Abstract
Rationale: Lysocardiolipin acyltransferase (LYCAT), a cardiolipin-remodeling enzyme regulating the 18:2 linoleic acid pattern of mammalian mitochondrial cardiolipin, is necessary for maintaining normal mitochondrial function and vascular development. We hypothesized that modulation of LYCAT expression in lung epithelium regulates development of pulmonary fibrosis.
Objectives: To define a role for LYCAT in human and murine models of pulmonary fibrosis.
Methods: We analyzed the correlation of LYCAT expression in peripheral blood mononuclear cells (PBMCs) with the outcomes of pulmonary functions and overall survival, and used the murine models to establish the role of LYCAT in fibrogenesis. We studied the LYCAT action on cardiolipin remodeling, mitochondrial reactive oxygen species generation, and apoptosis of alveolar epithelial cells under bleomycin challenge.
Measurements and Main Results: LYCAT expression was significantly altered in PBMCs and lung tissues from patients with idiopathic pulmonary fibrosis (IPF), which was confirmed in two preclinical murine models of IPF, bleomycin- and radiation-induced pulmonary fibrosis. LYCAT mRNA expression in PBMCs directly and significantly correlated with carbon monoxide diffusion capacity, pulmonary function outcomes, and overall survival. In both bleomycin- and radiation-induced pulmonary fibrosis murine models, hLYCAT overexpression reduced several indices of lung fibrosis, whereas down-regulation of native LYCAT expression by siRNA accentuated fibrogenesis. In vitro studies demonstrated that LYCAT modulated bleomycin-induced cardiolipin remodeling, mitochondrial membrane potential, reactive oxygen species generation, and apoptosis of alveolar epithelial cells, potential mechanisms of LYCAT-mediated lung protection.
Conclusions: This study is the first to identify modulation of LYCAT expression in fibrotic lungs and offers a novel therapeutic approach for ameliorating lung inflammation and pulmonary fibrosis.
Keywords: LYCAT, mitochondrial cardiolipin remodeling, bleomycin, IPF, apoptosis
At a Glance Commentary
Scientific Knowledge on the Subject
Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive lung fibrotic disease with poor prognosis. Despite recent advances, underlying IPF pathogenesis is still elusive and effective therapies are not currently available.
What This Study Adds to the Field
The role of lysocardiolipin acyltransferase (LYCAT), a cardiolipin-remodeling enzyme, in the development of pulmonary fibrosis is unknown. We show that LYCAT expression is significantly altered in peripheral blood mononuclear cells and lung tissues from patients with IPF, and LYCAT expression in peripheral blood mononuclear cells directly and significantly correlates with pulmonary function outcomes and overall survival. Using preclinical murine models of IPF, we show that LYCAT overexpression reduces several indices of lung fibrosis; conversely, down-regulation of native LYCAT expression accentuates fibrogenesis. Potential mechanisms of LYCAT-mediated lung protection may involve suppression of bleomycin-induced mitochondrial reactive oxygen species generation and apoptosis of alveolar epithelial cells.
Idiopathic pulmonary fibrosis (IPF), a chronic and progressive lung fibrotic disease, is characterized by the insidious onset of interstitial infiltrates in the lung parenchyma associated with progressive dyspnea, decreased exercise tolerance, and impaired pulmonary function (1, 2). Prognosis of patients with IPF is very poor with average life expectancy of 3–5 years after diagnosis (3). Despite extensive research efforts over the past decades, effective therapies are not yet available (4) with conventional therapies, such as corticosteroids, azathioprine, and cyclophosphamide, providing only marginal benefit (5, 6) and lung transplantation the only effective therapy to date. Emerging strategies to treat patients with IPF include inhibition of epithelial injury or enhancement of repair, treating with anticytokines, and inhibition of fibroblast proliferation or induction of fibroblast apoptosis. In light of the poor prognosis and lack of available antiinflammatory therapies, there is a pressing need for alternative approaches including evaluation of new targets and pathways in animal models of fibrosis. Accordingly, two bioactive lipids, lysophosphatidic acid and sphingosine-1-phosphate, and enzymes involved in their metabolism have been implicated in regulating the development of pulmonary fibrosis (7–11).
Recent studies suggest that gene variants, epigenetics, and environmental factors may contribute to the onset and development of pulmonary fibrosis. Genome-wide association studies (GWAS) have described single-nucleotide polymorphisms (SNPs) associated with genes encoding cyclooxygenase 2 (12), EGFR (13), telomerase (TERT and TERC) (14), IFN-γ G5644A (15), and MUC5B (16, 17) in IPF. We conducted a discovery GWAS involving a cohort of 542 European-American IPF cases (18), matched one-to-one by genetic ancestry to GWAS control subjects from dbGaP, to identify potential genetic variants in candidate genes associated with IPF susceptibility and severity (see Table E1 in the online supplement). Initial interrogation identified seven SNP clusters with low P values on chromosome 2, and in the preliminary data set the lysocardiolipin acyltransferase (LYCAT) gene had the highest ranking SNPs in the GWAS. Whereas these LYCAT SNPs failed to reach 10−9 significance in an IPF validation group consisting of 150 IPF cohorts, P values consistently were in the 10−4 to 10−7 range suggesting that this gene may likely modulate metabolic processes involved in IPF pathobiology.
LYCAT is a key enzyme that regulates the unique C18:2 linoleic acid pattern of mammalian mitochondrial cardiolipin necessary for binding to mitochondrial proteins, such as cytochrome C, and therefore is critical for normal mitochondrial electron transport and function (19, 20). Additionally, LYCAT regulates hematopoietic and endothelial lineages suggesting a potential role in vascular development (21). The role of LYCAT gene in the pathology of IPF is unknown; we now provide preclinical and human data supporting novel LYCAT involvement in IPF pathobiology. LYCAT expression was significantly altered in peripheral blood mononuclear cells (PBMCs) and in lung tissues from patients with IPF, which was confirmed in two preclinical murine models of IPF, bleomycin- and radiation-induced pulmonary fibrosis. LYCAT mRNA expression in PBMCs directly and significantly correlated with diffusing capacity of carbon monoxide (DlCO) and pulmonary function values as well as survival. In bleomycin- and radiation-induced pulmonary fibrosis murine models, hLYCAT overexpression reduced several indices of lung fibrosis, whereas conversely, down-regulation of native LYCAT expression (siRNA) accentuated fibrogenesis. In vitro studies supported that LYCAT overexpression suppressed bleomycin-mediated mitochondrial membrane potential, reactive oxygen species (ROS) generation, and apoptosis of alveolar epithelial cells suggesting a potential mechanism of LYCAT-mediated protection against lung inflammation and fibrosis. Preliminary results of this study have been previously reported in the form of abstracts (22, 23).
Methods
For details regarding materials and methods, see the online supplement.
Microarray Profiling and Analysis
The Affymetrix Human Exon 1.0 ST array (Affymetrix, Inc., Santa Clara, CA) (exon array) was used to profile whole-genome expression in a cohort of 45 patients with IPF. Briefly, the sample preparation and RNA isolation were based on standard molecular biology protocols. The labeling, microarray hybridization was performed at the University of Chicago Genomics Core Facility according to the manufacturer’s instructions. The exon array data were then normalized and summarized using the Affymetrix Power Tools. Before summarizing gene-level expression data, probes containing known polymorphisms (based on dbSNP v131) were removed as described previously (24). The log2 transformed gene-level (i.e., transcript clusters) expression data were then evaluated for differential expression using Significance Analysis of Microarray (25). Gene annotations were obtained from the Affymetrix NetAffy Analysis Center (http://www.affymetrix.com/). The microarray data have been deposited in the NCBI Gene Expression Omnibus (Accession Number: GSE38958) (17).
Human Subjects
The diagnosis of IPF was established based on American Thoracic Society/European Respiratory Society criteria, which is consistent with recent guidelines. Subjects were followed by physicians according to institutional practices. Pulmonary function tests, chest computed tomography, and lung biopsies were performed when clinically indicated. Patients had no evidence of autoimmune syndromes, malignancy, infections, or drug or occupational exposures associated with lung fibrosis. All patient information was maintained in a relational database. The study was approved by the institutional review board at each center and informed consent was obtained from all the patients before blood draw. Demographic and clinical characteristics of the patient cohorts are provided in Table E2.
Prioritization of Genes Deregulated by Intragenic Polymorphisms
We prioritized deregulated genes associated with IPF using a reanalysis of the GWAS at the gene level as opposed to SNP level, followed by mRNA expression validation (see Methods in the online supplement).
Statistical Analysis
Two-way analysis of variance (ANOVA) was used to compare the means of data from two or more different experimental groups. If significant difference was present by ANOVA (P < 0.05), a least significant differences test was performed post hoc. Subsequently, differences between groups were considered statistically significant when P values were less than 0.05. In some experiments data were subjected to statistical analysis using one-way ANOVA or two-tailed Student t test or Spearman correlation test. P values less than 0.05 were considered significant. Survival analysis was performed using the Cox proportional hazards model, implemented in the survival library in the R Statistical Package. A log-rank test P value less than 0.05 was deemed significant.
Results
LYCAT mRNA Expression Levels in PBMCs Directly Correlate with Lung Function and Survival in IPF
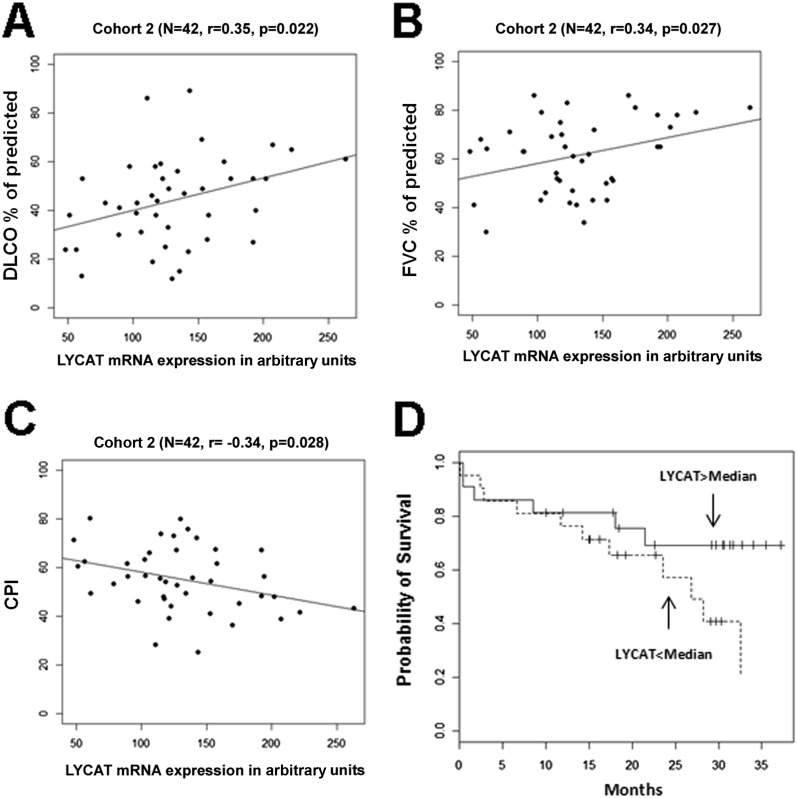
Our initial studies evaluated LYCAT expression in PBMC mRNA using the Affymetrix platform in two separate cohorts (n = 22 and n = 45) as previously reported (26). Patient characteristics are reported in Table E2. Compared with a cohort of normal healthy control subjects (n = 45), the mRNA levels of LYCAT were consistently lower in both IPF cohorts (P < 0.01; data not shown). To further elucidate a potential link between LYCAT mRNA levels in PBMCs and indices of lung function, LYCAT expression with DlCO% of predicted, FVC% of predicted, composite physiologic index and survival were correlated. Reduced LYCAT mRNA levels directly correlated with DlCO% predicted and FVC% predicted, indices of IPF severity (Figures 1A and 1B; see Figures E1A and E1B), and exhibited an inverse correlation with the composite physiologic index (Figure 1C; see Figure E1C). KM plot of high versus low LYCAT expression by Cox hazards regression analysis showed a significant probability of greater survival when LYCAT PBMC expression is greater than the median values (Figure 1D). These results clearly demonstrated a functional link of LYCAT mRNA expression to DlCO% predicted, FVC% predicted, composite physiologic index, and probability of survival of studied IPF cohorts.
Figure 1.
Expression of lysocardiolipin acyltransferase (LYCAT) correlates to the severity and survival of patients with idiopathic pulmonary fibrosis (IPF). (A–C) Expression of LYCAT in arbitrary units as a function of diffusing capacity of carbon monoxide (DlCO) % predicted (A), FVC% percent predicted (B), and composite physiologic index (CPI) (C) in patients with IPF (IPF cohort 2). Each point represents the value from one IPF patient (n = 42). The P value for each parameter is measured by Spearman rank correlation test. (D) Kaplan-Meier curves for patients with IPF with high and low levels of LYCAT expression. Patients were classified as having a high-level or a low-level mRNA expression, with the median of the expression across all the patients as the threshold value (LYCAT expression > median, n = 23; LYCAT expression < median, n = 22). Expression levels were measured using the Affymetrix Human Exon 1.0 ST Array. The P values are measured by log-rank test.
LYCAT Expression Is Increased in Lungs from Patients with IPF and in Murine Models of Fibrosis
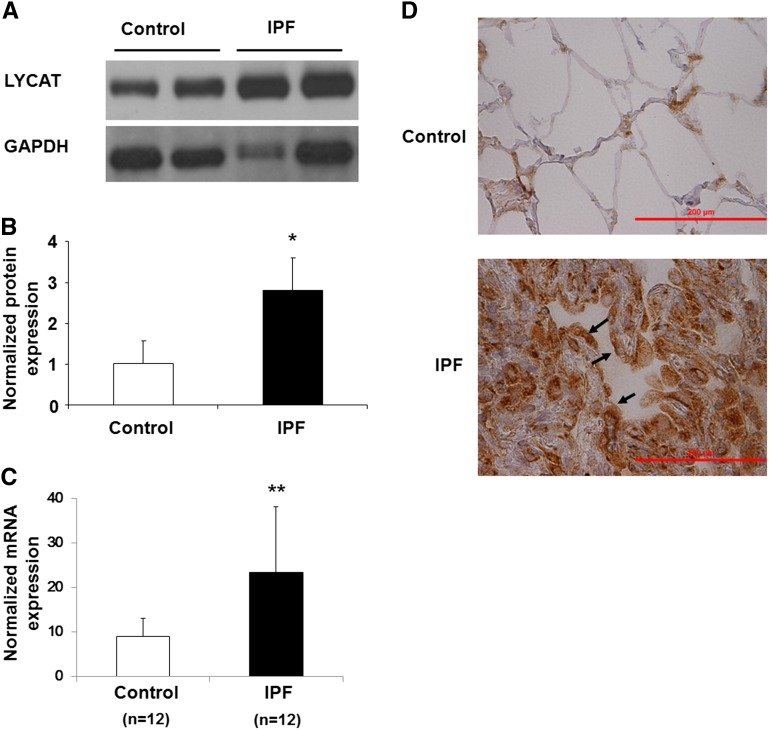
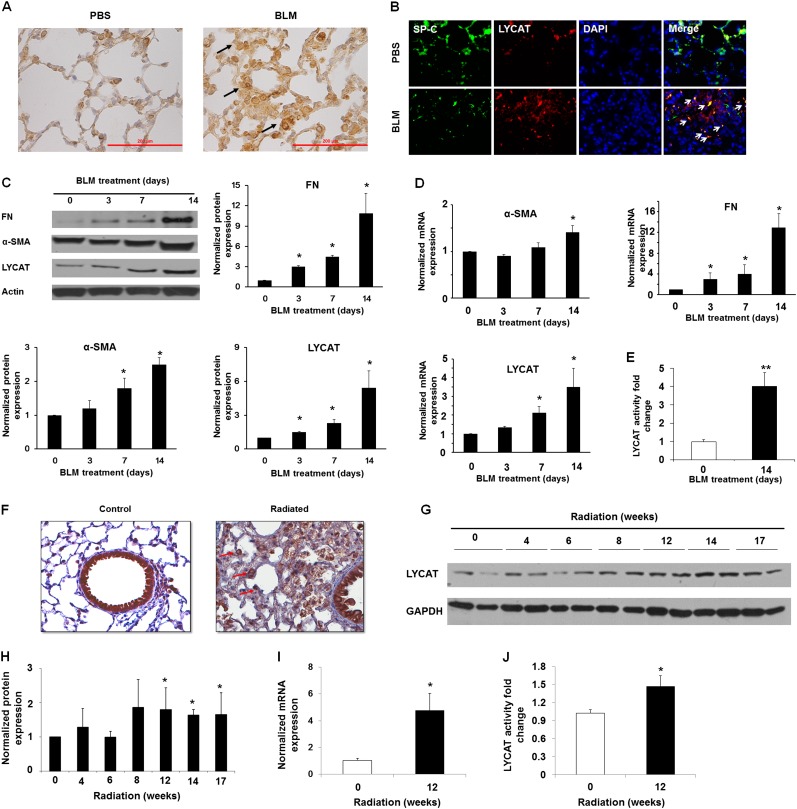
To investigate the potential role of LYCAT in pulmonary fibrosis, we examined frozen lung tissues from control subjects and human patients with IPF obtained from the Lung Tissue Research Consortium (Funded by NHLBI Lung Tissue Consortium, University of Colorado Health Sciences Center, Denver, CO) and bleomycin- and radiation-induced murine models of lung fibrosis. Both LYCAT mRNA and protein expression levels were increased (∼ threefold compared with control subjects) in IPF lung homogenates (Figures 2A–2C) and immunostaining of paraffin-embedded lung tissues revealed increased LYCAT immunoreactivity in IPF specimens compared with control subjects (Figure 2D). To further validate enhanced LYCAT expression in pulmonary fibrosis, two murine models of pulmonary fibrosis and lung injury were used. Direct airway instillation of bleomycin produces several key features of inflammation and lung fibrosis shared by mammalian IPF lungs; however, because bleomycin represents an acute lung inflammatory-fibrotic model, we complemented these studies with the preclinical model of radiation-induced pneumonitis and pulmonary fibrosis that replicates chronic fibrosis observed in mammalian IPF lung tissues (27). Bleomycin challenge induced LYCAT expression in alveolar epithelial cells as evidenced by colocalization with surfactant protein (SP)-C (Figures 3A and 3B) and increased LYCAT protein expression in lung tissue homogenates (Day 3–14) with concomitant up-regulation of α-smooth muscle actin (α-SMA) and fibronectin (FN) (Figure 3C), markers of myofibroblast differentiation (28, 29). Immunohistochemistry of lung tissues also showed enhanced colocalization of SP-C with α-SMA and LYCAT with α-SMA (see Figures E2A and E2B). The transcriptional up-regulation of LYCAT, FN, and α-SMA by bleomycin was confirmed by real-time reverse-transcriptase polymerase chain reaction (Figure 3D). LYCAT activity, as determined by conversion of monolysocardiolipin to cardiolipin, was enhanced in bleomycin-challenged lungs (Figure 3E). In contrast to the bleomycin model, increases in thoracic radiation-induced LYCAT expression in lung epithelial cells were delayed (Figures 3F–3I) (8–17 wk postirradiation). In vitro studies of LYCAT activity indicated a significant increase of LYCAT enzymatic activity in radiated lungs from mice (Figure 3J).
Figure 2.
Lysocardiolipin acyltransferase (LYCAT) is up-regulated in lung tissues obtained from patients with idiopathic pulmonary fibrosis (IPF). (A) Protein level of LYCAT in human lung tissue from IPF and control patients. The Western blot indicated the expression of LYCAT in lung tissue from the representative patients. (B) Normalized protein expression of LYCAT in human lung tissue. The intensity of each band after immunostaining with anti-LYCAT antibody was quantified and normalized with glyceraldehyde phosphate dehydrogenase (GAPDH). Data are expressed as mean ± SD, *P < 0.05 compared with control, n = 8 per group. (C) mRNA level of LYCAT in human lung tissue from IPF and control patients, and the mRNA level was quantified and normalized with GAPDH. Data are expressed as mean ± SD, **P < 0.01 compared with control, n = 12 per group. (D) Immunohistochemical staining was performed to determine the expression and localization of LYCAT in lung tissues from control subject and patients with IPF. Arrows indicate increased LYCAT expression in IPF specimens compared to control subjects. Scale bars = 200 μm. Experiments were performed twice.
Figure 3.
Lysocardiolipin acyltransferase (LYCAT) is up-regulated in lung tissues obtained from bleomycin (BLM)- and radiation-challenged mice. (A) Immunohistochemical staining with anti-LYCAT antibody was performed to determine the expression and localization of LYCAT in lung tissue from BLM-challenged mice. Arrows indicate increased LYCAT expression in mouse lung tissue treated with BLM. Scale bars = 200 μm. Experiments were performed twice. (B) Costaining of surfactant protein (SP)-C (green), LYCAT (red), DAPI (blue), and merge (yellow) in lung tissue from mice with phosphate-buffered saline (PBS) or BLM challenge. Arrows indicate increased LYCAT expression in lung epithelial cells after BLM treatment. The expression was examined by immunofluorescence microscopy using ×60 oil objective. (C) Protein level of LYCAT, fibronectin (FN), and α-smooth muscle actin (α-SMA). (D) mRNA level of LYCAT, FN, and α-SMA. (E) Fold change of LYCAT activity in lung tissue from BLM-challenged mice. Data are expressed as mean ± SEM, *P < 0.05, **P < 0.01 compared with BLM challenge Day 0, n = 5 per group. (F) Immunohistochemical staining was performed to determine the expression and localization of LYCAT in lung tissue from radiation-challenged mice (17 wk). Experiments were performed twice. Arrows indicate increased LYCAT expression in lung epithelial cells after radiation. Original magnification ×20. (G) Representative Western blot of LYCAT. (H) normalized LYCAT expression. (I) normalized mRNA level of LYCAT. (J) LYCAT activity in lung tissue from radiation-challenged mice (14 wk post-challenge). Data are expressed as mean ± SEM (n = 5). *P < 0.05 compared with mice without radiation. GAPDH = glyceraldehyde phosphate dehydrogenase.
LYCAT Overexpression Reduces Bleomycin- and Radiation-induced Lung Fibrosis in Mice
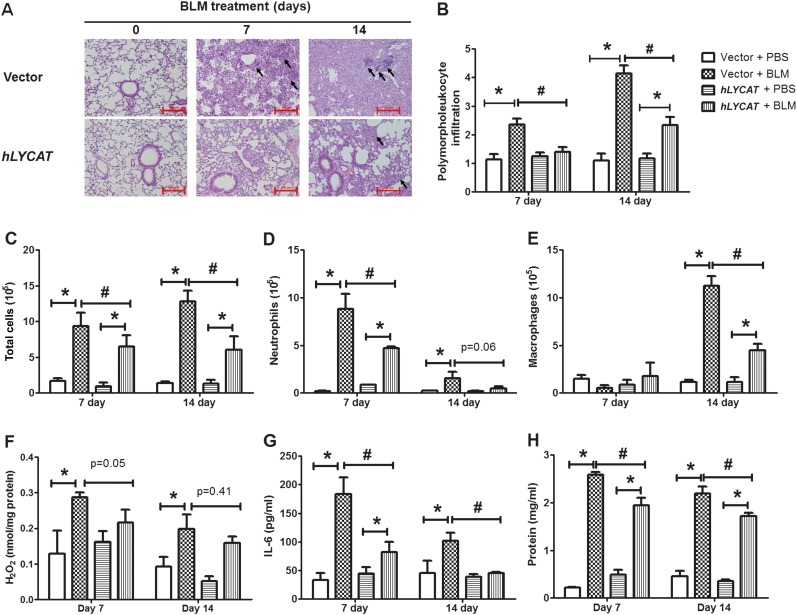
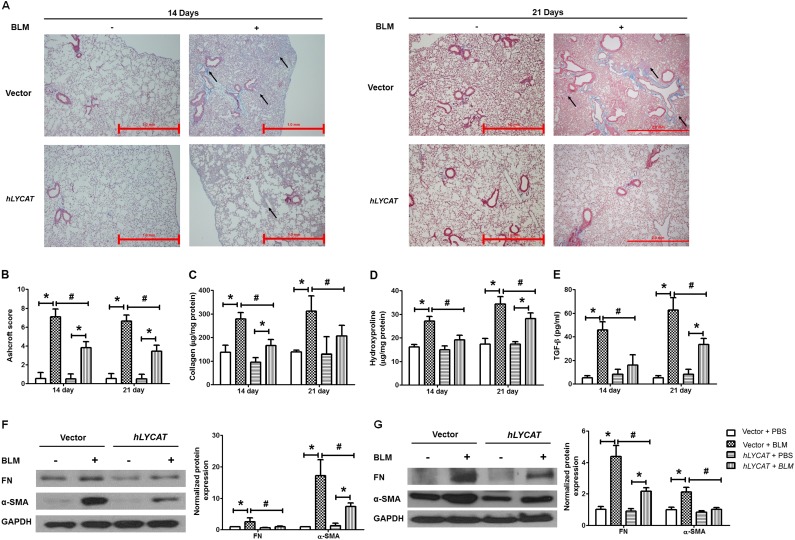
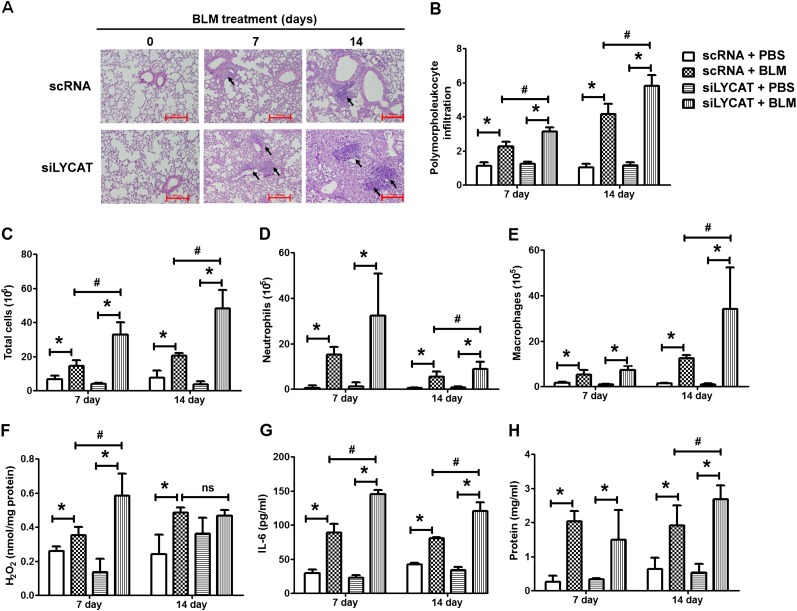
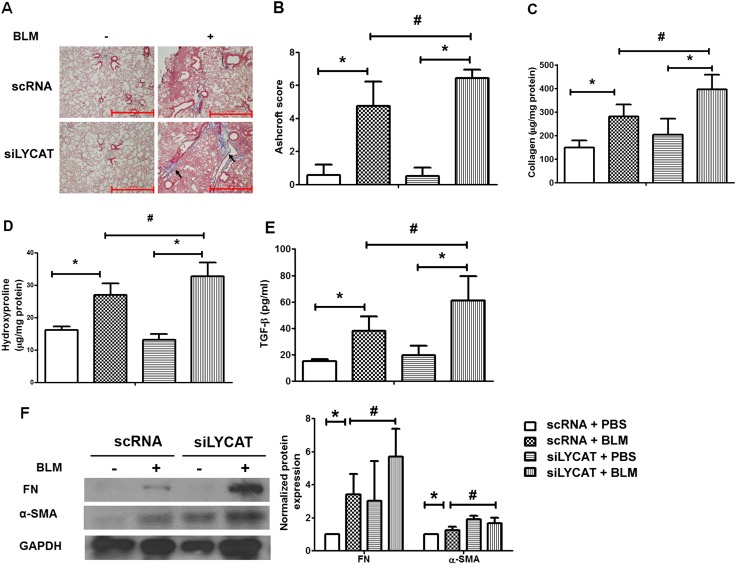
To determine the pathophysiologic role of increased LYCAT expression in vivo on fibrosis, LYCAT was overexpressed in mouse lungs by intratracheal instillation of a flag-tagged hLYCAT cDNA vector. The hLYCAT transgene was administered 3 days before bleomycin challenge followed by additional instillations once in 3 days post-bleomycin challenge. In the radiation model, the hLYCAT transgene was instilled after 7 weeks of post-radiation with additional instillations once in 3 days up to 17 weeks (see Figure E3A). Tracheal instillation of the hLYCAT transgene effectively overexpressed hLYCAT mRNA in lungs, which peaked on Day 3 and on Day 7 its mRNA expression was reduced significantly (see Figures E3D and E3E). The overexpressed Flag-tagged hLYCAT protein colocalized with SP-C (alveolar epithelial cells) as determined by immunohistochemistry (see Figures E3B and E3C). Bleomycin challenge predictably and significantly increased lung injury as evaluated by hematoxylin and eosin staining, a finding that was significantly ameliorated by hLYCAT overexpression (Figure 4A). Furthermore, bleomycin-induced inflammation as assessed by levels of ROS, infiltrating polymorphonuclear leukocytes, IL-6, and total protein in bronchoalveolar lavage (BAL) fluids were reduced in mice overexpressing hLYCAT compared with control subjects (Figures 4B–4H) despite the lack of tumor necrosis factor-α alterations (data not shown). Additionally, the effect of overexpression of hLYCAT on bleomycin-induced fibrosis was evaluated. As shown in Figure 5A, hLYCAT overexpression also reduced bleomycin-induced pulmonary fibrosis as determined by Masson trichrome staining for collagen and acid-soluble total collagen in whole lungs on Days 14 and 21 post-bleomycin challenge. Histopathologic quantification of pulmonary fibrosis (Ashcroft score) showed significant protection against pulmonary fibrosis conferred by increased hLYCAT expression (Figure 5B) with reduced collagen, hydroxyproline, transforming growth factor (TGF)-β, FN, and α-SMA expression on Days 14 and 21 post-bleomycin challenge (Figures 5C–5G). These data suggested that LYCAT expression represented a protective response to bleomycin-induced pulmonary fibrosis, observations confirmed in the radiation-mediated murine pulmonary fibrosis model. Mice receiving increased hLYCAT expression exhibited reduced lung injury, decreased infiltrating BAL polymorphonuclear leukocytes, BAL total protein, hydroxyproline, and collagen deposition post-radiation exposure (see Figures E4A–E4F). Additionally, downregulation of LYCAT expression in mouse lung by instillation of LYCAT siRNA augmented pulmonary fibrosis and lung injury mediated by either bleomycin or radiation (Figures 6 and 7; see Figures E5 and E6). These results provided strong evidence that expression of LYCAT regulated pulmonary lung injury and fibrosis in murine models of bleomycin- and radiation-induced pulmonary fibrosis.
Figure 4.
Overexpression of lysocardiolipin acyltransferase (LYCAT) attenuates bleomycin (BLM)-mediated inflammation and injury in mouse lung. C57BL/6J wild-type (male, 8 wk) receiving vector or hLYCAT plasmid were challenged with BLM (1.5 U/kg in 50 μl of phosphate-buffered saline [PBS]) or PBS intratracheally, and were killed on Day 0, 7, and 14, postadministration. Lungs were lavaged by PBS solution, and the bronchoalveolar lavage (BAL) fluids were analyzed as described in Methods. (A) Representative hematoxylin and eosin photomicrographs of lung sections obtained from wild-type mice receiving vector or hLYCAT plasmid at different time point post-BLM challenge (black arrows showing injury area). Original magnification ×10; scale bar = 200 μm. (B) Quantification of polymorphonuclear leukocytes infiltration in the lung tissue, (C) total cell number, (D) total neutrophils, (E) total macrophages, (F) H2O2 levels in BAL were normalized to total protein in BAL fluids, (G) IL-6 levels, and (H) total protein levels in BAL fluids. Data are expressed as mean ± SEM (n = 4–6). *P < 0.05; #P < 0.05.
Figure 5.
Overexpression of lysocardiolipin acyltransferase (LYCAT) attenuates bleomycin (BLM)-mediated fibrogenesis in mouse lung. (A) Representative images of trichrome staining of lung sections obtained from wild-type mice receiving vector or hLYCAT plasmid at different time point post-BLM challenge (arrows showing blue of collagen deposition area). Left panel, 14 days; right panel, 21 days post-BLM challenge. Original magnification ×4; scale bar = 1 mm. (B) Ashcroft score of the lung sections, (C) acid-soluble collagen in lung tissue was normalized to milligram protein of lung tissue lysates, (D) hydroxyproline content in lung tissue was normalized to milligram protein of lung tissue lysates, (E) transforming growth factor (TGF)-β concentration in bronchoalveolar lavage fluids, and (F and G) protein levels of α-smooth muscle actin (α-SMA) and fibronectin (FN) in lung tissue from mice receiving vector or hLYCAT plasmid at (F) 14 days and (G) 21 days post-BLM challenge. Data are expressed as mean ± SEM (n = 4–6). *P < 0.05; #P < 0.05. GAPDH = glyceraldehyde phosphate dehydrogenase.
Figure 6.
Reduced lysocardiolipin acyltransferase (LYCAT) expression accentuates bleomycin (BLM)-mediated inflammation and injury in mouse lung. C57BL/6J wild-type (male, 8 wk) receiving control (scRNA) or lycat siRNA (siLYCAT) were challenged with BLM (1.5 U/kg in 50 μl of phosphate-buffered saline [PBS]) or PBS intratracheally, and animals were killed on Day 0, 7, and 14, postadministration. Lungs were lavaged by PBS solution, and the brochoalveolar lavage (BAL) fluids were analyzed as described in Methods. (A) Representative hematoxylin and eosin photomicrographs of lung sections obtained from mice receiving scRNA or siLYCAT at different time point post-BLM challenge (arrows showing injury area). Original magnification ×10; scale bar = 200 μm. (B) Quantification of the polymorphonuclear infiltration in the lung tissue post-BLM challenge from the wild-type mice receiving scRNA or siLYCAT. (C) Total cell number, (D) total neutrophils, (E) total macrophages, (F) H2O2 levels in BAL were normalized to total protein in BAL fluids, (G) IL-6 levels, and (H) total protein levels in BAL fluids are expressed as mean ± SEM (n = 4–6). *P < 0.05; #P < 0.05.
Figure 7.
Reduced lysocardiolipin acyltransferase (LYCAT) expression accentuates bleomycin (BLM)-mediated fibrogenesis in mouse lung. (A) Representative images of trichrome staining of lung sections obtained from wild-type mice receiving scRNA or siLYCAT at Day 14 post-BLM challenge (arrows showing blue of collagen deposition area). (B) Ashcroft score of the lung sections, (C) acid-soluble collagen in lung tissue was normalized to milligram protein of lung tissue lysates, (D) hydroxyproline content in lung tissue was normalized to milligram protein of lung tissue lysates, (E) transforming growth factor (TGF)-β concentration in brochoalveolar lavage fluids, and (F) protein levels of α-smooth muscle actin (α-SMA) and fibronectin (FN) in lung tissue from mice receiving scRNA or siLYCAT at Day 14 post-BLM challenge. Data are expressed as mean ± SEM (n = 4–6). *P < 0.05; #P < 0.05. GAPDH = glyceraldehyde phosphate dehydrogenase; PBS = phosphate-buffered saline.
LYCAT Overexpression Attenuates Bleomycin-induced Apoptosis and Mitochondrial ROS Production and Restores Membrane Potential in Alveolar Epithelial Cells
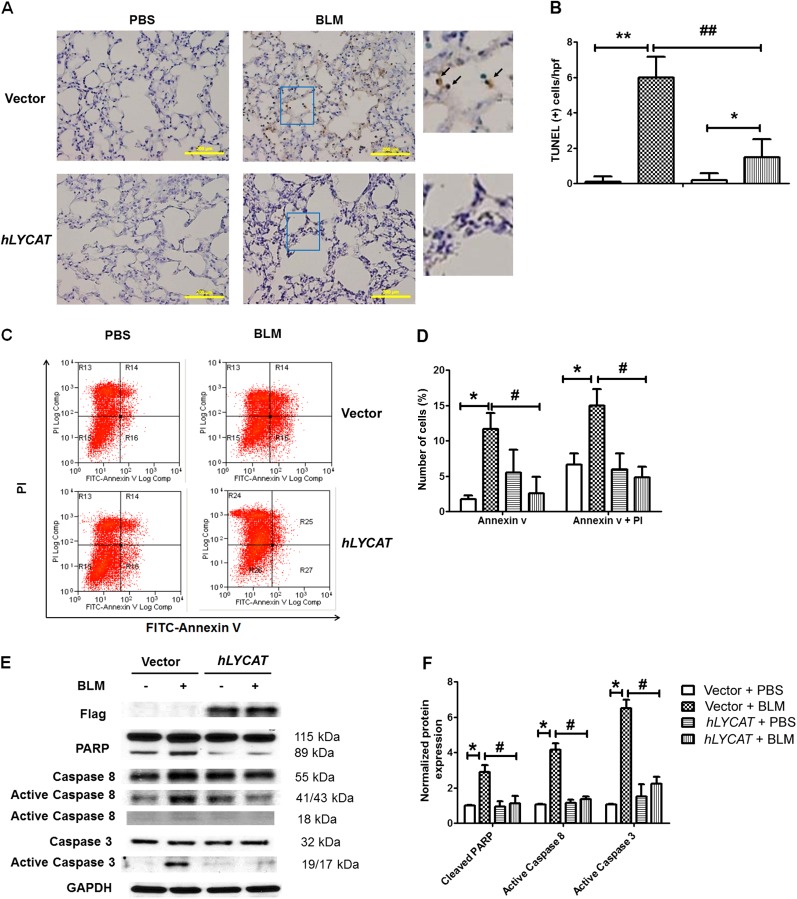
Alveolar epithelial injury is implicated in the pathogenesis of pulmonary fibrosis via release of mediators that activate fibroblasts and accelerate myofibroblast transformation via TGF-β–dependent signaling (30, 31). To define mechanisms of LYCAT-mediated protection against pulmonary fibrosis, we investigated the effect of overexpression of LYCAT on epithelial cell apoptosis. Analysis of mouse lung tissues for in vivo apoptosis within fibrotic foci revealed a significant reduction in TUNEL-positive staining in alveolar epithelial cells after overexpression of hLYCAT compared with control lungs (Figures 8A and 8B). We next examined in vitro responses to hLYCAT expression by using a MLE12 alveolar epithelial cell line. Consistent with the in vivo observation, overexpression of hLYCAT significantly reduced bleomycin-induced apoptosis as evidenced by reduced numbers of annexin-positive MLE12 cells as compared with control cells without hLYCAT overexpression (Figures 8C and 8D). Additionally, PARP degradation and cleavage of caspase 3 and 8 to lower-molecular-weight fractions, processes reflecting cellular apoptosis, were significantly reduced by hLYCAT overexpression in MLE 12 cells (Figures 8E and 8F).
Figure 8.
Lysocardiolipin acyltransferase (LYCAT) overexpression protects bleomycin (BLM)-induced apoptosis in lung alveolar epithelial cells. (A) Representative TUNEL-stained lung sections of alveolar epithelium area from BLM-challenged mice receiving vector or hLYCAT plasmid at Day 7 post-BLM challenge (1.5 U/kg). Right panel enlarged from the insert, and arrows indicated the TUNEL+ cells (brown). Scale bars = 200 μm. (B) Mean numbers of TUNEL+ cells per high-power field ± SEM in lung sections of mice receiving vector or hLYCAT plasmid on Day 7 post-BLM challenge (n = 5). (C and D) Overexpression of hLYCAT attenuated BLM-induced apoptosis in MLE12 cells. (C) Flow cytometry analysis of annexin V and propidium iodide (PI) dual staining of apoptotic MLE12 cells after overexpression of hLYCAT and BLM (10 mU/ml, 24 h) challenge. (D) Quantification of apoptotic cell post-BLM challenge. (E and F) Overexpression of hLYCAT attenuated BLM (10 mU/ml, 24 h) induced cleave of PARP and activation of caspase 3 and 8 in MLE12 cells. (E) Representative Western blot (F) quantification of BLM-induced cleavage of PARP, activation of caspase 8 and caspase 3 in MLE12 cells with and without overexpression of hLYCAT. *P < 0.05, **P < 0.01; #P < 0.05, ##P < 0.01. n = 4 per group. GAPDH = glyceraldehyde phosphate dehydrogenase; PBS = phosphate-buffered saline.
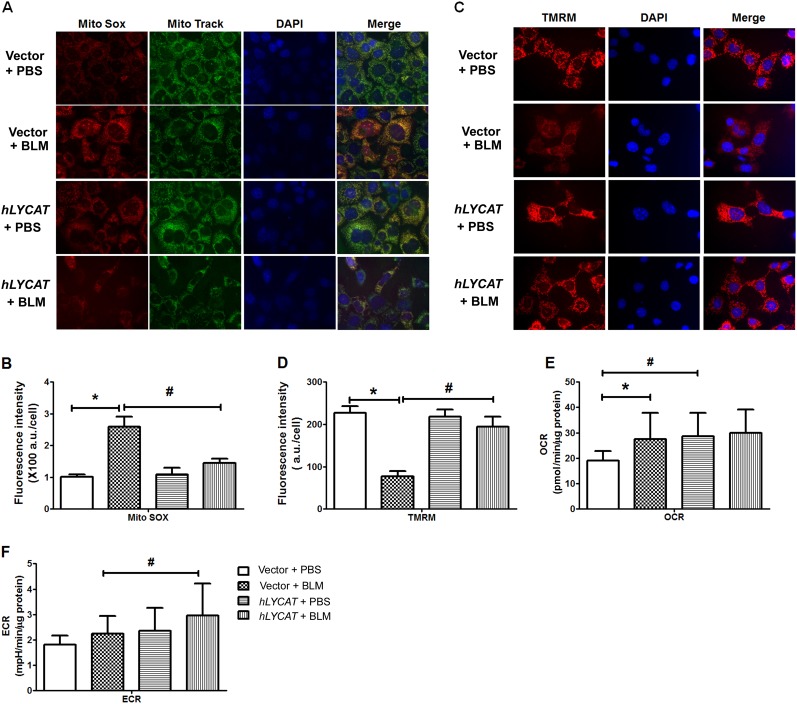
Because both bleomycin and radiation challenge increase ROS production and cellular oxidative burden (20), we next examined the effect of hLYCAT overexpression on total ROS and mitochondria-derived ROS production in epithelial cells. As expected, bleomycin challenge of MLE 12 cells increased total cellular ROS (data not shown) and mitochondrial ROS generation as evidenced by increased Mito-SOX fluorescence, which colocalized with the Mito-Tracker (Figures 9A and 9B), and overexpression of hLYCAT attenuated bleomycin-induced mitochondrial ROS content (Figures 9A and 9B). Next, the effect of hLYCAT on bleomycin-induced changes in mitochondrial membrane potential was investigated using tetramethylrhodamine methyl ester. As shown in Figures 9C and 9D, overexpression of hLYCAT restored bleomycin-mediated reduction of mitochondrial membrane potential to a level similar to that observed in control cells. To determine whether the suppression of mitochondrial ROS by LYCAT was caused by a general reduction in mitochondrial oxygen consumption, we assessed the oxygen consumption rate (OCR) and the extracellular acidification rate (ECAR; a surrogate measure for real-time extracellular lactate production) in epithelial cells exposed to bleomycin, with and without hLYCAT overexpression. Bleomycin caused a significant increase in mitochondrial oxygen consumption (control vs. bleomycin, P < 0.05; Figure 9E). Interestingly, LYCAT expression itself did not suppress mitochondrial oxygen consumption, but actually increased the OCR at basal conditions (control vs. hLYCAT, P < 0.05; Figure 9E). However, this higher baseline OCR in LYCAT overexpressing cells was not further affected by bleomycin treatment (Figure 9E). We also concomitantly assessed ECAR and found that LYCAT expression itself showed a trend toward higher ECAR, but this was not statistically significant (Figure 9F). These results further supported a protective role for hLYCAT overexpression in bleomycin-induced apoptosis in lung epithelium.
Figure 9.
Lysocardiolipin acyltransferase (LYCAT) overexpression attenuates bleomycin (BLM)-induced mitochondrial superoxide generation and mitochondrial membrane potential in MLE12 cells. MLE12 cell transfected with vector or hLYCAT plasmids were challenged with BLM (10 mU/ml, 24 h), and mitochondrial superoxide generation and mitochondrial membrane potential was measured as described in Methods. (A) Shown are representative images from three independent experiments. MitoSOX (red), MitoTracker (green), nuclear (blue), and merged image (yellow). (B) Quantification of mitochondrial superoxide production induced by BLM in MLE12 cells with and without hLYCAT overexpression. Data are expressed as mean ± SEM. (C) Shown are representative images of BLM-induced mitochondrial membrane potential from three independent experiments. Tetramethylrhodamine methyl ester (TMRM) (red) and DAPI (blue). (D) Quantification of TMRM fluorescence intensity induced by BLM in MLE12 cells with and without hLYCAT overexpression. Data are expressed as mean ± SEM (n = 4). (E and F) Effects of overexpression of LYCAT on oxygen consumption in MLE12 cells challenged with BLM. The quantification of oxygen consumption rate (OCR) (E) and extracellular acidification rate (ECAR) (F) were displayed as mean ± SD from three independent experiments (n = 3). *P < 0.05; #P < 0.05. PBS = phosphate-buffered saline.
LYCAT Overexpression Restores Bleomycin-induced Changes in Cardiolipin Fatty Acid Profile in Mouse Lungs
Because cardiolipin remodeling by LYCAT is believed to play an important role in repairing mitochondrial damage from oxidative stress, we investigated the role of hLYCAT overexpression in modulating the fatty acid composition of cardiolipin in mouse lungs before and after bleomycin challenge. Bleomycin challenge significantly decreased the cardiolipin level by approximately 50% and mol% of C18:1 and C18:2 fatty acids in mouse lung than those of control group (Table 1). In contrast to C18:1 and C18:2, the mol% of C18:0 was higher in mouse lung of bleomycin-challenged animals. Notably, the unsaturated to saturated fatty acid ratio and unsaturation index were lower in bleomycin-challenged animals indicating loss of unsaturated fatty acids, especially C18:1 and C18:2 fatty acids in the cardiolipin. Importantly, overexpression hLYCAT in mouse lung restored the cardiolipin and fatty acid levels similar to control animals without the bleomycin challenge. Furthermore, overexpression of hLYCAT also restored the unsaturated to saturated fatty acid ratio and unsaturation index in bleomycin-treated animals (Table 1). These results suggested remodeling of cardiolipin by hLYCAT in bleomycin-challenged mouse lung.
Table 1:
Cardiolipin Fatty Acid Composition and Levels in Lungs of Wild-Type and LYCAT-overexpressed Mice Treated with PBS and Bleomycin
| Fatty Acid | Vector + PBS |
Vector + Bleomycin |
hLYCAT + PBS |
hLYCAT + Bleomycin |
||||
|---|---|---|---|---|---|---|---|---|
| nmol/mg protein | mol % | nmol/mg protein | mol % | nmol/mg protein | mol % | nmol/mg protein | mol % | |
| C14:0 | 1.40 ± 0.02 | 4.60 ± 0.58 | 1.30 ± 0.66 | 9.00 ± 3.65 | 2.00 ± 0.68 | 6.40 ± 0.82 | 1.50 ± 0.55 | 4.80 ± 1.54 |
| C16:0 | 10.00 ± 0.97 | 33.00 ± 1.36 | 4.50 ± 1.20* | 31.00 ± 4.80 | 10.00 ± 2.50 | 33.00 ± 0.98 | 11.00 ± 1.80† | 37.00 ± 3.60 |
| C16:1 | 0.90 ± 0.17 | 2.90 ± 0.16 | 0.10 ± 0.11* | 0.90 ± 0.88 | 0.90 ± 0.03 | 3.00 ± 0.51 | 0.70 ± 0.32 | 2.00 ± 0.52 |
| C18:0 | 5.00 ± 0.85 | 16.00 ± 0.61 | 3.30 ± 0.08 | 24.00 ± 3.36* | 6.00 ± 1.17 | 19.00 ± 0.25 | 3.50 ± 0.42 | 12.00 ± 0.63† |
| C18:1 | 10.00 ± 1.50 | 33.00 ± 0.42 | 3.70 ± 0.03* | 26.00 ± 3.30* | 8.80 ± 1.63 | 29.00 ± 0.25 | 9.40 ± 1.10† | 32.00 ± 5.50† |
| C18:2 | 3.40 ± 0.69 | 11.08 ± 0.75 | 1.20 ± 0.01* | 9.00 ± 0.93* | 3.20 ± 0.63 | 10.00 ± 0.20 | 3.90 ± 0.06† | 13.00 ± 1.06† |
| Total fatty acids (nmol/mg protein) | 30.20 ± 4.21 |
14.20 ± 1.66* |
31.00 ± 6.63 |
30.00 ± 1.95† |
||||
| Total cardiolipin (nmol/mg protein) | 7.55 ± 1.05 |
3.50 ± 0.40* |
7.70 ± 1.65 |
7.51 ± 0.49† |
||||
| USR | 0.87 |
0.56 |
0.72 |
0.88 |
||||
| USI | 0.58 | 0.44 | 0.52 | 0.60 | ||||
Definition of abbreviations: C14:0 = myristic acid; C16:0 = palmitic acid; C16:1 = palmitoleic acid; C18:0 = stearic acid; C18:1 = oleic acid; C18:2 = linoleic acid; LYCAT = lysocardiolipin acyltransferase; PBS = phosphate-buffered saline; USI = unsaturation index; USR = unsaturated to saturated fatty acid ratio.
Lipid extraction, cardiolipin separation, and fatty acid analysis were done as described in the Methods. Each value is the average ± SD of three independent determinations. n = 3 per group.
Significantly different at P < 0.05 from the vector + PBS samples.
Significantly different from the vector + bleomycin samples.
Discussion
IPF is a highly debilitating disease with a life expectancy of only 3–5 years from the time of diagnosis and without available effective pharmacotherapies with the exception of lung transplantation. Therefore, there is a pressing need to identify novel therapeutic targets. In this context, using a combination of genetic and genomic strategies, we demonstrate for the first time a key role for the cardiolipin-remodeling enzyme, LYCAT, in inflammatory and fibrogenic responses using human and preclinical murine models of pulmonary fibrosis. Human studies revealed strong positive correlation between LYCAT expression in PBMCs from patients with IPF and lung function parameters, such as DlCO% predicted, FVC% predicted severity, and probability of survival. In contrast to decreased LYCAT mRNA expression in IPF PBMCs, LYCAT protein expression was elevated in lung tissues from patients with IPF, likely reflecting a beneficial response to the fibrotic process.
LYCAT or acyl-CoA:LYCAT (ALCAT1) is a key enzyme that has been implicated in remodeling the fatty acid composition of cardiolipin from saturated and monounsaturated to polyunsaturated (>60% linoleic acid) fatty acids in the mitochondria (32). Defective cardiolipin remodeling and loss of linoleic acid causes dilated cardiomyopathy in Barth syndrome, a genetic disorder characterized by mitochondrial dysfunction, growth retardation, and neutropenia (19, 33). We demonstrated here that overexpressed hLYCAT in mouse lung was actively involved in the remodeling of cardiolipin by restoring the fatty acid profiles of C18:1 and C18:2 unsaturated fatty acid profiles after bleomycin challenge (Table 1). In addition to remodeling of cardiolipin, lycat is involved in the development of hematopoietic and endothelial cell lineages in zebra fish (34, 35) and in vitro embryonic stem cell differentiation (36). In the current study, the pathophysiologic significance of enhanced LYCAT expression in pulmonary fibrosis and lung injury was evaluated using bleomycin and radiation models. In each model of lung fibrosis, immunohistochemical evaluation of lung sections revealed increased expression of LYCAT in alveolar epithelial cells with hLYCAT overexpression significantly reducing lung inflammation, ROS, total BAL protein content, IL-6 levels, and the burden of inflammatory cell infiltration into the alveolar space. Importantly, indices of lung fibrosis were similarly reduced, such as the deposition of extracellular matrix proteins, including collagen, hydroxyproline, and FN. In contrast, down-regulation of native LYCAT by siRNA strategies produced marked exacerbation of bleomycin- and radiation-induced fibrosis and lung injury, including collagen deposition. These results suggested that LYCAT expression was associated with a protective response to fibrogenic stimuli acting as a potential target to suppress pulmonary fibrogenesis and lung injury.
Although the etiology of IPF remain unknown, a variety of factors including TGF-β (37), matrix metalloproteinases (38), arachidonic acid metabolites (39), NOX4 (40), LPA/LPA1 and 2 (7, 30), sphingosine kinase/S1P signaling axis (10), β-arrestin (41), and caspase 1 (42) have all been implicated in the pathogenesis of pulmonary fibrosis and in inducing alveolar epithelial apoptosis and myofibroblast differentiation. Among these factors, increased ROS production resulting in oxidative stress seems to be an important molecular mechanism underlying the development of lung fibrosis (40). Importantly, in the current study LYCAT did not suppress mitochondrial oxygen consumption, but seemed to reduce mitochondrial ROS generation without compromising oxygen consumption. This suggested that LYCAT overexpression did not act as a general inhibitor of mitochondrial respiration but seemed to selectively suppress mitochondrial oxidant generation while increasing general mitochondrial oxidation. This increase in basal mitochondrial respiration with LYCAT seemed to prevent any acute bleomycin-induced increases in mitochondrial respiration and thus any acute bleomycin-induced oxidant generation. In addition to reducing bleomycin-induced mitochondrial ROS generation, hLYCAT overexpression in MLE-12 alveolar epithelial cells attenuated carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone (FCCP)-mediated mitochondrial ROS generation (see Figure E7) and bleomycin-induced mitochondrial depolarization (Figure 9C). LYCAT overexpression may therefore result in a metabolic priming of the cells that reduces their sensitivity to noxious mitochondrial stimuli, such as bleomycin challenge.
Excess generation of ROS via mitochondrial electron transport chain or Nox4 has been implicated in the development and progression of pulmonary fibrosis in bleomycin model and in human IPF (40). Furthermore, there is evidence with experimental animal models and human IPF that redox imbalance may be involved in the pathogenesis of pulmonary fibrosis (43). Decreased glutathione levels have been shown in IPF (44), and in vivo treatment with antioxidants or antioxidant enzymes has been shown to protect against bleomycin-induced lung damage and pulmonary fibrosis in animals (45–47). In this study, the expression of catalase, SOD2, and glutathione-dependent antioxidant enzymes, such as glutathione peroxidase 1 (GPX-1), and peroxiredoxins-3 (PRX-3) were determined. Although bleomycin challenge decreased the expression of catalase, GPX-1, PRX-3, and SOD2 in mouse lungs and MLE 12 cells, overexpression of hLYCAT did not significantly alter the expression of these antioxidant enzymes after bleomycin challenge (see Figure E8). However, in the absence of bleomycin, hLYCAT overexpression increased the expression of GPX-1 and SOD2 in mouse lungs and catalase, GPX-1, and PRX-3 in MLE 12 cells. Increased ROS production by bleomycin can lead to peroxidation of polyunsaturated fatty acids in lipids and lipid-derived free radicals, which have been observed in bleomycin-induced lung injury and pulmonary toxicity (48). Minimizing lipid peroxidation should be beneficial in reducing inflammation and fibrosis, and overexpression of hLYCAT significantly reduced accumulation of 4-hydroxynonenal–modified proteins in MLE 12 cells (see Figure E9). Further studies are necessary to link LYCAT expression and status of antioxidant and antioxidant enzyme expression in development and progression of lung inflammation and pulmonary fibrosis.
Another novel and interesting aspect of this study is the protective role of LYCAT against bleomycin-induced alveolar epithelial cell apoptosis as observed in in vivo and in vitro experiments. Emerging evidence supports the role of apoptosis in IPF and experimental pulmonary fibrosis (49–51). Of the three cell types in the fibrotic lung (alveolar epithelial, endothelial, and fibroblast) injury and apoptosis of alveolar epithelial cells contribute to fibrogenesis, whereas acquisition of an apoptosis-resistant myofibroblast phenotype contributes to progressive and irreversible fibrosis (52). Here we demonstrated that overexpression of hLYCAT in mouse lung and MLE 12 cells protected against bleomycin-induced alveolar epithelial cell apoptosis as evidenced by decrease in TUNEL positive, and annexin V (+)/PI (-) cells, and decreased caspase 3 and 8 activation and PARP cleavage. Furthermore, our results on caspase 3 and 8 activation suggested that bleomycin-mediated apoptosis of MLE 12 cells seemed to involve both intrinsic and extrinsic pathways. In fact, earlier studies have demonstrated the participation of both the intrinsic (mitochondrial) and extrinsic (Fas/FasL) pathways in bleomycin-induced apoptosis of lung epithelial cells (53, 54). Although the present study demonstrated a key role for LYCAT in conferring protection against bleomycin-induced ROS generation and apoptosis of alveolar epithelial cells, the action of LYCAT on conferring apoptosis resistance in myofibroblasts is unclear. Instillation of hLYCAT in mouse lung increased expression of hLYCAT protein in alveolar epithelial cells but not in fibroblasts and myofibroblasts. Overexpression of hLYCAT attenuated bleomycin-induced FN and α-SMA protein expression in the lung 14 and 21 days post-bleomycin challenge (Figures 5F and 5G) and TGF-β levels in BAL fluids. TGF-β is a potent profibrogenic cytokine that is known to induce apoptosis-resistant myofibroblast phenotype in vitro (55) and overexpression of hLYCAT in human lung fibroblasts modulated TGF-β–mediated FN and α-SMA expression (data not shown). Because mitochondrial ROS is known to activate latent TGF-β, and induce TGF-β gene expression and TGF-β signaling (56), the protection conferred by hLYCAT against bleomycin or radiation-induced pulmonary fibrosis could be most likely mediated at the alveolar epithelial and fibroblast cellular types and further investigations are required to address the role of hLYCAT in modulating the cross-talk between these two cells types and pulmonary fibrosis.
In summary, we present evidence for the first time that the expression of LYCAT, a cardiolipin-remodeling enzyme, was altered in human and murine models of fibrosis. In bleomycin- and radiation-induced preclinical fibrosis models, overexpression of hLYCAT conferred protection against lung fibrosis, whereas down-regulation of native LYCAT expression with siRNA exacerbated fibrogenesis. The mechanisms of hLYCAT-mediated protection included modulation of mitochondrial ROS generation and apoptosis in alveolar epithelial cells. Presumably, increased expression of LYCAT in IPF and animal models of pulmonary fibrosis indicated a protective cellular response to injury and fibrogenesis. Thus, modulation of LYCAT expression may offer a novel and promising therapeutic approach in minimizing or preventing further progression of pulmonary fibrosis.
Footnotes
Supported by National Institutes of Health grants P01 HL98050 (V.N. and J.G.N.G.), R01-GM094220 (J.R.), and HL099619 and HL101740 (I.N.) and by the Bernie Mac Foundation (J.G.N.G.) and Pulmonary Fibrosis Foundation (I.N.).
Author Contributions: V.N. and J.G.N.G. supervised the project. V.N., L.S.H., B.M., and J.G.N.G. designed experiments. L.S.H., B.M., A.H., and Y. Zhao performed animal experiments. L.S.H., P.V.U., and Y. Zhang performed in vitro experiments. L.S.H., E.V.B., S.R.K., T.O.G., and N.L.P. analyzed the cardiolipin in lung tissue. L.S.H. and B.M. analyzed data. T.Z., N.K., H.L., W.Z., S.-F.M., I.N., S.P.R., Y.A.L., and J.G.N.G. collected and analyzed the human patient samples. L.S.H., B.M., J.R., J.G.N.G., and V.N. wrote the manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201310-1917OC on April 29, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Selman M, King TE, Pardo A American Thoracic Society; European Respiratory Society; American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 2.Herazo-Maya JD, Kaminski N. Personalized medicine: applying 'omics' to lung fibrosis. Biomark Med. 2012;6:529–540. doi: 10.2217/bmm.12.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King TE., Jr Update in pulmonary medicine. Ann Intern Med. 1998;129:806–812. doi: 10.7326/0003-4819-129-10-199811150-00012. [DOI] [PubMed] [Google Scholar]
- 4.Baran CP, Fischer SN, Nuovo GJ, Kabbout MN, Hitchcock CL, Bringardner BD, McMaken S, Newland CA, Cantemir-Stone CZ, Phillips GS, et al. Transcription factor ets-2 plays an important role in the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:999–1006. doi: 10.1165/rcmb.2010-0490OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouros D, Antoniou KM. Current and future therapeutic approaches in idiopathic pulmonary fibrosis. Eur Respir J. 2005;26:693–702. doi: 10.1183/09031936.05.00145004. [DOI] [PubMed] [Google Scholar]
- 6.Hauber HP, Blaukovitsch M. Current and future treatment options in idiopathic pulmonary fibrosis. Inflamm Allergy Drug Targets. 2010;9:158–172. doi: 10.2174/187152810792231878. [DOI] [PubMed] [Google Scholar]
- 7.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 8.Huang LS, Berdyshev E, Mathew B, Fu P, Gorshkova IA, He D, Ma W, Noth I, Ma SF, Pendyala S, et al. Targeting sphingosine kinase 1 attenuates bleomycin-induced pulmonary fibrosis. FASEB J. 2013;27:1749–1760. doi: 10.1096/fj.12-219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang LS, Fu P, Patel P, Harijith A, Sun T, Zhao Y, Garcia JG, Chun J, Natarajan V. Lysophosphatidic acid receptor-2 deficiency confers protection against bleomycin-induced lung injury and fibrosis in mice. Am J Respir Cell Mol Biol. 2013;49:912–922. doi: 10.1165/rcmb.2013-0070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milara J, Navarro R, Juan G, Peiró T, Serrano A, Ramón M, Morcillo E, Cortijo J. Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax. 2012;67:147–156. doi: 10.1136/thoraxjnl-2011-200026. [DOI] [PubMed] [Google Scholar]
- 11.Oikonomou N, Mouratis MA, Tzouvelekis A, Kaffe E, Valavanis C, Vilaras G, Karameris A, Prestwich GD, Bouros D, Aidinis V. Pulmonary autotaxin expression contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol. 2012;47:566–574. doi: 10.1165/rcmb.2012-0004OC. [DOI] [PubMed] [Google Scholar]
- 12.Xaubet A, Fu WJ, Li M, Serrano-Mollar A, Ancochea J, Molina-Molina M, Rodriguez-Becerra E, Morell F, Rodríguez-Arias JM, Pereda J, et al. A haplotype of cyclooxygenase-2 gene is associated with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27:121–130. [PubMed] [Google Scholar]
- 13.Martinelli M, Ugolini G, Scapoli L, Rivetti S, Lauriola M, Mattei G, Rosati G, Montroni I, Manaresi A, Zattoni D, et al. The EGFR R521K polymorphism influences the risk to develop colorectal cancer. Cancer Biomark. 2010-2011;8:61–65. doi: 10.3233/DMA-2011-0826. [DOI] [PubMed] [Google Scholar]
- 14.Mushiroda T, Wattanapokayakit S, Takahashi A, Nukiwa T, Kudoh S, Ogura T, Taniguchi H, Kubo M, Kamatani N, Nakamura Y Pirfenidone Clinical Study Group. A genome-wide association study identifies an association of a common variant in TERT with susceptibility to idiopathic pulmonary fibrosis. J Med Genet. 2008;45:654–656. doi: 10.1136/jmg.2008.057356. [DOI] [PubMed] [Google Scholar]
- 15.Latsi P, Pantelidis P, Vassilakis D, Sato H, Welsh KI, du Bois RM. Analysis of IL-12 p40 subunit gene and IFN-gamma G5644A polymorphisms in Idiopathic Pulmonary Fibrosis. Respir Res. 2003;4:6. doi: 10.1186/1465-9921-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Noth I, Garcia JG, Kaminski N. A variant in the promoter of MUC5B and idiopathic pulmonary fibrosis. N Engl J Med. 2011;364:1576–1577. doi: 10.1056/NEJMc1013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Noth I, Zhang Y, Ma SF, Flores C, Barber M, Huang Y, Broderick SM, Wade MS, Hysi P, Scuirba J, et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1:309–317. doi: 10.1016/S2213-2600(13)70045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patil VA, Fox JL, Gohil VM, Winge DR, Greenberg ML. Loss of cardiolipin leads to perturbation of mitochondrial and cellular iron homeostasis. J Biol Chem. 2013;288:1696–1705. doi: 10.1074/jbc.M112.428938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Musatov A, Robinson NC. Susceptibility of mitochondrial electron-transport complexes to oxidative damage. Focus on cytochrome c oxidase. Free Radic Res. 2012;46:1313–1326. doi: 10.3109/10715762.2012.717273. [DOI] [PubMed] [Google Scholar]
- 21.Wang W, Ni L, Yu Q, Xiong J, Liu HC, Rosenwaks Z. Expression of the Lycat gene in the mouse cardiovascular and female reproductive systems. Dev Dyn. 2010;239:1827–1837. doi: 10.1002/dvdy.22300. [DOI] [PubMed] [Google Scholar]
- 22.Mathew B, Huang L, Noth I, Ma S-F, Kaminski N, Zhao Y, Wade MS, Berdyshev E, Siegler J, Jacobson JR, et al. Lysocardiolipin acyltransferase (LYCAT) is a novel candidate gene in radiation-induced pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:A6146. [Google Scholar]
- 23.Huang L, Mathew B, Fu P, Ma W, He D, Zhang W, Zhou T, Reddy S, Garcia JGN, Natarajan V. Lysocardiolipin acyltransferase (LYCAT) protects bleomycin-induced lung inflammation and fibrosis inmice. Am J Respir Crit Care Med. 2012;185:A5407. [Google Scholar]
- 24.Duan S, Zhang W, Bleibel WK, Cox NJ, Dolan ME. SNPinProbe_1.0: a database for filtering out probes in the Affymetrix GeneChip human exon 1.0 ST array potentially affected by SNPs. Bioinformation. 2008;2:469–470. doi: 10.6026/97320630002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herazo-Maya JD, Noth I, Richards T, Ma SF, Guardela BJ, Tseng GC, Lussier Y, Huang Y, Vij R, Lindell KO, et al. Peripheral blood mononuclear cells gene expression patterns predict survival in patients with idiopathic pulmonary fibrosis. Science Trans Med. 2013;5:205ra136. doi: 10.1126/scitranslmed.3005964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gorshkova I, Zhou T, Mathew B, Jacobson JR, Takekoshi D, Bhattacharya P, Smith B, Aydogan B, Weichselbaum RR, Natarajan V, et al. Inhibition of serine palmitoyltransferase delays the onset of radiation-induced pulmonary fibrosis through the negative regulation of sphingosine kinase-1 expression. J Lipid Res. 2012;53:1553–1568. doi: 10.1194/jlr.M026039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sappino AP, Schürch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990;63:144–161. [PubMed] [Google Scholar]
- 29.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122(6) Suppl:286S–289S. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- 30.Funke M, Zhao Z, Xu Y, Chun J, Tager AM. The lysophosphatidic acid receptor LPA1 promotes epithelial cell apoptosis after lung injury. Am J Respir Cell Mol Biol. 2012;46:355–364. doi: 10.1165/rcmb.2010-0155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Romestaing C, Han X, Li Y, Hao X, Wu Y, Sun C, Liu X, Jefferson LS, Xiong J, et al. Cardiolipin remodeling by ALCAT1 links oxidative stress and mitochondrial dysfunction to obesity. Cell Metab. 2010;12:154–165. doi: 10.1016/j.cmet.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chicco AJ, Sparagna GC. Role of cardiolipin alterations in mitochondrial dysfunction and disease. Am J Physiol Cell Physiol. 2007;292:C33–C44. doi: 10.1152/ajpcell.00243.2006. [DOI] [PubMed] [Google Scholar]
- 34.Xiong JW, Yu Q, Zhang J, Mably JD. An acyltransferase controls the generation of hematopoietic and endothelial lineages in zebrafish. Circ Res. 2008;102:1057–1064. doi: 10.1161/CIRCRESAHA.107.163907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patan S. Lycat and cloche at the switch between blood vessel growth and differentiation? Circ Res. 2008;102:1005–1007. doi: 10.1161/CIRCRESAHA.108.176446. [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Faloon PW, Tan Z, Lv Y, Zhang P, Ge Y, Deng H, Xiong JW. Mouse lysocardiolipin acyltransferase controls the development of hematopoietic and endothelial lineages during in vitro embryonic stem-cell differentiation. Blood. 2007;110:3601–3609. doi: 10.1182/blood-2007-04-086827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernandez IE, Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc. 2012;9:111–116. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 38.Dancer RC, Wood AM, Thickett DR. Metalloproteinases in idiopathic pulmonary fibrosis. Eur Respir J. 2011;38:1461–1467. doi: 10.1183/09031936.00024711. [DOI] [PubMed] [Google Scholar]
- 39.Kim KA, Park CY, Lim Y, Lee KH. Recent advances in particulate-induced pulmonary fibrosis; for the application of possible strategy experimentally and clinically. Curr Drug Targets. 2000;1:297–307. doi: 10.2174/1389450003349146. [DOI] [PubMed] [Google Scholar]
- 40.Crestani B, Besnard V, Boczkowski J. Signalling pathways from NADPH oxidase-4 to idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2011;43:1086–1089. doi: 10.1016/j.biocel.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Lovgren AK, Kovacs JJ, Xie T, Potts EN, Li Y, Foster WM, Liang J, Meltzer EB, Jiang D, Lefkowitz RJ, et al. β-arrestin deficiency protects against pulmonary fibrosis in mice and prevents fibroblast invasion of extracellular matrix. Sci Transl Med. 2011;3:74ra23. doi: 10.1126/scitranslmed.3001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu JF, Washko GR, Nakahira K, Hatabu H, Patel AS, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, et al. COPDGene Investigators. Statins and pulmonary fibrosis: the potential role of NLRP3 inflammasome activation. Am J Respir Crit Care Med. 2012;185:547–556. doi: 10.1164/rccm.201108-1574OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinnula VL, Myllärniemi M. Oxidant-antioxidant imbalance as a potential contributor to the progression of human pulmonary fibrosis. Antioxid Redox Signal. 2008;10:727–738. doi: 10.1089/ars.2007.1942. [DOI] [PubMed] [Google Scholar]
- 44.Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1989;139:370–372. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- 45.Yildirim Z, Kotuk M, Iraz M, Kuku I, Ulu R, Armutcu F, Ozen S. Attenuation of bleomycin-induced lung fibrosis by oral sulfhydryl containing antioxidants in rats: erdosteine and N-acetylcysteine. Pulm Pharmacol Ther. 2005;18:367–373. doi: 10.1016/j.pupt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Serrano-Mollar A, Closa D, Prats N, Blesa S, Martinez-Losa M, Cortijo J, Estrela JM, Morcillo EJ, Bulbena O. In vivo antioxidant treatment protects against bleomycin-induced lung damage in rats. Br J Pharmacol. 2003;138:1037–1048. doi: 10.1038/sj.bjp.0705138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li S, Yang X, Li W, Li J, Su X, Chen L, Yan G. N-acetylcysteine downregulation of lysyl oxidase activity alleviating bleomycin-induced pulmonary fibrosis in rats. Respiration. 2012;84:509–517. doi: 10.1159/000340041. [DOI] [PubMed] [Google Scholar]
- 48.Sato K, Tashiro Y, Chibana S, Yamashita A, Karakawa T, Kohrogi H. Role of lipid-derived free radical in bleomycin-induced lung injury in mice: availability for ESR spin trap method with organic phase extraction. Biol Pharm Bull. 2008;31:1855–1859. doi: 10.1248/bpb.31.1855. [DOI] [PubMed] [Google Scholar]
- 49.Farkas L, Gauldie J, Voelkel NF, Kolb M. Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am J Respir Cell Mol Biol. 2011;45:1–15. doi: 10.1165/rcmb.2010-0365TR. [DOI] [PubMed] [Google Scholar]
- 50.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122:2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheresh P, Kim SJ, Tulasiram S, Kamp DW. Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta. 2013;1832:1028–1040. doi: 10.1016/j.bbadis.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thannickal VJ, Horowitz JC. Evolving concepts of apoptosis in idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:350–356. doi: 10.1513/pats.200601-001TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallach-Dayan SB, Izbicki G, Cohen PY, Gerstl-Golan R, Fine A, Breuer R. Bleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathway. Am J Physiol Lung Cell Mol Physiol. 2006;290:L790–L796. doi: 10.1152/ajplung.00300.2004. [DOI] [PubMed] [Google Scholar]
- 54.Mungunsukh O, Griffin AJ, Lee YH, Day RM. Bleomycin induces the extrinsic apoptotic pathway in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2010;298:L696–L703. doi: 10.1152/ajplung.00322.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gnainsky Y, Kushnirsky Z, Bilu G, Hagai Y, Genina O, Volpin H, Bruck R, Spira G, Nagler A, Kawada N, et al. Gene expression during chemically induced liver fibrosis: effect of halofuginone on TGF-beta signaling. Cell Tissue Res. 2007;328:153–166. doi: 10.1007/s00441-006-0330-1. [DOI] [PubMed] [Google Scholar]
- 56.Jain M, Rivera S, Monclus EA, Synenki L, Zirk A, Eisenbart J, Feghali-Bostwick C, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling. J Biol Chem. 2013;288:770–777. doi: 10.1074/jbc.M112.431973. [DOI] [PMC free article] [PubMed] [Google Scholar]