Abstract
Rationale: Right heart failure is a cause of morbidity and mortality in common and rare heart and lung diseases. Exposure to traffic-related air pollution is linked to left ventricular hypertrophy, heart failure, and death. Relationships between traffic-related air pollution and right ventricular (RV) structure and function have not been studied.
Objectives: To characterize the relationship between traffic-related air pollutants and RV structure and function.
Methods: We included men and women with magnetic resonance imaging assessment of RV structure and function and estimated residential outdoor nitrogen dioxide (NO2) concentrations from the Multi-ethnic Study of Atherosclerosis, a study of individuals free of clinical cardiovascular disease at baseline. Multivariable linear regression estimated associations between NO2 exposure (averaged over the year prior to magnetic resonance imaging) and measures of RV structure and function after adjusting for demographics, anthropometrics, smoking status, diabetes mellitus, and hypertension. Adjustment for corresponding left ventricular parameters, traffic-related noise, markers of inflammation, and lung disease were considered in separate models. Secondary analyses considered oxides of nitrogen (NOx) as the exposure.
Measurements and Main Results: The study sample included 3,896 participants. In fully adjusted models, higher NO2 was associated with greater RV mass and larger RV end-diastolic volume with or without further adjustment for corresponding left ventricular parameters, traffic-related noise, inflammatory markers, or lung disease (all P < 0.05). There was no association between NO2 and RV ejection fraction. Relationships between NOx and RV morphology were similar.
Conclusions: Higher levels of NO2 exposure were associated with greater RV mass and larger RV end-diastolic volume.
Keywords: air pollutants, pulmonary circulation, heart ventricles, pulmonary hypertension
At a Glance Commentary
Scientific Knowledge on the Subject
Exposure to traffic-related air pollution has been linked to left ventricular hypertrophy, heart failure, and death. The lungs have substantial exposure to traffic-related pollutants; however, relationships between traffic-related air pollutants and right ventricular morphology have not been established.
What This Study Adds to the Field
Higher levels of traffic-related air pollution, estimated by exposure to oxides of nitrogen, are associated with greater right ventricular mass and larger volumes. This relationship was not dependent on differences in left ventricular mass or volumes, systemic inflammation, roadway noise, or lung disease.
Right heart failure is a cause of morbidity and mortality in obstructive and restrictive lung disease, left ventricular (LV) dysfunction, and pulmonary arterial hypertension (1–3). Right ventricular (RV) hypertrophy is also associated with increased risk for heart failure and cardiovascular death in community-dwelling adults without known cardiac disease at baseline (4). Despite important epidemiologic and clinical roles of the right ventricle, little is known about modifiable determinants of RV structure and function (5).
Traffic-related air pollution is linked to LV hypertrophy, heart failure, and cardiovascular death (6, 7). Air pollution may affect the left ventricle through inflammation, oxidative stress, and autonomic dysfunction and these mechanisms could also affect the right ventricle (8–10). The lungs have substantial exposure to traffic-related air pollution and inhalants, which may directly increase RV afterload and lead to disproportionately greater changes in the right ventricle compared with the left ventricle (11, 12). The impact of traffic-related air pollution on the right ventricle, however, is not well-studied.
We examined the relationship between nitrogen dioxide (NO2), a surrogate for traffic-related air pollution, and magnetic resonance imaging (MRI) measures of RV structure and function in a multiethnic cohort of adults free of clinical cardiovascular disease. We hypothesized that increased exposure to NO2 would be independently associated with greater RV mass and larger RV end-diastolic volume (RVEDV). Some of the results in these studies have been previously reported in the form of an abstract (13).
Methods
The Multi-ethnic Study of Atherosclerosis (MESA) is a multicenter prospective cohort study designed to investigate subclinical cardiovascular disease in whites, African-Americans, Hispanics, and Chinese-Americans (14). Exclusion criteria included clinical cardiovascular disease (physician-diagnosed heart attack, stroke, transient ischemic attack, heart failure, angina, current atrial fibrillation, any cardiovascular procedure), weight greater than 136 kg (300 lb), pregnancy, or impediment to long-term participation. The Environmental Protection Agency funded a large ancillary study to MESA, the Multi-ethnic Study of Atherosclerosis and Air Pollution (MESA Air), which added cohort-specific air pollution monitoring and modeling (15). The MESA-RV study was an ancillary study funded to interpret cardiac MRIs for RV function. Individual participants gave informed consent and the institutional review boards of participating institutions approved the protocols of MESA and all studies described herein.
Traffic-related Air Pollution Exposure
Participants’ residential address was assigned geographic coordinates using ArcGIS 9.1 software (ESRI, Redlands, CA) in conjunction with the Dynamap/2000 street network and geocoding database (Tele Atlas, Boston, MA). Using weighted averages of residential addresses over the year prior to cardiac MRI, individual outdoor home exposure to NO2 and NOx was estimated using spatiotemporal modeling and maximized by maximum likelihood (Figure 1) (16, 17). Estimates were fit using monitoring data from the Environmental Protection Agencies Air Quality System database and extensive cohort-specific air monitoring including home-based monitoring conducted as part of MESA Air (18). Geographic variables incorporated into the model included information on land use (e.g., industrial, residential); vegetative index; distance to various features (e.g., airports, coastline); road density; population density; elevation; urban topography; emissions sources; and dispersion model outputs integrating road position, traffic volume, diurnal traffic patterns, and meteorology.
Figure 1.
Representative map of Winston-Salem showing coarse and fine details of nitrogen dioxide predictions in parts per billion (ppb) from the spatiotemporal model including approximate Multi-ethnic Study of Atherosclerosis participant locations (jittered for privacy).
Cardiac MRI Measures
Methods for acquisition and interpretation of LV and RV MRI parameters have been previously reported (19, 20). Endocardial and epicardial borders of the RV were manually traced on short axis cine images at end-systole and end-diastole. The outflow tract was included in RV volume. Papillary muscles and trabeculae were included in RV volumes and excluded from RV mass, as is commonly done for LV mass (21, 22). RV end-systolic volume and RVEDV were calculated using Simpson's rule by summation of areas on each slice multiplied by the sum of slice thickness and image gap. RV mass was determined at end-diastole as the difference between RV free wall end-diastolic epicardial and endocardial volumes multiplied by the specific gravity of the heart (1.05 g/ml). RV ejection fraction was calculated by subtracting RV end-systolic volume from RVEDV and dividing this difference by RVEDV.
Covariables
Covariables including age, sex, race/ethnicity, height, weight, education, income, presence of hypertension or diabetes mellitus, fasting plasma glucose, cholesterol, systolic blood pressure, smoking status and pack-years, percent emphysema (obtained by chest computed tomography), and self-reported lung disease (asthma and/or emphysema) were measured as previously described (23). Because levels of air pollution within a neighborhood are correlated over time, self-reported time a participant lived in the index neighborhood (the residential neighborhood used to determine 1-year pollutant estimates) was used as a surrogate for exposure duration (8). Participants reported roadway noise as a “very serious problem,” “somewhat serious problem,” “minor problem,” or “not really a problem.”
Statistical Analysis
We used linear regression to characterize relationships between NO2 and RV parameters. All models were adjusted for height and weight, so it was not necessary to index RV parameters to account for differences in body size. Covariables were chosen a priori on the basis of known associations with ventricular size, heart disease, and comorbidities. In limited models, we adjusted for age, sex, race/ethnicity, height, and weight (24). In fully adjusted models, we also included MESA field center; markers of socioeconomic status (self-reported income and education); and cardiovascular risk factors including smoking status, smoking pack-years, hypertension, cholesterol, diabetes mellitus, and impaired glucose tolerance. In prespecified models, we further adjusted for LV parameters, self-reported roadway noise, markers of inflammation (C-reactive protein and interleukin-6), or lung structure (% emphysema) and self-reported lung disease in separate models.
The primary analysis examined the relationship between RV parameters and NO2 averaged over the year prior to cardiac MRI. Sensitivity analyses used fixed-year estimates of NO2 in 2000, 2001, and 2002 to ensure there was no error introduced by the timing of the MRI in relation to secular exposure trends. Secondary analyses in limited and fully adjusted models used NOx as the exposure of interest, which includes other components of the traffic-related air pollutant mix.
Several exploratory models further evaluated the relationship between NO2 and RV metrics. Duration and timing of exposure were considered using a sliding time window analysis (25). We estimated associations between NO2 and RV parameters in 5-year “time windows” (e.g., participants who lived in the index neighborhood for between 1 and 6 yr). The time window was then shifted by 1 year (e.g., participants who lived in the neighborhood between 2 and 7 yr) and new estimates of association and 95% confidence intervals (CI) were calculated. Overlapping 5-year periods avoid unstable estimates based on sparse data for a single calendar year and may more appropriately characterize the biologically relevant duration of exposure. Further exploratory models evaluated whether age, sex, or study site modified the association between NO2 and RV parameters. We performed sensitivity analyses adjusting for body mass index category (normal weight and category 1–3 overweight) instead of height and weight to evaluate for residual confounding by obesity. Analyses were performed using STATA 12.0 (StataCorp, College Station, TX).
Results
There were 6,814 men and women enrolled in MESA (see Figure E1 in the online supplement) of whom 5,098 underwent cardiac MRI and 5,004 (98%) had interpretable examinations for the left ventricle. Of 4,634 participants selected for MESA-RV, MRI reads were attempted in 4,484 participants before achieving the study goal of 4,204 participants (94% of attempted reads). Outdoor exposure to NO2 was estimated in 4,095 of these participants (97%). One hundred ninety-nine participants were excluded for missing covariables leaving 3,896 in the study sample. Table 1 shows characteristics of the study sample compared with those excluded. The mean age of the study sample was 61.4 years and 52.6% were women. Mean RV mass in the study sample was 21.1 ± 4.4 g, mean RVEDV was 124.2 ± 30.8 ml, and mean RV ejection fraction was 70.5 ± 6.4%. Mean NO2 was 21.8 ± 9.3 ppb with an interquartile range from 13.9 to 31.0 ppb. For individual cities the mean NO2 ranged from 10.1 to 32.7 ppb and the city-specific interquartile range ranged from 3.1 to 5.0 ppb (see Figure E2).
Table 1:
Characteristics of the Study Sample Compared with Excluded Participants
| Study Sample(n = 3,896) | Excluded (n = 2,918) | |
|---|---|---|
| Age, yr | 61.4 ± 10.1 | 63.2 ± 10.4 |
| Female, % | 52.6 | 53.2 |
| Race, % | ||
| White | 39.9 | 36.6 |
| Chinese | 12.5 | 10.8 |
| African-American | 25.6 | 30.6 |
| Hispanic | 22.0 | 22.0 |
| Height, cm | 166.4 ± 9.9 | 166.3 ± 10.2 |
| Weight, kg | 77.4 ± 16.2 | 80.3 ± 18.6 |
| Body mass index, kg/m2 | 27.8 ± 5.0 | 29.0 ± 6.0 |
| Educational attainment, % | ||
| No high school degree | 15.8 | 21.1 |
| High school degree | 18.1 | 18.3 |
| Some college | 16.1 | 16.7 |
| Bachelor's degree | 18.5 | 15.5 |
| Higher than bachelor's degree | 19.1 | 16.5 |
| Cigarette smoking status, % | ||
| Never | 52.6 | 47.3 |
| Former | 35.1 | 38.7 |
| Current | 12.4 | 14.0 |
| Pack-years of smoking | 10.8 ± 22.8 | 12.3 ± 21.4 |
| Hypertension, % | 42.5 | 48.4 |
| Systolic blood pressure, mm Hg | 125.3 ± 20.9 | 128.3 ± 22.1 |
| Diabetes mellitus, % | 12.3 | 15.3 |
| Fasting plasma glucose, mg/dl | 95.9 ± 28.2 | 99.3 ± 32.8 |
| Study Site, % | ||
| St. Paul | 16.0 | 15.2 |
| Los Angeles | 18.2 | 20.9 |
| Baltimore | 17.7 | 13.6 |
| Chicago | 14.1 | 21.1 |
| New York City | 20.3 | 10.7 |
| Winston-Salem | 13.8 | 18.5 |
| Stable residential neighborhood, % | ||
| >5 yr | 79.8 | 76.0 |
| >10 yr | 63.8 | 61.8 |
| NO2, ppb | 21.8 ± 9.3 | 21.8 ± 8.6* |
| NOx, ppb | 50.5 ± 26.9 | 50.4 ± 26.7* |
Definition of abbreviations: NO2 = nitrogen dioxide; ppb = parts per billion. Data are shown as mean ± SD or percent when appropriate.
A total of 1,055 participants with NO2 and NOx estimates not included in the study sample because of missing magnetic resonance imaging or covariables.
Higher NO2 was associated with greater RV mass (0.4 g for an interquartile increase in NO2) (Table 2). This relationship became stronger after adjustment for city (0.9 g for an interquartile increase in NO2) and after full adjustment for cardiovascular risk factors (1.0 g for an interquartile increase in NO2) (Figure 2). This amounted to an approximately 5% increase in RV mass for an interquartile increase in NO2. This significant association did not change with further adjustment for LV mass, traffic-related noise, inflammatory markers, or lung disease (Table 2; see Table E1).
Table 2:
Multivariable Linear Regression Estimating the Associations between NO2 Exposure and Right Ventricular Structure and Function
| Model | Per Interquartile Increase in NO2 |
||
|---|---|---|---|
| Difference | 95% CI | P Value | |
| RV mass, g | |||
| Limited model* | 0.4 | 0.2 to 0.7 | <0.001 |
| Limited model* + city | 0.9 | 0.3 to 1.4 | 0.002 |
| Full model† | 1.0 | 0.4 to 1.5 | 0.001 |
| Full model† + LV mass | 0.9 | 0.3 to 1.4 | 0.001 |
| RVEDV, ml | |||
| Limited model* | 2.9 | 1.4 to 4.7 | <0.001 |
| Limited model* + city | 2.7 | −0.9 to 6.2 | 0.14 |
| Full model† | 4.1 | 0.5 to 7.7 | 0.03 |
| Full model† + LVEDV | 2.7 | 0.0 to 5.4 | 0.05 |
| RVEF, % | |||
| Limited model* | −0.1 | −0.5 to 0.5 | 0.80 |
| Limited model* + city | −0.2 | −1.2 to 0.8 | 0.69 |
| Full model† | −0.2 | −1.2 to 0.8 | 0.72 |
| Full model† + LVEF | 0.0 | −1.0 to 0.9 | 0.92 |
Definition of abbreviations: CI = confidence interval; LV = left ventricular; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; NO2 = nitrogen dioxide; RV = right ventricular; RVEDV = right ventricular end-diastolic volume; RVEF = right ventricular ejection fraction.
Adjusted for age, sex, race/ethnicity, height, and weight.
Adjusted for age, sex, race/ethnicity, height, weight, city, education, income, smoking status, pack-years, hypertension, diabetes, cholesterol, and impaired glucose tolerance.
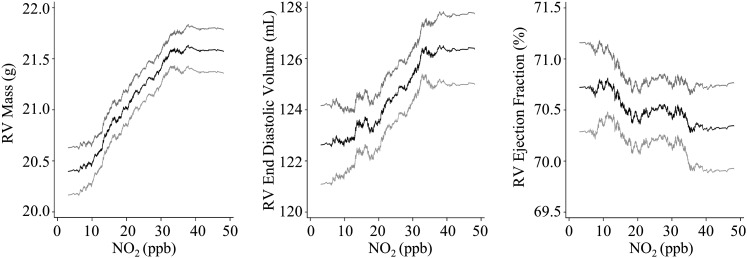
Figure 2.
Multivariable nonparametric smoothed relationship between nitrogen dioxide (NO2) in parts per billion (ppb) and right ventricular (RV) parameters with adjustment for age, sex, race/ethnicity, height, weight, city, education, income, smoking status, pack-years, hypertension, diabetes, cholesterol, and impaired glucose tolerance (black lines). Gray lines represent 95% confidence bounds.
Higher NO2 was associated with larger RVEDV (2.9 ml for an interquartile increase in NO2) (Table 2). This relationship became stronger after full adjustment for potential confounding by cardiovascular risk factors (4.1 ml for an interquartile increase in NO2) (Figure 2). This amounted to an approximately 3% increase in RVEDV for an interquartile increase in NO2. The significant association remained with further adjustment for LV end-diastolic volume, traffic-related noise, inflammatory markers, or lung disease (Table 2; see Table E1). NO2 was not associated with RV ejection fraction (Table 2, Figure 2).
Secondary analyses using NOx as the exposure of interest suggested relationships similar to those for NO2 but were in all cases modestly attenuated compared with NO2 (see Table E3). RVEDV was not consistently associated with NOx.
For participants with residential stability estimates (3,892 of 3,896 participants), sliding time window analyses indicated that participants who lived in the neighborhood several years before the MRI had incrementally stronger associations between NO2 and RV mass than did those who lived in the neighborhood for a shorter duration (Figure 3; see Table E2). An incremental increase in RVEDV with participant duration in the neighborhood was less clear (Figure 3; see Table E2). Choice of the NO2 reference period (calendar year 2000, 2001, or 2002) did not meaningfully impact the relationship between NO2 and RV parameters (see Table E4).
Figure 3.
Relationship between the number of years a participant lived in their neighborhood and the difference in right ventricular (RV) mass or end-diastolic volume (EDV) per interquartile increase in nitrogen dioxide (NO2): a sliding time window analysis of the full model. *P ≤ 0.05.
Participant age did not modify relationships between NO2 and RV parameters. The relationships of NO2 with RV mass may have been stronger in men (1.3 g [95% CI, 0.4 to 2.2 g] per interquartile increase in NO2) than women (0.6 g [95% CI, −0.1 to 1.3 g] per interquartile increase in NO2) (P for interaction = 0.03). Similarly, the relationship of NO2 with RVEDV may have been stronger in men (5.5 ml [95% CI, −0.3 to 11.2 ml] per interquartile increase in NO2) than women (2.1 ml [95% CI, −2.2 to 6.5 ml] per interquartile increase in NO2) (P for interaction = 0.04).
Participant city modified the relationship between NO2 and RV mass (P for interaction < 0.001), but not RVEDV (P for interaction = 0.33). Qualitative associations between NO2 and RV mass were in the same direction as the main association in St. Paul (6.4 g [95% CI, 4.1 to 8.8 g] per interquartile increase in NO2), Los Angeles (0.9 g [95% CI, −0.1 to 1.9 g] per interquartile increase in NO2), Baltimore (0.4 g [95% CI, −1.2 to 1.9 g] per interquartile increase in NO2), and Chicago (0.3 g [95% CI, −1.0 to 1.6 g] per interquartile increase in NO2). Qualitative associations were in the opposite direction as the main association in New York (−0.2 g [95% CI, −1.5 to 1.1 g] per interquartile increase in NO2) and Winston-Salem (−0.4 g [95% CI, −2.6 to 1.8 g] per interquartile increase in NO2). Because of the strong associations for St. Paul, we then excluded participants in cities with the greatest (St. Paul) and smallest (Winston-Salem) estimates of association between NO2 and RV mass. The estimate of association in this four-city sample was smaller but qualitatively similar to the main analysis (0.5 g [95% CI, −0.1 to 1.1 g] increase per interquartile increase in NO2; n = 2,738). Restricting this four-city sample to the sliding time window with the strongest association strengthened the relationship (1.3 g [95% CI, −0.1 to 2.7 g] increase per interquartile increase in NO2; n = 476).
A sensitivity analysis adjusting for body mass index category, instead of the standard adjustment by height and weight, did not change the results of any analysis.
Discussion
We have shown that higher estimates of long-term outdoor residential NO2 exposure are associated with greater RV mass and larger RVEDV in a multiethnic, multicity cohort of adults without clinical cardiovascular disease. MESA participants had a 1.0 g (5%) increase in RV mass and 4.1 ml (3%) increase in RVEDV with an interquartile increase in NO2. This difference in RV mass is quantitatively similar to that seen in LV mass in MESA participants with diabetes (2.4%) and in current smokers (5.3%), supporting biologic relevance (26, 27). RV hypertrophy in MESA participants is also associated with a three-fold increased risk of heart failure or cardiovascular death (4). This is the first report to suggest traffic-related air pollutants, of which NO2 is a well-recognized surrogate for the pollutant mix, is associated with morphologic changes in the right ventricle of the heart.
Our study provides initial insight into timing of this association. Duration of exposure to traffic-related air pollutants seems to be important. Participants who lived in the same neighborhood for several years had the strongest associations between NO2 and RV mass. This suggests a dose–response, may provide insight for duration of necessary exposure, and supports a causal relationship.
The finding of both increased RV mass and RVEDV may suggest that the exposure of interest increased RV afterload (28). Previous studies have suggested that air pollution increases endothelin-1, a potent pulmonary vasoconstrictor (29), which could lead to increased pulmonary vascular resistance, increased RV afterload, and ultimately RV hypertrophy and dilation. Alternatively, air pollutants can irritate the respiratory epithelium and lead to heterogeneous ventilation with decreased regional ventilation (30). Regional hypoxia can cause hypoxic pulmonary vasoconstriction, increased resistance, and RV enlargement (31). Increases in afterload may compound oxidative stress and autonomic dysfunction, which have been implicated in the relationship between air pollution and LV mass and could directly contribute to RV pathology (8–10, 32).
Other mechanisms are possible as well. Air pollution may up-regulate myocardial inflammatory genes and proteins in the RV (33). Although it is not feasible to study myocardial gene and protein profiles in such a large study of the general population, our findings remained after adjustment for C-reactive protein and interleukin-6 blood levels, which suggests that our findings were independent of systemic inflammation. Roadway noise, which accompanies traffic-related air pollution and may disrupt sleep, could mediate some aspects of the relationship between roadway proximity and heart disease (34). Adjusting for traffic-related noise did not attenuate relationships between NO2 and RV morphology in our analyses.
Air pollution has also been linked to obstructive lung disease severity, which could increase RV afterload leading to increased RV mass (35, 36). However, we have previously shown that increasing airflow obstruction is associated with decreased RVEDV in MESA (37). In addition, adjustment for structural or self-reported lung disease did not change relationships between NO2 and RV morphology in this analysis. Finally, LV mass may increase with traffic-related air pollution and LV hypertrophy can contribute to diastolic dysfunction and increased RV afterload, potentially explaining our results (6, 38). However, adjusting for the LV did not affect the results.
Relationships between NOx and RV morphology were similar, but mildly attenuated compared with those with NO2. In addition, the relationship between NOx and RVEDV was sensitive to adjustment. The NOx analyses reinforce that the observed relationships are consistent with associations of a pollutant mix, not a specific pollutant, and that relationships between these pollutants and RV mass are stronger than relationships with RVEDV.
The association between NO2 and RV mass was modified by city of residence. For example, participants in New York City did not seem to have a relationship between NO2 and RV mass despite the highest exposure to NO2. Heterogeneity by city is very common in air pollution research and was also seen in studies of LV mass and endothelial dysfunction (6, 39). Two key factors may contribute to city-specific heterogeneity. First, the validity of outdoor assessment of NO2 as a surrogate for individual exposure to traffic-related pollutants depends on the degree to which outdoor pollution contributes to indoor pollution (e.g., home infiltration coefficient, indoor sources) and the proportion of time a participant spends indoors, outdoors, and in different microenvironments (40). These complex relationships vary among cities as a function of culture, climate, cooking/ventilation patterns, and average building age, among other factors. Second, our estimates of NO2 are best conceptualized as a pattern of spatial decay consistent with some but not all traffic-related pollutants. For example, participants’ exposure to NO2 also reflects exposure to other hazardous air pollutants, such as benzene and several volatile organic compounds, levels of which may vary by city (41).
This study has limitations. Although we consider our exposure models to be a significant improvement over roadway proximity and nearest monitor analyses, measurement error and misclassification is likely present. Because error in exposure assignments is unlikely to be dependent on RV measurements, these errors may be nondifferential with bias toward the null, so actual relationships may even be stronger than we have shown. Residual or unmeasured confounding, particularly at the neighborhood level, could contribute to the results. This may be especially true in the adjustment for exposure duration because neighborhood residents with long-term stability may differ from short-term residents. In the adjustment for road noise, measured or modeled noise would have been preferable to self-reports, but was not available. Furthermore, our study was cross-sectional and causality cannot be confirmed. Finally, measurement of invasive pulmonary hemodynamics, which may have informed the mechanism underlying our results, was not feasible in almost 4,000 community-dwelling participants free of cardiovascular disease.
Conclusions
Higher estimated exposure to NO2 is associated with greater RV mass and larger RVEDV. This relationship is independent of markers of socioeconomic status, cardiovascular risk factors, left-sided cardiovascular disease, markers of inflammation, and lung disease. This is the first report to implicate traffic-related air pollution with changes in RV morphology. Air pollution may play a role in determining the RV response and outcomes in cardiopulmonary disease.
Acknowledgments
Acknowledgment
This manuscript was reviewed by the MESA Investigators for scientific content and consistency of data interpretation with previous MESA publications, and significant comments were incorporated before submission for publication. The authors thank the other investigators, staff, and participants of the MESA and MESA-Lung Studies for their valuable contributions. A full list of participating MESA Investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Supported by the National Institutes of Health (R01-HL086719, K24-HL103844, K24-ES013195, P30ES07033, R01-HL077612, N01-HC95159 through HC95165, N01-HC95169, and KL2TR000421). This publication was developed under a STAR research assistance agreement, No. RD831697 (MESA Air), awarded by the US Environmental protection Agency (EPA). It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors, and the EPA or National Institutes of Health do not endorse any products or commercial services mentioned in this publication.
Author Contributions: All authors participated in the conception and design of the research. J.D.K., C.L.C., A.A.S., V.C.V.H. developed the air pollution estimates. D.A.B. and J.A.L. oversaw the magnetic resonance imaging interpretation of right ventricular metrics. P.J.L. and S.M.K. analyzed and interpreted the data and drafted the report. All authors reviewed, revised, and approved the final version of the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Listen to accompanying podcast discussion at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201312-2298OC on March 4, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Thabut GG, Dauriat GG, Stern JB, Logeart D, Lévy A, Marrash-Chahla R, Mal H. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest. 2005;127:1531–1536. doi: 10.1378/chest.127.5.1531. [DOI] [PubMed] [Google Scholar]
- 2.Ghio SS, Gavazzi AA, Campana CC, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 3.D’Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;115:343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 4.Kawut SM, Barr RG, Lima JA, Praestgaard A, Johnson WC, Chahal H, Ogunyankin KO, Bristow MR, Kizer JR, Tandri H, et al. Right ventricular structure is associated with the risk of heart failure and cardiovascular death: the MESA-Right Ventricle Study. Circulation. 2012;126:1681–1688. doi: 10.1161/CIRCULATIONAHA.112.095216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, et al. National Heart, Lung, and Blood Institute Working Group on Cellular and Molecular Mechanisms of Right Heart Failure. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 6.Van Hee VC, Adar SD, Szpiro AA, Barr RG, Bluemke DA, Diez Roux AV, Gill EA, Sheppard L, Kaufman JD. Exposure to traffic and left ventricular mass and function: the Multi-Ethnic Study of Atherosclerosis. Am J Respir Crit Care Med. 2009;179:827–834. doi: 10.1164/rccm.200808-1344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 8.HEI Panel on the Health Effects of Traffic-Related Air PollutionTraffic-related air pollution: a critical review of the literature on emissions, exposure, and health effects. HEI Special Report 17. Boston, MA: Health Effects Institute; 2010 [Google Scholar]
- 9.Park SK, Auchincloss AH, O’Neill MS, Prineas R, Correa JC, Keeler J, Barr RG, Kaufman JD, Diez Roux AV. Particulate air pollution, metabolic syndrome, and heart rate variability: the multi-ethnic study of atherosclerosis (MESA) Environ Health Perspect. 2010;118:1406–1411. doi: 10.1289/ehp.0901778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mills NL, Amin N, Robinson SD, Anand A, Davies J, Patel D, de la Fuente JM, Cassee FR, Boon NA, Macnee W, et al. Do inhaled carbon nanoparticles translocate directly into the circulation in humans? Am J Respir Crit Care Med. 2006;173:426–431. doi: 10.1164/rccm.200506-865OC. [DOI] [PubMed] [Google Scholar]
- 12.Loennechen JP, Beisvag V, Arbo I, Waldum HL, Sandvik AK, Knardahl S, Ellingsen O. Chronic carbon monoxide exposure in vivo induces myocardial endothelin-1 expression and hypertrophy in rat. Pharmacol Toxicol. 1999;85:192–197. doi: 10.1111/j.1600-0773.1999.tb00091.x. [DOI] [PubMed] [Google Scholar]
- 13.Leary PJ, Barr RG, Bluemke JA, Hough CL, Kaufman JD, Szpiro AA, Kawut SM, Van Hee VC. The relationship of roadway proximity and NOx with right ventricular structure and function: the MESA-Right Ventricle and MESA-Air studies. Am J Respir Crit Care Med. 2013;187:A3976. [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR, Jr, Kronmal R, Liu K, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman JD, Adar SD, Allen RW, Barr RG, Budoff MJ, Burke GL, Casillas AM, Cohen MA, Curl CL, Daviglus ML, et al. Prospective study of particulate air pollution exposures, subclinical atherosclerosis, and clinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Am J Epidemiol. 2012;176:825–837. doi: 10.1093/aje/kws169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sampson PD, Szpiro AA, Sheppard L, Lindström J, Kaufman JD. Pragmatic estimation of a spatio-temporal air quality model with irregular monitoring data. Atmos Environ. 2011;45:6593–6606. [Google Scholar]
- 17.Szpiro AA, Sampson PD, Sheppard L, Lumley T, Adar SD, Kaufman JD. Predicting intraurban variation in air pollution concentrations with complex spatiotemporal dependencies. Environmetrics. 2010;21:606–631. doi: 10.1002/env.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen MA, Adar SD, Allen RW, Avol E, Curl CL, Gould T, Hardie D, Ho A, Kinney P, Larson TV, et al. Approach to estimating participant pollutant exposures in the Multi-Ethnic Study of Atherosclerosis and air pollution (MESA air) Environ Sci Technol. 2009;43:4687–4693. doi: 10.1021/es8030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chahal H, Johnson C, Tandri H, Jain A, Hundley WG, Barr RG, Kawut SM, Lima JA, Bluemke DA. Relation of cardiovascular risk factors to right ventricular structure and function as determined by magnetic resonance imaging (results from the multi-ethnic study of atherosclerosis) Am J Cardiol. 2010;106:110–116. doi: 10.1016/j.amjcard.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bluemke DA, Kronmal RA, Lima JA, Liu K, Olson J, Burke GL, Folsom AR. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel-Claussen J, Finn JP, Gomes AS, Hundley GW, Jerosch-Herold M, Pearson G, Sinha S, Lima JA, Bluemke DA. Left ventricular papillary muscle mass: relationship to left ventricular mass and volumes by magnetic resonance imaging. J Comput Assist Tomogr. 2006;30:426–432. doi: 10.1097/00004728-200605000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Winter MM, Bernink FJ, Groenink M, Bouma BJ, van Dijk AP, Helbing WA, Tijssen JG, Mulder BJ. Evaluating the systemic right ventricle by CMR: the importance of consistent and reproducible delineation of the cavity. J Cardiovasc Magn Reson. 2008;10:40. doi: 10.1186/1532-429X-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leary PJ, Barr RG, Bluemke DA, Bristow MR, Hough CL, Kronmal RA, Lima JA, McClelland RL, Tracy RP, Kawut SM. Von Willebrand factor and the right ventricle (the MESA-Right Ventricle study) Am J Cardiol. 2012;110:1846–1851. doi: 10.1016/j.amjcard.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawut SM, Lima JA, Barr RG, Chahal H, Jain A, Tandri H, Praestgaard A, Bagiella E, Kizer JR, Johnson WC, et al. Sex and race differences in right ventricular structure and function: the Multi-Ethnic Study of Atherosclerosis-Right Ventricle study. Circulation. 2011;123:2542–2551. doi: 10.1161/CIRCULATIONAHA.110.985515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauptmann M, Lubin JH, Rosenberg P, Wellmann J, Kreienbrock L. The use of sliding time windows for the exploratory analysis of temporal effects of smoking histories on lung cancer risk. Stat Med. 2000;19:2185–2194. doi: 10.1002/1097-0258(20000830)19:16<2185::aid-sim528>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Heckbert SR, Post W, Pearson GDN, Arnett DK, Gomes AS, Jerosch-Herold M, Hundley WG, Lima JA, Bluemke DA. Traditional cardiovascular risk factors in relation to left ventricular mass, volume, and systolic function by cardiac magnetic resonance imaging: the Multiethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;48:2285–2292. doi: 10.1016/j.jacc.2006.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sader S, Nian M, Liu P. Leptin: a novel link between obesity, diabetes, cardiovascular risk, and ventricular hypertrophy. Circulation. 2003;108:644–646. doi: 10.1161/01.CIR.0000081427.01306.7D. [DOI] [PubMed] [Google Scholar]
- 28.Leary PJ, Kurtz CE, Hough CL, Waiss MP, Ralph DD, Sheehan FH. Three-dimensional analysis of right ventricular shape and function in pulmonary hypertension. Pulm Circ. 2012;2:34–40. doi: 10.4103/2045-8932.94828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peretz A, Sullivan JH, Leotta DF, Trenga CA, Sands FN, Allen J, Carlsten C, Wilkinson CW, Gill EA, Kaufman JD. Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environ Health Perspect. 2008;116:937–942. doi: 10.1289/ehp.11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pietropaoli AP, Frampton MW, Hyde RW, Morrow PE, Oberdörster G, Cox C, Speers DM, Frasier LM, Chalupa DC, Huang LS, et al. Pulmonary function, diffusing capacity, and inflammation in healthy and asthmatic subjects exposed to ultrafine particles. Inhal Toxicol. 2004;16:59–72. doi: 10.1080/08958370490443079. [DOI] [PubMed] [Google Scholar]
- 31.Scherrer-Crosbie M, Steudel W, Hunziker PR, Foster GP, Garrido L, Liel-Cohen N, Zapol WM, Picard MH. Determination of right ventricular structure and function in normoxic and hypoxic mice: a transesophageal echocardiographic study. Circulation. 1998;98:1015–1021. doi: 10.1161/01.cir.98.10.1015. [DOI] [PubMed] [Google Scholar]
- 32.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest. 2009;135:794–804. doi: 10.1378/chest.08-0492. [DOI] [PubMed] [Google Scholar]
- 33.Villarreal-Calderon R, Dale G, Delgado-Chávez R, Torres-Jardón R, Zhu H, Herritt L, Gónzalez-Maciel A, Reynoso-Robles R, Yuan Y, Wang J, et al. Intra-city differences in cardiac expression of inflammatory genes and inflammasomes in young urbanites: a pilot study. J Toxicol Pathol. 2012;25:163–173. doi: 10.1293/tox.25.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim M, Chang SI, Seong JC, Holt JB, Park TH, Ko JH, Croft JB. Road traffic noise: annoyance, sleep disturbance, and public health implications. Am J Prev Med. 2012;43:353–360. doi: 10.1016/j.amepre.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Hansel NN, McCormack MC, Belli AJ, Matsui EC, Peng RD, Aloe C, Paulin L, Williams DL, Diette GB, Breysse PN. In-home air pollution is linked to respiratory morbidity in former smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:1085–1090. doi: 10.1164/rccm.201211-1987OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vonk-Noordegraaf A, Marcus JT, Holverda S, Roseboom B, Postmus PE. Early changes of cardiac structure and function in COPD patients with mild hypoxemia. Chest. 2005;127:1898–1903. doi: 10.1378/chest.127.6.1898. [DOI] [PubMed] [Google Scholar]
- 37.Grau M, Barr RG, Lima JA, Hoffman EA, Bluemke DA, Carr JJ, Chahal H, Enright PL, Jain A, Prince MR, et al. Percent emphysema and right ventricular structure and function: the Multi-Ethnic Study of Atherosclerosis-lung and Multi-Ethnic Study of Atherosclerosis-right ventricle studies. Chest. 2013;144:136–144. doi: 10.1378/chest.12-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, Baicu CF, Massie BM, Carson PE I-PRESERVE Investigators. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124:2491–2501. doi: 10.1161/CIRCULATIONAHA.110.011031. [DOI] [PubMed] [Google Scholar]
- 39.Krishnan RM, Adar SD, Szpiro AA, Jorgensen NW, Van Hee VC, Barr RG, O’Neill MS, Herrington DM, Polak JF, Kaufman JD. Vascular responses to long- and short-term exposure to fine particulate matter: MESA Air (Multi-Ethnic Study of Atherosclerosis and Air Pollution) J Am Coll Cardiol. 2012;60:2158–2166. doi: 10.1016/j.jacc.2012.08.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen RW, Adar SD, Avol E, Cohen M, Curl CL, Larson T, Liu LJ, Sheppard L, Kaufman JD. Modeling the residential infiltration of outdoor PM(2.5) in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Environ Health Perspect. 2012;120:824–830. doi: 10.1289/ehp.1104447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karner AA, Eisinger DS, Niemeier DA. Near-roadway air quality: synthesizing the findings from real-world data. Environ Sci Technol. 2010;44:5334–5344. doi: 10.1021/es100008x. [DOI] [PubMed] [Google Scholar]