Abstract
Human MED26 was originally purified in the cofactor required for the Sp1 activation complex (CRSP) as a 70-kDa component named CRSP70. This polypeptide was specific to metazoans and the “small” form of the Mediator complex. We report here that a Drosophila melanogaster homologue of MED26 similarly interacts with other components of the core Drosophila Mediator complex but not with the kinase module and is recruited to genes upon activation. Using a null allele of Med26, we show that Med26 is required for organismal viability but not for cell proliferation or survival. Clones lacking Med26 in the wing disc lead to loss of the adult wing margin and reduced expression of genes involved in wing margin formation. Surprisingly, when polytene chromosomes from the salivary gland were examined using antibodies to Med26, it was apparent that a fraction of the protein was associated with the chromocenter, which contains pericentric heterochromatin. This staining colocalizes with heterochromatin protein 1 (HP1). Immunoprecipitation experiments show that Med26 interacts with HP1. The interaction is mediated through the chromoshadow domain of HP1 and through the conserved motif in the carboxy terminus of the Med26 protein. This work is the first characterization of the metazoan-specific Mediator subunit in an animal model.
INTRODUCTION
Activation of transcription initiation in eukaryotic cells is an intricate process that involves formation of a preinitiation complex surrounding the start site of transcription.
This massive preinitiation complex contains more than 60 polypeptides that must come together at a precise time and place in the genome (1). Sequence-specific DNA binding proteins that directly interact with multiple components of the preinitiation complex orchestrate its assembly. One common conserved target, especially for transcriptional responses to signaling cascades, is the Mediator complex (2).
Mediator is a large multiprotein complex with a central core conserved from yeast to humans (3). In Saccharomyces cerevisiae, the complex contains 25 subunits. In metazoan cells, the complex contains a consensus set of more than 30 polypeptides, including metazoan-specific subunits. The core of Mediator is thought to be comprised of three modules, the “head,” “middle,” and “tail” modules (4). This core associates with either the kinase module, consisting of the MED12, MED13, cyclin-dependent kinase 8 (CDK8), and cyclin C subunits, or the MED26 subunit, forming the “large” and “small” Mediator complexes, respectively.
It is now clear that Mediator serves as a hub for signaling events and controls transcription in a complex way. Initial work on the metazoan complexes suggested that the kinase module containing the “large” complex was associated with repression of transcription (5), whereas the MED26 containing the “small” complex was thought to be associated with activation of transcription (6, 7). However, a growing body of work indicates that the CDK8-containing complex is also involved in activated transcription (8). In addition, it appears that both the CDK8 module and MED26 collaborate in the repression of transcription mediated by the REST complex (9, 10). Finally, both the CDK8 module and MED26 have been found to play a role in promoter escape and transcription elongation (11, 12). Specifically, in human cells, MED26 acts as a docking site for different transcription factors and its N-terminal domain appears first to interact with the initiation factor TFIID and then with factors facilitating release of a paused polymerase (11). Clearly, the regulation of transcription by Mediator is a multifaceted and complex process that is not fully understood.
Work in Drosophila melanogaster has shown that the kinase module plays important roles in developmental gene expression, including direct activation of target genes of the Wnt signaling pathway (13, 14). The subunits of the module are essential for organismal viability but not for cellular growth and proliferation (13, 14). More recent work indicates that there are distinct and separable roles for each of the subunits of the Cdk8 module in Drosophila development (15–19).
The role of the MED26 subunit in vivo is less studied. To better understand the relationship between the forms of Mediator in an animal model, we characterized the putative Drosophila homologue of MED26. We find that Drosophila Med26 is a bona fide subunit of the Mediator complex; it is recruited to activated genes and is required for proper wing margin development. Med26 is required for organismal survival but not for cellular proliferation or viability. Immunoprecipitation experiments show that the majority of Med26 is associated with a Mediator complex that lacks Med12 and Med13. In contrast, immunolocalization using salivary gland polytene chromosomes suggests that these two forms of Mediator may cooccupy many genes. Surprisingly, Med26 is also associated with pericentric heterochromatin and interacts with HP1, suggesting a role in gene silencing.
MATERIALS AND METHODS
Drosophila genetics.
The MB00704 insertion was obtained from the Bloomington Drosophila stock center and is described in Flybase. Excisions were generated by crossing to an SM6 chromosome carrying hs-Minos transposase (20) and heat shocking the progeny at 38.5°C for 1 h each day throughout larval and pupal development. The resulting SM6, hs-MiT; Med26MB00704/P(w+) females were crossed to Med26MB00704/P(w+) males, and 14 of 4,000 offspring were w−, indicating an excision of the MB00704 element that was viable over the original insertion.
The rescue construct was made from cDNA RE09558, obtained from the Drosophila Genomics Resource Center (DGRC). A premature stop codon in this clone was corrected by overlap PCR, introducing a 5′ StuI site and a 3′ BamHI site, and the cDNA was cloned into pBUF (DGRC), a vector that contains a 2-kb ubiquitin promoter and an N-terminal FLAG tag, as a StuI/EcoRV fragment followed by an EcoRV/BamHI fragment. The ubi-Med26 fragment was then cloned into pP[TargetB] (DGRC) using KpnI and NotI sites, to introduce P element ends and FLP recombination target (FRT) sites flanking ubi-Med26 and the w+ gene. The resulting construct was named FRTUbi-FLAGMed26. Transgenic flies were generated by Genetic Services, Inc. A stock carrying w, hs-FLP122; FRTUbi-FLAGMed26/TM3; Med26MB00704/Med26MB00704 was used to generate clones in wing discs, by heat shocking second or early third-instar larvae for 30 min at 38.5°C.
Antibodies.
Rabbit antibodies to Drosophila Med26 were generated to a glutathione S-transferase (GST) fusion of amino acids 1 to 135 produced in Escherichia coli. Mouse antibodies to Drosophila Med14 were generated to a GST fusion of amino acids 212 to 391 produced in E. coli. Taf4 monoclonal antibodies have been described (21). Mouse anti-Cut, mouse anti-Wg, mouse anti-HP1 (22), and mouse antitubulin are from the Developmental Studies Hybridoma Bank (DSHB). Guinea pig anti-Sens (23), mouse anti-Nubbin (24), rabbit anti-Med13 and guinea pig anti-Med12 (19), and guinea pig anti-Med17 (21) have been described. Mouse anti-Ser-5P RNA polymerase II (H14; Covance) is commercially available.
Immunohistochemistry.
In situ hybridization was performed as described previously (25) using a probe homologous to the coding region of the last exon of Med26. This probe was transcribed from a template that was produced by PCR from genomic DNA, introducing a T7 promoter sequence at the 3′ end. Adult wings were mounted in methyl salicylate-Canada balsam (1:2). Imaginal discs were stained as described previously (26). Antibodies used were rabbit anti-Med26 (1:50), mouse anti-Cut (1:10; DSHB), mouse anti-Wg (1:10; DSHB), guinea pig anti-Sens (1:1,000) (23), and mouse anti-Nubbin (1:10) (24). Salivary glands from Drosophila melanogaster w1118 or CXM40-4 (27) third-instar larvae were dissected in 0.7% NaCl or 50% Grace's medium. Glands were squashed, fixed, and stained as described previously (28).
Chromatin immunoprecipitation.
Drosophila Schneider line 2 cells (S2 cells) were heat shocked for 30 min at 37°C or left untreated. Cells were then cross-linked with 1.5% formaldehyde. Nuclei were isolated and lysed, and chromatin was sonicated to 500 to 1,000 bp in length as described previously (29). Chromatin was diluted and incubated with rabbit polyclonal sera against Med26 overnight. The chromatin-antibody mix was cleared and then incubated with protein A beads to isolate Med26-bound chromatin. Purified DNA was assayed by quantitative PCR (qPCR) to determine enrichment for genomic sites bound by Med26. Precipitated DNA was measured using SYBR green in a Chromo4 thermocycler. Five microliters of precipitated DNA or input DNA (diluted 1:10) was used in a 25-μl PCR mixture containing 20 mM Tris-HCl (pH 8.8), 10 mM (NH4)2SO4, 10 mM KCl, 2 mM MgSO4, 0.1% Triton X-100, 200 μM deoxynucleoside triphosphates (dNTPs), 1 U Taq DNA polymerase, 0.005 U Pfu DNA polymerase, 0.5 μM each primer, and 0.5× SYBR green. Gene-specific primer sequences used in the qPCR were as follows: Hsp23, CCAGGCCTTTTCATTCCCAC and GGGGCACAAACATCGACA; Hsp70, CTCGAATGTTCGCGAAAAGAGC and TAGCTTGTTCAGCTGCGCTTG.
Protein purification.
His-MBP-Med26ctd, His-MBP, and His-HP1 were expressed from plasmids pET-MBP-165ctd, pET-MBP, and pAK12, respectively, in BL21 pRARE DE3 cells in 100-ml cultures. Protein expression was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 h at 37°C. Cell pellets were resuspended in buffer A (50 mM Tris [pH 8.0], 100 mM NaCl, 1 mM dithiothreitol [DTT], 0.1% Triton X-100, 10% glycerol) containing 1 mg/ml lysozyme and incubated on ice for 20 min. NaCl was added to a final concentration of 500 mM and cells were sonicated. Lysates were centrifuged at 12,000 rpm, 4°C, for 15 min in an SS34 rotor. Supernatant was mixed with 400 μl packed nickel resin at 4°C for 1 h. Resin was washed twice with buffer B (50 mM Tris [pH 8.0], 500 mM NaCl, 1 mM DTT, 0.1% Triton X-100, 10% glycerol) and twice with buffer A. Proteins were eluted with 1 ml of buffer A containing 500 mM imidazole at 4°C for 10 min.
Gel filtration.
A total of 2.5 mg of Drosophila embryo nuclear extract (100 μl) in HEMG (25 mM HEPES, 0.1 mM EDTA, 1 mM DTT, 10% glycerol, 0.2 mM phenylmethylsulfonyl fluoride [PMSF], 0.15 M KCl, 12.5 mM MgCl2) was treated with Benzonase nuclease (EMD Millipore) on ice for 30 min before being loaded on a Superose 6 10/300 gel filtration column (GE Healthcare) equilibrated in 20 mM HEPES, 1 mM DTT, 0.1 mM EDTA, 150 mM NaCl. Twenty-five 1-ml fractions were collected. Fractions from the void volume through the 17-kDa standard were analyzed by immunoblotting for the presence of Med26 and Med17.
Pulldown experiments.
MBP-165ctd and MBP protein elutions were combined with 100 μl packed amylose resin (New England BioLabs) containing 1× protease inhibitors (SIGMAFAST EDTA-free protease inhibitor cocktail; Sigma-Aldrich) and were rotated at 4°C overnight. Resin was washed 5 times with buffer A and 3 times with 1× phosphate-buffered saline (PBS) containing 0.1% Triton X-100 and 1× protease inhibitor. Recombinant HP1 (100 μl) was combined with 25 μl packed MBP or MBP-165ctd resin in 1.5 ml 1× PBS containing 500 mM NaCl and rotated at 4°C for 1 h. Resin was washed twice with 1 ml 1× PBS containing 500 mM NaCl and three times with 1× PBS containing 0.1% Triton X-100. Proteins were eluted with 25 μl Laemmli sample buffer (Bio-Rad) and heated at 95°C for 10 min. Two identical sets of protein samples were electrophoresed on an Any kD mini-PROTEAN TGX acrylamide gel (Bio-Rad). The gel was cut in half, and half was stained with Coomassie and half was transferred to a nitrocellulose membrane for Western blotting. The membrane was probed with monoclonal mouse anti-HP1 antibody (C1A9) and goat anti-mouse Dylight 680-conjugated IgG (Thermo Scientific). Western blots were quantitated on an Odyssey infrared imaging system (LI-COR Biotechnology).
GST or GST fusions to HP1 were expressed in E. coli and immobilized on glutathione-Sepharose (GE Healthcare). A total of 100 μl of Drosophila embryo nuclear extract was diluted to 500 μl with 1× PBS and incubated with 50 μl glutathione-Sepharose containing each fusion protein. The nuclear extract and beads were incubated at 4°C for 4 h with constant turning. Resin was collected by centrifugation and washed 5 times with wash buffer containing 40 mM HEPES 7.6, 0.5 M KCl, 0.1 mM EDTA, 2 mM MgCl2, 1 mM beta-mercaptoethanol, 5% glycerol, 0.1% NP-40, 0.1 mg/ml egg white lysozyme, 0.2 mM PMSF.
Resin was collected by centrifugation and washed 2 times with PBS. Resin was collected by centrifugation and bound proteins were eluted with glutathione. Elutions were separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies to Med26.
Mediator coimmunoprecipitation.
A total of 500 μl of Drosophila embryo nuclear extract or a heparin fraction (H.4) (30) was combined with 500 μl 1× PBS, SIGMAFAST protease inhibitor, and 5 μl antibody (either rabbit anti-Med26, guinea pig anti-Med17, or rabbit anti-Med13). Samples were rotated at 4°C overnight. Debris was pelleted at 21,000 × g for 15 min at 4°C. Supernatant was combined with 100 μl protein A-Dynabeads (Invitrogen) and rotated at 4°C for 1.5 h. Beads were washed once with 1× PBS and six times with 1× PBS containing 500 mM NaCl and 0.1% Triton X-100. After a final wash with 1× PBS, beads were incubated on ice for 10 min with 30 μl Laemmli sample buffer (Bio-Rad) containing 50 mM DTT prior to heating at 95°C for 10 min. Proteins were electrophoresed on a 6% polyacrylamide SDS gel and transferred to a nitrocellulose membrane for Western blotting.
Mediator depletion.
Individual Mediator subunits were depleted from Drosophila Schneider line 2 cells (S2 cells) by RNA interference (RNAi) knockdown essentially as described previously (21) using 40 μg/ml double-stranded RNA (dsRNA). Cells were incubated with dsRNA for 3 days at 27°C in Schneider's insect medium containing 10% fetal bovine serum (Sigma). Cells were washed with 1× PBS, lysed in Laemmli sample buffer (Bio-Rad), and heated at 95°C for 10 min. Proteins were electrophoresed on a 6% polyacrylamide SDS gel and transferred to nitrocellulose membrane for Western blotting.
Heat shock protein mRNA qPCR.
S2 cells were treated with dsRNA targeting Med26 or Med17 or mock treated with dsRNA targeting the E. coli lacI gene. After 3.5 days, the cells were heat shocked at 37°C for 30 min. RNA was harvested immediately using TRIzol by following the manufacturer's instructions. RNA concentration was determined by UV absorption, and equal amounts of RNA were used in a cDNA synthesis reaction. RNA was combined with 0.3 μg random primers and 50 pmol oligo(dT) in 14.5 μl water. The sample was heated to 70°C for 10 min and then quickly cooled to 4°C. A total of 10.5 μl of reverse transcriptase (RT) mix was added to the primed RNA such that the final concentration of the reaction was 50 mM Tris-HCl (pH 8.3), 50 mM KCl, 3 mM MgCl2, 10 mM DTT, 400 μM dNTPs, and 200 units Moloney murine leukemia virus (MMLV) RnaseH− reverse transcriptase. The reaction mixture was incubated at 25°C for 30 min, followed by 60 min at 42°C and then 15 min at 70°C. cDNA was diluted 1:10 in TE (10 mM Tris 8.0, 0.1 mM EDTA). The qPCR conditions were the same as those used in the chromatin immunoprecipitation except they contained 2.5 μl of cDNA. Levels of heat shock protein (HSP) signal were normalized to the signal from ribosomal protein 49. Experiments were repeated with biological replicates at least twice. Gene-specific primer sequences are as follows: Hsp70, CAAAGTTGTAAGCGACGGCGGAA and TGTCTCCGGCTGTGGAGCGCA; Hsp23, GAAAGGATGGCTTCCAGGTC and GCCTCCTTGGGATTCTCCTT; and Rp49, CCACCAGTCGGATCGATATGC and CTCTTGAGAACGCAGGCGACC.
Western blotting.
The membranes were probed with primary antibodies described in the text. In Fig. 4B to D and 5G, primary antibodies were detected with goat anti-rabbit or goat anti-mouse Dylight 680- or Dylight 800-conjugated IgG (Thermo Scientific) or goat anti-guinea pig IRDye 700DX-conjugated IgG (Rockland Immunochemicals). Blots were imaged on an Odyssey infrared imaging system (LI-COR Biotechnology). In Fig. 1 and 5B, C, and E, primary antibodies were detected with horseradish peroxidase (HRP)-conjugated (Thermo Scientific) goat anti-rabbit, goat anti-mouse, or goat anti-guinea pig secondary antibodies by using ECL reagent (GE Life Sciences) and X-ray film.
FIG 4.
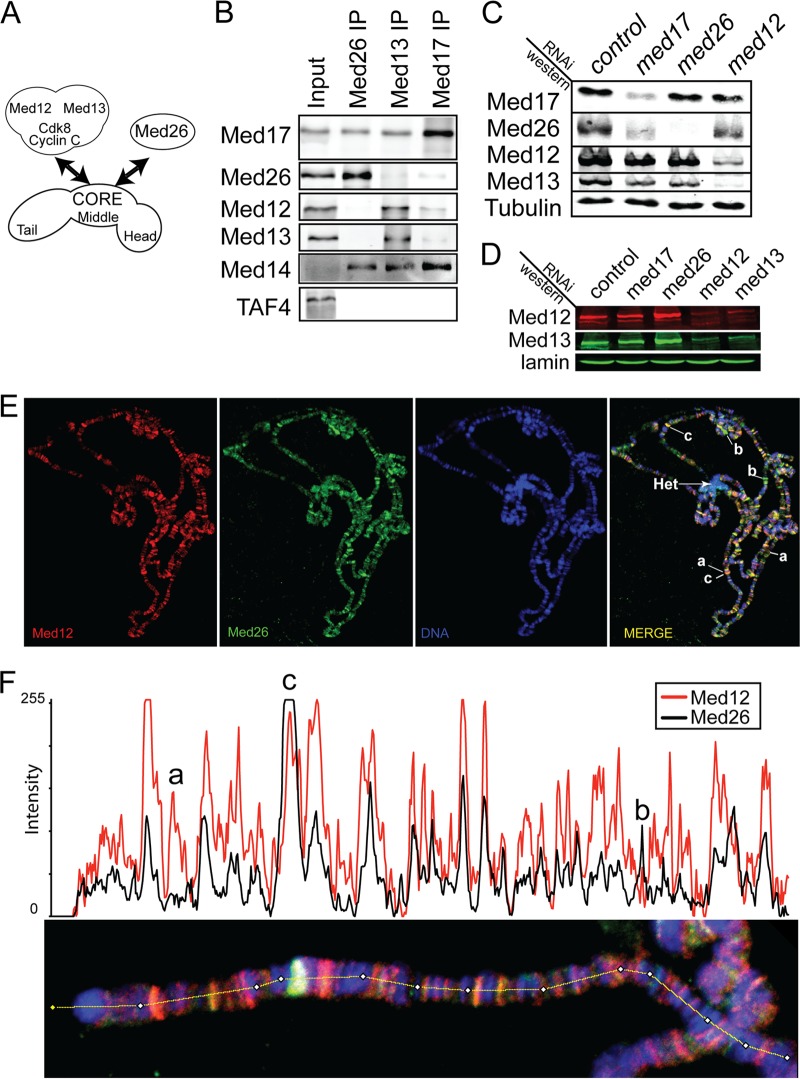
Med26 and Med12 are found in separate complexes. (A) Diagram of possible Mediator complex formations. (B) Western blot of precipitated Mediator subunits. Antibody used in the immunoprecipitation is listed at the top of each lane. Antibody used in the Western blot is listed at the left. (C, D) Western blots of total cellular protein after treatment with dsRNA. RNAi target is listed at the top of each lane. Antibody used in the Western blot is listed at the left. (E) Polytene chromosomes stained for Med12 (red) and Med26 (green) and counterstained for DNA (blue). The merged image is shown at the right. Examples of bands that contain only Med12 (a), only Med26 (b), or both (c) are indicated. Het indicates the chromocenter. (F) Graph of one arm of a polytene chromosome scanned using ImageJ software in either the red channel (Med12) or the green channel (Med26). Examples of bands that contain only Med12 (a), only Med26 (b), or both (c) are indicated above the graph. The path of the scan is indicated in the bottom image.
FIG 5.
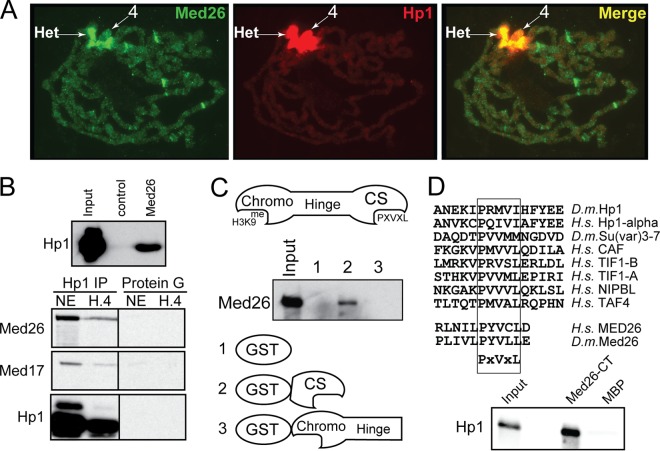
Med26 localizes to pericentric heterochromatin and binds HP1. (A) Polytene chromosomes stained for Med26 (green) or HP1 (red). Het indicates the chromocenter, and “4” indicates the 4th chromosome. (B) The top panel shows a Western blot of immunoprecipitated proteins. Antibody used in the immunoprecipitation is listed at the top of each lane. The blot was probed with C1A9 anti-HP1. The bottom panel shows a Western blot of immunoprecipitated proteins. Antibody used in the immunoprecipitation is listed at the top. The blot was probed with C1A9 (anti-HP1) and rabbit anti-Med26 or mouse anti-MED17. (C) The top panel shows a diagram of the HP1 domains. “Chromo” indicates the chromodomain, “CS” indicates the chromoshadow domain, “Hinge” indicates the hinge region that connects them. The middle panel shows a Western blot from an affinity chromatography experiment. Nuclear extract was loaded onto a column containing GST or GST fusions to HP1. Numbers above each lane correspond to the fusion proteins indicated below the gel. The bound proteins were eluted and probed with anti-Med26 antibodies. (D) The top panel shows an alignment of several proteins that interact with the chromoshadow domain of HP1. The extreme C terminus of Med26 contains the PXVXL motif. The bottom panel shows an affinity chromatography experiment. Purified HP1 protein was incubated with resin containing either MBP or MBP fused to the C terminus of Drosophila Med26. The bound proteins were eluted and probed with antibodies to HP1.
FIG 1.
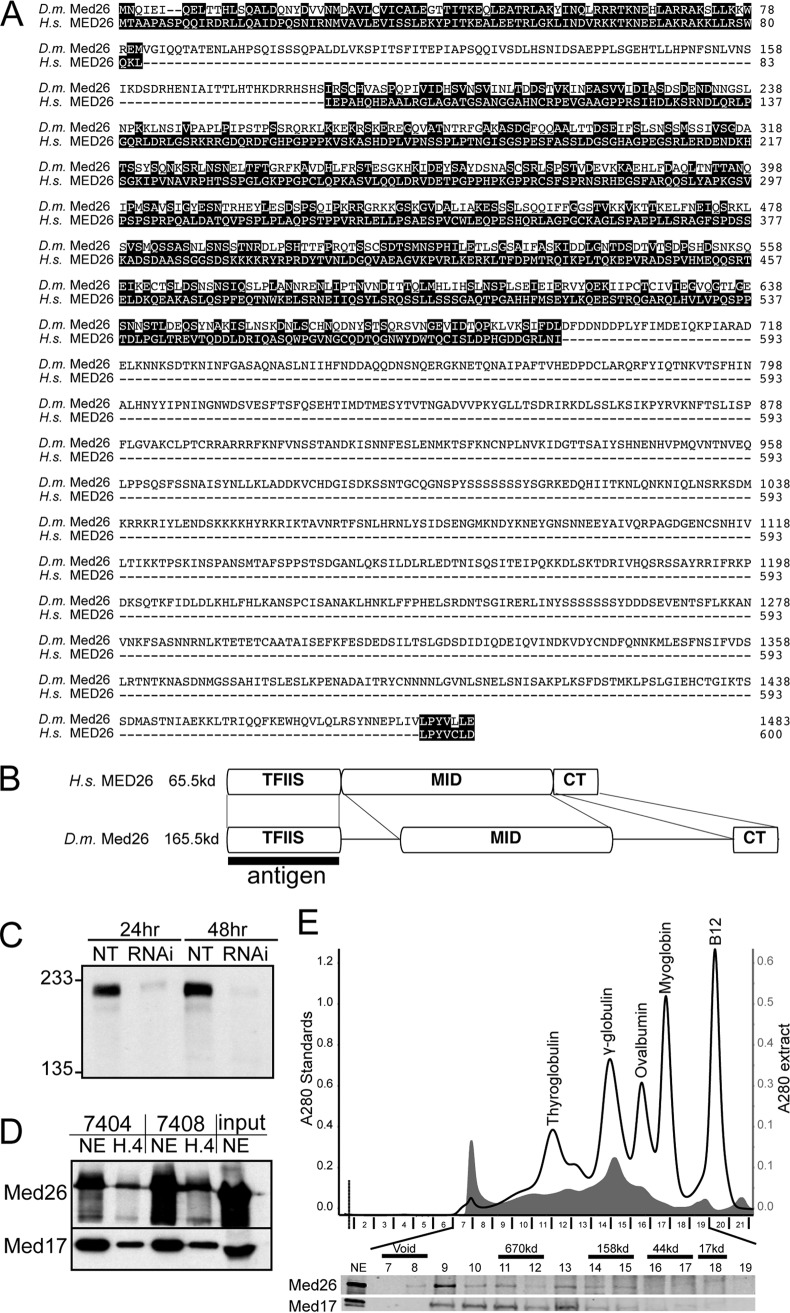
Med26 is conserved in Drosophila. (A) Alignment of Drosophila (D.m.) and human (H.s.) Med26 protein sequences. Similar residues are shown as white letters. (B) Diagram comparing the domain structure of Med26 from human (H.s.) and Drosophila (D.m.). The region used for antigen preparation is indicated. (C) Western blot of total protein from untreated S2 cells and cells treated for the indicated times with dsRNA directed against Med26. (D) Western blot of the proteins precipitated with antisera directed against Drosophila Med26. The top blot was developed with antibodies against Med26. The bottom blot was developed with antibodies against Med17. (E) Gel filtration analysis of Drosophila nuclear extract. Absorbance at 280 nm for extract is indicated in gray. A trace of absorbance at 280 nm for gel filtration standards is overlaid in black. The bottom panel shows an immunoblot of the fractions from the column.
RESULTS
Characterization of Drosophila Med26.
The Drosophila melanogaster homologue of human MED26 was first identified by the clear sequence identity in the amino terminus of the protein. A full alignment of the two proteins (Fig. 1A) reveals three regions that are related: the amino-terminal TFIIS-like domain, a middle region, and the extreme C terminus (Fig. 1B). One striking difference is that the annotated Drosophila Med26 protein is 100 kDa larger than mammalian MED26. The larger size of Med26 is conserved in all Drosophila genomes that have been sequenced.
We generated two rabbit polyclonal antisera to the amino terminus of the putative Drosophila Med26 protein in order to characterize this protein and its associated proteins. Immunoblots of extracts from Drosophila S2 cells or embryos using the antisera indicated that the Drosophila Med26 protein has an apparent molecular weight of 165 kDa, consistent with its annotated gene structure (Fig. 1C and D). Specificity of the antisera was verified by treating the cells with dsRNA targeting the annotated Med26 gene (Fig. 1C). We observed partial depletion of the immunoreactive band within 24 h of dsRNA treatment and almost complete depletion in 48 h, confirming that the antisera react with Drosophila Med26.
Given the difference in size of human and Drosophila Med26, we wanted to verify that this protein is a bona fide component of the Mediator complex. We immunoprecipitated Med26 from Drosophila embryo nuclear extracts or a fraction enriched for transcription factors (H.4) and probed for association with Med17. Med17 is a core subunit of the head module of the Mediator complex and is required for complex integrity in yeast (31, 32). Med17 is a common target of transcription activators in Drosophila (33). Using either of the two antisera, we were able to detect an association of Med26 with Med17, indicating that at least a portion of Med26 is associated with the Mediator complex. Gel filtration analysis of Drosophila embryo nuclear extract indicates that the majority of the Med26 protein exists in a large complex containing Med17 (Fig. 1E). Taken together, these results indicate that the annotated Med26 gene is likely the bona fide MED26 homologue.
Med26 is required for organismal viability but not for cell survival or proliferation.
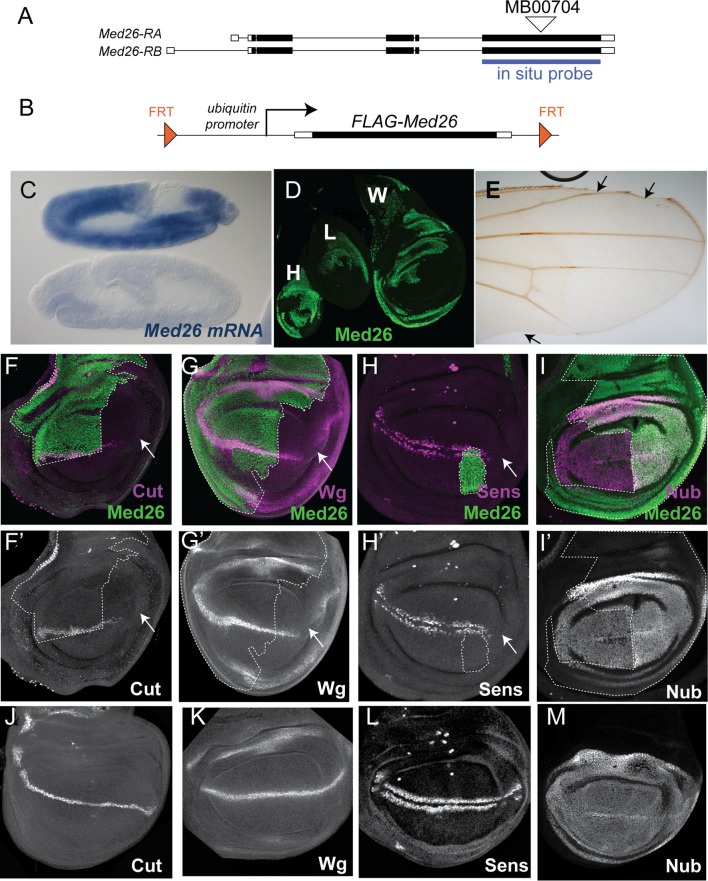
We identified a loss-of-function mutation in Med26 caused by an insertion of the Minos transposable element into a coding exon (Fig. 2A) (Flybase). The MB00704 insertion is homozygous lethal, and viable excision lines could be recovered after crossing in the Minos transposase, indicating that the insertion was responsible for the lethality (see Materials and Methods). In approximately 25% of stage 9 to 11 embryos produced from a cross between flies heterozygous for MB00704 and the wild-type allele, no Med26 mRNA could be detected by in situ hybridization with a probe to the last exon (Fig. 2C), suggesting that the Minos element disrupts the transcription or stability of Med26 mRNA. Although homozygous MB00704 embryos hatched, they died before the third larval instar. In order to generate mosaic clones homozygous for Med26, we generated a rescue construct in which FRT sites flanked a FLAG-tagged Med26 cDNA driven by the ubiquitin promoter (Fig. 2B). This transgene fully rescued the viability of MB00704 and could be removed in clones of cells by introducing a source of FLP recombinase. Large clones lacking any Med26 protein detectable by antibody staining were generated using FLP expressed under the control of the heat shock (hs) promoter (Fig. 2D), indicating that Med26MB00704 is a protein-null allele and that Med26 is not required for cell proliferation or survival in imaginal discs. However, induction of clones in the second larval instar caused extensive lethality at the pupal stage, and surviving adults were frequently missing parts of the wing margin (Fig. 2E).
FIG 2.
Identification and rescue of a Med26 mutation. (A) Diagram of the Med26 genomic locus. Med26-RA and Med26-RB are two alternatively spliced isoforms; exons are represented by white boxes, coding regions are indicated in black, and introns are shown as lines. The positions of the MB00704 Minos element insertion and of the probe used for in situ hybridization are indicated. (B) Diagram of the FRT Ubi-FLAG-Med26 rescue construct. (C) In situ hybridization with the Med26 probe on two germ band extended embryos derived from a cross of MedMB00704/+ heterozygous parents. The top embryo shows ubiquitous Med26 expression, while the bottom embryo lacks expression and is presumably a homozygous mutant. (D) Haltere (H), leg (L), and wing (W) imaginal discs with Med26MB00704 homozygous clones, stained with anti-Med26 (green). Mutant clones lacking any detectable Med26 staining can grow to a large size. (E) Adult wing in which Med26 mutant clones were induced, showing loss of regions of the wing margin (arrows). (F to M) Third larval instar wing imaginal discs with anterior to the left and dorsal up. Med26 is stained in green. The broken white line outlines the region containing Med26 in panels F, G, H, and I; unstained regions outside this line are homozygous null. Cut (magenta in panel F and white in panels F′ and J) and Wg (magenta in panel G and white in panels G′ and K) are lost from part of the dorsal-ventral boundary in Med26 mutant clones (arrows), but expression is still present in some mutant cells. (J and K) Wild-type imaginal discs. Sens (magenta in panel H and white in panels H′ and L) is still expressed in stripes on either side of the dorsal-ventral boundary despite the loss of Med26. There is some loss of expression on the posterior side of the disc indicated by the arrow. (L) Wild-type imaginal disc for comparison. Nub (magenta in panel I and white in panels I′ and M) shows reduced expression in Med26 mutant cells. Panel M shows a wild-type imaginal disc for comparison.
Gene regulation by Med26 in the wing disc.
To investigate the reason for the wing margin loss, we generated Med26 mutant clones in the wing imaginal disc and examined the effect on genes expressed at the dorsal-ventral boundary, which gives rise to the adult wing margin. cut and wingless (wg), two genes regulated by Notch signaling (34, 35), showed reduced expression in large Med26 mutant clones (Fig. 2, compare panel F′ with panel J and panel G′ with panel K). However, expression was not absent from all mutant cells, suggesting that these genes are not direct transcriptional targets of Med26 but are regulated by a nonautonomous mechanism influenced by Med26. In addition, sufficient Wg remained to activate its target gene senseless (sens) (36) almost normally in Med26 mutant tissue (Fig. 2, panels H and H′ versus panel L). Another gene important for specifying the wing pouch is nubbin (nub) (37). Nub protein levels were slightly reduced in the absence of Med26 (Fig. 2I). However, this is unlikely to explain the reduction in wg and cut expression, as Nub is reported to be a repressor of these genes (24).
Med26 associates with actively transcribed loci in Drosophila salivary glands.
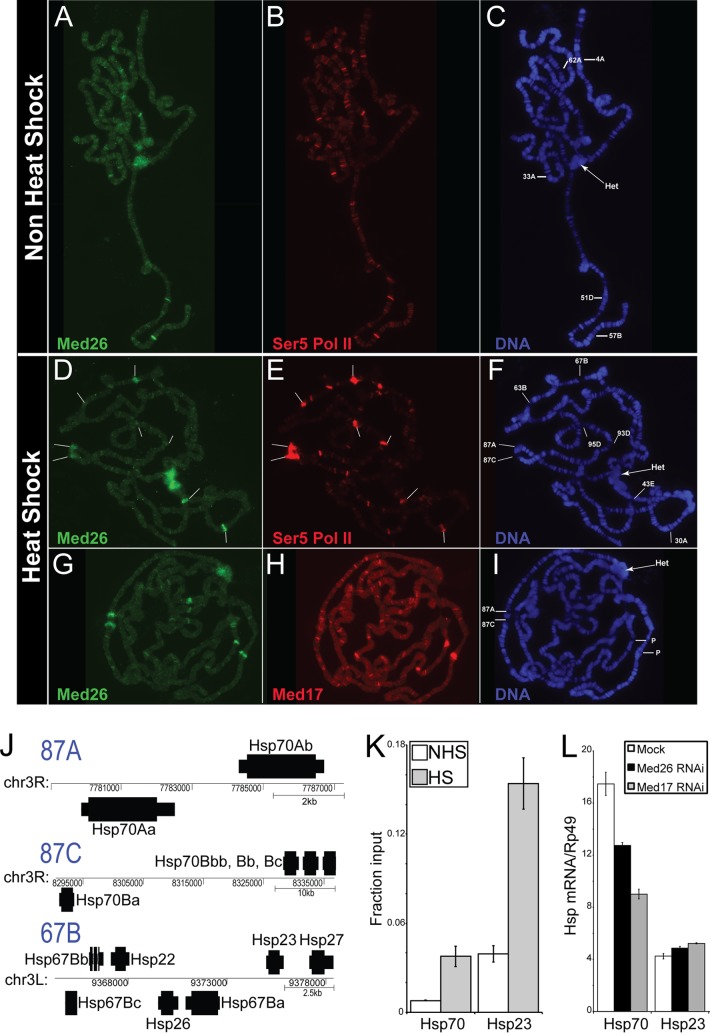
We used the antisera generated against Med26 to probe polytene chromosomes from third-instar larval salivary glands in an effort to identify loci bound by Med26. In addition, we costained with a monoclonal antibody to the C-terminal domain (CTD) of RNA polymerase II phosphorylated on serine 5 of the hepapeptide repeat (YSPTSPS). This antibody identifies active sites of transcription on polytene chromosomes (38). Med26 was associated with many of the actively transcribed genes, marked by phosphorylated RNA polymerase II, characteristic of the developmental puffs active during this period (Fig. 3A and B).
FIG 3.
Med26 localizes to active genes on polytene chromosomes. (A to F) Untreated polytene chromosomes (A to C) or heat-shocked polytene chromosomes (D to F) stained with Med26 (A, D), Ser5-phosphorylated RNA polymerase II (B, E), and 4′,6-diamidino-2-phenylindole (DAPI), showing DNA with developmental puffs (62A, 4A, 33A, 51D, and 57B in panel C) or HSP loci (63B, 67B, 87A, 87C, 95B, and 93D in panel F) and chromocenter (Het) indicated (C, F). (G to I) Heat-shocked polytene chromosomes stained with Med26 (G), Med17 (H), and DAPI-stained DNA with HSP70 loci (87A, 87C) and P elements bearing only HSEs indicated (I). (J) Diagram of the heat shock protein genes at 87A, 87C, and 67B. (K) qPCR analysis of chromatin precipitated with Med26 antisera under non-heat shock (NHS) or heat shock (HS) conditions. (L) Heat shock protein (Hsp) mRNA expression normalized to Rp49. Mock, mock-treated cells; Med26 RNAi, Med26-depleted cells; Med17 RNAi, Med17-depleted cells. The mRNA level relative to Rp49 is indicated on the y axis.
To determine if Med26 is recruited to induced genes upon stimulation, we used the well-characterized heat shock system. In response to a brief heat shock, the heat shock protein (HSP) loci become puffed and actively transcribed. This system has served as an important model for activated transcription. We observed clear inducible recruitment of Med26 to heat shock loci 87A, 87C, and 67B upon heat treatment (Fig. 3D and G) Costaining with antibodies for Med17 indicated that its recruitment is likely as a component of the Mediator complex (Fig. 3H). In agreement with previous work on heat shock factor (HSF) recruitment of Mediator in Drosophila, we observed both Med17 and Med26 recruited to P elements containing heat shock elements (HSEs) but no core promoter at chromosomal loci 30A and 43E (Fig. 3G and H) (27).
To examine Med26 recruitment to specific HSP genes, we used chromatin immunoprecipitation in cultured Drosophila cells. Because Med26 is recruited to 87A, 87C, and 67B upon heat shock, we immediately had candidate genes to investigate; 87A and 87C harbor the Hsp70 genes, and 67B contains the small heat shock genes (Fig. 3J). We targeted the Hsp70 and Hsp23 genes for analysis. Cells were cross-linked with formaldehyde under normal growing conditions or after heat shock. Chromatin associated with Med26 was precipitated using our polyclonal sera and then analyzed using quantitative PCR. We find that Med26 is recruited to both Hsp70 and Hsp23 upon heat shock. Heat-shocked cells show a 5-fold increase in Med26 in the promoter regions of both Hsp70 and Hsp23 (Fig. 3K).
To determine the effect of depletion of Med26 at specific genes, we depleted Med26 or Med17, a core Mediator subunit, from S2 cells and subjected the cells to a brief heat shock. We then assayed HSP RNA levels using quantitative PCR of cDNA prepared from these cells. We find that depletion of Med26 decreases the expression of Hsp70 about 25%, while depletion of Med17 causes a 50% decrease in Hsp70 mRNA (Fig. 3L). The effect is gene specific; the small heat shock gene Hsp23 is relatively unaffected by depletion of Med26 or Med17 under these same conditions.
Small and large Mediator complexes are conserved in Drosophila.
The Mediator complex is thought to exist in two dynamically related complexes. One complex contains the core subunits and Med26 and the other complex contains the core and the Cdk8 module (Fig. 4A). We tested this relationship in Drosophila using immunoprecipitation, protein stability experiments, and polytene chromosome staining. Using antisera to Med26 or to Med13 of the Cdk8 module, we immunoprecipitated Mediator complexes from Drosophila embryo nuclear extracts and probed for Med17 and MED14, as an indicator of the Mediator core complex. In addition, Med26, Med12, and Med13 were also tested (Fig. 4B).
Med12 and Med13 were undetectable in Mediator complexes precipitated with the Med26 antisera. However, we did observe Med17 and MED14 precipitation, indicating association with the core complex, as was seen in experiments discussed above. Mediator complexes immunoprecipitated with antisera against Med13 contained the core subunits and Med12 but only trace amounts of Med26. Finally, Med17 antiserum precipitated MED14, Med26, Med12, and Med13, verifying association of these subunits with the Mediator core, presumably in different complexes.
Subunit stability in large coactivator complexes has been used as an indication of complexes and subcomplexes present in the cell (39). The ease of RNA interference in Drosophila provides a method to investigate the stability of complex components to the loss of other components. We used dsRNA directed against Med26, Med12, and Med17 to probe the relationship of these Mediator complexes in the cell (Fig. 4C). Med17 is an important structural component of the head module, and loss of Med17 leads to instability of the entire head module in yeast (31, 32). As a control, we used a nonspecific dsRNA.
We found that the depletion of Med26 had little effect on the stability of Med17, Med12, or Med13. Depletion of Med17 caused instability of a large fraction of the Med26 in the cell. This indicates that Med26 stability depends on the integrity of the Med17 subunit and likely the head module. In contrast, depletion of Med17 has little effect on the stability of Med12 and Med13. Consistent with this observation, depletion of Med12 had no effect on the stability of Med17 or Med26 but caused a destabilization of Med13.
This result contrasts previous results examining the kinase module that found Med13 was stable upon loss of Med12 (16, 17). To confirm our findings, we repeated the experiment with dsRNA directed against both Med12 and Med13. Med12 and Med13 antibodies were derived from different host species, allowing detection on the same blot using the two different infrared detection channels of the Odyssey infrared imaging system. This ensures a direct comparison between Med12 and Med13 in an individual lane. We found that Med13 is destabilized in cells treated with Med12 dsRNA and that Med12 is destabilized in cells treated with Med13 dsRNA (Fig. 4D). This is consistent with the idea that there is an interdependence of the subunits in the kinase module for stability in these cells.
We used immunofluorescence of salivary gland polytene chromosomes to determine the distribution of Med26 and Med12 (a component of the kinase module). Surprisingly, both proteins were found at many sites on the polytene chromosomes (Fig. 4E). We observed three types of labeled sites: sites that contained only Med26, sites that contained only Med12, and sites that contained both. A closer examination of one chromosomal arm indicates that the majority of observable sites contained both Med26 and Med12 (Fig. 4F) although with various ratios.
Drosophila Med26 interacts with HP1 and pericentric heterochromatin.
During our examination of salivary gland polytene chromosomes, we noticed that, in contrast to the other Mediator subunits, Med26 antiserum consistently stained the pericentric heterochromatin of the chromocenter (Fig. 3 and 4). To positively identify this area of staining as the chromocenter, we costained with a monoclonal antibody to Drosophila HP1 (22), a well-characterized component of pericentric heterochromatin (Fig. 5A). Med26 and HP1 staining overlapped in the chromocenter. Interestingly, while HP1 also coats the heterochromatic chromosome 4, Med26 staining was not as uniform or strong on chromosome 4 as is seen in the chromocenter, indicating some specificity of Med26 for the chromocenter.
To verify the interaction, we performed immunoprecipitation experiments from nuclear extracts derived from 0- to 12-h embryos. Antiserum directed against Med26, but not preimmune serum, precipitated HP1 from nuclear extracts (Fig. 5B, top). Consistent with an interaction between Med26 and HP1, precipitation of HP1 using a coprecipitated Med26 from either nuclear extract or a chromatographic fraction enriched for transcription factors (H.4) (Fig. 5B, bottom). Both HP1 and Med26 also precipitate Med17.
To localize the region of HP1 responsible for interacting with Med26, we examined the ability of expressed subregions of HP1 to pull down Med26. HP1 protein contains three defined regions (Fig. 5C). The amino terminus contains a chromo domain that binds to methylated histone H3. The carboxy terminus contains a chromoshadow domain (CS) thought to bind to a PXVXL peptide sequence. A region termed the hinge region, which joins the two domains, is thought to bind RNA. To determine which region is responsible for the interaction with Med26, we created GST fusion proteins containing either the chromo and hinge regions or the chromoshadow domain. The GST fusion containing the chromoshadow domain was sufficient to precipitate Med26 from nuclear extracts, while the fusion containing the chromo domain and hinge region was not sufficient (Fig. 5C).
The HP1 chromoshadow domain binds a degenerate peptide sequence (PXVXL) found in many of its binding partners (Fig. 5D). Comparison of this motif with the conserved extreme carboxy terminus of the Med26 proteins from humans or Drosophila showed a related motif. To determine if this was sufficient to bind HP1, we created maltose binding protein (MBP) fusions containing the last 11 amino acids (PLIVLPYVLLE) of Drosophila Med26. MBPs containing this sequence but not MBP alone were capable of directly binding recombinant HP1 (Fig. 5D), indicating the sequence is sufficient for binding to HP1.
DISCUSSION
Higher eukaryotes have diversified the components of the Mediator complex to include subunits that are found only in metazoan cells. We have identified the metazoan-specific Med26 homologue in Drosophila and for the first time characterized this subunit in an animal model. We find that Med26 is essential for organismal survival but is dispensable for cellular proliferation and viability. This is reminiscent of the results for cells lacking the components of the kinase module Med12, Med13, Cdk8, and cyclin C (13, 14, 18). However, this contrasts with the results seen in cells lacking Med17, a component of the Mediator core, which is essential for cell growth and proliferation (14).
We find that the stability of Med26 in cultured cells depends on an intact Mediator head module, as depletion of Med17, an integral part of the head module, causes a loss of Med26 from the cell. In contrast, the stability of the kinase module does not depend on an intact head module. This suggests that the kinase module may behave independently from the Mediator core, while Med26 likely depends on the core, at least for its role as a transcriptional coactivator.
Interestingly, we find that in these cultured Drosophila S2 cells, the stability of Med12 and Med13 is interdependent. This contrasts the findings in Drosophila Kc cells (16) and in imaginal discs in vivo (17) where Med13 is stable upon loss of Med12. One possible explanation is the presence of a gene product that can substitute for Med12 in Kc cells and imaginal discs that is not present in S2 cells. In human cells, there are both Med12-like (MED12L) and Med13-like (MED13L) proteins in addition to the canonical subunits (40–43).
As expected, Drosophila Med26 behaves as a component of the active form of the Mediator complex. We find that Med26 and RNA polymerase II colocalize at transcriptionally active developmental puffs on polytene chromosomes. In addition, Med26 and Med17 are recruited to the heat shock genes upon stimulation. In good agreement with previous work on Mediator, transgenic loci that contain HSEs but no core promoter are sufficient for the recruitment in response to heat treatment (27). This is consistent with the idea that Med26 is a component of the activator-recruited Mediator complex. We also find a gene-specific requirement for intact Mediator for Hsp mRNA induction. While Hsp70 induction is affected by loss of either Med26 or Med17, the small heat shock genes are relatively unaffected.
Unexpectedly, Med26 and the Cdk8 module colocalize at many loci on polytene chromosomes. This could indicate that both factors can dynamically assemble on the same gene or that the multiple copies of each gene present in polytene chromosomes allow us to detect a coactivation cycle that involves exchange of Med26 and the kinase module. Indeed, our immunoprecipitation experiments from nuclear extracts suggest that the two complexes are almost mutually exclusive, supporting the latter notion. Alternatively, the promoter escape and elongation control roles for Med26 (11) and the Cdk8 module (12) could overlap, leading to their colocalization.
Finally, we find Med26 at pericentric heterochromatin and associated with the HP1 silencing protein. The association is not uniform, however, since the heterochromatic 4th chromosome does not show the same level of colocalization. This result is surprising given the well-documented role of Med26 in activated transcription. There are indications that in mammalian cells MED26 is associated with the REST complex involved in gene silencing (9). REST is known to use HP1 in the silencing complex, indicating the HP1-Med26 interaction is perhaps conserved in vertebrates and may be involved in silencing as well. However, in the REST system, both the Cdk8 module and MED26 participate in the silencing (9, 10). We have not observed Cdk8 components colocalizing with Med26 in pericentric heterochromatin.
This work represents the first investigation of MED26 in an intact animal model. Previous work in cell culture models indicated that MED26 is required for transcription elongation and cellular proliferation (11). While we find Med26 associated with active genes, it is not required for cell proliferation or survival, indicating that its role in transcription elongation is not essential or is confined to a subset of genes. Further work in both systems is required to understand these differences.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH to J.T.L. (GM25232), M.T.M. (GM085250), and J.E.T. (GM56131).
We thank Janis Werner for polytene staining in Fig. 3 and 5. We also thank Hugo Bellen, Steve Cohen, the DSHB, the DGRC, the Bloomington stock center, Robert Tjian for support and advice, and Richard Freiman for comments on the manuscript.
Footnotes
Published ahead of print 12 May 2014
REFERENCES
- 1.Lemon B, Tjian R. 2000. Orchestrated response: a symphony of transcription factors for gene control. Genes Dev. 14:2551–2569. 10.1101/gad.831000 [DOI] [PubMed] [Google Scholar]
- 2.Malik S, Roeder RG. 2010. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat. Rev. Genet. 11:761–772. 10.1038/nrg2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourbon HM. 2008. Comparative genomics supports a deep evolutionary origin for the large, four-module transcriptional mediator complex. Nucleic Acids Res. 36:3993–4008. 10.1093/nar/gkn349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lariviere L, Seizl M, Cramer P. 2012. A structural perspective on Mediator function. Curr. Opin. Cell Biol. 24:305–313. 10.1016/j.ceb.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 5.Akoulitchev S, Chuikov S, Reinberg D. 2000. TFIIH is negatively regulated by Cdk8-containing mediator complexes. Nature 407:102–106. 10.1038/35024111 [DOI] [PubMed] [Google Scholar]
- 6.Malik S, Gu W, Wu W, Qin J, Roeder RG. 2000. The USA-derived transcriptional coactivator PC2 is a submodule of TRAP/SMCC and acts synergistically with other PCs. Mol. Cell 5:753–760. 10.1016/S1097-2765(00)80254-3 [DOI] [PubMed] [Google Scholar]
- 7.Ryu S, Zhou S, Ladurner AG, Tjian R. 1999. The transcriptional cofactor complex CRSP is required for activity of the enhancer-binding protein Sp1. Nature 397:446–450. 10.1038/17141 [DOI] [PubMed] [Google Scholar]
- 8.Galbraith MD, Donner AJ, Espinosa JM. 2010. CDK8: a positive regulator of transcription. Transcription 1:4–12. 10.4161/trns.1.1.12373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding N, Tomomori-Sato C, Sato S, Conaway RC, Conaway JW, Boyer TG. 2009. MED19 and MED26 are synergistic functional targets of the RE1 silencing transcription factor in epigenetic silencing of neuronal gene expression. J. Biol. Chem. 284:2648–2656. 10.1074/jbc.M806514200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding N, Zhou H, Esteve PO, Chin HG, Kim S, Xu X, Joseph SM, Friez MJ, Schwartz CE, Pradhan S, Boyer TG. 2008. Mediator links epigenetic silencing of neuronal gene expression with x-linked mental retardation. Mol. Cell 31:347–359. 10.1016/j.molcel.2008.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi H, Parmely TJ, Sato S, Tomomori-Sato C, Banks CA, Kong SE, Szutorisz H, Swanson SK, Martin-Brown S, Washburn MP, Florens L, Seidel CW, Lin C, Smith ER, Shilatifard A, Conaway RC, Conaway JW. 2011. Human mediator subunit MED26 functions as a docking site for transcription elongation factors. Cell 146:92–104. 10.1016/j.cell.2011.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donner AJ, Ebmeier CC, Taatjes DJ, Espinosa JM. 2010. CDK8 is a positive regulator of transcriptional elongation within the serum response network. Nat. Struct. Mol. Biol. 17:194–201. 10.1038/nsmb.1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treisman J. 2001. Drosophila homologues of the transcriptional coactivation complex subunits TRAP240 and TRAP230 are required for identical processes in eye-antennal disc development. Development 128:603–615 [DOI] [PubMed] [Google Scholar]
- 14.Boube M, Faucher C, Joulia L, Cribbs DL, Bourbon HM. 2000. Drosophila homologs of transcriptional mediator complex subunits are required for adult cell and segment identity specification. Genes Dev. 14:2906–2917. 10.1101/gad.17900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janody F, Treisman JE. 2011. Requirements for mediator complex subunits distinguish three classes of notch target genes at the Drosophila wing margin. Dev. Dyn. 240:2051–2059. 10.1002/dvdy.22705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gobert V, Osman D, Bras S, Auge B, Boube M, Bourbon HM, Horn T, Boutros M, Haenlin M, Waltzer L. 2010. A genome-wide RNA interference screen identifies a differential role of the mediator CDK8 module subunits for GATA/ RUNX-activated transcription in Drosophila. Mol. Cell. Biol. 30:2837–2848. 10.1128/MCB.01625-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrera I, Janody F, Leeds N, Duveau F, Treisman JE. 2008. Pygopus activates Wingless target gene transcription through the mediator complex subunits Med12 and Med13. Proc. Natl. Acad. Sci. U. S. A. 105:6644–6649. 10.1073/pnas.0709749105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loncle N, Boube M, Joulia L, Boschiero C, Werner M, Cribbs DL, Bourbon HM. 2007. Distinct roles for Mediator Cdk8 module subunits in Drosophila development. EMBO J. 26:1045–1054. 10.1038/sj.emboj.7601566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janody F, Martirosyan Z, Benlali A, Treisman JE. 2003. Two subunits of the Drosophila mediator complex act together to control cell affinity. Development 130:3691–3701. 10.1242/dev.00607 [DOI] [PubMed] [Google Scholar]
- 20.Metaxakis A, Oehler S, Klinakis A, Savakis C. 2005. Minos as a genetic and genomic tool in Drosophila melanogaster. Genetics 171:571–581. 10.1534/genetics.105.041848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marr MT, II, Isogai Y, Wright KJ, Tjian R. 2006. Coactivator cross-talk specifies transcriptional output. Genes Dev. 20:1458–1469. 10.1101/gad.1418806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James TC, Elgin SC. 1986. Identification of a nonhistone chromosomal protein associated with heterochromatin in Drosophila melanogaster and its gene. Mol. Cell. Biol. 6:3862–3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nolo R, Abbott LA, Bellen HJ. 2000. Senseless, a Zn finger transcription factor, is necessary and sufficient for sensory organ development in Drosophila. Cell 102:349–362. 10.1016/S0092-8674(00)00040-4 [DOI] [PubMed] [Google Scholar]
- 24.Neumann CJ, Cohen SM. 1998. Boundary formation in Drosophila wing: Notch activity attenuated by the POU protein Nubbin. Science 281:409–413. 10.1126/science.281.5375.409 [DOI] [PubMed] [Google Scholar]
- 25.Ronchi E, Treisman J, Dostatni N, Struhl G, Desplan C. 1993. Down-regulation of the Drosophila morphogen bicoid by the torso receptor-mediated signal transduction cascade. Cell 74:347–355. 10.1016/0092-8674(93)90425-P [DOI] [PubMed] [Google Scholar]
- 26.Hazelett DJ, Bourouis M, Walldorf U, Treisman JE. 1998. decapentaplegic and wingless are regulated by eyes absent and eyegone and interact to direct the pattern of retinal differentiation in the eye disc. Development 125:3741–3751 [DOI] [PubMed] [Google Scholar]
- 27.Park JM, Werner J, Kim JM, Lis JT, Kim YJ. 2001. Mediator, not holoenzyme, is directly recruited to the heat shock promoter by HSF upon heat shock. Mol. Cell 8:9–19. 10.1016/S1097-2765(01)00296-9 [DOI] [PubMed] [Google Scholar]
- 28.Schwartz BE, Werner JK, Lis JT. 2004. Indirect immunofluorescent labeling of Drosophila polytene chromosomes: visualizing protein interactions with chromatin in vivo. Methods Enzymol. 376:393–404. 10.1016/S0076-6879(03)76026-1 [DOI] [PubMed] [Google Scholar]
- 29.Pennington KL, Marr SK, Chirn GW, Marr MT., II 2013. Holo-TFIID controls the magnitude of a transcription burst and fine-tuning of transcription. Proc. Natl. Acad. Sci. U. S. A. 110:7678–7683. 10.1073/pnas.1221712110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heberlein U, Tjian R. 1988. Temporal pattern of alcohol dehydrogenase gene transcription reproduced byDrosophila stage-specific embryonic extracts. Nature 331:410–415. 10.1038/331410a0 [DOI] [PubMed] [Google Scholar]
- 31.Takagi Y, Calero G, Komori H, Brown JA, Ehrensberger AH, Hudmon A, Asturias F, Kornberg RD. 2006. Head module control of mediator interactions. Mol. Cell 23:355–364. 10.1016/j.molcel.2006.06.007 [DOI] [PubMed] [Google Scholar]
- 32.Linder T, Zhu X, Baraznenok V, Gustafsson CM. 2006. The classical srb4-138 mutant allele causes dissociation of yeast Mediator. Biochem. Biophys. Res. Commun. 349:948–953. 10.1016/j.bbrc.2006.08.099 [DOI] [PubMed] [Google Scholar]
- 33.Park JM, Kim JM, Kim LK, Kim SN, Kim-Ha J, Kim JH, Kim YJ. 2003. Signal-induced transcriptional activation by Dif requires the dTRAP80 mediator module. Mol. Cell. Biol. 23:1358–1367. 10.1128/MCB.23.4.1358-1367.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Micchelli CA, Rulifson EJ, Blair SS. 1997. The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Development 124:1485–1495 [DOI] [PubMed] [Google Scholar]
- 35.Rulifson EJ, Blair SS. 1995. Notch regulates wingless expression and is not required for reception of the paracrine wingless signal during wing margin neurogenesis in Drosophila. Development 121:2813–2824 [DOI] [PubMed] [Google Scholar]
- 36.Parker DS, Jemison J, Cadigan KM. 2002. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development 129:2565–2576 [DOI] [PubMed] [Google Scholar]
- 37.Ng M, Diaz-Benjumea FJ, Cohen SM. 1995. Nubbin encodes a POU-domain protein required for proximal-distal patterning in the Drosophila wing. Development 121:589–599 [DOI] [PubMed] [Google Scholar]
- 38.Schwartz BE, Larochelle S, Suter B, Lis JT. 2003. Cdk7 is required for full activation of Drosophila heat shock genes and RNA polymerase II phosphorylation in vivo. Mol. Cell. Biol. 23:6876–6886. 10.1128/MCB.23.19.6876-6886.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright KJ, Marr MT, II, Tjian R. 2006. TAF4 nucleates a core subcomplex of TFIID and mediates activated transcription from a TATA-less promoter. Proc. Natl. Acad. Sci. U. S. A. 103:12347–12352. 10.1073/pnas.0605499103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato S, Tomomori-Sato C, Parmely TJ, Florens L, Zybailov B, Swanson SK, Banks CA, Jin J, Cai Y, Washburn MP, Conaway JW, Conaway RC. 2004. A set of consensus mammalian mediator subunits identified by multidimensional protein identification technology. Mol. Cell 14:685–691. 10.1016/j.molcel.2004.05.006 [DOI] [PubMed] [Google Scholar]
- 41.Nagase T, Kikuno R, Nakayama M, Hirosawa M, Ohara O. 2000. Prediction of the coding sequences of unidentified human genes. XVIII. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 7:273–281 [DOI] [PubMed] [Google Scholar]
- 42.Muncke N, Jung C, Rudiger H, Ulmer H, Roeth R, Hubert A, Goldmuntz E, Driscoll D, Goodship J, Schon K, Rappold G. 2003. Missense mutations and gene interruption in PROSIT240, a novel TRAP240-like gene, in patients with congenital heart defect (transposition of the great arteries). Circulation 108:2843–2850. 10.1161/01.CIR.0000103684.77636.CD [DOI] [PubMed] [Google Scholar]
- 43.Joensuu T, Hamalainen R, Yuan B, Johnson C, Tegelberg S, Gasparini P, Zelante L, Pirvola U, Pakarinen L, Lehesjoki AE, de la Chapelle A, Sankila EM. 2001. Mutations in a novel gene with transmembrane domains underlie Usher syndrome type 3. Am. J. Hum. Genet. 69:673–684. 10.1086/323610 [DOI] [PMC free article] [PubMed] [Google Scholar]