ABSTRACT
Nuclear factor κB (NF-κB) plays important roles in innate immune responses by regulating the expression of a large number of target genes involved in the immune and inflammatory response, apoptosis, cell proliferation, differentiation, and survival. To survive in the host cells, viruses have evolved multiple strategies to evade and subvert the host immune response. Herpes simplex virus 1 (HSV-1) bears a large DNA genome, with the capacity to encode many different viral proteins to counteract the host immune responses. In the present study, we demonstrated that HSV-1 protein kinase US3 significantly inhibited NF-κB activation and decreased the expression of inflammatory chemokine interleukin-8 (IL-8). US3 was also shown to hyperphosphorylate p65 at serine 75 and block its nuclear translocation. Two US3 mutants, K220M and D305A, still interacted with p65; however, they could not hyperphosphorylate p65, indicating that the kinase activity of US3 was indispensable for the function. The attenuation of NF-κB activation by HSV-1 US3 protein kinase may represent a critical adaptation to enable virus persistence within the host.
IMPORTANCE This study demonstrated that HSV-1 protein kinase US3 significantly inhibited NF-κB activation and decreased the expression of inflammatory chemokine interleukin-8 (IL-8). US3 hyperphosphorylated p65 at serine 75 to inhibit NF-κB activation. The kinase activity of US3 was indispensable for its hyperphosphorylation of p65 and abrogation of the nuclear translocation of p65. The present study elaborated a novel mechanism of HSV-1 US3 to evade the host innate immunity.
INTRODUCTION
Herpes simplex virus 1 (HSV-1), a member of the Alphaherpesvirinae subfamily, is a large, enveloped virus, with a linear, double-stranded (ds) DNA genome of about 152 kb. All members of the Alphaherpesvirinae subfamily encode a serine/threonine kinase called US3 that is not found in the other subfamilies (1). Although US3 is not essential for viral replication in cell culture, increasing evidence indicates that it is vital for viral fitness (1–5). Many biological functions have been directly ascribed to US3, including prevention of virus-induced apoptosis (6–11), nuclear egress, virion maturation (12–16), rearrangements of the cytoskeleton, promoting cell-to-cell spread of virus infection (17, 18), inhibiting histone deacetylation by phosphorylation of histone deacetylase 1 (HDAC-1) and HDAC-2, which otherwise silence gene expression (19–21), disrupting promyelocytic leukemia protein nuclear bodies (PML-NBs) (22), downregulating major histocompatibility complex class I (MHC-I) surface expression, and evasion of the host immune response (23). US3 is also reported to masquerade as cellular kinase Akt to phosphorylate tuberous sclerosis complex 2 (TSC2), leading to constitutive activation of mammalian target of rapamycin complex 1 (mTORC1) and enhancement of viral gene expression (24, 25).
In vitro studies suggested that HSV-1 US3 plays an important role in resistance to interferon (IFN). US3-deficient HSV-1 was more sensitive to alpha IFN (IFN-α) and induced stronger activation of IFN regulatory factor 3 (IRF3) (26, 27). Our recent work also demonstrated that US3 hyperphosphorylated IRF3 and inhibited IFN-β production (28). Liang et al. demonstrated that US3 protein kinase phosphorylated the α subunit of the IFN-γ receptor and subsequently led to inhibition of IFN-γ-induced IFN-stimulated gene (ISG) expression (29). Recently, US3 protein kinase was proven to be necessary and sufficient to suppress extracellular signal-regulated kinase (ERK) activity and subvert host mitogen-activated protein kinase (MAPK) signaling pathways (30). Furthermore, HSV-1 US3 cooperates with glycoprotein B to rapidly inhibit CD1d antigen presentation and natural killer T-cell activation (23). Unfortunately, the molecular mechanisms behind most of the functions of US3 are still poorly understood.
It is well documented that the transcription factor NF-κB plays important roles in the innate immune responses. Viral infection induces the activation of NF-κB, which mediates cytokine and chemokine production and regulation of apoptotic processes. Moreover, NF-κB regulates a large variety of genes involved in numerous physiological processes, including inflammation, immune cell development, cell survival, differentiation, proliferation, cellular stress responses, cell adhesion, and homoeostasis of the adaptive immune system (31–36). The NF-κB protein family comprises five members, including ReLA (p65) NF-κB1 (p50 and its precursor p105), NF-κB2 (p52 and its precursor p100), and ReLB and c-ReL, which share a structurally conserved N-terminal Rel homology domain (RHD) that is important for protein dimerization, DNA binding, interaction with inhibitor of NF-κB (IκB), and nuclear translocation (32, 37). Activation of NF-κB is a complex process induced by a variety of stimuli, including microbial and viral products, cytokines, DNA damage, oxidative stress, and radiation (38). Most NF-κB dimers are inactively sequestered in the cytoplasm because of their association with IκB proteins, the most common of which is IκBα. Upon stimulation, IκB proteins are phosphorylated to degradation by the IκB kinase (IKK) complex, which contains two catalytic subunits, IKKα and IKKβ, as well as a regulatory subunit, IKKγ (NF-κB essential modulator [NEMO]) (39, 40). This leads to liberation of the NF-κB p65/p50 heterodimers, their nuclear translocation, and NF-κB-dependent gene transcription. Numerous upstream signaling cascades converge on the IKK complex, which is therefore the central mediator of canonical NF-κB activation. The activation of NF-κB can induce the expression of IFN-β, MHC-I, and several inflammatory cytokines (for a review, see reference 41). And that is believed to protect hosts from viral pathogens. Hence, a wide variety of viruses counteract NF-κB activation with various strategies to evade the immune responses. Furthermore, viruses may modulate NF-κB signaling to enhance viral replication and prevent virus-induced apoptosis (42, 43). In most cases, these viruses encode proteins that disrupt or modulate immune responses by targeting specific aspects of the NF-κB signaling pathway.
Intensive studies have shown that viral genes carried by Epstein-Barr virus (EBV) (44–46), cytomegalovirus (47, 48), and varicella-zoster virus (VZV) (49) regulate the NF-κB pathway in a cell type-dependent manner. HSV-1 also encodes proteins to disturb the NF-κB pathway (50–53). ICP27, an immediate early gene product of HSV-1, has been shown to antagonize NF-κB signaling (54). VHS, a tegument protein which is carried in the virion and delivered into CD8+ dendritic cells (DCs), blocks the early-replication-independent activation of NF-κB during HSV-1 infection (55). The HSV-encoded late gene product γ134.5 protein inhibits activation of NF-κB in CD8+ DCs (56). The large genome of HSV-1 therefore enables the encoding of numerous proteins that modulate host innate immune responses. In the present study, we demonstrated that HSV-1 US3 dramatically downregulated NF-κB activation. US3 blocked tumor necrosis factor alpha (TNF-α)-induced nuclear translocation of p65 and decreased expression of inflammatory chemokines.
MATERIALS AND METHODS
Cells, viruses, and antibodies.
HEK293T cells and Vero cells were grown in Dulbecco's modified Eagle medium (DMEM; Gibco-BRL) supplemented with 10% fetal bovine serum (FBS) and 100 U/ml of penicillin and streptomycin. HeLa cells were maintained in Eagle's minimum essential medium (MEM; Gibco-BRL) supplemented with 10% FBS.
The wild-type (WT) HSV-1 F strain and its derivative HSV-1-US3-Flag, US3-Del-HSV-1, K220M-HSV-1, and D305A-HSV-1 recombinant viruses were propagated in Vero cells and titrated as described previously (28, 57).
The protease inhibitor mixture cocktail was purchased from CST (Boston, MA). Mouse anti-Myc (isotype IgG1), anti-hemagglutinin (HA) (isotype IgG2b), and anti-Flag (isotype IgG2b) monoclonal antibodies (MAbs) were purchased from ABmart (Shanghai, China). Mouse monoclonal IgG1 and IgG2b isotype control antibodies were purchased from eBioscience Inc. (San Diego, CA). Rabbit polyclonal anti-US3 was purchased from GL Biochem Ltd. (Shanghai, China). Rabbit polyclonal anti-IκBα and rabbit polyclonal anti-p65 were purchased from Proteintech (Wuhan, China), and mouse anti-β-actin MAb was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Human recombinant TNF-α (rTNF-α) was purchased from Biovision (San Francisco, CA).
Plasmid construction.
All enzymes, except for T4 DNA ligase (New England BioLabs, MA), used for cloning procedures were purchased from TaKaRa (Dalian, China). To construct US3-Flag, the US3 open reading frame (ORF) of HSV-1 F strain (GenBank accession no. GU734771.1) was amplified from plasmid US3-EYFP as previously described (58) and cloned into the BglII and EcoRI sites of the pCMV-Flag vector (Beyotime, Shanghai, China). Other plasmids were constructed similarly. Flag-NIK was amplified from the gift plasmid pcDNA3-NIK (59). Commercial reporter plasmids include NF-κB-Luc (Stratagene, La Jolla, CA) and RL-TK plasmid (Promega). Gift plasmids include the following: pCMV-p65-Flag (60), pFlag-IKKβ (61), pHA-IKKα (62), and pXP2-p-IL-8-Luc (63).
Transfection and DLR assays.
HEK293T cells were plated on 24-well dishes (Corning, NY) in DMEM (Gibco-BRL, MD) with 10% FBS at a density of 1 × 105 cells per well overnight before transfection as previously described (64). Cells were then cotransfected with 500 ng expression plasmid, 500 ng NF-κB-Luc, and 50 ng of RL-TK (internal control) to normalize transfection efficiency, as indicated by standard calcium phosphate precipitation (65, 66). Twenty-four hours posttransfection, cells were mock treated or treated with recombinant human TNF-α (10 ng/ml) or interleukin-1β (IL-1β) (10 ng/ml) for 6 h, and then dual-luciferase reporter (DLR) assays were performed as previously described (64) with a luciferase assay kit (Promega, Madison, WI). All reporter assays were completed at least in triplicate, and the results were shown as average values ± standard deviations (SD) from one representative experiment.
RNA isolation and semiquantitative RT-PCR.
Total RNA was extracted from HEK293T cells with TRIzol (Invitrogen, CA) according to the manufacturer's manual. Samples were digested with DNase I and subjected to reverse transcription. The cDNA was used as a template for semiquantitative PCR to investigate the expression pattern of human IL-8 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase). The detailed protocols have been previously described (64).
Immunofluorescence assays.
Immunofluorescence assays were performed as described previously (67). In brief, HeLa cells were transfected with the indicated plasmids for 24 h and then fixed in 4% paraformaldehyde, washed three times with phosphate-buffered saline (PBS), and permeabilized with 0.5% Triton X-100 in PBS for 10 min. The cells were rinsed with PBS and then incubated with PBS containing 5% bovine serum albumin (BSA) for 30 min at room temperature. Subsequently, cells were incubated with rabbit anti-p65 pAb (diluted 1:50) or with mouse anti-Flag MAb diluted (1:1,500) in PBS containing 0.5% BSA for 2 h at 37°C, followed by incubation with tetramethyl rhodamine isocyanate (TRITC)-conjugated goat anti-rabbit IgG (Pierce) and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (Sigma-Aldrich) in PBS containing 0.5% BSA for 1 h at 37°C. After each incubation step, cells were washed extensively with PBS. Samples were analyzed by fluorescence microscopy (Zeiss, Germany).
Co-IP assays.
Coimmunoprecipitation (co-IP) assays were performed as previously described (57). Briefly, HEK293T cells (5 × 106) were cotransfected with 10 μg of US3-Flag expression plasmids. Transfected cells were harvested at 24 h posttransfection and lysed on ice with 500 μl of lysis buffer. The lysate was incubated with 0.5 μg of the Flag antibodies and 30 μl of a 1:1 slurry of protein A/G Plus-agarose (Santa Cruz, CA) overnight at 4°C. The beads were washed four times with 1 ml of lysis buffer containing 500 mM NaCl, and Western blotting (WB) analysis was performed to detect endogenous p65 using p65 antibody. The co-IP assays were repeated twice, and typical blots are shown in the figures.
RESULTS
HSV-1 US3 inhibits NF-κB activation.
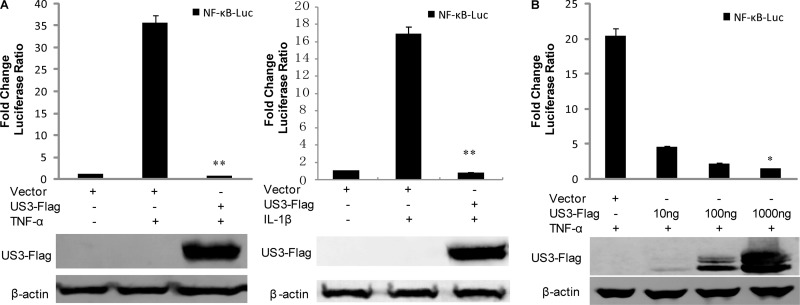
Given the importance of NF-κB signal transduction pathways in regulating the expression of functionally important immune molecules, we sought to determine whether US3 protein kinase modulated NF-κB activity. Proinflammatory cytokines induced by viral and bacterial infections (e.g., TNF-α, IL-1, dsRNA, and lipopolysaccharide [LPS]) and cellular stresses (e.g., phorbol esters and UV) activate the canonical NF-κB signaling pathway (34, 68, 69). NF-κB-Luc and RL-TK were cotransfected into HEK293T cells with or without US3-Flag. DLR assays were performed with cells treated with TNF-α or IL-1β. As expected, ectopic expression of US3 significantly inhibited both TNF-α- and IL-1β-induced NF-κB activation compared to the control vector (Fig. 1A). Additionally, US3 inhibited TNF-α-induced NF-κB promoter activity in a dose-dependent manner (Fig. 1B). The expression of the US3 protein was confirmed by WB analysis (Fig. 1B). Taken together, these results demonstrated that US3 dramatically reduced NF-κB activation.
FIG 1.
HSV-1 US3 inhibits TNF-α-induced NF-κB activation. (A) HEK293T cells were transfected with NF-κB-luc, together with RL-TK and pCMV-Flag control vector or US3-Flag. Twenty-four hours posttransfection, cells were treated with TNF-α or IL-1β and incubated for an additional 6 h. NF-κB-driven luciferase activity was determined by a dual-luciferase assay. (B) An increased amount of US3-Flag expression plasmid as indicated was transfected, and DLR assays were performed as described for panel A. The expression of US3 was analyzed by WB using anti-Flag and anti-β-actin monoclonal antibodies. The data represent means plus standard deviations for three replicates. Statistical analysis was performed using Student's t test. *, P < 0.05; **, P < 0.01.
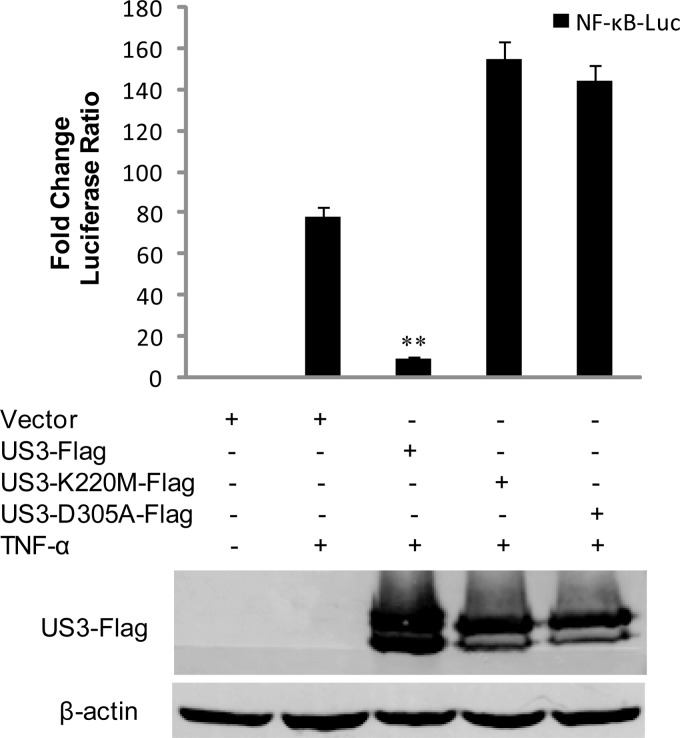
The kinase activity of US3 is essential for the inhibition of NF-κB activation.
HSV-1 US3 is a viral Ser/Thr kinase. To determine whether the kinase activity of US3 is required for the inhibition of NF-κB activation response, the kinase-dead (KD) US3 mutants K220M and D305A were generated. The expression plasmids were cotransfected into HEK293T cells to examine their ability to inhibit TNF-α-induced NF-κB reporter gene activities. In agreement with Fig. 1, expression of wild-type US3 strongly blocked TNF-α-mediated activation of NF-κB reporter. Expression of K220M or D305A, however, failed to repress the activation of NF-κB (Fig. 2). These results suggested that the kinase activity of US3 is essential for inhibiting NF-κB activation.
FIG 2.
The kinase activity of US3 is essential for its inhibition of NF-κB activation. Luciferase assays in HEK293T cells were performed as described for Fig. 1A to measure the activation of the NF-κB promoter following TNF-α stimulation in the presence of US3-Flag, K220M-Flag, or D305A-Flag. The expression of US3 and its mutants was verified by WB using mouse anti-Flag MAbs and anti-β-actin monoclonal antibodies. The data represent means plus standard deviations for three replicates. Statistical analysis was performed using Student's t test. **, P < 0.01.
HSV-1 US3 impedes the production of NF-κB-regulated cytokines IL-8.
The activation of NF-κB leads to production of antiviral cytokines and chemokines. To verify whether US3 downregulated NF-κB-regulated cytokines, IL-8-luciferase reporter assays were performed with an IL-8 reporter plasmid. HEK293T cells were transfected with IL-8-Luc and RL-TK in the presence or absence of US3-Flag. Twenty-four hours posttransfection, HEK293T cells were treated with TNF-α, and the luciferase activities in both p-IL-8-Luc-transfected cells were not affected by the control vector; however, US3 significantly inhibited IL-8-luciferase activity (Fig. 3A). To further determine the role of US3 in the inhibition of TNF-α-induced IL-8 production, IL-8 mRNA accumulation was measured by RT-PCR. As expected, mRNA levels of endogenous IL-8 were strongly upregulated by TNF-α stimulation (Fig. 3B), whereas US3 significantly reduced the accumulation of IL-8 mRNA. We also detected the accumulation of IL-8 mRNA under HSV-1 infection. As shown in Fig. 3C, the mRNA level of IL-8 was strongly upregulated in US3-del-HSV-1-infected cells compared with the wild-type HSV-1-infected cells. Moreover, cells infected with US3 kinase mutant viruses K220M-HSV-1 and D305A-HSV-1 induced higher IL-8 mRNA levels than that induced by WT HSV-1 infection. These results indicated that HSV-1 US3 blocked the production of NF-κB-regulated innate cytokines and the kinase activity of US3 is indispensable to prevent the production of NF-κB-regulated innate cytokines.
FIG 3.
HSV-1 US3 inhibits TNF-α-induced NF-κB cytokine expression. (A) HEK293T cells were cotransfected with pCMV-Flag control vector or US3-Flag expression plasmid along with pIL-8-Luc and RL-TK. Twenty-four hours posttransfection, cells were treated with TNF-α for 6 h and luciferase activity was measured. The expression of US3 was analyzed by WB using anti-Flag and anti-β-actin monoclonal antibodies. (B) HEK293T cells were transfected with pCMV-Flag control vector or US3-Flag. Twenty-four hours posttransfection, cells were mock treated or stimulated with TNF-α for 6 h before RT-PCR was performed using GAPDH and IL-8-primers. (C) HEK293T cells were mock infected or infected with WT HSV-1, US3-Del-HSV-1, K220M-HSV-1, D305A-HSV-1, and US3-repaired-HSV-1. Sixteen hours postinfection, cells were mock treated or treated with TNF-α for 6 h, and then the total RNA was analyzed by RT-PCR. The data represent means plus standard deviations for three replicates. Statistical analysis was performed using Student's t test. **, P < 0.01.
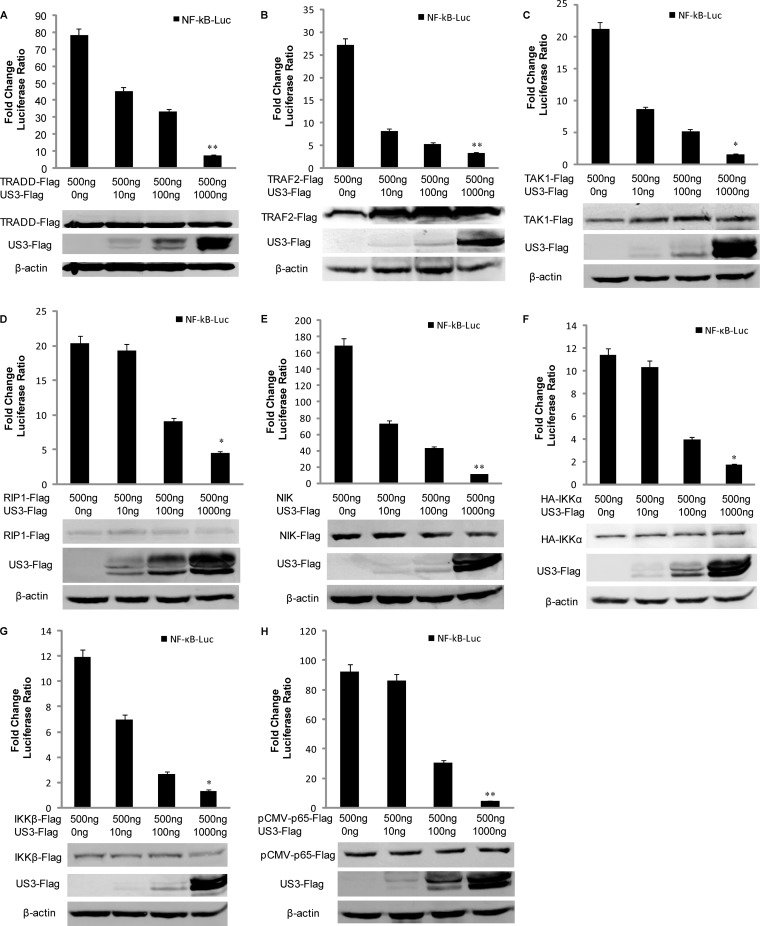
US3 inhibits NF-κB signaling pathway at the p65 level.
To further investigate at what level in the pathway US3 blocked NF-κB activity, we performed a dose-response assay with increasing amounts of Flag-tagged US3 and the expression plasmids of canonical NF-κB signaling pathway components, including TRADD (TNF receptor type 1-associated death domain), TRAF2 (TNF receptor-associated factor 2), TAK1 (TGF-β-activated kinase 1), RIP1 (receptor-interacting serine/threonine protein kinase 1), NIK (NF-κB-inducing kinase), IKKα, IKKβ, and p65. Expression of TRADD efficiently activated NF-κB-Luc reporter production 78-fold (Fig. 4A). And all the other expression constructs resulted in a 10- to 200-fold induction of the NF-κB-Luc reporter activity (Fig. 4B to H). NF-κB promoter activation driven by all of the expression constructs was inhibited by US3 in a dose-dependent manner (Fig. 4A to H). Collectively, these results suggested that US3 protein probably acted at or downstream from the level of p65 in the NF-κB signaling pathway.
FIG 4.
US3 inhibits the NF-κB signaling pathway at the p65 level. HEK293T cells were cotransfected with NF-κB-Luc, RL-TK, and indicated amount of US3 expression plasmid along with TRADD (A), TRAF2 (B), TAK1 (C), RIP1 (D), NIK (E), IKKα (F), IKKβ (G), or p65 (H) expression plasmids. Luciferase activity was analyzed as described for Fig. 1A. The data represent means plus standard deviations for three replicates. Statistical analysis was performed using Student's t test. **, P < 0.01; *, P < 0.05.
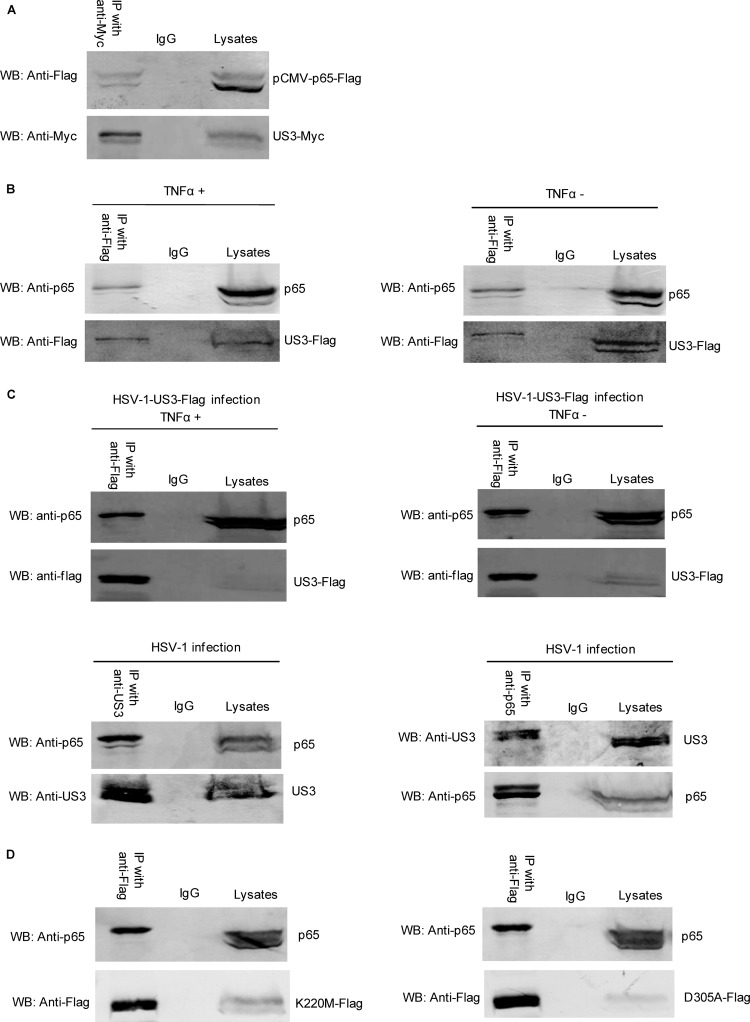
US3 interacts with endogenous p65.
In order to clarify the molecular mechanism of US3 to suppress NF-κB activation, we analyzed the potential interaction between p65 and US3. US3-Myc and pCMV-p65-Flag expression plasmids were cotransfected into HEK293T cells, and co-IP/WB analysis was performed with anti-Myc and anti-Flag MAbs. The ectopic expression of p65 was efficiently coimmunoprecipitated with US3 by anti-Myc MAbs (Fig. 5A).
FIG 5.
US3 interacts with endogenous p65. HEK293T cells were cotransfected with US3-Myc and pCMV-p65-Flag expression plasmids. (A) Thirty-six hours posttransfection, cells were harvested and lysed, and the samples were then subjected to immunoprecipitation assays using anti-Myc MAb (IP with anti-Myc) or nonspecific mouse monoclonal antibody (IgG). WBs were probed with the indicated Ab. (B) HEK293T cells were transfected with US3-Flag. Twenty-four hours posttransfection, cells were mock treated or treated with TNF-α and incubated for an additional 6 h, cells were harvested and lysed, and the samples were then subjected to immunoprecipitation assays using anti-Flag MAb (IP with anti-Flag) or nonspecific mouse monoclonal antibody (IgG). WBs were probed with the indicated Abs. (C) (Upper panel) HEK293T cells were infected with HSV-1-US3-Flag virus (multiplicity of infection [MOI], 10). Twenty-four hours postinfection, cells were mock treated or treated with TNF-α and incubated for 6 h. Immunoprecipitation assays were performed by anti-Flag (IP with anti-Flag) and WBs with anti-p65. (Lower panel) HEK293T cells were infected with WT HSV-1 (MOI = 10). Twenty-four hours postinfection, immunoprecipitation assays were performed by anti-US3 (IP with anti-US3) and WBs with anti-p65. The reciprocal IP was analyzed by anti-p65 (IP with anti-p65) and anti-US3. (D) HEK293T cells were transfected with K220M-Flag and D305A-Flag expression plasmids separately. Twenty-four hours posttransfection, cell lysates were immunoprecipitated with anti-Flag, or nonspecific mouse monoclonal antibody (IgG) WBs were probed with anti-p65.
We further investigated the interaction between US3 and endogenous p65; co-IP/WB analysis indicated that the endogenous p65 protein was efficiently coimmunoprecipitated with US3 by anti-Flag MAbs (Fig. 5B), and the interaction was independent of TNF-α stimulation. Furthermore, HEK293T cells were infected with HSV-1-US3-Flag to investigate the interaction between US3 and p65 in the context of viral infection with or without TNF-α stimulation. As a result, endogenous p65 was coimmunoprecipitated with US3, confirming the interaction between p65 and US3 (Fig. 5C, upper panel). We next infected the cells with wild-type HSV-1, and co-IP/WB analysis was performed with anti-p65/US3 and anti-US3/p65. The endogenous p65 was coimmunoprecipitated with US3 under viral infection (Fig. 5C, lower left panel). Moreover, the reciprocal IP reinforced their interaction (Fig. 5C, lower right panel).
Given that US3 kinase activity is important for the inhibition of NF-κB response, the US3 kinase mutants K220M and D305A were transfected into HEK293T cells separately. Co-IP/WB analysis indicated that the endogenous p65 protein was also efficiently coimmunoprecipitated with the two mutants (Fig. 5D). Thus, US3 interacted with endogenous p65, and kinase activity of US3 was dispensable for the interaction.
US3 hyperphosphorylates p65 at the site of serine 75.
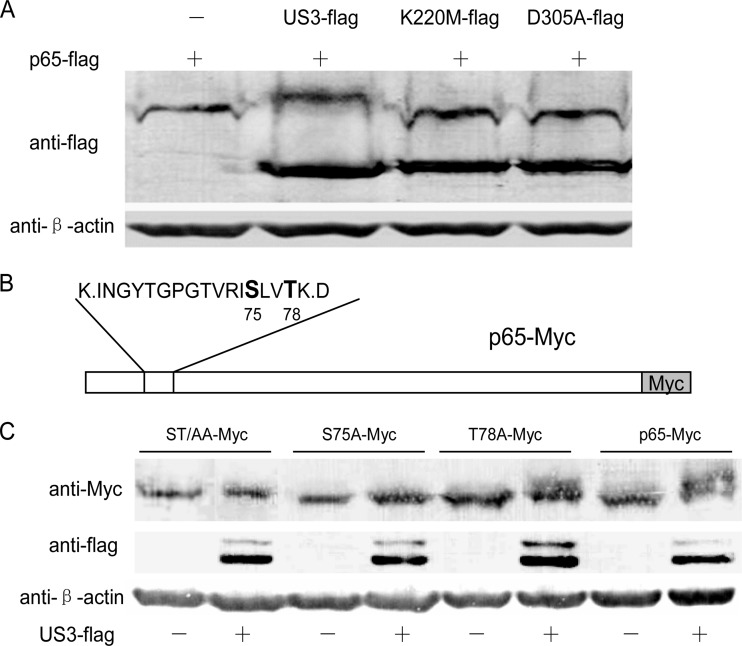
Subsequently, we investigated whether p65 was a substrate of US3 protein kinase. To test this hypothesis, HEK293T cells were transfected with pCMV-p65-Flag alone or with pCMV-US3-Flag, pCMV-K220M-Flag, or pCMV-D305A-Flag. As shown in Fig. 6A, p65-Flag was shown as a slower-migrating form under the expression of US3-Flag but not K220M-Flag or D305A-Flag, indicating that p65 was hyperphosphorylated by US3 and the kinase activity of US3 was indispensable.
FIG 6.
US3 hyperphosphorylates p65. (A) HEK293T cells were transfected with pCMV-p65-Flag alone or with pCMV-US3-Flag, pCMV-K220M-Flag, and pCMV-D305A-Flag separately. WBs were performed with anti-Flag and anti-β-actin monoclonal antibody. (B) Schematic diagram of the potential hyperphosphorylation sites (S75 and T78) of p65. (C) p65 and its three mutants (ST/AA-Myc, S75A-Myc, and T78A-Myc) were cotransfected with or without US3-Flag. Thirty-six hours posttransfection, cell lysates were subjected to WB analysis.
US3 usually targets Ser or Thr residues within motifs containing Arg or Lys. In order to identify the hyperphosphorylation sites of p65 by US3, the plasmids expressing p65-Myc and US3-Flag were cotransfected into HEK293T cells, and p65-Myc was immunoprecipitated by Myc MAb and subjected to SDS-PAGE analysis. The hyperphosphorylated p65 was cut and subjected to mass spectrometry. As shown in Fig. 6B, two potential hyperphosphorylation sites (S75 and T78) of p65 were identified. Three p65 mutants (ST/AA-Myc, S75A-Myc, and T78A-Myc) were then constructed to identify the exact hyperphosphorylation amino acids. As shown in Fig. 6C, T78A-Myc was also hyperphosphorylated by US3-Flag just like as p65-Myc, whereas ST/AA-Myc and S75A-Myc were not. The data indicated that p65 was hyperphosphorylated by US3 at the site of serine 75.
US3 prevents nuclear translocation of p65.
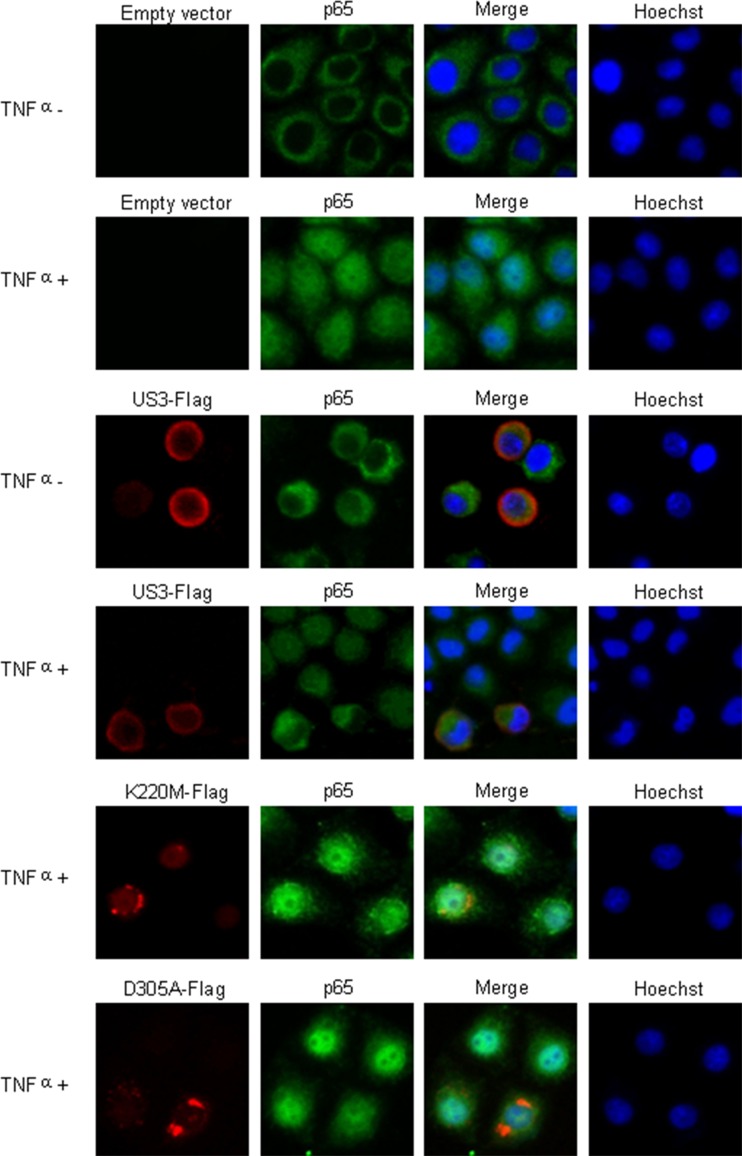
Nuclear translocation of p65 is crucial for the transcription of NF-κB. We hypothesized that hyperphosphorylation of p65 by US3 might abrogate the nuclear trafficking of this NF-κB subunit. In order to address this hypothesis, immunofluorescence was carried out to investigate whether US3 prevented the nuclear translocation of p65. HeLa cells were transfected with a US3-Flag plasmid or a control vector and stimulated with TNF-α for 30 min, followed by immunostaining of p65 and Flag-tagged US3. Although p65 predominantly located in the cytoplasm in mock-treated cells (Fig. 7, upper row), TNF-α treatment resulted in the nuclear translocation of p65. Ectopic expression of US3 prevented the nuclear translocation of p65 induced by TNF-α (Fig. 7). However, two mutants of US3 kinase, K220M and D305A, did not block the nuclear translocation of p65 induced by TNF-α (Fig. 7). These results demonstrated that the hyperphosphorylation of p65 by US3 was sufficient to prevent the nuclear accumulation of NF-κB and thereby to preclude its transcriptional activity.
FIG 7.
US3 prevents p65 nuclear translocation. HeLa cells were transfected with control vector, US3-Flag, K220M-Flag, and D305A-Flag expression plasmids. Twenty-four hours posttransfection, cells were treated with TNF-α or mock treated for 30 min as indicated. Cells were stained with mouse anti-Flag and rabbit anti-p65 pAb. FITC-conjugated goat anti-rabbit (green) and TRITC-conjugated goat anti-mouse (red) were used as the secondary antibodies. Cell nuclei (blue) were stained with Hoechst 33258. The images were obtained by fluorescence microscopy using a 40× objective.
DISCUSSION
In this study, HSV-1-encoded US3 has been demonstrated to be able to inhibit TNF-α-stimulated NF-κB signaling pathway. Given that the innate immune response is the first line of host antiviral systems, these results indicate that US3 plays an important role in immune evasion of the NF-κB signal transduction pathway. This pathway controls transcription of many immune molecules required to initiate an immune response to foreign pathogens, and so disruption of this pathway is likely to suppress critical immune effector capacity of the host cell.
It was previously reported that HSV-1 infection led to suppression of NF-κB activity (50–56), which was also described by other members of the herpesvirus family, including EBV, VZV, and Kaposi's sarcoma-associated herpesvirus (KSHV) (70–72). EBV BGLF4 is a member of the conserved herpesvirus kinases, having been found to phosphorylate several cellular and viral transcription factors, modulate their activities, and regulate downstream events. UXT is an NF-κB coactivator and interacts with NF-κB. BGLF4 phosphorylates UXT at the Thr3 residue. This modification interferes with the interaction between UXT and NF-κB. Thus, it attenuates NF-κB-mediated repression of the EBV lytic infection (70). VZV ORF61 is identified as an inhibitor of TNF-α-mediated activation of NF-κB within VZV-infected DCs, and its E3 ubiquitin ligase domain is essential (71). HSV-1 protein kinase US3 has been previously reported to be a potent inhibitor of IFN response, serving as one of several strategies used by HSV-1 to interrupt the innate immune system. It has been reported that removal of US3 increased IRF3 activation and Toll-like receptor 3 (TLR3) and IFN levels in infected monocytic cells (27). Our recent work has also clarified that the dimerization and nuclear translocation of IRF3 were blocked because of its hyperphosphorylation by US3 (28). In this study, HSV-1 US3 was demonstrated to block NF-κB activation and the expression of downstream cytokines. US3 also hyperphosphorylated p65 at serine 75 and abolished its nuclear translocation. A recent study showed that US3 inhibited TLR2-mediated activation of NF-κB through reducing TRAF6 polyubiquitination (73). However, the evidence was not convincing, as the effect of US3 on p65 was done only by DLR assays and the activation effect of p65 was very low. It also indicated that US3 may inhibit NF-κB activation via multiple strategies.
HSV-1 US3 is a protein kinase, which is conserved within alphaherpesvirus. K220 of HSV-1 US3 is critical for ATP binding, and D305 is critical for catalytic activity (74). The K220M and D305A mutants were constructed to investigate whether the kinase activity of US3 is necessary for the inhibitory activity against the NF-κB pathway. And our results show that the mutants could not block the activity of TNF-α-induced NF-κB promoter and hyperphosphorylate p65, indicating that the kinase activity was indispensable for the inhibitory activity.
It was reported that degradation of IκBα and nuclear translocation of NF-κB are not sufficient to promote a maximal NF-κB transcriptional response. Rather, the NF-κB complex must undergo additional posttranslational modifications involving site-specific phosphorylation (75). The p65/RelA subunit of NF-κB is a principal target for phosphorylation by various kinases, which function both in the cytoplasm and in the nucleus under differential induction by various stimuli (reviewed in depth in references 75 and 76). Both the RHD and the transcription activation domain (TAD) of p65 contain key sites (serine, threonine, or tyrosine that can be phosphorylated by kinases) that are specifically targeted by these kinases. One of the key phosphorylation events involves the action of the catalytic subunit protein kinase A (PKAc), which modifies the serine 276 residue that is located within the RHD of p65. This posttranslational modification regulates both the DNA-binding and oligomerization properties of NF-κB (77, 78). Phosphorylation of serine 276, however, is not exclusively mediated through PKAc. Whereas lipopolysaccharide (LPS) stimulation triggers PKAc action (79, 80), TNF-α stimulates phosphorylation of serine 276 through mitogen- and stress-activated kinase 1 (MSK1) (81), which enhances NF-κB transcriptional activity. Interestingly, PKAc phosphorylates p65 in the cytoplasm, whereas MSK1 functions in the nucleus. TNF-α induces phosphorylation of serine 311 within the RHD of p65 through the action of yet another kinase, PKCζ, and this modification similarly enhances the overall transcriptional response (82, 83). Direct phosphorylation of the NF-κB molecule at multiple sites has been shown to both positively and negatively regulate NF-κB activity. Phosphorylation of NF-κB is mediated by a variety of kinases (76), which could serve as potential targets for additional HSV-mediated modulation of the pathway.
Previous studies indicated that the alphaherpesvirus US3 kinases have minimal consensus phosphorylation sequence, which was characterized as (R)n-X-(S/T)-Z-Z (where n ≥ 2, X can be absent or Arg, Ala, Val, Pro, or Ser, and Z can be any amino acid except proline or an acidic residue) (10, 84). It is also reported that US3 protein kinases could masquerade as Akt and protein kinase A and phosphorylate the same substrates as they do (24, 74, 85). However, more evidence indicated that US3 has more substrates than originally predicted (16, 24).
In summary, the data presented herein describe the molecular mechanism of HSV-1 protein kinase US3 to abolish the NF-κB activation. Our results reveal a novel function for the US3 protein contained in the virion as an early inhibitor of the NF-κB signaling pathway. We provide convincing evidence that US3 efficiently inhibited p65-mediated transactivation by hyperphosphorylating p65 at serine 75 and preventing its nuclear translocation. Furthermore, we also demonstrate that the protein kinase activity of US3 is essential for the blockade of NF-κB activation, indicating that it is the process of phosphorylation that causes this pathway inhibition. These findings contribute to our understanding of the immune antagonism employed by HSV-1 US3 to dampen host antiviral signaling while potentially having implications for future development of therapeutic interventions to modulate HSV-1 pathogenesis, vaccine design, and gene therapies.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (81371795 and 81171584), the Major State Basic Research Development Program of China (973 Program) (2011CB504800 and 2010CB530100), and the Program for Changjiang Scholars and Innovative Research Team in Soochow University (PCSIRT, IRT1075) and Jiangsu Provincial Innovative Research Team.
We thank Rao Anjana (Immune Disease Institute, Harvard Medical School) and Gangmin Hur (Chungnam National University) for providing plasmids.
Footnotes
Published ahead of print 7 May 2014
REFERENCES
- 1.McGeoch DJ, Davison AJ. 1986. Alphaherpesviruses possess a gene homologous to the protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res. 14:1765–1777. 10.1093/nar/14.4.1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frame MC, Purves FC, McGeoch DJ, Marsden HS, Leader DP. 1987. Identification of the herpes simplex virus protein kinase as the product of viral gene US3. J. Gen. Virol. 68(Part 10):2699–2704 [DOI] [PubMed] [Google Scholar]
- 3.Hanks SK, Quinn AM, Hunter T. 1988. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 241:42–52. 10.1126/science.3291115 [DOI] [PubMed] [Google Scholar]
- 4.Heineman TC, Seidel K, Cohen JI. 1996. The varicella-zoster virus ORF66 protein induces kinase activity and is dispensable for viral replication. J. Virol. 70:7312–7317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Purves FC, Longnecker RM, Leader DP, Roizman B. 1987. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J. Virol. 61:2896–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leopardi R, Van Sant C, Roizman B. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. U. S. A. 94:7891–7896. 10.1073/pnas.94.15.7891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geenen K, Favoreel HW, Olsen L, Enquist LW, Nauwynck HJ. 2005. The pseudorabies virus US3 protein kinase possesses anti-apoptotic activity that protects cells from apoptosis during infection and after treatment with sorbitol or staurosporine. Virology 331:144–150. 10.1016/j.virol.2004.10.027 [DOI] [PubMed] [Google Scholar]
- 8.Hata S, Koyama AH, Shiota H, Adachi A, Goshima F, Nishiyama Y. 1999. Antiapoptotic activity of herpes simplex virus type 2: the role of US3 protein kinase gene. Microbes Infect. 1:601–607. 10.1016/S1286-4579(99)80059-8 [DOI] [PubMed] [Google Scholar]
- 9.Ogg PD, McDonell PJ, Ryckman BJ, Knudson CM, Roller RJ. 2004. The HSV-1 Us3 protein kinase is sufficient to block apoptosis induced by overexpression of a variety of Bcl-2 family members. Virology 319:212–224. 10.1016/j.virol.2003.10.019 [DOI] [PubMed] [Google Scholar]
- 10.Benetti L, Roizman B. 2004. Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis. Proc. Natl. Acad. Sci. U. S. A. 101:9411–9416. 10.1073/pnas.0403160101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munger J, Roizman B. 2001. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc. Natl. Acad. Sci. U. S. A. 98:10410–10415. 10.1073/pnas.181344498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagenaar F, Pol JM, Peeters B, Gielkens AL, de Wind N, Kimman TG. 1995. The US3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus. J. Gen. Virol. 76(Part 7):1851–1859 [DOI] [PubMed] [Google Scholar]
- 13.Reynolds AE, Wills EG, Roller RJ, Ryckman BJ, Baines JD. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939–8952. 10.1128/JVI.76.17.8939-8952.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schumacher D, Tischer BK, Trapp S, Osterrieder N. 2005. The protein encoded by the US3 orthologue of Marek's disease virus is required for efficient de-envelopment of perinuclear virions and involved in actin stress fiber breakdown. J. Virol. 79:3987–3997. 10.1128/JVI.79.7.3987-3997.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wisner TW, Wright CC, Kato A, Kawaguchi Y, Mou F, Baines JD, Roller RJ, Johnson DC. 2009. Herpesvirus gB-induced fusion between the virion envelope and outer nuclear membrane during virus egress is regulated by the viral US3 kinase. J. Virol. 83:3115–3126. 10.1128/JVI.01462-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mou F, Forest T, Baines JD. 2007. US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J. Virol. 81:6459–6470. 10.1128/JVI.00380-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demmin GL, Clase AC, Randall JA, Enquist LW, Banfield BW. 2001. Insertions in the gG gene of pseudorabies virus reduce expression of the upstream Us3 protein and inhibit cell-to-cell spread of virus infection. J. Virol. 75:10856–10869. 10.1128/JVI.75.22.10856-10869.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Favoreel HW, Van Minnebruggen G, Adriaensen D, Nauwynck HJ. 2005. Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread. Proc. Natl. Acad. Sci. U. S. A. 102:8990–8995. 10.1073/pnas.0409099102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morimoto T, Arii J, Tanaka M, Sata T, Akashi H, Yamada M, Nishiyama Y, Uema M, Kawaguchi Y. 2009. Differences in the regulatory and functional effects of the Us3 protein kinase activities of herpes simplex virus 1 and 2. J. Virol. 83:11624–11634. 10.1128/JVI.00993-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poon AP, Gu H, Roizman B. 2006. ICP0 and the US3 protein kinase of herpes simplex virus 1 independently block histone deacetylation to enable gene expression. Proc. Natl. Acad. Sci. U. S. A. 103:9993–9998. 10.1073/pnas.0604142103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walters MS, Kinchington PR, Banfield BW, Silverstein S. 2010. Hyperphosphorylation of histone deacetylase 2 by alphaherpesvirus US3 kinases. J. Virol. 84:9666–9676. 10.1128/JVI.00981-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung M, Finnen RL, Neron CE, Banfield BW. 2011. The alphaherpesvirus serine/threonine kinase US3 disrupts promyelocytic leukemia protein nuclear bodies. J. Virol. 85:5301–5311. 10.1128/JVI.00022-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao P, Pham HT, Kulkarni A, Yang Y, Liu X, Knipe DM, Cresswell P, Yuan W. 2011. Herpes simplex virus 1 glycoprotein B and US3 collaborate to inhibit CD1d antigen presentation and NKT cell function. J. Virol. 85:8093–8104. 10.1128/JVI.02689-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chuluunbaatar U, Roller R, Feldman ME, Brown S, Shokat KM, Mohr I. 2010. Constitutive mTORC1 activation by a herpesvirus Akt surrogate stimulates mRNA translation and viral replication. Genes Dev. 24:2627–2639. 10.1101/gad.1978310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuluunbaatar U, Mohr I. 2011. A herpesvirus kinase that masquerades as Akt: you don't have to look like Akt, to act like it. Cell Cycle 10:2064–2068. 10.4161/cc.10.13.16242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piroozmand A, Koyama AH, Shimada Y, Fujita M, Arakawa T, Adachi A. 2004. Role of Us3 gene of herpes simplex virus type 1 for resistance to interferon. Int. J. Mol. Med. 14:641–645 [PubMed] [Google Scholar]
- 27.Peri P, Mattila RK, Kantola H, Broberg E, Karttunen HS, Waris M, Vuorinen T, Hukkanen V. 2008. Herpes simplex virus type 1 Us3 gene deletion influences toll-like receptor responses in cultured monocytic cells. Virol. J. 5:140. 10.1186/1743-422X-5-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Wang K, Lin R, Zheng C. 2013. Herpes simplex virus 1 serine/threonine kinase US3 hyperphosphorylates IRF3 and inhibits beta interferon production. J. Virol. 87:12814–12827. 10.1128/JVI.02355-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang L, Roizman B. 2008. Expression of gamma interferon-dependent genes is blocked independently by virion host shutoff RNase and by US3 protein kinase. J. Virol. 82:4688–4696. 10.1128/JVI.02763-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chuluunbaatar U, Roller R, Mohr I. 2012. Suppression of extracellular signal-regulated kinase activity in herpes simplex virus 1-infected cells by the Us3 protein kinase. J. Virol. 86:7771–7776. 10.1128/JVI.00622-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karin M, Greten FR. 2005. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 5:749–759. 10.1038/nri1703 [DOI] [PubMed] [Google Scholar]
- 32.Gilmore TD. 2006. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 25:6680–6684. 10.1038/sj.onc.1209954 [DOI] [PubMed] [Google Scholar]
- 33.Hatada EN, Krappmann D, Scheidereit C. 2000. NF-kappaB and the innate immune response. Curr. Opin. Immunol. 12:52–58. 10.1016/S0952-7915(99)00050-3 [DOI] [PubMed] [Google Scholar]
- 34.Ghosh S, May MJ, Kopp EB. 1998. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225–260. 10.1146/annurev.immunol.16.1.225 [DOI] [PubMed] [Google Scholar]
- 35.Hayden MS, Ghosh S. 2008. Shared principles in NF-kappaB signaling. Cell 132:344–362. 10.1016/j.cell.2008.01.020 [DOI] [PubMed] [Google Scholar]
- 36.Hayden MS, West AP, Ghosh S. 2006. NF-kappaB and the immune response. Oncogene 25:6758–6780. 10.1038/sj.onc.1209943 [DOI] [PubMed] [Google Scholar]
- 37.Hayden MS, Ghosh S. 2004. Signaling to NF-kappaB. Genes Dev. 18:2195–2224. 10.1101/gad.1228704 [DOI] [PubMed] [Google Scholar]
- 38.Vallabhapurapu S, Karin M. 2009. Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 27:693–733. 10.1146/annurev.immunol.021908.132641 [DOI] [PubMed] [Google Scholar]
- 39.Baeuerle PA. 1998. IkappaB-NF-kappaB structures: at the interface of inflammation control. Cell 95:729–731. 10.1016/S0092-8674(00)81694-3 [DOI] [PubMed] [Google Scholar]
- 40.Baeuerle PA, Baltimore D. 1988. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science 242:540–546. 10.1126/science.3140380 [DOI] [PubMed] [Google Scholar]
- 41.Pahl HL. 1999. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18:6853–6866. 10.1038/sj.onc.1203239 [DOI] [PubMed] [Google Scholar]
- 42.Hiscott J, Kwon H, Genin P. 2001. Hostile takeovers: viral appropriation of the NF-kappaB pathway. J. Clin. Invest. 107:143–151. 10.1172/JCI11918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santoro MG, Rossi A, Amici C. 2003. NF-kappaB and virus infection: who controls whom. EMBO J. 22:2552–2560. 10.1093/emboj/cdg267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laherty CD, Hu HM, Opipari AW, Wang F, Dixit VM. 1992. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J. Biol. Chem. 267:24157–24160 [PubMed] [Google Scholar]
- 45.Stewart S, Dawson CW, Takada K, Curnow J, Moody CA, Sixbey JW, Young LS. 2004. Epstein-Barr virus-encoded LMP2A regulates viral and cellular gene expression by modulation of the NF-kappaB transcription factor pathway. Proc. Natl. Acad. Sci. U. S. A. 101:15730–15735. 10.1073/pnas.0402135101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sylla BS, Hung SC, Davidson DM, Hatzivassiliou E, Malinin NL, Wallach D, Gilmore TD, Kieff E, Mosialos G. 1998. Epstein-Barr virus-transforming protein latent infection membrane protein 1 activates transcription factor NF-kappaB through a pathway that includes the NF-kappaB-inducing kinase and the IkappaB kinases IKKalpha and IKKbeta. Proc. Natl. Acad. Sci. U. S. A. 95:10106–10111. 10.1073/pnas.95.17.10106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moutaftsi M, Brennan P, Spector SA, Tabi Z. 2004. Impaired lymphoid chemokine-mediated migration due to a block on the chemokine receptor switch in human cytomegalovirus-infected dendritic cells. J. Virol. 78:3046–3054. 10.1128/JVI.78.6.3046-3054.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yurochko AD, Kowalik TF, Huong SM, Huang ES. 1995. Human cytomegalovirus upregulates NF-kappa B activity by transactivating the NF-kappa B p105/p50 and p65 promoters. J. Virol. 69:5391–5400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones JO, Arvin AM. 2006. Inhibition of the NF-kappaB pathway by varicella-zoster virus in vitro and in human epidermal cells in vivo. J. Virol. 80:5113–5124. 10.1128/JVI.01956-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amici C, Rossi A, Costanzo A, Ciafre S, Marinari B, Balsamo M, Levrero M, Santoro MG. 2006. Herpes simplex virus disrupts NF-kappaB regulation by blocking its recruitment on the IkappaBalpha promoter and directing the factor on viral genes. J. Biol. Chem. 281:7110–7117. 10.1074/jbc.M512366200 [DOI] [PubMed] [Google Scholar]
- 51.Daubeuf S, Singh D, Tan Y, Liu H, Federoff HJ, Bowers WJ, Tolba K. 2009. HSV ICP0 recruits USP7 to modulate TLR-mediated innate response. Blood 113:3264–3275. 10.1182/blood-2008-07-168203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel A, Hanson J, McLean TI, Olgiate J, Hilton M, Miller WE, Bachenheimer SL. 1998. Herpes simplex type 1 induction of persistent NF-kappa B nuclear translocation increases the efficiency of virus replication. Virology 247:212–222. 10.1006/viro.1998.9243 [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Wang K, Wang S, Zheng C. 2013. Herpes simplex virus 1 E3 ubiquitin ligase ICP0 protein inhibits tumor necrosis factor alpha-induced NF-kappaB activation by interacting with p65/RelA and p50/NF-kappaB1. J. Virol. 87:12935–12948. 10.1128/JVI.01952-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim JC, Lee SY, Kim SY, Kim JK, Kim HJ, Lee HM, Choi MS, Min JS, Kim MJ, Choi HS, Ahn JK. 2008. HSV-1 ICP27 suppresses NF-kappaB activity by stabilizing IkappaBalpha. FEBS Lett. 582:2371–2376. 10.1016/j.febslet.2008.05.044 [DOI] [PubMed] [Google Scholar]
- 55.Cotter CR, Kim WK, Nguyen ML, Yount JS, Lopez CB, Blaho JA, Moran TM. 2011. The virion host shutoff protein of herpes simplex virus 1 blocks the replication-independent activation of NF-kappaB in dendritic cells in the absence of type I interferon signaling. J. Virol. 85:12662–12672. 10.1128/JVI.05557-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin H, Ma Y, Yan Z, Prabhakar BS, He B. 2012. Activation of NF-kappaB in CD8+ dendritic cells ex vivo by the gamma134.5 null mutant correlates with immunity against herpes simplex virus 1. J. Virol. 86:1059–1068. 10.1128/JVI.06202-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xing J, Wang S, Lin F, Pan W, Hu CD, Zheng C. 2011. Comprehensive characterization of interaction complexes of herpes simplex virus type 1 ICP22, UL3, UL4, and UL20.5. J. Virol. 85:1881–1886. 10.1128/JVI.01730-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xing J, Wang S, Li Y, Guo H, Zhao L, Pan W, Lin F, Zhu H, Wang L, Li M, Zheng C. 2011. Characterization of the subcellular localization of herpes simplex virus type 1 proteins in living cells. Med. Microbiol. Immunol. 200:61–68. 10.1007/s00430-010-0175-9 [DOI] [PubMed] [Google Scholar]
- 59.Jiang X, Takahashi N, Matsui N, Tetsuka T, Okamoto T. 2003. The NF-kappa B activation in lymphotoxin beta receptor signaling depends on the phosphorylation of p65 at serine 536. J. Biol. Chem. 278:919–926. 10.1074/jbc.M208696200 [DOI] [PubMed] [Google Scholar]
- 60.Severa M, Coccia EM, Fitzgerald KA. 2006. Toll-like receptor-dependent and -independent viperin gene expression and counter-regulation by PRDI-binding factor-1/BLIMP1. J. Biol. Chem. 281:26188–26195. 10.1074/jbc.M604516200 [DOI] [PubMed] [Google Scholar]
- 61.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. 1997. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science 278:860–866. 10.1126/science.278.5339.860 [DOI] [PubMed] [Google Scholar]
- 62.Park KA, Byun HS, Won M, Yang KJ, Shin S, Piao L, Kim JM, Yoon WH, Junn E, Park J, Seok JH, Hur GM. 2007. Sustained activation of protein kinase C downregulates nuclear factor-kappaB signaling by dissociation of IKK-gamma and Hsp90 complex in human colonic epithelial cells. Carcinogenesis 28:71–80. 10.1093/carcin/bgl094 [DOI] [PubMed] [Google Scholar]
- 63.Gao S, Song L, Li J, Zhang Z, Peng H, Jiang W, Wang Q, Kang T, Chen S, Huang W. 2012. Influenza A virus-encoded NS1 virulence factor protein inhibits innate immune response by targeting IKK. Cell. Microbiol. 14:1849–1866. 10.1111/cmi.12005 [DOI] [PubMed] [Google Scholar]
- 64.Zhu H, Zheng C, Xing J, Wang S, Li S, Lin R, Mossman KL. 2011. Varicella-zoster virus immediate-early protein ORF61 abrogates the IRF3-mediated innate immune response through degradation of activated IRF3. J. Virol. 85:11079–11089. 10.1128/JVI.05098-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jordan M, Schallhorn A, Wurm FM. 1996. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 24:596–601. 10.1093/nar/24.4.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, Lei C, He X, Zhang L, Tien P, Shu HB. 2008. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29:538–550. 10.1016/j.immuni.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 67.Xing J, Wu F, Pan W, Zheng C. 2010. Molecular anatomy of subcellular localization of HSV-1 tegument protein US11 in living cells. Virus Res. 153:71–81. 10.1016/j.virusres.2010.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Campbell KJ, Perkins ND. 2006. Regulation of NF-kappaB function. Biochem. Soc. Symp. 2006:165–180 [DOI] [PubMed] [Google Scholar]
- 69.Herbein G, O'Brien WA. 2000. Tumor necrosis factor (TNF)-alpha and TNF receptors in viral pathogenesis. Proc. Soc. Exp. Biol. Med. 223:241–257. 10.1046/j.1525-1373.2000.22335.x [DOI] [PubMed] [Google Scholar]
- 70.Chang LS, Wang JT, Doong SL, Lee CP, Chang CW, Tsai CH, Yeh SW, Hsieh CY, Chen MR. 2012. Epstein-Barr virus BGLF4 kinase downregulates NF-kappaB transactivation through phosphorylation of coactivator UXT. J. Virol. 86:12176–12186. 10.1128/JVI.01918-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sloan E, Henriquez R, Kinchington PR, Slobedman B, Abendroth A. 2012. Varicella-zoster virus inhibition of the NF-kappaB pathway during infection of human dendritic cells: role for open reading frame 61 as a modulator of NF-kappaB activity. J. Virol. 86:1193–1202. 10.1128/JVI.06400-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seo T, Park J, Lim C, Choe J. 2004. Inhibition of nuclear factor kappaB activity by viral interferon regulatory factor 3 of Kaposi's sarcoma-associated herpesvirus. Oncogene 23:6146–6155. 10.1038/sj.onc.1207807 [DOI] [PubMed] [Google Scholar]
- 73.Sen J, Liu X, Roller R, Knipe DM. 2013. Herpes simplex virus US3 tegument protein inhibits Toll-like receptor 2 signaling at or before TRAF6 ubiquitination. Virology 439:65–73. 10.1016/j.virol.2013.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deruelle MJ, Favoreel HW. 2011. Keep it in the subfamily: the conserved alphaherpesvirus US3 protein kinase. J. Gen. Virol. 92:18–30. 10.1099/vir.0.025593-0 [DOI] [PubMed] [Google Scholar]
- 75.Chen LF, Greene WC. 2004. Shaping the nuclear action of NF-kappaB. Nat. Rev. Mol. Cell Biol. 5:392–401. 10.1038/nrm1368 [DOI] [PubMed] [Google Scholar]
- 76.Viatour P, Merville MP, Bours V, Chariot A. 2005. Phosphorylation of NF-kappaB and IkappaB proteins: implications in cancer and inflammation. Trends Biochem. Sci. 30:43–52. 10.1016/j.tibs.2004.11.009 [DOI] [PubMed] [Google Scholar]
- 77.Mosialos G, Gilmore TD. 1993. v-Rel and c-Rel are differentially affected by mutations at a consensus protein kinase recognition sequence. Oncogene 8:721–730 [PubMed] [Google Scholar]
- 78.Ganchi PA, Sun SC, Greene WC, Ballard DW. 1993. A novel NF-kappa B complex containing p65 homodimers: implications for transcriptional control at the level of subunit dimerization. Mol. Cell. Biol. 13:7826–7835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. 1997. The transcriptional activity of NF-kappaB is regulated by the IkappaB-associated PKAc subunit through a cyclic AMP-independent mechanism. Cell 89:413–424. 10.1016/S0092-8674(00)80222-6 [DOI] [PubMed] [Google Scholar]
- 80.Zhong H, May MJ, Jimi E, Ghosh S. 2002. The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol. Cell 9:625–636. 10.1016/S1097-2765(02)00477-X [DOI] [PubMed] [Google Scholar]
- 81.Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G. 2003. Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J. 22:1313–1324. 10.1093/emboj/cdg139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Leitges M, Sanz L, Martin P, Duran A, Braun U, Garcia JF, Camacho F, Diaz-Meco MT, Rennert PD, Moscat J. 2001. Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol. Cell 8:771–780. 10.1016/S1097-2765(01)00361-6 [DOI] [PubMed] [Google Scholar]
- 83.Duran A, Diaz-Meco MT, Moscat J. 2003. Essential role of RelA Ser311 phosphorylation by zetaPKC in NF-kappaB transcriptional activation. EMBO J. 22:3910–3918. 10.1093/emboj/cdg370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Purves FC, Deana AD, Marchiori F, Leader DP, Pinna LA. 1986. The substrate specificity of the protein kinase induced in cells infected with herpesviruses: studies with synthetic substrates [corrected] indicate structural requirements distinct from other protein kinases. Biochim. Biophys. Acta 889:208–215. 10.1016/0167-4889(86)90106-0 [DOI] [PubMed] [Google Scholar]
- 85.Daikoku T, Yamashita Y, Tsurumi T, Maeno K, Nishiyama Y. 1993. Purification and biochemical characterization of the protein kinase encoded by the US3 gene of herpes simplex virus type 2. Virology 197:685–694. 10.1006/viro.1993.1644 [DOI] [PubMed] [Google Scholar]