Abstract
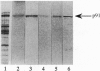
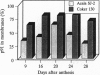
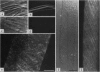
Sucrose synthase (SuSy; EC 2.4.1.13; sucrose + UDP reversible UDPglucose + fructose) has always been studied as a cytoplasmic enzyme in plant cells where it serves to degrade sucrose and provide carbon for respiration and synthesis of cell wall polysaccharides and starch. We report here that at least half of the total SuSy of developing cotton fibers (Gossypium hirsutum) is tightly associated with the plasma membrane. Therefore, this form of SuSy might serve to channel carbon directly from sucrose to cellulose and/or callose synthases in the plasma membrane. By using detached and permeabilized cotton fibers, we show that carbon from sucrose can be converted at high rates to both cellulose and callose. Synthesis of cellulose or callose is favored by addition of EGTA or calcium and cellobiose, respectively. These findings contrast with the traditional observation that when UDPglucose is used as substrate in vitro, callose is the major product synthesized. Immunolocalization studies show that SuSy can be localized at the fiber surface in patterns consistent with the deposition of cellulose or callose. Thus, these results support a model in which SuSy exists in a complex with the beta-glucan synthases and serves to channel carbon from sucrose to glucan.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrawis A., Solomon M., Delmer D. P. Cotton fiber annexins: a potential role in the regulation of callose synthase. Plant J. 1993 Jun;3(6):763–772. doi: 10.1111/j.1365-313x.1993.00763.x. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Carpita N. C., Delmer D. P. Concentration and metabolic turnover of UDP-glucose in developing cotton fibers. J Biol Chem. 1981 Jan 10;256(1):308–315. [PubMed] [Google Scholar]
- Carpita N. C., Gibeaut D. M. Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993 Jan;3(1):1–30. doi: 10.1111/j.1365-313x.1993.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Clarke S. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu Rev Biochem. 1992;61:355–386. doi: 10.1146/annurev.bi.61.070192.002035. [DOI] [PubMed] [Google Scholar]
- Delmer D. P., Amor Y. Cellulose biosynthesis. Plant Cell. 1995 Jul;7(7):987–1000. doi: 10.1105/tpc.7.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P., Ohana P., Gonen L., Benziman M. In Vitro Synthesis of Cellulose in Plants: Still a Long Way to Go! Plant Physiol. 1993 Oct;103(2):307–308. doi: 10.1104/pp.103.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P., Solomon M., Read S. M. Direct Photolabeling with [P]UDP-Glucose for Identification of a Subunit of Cotton Fiber Callose Synthase. Plant Physiol. 1991 Feb;95(2):556–563. doi: 10.1104/pp.95.2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P. The Regulatory Properties of Purified Phaseolus aureus Sucrose Synthetase. Plant Physiol. 1972 Oct;50(4):469–472. doi: 10.1104/pp.50.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P. The purification and properties of sucrose synthetase from etiolated Phaseolus aureus seedlings. J Biol Chem. 1972 Jun 25;247(12):3822–3828. [PubMed] [Google Scholar]
- Dietrich R. A., Delaney T. P., Uknes S. J., Ward E. R., Ryals J. A., Dangl J. L. Arabidopsis mutants simulating disease resistance response. Cell. 1994 May 20;77(4):565–577. doi: 10.1016/0092-8674(94)90218-6. [DOI] [PubMed] [Google Scholar]
- Elthon T. E., McIntosh L. Identification of the alternative terminal oxidase of higher plant mitochondria. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8399–8403. doi: 10.1073/pnas.84.23.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler C. H., Rao N. R., Roberts E. M., Huang J. Y., Upchurch D. R., Trolinder N. L. Cultured Ovules as Models for Cotton Fiber Development under Low Temperatures. Plant Physiol. 1991 Jan;95(1):88–96. doi: 10.1104/pp.95.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Read S. M., Bussell J., Thelen M., Lin F. C., Brown R. M., Delmer D. P. UDP-Glucose: (1-->3)-beta-Glucan Synthases from Mung Bean and Cotton: Differential Effects of Ca and Mg on Enzyme Properties and on Macromolecular Structure of the Glucan Product. Plant Physiol. 1987 Apr;83(4):1054–1062. doi: 10.1104/pp.83.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S. R., Northcote D. H. In vitro glucan synthesis by membranes of celery petioles: the role of the membrane in determining the type of linkage formed. J Cell Sci Suppl. 1985;2:1–11. doi: 10.1242/jcs.1985.supplement_2.1. [DOI] [PubMed] [Google Scholar]
- Kudlicka K., Brown R. M., Jr, Li L., Lee J. H., Shin H., Kuga S. [beta]-Glucan Synthesis in the Cotton Fiber (IV. In Vitro Assembly of the Cellulose I Allomorph). Plant Physiol. 1995 Jan;107(1):111–123. doi: 10.1104/pp.107.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lever M. A new reaction for colorimetric determination of carbohydrates. Anal Biochem. 1972 May;47(1):273–279. doi: 10.1016/0003-2697(72)90301-6. [DOI] [PubMed] [Google Scholar]
- Lin F. C., Brown R. M., Jr, Drake R. R., Jr, Haley B. E. Identification of the uridine 5'-diphosphoglucose (UDP-Glc) binding subunit of cellulose synthase in Acetobacter xylinum using the photoaffinity probe 5-azido-UDP-Glc. J Biol Chem. 1990 Mar 25;265(9):4782–4784. [PubMed] [Google Scholar]
- Maltby D., Carpita N. C., Montezinos D., Kulow C., Delmer D. P. beta-1,3-Glucan in Developing Cotton Fibers: Structure, Localization, and Relationship of Synthesis to That of Secondary Wall Cellulose. Plant Physiol. 1979 Jun;63(6):1158–1164. doi: 10.1104/pp.63.6.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinert M. C., Delmer D. P. Changes in biochemical composition of the cell wall of the cotton fiber during development. Plant Physiol. 1977 Jun;59(6):1088–1097. doi: 10.1104/pp.59.6.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow D. L., Lucas W. J. (1-->3)-beta-d-Glucan Synthase from Sugar Beet : I. Isolation and Solubilization. Plant Physiol. 1986 May;81(1):171–176. doi: 10.1104/pp.81.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana P., Delmer D. P., Volman G., Steffens J. C., Matthews D. E., Benziman M. beta-Furfuryl-beta-Glucoside: An Endogenous Activator of Higher Plant UDP-Glucose:(1-3)-beta-Glucan Synthase : Biological Activity, Distribution, and in Vitro Synthesis. Plant Physiol. 1992 Feb;98(2):708–715. doi: 10.1104/pp.98.2.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovádi J. Physiological significance of metabolic channelling. J Theor Biol. 1991 Sep 7;152(1):1–22. [PubMed] [Google Scholar]
- Ross P., Mayer R., Benziman M. Cellulose biosynthesis and function in bacteria. Microbiol Rev. 1991 Mar;55(1):35–58. doi: 10.1128/mr.55.1.35-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlupmann H., Bacic A., Read S. M. Uridine Diphosphate Glucose Metabolism and Callose Synthesis in Cultured Pollen Tubes of Nicotiana alata Link et Otto. Plant Physiol. 1994 Jun;105(2):659–670. doi: 10.1104/pp.105.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. R., Ferl R. J., Baier J., St Clair D., Carson C., McCarty D. R., Hannah L. C. Structural features of the maize sus1 gene and protein. Plant Physiol. 1994 Dec;106(4):1659–1665. doi: 10.1104/pp.106.4.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su J. C., Preiss J. Purification and properties of sucrose synthase from maize kernels. Plant Physiol. 1978 Mar;61(3):389–393. doi: 10.1104/pp.61.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias R. B., Boyer C. D., Shannon J. C. Alterations in Carbohydrate Intermediates in the Endosperm of Starch-Deficient Maize (Zea mays L.) Genotypes. Plant Physiol. 1992 May;99(1):146–152. doi: 10.1104/pp.99.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff D. M. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969 Dec;32(3):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- Wu A., Harriman R. W., Frost D. J., Read S. M., Wasserman B. P. Rapid Enrichment of CHAPS-Solubilized UDP-Glucose: (1,3)-beta-Glucan (Callose) Synthase from Beta vulgaris L. by Product Entrapment : Entrapment Mechanisms and Polypeptide Characterization. Plant Physiol. 1991 Oct;97(2):684–692. doi: 10.1104/pp.97.2.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Wasserman B. P. Limited proteolysis of (1,3)-beta-glucan (callose) synthase from Beta vulgaris L: topology of protease-sensitive sites and polypeptide identification using Pronase E. Plant J. 1993 Oct;4(4):683–695. doi: 10.1046/j.1365-313x.1993.04040683.x. [DOI] [PubMed] [Google Scholar]
- Xu D. P., Sung S. J., Loboda T., Kormanik P. P., Black C. C. Characterization of Sucrolysis via the Uridine Diphosphate and Pyrophosphate-Dependent Sucrose Synthase Pathway. Plant Physiol. 1989 Jun;90(2):635–642. doi: 10.1104/pp.90.2.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrenner R., Salanoubat M., Willmitzer L., Sonnewald U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). Plant J. 1995 Jan;7(1):97–107. doi: 10.1046/j.1365-313x.1995.07010097.x. [DOI] [PubMed] [Google Scholar]