Abstract
Lens opacification or cataract reduces vision in over 80 million people worldwide and blinds 18 million. These numbers will increase dramatically as both the size of the elderly demographic and the number of those with carbohydrate metabolism-related problems increase. Preventative measures for cataract are critical because the availability of cataract surgery in much of the world is insuficient. Epidemiologic literature suggests that the risk of cataract can be diminished by diets that are optimized for vitamin C, lutein/zeaxanthin, B vitamins, omega-3 fatty acids, multivitamins, and carbohydrates: recommended levels of micronutrients are salutary. The limited data from intervention trials provide some support for observational studies with regard to nuclear – but not other types of – cataracts. Presented here are the beneficial levels of nutrients in diets or blood and the total number of participants surveyed in epidemiologic studies since a previous review in 2007.
Keywords: aging, carbohydrate, carotenoids, cataract, eye, glycation, glycemic index, lens, omega fats, vitamins
INTRODUCTION
Vision is the most precious of the five senses. Because vision impairment is a common and virtually inevitable debility among the aged, afflicting 285 million people worldwide, it is not surprising that loss of vision is among the greatest fears of the elderly. It has been estimated that over 68% of people over 79 years of age have some form of lens opacification or cataract. In those at least 50 years of age, cataract prevalence is greater than the combined prevalences of glaucoma and age-related macular degeneration.1 In 2007, the World Health Organization estimated that cataracts are responsible for blindness in 39% of the 37 million blind people worldwide. Rates of blindness due to cataract vary widely from country to country because of disparities in financial resources, the availability of ophthalmologists, the perceived need to improve vision, and genetic and environmental factors.2–4 These barriers highlight the importance of providing a means to prevent or delay the formation of cataract.
A significant body of research indicates that nutritional intervention may offer a way to diminish the risk of cataract. Much of the early research on the role of diet in cataract focused on antioxidants, but this has since expanded to include macronutrients such as fatty acids and carbohydrates.5–12 The role of nutrients in eye health has also been explored in a variety of model systems, including in vitro, cell culture, animal, and human studies.13–17
The present review has four objectives. The first is to inform the reader about current understanding of the etiology of age-related cataract and why nutrients are likely to affect risk of cataract. The second is to provide a summary that is updated since a previous review.5 This summary is presented in entirety, in graphic form, but its length requires that much of it be presented online, as Supporting Information (Figures S1–S41, Tables S1 and S2). Thus, the number of studies that attempted to find a relationship between intake of a specific nutrient and a form of cataract is mentioned, but null data are not fully described in the text, and supplementary figures were kept to a minimum by excluding figures for nutrients for which only null data were obtained. However, to provide a complete summary, a bibliography pertaining to those nutrients for which the effect of a nutrient on cataract risk is null is included in Table S2. As an exception, findings from randomized double-blinded intervention trials are reported in the text, even when they are null, due to the importance of data from this type of trial. The third objective of this review is to introduce in the main text the newest class of nutrients to be related to risk of cataract, i.e., carbohydrates. The fourth objective is to present, when available, levels of nutrients that were correlated with risk of cataracts and the approximate total number of subjects surveyed for each nutrient.
WHAT ARE CATARACTS?
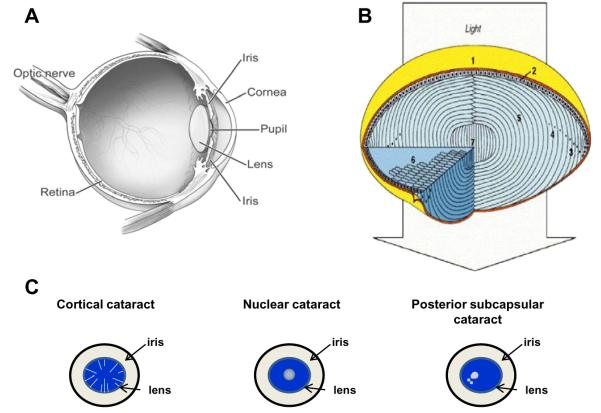
The primary function of the eye lens is to collect and focus light on the retina. To do so, the lens must remain clear throughout life. The lens is located posterior to the cornea and iris (Figure 1A). It is avascular, receiving nutriture from the aqueous humor.18 Although the clarity of the lens is frequently interpreted as indicative of an absence of structure, the lens is exquisitely organized (Figure 1B). A single layer of epithelial cells is found directly under the anterior surface of the collagenous capsule in which the lens is found. The epithelial cells at the germinative region divide, migrate posteriorly, and differentiate into lens fibers. The transparency of the lens is made possible by a tight configuration of lens fibers, which are mostly devoid of cellular organelles. The primary gene products of the fibers are called crystallins. In the young lens, the flexibility of the fibers, particularly in the cortical areas, facilitates lens accommodation, allowing the eye to focus on images both near and far.19 New cells are formed throughout life, but older cells usually are not lost. Instead, they are compressed and compacted into the center or nucleus of the lens.20 There is a coincident dehydration of the fibers. Metabolism is different in the epithelium/subcapsular tissue, the cortex, and the nucleus of the lens. This is enforced by the shedding of nuclei from fiber cells in the inner cortex and nucleus.
Figure 1.
Lens biology and cataract formation. A) The lens is located behind the cornea and in front of the retina.18 B) (1) The lens of the eye is enclosed by the lens capsule. (2) The anterior surface of the lens (top) consists of a single layer of epithelial cells. Cuboidal epithelial cells near the equator proliferate throughout life and elongate (3) anteriorly and posteriorly until their ends reach the two lens poles (top and bottom). At this stage, the hexagonal fiber cells degrade their organelles (4, 6). The nuclei are indicated as black dots. Thus, the lens consists primarily of long fiber cells devoid of cytoplasmic organelles (5). The core of the lens (lens nucleus) is composed of the primary fiber cells (7). [Reproduced from Perng et al.20 with permission from Elsevier.] C) Schematics illustrating the locations of cortical, nuclear, and posterior subcapsular opacities. In cortical cataract, opaque streaks are observed in the cortex, circumscribing the lens, while in nuclear cataract, the opacity is in the center on the lens. Posterior subcapsular cataract describes an opacity at the back of the lens that have dot opacities in the cortex as well.
Throughout life, the lens undergoes biochemical, physiological, and functional changes. Most of these changes result in a less-flexible lens with limited accommodative capability. As the lens ages, its proteins are photooxidatively damaged, and they crosslink, aggregate, precipitate, and accumulate in lens opacities.21 Dysfunction of the lens due to opacification is called cataract. The term “age-related” cataract is used to distinguish lens opacification associated with old age from iatrogenic opacification associated with other causes, such as congenital and metabolic disorders, medication-induced opacification, trauma, or high-energy radiation. This review focuses on age-related cataract.
Clinically, opacities are evaluated separately in the cortical, nuclear, and posterior subcapsular (PSC) regions (Figure 1C) of the lens because they are phenotypically distinguishable. They may be described as “mixed” if the opacity is present in more than one location. Separation of grading into different zones of the lens is also important because cataracts at each location are thought to have some differences in etiology.22 However, some ophthalmologic exams do not distinguish one type of cataract from another. Outcomes of such exams are described herein as “any” type of cataract. There are several systems for evaluating and grading cataracts. Most of these use an assessment of extent of density and location of the opacity.23,24 Attesting to differences in the etiologies of nuclear, cortical, and PSC cataracts, good nutrition is often related to diminished risk of nuclear cataracts, but less often to reduced risk of cortical or PSC cataracts. Because of these differences in etiology, relations between nutrient status and risk of each type of cataract or extraction are discussed separately. The extraction outcome may result in a smaller (nutrient) effect because different surgeons or clients choose to remove cataracts at different levels of cataract maturity. Although not directly associated with all opacities, coloration or brunescence is also quantified, since brunescence diminishes visual function.25
Nuclear and cortical opacities constitute the majority of cataracts, but nuclear opacities are usually of greater concern because they interfere directly with the passage of light along the visual axis. PSC opacities occur far less frequently but are also located along the visual axis.
CATARACT RISK FACTORS AND ETIOLOGY
Age, gender, educational status, smoking, diabetes, and obesity are all risk factors for cataract. Men are more prone to PSC cataracts, while women are more likely to get cortical cataracts. Caucasians and those with a college degree have a decreased risk of cortical cataract compared with non-Caucasians and those with a high school education or less, respectively.26
The lens is especially prone to opacification because of its high protein content (the solid mass of the lens is about 98% protein) and minimal turnover or replenishment of these proteins as the lens ages. During the aging process, these proteins are subject to chronic stresses from exposure to incident visible and ultraviolet light as well as other forms of high-energy radiation. Recently, it was observed in African diabetics that exposure to sun light was strongly associated with an increased risk of cataract.27 Consistent with an impact of incident radiation, decreasing latitude of residence and further deterioration of the ozone layer are expected to increase the risk of cataract surgery and cortical cataract, respectively.28 A summary of the literature regarding ultraviolet light and risk of cataract is provided in the review by McCarty and Taylor.29 Artificial ultraviolet radiation, such as that produced by tanning beds, fluorescent lamps, or excimer lasers, may also increase the risk of cataract, especially among aged, medicated adults using compounds such as steroids, thyroid hormone, or estrogen.26,30–33
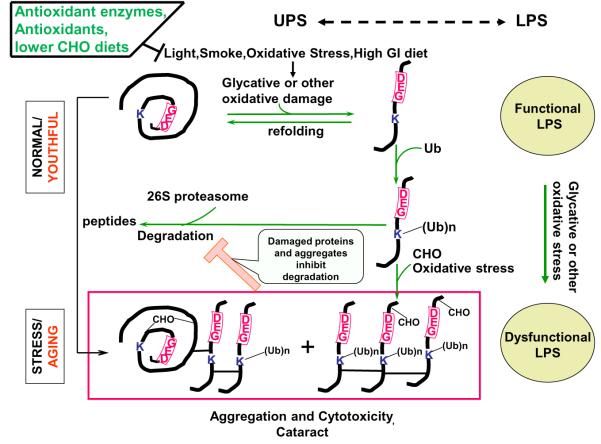
Excessive oxygen and its reactive metabolites, along with many other moieties, have been related to enhanced risk of cataract. Cataract was observed in patients treated with hyperbaric oxygen therapy, in lenses exposed to hyperbaric oxygen in vitro, in mice that survived exposure to 100% oxygen twice weekly for 3 h, as well as in guinea pigs exposed to hyperbaric oxygen. Upon oxidative stress, elevated levels of glutathione disulfides, the oxidized form of the antioxidant glutathione (GSH), were noted, along with a decrease in levels of reduced GSH. Comparable chemistry explains the accumulation of disulfide-linked proteins upon oxidative stress and aging in the lens (Figure 2).34
Figure 2.
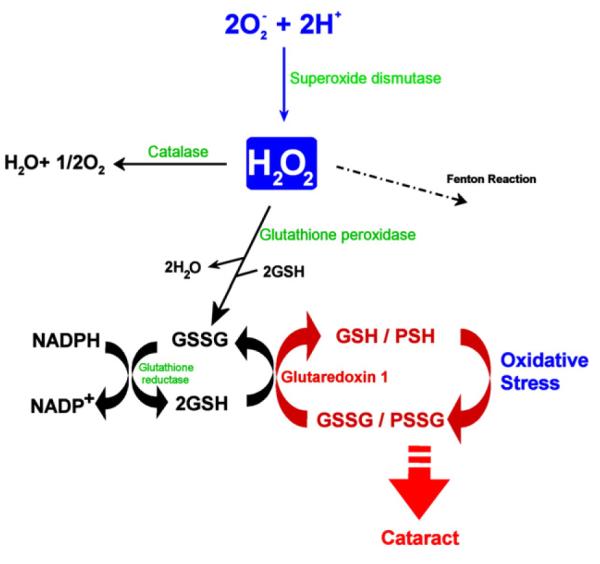
Antioxidative defense systems and glutaredoxin 1 function in the lens. H2O2 generated by the dismutation of superoxide anion is degraded by several pathways, including catalase, glutathione peroxidase, and the Fenton reaction. A decreased SH/S–S ratio by oxidation can be reversed by glutathione reductase or glutaredoxin 1; the latter specifically reduces proteinthiol mixed disulfides. [Reproduced from Meyer et al.39 with permission from Elsevier.]
Smoking and tobacco chewing induce oxidative stress and have been associated with diminished levels of antioxidants, including ascorbate, tocopherols, and carotenoids, as well as with exacerbated nuclear sclerosis and enhanced risk of cataract, especially nuclear cataract, as reviewed by Kelly et al.35 and Richter et al.36 Further-more, in the Age-Related Eye Disease Study (AREDS) cohort, a multicenter clinical trial of over 4,000 elderly (55–80 years of age) men and women randomized to receive either a placebo or a cocktail of 500 mg of vitamin C, 400 IU of vitamin E, and 15 mg of β-carotene for an average of 6.3 years, former smokers had a higher risk of cataract surgery than nonsmokers, and current smokers had a higher risk of cortical cataract.26 Consistent with an oxidative stress-related etiology of cataract, there was also a diminished risk of cataract in male physician smokers who used multivitamins.37 However, increasing consumption of all antioxidants is not always beneficial among smokers, as indicated by an increased risk of lung cancer among smokers supplemented with β-carotene.38
Antioxidants, antioxidant enzymes, and proteases as defenses against lens damage
Several interrelated capacities offer protection against photooxidative insults. Antioxidants, including ascorbate, carotenoids, vitamin E (reviewed by Taylor7), and antioxidant enzymes, protect proteins and other constituents against oxidative stress (Figure 2).39 Additional defenses include proteolytic and repair processes that degrade and eliminate damaged proteins or aid in the repair of damaged biomolecules (Figure 3).
Figure 3.
Proposed interaction between lens proteins, oxidants, light, smoking, antioxidants, antioxidant enzymes, and proteases. Lens proteins are subject to alteration by light and oxygen but are protected indirectly by antioxidant enzymes and directly by antioxidants. A higher-glycemic-index diet also enhances oxidation and/or glycation-induced protein damage. When levels of damaged proteins are low, the ubiquitin–proteasome system (UPS) (center) and the lysosomal proteolytic system (LPS) (right side, ovals) can degrade the damaged proteins, thereby averting toxicity (top). Under chronic stress, glycated (CHO), ubiquitinated (Ub), or otherwise oxidatively modified proteins accumulate. Some may oligomerize and crosslink, forming the higher-mass aggregates that precipitate in cataracts if there is insufficient proteasomal (including deubiquitinating) activity.
Antioxidants
The lens is primarily an aqueous environment. Aqueous-soluble antioxidants such as ascorbate and GSH are far more concentrated in the lens than in the plasma. Therefore, it might be anticipated that aqueous antioxidants would confer the greatest benefit with regard to reducing the risk of cataract (Figures 2 and 3). Observational studies support this concept (see sections on epidemiologic studies below). Additionally, GSH levels diminish in the older and cataractous lens.40
Antioxidant enzymes
The lens also contains multiple antioxidant enzymes, including catalase, superoxidase dismutase, reductases, and enzymes of the GSH redox cycle. These enzymes interact with oxygen, other oxidants, and antioxidants (i.e., GSH is a substrate for glutathione peroxidase) to decrease the oxidative burden. However, the activities of many antioxidant enzymes themselves become modified and compromised during aging and cataract formation. This contributes to a vicious cycle of stress followed by damage to bulk proteins (e.g., by oxidation of catalytically essential cysteines), antioxidants, and damage removal systems, resulting in exacerbated stress (Figure 3).41–43 Besides oxidation, protein glycation, deamidation, and transglutamination all contribute to crosslinking and subsequent insolubility of proteins44–46 and to lens opacification.47–49 Corroborating this oxidation-based hypothesis, the industrial antioxidant 0.4% butylated hydroxytoluene incorporated into the diets of galactosefied (50% of diet) rats diminished the prevalence of cataract.50
Protein editing as a defense
Proteolytic systems can be considered additional defense capacities that mark and remove cytotoxic, damaged, or obsolete proteins from lenses and other eye tissues.51,52 In young lens tissue, degradation by such proteolytic systems maintains damaged proteins at harmless levels (Figure 3 top).
Several studies indicate interactions between anti-oxidant and proteolytic defense systems. There is a direct sparing effect of ascorbate on photooxidatively induced compromises of proteolytic function. GSH also spares the activity of enzymes involved in the conjugation of ubiquitin to substrates, thus prolonging ubiquitin-dependent proteloysis.53,54 However, during aging or oxidative stress, most of these enzymatic capabilities are found in a state of reduced activity. The observed accumulation of oxidized (and/or otherwise modified) proteins in older lenses is consistent with the diminished capacity of these protective systems to keep pace with the insults that damage lens proteins.54,55 Thus, age-related compromises in the activity of antioxidant enzymes, diminished concentrations of antioxidants, and reduced activity of protein editing-capacities lead to attenuated protection against oxidative insults. This leaves the long-lived proteins and other constituents vulnerable to damage, resulting in cataract.52
Molecular chaperones provide another capacity to delay cataract-related protein precipitation, as reviewed by Shang and Taylor.56
NEWER EPIDEMIOLOGIC STUDIES THAT RELATE CAROTENOIDS AND VITAMINS C, E, AND B TO RISK OF CATARACT
Many epidemiologic studies have attempted to determine which nutrients offer protection against cataract.5 The most recent clinically relevant data are summarized in the text as well as in the graphs available online as Supporting Information (Figures S1–S41) and the additional references for this material (Table S1). For some nutrients, the data do not suggest any effect on certain types of cataract; references for these data can be found in Table S2, available in the Supporting Information online.
Vitamin C
Present in the lens and aqueous humor at more than 50-fold the concentration found in plasma, ascorbate is probably the most effective and least toxic antioxidant identifed in mammalian systems. It not only scavenges free radicals but can also regenerate vitamin E and GSH to further increase the antioxidant capacity of a cell. A significant amount of literature indicates that there are age-related decrements in ascorbate levels in the lens, and that this may be related to compromises in lens function.57,58 Conversely, enhancing ascorbate supplies may potentially provide benefit. In subjects consuming the recommended intake of vitamin C, 75 mg/day, blood ascorbate levels were approximately 12 μg/mL, and increased consumption of vitamin C in this population resulted in even higher blood ascorbate levels.59 There is also almost 50 times as much ascorbate in the lens as in the plasma, as indicated by another study in which supplementation with 2 g of vitamin C per day was associated with a nearly 40-fold increase in lens ascorbate, to >4 mM.57 Importantly, increased plasma vitamin C has also been associated with lower levels of plasma thiobarbituric acid reactive substances (TBARS), a marker of oxidative stress (P < 0.01).60 Although it has been suggested in a mouse model that vitamin C may mediate cataract formation through glycation of carbohydrates, data from other animal models and from human studies do not support this observation.15 It is therefore not surprising that elevated vitamin C status is robustly related to diminished risk of cataract in many epidemiologic studies (Figures S1–S5, S7–S8). Based upon relations between diet and levels of vitamin C in the aqueous humor or lens, it appears that intake beyond approximately 200 mg/day is associated with limited risk reduction.
Prior to 2007, data from over 110,000 subjects were analyzed to help determine the role of vitamin C in lens health (Figures S1–S10). The consensus among these studies is that blood levels of at least 49 μM or intake of 135 mg/day may reduce the risk of cortical, nuclear, and PSC cataract (Figures S1, S3–S4, S6–S7).5 Data collected since 2007 support these findings and suggest that vitamin C is most effective against nuclear cataracts, reducing the risk of this cataract with as little as 3 μM in the blood or intake of less than 2 mg/day, though some studies failed to find an effect of vitamin C (Table S2).
A recent cross-sectional study of 1,443 rural Indians over the age of 50 (INDEYE study) indicated that people with plasma vitamin C concentrations in the highest compared with the lowest tertile had approximately 40% decreased odds (odds ratio [OR] = 0.62; 95% confidence interval [CI]: 0.40–0.96) of cortical cataract (Figure S1).61 Cross-sectional analysis of a larger cohort of elderly Indians (n = 5,638) supports the benefits of vitamin C in lens health. Analysis of the entire cohort revealed that those with the highest plasma levels of vitamin C had a 35% reduced risk of cortical cataract (95%CI: 0.50–0.85) compared with those with the lowest plasma levels. This effect appeared to be driven by participants living in the southern (OR = 0.63; 95%CI: 0.47–0.86) rather than the northern (OR = 0.74; 95%CI: 0.45–1.20) part of India (Figure S1).62 This geographic difference is of interest because there is a geographic “cataract belt” of high cataract prevalence in the eastern Indian provinces of Bihar, Jharkhand, and Orissa. The benefits of vitamin C are supported by prospective analysis in the Nutrition Vision Project (NVP), a subset of the Nurses’ Health Study, which showed that, among women aged ≤60 years, consumption of at least 363 mg/day vitamin C was associated with a 57% decreased risk of developing a cortical cataract compared with women who consumed less than 140 mg/day vitamin C (Figure S2). Moreover, women who took supplemental vitamin C for at least 10 years had significantly fewer cortical lens opacities than those who did not supplement (OR = 0.40; 95%CI: 0.18–0.87) (Figure S2).63
A robust analysis of observational studies indicates that vitamin C intake is also likely to be most effective in reducing the risk of nuclear cataract. Decreases in risk of approximately 40% have been reported in a majority of studies for intakes above approximately 135 mg/day or blood concentrations of 6 μM. Long-term elevated intake or use of supplements was also associated with a decreased risk of nuclear cataract (Figures S3–S5). In the INDEYE study, those with plasma vitamin C concentrations in the highest compared with the lowest tertile had an OR of 0.62 (95%CI: 0.40–0.96) for nuclear cataract (Figure S4).61 Ravindran et al.62 also found that those with the highest plasma levels of vitamin C had a reduced risk of nuclear cataract compared with those with the lowest levels (OR = 0.58; 95%CI: 0.47–0.72). Notably, benefit was observed in participants from northern (OR = 0.52; 95%CI: 0.38–0.72) and from southern (OR = 0.69; 95%CI: 0.54–0.89) India (Figure S4). Protective effects of vitamin C against nuclear opacities were observed in prospective studies as well. Risk ratios ranged from 0.30 to 0.55 for nuclear cataract among persons with an intake of 140 mg/day compared with those with a lower intake (Figure S5). The aggregate of retrospective studies regarding PSC prior to 2007 suggests that elevating intake and plasma levels of vitamin C may confer weak protection. Risk ratios varied from 0.09 to 0.53 among the approximately 7,900 people studied, namely among those with an intake of at least 491 mg/day or blood levels above 49 μM (Figure S6).5 This was corroborated in the INDEYE study (OR = 0.59; 95%CI: 0.35–0.99) and in a cross-sectional analysis of 5,638 elderly Indians (OR = 0.53; 95%CI: 0.42–0.66) in both the northern (OR = 0.44; 95%CI: 0.32–0.61) and the southern (OR = 0.69; 95%CI: 0.54–0.89) study centers (Figure S6).61,62
The INDEYE study reported a beneficial effect of circulating vitamin C on risk of mixed cataract (OR = 0.64; 95%CI: 0.48–0.85). However, was no signicant relationship between vitamin C intake and risk of any type of cataract in the Women’s Health Study (WHS), a randomized, double-blinded, placebo-controlled trial that analyzed the effect of aspirin, vitamin E, and β-carotene on cardiovascular disease in 35,551 female health professionals at least 45 years of age.61,64
More recently, in India, high vitamin C plasma levels (OR = 0.61; 95%CI: 0.51–0.74) as well as intake (OR = 0.78; 95%CI: 0.62–0.98) reduced the risk of “any” type of cataract (Figure S7). The benefit of circulating vitamin C was observed in both the northern (OR = 0.55; 95%CI: 0.41–0.74) and the southern (OR = 0.71; 95%CI: 0.57–0.89) study centers (Figure S7).62
Older retrospective and prospective data also indicate that vitamin C may help reduce the risk of “any” cataract and cataract extraction (Figures S8–S10).5 Analysis of 4,001 subjects aged between 60 years and 74 years from the National Health and Nutrition Examination Survey II indicates that serum ascorbic acid levels were inversely associated with self-reported cataract (OR = 0.74; 95%CI: 0.56–0.97), and each increase of 1 mg/dL ascorbic acid was associated with a 26% decrease in cataract risk (P = 0.03).65 In a prospective study, Yoshida et al.66 found that men and women who consumed more than 212 mg of vitamin C per day were less likely to report “any” type of cataract compared with those who consumed less than 83 mg/day (Figure S8). In this cohort, vitamin C intake was also inversely related to risk of cataract extraction in women (Figure S10).
The positive data above fueled enthusiasm for intervention trials like the recent continuation of the Physicians’ Health Study (PHS II).5 However, among 11,545 male physicians aged 40–84 years, supplementation either every other day with 400 IU of vitamin E or daily with 500 mg of vitamin C had no effect on risk of cataract or cataract extraction.67
Concern about vitamin C supplementation was raised by findings in the Swedish Mammography Cohort (“Swedish” in the figures) of 24,593 women aged 49–83 years, which showed that vitamin C supplementation for longer than 10 years was associated with a 25% increase in the risk of cataract extraction (95% CI: 1.00–1.50) (Figure S10), even after correcting for reverse causality (by excluding women in the first 5 years postsurgery). Among women aged 60 years and older, supplementation with vitamin C was associated with a 38% increased risk of cataract extraction (95%CI: 1.12–1.69) (Figure S10). Women using corticosteroids along with vitamin C supplements had a 97% greater risk of extraction (95%CI: 1.35–2.88) (Figure S10),68 but this finding is not surprising because corticosteroids are known cataractogens.
Vitamin E
Vitamin E is a lipid-soluble antioxidant with diverse physiological roles involving maintenance of membrane integrity, inflammation, lipid metabolism, and antioxidant capabilities that include GSH recycling and attenuation of galactose- and aminotriazole-induced cataracts in animals.5,7,69 Vitamin E encompasses a family of molecules known as tocopherols. Concentrations of tocopherols in the whole lens are in the micromolar range (1,940 ng/g), and mechanisms that relate lens and dietary levels of tocopherols remain to be elucidated. Because most tocopherols are found in membranes, particularly in younger tissues, the concentrations in membranes may be orders of magnitude higher than what has been reported in blood. The majority of studies focus specifically on α-tocopherol because this is the most biologically active tocopherol. α-tocopherol, found at high levels in olives and sunflower seeds, is the major tocopherol in the European diet, whereas γ-tocopherol, the main isomer in soy and corn, is the major tocopherol in the American diet.5,7
Numerous observational and intervention studies investigated the effects of vitamin E in concert with other nutrients (see below). Discussed here are studies that included a total of over 220,000 subjects and that were able to parse the effect of vitamin E on risk of cataract. Most of these studies, comprising approximately 166,000 subjects, were completed prior to 2007 and failed to find a strong association between vitamin E intake or blood levels and risk of cataract (Figures S11–S15, Tables S1 and S2).5 However, from those studies that showed an inverse relationship, it would appear that blood levels of at least 41 μM or an intake of at least 20 mg/day could reduce the risk of nuclear cataract. There appears to be no consistent effect of vitamin E on risk of PSC cataract, but blood levels of at least 33 μM or an intake of 5 mg/day may increase risk. In studies completed since 2007 (approximately 56,000 subjects enrolled), there also appeared to be no effect of vitamin E on risk of cataract in any part of the lens. This includes retrospective, cross-sectional studies as well as prospective studies regarding risk of cataract associated with vitamin E intake, plasma levels, or supplementation (Table S2).70,71
There was also no beneficial effect after 4 years of vitamin E supplementation on the incidence or progression of cortical cataract in the Vitamin E, Cataract and Age-related Maculopathy Study (VECAT), a randomized, double-blind, placebo-controlled trial in which subjects were given either 500 IU of vitamin E per day or placebo.72 Similarly, there was no effect of vitamin E on risk of cortical cataract in the WHS, in which women were supplemented with 600 IU of vitamin E every 2 days, or in the second cohort of the PHS after supplementation with 400 IU of vitamin E every other day.67,73 A few retrospective and prospective studies, however, suggested there might be beneficial effects of higher plasma levels of vitamin E or intakes of at least 90.8 mg/day (along with other nutrients) compared with intakes of less than 6.7 mg/day on risk of nuclear opacity (Figures S13 and S14).5 The NVP also reported trends of decreasing risk of nuclear opacity (P = 0.03) and decreased progression of opacity (P = 0.006) with increased duration of vitamin E supplementation in women.74 However, the VECAT, WHS, and PHS II intervention studies found no effect of vitamin E on the risk of nuclear cataract.
There was a 42% reduction in risk (OR = 0.58; 95% CI: 0.36–0.94) of mixed cataract in subjects with high blood levels of vitamin E in the INDEYE study (Figure S16).61 Consistent with findings regarding mixed cataract, patients with “any” cataract had lower serum α-tocopherol levels compared with noncataractous controls (P < 0.001), and for those greater than 61 years of age, senile cataract was associated with lower serum levels of α-tocopherol.75,76 Smokers in India also showed inverse associations between risk of “any” cataract and serum vitamin E levels (P = 0.0001).77 Salutary effects are further corroborated in prospective analysis of the WHS (OR = 0.86; 95%CI: 0.74–1.00) (Figure S17), and supple-mentation for 10 years also appeared to offer protection against “any” cataract.
Although two older retrospective studies reported a reduction in cataract extraction with vitamin E supplementation (Figure S18), prospective studies PHS II and WHS and the multiyear Alpha Tocopherol Beta Carotene intervention (in which male smokers were supplemented with 20 mg of β-carotene per day) reported no such advantage.5,67,73 Prospective analysis of the Blue Mountains Eye Study indicated that vitamin E may even increase the risk of cataract extraction (OR = 1.55; 95% CI: 1.02–2.38).71
Carotenoids and vitamin A
The 400 carotenoids, like vitamin E, are natural lipid-soluble antioxidants. Elevated carotenoid intake has been associated with several health benefits and, in some cases, with decreased risk of cataract (Figures S19–S31).5,78,79 Most early work focused on β-carotene, levels of which are limited in human lenses (<0.1 ng/g). Instead, the major lens carotenoids are lutein and zeaxanthin (13.8 ng/g combined). These also are the most prevalent carotenoids in the retina. Also present in the lens are retinol (38.1 ng/g) and retinol ester (25.6 ng/g). Studies investigating status of various carotenoids and risk of cataract in over 220,000 subjects are discussed below.
Lutein and zeaxanthin
Most retrospective and prospective studies (comprising over 16,000 subjects) indicate that intake or blood levels of lutein and zeaxanthin do not modulate risk of cortical, nuclear, or PSC cataract (Figures S19 and S20, Table S2), although Karppi et al.80 did find that, among elderly Finnish subjects, those with the highest plasma levels of lutein (RR = 0.58; 95%CI: 0.35–0.98) and zeaxanthin (relative risk [RR] = 0.59; 95%CI: 0.35–0.99) had a reduced risk of nuclear cataract (Figure S19). Older prospective data from Beaver Dam, Pathologies Oculaires Liées á l’Age (POLA), and the NVP (Figure S20)5 also suggest that elevated lutein and zeaxanthin status is associated with diminished risk of nuclear cataract. A cross-sectional analysis of 1,443 Indian subjects supports this data and revealed that high zeaxanthin blood levels were protective against nuclear cataract (P < 0.03),61 but this was not observed in a Carotenoids in Age-Related Eye Disease Study (CAREDS) analysis (Figure S20).81
Data are slightly more encouraging when “any” cataract is the endpoint. A large cross-sectional analysis observed that Indians with the highest blood levels of lutein (OR = 0.79; 95%CI: 0.63–0.99) and zeaxanthin (OR = 0.76; 95%CI: 0.58–0.99) had less risk of “any” type of cataract (Figure S21).62 Similarly, Dherani et al.61 also found lower odds of mixed cataract (OR = 0.66; 95%CI: 0.45–0.96) among those with the highest blood levels of zeaxanthin (Figure S21). A study of 177 institutionalized elderly also found that high intakes of zeaxanthin were associated with decreased risk of cataract (OR = 0.96; 95%CI: 0.91–0.99) (Figure S21).82 This is supported by a cross-sectional study of 376 subjects in whom serum lutein (but not zeaxanthin) correlated with lens optical density (P = 0.001), a surrogate marker of lens function.83 Additionally, the WHS found that, compared with women who had the lowest intakes of lutein and zeaxanthin, women with the highest levels had a reduced risk of “any” cataract as diagnosed by a physician (RR = 0.82; 95%CI: 0.71–0.95) (Figure S22).64 Interestingly, a small pilot study of only 17 cataract patients showed that, compared with placebo, supplementation with 15 mg of lutein three times a week for 2 years improved visual acuity.84 Another small study, however, indicated adverse effects of serum lutein and zeaxanthin in people under the age of 61 with age-related cataracts.76
To clarify the role of lutein and zeaxanthin in cataract risk, the Age-Related Eye Disease Study 2 (AREDS2) followed 3,159 elderly (50–85 years of age) men and women for 5 years. Supplementation with 10 mg of lutein and 2 mg of zeaxanthin had no effect on risk of any type of cataract (Figure S22), nor did it improve visual acuity. However, a subgroup analysis did show a beneficial effect of lutein and zeaxanthin on cataract risk (HR = 0.70; 95%CI: 0.53–0.94) in those patients with the lowest baseline intake of these carotenoids (Figure S22).85
Finally, using cataract extraction as the endpoint, all prospective studies except the Nurses’ Health Study (NHS) found no effect of lutein and zeaxanthin (Figure S23, Table S2).5,86 In AREDS2, supplementation with lutein and zeaxanthin had no effect on risk of cataract extraction in the primary analysis, but supplementation did reduce extraction risk (HR = 0.68; 95%CI: 0.48–0.96) in patients with low baseline intakes of lutein and zeaxanthin (Figure S23).85 These observations, along with those related to risk of any type of cataract, suggest that lutein and zeaxanthin may be beneficial for undernourished populations, but this needs to be addressed in additional trials.
Beta-carotene, α-carotene, lycopene, cryptoxanthin, and total carotenoids
β-carotene exhibits strong antioxidant activity at low partial pressures of oxygen, similar to the approximately 20 torr partial pressure of oxygen in the core of the lens. However, data from over 17,000 subjects, including the Alpha Tocopherol Beta Carotene intervention, indicate that this carotenoid does not affect risk of cortical, nuclear, PSC, or “any” cataract or risk of cataract extraction (Table S2).5,61,62,71 Intakes or blood levels of α-carotene, lycopene, cryptoxanthin, and total carotenoids were also evaluated for possible relations with risk of onset or risk of progression of various forms of cataract, but records from as many as 190,000 subjects indicate little effect of these nutrients (Table S2).
Vitamin A/retinol
Records for over 10,000 people have been examined to clarify relations between vitamin A or retinol status and risk of cataracts. Consistent with an early study,5 both Dherani et al.61 (OR = 0.56; 95%CI: 0.33–0.96) and Ravindran et al.62 (OR = 0.69; 95%CI: 0.56–0.84) found that those with the highest blood levels of retinol had a reduced risk of nuclear cataract (Figure S24). Recently, Klein et al.87 found, in prospective studies, that those who supplemented with vitamin A had a reduced risk of cortical cataract (OR = 0.42; 95%CI: 0.24–0.73) (Figure S25).
Ravindran et al.62 found that those with the highest blood levels of retinol had a reduced risk of PSC cataract (OR = 0.65; 95%CI: 0.50–0.85) and “any” type of cataract (OR = 0.72; 95%CI: 0.59–0.88), as Dherani et al.61 found for mixed cataract (OR = 0.58; 95%CI: 0.37–0.91) (Figure S24). In the European Prospective Study Investigation in Cancer and Nutrition (EPIC),high intakes of vitamin A were also associated with increased risk of “any” cataract (IRR [incidence risk ratio] = 1.28; 95%CI: 1.07–1.54) (Figure S25).88
B vitamins: riboflavin, thiamin, folate, and vitamin B12
Analysis of over 12,000 subjects indicates that intake of at least 2 μg of riboflavin may help reduce the risk of cortical and nuclear cataract, particularly in malnourished populations (Figures S26–S29, Table S2).5 In general, higher thiamin and niacin status was associated with lower risk of some cataract endpoints.
Older data regarding the role of folate in eye health are not clear.5 Data from the Elderly Nutrition and Health Survey in Taiwan indicated that, relative to men – but not women – who were folate insufficient (<14 μM blood level), those with adequate folate status had a reduced risk of “any” type of cataract (OR = 0.67; 95%CI: 0.44–0.98) (Figure S28). This effect was driven by those over the age of 75.89 Although the EPIC did not find any association between risk of “any” type of cataract and intakes of thiamin, riboflavin, niacin, or folate, it did report an increased risk among those with elevated vitamin B12 intake (IRR = 1.29; 95%CI: 1.06–1.56) (Figure S28).88
Studies on antioxidant combinations and multivitamins, including intervention trials
People eat whole diets composed of many nutrients. To attempt to approximate the combined effects of multiple nutrients and to assess the value of consuming multivitamins, cataract risk has been related to intake of multivitamins or antioxidant indices in over 26,000 subjects (Figures S30–S37, Table S2).
Retrospective as well as prospective studies suggest that multivitamin use, antioxidant intake (>125 mg/day vitamin C, >8.4 mg/day vitamin E, >5,677 IU/day carotenoids), and high antioxidant blood levels (>40 μM vitamin C, >21 μM vitamin E, >1.7 μM carotenoids) reduce the risk of cortical cataract (Figures S30 and S31).90 This was recently corroborated by Klein et al.,91 who reported a 23% reduction in risk of progression of cortical cataract among those who used multivitamins in the Beaver Dam Eye Study (OR = 0.77; 95%CI: 0.62–0.95) (Figure S31). None of the large intervention trials, however, have shown an effect of multivitamins on the risk of cortical cataract (Figure S32, Table S2).5
Nuclear opacities have also been the subject of active investigation with regard to multivitamins. Prospective and intervention studies demonstrate that antioxidant combinations and multivitamins may be effective in abating the risk of nuclear opacities (Figures S33–S35, Table S2),5 findings that are supported by recent analyses from the Clinical Trial of Nutritional Supplements and Age-Related Cataract (CTNS) (OR = 0.66; 95%CI: 0.50–0.88) (Figure S35).92 Specifically, use of the multivitamin Centrum® (Pfizer, New York, NY) in AREDS was associated with a 17% decrease in risk of nuclear cataract in prospective analyses and with a 25% decrease in risk of nuclear cataract in the intervention arm of the trial (Figures S34 and S35).26,93 The beneficial effects of Centrum®, which were not observed with the cocktail of vitamin C, vitamin E, β-carotene, and zinc that was prescribed in AREDS, suggest that the AREDS cocktail was missing components that are found in Centrum®.
Unlike findings for nuclear cataract, data from the CTNS suggest that multivitamins may increase the risk of PSC cataract (OR = 2.00; 95%CI: 1.35–2.98).92 These data, together with data from the 8-year PHS II, show there is still no clear mandate for using antioxidant supplements to diminish the risk of cataract or cataract extraction.67,92
In conclusion, it should be appreciated that most of the subjects in these studies had higher socioeconomic status and were well fed. It appears that, for the chronically undernourished, supplements may provide benefit. However, any recommendation for use of antioxidants to diminish the risk of cataract in well-fed people who consume adequate levels of fruits and vegetables is based as much upon the idea that supplements that provide the recommended dietary allowance or the dietary reference intake of micronutrients do not harm, as much as on findings that they actually help.
EPIDEMIOLOGIC STUDIES THAT RELATE INTAKE OF CARBOHYDRATE TO RISK OF CATARACT
Glycation of proteins compromises cellular defenses and is also highly relevant to cataract risk, given the large proportion of the American diet that comprises carbohydrates.45 Carbohydrate intake has increased considerably in the last 30 years, as have rates of obesity. Intake and quality of carbohydrates have been shown to be related to risk of cardiovascular disease and type 2 diabetes, both of which are comorbidities of cataract.12 Reactions between carbohydrates and proteins can form Schiff bases, Maillard products, and advanced glycation end products in a process commonly referred to as “browning.”25 Sequelae of these reactions are aggregation and precipitation of proteins that are associated with cataractogenesis, age-related macular degeneration, and other debilities (Figure 2).12,44,45
Consistent with the biochemistry of carbohydrate metabolism, people with lower fasting glucose levels show diminished incidence and delayed progression of all cataract subtypes.94 Supporting these findings, a study of 124 subjects showed that nondiabetics with elevated serum fructose, a highly “glycating” sugar, have increased risk of cataract (P < 0.05).95 Additionally, diabetics experience large fluctuations in glucose and have vastly higher levels of PSC cataract and surgery. Risk of these types of cataract was also related to obesity.26,96
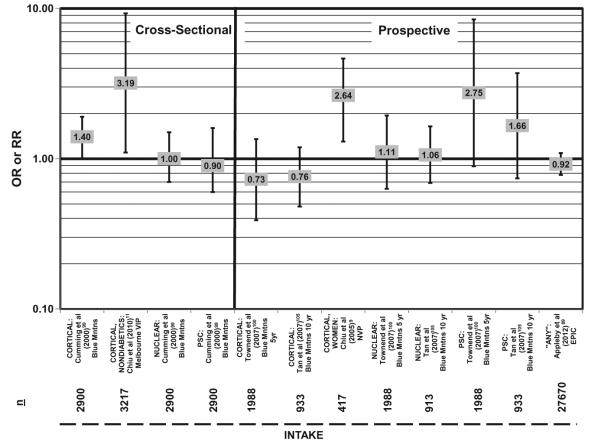
Six cohorts have been used to study the associations between eye health and the amount of carbohydrate intake or the quality of carbohydrate, and none report a beneficial effect of a high-carbohydrate diet.88,97 The NVP found that women consuming more than 200 g of carbohydrate per day were far more likely to develop cortical opacity than women consuming less than 185 g of carbohydrate per day (OR = 2.64; 95%CI: 1.30–4.64) (Figure 4).9 The NVP also reported a trend for increased risk of cortical opacity with increased consumption of carbohydrate (P = 0.005 for trend).9 Data from 3,217 subjects from the Melbourne Visual Impairment Project corroborate the NVP results and indicate that nondiabetics who consumed more than 181 g/day had more than a threefold greater risk of cortical cataract compared with those who consumed the least amount of carbohydrates (95%CI: 1.10–9.27) (Figure 4).11
Figure 4.
Relationship between cortical, nuclear, or posterior subcapsular cataract (PSC) and high versus low intake of carbohydrate: cross-sectional and prospective studies. n = number of subjects analyzed in each cohort.
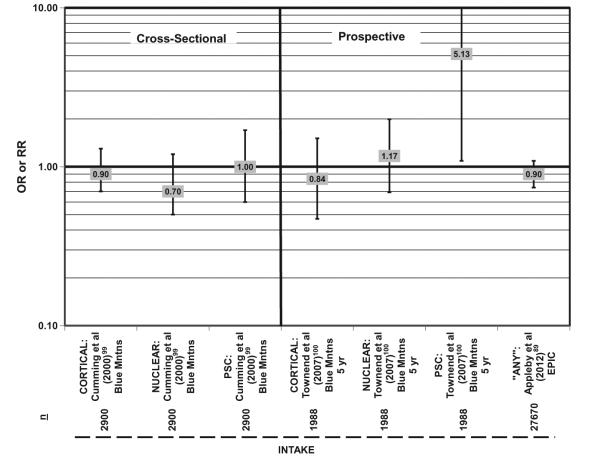
Given the associations between carbohydrate and health, it is logical to ask if the results may be due to dietary fiber. Cross-sectional analysis indicates that fiber intake does not influence risk of cortical, nuclear, or PSC cataract, although a prospective analysis found that intake of 23–29 g of fiber per day was associated with increased risk of PSC cataract (OR = 5.13; 95%CI: 1.09–24.24) compared with the least amount of intake (Figure 5).88,98,99
Figure 5.
Relationship between cortical, nuclear, or posterior subcapsular cataract (PSC) and high versus low intake of dietary fiber: cross-sectional and prospective studies. n = number of subjects analyzed in each cohort.
In an attempt to determine if different types of carbohydrate affect lens clarity differentially, risk of cataract was also related to the dietary glycemic index. The glycemic index describes how rapidly blood glucose levels increase after intake of a food, relative to the rise in blood glucose levels following consumption of a standard food with the same amount of carbohydrate (such as glucose or white bread). The dietary glycemic index averages this value for the whole diet. Recent studies have shown that consumption of a low-glycemic-index diet is associated with a reduction in risk of age-related macular degeneration, diabetes, cardiovascular disease, and renal disease.97,100–103
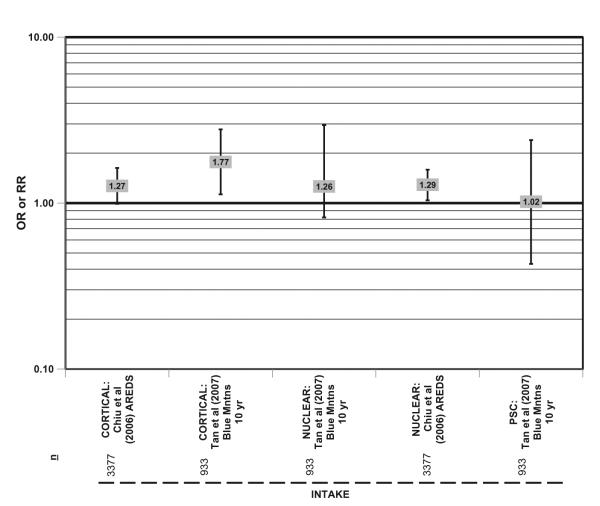
In the Blue Mountains cohort of 933 subjects, those who consumed carbohydrates of the highest glycemic index were more likely to develop cortical cataracts in 10 years than those who consumed carbohydrates of the lowest glycemic index (HR = 1.77; 95% CI: 1.13–2.78) (Figure 6).104 A similar trend was observed in 3,377 subjects in AREDS (Figure 6).10 AREDS also showed that those who consumed carbohydrates of the highest glycemic index also had an increased risk of nuclear cataract (OR = 1.29; 95%CI: 1.04–1.59; P for trend = 0.02) compared with those who consumed carbohydrates of the lowest glycemic index (Figure 6). The different relationships between dietary carbohydrate and nuclear and cortical cataract are consistent with the different etiologies of these types of cataract.
Figure 6.
Relationship between cortical, nuclear, or posterior subcapsular cataract (PSC) and intake of a high glycemic index diet: prospective studies. n = number of subjects analyzed in each cohort.
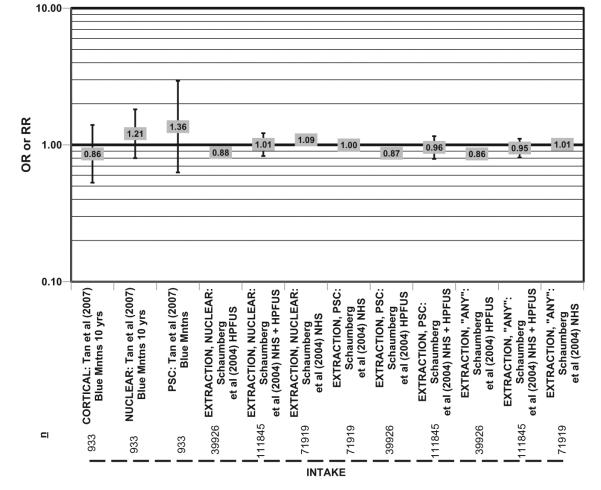
Glycemic load, defined as the weighted average of the glycemic indices of individual foods, multiplied by the percentage of energy as carbohydrate, is an outcome measure that combines the amount of carbohydrate consumed with the glycemic index of that particular carbohydrate.105 The Blue Mountain Eye Study104 and Schaumberg et al.,106 using combined data from the NHS and the Health Professionals Follow-Up Study (HPFUS) cohorts, which totaled 111,845 men and women, analyzed the effect of glycemic load on risk of reported cataract but found no significant associations (Figure 7). Curiously, when these two cohorts were evaluated separately, men with the highest glycemic load in the HPFUS had a 12% and 14% reduction in risk of extraction of nuclear cataract and “any” cataract, respectively (Figure 7).106 Importantly, the NHS and HPFUS relied on self-report of cataract, whereas participants in AREDS and the NVP were clinically evaluated and had their cataracts graded by ophthalmologists, which may account for some discrepancy between these studies. Obviously, with too few studies on this topic, but with growing levels of carbohydrates in the diet, this area of research deserves more attention.
Figure 7.
Relationship between cortical, nuclear, or posterior subcapsular (PSC) or “any” type of cataract extraction and intake of a high glycemic load diet: prospective studies. n = number of subjects analyzed in each cohort.
EPIDEMIOLOGIC STUDIES THAT RELATE INTAKE OF LIPIDS TO RISK OF CATARACT
Lens cell membranes have unusually high cholesterol ratios, an unusual lipid composition, and critical functions in intracellular communication and are required to function for decades with little option for replacement upon damage. In light of this, one might expect fat status to be related to risk of various forms of cataract. An overview of the epidemiologic records from over 17,000 subjects (Figures S38–S40, Tables S1 and S2), however, suggests the contrary.5 There appear to be few associations between fat intake from cholesterol, trans fats, or animal or vegetable sources and various forms of cataract other than the ones that follow here. 1) In the EPIC, risk of “any” type of cataract increased with elevated blood levels of saturated fat (RR = 1.19; 95%CI: 1.01–1.40) and cholesterol (RR = 1.23; 95%CI: 1.01–1.50).88 2) In the Blue Mountains Eye Study, a 30% decreased risk of cortical cataract was found for subjects who consumed more than 6.8 g/day of polyunsaturated fats.98 3) In contrast, however, a 2.3-fold increased risk of nuclear cataract was found in the NVP/NHS for persons with a higher intake of the same fats (Figure S38).107 4) A 42% lower risk of nuclear cataract was found in consumers of 0.5–1.42 g/day of omega-3 fatty acids (found in flaxseed, walnuts, salmon, shrimp, and many other seafoods).99 5) A 17% or 12% decreased risk of nuclear or “any” cataract extraction, respectively, was found in the NHS (Figure S39) among those with elevated intake of omega-3 fatty acids (specifically, eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]).108 6) In contrast to data for the complete NHS, there was a 2.2-fold increase in the risk of “any” cataract for those with high intake of omega-3 fatty acids in the NVP (Figure S39).107 7) In a large 6-year study, Martinez-Lapiscina et al.8 found that subjects with 0.05% and 0.2% of total energy intake as omega-6 fatty acids (found predominantly in oils such as saflower, sunflower, corn, and soybean oils) had a reduced risk of “any” cataract (OR = 0.54; 95%CI: 0.29–0.99) compared with those with the lowest intake (Figure S40). 8) There was a trend of increased risk of extraction of “any” type of cataract with increased consumption of linoleic acid in the NHS (P = 0.04).108 The increased consumption of linoleic acid (but not arachidonic acid) may explain the increased risk of nuclear cataract observed in those with high polyunsaturated fatty acid intake (Figures S38 and S40).
EPIDEMIOLOGIC STUDIES THAT RELATE INTAKE OF PROTEIN TO RISK OF CATARACT
All of the data regarding relations between dietary protein and eye health are derived from the 4,888 subjects in the Blue Mountains Eye Study and the 27,670 subjects in the EPIC. Retrospective analyses indicate that subjects who consumed approximately 99 g of protein per day had a 50% reduction in risk of nuclear cataract (95%CI: 0.30–0.80) compared with those who consumed the least amount of protein per day, and there was a trend of increasing intake associated with decreased risk (P = 0.009) (Figure S41).98 In the same population, 5-year incidence of PSC cataract was decreased in those who consumed approximately 107 g of protein per day (OR = 0.28; 95%CI: 0.10–0.76) compared with those who consumed the least amount, and increasing amounts of protein were associated with decreased risk (P = 0.015).99 Interestingly, in EPIC, those subjects who had the highest intake of protein had an increased risk of “any” type of cataract (IRR = 1.30; 95%CI: 1.10–1.55) (Figure S41).88 It is not clear why an increased intake of protein would confer risk, but increased protein intake may reflect increased total energy intake, which was associated with a trend of increased cataract risk (P = 0.044).
There have also been recent analyses of the effects of overall dietary patterns on cataract risk. A cross-sectional analysis of 240 African diabetic men reported that those who consumed a Mediterranean diet (which emphasizes polyunsaturated fats, fruits, and vegetables) had less risk of cataract (P < 0.005).27 In EPIC, decreased consumption of meat was associated with reduced risk of cataract (P < 0.001).88 Additionally, those over the age of 65 who consumed diets rich in fish and vegetables rather than meat had a 23% reduced risk of cataract (IRR = 0.77; 95%CI: 0.61–0.98), and vegetarians of all ages also had a reduced risk of cataract (IRR = 0.74; 95%CI: 0.63–0.86).88
CONCLUSION
Preventative measures to avoid cataract remain a crucial unmet medical need, especially important for the millions of people without access to cataract surgery. The problem is becoming more urgent as the elderly population burgeons beyond the healthcare resources that can be supported by the current working population, and as health problems associated with carbohydrate-dense diets accelerate. Robust observational data from well over 250,000 people suggest that maintaining a protein intake of 100–150 g/day and a vitamin C intake of approximately 135 mg/day (nearly twice the recommended level) while avoiding frequent large intakes of simple carbohydrates (i.e., chronic consumption of super-sized sweetened beverages) is prudent. Observational data also suggest that optimizing intakes of lutein, zeaxanthin, B vitamins, and multivitamin supplements may be beneficial in preserving lens health, particularly with regard to diminishing risk of nuclear and, possibly, cortical cataract. It has been difficult to tease apart the effects of single – or even groups of a few – nutrients, possibly indicating there are synergistic effects of multiple nutrients with regard to eye health. Importantly, reports regarding adverse effects of eating micronutrient-rich diets are rare. Despite these encouraging observational data,most of the clinical trials that evaluated the effect of vitamin C, vitamin E, carotenoids, and multivitamins (or antioxidant combinations) on specific types of cataracts or on cataract extraction failed to establish or confirm causal associations and benefit. Whereas the data for nuclear cataract in intervention trials are encouraging, the potential for increased risk of PSC is an important caution because PSC cataracts obscure the central field of vision. The lack of salutary effects of multivitamins in the relatively short-term trials may imply that only long-term intake from foods or supplementation (such as the AREDS Centrum® data) can provide clinical benefit. Additional long-term studies to clarify the types of cataract most affected by specific nutrients, dietary patterns, or environmental influences will improve life quality for the elderly and help reduce the substantial burden of cataract on public health resources.
Supplementary Material
Acknowledgments
We would like to thank CJ Chiu for his help compiling data.
Funding. This research was funded by USDA 1950-510000-060-01A, Johnson and Johnson Focused Giving, the American Health Assistance Foundation, and a generous gift from Alcon via Dr. John Lang to AT. The work was also supported by EY RO1 13250 and EY RO1 21212 from NIH. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the US Department of Agriculture.
Footnotes
Declaration of interestg. The authors have no relevant interests to declare.
REFERENCES
- 1.McGwin G, Khoury R, Cross J, et al. Vision impairment and eye care utilization among Americans 50 and older. Curr Eye Res. 2010;35:451–458. doi: 10.3109/02713681003664931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . Global Initiative for the Elimination of Avoidable Blindness: Action Plan 2006–2011. WHO Press; Geneva: 2007. [Google Scholar]
- 3.Tabin G, Chen M, Espandar L. Cataract surgery for the developing world. Curr Opin Ophthalmol. 2008;19:55–59. doi: 10.1097/ICU.0b013e3282f154bd. [DOI] [PubMed] [Google Scholar]
- 4.Athanasiov PA, Edussuriya K, Senaratne T, et al. Cataract in central Sri Lanka: cataract surgical coverage and self-reported barriers to cataract surgery. Clin Experiment Ophthalmol. 2009;37:780–784. doi: 10.1111/j.1442-9071.2009.02152.x. [DOI] [PubMed] [Google Scholar]
- 5.Chiu CJ, Taylor A. Nutritional antioxidants and age-related cataract and maculopathy. Exp Eye Res. 2007;84:229–245. doi: 10.1016/j.exer.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Siegal M, Chiu CJ, Taylor A. Antioxidant status and risk for cataract. In: Bendich A, Deckelbaum RJ, editors. Preventive Nutrition: The Comprehensive Guide for Health Professionals. 3rd Humana Press Inc.; Totowa, NJ: 2005. pp. 463–503. [Google Scholar]
- 7.Taylor A. Nutritional and environmental influences on risk for cataract. In: Taylor A, editor. Nutritional and Environmental Influences on the Eye. CRC Press; Boca Raton, FL: 1999. pp. 458–487. [Google Scholar]
- 8.Martinez-Lapiscina EH, Martinez-Gonzalez MA, Guillen Grima F, et al. Dietary fat intake and incidence of cataracts: the SUN Prospective study in the cohort of Navarra, Spain [in Spanish] Med Clin (Barc) 2010;134:194–201. doi: 10.1016/j.medcli.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 9.Chiu CJ, Morris MS, Rogers G, et al. Carbohydrate intake and glycemic index in relation to the odds of early cortical and nuclear lens opacities. Am J Clin Nutr. 2005;81:1411–1416. doi: 10.1093/ajcn/81.6.1411. [DOI] [PubMed] [Google Scholar]
- 10.Chiu CJ, Milton RC, Gensler G, et al. Dietary carbohydrate intake and glycemic index in relation to cortical and nuclear lens opacities in the Age-Related Eye Disease Study. Am J Clin Nutr. 2006;83:1177–1184. doi: 10.1093/ajcn/83.5.1177. [DOI] [PubMed] [Google Scholar]
- 11.Chiu CJ, Robman L, McCarty CA, et al. Dietary carbohydrate in relation to cortical and nuclear lens opacities in the Melbourne Visual Impairment Project. Invest Ophthalmol Vis Sci. 2010;51:2897–2905. doi: 10.1167/iovs.08-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu C-J, Liu S, Willett WC, et al. Informing food choices and health outcomes by use of the dietary glycemic index. Nutr Rev. 2011;69:231–242. doi: 10.1111/j.1753-4887.2011.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu SY, Leske MC. Antioxidants and cataract formation: a summary review. Int Ophthalmol Clin. 2000;40:71–81. doi: 10.1097/00004397-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Taylor A, Jacques PF, Epstein EM. Relations among aging, antioxidant status, and cataract. Am J Clin Nutr. 1995;62(Suppl 6):S1439–S1447. doi: 10.1093/ajcn/62.6.1439S. [DOI] [PubMed] [Google Scholar]
- 15.Fan X, Reneker LW, Obrenovich ME, et al. Vitamin C mediates chemical aging of lens crystallins by the Maillard reaction in a humanized mouse model. Proc Natl Acad Sci U S A. 2006;103:16912–16917. doi: 10.1073/pnas.0605101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chitchumroonchokchai C, Bomser JA, Glamm JE, et al. Xanthophylls and α-tocopherol decrease UVB-induced lipid peroxidation and stress signaling in human lens epithelial cells. J Nutr. 2004;134:3225–3232. doi: 10.1093/jn/134.12.3225. [DOI] [PubMed] [Google Scholar]
- 17.Cheng R, Feng Q, Ortwerth BJ. LC-MS display of the total modified amino acids in cataract lens proteins and in lens proteins glycated by ascorbic acid in vitro. Biochim Biophys Acta. 2006;1762:533–543. doi: 10.1016/j.bbadis.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 18.National Eye Institute of the National Institutes of Health Eye diagram. Available at: http://www.nei.nih.gov/photo/keyword.asp?conditions = Normal +Eye+Images&match = all. Electronic resource no. NEA04. Accessed June 11, 2010.
- 19.Vrensen GF. Early cortical lens opacities: a short overview. Acta Ophthalmol. 2009;87:602–610. doi: 10.1111/j.1755-3768.2009.01674.x. [DOI] [PubMed] [Google Scholar]
- 20.Perng MD, Sandilands A, Kuszak J, et al. The intermediate filament systems in the eye lens. Methods Cell Biol. 2004;78:597–624. doi: 10.1016/s0091-679x(04)78021-8. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava OP. Age-related increase in concentration and aggregation of degraded polypeptides in human lenses. Exp Eye Res. 1988;47:525–543. doi: 10.1016/0014-4835(88)90092-9. [DOI] [PubMed] [Google Scholar]
- 22.Taylor A, Nowell T. Oxidative stress and antioxidant function in relation to risk for cataract. In: Sies H, editor. Antioxidants in Disease Mechanisms and Therapy. Advances in Pharmacology. Academic Press; San Diego: 1997. pp. 515–536. [DOI] [PubMed] [Google Scholar]
- 23.Chylack LT, Jr, Wolfe JK, Singer DM, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993;111:831–836. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 24.Taylor HR, West SK. A simple system for the clinical grading of lens opacities. Yan Ke Xue Bao. 1988;4:14–18. [PubMed] [Google Scholar]
- 25.Chylack LT., Jr. Function of the lens and methods of quantifying cataract. In: Taylor A, editor. Nutritional and Environmental Influences on the Eye. Vol. 1999. CRC Press; Boca Raton, FL: pp. 25–52. [Google Scholar]
- 26.Chang JR, Koo E, Agron E, et al. Risk factors associated with incident cataracts and cataract surgery in the Age-Related Eye Disease Study (AREDS): AREDS report number 32. Ophthalmology. 2011;118:2113–2119. doi: 10.1016/j.ophtha.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moise MM, Benjamin LM, Doris TM, et al. Role of Mediterranean diet, tropical vegetables rich in antioxidants, and sunlight exposure in blindness, cataract and glaucoma among African type 2 diabetics. Int J Ophthalmol. 2012;5:231–237. doi: 10.3980/j.issn.2222-3959.2012.02.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.West SK, Longstreth JD, Munoz BE, et al. Model of risk of cortical cataract in the US population with exposure to increased ultraviolet radiation due to stratospheric ozone depletion. Am J Epidemiol. 2005;162:1080–1088. doi: 10.1093/aje/kwi329. [DOI] [PubMed] [Google Scholar]
- 29.McCarty CA, Taylor HR. A review of the epidemiologic evidence linking ultraviolet radiation and cataracts. Dev Ophthalmol. 2002;35:21–31. doi: 10.1159/000060807. [DOI] [PubMed] [Google Scholar]
- 30.Mansour AM, Ghabra M. Cataractogenesis after repeat laser in situ keratomileusis. Case Rep Ophthalmol. 2012;3:262–265. doi: 10.1159/000342134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walls HL, Walls KL, Benke G. Eye disease resulting from increased use of fluorescent lighting as a climate change mitigation strategy. Am J Public Health. 2011;101:2222–2225. doi: 10.2105/AJPH.2011.300246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costagliola C, Menzione M, Chiosi F, et al. Retinal phototoxicity induced by hydrochlorothiazide after exposure to a UV tanning device. Photochem Photobiol. 2008;84:1294–1297. doi: 10.1111/j.1751-1097.2008.00404.x. [DOI] [PubMed] [Google Scholar]
- 33.Weatherall M, Clay J, James K, et al. Dose-response relationship of inhaled corticosteroids and cataracts: a systematic review and meta-analysis. Respirology. 2009;14:983–990. doi: 10.1111/j.1440-1843.2009.01589.x. [DOI] [PubMed] [Google Scholar]
- 34.West SK. Smoking and the risk of eye diseases. In: Taylor A, editor. Nutritional and Environmental Influences on the Eye. Vol. 1999. CRC Press; Boca Raton, FL: pp. 151–164. [Google Scholar]
- 35.Kelly SP, Thornton J, Edwards R, et al. Smoking and cataract: review of causal association. J Cataract Refract Surg. 2005;31:2395–2404. doi: 10.1016/j.jcrs.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 36.Richter GM, Choudhury F, Torres M, et al. Risk factors for incident cortical, nuclear, posterior subcapsular, and mixed lens opacities: the Los Angeles Latino Eye Study. Ophthalmology. 2012;119:2040–2047. doi: 10.1016/j.ophtha.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seddon JM, Christen WG, Manson JE, et al. The use of vitamin supplements and the risk of cataract among US male physicians. Am J Public Health. 1994;84:788–792. doi: 10.2105/ajph.84.5.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Virtamo J, Pietinen P, Huttunen JK, et al. Incidence of cancer and mortality following α-tocopherol and β-carotene supplementation: a postintervention follow-up. JAMA. 2003;290:476–485. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- 39.Meyer LM, Lofgren S, Ho YS, et al. Absence of glutaredoxin1 increases lens susceptibility to oxidative stress induced by UVR-B. Exp Eye Res. 2009;89:833–839. doi: 10.1016/j.exer.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 40.Reddy VN. Glutathione and its function in the lens – an overview. Exp Eye Res. 1990;50:771–778. doi: 10.1016/0014-4835(90)90127-g. [DOI] [PubMed] [Google Scholar]
- 41.Xing KY, Lou MF. Effect of age on the thioltransferase (glutaredoxin) and thioredoxin systems in the human lens. Invest Ophthalmol Vis Sci. 2010;51:6598–6604. doi: 10.1167/iovs.10-5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donma O, Yorulmaz E, Pekel H, et al. Blood and lens lipid peroxidation and antioxidant status in normal individuals, senile and diabetic cataractous patients. Curr Eye Res. 2002;25:9–16. doi: 10.1076/ceyr.25.1.9.9960. [DOI] [PubMed] [Google Scholar]
- 43.Garner MH, Spector A. Selective oxidation of cysteine and methionine in normal and senile cataractous lenses. Proc Natl Acad Sci U S A. 1980;77:1274–1277. doi: 10.1073/pnas.77.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weikel KA, Fitzgerald P, Shang F, et al. Natural history of age-related retinal lesions that precede AMD in mice fed high or low glycemic index diets. Invest Ophthalmol Vis Sci. 2012;53:622–632. doi: 10.1167/iovs.11-8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchiki T, Weikel KA, Jiao W, et al. Glycation-altered proteolysis as a pathobiologic mechanism that links dietary glycemic index, aging, and age-related disease (in non diabetics) Aging Cell. 2012;11:1–13. doi: 10.1111/j.1474-9726.2011.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor A. Mechanistically linking age-related diseases and dietary carbohydrate via autophagy and the ubiquitin proteolytic systems. Autophagy. 2012;8:1404–1406. doi: 10.4161/auto.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monnier VM, Cerami A. Detection of nonenzymatic browning products in human lens. Biochim Biophys Acta. 1983;760:97–103. doi: 10.1016/0304-4165(83)90129-0. [DOI] [PubMed] [Google Scholar]
- 48.Fuentealba D, Friguet B, Silva E. Advanced glycation endproducts induce photocrosslinking and oxidation of bovine lens proteins through type-I mechanism. Photochem Photobiol. 2009;85:185–194. doi: 10.1111/j.1751-1097.2008.00415.x. [DOI] [PubMed] [Google Scholar]
- 49.Wan XH, Lee EH, Koh HJ, et al. Enhanced expression of transglutaminase 2 in anterior polar cataracts and its induction by TGF-β in vitro. Br J Ophthalmol. 2002;86:1293–1298. doi: 10.1136/bjo.86.11.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srivastava SK, Ansari NH. Prevention of sugar-induced cataractogenesis in rats by butylated hydroxytoluene. Diabetes. 1988;37:1505–1508. doi: 10.2337/diab.37.11.1505. [DOI] [PubMed] [Google Scholar]
- 51.Shang F, Taylor A. Ubiquitin-proteasome pathway and cellular responses to oxidative stress. Free Radic Biol Med. 2011;51:5–16. doi: 10.1016/j.freeradbiomed.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shang F, Gong X, Palmer HJ, et al. Age-related decline in ubiquitin conjugation in response to oxidative stress in the lens. Exp Eye Res. 1997;64:21–30. doi: 10.1006/exer.1996.0176. [DOI] [PubMed] [Google Scholar]
- 53.Shang F, Taylor A. Oxidative stress and recovery from oxidative stress are associated with altered ubiquitin conjugating and proteolytic activities in bovine lens epithelial cells. Biochem J. 1995;307:297–303. doi: 10.1042/bj3070297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jahngen-Hodge J, Obin MS, Gong X, et al. Regulation of ubiquitin-conjugating enzymes by glutathione following oxidative stress. J Biol Chem. 1997;272:28218–28226. doi: 10.1074/jbc.272.45.28218. [DOI] [PubMed] [Google Scholar]
- 55.Cuervo AM, Dice JF. How do intracellular proteolytic systems change with age? Front Biosci. 1998;3:d25–d43. doi: 10.2741/a264. [DOI] [PubMed] [Google Scholar]
- 56.Shang F, Taylor A. Roles for the ubiquitin-proteasome pathway in protein quality control and signaling in the retina: implications in the pathogenesis of age-related macular degeneration. Mol Aspects Med. 2012;33:446–466. doi: 10.1016/j.mam.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor A, Jacques PF, Nadler D, et al. Relationship in humans between ascorbic acid consumption and levels of total and reduced ascorbic acid in lens, aqueous humor, and plasma. Curr Eye Res. 1991;10:751–759. doi: 10.3109/02713689109013869. [DOI] [PubMed] [Google Scholar]
- 58.Berger J, Shepard D, Morrow F, et al. Relationship between dietary intake and tissue levels of reduced and total vitamin C in the nonscorbutic guinea pig. J Nutr. 1989;119:734–740. doi: 10.1093/jn/119.5.734. [DOI] [PubMed] [Google Scholar]
- 59.Pollard J, Wild CP, White KL, et al. Comparison of plasma biomarkers with dietary assessment methods for fruit and vegetable intake. Eur J Clin Nutr. 2003;57:988–998. doi: 10.1038/sj.ejcn.1601634. [DOI] [PubMed] [Google Scholar]
- 60.Tarwadi KV, Chiplonkar SA, Agte V. Dietary and nutritional biomarkers of lens degeneration, oxidative stress and micronutrient inadequacies in Indian cataract patients. Clin Nutr. 2008;27:464–472. doi: 10.1016/j.clnu.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 61.Dherani M, Murthy GV, Gupta SK, et al. Blood levels of vitamin C, carotenoids and retinol are inversely associated with cataract in a North Indian population. Invest Ophthalmol Vis Sci. 2008;49:3328–3335. doi: 10.1167/iovs.07-1202. [DOI] [PubMed] [Google Scholar]
- 62.Ravindran RD, Vashist P, Gupta SK, et al. Inverse association of vitamin C with cataract in older people in India. Ophthalmology. 2011;118:1958.e2–1965.e2. doi: 10.1016/j.ophtha.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor A, Jacques PF, Chylack LT, Jr, et al. Long-term intake of vitamins and carotenoids and odds of early age-related cortical and posterior subcapsular lens opacities. Am J Clin Nutr. 2002;75:540–549. doi: 10.1093/ajcn/75.3.540. [DOI] [PubMed] [Google Scholar]
- 64.Christen WG, Liu S, Glynn RJ, et al. Dietary carotenoids, vitamins C and E, and risk of cataract in women: a prospective study. Arch Ophthalmol. 2008;126:102–109. doi: 10.1001/archopht.126.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simon JA, Hudes ES. Serum ascorbic acid and other correlates of self-reported cataract among older Americans. J Clin Epidemiol. 1999;52:1207–1211. doi: 10.1016/s0895-4356(99)00110-9. [DOI] [PubMed] [Google Scholar]
- 66.Yoshida M, Takashima Y, Inoue M, et al. Prospective study showing that dietary vitamin C reduced the risk of age-related cataracts in a middle-aged Japanese population. Eur J Nutr. 2007;46:118–124. doi: 10.1007/s00394-006-0641-8. [DOI] [PubMed] [Google Scholar]
- 67.Christen WG, Glynn RJ, Sesso HD, et al. Age-related cataract in a randomized trial of vitamins E and C in men. Arch Ophthalmol. 2010;128:1397–1405. doi: 10.1001/archophthalmol.2010.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rautiainen S, Lindblad BE, Morgenstern R, et al. Vitamin C supplements and the risk of age-related cataract: a population-based prospective cohort study in women. Am J Clin Nutr. 2010;91:487–493. doi: 10.3945/ajcn.2009.28528. [DOI] [PubMed] [Google Scholar]
- 69.Galli F, Azzi A. Present trends in vitamin E research. Biofactors. 2010;36:33–42. doi: 10.1002/biof.75. [DOI] [PubMed] [Google Scholar]
- 70.Mares JA, Voland R, Adler R, et al. Healthy diets and the subsequent prevalence of nuclear cataract in women. Arch Ophthalmol. 2010;128:738–749. doi: 10.1001/archophthalmol.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tan AG, Mitchell P, Flood VM, et al. Antioxidant nutrient intake and the long-term incidence of age-related cataract: the Blue Mountains Eye Study. Am J Clin Nutr. 2008;87:1899–1905. doi: 10.1093/ajcn/87.6.1899. [DOI] [PubMed] [Google Scholar]
- 72.McNeil JJ, Robman LD, Tikellis G, et al. Vitamin E supplementation and cataract:randomized controlled trial. Ophthalmology. 2004;111:75–84. doi: 10.1016/j.ophtha.2003.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Christen WG, Glynn RJ, Chew EY, et al. Vitamin E and age-related cataract in a randomized trial of women. Ophthalmology. 2008;115:822.e1–829.e1. doi: 10.1016/j.ophtha.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 74.Jacques P, Taylor A, Moeller S, et al. Long-term nutrient intake and five-year change in nuclear lens opacities. Arch Ophthalmol. 2005;123:517–526. doi: 10.1001/archopht.123.4.517. [DOI] [PubMed] [Google Scholar]
- 75.Nourmohammadi I, Modarress M, Khanaki K, et al. Association of serum α-tocopherol, retinol and ascorbic acid with the risk of cataract development. Ann Nutr Metab. 2008;52:296–298. doi: 10.1159/000148189. [DOI] [PubMed] [Google Scholar]
- 76.Olmedilla B, Granado F, Blanco I, et al. Serum status of carotenoids and tocopherols in patients with age-related cataracts: a case-control study. J Nutr Health Aging. 2002;6:66–68. [PubMed] [Google Scholar]
- 77.Mosad SM, Ghanem AA, El-Fallal HM, et al. Lens cadmium, lead, and serum vitamins C, E, and beta carotene in cataractous smoking patients. Curr Eye Res. 2010;35:23–30. doi: 10.3109/02713680903362880. [DOI] [PubMed] [Google Scholar]
- 78.Schalch W, Dayhaw-Barker P, Barker FM., II . The carotenoids of the human retina. In: Taylor A, editor. Nutritional and Environmental Influences on the Eye. CRC Press; Boca Raton, FL: 1999. pp. 215–250. [Google Scholar]
- 79.Donaldson MS. A carotenoid health index based on plasma carotenoids and health outcomes. Nutrients. 2011;3:1003–1022. doi: 10.3390/nu3121003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karppi J, Laukkanen JA, Kurl S. Plasma lutein and zeaxanthin and the risk of age-related nuclear cataract among the elderly Finnish population. Br J Nutr. 2012;108:148–154. doi: 10.1017/S0007114511005332. [DOI] [PubMed] [Google Scholar]
- 81.Moeller S, Voland R, Tinker L, et al. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in Age-Related Eye Disease Study (CAREDS), an ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2008;126:354–364. doi: 10.1001/archopht.126.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rodriguez-Rodriguez E, Ortega RM, Lopez-Sobaler AM, et al. The relationship between antioxidant nutrient intake and cataracts in older people. Int J Vitam Nutr Res. 2006;76:359–366. doi: 10.1024/0300-9831.76.6.359. [DOI] [PubMed] [Google Scholar]
- 83.Berendschot TT, Broekmans WM, Klopping-Ketelaars IA, et al. Lens aging in relation to nutritional determinants and possible risk factors for age-related cataract. Arch Ophthalmol. 2002;120:1732–1737. doi: 10.1001/archopht.120.12.1732. [DOI] [PubMed] [Google Scholar]
- 84.Olmedilla B, Granado F, Blanco I, et al. Lutein, but not α-tocopherol, supplementation improves visual function in patients with age-related cataracts: a 2-y double-blind, placebo-controlled pilot study. Nutrition. 2003;19:21–24. doi: 10.1016/s0899-9007(02)00861-4. [DOI] [PubMed] [Google Scholar]
- 85.Age-Related Eye Disease Study 2 (AREDS) Research Group. Chew EW, SanGiovanni JP, et al. Lutein/zeaxanthin for the treatment of age-related cataract: AREDS2 randomized trial report no. 4. JAMA Ophthalmol. 2013;131:843–850. doi: 10.1001/jamaophthalmol.2013.4412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chasan-Taber L, Willett WC, Seddon JM, et al. A prospective study of carotenoid and vitamin A intakes and risk of cataract extraction in US women. Am J Clin Nutr. 1999;70:509–516. doi: 10.1093/ajcn/70.4.509. [DOI] [PubMed] [Google Scholar]
- 87.Klein BE, Knudtson MD, Lee KE, et al. Supplements and age-related eye conditions:the Beaver Dam Eye Study. Ophthalmology. 2008;115:1203–1208. doi: 10.1016/j.ophtha.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 88.Appleby PN, Allen NE, Key TJ. Diet, vegetarianism, and cataract risk. Am J Clin Nutr. 2012;93:1128–1135. doi: 10.3945/ajcn.110.004028. [DOI] [PubMed] [Google Scholar]
- 89.Chen KJ, Pan WH, Huang CJ, et al. Association between folate status, diabetes, antihypertensive medication and age-related cataracts in elderly Taiwanese. J Nutr Health Aging. 2011;15:304–310. doi: 10.1007/s12603-010-0282-8. [DOI] [PubMed] [Google Scholar]
- 90.Jacques PF, Chylack LT., Jr. Epidemiologic evidence of a role for the antioxidant vitamins and carotenoids in cataract prevention. Am J Clin Nutr. 1991;53(Suppl 1):S352–S355. doi: 10.1093/ajcn/53.1.352S. [DOI] [PubMed] [Google Scholar]
- 91.Klein R, Knudtson MD, Cruickshanks KJ, et al. Further observations on the association between smoking and the long-term incidence and progression of agerelated macular degeneration: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126:115–121. doi: 10.1001/archopht.126.1.115. [DOI] [PubMed] [Google Scholar]
- 92.Clinical Trial of Nutrional Supplements and Age-Related Cataract Study Group. Maraini G, Sperduto RD, Ferris F, et al. A randomized, double-masked, placebocontrolled clinical trial of multivitamin supplementation for age-related lens opacities. Clinical Trial of Nutritional Supplements and Age-Related Cataract report no. 3. Ophthalmology. 2008;115:599.e1–607.e1. doi: 10.1016/j.ophtha.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 93.Milton RC, Sperduto RD, Clemons TE, et al. Centrum use and progression of age-related cataract in the Age-Related Eye Disease Study: a propensity score approach. AREDS report no. 21. Ophthalmology. 2006;113:1264–1270. doi: 10.1016/j.ophtha.2006.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kanthan GL, Mitchell P, Burlutsky G, et al. Fasting blood glucose levels and the long-term incidence and progression of cataract – the Blue Mountains Eye Study. Acta Ophthalmol. 2011;89:e434–e438. doi: 10.1111/j.1755-3768.2011.02149.x. [DOI] [PubMed] [Google Scholar]
- 95.Gul A, Rahman MA, Hasnain SN. Role of fructose concentration on cataractogenesis in senile diabetic and non-diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2009;247:809–814. doi: 10.1007/s00417-008-1027-9. [DOI] [PubMed] [Google Scholar]
- 96.Noble D, Mathur R, Dent T, et al. Risk models and scores for type 2 diabetes: systematic review. BMJ. 2011;343:d7163. doi: 10.1136/bmj.d7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chiu CJ, Taylor A. Dietary hyperglycemia, glycemic index and metabolic retinal diseases. Prog Retin Eye Res. 2011;30:18–53. doi: 10.1016/j.preteyeres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cumming RG, Mitchell P, Smith W. Diet and cataract: the Blue Mountains Eye Study. Ophthalmology. 2000;107:450–456. doi: 10.1016/s0161-6420(99)00024-x. [DOI] [PubMed] [Google Scholar]
- 99.Townend BS, Townend ME, Flood V, et al. Dietary macronutrient intake and five-year incident cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2007;143:932–939. doi: 10.1016/j.ajo.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 100.Semba RD, Fink JC, Sun K, et al. Carboxymethyl-lysine, an advanced glycation end product, and decline of renal function in older community-dwelling adults. Eur J Nutr. 2009;48:38–44. doi: 10.1007/s00394-008-0757-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ferland A, Brassard P, Lemieux S, et al. Impact of high-fat/low-carbohydrate, high-, low-glycaemic index or low-caloric meals on glucose regulation during aerobic exercise in type 2 diabetes. Diabet Med. 2009;26:589–595. doi: 10.1111/j.1464-5491.2009.02734.x. [DOI] [PubMed] [Google Scholar]
- 102.McKeown NM, Meigs JB, Liu S, et al. Dietary carbohydrates and cardiovascular disease risk factors in the Framingham Offspring cohort. J Am Coll Nutr. 2009;28:150–158. doi: 10.1080/07315724.2009.10719766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Riegersperger M, Sunder-Plassmann G. How to prevent progression to end stage renal disease. J Ren Care. 2007;33:105–107. doi: 10.1111/j.1755-6686.2007.tb00053.x. [DOI] [PubMed] [Google Scholar]
- 104.Tan J, Wang JJ, Flood V, et al. Carbohydrate nutrition, glycemic index, and the 10-y incidence of cataract. Am J Clin Nutr. 2007;86:1502–1508. doi: 10.1093/ajcn/86.5.1502. [DOI] [PubMed] [Google Scholar]
- 105.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 106.Schaumberg DA, Liu S, Seddon JM, et al. Dietary glycemic load and risk of age-related cataract. Am J Clin Nutr. 2004;80:489–495. doi: 10.1093/ajcn/80.2.489. [DOI] [PubMed] [Google Scholar]
- 107.Lu M, Taylor A, Chylack LT, Jr, et al. Dietary fat intake and early age-related lens opacities. Am J Clin Nutr. 2005;81:773–779. doi: 10.1093/ajcn/81.4.773. [DOI] [PubMed] [Google Scholar]
- 108.Lu M, Cho E, Taylor A, et al. Prospective study of dietary fat and risk of cataract extraction among US women. Am J Epidemiol. 2005;161:948–959. doi: 10.1093/aje/kwi118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.