 were cultured for 48 h. Thereafter, IL-4 (A), IL-13 (B), and IL-33 (C) were detected to analyze the modulation of IDO1 on cytokine production of ESCs. Herein, Normal ESC: ESC from endometriosis-free patients; Ectopic ESC: ESC from endometriosis-derived endometriotic tissue; Vector control: ectopic ESC treated with vector-only plasmid SD11; IDO1 interference: ectopic ESC transfected with SD11-IDO1 shRNA. The columns represent the means ± SD of 12 different experiments. *P<0.05; **P<0.01.
sST-2 reversed impaired phagocytic ability and M2 phenotype of macrophages driven by IDO1 in ectopic ESCs
To further address the effect of the IDO1 promotion of IL-33 expression, the co-culture units were incubated with rIL-33 or sST2. Because no significant difference in phenotype or cytokine production was observed in the direct and indirect co-culture units, we discuss the results of the direct ESC-macrophage co-culture unit here. sST2 treatment resulted in markedly decreased HLA-DR and CD11c expression levels in the macrophages as well as an increased phagocytic ability of the macrophages from the ectopic ESC-macrophage units (Figures 6, 7A and 7B; group h vs. group e, P<0.05). Meanwhile, the expression levels of CD163 and CD209 in the macrophages derived from the ectopic ESC-macrophage units were also reduced after the treatment with sST2 (Figure 7C and 7D; P<0.05). Additionally, after rIL-33 stimulation, the macrophage of the IDO1 interference-macrophage units exerted a similar phenotype to the macrophages from the ectopic ESC-macrophage units without rIL-33 stimulation (Figures 6 and 7; group e vs. group l, P>0.05).
Figure 6.
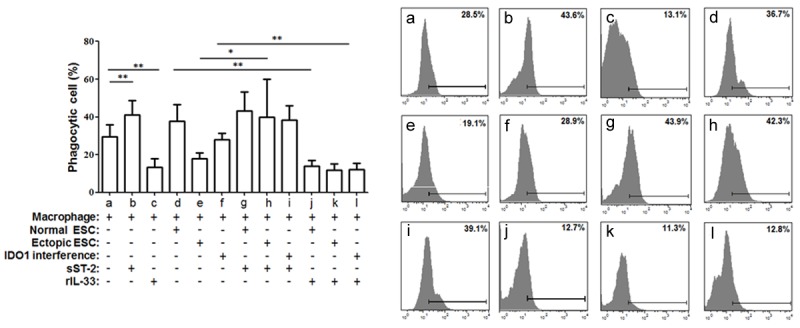
IL-33 from ectopic ESCs altered macrophage phagocytosis in ESC-macrophage units. The direct co-culture unit was treated with rIL-33 (10 ng/ml) or sST2 (100 ng/ml) for 48 h. Ectopic ESCs suppressed the phagocytic ability of monocyte-derived macrophages through higher expression of IDO1, while sST2 reversed this effect. Herein, Normal ESC: ESC from endometriosis-free patients; Ectopic ESC: ESC from endometriosis-derived endometriotic tissue; IDO1 interference: ectopic ESC transfected with SD11-IDO1 shRNA; Phagocytic cells (%): the ratio of macrophages that ingested fluorescent beads. Representative data are shown as well as the means ± SD of 15 different experiments. *P<0.05; **P<0.01.
Figure 7.
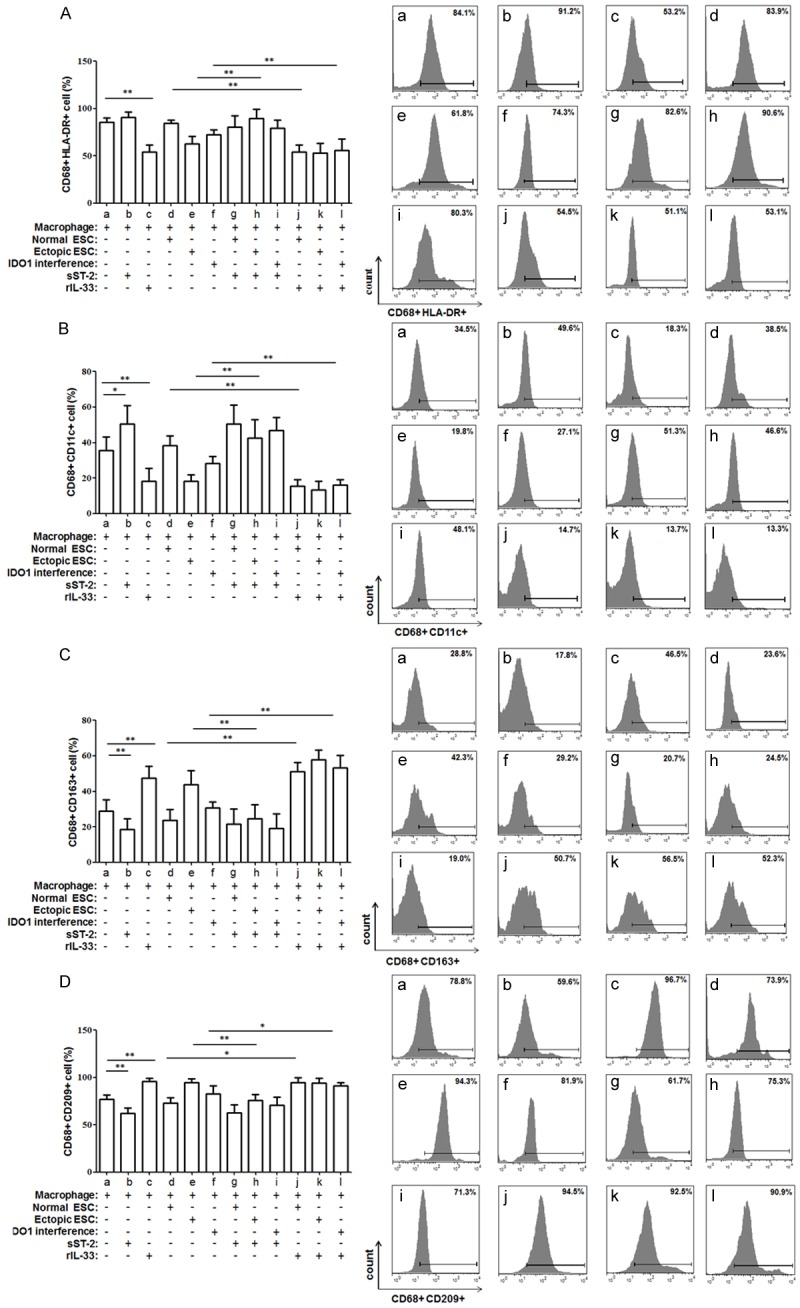
sST2 inhibits the ectopic ESC-stimulated macrophage immunophenotype differentiation. Flow cytometry was employed to detect the HLA-DR (A), CD11c (B), CD163 (C), and CD209 (D) expression of CD68+ macrophages from co-culture units. Inhibition of IL-33 by sST2 reversed the HLA-DR and CD11c down-regulation and CD206 and CD209 up-regulation stimulated by ectopic ESCs. Herein, Normal ESC: ESC from endometriosis-free patients; Ectopic ESC: ESC from endometriosis-derived endometriotic tissue; IDO1 interference: ectopic ESC transfected with SD11-IDO1 shRNA. Representative data are shown as well as the means ± SD of 15 different experiments. *P<0.05; **P<0.01.
IDO1 induction of IL-33 in ectopic ESCs led macrophages to display the tolerant cytokine profile
Finally, we evaluated the effect of IL-33 induction by IDO1 on macrophage cytokine production in the supernatant of co-culture unit. There was no significant difference in the ESC cytokine production levels of IL-10, IL-23 or TGF-β1 when they were cultured with rIL-33 or sST2 (Supplementary Figure 1; P>0.05). The IL-10, IL-12p70 and TGF-β1 secretion of the ectopic ESC-macrophage units did not differ significantly from the rIL-33-treated IDO1 interference-macrophage units (Figure 8A-C; group e vs. group l, P>0.05). The elevated levels of IL-10 and TGF-β1 induced by ectopic ESCs were also reversed by sST2, while the decreased IL-12p70 induced by ectopic ESCs was up-regulated after sST2 treatment (Figure 8A-C; group h vs. group e, P<0.05).
Figure 8.
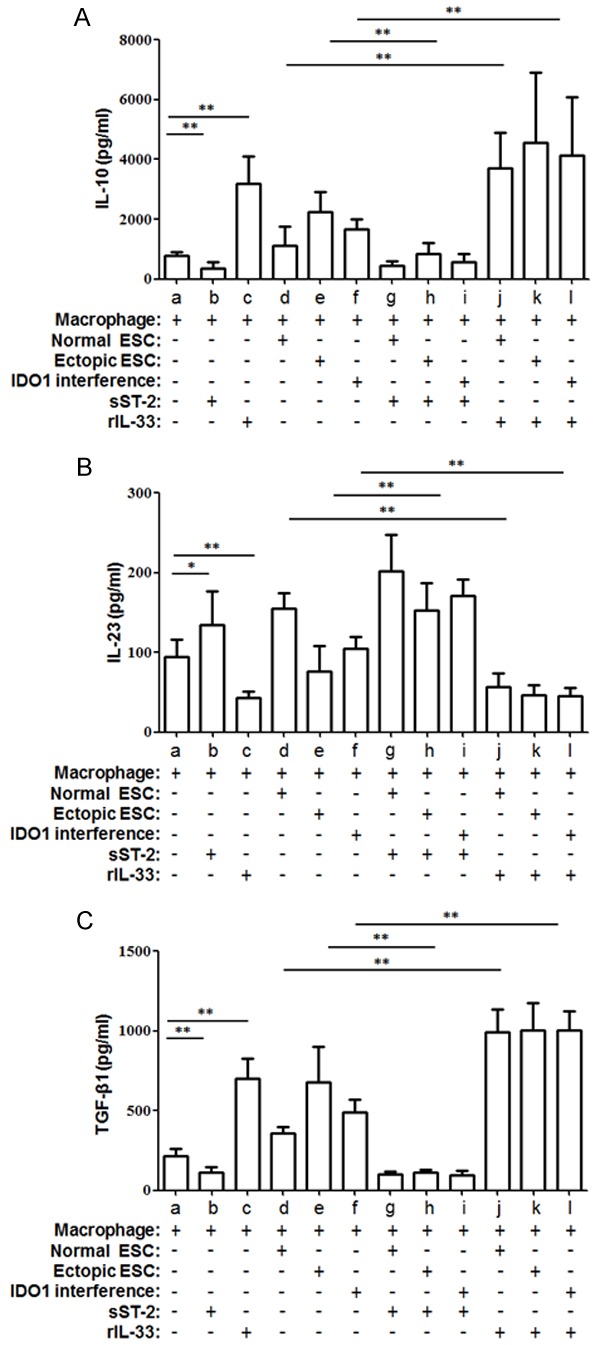
Cytokine excretion of co-culture units was orchestrated by IL-33 derived from ectopic ESC. IL-10 (A), IL-12p70 (B) and TGF-β1 (C) excretion was accommodated by SD11-IDO1 shRNA transfection in ectopic ESC-macrophage units; and this change was restored by IL-33 inhibitor- sST2. Herein, Normal ESC: ESC from endometriosis-free patients; Ectopic ESC: ESC from endometriosis-derived endometriotic tissue; IDO1 interference: ectopic ESC transfected with SD11-IDO1 shRNA. Data are shown as the means ± SD of 15 different experiments. *P<0.05; **P<0.01.
Similar to the results of the cytokine profile, sST2 down-regulated IL-10 and TGF-β1 expression in the macrophages from the ectopic ESC-macrophage units (Figure 9A; P<0.01), and there was no difference in the expression levels of IL-10, IL-12p70 or TGF-β1 in macrophages from rIL-33 treated-IDO1 interference-macrophage units compared to the ectopic ESC-macrophage units (Figure 9A-C; group e vs. group l, P>0.05).
Figure 9.
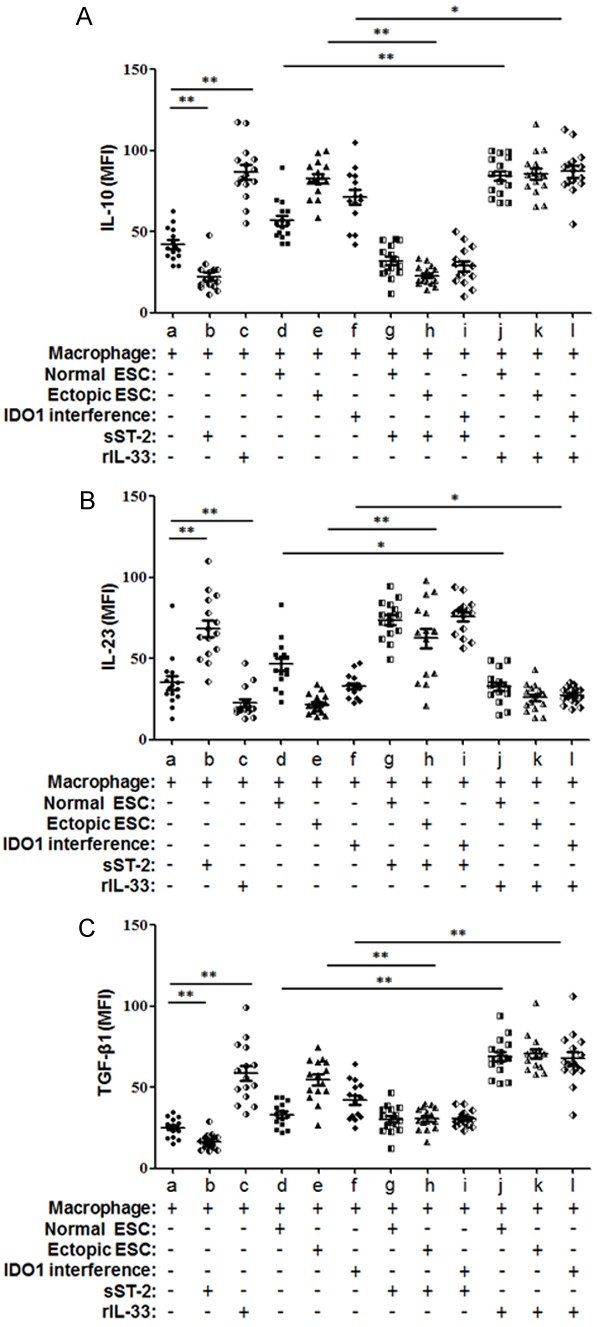
Flow cytometric analysis of intracellular molecules in CD68+ macrophage. The expression of three cytokines, IL-10 (A), IL-12p70 (B) and TGF-β1 (C), are presented as median fluorescent intensity (MFI) as determined by flow cytometry. Herein, Normal ESC: ESC from endometriosis-free patients; Ectopic ESC: ESC from endometriosis-derived endometriotic tissue; IDO1 interference: ectopic ESC transfected with SD11-IDO1 shRNA. Data are shown as the means ± SD of different experiments. *P<0.05; **P<0.01.
Discussion
Although endometriosis has been extensively studied, its etiology remains an enigma. While Sampson’s theory of retrograde menstruation is widely accepted [10], many investigators focused their attention on the role of peritoneal fluid (PF) cellular population and soluble cytokines [11]. However, the factors that favor the involvement of PF macrophages and their secretory cytokines in endometriosis-associated inflammatory responses are poorly understood. IDO1 has been well identified as an immune modulator [12], and its increased expression in endometriosis-derived ESCs drew our interest toward the dynamic interplay between ESCs and macrophages in the peritoneal cavity.
In our study, we found that the presence of endometrial cells in the peritoneal cavity of women with endometriosis may be the result of insufficient activity of macrophages. Soluble factors mediate the interaction of ESCs with macrophages; increased expression of IDO1 in ectopic ESCs upregulated IL-33 expression and contributed to the inducement of macrophage tolerance. Furthermore, modification of the secretion of cytokines from macrophages may further favor the implantation and growth of endometrial tissue.
Macrophages are the predominant cells in the PF and are accompanied by other cells, such as lymphocytes, natural killer (NK) cells and mesothelial cells. After PBMCs migrate into the peritoneal cavity, they become PF macrophages, which function locally in phagocytosis, antigen presentation and cytokine production [13]. In endometriosis, the inactivation of PF macrophages with respect to phagocytosis and cytotoxicity of refluxed endometrial tissue has been previously reported [14]. The scavenger function of macrophages is mediated by the activation of matrix metalloproteinases (MMPs), which degrade the extracellular matrix of retrograded endometrium, and the expression of scavenger receptors (SR), which facilitate the engulfment of cellular debris. It has been suggested that the expression levels of MMP-9, SR-BIII (CD36) [15,16], PGE2, and Annexin A2 [17] can affect the phagocytic capability of peritoneal macrophages in patients with endometriosis. Our present data demonstrated that IDO1 overexpression in ESCs decreased the scavenger function of macrophages. We previously identified the correlation between IDO1 and MMPs and COX-2 in ESCs [9]. We proposed that, in addition to autologous MMPs and PGE2 in macrophages, activated MMPs and PGE2 secreted by ESCs in the peritoneal cavity could also play a role in macrophage phagocytosis. The activation of MMPs and PGE2 caused by IDO1 in ESCs can also lead to the impaired phagocytosis of macrophages. As a result, macrophages fail to clear the peritoneal ectopic endometrium; which plays an important role in the development of endometriosis.
Macrophages commonly alter their function in response to changing microenvironments. M1s are typically induced in response to tissue damage or pathogen exposure (such as TNF, LPS and IFN-γ). They are characterized by high IL-12, IL-23 and low IL-10 expression [2]. In contrast, M2s are a heterogeneous population of macrophages proposed to be induced by a variety of stimuli, including IL-4, IL-13, IL-33 and M-CSF, and they generally share characteristics, such as higher CD163 and CD206 marker expression and higher IL-12 and IL-23 production [18]. However, the altered phenotype applied for the classification of macrophages is still under debate, and the M1/M2 classification of macrophage phenotypes is not an exhaustive description of the many different macrophages present in the different tissue milieus. Based on the conclusion that the phenotypes of PBMC and PF macrophages did not differ significantly in women with and without endometriosis [19], we were able to use PBMC-derived macrophages to imitate the PF macrophages. We co-cultured ESCs and macrophages to determine if IDO1 expression in ectopic ESCs could have any effect on the immune function of macrophages. After incubation of the macrophages with ectopic ESCs, the macrophages were polarized to the M2 phenotype with increased expression of CD163 and CD209, increased production of IL-10 and TGF-β1, decreased expression of HLA-DR and CD11c level and decreased secretion of IL-12/23. The elevated CD163 (a scavenger receptor) and CD209 (C-type lectin receptor) are the adhesion molecules that could be the marker of the subset of regulatory macrophages induced by ectopic ESCs in endometriosis [20,21]. Lower HLA-DR and CD11c (co-stimulatory molecules) levels, which represent the antigen-presenting ability of macrophages, suggest that the antigen presentation of retrograde endometrial tissue might be weakened in the peritoneal cavity; thus, HLA-DR and CD11c have also been considered to participate in the initiation and progression of endometriosis. Furthermore, the ectopic ESCs-induced M2 polarization could be reversed by IDO1 interference in ectopic ESCs, but not totally. It indicated that higher expression of IDO1 in ectopic ESCs might be a candidate molecule, but definitely not the only one, leading to the induction of macrophage tolerance during the peritoneal ESC-macrophage interaction, thereby participating in the impairment of peritoneal macrophage in endometriosis. In addition to IDO1, as mentioned before, MMP-9, SR-BIII (CD36), PGE2, and Annexin A2 could also mediate in the differentiation of peritoneal macrophages in patients with endometriosis. They jointly induced the immune dysfunctional macrophage, especially with an attenuated phagocytic ability and elevated regulatory cytokine secretion, which fail to remove the retrograded endometrial debris in peritoneal cavity, and initiate the occurrence of endometriosis.
Because there was no difference in macrophage phenotype and cytokine production between the contact and non-contact co-culture units, soluble factors rather than immediate contact was speculated to cause the M2 switch by IDO1 in ectopic ESCs. We measured the common stimuli of the M2 phenotype (IL-4, IL-13 and IL-33), and IL-33 was found to be responsible for M2 polarization by IDO1 in ESCs. IL-33 is a novel member of the IL-1 family and was identified as the ligand for the orphan receptor ST2 (IL-1RL1). Due to differential splicing of the ST2 mRNA, ST2 exists in two forms: a transmembrane full-length form (ST2L) and a soluble, secreted form (sST2). Soluble ST2 acts as a decoy receptor for IL-33 [22]. IL-33 has been reported to be expressed in human ESCs and to participate in the decidualization of ESCs [23]. Furthermore, the serum and peritoneal IL-33 levels have been shown to be increased in women with endometriosis, especially in cases of deeply infiltrating endometriosis. IL-33 may even act as a profibrotic mediator involved in the pathogenesis of the fibrotic component of endometriosis [24]. Our findings provided a new role of IL-33 in the pathogenesis of endometriosis. Increased IDO1 expression in ectopic ESCs induced the secretion of IL-33, further reduced the phagocytic ability of macrophages and promoted M2 polarization in co-culture units. The effects of IL-33 could be reversed by sST2, which resulted in lower CD163 and CD209 expression and lower IL-10 and TGF-β1 production. These observations are in accordance with previous reports that abnormal ESCs may have an unexpected role in regulating local immunity [25]. The M2 macrophage has been reported to ameliorate type 1 inflammatory responses and adaptive immunity, to promote type 2 immune responses, and even to regulate tissue repair [26]. M2 peritoneal macrophages were closely related to the development of endometriosis in a murine endometriosis model in that they induced angiogenesis in lesions [5]. Additionally, M2 macrophages could play a principal role in ESC proliferation and lead to the development of pelvic endometriosis [4]. Thus, the interaction between M2 macrophages and ESCs may have an indispensable role in the development of endometriosis. However, IDO1 overexpression could also induce the exhaustion of local tryptophan and metabolites of the kynurenine pathway, which could also cause immunosuppressive effects on immune cells [27]. Therefore, IL-33 might not be the only molecule that mediates M2 polarization in the co-culture system, and further studies are needed to clarify the mechanism of M2 polarization.
The peritoneal macrophage can be stimulated by soluble factors derived from ESCs and can be differentiated in response to changing microenvironments. In addition, macrophage-derived cytokines, such as IL-10 and TGF-β1, are also believed to be associated with endometriosis development. IL-10 produced by M2 dampens the immune response and limits inflammation [28]. Additionally, the impairment of immune cells enables the survival of the ectopic endometrium and is considered to be important in the initiation of endometriosis. TGF-β1 is recognized as a key profibrotic cytokine in endometriosis. It plays an important role in cell growth and angiogenesis. Additionally, TGF-β1 is known to be a potent inhibitor of NK cell activity; that inhibition leads to a decrease in the clearance of regurgitated endometrial cells in the pelvic cavity [29].
In an ESC-macrophage co-culture system, we found that IDO1 regulation in ESCs modulated macrophage phenotypes and cytokine secretion via IL-33. In the ectopic ESC-macrophage co-culture unit, macrophages have lower phagocytic ability, and macrophages exhibit M2 polarization. Decreased expression of HLA-DR and CD11c suggested a depressed antigen presentation capacity of macrophages induced by IDO1. Meanwhile, the raised IL-10 and TGF-β1 levels may play a signifi cant counter-regulatory role in dampening the activation of the peritoneal immune cells and have been found to be related to the growth and angiogenesis of endometriotic implants. However, the following contradiction exists and has been previously described: higher IDO1 levels in ESCs could down-regulate the scavenging ability of macrophages. Nevertheless, the M2 macrophage was characterized as having a higher phagocytic ability. We speculated that, although M2s share general characteristics, they are still a heterogeneous population of macrophages with different features in response to different local milieus. The exact reason for this should be investigated further.
On the whole, endometriosis progression can be recognized as the product of evolving cross-talk between ectopic ESCs and macrophages within the peritoneal cavity. A high level of IDO1 in ectopic ESCs of women with endometriosis can modulate adjacent macrophages through soluble factors, such as IL-33, rather than through immediate contact, to generate a supportive microenvironment. Dysfunctional macrophages not only have impaired phagocytic abilities but also have a decreased ability to present antigens to T cells, this ability is decreased due to a reduction in HLA-DR and CD11c. In contrast, cytokines released by modified macrophages, including IL-10 and TGF-β1, promote endometrial tissue growth and alter the polarization of further recruited macrophages. With the potential role of IDO1 in endometriosis and its application in clinical trials of cancer therapy [30], further work should be undertaken to identify IDO1-based therapies for endometriosis.
Acknowledgements
This work was supported by Natural Science Foundation of China (No. 81270677 to X.-Y.Z.), Foundation for Talents of Shanghai funded by Shanghai Municipal Human Resources and Social Security Bureau (No. 2009008 to X.-Y.Z.), Program for Creative Talents Education of Key Discipline of Fudan University (to J.M.).
Disclosure of conflict of interest
None to declare.
Supporting Information
References
- 1.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–9. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Itoh F, Komohara Y, Takaishi K, Honda R, Tashiro H, Kyo S, Katabuchi H, Takeya M. Possible involvement of signal transducer and activator of transcription-3 in cell-cell interactions of peritoneal macrophages and endometrial stromal cells in human endometriosis. Fertil Steril. 2013;99:1705–13. doi: 10.1016/j.fertnstert.2013.01.133. [DOI] [PubMed] [Google Scholar]
- 5.Bacci M, Capobianco A, Monno A, Cottone L, Di Puppo F, Camisa B, Mariani M, Brignole C, Ponzoni M, Ferrari S, Panina-Bordignon P, Manfredi AA, Rovere-Querini P. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am J Pathol. 2009;175:547–56. doi: 10.2353/ajpath.2009.081011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lob S, Konigsrainer A, Schafer R, Rammensee HG, Opelz G, Terness P. Levo- but not dextro-1-methyl tryptophan abrogates the IDO activity of human dendritic cells. Blood. 2008;15:2152–4. doi: 10.1182/blood-2007-10-116111. [DOI] [PubMed] [Google Scholar]
- 7.Mándi Y, Vécsei L. The kynurenine system and immunoregulation. J Neural Transm. 2012;119:197–209. doi: 10.1007/s00702-011-0681-y. [DOI] [PubMed] [Google Scholar]
- 8.Mei J, Jin LP, Ding D, Li MQ, Li DJ, Zhu XY. Inhibition of IDO1 suppresses cyclooxygenase-2 and matrix metalloproteinase-9 expression and decreases proliferation, adhesion and invasion of endometrial stromal cells. Mol Hum Reprod. 2012;18:467–76. doi: 10.1093/molehr/gas021. [DOI] [PubMed] [Google Scholar]
- 9.Mei J, Li MQ, Ding D, Li DJ, Jin LP, Hu WG, Zhu XY. Indoleamine 2,3-dioxygenase-1 (IDO1) enhances survival and invasiveness of endometrial stromal cells via the activation of JNK signaling pathway. Int J Clin Exp Pathol. 2013;6:431–44. [PMC free article] [PubMed] [Google Scholar]
- 10.Spuijbroek MD, Dunselman GA, Menheere PP, Evers JL. Early endometriosis invades the extracellular matrix. Fertil Steril. 1992;58:929–33. doi: 10.1016/s0015-0282(16)55437-5. [DOI] [PubMed] [Google Scholar]
- 11.Siristatidis C, Nissotakis C, Chrelias C, Iacovidou H, Salamalekis E. Immunological factors and their role in the genesis and development of endometriosis. J Obstet Gynaecol Res. 2006;32:162–70. doi: 10.1111/j.1447-0756.2006.00373.x. [DOI] [PubMed] [Google Scholar]
- 12.Soliman H, Mediavilla-Varela M, Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer J. 2010;16:354–9. doi: 10.1097/PPO.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young VJ, Brown JK, Saunders PT, Horne AW. The role of the peritoneum in the pathogenesis of endometriosis. Hum Reprod Update. 2013;19:558–69. doi: 10.1093/humupd/dmt024. [DOI] [PubMed] [Google Scholar]
- 14.Chuang PC, Lin YJ, MH Wu, Wing LY, Shoji Y, Tsai SJ. Inhibition of CD36-dependent phagocytosis by prostaglandin E2 contributes to the development of endometriosis. Am J Pathol. 2010;176:850–60. doi: 10.2353/ajpath.2010.090551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu MH, Shoji Y, Wu MC, Chuang PC, Lin CC, Huang MF, Tsai SJ. Suppression of matrix metalloproteinase-9 by prostaglandin E(2) in peritoneal macrophage is associated with severity of endometriosis. Am J Pathol. 2005;167:1061–9. doi: 10.1016/S0002-9440(10)61195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuang PC, Wu MH, Shoji Y, Tsai SJ. Down-regulation of CD36 results in reduced phagocytic ability of peritoneal macrophages of women with endometriosis. J Pathol. 2009;219:232–41. doi: 10.1002/path.2588. [DOI] [PubMed] [Google Scholar]
- 17.Wu MH, Chuang PC, Lin YJ, Tsai SJ. Suppression of annexin A2 by prostaglandin E2 impairs phagocytic ability of peritoneal macrophages in women with endometriosis. Hum Reprod. 2013;28:1045–53. doi: 10.1093/humrep/det003. [DOI] [PubMed] [Google Scholar]
- 18.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Maeda N, Izumiya C, Kusume T, Oguri H, Kawashima M, Hayashi K, Nomura A, Yamashita C, Fukaya T. Decreased human leukocyte antigen-DR expression in the lipid raft by peritoneal macrophages from women with endometriosis. Fertil Steril. 2008;89:52–9. doi: 10.1016/j.fertnstert.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 20.Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, Bar-Or A, Antel JP. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–27. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- 21.Ko SY, Ladanyi A, Lengyel E, Naora H. Expression of the homeobox gene HOXA9 in ovarian cancer induces peritoneal macrophages to acquire an M2 tumor-promoting phenotype. Am J Pathol. 2014;184:271–81. doi: 10.1016/j.ajpath.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011;7:321–9. doi: 10.1038/nrrheum.2011.53. [DOI] [PubMed] [Google Scholar]
- 23.Salker MS, Nautiyal J, Steel JH, Webster Z, Sućurović S, Nicou M, Singh Y, Lucas ES, Murakami K, Chan YW, James S, Abdallah Y, Christian M, Croy BA, Mulac-Jericevic B, Quenby S, Brosens JJ. Disordered IL-33/ST2 activation in decidualizing stromal cells prolongs uterine receptivity in women with recurrent pregnancy loss. PLoS One. 2012;7:e52252. doi: 10.1371/journal.pone.0052252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santulli P, Borghese B, Chouzenoux C, Vaiman D, Borderie D, Streuli I, Goffinet F, de Ziegler D, Weill B, Batteux F, Chapron C. Serum and peritoneal Interleukin-33 levels are elevated in deeply infiltrating endometriosis. Hum Reprod. 2012;27:2001–9. doi: 10.1093/humrep/des154. [DOI] [PubMed] [Google Scholar]
- 25.Wang XQ, Yu J, Luo XZ, Shi YL, Wang Y, Wang L, Li DJ. The high level of RANTES in the ectopic milieu recruits macrophages and induces their tolerance in progression of endometriosis. J Mol Endocrinol. 2010;45:291–9. doi: 10.1677/JME-09-0177. [DOI] [PubMed] [Google Scholar]
- 26.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–9. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 27.King NJ, Thomas SR. Molecules in focus: indoleamine 2,3-dioxygenase. Int J Biochem Cell Biol. 2007;39:2167–72. doi: 10.1016/j.biocel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 29.Lee HJ, Kim H, Ku SY, Kim SH, Kim JG. Transforming growth factor-β1 gene polymorphisms in Korean women with endometriosis. Am J Reprod Immunol. 2011;6:428–34. doi: 10.1111/j.1600-0897.2011.01009.x. [DOI] [PubMed] [Google Scholar]
- 30.Opitz CA, Litzenburger UM, Opitz U, Sahm F, Ochs K, Lutz C, Wick W, Platten M. The indoleamine-2,3-dioxygenase (IDO) inhibitor 1-methyl-D-tryptophan upregulates IDO1 in human cancer cells. PLoS One. 2011;6:e19823. doi: 10.1371/journal.pone.0019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


