Abstract
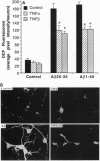
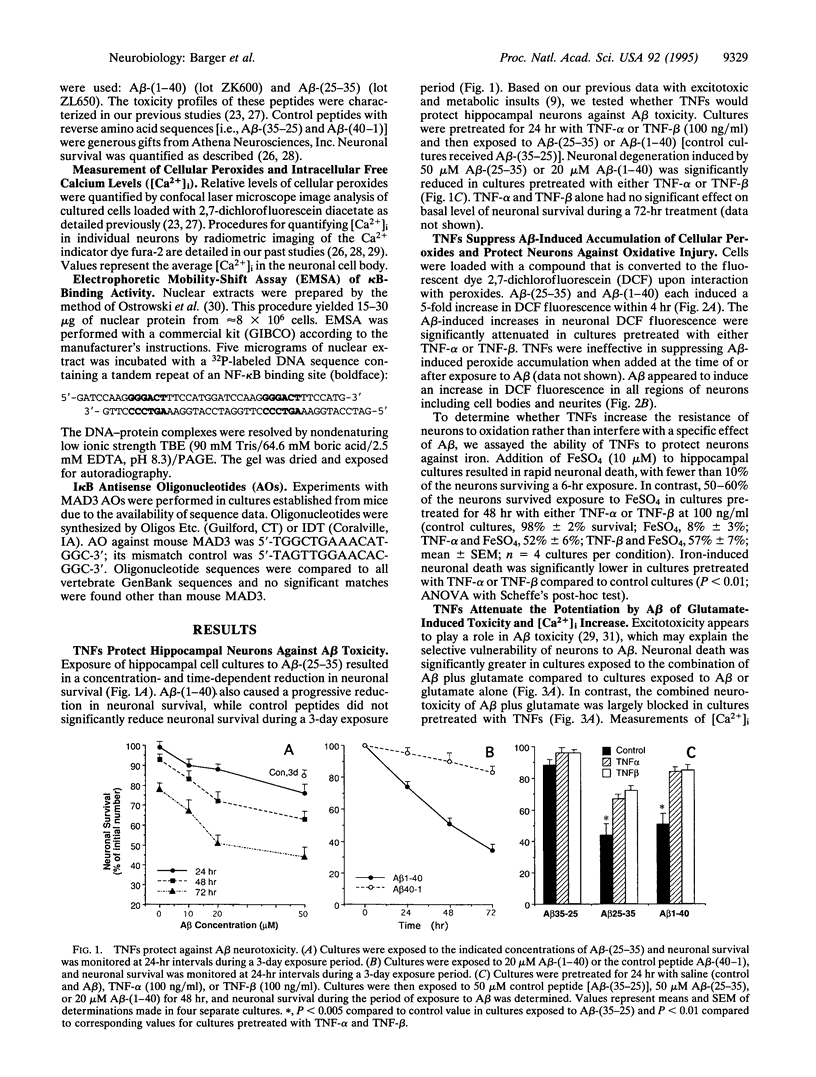
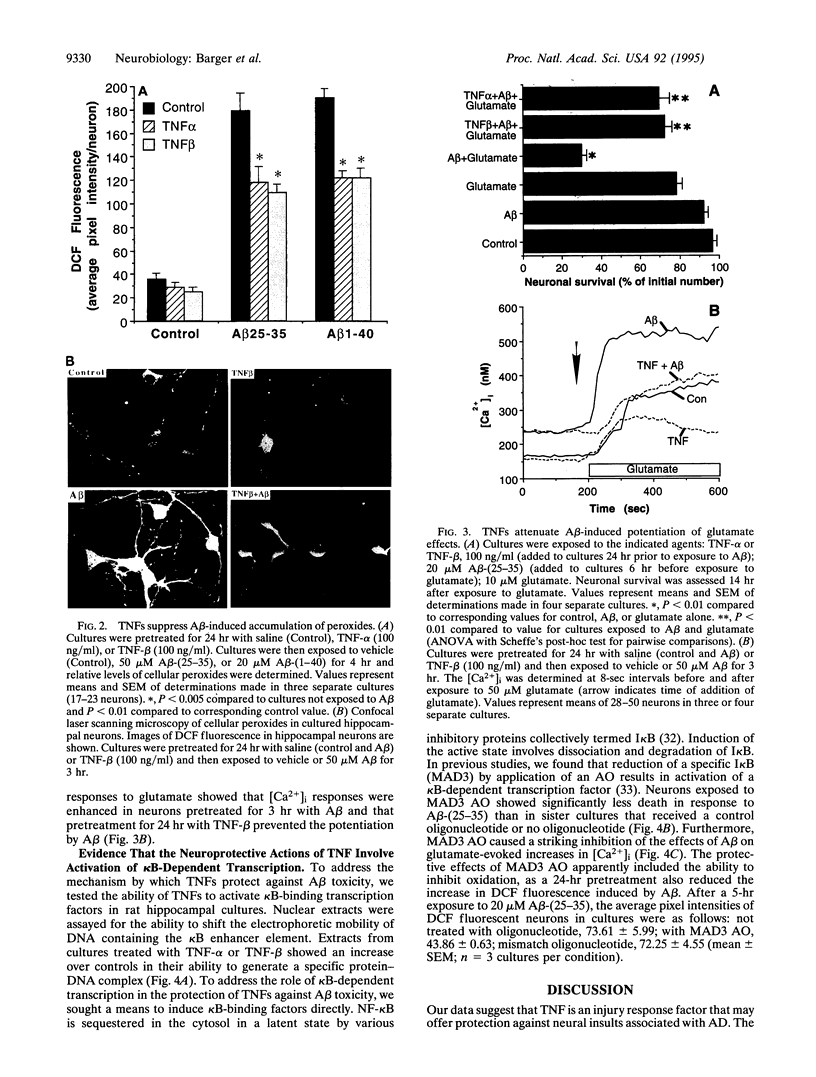
In Alzheimer disease (AD) the amyloid beta-peptide (A beta) accumulates in plaques in the brain. A beta can be neurotoxic by a mechanism involving induction of reactive oxygen species (ROS) and elevation of intracellular free calcium levels ([Ca2+]i). In light of evidence for an inflammatory response in the brain in AD and reports of increased levels of tumor necrosis factor (TNF) in AD brain we tested the hypothesis that TNFs affect neuronal vulnerability to A beta. A beta-(25-35) and A beta-(1-40) induced neuronal degeneration in a concentration- and time-dependent manner. Pretreatment of cultures for 24 hr with TNF-beta or TNF-alpha resulted in significant attenuation of A beta-induced neuronal degeneration. Accumulation of peroxides induced in neurons by A beta was significantly attenuated in TNF-pretreated cultures, and TNFs protected neurons against iron toxicity, suggesting that TNFs induce antioxidant pathways. The [Ca2+]i response to glutamate (quantified by fura-2 imaging) was markedly potentiated in neurons exposed to A beta, and this action of A beta was suppressed in cultures pretreated with TNFs. Electrophoretic mobility-shift assays demonstrated an induction of a kappa beta-binding activity in hippocampal cells exposed to TNFs. Exposure of cultures to I kappa B (MAD3) antisense oligonucleotides, a manipulation designed to induce NF-kappa B, mimicked the protection by TNFs. These data suggest that TNFs protect hippocampal neurons against A beta toxicity by suppressing accumulation of ROS and Ca2+ and that kappa B-dependent transcription is sufficient to mediate these effects. A modulatory role for TNF in the neurodegenerative process in AD is proposed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisen P. S., Davis K. L. Inflammatory mechanisms in Alzheimer's disease: implications for therapy. Am J Psychiatry. 1994 Aug;151(8):1105–1113. doi: 10.1176/ajp.151.8.1105. [DOI] [PubMed] [Google Scholar]
- Araujo D. M., Cotman C. W. Beta-amyloid stimulates glial cells in vitro to produce growth factors that accumulate in senile plaques in Alzheimer's disease. Brain Res. 1992 Jan 8;569(1):141–145. doi: 10.1016/0006-8993(92)90380-r. [DOI] [PubMed] [Google Scholar]
- Behl C., Davis J. B., Lesley R., Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994 Jun 17;77(6):817–827. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Chao C. C., Molitor T. W., Hu S. Neuroprotective role of IL-4 against activated microglia. J Immunol. 1993 Aug 1;151(3):1473–1481. [PubMed] [Google Scholar]
- Cheng B., Christakos S., Mattson M. P. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994 Jan;12(1):139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993 Oct 29;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Das S., Potter H. Expression of the Alzheimer amyloid-promoting factor antichymotrypsin is induced in human astrocytes by IL-1. Neuron. 1995 Feb;14(2):447–456. doi: 10.1016/0896-6273(95)90300-3. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Lee S. C., Mattiace L. A., Yen S. H., Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer's disease. Glia. 1993 Jan;7(1):75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- Fillit H., Ding W. H., Buee L., Kalman J., Altstiel L., Lawlor B., Wolf-Klein G. Elevated circulating tumor necrosis factor levels in Alzheimer's disease. Neurosci Lett. 1991 Aug 19;129(2):318–320. doi: 10.1016/0304-3940(91)90490-k. [DOI] [PubMed] [Google Scholar]
- Gelbard H. A., Dzenko K. A., DiLoreto D., del Cerro C., del Cerro M., Epstein L. G. Neurotoxic effects of tumor necrosis factor alpha in primary human neuronal cultures are mediated by activation of the glutamate AMPA receptor subtype: implications for AIDS neuropathogenesis. Dev Neurosci. 1993;15(6):417–422. doi: 10.1159/000111367. [DOI] [PubMed] [Google Scholar]
- Gilmore T. D., Morin P. J. The I kappa B proteins: members of a multifunctional family. Trends Genet. 1993 Dec;9(12):427–433. doi: 10.1016/0168-9525(93)90106-r. [DOI] [PubMed] [Google Scholar]
- Goldgaber D., Harris H. W., Hla T., Maciag T., Donnelly R. J., Jacobsen J. S., Vitek M. P., Gajdusek D. C. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7606–7610. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman Y., Mattson M. P. Secreted forms of beta-amyloid precursor protein protect hippocampal neurons against amyloid beta-peptide-induced oxidative injury. Exp Neurol. 1994 Jul;128(1):1–12. doi: 10.1006/exnr.1994.1107. [DOI] [PubMed] [Google Scholar]
- Goodman Y., Steiner M. R., Steiner S. M., Mattson M. P. Nordihydroguaiaretic acid protects hippocampal neurons against amyloid beta-peptide toxicity, and attenuates free radical and calcium accumulation. Brain Res. 1994 Aug 15;654(1):171–176. doi: 10.1016/0006-8993(94)91586-5. [DOI] [PubMed] [Google Scholar]
- Griffin W. S., Stanley L. C., Ling C., White L., MacLeod V., Perrot L. J., White C. L., 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K., Carney J. M., Mattson M. P., Aksenova M., Harris M., Wu J. F., Floyd R. A., Butterfield D. A. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman M., Shalit F., Roth-Deri I., Gutman B., Brodie C., Kott E., Sredni B. Correlation of cytokine secretion by mononuclear cells of Alzheimer patients and their disease stage. J Neuroimmunol. 1994 Jul;52(2):147–152. doi: 10.1016/0165-5728(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Kabrun N., Enrietto P. J. The Rel family of proteins in oncogenesis and differentiation. Semin Cancer Biol. 1994 Apr;5(2):103–112. [PubMed] [Google Scholar]
- Koh J. Y., Yang L. L., Cotman C. W. Beta-amyloid protein increases the vulnerability of cultured cortical neurons to excitotoxic damage. Brain Res. 1990 Nov 19;533(2):315–320. doi: 10.1016/0006-8993(90)91355-k. [DOI] [PubMed] [Google Scholar]
- Kolesnick R., Golde D. W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994 May 6;77(3):325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Lewis M., Tartaglia L. A., Lee A., Bennett G. L., Rice G. C., Wong G. H., Chen E. Y., Goeddel D. V. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2830–2834. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Clark R. K., McDonnell P. C., Young P. R., White R. F., Barone F. C., Feuerstein G. Z. Tumor necrosis factor-alpha expression in ischemic neurons. Stroke. 1994 Jul;25(7):1481–1488. doi: 10.1161/01.str.25.7.1481. [DOI] [PubMed] [Google Scholar]
- Marshak D. R., Pesce S. A., Stanley L. C., Griffin W. S. Increased S100 beta neurotrophic activity in Alzheimer's disease temporal lobe. Neurobiol Aging. 1992 Jan-Feb;13(1):1–7. doi: 10.1016/0197-4580(92)90002-f. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Barger S. W., Begley J. G., Mark R. J. Calcium, free radicals, and excitotoxic neuronal death in primary cell culture. Methods Cell Biol. 1995;46:187–216. doi: 10.1016/s0091-679x(08)61930-5. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Cheng B., Davis D., Bryant K., Lieberburg I., Rydel R. E. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992 Feb;12(2):376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P., Tomaselli K. J., Rydel R. E. Calcium-destabilizing and neurodegenerative effects of aggregated beta-amyloid peptide are attenuated by basic FGF. Brain Res. 1993 Sep 3;621(1):35–49. doi: 10.1016/0006-8993(93)90295-x. [DOI] [PubMed] [Google Scholar]
- Meda L., Cassatella M. A., Szendrei G. I., Otvos L., Jr, Baron P., Villalba M., Ferrari D., Rossi F. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995 Apr 13;374(6523):647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- Mokuno K., Ohtani K., Suzumura A., Kiyosawa K., Hirose Y., Kawai K., Kato K. Induction of manganese superoxide dismutase by cytokines and lipopolysaccharide in cultured mouse astrocytes. J Neurochem. 1994 Aug;63(2):612–616. doi: 10.1046/j.1471-4159.1994.63020612.x. [DOI] [PubMed] [Google Scholar]
- Ostrowski J., Sims J. E., Sibley C. H., Valentine M. A., Dower S. K., Meier K. E., Bomsztyk K. A serine/threonine kinase activity is closely associated with a 65-kDa phosphoprotein specifically recognized by the kappa B enhancer element. J Biol Chem. 1991 Jul 5;266(19):12722–12733. [PubMed] [Google Scholar]
- Prehn J. H., Backhauss C., Krieglstein J. Transforming growth factor-beta 1 prevents glutamate neurotoxicity in rat neocortical cultures and protects mouse neocortex from ischemic injury in vivo. J Cereb Blood Flow Metab. 1993 May;13(3):521–525. doi: 10.1038/jcbfm.1993.67. [DOI] [PubMed] [Google Scholar]
- Schreck R., Albermann K., Baeuerle P. A. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells (a review). Free Radic Res Commun. 1992;17(4):221–237. doi: 10.3109/10715769209079515. [DOI] [PubMed] [Google Scholar]
- Schwartz M., Solomon A., Lavie V., Ben-Bassat S., Belkin M., Cohen A. Tumor necrosis factor facilitates regeneration of injured central nervous system axons. Brain Res. 1991 Apr 5;545(1-2):334–338. doi: 10.1016/0006-8993(91)91309-o. [DOI] [PubMed] [Google Scholar]
- Sheng J. G., Mrak R. E., Griffin W. S. S100 beta protein expression in Alzheimer disease: potential role in the pathogenesis of neuritic plaques. J Neurosci Res. 1994 Nov 1;39(4):398–404. doi: 10.1002/jnr.490390406. [DOI] [PubMed] [Google Scholar]
- Shohami E., Novikov M., Bass R., Yamin A., Gallily R. Closed head injury triggers early production of TNF alpha and IL-6 by brain tissue. J Cereb Blood Flow Metab. 1994 Jul;14(4):615–619. doi: 10.1038/jcbfm.1994.76. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Farrah T., Goodwin R. G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994 Mar 25;76(6):959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Carney J. M., Tatsumo T., Stadtman E. R., Floyd R. A., Markesbery W. R. Protein oxidation in aging brain. Ann N Y Acad Sci. 1992 Nov 21;663:110–119. doi: 10.1111/j.1749-6632.1992.tb38654.x. [DOI] [PubMed] [Google Scholar]
- Tchelingerian J. L., Quinonero J., Booss J., Jacque C. Localization of TNF alpha and IL-1 alpha immunoreactivities in striatal neurons after surgical injury to the hippocampus. Neuron. 1993 Feb;10(2):213–224. doi: 10.1016/0896-6273(93)90312-f. [DOI] [PubMed] [Google Scholar]